Abstract
Influenza pandemics, defined as global outbreaks of the disease due to viruses with new antigenic subtypes, have exacted high death tolls from human populations. The last two pandemics were caused by hybrid viruses, or reassortants, that harbored a combination of avian and human viral genes. Avian influenza viruses are therefore key contributors to the emergence of human influenza pandemics. In 1997, an H5N1 influenza virus was directly transmitted from birds in live poultry markets in Hong Kong to humans. Eighteen people were infected in this outbreak, six of whom died. This avian virus exhibited high virulence in both avian and mammalian species, causing systemic infection in both chickens and mice. Subsequently, another avian virus with the H9N2 subtype was directly transmitted from birds to humans in Hong Kong. Interestingly, the genes encoding the internal proteins of the H9N2 virus are genetically highly related to those of the H5N1 virus, suggesting a unique property of these gene products. The identification of avian viruses in humans underscores the potential of these and similar strains to produce devastating influenza outbreaks in major population centers. Although highly pathogenic avian influenza viruses had been identified before the 1997 outbreak in Hong Kong, their devastating effects had been confined to poultry. With the Hong Kong outbreak, it became clear that the virulence potential of these viruses extended to humans.
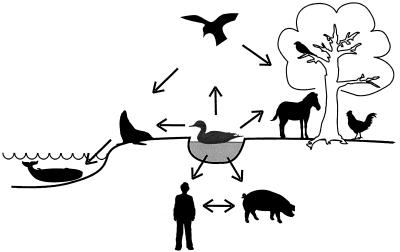
Influenza A viruses have been isolated from a variety of animals, including humans, pigs, horses, sea mammals, and birds 201 (Fig. 1). Phylogenetic studies of these viruses have revealed species-specific lineages of viral genes, as well as genes that have crossed species barriers. Such studies have also revealed that aquatic birds are the source of all influenza viruses in other animal species. Influenza viruses do not cause lethal disease in wild aquatic birds, indicating that they have achieved an optimal level of adaptation in this natural reservoir. Avian influenza viruses have been transmitted to pigs, horses, and even sea mammals. Although humans and nonhuman primates can be experimentally infected with avian viruses 14, 124, the limited viral replication in these hosts has led to the notion that avian influenza viruses are not directly transmitted to humans in nature.
FIG. 1.
“Habitat” of influenza A viruses. Ecological and phylogenetic studies suggest that wild waterfowl are the principal reservoirs for influenza A viruses, which occasionally are transmitted to other host animals such as horses, pigs, and chickens, leading to influenza outbreaks among these species. Some of the viruses may become established in these new hosts and cause epidemics and epizootics. Viruses are transmitted among these new host animals (e.g., between humans and pigs or between chickens and humans, as occurred in 1997 in Hong Kong).
Influenza pandemics, defined as global outbreaks of the disease due to viruses with new antigenic subtypes, have exacted a high death toll from human populations. The most devastating pandemic, the so-called Spanish influenza of 1918 to 1919, resulted from an H1N1 virus and caused the deaths of at least 20 million people worldwide 39. Other much less catastrophic pandemics occurred in 1957 (Asian influenza [H2N2 virus]), 1968 (Hong Kong influenza [H3N2 virus]), and 1977 (Russian influenza [H1N1 virus]) 125, 149. It is noteworthy that both the Asian and Hong Kong outbreaks were caused by hybrid viruses, or reassortants, that harbored a combination of avian and human viral genes. Avian influenza viruses are therefore key contributors to the emergence of human influenza pandemics.
In May 1997, an H5N1 influenza virus was isolated from a 3-year-old boy in Hong Kong 222, who died of extensive influenza pneumonia complicated by Reye's syndrome. By the end of 1997, a total of 18 cases of human influenza had been identified, all caused by the same H5N1 virus. Six of the patients died 31. H5 influenza virus had never been isolated from humans, raising concern over the possibility of a major influenza pandemic among the world's immunologically naive populations. The H5N1 isolates were not reassortants like the 1957 and 1968 pandemic strains; instead, all of the viral genes had originated from an avian virus 34, 184.
Although highly pathogenic avian influenza viruses had been identified before the 1997 outbreak in Hong Kong, their devastating effects had been confined to poultry. With the Hong Kong outbreak, it became clear that the virulence potential of these viruses extended to humans. Here we review current knowledge on the ecology, interspecies transmission, and pathogenicity of avian influenza viruses and discuss the human health threats posed by these pathogens.
CLASSIFICATION
Influenza viruses, which belong to the Orthomyxoviridae family, are classified as A, B, and C based on antigenic differences in their nucleoprotein (NP) and matrix (M1) protein. All avian influenza viruses are classified as type A. Further subtyping is based on the antigenicity of two surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA). Currently, 15 HA and 9 NA subtypes have been identified among influenza A viruses 125, 159. The amino acid sequences of the HA1 region, which is responsible for HA antigenicity, differ from subtype to subtype by 30% or more 159.
Although viruses with all HA and NA subtypes are found in avian species, viral subtypes of mammalian influenza viruses are limited (Table 1). Those which have circulated widely in humans are restricted to three HA and two NA subtypes (i.e., H1, H2, and H3 and N1 and N2 subtypes). Most of the human influenza A viruses are classified as H1N1, H2N2 (which circulated from 1957 to 1968 only), and H3N2; however, H1N2 reassortant viruses have circulated in humans in China 66. Pigs are susceptible to all subtypes of avian influenza viruses in experimental settings 95, but only the H1 and H3 and N1 and N2 subtypes, including a unique H1N2 reassortant, have been isolated from pigs in nature, with the exception of a recent H1N7 isolate 20, 21. Two different subtypes of influenza A viruses (H7N7 and H3N8) have been isolated from horses; the former virus is commonly known as equine type 1, and the latter is known as equine type 2. Both viruses produce similar disease signs in horses, but the infections produced by equine type 2 virus are typically more severe. Although equine type 1 virus has not been isolated from horses since 1978, serological surveys indicate that it may still be circulating in some parts of the world 199. The limited array of HA and NA subtypes that infect mammalian hosts suggests that still obscure genetic and biological factors determine the subtype specificities of influenza A viruses in nature.
TABLE 1.
Representative strains of each HA and NA subtype of influenza A virus found in different animal species
| Subtype | Strain found ina:
|
|||
|---|---|---|---|---|
| Humans | Swine | Horses | Birds | |
| HA | ||||
| H1 | A/Puerto Rico/8/34 (H1N1) | A/Swine/Iowa/15/30 (H1N1) | A/Duck/Alberta/35/76 (H1N1) | |
| H2 | A/Singapore/1/57 (H2N2) | A/Duck/Germany/1215/73 (H2N3) | ||
| H3 | A/Hong Kong/1/68 (H3N2) | A/Swine/Taiwan/70 (H3N2) | A/Equine/Miami/1/63 (H3N8) | A/Duck/Ukraine/1/63 (H3N8) |
| H4 | A/Duck/Czechoslovakia/56 (H4N6) | |||
| H5 | [A/Hong Kong/156/97 (H5N1)] | A/Tern/South Africa/61 (H5N3) | ||
| H6 | A/Turkey/Massachusetts/3740/65 (H6N2) | |||
| H7 | [A/England/268/96 (H7N7)] | A/Equine/Prague/1/56 (H7N7) | A/Fowl plague virus/Dutch/27 (H7N7) | |
| H8 | A/Turkey/Ontario/6118/68 (H8N4) | |||
| H9 | A/Turkey/Wisconsin/1/66 (H9N2) | |||
| H10 | A/Chicken/Germany/N/49 (H10N7) | |||
| H11 | A/Duck/England/56 (H11N6) | |||
| H12 | A/Duck/Alberta/60/76 (H12N5) | |||
| H13 | A/Gull/Maryland/704/77 (H13N6) | |||
| H14 | A/Duck/Gurjev/263/82 (H14N?) | |||
| H15 | A/Duck/Australia/341/83 (H15N?) | |||
| NA | ||||
| N1 | A/Puerto Rico/8/34 (H1N1) | A/Swine/Iowa/15/30 (H1N1) | A/Chicken/Scotland/59 (H5N1) | |
| N2 | A/Singapore/1/57 (H2N2) | A/Swine/Taiwan/70 (H3N2) | A/Turkey/Massachusetts/3740/65 (H6N2) | |
| N3 | A/Tern/South Africa/61 (H5N3) | |||
| N4 | A/Turkey/Ontario/6118/68 (H8N4) | |||
| N5 | A/Shearwater/Australia/1/72 (H6N5) | |||
| N6 | A/Duck/Czechoslovakia/56 (H4N6) | |||
| N7 | [A/England/268/96 (H7N7)] | [A/Swine/England/191973/92 (H1N7)] | A/Equine/Prague/1/56 (H7N7) | A/Fowl plague virus/Dutch/27 (H7N7) |
| N8 | A/Equine/Miami/1/63 (H3N8) | A/Duck/Ukraine/1/63 (H3N8) | ||
| N9 | A/Duck/Memphis/546/74 (H11N9) | |||
Viruses in brackets did not establish in the hosts.
The nomenclature system applied to influenza viruses includes the host of origin (excluding humans), geographical site of origin, strain number, and year of isolation. The antigenic description of the HA and NA is given last, in parentheses [e.g., A/Chicken/Pennsylvania/1370/83 (H5N2), A/Hong Kong/156/97 (H5N1)].
HISTORY AND PROPERTIES
History
The “fowl plague,” now known to be caused by highly pathogenic avian influenza viruses, was first described in 1878 145 as a disease affecting chickens in Italy. The causative agent was eventually isolated from a chicken in 1902 [A/Chicken/Brescia/1902 (H7N7)], marking the first documented isolation of influenza virus. Similar outbreaks were observed in Europe and then worldwide, with subsequent isolation of several fowl plague viruses (H7 subtypes). By contrast, the first human influenza virus was not isolated until 1933 178. Propagation of the virus in embryonated chicken eggs greatly improved the efficiency of virus isolation, allowing the preparation of high-titer virus stocks 24. In 1941, Hirst 75 discovered the hemagglutination activity of influenza virus, and in 1955, Schafer 164 demonstrated that fowl plague virus was a member of the influenza A virus group. The emergence of human pandemic strains in 1957 and in 1968 prompted extensive study of the ecology of influenza viruses in animals as a means of gaining insight into the origin of pandemic strains 84. During this surveillance, many nonpathogenic avian influenza A viruses were isolated from avian species, including wild birds, captive and caged birds, and domestic ducks, chickens, and turkeys 74, 177. These isolations led to the realization that such viruses are widely distributed among birds and probably ubiquitous in waterfowl. Highly pathogenic strains belonging to H5 subtypes were subsequently found in chickens in Scotland [A/Chicken/Scotland/59 (H5N1)] and terns [A/Tern/South Africa/61 (H5N3)] 15.
These findings defined two types of avian influenza A viruses based on their virulence: a highly virulent type that causes fowl plagues, and an avirulent type that causes only mild disease or asymptomatic infection. In rare instances, however, viruses with low pathogenicity in the laboratory cause outbreaks of severe disease in the field. Such viruses are economically important. For example, in Minnesota in 1978, over 140 turkey flocks were infected with influenza virus of low pathogenicity, resulting in an estimated loss of over 5 million dollars 148. Nonetheless, the morbidity and mortality associated with these viruses tend to be much lower than those caused by lethal viruses.
Over the last 30 years, highly virulent avian influenza viruses have caused outbreaks in poultry in Australia (1976 [H7] 7, 1985 [H7] 40, 131, 1992 [H7] 144, 1995 [H7], and 1997 [H7]), England (1979 [H7] 218 and 1991 [H5] 1), the United States (1983 to 1984 [H5] 44), Ireland (1983 to 1984 [H5] 88), Germany (1979 [H7] 158), Mexico (1994 to 1995 [H5] 49, 79), Pakistan (1995 [H7] 144), Italy (1997 [H5]), and Hong Kong (1997 [H5] 34). These events clearly demonstrate that, without exception, all of the pathogenic avian influenza A viruses are of the H5 or H7 subtype, although the reason for this subtype specificity remains unknown. There appears to be no association of NA subtypes with virulent viruses. Two additional subtypes, H4 [A/Chicken/Alabama/7395/75 (H4N8) 83] and H10 [A/Chicken/Germany/N/49 (H10N7)], were isolated from chickens during severe fowl plague-like outbreaks; however, these viruses are not lethal to experimentally infected chickens. In 1997, a fowl plague outbreak occurred in Hong Kong and was subsequently linked to human infections due to the same avian virus (see below).
Biological Properties
Viral proteins.
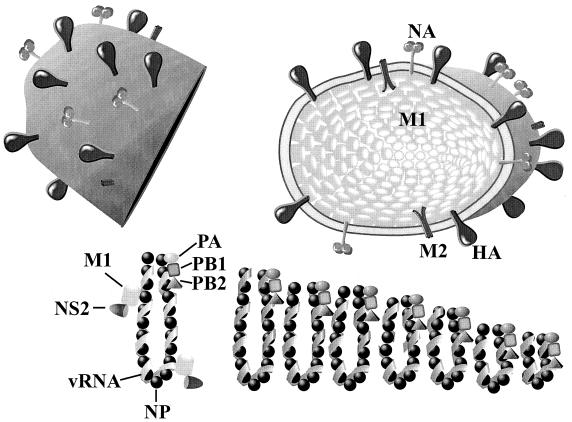
The structures of influenza A viruses are quite similar (reviewed in reference 109. By electron microscopy, the viruses are pleomorphic, including virions that are roughly spherical (approximately 120 nm in diameter) and filamentous. Two distinct types of spikes (approximately 16 nm in length), corresponding to the HA and NA molecules, reside on the surface of the virions. The HA spike appears rod shaped and protrudes from the envelope as a trimer 216; the NA spike is a mushroom-shaped tetramer 36. These two glycoproteins are anchored to the lipid envelope derived from the plasma membrane of host cells by short sequences of hydrophobic amino acids (transmembrane region) (Fig. 2). HA is a type I glycoprotein (containing an N-terminal ectodomain and a C-terminal anchor), while NA is a type II glycoprotein (containing an N-proximal anchor and a C-terminal ectodomain). HA enables the virion to attach to cell surface sialyloligosaccharides 140 and is responsible for its hemagglutinating activity 75. HA elicits virus-neutralizing antibodies that are important in protection against infection. NA is a sialidase 57 that prevents virion aggregation by removing cell and virion surface sialic acid (the primary moiety in sialyloligosaccharides recognized by HA) 140. Antibodies to NA are also important in protecting hosts 209.
FIG. 2.
Structure of influenza A virus virions. Two glycoprotein spikes, HA and NA, and the M2 protein are embedded in the lipid bilayer derived from the host plasma membrane. The RNP consists of a viral RNA segment associated with the NP and the three polymerase proteins (PA, PB1, and PB2). The M1 protein is associated with both RNP and the viral envelope, while NS2 is associated with RNP through interaction with M1. NS1 is the only nonstructural protein of influenza A virus.
In addition to HA and NA, a limited number of M2 proteins are integrated into the virions 223. They form tetramers, have H+ ion channel activity, and, when activated by the low pH in endosomes, acidify the inside of the virion, facilitating its uncoating 146. M1 protein that lies within the envelope is thought to function in assembly and budding. Eight segments of single-stranded RNA molecules (negative sense, or complementary to mRNA) are contained within the viral envelope, in association with NP and three subunits of viral polymerase (PB1, PB2, and PA), which together form a ribonucleoprotein (RNP) complex that participates in RNA replication and transcription. NS2 protein, now known to exist in virions 152, 220, is thought to play a role in the export of RNP from the nucleus 137 through interaction with M1 protein 198. NS1 protein, the only nonstructural protein of influenza A viruses, has multiple functions, including regulation of splicing and nuclear export of cellular mRNAs as well as stimulation of translation (reviewed in reference 109. Its major function seems to be to counteract the interferon activity of the host, since an NS1 knockout virus was viable although it grew less efficiently than the parent virus in interferon-nondefective cells 50.
Life cycle.
After binding to sialic acid-containing receptors on the membrane surface, the virus enters the cell by receptor-mediated endocytosis. A low pH in the endosome induces a conformational change in HA 23, resulting in membrane fusion between the viral envelope and the endosomal membrane. Within the endosome, the M2 proton channel exposes the viral core to low pH, resulting in dissociation of M1 from RNP and leading to a release of RNP to the cytoplasm. RNP is then transported to the nucleus, most probably by nuclear localization signals in proteins composed of the RNP complex (PB1 129, PB2 122, and NP 132, 197).
The mechanism of viral RNA transcription is unique. The 5′ cap from cellular mRNAs is cleaved by a viral endonuclease and used as a primer for transcription by the viral transcriptase 106, 147. Six of eight RNA segments are transcribed into mRNAs in a monocistronic manner and translated into HA, NA, NP, PB1, PB2, and PA. By contrast, two RNA segments are each transcribed to two mRNAs by splicing (reviewed in reference 109. For both the M and NS genes, these mRNAs are translated in different reading frames, generating M1 and M2 proteins and NS1 and NS2 proteins, respectively. It is believed that the increased concentration of free NP triggers the shift from mRNA synthesis to cRNA and vRNA synthesis 174. Newly synthesized vRNAs are encapsidated with NP in the nucleus, where they function as templates for secondary transcription of viral mRNAs.
