Abstract
The anaerobic protozoa Giardia duodenalis, Trichomonas vaginalis, and Entamoeba histolytica infect up to a billion people each year. G. duodenalis and E. histolytica are primarily pathogens of the intestinal tract, although E. histolytica can form abscesses and invade other organs, where it can be fatal if left untreated. T. vaginalis infection is a sexually transmitted infection causing vaginitis and acute inflammatory disease of the genital mucosa. T. vaginalis has also been reported in the urinary tract, fallopian tubes, and pelvis and can cause pneumonia, bronchitis, and oral lesions. Respiratory infections can be acquired perinatally. T. vaginalis infections have been associated with preterm delivery, low birth weight, and increased mortality as well as predisposing to human immunodeficiency virus infection, AIDS, and cervical cancer. All three organisms lack mitochondria and are susceptible to the nitroimidazole metronidazole because of similar low-redox-potential anaerobic metabolic pathways. Resistance to metronidazole and other drugs has been observed clinically and in the laboratory. Laboratory studies have identified the enzyme that activates metronidazole, pyruvate:ferredoxin oxidoreductase, to its nitroso form and distinct mechanisms of decreasing drug susceptibility that are induced in each organism. Although the nitroimidazoles have been the drug family of choice for treating the anaerobic protozoa, G. duodenalis is less susceptible to other antiparasitic drugs, such as furazolidone, albendazole, and quinacrine. Resistance has been demonstrated for each agent, and the mechanism of resistance has been investigated. Metronidazole resistance in T. vaginalis is well documented, and the principal mechanisms have been defined. Bypass metabolism, such as alternative oxidoreductases, have been discovered in both organisms. Aerobic versus anaerobic resistance in T. vaginalis is discussed. Mechanisms of metronidazole resistance in E. histolytica have recently been investigated using laboratory-induced resistant isolates. Instead of downregulation of the pyruvate:ferredoxin oxidoreductase and ferredoxin pathway as seen in G. duodenalis and T. vaginalis, E. histolytica induces oxidative stress mechanisms, including superoxide dismutase and peroxiredoxin. The review examines the value of investigating both clinical and laboratory-induced syngeneic drug-resistant isolates and dissection of the complementary data obtained. Comparison of resistance mechanisms in anaerobic bacteria and the parasitic protozoa is discussed as well as the value of studies of the epidemiology of resistance.
A small group of diverse, parasitic protozoa has significant impact on the mucosal health of humans. Historically these organisms have included Giardia duodenalis, Trichomonas vaginalis, and Entamoeba histolytica. More recently, gastrointestinal infections have been associated with Blastocystis hominis, Cryptosporidium parvum, Isospora spp., and Cyclospora spp., although these organisms are of contentious phylogeny. Collectively, they infect over one billion people each year. G. duodenalis, E. histolytica, B. hominis, and C. parvum parasitize the gastrointestinal tract, where they cause diarrhea, dysentery, and associated symptoms. E. histolytica can also invade the gut epithelium and subsequently other organs, where it forms abscesses which can be fatal if left untreated. T. vaginalis is sexually transmitted and adheres to the epithelium of the vagina, resulting in a range of symptoms from vaginitis and discharge through pelvic inflammatory disease to preterm delivery and low birth weight concomitant with increased mortality.
The basic metabolism of these protozoa appears to be anaerobic and microaerotolerant, although differing in extent in each case. Anaerobicity protects the key, low-redox-potential metabolic enzyme pyruvate:ferredoxin oxidoreductase and ferredoxin, which also activate the nitroimidazole drug metronidazole, the front line of defense against G. duodenalis, E. histolytica, and T. vaginalis. Metabolic pathways such as these, which are found in anaerobic bacteria that are also susceptible to metronidazole but not in other eukaryotes, strengthen the idea that these protozoa are very early diverging in the eukaryotic lineage. There are no recommended, successful therapeutic strategies for C. parvum as yet, and little is known about mechanisms of drug resistance for this parasite or for Blastocystis, Isospora, and Cyclospora spp. Less common gastrointestinal infections, particularly by other flagellates and coccidians, may also include protozoans with uncharacterized anaerobic metabolisms. This review concentrates on data which have been published or upgraded after earlier extensive reviews on specific organisms and which are quoted in the text.
GIARDIA DUODENALIS
G. duodenalis, also known as Giardia lamblia and Giardia intestinalis, is a flagellated diplomonad and the most commonly detected flagellate and protozoan in the intestinal tract 123. The trophozoites were first noted by von Leeuwenhoek in 1681 in his own stools. A morphologically identical organism infects over 40 animal species 103 and is regarded as zoonotic by the World Health Organization (WHO). There is evidence, however, that host range can be restricted 190, 205, and there is a lack of evidence for the extent of zoonosis 209, so that animal reservoirs of human outbreaks have not been clearly identified, although they are possible 82, 91. In cold climates, the cysts which are the infective form of the parasite can survive in water sources for months 226. Although large outbreaks have been linked to contaminated water 123, the fecal-oral route is regarded as the major source of infection, particularly in countries where the water is warm or there is little drainage and no reticulated water supply 143. This premise is supported by the prevalence of giardiasis in child care centers and nursing homes for the elderly 39, 136, 232. The WHO estimated that 280 million people are infected each year. The incidence may be very high in children in developing countries and decreased to 2 to 7% in some industrialized nations 59, 123. Incidence varies significantly with locale 63, 65. More recently, the WHO has estimated that 3,000 million people live in unsewered environments in developing countries and the rate of giardiasis among them approaches 30%, suggesting that there are closer to 1,000 million cases of giardiasis at any one time, contributing to 2.5 million deaths annually from diarrheal disease (WHO press release, 1998).
A study of 200 patients with diarrhea in a 2-week window at a drought rehabilitation camp in Korem, Ethiopia, showed that 98% of apyrexic patients carrying trophozoites of Giardia or Entamoeba had dysentery 45. Giardia is the most common cause of chronic diarrhea in travelers 59, 232, 239. Although infections vary from asymptomatic through diarrhea and weight loss to profound weight loss and failure to thrive, there are currently no genetic markers or classification systems to distinguish pathogenic from nonpathogenic strains, notwithstanding various isoenzyme 6, 129, 190, DNA fingerprinting 203, 207, and electrophoretic karyotyping 220, 221 techniques to identify isolates. Nonclonal infections add to the complexity of identifying pathogenic or virulent strains 199, 207.
The organism is ingested as the inactive cyst form, which excysts to the active trophozoite form in the duodenum after passage through the acid environment of the stomach. Trophozoites graze on the duodenal or jejunal mucosa and reproduce by binary fission 1, 54, 103, after which they encyst, probably induced by passage through the local environment, including low cholesterol levels 71, 120. Detection methods in both patients and water supplies have recently been reviewed 2, 112, 123, 226.
Giardia Infection
The incubation period is 1 to 2 weeks after ingestion, following which various grades of symptoms, including nausea, stomach cramps, diarrhea, and vomiting, ensue. The acute stage can last from 3 to 4 days but can persist for much longer 1. In children, a failure-to-thrive syndrome may occur 59, and in the developing world, giardiasis is an important cause of morbidity 29. Persistent infection and diarrhea may occur in immunocompromised individuals, e.g., immunoglolubin A (IgA) deficiency or variable immunodeficiency, but is less obvious in cases of AIDS 1, 59, 82.
Infestation by the organism causes decreased small intestinal brush border surface area, microvillus and villus atrophy, enterocyte immaturity, disaccharidase and luminal enzyme deficiencies, and malabsorption of electrolytes, a multifactorial pathogenesis which is not well understood 29. A number of other symptoms have been associated with giardiasis 82, but these have not been routinely or consistently found.
Similarly, granulomatous hepatitis and cholangitis have been associated with chronic diarrhea attributed to giardiasis 163. A more recent survey of 25 patients with giardiasis and high liver enzyme values demonstrated return to normal function after antiparasite therapy 182. This involvement may be consistent with poor fatty acid uptake and steatorrhea in giardiasis 59; previous anecdotal observations regarding other symptoms may therefore have a stronger foundation, but vary with the host patient (e.g., immunological status), parasite burden, or strain.
Such a benchmark example was recently described for a Giardia isolate which was found to be lethal for sulfur-crested cockatoos 204. This strain was genetically similar to human isolates and was infective for mice, in which it established a chronic infection with high parasite burdens; the mice also lost up to 20% of their body weight, mimicking the human failure-to-thrive syndrome 204, 205. This is the first report of a bird isolate infecting mammals. Similarly, it is not known why symptoms range from a carrier state to failure-to-thrive syndrome, although a gene was recently reported which has 57% homology with the sarafotoxins, which cause stomach cramps, nausea, diarrhea, and vomiting 37.
The Giardia toxin homologue is only present in some isolates and subject to the silencing of telomeric position effects, where expression can also be induced to very high levels 219. This region of the genome is also mobile among chromosomes. The gene is a member of a family of genes which vary via a cassette-like mechanism 38, 215, 220 and is not present in all isolates 219. Such complex expression patterns of genes encoding diverse toxin and cell-signaling molecules may explain the variability in symptoms.
A recent report by the European Commission 154 suggested that Giardia be targeted for vaccine development, which may complement the use of drug treatment.
Genes from Giardia were first cloned in 1987 202. A recent review has described the plasticity in the organization and structure of the Giardia genome analyzed by electrophoretic karyotyping, chromosome mapping, and strain analysis, showing variable ploidy, aneuploidy, and accessory chromosomes 220. The proximal subtelomeric regions are particularly dynamic, with recombination occurring among gene blocks (telomere gene units) inserted into the repeated telomeric ribosomal DNA (rDNA) arrays and also within cassettes in cysteine-rich protein genes encoded in those gene units. The gene units are under the silencing control of a telomere position effect, are mobile within the genome, and vary among isolates 37, 219, 220.
Giardia Metabolism
Giardia is amitochondrial and asexual. Its metabolism is glycolytic and fermentative 22, 92, 110, 131, with the terminal part of the glycolytic pathway being executed by the anaerobic bacterial homologue pyruvate:ferredoxin oxidoreductase (PFOR) replacing pyruvate dehydrogenase found in aerobic organisms 22, 196. The protein has been purified and characterized 196. ATP is generated by substrate—level phosphorylation; the tricarboxylic acid pathway found in the mitochondrion of aerobic eukaryotes is absent, although malate dehydrogenases are present. The gene for malic enzyme has been cloned 168. Electrons from PFOR are transferred to ferredoxin(s), of which three have been described 194; the major one has been sequenced and its gene has been cloned (P. Upcroft, J. A. Upcroft, D. M. Brown, and C. Kennett, unpublished data). Some enzymes in the glycolytic pathway are dependent on PPi instead of ATP, suggesting a more primitive use of PPi bond energy 127. No cytochromes have been found in Giardia. A terminal oxidase which converts oxygen directly to water scavenges oxygen from this microaerotolerant organism to protect the anaerobic PFOR and ferredoxins 24. This 46-kDa NADH oxidase is the only aerobic enzyme so far described in Giardia and is a homologue of the enzyme found in anaerobic bacteria. This same enzyme is also found in the obligate anaerobic archaebacterium Methanococcus jannaschii, in which it is one of only two genes encoding aerobic functions 27, the second being NADH:ubiquinone oxidoreductase, which is also likely to occur in Giardia. A gene for a third likely aerobic function has recently been described for M. jannaschii, neelaredoxin, a small non-heme blue iron protein which is part of an oxygen sensing pathway and has superoxide dismutase activity in Desulfovibrio gigas and other anaerobes 178. In Giardia, PFOR is assisted by another 2-oxoacid oxidoreductase and by an arginine dihydrolase pathway to provide alternative sources of energy 22, 98, 196, 216; the gene encoding one of these eubacterial-like PFORs has been cloned 196. The gene for acetyl coenzyme A (acetyl-CoA) synthetase has been cloned and is a member of a family of similar genes of limited taxonomic distribution 169. A malate dehydrogenase gene representative of the eukaryotic cytosolic group has been cloned and is related to the same gene from T. vaginalis, leading to the suggestion that mitochondrial properties were lost in the common ancestor of both groups 165; an alternative and more parsimonious interpretation is that similar cell fusions generated the first eukaryotes and Giardia retained predominantly the anaerobic gene repertoire, while others pursued the aerobic, mitochondrial evolutionary route 220. Final endproducts of fermentation in Giardia are CO2, acetate, alanine, and ethanol, which vary according to growth conditions 22, 50, 145.
The conventional mechanisms of oxidative stress management, including superoxide dismutase (SOD), catalase, peroxidase, and glutathione cycling, are absent from Giardia. They are replaced by a broad-range prokaryotic thioredoxin reductase-like disulfide reductase and the low-molecular-weight thiols cysteine, thioglycolate, sulfite, and CoA, the NADH oxidase, and a membrane-associated NADH peroxidase 22. Genome sequencing projects will probably yield information for other genes encoding functions which have not been assayed yet or have no assays, like the neelaredoxin of M. jannaschii. This information will complement the biochemical data, since the 30 to 50% novel genes found in each of the genomes so far studied suggest that functional studies will remain necessary to understand the diversity of biochemistry in most organisms. To draw an extreme analogy in an era of frenzied evolutionary hypothesizing, it is of note that M. jannaschii also appears to have a genome of variable ploidy 122.
Giardia Drug Treatment and Resistance
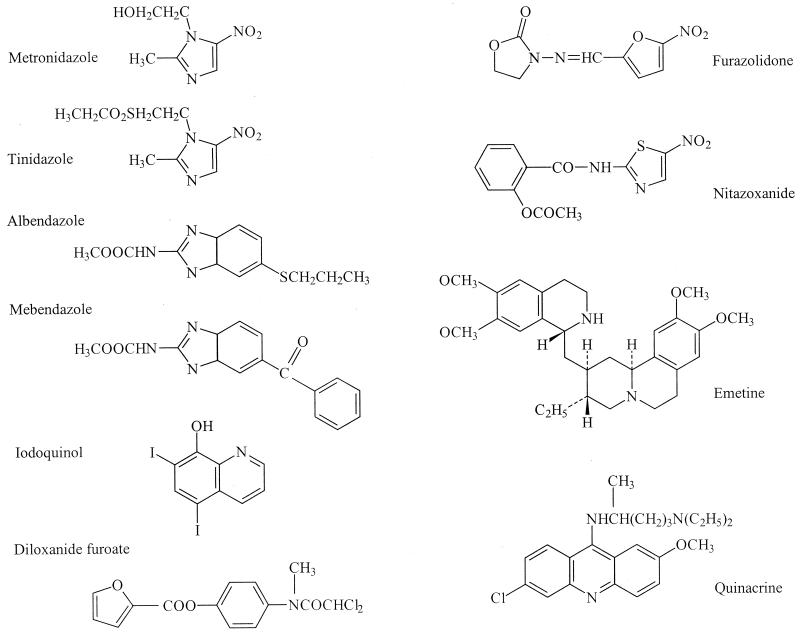
Current Medical Diagnosis & Treatment 1999 191 lists tinidazole, metronidazole, furazolidone, and quinacrine for treatment of giardiasis in the United States, with success rates of less than 90% (furazolidone less than 80%), followed by albendazole (along with paromomycin), with success rates of 10 to 95%, as a possible treatment. Similar recommendations are found in Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases 82, the Oxford Textbook of Medicine 81, and Harrison's Principles of Internal Medicine 136 (Fig. 1).
