Abstract
Purpose
To determine if enhanced flow cytometry (CellPrint™) can identify intracellular proteins of lithium responsiveness in monocytes and CD4+ lymphocytes from patients with bipolar disorder.
Methods
Eligible bipolar I or II patients were openly treated with lithium for 16-weeks. Baseline levels of Bcl2, BDNF, calmodulin, Fyn, phospho-Fyn/phospho-Yes, GSK3β, phospho-GSK3αβ, HMGB1, iNOS, IRS2, mTor, NLPR3, PGM1, PKA C-α, PPAR-γ, phospho-RelA, and TPH1 in monocytes and CD4+ lymphocytes of lithium responders and non-responders were measured with CellPrint™. Their utility of discriminating responders from non-responders was explored. Protein-protein network and pathway enrichment analyses were conducted.
Results
Of the 24 intent-to-treat patients, 12 patients completed the 16-week study. Eleven of 13 responders and 8 of 11 non-responders were available for this analysis. The levels of the majority of analytes in lithium responders were lower than non-responders in both cell types, but only the level of GSK3β in monocytes was significantly different (p = 0.034). The combination of GSK3β and phospho-GSK3αβ levels in monocytes correctly classified 11/11 responders and 5/8 non-responders. Combination of GSK3β, phospho-RelA, TPH1 and PGM1 correctly classified 10/11 responders and 6/7 non-responders, both with a likelihood of ≥ 85%. Prolactin, leptin, BDNF, neurotrophin, and epidermal growth factor/epidermal growth factor receptor signaling pathways are involved in the lithium treatment response. GSK3β and RelA genes are involved in 4 of 5 these pathways.
Conclusion
CellPrint™ flow cytometry was able to detect differences in multiple proteins in monocytes and CD4+ lymphocytes between lithium responders and non-responders. A large study is warranted to confirm or refute these findings.
Keywords: lithium, bipolar disorder, blood mononuclear cell, biomarkers
Introduction
Lithium is still a gold standard, first-line medication for bipolar disorder (BD).1 In the acute treatment of BD, about 1/3 to 2/3 of patients may respond (≥50% improvement from baseline) to lithium.2–4 For long-term treatment, about 1/3 of patients were reported as “excellent lithium responders”.5–9 Compared to other mood stabilizers, lithium is very inexpensive. Compared to most antipsychotic mood stabilizer, lithium has lower risk for somnolence/sedation and metabolic burden.10,11,12 Lithium also has neuroprotective effects and anti-suicidal properties which other mood stabilizers do not have.13,14 However, the unique effects and low cost of lithium have not prevented its use from declining.15 The declined use of lithium is multifactorial. Lithium has very low market value compared to highly marketed antipsychotics and anticonvulsants. Requirement of frequent monitoring of its potential side effects on thyroid and renal functions also deters its use. There is also no reliable predictor of lithium treatment response although some demographics and clinical correlates were associated with lithium treatment response.2,16,17 The current “trial and error” approach for prescribing psychotropics further limits its use. Therefore, finding an objective biomarker(s) for lithium response will not only reduce unnecessary lithium exposure to and inconvenient monitoring for non-responders, but also potentially increase its use in responders.
The results of studies with human induced pluripotent stem cells (iPSC) suggested that lithium responders and non-responders have different identifiable molecular and electrophysiological biomarkers.18–19 However, previous efforts to discover reliable biomarker(s) at genomic, transcriptomic, and proteomic levels for predicting lithium response have not yet yielded a robust test for clinical use.16,20,21 The unsuccessful attempts are likely to be multifactorial: 1) A very small risk of each gene SNP (single nucleotide polymorphism) attributes to a complex disease like BD;21 2) Nucleic acid level is a poor predictor of protein level;22–25 3) RNA analysis and even certain protein analyses cannot assess the post-translational modifications key to protein activity, such as phosphorylation or methylation;26–28 and 4) Many techniques, such as plasma studies, Western blot, microarray, and quantitative PCR, typically evaluate the averaged productive capabilities of heterogeneous cells with diverse expression profiles, which undermines the precision and predictive capability of the measurements.29,30 Together, these deficits suggest that there may be limitations to commonly used methodologies in searching for biomarkers of lithium response.
Flow cytometry circumvents many of the issues described above and is a well-established and a user-friendly method for single cell protein analysis. It is used to simultaneously measure multiple analyte expression patterns, including protein expression levels and protein post-translational modifications, in individual cells.31 Flow cytometry is a powerful tool for clinical and research and is used in hematology, immunology, virology, bacteriology, molecular biology, oncology, and infectious disease32–35 as well as other complex diseases.36,37 In psychiatry, BD is a complex disease and molecular studies assessing intracellular protein expression levels in individual blood cells may be successful in finding lithium response biomarkers.38–40
However, traditional flow cytometry can suffer from poor signal to noise when analyzing intracellular or rare events.41 Tyramine-based signal-amplified flow cytometry increases intracellular detection sensitivity for 10 fold.42,43 CellPrint Biotechnology, LLC (the CellPrint) has developed a tyramide-based catalytic deposition labeling procedure for flow cytometry called CellPrint™. CellPrint flow cytometry improves signal to noise ratios by 10-100-fold and dynamic range by 20-fold for intracellular and surface protein detection compared to standard flow cytometric staining methods while retaining the ability to assess cellular subtypes.42–47 CellPrint flow cytometry is able to detect expression levels of low abundance molecules as well as phosphorylated proteins and has enabled quantification of a wide variety of analytes from numerous cell types (see Supplemental Materials). The platform has been used to evaluate numerous diseases for drug and biomarker development, clinical diagnostics, and research purposes. Importantly, CellPrint flow cytometry is capable of measuring subtly different expression patterns of intracellular analytes between individuals. The aim of this feasibility study was to use the CellPrint flow cytometry to measure the levels of multiple intracellular proteins in CD4+ lymphocytes and monocytes between lithium responders and lithium non-responders and to explore potential biomarker(s) for predicting lithium treatment response.
Methods
Study Design
This study was an open-label, 16-week study of lithium monotherapy in the treatment of bipolar I or II disorder with a screening period for up to 4-weeks (NCT02909504). The protocol was approved by the Institutional Review Board for Human Investigation of the University Hospitals Cleveland Medical Center. Eligible patients received lithium treatment for up to 16 weeks and were seen at weeks 0 (baseline), 1, 2, 4, 6, 8, 12, and 16 to complete assessments including efficacy and safety measures. Any psychotropic medications (with the exception of allowed rescue medications) were tapered off by week 4. The participants were discontinued from the trial if their mood worsened to a significant degree as judged by a research psychiatrist and/or could not discontinue the unpermitted concomitant medication(s). All patients who discontinued the study due to any reason received 3 monthly routine clinical care gratis visits over a 3-month period at no cost. Blood samples were collected from all participants for routine laboratory and flow cytometric analyses. The inclusion and exclusion criteria of participants, study procedures including diagnosis, and efficacy and safety assessments are included in Supplemental Materials.
Blood Sample
De-identified blood samples were collected at screening/baseline and the end of study and transferred to the CellPrint within 3 hours of the blood draw for flow cytometry and to the hospital laboratory for electrolytes, hepatic function, kidney function, thyroid stimulating hormone, and complete blood count. Peripheral monocytes and CD4+ T lymphocytes were isolated by ficoll/hypaque discontinuous gradient centrifugation and cryopreserved for subsequent batch analysis.
Antibodies and Cytometric Analyses
After sample accrual was completed for all participants, the frozen samples were thawed for cell-specific molecular expression analysis with the CellPrint flow cytometry, but the key to each sample was remained in the clinical site before the completion of cytometric analysis. CD4+ T cells and monocytes were labeled using standard staining procedures recommended by the antibody manufacturer (www.biolegend.com) and were not amplified by the CellPrint. CellPrint staining was only applied to the intracellular analytes. The fundamental procedures of CellPrint staining have been outlined previously.42–47After CellPrint staining, the median fluorescence intensities (MFI) were recorded for each of the analytes assayed on a BD Accuri C6 flow cytometer. A fluorescence minus one (FMO) control was included to account for background noise levels and to identify positive signal. Cytometric analyses were accomplished by the technologists at the CellPrint who were blind to the clinical status of the patients.
Antibodies to 17 analytes were obtained from commercial sources. The purchased antibodies were evaluated by the CellPrint team with its proprietary quality control methods. Only antibodies that passed the criteria of the CellPrint were included in the study. The analysis interrogated a spectrum of pathways/functions (Supplemental Table 1). The analytes are involved in apoptosis (Bcl2), calcium transport (Calmodulin), cell signaling [(GSK3β, phospho-GSK3α(Tyr279)β(Tyr216), iNOS, mTor)], metabolic enzymes (PGM1, TPH1), inflammation (HMGB1, NLPR3), kinase (phospho-Fyn (Y530)/Yes(Y537), PKA C-α), neurotrophic factors (BDNF), receptors (IRS2, PPAR-γ), transcriptional factors [(NFkB phospho-p65(Ser536), phospho-RelA].
