Abstract
Inadequate response to antidepressant treatment, in a significant proportion of patients diagnosed with Major Depressive Disorder, contributes to the large burden of disability associated with the disease; thus, predicting treatment response is one of the most important challenge for clinicians who deal with depressed patients. The cytokine hypothesis of depression suggests that altered pheripheral cytokine levels are involved in the pathophysiology of depressive disorder and in modulating response to treatment. Present meta-analysis aimed to investigate the association between cytokine levels at baseline and response to antidepressant therapies. Authors performed a systematic search of PubMed and Embase databases for studies published between 2010 and January 2021: of 3345 identified records, 31 studies met the inclusion criteria for the qualitative synthesis, whereas 19 studies were eligible for quantitative analysis. Patients who failed to respond to antidepressant had aberrant inflammatory process, namely higher baseline levels of C-Reactive Protein and Interleukine-8, which is associated with treatment outcome in Major Depressive Disorder. Despite these promising results, further investigations are needed in order to replicate the data and to examine the potential role of inflammatory marker as a novel predictive tool for pharmacological treatment of depressive disorder.
Keywords: major depressive disorder, antidepressant response, inflammation, cytokines, inflammatory biomarker
Introduction
Major Depressive Disorder (MDD) is a chronic, disabling medical illness, that affects over 320 million people and represents one of the most common cause of disability worldwide.1 Despite the progress in psychopharmacology field, only 30% of patients achieve symptomatology remission with the first prescribed antidepressant and, to date, the selection of antidepressant medication is still based on a trial-and-error approach that could require several attempts.2 Treatment-resistant depression (TRD) is a complex phenomenon reflecting a variety of depressive subtypes, psychiatric comorbidity, need of adjunctive polypharmacotherapy,3–5 and coexisting medical illnesses;6 furthermore, it represents the highest direct and indirect medical costs among MDD patients: individuals with TRD are indeed twice as likely to be hospitalized and the cost of this hospitalization is more than six times the mean total cost for depressed patients who are not treatment-resistant.7
Many clinicians and researchers proposed that outcome of depression, such as other psychiatric disorder, could be improved by personalizing treatment with the use of biomarker.8–12 The basic definition of a biomarker is “a defined characteristic that is measured as an indicator of normal biological process, pathogenic process or response to an exposure or intervention” (FDA-NIH Biomarker Working Group). To be useful in clinical practice a biomarker should be non-invasive, easily measured, and inexpensive; moreover, it should be from a readily available source, such as blood or urine, and should have high sensitivity and specificity.13
Emerging evidence implicate aberrant inflammatory processes in the pathogenesis of MDD and mechanisms of antidepressant response.14 Cytokines mediate signals between immune cells, initiating and coordinating the cascade of immunological response. In the Central Nervous System (CNS), peripheral immune response is amplified by local inflammatory network, including cytokine production and inflammatory signal transduction pathways.15 Altered inflammation has been associated with monoamine dysregulation, with increased levels of pro-inflammatory cytokines inducing indolamine 2,3-dioxygenase (IDO) that converts tryptophan in kynurenine, thereby driving this essential aminoacid away from serotonin synthesis.16 Furthermore, a persistent up-regulation of pro-inflammatory cytokines also impact on neurotransmitter reuptake, activate the hypothalamic-pituitary-adrenal (HPA) axis and decrease neuroplasticity, contributing to the onset of depressive symptomatology.15,17 Patients with MDD have been shown to have increased levels of circulating cytokines; numerous studies have indeed reported increased levels of interleukin (IL)-1, IL-6, Tumor Necrosis Factor (TNF)-α, and C-reactive Protein (CRP) in serum/plasma and cerebrospinal fluid of depressed patients.18–20 On the other hand, anti-inflammatory cytokines, such as IL-4 and IL-10, were shown to be lower in patients diagnosed with MDD.21 Moreover, baseline inflammatory markers have been reported to be associated to antidepressant response; namely, higher value of pro-inflammatory cytokines were detected in serum/plasma of depressed patients who failed to respond to antidepressant medication.18,22,23
In light of the above, it is clear that the association between Major Depressive Disorder and altered peripheral cytokines levels have been examined in several studies and meta-analysis, but only very few meta-analysis have investigated the association between baseline cytokines levels and treatment outcome and no clear picture emerged.24,25 To date, the meta-analysis performed by Liu and colleagues is the most comprehensive. Liu’s working group highlighted that, despite so many cytokines have been investigated, most of the association were not significant and only levels of IL-8 were associated with antidepressant response.25 Given the large number of emerging studies investigating the possible effect of inflammatory markers on treatment response in patients with MDD, authors were of the opinion that an updated systematic search of literature with meta-analysis on the association between baseline cytokine levels and response to treatment in MDD is much warrant.
Materials and Methods
A literature search was conducted from PubMed and EmBase databases from 2010 to January 2021 using the combination of terms Cytokine, and it synonymous, AND Depressive Disorder, and its synonymous, AND Antidepressant, and its synonymous. Inclusion criteria were the following:
Study conducted on patients aged over 18 years old, diagnosed with Major Depressive Disorder according to the Diagnostic and Statistical Manual of Mental Disorder (DSM) or the International Classification of Disease (ICD);
Study including any kind of pre-treatment assessment of inflammatory biomarker;
Antidepressant medication must be continued for at least 4 weeks;
Use of standardized post-treatment symptom evaluation tool with a reported cut-off value dividing patients into responder and non-responder.
Studies conducted on bipolar patients and studies in which any anti-inflammatory treatment was prescribed were excluded from the analysis; furthermore, studies that not provide data of mean concentration of inflammatory biomarker at baseline and during the follow-up, for both group of patients, were excluded from the quantitative synthesis. The details of the search strategy are shown in Figure 1.
Figure 1.