FIG. 1.
Structures of the drugs commonly used against the anaerobic protozoa. Structures are redrawn from the Merck Index except for nitazoxanide, which was redrawn for direct comparison with the nitroimidazoles, nitrofurans, and benzimidazoles (166).
Quinacrine was the first effective antigiardial 79 until it was supplanted by the nitroimidazole metronidazole 218. Quinacrine is a substituted acridine and was introduced as an antimalarial in the mid-1930s. Quinacrine is not recommended in Australia because of its side effects, although it is still used in France and has been used effectively in cases of nitroimidazole failure 200. Metronidazole has been regarded as the drug of choice, with the derivative tinidazole being an alternative 18. Single-high-dose regimens and regimens lasting 3, 5, and 7 days with lower doses have variously been recommended 18, 191. Clinical resistance prevalence levels as high as 20% have been reported 19, 59, with recurrence rates as high as 90% 240. Resistant organisms have been isolated from patients refractory to metronidazole and the nitrofuran furazolidone; there is a large range of susceptibility to these drugs, and the development of resistance has been demonstrated in patients 59, 208, 218; L. Favennec, personal communication). Cross-resistance to tinidazole has been demonstrated with metronidazole-resistant Giardia strains 208, 218 (Fig. 2).
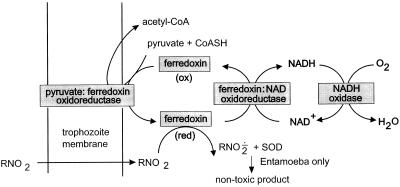
FIG. 2.
Proposed fundamental metabolism of the anaerobic protozoa, with PFOR replacing the pyruvate dehydrogenase of aerobic organisms. Electrons derived from oxidative decarboxylation of pyruvate (catalyzed by PFOR) are transferred to ferredoxin (oxidized [ox]), which subsequently donates electrons to NAD(P)+ (catalyzed by putative ferredoxin:NAD oxidoreductase), regenerating intracellular pools of NAD(P)H and oxidized ferredoxin. In the process of ferredoxin reduction, acetyl-CoA is generated from pyruvate and reduced CoA (CoASH). Alternatively, ferredoxin has a sufficiently low redox potential to reduce (activate) metronidazole (R-NO2) to its toxic nitroradical RNO2· via reduced (red) ferredoxin (194, 196). NADH oxidase then serves as the terminal oxidase in the transfer of electrons to O2, producing H2O. NADH oxidase also has the potential to reduce furazolidone to its toxic nitro radical. Adapted from Giardia data in references 22 to 25. The PFOR reaction occurs in the hydrogenosome in T. vaginalis. There are likely to be other coupled reactions, including alternative oxidoreductases, ferredoxins, and flavodoxins (22, 25, 117, 194, 216).
Furazolidone was often recommended for children because of its availability as a suspension 18, 93. Furazolidone-resistant Giardia strains developed in vitro adapted more readily to quinacrine resistance 200. Furazolidone is not available in some countries, including Australia. Metronidazole, quinacrine, and furazolidone are not tolerated with alcohol because of a disulfiram-like reaction 93. Tinidazole is not available in the United States.
Albendazole was first introduced in 1982 as an anthelmintic. The first published report of (successful) use of albendazole against Giardia was in 1986 243, 244, with other benzimadazoles also being tested prior to 1990 and having mixed success 18. The first large-scale human study was in Bangladesh, where the average efficacy of albendazole was 62 to 95%, compared with 97% for metronidazole 77. The higher levels of efficacy with albendazole require multiple doses. These figures are consistent with recent, smaller studies and reports of albendazole treatment failures 21, 99. The single high doses of metronidazole or tinidazole are an advantage for compliance. Albendazole eventually may be accepted as an alternative to metronidazole, particularly in cases of metronidazole failure, if current levels of albendazole resistance or treatment failure do not increase further. Like metronidazole, resistance of Giardia to albendazole has been reported to occur readily in vitro 111, 206. Albendazole resistance also developed more readily in a furazolidone-resistant strain, and therefore these strains are multidrug resistant 206, 218. A giardiasis patient with parasites refractory to metronidazole and albendazole administered separately was successfully treated with these drugs administered together (unpublished data). Toxicity and side effects have been discussed at length (e.g., references 18 and 93.
Other drugs have been used to treat giardiasis with lower levels of success 18, 166. New 5-nitroimidazoles have been developed in anticipation of further deterioration in susceptibility of clinical cases to the few effective drugs available 201.
Purified Giardia PFOR and ferredoxin have been shown to activate metronidazole in vitro 193, 194, 196, 218. PFOR is downregulated up to fivefold in metronidazole-resistant Giardia. This is consistent with the reduction in activation of the drug to its nitroso and other radicals in anaerobes by low-redox-potential electron transfer reactions 196. PFOR was not downregulated in furazolidone-resistant Giardia, and this strain was not affected by quinacrine. The next electron acceptor in the transport chain, ferredoxin I, is also downregulated about sevenfold, determined by in vitro assay in the same strain, but only twofold by Western blotting, suggesting that another component may be regulating the acceptor activity in the cytosolic extract 117, 218. In Helicobacter pylori, PFOR appears to be downregulated by mutations in an NADPH nitroreductase which also render the bacterium metronidazole resistant 73. Increased efflux of metronidazole is also involved in protecting the parasite 208, 215–218. PFOR is not totally downregulated, as in highly resistant T. vaginalis, probably because the enzyme is essential to the glycolytic pathway. In vivo assays suggested that PFOR is downregulated further than the assays executed on extracted enzyme 51, 52, implying that a second level of enzyme control may also be acting in the resistant cells 196, 218.
A second 2-oxoacid oxidoreductase supplants the reduced PFOR activity 196, 216. This activity has different substrate requirements, principally 2-ketobutyrate, does not neccessarily donate electrons to ferredoxins, and appears to bypass the activation of metronidazole because of a higher redox potential 216. Albendazole resistance was easier to induce in metronidazole-resistant Giardia 206, producing a multidrug resistance phenotype. Although the cytoskeletal structure was changed in albendazole-resistant lines, the common mutation seen in other organisms resistant to albendazole in β-tubulin at amino acid position 200 was not observed, suggesting an alternative mechanism of resistance.
Furazolidone resistance has also been induced in Giardia 22, 195, and NADH oxidase has been shown to activate it to its free radical state 22, 24. These lines were also more easily induced to quinacrine resistance, generating a second type of multidrug resistance 200. Although it has been suggested that the primary mode of action of quinacrine is DNA binding, the innate fluorescence of quinacrine was used to show that it does not target the nucleus of Giardia 200. Sensitive cells showed cytoplasmic membrane fragility. It is actively excluded from quinacrine-resistant Giardia cells and inhibits NADH oxidase 23, the same enzyme that activates furazolidone.
ENTAMOEBA HISTOLYTICA
The trophozoite stage of E. histolytica was first described by Lösch in 1875, and the cyst form was first described by Quincke and Roos in 1893 109, 123, 157. The cysts survive in water and food and are the infective form upon ingestion. At neutral or slightly alkaline pH, the cysts turn into active metacystic trophozoites, which mature into trophozoites in the large intestine 123. The cycle is completed by formation of cysts in the intestinal tract.
Approximately 500 million people are infected with E. histolytica at any one time 173. About 10% of these have clinical symptoms, while in approximately 10% of these cases the parasite invades the intestinal mucosa. The WHO estimates that Entamoeba causes severe disease in 48 million people each year, killing 70,000 each year 229, while other estimates place this figure closer to 100,000 157, 173. Recently, the differentiation of E. histolytica into two species, the nonpathogenic and noninvasive E. dispar and the pathogenic and invasive E. histolytica, originally proposed by Brumpt in 1925, has regained acceptance, based principally on isoenzyme typing 48, 157 but supported by gene sequencing data 143. This conclusion is also supported by ultrastructural and cytopathogenicity studies on two representative strains 55. One interpretation of this new classification is that every individual infected with E. histolytica would suffer disease symptoms of dysentery involving invasion of the intestinal mucosa, while in up to 20% the parasite invades beyond the mucosa to other internal organs, including the liver and brain, assuming that half the infections are caused by E. histolytica, although this may be closer to one third in some areas 78. If untreated, brain abscesses, and up to 20% of cases involving abscesses in other organs, are fatal. This classification appears to be a very reasonable working interpretation based upon the disease symptoms. If facets of pathogenicity are later found to be graded between the two species or other intermediate “species” are found, appropriate reinterpretation or subclassification may need to be considered, e.g., a high rate of occult infection with E. histolytica has recently been observed in Mexican children without dysentery, using analytical tools which reinstated the E. histolytica and E. dispar species 139 Earlier work also suggested a large range in the frequency of asymptomatic infections with E. histolytica, the mechanism of which remains unknown 157. Similarly, an asymptomatic carrier in Italy appears to have infected five family members, one of whom died 67.
Entamoeba Infection
The incubation period after ingestion of cysts can vary from days to months or even longer. Symptoms can range from acute amoebic colitis, which usually presents as frequent bloody stools over several weeks with fever, through profuse bloody diarrhea with fever, to amoeboma with dysentery, and finally invasion to other organs. This last stage has been equated with metastasis of colon tumors 106. Progress towards a possible vaccine has recently been reviewed 183.
Genes for cytoskeletal proteins, dominant antigens, multidrug resistance glycoproteins, adhesion molecules, including the Gal/GalNac lectin 157, and lytic factors have been cloned 125. An extracellular matrix binding protein gene encodes a multifunctional alcohol dehydrogenase and acetaldehyde dehydrogenase, homologous to the adhE gene of Escherichia coli 26. Attempts at defining an electrophoretic karyotype have had mixed success 10, 11, 47, 148, with the most recent data suggesting variation among isolates, polyploidy, and 31 to 35 chromosomes ranging from 0.3 to 2.2 Mb 231. rDNA appears to be on extrachromosomal circles 47, but it has been proposed that rDNA is chromosomally located as well 11.
Entamoeba Metabolism
Entamoeba's metabolism appears similar to that of Giardia and Trichomonas, that is, anaerobic, fermentative, and bacterial in nature, but lacking the hydrogenosome organelle of the latter organism 158. However, Entamoeba has been described as a facultative aerobe in that it can grow in 5% oxygen 125. This may be better described as aerotolerant or microaerotolerant, because we were unable to find any publication that demonstrated growth rate improvement aerobically. Oxygen and its breakdown products are toxic to Entamoeba 13, 70, and at 5% atmospheric oxygen there is very little oxygen in the medium itself because it is reduced 13. The parasite produces SOD, catalase, and peroxidase for detoxification 36, 187. Many of the glycolytic enzymes are reversible 127, 158 and utilize the energy of the pyrophosphate bond, not ATP. To date, only one 2-oxoacid oxidoreductase, PFOR, has been detected in Entamoeba, and it is predominantly membrane bound 167, 189, 216. This enzyme also utilizes alternative substrates such as α-ketobutyrate, α-ketoglutarate, and oxaloacetate. The gene encoding PFOR has been cloned 164, 189. Unlike Giardia, Entamoeba produces SOD, and two different SOD activities have been detected 167. Entamoeba lacks glutathione reductase activity and does not synthesize glutathione 57. The major low-molecular-weight thiol is cysteine, although it has recently been shown that Entamoeba can take up glutathione from the medium and convert it to bisglutathionyl-spermidine (trypanothione) 142, a thiol derivative first found in trypanosomes 58. Novel cysteine synthase genes have been cloned from Entamoeba 140. An Entamoeba organelle of unknown function to which the chaperonin Hsp60 homes has recently been described 121, 192; whether this represents a degenerate mitochondrion, a novel organelle derived from an alternative symbiosis, or the product of an early fusion(s) to generate eukaryotes remains to be determined. An example that multiple symbioses and/or fusions can be considered is the weevil Sitophilus oryzae, which has three intracellular organelle genomes, mitochondrial, the γ3-proteobacterial S. oryzae principal endosymbiont, and the α-proteobacterial Wolbachia genome 80. Such symbioses appear to be quite common in biology, even among bacteria 41.
Entamoeba Drug Treatment and Resistance
Emetine was first demonstrated to be an effective amoebicide in 1912 97. A miscellany of drugs in addition to emetine have since been reported to be effective to various degrees. Metronidazole has become the drug of choice following recognition of its amoebicidal properties in the mid-1960s.
Emetine (or dehydroemetine) hydrochloride is a potent tissue amoebicide. Although it continues to be a life-saving drug in appropriate situations, this drug does have serious side effects 97. The drug is slowly excreted into the gut and urine, allowing high concentrations to build up in the liver, heart, and other viscera. To illustrate, emetine may be detectable in urine 40 days after the termination of treatment. Emetine is administered by daily intramuscular or deep subcutaneous injections for a maximum of 10 days, with local pain and tenderness at the sites of injection being common. If given orally, the drug usually causes vomiting, and very little is absorbed. Given intravenously, it is dangerously cardiotoxic 97.
There is evidence that multidrug resistance transporters may be involved in emetine and colchicine accumulation in a drug-resistant selected line, because uptake was lower in the resistant line and verapamil increased drug accumulation to that seen in the parental line 9, 20. Six P-glycoprotein-like genes have been cloned: four are expressed in a drug-resistant line, and there is differential expression 72.
The synthetic 5-nitroimidazole metronidazole, on the other hand, is remarkably safe in comparison to emetine and is now the recommended drug for the treatment of amoebiasis. It is also effective against a wide range of anaerobic bacteria 64 because of similar low-redox-activating enzymes. Oral doses of metronidazole are readily absorbed and can be found in most body fluids with few side effects. It is now so widely used both therapeutically and prophylactically for numerous major and minor ailments that exposure of Entamoeba to this drug worldwide is guaranteed. Inappropriate short-term exposure and exposure to sublethal levels of metronidazole are normally prescribed for prophylaxis and are precisely the conditions under which drug resistance is induced 208.