Later in infection, the principal translation products are M1, HA, and NA. HA and NA are glycosylated in the rough endoplasmic reticulum, further processed in the Golgi apparatus, and then transported to the cell surface, where they integrate into the cell membrane. Nuclear localization of M1 and NS2 proteins is essential for the migration of RNP out of the nucleus for assembly into progeny viral particles in the cytoplasm 114, 137. The RNP-M1 complex presumably interacts with M1 proteins that are associated with the plasma membrane and then buds outward through the cell membrane, enclosing itself within a bubble of membrane (envelope) studded with both the HA and NA glycoproteins.
Antigenic Variation
The antigenicity of influenza viruses changes gradually by point mutation (antigenic drift) or drastically by genetic reassortment (antigenic shift) 125. Immunological pressure on HA and NA is thought to drive antigenic drift. Such changes in the antigenicity of human influenza virus necessitate the replacement of vaccine strains every several years. Antigenic drift has also been detected among avian influenza viruses, but to a lesser extent than in human viruses 4, 96, possibly because of limited immunological pressure in short-lived birds. Studies with the human H3 virus HA revealed that only a single point mutation in the antigenic site can alter the structure of this glycoprotein, leading to antigenic variation 214–216.
Antigenic shift is caused by either direct transmission of nonhuman influenza viruses to humans or the reassortment of genes from two different influenza viruses that have infected a single cell 208. Theoretically, 256 different combinations of RNA can be produced from the shuffling of the eight different genomic segments of the virus. Genetic reassortment is well documented both in vitro and in vivo under laboratory conditions 207. More importantly, mixed infections occur relatively frequently in nature and can lead to genetic reassortment 9, 72, 221. Reemergence of a previously circulating virus is another mechanism by which antigenic shift can occur. For example, the H1N1 Russian influenza virus, which was circulating in the 1950s 127, 171, subsequently reemerged in 1977 in human populations that were immunologically naive to this subtype of virus, especially younger persons, who had not been infected with H1N1 virus in the past 107.
Reservoirs and Evolutionary Pathways
All 15 HA and 9 NA subtypes of influenza A virus are maintained in aquatic bird populations, mainly ducks, shorebirds, and gulls 201. In waterfowl, influenza viruses replicate preferentially in the intestinal tract, resulting in excretion of high-titer viruses in the feces. Thus, transmission of influenza viruses via the fecally contaminated water-oral route is a major mechanism of virus dissemination among aquatic birds.
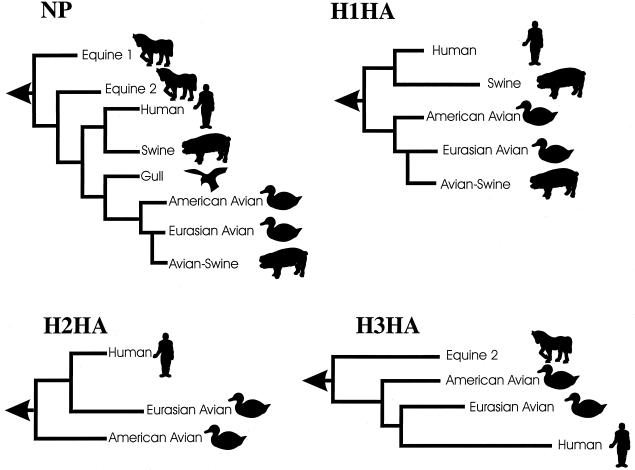
Phylogenetic analyses of the NP genes from a variety of hosts inhabiting different geographical regions show that influenza A viruses have evolved into seven host-specific lineages: two in horses (old [equine 1] and recent [equine 2]), one in gulls, one in North American birds, one in Eurasian birds (including avian-like swine virus), one in swine, and one in humans 53 (Fig. 3). The human and classical swine viruses form a genetic “sister group” relationship, based on a common ancestor that appears to be directly derived from an avian virus.
FIG. 3.
Phylogenetic relationships among influenza A virus genes. These generalized phylogenetic trees are derived from references 53 (NP, rooted to influenza virus NP), 80 (H1 HA, rooted to H2 HA), 164 (H2 HA, rooted to H5 HA), and 11 (H3 HA, rooted to H4 HA). Horizontal distances are proportional to the number of nucleotide differences needed to join the gene sequences, while vertical lines are used for spacing branches and labels. The arrow at the left of each tree represents the node connecting the influenza B virus homologue. Equine 1, H7N7 subtype (e.g., A/Equine/Prague/56); Equine 2, H3N8 subtype (e.g., A/Equine/Miami/63); Human NP, H1N1, H2N2 and H3N2 subtypes; Swine, classic swine viruses of the H1N1 subtype (e.g., A/Swine/Iowa/15/30); Gull, H13 gull viruses; Avian-Swine, European swine viruses derived from an avian virus.
Studies of each gene segment in avian species have revealed two geographically separate sublineages (Eurasian and American) 42 (Fig. 3), indicating that birds migrating between the Northern and Southern Hemispheres play a key role in the transmission of avian influenza viruses. Influenza viruses in aquatic birds appear to be approaching or to have reached an optimal state of adaptation, wherein amino acid changes provide no selective advantage 53. These observations, together with the fact that waterfowl infected with influenza viruses rarely show disease signs, indicate that influenza viruses have achieved evolutionary equilibrium in these birds, making them the ultimate natural reservoir.
PATHOGENESIS
Infection and Transmission in Natural Hosts
Many domestic and wild avian species are infected with influenza viruses. These include chickens, turkeys, ducks, guinea fowl, domestic geese, quail, pheasants, partridge, mynah birds, passerines, psittacines, budgerigars, gulls, shorebirds, seabirds, and emu (reviewed in references 43 and 204. Some infected birds show symptoms of influenza, while others do not. Among domestic avian species, turkeys are the most frequently involved in outbreaks of influenza; chickens have also been involved but less frequently.
Avian influenza A viruses produce an array of syndromes in birds, ranging from asymptomatic to mild upper respiratory infections to loss of egg production to rapidly fatal systemic disease 43. The severity of disease depends on multiple factors, including the virulence of the virus, the immune status and diet of the host, accompanying bacterial infections, and stresses imposed on the host. Depending on their pathogenicity in chickens and turkeys, avian influenza A viruses are classified as virulent (capable of causing fowl plague) or avirulent (causing mild or asymptomatic disease). Most avian influenza viruses isolated in the field are avirulent; virulent viruses have never been isolated from apparently healthy waterfowl, with the exception of pathogenic isolates collected from ducks or geese near a chicken influenza outbreak 88, 158.
Even when highly pathogenic for one avian species, influenza A viruses may not be pathogenic for another avian species 2. In fact, ducks are typically resistant to viruses that are lethal in chickens. For example, A/Turkey/Ireland/1378/85 (H5N8), which readily kills chickens and turkeys, does not cause disease symptoms in ducks, even though it can be detected in a variety of internal organs and in the blood of infected birds 88. Host factors determining susceptibility to influenza viruses remain to be identified.
Influenza viruses are secreted from the intestinal tract into the feces of infected birds 98, 210. The modes of transmission can be either direct or indirect; they include contact with aerosol and other virus-contaminated materials. Since infected birds excrete large amounts of virus in their feces, many different items can become contaminated (e.g., feed, water, equipment, and cages) and contribute to dissemination of the virus. Waterborne transmission may provide a mechanism for the year-to-year perpetuation of avian influenza viruses in natural waterfowl habitats. Avian influenza viruses were readily isolated from Alaskan lakes that serve as breeding sites for migrating waterfowl 81.
Pathology
The typical signs and symptoms manifested by poultry infected with highly pathogenic avian influenza viruses include decreased egg production, respiratory signs, rales, excessive lacrimation, sinusitis, cyanosis of unfeathered skin (especially the combs and wattles), edema of the head and face, ruffled feathers, diarrhea, and nervous system disorders (Table 2). The number of presenting features depends on the species and age of the bird, the strain of virus, and accompanying bacterial infections 43, 204. Occasionally, a bird will die without showing any signs of illness 1, 217.
TABLE 2.
Properties of virulent and avirulent avian influenza A viruses
| Property | Avirulent strains | Virulent strains |
|---|---|---|
| HA subtype | H1–H15 | H5, H7 |
| Replication | Respiratory and/or intestinal tracts | Most organs |
| Disease | Inapparent or mild: respiratory signs, depression, diarrhea, decreased egg production | systemic or “fowl plague” symptoms: depression; listlessness; ruffled feathers, swelling around the eyes; cessation of egg laying; respiratory signs; excessive lacrimation; sinusitis; edema of the head, face, neck and legs; cyanosis of unfeathered skin, particularly the combs and wattles; diarrhea; nervous system disorders |
| Mortality | Low | High (up to 100%) |
| Plaque formation in cell culture without exogenous protease | No | Yes |
| HA cleavage by intracellular proteases | No | Yes |
| HA cleavage site sequence | Single arginine | Multiple basic amino acids |
| Endoproteases responsible for HA cleavagea | Factor Xa-like protease (in ovo); plasmin (?), protease Clara (?), trypsin (?), bacterial protease (?) (in vivo) | Intracellular proteases expressed in most tissues (subtilisin-like proteases such as furin and PC6) |
Proteases that can proteolytically activate the avirulent virus HAs can do so also for the virulent virus HAs.
The gross and histological lesions in chickens inoculated with highly pathogenic viruses are quite similar but do show some strain variation 2, 119, 187. Some of the differences among reported cases may reflect differences in experimental conditions, including the route of inoculation, the breed and age of the chickens, and the dose of virus. Swelling of the microvascular endothelium, systemic congestion, multifocal hemorrhages, perivascular mononuclear cell infiltration, and thrombosis are commonly seen in chickens infected with highly virulent viruses. Such viruses replicate efficiently in the vascular endothelium and perivascular parenchymatous cells, a property that appears important for viral dissemination and systemic infection 105, 182. Viral antigens are found in necrotic cardiac myocytes in addition to cells in other organs with necrotic and inflammatory changes 105. Thus, involvement of the cardiovascular system plays an important role in the pathogenesis of avain influenza virus infection.
Hemagglutinin as a Determinant of Pathogenicity
Although the virulence of avian influenza virus is polygenic, the HA glycoprotein plays a pivotal role 51, 180. It initiates infection by mediating virus binding to cell receptors and by promoting release of the viral RNP through membrane fusion 213. Posttranslational proteolytic activation of the precursor HA molecule (HA0) into HA1 and HA2 subunits by host proteases generates a fusogenic domain at the amino terminus of HA2, which mediates fusion between the viral envelope and the endosomal membrane. Hence, proteolytic activation of the HA molecule is essential for infectivity 102, 110 and for spread of the virus through the host's body 51.
The HAs of avirulent avian influenza viruses are usually cleaved only in a limited number of cell types, so that the viruses cause only localized infections in the respiratory or intestinal tract, or both, resulting in mild or asymptomatic infections. By contrast, the HAs of virulent avian viruses are cleaved in a broad range of different host cells and therefore are capable of causing lethal systemic infection in poultry. In tissue culture, the HAs of virulent viruses are cleaved in the absence of exogenous proteases, such as trypsin, whereas those of avirulent viruses are not, indicating a difference in sensitivity of the two HAs to endogenous cellular proteases 18. These findings implicate HA cleavability as one of the major determinants of the tissue tropism of influenza viruses and suggest that differences in the tissue distribution of proteases and HA susceptibility to these enzymes determine the outcome of virus infection.
Sequence Requirement for High Hemagglutinin Cleavability and Virulence
Two structural features determine HA cleavability: the amino acid sequence at the cleavage site and the carbohydrate in the vicinity of the cleavage site. Amino acid sequence comparisons between naturally occurring avirulent and virulent avian influenza viruses have shown that HAs with restricted cleavability (avirulent type) usually have a single arginine (R) whereas those with high cleavability (virulent type) have multiple basic residues situated immediately upstream of the cleavage site 17 (Table 3). The majority of influenza A viruses have R at the carboxyl terminus of HA1 and glycine (G) at the amino terminus of HA2, although some have lysine (K) at the former position 63, 93. Among H5 viruses, proline (P) and glutamine (Q), located proximally upstream of the HA1 carboxyl terminus, are also conserved. Between the Q and G residues lies a region designated the connecting peptide (-P-Q-X-...-X-R//G-, where // indicates the cleavage site between HA1 and HA2 and X is any nonbasic amino acid). The sequence in this region varies in its amino acid composition and in the number of amino acids it contains, depending on the virus strain. All naturally isolated avirulent H5 viruses have four amino acids in the connecting peptide; most have R-E-T-R (very rarely K-Q-T-R, R-E-T-K, I-G-E-R, and R-E-A-R 173), while virulent-type HAs contain B-X-B-R (where B is any basic amino acid) in the absence of a nearby carbohydrate side chain [as exemplified by the virulent A/Chicken/Scotland/59 (H5N1) virus]. If the carbohydrate moiety is present, two amino acid insertions, X-X-B-X-B-R, or an alteration of the conserved proline or glutamine to a basic residue, B(X)-X(B)-B-X-B-R, is required. Otherwise, the virus is nonpathogenic, as exemplified by the avirulent A/Chicken/Pennsylvania/1/83 (H5N2) strain 87. Thus, interplay between cleavage site sequences and a nearby carbohydrate side chain determines the susceptibility of the HA to ubiquitous proteases (i.e., its cleavability); the carbohydrate side chain interferes with the accessibility of the HA to proteases 41, 91, 92.
TABLE 3.
HA cleavage site sequence of avian influenza virus isolates
| Subtype | Isolate | Amino acid sequence a ←HA1 | | HA2→ |
|---|---|---|
| H5 | ||
| Avirulent | ||
| Most avirulent | P Q - - - - R E T R G L | |
| Recent avirulent in United States | P Q - - - - R K T R G L | |
| A/Chicken/Pennsylvania/1/83 (H5N2) | P Q - - - - K K K R G L | |
| Virulent | ||
| A/Chicken/Scotland/59 (H5N1) | P Q - - - - R K K R G L | |
| A/Tern/South Africa/61 (H5N3) | P Q R E T R R Q K R G L | |
| A/Turkey/Ontario/7732/66 (H5N9) | P Q - - (R) R R K K R G L | |
| A/Chicken/Pennsylvania/1370/83 (H5N2) | P Q - - - - K K K R G L | |
| A/Turkey/Ireland/1378/85 (H5N8) | P Q - - R K R K K R G L | |
| A/Turkey/England/50-92/91 (H5N1) | P Q - - R K R K T R G L | |
| A/Chicken/Queretaro-19/95 (H5N2) | P Q - - R K R K T R G L | |
| A/Chicken/Queretaro-20/95 (H5N2) | P Q R K R K R K T R G L | |
| A/Chicken/Hong Kong/258/97 (H5N1) | P Q R E R R R K K R G L | |
| A/Hong Kong/156/97 (H5N1) | P Q R E R R R K K R G L | |
| A/Chicken/Italy/1487/97 (H5N2) | P Q - - R R R K K R G L | |
| H7 | ||
| Avirulent | P E X P - - - - K X R G L | |
| Virulent | ||
| A/Chicken/Brescia/02 (H7N7) | P E P S - K K R K K R G L | |
| A/Fowl plague virus/Dutch/27 (H7N7) | P E P P - K K R R K R G L | |
| A/Fowl plague virus/Dobson/27 (H7N7) | P E L P - K K R R K R G L | |
| A/Fowl plague virus/Rostock/34 (H7N1) | P E P S - K K R K K R G L | |
| A/Fowl plague virus/Egypt/45 (H7N1) | F S - K K R R K R G L | |
| A/Turkey/England/63 (H7N3) | P E T P - - K R R R R G L | |
| A/Chicken/Victoria/76 (H7N7) | P E I P - K K R E K R G L | |
| A/Turkey/England/199/79 (H7N7) clone 1 | P E I P - K K R E K R G L | |
| A/Turkey/England/199/79 (H7N7) clone 2 | P E I P - - K K R K R G L | |
| A/Chicken/Leipzig/79 (H7N7) | P E I P - - K K K K R G L | |
| A/Goose/Leipzig/137-8/79 (H7N7) | P E I P - - K R K K R G L | |
| A/Goose/Leipzig/187-7/79 (H7N7) | P E I P K K K K K K R G L | |
| A/Goose/Leipzig/192-7/79 (H7N7) | P E I P - K K R K K R G L | |
| A/Chicken/Victoria/85 (H7N7) | P E I P - K K R E K R G L | |
| A/Chicken/Victoria/92 (H7N3) | P E I P - - K K K K R G L | |
| A/Chicken/Queensland/95 (H7N3) | P E I P - - R K R K R G L | |
| A/Chicken/Pakistan/1369/95 (H7N2) | P E I P - - K R R K R G L | |
| A/Chicken/Pakistan/447-4/95 (H7N3) | P E I P - K R K R K R G L |
Some clones have additional R (shown in parentheses).