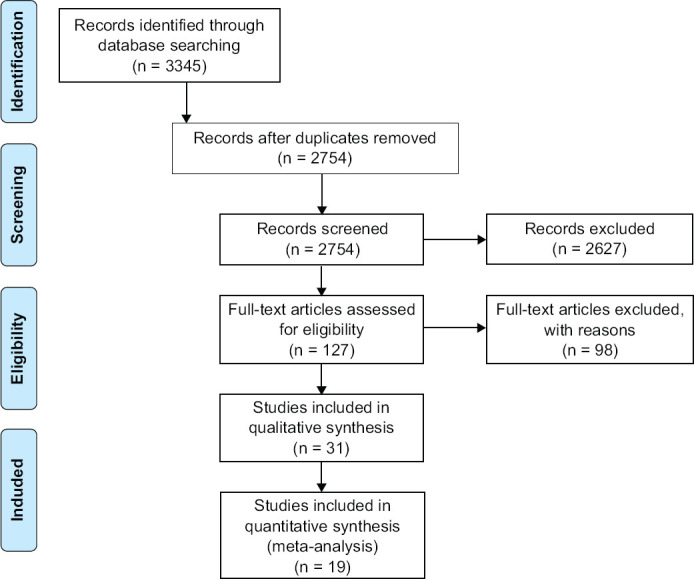
PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) Flow Chart
Data extracted include: means, standard deviation (SD) and sample size for both responder and non-responder, in addition to methodological and participants’ characteristics. When data were provided as log-transformed values, the raw scale was transformed according to Higgins and colleagues’ method one.26 When authors did not provide original data, they were also extracted from bar chart using Engauge Digitazier software.27
For the quality assessment of the studies included in the quantitative synthesis, authors adapted a quality tool referring to the Newcastle-Ottawa Scale for observational studies28 together with the Cochrane common classification scheme for bias. To assess the methodological quality of the included studies, the adapted quality tool contained the following parameters:
Sample size ≥ 40 (yes = 1; no = 0);
Severity of depression reported at baseline and at follow-up (yes = 1; no = 0);
Assessment for potential confounder (age, gender, smoking, body mass index) (for each confounder: yes = 1; no = 0. Maximum 4 points for this parameters);
Drug-naïve patients or patients who experienced a wash-out period (yes = 1; no = 0);
Attrition rate ≥ 20% (yes = 1; no = 0);
The need to use data extraction software (yes = 0; no = 1);
Detailed description of laboratory test and procedures used for the determination of inflammatory markers concentration (yes = 1; no = 0)
As can be deducted, the total quality score may vary from 0 to 10, with a higher score standing for a higher quality of the study.
Meta-analysis was conducted, using Review Manager Software (RevMan—Version 5.4), only when each cytokine was investigated in at least three studies. The effect size (ES) was provided estimating standard mean difference for each cytokine with a confidence interval (CI) of 95%. To assess heterogeneity across the studies, authors performed the Cochran Q test and quantified the heterogeneity with the I^2 statistic.29 An I^2 < 25% indicates a low grade of heterogeneity across studies, whereas an I^2 ranging from 25 to 75% was deemed to have a moderate grade of heterogeneity, and an I^2 > 75% indicates a high degree of heterogeneity. Whenever the heterogeneity across studies was low/moderate (< 50%), authors pooled the ES using a fixed-model effect, whereas for an I^2 > 50% the ES was calculated using a random-model effect. An ES of 0.2 was considered low, 0.5 moderate and 0.8 large.30 Publication bias were inspected checking funnel plot for asymmetry. A p-value < 0.05 was considered statistically significant.
Results
A total of 3345 studies were identified through databases searching as potentially eligible for the purpose of the present study. Articles were examined independently by two researchers and a third researcher was involved whenever there was a dissenting opinion among the main investigators. A total of 127 articles were identified as potentially eligible based on title and abstract; of these only 31 studies were retrieved for qualitative synthesis after full-text review, whereas 19 studies met the inclusion criteria for meta-analysis. Table 1 shows the main characteristics of the included studies; articles included in the meta-analysis are reported in bold (Table 1).
Table 1. Characteristic of the Included Studies.
| Studies | Sample Size | Markers | Response definition | Responder/Non-responder | Quality assessment | Results |
| Bot et al, 2011 | 149 (M=105; F=44) |
CRP, IL-6, TNFα | Reduction BDI-II ≥ 50% | 73/76 | 5 | No statistically significant differences |
| Fornaro et al, 2011 | 16 (M=4; F=12) |
IL-6 | Reduction HAMD-17 ≥ 50% | 9/7 | 6 | Higher in non-responder (p < 0.05) |
| Chang et al, 2012 | 149 (M=42; F=102) |
CRP | Reduction HAMD-21 ≥ 50% | 64/85 | - | Higher in non-responder (p = 0.020) |
| Fornaro et al, 2013 | 30 (M=8; F=22) |
IL-1β, IL-2, IL-4, IL-10, IL-12, INFγ, TNFα | Reduction HAMD-17 ≥ 50% | 12/18 | 6 | IL-1β higher in responder (p = 0.032) |
| Li et al, 2013 | 61 (M=11; F=50) |
TNFα | Reduction HAMD-17 ≥ 50% | 49/12 | 10 | Higher in non-responder (p < 0.05) |
| Yoshimura et al, 2013 | 118 (M=67; F=51) |