Indeed, using stepwise incremental increases in drug dose, we have induced metronidazole resistance in two axenic lines of E. histolytica 167. Parasites of strains HM-I:IMSS (isolated from a rectal ulcer from an adult male in Mexico City in 1967) and HTH-56:MUTM (established in culture from an amoebic abscess from an adult in Bangkok in 1992) currently grow in our laboratory in 10 μM metronidazole, a concentration of drug which is normally lethal to parasites in vitro 68. A minimum lethal dose for this strain has been reported as 11.6 μM over 72 h, and a 50% inhibitory dose of 29.7 μM has been reported, while the reported susceptibility of the HK-9 strain ranges from a 50% effective dose of 2 μM to a lethal dose of 11.6 μM over 100 h 167. Expression of the iron-containing SOD was increased three- to five-fold in metronidazole-resistant parasites, and a second SOD activity disappeared 167. Unlike in Giardia and Trichomonas, PFOR activity did not decrease significantly. The work with the iron-containing SOD has been confirmed at the gene expression level by Wassmann et al. 227, with parasites resistant to 40 μM metronidazole; peroxiredoxin expression was also increased, and ferredoxin 1 expression was decreased.
Unique in that it is effective both in the bowel lumen and in tissues, metronidazole has been reported to eradicate only up to 50% of luminal infections 191. This statement has support from a study of 36 patients with amoebic liver abscess for whom the hepatic lesions were cleared; but 20 were recolonized in the intestine, 16 asymptomatically. This was ascribed to the pharmacokinetics of metronidazole cycling in the liver and the action of metronidazole against trophozoites but not invariable eradication of cysts, creating E. histolytica carrier states 90, 93. These figures are considerably higher than generally published, although no single treatment has claims of 100% efficacy; 90% success after 5 to 10 days of metronidazole treatment is regarded as adequate by some workers. Alternatively, other workers have regarded that this level of efficacy suggests resistance and the need for multiple drug treatment 157. Because the organism is difficult to culture axenically from patients, there are insufficient reliable data regarding resistance levels, although a recent study indicates decreased susceptibility in clinical isolates from Chandigarh (R. Sehgal, unpublished data). Therefore, current recommendations suggest the use of metronidazole or tinidazole plus the luminal amoebicide diloxanide furoate or iodoquinol, with other combinations (including paromomycin, tetracycline, and chloroquine) depending on the severity of the infection and site, i.e., whether it is intraluminal, invasive, or abscessed 157, 191. Iodoquinol and diloxanide furoate have unknown mechanisms of action 93. Like diloxanide furoate, paromomycin is poorly absorbed. There are no reports of the very high levels of resistance to metronidazole found in Trichomonas or Giardia.
Similar to the interpretation of pathogenic versus nonpathogenic strains or species of Entamoeba, coupled with treatment failure due to resistance, there is disagreement over which cases should receive treatment. Clearly, anyone with serious disease requires adequate treatment with the available drugs. It has been strongly argued that metronidazole should not be used prophylactically to preserve the usefulness and potency of the drug 76, 208, 218. Sargeaunt 170 is quite adamant that only E. histolytica is pathogenic, obviating the need to treat E. dispar carriers, while Diamond and Clark 48 caution against witholding treatment from asymptomatic individuals because E. histolytica has been found in these carriers. Burchard 28 advises treatment of E. histolytica only, but that all cyst passers be treated in the absence of differentiation. Results of a treatment and placebo trial in Mexico were interpreted to mean that money spent treating carriers in this country could be better spent on treating cases of amoebic disease 66. All these opinions appear to be correct in their context, in the absence of adequate funds and new, effective antiprotozoal drugs, and in lieu of eradication.
TRICHOMONAS VAGINALIS
Trichomoniasis is a common, sexually transmitted disease caused by the flagellated protozoan parasite T. vaginalis 160. T. vaginalis was first described by Donné in 1836 147. The trophozoites have four free anterior flagella (one along the outer edge of an undulating membrane) and one recurrent; there is no known cyst form. Trophozoites divide by binary fission 30. The most frequent form of nonvenereal infection is perinatal.
The WHO has estimated that 180 million infections are acquired annually worldwide. The estimates for North America alone are between 5 and 8 million new infections each year, with an estimated rate of asymptomatic cases as high as 50% 87, 147. T. vaginalis has been isolated from 14 to 60% of male partners of infected women and 67 to 100% of partners of infected men. Up to 58% of young men at risk for sexually transmitted diseases are reported to have been infected 172. In Nigeria, 37% of female students at a higher education institute were recently reported to have trichomoniasis 7, which is comparable to the data from an American study 172. Institutional crowding in itself is insufficient for transmission 96. In rural South Africa, 65% of pregnant women attending an antenatal class were reported to have trichomoniasis in 1981 88, 171 and 49% in 1989 141. In another more recent South African rural survey, this figure was 41% 230. Among Australian aboriginals from whom genital swab samples had been taken, 17% were positive for trichomonads, while the rate was less than 1% for nonaboriginals 225. The prevalence of trichomoniasis in women attending a sexually transmitted diseases clinic in Ulaanbaatar, Mongolia, was 67% in 1998 175. Thirty-three percent of men at a sexually transmitted disease and dermatology clinic in Malawi were T. vaginalis positive, of whom 20.8% were symptomatic and had a sixfold increase in human immunodeficiency virus (HIV) concentration in their semen 83, 87. These data emphasize earlier suggestions of the association between HIV/AIDS and trichomoniasis 4, 56, 128, 181.
Trichomonal infection in women ranges from an asymptomatic carrier state to profound, acute, inflammatory disease 160. The parasite principally infects the squamous epithelium of the genital tract but can be recovered from the urethra and has been found in the fallopian tubes and the pelvis 74, 147. In males, T. vaginalis causes urethritis and prostatitis 100. Pneumonia, bronchitis, and oral lesions have also been well documented. Respiratory infections are acquired perinatally from infected mothers 86. In children, trichomonads can infect the urinary tract as well as the vagina. T. vaginalis infections have been linked to sterility problems, but there are no unequivocal data, and to adverse pregnancy outcomes 4. On the other hand, Trichomonas foetus in cattle causes infertility, resulting in considerable economic loss 40, 84. Recent, prospective epidemiological studies have shown that among 14,000 women examined in university-affiliated hospitals in the United States, T. vaginalis infection is associated with low birth weight and preterm delivery (40%) 42, 171. These in turn are closely associated with high mortality, particularly in the black minorities. T. vaginalis infection also predisposes carriers to HIV/AIDS 4, 128, 181, and a study of 19,000 women in Finland has recently indicated a high relative risk of cervical cancer with Trichomonas infection 223.
T. vaginalis uses lectin-like adhesins to attach to the epithelial mucin, proteases to degrade the mucin, and flagellar motility to colonize the underlying epithelial cells 53, 104. A number of adhesin genes have been cloned and appear to encode adapted metabolic enzymes. Early studies indicated considerable morphological variety among T. vaginalis isolates 85. This variety was supported in limited studies of protease diversity 137, a 270-kDa surface protein that varies phenotypically 3, and monoclonal antibodies to surface antigens 61, but does not appear to have been pursued in any further detail.
The karyotype of T. vaginalis has been described as haploid, with six chromosomes 49, and diploid, with 12 chromosomes 238. Although high-molecular-weight DNA has been extracted and genes have been cloned, no consistent electrophoretic karyotype has been described because of apparent DNA degradation during lysis block preparation, unusual chromosome topology, or large size preventing migration into the agarose.
Trichomonas Metabolism
T. vaginalis lacks mitochondria and other characteristics of higher eukaryotic respiratory metabolism such as cytochromes and oxidative phosphorylation 51, 52. Nutrients are taken up by transport through the cell membrane and by phagocytosis. Energy requirements are provided by glycolysis of glucose to glycerol and succinate in the cytoplasm, followed by further conversion of pyruvate and malate to hydrogen and acetate in an organelle called the hydrogenosome 132, 133. Lactate dehydrogenase is present; the gene has been cloned and is derived from the gene for malate dehydrogenase 234. The hydrogenosome contains electron transport components linked to PFOR, hydrogenase, and an undefined oxidase (terminal?). Hydrogenosomes also store calcium 89. The hydrogenosome may be a degenerate mitochondrion 15, 132, 133, an organelle derived from a symbiosis with a bacterium related to those that generated mitochondria 95, 124, 130, or a relic of early fusions between groups such as the archaebacteria and eubacteria 75 that generated the earliest eukaryotes 220. Sequencing of the genome of the α-proteobacterial, intracellular parasite Rickettsia prowazekii, phylogenetically closely related to the originator of the mitochondrion, casts doubt on the first two possibilities, since its reduced genome does not contain the anaerobic pathways found in hydrogenosomes 134. This leaves the origin of the hydrogenosome different from that of the mitochondrion, via another type of genome reduction not found in Rickettsia today, or as a product of multiple, transitory, or ephemeral symbioses being tested in the early fusions that generated the first eukaryotes 220. Mycoplasma hominis has been reported to be a common parasite of T. vaginalis also 152. Although this interpretation has been challenged 188, there appears to be a strong association of these two organisms 153, which suggests that symbioses may have been common in the evolution of Trichomonas.
Although Trichomonas is regarded as an anaerobe, there is a report that growth rates increase at levels of oxygen that are barely detectable by mass spectrometry 146. These levels of oxygen (0.025% atmospheric oxygen) are in the range that E. coli, for example, has switched from aerobic, through scavenging, terminal cytochrome oxidases, finally to anaerobic metabolism 197. It is unclear why an organism with such exquisite sensitivity to increased growth rates at such low oxygen concentrations has not exploited the concomitant potentially large increase in ATP production demonstrated in the other aerobes. On the other hand, growth response appears to be far more sensitive to CO2 concentration 146, which suggests that metabolic pathways are present that have not been defined as yet; extremely low O2 utilization may reflect coupled scavenging or protection pathways that are not revealed in the culture conditions currently employed. A more recent paper suggests that oxygen is converted into hydrogen peroxide via a cytosolic NADPH oxidase 35. There is no evidence for O2 utilization in Giardia, although O2 has been shown to be consumed 110, 229. Alternatively, it has been shown that O2 can be converted directly to H2O by NADH oxidase to protect the key anaerobic enzymes in the glycolytic pathway, PFOR and ferredoxin, for example 22, 24. These components have been purified and their activity has been demonstrated in vitro 194, 196. It is of interest that the strict archaebacterial anaerobe M. jannaschii has only two aerobic enzyme genes encoded in its genome 27, one of which is NADH oxidase, which is closely related to the giardial enzyme 196. Purification of the appropriate metabolic enzymes from Trichomonas may resolve our understanding of the possible roles of O2 in its metabolism and in metronidazole resistance (see below).
Trichomonas Drug Treatment and Resistance
Although T. vaginalis was discovered in 1836 and has been known to cause vaginitis since 1916, it was not until 1957 that an effective cure, metronidazole, was discovered. Drug resistance was first reported soon after in 1962 118, although metronidazole is still the drug of choice 94, 177. Cross-resistance between different nitroimidazoles has been reported 135 and is consistent with earlier studies 126, 135. Metronidazole-resistant isolates were sensitive to the nitrofuran furazolidone 135, consistent with these drugs not being cross-resistant in multidrug-resistant Giardia. A metronidazole-resistant clinical isolate has been described which shows only partial cross-resistance to tinidazole 224.
There has been reluctance to utilize metronidazole for trichomoniasis in pregnant women, particularly in the United States, because of weakly mutagenic effects in bacteria and carcinogenic effects detected in rodents 43, 44, 171, 180. However, no teratogenic effects have been detected during the long use of metronidazole in humans, and there is an easing of this stand because of the association of trichomoniasis with preterm deliveries, low birth weight, and increased infant mortality 42, 171 and predisposition to HIV/AIDS 4, 128, 181. It is now regarded as acceptable to use metronidazole, particularly in the last two trimesters of birth, to treat trichomoniasis, and possibly in the first trimester if warranted 31, 42, 43, 44, 149, 171, 174, 177.
Aerobic versus Anaerobic Drug Resistance
Although there is circumstantial evidence for changes in the structure of the hydrogenosome in drug-resistant T. vaginalis, it is unclear whether they are primary events or secondary to the advent of resistance 94. Earlier work suggested that the hydrogenosome could be lost in drug-resistant cells, but recent data have shown that the organelle remains in a modified form (J. Kulda, unpublished data). There is, however, evidence for changes in upstream regulatory regions of the ferredoxin gene in four drug-resistant strains 151. Intracellular levels of the ferredoxin protein and its mRNA were reduced by approximately 50%; whether this is adequate to confer resistance is not known. A Czechoslovakian study in 1988 showed a decrease in PFOR and hydrogenase activity, but it is unknown whether these are primary or pleiotropic events 33, 34. Very few new data have appeared since 52. In principle, both PFOR and hydrogenase could activate the 5-nitroimidazoles via a ferredoxin or perhaps flavodoxin intermediate.
It has been proposed that clinical cases of drug-resistant T. vaginalis occur as aerobic resistance because O2 is required in some way to detoxify metronidazole 33, 34, 52; resistance has been accounted for as a decreased affinity for O2 by the respiratory system. Results of electron spin resonance spectrometry 235 suggested that one metronidazole-resistant isolate was deficient in O2 scavenging. Further work with membrane inlet mass spectrometry indicated a diminished affinity for O2 by a hydrogenosome-containing fraction. However, the most recent data 52 indicate that hydrogenase activity is lowered in two clinically resistant isolates, which is a phenomenon (along with decreased PFOR activity) previously attributed to anaerobic resistance 33, 34, 94. Furthermore, the proposed decrease in oxidase activity is inconsistent in these isolates and the decrease in H2 production is also inconsistent with earlier work 33, 34. Yarlett et al. 235 suggested that decreased oxidase activity associated with the hydrogenosome could be responsible for a possibly higher O2 concentration inside the hydrogenosome; the O2 could then cycle with the reduced free-radical forms of the nitroimidazoles, decreasing their effective concentration. In the most recent work by the same group 52, in which the changes in oxidase levels are modest compared with the hydrogenases, one of the methods for the purification of the NADH and NADPH oxidases also included the much more abundant cytoplasmic oxidases 113 that apparently are not correlated with resistance 33, 34. It is therefore unclear at this stage whether a discrete hydrogenosome-specific oxidase activity is involved with drug resistance. The mechanisms proposed for drug resistance in general need clarification with standardized techniques and common strains.
As resistance levels increase in parasites that have been selected for resistance by increasing the levels of metronidazole that they will tolerate, there is a progressive decrease in PFOR 101. Ferredoxin levels, hydrogenosomal malic enzyme, and NAD: ferredoxin oxidoreductase also decrease. T. vaginalis enhances lactate fermentation and glycolysis to compensate. Highly resistant lines have no detectable PFOR activity, PFOR mRNA, or ferredoxin mRNA 25; unpublished data). Alternate 2-oxoacid oxidoreductases have now been described which increase in activity during stepwise selection for increase in drug resistance 25, 216. As in Giardia, these alternative oxidoreductases have different substrate requirements, do not necessarily donate electrons to ferredoxin, and appear not to activate metronidazole, thereby circumventing the downregulated PFOR to provide alternative sources of energy. The arginine dihydrolase pathway also provides a source of energy for the parasite 114, 236.
