Abstract
Purpose
To study the association of clinical factors and optical coherence tomography (OCT) retinal imaging with axial length (AL) and AL growth in preterm infants
Methods
Among a subgroup of infants from the prospective BabySTEPS study who were screened for retinopathy of prematurity (ROP) and had both AL measured and OCT imaging performed, we analyzed data collected prior to 42 weeks postmenstrual age (PMA) and prior to ROP treatment. Using linear mixed effects models, we evaluated associations between AL and AL growth with gestational age (GA), birthweight, PMA, sex, race, multiparity, maximum ROP stage, and OCT features.
Results
We included 66 infants (132 eyes), mean GA = 27.6 weeks (SD = 2.3; range: 23.0–34.4) and mean birthweight = 961 g (SD = 269, range: 490–1580). In the final predictive model, longer AL was associated with earlier GA, higher birthweight, later PMA, non-White race, and thicker subfoveal choroid (all p values ≤ 0.01). AL increased linearly up to 42 weeks PMA. There was no difference in AL growth rate by GA, sex, race, multiparity, maximum ROP severity, central foveal thickness, or subfoveal choroidal thickness (all p values > 0.05); but AL growth rate was slower in infants with lower birthweight (p = 0.01).
Conclusions
Among preterm infants, those with earlier GA, higher birthweight, later PMA, non-White race, and thicker subfoveal choroid had the longest AL. AL increased linearly up to 42 weeks PMA and lower birthweight was associated with slower AL growth. These findings may improve the accuracy of measurements taken on preterm infants using imaging techniques affected by AL (e.g., measuring lateral dimensions on OCT).
Trial registration https://clinicaltrials.gov/ct2/show/NCT02887157, date of registration: August 25, 2016
Keywords: Axial length, Axial length growth, Optical coherence tomography, Preterm infants, Premature infants, Retinopathy of prematurity
Introduction
Optical coherence tomography (OCT) imaging has revolutionized the practice of ophthalmology by providing high-resolution, cross-sectional images of the eye. OCT imaging is non-contact, does not require visible light, and is relatively quick to perform, making it well-suited for imaging infant eyes. While not routinely used to screen infants for retinopathy of prematurity (ROP), bedside OCT imaging of preterm infants has been able to elucidate findings not seen clinically using binocular indirect ophthalmoscopy and may help guide treatment decisions. Among preterm infants, OCT images have been shown to correlate with major histologic findings [1], to demonstrate delayed maturation of photoreceptors in very preterm compared to age-matched term infants [2], and to identify foveal involvement and help differentiate retinal detachment from retinoschisis among eyes with stage 4 ROP [3].
To increase the utility of OCT imaging, obtaining accurate measurements and understanding the relationships between eye size and ocular structures are important. Because axial length (AL) changes the magnification of lateral dimensions of retinal structures on OCT imaging [4], to calibrate OCT imaging to obtain accurate measurements, it is important to understand the “standard” AL and AL growth in eyes of preterm infants. It is known that AL increases with age, and the greatest increase in AL of the human eye occurs in the youngest age groups [5]. In studies of preterm infants in their first year of life, AL has been shown to have a linear growth pattern, both among eyes that do and do not develop ROP [6–9]. One study found that compared to age-matched full-term infants, the AL of preterm infants is shorter up to 7 years of age, but this difference diminished over time, and there was no difference by 8–10 years of age [10]. Previous studies among preterm infants have found associations between increasing AL and later gestational age (GA) [11, 12], higher birthweight [10–12], and male sex [8] but mixed results regarding an association between AL and higher stage/severity of ROP [7–9, 12, 13]. Among preterm infants, the relationship between AL and OCT measurements of ocular structures (e.g., subfoveal choroidal thickness and central foveal thickness) is unknown.
The purpose of this study was to evaluate the association of clinical factors (i.e., prematurity and severity of ROP) and OCT measurements of ocular structures (i.e., subfoveal choroidal thickness and central foveal thickness) with AL and AL growth in preterm infants.
Methods
This study was approved by the Duke Health System Institutional Review Board and adhered to the tenets of the Declaration of Helsinki.