IL-6 | Reduction HAMD-17 ≥ 50% | 62/56 | 7 | Higher in responder (p = 0.031) |
| Uher et al, 2014 | 241 (M=15; F=226) |
CRP | Reduction MADRS ≥ 50% | 120/121 | - | Higher in non-responder to Escitalopram (p = 0.001) |
| Zoga et al, 2014 | 40 (M=0; F=40) |
CRP, TNFα, INFγ | Reduction HDRS ≥ 50% | 40/0 | - | No statistically significant differences |
| Brunoni et al, 2015 | 73 (M=32; F=41) |
IL-2, IL-4, IL-6, IL-10, IL-17, TNFα, INFγ | Reduction MADRS ≥ 50% | 34/39 | - | No statistically significant differences |
| Gupta et al, 2016 | 30 (M=15; F=15) |
TNFα | Reduction HAMD ≥ 50% or HAMD ≥ 7 | 25/5 | 4 | Higher in responder (p < 0.05) |
| Manoharan et al, 2016 | 73 (M=27; F=46) |
IL-6 | Reduction HAMD-17 ≥ 50% | 39/34 | 10 | No statistically significant differences |
| Myung et al, 2016 | 66 (M=16; F=50) |
Eotaxin, sCD40L, MCP-1, IL-8, TNFα | Reduction HAMD-17 ≥ 50% | 40/26 | 8 | No statistically significant differences |
| Ormstad et al, 2016 | 43 (M=8; F=35) |
CRP, IL-1, MIP-1, IL-6, IL-8, TNFα, GM-CSF | Reduction IDS > del 50% | 24/19 | 10 | IL-8 and GM-CSF higher in non-responder (p = 0.027 and 0.034 respectively) |
| Schimdt et al, 2016 | 30 (M=13; F=17) |
IL-2, IL-4, IL-5, IL-10, IL-12, IL-13, GM-CSF, INFγ, TNFα, CRP | Reduction HAMD-17 ≥ 50% | 15/15 | 9 | TNFα, INFγ, GM-CSF, IL-12, IL-10, IL-4, IL-2 higher in responder (all p < 0.05) |
| Gadad et al, 2017 | 94 (M=26; F=68) |
TNFα | QIDS-SR ≥ 8 | 47/47 | 4 | No statistically significant differences |
| Gupta et al, 2017 | 60 (M=25; F=35) |
TNFα | Reduction HAMD ≥ 50% or HAMD ≥ 7 | 54/6 | 5 | No statistically significant differences |
| Hasabe et al, 2017 | 58 (M=19; F=39) |
IL-6, CRP | Reduction MADRS ≥ 50% | 28/30 | 5 | Lower levels of IL-6 correlated with a better response (p = 0.029) |
| Jha et al, 2017 | 106 (M=32; F=74) |
CRP | QIDS-SR < 6 | 45/61 | - | Higher level in non-responder to SSRI monotherapy |
| Milenkovic et al, 2017 | 47 (M=23; F=24) |
Eotaxin1, Eotaxin3, MIP-1α, MIP-1β, MCP-1, MCP-4, MDC, IP-10, TARC | Reduction HAMD-17 ≥ 50% | 31/16 | 8 | No statistically significant differences |
| Mocking et al, 2017 | 70 (M=24; F=46) |
CPR | Reduction HDRS ≥ 50% | 48/22 | - | Higher levels in responder (p = 0.008) |
| Ricken et al, 2017 | 95 (M=38; F=57) |
IL-2, IL-4, IL-6, IL-8, IL-10, TNF-α, INF-γ, GM-CSF | Reduction HAMD-17 ≥ 50% | 43/52 | 5 | Higher levels of IL-2 in responder Higher levels of IL-6 in non-responder (all p < 0.05) |
| Brunoni et al, 2018 | 178 (M=60; F=118) |
IL-18, IL-33, IL-1β, IL-6, IL-10, TNFα | Reduction HDRS ≥ 50% | 84/94 | - | No statistically significant differences |
| Chen et al, 2018 | 91 (M=35; F=56) |
INFγ, TNFα, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, GM-CSF | HDRS-21 ≥ 7 | 44/47 | 6 | Higher levels of IL-4 in non-responder |
| Jha et al, 2018 | 166 (M=49; F=117) |
IL-17 | QIDS-SR < 6 | 83/83 | - | Higher level associated with a worse response to SSRI monotherapy |
| Reininghaus et al, 2018 | 87 (M=38; F=49) |
IL-6, CRP | BDI-II > 29 to BDI-II < 14 | 48/39 | 9 | No statistically significant differences |
| Carboni et al, 2019 | 210 (M=74; F=136) |
TNFα, IL-6, IL-10, CRP | Reduction HAMD ≥ 50% | 147/63 | - | Higher levels of IL-10 and TNFα in responder |
| Jun et al, 2019 | 75 (M=26; F=49) |
CRP | Reduction HAMD ≥ 50% | 59/16 | - | Higher levels in non-responder (p = 0.003) |
| Choi et al, 2020 | 1086 (M=490; F=596) |
CRP | HDRS ≥ 7 | 490/596 | 6 | Higher levels in non-responder (p = 0.0027) |
| Fond et al, 2020 | 139 (M=53; F=86) |
CRP | CDSS < 6 | 42/97 | - | No statistically significant differences |
| Jha et al, 2020 | 220 (M=75; F=145) |
CRP | HAMD-17 ≥ 7 | 84/136 | - | Higher levels in female non-responder |
| Kruse et al, 2020 | 40 (M=18; F=22) |
IL-8, IL-6, IL-10, TNFα, CRP | Reduction HAMD ≥ 50% | 20/20 | 5 | No statistically significant differences |
As is clear from Table 1, results from qualitative synthesis are inconclusive and often at odds with each other; moreover, many authors failed to demonstrate a statistically significant association between pre-treatment levels of inflammatory markers and response to antidepressant therapy. Results from meta-analysis are displayed in Table 2; statistically significant results are reported in bold.