It has been proposed that aerobic drug resistance occurs in infected patients and that the more highly anaerobic resistance can only be generated in the laboratory 101. However, the first proven drug-resistant Australian isolate was highly resistant to metronidazole when also tested anaerobically; therefore, anaerobic resistance can clearly develop in Trichomonas infections of humans 224; unpublished data). This is cause for concern, since it has been regarded that anaerobic resistance is unlikely to develop in naturally infected hosts because of the multiple steps that have so far been required to generate anaerobic resistance in the laboratory 101. Two strains have been isolated that are resistant under anaerobic conditions but do not grow aerobically (unpublished data). This is in contrast to previous data which suggest that there is a stepwise progression from aerobic to anaerobic resistance 101 and imply that there is more than one route to resistance. The earlier conflicting data may also be explained by such differences in strains and the differential regulation of metabolic pathways involved in resistance mechanisms.
Although reports of drug-resistant Trichomonas have appeared regularly since the phenomenon was first documented in 1962 118, the occurrences have not increased to epidemic proportions. It has been assumed that resistance may debilitate the parasite and impede its transfer among sexually active partners, leaving the resistance a problem for the carrier alone. Treatment failure is not uncommon 118, 147, 159, 177, 191. In two clinics in the United States (Philadelphia and Detroit), the number of highly resistant cases has been reported as 1 per 2,000 to 3,000 trichomoniasis cases treated prior to 1996 180. However, this number has increased to 17 in 1997 and 1998, with a clear upward trend, and some cases appear incurable 180. If Lossick's 118 frequency of refractory cases resistant to the highest recommended doses of metronidazole is applied to the 1999 data, 5 to 10% of trichomoniasis isolates now show some level of resistance, e.g., need more than one series of treatments. This is consistent with Centers for Disease Control and Prevention data of 1989 147. Whether continued drug selection has now allowed increased transmission, more resistant organisms, or the emergence of a more serious sexually transmitted disease problem remains to be monitored.
CLINICAL AND LABORATORY-GENERATED DRUG-RESISTANT ISOLATES
Laboratory-based studies which have developed systems for inducing drug resistance complement analysis of clinical isolates of T. vaginalis, G. duodenalis, and E. histolytica 218. Stepwise incremental increases in drug resistance induced by a variety of drug regimens, including increases in drug concentration at sublethal levels or short multiple exposures to lethal levels, that are administered with and without mutagens, have provided considerable information about the ability of parasites to mount a resistance defense and the mechanisms involved 22, 24, 25, 33, 34, 101, 193, 194, 195, 196, 200, 201, 206, 208, 216, 217, 218. With no examples of horizontal, transposon, or plasmid transfer of drug resistance in the anaerobic protozoa, this has been a valid and extremely useful approach to determine mechanisms of resistance. However, a double-stranded RNA virus in Giardia 237 and Trichomonas 3, 186 and the mini-circular plasmid form of a transposable element in Giardia 215 suggest the possibility of transfer. Perhaps the only drawback is the lack of host-parasite interaction in the development of resistance; in many instances, however, any interaction in the maintenance or modulation of resistance could be ascertained later with in vivo studies. Furthermore, mechanisms uncovered in the laboratory can then be readily analyzed for their appearance in clinical isolates; intermediate stages of resistance can be compared for their likelihood to progress to much higher levels. The great advantage of in vitro studies is that the parental, sensitive parasites and the selected, resistant parasites are genetically syngeneic, so that resistance is developed in the same genetic background, and hence confounding or alternate mechanisms do not complicate mechanistic interpretations, a situation which has arisen with malaria, for example 62, 156. A second advantage for in vitro studies with the anaerobic protozoa addressed here is that they have no known sexual stages, so that multifactorial or polygenic resistance traits must arise in the same parasite and can be monitored over time and incremental increases in drug concentration. A third advantage is the ability to determine the resistance capabilities of the parasite before they arise as an epidemiological or public health problem at large; prior knowledge and predictive values of resistance capabilities and mechanisms for malaria, for example, may have alleviated and forestalled the major, and currently insurmountable, resistance problems that there are today 17, 138.
There is only one example of the inability to develop resistance in vitro to an antiparasitic drug, that being development of chloroquine resistance in Plasmodium falciparum malaria in two regions, Thailand and South America. Resistance to chloroquine was subsequently transferred or transmitted worldwide 184. Laboratory-induced resistance has not been demonstrated convincingly as yet, although it was readily achieved for Plasmodium berghei 150; this is more likely due to a difficulty in screening sufficient numbers of parasites than an inability to induce resistance. A bacterial example of the inability to develop resistance is Bacteroides fragilis, which is regarded as a “gold standard” for anaerobic bacterial resistance to metronidazole. Less than 1% resistance has been detected in clinical isolates, and these are not at high drug concentrations 155; it is therefore assumed that resistance to metronidazole in anaerobes is difficult to find or achieve 155. This is clearly not the case for anaerobic protozoa. For example, metronidazole resistance in Trichomonas was detected within 1 year of initial drug release. Although resistance may presently be a major problem for the individual harboring such organisms, epidemics of resistant organisms have not been demonstrated so far. Nor is it true for all anaerobic bacteria, e.g., Helicobacter pylori isolates from some parts of the world show high incidences of metronidazole resistance 12.
Such arguments also highlight the history of chloroquine usage and the past lack of resistant P. falciparum, for example, but do not reflect the relentless development of resistance to antimicrobials in microorganisms, including Plasmodium 156. Although the onset of resistance to multiple drugs in Plasmodium has been attributed to sorting of genes conferring different aspects of resistance, alternative interpretations have been proposed; comparing the diversity in different genes, Rich et al. 162 suggest that current isolates examined worldwide may be derived from a very recent (thousands of years) single, clonal propagation and that sexual exchange plays a much less important role than mitotic exchange in generation of diversity. Therefore, even in sexual protozoa, drug resistance may have propagated in a manner similar to that in asexual organisms. This is also consistent with the spread of resistance to chloroquine from two regions 184. Selection under continuous drug pressure also appears to have generated traits (accelerated resistance to multiple drugs) in Plasmodium that increase the innate ability to initiate rapid and high resistance to novel compounds that is not the result of sexual gene assortment 156 .
Recently, multiple, simultaneous resistance of B. fragilis to metronidazole, coamoxiclav, and imipinem was described for a patient after surgical procedures 198 . Resistance to metronidazole in a series of hospital isolates has been attributed to novel transferable genes (nimA to nimD) 161 that encode or regulate a metronidazole oxidoreductase 32. nim genes have been found in other B. fragilis isolates as well as other anaerobes 119. Whether this is the beginning of an increase in resistance mechanisms, as has been observed in malaria for a number of other drugs 156, is of great significance. An example of metroninidazole resistance being clearly on the increase is Helicobacter pylori; isolates from countries in which metronidazole can be easily obtained without prescription commonly develop resistance through secondary selection in the gut 12.
Comparison of Giardia isolates has shown a range of susceptibilities to metronidazole and furazolidone 208. However, the relevance of point mutations in drug resistance among these isolates is difficult to determine because of considerable genetic diversity; there is up to 30% divergence in the sequence of some genes encoding housekeeping enzymes, and up to 50% for intergenic spacers containing regulatory elements 37, 38, 185, 203, 220, 221. The range of variation in enzymatic activity (unpublished data) also makes interpretation of their role in resistance more difficult. This is further complicated by the presence of mixed genotypes in human infections and differential selection during in vitro culture 207. Major changes, such as chromosomal deletion and complete loss of function, e.g., the elimination of hydrogenosomal enzymes in Trichomonas, render correlations much more significant and simplify interpretation. Isogenic strains are therefore essential to follow induced changes and the development of high levels of resistance 218 when there is high genetic diversity. Stepwise induction of resistance in vitro has led to the unusual situation in which strains are more resistant than those so far detected in the wild 101, 218. We are therefore warned in advance of the parasite's resistance capabilities in a number of ways, including resistance mechanisms at the molecular level, common or coordinately regulated pathways of drug activation and drug resistance, the number of enzymatic steps involved, and multiple drug resistance. Knowledge of these resistance mechanisms is complemented by development of ways of circumventing these events, identification of new targets, and development of new drugs (Upcroft et al., unpublished data), and delivery systems which will overcome or bypass them. If novel parasite resistance mechanisms arise in the host, a considerable background knowledge has therefore been generated to determine at which level in the organism this may occur and the relationship to known mechanisms. A framework for a rational approach to drug design and therapy can be envisioned 208, 209, 218; unpublished data).
In a bacterial comparison, the importance of using isogenic strains for analyzing drug resistance and virulence in Mycobacterium tuberculosis, another organism which appears not to use plasmid-mediated transfer of resistance determinants, has been emphasized recently 241, 242. Isogenic strains are being used in combination with the natural variation in drug susceptibilities of different Mycobacterium species and gene transfer studies to unravel the range of mechanisms now evident in sensitivity and resistance, as well as the participation of subtle pleiotropic enzymatic pathways which augment high levels of resistance 242. The genome sequence of two different strains will also aid in analyzing loss-of-function and recessive mutations involved in the development of resistance 46, 241.
EPIDEMIOLOGY OF RESISTANCE
Although our understanding of drug resistance in even one bacterium is still incomplete, significant detail about mechanisms such as penicillin resistance and drug transporters at the molecular level has allowed new approaches to be instigated. One of these is the epidemiology and population dynamics of resistance extending beyond the enumeration or prevalence of resistant organisms, which may allow predictive outcomes for the optimized use of current drugs and introduction of new drugs 107, 108. Our intuitive expectation of drug use and subsequent resistance development may not parallel the real capabilities of an organism, e.g., the long-held supposition that the development of resistance is always a burden for the organism and that removal of drug pressure will allow return of sensitive organisms by natural selection is clearly not universally correct 5, 105, 115, 116. In one study of Salmonella strains resistant to three antibiotics and thus rendered avirulent, compensatory mutations to virulence occurred more frequently than backmutations to drug sensitivity 16. Similarly, modeling and subsequent experimental testing can provide unexpected outcomes for development of resistance and multidrug resistance that may change the way drugs are rotated in hospital use to prevent the acquisition of resistance and the spread of these strains in the community 8, 14, 69, 107, 108. Such studies are in their infancy for the anaerobic protozoa, but every study of resistant organisms and the mechanisms and spread of resistance adds information to a more complete understanding of the capabilities of these organisms and the dynamics of the host-parasite relationship.
Direct comparison of the anaerobic protozoa with bacteria has been very valuable, particularly in defining the fundamental metabolic pathways and enzymes involved in activation of drugs and subsequent resistance. This has placed them in an evolutionary position that differs from the aerobic eukaryotes, which has been argued above as being early diverging and distinct from those organisms that now have mitochondria. There are also considerable differences, e.g., detoxification pathways in which SOD, catalase, and peroxidase may be present or absent and unusual organelles in Trichomonas and Entamoeba. The work with Giardia and Entamoeba also highlights that each of the protozoa subsequently regulates its essential metabolism, glycolysis, idiosyncratically under drug pressure, e.g., whether PFOR (and ferredoxin) is downregulated and to what extent. As we determine the more subtle aspects of the coupled pathways and differences in bypass and supplementary metabolic systems, such as alternative oxidoreductases, the varieties of ferredoxins and flavodoxins, and detoxification systems, the extent of the diversity in these anaerobes will become even more discernable. Initially grouping them together has been a valuable exercise, but determining these differences will probably add even more to our understanding of the few anaerobic protozoa that have been examined in any real detail. This process will also evoke novel drug targets and drugs for improved control, a challenge for the future.
ACKNOWLEDGMENTS
We thank the National Health and Medical Research Council of Australia and the Australian Research Council for grant support.
