Summary
Background
Drug resistance threatens global tuberculosis control. We aimed to examine mortality in patients with tuberculosis from high-burden countries, according to concordance or discordance of results from drug susceptibility testing done locally and whole-genome sequencing (WGS).
Methods
In this multicentre cohort study, we collected pulmonary Mycobacterium tuberculosis isolates and clinical data from individuals with tuberculosis from antiretroviral therapy programmes and tuberculosis clinics in Côte d’Ivoire, Democratic Republic of the Congo, Kenya, Nigeria, Peru, South Africa, and Thailand, stratified by HIV status and drug resistance. Sites tested drug susceptibility using routinely available methods. WGS was done on Illumina HiSeq 2500 in the USA and Switzerland, and TBprofiler was used to analyse the genomes. We included individuals aged 16 years or older with pulmonary tuberculosis (bacteriologically confirmed or clinically diagnosed). We analysed mortality in multivariable logistic regression models adjusted for sex, age, HIV status, history of tuberculosis, and sputum positivity.
Findings
Between Sept 1, 2014, and July 4, 2016, of 634 patients included in our previous analysis, we included 582 patients with tuberculosis (median age 33 years [IQR 27–43], 225 [39%] women, and 247 [42%] HIV-positive). Based on WGS, 339 (58%) isolates were pan-susceptible, 35 (6%) monoresistant, 146 (25%) multidrug-resistant, and 24 (4%) pre-extensively drug-resistant (pre-XDR) or XDR. The analysis of mortality was based on 530 patients; 63 (12%) died and 77 (15%) patients received inappropriate treatment. Mortality ranged from 6% (18 of 310) in patients with pan-susceptible tuberculosis to 39% (nine of 23) in patients with pre-XDR or XDR tuberculosis. The adjusted odds ratio for mortality was 4·92 (95% CI 2·47–9·78) among undertreated patients, compared with appropriately treated patients.
Interpretation
In seven countries with a high burden of tuberculosis, we observed discrepancies between drug resistance patterns obtained locally and WGS. The underdiagnosis of drug resistance resulted in inappropriate treatment and higher mortality. WGS can provide accurate and detailed drug resistance information required to improve the outcomes of drug-resistant tuberculosis in high-burden settings. Our results support WHO’s call for point-of-care tests based on WGS.
Funding
National Institutes of Allergy and Infectious Diseases, Swiss National Science Foundation, and Swiss National Center for Mycobacteria.
Introduction
Tuberculosis is caused by bacteria of the Mycobacterium tuberculosis complex and is the leading cause of death by a single infectious agent worldwide.1 In 2019, ten million people were estimated to have developed active tuberculosis, of whom 8% also had HIV. In the same year, around 1·2 million people died from tuberculosis, including 208 000 people with HIV.1 Tuberculosis accounts for approximately 40% of HIV and AIDS-related adult deaths, and half of these remain undiagnosed.2
The emergence of drug-resistant M tuberculosis strains threatens tuberculosis control. In 2019, 3% of new tuberculosis cases worldwide were estimated to be multidrug-resistant (MDR) tuberculosis, and 18% of individuals who had been previously treated had MDR tuberculosis.1 People with HIV are at greater risk of acquiring MDR tuberculosis than people who are HIV-negative.3 Also, treatment outcomes in people with HIV and MDR tuberculosis are worse than among HIV-negative patients with MDR tuberculosis.3 Pre-extensively drug-resistant (pre-XDR) or XDR tuberculosis poses additional challenges for treatment and control of the disease.4 Strategies to control and prevent drug-resistant tuberculosis include surveillance, rapid drug susceptibility testing, and ensuring the completion of an appropriate treatment regimen. The limited access to detailed drug susceptibility testing and effective second-line anti-tuberculosis drugs, insufficient adherence and drug dosages, and comorbidities challenge the management of drug-resistant tuberculosis in low-income and middle- income countries.2,5–7
The present study is part of a research programme investigating drug-resistant tuberculosis of the International epidemiology Databases to Evaluate AIDS.8 In a previous analysis, we compared the results of drug susceptibility testing from high-burden countries in Africa, Asia, and Latin America with phenotypic drug susceptibility testing results from the Swiss National Center for Mycobacteria.9 We found that the accuracy of testing done at participating sites was moderate, and that discordant results and inappropriate treatment were associated with increased mortality. The Swiss reference laboratory tested drug resistance to six drugs only: isoniazid, rifampicin, pyrazinamide, ethambutol, amikacin, and moxifloxacin. Therefore, other resistances could have been missed, including resistance to streptomycin, kanamycin, ethionamide, levofloxacin, or newer drugs.