Table 2. Meta-analysis of Studies Measuring Baseline Cytokine Levels in Responder and Non-responder.
| Citochina | Responder (N) | Non Responder (N) | ES (IC 95%) | p-value | I2(%) |
| IL-1β | 89 | 75 | 0.19 [−0.49, 0.86] | 0.64 | 74 |
| IL-2 | 152 | 155 | 0.48 [−0.07, 1.03] | 0.09 | 81 |
| IL-4 | 114 | 132 | –0.14 [−0.48, 0.20] | 0.41 | 38 |
| IL-6 | 374 | 362 | 0.02 [−0.13, 0.17] | 0.79 | 1 |
| IL-8 | 151 | 144 | –0.27 [−0.49, −0.05] | 0.02 | 10 |
| IL-10 | 134 | 152 | –0.00 [−0.24, 0.23] | 0.98 | 29 |
| TNFα | 380 | 282 | –0.03 [−0.33, 0.27] | 0.83 | 66 |
| INFγ | 110 | 136 | –0.06 [−0.31, 0.19] | 0.64 | 45 |
| PCR | 698 | 795 | –0.18 [−0.28, –0.08] | 0.0006 | 0 |
| GM-CSF | 123 | 137 | 0.09 [−0.16, 0.33] | 0.48 | 0 |
Four studies investigated the possible role of IL-1β as a predictor of treatment response, of which three studies are eligible for the quantitative synthesis. Fornaro and his colleagues observed higher levels of IL-1β in patients showing a better response to antidepressant treatment (p = 0.032); furthermore, they highlighted an IL-1β/IL-10 ratio lower in non-responder (2.32 ± 0.92 vs 1.73 ± 0.58; p = 0.040), suggesting that an altered Th1/Th2 ratio could be responsible, to some extent, of the variability observed in response to antidepressant medication.31 Similar results were reported in a recent study by Chen and co-authors.32 Baseline IL-1β levels did not differ between responder (n = 89) and non-responder (n = 75) across the include studies (ES = 0.19 [−0.49, 0.86]; p = 0.64).
Baseline IL-2 levels were investigated in five studies, of which four were included in meta-analysis comprising 152 responders and 155 non-responders. Although some authors demonstrated higher levels of IL-2 in those patients with a better response to therapy,32,33 from meta-analysis no statistically significant differences emerged (ES = 0.48 [−0.07, 1.03]; p = 0.09).
The possible association between IL-4 baseline levels and response to antidepressant were investigated has been explored in 5 studies. The data in literature are inconclusive, with some authors demonstrating higher levels of IL-4 in responders33 in striking contrast with other authors who have pointed higher baseline levels in those patients who failed to respond to antidepressant treatment.32 Across the four studies included in meta-analysis, no statistically significant difference between the two groups emerged (ES = −0.14 [−0.48, 0.20]; p = 0.41).
IL-6 is one of the most investigated cytokines in MDD, being subject to several studies:
of the 13 studies included in the qualitative analysis, 10 were selected for meta-analysis, for a total of 736 patients (M = 359; F = 377), of which 374 responders and 362 non-responders. Although several studies have highlighted how non-responder patients have significantly higher IL-6 levels than responders,34,35 and how lower values of this cytokine are associated with a better response to antidepressant therapy,36 the data currently present in the literature do not allow to draw unambiguous conclusions. Indeed, from quantitative analysis of the data available no statistically significant differences between the two groups of patients emerged (ES = 0.02 [−0.13, 0.17]; p = 0.79).
Five studies evaluated baseline plasma levels of IL-8 in a total of 335 patients (M = 115; F = 220), including 171 responders and 164 non-responders.
MDD patients who showed a worse treatment response at the endpoint had significantly higher levels of IL-8 at baseline, with a moderate effect size (ES = −0.27 [−0.49, −0.05]; p = 0.02). Heterogeneity across the included studies was low (I^2 = 10%), and visual inspection of funnel plot shows a quite symmetrical distribution of studies, thus allowing to exclude important publication bias (Figure 2).
Figure 2.
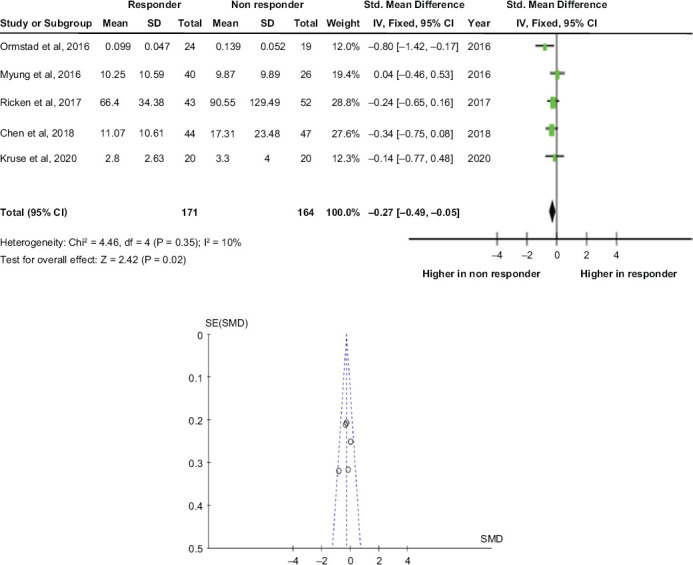
Baseline IL-8 and Treatment Response: Forest Plot and Funnel Plot
In a study, conducted on 30 patients with depression, Schmidt and collaborators demonstrated that patients responding to antidepressant therapy had higher baseline levels of IL-10 than non-responding patients (p = 0.001).33 This result seems to be confirmed by a recent study, conducted on 210 patients from two randomized placebo-controlled clinical studies, in which the authors showed a better response to antidepressant therapy in the group of patients who had higher baseline levels of IL-10 (p = 0.009).37 Despite these promising results, quantitative analysis did no shows any significant differences between the two group of patients (ES = −0.00 [−0.24, 0.23]; p = 0.98).