REFERENCES
- 1.Adam R D. The biology of Giardia spp. Microbiol Rev. 1991;55:706–732. doi: 10.1128/mr.55.4.706-732.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldeen W E, Carroll K, Robinson A, Morrison M, Hale D. Comparison of nine commercially available enzyme-linked immunosorbent assays for detection of Giardia lamblia in fecal specimens. J Clin Microbiol. 1998;36:1338–1340. doi: 10.1128/jcm.36.5.1338-1340.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alderete J F. Iron modulates phenotypic variation and phosphorylation of P270 in double-stranded RNA virus-infected Trichomonas vaginalis. Infect Immun. 1999;67:4298–4302. doi: 10.1128/iai.67.8.4298-4302.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alderete J F, Lehker M W, Arroyo R. The mechanisms and molecules involved in cytoadherence and pathogenesis of Trichomonas vaginalis. Parasitol Today. 1995;11:70–74. [Google Scholar]
- 5.Andersson D I, Levin B R. The biological cost of antibiotic resistance. Curr Opin Microbiol. 1999;2:489–493. doi: 10.1016/s1369-5274(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 6.Andrews R H, Monis P T, Ey P L, Mayrhofer G. Comparison of the levels of intra-specific genetic variation within Giardia muris and Giardia intestinalis. Int J Parasitol. 1998;28:1179–1185. doi: 10.1016/s0020-7519(98)00097-6. [DOI] [PubMed] [Google Scholar]
- 7.Anisoke J C, Onwuliri C O, Inyang R E, Akoh J I, Nwoke B E, Adeiyongo C M, Okoye S N, Akogun O B. Trichomoniasis amongst students of a higher institution in Nigeria. Appl Parasitol. 1993;34:19–25. [PubMed] [Google Scholar]
- 8.Austin D J, Kakehashi M, Anderson R M. The transmission dynamics of antibiotic-resistant bacteria: the relationship between resistance in commensal organisms and antibiotic consumption. Proc R Soc Lond B. 1997;264:1629–1638. doi: 10.1098/rspb.1997.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayala P, Samuelson J, Wirth D, Orozco E. Entamoeba histolytica: physiology of multidrug resistance. Exp Parasitol. 1990;71:169–175. doi: 10.1016/0014-4894(90)90019-9. [DOI] [PubMed] [Google Scholar]
- 10.Báez-Camargo M, Flores-Soto E, Orozco E. Improved molecular karyotype of Entamoeba histolytica. Arch Med Res. 1992;23:7–9. [PubMed] [Google Scholar]
- 11.Báez-Camargo M, Riverón AM, Delgadillo DM, Flores E, Sánchez T, Garcia-Rivera G, Orozco E. Entamoeba histolytica: gene linkage groups and relevant features of its karyotype. Mol Gen Genet. 1996;253:289–296. doi: 10.1007/pl00008595. [DOI] [PubMed] [Google Scholar]
- 12.Banatvala N, Davies G R, Abdi Y, Clements L, Rampton D S, Hardie J M, Feldman R A. High prevalence of Helicobacter pylori metronidazole resistance in migrants to east London: relation with previous nitroimidazole exposure and gastroduodenal disease. Gut. 1994;35:1562–1566. doi: 10.1136/gut.35.11.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Band R N, Cirrito H. Growth response of axenic Entamoeba histolytica to hydrogen, carbon dioxide, and oxygen. J Protozool. 1979;26:282–286. doi: 10.1111/j.1550-7408.1979.tb02781.x. [DOI] [PubMed] [Google Scholar]
- 14.Baquero F, Negri M C, Morosini M I, Blázquez J. The antibiotic selective process: concentration-specific amplification of low-level resistant populations. Ciba Found Symp. 1997;207:93–111. doi: 10.1002/9780470515358.ch7. [DOI] [PubMed] [Google Scholar]
- 15.Biagini G A, Finlay B J, Lloyd D. Evolution of the hydrogenosome. FEMS Microbiol Lett. 1997;155:133–140. doi: 10.1016/s0378-1097(97)00333-9. [DOI] [PubMed] [Google Scholar]
- 16.Björkman J, Hughes D, Andersson D I. Virulence of antibiotic-resistant Salmonella typhimurium. Proc Natl Acad Sci USA. 1998;95:3949–3953. doi: 10.1073/pnas.95.7.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bloland P B, Ettling M. Making malaria-treatment policy in the face of drug resistance. Ann Trop Med Parasitol. 1999;93:5–23. doi: 10.1080/00034989958753. [DOI] [PubMed] [Google Scholar]
- 18.Boreham P F L. The current status of chemotherapy for giardiasis. In: Thompson R C A, Reynoldson J A, Lymbery A J, editors. Giardia: from molecules to disease. Cambridge, U.K: CAB International; 1994. pp. 317–328. [Google Scholar]
- 19.Boreham P F L, Phillips R E, Shepherd R W. Altered uptake of metronidazole in vitro by stocks of Giardia intestinalis with different drug sensitivities. Trans R Soc Trop Med Hyg. 1988;82:104–106. doi: 10.1016/0035-9203(88)90278-7. [DOI] [PubMed] [Google Scholar]
- 20.Borst P, Ouellette M. New mechanisms of drug resistance in parasitic protozoa. Annu Rev Microbiol. 1995;49:427–460. doi: 10.1146/annurev.mi.49.100195.002235. [DOI] [PubMed] [Google Scholar]
- 21.Brasseur P, Favennec L. Two cases of giardiasis unsuccessfully treated by albendazole. Parasite. 1995;2:422. [PubMed] [Google Scholar]
- 22.Brown D M, Upcroft J A, Edwards M R, Upcroft P. Anaerobic bacterial metabolism in the ancient eukaryote Giardia duodenalis. Int J Parasitol. 1998;28:149–164. doi: 10.1016/s0020-7519(97)00172-0. [DOI] [PubMed] [Google Scholar]
- 23.Brown D M, Upcroft J A, Upcroft P. Free radical detoxification in Giardia duodenalis. Mol Biochem Parasitol. 1995;72:47–56. doi: 10.1016/0166-6851(95)00065-9. [DOI] [PubMed] [Google Scholar]
- 24.Brown D M, Upcroft J A, Upcroft P. A H2O-producing NADH oxidase from the protozoan parasite Giardia duodenalis. Eur J Biochem. 1996;241:155–161. doi: 10.1111/j.1432-1033.1996.0155t.x. [DOI] [PubMed] [Google Scholar]
- 25.Brown D M, Upcroft J A, Dodd H N, Chen N, Upcroft P. Alternative 2-ketoacid oxidoreductase activities in Trichomonas vaginalis. Mol Biochem Parasitol. 1999;98:203–214. doi: 10.1016/s0166-6851(98)00169-8. [DOI] [PubMed] [Google Scholar]
- 26.Bruchhaus I, Tannich E. Purification and molecular characterization of the NAD(+)-dependent acetaldehyde/alcohol dehydrogenase from Entamoeba histolytica. Biochem J. 1994;303:743–748. doi: 10.1042/bj3030743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bult C J, et al. Complete genome sequence of the methanogenic archaeon Methanococcus jannaschii. Science. 1996;273:1017–1140. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 28.Burchard G D. Therapy for malaria and amoebiasis. Immun Infekt. 1994;22:45–47. [PubMed] [Google Scholar]
- 29.Buret A. Pathogenesis—how does Giardia cause disease? In: Thompson R C A, Reynoldson J A, Lymbery A J, editors. Giardia: from molecules to disease. Cambridge, U.K: CAB International; 1994. pp. 293–315. [Google Scholar]
- 30.Burgess D E. Trichomonads and intestinal flagellates. In: Collier L, Balows A, Sussman M, editors. Topley and Wilson's microbiology and microbial infections. 9th ed. 5: parasitology. London, U.K: Arnold; 1998. pp. 203–214. [Google Scholar]
- 31.Burtin P, Taddio A, Ariburnu O, Einarson T R, Koren G. Safety of metronidazole in pregnancy: a meta-analysis. Am J Obstet Gynecol. 1995;172:525–529. doi: 10.1016/0002-9378(95)90567-7. [DOI] [PubMed] [Google Scholar]
- 32.Carlier J-P, Sellier N, Rager M-N, Reysset G. Metabolism of a 5-nitroimidazole in susceptible and resistant isogenic strains of Bacteroides fragilis. Antimicrob Agents Chemother. 1997;41:1495–1499. doi: 10.1128/aac.41.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Čerkasovová A, Čerkasov J, Kulda J. Resistance of trichomonads to metronidazole. Acta Univ Carol Biol. 1988;30:485–503. [Google Scholar]
- 34.Čerkasovová A, Novák J, Čerkasov J, Kulda J, Tachezy J. Metabolic properties of Trichomonas vaginalis resistant to metronidazole under anaerobic conditions. Acta Univ Carol Biol. 1988;30:505–512. [Google Scholar]
- 35.Chapman A, Linstead D J, Lloyd D. Hydrogen peroxide is a product of oxygen consumption by Trichomonas vaginalis. J Biosci. 1999;24:339–344. [Google Scholar]
- 36.Chen J, Huang X, Liu Y, Dai G, Chen W. Detoxicating enzymes of Entamoeba histolytica and their detoxifying roles. Chin Med J (Engl Ed) 1996;109:792–794. [PubMed] [Google Scholar]
- 37.Chen N, Upcroft J A, Upcroft P. A Giardia duodenalis gene encoding multiple repeats of a toxin homologue. Parasitology. 1995;111:423–431. doi: 10.1017/s0031182000065926. [DOI] [PubMed] [Google Scholar]
- 38.Chen N, Upcroft J A, Upcroft P. A new cysteine-rich protein-encoding gene family in Giardia. Gene. 1996;169:33–38. doi: 10.1016/0378-1119(95)00759-8. [DOI] [PubMed] [Google Scholar]
- 39.Cheney C P, Wong R K H. Acute infectious diarrhea. Med Clin N Am. 1993;77:1169–1196. doi: 10.1016/s0025-7125(16)30216-4. [DOI] [PubMed] [Google Scholar]
- 40.Corbeil L B. Vaccination strategies against Tritrichomonas foetus. Parasitol Today. 1994;10:103–106. doi: 10.1016/0169-4758(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 41.Corsaro D, Venditti D, Padula M, Valassina M. Intracellular life. Crit Rev Microbiol. 1999;25:39–79. doi: 10.1080/10408419991299167. [DOI] [PubMed] [Google Scholar]
- 42.Cotch M F, Pastorek II J G, Nugent R P, Hillier S L, Gibbs R S, Martin D H, Eschenbach D A, Edelman R, Carey J C, Regan J A, Krohn M A, Klebanoff M A, Rao A V, Rhoads G G the Vaginal Infections and Prematurity Study Group. Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex Transm Dis. 1997;24:353–360. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Coustan D R. Use of metronidazole during pregnancy. Ped Infect Dis J. 1999;18:79. doi: 10.1097/00006454-199901000-00026. [DOI] [PubMed] [Google Scholar]
- 44.Coustan D R, Mochizuki T R. Handbook for prescribing medications during pregnancy. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1998. Metronidazole; pp. 297–298. [Google Scholar]
- 45.Desenclos J C, Zergabachew A, Desmoulins B, Chouteau L, Desve G, Admassu M. Clinical, microbiological and antibiotic susceptibility patterns of diarrhoea in Korem, Ethiopia. J Trop Med Hyg. 1988;91:296–301. [PubMed] [Google Scholar]
- 46.De Smet K A L. Mycobacterium tuberculosis: beyond genome sequencing. Trends Microbiol. 1997;5:429–431. doi: 10.1016/S0966-842X(97)01132-3. [DOI] [PubMed] [Google Scholar]
- 47.Dhar S K, Choudhury N R, Mittal V, Bhattacharya A, Bhattacharya S. Replication initiates at multiple dispersed sites in the ribosomal DNA plasmid of the protozoan parasite Entamoeba histolytica. Mol Cell Biol. 1996;16:2314–2324. doi: 10.1128/mcb.16.5.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diamond L S, Clark C G. A redescription of Entamoeba histolytica Schaudinn, 1903 (Emended Walker, 1911) separating it from Entamoeba dispar Brumpt, 1925. J Eukaryot Microbiol. 1993;40:340–344. doi: 10.1111/j.1550-7408.1993.tb04926.x. [DOI] [PubMed] [Google Scholar]
- 49.Drmota T, Král J. Karyotype of Trichomonas vaginalis. Eur J Protistol. 1997;33:131–135. [Google Scholar]
- 50.Edwards M R, Gilroy F V, Jiminez B M, O'Sullivan W J. Alanine is a major end product of metabolism by Giardia lamblia: a proton nuclear magnetic resonance study. Mol Biochem Parasitol. 1989;37:19–26. doi: 10.1016/0166-6851(89)90098-4. [DOI] [PubMed] [Google Scholar]
- 51.Ellis J E, Cole D, Lloyd D. Influence of oxygen on the fermentative metabolism of metronidazole-sensitive and resistant strains of Trichomonas vaginalis. Mol Biochem Parasitol. 1992;56:79–88. doi: 10.1016/0166-6851(92)90156-e. [DOI] [PubMed] [Google Scholar]
- 52.Ellis J E, Wingfield J M, Cole D, Boreham P F L, Lloyd D. Oxygen affinities of metronidazole-resistant and -sensitive stocks of Giardia intestinalis. Int J Parasitol. 1992;23:35–39. doi: 10.1016/0020-7519(93)90095-g. [DOI] [PubMed] [Google Scholar]
- 53.Engbring J A, O'Brien J L, Alderete J F. Trichomonas vaginalis adhesin proteins display molecular mimicry to metabolic enzymes. In: Kahane I, Ofek I, editors. Towards anti-adhesion therapy for microbial diseases. New York, N.Y: Plenum Press; 1996. pp. 207–223. [DOI] [PubMed] [Google Scholar]
- 54.Erlandsen S L, Meyer E A. Giardia and giardiasis: biology, pathogenesis, and epidemiology. New York, N.Y: Plenum Press; 1984. [Google Scholar]
- 55.Espinosa-Cantellano M, González-Robles A, Chávez B, Castañón G, Argüello C, Lázaro-Haller A, Martínez-Palomo Entamoeba dispar: ultrastructure, surface properties and cytopathic effect. J Eukaryot Microbiol. 1998;45:265–272. doi: 10.1111/j.1550-7408.1998.tb04535.x. [DOI] [PubMed] [Google Scholar]
- 56.Estambale B B A, Knight R. Protozoan infections and HIV-1 infection: a review. East Afr Med J. 1992;69:373–377. [PubMed] [Google Scholar]
- 57.Fahey R C, Newton G L, Arrick B, Overdank-Bogart T, Aley S B. Entamoeba histolytica: a eukaryote without glutathione metabolism. Science. 1984;224:70–72. doi: 10.1126/science.6322306. [DOI] [PubMed] [Google Scholar]
- 58.Fairlamb A H, Cerami A. Metabolism and functions of trypanothione in the Kinetoplastida. Annu Rev Microbiol. 1992;46:695–729. doi: 10.1146/annurev.mi.46.100192.003403. [DOI] [PubMed] [Google Scholar]
- 59.Farthing M J G. Giardiasis. Gastroenterol Clin N Am. 1996;25:493–515. doi: 10.1016/s0889-8553(05)70260-0. [DOI] [PubMed] [Google Scholar]
- 60.Reference deleted.