Supplemental Table 1. Functions of 17 Analytes Included in the Study.
| Analytes | Full name | Function(s) |
| Bcl-2 | B-cell lymphoma 2 | B-cell lymphoma 2 type proteins are key regulators of the intrinsic or mitochondrial pathway for cell apoptosis and proliferation. |
| BDNF | brain-derived neurotrophic factor | Brain-derived neurotrophic factor plays an important role in neuronal survival and growth and neuronal plasticity, which are essential for learning and memory. Decreased levels of BDNF are associated with neurodegenerative diseases with neuronal loss. |
| Calmodulin | calcium-modulated protein | Cellular signaling; Calcium transport |
| Fyn | Kinase; Associated with T-cell and neuronal signaling in development and normal cell physiology | |
| GSK3β | Glycogen synthase kinase 3 beta (Ser9) | GSK3 is expressed serine/threonine kinase as GSK-3α and GSK-3β isoforms, both active under basal conditions and inactivated upon phosphorylation by different upstream kinases. GSK-3 is involved in several signaling pathways controlling many different key functions, involved in several cancers, apoptosis neurodegenerative and liver diseases, oxidative stress and autophagic cell death. |
| HMGB1 | High mobility group box 1 protein | High mobility group box 1 protein is a DNA binding protein involved in maintenance of nucleosome structure and regulation of gene transcription. Extracellular HMGB1 binds with several different receptors and interactors to mediate the proliferation, differentiation, mobilization, and senescence of hematopoietic stem cells (HSCs). HMGB1 is also involved in the formation of the inflammatory bone marrow (BM) microenvironment by activating proinflammatory signaling pathways. |
| iNOS | inducible isoform nitric oxide synthase | Cellular signaling; Modulating vascular tone, insulin secretion, airway tone, and peristalsis; involved in angiogenesis and neural development. |
| IRS2 | Insulin receptor substrate 2 | A cytoplasmic signaling molecule that mediates effects of insulin, insulin-like growth factor 1, and other cytokines by acting as a molecular adaptor between diverse receptor tyrosine kinases and downstream effectors. |
| mTor | The mammalian target of rapamycin | Kinase; Regulate cell growth, cell proliferation, cell motility, cell survival, protein synthesis, autophagy, and transcription. |
| NFKB phospho-P65 (phospho-RelA) | phosphorylated nuclear factor NF-kappa-B p65(Ser536) subunit | An essential transcription factor complex involved in all types of cellular processes including cellular metabolism and chemotaxis; Modulating immune responses; Activation of RelA associated with multiple types of cancer; Phosphorylation and acetylation of RelA are crucial post-translational modifications required for its activation. |
| NLRP3 | NACHT, LRR and PYD domains-containing protein 3 | Expressed predominantly in macrophages and as a component of the inflammasome, a component of innate immune system. |
| phospho-Fyn/Yes | phosphorylated-Fyn (Y530)/phosphorylated Yes (Y537) | The Src family of tyrosine kinases includes src, fyn, and yes and is involved in many signaling pathways such as T and B cell receptor signaling, integrin-mediated signaling, growth factor and cytokine receptor signaling. |
| PGM1 | Phosphoglucomutase 1 | PGM1 is an evolutionarily conserved enzyme that regulates the bi-directional interconversion of glucose 1-phosphate (G-1-P) and glucose 6-phosphate (G-6-P): Involved in glycolysis in one direction and synthesis of glycogen. |
| phospho-GSK3αβ | phosphorylated glycogen synthase kinase 3 alpha(Tyr279)beta(Tyr216) | Cellular signaling (see GSK3β) |
| PKA C-α | Protein kinase A catalytic subunit alpha | PKA is also known as cAMP-dependent protein kinase. Its functions include regulation of glycogen, glucose, and lipid metabolism. |
| PPAR-γ | peroxisome proliferator-activated receptor gamma | Also knowns a glitazone receptor; Regulating fatty acid storage and glucose metabolism |
| TPH1 | Tryptophan hydroxylase 1 | The rate-limiting enzyme in the biosynthesis of serotonin. |
Upon completing the flow cytometry analyses by the CellPrint, the MFI data were sent to the data management statistical analysis unit of the Mood Disorders Program. The key to each subject (responders or non-responder) was provided to the statistic team and used to assess any differences in responders and non-responders.
Raw Data Normalization
The raw data generated by the flow cytometer are the MFI for each analyte stained with the CellPrint. In the present study, the MFI of each analyte of responders and non-responders was “normalized” with fold change/difference (FC). The FC of each analyte was calculated with a formula of 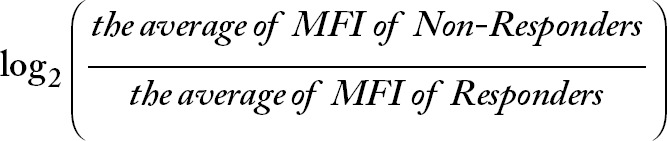 . Therefore, a positive value of the log2(FC) is indicative of a higher expression level in non-responders than in responders, and a negative value of log2(FC) is indicative of a lower expression level in non-responders than in responders.
. Therefore, a positive value of the log2(FC) is indicative of a higher expression level in non-responders than in responders, and a negative value of log2(FC) is indicative of a lower expression level in non-responders than in responders.
Statistical Analysis
The categorical data were analyzed with Chi-square or Fisher Exact tests, and continuous variables were analyzed with T-test. Demographics and historical correlates of lithium responders and non-responders were analyzed according to the nature of a variable. A ≥ 50% reduction in Montgomery Asberg Rating Scale (MADRS and/or Young Mania Rating Scale (YMRS) from baseline to the end of week 16 was used to define a responder. The last observation carried forward strategy was used for patients who did not complete the study. The cytometric MFI data for the analytes in both monocyte and CD4+ lymphocyte cell types between responders and non-responders at baseline were analyzed with unpaired t-test. The MFI data of individual analytes were used to compute their statistical association and interactions with the lithium response outcome. The log2(FC) data were used along with MFI data of the analytes to study the relationships between analytes and lithium response.
For prediction analysis, we used the FC of the top 6 analytes to estimate the probability for being a responder. The probability was estimated with logistic regression of logbP/1-P = β0 + β1×1 + β2×2 …. The beta is an estimated coefficient in the regression. The x is the FC of an analyte.
Protein-to-protein Interaction and Pathway Analysis
In order to provide the system-level interpretation of the analytes and lithium response outcome, the FCs of all proteins in monocytes and CD4+ lymphocytes were used for the protein-protein interaction (PPI) network analysis. Network-based analysis of diverse phenotypes demonstrate that the proteins that are implicated in similar phenotypes are clustered together in cellular networks.48 In order to identify the highly connected modules (functional module) that are centered around the studied proteins, we used network propagation algorithms49 with the studied proteins as the seeds. For every pair of proteins in the study, the shortest path was computed. Networks were generated with random walk with restart at a seed. The BioGRID database, in which there are 8839 proteins and 67056 interactions,50 was used to generate PPI network and sub-networks.
After the modules were identified, we performed pathway enrichment analysis on the induced PPI network and subnetworks. For this purpose, we used a hypergeometric model to assess the significance of the pathways in the Wiki Pathways dataset. Pathway enrichment analysis was conducted based on the Software of Emich.51
Sample Size Calculation
The sample size calculation was not attempted because this was the first time of using this technology in this population. The initial plan was to enroll 50 patients, but only about half of the original targets were enrolled and completed the study. The study had to stop because of insufficient funding and the nature of a feasibility study.
Results
Demographics and Historical Correlates
Thirty-one patients consented to the study, twenty-five received lithium, and twenty-four returned for at least one post-baseline visit (intent to treat). Twelve patients presented with an index episode of depression (MADRS ≥ 12, YMRS < 12), 12 patients presented with mixed symptoms (MADRS ≥ 12, YMRS ≥ 12), and none met the criteria for “pure” mania/hypomania (MADRS < 12, YMRS ≥ 12). Forty-two percent were male, and 71% were diagnosed with bipolar I disorder (Table 1). Sixteen patients made it to the 8-week time in the study and 12 patients completed the whole 16-week study.
Table 1. Demographics and Historical Correlates of the Responders and Non-Responders.
| Lithium responder (N = 13) |
Lithium non-responder (N = 11) |
|||
| Mean | SD | Mean | SD | |
| Age | 35.35 | 12.57 | 40.21 | 12.82 |
| N | % | N | % | |
| Gender (male) | 6 | 46.2 | 4 | 36.3 |
| Race (white) | 9 | 69.2 | 7 | 63.6 |
| Marital Status (married) | 3 | 23.1 | 1 | 9.1 |
| Education level (≥4 year college) | 4 | 30.8 | 6 | 55.4 |
| Employment Status (employed) | 7 | 53.9 | 5 | 45.5 |
| Bipolar Disorder Subtype I | 10 | 76.9 | 7 | 63.6 |
| History of Rapid Cycling (yes) | 6 | 46.2 | 1 | 9.1 |
| History of abuse | ||||
| Verbal | 9 | 69.2 | 8 | 72.7 |
| Physical | 5 | 38.5 | 8 | 72.7 |
| Sexual | 7 | 53.8 | 4 | 36.4 |
| Past history of suicide attempt(s) (yes) | 1 | 7.7 | 1 | 9.1 |
| Current Comorbidity | ||||
| Generalized anxiety disorder | 9 | 69.2 | 6 | 54.5 |
| Social phobia | 7 | 53.8 | 5 | 45.4 |
| Panic disorder | 3 | 23.1 | 1 | 9.1 |
| Current DSM-5 alcohol use disorder | 2 | 15.4 | 2 | 18.2 |
| Current DSM-5 drug use disorder | 4 | 30.8 | 6 | 54.4 |
| Lifetime * Alcohol Use Disorder | 7 | 53.8 | 6 | 54.4 |
| Lifetime * Cannabis Use Disorder | 5 | 38.5 | 4 | 36.4 |
| Lifetime * Other Drug Use Disorder | 3 | 23.1 | 3 | 27.2 |
| Medications for the Current Episode | ||||
| Anticonvulsants | 2 | 15.4 | 0 | 0 |
| Antipsychotics | 2 | 15.4 | 3 | 27.2 |
| Antidepressants | 2 | 15.4 | 5 | 45.5 |
| Anxiolytics/hypnotics | 1 | 7.7 | 4 | 36.4 |
| Lithium | 2 | 15.4 | 3 | 27.2 |
| Number of Previous Medication Trials | ||||
| 0 | 1 | 7.7 | 0 | 0 |
| 1 | 4 | 30.8 | 2 | 18.2 |
| 2 | 3 | 23.1 | 3 | 27.2 |
| 3 | 1 | 7.7 | 1 | 9.1 |
| > 4 | 4 | 30.8 | 5 | 45.5 |
Primary Outcome
Among the 24 patients who received lithium treatment, 13 were classified as treatment responders, and 11 were treatment non-responders. Demographics and clinical correlates were comparable between the 2 groups (Table 1). The lithium levels were 0.68 ± 0.19 mEq/L in responders, and 0.81 ± 0.26 mEq/L in non-responders. There was no significant difference in lithium levels between two groups (p = 0.20).
Changes in Symptom Severity from Baseline to End of the Study
At baseline, with the exception of CGI-S, there were no significant differences between responders and non-responders in rating scale scores (Table 2). Among the secondary outcome measures (Table 2), MADRS total scores in the responder group from baseline to the end of 16 weeks were significantly reduced compared to change in the scores of the non-responder group. The change in YMRS scores from baseline to week 8 and week 16 in the responder group was significantly lower compared to that in the non-responder group. The other secondary outcome measures were not significantly different between the two groups.