Many authors found no statistically significant differences between baseline TNFα levels in responder and non-responder patients. In two studies, on the other hand, higher values were found in patients who had a good response to antidepressant therapy,33,38 a result that seems to be confirmed by the evidence of a positive correlation between baseline TNFα levels and response to antidepressant therapy.37 The potential role of TNFα as a predictor of response to antidepressant was investigated in 11 studies, on a total of 662 patients (M = 292; F = 370) including 380 responders and 282 non-responders: no statistically significant differences between the two groups of patients was observed (ES = −0.03 [−0.33, 0.27]; p = 0.83).
For what concern the role of INFγ, it was investigated six studies, of which four met the inclusion criteria for the meta-analysis. Plasma concentrations of INFγ were evaluated on a total of 246 patients (M = 84; F = 162) of which 110 showed a satisfactory response to antidepressant therapy and 136 were considered non-responsive to therapy. the quantitative analysis of the included studies did not show statistically significant differences between the two groups of patients (ES = −0.06 [−0.31, 0.19]; p = 0.64).
Several authors, especially in recent years, have focused on the study of C-reactive Protein (CRP) as a possible predictor of response to antidepressant therapy in patients with MDD. From the qualitative analysis, it is clear that different research groups have shown higher baseline CRP values in non-responder patients.39–41 In greater detail, Uher and collaborators showed that high baseline CRP values were associated with a worse response to Escitalopram compared to patients treated with Nortriptyline.42 This finding seems to be confirmed by a subsequent study, which found that higher CRP values were associated with a worse response to SSRI monotherapy than to SSRI + Bupropion combination.43 In a subsequent study, Jha and collaborators highlighted how gender is an important factor in modulating the association between baseline CRP values and response to antidepressant therapy. In fact, according to what the authors reported, high CRP values would be associated with a worse response to antidepressant therapy only in females, while in males there would be an inversion of this trend.44 In contrast to the results described so far, Mocking’s working group, in a study conducted on 70 patients with DDM, found higher CRP values in responder patients than in non-responders.45
Seven studies were included in meta-analysis: baseline CRP levels were assessed on a total of 1493 patients (M = 691; F = 802), including 698 responders and 795 non-responders. From quantitative analysis a statistically significant association emerged between the baseline CRP values and response to antidepressant therapy, with higher values in non-responder patients, with a small effect size (ES = −0.18 [−0.28, −0.08]; p = 0.0006). No heterogeneity across studies was observed (I^2 = 0%) and funnel plot shows no evidence of publication bias.
Currently available data on the possible role of GM-CSF as a marker response antidepressant response are inconclusive and in contrast to each other. While some authors have found higher levels of this cytokine in non-responder patients,46 other have highlighted how patients who were subsequent responder have higher baseline levels of GM-CSF than patients who do not have an adequate response.33 Baseline GM-CSF levels were assessed on a total of 260 patients with depression (M = 94; F = 166), of which 123 responders and 137 non-responders: no statistical association emerged from meta-analysis (ES = 0.09 [−0.16, 0.33]; p = 0.48).
From qualitative synthesis, a correlation between elevated levels of IL-12 and a better response to antidepressant therapy emerged, as highlighted in study conducted by Schmidt’s working group.32 Furthermore, in a recent study conducted on 166 patients with DDM, authors showed that elevated baseline levels of IL-17 are associated with a worse response to monotherapy with SSRI, but with a better response to the combination of SSRI and Bupropion.47 No differences of baseline levels of Eotaxin, sCD40L, MCP-1, MIP-1, IL-13, MCP-4, MDC, IP-10, TARC, IL-18, IL-33, and IL-5 were observed between responders and non-responders.32,46,48–50
Discussion
As previously stated, this review with meta-analysis was conducted with the aim of investigating the existence of a possible correlation between circulating levels of inflammatory cytokines and antidepressant response, for the purpose of identifying a possible predictive marker of treatment response that could guide the clinician in the prescription of a personalized therapy for the single individual. It was found that patients with DDM, responsive to antidepressant therapy, have lower baseline levels of IL-8 and CRP than non-responders. These results confirm, to some extent, Liu and collaborators findings: the authors have indeed found higher circulating levels of IL-8 in patients with inadequate response to conventional antidepressant therapies, but did not find any significant differences in baseline CRP levels.25
IL-8 is a well-known chemotactic factor for neutrophils which, at the peripheral level, is produced by monocytes, lymphocytes and endothelial cells, while at the central level it is released mainly by microglia cells. The exact function of IL-8 is not yet fully known: it seems to have both pro-inflammatory and anti-inflammatory action depending on its concentration. High concentrations of IL-8 would inhibit the infiltration of neutrophils into the site of infection, thus exerting an anti-inflammatory effect. This dual function could explain, at least to some extent, the inconsistency of the association found between its circulating levels and the depressive disorder.51 In a recent study on 40 DDM patients, higher baseline IL-8 values have been associated with a worse treatment outcome only in female patients.52 This gender difference could be traced to the increased secretion of IL-8 due to estradiol which seems to stimulate the production of this cytokine in human immature dendritic cell cultures.53
C-Reactive Protein is an acute phase protein whose production by liver cells is induced by some pro-inflammatory cytokines, especially IL-6, in response to infections, inflammatory processes and tissue damage. The association between higher CRP and MDD has been documented by several authors.54,55 In particular, CRP was found to be elevated in patients with treatment-resistant depression and in patients who had suffered trauma in childhood;19 furthermore, elevated CRP values seem to correlate with a prevalence of vegetative symptoms, such as psychomotor slowdown and insomnia.56 As previously pointed out, the analysis of the available data revealed a statistically significant association between baseline levels of CRP and antidepressant response, with higher values found in non-responders. Specifically, several authors have highlighted how elevated CRP values are associated with a worse response to therapy with selective serotonin reuptake inhibitors.20,42,44 The effects of inflammatory markers on serotonin transport and tryptophan metabolism, through the activation of the IDO enzyme, could at least partially explain the resistance to SSRIs observed by the aforementioned authors. Jha’s working group recently highlighted how the response to antidepressant therapy is negatively influenced by baseline CRP values only in female patients.44 Gender differences in the immune system have been known for some time and are due to both genetic and hormonal factors. Female subjects generally have higher levels of inflammatory markers than men;57 moreover, in women there is an imbalance of the immune response towards Th2 response, contrary to what happens in the male sex in which the Th1/Th2 ratio is unbalanced towards Th1 response. SSRIs reduce the production of cytokines produced by Th2 lymphocytes, while drugs with noradrenergic action inhibit the Th1 mediated response.58 This antidepressant drugs effect could explain, at least in some extent, the gender differences between baseline CRP levels and treatment outcome.