- 61.Fiori P L, Rappelli P, Manca C, Mattana A, Cappuccinelli P. Phenotypic variation of surface antigenic determinants in Trichomonas vaginalis detected by monoclonal antibodies. Microbiologica. 1992;15:227–235. [PubMed] [Google Scholar]
- 62.Foote S J, Cowman A F. The mode of action and the mechanism of resistance to antimalarial drugs. Acta Trop. 1994;56:157–171. doi: 10.1016/0001-706x(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 63.Fraser D, Dagan R, Naggan L, Greene V, el-On J, Abu-Rbiah Y, Deckelbaum R J. Natural history of Giardia lamblia and Cryptosporidium infections in a cohort of Israeli Bedouin infants: a study of a population in transition. Am J Trop Med Hyg. 1997;57:544–549. doi: 10.4269/ajtmh.1997.57.544. [DOI] [PubMed] [Google Scholar]
- 64.Freeman C D, Klutman N E, Lamp K C. Metronidazole: a therapeutic review and update. Drugs. 1997;54:679–708. doi: 10.2165/00003495-199754050-00003. [DOI] [PubMed] [Google Scholar]
- 65.Garcia L S. Giardiasis. In: Collier L, Balows A, Sussman M, editors. , Topley and Wilson' s microbiology and microbial infections. 9th ed. 5: parasitology. London, U.K: Arnold; 1998. pp. 193–202. [Google Scholar]
- 66.Garduño-Espinosa J, Martinez-Garcia M C, Valadez-Salazar A, Padilla G, Cedillo-Rivera R, Muñoz O. Cost-effectiveness analysis of treatment of E. histolytica/E. dispar cyst carriers. Arch Med Res. 1997;28(Spec. No.):293–294. [PubMed] [Google Scholar]
- 67.Gatti S, Cevini C, Bruno A, Novati S, Scaglia M. Transmission of Entamoeba histolytica within a family complex. Trans R Soc Trop Med Hyg. 1995;89:403–405. doi: 10.1016/0035-9203(95)90029-2. [DOI] [PubMed] [Google Scholar]
- 68.Gault M J, Reiner D S, Gillin F D. Tolerance of axenically cultured Entamoeba histolytica and Giardia lamblia to a variety of antimicrobial agents. Trans R Soc Trop Med Hyg. 1985;79:60–62. doi: 10.1016/0035-9203(85)90236-6. [DOI] [PubMed] [Google Scholar]
- 69.Gaynes R, Monnet D. The contribution of antibiotic use on the frequency of antibiotic resistance in hospitals. Ciba Found Symp. 1997;207:47–60. doi: 10.1002/9780470515358.ch4. [DOI] [PubMed] [Google Scholar]
- 70.Gillin F D, Diamond L S. Entamoeba histolytica and Entamoeba invadens: effects of temperature and oxygen tension on growth and survival. Exp Parasitol. 1980;49:328–338. doi: 10.1016/0014-4894(80)90069-7. [DOI] [PubMed] [Google Scholar]
- 71.Gillin F D, Reiner D S, McCaffery J M. Cell biology of the primitive eukaryote Giardia lamblia. Annu Rev Microbiol. 1996;50:679–705. doi: 10.1146/annurev.micro.50.1.679. [DOI] [PubMed] [Google Scholar]
- 72.Gomez M D, Perez D G, Ayala P, Samuelson J, Orozco E. Physiology and molecular biology of multidrug resistance in Entamoeba histolytica. Arch Med Res. 1996;27:421–425. [PubMed] [Google Scholar]
- 73.Goodwin A, Kersulyte D, Sisson G, Veldhuyzen van Zanten S J, Berg D E, Hoffman P S. Metronidazole resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NADPH nitroreductase. Mol Microbiol. 1998;28:383–393. doi: 10.1046/j.1365-2958.1998.00806.x. [DOI] [PubMed] [Google Scholar]
- 74.Gupta P K, Frost J K. Cytopathology and histopathology of the female genital tract in Trichomonas vaginalis infection. In: Honigberg B M, editor. Trichomonads parasitic in humans. New York, N.Y: Springer-Verlag; 1990. pp. 274–290. [Google Scholar]
- 75.Gupta R S, Golding G B. The origin of the eukaryotic cell. Trends Biochem Sci. 1996;21:166–171. [PubMed] [Google Scholar]
- 76.Hager W D, Rapp R P. Metronidazole. Obstet Gynecol Clin N Am. 1992;19:497–510. [PubMed] [Google Scholar]
- 77.Hall A, Nahar Q. Albendazole as a treatment for infection with Giardia duodenalis in children in Bangladesh. Trans R Soc Trop Med Hyg. 1993;87:84–86. doi: 10.1016/0035-9203(93)90435-s. [DOI] [PubMed] [Google Scholar]
- 78.Haque R, Ali I M, Petri W A., Jr Prevalence and immune response to Entamoeba histolytica infection in preschool children in Bangladesh. Am J Trop Med Hyg. 1999;60:1031–1034. doi: 10.4269/ajtmh.1999.60.1031. [DOI] [PubMed] [Google Scholar]
- 79.Hartman H R, Kyser F A. Giardiasis and its treatment. J Am Med Assoc. 1941;116:2835–2839. [Google Scholar]
- 80.Heddi A, Grenier A M, Khatchadourian C, Charles H, Nardon P. Four intracellular genomes direct weevil biology: nuclear, mitochondrial, principal endosymbiont, and Wolbachia. Proc Natl Acad Sci USA. 1999;96:6814–6819. doi: 10.1073/pnas.96.12.6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heyworth M F. Giardiasis. In: Weatherall D J, Ledingham J G G, Warrell D A, editors. Oxford textbook of medicine. Oxford, U.K: Oxford University Press; 1996. pp. 878–880. [Google Scholar]
- 82.Hill D R. Giardia lamblia. In: Mandell GL, Bennett J E, Dolin R, editors. Mandell, Douglas and Bennett's principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone; 1995. pp. 2487–2493. [Google Scholar]
- 83.Hobbs M M, Kazembe P, Reed A W, Miller W C, Nkata E, Zimba D, Costello Daly C, Chakraborty H, Cohen M S, Hoffman I. Trichomonas vaginalis as a cause of urethritis in Malawian men. Sex Transm Dis. 1999;26:381–387. doi: 10.1097/00007435-199908000-00003. [DOI] [PubMed] [Google Scholar]
- 84.Honigberg B M. Trichomonads of veterinary importance. In: Kreier J P, editor. Parasitic protozoa. II. New York, N.Y: Academic Press; 1978. pp. 163–273. [Google Scholar]
- 85.Honigberg B M. Trichomonads of importance in human medicine. In: Kreier J P, editor. Parasitic protozoa. II. New York, N.Y: Academic Press; 1978. pp. 275–454. [Google Scholar]
- 86.Honigberg B M. Trichomonads found outside the urogenital tract of humans. In: Honigberg B M, editor. Trichomonads parasitic in humans. New York, N.Y: Springer-Verlag; 1990. [Google Scholar]
- 87.Hook E W., III Trichomonas vaginalis—no longer a minor STD. Sex Transm Dis. 1999;26:288–389. doi: 10.1097/00007435-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 88.Hoosen A A, Ross S M, Mulla M J, Patel M. The incidence of selected vaginal infections among pregnant urban blacks. S Afr Med J. 1981;59:827–829. [PubMed] [Google Scholar]
- 89.Humphreys M, Ralphs J, Durrant L, Lloyd D. Confocal laser scanning microscopy of trichomonads: hydrogenosomes store calcium and show a membrane potential. Eur J Protistol. 1998;34:356–362. [Google Scholar]
- 90.Irusen E M, Jackson T F, Simjee A E. Asymptomatic intestinal colonization by pathogenic Entamoeba histolytica in amebic liver abscess: prevalence, response to therapy, and pathogenic potential. Clin Infect Dis. 1992;14:889–893. doi: 10.1093/clinids/14.4.889. [DOI] [PubMed] [Google Scholar]
- 91.Isaac-Renton J L, Cordeiro C, Sarafis K, Shahriari H. Characterization of Giardia duodenalis isolates from a waterborne outbreak. J Infect Dis. 1993;167:431–440. doi: 10.1093/infdis/167.2.431. [DOI] [PubMed] [Google Scholar]
- 92.Jarroll E L, Müller P J, Meyer E A, Morse S A. Lipid and carbohydrate metabolism of Giardia lamblia. Mol Biochem Parasitol. 1981;2:187–196. doi: 10.1016/0166-6851(81)90099-2. [DOI] [PubMed] [Google Scholar]
- 93.Jernigan J A, Pearson R D. Antiparasitic agents. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas and Bennett's principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone; 1995. pp. 458–492. [Google Scholar]
- 94.Johnson P J. Metronidazole and drug resistance. Parasitol Today. 1993;9:183–186. doi: 10.1016/0169-4758(93)90143-4. [DOI] [PubMed] [Google Scholar]
- 95.Johnson P J, Lahti C J, Bradley P J. Biogenesis of the hydrogenosome in the anaerobic protist Trichomonas vaginalis. J Parasitol. 1993;79:664–670. [PubMed] [Google Scholar]
- 96.Klausner J D, Baer J T, Contento K M, Bolan G. Investigation of a suspected outbreak of vaginal trichomoniasis among female inmates. Sex Transm Dis. 1999;26:335–338. doi: 10.1097/00007435-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 97.Knight R. The chemotherapy of amoebiasis. J Antimicrob Chemother. 1980;6:577–593. doi: 10.1093/jac/6.5.577. [DOI] [PubMed] [Google Scholar]
- 98.Knodler L A, Sekyere E O, Stewart T S, Schofield P J, Edwards M R. Cloning and expression of a prokaryotic enzyme, arginine deiminase, from a primitive eukaryote Giardia intestinalis. J Biol Chem. 1998;273:4470–4477. doi: 10.1074/jbc.273.8.4470. [DOI] [PubMed] [Google Scholar]
- 99.Kollaritsch H, Jeschko E, Wiedermann G. Albendazole is highly effective against cutaneous larva migrans but not against Giardia infection: results of an open pilot trial in travellers returning from the tropics. Trans R Soc Trop Med Hyg. 1993;87:689. doi: 10.1016/0035-9203(93)90296-3. [DOI] [PubMed] [Google Scholar]
- 100.Krieger J N. Epidemiology and clinical manifestations of urogenital trichomoniasis in men. In: Honigberg B M, editor. Trichomonads parasitic in humans. New York, N.Y: Springer-Verlag; 1990. pp. 235–245. [Google Scholar]
- 101.Kulda J. Trichomonads, hydrogenosomes and drug resistance. Int J Parasitol. 1999;29:199–212. doi: 10.1016/s0020-7519(98)00155-6. [DOI] [PubMed] [Google Scholar]
- 102.Reference deleted.
- 103.Kulda J, Nohýnková E. Flagellates of the human intestine and of intestines of other species. In: Kreier J P, editor. Parasitic protozoa. II. New York, N.Y: Academic Press; 1978. pp. 1–138. [Google Scholar]
- 104.Lehker M W, Sweeney D. Trichomonad invasion of the mucous layer requires adhesins, mucinases, and motility. Sex Transm Infect. 1999;75:231–238. doi: 10.1136/sti.75.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lenski R E. The cost of antibiotic resistance—from the perspective of a bacterium. Ciba Found Symp. 1997;207:131–151. doi: 10.1002/9780470515358.ch9. [DOI] [PubMed] [Google Scholar]
- 106.Leroy A, Mareel M, De Bruyne G, Bailey G, Nelis H. –1995. Metastasis of Entamoeba histolytica compared to colon cancer: one more step in invasion. Invasion Metastasis. 1994;14:177–191. [PubMed] [Google Scholar]
- 107.Levin B R, Lipsitch M, Bonhoeffer S. Population biology, evolution, and infectious disease: convergence and synthesis. Science. 1999;283:806–809. doi: 10.1126/science.283.5403.806. [DOI] [PubMed] [Google Scholar]
- 108.Levin B R, Lipsitch M, Perrot V, Schrag S, Antia R, Simonsen L, Moore Walker N, Stewart F M. The population genetics of antibiotic resistance. Clin Infect Dis. 1997;24(Suppl. 1):S9–S16. doi: 10.1093/clinids/24.supplement_1.s9. [DOI] [PubMed] [Google Scholar]
- 109.Li E, Stanley S L., Jr Amebiasis. Gastroenterol Clin N Am. 1996;25:471–492. doi: 10.1016/s0889-8553(05)70259-4. [DOI] [PubMed] [Google Scholar]
- 110.Lindmark D G. Energy metabolism of the anaerobic protozoon Giardia lamblia. Mol Biochem Parasitol. 1980;1:1–12. doi: 10.1016/0166-6851(80)90037-7. [DOI] [PubMed] [Google Scholar]
- 111.Lindquist H D. Induction of albendazole resistance in Giardia lamblia. Microb Drug Resist. 1996;2:433–434. doi: 10.1089/mdr.1996.2.433. [DOI] [PubMed] [Google Scholar]
- 112.Lindquist H D, Dufour A P, Wymer L J, Schaefer F W., III Criteria for evaluation of proposed protozoan detection. J Microbiol Methods. 1998;37:33–43. doi: 10.1016/s0167-7012(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 113.Linstead D J, Bradley S. The purification and properties of two soluble reduced nicotinamide:acceptor oxidoreductases from Trichomonas vaginalis. Mol Biochem Parasitol. 1988;27:125–134. doi: 10.1016/0166-6851(88)90032-1. [DOI] [PubMed] [Google Scholar]
- 114.Linstead D, Cranshaw M A. The pathway of arginine catabolism in the parasitic flagellate Trichomonas vaginalis. Mol Biochem Parasitol. 1983;8:241–252. doi: 10.1016/0166-6851(83)90046-4. [DOI] [PubMed] [Google Scholar]
- 115.Lipsitch M, Levin B R. The population dynamics of antimicrobial chemotherapy. Antimicrob Agents Chemother. 1997;41:363–373. doi: 10.1128/aac.41.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lipsitch M, Levin B R. The within-host population dynamics of antibacterial chemotherapy: conditions for the evolution of resistance. Ciba Found Symp. 1997;207:112–130. doi: 10.1002/9780470515358.ch8. [DOI] [PubMed] [Google Scholar]
- 117.Liu S M, Brown D M, O'Donoghue P, Upcroft P, Upcroft J A. Ferredoxin involvement in metronidazole resistance of Giardia duodenalis. Mol Biochem Parasitol. 2000;108:137–140. doi: 10.1016/s0166-6851(00)00194-8. [DOI] [PubMed] [Google Scholar]
- 118.Lossick J G. Therapy of urogenital trichomoniasis. In: Honigberg B M, editor. Trichomonads parasitic in humans. New York, N.Y: Springer-Verlag; 1990. pp. 324–341. [Google Scholar]
- 119.Lubbe M M, Stanley K, Chalkley L J. Prevalence of nim genes in anaerobic/facultative anaerobic bacteria isolated in South Africa. FEMS Microbiol Lett. 1999;172:79–83. doi: 10.1111/j.1574-6968.1999.tb13453.x. [DOI] [PubMed] [Google Scholar]