Whole-genome sequencing (WGS) can simultaneously provide information on resistance to first-line and second-line drugs, for which drug-resistance-conferring mutations are known. WGS has the potential to overcome many of the limitations of conventional drug susceptibility testing with higher throughput.10 We and others showed that drug susceptibility predicted from M tuberculosis genomes correlates with phenotypic drug susceptibility testing.11,12 WHO recommends WGS for drug resistance surveillance and is evaluating sequencing technologies for routine drug susceptibility testing. 1,13 Here, we aimed to compare the drug resistance patterns routinely obtained in seven countries with a high tuberculosis burden with the results from WGS, and examined the mortality associated with discordant resistance profiles using WGS as the reference.
Methods
Study design and participants
We did a multicentre cohort study. As described in detail elsewhere, 9 we recruited patients from antiretroviral therapy programmes and tuberculosis clinics in their corresponding catchment areas in Côte d’Ivoire, Democratic Republic of the Congo, Kenya, Nigeria, Peru, South Africa, and Thailand. In South Africa, we used strain collections held at the University of Cape Town (Cape Town, South Africa). All patients had bacteriologically confirmed, or clinically diagnosed tuberculosis. We included individuals aged 16 years or older with pulmonary tuberculosis. We excluded patients for whom no viable isolate was available, patients with extrapulmonary tuberculosis only, patients with missing data that were necessary for the analyses, and patients for whom the M tuberculosis genome could not be sequenced (appendix p 2). Recruitment was stratified by HIV status and drug resistance as defined at local clinics. We collected demographic and clinical characteristics of participants using a standardised questionnaire. M tuberculosis isolates were subcultured at the recruitment sites.
The Cantonal Ethics Committee in Bern, Switzerland, and local institutional review boards approved the study. Written informed consent was obtained at all sites, except in South Africa, where consent was not required for the use of archived samples.
Procedures
The local laboratories tested molecular or phenotypic drug susceptibility according to routine procedures. DNA was extracted from isolates using standard protocols.14 Libraries were prepared using the Illumina Nextera XT kit (Illumina, San Diego, CA, USA) and sequenced on Illumina HiSeq 2500 at the Department of Biosystems Science and Engineering of the Swiss Federal Institute of Technology in Basel, Switzerland and the Broad Institute in Cambridge, MA, USA. Sequences had 101, 138, or 151 bp paired-end reads. After Illumina adaptors were clipped and low-quality reads trimmed with Trimmomatic, version 0.38, reads shorter than 36 bp were excluded. The minimum read depth at each position was 10 × in 99% of the genome (IQR 99–99, range 77–100; seven genomes were less than 90%). BCFtools, version 1.11 mpileup was used to map the reads to the H37Rv reference genome. We included reads with a minimum mapping quality of eight. We screened one isolate per patient for anti-tuberculosis drug resistance mutations using the TBprofiler, version 2.8.2 pipeline.10,15 The pipeline aligns reads to the reference genome using BWA, version 0.7.17 and calls variants with SAMtools, version 1.9.10,16–18 The variants were then compared to a drug resistance database. Single-nucleotide polymorphisms, insertions, and deletions responsible for resistance to 19 anti-tuberculosis drugs were identified:10,15,19 streptomycin, para-aminosalicylic acid, isoniazid, pyrazinamide, cycloserine, kanamycin, ethionamide, ethambutol, amikacin, rifampicin, capreomycin, ofloxacin, ciprofloxacin, moxafloxacin, levofloxacin, linezolid, bedaquiline, clofazimine, and delamanid. A coverage of ten reads was needed to call a polymorphism. We considered all drug resistance alleles with a variant frequency equal to or higher than 90%.
WHO defines monoresistance as resistance to one of the first-line drugs (ie, isoniazid, pyrazinamide, ethambutol, and rifampicin).1,13 MDR tuberculosis is defined as resistance to both isoniazid and rifampicin. Pre-XDR tuberculosis is defined as resistance to isoniazid and rifampicin plus fluoroquinolones or one of the three second-line injectable drugs (ie, amikacin, ciprofloxacin, or kanamycin). XDR tuberculosis is defined as drug resistance against isoniazid, rifampicin, fluoroquinolones, and at least one of the three second-line injectable drugs.