Compared to the plasma IL-8 assay, CRP quantification has some advantages, including lower cost, greater sample stability both at room temperature and refrigerated, shorter reporting time and less laborious laboratory procedures. CRP, even more than IL-8, could therefore represent a pragmatic clinical marker as it is easily available, cheap, relatively stable and its dosage is not influenced by either the time of collection or food intake. In the light of the above, it seems legitimate to think that CRP could represent a valid tool to help clinicians choosing the most appropriate antidepressant therapy for each patient. Although the results obtained so far are promising, further studies are needed in order to test the replicability of the data, also extending the investigation to other classes of antidepressants and, hopefully, also evaluating whether high values of the inflammatory marker may somehow influence the tolerability of drugs.
There are several limitations in this study. First of all, due to small number of studies included in each analysis, it is not possible to exclude the possibility of publication bias and small study-effect, despite the inspection of funnel plot. Furthermore, the quality of the included studies varies significantly and many studies were at high/moderate risk of bias.
Conclusions
The theme of the personalization of psychopharmacological therapy is as alive as ever in recent years, and succeeding in identifying an easily assessable marker, represents the main objective to which future studies should focus on in future studies, trying to ensure the patient more tailored solutions and therefore more effective and safe therapies, also reducing healthcare costs.59 Although the results obtained so far are promising, the scientific community still appears far from identifying such a parameter. It also seems reasonable to believe that future studies, rather than looking for single predictive markers of non-response, should be aimed at finding combinations of markers that take consideration the wide heterogeneity of Major Depressive Disorder.
References
- 1.Depression and other common mental disorders: global health estimates. Geneva. 2017 WHO. [Google Scholar]
- 2.Rush AJ et al. An evaluation of the Quick Inventory of Depressive Symptomatology and the Hamilton Rating Scale for Depression: A sequenced treatment alternatives to relieve depression trial report. Biol Psychiatry . 2006;54:493–501. doi: 10.1016/j.biopsych.2005.08.022. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diurni M, Baranzini F, Costantini C, Poloni N, Vender S, Callegari C. Effetti metabolici dei farmaci antipsicotici di seconda generazione in pazienti drug-naïve: uno studio preliminare [Metabolic side effects of second generation antipsychotics in drug-naïve patients: a preliminary study] Riv Psichiatr . 2009;44(3):176–8. Italian. PMID: 20066804. [PubMed] [Google Scholar]
- 4.Ostuzzi G, Mazzi MA, Terlizzi S, Bertolini F, Aguglia A, Bartoli F, Bortolaso P, Callegari C, Caroleo M, Carrà G, Corbo M, D’Agostino A, Gastaldon C, Lucii C, Magliocco F, Martinotti G, Nosé M, Ostinelli EG, Papola D, Piccinelli MP ... STAR Network Investigators. Factors associated with first-versus second-generation long-acting antipsychotics prescribed under ordinary clinical practice in Italy. PloS One . 2018;13(8):e0201371. doi: 10.1371/journal.pone.0201371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poloni N, Ielmini M, Caselli I, Lucca G, Gasparini A, Gasparini A, Lorenzoli G, Callegari C. Oral Antipsychotic Versus Long-Acting Injections Antipsychotic in Schizophrenia Spectrum Disorder: a Mirror Analysis in a Real-World Clinical Setting. Psychopharmacol Bull . 2019;49(2):17–27. PMID: 31308579; PMCID: PMC6598776. [PMC free article] [PubMed] [Google Scholar]
- 6.Gaynes BN et al. Defining treatment-resistant depression. Depress Anxiety . 2019:1–12. doi: 10.3390/biom10060947. doi: [DOI] [PubMed] [Google Scholar]
- 7.Ivanova JI et al. Direct and indirect costs of employees with treatment-resistant and non-treatment-resistant major depressive disorder. Current Medical Research and Opinion . 2010;26(10):2475–2484. doi: 10.1185/03007995.2010.517716. [DOI] [PubMed] [Google Scholar]
- 8.Simon GE, Perlis RH. Personalized medicine for depression: can we match patients with treatment? Am J Psychiatry . 2010;167:1445–1455. doi: 10.1176/appi.ajp.2010.09111680. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ielmini M et al. The utility of pharmacogenetic testing to support the treatment of bipolar disorder. Pharmacogenom. Personal Med . 2018;11:35–42. doi: 10.2147/PGPM.S160967. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pagani R et al. Twenty years of Lithium pharmacogenetics: A systematic review. 2019:42–50. doi: 10.1016/j.psychres.2019.05.036. DOI: [DOI] [PubMed] [Google Scholar]
- 11.Callegari C, Isella C, Caselli I, Poloni N, Ielmini M. Pharmacogenetic Tests in Reducing Accesses to Emergency Services and Days of Hospitalization in Bipolar Disorder: A 2-Year Mirror Analysis. J Pers Med . 2019;9(2):22. doi: 10.3390/jpm9020022. doi: PMID: 31052247; PMCID: PMC6617043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ielmini M. Pharmacogenetics: The Perspective of a Routine Use of Pharmacogenetic Testing in Psychiatric Clinical Practice. Psychiatry Research . 2021;305 doi: 10.1016/j.psychres.2021.114236. n. pag. Web. [DOI] [PubMed] [Google Scholar]