Additionally, among H5N2 viruses recently isolated from shorebirds and emus in the United States, the avirulent viruses, whose HA cleavage site was R-K-T-R 143, 162, had the potential for virulence. That is, the virus was avirulent in the presence of a nearby carbohydrate side chain but virulent in its absence 77. Highly virulent viruses with R-K-R-K-T-R at the HA cleavage site were isolated in England [A/Turkey/England/50-92/91 (H5N1)] 217 and in Mexico [A/Chicken/Queretaro/95 (H5N2)] 49, 79, indicating that the basic residue at the second position from the carboxyl terminus of HA1 is not fully required for the virulence phenotype as long as the third residue from the carboxyl terminus is a basic residue.
Similarly, among H7 viruses, conserved residues are found immediately upstream of the HA1 carboxyl terminus: -P-E-X-P-X-…-X-R//G- or P-E-P-S-X-…-X-R//G-. Residues between the P or S and G are considered the connecting peptide. Most naturally occurring avirulent H7 viruses have a K-X-R motif. Sequence alignment of the connecting peptides from virulent viruses and avirulent mutants indicate that R-X-B-R is the minimal sequence requirement for H7 virulent-type HAs 193. Whether K at the fourth position upstream of the cleavage site is acceptable for high HA cleavability depends on the HA. Avirulent mutants of A/Fowl/Victoria/75 have K-K-K-E-K-R at the cleavage site 193, while virulent isolates [A/Chicken/Leipzig/79 (H7N7) and A/Goose/Leipzig/187–7/79 (H7N7)] have HA cleavage site sequences K-K-K-K-R or K-K-K-K-K-K-R, respectively 158. Acidic amino acids (E) at position -3, as found in avirulent mutants of A/Fowl/Victoria/75, may negatively influence protease recognition and proteolytic catalysis.
A reverse-genetics approach, which generated viruses with mutations at the HA cleavage site but otherwise identical genetic backgrounds, provided a direct correlation between HA cleavability and the virulence of avian influenza viruses. That is, an alteration of only the HA cleavage site sequence from a virulent type (R-R-R-K-K-R) to a typical avirulent type (R-E-T-R) led to a major reduction of virulence 76.
To test the hypothesis that human influenza viruses become highly pathogenic by acquiring high HA cleavability, multiple basic amino acids were introduced at the cleavage site of human H3 virus HAs 85, 135. The mutated HAs were cleaved by intracellular proteases, expressed at the cell surface, and hemadsorbed, suggesting that human virus HAs can be converted to those with high cleavability without any deleterious effects. However, the lack of viruses with multiple basic residues at the cleavage site (other than H5 and H7) suggests the presence of structural features that limit the number of such residues at this site.
In addition, virulent and avirulent viruses appear to differ in M2 ion channel activity. Reassortants produced with such viruses always possess the M gene from the virulent virus if the HA is from the virulent virus 16, 133. This finding can be explained by possible differences between the two viruses in the ability of M2 to prevent a low-pH-induced conformational change in the intracellularly cleaved HA of virulent viruses 67, 188, although the molecular basis for this difference remains unclear.
Host Cell Proteases Responsible for Hemagglutinin Cleavage
Two groups of proteases appear responsible for HA cleavage 100, 160 (Table 2). One includes enzymes capable of cleaving avirulent-type HAs with only a single arginine at the cleavage site, as well as virulent-type HAs with multiple basic residues at this site. Such proteases are often called trypsin-like enzymes. In cells growing in tissue culture, multiple replication cycles for avirulent avian viruses and mammalian viruses (with the exception of H7N7 equine viruses) can be achieved by adding exogenous proteases, such as trypsin 102, 191. With some strains, the human WSN virus (H1N1), for example, plasmin can proteolytically activate the HA 55. The mechanism of activation appears quite novel, in that the NA molecule binds and sequesters plasminogen, the precursor of plasmin, thereby increasing local concentrations of the protease 55. Because plasminogen is ubiquitous, viruses possessing a plasminogen-binding NA can readily exploit plasmin for HA cleavage in a variety of organs. Thus, the ability of the NA to bind plasminogen represents a determinant of virulence among influenza viruses.
All avian influenza viruses grow well in embryonated chicken eggs, in which protease similar to the blood-clotting factor Xa, a member of the prothrombin family, is responsible for HA cleavage in allantoic fluid, permitting virus replication in ovo 56. A tryptase with similar substrate specificity is secreted from Clara cells, which reside in the bronchial epithelium of rats and mice 99. Proteases analogous to the Clara tryptase have not been identified in the respiratory tracts of humans and birds; in fact, Clara cells are not prevalent in human respiratory tracts, suggesting that other proteases are probably responsible for HA cleavage. Bacterial proteases can also directly or indirectly activate HAs. Serine proteases secreted by some bacteria, such as Staphylococcus aureus, cleave HAs with a single arginine at the cleavage site in vitro and in mouse lungs (direct HA activation) 165, 189. Moreover, bacterial staphylokinase and streptokinase can proteolytically activate plasminogen to plasmin, which in turn cleaves the HA (indirect HA activation). These findings may explain the development of pneumonia after combined viral-bacterial infection. Although avirulent avian influenza viruses can replicate in the intestinal tracts of birds, the proteases activating their HAs have not been identified.
The second group of proteases cleave only virulent-type HAs with multiple basic residues at the cleavage site. They must be ubiquitous, because virulent viruses can replicate in most tissues and cells. Located in either the medial or trans-Golgi apparatus, this group of enzymes is calcium dependent and has an acidic pH optimum 196. Many bioactive peptides and proteins are produced from large precursors through endoproteolysis, a process that usually occurs at paired or multiple basic amino acids, mediated by mammalian subtilisin-related endoproteases 6, 172 such as furin, the product of the fur gene. Furin, discovered in the region immediately upstream of the human c-fes/fps proto-oncogene 153, is ubiquitously expressed and catalyzes the processing of precursor proteins through constitutive pathways 128. First identified as a cleavage enzyme for the HAs of virulent avian viruses, furin recognizes the B-X-B-R motif 181,195. Its cleavage site is immediately downstream of arginine at the B-X-X-R or B-X-X-X-B-R motif, depending on other structural features of the substrates 19, 120. The HAs of avirulent H5 viruses typically contain R-E-T-R at the cleavage site, raising the possibility that avirulent H5 viruses could be converted to virulent strains if the carbohydrate side chain near the cleavage site is lost. Another mammalian subtilisin-related endoprotease, PC6, which is expressed in many tissues and has a sequence requirement similar to that of furin, can also activate virulent avian viruses, indicating the presence of multiple HA cleavage enzymes in mammals 78.
Outbreaks of Avian Influenza
Several outbreaks of avian influenza during the last two decades have contributed to our understanding of the epizootiology and pathogenesis of this disease.
Pennsylvanian outbreaks.
In April 1983, an avirulent H5N2 influenza A virus (A/Chicken/Pennsylvania/1/83) appeared in chickens in Pennsylvania. Later, in October 1983, virulent influenza viruses (e.g., A/Chicken/Pennsylvania/1370/83) were isolated from chickens after they had decimated numerous flocks (>80% mortality). The virus was eventually eradicated by the destruction of over 17 million birds at a cost of over 61 million dollars 44.
Sequencing studies and RNA-RNA hybridization analyses revealed that the genes of these viruses were derived not from a virulent H5 influenza virus responsible for previous influenza outbreaks but from an avirulent virus maintained in North America 10, 89. In 1985 to 1986, H5N2 avirulent viruses were again isolated from poultry in Pennsylvania and subsequently in other states. Extensive epizoological studies by the U.S. Department of Agriculture finally traced the virus to birds in live-poultry markets in New York City, New Jersey, and Miami 90. Genetic and antigenic analyses demonstrated their close relationship to the H5N2 viruses isolated in Pennsylvania in 1983 to 1984, suggesting that live-poultry markets serve as an important reservoir for avian influenza viruses. This concern was substantiated by the Hong Kong outbreak in 1997.
Oligonucleotide mapping of RNAs from the virulent and avirulent Pennsylvania avian viruses indicated that point mutations were responsible for the acquisition of virulence 10, while a study of reassortant viruses identified the HA gene as a critical determinant of virulence 206. Subsequent comparison of the entire HA amino acid sequences of avirulent, virulent, and revertant viruses demonstrated a single amino acid substitution at residue 13 (Thr to Lys), which is thought to be responsible for the acquisition of virulence 41, 87. This substitution is remarkable in that it coincided with the loss of an oligosaccharide side chain. On the three-dimensional structure of HA, this change is located in the middle of the stalk in the vicinity of the cleavage site between HA1 and HA2, suggesting that the deleted carbohydrate side chain normally blocks the access of cellular proteases to the cleavage site 87. A unique aspect of this outbreak was that the HAs of the avirulent viruses initially isolated from chickens already possessed multiple basic amino acids (K-K-K-R) at the cleavage site, a primary requirement for the virulence phenotype; however, cleavage was blocked by a nearby carbohydrate side chain. Thus, once the carbohydrate side chain of the avirulent virus was lost, the HA became highly cleavable, leading to the acquisition of virulence.
In 1993, avirulent H5N2 viruses were again isolated in live-bird markets in Pennsylvania, as well as Florida. Phylogenetic analysis of the HA genes of these isolates indicated that they shared a common ancestor with an H5N2 virus isolated from shorebirds (ruddy turnstones) in 1991 and did not originate from the Pennsylvania virus that caused the 1983 to 1984 outbreak, suggesting that the virus had been introduced from wild waterfowl into chickens 162. The HA of these avirulent viruses contained an atypical cleavage site sequence (R-K-T-R), requiring only a single mutation for conversion to high HA cleavability 77. This isolate and the avirulent 1983 Pennsylvania virus caution us that potentially hazardous influenza viruses are more prevalent in nature than one would suspect from documented outbreaks.
Mexican outbreak.
In October 1993, there was a drop in egg production and an increase in mortality among Mexican chickens that was associated with an H5N2 avian influenza virus. The virus (e.g., A/Chicken/Mexico/26654-1374/94), which caused only a mild respiratory syndrome in specific-pathogen-free chickens, eventually spread throughout the country. Eradication of the virus was not considered an economically sound option by the poultry industry, a decision that afforded opportunities to monitor the biological and molecular changes that accompany the acquisition of virulence by influenza viruses in a natural setting. By the end of 1994, the virus had acquired mutations that rendered the HA highly cleavable, resulting in a conversion to moderate virulence, characterized by an increased death rate among experimentally infected chickens (e.g., with A/Chicken/Puebla/8624-602/94). Within a few months, a more pathogenic virus was isolated (A/Chicken/Queretaro/14588-19/95) that caused 100% mortality. Both the moderately and highly pathogenic isolates contained insertions and a substitution of basic residues in the HA connecting peptide: R-K-R-K-T-R versus R-E-T-R in the original avirulent isolate 49, 79, 187.
Phylogenetic analysis of viruses isolated during the Mexican outbreak revealed two geographically related lineages that each contained virulent viruses 49, suggesting the independent emergence of pathogenic viruses from avirulent viruses. Further study of the H5 HAs indicated that the epizootic virus had originated from a single virus of the American lineage that had been introduced into Mexican chickens 49, 79. Since the virus is genetically most closely related to a 1991 shorebird isolate in the United States [A/Ruddy turnstone/Delaware/244/91 (H5N2)], an ancestral virus may have been introduced into Mexico by migrating waterfowl. This outbreak demonstrates that pathogenic mutants can emerge in nature from a typical avirulent virus (R-E-T-R at the HA cleavage site).
Mechanism for the Emergence of Virulent Viruses
The acquisition of high HA cleavability is an essential event in the conversion of avirulent avian influenza viruses to virulent strains. Since only a limited number of mutations are needed to convert an avirulent phenotype to a more virulent one, the most plausible mechanism for this change is the introduction of mutations by error-prone RNA polymerase followed by the selection of viruses with highly cleavable HAs. Attempts to test this possibility in trypsin-free chicken embryo fibroblast cultures, using an avirulent virus isolated during the 1983 to 1984 Pennsylvania outbreak, were successful 134. All of the mutants examined had increased numbers of basic amino acids at the HA cleavage site but lacked alterations in the glycosylation site at Asn-11, in contrast to findings in naturally generated virulent viruses in the Pennsylvania outbreaks. Thus, selective pressures exerted in cell culture differ from those in natural settings.
The finding that virulent mutants can be readily selected in 14-day-old but not 10-day-old embryonated chicken eggs inoculated with the 1983 Pennsylvania avirulent isolate 22, 77 provides a clue to the mechanism of emergence of virulent avian viruses in nature. Virulent variants predominated after only three passages of the avirulent virus in 14-day-old eggs, indicating a replicative advantage of virulent viruses in older eggs 77. Sequencing of the virulent-type HA revealed a loss of the glycosylation site at Asn-11, the same mutation found in virulent Pennsylvania isolates. However, whether older eggs play a role in the selection of virulent mutants in nature is unclear.
How do avirulent viruses acquire multiple basic residues at the HA cleavage site? One postulated mechanism is direct duplication of a part of the purine-rich region at the cleavage site 144. Additionally, RNA recombination resulting in a large insertion of 28S host rRNA 94 or a viral NP gene 138 sequence immediately upstream of the HA cleavage site leads to the generation of a highly cleavable HA, although counterparts of these mutants have yet to be identified in nature.
INTERSPECIES TRANSMISSION AND HOST RANGE RESTRICTION
Ample evidence suggests that all mammalian influenza viruses originated from ancestral precursors in wild waterfowl 201, and examples of bird-to-mammal transmission of influenza viruses have been reported 26, 52, 64, 70, 71, 103, 113, 166. Here we review modes of interspecies transmission of influenza A virus, emphasizing bird-to-mammal routes and host range restrictions that normally prevent the spread of influenza virus across interspecies barriers.
Transmission to Mammals
Transmission to pigs.
In 1979, an H1N1 avian virus was transmitted to pigs in Europe and is still circulating among these animals 142, 166. An H1N1 virus was also transmitted from birds to pigs in southern China in 1996 59, but it does not appear to be circulating in these hosts at present. Thus far, viruses other than the H1, H3, N1, N2, and N7 subtypes have not been isolated from pigs.
Transmission of influenza virus from humans to pigs has been reported many times 28, 97, 130. Interestingly, these human viruses were all of the H3 subtype. In Europe, avian H1N1 and human H3N2 viruses cocirculated in pigs, beginning in 1979, and eventually reassorted in this host 29, generating a virus with its HA and NA genes from the human H3N2 virus and the remainder from the avian virus. This reassortant was then transmitted to children in the Netherlands 33, demonstrating that genetic reassortment, occurring between avian and human viruses in pigs, can give rise to hybrid strains capable of infecting humans. Swine H3N2 influenza viruses currently circulating in Europe appear to be the descendants of such reassortants. By one estimate, H1N1 swine viruses may have been transmitted to 76% of young persons under 20 years of age who had contact with pigs 212. This incident and similar ones support the theory that pigs serve as “mixing vessels” for the generation of avian-human reassortants with the potential to cause human pandemics 169. In 1992, an influenza virus with an unusual subtype (H1N7) was isolated from pigs in England 20, 21. Two of its genes (NA and M) appear to be of equine origin, while the others seem to have originated in humans. Thus, pigs are susceptible to a variety of influenza viruses and should be monitored regularly as part of an early-warning system to detect viruses with pandemic traits.
Interestingly, the receptor specificity of the avian H1N1 virus that was transmitted to pigs in Europe changed during replication in this animal 80. Although the avian virus was not available for study before its transmission to pigs, it most probably recognized the NeuAcα2,3Gal linkage like other avian viruses 155, 157 (see below). The viruses isolated from pigs between 1979 and 1984 recognized both NeuAcα2,3Gal and NeuAcα2,6Gal, while those isolated after 1985 recognized only NeuAcα2,6Gal, resembling the receptor specificity of human viruses 155, 157. This finding suggests a mechanism by which avian viruses could acquire the HA properties needed for transmission to humans.
Transmission to horses.
In 1989 to 1990, a severe outbreak of respiratory disease with high mortality (20% in some herds) occurred in horses in northeastern China. The causative virus was classified as an influenza virus H3N8 subtype antigenically distinguishable from the prototypic H3N8 equine virus (A/Equine/Miami/63). China had been free of equine influenza viruses for some time prior to this outbreak. Phylogenetic analyses of the eight genes of the H3N8 equine isolate indicated that the virus was recently derived from an avian virus and not from viruses that were circulating in horses in other parts of the world 64, 65.
Transmission to seals.
In 1979 to 1980, approximately 20% of harbor seals (Phoca vitulina) along the northeastern coast of the United States died of a severe respiratory infection with features typical of primary viral pneumonia 52. Influenza A viruses, which were antigenically and genetically related to avian viruses, were isolated from the infected seals and subtyped as H7N7 (A/Seal/Massachusetts/1/80). Although phylogenetic analysis of the isolates indicated their recent introduction from avian species, they replicated well in mammals but poorly in birds 203, probably because of their rapid adaptation to seals. Several field workers who had close contact with the seals developed conjunctivitis, although serum HI antibodies to the virus were not detected in these individuals 202. The virus caused systemic infection in squirrel monkeys and was isolated from the spleen, liver, muscles, and lungs of a monkey that died of viral pneumonia 123.