Table 2. Changes in Depression, Anxiety and Manic Symptom Severity from Baseline to Week 8 and Week 16 in Lithium Responders and lithium non-Responders.
| Assessment | Baseline M ± SD |
Week 8 M ± SD |
Lithium Responders Change from baseline M ± SD |
Week 16 M ± SD |
Change from baseline M ± SD |
Baseline M ± SD |
Week 8 M ± SD |
Lithium non-Responders Change from baseline M ± SD |
Week 16 M ± SD |
Change from baseline M ± SD |
Δ Diff at Week 8: Res vs. Non-Res P value |
Δ Diff at Week 16: Res vs. Non-Res P value |
| Blinded assessments | ||||||||||||
| MADRS | 21.69 ± 6.33 | 8.55 ± 7.92 | −13.64 ± 11.39 | 5.25 ± 2.49 | −17.63 ± 8.68 | 26.00 ± 6.32 | 16.40 ± 12.26 | −8.00 ± 12.79 | 19.25 ± 5.85 | −4.75 ± 10.78 | 0.3909 | 0.0485 |
| YMRS | 12.08 ± 6.03 | 3.36 ± 2.34 | −8.00 ± 6.25 | 2.88 ± 1.89 | −8.63 ± 5.66 | 12.45 ± 7.01 | 14.00 ± 4.06 | 2.00 ± 4.85 | 10.75 ± 1.71 | 0.00 ± 3.74 | 0.0070 | 0.0211 |
| HAMA | 16.62 ± 5.72 | 8.09 ± 5.43 | −8.09 ± 0.75 | 7.25 ± 6.61 | −8.63 ± 8.50 | 20.18 ± 7.03 | 16.60 ± 8.08 | −2.80 ± 11.15 | 18.40 ± 5.41 | −1.00 ± 11.27 | 0.2467 | 0.1911 |
| Non-blinded assessment | ||||||||||||
| CGI-S | *3.15 ± 0.80 | 1.42 ± 0.51 | −1.75 ± 0.75 | 1.50 ± 0.53 | −1.63 ± 0.92 | *4.09 ± 0.54 | 2.40 ± 1.34 | −1.40 ± 1.67 | 2.50 ± 1.00 | −1.50 ± 1.00 | 0.5512 | 0.8328 |
| Self-reported assessment | ||||||||||||
| QIDS-16-SF | 12.67 ± 4.66 | 5.00 ± 3.19 | −8.00 ± 4.03 | 3.14 ± 2.61 | −9.00 ± 2.71 | 15.27 ± 2.61 | 6.40 ± 4.04 | −9.40 ± 3.72 | 9.25 ± 3.69 | −7.00 ± 2.58 | 0.5202 | 0.2620 |
Note: *Significantly different between responders and non-responders (p = 0.0033).
Abbreviations: CGI-S, Clinical Global Impression-Severity; DIFF, difference; HAMA, Hamilton Anxiety Rating Scale; M, mean; MADRS, Montgomery-Asberg Depression Rating Scale; Non-Res, non-responders; QIDS-16-SF, the 16 item Quick Inventory of Depressive Symptomatology Self Report; Res, responders; SD, standard deviation; YMRS, Young Mania Rating Scale.
MFI of Analytes
The blood samples of 11 responders and 8 non-responders were available for the analysis. In monocytes, the mean level of GSK3β was significantly higher in lithium non-responders than in responders (p = 0.034), and phosphorylated GSK3αβ was higher, but not significant in non-responders (p = 0.093) (Table 3). In CD4+ lymphocytes, the mean level phosphorylated GSK3αβ was also higher, but not significant in non-responders than in responders (p = 0.086) (Table 3).
Table 3. Comparison of 17 Analyte Levels (median fluorescent intensity) between Lithium Responders and Non-Responders in CD4+ Lymphocytes and Monocytes.
| Analytes | Non-responders (n = 8) Mean ± SD |
CD4+Lymphocytes Responders (n = 11) Mean ± SD |
Responders vs. non-responders p-value |
Non-responders (n = 8) Mean ± SD |
Monocytes Responders (n = 11) Mean ± SD |
Responders vs. non-responders p-value |
| Bcl-2 | 21950 ± 5229 | 22105 ± 2790 | 0.940 | 20910 ± 6349 | 18817 ± 5556 | 0.467 |
| BDNF | 21254 ± 5124 | 22346 ± 2559 | 0.592 | 37167 ± 8915 | 38955 ± 5577 | 0.627 |
| Calmodulin | 18497 ± 7132 | 17243 ± 6350 | 0.698 | 27940 ± 14323 | 26059 ± 14304 | 0.781 |
| Fyn | 18639 ± 5032 | 18717 ± 30740 | 0.970 | 31566 ± 8759 | 30122 ± 7244 | 0.709 |
| phospho-Fyn/Yes | 9175 ± 5862 | 8706 ± 5182 | 0.859 | 20637 ± 9078 | 19453 ± 11467 | 0.805 |
| GSK3β | 9418 ± 5293 | 6338 ± 3417 | 0.141 | 11633 ± 5317 | 7078 ± 3312 | 0.034 |
| phospho-GSK3αβ | 1162 ± 490 | 806 ± 210 | 0.086 | 1738 ± 716 | 1255 ± 468 | 0.093 |
| HMGB1 | 15804 ± 5358 | 15615 ± 2407 | 0.928 | 23985 ± 7443 | 23131 ± 5987 | 0.793 |
| iNOS | 16244 ± 5569 | 15950 ± 3233 | 0.896 | 20612 ± 8332 | 20694 ± 7223 | 0.982 |
| IRS2 | 15832 ± 6377 | 13208 ± 5458 | 0.363 | 27717 ± 11305 | 24017 ± 11502 | 0.495 |
| mTor | 13474 ± 5121 | 12276 ± 4332 | 0.600 | 13544 ± 6156 | 12049 ± 6056 | 0.607 |
| NLRP3 | 21664 ± 5375 | 21672 ± 2320* | 0.997 | 37776 ± 8503 | 35738 ± 7961 | 0.604 |
| NFkB p-P65 | 9110 ± 7785 | 6549 ± 4769 | 0.428 | 4749 ± 2954 | 2855 ± 1809 | 0.137 |
| PGM1 | 15517 ± 5018 | 16522 ± 3614 | 0.638 | 21509 ± 7459 | 24531 ± 7620 | 0.401 |
| PKA C-α | 11286 ± 5725 | 9204 ± 3457 | 0.380 | 17893 ± 11267 | 14138 ± 7903 | 0.340 |
| PPAR-γ | 13718 ± 4335 | 12304 ± 3013 | 0.443 | 24844 ± 8176 | 22921 ± 6906 | 0.598 |
| TPH1 | 8360 ± 5830 | 8256 ± 4142 | 0.968 | 17198 ± 8267** | 18793 ± 6841 | 0.678 |
Note: *10 samples were available; **Seven samples were available.
Abbreviations: Bcl-2, B-cell lymphoma 2; BDNF, brain-derived neurotrophic factor; Calmodulin, calcium-modulated protein; GSK3β, glycogen synthase kinase 3 beta (Ser9); Phospho-GSK-3αβ, phosphorylated glycogen synthase kinase 3 alpha(Tyr279)beta(Tyr216); HMGB1, high mobility group box 1 protein; iNOS, inducible isoform nitric oxide synthase; IRS2, insulin receptor substrate 2; mTor, the mammalian target of rapamycin; NFkB p-P65, phosphorylated nuclear factor NF-kappa-B p65(Ser536) subunit (phospho-RelA); NLRP3, NACHT, LRR and PYD domains-containing protein 3; Phospho-Fyn Yes, phosphorylated-Fyn (Y530)/phosphorylated Yes(Y537); PGM1, phosphoglucomutase 1; PKA C-α, protein kinase A catalytic subunit alpha; PPAR-γ, peroxisome proliferator-activated receptor gamma; TPH1, tryptophan hydroxylase 1.
FC of Analytes
In monocytes, the FC of BDNF, PGM1 or TPH1 levels between non-responders and responder was negative. The FCs for TPH1 and PGM1 were > −0.1 (Figure 1). The FC of IRS2, PPAR-γ, Bcl2, mTor, PKA C-α, GSK3β, phospho-GSK3αβ, phospho-RelA, or calmodulin levels between the groups were ≥ 0.1. The FC between non-responders and responders was 0.72 for GSK3β, 0.47 for phospho-GSK3αβ, and 0.73 for phospho-RelA, respectively.
Figure 1.
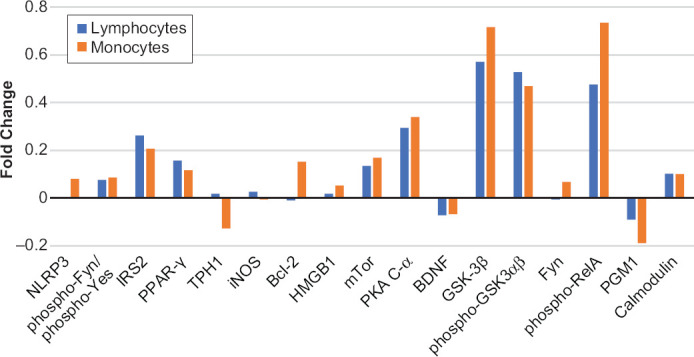
Fold change (FC) of 17 Analytes at Baseline between Lithium Responders and Non-Responders in CD4+ Lymphocytes (blue bars) and Monocytes (brown bars). FC = log2 (median fluorescent intensity of non-responders/median fluorescent intensity of responders)
Note: Positive value is indicative of a higher level of protein expression in lithium non-responders than in lithium responders. Negative value is indicative of a lower level of protein expression in lithium non-responders than in lithium responders.
Abbreviations: Bcl-2, B-cell lymphoma 2; BDNF, brain-derived neurotrophic factor; Calmodulin, calcium-modulated protein; GSK-3β, glycogen synthase kinase 3 beta (Ser9); HMGB1, high mobility group box 1 protein; iNOS, inducible isoform nitric oxide synthase; IRS2, insulin receptor substrate 2; mTor, the mammalian target of rapamycin; Phospho-RelA, phosphorylated nuclear factor NF-kappa-B p65(Ser536) subunit; NLRP3, NACHT, LRR and PYD domains-containing protein 3; Phospho-Fyn/Yes, phosphorylated-Fyn (Y530)/phosphorylated Yes(Y537); PGM1, phosphoglucomutase 1; Phospho-GSK3αβ, phosphorylated glycogen synthase kinase 3 alpha(Tyr279)beta(Tyr216); PKA C-α, protein kinase A catalytic subunit alpha; PPAR-γ, peroxisome proliferator-activated receptor gamma; TPH1, tryptophan hydroxylase 1.