This study was conducted as part of a prospective study, BabySTEPS (clinical trials.gov identifier: NCT02887157). As part of the BabySTEPS study, infants undergoing routine ROP screening in the neonatal intensive care unit at Duke University Hospital were prospectively enrolled, OCT images were collected, and in a subset of infants, ALs were measured on the same day. The imaging process and system used have been previously described [14]. Briefly, certified imagers imaged non-sedated infants lying supine using an investigational hand-held swept-source OCT system (Department of Biomedical Engineering, Duke University, Durham, NC) with one of two non-contact, ultra-compact (UC) probes: the UC2 captured 6.93 × 6.39 mm scans with 512 A-scans per each of 112 B-scans from September 13, 2016, to October 2, 2018, and the UC3 captured 10 × 10 mm scans with 1000 A-scans per each of 256 B-scans from October 9, 2018, onward. The center foveal scan for each eye was identified by a certified grader and segmented using a custom semi-automated proprietary infant-specific software, the Duke OCT Retinal Analysis Program Marking Code Baby version 2.0 (MATLAB R2017b; Mathworks, Natick, MA). After routine ROP screening was performed with binocular indirect ophthalmoscopy by a fellowship-trained pediatric ophthalmologist, an A-scan biometer (Sonomed Master-Vu® universal serial bus (USB) ultrasound system, Lake Success, NY) was used to measure the AL of both eyes. At the beginning of each day, prior to acquiring ALs, the A-scan was calibrated per the manufacturer’s operators’ manual. The 10 MHz A-scan probe was connected by a USB cable to a laptop computer. To measure AL, the manual mode was selected, and a sanitized A-scan probe was gently placed on the anesthetized infant’s cornea, along the visual axis until an image with a steeply rising retinal spike was captured. If more than one scan was captured, we used an average of the 2 scans.
For this study, we included all infants who had both OCT images acquired and ALs measured prior to 42 weeks postmenstrual age (PMA). Both eyes were included. We excluded any data collected ≥ 42 weeks PMA and any data collected after an eye was treated for ROP. We included infant demographics, AL, and the following OCT measurements: retinal thickness at the foveal center and subfoveal choroidal thickness (center 1 mm).
All statistical analysis was performed using R (v 3.5.1, R Foundation for Statistical Computing, Vienna, Austria). We evaluated the association between AL and each of the following variables: GA, birthweight, PMA at the time of OCT imaging and AL measurements, sex, race (White, non-White), multiparity (singleton, multiple gestation), highest ROP stage ever present (stages 0, 1, 2, ≥ 3), and OCT measurements (central foveal and subfoveal choroidal thicknesses) using linear mixed effect models, where random effects for subject and eye were used to account for inter-eye correlation within a subject and repeated measure correlation for an eye. Simple associations with AL were tested with a univariate model and a multivariate model adjusted for PMA. A final predictive model for AL was developed using the forward selection approach using a p < 0.05 significance level for covariate inclusion. We calculated AL growth rate by modeling PMA as a continuous variable and evaluated the effect of each covariate on AL growth rate using a model that included the covariate, PMA, and the interaction between them (to compare rates of AL growth across groups). Continuous covariates were divided into subsets to provide model estimates for AL at PMA = 40 weeks and AL growth rate (in mm/week) for each subset, while tests for statistical significance were performed using continuous covariates when applicable.
Results
Sixty-six infants (132 eyes) undergoing routine ROP screening fulfilled inclusion criteria for this study. Mean GA was 27.6 weeks (standard deviation (SD) = 2.3; range: 23.0–34.4), mean birthweight was 961 g (SD = 269, range: 490–1580), 50% were males, 50% were White, and 42.4% Black (Table 1). Four (6.1%) infants (8 eyes) were treated for ROP at a mean PMA = 36.2 weeks (SD 1.1; range: 35.3–38.0). The highest stage of ROP ever present prior to 42 weeks PMA was stage 0 in 45 (34%) eyes, stage 1 in 25 (19%) eyes, stage 2 in 48 (36%) eyes, stage 3 in 13 (10%) eyes, and stage 4 in 1 (0.8%) eye (Table 1).
Table 1.
Demographic and ocular characteristics of study population
| Infant Characteristics (n = 66) | ||
|---|---|---|
|
| ||
| n | % | |
| Gestational age (weeks) | ||
| 23 | 2 | 3.0 |
| 24 | 9 | 13.6 |
| 25 | 5 | 7.6 |
| 26 | 10 | 15.2 |
| 27 | 6 | 9.1 |
| 28 | 14 | 21.2 |
| 29 | 12 | 18.2 |
| 30 | 4 | 6.1 |
| > 30 | 4 | 6.1 |
| Birthweight (grams) | ||
| ≤ 750 | 19 | 28.8 |
| > 750 to ≤ 1000 | 17 | 25.8 |
| > 1000 to ≤ 1250 | 23 | 34.8 |
| > 1250 | 7 | 10.6 |
| Sex | ||
| Female | 33 | 50.0 |
| Male | 33 | 50.0 |
| Race | ||
| White | 33 | 50.0 |
| Black | 28 | 42.4 |
| Asian | 2 | 3.0 |
| Others | 3 | 4.5 |
| Multiparity | ||
| Singleton | 46 | 69.7 |
| Multiple gestation | 20 | 30.3 |
| Eye characteristics (n = 132) | ||
| Highest retinopathy of prematurity stage ever present before 42 weeks postmenstrual age | n | % |
| 0 | 45 | 34.1 |
| 1 | 25 | 18.9 |
| 2 | 48 | 36.4 |
| 3 | 13 | 9.8 |
| 4 | 1 | 0.8 |
| Treated for retinopathy of prematurity | 8 | 6.1 |
Each eye had AL measured longitudinally on average 4 times (SD = 2.3; range: 1–12). The mean PMA of the first and last AL measurements were 33.0 (SD = 2.0; range: 30.0–38.7) and 38.3 (SD = 2.8; range: 30.4–41.7) weeks, respectively. In the univariate analysis, longer AL was associated with later GA, higher birthweight, later PMA, highest ROP stage ever present, thicker central fovea, and thicker subfoveal choroid (all p values < 0.001, Table 2). When adjusted for PMA, longer AL was associated only with later GA (p = 0.03), higher birthweight (p < 0.001), non-White race (p = 0.014), and thicker subfoveal choroid (p < 0.001) (Table 2). In the final predictive model including GA, birthweight, PMA, race, and subfoveal choroidal thickness; longer AL was associated with earlier GA (p < 0.001), non-White race (p < 0.001), thicker subfoveal choroid (p = 0.003), higher birthweight (p = 0.001), and later PMA (p = 0.004), where the birthweight effect increases with later PMA (p = 0.01) (Table 3). For PMA < 42 weeks, AL can be estimated using our final predictive model by inputting GA, birthweight, PMA, race, and subfoveal choroidal thickness (Electronic supplementary information).