In 1982 to 1983, H4N5 influenza A viruses were recovered from harbor seals dying of viral pneumonia on the New England coast 71. Again, these seal isolates were antigenically and genetically most closely related to avian viruses. Unlike the H7N7 seal viruses, the new isolates replicated in the intestinal tracts of ducks, a defining characteristic of avian viruses, suggesting that they were still evolving in their adaptation from avian species to mammals. Then, in 1991 and 1992, H4N6 and H3N3 influenza viruses were isolated from seals that had died of pneumonia 26. They also were closely related to avian viruses, indicating that the transmission of avian influenza viruses to seals is not a rare event.
Transmission to whales and mink.
Viruses of the H13N2 and H13N9 subtypes, which are typically found in avian species, were isolated from the lungs and hilar nodes of a pilot whale 32, 70. An H1N3 virus had previously been isolated from the lungs and livers of whales 113. In 1984, viruses of avian origin (H10N4) were isolated from domestic mink with severe respiratory disease in Sweden 103. Before this outbreak, mink were experimentally shown to be susceptible to avian influenza viruses of several HA and NA subtypes 118, 136. These incidents provided important evidence that an avian influenza virus can cause severe disease in mammalian populations in nature without undergoing discernible alterations of its genetic complement.
Transmission to humans (excluding the Hong Kong outbreak of 1997).
In general, avian influenza viruses do not replicate efficiently in humans, indicating that direct transmission of avian viruses to humans would be a rare event. For example, high doses of avian influenza viruses were required to produce a quantifiable level of replication in human volunteers 14. During the 1983 to 1984 Pennsylvania outbreak in poultry, no cases of influenza-like illness were reported among workers exposed to highly pathogenic avian viruses. It has long been believed that the growth restriction of avian influenza viruses in humans prevents the emergence of new pandemic strains via direct avian-to-human transmission. A few isolated cases argue against this notion. In 1996, an avian H7 influenza virus was isolated from a woman with conjunctivitis [A/England/268/96 (H7N7)] 108. The source of the virus was considered to be waterfowl, since she tended a collection of 26 ducks of various breeds that mixed freely with feral waterfowl on a small lake. The entire HA gene of this human isolate showed close homology to an H7N7 virus isolate from a turkey in Ireland in 1995 5. A virus of the same subtype has also been isolated from a man suffering from infectious hepatitis, although the virus was not considered to be the causative agent 27.
Molecular Determinants of Host Range Restriction
Although interspecies transmission of influenza viruses has been demonstrated repeatedly, as described above, certain host range restrictions apply. For example, avian influenza viruses do not replicate efficiently in humans 14 or in nonhuman primates 124; similarly, human influenza viruses do not grow in ducks 73. Very little is known about the viral and host factors that determine the species range of influenza viruses or about the mechanisms by which host barriers are crossed. Here we review the roles of individual viral gene products in establishing host range restrictions.
HA.
The HA glycoprotein is clearly a major determinant of host range restriction, primarily because of its role in host cell recognition. The receptor specificity of influenza virus varies according to the host animal from which the virus was isolated. In humans, influenza viruses preferentially recognize sialyloligosaccharides terminated by N-acetylsialic acid linked to galactose by the α2,6 linkage (NeuAcα2,6Gal), whereas avian and equine viruses recognize N-acetylsialic acid linked to galactose by the α2,3 linkage (NeuAcα2,3Gal) 37, 47, 117, 154–156, 185. Correspondingly, the predominant sialic acid-galactose linkages of sialyloligosaccharides in epithelial cells at viral replication sites differ by the host animal species. For example, the epithelial cells in the human trachea contain mainly NeuAcα2,6Gal 38 whereas those in the horse trachea and duck intestine (where avian viruses replicate) contain mainly NeuAcα2,3Gal linkages 80. Interestingly, the epithelial cells in the pig trachea contain both NeuAcα2,6Gal and NeuAcα2,3Gal linkages 80, explaining why this animal is highly susceptible to both human and avian influenza viruses. Thus, the host specificities of influenza viruses could be affected by the abundance of these two types of sialic acid-galactose linkages on cell surface sialyloligosaccharides. Such differences in specificity are determined by the structure of the HA receptor-binding site: for example, Leu-226 in human H3 viruses, instead of Gln-226 as in avian and horse viruses, confers NeuAcα2,6Gal specificity 126, 156. Several other amino acids have also been implicated as determinants of HA receptor specificity 37, 47, 80, 82, 117, 154–156, 186, 211, 216.
The HA gene of the pandemic 1968 Hong Kong virus was derived from an avian virus 170. Analysis of the predicted amino acid sequence of the hypothetical precursor strain that immediately preceded the pandemic strain indicated that fewer than six amino acids in the HA changed during the avian-human transition 11. Each of these alterations modified the head portion of the HA molecule, including the area surrounding the receptor-binding pocket. One of the mutations (Glu to Leu) affected position 226, as described above. Three others occurred at positions 62, 144, and 193, which are among the most variable sites in the molecule 11, each possessing three or four different amino acids that differ among human virus H3 HAs. One interpretation is that these mutations reflect adaptation to a new host. Alternatively, since all of the changes occurred within an antigenic site, they may represent the early stages of antigenic drift, before detection of the virus.
NA.
The NA also plays a role in host range discrimination 73; for example, a reassortant virus that contains all duck virus genes except for a human virus NA does not grow in ducks. As with HA, the substrate specificity of NA seems to contribute to efficient virus replication. As one example, NA of an N2 avian virus, although highly specific for hydrolyzation of the NeuAcα2,3Gal linkage, acquired NeuAcα2,6Gal specificity during its evolution in humans 8. This acquisition corresponds to the specificity of the human virus HA, emphasizing the importance of both glycoproteins in promoting efficient replication in animals. However, the requirement for substrate specificity does not appear to be as stringent for NA as for HA, since NAs of human N2 viruses still recognized only the NeuAcα2,3Gal linkage soon after their introduction from birds 8, 104 whereas the HAs were preferentially specific for NeuAcα2,6Gal 155, 157.
Other gene products.
The gene encoding the internal proteins of influenza A viruses are also likely to influence host specificity. Reassortant viruses possessing internal proteins encoded by avian virus genes, with all other genes coming from a human virus, show attenuated growth in squirrel monkeys compared with that of the parental human viruses 179, 192. Similarly, reassortants with the fowl plague virus HA and M genes and other genes from a human virus (including NP) failed to replicate in chickens and in chicken embryo fibroblasts but replicated well in cells of mammalian origin (MDCK cells) 168. These data suggest that NP and other internal proteins participate in host range restriction. Similarly, the introduction of an avian virus PB1 into a human virus resulted in attenuation of viral replicative ability in MDCK cells and in squirrel monkeys but not in chicken kidney cells 179. A single-gene reassortant virus (PB2 gene from an avian virus and all others from a human virus) displayed a host range restriction phenotype characterized by efficient replication in avian but not mammalian cells. A single amino acid in the PB2 protein (Glu-627 in avian virus and Lys-627 in human viruses) was responsible for the host cell restriction 183. PB2 also restricts the replication of a fowl plague virus in certain cell lines 3. These data can be interpreted either as incompatibility among the viral gene products or as a contribution of these gene products to host range restriction.
INFLUENZA PANDEMICS AND OUTBREAK OF H5N1 VIRUS IN HONG KONG IN 1997
Origin of Pandemic Viruses That Emerged in the 20th Century
Four human influenza pandemics have occurred in this century. Here we briefly review the salient aspects of these global outbreaks, focusing on the origin and biological properties of the causative viruses.
Spanish influenza.
The biological properties of the virus responsible for “Spanish influenza” (H1N1) in 1918 to 1919 have not been studied, owing to the lack of viral isolates. Phylogenetic and seroarcheological studies suggest that all genes of the 1918 pandemic strain were closely related to those of H1N1 swine viruses 53, 201, although sequence data to support this notion were lacking until 1997, when Taubenberger et al. (190), using reverse transcription-PCR, succeeded in obtaining nucleotide sequences of this pandemic virus from a formalin-fixed, paraffin-embedded, lung tissue sample from an influenza victim. Phylogenetic analysis of sequences encoding the HA, NA, NP, M1, and M2 proteins disclosed a genetic relationship between the pandemic strain and H1N1 human and swine influenza A viruses. Further study of the HA gene indicated the absence of multiple basic amino acids at the cleavage site 151, 190, in contrast to findings in virulent avian viruses. Although the virus is most probably derived from birds 201, it will be difficult to determine whether pigs served as an intermediate host, at least until sufficient sequence information on early swine H1N1 viruses becomes available. The only virulence determinants of influenza virus that we understand involve genes encoding HA (high cleavability due to multiple basic amino acids at the cleavage site) 101, NA (plasminogen-binding activity) (55), or NS1 (interferon-counteracting activity) 50. Thus, unless sequence analyses of the HA, NA, and NS1 genes of this H1N1 virus suggest some unique features about these activities, it is unlikely that the extreme virulence of the 1918 strain will ever be understood.
Asian and Hong Kong influenza.
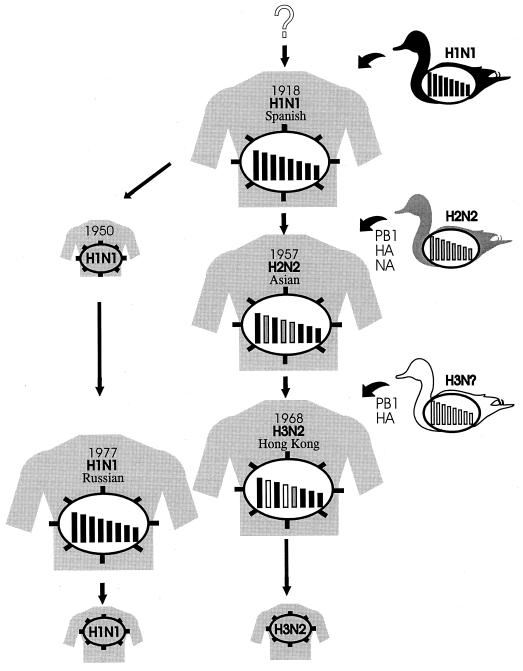
The human pandemic viruses, such as those responsible for Asian influenza (H2N2) in 1957 and Hong Kong influenza (H3N2) in 1968, share two defining features: they first emerged in southeastern Asia (35, 150), and they were antigenically distinct from the influenza viruses then circulating in humans. Genetic studies, together with biochemical analyses, indicated that the Asian and Hong Kong pandemic strains were generated by reassortment between human and avian viral genes (Fig. 4). The causative virus in the 1957 pandemic possessed three genes (PB1, HA [H2], and NA [N2]) from an avian virus and all remaining genes from a circulating human H1N1 virus 86, 170, which disappeared soon after the H2N2 virus emerged. The pandemic H3N2 virus identified in Hong Kong in 1968 was a reassortant with avian PB1 and HA (H3) genes and six other genes from a human H2N2 virus 86,170. The latter virus could no longer be detected in humans after the H3N2 strains appeared. Phylogenetic studies indicated that the avian viral genes in these pandemic strains were derived from viruses of Eurasian lineage, in accord with epidemiological findings suggesting that the viruses originated in southern China. Why the viruses previously circulating in the human population vanished with the emergence of the new viruses remains uncertain. Although many people died during these pandemics, the causative viruses do not seem to be extraordinarily pathogenic, unlike the 1918 strain. The increased death rate associated with these viruses was probably a result of the lack of immunity to these viruses in human populations.
FIG. 4.
Possible origins of pandemic influenza viruses. Phylogenetic studies suggest that an avian influenza virus was transmitted to humans, leading to the 1918 pandemic. A reassortant virus possessing its PB1, HA, and NA genes from a Eurasian avian virus, with the remainder coming from an H1N1 human virus, caused the Asian pandemic of 1957. The H1N1 virus subsequently disappeared from humans. In 1968, a reassortant possessing its PB1 and HA genes from a Eurasian avian virus and the remainder from an H2N2 human virus emerged, followed by the disappearance of the H2N2 virus. In 1977, a virus genetically almost identical to those circulating in humans in 1950 appeared and spread among children and young adults. The H1N1 and H3N2 viruses are now cocirculating in humans.
Russian influenza.
The causative agent of the Russian influenza pandemic in 1977 was essentially identical to viruses circulating among humans in the 1950s 127, 171 (Fig.. 4). Given that influenza viruses continually evolve in animals, it is highly unlikely that this virus was maintained in an animal host for over 20 years without changes. Thus, one logical conclusion is that the virus was maintained in a freezer until it somehow was introduced into human populations. The relatively low death rate in this pandemic can be attributed to the immunity of persons over 20 years of age who had been infected with the virus when it circulated earlier in the century.
Outbreak of H5N1 Virus in Hong Kong in 1997
In May 1997, an H5N1 influenza virus (A/Hong Kong/156/97) was isolated from a 3-year-old boy in Hong Kong 34, 184. The patient died of extensive influenza pneumonia complicated by Reye's syndrome. By the end of 1997, a total of 18 Hong Kong residents were infected with H5N1 influenza viruses, 6 of whom died 31. Because human populations lacked immunity to the H5 influenza virus subtype, there was great concern about the possibility of a major pandemic due to this unusual virus. The human H5N1 isolates were not reassortants like the 1957 and 1968 pandemic strains; instead, all of the viral genes had originated from an avian virus 34, 184.
Patients.
The 18 human cases were not confined geographically or to a specific age group. Rather, they developed in both children and adults with ages ranging from 1 to 60 years 31. In 7 of the 18 cases, there were histories of possible exposure to poultry: the patients either had bought chickens before they became ill or had worked in proximity to chicken stalls near their homes 121. The clinical features of the first 12 cases included onset with fever and upper respiratory tract infection, typical of classic influenza 222. Seven of the patients had severe complications, most prominently pneumonia, gastrointestinal manifestations, elevated liver enzyme levels, and renal failure. In general, children fared better than adults. With one exception, patients younger than 13 years recovered from their illness whereas older patients had severe disease that resulted in five deaths. Epidemiological studies suggested mainly direct transmission of the virus from birds to humans; serological evidence of human-to-human transmission was limited to a few cases only 121. Serologic surveillance also indicated limited transmission of H5N1 virus in Hong Kong residents 161. Although isolates from cousins were genetically very similar (only 4 nucleotide differences out of approximately 6,000 nucleotides compared) 48, it is not clear whether one cousin acquired the virus from the other or whether they both were infected with virus from a common source. The lack of evidence for human-to-human transmission of this virus in the majority of cases suggests that the virus had not fully adapted to its human host. Most probably, additional mutations introduced through continued replication in humans or perhaps reassortment with a currently circulating human virus will be required to produce a highly virulent and contagious virus.
Where did the 1997 Hong Kong virus originate? From late March to early May 1997, an H5N1 influenza killed more than 6,500 chickens (75% mortality rate) on three farms in the New Territories, Hong Kong SAR (Special Administrative Region), China 219. Epidemiological and phylogenetic analyses suggested that the human H5N1 isolates had originated from an avian virus prior to this outbreak in poultry 34, 182. Surveillance of Hong Kong poultry markets for H5N1 viruses in December 1997 detected the virus in approximately 20% of fecal samples from chickens and 2% of fecal samples from ducks and geese (176). One virus-positive market that served as a wholesale outlet for the city may have been responsible for the contamination of other live-poultry markets. Each of the H5N1 isolates was lethal when tested in chickens. These findings implicate virus-contaminated birds in live-poultry markets as the source of the H5N1 influenza outbreak in Hong Kong.
A potential lead to the origin of the H5N1 virus came from isolation of a similar virus from geese dying of an influenza-like disease in Guandong Province, China 176, 219. However, experimental infection of geese with this isolate did not reproduce the disease, indicating that the H5N1 virus was not the causative agent in the outbreak. Phylogenetic studies showed that the HA of this isolate, but not other genes, shares an immediate ancestor with the Hong Kong H5N1 virus 219. The HA of another H5N1 virus isolated from a waterfowl in a wholesale live-poultry market in Hong Kong in the beginning of 1999 was genetically closely related to the H5N1 viruses isolated from patients in Hong Kong in 1997. These findings suggest a precursor role for these viruses, at least for the HA. An ancestral nonpathogenic strain for the 1997 Hong Kong outbreak has not been isolated, as found in the Pennsylvania and the 1993 Mexican outbreaks.
Properties of the causative virus.