In CD4+ lymphocytes, the FC of BDNF or PGM1 level between non-responders and responder was also negative (Figure 1). However, the differences were less than −0.1 and insignificant. The FCs of other analytes were positive. Only IRS2, PPAR-γ, mTor, PKA C-α, GSK3β, phospho-GSK3αβ, phospho-RelA, and calmodulin had FC ≥ 0.1. The FCs of phospho-GSK3αβ and GSK3β between two groups were 0.57 and 0.53, respectively.
Classification of Responders and Non-Responders according to MFI
Classification of responders and non-responders according to the absolute value of the MFI was explored with single or paired analytes. Figure 2 illustrates use of the expression levels of GSK3β and phospho-GSK3αβ in monocytes to classify lithium responders and non-responders. If “arbitrarily” using GSK3β expression level at 1 × 104 alone as a cut-off, 9/11 (82%) correctly classified as responders and 5/8 (63%) correctly classified as non-responders. If using the levels of phospho-GSK3αβ at ≤ 1500 alone, 9/11 (82%) correctly classified as responders and 4/8 (50%) correctly classified as non-responders. If using the correlation between the levels of GSK3α and phospho-GSK3αβ (yellow dash line), the classification of responders reached 11/11 (100%). In CD4+ lymphocytes, phospho-GSK3αβ level alone could correctly classify 5/8 non-responders and 9/11 responders. If using the correlation between the levels of GSK3β and phospho-GSK3αβ, the classification of responders was 10/11.
Figure 2.
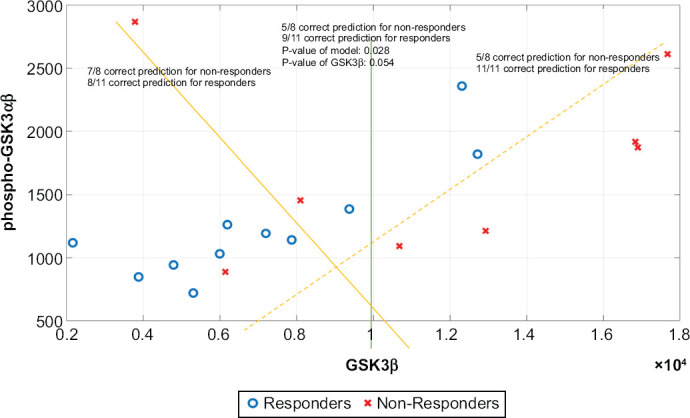
Classification of Lithium Responders and Non-responders According to Cut-off Levels of GSK3β and Phospho-GSK3αβ in Monocytes. Y-axis is the Median Fluorescent Intensities of Phosphorylated GSK3αβ, and X-axis is the Median Fluorescent Intensities of GSK3β
Regression Analysis of Probability Estimate of Being a Responder or Non-Responder
In monocytes, the probability of being a responder or non-responder among all studied patients was estimated with logistic regression with GSK3β, phospho-GSK3αβ, phospho-RelA, PGM1, TPH1, and BDNF (Table 4). As shown in Table 4, the probability of being a responder varied depending on what protein and how many proteins were in the model. With GSK3β and phospho-GSK3αβ in the model, 9/11 responders had ≥ 60% probability of being correctly classified as a responder whereas 2/8 non-responders had ≥ 60% probability of being incorrectly classified as a responder. The difference in probability of being responders was significantly different (p = 0.020). However, the difference in the ≥ 80% probability of being a responder between true responders and non-responders was not significantly different.
Table 4. The Rates of ≥ 60% or ≥ 80% Probability as a Responder among the Responders and Non-responders with Different Analytes in Monocytes and CD4+ Lymphocytes in the Logistic Regression Models.
| ≥ 60% probability as Being Predicted a Responder | ≥ 80% probability as Being Predicted a Responder | |||||||||||
| True Responders | True Non-Responders | True Responders | True Non-Responders | |||||||||
| Analytes in the model | Predicted as Res. N | Total N | % | Predicated as Res. N | Total N | % | Predicted as Res. N | Total N | % | Predicted as Res. N | Total N | % |
| Monocytes | ||||||||||||
| GSK3β, phospho-GSK3αβ | 9 | 11 | 81.8 | 2 | 8 | 25.0 | 4 | 11 | 36.4 | 1 | 8 | 12.5 |
| GSK3β, PGM1 | 10 | 11 | 90.9 | 2 | 8 | 25.0 | 7 | 11 | 66.6 | 0 | 8 | 0.0 |
| Phospho-RelA, PGM1 | 6 | 11 | 54.5 | 2 | 8 | 25.0 | 3 | 11 | 27.3 | 2 | 8 | 25.0 |
| GSK3β, phospho-GSK3αβ, phospho-RelA | 9 | 11 | 81.8 | 2 | 8 | 25.0 | 4 | 11 | 36.4 | 1 | 8 | 12.5 |
| GSK3β, phospho-GSK3αβ, phospho-RelA, PGM1 | 10 | 11 | 90.9 | 2 | 8 | 25.0 | 7 | 11 | 66.6 | 0 | 8 | 0.0 |
| GSK3β, phospho-RelA, PGM1, TPH1 | 10 | 11 | 90.9 | 1 | 7 | 14.3 | 10 | 11 | 90.9 | 0 | 7 | 0.0 |
| GSK3β, phospho-RelA, PGM1, BDNF | 9 | 11 | 81.8 | 2 | 8 | 25.0 | 7 | 11 | 66.6 | 0 | 8 | 0.0 |
| Lymphocytes | ||||||||||||
| GSK3β, phospho-GSK3αβ | 8 | 11 | 72.3 | 4 | 8 | 50.0 | 3 | 11 | 27.3 | 1 | 8 | 12.5 |
| GSK3β, PGM1 | 10 | 11 | 90.9 | 2 | 8 | 25.0 | 6 | 11 | 54.5 | 1 | 8 | 12.5 |
| GSK3β, phospho-RelA, PGM1, TPH1 | 10 | 11 | 90.9 | 1 | 7 | 14.3 | 9 | 11 | 81.8 | 1 | 7 | 14.3 |
| GSK3β, phospho-RelA, PGM1, BDNF | 9 | 11 | 81.8 | 2 | 8 | 25.0 | 7 | 11 | 66.6 | 1 | 8 | 12.5 |
Abbreviations: BDNF, brain-derived neurotrophic factor; GSK-3β, glycogen synthase kinase 3 beta (Ser9); N, number; Phospho-GSK-3αβ, phosphorylated glycogen synthase kinase 3 alpha(Tyr279)beta(Tyr216); Phospho-RelA, phosphorylated nuclear factor NFkB p65(Ser536) subunit; PGM1, phosphoglucomutase 1; Res, responder; TPH1, tryptophan hydroxylase 1.
In contrast, when GSK3β, phospho-RelA, TPH1, and PGM1 were in the model, 10/11 of responders had ≥ 60% probability of being correctly classified as a responder compared to 1/7 non-responders of being incorrectly classified as a responder (Table 4). The difference between two groups was significantly different (p = 0.002). Similarly, 10/11 of responders had ≥ 80% probability of being correctly classified as a responder, however, none of the non-responders had ≥ 80% probability of being incorrectly classified as a responder. The difference was significantly different (p = 0.0001).
In CD4+ lymphocytes (Table 4), the probability of being a responder also varied depending on what protein and how many proteins were in the model. When GSK3β, phospho-RelA, TPH1, and PGM1 in the model, 10/11 of true responders had ≥ 60% probability of being correctly classified as a responder compared to 1/7 non-responders being incorrectly classified as a responder (Table 4). The difference between two groups was significantly different (p = 0.002). Similarly, 9/11 of true responders and 1/7 of non-responders had ≥ 80% probability of being predicted as a responder. The difference was also significantly different (p = 0.009).
Protein-Protein Interaction Network and Pathway Analysis
The PPI network of 14 proteins related to the 17 analytes in monocytes was generated (Figure 3). These 14 proteins are in a network of a total 71 proteins. In monocytes, with the exception of TPH1, PGM1, and BDNF, the other 11 proteins had a higher level of expression in lithium non-responders than responders (Figure 3). In lymphocytes, about a half of studied proteins had a higher level of expression in lithium non-responders than responders (data not shown). There were significant overlaps of the expression levels of those proteins in both cell types.
Figure 3.
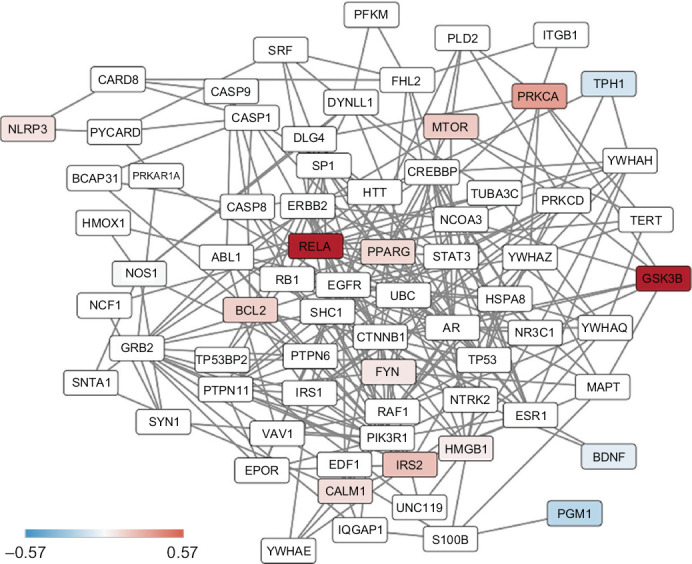
Results of Protein-protein Interaction Network of 14 Proteins in Monocytes
Note: The color of the nodes represents the magnitude of differences in protein expression levels between lithium non-responders and responders at the baseline as measured with the 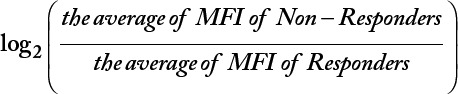 . The white nodes are proteins that were not measured in the study but they are on the shortest paths between pairs of proteins that are included in the study.