Table 2.
Modeling associations between axial length and various variables: univariate model and a model adjusted for postmenstrual age
| Variables | Effect/level | Univariate analysis |
Adjusted for postmenstrual age |
||
|---|---|---|---|---|---|
| Coefficient/mean Estimate (standard error) | p Value | Coefficient/mean estimate (standard error) | p Value | ||
| Clinical features | |||||
| Postmenstrual age (weeks) | Per week increase | 0.22 (0.01) | < 0.001 * | n/a | n/a |
| Gestational age (weeks) | Per week increase | 0.17 (0.03) | < 0.001 * | 0.07 (0.03) | 0.029 * |
| Birth-weight (grams) | Per 100 gram increase | 0.18 (0.02) | < 0.001 * | 0.14 (0.02) | < 0.001 * |
| Highest retinopathy of prematurity stage ever presenta | Stage 0 (reference) | 14.70 (0.11) | - | 15.18 (0.09) | - |
| Stage 1 | 15.34 (0.15) | < 0.001 * | 15.23 (0.11) | 0.65 | |
| Stage 2 | 15.74 (0.13) | < 0.001 * | 15.06 (0.10) | 0.23 | |
| ≥ Stage 3 | 16.03 (0.23) | < 0.001 * | 15.01 (0.19) | 0.39 | |
| Race | White (reference) | 14.96 (0.12) | - | 14.96 (0.11) | - |
| Non-White | 15.28 (0.12) | 0.072 | 15.34 (0.11) | 0.014 * | |
| Multiparity | Singleton (reference) | 15.04 (0.11) | - | 15.16 (0.09) | - |
| Multiple gestation | 15.28 (0.16) | 0.21 | 15.12 (0.14) | 0.78 | |
| Sex | Male (reference) | 15.05 (0.12) | - | 15.16 (0.11) | - |
| Female | 15.19 (0.13) | 0.45 | 15.13 (0.11) | 0.83 | |
| Optical coherence tomography features | |||||
| Central Foveal Thickness (μm) | Per 100μm increase | 0.38 (0.05) | < 0.001 * | -0.03 (0.05) | 0.54 |
| Subfoveal Choroidal Thickness (μm) | Per 100μm increase | 0.59 (0.06) | < 0.001 * | 0.20 (0.05) | <0.001 * |
n/a, not applicable (i.e. not included in the model). Bolded values(*) denote statistical significance at p < 0.05
Overall anova p values for retinopathy of prematurity stage for univariate and adjusted analyses are < 0.001 and 0.44, respectively
Table 3.
The final predictive model for axial lengtha
| Variables | Effect/level | Model coefficient (standard error) | p Valueb |
|---|---|---|---|
| Intercept | 15.29 (0.77) | < 0.001 * | |
| Gestational age (weeks) | Per week increase | − 0.12 (0.03) | < 0.001 * |
| Postmenstrual age (PMA, weeks) | Per week increase | 0.11 (0.04) | 0.004 * |
| Birth weight (grams) | Per 100 gram increase | 0.12 (0.04) | 0.001 * |
| PMA × birthweight (interaction term) | 0.01 (0.004) | 0.01 * | |
| Race | White (reference) | - | - |
| Non-White | 0.43 (0.11) | < 0.001 * | |
| Subfoveal Choroidal thickness (μm) | Per 100μm increase | 0.15 (0.05) | 0.003 * |
Bolded values(*) denote statistical significance at p < 0.05
PMA is normalized such that the model intercept is at 30 weeks PMA
All p values test whether coefficients are different from zero or not
Overall, AL increased linearly up to 42 weeks PMA. At PMA = 40 weeks, mean AL was 16.07 mm (95% confidence interval (CI): 15.89–16.24) and mean AL growth rate was 0.22 mm/week (95% CI: 0.20–0.24). In the model including the covariate, PMA, and their interaction (to compare rates of AL growth across groups), there was no statistically significant difference in rate of AL growth by GA (p = 0.14), sex (p = 0.14), highest ROP stage ever present (p = 0.08), race (p = 0.29), multiparity (p = 0.70), central foveal thickness (p = 0.26), or subfoveal choroidal thickness (p = 0.63); but there was slower AL growth for those with lower birthweight (p = 0.02) (Table 4, Fig. 1). While there was no statistically significant difference in rate of AL growth by highest ROP stage ever present (p = 0.08), the rate of AL growth for stage 0–2 ROP ranged from 0.20 to 0.26 mm/week, while the rate of AL growth for stage ≥ 3 ROP was 0.12 mm/week.