We compared the drug resistance profiles obtained at sites using routine drug susceptibility testing to drug resistance patterns obtained from whole-genome sequences. We considered any drug resistance obtained from the tests that a patient underwent locally. Drug resistance profiles were defined as concordant or discordant according to the resistance categories defined by WHO.1 Discordant results were further categorised into discordant results potentially leading to undertreatment, or potentially leading to overtreatment (appendix p 6).1,13 Discordances with no clear implications for treatment were defined as other discordances. We assessed the appropriateness of prescribed anti- tuberculosis treatment according to WHO guidelines (appendix p 7).1,13 Effective drugs were defined as drugs to which no drug-resistance-conferring mutations were observed in WGS (appendix p 8). The prescription of less than three effective drugs was defined as undertreatment, except for patients with isoniazid-resistant or rifampicin- resistant isolates. In these patients, a regimen comprising fewer than four effective drugs was considered as undertreatment, according to WHO guidelines. Overtreatment included second-line drugs given to patients for whom first-line regimens would have been appropriate. The classification of regimens is shown in the appendix (p 11).
Statistical analysis
We used descriptive statistics for patient characteristics by levels of drug resistance based on WGS. We compared the following drug resistance categories: pan-susceptible tuberculosis, monoresistant tuberculosis (any monoresistance), MDR tuberculosis, pre-XDR or XDR tuberculosis, any isoniazid-resistant tuberculosis (including isoniazid-monoresistant, MDR, and pre-XDR or XDR tuberculosis), any rifampicin-resistant tuberculosis (including rifampicin-monoresistant, MDR, and pre-XDR or XDR tuberculosis). Patients with missing data for treatment regimen, treatment outcome, ongoing treatment, or sputum microscopy were excluded from the analysis of mortality.
Four logistic regression models were calculated to assess the effects of: any drug resistance; drug resistance categories; discordant diagnoses; and treatment appropriateness on mortality. Logistic regression models were adjusted for sex, age, HIV status, history of tuberculosis, and sputum positivity. The country of origin was included as a random effect on the intercept.20 We did three sensitivity analyses. First, we repeated all logistic regression analyses after restricting the data to drug resistances that could be diagnosed with the locally available tests. We thus excluded drug resistances that were missed due to unavailable testing methods. Second, we repeated the logistic regression for mortality by treatment appro priateness, excluding patients with pre-XDR or XDR tuberculosis. Third, we examined the effect of different variant frequency cutoffs on each logistic regression (≥0% and 100%). All analyses were done in R, version 3.6.1, or Python, version 3.7.6.21,22
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Between Sept 1, 2014, and July 4, 2016, of the 634 patients included in our previous analysis,9 we were unable to sequence 52 (8%) isolates due to poor bacterial growth, DNA quality, or failures in the library preparation (appendix p 2). We therefore included 582 patients with tuberculosis, 406 (70%) from Africa, 93 (16%) from Latin America, and 83 (14%) from Asia. 172 (30%) patients came from South Africa, 94 (16%) from Côte d’Ivoire, 93 (16%) from Peru, 83 (14%) from Thailand, 59 (10%) from Democratic Republic of the Congo, 53 (9%) from Nigeria, and 28 (5%) from Kenya (table 1). The median age was 33 years (IQR 27–43), 225 (39%) were women, and 247 (42%) were HIV-positive. Six M tuberculosis lineages were represented: 24 (4%) cases of L1, 135 (23%) L2, 18 (3%) L3, 403 (69%) L4, one (<1%) L5, and one (<1%) L6.