. The white nodes are proteins that were not measured in the study but they are on the shortest paths between pairs of proteins that are included in the study.
Abbreviation: BCL2, B-cell lymphoma 2; BDNF, brain-derived neurotrophic factor; CALM1, calcium-modulated protein; GSK3B, glycogen synthase kinase 3 beta; HMGB1, high mobility group box 1 protein; IRS2, insulin receptor substrate; MTOR, mammalian target of rapamycin; NLPR3, NACHT, LRR and PYD domains-containing protein 3; NOS1, inducible isoform nitric oxide synthase; PGM1, phosphoglucomutase 1; PPARG, peroxisome proliferator-activated receptor gamma; PRKCA, protein kinase A catalytic subunit alpha; RELA, nuclear factor NFkB p65 subunit; TPH1, tryptophan hydroxylase 1.
Using the PPI network generated with 14 proteins in the study, we found that genes coding for the proteins in the network are involved in prolactin signaling pathway, leptin signaling pathway, BDNF signaling pathway, neurotrophin signaling pathway, and EGF (epidermal growth factor)/EGFR(epidermal growth factor receptor) signaling pathway (Table 5). Both GSK3β and RelA genes are involved in prolactin signaling pathway, leptin signaling pathway, BDNF signaling pathway, and neurotrophin signaling pathway.
Table 5. Results of Genes Coding 14 Proteins in the Study Based on Pathway Enrichment Analysis on the Protein-Protein Networks of the 14 Proteins.
| Pathway | Genes | P-value |
| Prolactin Signaling Pathway | FYN; GSK3β; MTOR; RELA; IRS2; ITGB1; IRS1; SHC1; STAT3; IRS2; PTPN11; PIK3R1; YWHAZ; VAV1; ERBB2; GRB2; PTPN6; RAF1 | E-26 |
| Leptin signaling pathway | FYN; GSK3β; RELA; MTOR; IRS1; SHC1; STAT3; PTPN11; PIK3R1; ESR1; SP1; ERBB2; GRB2; RAF1 | E-21 |
| Brain-Derived Neurotrophic Factor (BDNF) signaling pathway | GSK3β; IRS2; MTOR; RELA; FYN; BDNF; NTRK2; NCF1; IRS1; SHC1; PRKCD; STAT3; PTPN11; PIK3R1; SYN1; CTNNB1; GRB2; MAPT; RAF1 | E-25 |
| Neurotrophin signaling pathway | CALM1; IRS2; BCL2; BDNF; GSK3β; RELA; YWHAE; NTRK2; IRS1; SHC1; PRKCD; PTPN11; PIK3R1; YWHAZ; YWHAQ; ABL1; GRB2; RAF1; TP53; YWHAH | E-28 |
| EGF/EGFR Signaling Pathway | PRKCα; MTOR; SHC1; NCOA3; PRKCD; STAT3; PTPN11; IQGAP1; PIK3R1; VAV1; EGFR; PLD2; SP1; ERBB2; ABL1; GRB2; RAF1 | E-20 |
Note: The bold genes are implicated in the current study.
Abbreviations: BCL2, B-cell lymphoma 2; BDNF, brain-derived neurotrophic factor; CALM1, calcium-modulated protein; GSK3β, glycogen synthase kinase 3 beta; IRS2, insulin receptor substrate; MTOR, mammalian target of rapamycin; RELA, nuclear factor NF-kappa-B p65 subunit; PRKCα, protein kinase A catalytic subunit.
Discussion
This feasibility study suggests that tyramine-based signal-amplified flow cytometry like the CellPrint may be capable of detecting intracellular proteins for predicting lithium response in BD. It is sensitive enough to detect significant differences in some intracellular proteins from specific peripheral blood cell subtypes between lithium responders and non-responders. Some differences between responders and non-responders may be used alone or combinations to classify lithium treatment response (Figure 2). Definitely, these findings need to be confirmed with large sample studies. The differences in GSK3β, phospho-GSK3αβ, and phospho-RelA were the largest among the analytes, suggesting that these 3 proteins should be considered for future studies. However, the logistic regression results suggest that combination of proteins even without significant difference between responders and non-responders may increase sensitivity and specificity for prediction of lithium response. The PPI network and pathway enrichment analysis suggest that lithium affects multiple pathways through GSK3β and RelA.
The FC results suggest that lithium responders and non-responders have differences in expression levels of several proteins (Figure 1 and Table 3). A larger FC of some proteins in monocytes compared to lymphocytes or vice versa suggests that different cell types may provide different information on the same protein between the groups and underscored the importance of analyzing specific cell subtypes. Although these peripheral changes may not reflect the changes of neurons in the brain, the connection between central nervous system and immune system suggest that changes in peripheral blood mononuclear cells may be used as surrogates for biomarker studies of lithium treatment response.52–59 More studies are needed to assess the utility of protein levels in lymphocytes and/or monocyte to predict lithium treatment response. Until then, it seems reasonable to include these two cell types in future studies with flow cytometry.
Lithium can cause changes in many proteins in different tissues18,60 The results of this study suggest that flow cytometric analysis with a small number of analytes may detect protein signal separation in blood mononuclear cells between the responders and non-responders. Our selection of analytes was based on previous studies of BD covering a spectrum of pathways/functions including apoptosis, calcium transport, cell signaling, metabolic enzymes, kinase, neurotrophic factors, receptors, and transcriptional factors. GSK, the most recognized factor of lithium response20,60 did show differential effects between lithium responders and non-responders although the levels of other proteins between the two groups were not significantly different (Figure 1 and Table 3). This finding is somewhat consistent with previous studies.6,11 However, some proteins such as BDNF, PKA, and Bcl-26,11,60 have been recognized as important factors of the etiology of bipolar disorder and lithium treatment response, yet they did not show a differential effect between lithium responders and non-responders. The reason for these inconsistent findings with previous studies may be due to the statistical power of this pilot study or the sensitivity of our cytometric analysis. Alternatively, the relationship between lithium response and expression level of these analytes may be important in cell types not analyzed in this study, but not in peripheral blood monocytes and lymphocytes. Also, it may be that the expression levels of these analytes is not the primary determinant of the activity of these analytes for lithium treatment response.
A large difference between responders and non-responder in the expression level of phospho-RelA, a subunit of NFkB, is somewhat unexpected. This is the first study of patients with BD showing a large difference in phospho-RelA levels at baseline between lithium responders and non-responders. As a transcription factor, the NFkB system plays a key role in regulating immune development, immune response, inflammation, cell cycles, proliferation, cancer, and cell death.61,62 Increased levels of NFkB in the brain63,64 and peripheral blood cells of patients with BD have been reported.54 Serum/plasma studies have also shown that cytokines are involved in BD and lithium treatment response.29 Lithium can down-regulate NFkB through inhibition of GSK3β.65–69 Some studies have shown that lithium has an anti-inflammatory effect that is believed to be the mechanism of the efficacy of lithium.70 Therefore, it may not be surprising to find that phospho-RelA, a downstream target of GSK3β and an upstream regulating factor of gene expression of proteins related to anti-inflammation and pro-inflammation, had a differential effect on lithium responsiveness.
The results of PPI network suggest that there are other proteins potentially involved in the lithium treatment response that were not included in the current analysis. The role of those proteins in predicting lithium response needs further exploration. The results of pathway analysis suggest that many genes coding proteins in different pathways are involved in lithium response. BDNF and neurotrophin signaling pathways are well known for their involvement in lithium response and pathophysiology of BD.60 The other pathways are less well known for their roles in BD and lithium treatment response. Genome wide association studies have not yielded robust results of the genetic basis for lithium response16,20,21 According to an ingenuity pathway analysis generated with SNPs from GWAS and genetic association studies, genes coding GSK3β were associated with all top 10 diseases and phenotypes related to a glutamatergic network mediating lithium response.71 Moreover, GSK3β directly interacts at least 265 proteins.72 Therefore, a technology measuring multiple upstream and downstream target proteins of GSK3β by using enhanced flow cytometry like the present study may find biomarker(s) and locate genes in predicting lithium treatment response.
Regardless of what proteins and/or genes might be useful to predict lithium treatment response, this feasibility study suggests that tyramine-based, signal-amplified flow cytometry like the CellPrint may help elucidate lithium response biomarkers consisting of multiple intracellular proteins better than a “trial-and-error” approach used in current clinical practice. In previous clinical trials, the clinically observed responders from lithium ranged from 32% to 63%.2–4 The logistic regression results from the current study (Table 4) suggest a panel of proteins can predict acute lithium treatment response with a probability of higher than that of clinical observation. Larger studies are warranted to replicate these findings. More importantly, like any other potential biomarker(s) for diagnosis and treatment of BD, the field needs to define what sensitivity and specificity are acceptable for a biomarker(s) being used for clinical practice.73
Flow cytometry has been used in previous studies of lithium response,54,74–78 but this is the first study using tyramine-based, signal-amplified flow cytometry to measure multiple intracellular proteins in both quesicient CD4+ lymphocytes and monocytes of patients with BD. Although our results are promising, there are several caveats that would improve follow-on studies. This study is limited because of a small sample size and low completion rate. Some markers might show significant expression differences between lithium responders and non-responders with a large sample size. Moreover, the duration of this study is relatively short compared to long-term maintenance studies although it is longer than most acute treatment studies of 6-8 weeks for bipolar depression and 3-4 weeks for bipolar mania.79 It is unclear if these short-term responders will be long-term responders. A large longitudinal study found that about 26% of bipolar I patients stabilized with lithium in the acute treatment discontinued the study due to mood relapse or intolerable side effect during 2 year follow-up study period,2 suggesting that a large number of patients who respond to acute lithium treatment are likely to remain stable for a long period. In addition, any treatment goal is to reach remission, not response. Due to the small sample size, we had to use “response” as a primary outcome to give us relatively “balanced” groups. Future studies using response and remission rates are necessary to divide groups for cytometric analyses. As the study was conducted in an outpatient setting and no “pure” manic patient was included, this selection bias might also confound the results.
Conclusion
The results from this feasibility study suggest that tyramine-based, signal-amplified flow cytometry like the CellPrint to measure multiple proteins in the peripheral blood cells of bipolar patients may identify biomarker(s) for predicting lithium response. Large sample size studies are warranted to support or refute our findings.