Table 4.
Axial length and rate of axial length growth at a postmenstrual age of 40 weeks by subsets of various variablesa
| Variable | Subsets | Intercept (95% CI) | Slope (95% CI) | p Valueb |
|---|---|---|---|---|
| Gestational age (weeks) | 23–26 | 15.66 (15.39–15.93) | 0.20 (0.18–0.23) | 0.14 |
| 27–28 | 16.37 (16.07–16.67) | 0.25 (0.21–0.28) | ||
| ≥ 29 | 16.26 (15.95–16.56) | 0.23 (0.18–0.28) | ||
| Birth weight (grams) | ≤ 750 | 15.44 (15.17–15.71) | 0.19 (0.16–0.22) | 0.02 * |
| > 750 to ≤ 1000 | 16.09 (15.80–16.39) | 0.24 (0.20–0.28) | ||
| > 1000 to ≤ 1250 | 16.34 (16.08–16.61) | 0.23 (0.19–0.27) | ||
| > 1250 | 16.97 (16.43–17.51) | 0.28 (0.19–0.37) | ||
| Highest retinopathy of prematurity stage ever present | Stage 0 | 16.32 (16.04–16.61) | 0.26 (0.23–0.30) | 0.08 |
| Stage 1 | 16.04 (15.70–16.38) | 0.20 (0.15–0.26) | ||
| Stage 2 | 15.95 (15.73–16.16) | 0.21 (0.17–0.25) | ||
| ≥ Stage 3 | 15.93 (15.57–16.30) | 0.12 (− 0.04–0.28) | ||
| Sex | Male | 16.15 (15.89–16.40) | 0.24 (0.21–0.26) | 0.14 |
| Female | 15.99 (15.74–16.24) | 0.21 (0.18–0.24) | ||
| Race | White | 15.91 (15.68–16.15) | 0.23 (0.21–0.26) | 0.29 |
| Non-White | 16.20 (15.95–16.45) | 0.21 (0.18–0.24) | ||
| Multiparity | Singleton | 16.07 (15.85–16.29) | 0.22 (0.20–0.25) | 0.70 |
| Multiple gestation | 16.05 (15.75–16.35) | 0.23 (0.20–0.26) | ||
| Central foveal thickness (μm) | ≤ 0.156 | 16.30 (16.04–16.57) | 0.26 (0.21–0.30) | 0.26 |
| > 0.156–0.212 | 15.99 (15.67–16.31) | 0.21 (0.17–0.26) | ||
| > 0.212–0.312 | 15.99 (15.68–16.31) | 0.23 (0.17–0.28) | ||
| > 0.312 | 15.96 (15.72–16.20) | 0.19 (0.14–0.25) | ||
| Subfoveal choroidal thickness (μm) | ≤ 0.164 | 15.79 (15.47–16.12) | 0.20 (0.16–0.25) | 0.63 |
| > 0.164–0.216 | 15.90 (15.63–16.18) | 0.21 (0.17–0.25) | ||
| > 0.216–0.290 | 15.93 (15.70–16.16) | 0.21 (0.16–0.26) | ||
| > 0.290 | 16.24 (16.01–16.46) | 0.19 (0.14–0.24) |
CI confidence interval
Estimates from model included covariate of interest, postmenstrual age, and the interaction between them.
p Value comparing differences in rate of axial length growth across levels of each parameter, bolded values(*) denote statistical significance at p < 0.05.
Fig. 1.