Table 1:
Patient characteristics by resistance profiles obtained by whole-genome sequencing
| Pan-susceptible | Any resistance | p value | Monoresistance |
Polyresistance |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Isoniazid | Pyrazinamide | Ethambutol | Rifampicin | All | Multidrug-resistant | Pre-XDR or XDR | Other | ||||
| Total | 339 | 243 | ·· | 35 | 8 | 2 | 1 | 24 | 208 | 146 | 24 | 38 |
| Sex | ·· | ·· | 0·99 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
| Women | 131 (39%) | 94 (39%) | ·· | 10 (29%) | 3 (38%) | 0 (0%) | 1 (100%) | 6 (25%) | 84 (40%) | 56 (38%) | 13 (54%) | 15 (39%) |
| Men | 208 (61%) | 149 (61%) | ·· | 25 (71%) | 5 (63%) | 2 (100%) | 0 | 18 (75%) | 124 (60%) | 90 (62%) | 11 (46%) | 23 (61%) |
| Age, years | ·· | ·· | 0·0067 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| At diagnosis | 35 (28–45) | 32 (25–40) | ·· | 32 (25–40) | 40 (31–49) | 26 (25–28) | 36 (36–36) | 29 (25–39) | 32 (26–40) | 31 (25–39) | 30 (25–34) | 36 (29–44) |
| HIV status | ·· | ·· | <0·0001 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
| HIV-negative | 169 (50%) | 166 (68%) | ·· | 23 (66%) | 7 (88%) | 1 (50%) | 0 | 15 (63%) | 143 (69%) | 103 (71%) | 14 (58%) | 26 (68%) |
| HIV-positive | 170 (50%) | 77 (32%) | ·· | 12 (34%) | 1 (13%) | 1 (50%) | 1 (100%) | 9 (38%) | 65 (31%) | 43 (29%) | 10 (42%) | 12 (32%) |
| Mycobacterium tuberculosis lineage | ·· | ·· | 0·039 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
| L1 | 18 (5%) | 6 (2%) | ·· | 2 (6%) | 0 | 0 | 0 | 2 (8%) | 4 (2%) | 1 (1%) | 0 | 3 (8%) |
| L2 | 79 (23%) | 56 (23%) | ·· | 7 (20%) | 3 (38%) | 1 (50%) | 0 | 3 (13%) | 49 (24%) | 23 (16%) | 8 (33%) | 18 (47%) |
| L3 | 15 (4%) | 3 (1%) | ·· | 0 | 0 | 0 | 0 | 0 | 3 (1%) | 2 (1%) | 1 (4%) | 0 |
| L4 | 225 (66%) | 178 (73%) | ·· | 26 (74%) | 5 (63%) | 1 (50%) | 1 (100%) | 19 (79%) | 152 (73%) | 120 (82%) | 15 (63%) | 17 (45%) |
| L5 | 1 (<1%) | 0 | ·· | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| L6 | 1 (<1%) | 0 | ·· | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Country | ·· | ·· | <0·0003 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | |
| Côte d’Ivoire | 46 (14%) | 48 (20%) | ·· | 5 (14%) | 2 (25%) | 0 | 1 (100%) | 2 (8%) | 43 (21%) | 39 (27%) | 3 (13%) | 1 (3%) |
| Democratic Republic of the Congo | 29 (9%) | 30 (12%) | ·· | 1 (3%) | 0 | 0 | 0 | 1 (4%) | 29 (14%) | 19 (13%) | 8 (33%) | 2 (5%) |
| Kenya | 21 (6%) | 7 (3%) | ·· | 1 (3%) | 1 (13%) | 0 | 0 | 0 | 6 (3%) | 5 (3%) | 0 | 1 (3%) |
| Nigeria | 19 (6%) | 34 (14%) | ·· | 6 (17%) | 0 | 0 | 0 | 6 (25%) | 28 (13%) | 20 (14%) | 4 (17%) | 4 (11%) |
| Peru | 57 (17%) | 36 (15%) | ·· | 2 (6%) | 2 (25%) | 0 | 0 | 0 | 34 (16%) | 28 (19%) | 2 (8%) | 4 (11%) |
| South Africa | 111 (33%) | 61 (25%) | ·· | 15 (43%) | 0 | 1 (50%) | 0 | 14 (58%) | 46 (22%) | 28 (19%) | 7 (29%) | 11 (29%) |
| Thailand | 56 (17%) | 27 (11%) | ·· | 5 (14%) | 3 (38%) | 1 (50%) | 0 | 1 (4%) | 22 (11%) | 7 (5%) | 0 | 15 (39%) |
| History of tuberculosis | ·· | ·· | <0·0001 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