Acknowledgments
This study was supported by an independent investigator grant from the Brain and Behavior Research Foundation.
Supplemental Materials
CellPrint Flow Cytometry
The flow cytometry developed by CellPrint Biotechnology, LLC uses a catalytic deposition labeling procedure called CellPrint™. The CellPrint flow cytometry improves signal to noise ratios by 10-100-fold and dynamic range by 20-fold for intracellular and surface protein detection compared to standard flow cytometric staining methods while retaining the ability to assess cellular subtypes.1–5 The CellPrint flow cytometry is able to detect expression levels of low abundance molecules as well as phosphorylated proteins and has enabled quantification of a wide variety of analytes from numerous cell types including platelets,6,7 lymphocytes,4,8 stem cells,9,10 leukemic cells,11–14 and cultured peripheral blood mononuclear cells.3
Participants
For inclusion in this study, subjects must have met all of the following criteria: 1) Male or female who was 18-70 years old and able to provide informed consent before beginning any study-specific procedures; 2) Met current DSM-5 criteria for bipolar I or II disorder as assessed by the MINI and structured research diagnostic interview by a research psychiatrist; 3) At any symptomatic phase of bipolar I or II disorder including depressive, manic or hypomanic with Global Clinical Impression-Severity for Bipolar Disorder (CGI-S-BD) ≥3; 4) Willing to take lithium and have blood drawn; 5) If a sexually active female was of childbearing potential, she must use a reliable method of contraception and had a negative urine pregnancy test before starting lithium.
Any of the following was regarded as a criterion for exclusion from the study: 1) Unwilling to comply with study requirements or have blood drawn; 2) Renal impairment (serum creatinine >1.5 mg/dL); 3) Thyroid stimulating hormone (TSH) over >20% above the upper normal limit (participants maintained on thyroid medication must be euthyroid for at least 3 months before Visit 1; 4) Had other contraindication to lithium and had previous intolerable side effect from lithium; 5) Patients who required inpatient care for psychiatric problems including requiring immediate acute detoxification for alcohol and drug related issues; 6) Pregnancy as determined by serum pregnancy test or breastfeeding; 7) History of nonresponse to lithium at doses ≥ 900 mg/d for ≥8 weeks; 8) Patients with chronic medical conditions such as diabetes, cardiovascular disease, immune diseases, infectious diseases and neurological disorders; 9) Active suicidal ideation with a plan or intent, or a suicide attempt within past 6 months or more than 2 suicide attempts within the past 2 years; 10) Currently on lithium.
Study Procedures
Screening Phase
After obtaining the informed consent, all potential research participants were assessed with a systemic research diagnostic interview by a research psychiatrist15 and a structured interview with MINI for DSM-5 (Mini International Neuropsychiatric Interview for DSM-5).16 CGI-S-BD,17 QIDS-16 SR (the 16 item Quick Inventory of Depressive Symptomatology – Self-Report),18 Cumulative Illness Rating Scale,19 vital signs, physical examination and medical history were performed, and blood and urine samples were collected. The screening phase is up to 4 weeks in duration. For those who met the diagnostic inclusion criteria and did not have any exclusion criterion, they were enrolled into the study for baseline and follow-us visits.
Treatment Phase
Eligible patients received lithium 300 mg per day for 3 nights and then increased to 600 mg/d as tolerated. After at least 5 days of treatment with lithium 600 mg/d, a lithium level was done. If necessary, titration in 300 mg increments every 7days as tolerated took place to achieve blood lithium levels 0.6–1.2 mEq/L. Any psychotropic medications (with the exception of allowed rescue medications) were tapered off by week 4. The study psychiatrist worked with each individual participant concerning how quickly and to what schedule the prohibited medication(s) was weaned off. Participants were closely monitored, and came in for unscheduled visits if necessary (typically 1 visit per week, though visits could be as frequent as required), and were encouraged to contact the clinic if they had questions or concerns. The participant was discontinued from the trial if their mood worsened to a significant degree judged by a research psychiatrist and/or they could not discontinue unpermitted concomitant medication(s).
Permitted Concomitant Medications
A benzodiazepine and/or a hypnotic medication was allowed for anxiety or insomnia throughout the study. No other mood stabilizer(s), anticonvulsant, antidepressant, or antipsychotic was allowed during the study after week 4.
Assessments
At baseline, psychiatric assessments were administered. Symptom severity was measured with Montgomery Depression Rating Scale (MADRS) for depression,20 Young Mania Rating Scale (YMRS) for mania/hypomania,21 Clinical Global Impression-Efficacy Index (CGI-EI) for overall effectiveness,22 Hamilton anxiety score (HAM-A) for anxiety,23 Snaith-Hamilton Pleasure Scale (SHAPS) for anhedonia.24 The disability was measured with SDS (Sheehan Disability Scale),25 and quality of life was measured with Quality of Life Enjoyment and Satisfaction Questionnaire – Short Form (Q-LES-Q-SF).26 Iowa Fatigue Scale (IFS) was used to measure the severity of fatigue.27 For those who met the criteria for a current substance use disorder (SUD), the Time Line Follow back (TLFB)28 was used to measure the severity of SUD. Safety was measured with Frequency, Intensity, and Burden of Side Effects Ratings/Global Rating of Side Effects Burden (FIBSER)29 and Columbia Suicide-Severity Rating Scale (C-SSRS).30 Vital signs, MADRS, YMRS, HAM-A, QIDS-16-SF, CGI-S-BD, SDS, Q-LES-Q-SF, IFS, FIBSR/GRSEB, and C-SSRS were repeated week 1, 2, 4, 6, 8, 12, and 16. At the end of the study, the CIRS, physical examination and medical history, and laboratory tests were repeated.
References
- 1.Yatham LN, Kennedy SH, Parikh SV et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord . 2018;20(2):97–170. doi: 10.1111/bdi.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin Y, Maihofer AX, Stapp E et al. Clinical Predictors of Non-Response to Lithium Treatment in The Pharmacogenomics of Bipolar Disorder (PGBD) Study. Bipolar Disorder . 2021 Apr 2; doi: 10.1111/bdi.13078. doi:10.1111/bdi.13078. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.van der Loos ML, Mulder PG, Hartong EG et al. Efficacy and safety of lamotrigine as add-on treatment to lithium in bipolar depression: a multicenter, double-blind, placebo-controlled trial. J Clin Psychiatry . 2009;70(2):223–231. doi: 10.4088/jcp.08m04152. [DOI] [PubMed] [Google Scholar]
- 4.Young AH, McElroy SL, Bauer M et al. A double-blind, placebo-controlled study of quetiapine and lithium monotherapy in adults in the acute phase of bipolar depression (EMBOLDEN I) J Clin Psychiatry . 2010;71(2):150–162. doi: 10.4088/JCP.08m04995gre. [DOI] [PubMed] [Google Scholar]
- 5.Baldessarini RJ, Tondo L. Does lithium treatment still work? Evidence of stable responses over three decades. Arch Gen Psychiatry . 2000;57(2):187–190. doi: 10.1001/archpsyc.57.2.187. [DOI] [PubMed] [Google Scholar]
- 6.Alda M. Lithium in the treatment of bipolar disorder: pharmacology and pharmacogenetics. Mol Psychiatry . 2015;20(6):661–670. doi: 10.1038/mp.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rybakowski JK, Chlopocka-Wozniak M, Suwalska A. The prophylactic effect of long-term lithium administration in bipolar patients entering treatment in the 1970s and 1980s. Bipolar Disord . 200;3(2):63–67. doi: 10.1034/j.1399-5618.2001.030203.x. [DOI] [PubMed] [Google Scholar]
- 8.Garnham J, Munro A, Slaney C et al. Prophylactic treatment response in bipolar disorder: results of a naturalistic observation study. J Affect Disord . 2007;104(1–3):185–190. doi: 10.1016/j.jad.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Tondo L, Alda M, Bauer M et al. Clinical use of lithium salts: guide for users and prescribers. Int J Bipolar Disord . 2019;7(1):16. doi: 10.1186/s40345-019-0151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao K, Goto T, Yuan C et al. A pilot study of the effectiveness of lithium versus quetiapine immediate release monotherapy in patients with bipolar spectrum disorders. J Clin Psychopharmacol . 2018;38(5):422–434. doi: 10.1097/JCP.0000000000000927. [DOI] [PubMed] [Google Scholar]
- 11.Gao K, Yuan C, Wu R et al. Important clinical features of atypical antipsychotics in acute bipolar depression that inform routine clinical care: a review of pivotal studies with number needed to treat. Neurosci Bull . 2015;31(5):572–588. doi: 10.1007/s12264-014-1534-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tohen M, Greil W, Calabrese JR et al. Olanzapine versus lithium in the maintenance treatment of bipolar disorder: a 12-month, randomized, double-blind, controlled clinical trial. Am J Psychiatry . 2005;162(7):1281–1290. doi: 10.1176/appi.ajp.162.7.1281. [DOI] [PubMed] [Google Scholar]
- 13.Can A, Schulze TG, Gould TD. Molecular actions and clinical pharmacogenetics of lithium therapy. Pharmacol Biochem Behav . 2014;123:3–16. doi: 10.1016/j.pbb.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tondo L, Baldessarini RJ. Antisuicidal effects in mood disorders: are they unique to lithium. Pharmacopsychiatry . 2018;51(5):177–188. doi: 10.1055/a-0596-7853. [DOI] [PubMed] [Google Scholar]
- 15.Rybakowski JK. Challenging the negative perception of lithium and optimizing its long-term administration. Front Mol Neurosci . 2018;11:349. doi: 10.3389/fnmol.2018.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao K, Calabrese JR. Pharmacogenetics of lithium response: close to clinical practice. Lancet . 2016;387(10023):1034–1036. doi: 10.1016/S0140-6736(16)00147-1. [DOI] [PubMed] [Google Scholar]