Sequence analysis of the human H5N1 isolates indicated that all of the genes have an avian origin and are not reassortants 34, 184. They contain multiple basic amino acids (R-E-R-R-R-K-K-R) at the HA cleavage site and thus fulfill one of the requirements for high virulence in chickens. However, they are biologically heterogeneous in terms of the plaque sizes they produce in MDCK cells, as well as their pathogenicity in mice 48, 112. Some of the human H5N1 isolates were highly lethal in mice (the dose required to kill 50% of mice [LD50] was approximately 0.3 PFU) and could replicate in a variety of organs, including the brain 48, 112. Other isolates showed moderate virulence (LD50 ≥ 103 PFU) and replicated only in respiratory organs. Nonetheless, all human and avian H5N1 isolates from Hong Kong were lethal to chickens (LD50 ≈ 102 PFU) and replicated well in a variety of organs, including the brain and endothelial cells 182, a property shared by other virulent avian influenza viruses 105.
In an effort to gain insight into the molecular basis of viral adaptation in humans, sequences of the Hong Kong isolates were compared with those of H5 chicken, duck, and goose viruses from live-poultry markets 176. Like the human viruses, all of the avian isolates contained multiple basic amino acids at the HA cleavage site, but there were no amino acid differences that immediately suggested the basis for viral adaptation in humans. Further analysis with monoclonal antibodies disclosed two discrete antigenic groups, which correlated with genetic findings and the presence or absence of a carbohydrate at residue 158 adjacent to the receptor-binding site on HA 176. However, the results of antigenic and genetic studies did not correspond to virus classifications based on virulence in mice 48. Also, both the index human and avian isolates preferentially bound to NeuAcα2,3Gal- rather than NeuAcα2,6Gal-containing receptors 116, although epithelial cells lining the human trachea contain mainly NeuAcα2,6Gal glycoconjugates 38. Thus, there appears to be some “leakiness” of the receptor specificities of influenza viruses in host range restriction. This finding is not unexpected, since the receptor specificity of influenza viruses is only “preferential” and conventional human viruses do bind NeuAcα2,3Gal (although they do so only poorly). Since the earliest isolates from the 1957 and 1968 pandemics preferentially recognize NeuAcα2,6Gal over NeuAcα2,3Gal 155, 157, even though their HAs were derived from an avian virus, the findings with the H5N1 Hong Kong viruses suggest that conversion of the receptor specificity of the avian virus HA from NeuAcα2,3Gal to NeuAcα2,6Gal is a necessary step leading to the efficient transmission of avian-like influenza viruses in human hosts.
The H5N1 chicken and human viruses contained a 19-amino-acid deletion in the NA stalk that may have decreased the functionality of the enzymatic activity 30, 34, 45, 116, 184. Whether this deletion is involved in the pathogenicity of the H5N1 viruses is unclear, although a similar change might be required for the adaptation of influenza viruses from wild aquatic birds to chickens.
Antiviral drugs and vaccines.
When the number of human H5N1 infections began to increase in late 1997, amantadine was rapidly imported into Hong Kong. This anti-influenza drug prevents viral infection by interfering with M2 ion channel activity, thus inhibiting virus uncoating 67, 146. When given experimentally to chickens, amantadine affords complete protection against viral infection 205. Withdrawal of the drug and rechallenge with the lethal virus results in the deaths of all chickens. A single mutation confers resistance to the drug, as demonstrated in studies with both human and avian influenza viruses 58. As one example, virus-infected chickens treated with amantadine survived but shed drug-resistant viruses that were lethal to contact birds 12, 13, 205. Hence, amantadine offers prophylaxis against influenza virus infection but is not ideal for treatment of active disease. Apart from experience in nursing homes and similar institutions 68, there is little published information on the efficacy of amantadine when used extensively in large populations of patients. Several NA inhibitors are also being tested in clinical trials 25, 69, 194. Although viruses with resistance to these compounds do appear in vitro (reviewed in reference 25) and in vivo 61, they emerge less frequently than do mutants with amantadine resistance 54. One of these compounds (zanamivir) was shown to be efficacious in reducing the viral load and mortality in mice infected with the H5N1 virus 62.
The lethality of the H5N1 viruses isolated in Hong Kong posed difficulties in producing vaccines. Not only did their virulence threaten personnel in vaccine production, but also it compromised efforts to obtain high-quality allantoic fluid with acceptable virus titers from embryonated eggs, the current standard in vaccine production. Thus, health officials elected to exploit an avirulent H5N1 virus whose antigenicity is similar to that of the Hong Kong virus. Additionally, using reverse genetics (technology allowing the generation of influenza virus containing a gene derived from cloned cDNA) 46, investigators produced avirulent mutants of the Hong Kong virus, which contain a typical avirulent virus HA sequence at the cleavage site instead of the usual series of basic amino acids 111. This avirulent mutant is ideal for vaccine production because it is antigenically identical to the virulent parent. However, the production of such viruses is time-consuming, so that a naturally isolated avirulent virus, antigenically similar to the causative virus, continues to be the first choice for rapid production of vaccine following a major outbreak of influenza.
Lessons learned.
The recent Hong Kong influenza outbreak demonstrated that avian influenza viruses can cause severe disease in humans without any reassortment with a human virus and without any apparent intermediate mammalian host, such as swine. This incident warns us that any subtype of influenza A virus could be a latent pandemic strain. Thus, it becomes important to separate animals that could serve as vehicles for the interspecies transmission of virus. In Hong Kong, chickens and waterfowl (e.g., ducks and geese) are now sold in different markets and ducks and geese are now slaughtered at the markets. Since these and other preventive measures were instigated, there have been no further cases of H5N1 influenza in humans residing in Hong Kong or surrounding regions. The conventional wisdom has been that avian influenza A viruses are not directly transmitted to humans. The infection of Hong Kong residents argues against this notion. Indeed, we would be wise to regard all influenza viruses, whether virulent or avirulent, as possible zoonotic agents and occupational biohazards and begin to devise contingency plans for their containment in both rural and urban areas of the world.
There are many unanswered questions posed by this outbreak. Where did the virus originate? What was the mode of transmission from birds to humans? Has the H5N1 virus disappeared from Hong Kong and neighboring regions? How prevalent are avian viruses capable of being transmitted from birds to humans and then causing severe disease? What genetic traits made this virus so transmissible to humans? Any efforts to answer these and other pertinent questions will depend on the results of comprehensive studies now under way.
Transmission of H9N2 Viruses from Birds to Humans
In March 1999 in Hong Kong, an H9N2 avian influenza virus was isolated from two children with mild influenza symptoms. Genetic studies showed that all the genes other than the HA and NA were closely related to those of the H5N1 viruses, suggesting the role of these genes in efficient transmission of the virus from birds to humans 60, 141. In fact, surveillance of live-poultry markets in Hong Kong in December 1997 revealed the presence of H9N2 viruses in addition to H5N1 viruses in birds in these markets.
Possible Mechanisms for the Generation of Pandemic Influenza Viruses
Future influenza pandemics will probably be caused by an avian virus possessing an HA surface protein to which humans lack immunity. Whether this virus will be introduced directly or indirectly into human populations is uncertain, but at least two mechanisms seem plausible.
Roles of pigs.
If the next pandemic strain proves to be a reassortant virus like those in 1957 and 1968, a single animal will have to be infected with two different viruses, one avian and one human. Because of host range restrictions governing the transmission of the majority of avian influenza viruses in humans but not in pigs, the latter animal has become the principal candidate for the role of intermediary, or mixing vessel, in genetic reassortment steps leading to efficient replication of viruses with avian virus HAs in humans 169, 170. Genetic reassortment between H1N1 avian and H3N2 human viruses in European pigs 29 and their transmission to children in The Netherlands 33 supports this hypothesis (Fig. 5, model A).
FIG. 5.
Models for the generation of pandemic influenza virus strains in pigs. In the classical genetic reassortment model (A), avian and human viruses bind respectively to NeuAcα2,3Gal and NeuAcα2,6Gal (α2,3 and α2,6) linkages in the pig trachea, setting the stage for the emergence of a reassortant that infects a large fraction of the human population. The segments in the center of each particle represent the viral genome. The reassortant HA gene (black) is derived from an avian virus. In the adaptation model (B), avian viruses acquire the ability to replicate efficiently in humans during adaptation to the NeuAcα2,6Gal linkage in pigs. This change is mediated by a mutation in the HA gene. Alternatively, an avian influenza virus is transmitted directly to humans, where it reassorts with a human virus (C) or acquires the ability to recognize the NeuAcα2,6Gal linkage after direct introduction from birds (D), leading to efficient replication in humans. Reprinted from reference 80 with permission of the American Society for Microbiology.
Evidence that the receptor specificity of an H1N1 avian influenza virus changed during replication of the virus in pigs 80 suggests a mechanism by which pigs could serve as intermediate hosts for the adaptation of avian viruses to humans (Fig. 5, model B). It should be stressed that models A and B are not mutually exclusive; indeed, alteration of receptor specificity during replication of an avian virus in pigs may occur both before and after reassortment with a human virus. Alternatively, an avian virus may become adapted in pigs to the extent that it would not require reassortment with a human virus for efficient replication in humans.
Direct transmission.
The 1997 Hong Kong outbreak was unique in that it suggested additional models for the generation of pandemic strains from avian viruses: direct transmission and reassortment (Fig. 5, model C) or adaptation (Fig. 5, model D) in humans. Whether these mechanisms operate with only a limited number of avian viruses or apply more widely than previously thought remains in question.
CONCLUDING REMARKS
Fowl plague caused by highly pathogenic avian influenza A viruses is a constant threat to the poultry industry, but until the Hong Kong influenza outbreak, there was no zoonotic evidence that avian viruses could be transmitted directly to humans. The identification of avian viruses in humans underscores the potential of these and similar strains to cause devastating influenza outbreaks in major population centers. Since changes in the world's agricultural and marketing practices, especially those in China 169, are not likely to change in the near future, it will be critical to identify the molecular determinants that allow efficient transmission and replication of avian influenza viruses in humans, so that probable pandemics can be anticipated well before the death toll begins to mount.
Southern China is considered to be an influenza epicenter based on its role as the site of two previous pandemics (Asian and Hong Kong influenza) 175. The large mix of people, pigs, and ducks in this region affords an ideal environment for the generation of reassortant influenza viruses. Since methods for predicting human influenza outbreaks on the basis of the molecular features of precursor viruses are still rudimentary, health officials should begin to consider the production of emergency vaccines against all 15 existing HA subtypes of influenza virus. Also, given the existence of a vast number of influenza viruses in the aquatic wild-bird reservoir, we must accept the fact that they always pose pandemic threats. Thus, it is recomended that poultry (chickens, turkeys, etc.), domesticated ducks, and pigs be kept in ecologically controlled, secure houses with limited access to wild birds 200.
ACKNOWLEDGMENTS
We thank John Gilbert and Krisna Wells for editing the manuscript. We also thank Yuko Kawaoka for illustrations.
Support for this work came from National Institute of Allergy and Infectious Diseases Public Health Service research grants.
REFERENCES
- 1.Alexander D J, Lister S A, Johnson M J, Randall C J, Thomas P J. An outbreak of highly pathogenic avian influenza in turkeys in Great Britain in 1991. Vet Rec. 1993;132:535–536. doi: 10.1136/vr.132.21.535. [DOI] [PubMed] [Google Scholar]
- 2.Alexander D J, Parsons G, Manvell R J. Experimental assessment of the pathogenicity of eight avian influenza A viruses of H5 subtype for chickens, turkeys, ducks and quail. Avian Pathol. 1986;15:647–662. doi: 10.1080/03079458608436328. [DOI] [PubMed] [Google Scholar]
- 3.Almond J W. A single gene determines the host range of influenza virus. Nature (London) 1977;270:617–618. doi: 10.1038/270617a0. [DOI] [PubMed] [Google Scholar]
- 4.Austin F J, Webster R G. Antigenic mapping of an avian H1 influenza virus haemagglutinin and interrelationships of H1 viruses from humans, pigs and birds. J Gen Virol. 1986;67:983–992. doi: 10.1099/0022-1317-67-6-983. [DOI] [PubMed] [Google Scholar]
- 5.Banks J, Speidel E, Alexander D J. Characterisation of an avian influenza A virus isolated from a human—is an intermediate host necessary for the emergence of pandemic influenza viruses? Arch Virol. 1998;143:781–787. doi: 10.1007/s007050050329. [DOI] [PubMed] [Google Scholar]
- 6.Barr P J. Mammalian subtilisins: the long-sought dibasic processing endoproteases. Cell. 1991;66:1–3. doi: 10.1016/0092-8674(91)90129-m. [DOI] [PubMed] [Google Scholar]
- 7.Bashiruddin J B, Gould A R, Westbury H A. Molecular pathotyping of two avian influenza viruses isolated during the Victoria 1976 outbreak. Aust Vet J. 1992;69:140–142. doi: 10.1111/j.1751-0813.1992.tb07485.x. [DOI] [PubMed] [Google Scholar]
- 8.Baum L G, Paulson J C. The N2 neuraminidase of human influenza virus has acquired a substrate specificity complementary to the hemagglutinin receptor specificity. Virology. 1991;180:10–15. doi: 10.1016/0042-6822(91)90003-t. [DOI] [PubMed] [Google Scholar]
- 9.Bean W J, Cox N J, Kendal A P. Recombination of human influenza A viruses in nature. Nature (London) 1980;284:638–640. doi: 10.1038/284638a0. [DOI] [PubMed] [Google Scholar]
- 10.Bean W J, Kawaoka Y, Wood J M, Pearson J E, Webster R G. Characterization of virulent and avirulent A/Chicken/Pennsylvania/83 influenza A viruses: potential role of defective interfering RNAs in nature. J Virol. 1985;54:151–160. doi: 10.1128/jvi.54.1.151-160.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bean W J, Schell M, Katz J, Kawaoka Y, Naeve C, Gorman O, Webster R G. Evolution of the H3 influenza virus hemagglutinin from human and nonhuman hosts. J Virol. 1992;66:1129–1138. doi: 10.1128/jvi.66.2.1129-1138.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bean W J, Threlkeld S C, Webster R G. Biologic potential of amantadine-resistant influenza A virus in an avian model. J Infect Dis. 1989;159:1050–1056. doi: 10.1093/infdis/159.6.1050. [DOI] [PubMed] [Google Scholar]
- 13.Beard C W, Brugh M, Webster R G. Emergence of amantadine-resistant H5N2 avian influenza virus during a simulated layer flock treatment program. Avian Dis. 1987;31:533–537. [PubMed] [Google Scholar]