- 13.Bennet MR, Devarajan P. Charles L. Edelstein - Academic Press; 2011. Chapter 1 - Characteristics of an ideal biomarker of kidney disease. [Google Scholar]
- 14.Miller AH, Maletiv V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry . 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience . 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reininghaus B et al. Changes in the tryptophan-kynurenine axis in association to therapuetic response in clinically depressed patients undergoing psychiatric rehabilitation. Psychoneuroendocrinology . 2018;94:25–30. doi: 10.1016/j.neuroscience.2013.04.060. doi: [DOI] [PubMed] [Google Scholar]
- 17.Lichtblau N et al. Cytokines as a biomarkers in depressive disorder: current standing and prospects. Int Rev of Psychiatry . 2013;25:592–603. doi: 10.3109/09540261.2013.813442. [DOI] [PubMed] [Google Scholar]
- 18.Dowlati Y et al. A meta-analysis of cytokines in major depression. 201(67):446–457. doi: 10.3109/09540261.2013.813442. doi: [DOI] [PubMed] [Google Scholar]
- 19.Howren, Lamkin DM, Suls J. Association of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med . 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. doi: [DOI] [PubMed] [Google Scholar]
- 20.Chamberlain SR et al. Treatment-resistant depression and peripheral C-reactive protein. The British Journal of Psychiatry. 2019;214:11–19. doi: 10.1192/bjp.2018.66. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiles SA et al. A meta-analysis of differences in IL-6 and IL-10 between people with and without depression: exploring the causes of heterogeneity. Brain Behav Immun . 2012;26:1180–1188. doi: 10.1016/j.bbi.2012.06.001i. DOI: [DOI] [PubMed] [Google Scholar]
- 22.Yoshimura R et al. Higher plasma interleukin-6 (IL-6) is associated with SSRI- or SNRI-refractory depression. Prog Neuro-Psychopharmacol Biol Psychiatry . 2009;33:722–726. doi: 10.1016/j.pnpbp.2009.03.020. DOI: [DOI] [PubMed] [Google Scholar]
- 23.Lanquillon S et al. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology . 2000;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. DOI: [DOI] [PubMed] [Google Scholar]
- 24.Strawbridge R et al. Inflammation and clinical response to treatment in depression: a meta-analysis. Eur Neuropsychopharmacol . 2015;25:1532–1543. doi: 10.1016/j.euroneuro.2015.06.007. DOI: [DOI] [PubMed] [Google Scholar]
- 25.Liu JJ et al. Peripheral cytokine levels and response to antidepressant treatment in depression: a systematic review and meta-analysis. Molecular Psychiatry . 2019 doi: 10.1038/s41380-019-0474-5. doi: [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP et al. Measuring inconsistency in meta-analysis. BMJ . 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell M, Muftakhidinov B, Winchen T. Enagauge Digitizer Software. n.d. http://markummitchell.github.io/engauge-digitizer. [Google Scholar]
- 28.Stang A. Critical evaluation of the Newcastle-Ottawa Scale for the assessment of the quality of nonrandomized studies in meta-analysis. Eur J Epidemiol . 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, White IR, Anzures-Cabrera J. Meta-analysis of skewd data: combining results reported on log-transformed or raw scale. Stat Med . 2008;27:6072–6092. doi: 10.1002/sim.3427. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen J. A power primer. Psychol Bull . 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. DOI: [DOI] [PubMed] [Google Scholar]
- 31.Fornaro M et al. Might different cytokine trends in depressed patients receiving duloxetine indicate differential biological background. Journal of Affective Disorders . 2013;145:300–307. doi: 10.1016/j.jad.2012.08.007. DOI: [DOI] [PubMed] [Google Scholar]
- 32.Chen CY et al. Differences in immunomodulatory properties between venlafaxine and paroxetine in patients with major depressive disorder. Psychoneuroendocrinology . 2018:108–118. doi: 10.1016/j.bbi.2011.07.239. doi: [DOI] [PubMed] [Google Scholar]
- 33.Schmidt FM et al. Pro- and anti-inflammatory cytokines, but not CRP, are inversely correlated with severity and symptoms of major depression. Psychiatry Research . 2016 doi: 10.1016/j.psychres.2016.02.052. doi: [DOI] [PubMed] [Google Scholar]
- 34.Ricken R et al. Cytokine serum levels remain unchanged during lithium augmentation of antidepressant in major depression. Journal of Psychiatric Researc . 2017 doi: 10.1016/j.jpsychires.2017.10.002. DOI: [DOI] [PubMed] [Google Scholar]
- 35.Fornaro M et al. Increase in IL-6 levels among major depressive disorder patients after 6-week treatment with duloxetine 60 mg/die: a preliminary observation. Neuropsychiatric Disease and Treatment . 2011;7:51–56. doi: 10.1016/j.jad.2012.08.007. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasabe K et al. Adjunctive N-acetylcysteine in depression: exploration of interleukin-6, C-reactive protein and brain-derived neurotrophic factor. Acta Neuropsychiatrica . 2017 doi: 10.1017/neu.2017.2. DOI: [DOI] [PubMed] [Google Scholar]
- 37.Carboni L et al. Biomarkers for response in major depression: comparing paroxetine and venlafaxine from two randomised palcebo-controlled clinical studies. Translational Psychiatry . 2019;9:182. doi: 10.1038/s41398-019-0521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta R et al. Effect of Mirtazapine Treatment on Serum levels of Brain-Derived Neurotrophic Factor and Tumor Necrosis Factor- in Patients of Major Depressive Disorder with Severe Depression. Psychopharmacology . 2016;97:184–188. doi: 10.1159/000444220. doi: [DOI] [PubMed] [Google Scholar]