- 17.Hui TP, Kandola A, Shen L et al. A systematic review and meta-analysis of clinical predictors of lithium response in bipolar disorder. Acta Psychiatr Scand . 2019;140(2):94–115. doi: 10.1111/acps.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mertens J, Wang QW, Kim Y et al. Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder. Nature . 2015;527(7576):95–99. doi: 10.1038/nature15526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stern S, Santos R, Marchetto MC et al. Neurons derived from patients with bipolar disorder divide into intrinsically different sub-populations of neurons, predicting the patients’ responsiveness to lithium. Mol Psychiatry . 2018;23(6):1453–1465. doi: 10.1038/mp.2016.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao K, Calabrese JR. The mechanisms of action of lithium in bipolar disorder. Road to Novel Therapeutics . 2021:357–364. In neurobiology of bipolar disorder. Academic Press. [Google Scholar]
- 21.Vieta E, Berk M, Schulze TG et al. Bipolar disorders. Nat Rev Dis Primers . 2018;4:18008. doi: 10.1038/nrdp.2018.8. [DOI] [PubMed] [Google Scholar]
- 22.Favre N, Bordmann G, Rudin W. Comparison of cytokine measurements using ELISA, ELISPOT and semi-quantitative RT-PCR. J Immunol Methods . 1997;204(1):57–66. doi: 10.1016/s0022-1759(97)00033-1. [DOI] [PubMed] [Google Scholar]
- 23.Zhang B, Wang J, Wang X et al. Proteogenomic characterization of human colon and rectal cancer. Nature . 2014;513:382–387. doi: 10.1038/nature13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hukelmann JL, Anderson KE, Sinclair LV et al. The cytotoxic T cell proteome and its shaping by the kinase mTOR. Nat Immunol . 2016;17:104–112. doi: 10.1038/ni.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darmanis S, Gallant CJ, Marinescu VD et al. Simultaneous multiplexed measurement of RNA and proteins in single cells. Cell Rep . 2016;14:380–389. doi: 10.1016/j.celrep.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krutzik PO, Nolan GP. Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A . 2003;55(2):61–70. doi: 10.1002/cyto.a.10072. [DOI] [PubMed] [Google Scholar]
- 27.Coppin E, Malergue F, Thibult ML et al. Flow cytometric analysis of intracellular phosphoproteins in human monocytes. Cytometry B Clin Cytom . 2017;92(3):207–210. doi: 10.1002/cyto.b.21207. [DOI] [PubMed] [Google Scholar]
- 28.Lago SG, Tomasik J, van Rees GF et al. Exploring the neuropsychiatric spectrum using high-content functional analysis of single-cell signaling networks. Mol Psychiatry . 2020;25(10):2355–2372. doi: 10.1038/s41380-018-0123-4. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez SD, Williams AJ, Blacker CJ et al. Putative biological predictors of treatment response in bipolar disorders. Personalized Medicine in Psychiatry . 2017;1:39–58. [Google Scholar]
- 30.van den Ameele S, van Diermen L, Staels W et al. The effect of mood-stabilizing drugs on cytokine levels in bipolar disorder: A systematic review. J Affect Disord . 2016;203:364–373. doi: 10.1016/j.jad.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 31.Blundell MP, Sanderson SL, Long TA. Flow cytometry as an important tool in proteomic profiling. Methods Mol Biol . 2021;v2261:213–227. doi: 10.1007/978-1-0716-1186-9_13. [DOI] [PubMed] [Google Scholar]
- 32.McKinnon KM. Flow Cytometry: An Overview. Curr Protoc Immunol . 2018;120:5.1.1–5.1.11. doi: 10.1002/cpim.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delmonte OM, Fleisher TA. Flow cytometry: surface markers and beyond. J Allergy Clin Immunol . 2019;143(2):528–537. doi: 10.1016/j.jaci.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Suo Y, Gu Z, Wei X. Advances of in vivo flow cytometry on cancer studies. Cytometry A . 2020;97(1):15–23. doi: 10.1002/cyto.a.23851. [DOI] [PubMed] [Google Scholar]
- 35.Pillai V, Dorfman DM. Flow cytometry of nonhematopoietic neoplasms. Acta Cytol . 2016;60(4):336–343. doi: 10.1159/000448371. [DOI] [PubMed] [Google Scholar]
- 36.Bank IE, Timmers L, Gijsberts CM et al. The diagnostic and prognostic potential of plasma extracellular vesicles for cardiovascular disease. Expert Rev Mol Diagn . 2015;15(12):1577–1588. doi: 10.1586/14737159.2015.1109450. [DOI] [PubMed] [Google Scholar]
- 37.Soma P, Swanepoel AC, du Plooy JN et al. Flow cytometric analysis of platelets type 2 diabetes mellitus reveals ‘angry’ platelets. Cardiovasc Diabetol . 2016;15:52. doi: 10.1186/s12933-016-0373-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ayati M, Chance MR, Koyuturk M. Co-phosphorylation networks reveal subtype-specific signaling modules in breast cancer. Bioinformatics . 2020;btaa678 doi: 10.1093/bioinformatics/btaa678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ayati M, Wiredja D, Schlatzer D et al. CoPhosK: A method for comprehensive kinase substrate annotation using co-phosphorylation analysis. PLoS Computational Biology . 2019;15(2):e1006678. doi: 10.1371/journal.pcbi.1006678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bebek G, Koyutürk M, Price ND et al. Network biology methods integrating biological data for translational science. Brief Bioinform . 2012;13(4):446–459. doi: 10.1093/bib/bbr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han Y, Gu Y, Zhang AC et al. Review: imaging technologies for flow cytometry. Lab Chip . 2016;16(24):4639–4647. doi: 10.1039/c6lc01063f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karkmann U, Radbruch A, Hölzel V et al. Enzymatic signal amplification for sensitive detection of intracellular antigens by flow cytometry. J Immunol Methods . 1999;230(1–2):113–120. doi: 10.1016/s0022-1759(99)00130-1. [DOI] [PubMed] [Google Scholar]
- 43.Clutter MR, Heffner GC, Krutzik PO et al. Tyramide signal amplification for analysis of kinase activity by intracellular flow cytometry. Cytometry A . 2010;77(11):1020–1031. doi: 10.1002/cyto.a.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaplan D, Smith D. Enzymatic amplification staining for flow cytometric analysis of cell surface molecules. Cytometry . 2000;40(1):81–5. doi: 10.1002/(sici)1097-0320(20000501)40:1<81::aid-cyto11>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan D, Husel W, Meyerson H. Immunophenotypic analysis with enhanced sensitivity of detection by enzymatic amplification staining. Clin Lab Med . 2001;21(4):763–778. [PubMed] [Google Scholar]
- 46.Kaplan D. Enzymatic amplification staining for single cell analysis: applied to in situ hybridization. J Immunol Methods . 2003;283(1–2):1–7. doi: 10.1016/j.jim.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Kaplan D, Meyerson H, Husel W et al. D cyclins in lymphocytes. Cytometry A . 2005;63(1):1–9. doi: 10.1002/cyto.a.20103. [DOI] [PubMed] [Google Scholar]
- 48.Zhou X, Menche J, Barabási AL et al. Human symptoms-disease network. Nat Commun . 2014;5:4212. doi: 10.1038/ncomms5212. [DOI] [PubMed] [Google Scholar]
- 49.Cowen L, Ideker T, Raphael BJ et al. Network propagation: a universal amplifier of genetic associations. Nat Rev Genet . 2017;18(9):551–562. doi: 10.1038/nrg.2017.38. [DOI] [PubMed] [Google Scholar]
- 50.Oughtred R, Stark C, Breitkreutz BJ et al. The BioGRID interaction database: 2019 update. Nucleic Acids Res . 2019;47(D1):D529–D541. doi: 10.1093/nar/gky1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen EY, Tan CM, Kou Y et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics . 2013;14:128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanabe K, Ang CE, Chanda S et al. Transdifferentiation of human adult peripheral blood T cells into neurons. Proc Natl Acad Sci U S A . 2018;115(25):6470–6475. doi: 10.1073/pnas.1720273115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang X, Wang S, Bai Y et al. Conversion of adult human peripheral blood mononuclear cells into induced neural stem cell by using episomal vectors. Stem Cell Res . 2016;16(2):236–242. doi: 10.1016/j.scr.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 54.Barbosa IG, Nogueira CR, Rocha NP et al. Altered intracellular signaling cascades in peripheral blood mononuclear cells from BD patients. J Psychiatr Res . 2013;47(12):1949–1954. doi: 10.1016/j.jpsychires.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 55.Drexhage RC, Hoogenboezem TH, Versnel MA, Berghout A, Nolen WA, Drexhage HA. The activation of monocyte and T cell networks in patients with bipolar disorder. Brain Behav Immun . 2011;25(6):1206–1213. doi: 10.1016/j.bbi.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 56.Pandey GN, Ren X, Rizavi HS et al. Abnormal gene expression of proinflammatory cytokines and their receptors in the lymphocytes of patients with bipolar disorder. Bipolar Disord . 2015;17:636–644. doi: 10.1111/bdi.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maffioletti E, Cattaneo A, Rosso G et al. Peripheral whole blood microRNA alterations in major depression and bipolar disorder. J Affect Disord . 2016;200:250–258. doi: 10.1016/j.jad.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 58.Miklowitz DJ, Portnoff LC, Armstrong CC et al. Inflammatory cytokines and nuclear factor-kappa B activation in adolescents with bipolar and major depressive disorders. Psychiatry Res . 2016;41:315–322. doi: 10.1016/j.psychres.2016.04.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roux M, Dosseto A. From direct to indirect lithium targets: a comprehensive review of omics data. Metallomics . 2017;9(10):1326–1351. doi: 10.1039/c7mt00203c. [DOI] [PubMed] [Google Scholar]
- 60.Wu R, Fan J, Zhao J et al. The relationship between neurotrophins and bipolar disorder. Expert Rev Neurother . 2014;14(1):51–65. doi: 10.1586/14737175.2014.863709. [DOI] [PubMed] [Google Scholar]
- 61.Christian F, Smith EL, Carmody RJ. The regulation of NF–κB subunits by phosphorylation. Cells . 2016;5(1):12. doi: 10.3390/cells5010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitchell S, Vargas J, Hoffmann A. Signaling via the NFκB system. Wiley Interdiscip Rev Syst Biol Med . 2016;8(3):227–241. doi: 10.1002/wsbm.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun Y, Zhang L, Johnston NL et al. Serial analysis of gene expression in the frontal cortex of patients with bipolar disorder. Br J Psychiatry Suppl . 2001;41:s137–s141. doi: 10.1192/bjp.178.41.s137. [DOI] [PubMed] [Google Scholar]