- 14.Beare A S, Webster R G. Replication of avian influenza viruses in humans. Arch Virol. 1991;119:37–42. doi: 10.1007/BF01314321. [DOI] [PubMed] [Google Scholar]
- 15.Becker W B. The isolation and classification of tern virus: influenza virus A/Tern/South Africa/61. J Hyg. 1966;64:309–320. doi: 10.1017/s0022172400040596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonin J, Scholtissek C. Mouse neurotropic recombinants of influenza A viruses. Arch Virol. 1983;75:255–268. doi: 10.1007/BF01314891. [DOI] [PubMed] [Google Scholar]
- 17.Bosch F X, Garten W, Klenk H-D, Rott R. Proteolytic cleavage of influenza virus hemagglutinins: primary structure of the connecting peptide between HA1 and HA2 determines proteolytic cleavability and pathogenicity of avian influenza viruses. Virology. 1981;113:725–735. doi: 10.1016/0042-6822(81)90201-4. [DOI] [PubMed] [Google Scholar]
- 18.Bosch F X, Orlich M, Klenk H-D, Rott R. The structure of the hemagglutinin, a determinant for the pathogenicity of influenza viruses. Virology. 1979;95:197–207. doi: 10.1016/0042-6822(79)90414-8. [DOI] [PubMed] [Google Scholar]
- 19.Brennan S O, Nakayama K. Cleavage of proalbumin peptides by furin reveals unexpected restrictions at the P2 and P′1 sites. FEBS Lett. 1994;347:80–84. doi: 10.1016/0014-5793(94)00511-7. [DOI] [PubMed] [Google Scholar]
- 20.Brown I H, Alexander D J, Chakraverty P, Harris P A, Manvell R J. Isolation of an influenza A virus of unusual subtype (H1N7) from pigs in England, and the subsequent experimental transmission from pig to pig. Vet Microbiol. 1994;39:125–134. doi: 10.1016/0378-1135(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 21.Brown I H, Hill M L, Harris P A, Alexander D J, McCauley J W. Genetic characterisation of an influenza A virus of unusual subtype (H1N7) isolated from pigs in England. Arch Virol. 1997;142:1045–1050. doi: 10.1007/s007050050140. [DOI] [PubMed] [Google Scholar]
- 22.Brugh M, Beck J R. Proceedings of the Third International Symposium on Avian Influenza. 1992. Recovery of minority subpopulation of highly pathogenic avian influenza virus; pp. 166–174. [Google Scholar]
- 23.Bullough P A, Hughson F M, Skehel J J, Wiley D C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature (London) 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- 24.Burnet F M. Influenza virus on the developing egg. Br J Exp Pathol. 1936;17:282. [Google Scholar]
- 25.Calfee D P, Hayden F G. New approaches to influenza chemotherapy—neuraminidase inhibitors. Drugs. 1998;56:537–553. doi: 10.2165/00003495-199856040-00003. [DOI] [PubMed] [Google Scholar]
- 26.Callan R J, Early G, Kida H, Hinshaw V S. The appearance of H3 influenza viruses in seals. J Gen Virol. 1995;76:199–203. doi: 10.1099/0022-1317-76-1-199. [DOI] [PubMed] [Google Scholar]
- 27.Campbell C H, Webster R G, Breese S S J. Fowl plague virus from man. J Infect Dis. 1970;122:513–516. doi: 10.1093/infdis/122.6.513. [DOI] [PubMed] [Google Scholar]
- 28.Castrucci M R, Campitelli L, Ruggieri A, Barigazzi G, Sidoli L, Daniels R, Oxford J S, Donatelli I. Antigenic and sequence analysis of H3 influenza virus haemagglutinins from pigs in Italy. J Gen Virol. 1994;75:371–379. doi: 10.1099/0022-1317-75-2-371. [DOI] [PubMed] [Google Scholar]
- 29.Castrucci M R, Donatelli I, Sidoli L, Barigazzi G, Kawaoka Y, Webster R G. Genetic reassortment between avian and human influenza A viruses in Italian pigs. Virology. 1993;193:503–506. doi: 10.1006/viro.1993.1155. [DOI] [PubMed] [Google Scholar]
- 30.Castrucci M R, Kawaoka Y. Biologic importance of neuraminidase stalk length in influenza A virus. J Virol. 1993;67:759–764. doi: 10.1128/jvi.67.2.759-764.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Update. Isolation of avian influenza A (H5N1) viruses from humans—Hong Kong, 1997–1998. Morb Mortal Wkly Rep. 1998;46:1245–1247. [PubMed] [Google Scholar]
- 32.Chambers T M, Yamnikova S, Kawaoka Y, Lvov D K, Webster R G. Antigenic and molecular characterization of subtype H13 hemagglutinin of influenza virus. Virology. 1989;172:180–188. doi: 10.1016/0042-6822(89)90119-0. [DOI] [PubMed] [Google Scholar]
- 33.Claas E C J, Kawaoka Y, De Jong J C, Masurel N, Webster R G. Infection of children with avian-human reassortant influenza virus from pigs in Europe. Virology. 1994;204:453–457. doi: 10.1006/viro.1994.1553. [DOI] [PubMed] [Google Scholar]
- 34.Claas E J, Osterhaus A E, Van Beek R, De Jong J C, Rimmelzwaan G F, Senne D A, Krauss S, Shortridge K F, Webster R G. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 35.Cockburn W C, Delon P J, Ferreira W. Origin and progress of the 1968–1969 Hong Kong influenza epidemic. Bull W H O. 1969;41:345–353. [PMC free article] [PubMed] [Google Scholar]
- 36.Colman P M, Laver W G, Varghese J N, Baker A T, Tulloch P A, Air G M, Webster R G. The three-dimensional structure of a complex of influenza virus neuraminidase and an antibody. Nature (London) 1987;326:358–363. doi: 10.1038/326358a0. [DOI] [PubMed] [Google Scholar]
- 37.Connor R J, Kawaoka Y, Webster R G, Paulson J C. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology. 1994;205:17–23. doi: 10.1006/viro.1994.1615. [DOI] [PubMed] [Google Scholar]
- 38.Couceiro J N S S, Paulson J C, Baum L G. Influenza virus strains selectively recognize sialyloligosaccharides on human respiratory epithelium: the role of the host cell in selection of hemagglutinin receptor specificity. Virus Res. 1993;29:155–165. doi: 10.1016/0168-1702(93)90056-s. [DOI] [PubMed] [Google Scholar]
- 39.Crosby A W. America's forgotten pandemic: the influenza of 1918. Cambridge, United Kingdom: Cambridge University Press; 1989. [Google Scholar]
- 40.Cross G M. Proceedings of the Second International Symposium on Avian Influenza. 1987. The status of avian influenza in poultry in Australia; pp. 96–103. [Google Scholar]
- 41.Desphande K L, Fried V A, Ando M, Webster R G. Glycosylation affects cleavage of an H5N2 influenza virus hemagglutinin and regulates virulence. Proc Natl Acad Sci USA. 1987;84:36–40. doi: 10.1073/pnas.84.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donis R O, Bean W J, Kawaoka Y, Webster R G. Distinct lineages of influenza virus H4 hemagglutinin genes in different regions of the world. Virology. 1989;169:408–417. doi: 10.1016/0042-6822(89)90166-9. [DOI] [PubMed] [Google Scholar]
- 43.Easterday B C, Hinshaw V S, Halvorson D A. Influenza. In: Calnek B W, Barnes H J, Beard C W, McDougald L R, Saif Y M, editors. Diseases of poultry. Ames: Iowa State University Press; 1997. pp. 583–605. [Google Scholar]
- 44.Eckroade R J, Bachin L A S. Proceedings of the Second International Symposium on Avian Influenza. 1987. Avian influenza in Pennsylvania: the beginning; pp. 22–32. [Google Scholar]
- 45.Els M C, Air G M, Murti K G, Webster R G, Laver W G. An 18 amino acid deletion in an influenza neuraminidase. Virology. 1985;142:241–248. doi: 10.1016/0042-6822(85)90332-0. [DOI] [PubMed] [Google Scholar]
- 46.Enami M, Luytjes W, Krystal M, Palese P. Introduction of site-specific mutations into the genome of influenza virus. Proc Natl Acad Sci USA. 1990;87:3802–3805. doi: 10.1073/pnas.87.10.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gambaryan A S, Tuzikov A B, Piskarev V E, Yamnikova S S, Lvov D K, Robertson J S, Bovin N V, Matrosovich M N. Specification of receptor-binding phenotypes of influenza virus isolates from different hosts using synthetic sialylglycopolymers: non-egg-adapted human H1 and H3 influenza A and influenza B viruses share a common high binding affinity for 6′-sialyl(N-acetyllactosamine) Virology. 1997;232:345–350. doi: 10.1006/viro.1997.8572. [DOI] [PubMed] [Google Scholar]
- 48.Gao P, Watanabe S, Ito T, Goto H, Wells K, McGregor M, Cooley A J, Kawaoka Y. Biological heterogeneity, including systemic replication in mice, of H5N1 influenza A virus isolates from humans in Hong Kong. J Virol. 1999;73:3184–3189. doi: 10.1128/jvi.73.4.3184-3189.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia M, Crawford J M, Latimer J W, Rivera-Cruz E, Perdue M L. Heterogeneity in the haemagglutinin gene and emergence of the highly pathogenic phenotype among recent H5N2 avian influenza viruses from Mexico. J Gen Virol. 1996;77:1493–1504. doi: 10.1099/0022-1317-77-7-1493. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Sastre A, Egorov A, Matassov D, Brandt S, Levy D E, Durbin J E, Palese P, Muster T. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology. 1998;252:324–330. doi: 10.1006/viro.1998.9508. [DOI] [PubMed] [Google Scholar]
- 51.Garten W, Klenk H-D. Understanding influenza virus pathogenicity. Trends Microbiol. 1999;7:99–100. doi: 10.1016/s0966-842x(99)01460-2. [DOI] [PubMed] [Google Scholar]
- 52.Geraci J R, St. Aubin D J, Barker I K, Webster R G, Hinshaw V S, Bean W J, Ruhnke H L, Prescott J H, Early G, Baker A S, Madoff S, Schooley R T. Mass mortality of harbor seals: pneumonia associated with influenza A virus. Science. 1982;215:1129–1131. doi: 10.1126/science.7063847. [DOI] [PubMed] [Google Scholar]
- 53.Gorman O T, Bean W J, Kawaoka Y, Donatelli I, Guo Y, Webster R G. Evolution of influenza A virus nucleoprotein genes: implications for the origins of H1N1 human and classical swine viruses. J Virol. 1991;65:3704–3714. doi: 10.1128/jvi.65.7.3704-3714.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goto H, Bethell R C, Kawaoka Y. Mutations affecting the sensitivity of the influenza virus neuraminidase to 4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid. Virology. 1997;238:265–272. doi: 10.1006/viro.1997.8810. [DOI] [PubMed] [Google Scholar]
- 55.Goto H, Kawaoka Y. A novel mechanism for the acquisition of virulence by a human influenza A virus. Proc Natl Acad Sci USA. 1998;95:10224–10228. doi: 10.1073/pnas.95.17.10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gotoh B, Ogasawara T, Toyoda T, Inocencio N M, Hamaguchi M, Nagai Y. An endoprotease homologous to the blood clotting factor X as a determinant of viral tropism in chick embryo. EMBO J. 1990;9:4189–4195. doi: 10.1002/j.1460-2075.1990.tb07643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gottschalk A. The specific enzyme of influenza virus and Vibrio cholerae. Biochim Biophys Acta. 1957;23:645–646. doi: 10.1016/0006-3002(57)90389-x. [DOI] [PubMed] [Google Scholar]
- 58.Grambas S, Bennett M S, Hay A J. Influence of amantadine resistance mutations on the pH regulatory function of the M2 protein of influenza A viruses. Virology. 1992;191:541–549. doi: 10.1016/0042-6822(92)90229-i. [DOI] [PubMed] [Google Scholar]
- 59.Guan Y, Shortridge K F, Krauss S, Li P H, Kawaoka Y, Webster R G. Emergence of avian H1N1 influenza viruses in pigs in China. J Virol. 1996;70:8041–8046. doi: 10.1128/jvi.70.11.8041-8046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guan Y, Shortridge K F, Krauss S, Webster R G. Molecular characterization of H9N2 influenza viruses: were they the donors of the “internal” genes of H5N1 viruses in Hong Kong? Proc Natl Acad Sci USA. 1999;96:9363–9367. doi: 10.1073/pnas.96.16.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gubareva L V, Matrosovich M N, Brenner M K, Bethell R C, Webster R G. Evidence for zanamivir resistance in an immunocompromised child infected with influenza B virus. J Infect Dis. 1998;178:1257–1262. doi: 10.1086/314440. [DOI] [PubMed] [Google Scholar]
- 62.Gubareva L V, McCullers J A, Bethell R C, Webster R G. Characterization of influenza A/HongKong/156/97 (H5N1) virus in a mouse model and protective effect of zanamivir on H5N1 infection in mice. J Infect Dis. 1998;178:1592–1596. doi: 10.1086/314515. [DOI] [PubMed] [Google Scholar]
- 63.Günther I, Glatthaar B, Döller G, Garten W. A H1 hemagglutinin of a human influenza A virus with a carbohydrate-modulated receptor binding site and an unusual cleavage site. Virus Res. 1993;27:147–160. doi: 10.1016/0168-1702(93)90078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo Y, Wang M, Kawaoka Y, Gorman O, Ito T, Saito Y, Webster R G. Characterization of a new avian-like influenza A virus from horses in China. Virology. 1992;188:245–255. doi: 10.1016/0042-6822(92)90754-d. [DOI] [PubMed] [Google Scholar]
- 65.Guo Y, Wang M, Zheng G-S, Li W, Kawaoka Y, Webster R G. Seroepidemiological and molecular evidence for the presence of two H3N8 equine influenza viruses in China in 1993–94. J Gen Virol. 1995;76:2009–2014. doi: 10.1099/0022-1317-76-8-2009. [DOI] [PubMed] [Google Scholar]
- 66.Guo Y, Xu X, Cox N J. Human influenza A (H1N2) viruses isolated from China. J Gen Virol. 1992;73:383–388. doi: 10.1099/0022-1317-73-2-383. [DOI] [PubMed] [Google Scholar]
- 67.Hay A J, Wolstenholme A J, Skehel J J, Smith M H. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 1985;4:3021–3024. doi: 10.1002/j.1460-2075.1985.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hayden F G, Hay A J. Emergence and transmission of influenza A viruses resistant to amantadine and rimantadine. Curr Top Microbiol Immunol. 1992;176:119–130. doi: 10.1007/978-3-642-77011-1_8. [DOI] [PubMed] [Google Scholar]
- 69.Hayden F G, Osterhaus A E, Treanor J J, Fleming D M, Aoki F Y, Nicholson K G, Bohnen A M, Hirst H M, Keene O, Wightman K. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza virus infections. N Engl J Med. 1997;337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- 70.Hinshaw V S, Bean W J, Geraci J R, Fiorelli P, Early G, Webster R G. Characterization of two influenza A viruses from a pilot whale. J Virol. 1986;58:655–656. doi: 10.1128/jvi.58.2.655-656.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hinshaw V S, Bean W J, Webster R G, Rehg J E, Fiorelli P, Early G, Geraci J R, St. Aubin D J. Are seals frequently infected with avian influenza viruses? JVirol. 1984;51:863–865. doi: 10.1128/jvi.51.3.863-865.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hinshaw V S, Bean W J, Webster R G, Sriram G. Genetic reassortment of influenza A viruses in the intestinal tract of ducks. Virology. 1980;102:412–419. doi: 10.1016/0042-6822(80)90108-7. [DOI] [PubMed] [Google Scholar]
- 73.Hinshaw V S, Webster R G, Naeve C W, Murphy B R. Altered tissue tropism of human-avian reassortant influenza viruses. Virology. 1983;128:260–263. doi: 10.1016/0042-6822(83)90337-9. [DOI] [PubMed] [Google Scholar]
- 74.Hinshaw V S, Webster R G, Turner B. The perpetuation of orthomyxoviruses and paramyxoviruses in Canadian waterfowl. Can J Microbiol. 1980;26:622–629. doi: 10.1139/m80-108. [DOI] [PubMed] [Google Scholar]
- 75.Hirst G K. Agglutination of red cells by allantoic fluid of chick embryos infected with influenza virus. Science. 1941;94:22–23. doi: 10.1126/science.94.2427.22. [DOI] [PubMed] [Google Scholar]
- 76.Horimoto T, Kawaoka Y. Reverse genetics provides direct evidence for a correlation of hemagglutinin cleavability and virulence of an avian influenza A virus. J Virol. 1994;68:3120–3128. doi: 10.1128/jvi.68.5.3120-3128.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Horimoto T, Kawaoka Y. Molecular changes in virulent mutants arising from avirulent avian influenza viruses during replication in 14-day-old embryonated eggs. Virology. 1995;206:755–759. doi: 10.1016/s0042-6822(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 78.Horimoto T, Nakayama K, Smeekens S P, Kawaoka Y. Proprotein-processing endoproteases PC6 and furin both activate hemagglutinin of virulent avian influenza viruses. J Virol. 1994;68:6074–6078. doi: 10.1128/jvi.68.9.6074-6078.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Horimoto T, Rivera E, Pearson J, Senne D, Krauss S, Kawaoka Y, Webster R G. Origin and molecular changes associated with emergence of a highly pathogenic H5N2 influenza virus in Mexico. Virology. 1995;213:223–230. doi: 10.1006/viro.1995.1562. [DOI] [PubMed] [Google Scholar]
- 80.Ito T, Couceiro J S, Kelm S, Baum L G, Krauss S, Castrucci M R, Donatelli I, Kida H, Paulson J C, Webster R G, Kawaoka Y. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J Virol. 1998;72:7367–7373. doi: 10.1128/jvi.72.9.7367-7373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ito T, Okazaki K, Kawaoka Y, Takada A, Webster R G, Kida H. Perpetuation of influenza A viruses in Alaskan waterfowl reservoirs. Arch Virol. 1995;140:1163–1172. doi: 10.1007/BF01322743. [DOI] [PubMed] [Google Scholar]