- 120.Lujan H D, Mowatt M R, Nash T E. Mechanisms of Giardia lamblia differentiation into cysts. Microbiol Mol Biol Rev. 1997;61:294–304. doi: 10.1128/mmbr.61.3.294-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mai Z, Ghosh S, Frisardi M, Rosenthal B, Rogers R, Samuelson J. Hsp60 is targeted to a cryptic mitochondrion-derived organelle (“crypton”) in the microaerophilic protozoan parasite Entamoeba histolytica. Mol Cell Biol. 1999;19:2198–2205. doi: 10.1128/mcb.19.3.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Malandrin L, Huber H, Bernander R. Nucleoid structure and partition in Methanococcus jannaschii: an archaeon with multiple copies of the chromosome. Genetics. 1999;152:1315–1323. doi: 10.1093/genetics/152.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Marshall M M, Naumovitz D, Ortega Y, Sterling C R. Waterborne protozoan pathogens. Clin Microbiol Rev. 1997;10:67–85. doi: 10.1128/cmr.10.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Martin W, Müller M. The hydrogen hypothesis for the first eukaryote. Nature. 1998;392:37–41. doi: 10.1038/32096. [DOI] [PubMed] [Google Scholar]
- 125.Martínez-Palomo A, Espinosa Cantellano M. Intestinal ameobae. In: Collier L, Balows A, Sussman M, editors. Topley and Wilson's microbiology and microbial infections. 9th ed. 5:parasitology. London, U.K: Arnold; 1998. pp. 157–177. [Google Scholar]
- 126.Meingassner J G, Mieth H. Cross-resistance of trichomonads to 5-nitroimidazole-derivatives. Experientia. 1976;32:183–184. doi: 10.1007/BF01937754. [DOI] [PubMed] [Google Scholar]
- 127.Mertens E. ATP versus pyrophosphate: glycolysis revisited in parasitic protists. Parasitol Today. 1993;9:122–126. doi: 10.1016/0169-4758(93)90169-g. [DOI] [PubMed] [Google Scholar]
- 128.Meysick K, Garber G E. Trichomonas vaginalis. Curr Opin Infect Dis. 1995;8:22–25. [Google Scholar]
- 129.Monis P T, Andrews R H. Molecular epidemiology: assumptions and limitations of commonly applied methods. Int J Parasitol. 1998;28:981–987. doi: 10.1016/s0020-7519(98)00042-3. [DOI] [PubMed] [Google Scholar]
- 130.Moreira D, Lopez-Garcia P. Symbiosis between methanogenic archaea and delta-proteobacteria as the origin of eukaryotes: the syntrophic hypothesis. J Mol Evol. 1998;47:517–530. doi: 10.1007/pl00006408. [DOI] [PubMed] [Google Scholar]
- 131.Müller M. Energy metabolism of protozoa without mitochondria. Annu Rev Microbiol. 1988;42:465–488. doi: 10.1146/annurev.mi.42.100188.002341. [DOI] [PubMed] [Google Scholar]
- 132.Müller M. The hydrogenosome. J Gen Microbiol. 1993;139:2879–2889. doi: 10.1099/00221287-139-12-2879. [DOI] [PubMed] [Google Scholar]
- 133.Müller M. Enzymes and compartmentation of core energy metabolism of anaerobic protists—a special case in eukaryotic evolution? In: Coombs G H, Vickerman K, Sleigh M A, Warren A, editors. Evolutionary relationships among protozoa. London, U.K: Chapman and Hall; 1998. pp. 108–132. [Google Scholar]
- 134.Müller M, Martin W. The genome of Rickettsia prowazekii and some thoughts on the origin of mitochondria and hydrogenosomes. Bioessays. 1999;21:377–381. doi: 10.1002/(SICI)1521-1878(199905)21:5<377::AID-BIES4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 135.Narcisi E M, Secor W E. In vitro effect of tinidazole and furazolidone on metronidazole-resistant Trichomonas vaginalis. Antimicrob Agents Chemother. 1996;40:1121–1125. doi: 10.1128/aac.40.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nash T E, Weller P F. Protozoal intestinal infections and trichomoniasis. In: Fauci A S, Braunwald E, Isselbacher K J, Wilson J D, Martin J B, Kasper D L, Hauser S L, Longo D L, editors. Harrison's principles of internal medicine. 14th ed. New York, N.Y: McGraw-Hill; 1998. pp. 1202–1203. [Google Scholar]
- 137.Neale K A, Alderete J F. Analysis of the proteinases of representative Trichomonas vaginalis isolates. Infect Immun. 1990;58:157–162. doi: 10.1128/iai.58.1.157-162.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Newton P, White N. Malaria: new developments in treatment and prevention. Annu Rev Med. 1999;50:179–192. doi: 10.1146/annurev.med.50.1.179. [DOI] [PubMed] [Google Scholar]
- 139.Newton-Sanchez O A, Sturm-Ramirez K, Romero-Zamora J L, Santos-Preciado J I, Samuelson J. High rate of occult infection with Entamoeba histolytica among non-dysenteric Mexican children. Arch Med Res. 1997;28(Spec. No.):311–313. [PubMed] [Google Scholar]
- 140.Nozaki T, Asai T, Kobayashi S, Ikegami F, Noji M, Sato K, Takeuchi T. Molecular cloning and characterization of the genes encoding two isoforms of cysteine synthase in the enteric protozoan parasite Entamoeba histolytica. Mol Biochem Parasitol. 1998;97:33–44. doi: 10.1016/s0166-6851(98)00129-7. [DOI] [PubMed] [Google Scholar]
- 141.O'Farrell N, Hoosen A A, Kharsany A B, van den Ende J. Sexually transmitted pathogens in pregnant women in a rural South African community. Genitourin Med. 1989;65:276–280. doi: 10.1136/sti.65.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ondarza R N, Iturbe A, Hurtado G, Tamayo E, Ondarza M, Hernandez E. Entamoeba histolytica: a eukaryote with trypanothione metabolism instead of glutathione metabolism. Biotechnol Appl Biochem. 1999;30:47–52. [PubMed] [Google Scholar]
- 143.Ortega Y R, Adam R D. Giardia: overview and update. Clin Infect Dis. 1997;25:545–550. doi: 10.1086/513745. [DOI] [PubMed] [Google Scholar]
- 144.Ortner S, Clark C G, Binder M, Scheiner O, Wiedermann G, Duchêne M. Molecular biology of the hexokinase isoenzyme pattern that distinguishes pathogenic Entamoeba histolytica from nonpathogenic Entamoeba dispar. Mol Biochem Parasitol. 1997;86:85–94. doi: 10.1016/s0166-6851(97)90008-6. [DOI] [PubMed] [Google Scholar]
- 145.Paget T A, Kelly M L, Jarroll E L, Lindmark D G, Lloyd D. The effects of oxygen on fermentation in the intestinal protozoal parasite Giardia lamblia. Mol Biochem Parasitol. 1993;57:65–72. doi: 10.1016/0166-6851(93)90244-r. [DOI] [PubMed] [Google Scholar]
- 146.Paget T A, Lloyd D. Trichomonas vaginalis requires traces of oxygen and high concentrations of carbon dioxide for optimal growth. Mol Biochem Parasitol. 1990;41:65–72. doi: 10.1016/0166-6851(90)90097-6. [DOI] [PubMed] [Google Scholar]
- 147.Petrin D, Delgaty K, Bhatt R, Garber G. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300–317. doi: 10.1128/cmr.11.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Petter R, Rozenblatt S, Schechtman D, Wellems T E, Mirelman D. Electrophoretic karyotype and chromosome assignments for a pathogenic and nonpathogenic strain of Entamoeba histolytica. Infect Immun. 1993;61:3574–3577. doi: 10.1128/iai.61.8.3574-3577.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Piper J M, Mitchell E F, Ray W A. Prenatal use of metronidazole and birth defects: no association. Obstet Gynecol. 1993;82:348–352. [PubMed] [Google Scholar]
- 150.Platel D F, Mangou F, Tribouley-Duret J. Role of glutathione in the detoxification of ferriprotoporphyrin IX in chloroquine resistant Plasmodium berghei. Mol Biochem Parasitol. 1999;98:215–223. doi: 10.1016/s0166-6851(98)00170-4. [DOI] [PubMed] [Google Scholar]
- 151.Quon D V, d'Oliveira C E, Johnson P J. Reduced transcription of the ferredoxin gene in metronidazole-resistant Trichomonas vaginalis. Proc Natl Acad Sci USA. 1992;89:4402–4406. doi: 10.1073/pnas.89.10.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rappelli P, Addis M F, Carta F, Fiori P L. Mycoplasma hominis parasitism of Trichomonas vaginalis. Lancet. 1998;352:1286. doi: 10.1016/S0140-6736(98)00041-5. [DOI] [PubMed] [Google Scholar]
- 153.Rappelli P, Addis M F, Carta F, Fiori P L. Mycoplasma hominis parasitism of Trichomonas vaginalis. Lancet. 1998;352:2023. doi: 10.1016/S0140-6736(98)00041-5. [DOI] [PubMed] [Google Scholar]
- 154.Reference deleted.
- 155.Rasmussen B A, Bush K, Tally F P. Antimicrobial resistance in anaerobes. Clin Infect Dis. 1997;24(Suppl. 1):S110–S120. doi: 10.1093/clinids/24.supplement_1.s110. [DOI] [PubMed] [Google Scholar]
- 156.Rathod P K, McErlean T, Lee P-C. Variations in frequencies of drug resistance in Plasmodium falciparum. Proc Natl Acad Sci USA. 1997;94:9389–9393. doi: 10.1073/pnas.94.17.9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ravdin J I, Petri W A., Jr . Entamoeba histolytica (amebiasis) In: Mandell G L, Bennett J E, Dolin R, editors. Mandell, Douglas and Bennett's principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone; 1995. pp. 2395–2408. [Google Scholar]
- 158.Reeves R E. Metabolism of Entamoeba histolytica Schaudinn, 1903. Adv Parasitol. 1984;23:105–142. doi: 10.1016/s0065-308x(08)60286-9. [DOI] [PubMed] [Google Scholar]
- 159.Rein M F. Trichomonas vaginalis. In: Mandell G L, Bennett J E, Dolin R, editors. Mandell, Douglas and Bennett's principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone; 1995. pp. 2493–2497. [Google Scholar]
- 160.Rein M F, Müller M. Trichomonas vaginalis and trichomoniasis. In: Holmes K K, Mårdh P-A, Sparling P F, Wiesner P J, editors. Sexually transmitted diseases. New York, N.Y: McGraw-Hill; 1990. pp. 481–492. [Google Scholar]
- 161.Reysset G. Genetics of 5-nitroimidazole resistance in Bacteroides species. Anaerobe. 1996;2:59–69. doi: 10.1006/anae.1996.0008. [DOI] [PubMed] [Google Scholar]
- 162.Rich S M, Hudson R R, Ayala F J. Plasmodium falciparum antigenic diversity: evidence of clonal population structure. Proc Natl Acad Sci USA. 1997;94:13040–13045. doi: 10.1073/pnas.94.24.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Roberts-Thomson I C, Anders R F, Bhathal P S. Granulomatous hepatitis and cholangitis associated with giardiasis. Gastroenterology. 1982;83:480–483. [PubMed] [Google Scholar]
- 164.Rodríguez M A, Hidalgo M E, Sánchez T, Orozco E. Cloning and characterization of the Entamoeba histolytica pyruvate:ferredoxin oxidoreductase gene. Mol Biochem Parasitol. 1996;78:273–277. doi: 10.1016/s0166-6851(96)02613-8. [DOI] [PubMed] [Google Scholar]
- 165.Roger A J, Morrison H G, Sogin M L. Primary structure and phylogenetic relationships of a malate dehydrogenase gene from Giardia lamblia. J Mol Evol. 1999;48:750–755. doi: 10.1007/pl00006519. [DOI] [PubMed] [Google Scholar]
- 166.Romero Cabello R, Guerrero L R, Muñoz Garcia M R, Geyne Cruz A. Nitazoxanide for the treatment of intestinal protozoan and helminthic infections in Mexico. Trans R Soc Trop Med Hyg. 1997;91:701–703. doi: 10.1016/s0035-9203(97)90531-9. [DOI] [PubMed] [Google Scholar]
- 167.Samarawickrema N, Upcroft J A, Brown D M, Thammapalerd N, Upcroft P. Superoxide dismutase and pyruvate:ferredoxin oxidoreductase involvement in mechanisms of metronidazole resistance in Entamoeba histolytica. J Antimicrob Chemother. 1997;40:833–840. doi: 10.1093/jac/40.6.833. [DOI] [PubMed] [Google Scholar]
- 168.Sanchez L B, Hashimoto T, Müller M. Sequence of a malic enzyme gene of Giardia lamblia. Mol Biochem Parasitol. 1996;82:145–151. doi: 10.1016/0166-6851(96)02728-4. [DOI] [PubMed] [Google Scholar]
- 169.Sanchez L B, Morrison H G, Sogin M L, Müller M. Cloning and sequencing of an acetyl-CoA synthetase (ADP-forming) gene from the amitochondriate protist, Giardia lamblia. Gene. 1999;233:225–231. doi: 10.1016/s0378-1119(99)00134-1. [DOI] [PubMed] [Google Scholar]
- 170.Sargeaunt P G. Entamoeba histolytica and E. dispar. Trans R Soc Trop Med Hyg. 1994;88:254–255. doi: 10.1016/0035-9203(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 171.Saurina G R, McCormack W M. Trichomoniasis in pregnancy. Sex Transm Dis. 1997;24:361–362. doi: 10.1097/00007435-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 172.Saxena, S. B., and R. R. Jenkins. Prevalence of Trichomonas vaginalis in men at high risk for sexually transmitted diseases. Sex. Transm. Dis. 18:138–142. [DOI] [PubMed]
- 173.Schmunis G A, López Antuñano F J. World-wide importance of parasites. In: Collier L, Balows A, Sussman M, editors. Topley and Wilson's microbiology and microbial infections. 9th ed. 5:parasitology. London, U.K: Arnold; 1998. pp. 19–38. [Google Scholar]
- 174.Schwebke J R. Metronidazole: utilization in the obstetric and gynecologic patient. Sex Transm Dis. 1995;22:370–376. [PubMed] [Google Scholar]
- 175.Schwebke J R, Aira T, Jordan N, Jolly P E, Vermund S H. Sexually transmitted diseases in Ulaanbaatar, Mongolia. Int J STD AIDS. 1998;9:354–358. doi: 10.1258/0956462981922269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Reference deleted.