- 64.Rao JS, Rapoport SI. Mood-stabilizers target the brain arachidonic acid cascade. Curr Mol Pharmacol . 2009;2(2):207–214. doi: 10.2174/1874467210902020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Troib A, Azab AN. Effects of psychotropic drugs on Nuclear Factor kappa B. Eur Rev Med Pharmacol Sci . 2015;19(7):1198–1208. [PubMed] [Google Scholar]
- 66.Hofmann C, Dunger N, Schölmerich J et al. Glycogen synthase kinase 3-β: a master regulator of toll-like receptor-mediated chronic intestinal inflammation. Inflamm Bowel Dis . 2010;16(11):1850–1858. doi: 10.1002/ibd.21294. [DOI] [PubMed] [Google Scholar]
- 67.Hoeflich KP, Luo J, Rubie EA et al. Requirement for glycogen synthase kinase-3beta in cell survival and NF–kappaB activation. Nature . 2000;406(6791):86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 68.Xia Y, Rao J, Yao A et al. Lithium exacerbates hepatic ischemia/reperfusion injury by inhibiting GSK-3β/NF-κB-mediated protective signaling in mice. Eur J Pharmacol . 2012;697(1–3):117–125. doi: 10.1016/j.ejphar.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 69.Li H, Huang K, Liu X et al. Lithium chloride suppresses colorectal cancer cell survival and proliferation through ROS/GSK-3beta/NF-kappaB signaling pathway. Oxid Med Cell Longev . 2014;2014:241864. doi: 10.1155/2014/241864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nassar A, Azab AN. Effects of lithium on inflammation. ACS Chem Neurosci . 2014;5(6):451–458. doi: 10.1021/cn500038f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Higgins GA, Allyn-Feuer A, Barbour E et al. A glutamatergic network mediate lithium response in bipolar disorder as defined by epigenome pathway analysis. Pharmacogemonics . 2015;16(14):1547–1563. doi: 10.2217/pgs.15.106. [DOI] [PubMed] [Google Scholar]
- 72.Szklarczyk D, Santos A, von Mering C et al. STITCH 5: augmenting protein-chemical interaction networks with tissue and affinity data. Nucleic Acids Res . 2016;44(D1):D380–D384. doi: 10.1093/nar/gkv1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao K. Can obesity in a major depressive episode be considered as a biomarker for bipolar spectrum disorders. Bipolar Disord . 2019;21(1):11–12. doi: 10.1111/bdi.12705. [DOI] [PubMed] [Google Scholar]
- 74.Guloksuz S, Cetin EA, Cetin T et al. Cytokine levels in euthymic bipolar patients. J Affect Disord . 2010;126(3):458–462. doi: 10.1016/j.jad.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 75.Hayashi Y, Nihonmatsu-Kikuchi N, Hisanaga S et al. Neuropathological similarities and differences between schizophrenia and bipolar disorder: a flow cytometric postmortem brain study. PLoS One . 2012;7(3):e33019. doi: 10.1371/journal.pone.0033019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brietzke E, Stertz L, Fernandes BS et al. Comparison of cytokine levels in depressed, manic and euthymic patients with bipolar disorder. J Affect Disord . 2009;116(3):214–217. doi: 10.1016/j.jad.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 77.Wieck A, Grassi-Oliveira R, do Prado CH et al. Differential neuroendocrine and immune responses to acute psychosocial stress in women with type 1 bipolar disorder. Brain Behav Immun . 2013;34:47–55. doi: 10.1016/j.bbi.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 78.do Prado CH, Rizzo LB, Wieck A et al. Reduced regulatory T cells are associated with higher levels of Th1/TH17 cytokines and activated MAPK in type 1 bipolar disorder. Psychoneuroendocrinology . 2013;38(5):667–676. doi: 10.1016/j.psyneuen.2012.08.005. PMID: 22989476. [DOI] [PubMed] [Google Scholar]
- 79.Bai Y, Liu T, Xu A et al. Comparison of common side effects from mood stabilizers and antipsychotics between pediatric and adult patients with bipolar disorder: a systematic review of randomized, double-blind, placebo-controlled trials. Expert Opin Drug Saf . 2019;18(8):703–717. doi: 10.1080/14740338.2019.1632832. [DOI] [PubMed] [Google Scholar]
References
- 1.Kaplan D, Smith D. Enzymatic amplification staining for flow cytometric analysis of cell surface molecules. Cytometry . 2000;40(1):81–85. doi: 10.1002/(sici)1097-0320(20000501)40:1<81::aid-cyto11>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan D, Husel W, Meyerson H. Immunophenotypic analysis with enhanced sensitivity of detection by enzymatic amplification staining. Clin Lab Med . 2001;21(4):763–778. [PubMed] [Google Scholar]
- 3.Kaplan D. Enzymatic amplification staining for single cell analysis: applied to in situ hybridization. J Immunol Methods . 2003;283(1–2):1–7. doi: 10.1016/j.jim.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan D, Meyerson H, Husel W et al. D cyclins in lymphocytes. Cytometry A . 2005;63(1):1–9. doi: 10.1002/cyto.a.20103. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan D. Enzymatic amplification staining for cell surface antigens. Curr Protoc Cytom . 2003;Chapter 6(Unit 6.14) doi: 10.1002/0471142956.cy0614s23. [DOI] [PubMed] [Google Scholar]
- 6.Lewandowska K, Kaplan D, Husel W. CD34 expression on platelets. Platelets . 2003;14(2):83–87. doi: 10.1080/0953710031000080577. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell WB, Pinheiro MP, Boulad N et al. Effect of thrombopoietin receptor agonists on the apoptotic profile of platelets in patients with chronic immune thrombocytopenia. Am J Hematol . 2014;89(12):E228–E234. doi: 10.1002/ajh.23832. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan D, Smith D, Meyerson H et al. CD5 expression by B lymphocytes and its regulation upon Epstein-Barr virus transformation. Proc Natl Acad Sci U S A . 2001;98(24):13850–13853. doi: 10.1073/pnas.241509398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan D, Kaye N, Liu F et al. The functional duality of HoxB4 in hematopoietic reconstituting cells. Cytometry A . 2013;83(1):127–133. doi: 10.1002/cyto.a.22059. [DOI] [PubMed] [Google Scholar]
- 10.Lazarus HM, Sommers SR, Arfons LM et al. Spontaneous autologous graft-versus-host disease in plasma cell myeloma autograft recipients: flow cytometric analysis of hematopoietic progenitor cell grafts. Biol Blood Marrow Transplant . 2011;17(7):970–978. doi: 10.1016/j.bbmt.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan D, Meyerson H, Lewandowska K. High resolution immunophenotypic analysis of chronic lymphocytic leukemic cells by enzymatic amplification staining. Am J Clin Pathol . 2001;116(3):429–436. doi: 10.1309/KXQ7-LHKC-CYQ8-R70W. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan D, Sun Z, Tallman MS et al. Prognostic information and biological insights in chronic lymphocytic leukemia by high-resolution immunophenotypic analysis of ZAP70. Cytometry A . 2014;85(9):798–808. doi: 10.1002/cyto.a.22485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyerson HJ, MacLennan G, Husel W et al. D cyclins in CD5+ B-cell lymphoproliferative disorders: cyclin D1 and cyclin D2 identify diagnostic groups and cyclin D1 correlates with ZAP-70 expression in chronic lymphocytic leukemia. Am J Clin Pathol . 2006;125:241–250. doi: 10.1309/7C2V-V961-P60R-MLHD. [DOI] [PubMed] [Google Scholar]
- 14.Gong S, Osei ES, Kaplan D et al. CD317 is over-expressed in B-cell chronic lymphocytic leukemia, but not B-cell acute lymphoblastic leukemia. Int J Clin Exp Pathol . 2015;8(2):1613–1621. [PMC free article] [PubMed] [Google Scholar]
- 15.Gao K, Tolliver BK, Kemp DE et al. Differential interactions between comorbid anxiety disorders and substance use disorder in rapid cycling bipolar I or II disorder. J Affect Disord . 2008;110:167–173. doi: 10.1016/j.jad.2007.12.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheehan DV. 2016 M.I.N.I. Mini International Neuropsychiatric Interview, version 7.02, for DSM-5. https://harmresearch.org/index.php/mini-international-neuropsychiatric-interview-mini/#How%20to%20Cite%20the%20MINI. [Google Scholar]
- 17.Spearing M, Post R, Leverich G et al. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry research . 1997;73(3):159–171. doi: 10.1016/s0165-1781(97)00123-6. [DOI] [PubMed] [Google Scholar]
- 18.Rush AJ, Trivedi MH, Ibrahim HM et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry . 2003;54(5):573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 19.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc . 1968;16(5):622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 20.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry . 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 21.Young R, Biggs J, Ziegler V et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry . 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 22.Guy W. Washington, DC: U.S. Government Printing Office; 1976. ECDEU Assessment Manuel for Psychopharmacology, Revised. [Google Scholar]
- 23.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol . 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 24.Snaith RP, Hamilton M, Morley S et al. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry . 1995;167(1):99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- 25.Leon A, Olfson M, Portera L et al. Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. Int J Psychiatry Med . 1997;27(2):93–105. doi: 10.2190/T8EM-C8YH-373N-1UWD. [DOI] [PubMed] [Google Scholar]
- 26.Endicott J, Nee J, Harrison W et al. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull . 1993;29(2):321–326. [PubMed] [Google Scholar]
- 27.Hartz A, Bentler S, Watson D. Measuring fatigue severity in primary care patients. J Psychosom Res . 2003;54:515–521. doi: 10.1016/s0022-3999(02)00600-1. [DOI] [PubMed] [Google Scholar]
- 28.Sobell LC, Sobell MB. New Jersey: Humana Press; 1996. Timeline Followback: a technique for assessing self-reported alcohol consumption; pp. 41–72. In: Litten RZ, Allen JP eds. Measuring alcohol consumption: psychosocial and biological methods. [Google Scholar]
- 29.Wisniewski SR, Rush AJ, Balasubramani GK et al. Self-rated global measure of the frequency, intensity, and burden of side effects. J Psychiatr Pract . 2006;12:71–79. doi: 10.1097/00131746-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Posner K, Oquendo MA, Gould M et al. Columbia Classification Algorithm of Suicide Assessment (C-CASA): classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry . 2007;164(7):1035–1043. doi: 10.1176/appi.ajp.164.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]