- 82.Ito T, Suzuki Y, Takada A, Kawamoto A, Otsuki K, Masuda H, Yamada M, Suzuki T, Kida H, Kawaoka Y. Differences in sialic acid-galactose linkages in the amnion and allantois of chicken eggs influence human influenza virus receptor specificity and varian selection. J Virol. 1997;71:3357–3362. doi: 10.1128/jvi.71.4.3357-3362.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johnson D C, Maxfield B G, Moulthrop J I. Epidemiologic studies of the 1975 avian influenza outbreak in chickens in Alabama. Avian Dis. 1976;21:167–177. [PubMed] [Google Scholar]
- 84.Kaplan M, Beveridge W I. WHO coordinated research on the role of animals in influenza epidemiology: introduction. Bull W H O. 1972;47:439–448. [PMC free article] [PubMed] [Google Scholar]
- 85.Kawaoka Y. Structural features influencing hemagglutinin cleavability in a human influenza A virus. J Virol. 1991;65:1195–1201. doi: 10.1128/jvi.65.3.1195-1201.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kawaoka Y, Krauss S, Webster R G. Avian-to-human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J Virol. 1989;63:4603–4608. doi: 10.1128/jvi.63.11.4603-4608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kawaoka Y, Naeve C W, Webster R G. Is virulence of H5N2 influenza viruses in chickens associated with loss of carbohydrate from the hemagglutinin? Virology. 1984;139:303–316. doi: 10.1016/0042-6822(84)90376-3. [DOI] [PubMed] [Google Scholar]
- 88.Kawaoka Y, Nestorowicz A, Alexander D J, Webster R G. Molecular analyses of the hemagglutinin genes of H5 influenza viruses: origin of a virulent turkey strain. Virology. 1987;158:218–227. doi: 10.1016/0042-6822(87)90256-x. [DOI] [PubMed] [Google Scholar]
- 89.Kawaoka Y, Webster R G. Evolution of the A/Chicken/Pennsylvania/83 (H5N2) influenza virus. Virology. 1985;146:130–137. doi: 10.1016/0042-6822(85)90059-5. [DOI] [PubMed] [Google Scholar]
- 90.Kawaoka Y, Webster R G. Molecular mechanism of acquisition of virulence in influenza virus in nature. Microb Pathog. 1988;5:311–318. doi: 10.1016/0882-4010(88)90032-0. [DOI] [PubMed] [Google Scholar]
- 91.Kawaoka Y, Webster R G. Sequence requirements for cleavage activation of influenza virus hemagglutinin expressed in mammalian cells. Proc Natl Acad Sci USA. 1988;85:324–328. doi: 10.1073/pnas.85.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kawaoka Y, Webster R G. Interplay between carbohydrate in the stalk and the length of the connecting peptide determines the cleavability of influenza virus hemagglutinin. J Virol. 1989;63:3296–3300. doi: 10.1128/jvi.63.8.3296-3300.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kawaoka Y, Yamnikova S, Chambers T M, Lvov D K, Webster R G. Molecular characterization of a new hemagglutinin, subtype H14, of influenza A virus. Virology. 1990;179:759–767. doi: 10.1016/0042-6822(90)90143-f. [DOI] [PubMed] [Google Scholar]
- 94.Khatchikian D, Orlich M, Rott R. Increased viral pathogenicity after insertion of a 28S ribosomal RNA sequence into the haemagglutinin gene of an influenza virus. Nature (London) 1989;340:156–157. doi: 10.1038/340156a0. [DOI] [PubMed] [Google Scholar]
- 95.Kida H, Ito T, Yasuda J, Shimizu Y, Itakura C, Shortridge K F, Kawaoka Y, Webster R G. Potential for transmission of avian influenza viruses to pigs. J Gen Virol. 1994;75:2183–2188. doi: 10.1099/0022-1317-75-9-2183. [DOI] [PubMed] [Google Scholar]
- 96.Kida H, Kawaoka Y, Naeve C W, Webster R G. Antigenic and genetic conservation of H3 influenza in wild ducks. Virology. 1987;159:109–119. doi: 10.1016/0042-6822(87)90353-9. [DOI] [PubMed] [Google Scholar]
- 97.Kida H, Shortridge K F, Webster R G. Origin of the hemagglutinin gene of H3N2 influenza viruses from pigs in China. Virology. 1988;162:160–166. doi: 10.1016/0042-6822(88)90405-9. [DOI] [PubMed] [Google Scholar]
- 98.Kida H, Yanagawa R, Matsuoka Y. Duck influenza lacking evidence of disease signs and immune response. Infect Immun. 1980;30:547–553. doi: 10.1128/iai.30.2.547-553.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kido H, Yokogoshi Y, Sakai K, Tashiro M, Kishino Y, Fukutomi A, Katunuma N. Isolation and characterization of a novel trypsin-like protease found in rat bronchiolar epithelial Clara cells. A possible activator of the viral fusion glycoprotein. J Biol Chem. 1992;267:13573–13579. [PubMed] [Google Scholar]
- 100.Klenk H-D, Garten W. Host cell proteases controlling virus pathogenicity. Trends Microbiol. 1994;2:39–43. doi: 10.1016/0966-842x(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 101.Klenk H-D, Rott R. The molecular biology of influenza virus pathogenicity. Adv Virus Res. 1988;34:247–281. doi: 10.1016/S0065-3527(08)60520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Klenk H-D, Rott R, Orlich M, Blodorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975;68:426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- 103.Klingeborn B, Englund L, Rott R, Juntti N, Rockborn G. An avian influenza A virus killing a mammalian species—the mink. Arch Virol. 1985;86:347–351. doi: 10.1007/BF01309839. [DOI] [PubMed] [Google Scholar]
- 104.Kobasa D, Kodihalli S, Luo M, Castrucci M R, Donatelli I, Suzuki Y, Suzuki T, Kawaoka Y. Amino acid residues contributing to the substrate specificity of the influenza A virus neuraminidase. J Virol. 1999;73:6743–6751. doi: 10.1128/jvi.73.8.6743-6751.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kobayashi Y, Horimoto T, Kawaoka Y, Alexander D J, Itakura C. Pathological studies of chickens experimentally infected with two highly pathogenic avian influenza viruses. Avian Pathol. 1996;25:285–304. doi: 10.1080/03079459608419142. [DOI] [PubMed] [Google Scholar]
- 106.Krug R M, Broni B A, Bouloy M. Are the 5′ ends of influenza viral mRNAs synthesized in vivo donated by host mRNAs? Cell. 1979;18:329–334. doi: 10.1016/0092-8674(79)90052-7. [DOI] [PubMed] [Google Scholar]
- 107.Kung H C, Jen K F, Yuan W C. Influenza in 1977: recurrence of influenza virus A subtype H1N1. Bull W H O. 1978;56:913–918. [PMC free article] [PubMed] [Google Scholar]
- 108.Kurtz J, Manvell R J, Banks J. Avian influenza virus isolated from a woman with conjunctivitis. Lancet. 1996;348:901–902. doi: 10.1016/S0140-6736(05)64783-6. [DOI] [PubMed] [Google Scholar]
- 109.Lamb R A, Krug R M. Orthomyxoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1353–1395. [Google Scholar]
- 110.Lazarowitz S G, Choppin P W. Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology. 1975;68:440–454. doi: 10.1016/0042-6822(75)90285-8. [DOI] [PubMed] [Google Scholar]
- 111.Li S Q, Liu C G, Klimov A, Subbarao K, Perdue M L, Mo D, Ji Y Y, Woods L, Hietala S, Bryant M. Recombinant influenza A virus vaccines for the pathogenic human A Hong Kong 97 (H5N1) viruses. J Infect Dis. 1999;179:1132–1138. doi: 10.1086/314713. [DOI] [PubMed] [Google Scholar]
- 112.Lu X H, Tumpey T M, Morken T, Zaki S R, Cox N J, Katz J M. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J Virol. 1999;73:5903–5911. doi: 10.1128/jvi.73.7.5903-5911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lvov D K, Zhdanov V M, Sazonov A A, Braude N A, Vladimirtceva E A, Agafonova L V, Skijanskaja E I, Kaverin N V, Reznik V I, Pysina T V, Oserovic A M, Berzin A A, Mjasnikova I A, Podcernjaeva R Y, Klimenko S M, Andrejev V P, Yakhno M A. Comparison of influenza viruses isolated from man and from whales. Bull W H O. 1978;56:923–930. [PMC free article] [PubMed] [Google Scholar]
- 114.Martin K, Helenius A. Nuclear transport of influenza virus ribonucleoproteins: the viral matrix protein (M1) promotes export and inhibits import. Cell. 1991;67:117–130. doi: 10.1016/0092-8674(91)90576-k. [DOI] [PubMed] [Google Scholar]
- 115.Marwick C. Influenza vaccine virus strains chosen. JAMA. 1998;279:1150–1151. [PubMed] [Google Scholar]
- 116.Matrosovich M, Zhou N, Kawaoka Y, Webster R. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J Virol. 1999;73:1146–1155. doi: 10.1128/jvi.73.2.1146-1155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Matrosovich M N, Gambaryan A S, Teneberg S, Piskarev V E, Yamnikova S S, Lvov D K, Robertson J S, Karlsson K A. Avian influenza a viruses differ from human viruses by recognition of sialyloligosaccharides and gangliosides and by a higher conservation of the HA receptor-binding site. Virology. 1997;233:224–234. doi: 10.1006/viro.1997.8580. [DOI] [PubMed] [Google Scholar]
- 118.Matsuura Y, Yanagawa R, Noda H. Experimental infection of mink with influenza A viruses. Arch Virol. 1979;62:71–76. doi: 10.1007/BF01314905. [DOI] [PubMed] [Google Scholar]
- 119.Mo I P, Brugh M, Fletcher O J, Rowland G N, Swayne D E. Comparative pathology of chickens experimentally inoculated with avian influenza viruses of low and high pathogenicity. Avian Dis. 1997;41:125–136. [PubMed] [Google Scholar]
- 120.Molloy S S, Bresnahan P A, Leppla S H, Klimpe K R, Thomas G. Human furin is a calcium-dependent serin endoprotease that recognize the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J Biol Chem. 1992;267:16396–16402. [PubMed] [Google Scholar]
- 121.Mounts A W, Kwong H, Izurieta H S, Ho Y Y, Au T K, Lee M, Bridges C B, Williams S W, Mak K H, Katz J M, Thompson W W, Cox N J, Fukuda K. Case-control study of risk factors for avian influenza A (H5N1) disease, Hong Kong, 1997. J Infect Dis. 1999;180:505–508. doi: 10.1086/314903. [DOI] [PubMed] [Google Scholar]
- 122.Mukaigawa J, Nayak D P. Two signals mediate nuclear localization of influenza virus (A/WSN/33) polymerase basic protein 2. J Virol. 1991;65:245–253. doi: 10.1128/jvi.65.1.245-253.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Murphy B R, Harper J S, Sly D L, London W T, Miller N T, Webster R G. Evaluation of the A/Seal/Mass/1/80 virus in squirrel monkeys. Infect Immun. 1983;42:424–426. doi: 10.1128/iai.42.1.424-426.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Murphy B R, Hinshaw V S, Sly D L, London W T, Hosier N T, Wood F T, Webster R G, Chanock R M. Virulence of avian influenza A viruses for squirrel monkeys. Infect Immun. 1982;37:1119–1126. doi: 10.1128/iai.37.3.1119-1126.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Murphy B R, Webster R G. Orthomyxoviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1397–1445. [Google Scholar]
- 126.Naeve C W, Hinshaw V S, Webster R G. Mutations in the hemagglutinin receptor-binding site can change the biological properties of an influenza virus. J Virol. 1984;51:567–569. doi: 10.1128/jvi.51.2.567-569.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nakajima K, Desselberger U, Palese P. Recent human influenza A (H1N1) viruses are closely related genetically to strains isolated in 1950. Nature (London) 1978;274:334–339. doi: 10.1038/274334a0. [DOI] [PubMed] [Google Scholar]
- 128.Nakayama K. Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem J. 1997;327:625–635. doi: 10.1042/bj3270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nath S T, Nayak D P. Function of two discrete regions is required for nuclear localization of polymerase basic protein 1 of A/WSN/33 influenza virus (HIN1) Mol Cell Biol. 1990;10:4139–4145. doi: 10.1128/mcb.10.8.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nerome K, Kanegae Y, Shortridge K F, Sugita S, Ishida M. Genetic analysis of porcine H3N2 viruses originating in southern China. J Gen Virol. 1995;76:613–624. doi: 10.1099/0022-1317-76-3-613. [DOI] [PubMed] [Google Scholar]
- 131.Nestorowicz A, Kawaoka Y, Bean W J, Webster R G. Molecular analysis of the hemagglutinin genes of Australian H7N7 influenza viruses: role of passerine birds in maintenance or transmission? Virology. 1987;160:411–418. doi: 10.1016/0042-6822(87)90012-2. [DOI] [PubMed] [Google Scholar]
- 132.Neumann G, Castrucci M R, Kawaoka Y. Nuclear import and export of influenza virus nucleoprotein. J Virol. 1997;71:9690–9700. doi: 10.1128/jvi.71.12.9690-9700.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ogawa T, Ueda M. Genes involved in the virulence of an avian influenza virus. Virology. 1981;113:304–313. doi: 10.1016/0042-6822(81)90157-4. [DOI] [PubMed] [Google Scholar]
- 134.Ohuchi M, Orlich M, Ohuchi R, Simpson B E J, Garten W, Klenk H-D, Rott R. Mutations at the cleavage site of the hemagglutinin alter the pathogenicity of influenza virus A/chick/Penn/83 (H5N2) Virology. 1989;168:274–280. doi: 10.1016/0042-6822(89)90267-5. [DOI] [PubMed] [Google Scholar]
- 135.Ohuchi R, Ohuchi M, Garten W, Klenk H-D. Human influenza virus hemagglutinin with high sensitivity to proteolytic activation. J Virol. 1991;65:3530–3537. doi: 10.1128/jvi.65.7.3530-3537.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Okazaki K, Yanagawa R, Kida H. Contact infection of mink with 5 subtypes of avian influenza virus. Arch Virol. 1983;77:265–269. doi: 10.1007/BF01309274. [DOI] [PubMed] [Google Scholar]
- 137.O'Neill R E, Talon J, Palese P. The influenza virus NEP (NS2 protein) mediates the nuclear export of viral ribonucleoproteins. EMBO J. 1998;17:288–296. doi: 10.1093/emboj/17.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Orlich M, Gottwald H, Rott R. Nonhomologous recombination between the hemagglutinin gene and the nucleoprotein gene of an influenza virus. Virology. 1994;204:462–465. doi: 10.1006/viro.1994.1555. [DOI] [PubMed] [Google Scholar]
- 139.Palese P, Tobita K, Ueda M, Compans R W. Characterization of temperature-sensitive influenza virus mutants defective in neuraminidase. Virology. 1974;61:397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- 140.Paulson J C. Interactions of animal viruses with cell surface receptors. In: Connor M, editor. The receptors. Orlando, Fla: Academic Press, Inc.; 1985. pp. 131–219. [Google Scholar]
- 141.Peiris M, Yam W C, Chan K H, Ghose P, Shortridge K F. Influenza A H9N2: aspects of laboratory diagnosis. J Clin Microbiol. 1999;37:3426–3427. doi: 10.1128/jcm.37.10.3426-3427.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pensaert M, Ottis K, Vandeputte J, Kaplan M M, Bachmann P A. Evidence for the natural transmission of influenza A virus from wild ducks to swine and its potential importance for man. Bull W H O. 1981;59:75–78. [PMC free article] [PubMed] [Google Scholar]
- 143.Perdue M L, Garcia M, Beck J, Brugh M, Swayne D E. An Arg-Lys insertion at the hemagglutinin cleavage site of an H5N2 avian influenza isolate. Virus Genes. 1996;12:77–84. doi: 10.1007/BF00370003. [DOI] [PubMed] [Google Scholar]
- 144.Perdue M L, Garcia M, Senne D. Virulence-associated sequence duplication at the hemagglutinin cleavage site of avian influenza viruses. Virus Res. 1997;49:173–186. doi: 10.1016/s0168-1702(97)01468-8. [DOI] [PubMed] [Google Scholar]
- 145.Perroncito E. Epizoozia tifoide nei gallinacei. Ann Acad Agric. 1878;21:87. [Google Scholar]
- 146.Pinto L H, Holsinger L J, Lamb R A. Influenza virus M2 protein has ion channel activity. Cell. 1992;69:517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- 147.Plotch S J, Bouloy M, Ulmanen I, Krug R M. A unique cap (m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell. 1981;23:847–858. doi: 10.1016/0092-8674(81)90449-9. [DOI] [PubMed] [Google Scholar]
- 148.Poss P E, Halvorson D A. Proceedings of the 2nd International Symposium on Avian Influenza. U.S. Animal Health Association; 1987. The nature of avian influenza in turkeys in Minnesota; pp. 112–117. [Google Scholar]
- 149.Potter C W. Chronicle of influenza pandemics. In: Nicholson K G, Webster R G, Hay A J, editors. Textbook of influenza. Oxford, United Kingdom: Blackwell Scientific Publications, Ltd.; 1998. pp. 3–18. [Google Scholar]
- 150.Pyle G F, Patterson K D. Influenza diffusion in European history: patterns and paradigms. Ecol Dis. 1984;2:173–184. [Google Scholar]
- 151.Reid A H, Fanning T G, Hultin J V, Taubenberger JK. Origin and evolution of the 1918 “Spanish” influenza virus hemagglutinin gene. Proc Natl Acad Sci USA. 1999;96:1651–1656. doi: 10.1073/pnas.96.4.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Richardson J C, Akkina R K. NS2 protein of influenza virus is found in purified virus and phosphorylated in infected cells. Arch Virol. 1991;116:69–80. doi: 10.1007/BF01319232. [DOI] [PubMed] [Google Scholar]