- 177.Sherrard J. National guideline for the management of Trichomonas vaginalis. Sex Transm Infect. 1999;75(Suppl. 1):S21–S23. [PubMed] [Google Scholar]
- 178.Silva G, Oliveira S, Gomes C M, Pacheco I, Liu M Y, Xavier A V, Teixeira M, LeGall J, Rodrigues-Pousada C. Desulfovibrio gigas neelaredoxin—a novel superoxide dismutase integrated in a putative oxygen sensory operon of an anaerobe. Eur J Biochem. 1999;259:235–243. doi: 10.1046/j.1432-1327.1999.00025.x. [DOI] [PubMed] [Google Scholar]
- 179.Soave R. Cyclospora: an overview. Clin Infect Dis. 1996;23:429–437. doi: 10.1093/clinids/23.3.429. [DOI] [PubMed] [Google Scholar]
- 180.Sobel J D, Nagappan V, Nyirjesy P. Metronidazole-resistant vaginal trichomoniasis—an emerging problem. N Engl J Med. 1999;341:292–293. doi: 10.1056/NEJM199907223410417. [DOI] [PubMed] [Google Scholar]
- 181.Sorvillo F, Kerndt P. Trichomonas vaginalis and amplification of HIV-1 transmission. Lancet. 1998;351:213–214. doi: 10.1016/S0140-6736(05)78181-2. [DOI] [PubMed] [Google Scholar]
- 182.Sotto A, Alvarez J L, Garcia B, Pomar F, Cendan A. Acute hepatic lesion caused by Giardia lamblia. Rev Esp Enferm Dig. 1990;77:24–28. [PubMed] [Google Scholar]
- 183.Stanley S L., Jr Progress towards development of a vaccine for amebiasis. Clin Microbiol Rev. 1997;10:637–649. doi: 10.1128/cmr.10.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Su X-Z, Kirkman L A, Fujioka H, Wellems T E. Complex polymorphisms in an ∼330 kDa protein are linked to chloroquine-resistant P. falciparum in Southeast Asia and Africa. Cell. 1997;91:593–603. doi: 10.1016/s0092-8674(00)80447-x. [DOI] [PubMed] [Google Scholar]
- 185.Swarbrick A, Lim R L H, Upcroft J A, Stewart T S. Nucleotide variation in the cytidine triphospate synthetase gene of Giardia duodenalis. J Eukaryot Microbiol. 1998;44:531–534. doi: 10.1111/j.1550-7408.1997.tb05957.x. [DOI] [PubMed] [Google Scholar]
- 186.Tai J H, Su H M, Shaio M F, Wang C C. The divergence of Trichomonas vaginalis among isolates of Trichomonas vaginalis. Exp Parasitol. 1993;76:278–286. doi: 10.1006/expr.1993.1033. [DOI] [PubMed] [Google Scholar]
- 187.Tannich E, Bruchhaus I, Walter R D, Horstmann R D. Pathogenic and nonpathogenic Entamoeba histolytica: identification and molecular cloning of an iron-containing superoxide dismutase. Mol Biochem Parasitol. 1991;49:61–71. doi: 10.1016/0166-6851(91)90130-x. [DOI] [PubMed] [Google Scholar]
- 188.Taylor-Robinson D. Mycoplasma hominis parasitism of Trichomonas vaginalis. Lancet. 1998;352:2022–2023. doi: 10.1016/S0140-6736(05)61371-2. [DOI] [PubMed] [Google Scholar]
- 189.Thammapalerd N, Kotimanusvanij D, Duchêne M, Upcroft J A, Mitchell R, Healey A, Samarawickrema N, Tharavanij S, Wiedermann G, Upcroft P. Pyruvate:ferredoxin oxidoreductase from Entamoeba histolytica recognised by a monoclonal antibody. Southeast Asian J Trop Med Public Health. 1996;27:63–70. [PubMed] [Google Scholar]
- 190.Thompson R C A, Reynoldson J A, Mendis A H W. Giardia and giardiasis. Adv Parasitol. 1993;32:71–160. doi: 10.1016/s0065-308x(08)60207-9. [DOI] [PubMed] [Google Scholar]
- 191.Tierney L M Jr, McPhee S J, Papadakis M A, editors. Current medical diagnosis and treatment 1999. Stamford, Conn: Appleton & Lange; 1999. [Google Scholar]
- 192.Tovar J, Fischer A, Clark C G. The mitosome, a novel organelle related to mitochondria in the amitochondrial parasite Entamoeba histolytica. Mol Microbiol. 1999;32:1013–1021. doi: 10.1046/j.1365-2958.1999.01414.x. [DOI] [PubMed] [Google Scholar]
- 193.Townson S M, Boreham P F L, Upcroft P, Upcroft J A. Resistance to the nitroheterocyclic drugs. Acta Trop. 1994;56:173–194. doi: 10.1016/0001-706x(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 194.Townson S M, Hanson G R, Upcroft J A, Upcroft P. A purified ferredoxin from Giardia duodenalis. Eur J Biochem. 1994;220:439–446. doi: 10.1111/j.1432-1033.1994.tb18641.x. [DOI] [PubMed] [Google Scholar]
- 195.Townson S M, Laqua H, Upcroft P, Boreham P F L, Upcroft J A. Induction of metronidazole and furazolidone resistance in Giardia. Trans R Soc Trop Med Hyg. 1992;86:521–522. doi: 10.1016/0035-9203(92)90095-t. [DOI] [PubMed] [Google Scholar]
- 196.Townson S M, Upcroft J A, Upcroft P. Characterisation and purification of pyruvate:ferredoxin oxidoreductase from Giardia duodenalis. Mol Biochem Parasitol. 1996;79:183–193. doi: 10.1016/0166-6851(96)02661-8. [DOI] [PubMed] [Google Scholar]
- 197.Tseng C-P, Albrecht J, Gunsalus R P. Effect of microaerophilic cell growth conditions on expression of the aerobic (cyoABCDE and cydAB) and anaerobic (narGHJI, frdABCD, and dmsABC) respiratory pathway genes in Escherichia coli. J Bacteriol. 1996;178:1094–1098. doi: 10.1128/jb.178.4.1094-1098.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Turner P, Edwards R, Weston V, Gazis A, Ispahani P, Greenwood D. Simultaneous resistance to metronidazole, co-amoxiclav, and imipinem in clinical isolate of Bacteroides fragilis. Lancet. 1995;345:1275–1277. doi: 10.1016/s0140-6736(95)90927-3. [DOI] [PubMed] [Google Scholar]
- 199.Upcroft J A, Boreham P F L, Campbell R W, Shepherd R W, Upcroft P. Biological and genetic analysis of a longitudinal collection of Giardia samples derived from humans. Acta Trop. 1995;60:35–46. doi: 10.1016/0001-706x(95)00100-s. [DOI] [PubMed] [Google Scholar]
- 200.Upcroft J A, Campbell R W, Upcroft P. Quinacrine-resistant Giardia duodenalis. Parasitology. 1996;112:309–313. doi: 10.1017/s0031182000065823. [DOI] [PubMed] [Google Scholar]
- 201.Upcroft J A, Campbell R W, Benakli K, Upcroft P, Vanelle P. The efficacy of new 5-nitroimidazoles against metronidazole-susceptible and -resistant anaerobic protozoa. Antimicrob Agents Chemother. 1999;43:73–76. doi: 10.1128/aac.43.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Upcroft J A, Capon A J, Dharmkrong-At A, Healey A, Boreham P F L, Upcroft P. Giardia intestinalis antigens expressed in Escherichia coli. Mol Biochem Parasitol. 1987;26:267–276. doi: 10.1016/0166-6851(87)90079-x. [DOI] [PubMed] [Google Scholar]
- 203.Upcroft J A, Healey A, Mitchell R W, Upcroft P. A new rDNA repeat unit in human Giardia. J Eukaryot Microbiol. 1994;41:639–642. doi: 10.1111/j.1550-7408.1994.tb01526.x. [DOI] [PubMed] [Google Scholar]
- 204.Upcroft J A, McDonnell P A, Gallagher A N, Chen N, Upcroft P. Lethal Giardia from a wild-caught sulphur-crested cockatoo (Cacatua galerita) established in vitro chronically infects mice. Parasitology. 1997;114:407–412. doi: 10.1017/s0031182096008724. [DOI] [PubMed] [Google Scholar]
- 205.Upcroft J A, McDonnell P A, Upcroft P. Virulent avian Giardia duodenalis pathogenic for mice. Parasitol Today. 1998;14:281–284. doi: 10.1016/s0169-4758(98)01262-9. [DOI] [PubMed] [Google Scholar]
- 206.Upcroft J A, Mitchell R, Chen N, Upcroft P. Albendazole resistance in Giardia is correlated with cytoskeletal changes but not with a mutation at amino acid 200 in β-tubulin. Microb Drug Resist. 1996;2:303–308. doi: 10.1089/mdr.1996.2.303. [DOI] [PubMed] [Google Scholar]
- 207.Upcroft J A, Upcroft P. Two distinct varieties of Giardia in a mixed infection from a single human patient. J Eukaryot Microbiol. 1994;41:189–194. doi: 10.1111/j.1550-7408.1994.tb01495.x. [DOI] [PubMed] [Google Scholar]
- 208.Upcroft J A, Upcroft P. Drug resistance and Giardia. Parasitol Today. 1993;9:187–190. doi: 10.1016/0169-4758(93)90144-5. [DOI] [PubMed] [Google Scholar]
- 209.Upcroft J A, Upcroft P. Metronidazole and drug resistance. Parasitol Today. 1993;9:417–418. doi: 10.1016/0169-4758(93)90049-l. [DOI] [PubMed] [Google Scholar]
- 210.Reference deleted.
- 211.Reference deleted.
- 212.Reference deleted.
- 213.Reference deleted.
- 214.Reference deleted.
- 215.Upcroft J A, Upcroft P. My favourite cell: Giardia. BioEssays. 1998;20:256–263. doi: 10.1002/(SICI)1521-1878(199803)20:3<256::AID-BIES9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 216.Upcroft J A, Upcroft P. Keto-acid oxidoreductases in the anaerobic protozoa. J Eukaryot Microbiol. 1999;46:447–449. doi: 10.1111/j.1550-7408.1999.tb04628.x. [DOI] [PubMed] [Google Scholar]
- 217.Upcroft P. Multiple drug resistance in the pathogenic protozoa. Acta Trop. 1994;56:195–212. doi: 10.1016/0001-706x(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 218.Upcroft P. Drug resistance in Giardia: clinical versus laboratory isolates. Drug Resist Updates. 1998;1:166–168. doi: 10.1016/s1368-7646(98)80035-6. [DOI] [PubMed] [Google Scholar]
- 219.Upcroft P, Chen N, Upcroft J A. Telomeric organization of a variable and inducible toxin gene family in the ancient eukaryote Giardia duodenalis. Genome Res. 1997;7:37–47. doi: 10.1101/gr.7.1.37. [DOI] [PubMed] [Google Scholar]
- 220.Upcroft P, Upcroft J A. Organization and structure of the Giardia genome. Protist. 1999;150:17–23. doi: 10.1016/S1434-4610(99)70005-2. [DOI] [PubMed] [Google Scholar]
- 221.Upcroft P, Upcroft J A. Lessons from Giardia on genome analysis of protozoan parasites. Int J Parasitol. 1999;29:1389–1391. [Google Scholar]
- 222.Reference deleted.
- 223.Viikki M, Pukkala E, Nieminen P, Hakama M. Gynaecological infections as risk determinants of subsequent cervical neoplasia. Acta Oncol. 2000;39:71–75. doi: 10.1080/028418600431003. [DOI] [PubMed] [Google Scholar]
- 224.Voolmann T, Boreham P. Metronidazole resistant Trichomonas vaginalis in Brisbane. Med J Aust. 1993;159:490. doi: 10.5694/j.1326-5377.1993.tb137978.x. [DOI] [PubMed] [Google Scholar]
- 225.Voolman T, Morey F, Rich G. Trichomoniasis is a problem in aboriginal women—fact or fiction? Venereology. 1995;8:34–36. [Google Scholar]
- 226.Wallis P M. Abiotic transmission—is water really significant? In: Thompson R C A, Reynoldson J A, Lymbery A J, editors. Giardia: from molecules to disease. Wallingford, U.K: CAB International; 1994. pp. 99–122. [Google Scholar]
- 227.Wassmann C, Hellberg A, Tannich E, Bruchhaus I. Metronidazole resistance in the protozoan parasite Entamoeba histolytica is associated with increased expression of iron-containing superoxide dismutase and peroxiredoxin and decreased expression of ferredoxin 1 and flavin reductase. J Biol Chem. 1999;274:26051–26056. doi: 10.1074/jbc.274.37.26051. [DOI] [PubMed] [Google Scholar]
- 228.Reference deleted.
- 229.Weinbach E C, Elwood-Claggett C, Keister D B, Diamond L S, Kon H. Respiratory metabolism of Giardia lamblia. J Parasitol. 1980;66:347–350. [PubMed] [Google Scholar]
- 230.Wilkinson D, Abdool Karim S S, Harrison A, Lurie M, Colvin M, Connolly C, Sturm A W. Unrecognized sexually transmitted infections in rural South African women: a hidden epidemic. Bull World Health Org. 1999;77:22–28. [PMC free article] [PubMed] [Google Scholar]
- 231.Willhoeft U, Tannich E. The electrophoretic karyotype of Entamoeba histolytica. Mol Biochem Parasitol. 1999;99:41–53. doi: 10.1016/s0166-6851(98)00178-9. [DOI] [PubMed] [Google Scholar]
- 232.Wittner M, Tanowitz H B. Intestinal parasites in returned travellers. Med Clin N Am. 1992;76:1433–1448. doi: 10.1016/s0025-7125(16)30295-4. [DOI] [PubMed] [Google Scholar]
- 233.World Health Organization. Intestinal parasites control: Burden and trends. Geneva, Switzerland: WHO Division of Control of Tropical Diseases, World Health Organization; 1998. [Google Scholar]
- 234.Wu G, Fiser A, ter Kuile B, Sali A, Müller M. Convergent evolution of Trichomonas vaginalis lactate dehydrogenase from malate dehydrogenase. Proc Natl Acad Sci USA. 1999;96:6285–6290. doi: 10.1073/pnas.96.11.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yarlett N, Yarlett N C, Lloyd D. Metronidazole-resistant clinical isolates of Trichomonas vaginalis have lowered oxygen affinities. Mol Biochem Parasitol. 1986;19:111–116. doi: 10.1016/0166-6851(86)90115-5. [DOI] [PubMed] [Google Scholar]
- 236.Yarlett N, Martinez M P, Moharrami M A, Tachezy J. The contribution of the arginine dihydrolase pathway to energy metabolism by Trichomonas vaginalis. Mol Biochem Parasitol. 1996;78:117–125. doi: 10.1016/s0166-6851(96)02616-3. [DOI] [PubMed] [Google Scholar]
- 237.Yu D C, Wang A L, Wang C C. Amplification, expression, and packaging of a foreign gene by giardiavirus in Giardia lamblia. J Virol. 1996;70:8752–8757. doi: 10.1128/jvi.70.12.8752-8757.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yuh Y-S, Liu J-Y, Shaio M-F. Chromosome number of Trichomonas vaginalis. J Parasitol. 1997;83:551–553. [PubMed] [Google Scholar]
- 239.Yung A P, Ruff T A. Travel medicine 2: upon return. Med J Aust. 1994;160:206–212. [PubMed] [Google Scholar]
- 240.Zaat J O, Mank T G, Assendelft W J. A systematic review on the treatment of giardiasis. Trop Med Int Health. 1997;2:63–82. doi: 10.1046/j.1365-3156.1997.d01-132.x. [DOI] [PubMed] [Google Scholar]
- 241.Zhang Y. Life without KatG. Trends Microbiol. 1996;4:415–416. doi: 10.1016/0966-842x(96)30030-9. [DOI] [PubMed] [Google Scholar]
- 242.Zhang Y, Dhandayuthapani S, Deretic V. Molecular basis for the exquisite sensitivity of Mycobacterium tuberculosis to isoniazid. Proc Natl Acad Sci USA. 1996;93:13212–13216. doi: 10.1073/pnas.93.23.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhang Y-Q, Wang Z-Y, Lu S-Q, Feng M-L. A familial infection of giardiasis. Chin Med J (Engl Ed) 1986;99:417–419. [PubMed] [Google Scholar]
- 244.Zhong H-L, Cao W-J, Rossignol J F, Feng M-L, Hu R-Y, Gan S-B, Tan W. Albendazole in nematode, cestode, trematode and protozoan (Giardia) infections. Chin Med J (Engl Ed) 1986;99:912–915. [PubMed] [Google Scholar]