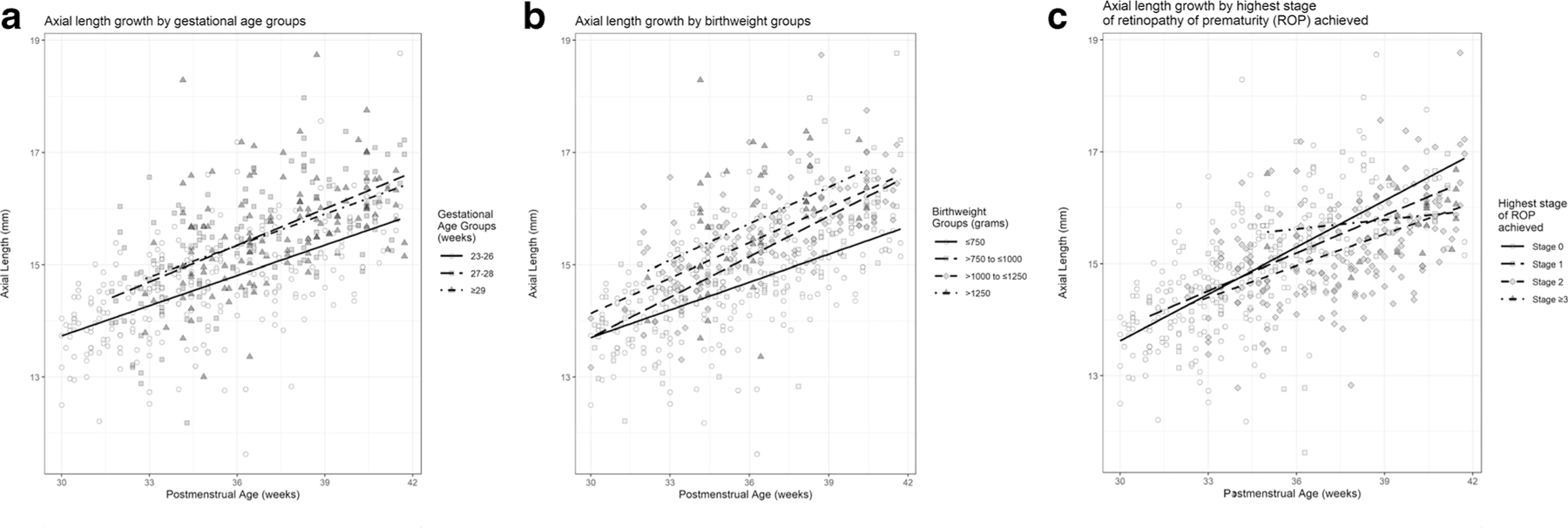
Rate of axial length growth by gestational age, birthweight, and highest retinopathy of prematurity (ROP) stage (a) There is no difference in the rate of axial length growth between various subsets of gestational age (p = 0.14). (b) There is a statistically significant difference in the rate of axial length growth between subsets of birthweight, where the rate of axial length growth is slower for those with lower birthweight (p = 0.02). Due to a significant interaction between postmenstrual age and birthweight (p = 0.011), the birthweight effect increases with later postmenstrual age. (c) While there was no statistically significant difference in the rate of axial length growth by highest ROP stage ever present (p = 0.08), the rate of axial length growth for stage 0–2 ROP ranged from 0.20 to 0.26mm/week, while the rate of axial length growth for stage ≥ 3 ROP was 0.12 mm/week. All graphs include the interaction term of each variable with postmenstrual age
Discussion
We found that among preterm infants at risk for ROP, longer AL was associated with earlier GA, non-White race, a thicker subfoveal choroid, higher birthweight, and later PMA, where the birthweight effect increases with later PMA. We have developed an online calculator to estimate AL from the inputs of GA, birthweight, PMA, race, and subfoveal choroidal thickness. We found that up to 42 weeks PMA, AL increased linearly, and there was no difference in AL growth rate by GA, highest ROP stage, sex, race, multiparity, central foveal thickness, or subfoveal choroidal thickness; but those with lower birthweight had slower AL growth.
GA is associated with AL. This association held true even after adjusting for PMA and creating our final predictive model, which also included birthweight, race, and subfoveal choroidal thickness. Interestingly, in both the univariate analysis and the model adjusted for PMA, later GA was associated with longer AL; however, in our final predictive model, earlier GA was associated with longer AL. While our data supports the positive association between GA and AL, the negative association we found in our final predictive model could be due to multicollinearity, i.e., more than one variable in our final model is highly linearly related, for example, GA is highly correlated to birthweight. The likely positive association between GA and AL is supported by other studies which have found AL to be positively associated with GA in young children [11, 12].
Higher birthweight is associated with longer AL. This association held true even after adjusting for PMA and creating our final predictive model, which also included GA, race, and subfoveal choroidal thickness. Interestingly, our final predictive model included an interaction term between PMA and birthweight, meaning that the birthweight effect increases with later PMA. Our finding is supported by other studies which have found AL to be positively associated with birthweight in young children, including former preterm infants with and without ROP and term infants [10–12].
We found that among preterm infants, non-White race (predominantly Black in our study) is associated with longer AL. This association held true when adjusting for PMA and creating our final predictive model, which also included GA, birthweight, and subfoveal choroidal thickness. We included race in our analysis because severe ROP is more commonly seen in Whites than in Blacks [15–18]. Other studies looking at AL among preterm infants did not report the race distribution of their study populations, so we cannot directly compare our results to those of other studies [6–9]. As our study is the first to report an association between AL and race, this finding should be validated in future studies.
While a previous study has reported an association between AL and sex, we did not find this association. One study found that among preterm infants, males had longer ALs even after adjusting for birthweight, PMA, and individual variation in number of AL readings [8].