- 39.Chang HH et al. Treatment response and cognitive impairment in major depression: Association with C-reactive protein. Brain, Behavior, and Immunity. 2012:90–95. doi: 10.1016/j.bbi.2011.07.239. doi: [DOI] [PubMed] [Google Scholar]
- 40.Jun Z et al. Baseline sereum C-reactive protein levels may predict antidepressant treatment response in patients with major depressive disorder. Journal of Affective Disorder . 2019;250:432–438. doi: 10.1016/j.jad.2019.03.001. doi: [DOI] [PubMed] [Google Scholar]
- 41.Choi W et al. Interactive effects of systemic inflammation and life stressors on treatment response of depressive disorders. Brain, Behavior, and Immunity . Brain Sci . 20202021:12. doi: 10.1016/j.bbi.2021.01.029. doi: [DOI] [PubMed] [Google Scholar]
- 42.Uher R et al. An Inflammatory Biomarker as a Differential Predictor of Outcome of Depression Treatment with Escitalopram and Nortriptyline. Am J Psychiatry . 2014;171:1278–1286. doi: 10.1176/appi.ajp.2014.14010094. [DOI] [PubMed] [Google Scholar]
- 43.Jha MK et al. Can C-Reactive Protein Inform Antidepressant Medication Selection in Depressed Outpatients? Findings from the CO-MED Trial. Psychoneuroendocrinology . 2018;78:105–113. doi: 10.1016/j.psyneuen.2017.01.023. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jha MK et al. Sex differences in the association of baseline c-reactive protein (CRP) and acute-phase treatment outcomes in major depressive disorder: Findings from the EMBARC study. J Psychiatr Res . 2019;113:165–171. doi: 10.1016/j.jpsychires.2019.03.013. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mocking RJT et al. Biological profiling of prospective antidepressant response in major depressive disorder: Association with (neuro)inflammation, fatty acid metabolism, and amygdala-reactivity. Psychoneuroendocrinology . 2017;79:84–92. doi: 10.1016/j.psyneuen.2017.02.019. doi: [DOI] [PubMed] [Google Scholar]
- 46.Ormstad H et al. Increased plasma levels of competing amino acids, rather than lowered plasma tryptophan levels, are associated with non-response to treatment in major depression. European Neuropsychopharmacology . 2016 doi: 10.1016/j.euroneuro.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Jha MK et al. Interleukin 17 Selectively Predicts Better outcomes with Bupropion-SSRI Combination: Novel T Cell Biomarker for Antidepressant Medication Selection. Brain Behav Immun . 2017;66:103–110. doi: 10.1016/j.bbi.2017.07.005. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milenkovic VM et al. Macrophage-Derived Chemokine: A Putative Marker of Pharmacological Therapy Response in Major Depression? Neuroimmunomodulation . 2017 doi: 10.1159/000479739. DOI: [DOI] [PubMed] [Google Scholar]
- 49.Brunoni AR et al. Plasma biomarkers in a placebo-controlled trial comparing tDCS and escitalopram efficacy in major depression. Progress in Neuropsychopharmacology & Biological Psychiatry . 2018:211–217. doi: 10.1016/j.pnpbp.2018.06.003. doi: [DOI] [PubMed] [Google Scholar]
- 50.Myung W et al. Serum Cytokine Levels in Major Depressive Disorder and Its Role in Antidepressant Response. Psychiatry Investig . 2016;13(6):644–651. doi: 10.4306/pi.2016.13.6.644. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lehto SM et al. Serum chemokine levels in major depressive disorder. Psychoneuroendocrinology . 2010;35:226–232. doi: 10.1016/j.psyneuen.2009.06.007. DOI: [DOI] [PubMed] [Google Scholar]
- 52.Kruse JL et al. Inflammation and depression treatment response to electroconvulsive therapy: Sex-specicific role of interleukin-8. Brain, Behavior, and Immunity . 2020 doi: 10.1016/j.bbi.2020.05.069. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bengtsson AK et al. 17beta-estradiol (E2) modulates cytokine and chemokine expression in human monocyte-derived dendritic cells. Blood . 2004;104(5):1404–1410. doi: 10.1182/blood-2003-10-3380. DOI: [DOI] [PubMed] [Google Scholar]
- 54.Cepeda MS, Stang P, Makadia R. Depression is associated with high levels of C-Reactive Protein and low levels of Fractional Exhaled Nitric Oxide: results from the 2007–2012 National Health and Nutrition Examination Surveys. J Clin Psychiatry . 2016;77:12. doi: 10.4088/JCP.15m10267. DOI: [DOI] [PubMed] [Google Scholar]
- 55.Raison CL, Miller AH. Is Depression an Inflammatory Disorder? Curr Psychiatry Rep . 2011;13(6):467–475. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duivis HE et al. Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation: findings of the Netherlands Study of Depression and Anxiety (NESDA) Psychoneuroendocrinology . 2013;38(9):1573–1585. doi: 10.1016/j.psyneuen.2013.01.002. DOI: [DOI] [PubMed] [Google Scholar]
- 57.Klein SL, Flanagan KL. Sex differences in immune response. Nature Reviews Immunology . 2016;16:626. doi: 10.1038/nri.2016.90. DOI: [DOI] [PubMed] [Google Scholar]
- 58.Martino M et al. Immunomodulation mechanism of antidepressant: interactions between serotonin/norephinephrine balance and Th1/Th2 balance. Curr Neuropharmacol . Brain Sci . 2012;2021;10:97–123. 12. doi: 10.2174/157015912800604542. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Panella L, Volontè L, Poloni N, Caserta A, Ielmini M, Caselli I, Lucca G, Callegari C. Pharmacogenetic Testing in Acute and Chronic Pain: A Preliminary Study. Medicina (Kaunas). 2019;55(5):147. Brain Sci . 2021;12 doi: 10.3390/medicina55050147. doi: PMID: 31100953; PMCID: PMC6572509. [DOI] [PMC free article] [PubMed] [Google Scholar]