- 153.Roebroek A J, Schalken J A, Leunissen J A, Onnekink C, Bloemers H P, van de Ven W J. Evolutionary conserved close linkage of the c-fes/fps proto-oncogene and genetic sequences encoding a receptor-like protein. EMBO J. 1986;5:2197–2202. doi: 10.1002/j.1460-2075.1986.tb04484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rogers G N, D'Souza B L. Receptor binding properties of human and animal H1 influenza virus isolates. Virology. 1989;173:317–322. doi: 10.1016/0042-6822(89)90249-3. [DOI] [PubMed] [Google Scholar]
- 155.Rogers G N, Paulson J C. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983;127:361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- 156.Rogers G N, Paulson J C, Daniels R S, Skehel J J, Wilson I A, Wiley D C. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature (London) 1983;304:76–78. doi: 10.1038/304076a0. [DOI] [PubMed] [Google Scholar]
- 157.Rogers G N, Pritchett T J, Lane J L, Paulson J C. Differential sensitivity of human, avian, and equine influenza A viruses to a glycoprotein inhibitor of infection: selection of receptor specific variants. Virology. 1983;131:394–408. doi: 10.1016/0042-6822(83)90507-x. [DOI] [PubMed] [Google Scholar]
- 158.Röhm C, Süss J, Pohle V, Webster R G. Different hemagglutinin cleavage site variants of H7N7 in an influenza outbreak in chickens in Leipzig, Germany. Virology. 1996;218:253–257. doi: 10.1006/viro.1996.0187. [DOI] [PubMed] [Google Scholar]
- 159.Röhm C, Zhou N A, Süss J C, Mackenzie J, Webster R G. Characterization of a novel influenza hemagglutinin, H15: criteria for determination of influenza A subtypes. Virology. 1996;217:508–516. doi: 10.1006/viro.1996.0145. [DOI] [PubMed] [Google Scholar]
- 160.Rott R, Klenk H D, Nagai Y, Tashiro M. Influenza viruses, cell enzymes, and pathogenicity. Am J Respir Crit Care Med. 1995;152:S16–S19. doi: 10.1164/ajrccm/152.4_Pt_2.S16. [DOI] [PubMed] [Google Scholar]
- 161.Rowe T, Abernathy R A, Hu-Primmer J, Thompson W W, Lu X H, Lim W, Fukuda K, Cox N J, Katz J M. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37:937–943. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Saito T, Horimoto T, Kawaoka Y, Senne D A, Webster R G. Emergence of a potentially pathogenic H5N2 influenza virus in chickens. Virology. 1994;201:277–284. doi: 10.1006/viro.1994.1292. [DOI] [PubMed] [Google Scholar]
- 163.Schäfer J R, Kawaoka Y, Bean W J, Süss J, Senne D, Webster R G. Origin of the pandemic 1957 H2 influenza A virus and the persistence of its possible progenitors in the avian reservoir. Virology. 1993;194:781–788. doi: 10.1006/viro.1993.1319. [DOI] [PubMed] [Google Scholar]
- 164.Schafer W. Sero-immunologic studies on incomplete forms of the virus of classical fowl plague (German) Arch Exp Vet Med. 1955;9:218–230. [Google Scholar]
- 165.Scheiblauer H, Reinacher M, Tashiro M, Rott R. Interactions between bacteria and influenza A virus in the development of influenza pneumonia. J Infect Dis. 1992;166:783–791. doi: 10.1093/infdis/166.4.783. [DOI] [PubMed] [Google Scholar]
- 166.Scholtissek C, Burger H, Bachmann P A, Hannoun C. Genetic relatedness of hemagglutinins of the H1 subtype of influenza A viruses isolated from swine and birds. Virology. 1983;129:521–523. doi: 10.1016/0042-6822(83)90194-0. [DOI] [PubMed] [Google Scholar]
- 167.Scholtissek C, Burger H, Kistner O, Shortridge K F. The nucleoprotein as a possible major factor in determining host specificity of influenza H3N2 viruses. Virology. 1985;147:287–294. doi: 10.1016/0042-6822(85)90131-x. [DOI] [PubMed] [Google Scholar]
- 168.Scholtissek C, Koennecke I, Rott R. Host-range recombinants of fowl plague (influenza A) virus. Virology. 1978;91:79–85. doi: 10.1016/0042-6822(78)90356-2. [DOI] [PubMed] [Google Scholar]
- 169.Scholtissek C, Naylor E. Fish farming and influenza pandemics. Nature (London) 1988;331:215. doi: 10.1038/331215a0. [DOI] [PubMed] [Google Scholar]
- 170.Scholtissek C, Rohde W, Von Hoyningen V, Rott R. On the origin of the human influenza virus subtype H2N2 and H3N2. Virology. 1978;87:13–20. doi: 10.1016/0042-6822(78)90153-8. [DOI] [PubMed] [Google Scholar]
- 171.Scholtissek C, Von Hoyningen V, Rott R. Genetic relatedness between the new 1977 epidemic strains (H1N1) of influenza and human influenza strains isolated between 1947 and 1957. Virology. 1978;89:613–617. doi: 10.1016/0042-6822(78)90203-9. [DOI] [PubMed] [Google Scholar]
- 172.Seidah N G, Chretien M. Eukaryotic protein processing: endoproteolysis of precursor proteins. Curr Opin Biotechnol. 1997;8:602–607. doi: 10.1016/s0958-1669(97)80036-5. [DOI] [PubMed] [Google Scholar]
- 173.Senne D A, Panigrahy B, Kawaoka Y, Pearson J E, Süss J, Lipkind M, Kida H, Webster R G. Survey of the hemagglutinin (HA) cleavage site sequence of H5 and H7 avian influenza viruses: amino acid sequence at the HA cleavage site as a marker of pathogenicity potential. Avian Dis. 1996;40:425–437. [PubMed] [Google Scholar]
- 174.Shapiro G I, Krug R M. Influenza virus RNA replication in vitro: synthesis of viral template RNAs and virion RNAs in the absence of an added primer. J Virol. 1988;62:2285–2290. doi: 10.1128/jvi.62.7.2285-2290.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shortridge K F, Stuart-Harris C H. An influenza epicenter? Lancet. 1982;ii:812–813. doi: 10.1016/s0140-6736(82)92693-9. [DOI] [PubMed] [Google Scholar]
- 176.Shortridge K F, Zhou N N, Guan Y, Gao P, Ito T, Kawaoka Y, Kodihalli S, Krauss S, Markwell D, Murti K G, Norwood M, Senne D, Sims L, Takada A, Webster R G. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology. 1998;252:331–342. doi: 10.1006/viro.1998.9488. [DOI] [PubMed] [Google Scholar]
- 177.Slemons R D, Johnson D C, Osborn J S, Hayes F. Type-A influenza viruses isolated from wild free-flying ducks in California. Avian Dis. 1974;18:119–125. [PubMed] [Google Scholar]
- 178.Smith W, Andrewes C H, Laidlaw P P. A virus obtained from influenza patients. Lancet. 1933;i:66–68. [Google Scholar]
- 179.Snyder M H, Buckler-White A J, London W T, Tierney E L, Murphy B R. The avian influenza virus nucleoprotein gene and a specific constellation of avian and human virus polymerase genes each specify attenuation of avian/human influenza A/Pintail/79 reassortant viruses from monkeys. J Virol. 1987;61:2857–2863. doi: 10.1128/jvi.61.9.2857-2863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Steinhauer D A. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology. 1999;258:1–20. doi: 10.1006/viro.1999.9716. [DOI] [PubMed] [Google Scholar]
- 181.Stieneke-Grober A, Vey M, Angliker H, Shaw E, Thomas G, Roberts C, Klenk H-D, Garten W. Influenza virus hemagglutinin with multibasic cleavage site is activated by furin, a subtilisin-like endoprotease. EMBO J. 1992;11:2407–2414. doi: 10.1002/j.1460-2075.1992.tb05305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Suarez D L, Perdue M L, Cox N, Rowe T, Bender C, Huang J, Swayne D E. Comparisons of highly virulent H5N1 influenza A viruses isolated from humans and chickens from Hong Kong. J Virol. 1998;72:6678–6688. doi: 10.1128/jvi.72.8.6678-6688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Subbarao E K, London W, Murphy B R. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J Virol. 1993;67:1761–1764. doi: 10.1128/jvi.67.4.1761-1764.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, Hemphill M, Rowe T, Shaw M, Xu X, Fukuda K, Cox N. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 185.Suzuki Y. Gangliosides as influenza virus receptors. Variation of influenza viruses and their recognition of the receptor sialo-sugar chains. Prog Lipid Res. 1994;33:429–457. doi: 10.1016/0163-7827(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 186.Suzuki Y, Kato H, Naeve C W, Webster R G. Single-amino-acid substitution in an antigenic site of influenza virus hemagglutinin can alter the specificity of binding to cell membrane-associated gangliosides. J Virol. 1989;63:4298–4302. doi: 10.1128/jvi.63.10.4298-4302.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Swayne D E, Perdue M L, Garcia M, Rivera-Cruz E, Brugh M. Pathogenicity and diagnosis of H5N2 Mexican avian influenza viruses in chickens. Avian Dis. 1997;41:335–346. [PubMed] [Google Scholar]
- 188.Takeuchi K, Lamb R A. Influenza virus M2 protein ion channel activity stabilizes the native form of fowl plague virus hemagglutinin during intracellular transport. J Virol. 1994;68:911–919. doi: 10.1128/jvi.68.2.911-919.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tashiro M, Ciborowski P, Klenk H-D, Pulverer G, Rott R. Role of staphylococcus protease in the development of influenza pneumonia. Nature (London) 1987;325:536–537. doi: 10.1038/325536a0. [DOI] [PubMed] [Google Scholar]
- 190.Taubenberger J K, Reid A H, Krafft A E, Bijwaard K E, Fanning T G. Initial genetic characterization of the 1918 “Spanish” influenza virus. Science. 1997;275:1793–1796. doi: 10.1126/science.275.5307.1793. [DOI] [PubMed] [Google Scholar]
- 191.Tobita K, Sugiura A, Enomoto C, Furuyama M. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med Microbiol Immunol. 1975;162:9–14. doi: 10.1007/BF02123572. [DOI] [PubMed] [Google Scholar]
- 192.Treanor J J, Snyder M H, London W T, Murphy B R. The B allele of the NS gene of avian influenza viruses, but not the A allele, attenuates a human influenza A virus for squirrel monkeys. Virology. 1989;171:1–9. doi: 10.1016/0042-6822(89)90504-7. [DOI] [PubMed] [Google Scholar]
- 193.Vey M, Orlich M, Adler S, Klenk H-D, Rott R, Garten W. Hemagglutinin activation of pathogenic avian influenza viruses of serotype H7 requires the protease recognition motif R-X-K/R-R. Virology. 1992;188:408–413. doi: 10.1016/0042-6822(92)90775-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Von Itzstein M, Wu W-Y, Kok G B, Pegg M S, Dyason J C, Jin B, Van Phan T, Smythe M L, White H F, Oliver S W, Colman P M, Varghese J N, Ryan D M, Woods J M, Bethell R C, Hotham V J, Cameron J M, Penn C R. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature (London) 1993;363:418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- 195.Walker J A, Molloy S S, Thomas G, Sakaguchi T, Yoshida T, Chambers T M, Kawaoka Y. Sequence specificity of furin, a proprotein-processing endoprotease, for the hemagglutinin of a virulent avian influenza virus. J Virol. 1994;68:1213–1218. doi: 10.1128/jvi.68.2.1213-1218.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Walker J A, Sakaguchi T, Matsuda Y, Yoshida T, Kawaoka Y. Location and character of the cellular enzyme that cleaves the hemagglutinin of a virulent avian influenza virus. Virology. 1992;190:278–287. doi: 10.1016/0042-6822(92)91214-f. [DOI] [PubMed] [Google Scholar]
- 197.Wang P, Palese P, O'Neill RE. The NPI-1/NPI-3 (Karyopherin α) binding site on the influenza A virus nucleoprotein NP is a nonconventional nuclear localization signal. J Virol. 1997;71:1850–1856. doi: 10.1128/jvi.71.3.1850-1856.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ward A C, Castelli L A, Lucantoni A C, White J F, Azad A A, Macreadie I G. Expression and analysis of the NS2 protein of influenza A virus. Arch Virol. 1995;140:2067–2073. doi: 10.1007/BF01322693. [DOI] [PubMed] [Google Scholar]
- 199.Webster R G. Are equine 1 influenza viruses still present in horses? Equine Vet J. 1993;25:537–538. doi: 10.1111/j.2042-3306.1993.tb03009.x. [DOI] [PubMed] [Google Scholar]
- 200.Webster R G. Influenza virus: transmission between species and relevance to emergence of the next human pandemic. Arch Virol. 1997;142(Suppl. 13):105–113. doi: 10.1007/978-3-7091-6534-8_11. [DOI] [PubMed] [Google Scholar]
- 201.Webster R G, Bean W J, Gorman O T, Chambers T M, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Webster R G, Geraci J R, Petursson G, Skirnisson K. Conjunctivitis in human beings caused by influenza A virus of seals. N Engl J Med. 1981;304:911. doi: 10.1056/NEJM198104093041515. [DOI] [PubMed] [Google Scholar]
- 203.Webster R G, Hinshaw V S, Bean W J, Van Wyke K L, Geraci J R, St. Aubin D J, Petursson G. Characterization of an influenza A virus from seals. Virology. 1981;113:712–724. doi: 10.1016/0042-6822(81)90200-2. [DOI] [PubMed] [Google Scholar]
- 204.Webster R G, Kawaoka Y. Avian influenza. Crit Rev Poult Biol. 1988;1:211–246. [Google Scholar]
- 205.Webster R G, Kawaoka Y, Bean W J, Beard C W, Brugh M. Chemotherapy and vaccination: a possible strategy for the control of highly virulent influenza virus. J Virol. 1985;55:173–176. doi: 10.1128/jvi.55.1.173-176.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Webster R G, Kawaoka Y, Bean W J., Jr Molecular changes in A/Chicken/Pennsylvania/83 (H5N2) influenza virus associated with acquisition of virulence. Virology. 1986;149:165–173. doi: 10.1016/0042-6822(86)90118-2. [DOI] [PubMed] [Google Scholar]
- 207.Webster R G, Laver W G. Antigenic variation of influenza viruses. In: Kilbourne E D, editor. The influenza viruses and influenza. New York, N.Y: Academic Press, Inc.; 1975. pp. 270–314. [Google Scholar]
- 208.Webster R G, Laver W G, Air G M, Schild G C. Molecular mechanisms of variation in influenza viruses. Nature (London) 1982;296:115–121. doi: 10.1038/296115a0. [DOI] [PubMed] [Google Scholar]
- 209.Webster R G, Reay P A, Laver W G. Protection against lethal influenza with neuraminidase. Virology. 1988;164:230–237. doi: 10.1016/0042-6822(88)90640-x. [DOI] [PubMed] [Google Scholar]
- 210.Webster R G, Yakhno M A, Hinshaw V S, Bean W J, Murti K G. Intestinal influenza: replication and characterization of influenza viruses in ducks. Virology. 1978;84:268–278. doi: 10.1016/0042-6822(78)90247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Weis W, Brown J H, Cusack S, Paulson J C, Skehel J J, Wiley D C. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature (London) 1988;333:426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- 212.Wells D L, Hopfensperger D J, Arden N H, Harmon M W, Davis J P, Tipple M A, Schonberger L B. Swine influenza virus infections. Transmission from ill pigs to humans at a Wisconsin agricultural fair and subsequent probable person-to-person transmission. JAMA. 1991;265:478–481. doi: 10.1001/jama.265.4.478. [DOI] [PubMed] [Google Scholar]
- 213.White J, Kartenbeck J, Helenius A. Membrane fusion activity of influenza virus. EMBO J. 1982;1:217–222. doi: 10.1002/j.1460-2075.1982.tb01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wiley D C, Skehel J J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- 215.Wiley D C, Wilson I A, Skehel J J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature (London) 1981;289:373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- 216.Wilson I A, Skehel J J, Wiley D C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3A resolution. Nature (London) 1981;289:366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- 217.Wood G W, Banks J, McCauley J W, Alexander D J. Deduced amino acid sequences of the haemagglutinin of H5N1 avian influenza virus isolates from an outbreak in turkeys in Norfolk, England. Arch Virol. 1994;134:185–194. doi: 10.1007/BF01379117. [DOI] [PubMed] [Google Scholar]
- 218.Wood G W, McCauley J W, Bashiruddin J B, Alexander D J. Deduced amino acid sequences at the haemagglutinin cleavage site of avian influenza A viruses of H5 and H7 subtypes. Arch Virol. 1993;130:209–217. doi: 10.1007/BF01319010. [DOI] [PubMed] [Google Scholar]
- 219.Xu X Y, Subbarao K, Cox N J, Guo Y J. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: Similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology. 1999;261:15–19. doi: 10.1006/viro.1999.9820. [DOI] [PubMed] [Google Scholar]
- 220.Yasuda J, Nakada S, Kato A, Toyoda T, Ishihama A. Molecular assembly of influenza virus: association of the NS2 protein with virion matrix. Virology. 1993;196:249–255. doi: 10.1006/viro.1993.1473. [DOI] [PubMed] [Google Scholar]
- 221.Young J F, Palese P. Evolution of human influenza A viruses in nature. Recombination contributes to genetic variation of H1N1 strains. Proc Natl Acad Sci USA. 1979;76:6547–6551. doi: 10.1073/pnas.76.12.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yuen K Y, Chan P S, Peiris M, Tsang D C, Que T L, Shortridge K F, Cheung P T, To W K, Ho E F, Sung R, Cheng A B, Mena I members of the H5N1 Study Group. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 223.Zebedee S L, Lamb R A. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J Virol. 1988;62:2762–2772. doi: 10.1128/jvi.62.8.2762-2772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]