There are mixed published findings with regard to an association between AL and ROP severity. One study that looked at infants with a PMA from 28 to 51 weeks found that only between a PMA of 34–37 weeks did those with ROP compared to without ROP have a shorter AL (evaluation between AL and ROP severity was not reported) [9]. Another study found that even after controlling for PMA, birthweight, sex, variable numbers of AL readings, and head diameter, eyes with more severe ROP had shorter ALs [8]. In contrast, another study found that when the effects of GA and birthweight were accounted for, there was no difference in AL between eyes with different ROP stages [7]. An additional study also found no difference in AL between ROP stages, except for longer AL found among stage 3 eyes that received cryotherapy (but not laser therapy) [13]. In a study of 5–15-year-olds, there was no difference in AL between preterm children with versus without a history of ROP, but preterm children (with or without a history of ROP) had a shorter AL compared to their age-matched full-term controls [19]. Among myopic adults, those with a history of ROP (compared to those born full-term) have been shown to have a shorter AL [20]. In our univariate analysis, having developed a higher ROP stage was associated with a shorter AL, but this association was not significant when adjusted for PMA, and its association did not reach a level of significance level to be included in our final predictive model.
When adjusted for PMA, subfoveal choroidal thickness (as measured by OCT) in preterm infants lying supine is associated with AL, while central foveal thickness is not. The association between those with a thicker subfoveal choroid having a longer AL held true both when adjusting for PMA and when creating our final predictive model, which also included GA, birthweight, and non-White race. Our current findings are supported by our previous study, using spectral domain OCT, in which subfoveal choroidal thickness increased from 31 to 42 weeks PMA among preterm infants [21]. Our findings contrast with multiple studies that demonstrate an inverse correlation between choroidal thickness and AL in children; however it is important to recognize that none of these prior studies included children under 5 years of age. Among Chinese children 7–13 years old, one study found that choroidal, but not retinal, thickness was closely correlated with AL, where thinner central foveal choroid was associated with longer AL [22]. Following these children over the next year, the authors did not find an association between increasing AL and choroidal thinning but did find that increasing AL and choroidal thinning were associated with a myopic shift [23]. An additional study among Chinese school children 6–19 years old found in multiple regression analysis that thinner central foveal choroid was associated with younger age, longer AL, and higher myopia [24]. Evidence suggests that changes in AL and biometric measures occur most rapidly within 12 months prior and following myopia onset [25]. Another study found that among children 10–15 years old, overall there was an increase in subfoveal choroidal thickness longitudinally over 18 months, although there was a significant association between the change in choroidal thickness and the change in AL over time where those with faster AL growth had less choroidal thickening over time [26]. In a study of choroidal structure in 5–15-year-olds, despite a difference in AL between preterm children (with or without a history of ROP) and their age-matched full-term controls, they found no significant difference in subfoveal choroidal thickness between these groups and no association between subfoveal choroidal thickness and axial length [19]. Our study differs from the previous studies in that we are the first study to look at associations between OCT measurements and AL of preterm infants very early in life (i.e., prior to 42 weeks PMA). Thus, our data differ from and are not comparable to published data from older (i.e., 5–19 years old) children at a timepoint at which a myopic shift may be occurring. We believe that at this very early age, which is prior to the onset of myopic shift, a thicker subfoveal choroid is associated with longer axial length.
Among preterm infants at risk for ROP, AL has consistently been reported to increase linearly over time up to 52 weeks PMA [6–9], even though the reported rate of AL growth has varied between studies. Like previous studies, we found AL to increase linearly up to 42 weeks PMA. Among studies that included infants with and without ROP, the average rate of AL growth ranged from 0.16 to 0.18 mm/week [7–9]. Overall, our study found an average rate of AL growth of 0.22 mm/week.
The rate of AL growth appears slower with lower birthweight and may be associated with increasing ROP stage/severity. Two previous studies among preterm infants have looked at AL growth across groups (i.e., by sex or highest ROP stage). One study which looked at a similar time frame (PMA = 32–41 weeks) as our study did not find a difference in AL growth rate by sex or highest ROP stage (but did see a trend for slower growth among eyes that attained stage 3 ROP compared to no ROP) [8]. Another study, which evaluated infants from 32 to 52 weeks PMA, noted that AL growth rate differed between ROP stages, where infants that developed threshold disease and were lasered had the slowest AL growth rate [7]. While we did not find a statistically significant difference in rate of AL growth by highest ROP stage ever present (p = 0.08), the rate of AL growth for those that attained stage ≥ 3 ROP was almost half of that for those that attained stage 0–2 ROP (i.e,. 0.12 mm/week versus 0.20–0.26 mm/week, respectively). We suspect the low number of eyes with severe ROP in our study limited our statistical power. While both previous studies [7, 8] and our results indicate that a higher ROP stage (in particular stage 3 or higher) is likely associated with slower AL growth, it is important to keep in mind that both of the previous studies included eyes after they were treated for ROP, whereas our study did not. While we found no difference in the rate of AL growth by GA, ROP stage, sex, race, multiparity, central foveal thickness, or subfoveal choroidal thickness, we did find a slower rate of AL growth for those with lower birthweight (p = 0.015). As our study is the first to report a slower rate of AL growth for eyes among preterm infants with lower birthweight, this finding should be validated in future studies.
Our study had limitations. Because our analysis included only data collected up to 42 weeks PMA, our results are not generalizable beyond this age. Because AL measurements were performed using a contact method (versus immersion), we cannot rule out some measurement variability despite attempting not to place extra pressure on the eye, inadvertently decreasing AL. In addition, average AL measurements may be more accurate than single measurements. While we did not routinely perform multiple AL measurements at each examination, as was the norm in other studies [6–9], our study coordinator was trained to capture images only when she noted a steeply rising retinal spike. While some OCT imaging sessions were excluded due to image quality insufficient for choroid measurements, the imaging was performed with an investigational swept-source OCT centered on 1050-nm wavelength which gave better penetration to the outer choroid margin, thus allowing better resolution (i.e., more precise measurements). Because we excluded eyes after receiving treatment for ROP, we could not characterize AL growth following treatment for ROP.
Conclusion
Among preterm infants, those with higher birthweight, non-White (in particular Black) race, and thicker subfoveal choroid by OCT have the longest AL. AL increases linearly up to 42 weeks PMA, and the AL growth rate is not affected by sex, ROP stage, race, multiparity, central foveal thickness, or subfoveal choroidal thickness; but those with lower birthweight have a slower AL growth rate. These findings may improve the accuracy of measurements taken on preterm infants using imaging techniques affected by AL (e.g., measuring lateral dimensions on OCT).
Supplementary Material
Key Messages:
Previous studies among preterm infants have shown that axial length increases linearly in the first year of life and have found associations between longer axial length and later gestational age, higher birthweight, and male sex.
We found that among preterm infants, in addition to higher birthweight, longer axial length was associated with non-White race and a thicker subfoveal choroid as measured by optical coherence tomography.
We found that among preterm infants, lower birthweight was associated with a slower rate of axial length growth.
Acknowledgements
The authors thank Katrina P. Winter, BS for performing segmentation correction and helping with data analysis.
Funding
This study was supported by funding from the National Institutes of Health (NIH) Grants R01 EY025009 and P30 EY005722. Contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the NIH. The sponsors or funding organizations had no role in the design or conduct of this research.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00417-021-05158-4.
Availability of data and materials A summary, de-identified data set will be made available upon request through direct inquiries to the study principal investigator (C.A.T.) or the coordinating center a year after relevant print publication.
Code availability Proprietary software application
Declarations
Ethics approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Duke Health System Institutional Review Board (IRB number: Pro00069721). The study is listed at ClinicalTrials.gov (NCT 02887157).
Consent to participate Written informed consent was obtained from a parent/legal guardian of all individual participants prior to study participation.
Consent for publication Written informed consent was obtained from a parent/legal guardian of all individual participants prior to study participation.
Conflict of interest Dr. Toth receives royalties through her university from Alcon. She is a co-founder and equity owner of Theia Imaging, LLC (Chapel Hill, NC). Through her university, she also has unlicensed and pending patents regarding OCT technology and methods.
References
- 1.Vajzovic L, Hendrickson AE, O’Connell RV, Clark LA, Tran-Viet D, Possin D, Chiu SJ, Farsiu S, Toth CA (2012) Maturation of the human fovea: correlation of spectral-domain optical coherence tomography findings with histology. Am J Ophthalmol 154:779–89 e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vajzovic L, Rothman AL, Tran-Viet D, Cabrera MT, Freedman SF, Toth CA (2015) Delay in retinal photoreceptor development in very preterm compared to term infants. Invest Ophthalmol Vis Sci 56: 908–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X, Prakalapakorn SG, Freedman SF, Vajzovic L, Toth CA. (2019) Differentiating retinal detachment and retinoschisis using handheld optical coherence tomography in stage 4 retinopathy of prematurity. JAMA Ophthalmol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linderman R, Salmon AE, Strampe M, Russillo M, Khan J, Carroll J (2017) Assessing the accuracy of foveal avascular zone measurements using optical coherence tomography angiography: segmentation and scaling. Transl Vis Sci Technol 6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon RA, Donzis PB (1985) Refractive development of the human eye. Arch Ophthalmol 103:785–789 [DOI] [PubMed] [Google Scholar]
- 6.Cook A, White S, Batterbury M, Clark D (2003) Ocular growth and refractive error development in premature infants without retinopathy of prematurity. Invest Ophthalmol Vis Sci 44:953–960 [DOI] [PubMed] [Google Scholar]
- 7.Cook A, White S, Batterbury M, Clark D (2008) Ocular growth and refractive error development in premature infants with or without retinopathy of prematurity. Invest Ophthalmol Vis Sci 49:5199–5207 [DOI] [PubMed] [Google Scholar]
- 8.Laws DE, Haslett R, Ashby D, O’Brien C, Clark D (1994) Axial length biometry in infants with retinopathy of prematurity. Eye (Lond) 8(Pt 4):427–430 [DOI] [PubMed] [Google Scholar]
- 9.Ozdemir O, Ozen Tunay Z, Erginturk AD (2016) Growth of biometric components and development of refractive errors in premature infants with or without retinopathy of prematurity. Turk J Med Sci 46:468–473 [DOI] [PubMed] [Google Scholar]
- 10.Fieß A, Kolb-Keerl R, Knuf M, Kirchhof B, Blecha C, Oberacher-Velten I, Muether PS, Bauer J (2017) Axial length and anterior segment alterations in former preterm infants and full-term neonates analyzed with scheimpflug imaging. Cornea 36:821–827 [DOI] [PubMed] [Google Scholar]
- 11.Ozdemir M, Koylu S (2009) Ocular growth and morbidity in preterm children without retinopathy of prematurity. Jpn J Ophthalmol 53:623–628 [DOI] [PubMed] [Google Scholar]
- 12.Zhu X, Zhao R, Wang Y, Ouyang L, Yang J, Li Y, Pi L (2017) Refractive state and optical compositions of preterm children with and without retinopathy of prematurity in the first 6 years of life. Medicine (Baltimore) 96:e8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kent D, Pennie F, Laws D, White S, Clark D (2000) The influence of retinopathy of prematurity on ocular growth. Eye (Lond) 14(Pt 1):23–29 [DOI] [PubMed] [Google Scholar]
- 14.Mangalesh S, McGeehan B, Tai V, Chen X, Tran-Viet D, Vajzovic L, Viehland C, Izatt JA, Cotten CM, Freedman SF, Maguire M, Toth CA, Baby SG. (2020) Macular optical coherence tomography characteristics at 36 weeks postmenstrual age in infants examined for retinopathy of prematurity. Ophthalmol Retina [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Husain SM, Sinha AK, Bunce C, Arora P, Lopez W, Mun KS, Reddy MA, Adams GG (2013) Relationships between maternal ethnicity, gestational age, birth weight, weight gain, and severe retinopathy of prematurity. J Pediatr 163:67–72 [DOI] [PubMed] [Google Scholar]
- 16.Ng YK, Fielder AR, Shaw DE, Levene MI (1988) Epidemiology of retinopathy of prematurity. Lancet 2:1235–1238 [DOI] [PubMed] [Google Scholar]
- 17.Saunders RA, Donahue ML, Christmann LM, Pakalnis AV, Tung B, Hardy RJ, Phelps DL (1997) Racial variation in retinopathy of prematurity. The cryotherapy for retinopathy of prematurity cooperative group. Arch Ophthalmol 115:604–608 [DOI] [PubMed] [Google Scholar]
- 18.Tadesse M, Dhanireddy R, Mittal M, Higgins RD (2002) Race, Candida sepsis, and retinopathy of prematurity. Biol Neonate 81: 86–90 [DOI] [PubMed] [Google Scholar]
- 19.Lavric A, Tekavcic Pompe M, Markelj S, Ding J, Mahajan S, Khandelwal N, Agrawal R (2019) Choroidal structural changes in preterm children with and without retinopathy of prematurity. Acta Ophthalmol [DOI] [PubMed] [Google Scholar]
- 20.Baker PS, Tasman W (2008) Myopia in adults with retinopathy of prematurity. Am J Ophthalmol 145:1090–1094 [DOI] [PubMed] [Google Scholar]
- 21.Moreno TA, O’Connell RV, Chiu SJ, Farsiu S, Cabrera MT, Maldonado RS, Tran-Viet D, Freedman SF, Wallace DK, Toth CA (2013) Choroid development and feasibility of choroidal imaging in the preterm and term infants utilizing SD-OCT. Invest Ophthalmol Vis Sci 54:4140–4147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin P, Zou H, Zhu J, Xu X, Jin J, Chang TC, Lu L, Yuan H, Sun S, Yan B, He J, Wang M, He X (2016) Choroidal and retinal thickness in children with different refractive status measured by swept-source optical coherence tomography. Am J Ophthalmol 168: 164–176 [DOI] [PubMed] [Google Scholar]
- 23.Jin P, Zou H, Xu X, Chang TC, Zhu J, Deng J, Lv M, Jin J, Sun S, Wang L, He X (2019) Longitudinal changes in choroidal and retinal thicknesses in children with myopic shift. Retina 39:1091–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong S, He X, Deng J, Lv M, Jin J, Sun S, Yao C, Zhu J, Zou H, Xu X (2017) Choroidal thickness in 3001 Chinese children aged 6 to 19 years using swept-source OCT. Sci Rep 7:45059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiang F, He M, Morgan IG (2012) Annual changes in refractive errors and ocular components before and after the onset of myopia in Chinese children. Ophthalmology 119:1478–1484 [DOI] [PubMed] [Google Scholar]
- 26.Read SA, Alonso-Caneiro D, Vincent SJ, Collins MJ (2015) Longitudinal changes in choroidal thickness and eye growth in childhood. Invest Ophthalmol Vis Sci 56:3103–3112 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