| No | 269 (79%) | 104 (43%) | ·· | 13 (37%) | 7 (88%) | 1 (50%) | 1 (100%) | 4 (17%) | 91 (44%) | 56 (38%) | 5 (21%) | 30 (79%) |
| Yes | 70 (21%) | 139 (57%) | ·· | 22 (63%) | 1 (13%) | 1 (50%) | 0 | 20 (83%) | 117 (56%) | 90 (62%) | 19 (79%) | 8 (21%) |
| Treatment outcomes | ·· | ·· | <0·0001 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
| Success | 248 (73%) | 129 (53%) | ·· | 16 (46%) | 5 (63%) | 0 | 1 (100%) | 10 (42%) | 113 (54%) | 76 (52%) | 11 (46%) | 26 (68%) |
| Mortality | 19 (6%) | 45 (19%) | ·· | 6 (17%) | 1 (13%) | 1 (50%) | 0 | 4 (17%) | 39 (19%) | 24 (16%) | 9 (38%) | 6 (16%) |
| Treatment failure | 11 (3%) | 10 (4%) | ·· | 3 (9%) | 0 | 1 (50%) | 0 | 2 (8%) | 7 (3%) | 5 (3%) | 2 (8%) | 0 |
| Lost to follow-up | 26 (8%) | 29 (12%) | ·· | 5 (14%) | 0 | 0 | 0 | 5 (21%) | 24 (12%) | 22 (15%) | 0 | 2 (5%) |
| Transfer | 13 (4%) | 15 (6%) | 2 (6%) | 0 | 0 | 0 | 2 (8%) | 13 (6%) | 10 (7%) | 2 (8%) | 1 (3%) | |
| Ongoing, unknown | 22 (6%) | 15 (6%) | ·· | 3 (9%) | 2 (25%) | 0 | 0 | 1 (4%) | 12 (6%) | 9 (6%) | 0 | 3 (8%) |
| Sputum | ·· | ·· | 0·089 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
| Positive | 264 (78%) | 205 (84%) | ·· | 25 (71%) | 7 (88%) | 1 (50%) | 1 (100%) | 16 (67%) | 180 (87%) | 129 (88%) | 17 (71%) | 34 (89%) |
| Negative | 68 (20%) | 36 (15%) | ·· | 10 (29%) | 1 (13%) | 1 (50%) | 0 | 8 (33%) | 26 (13%) | 17 (12%) | 6 (25%) | 3 (8%) |
Data are n (%) or median (IQR). p values show the difference between pan-susceptible and any resistance, obtained with the χ2 test (L5 and L6 were excluded and for age the t test was used). The category other included the following drug resistances: cycloserine (n=1); ethionamide (n=5); streptomycin (n=9); ethambutol and rifampicin (n=1); ethambutol and streptomycin (n=1); isoniazid and ethionamide (n=14); isoniazid and pyrazinamide (n=1); isoniazid and streptomycin (n=1); ethambutol, isoniazid, and streptomycin (n=1); isoniazid, ethionamide, and streptomycin (n=1); rifampicin, pyrazinamide, streptomycin, and ethionamide (n=1); isoniazid, levofloxacin, moxifloxacin, ofloxacin, para-aminosalicylic acid, and ciprofloxacin (n=1); ethambutol, rifampicin, levofloxacin, moxifloxacin, ofloxacin, ciprofloxacin, and streptomycin (n=1). XDR=extensively drug-resistant. Due to rounding, some group percentage totals are more than 100%.
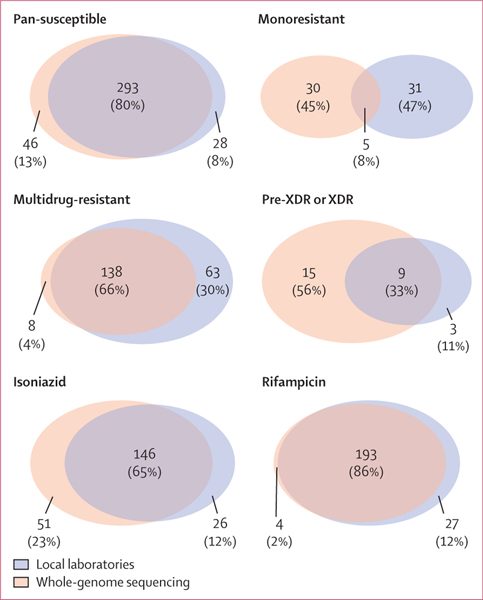
Based on WGS, 339 (58%) isolates were pan-susceptible and 35 (6%) were monoresistant: 24 rifampicin, eight isoniazid, two pyrazinamide, and one ethambutol mono- resistant isolates. There were 208 (36%) polyresistant isolates, including 146 (25%) MDR, 24 (4%) pre-XDR or XDR isolates, and 38 (7%) other types of polyresistances (table 1; figure 1). Among the 24 patients with pre-XDR or XDR, nine had resistance to fluoroquinolones, six to injectable drugs, and nine to both.
Figure 1: Distribution of diagnosed drug resistance between whole-genome sequencing and local drug susceptibility testing.
The categories include pan-susceptible, monoresistant (any monoresistance), multidrug-resistant, pre-XDR or XDR, any isoniazid-resistant, or any rifampicin-resistant tuberculosis. Due to rounding, some group percentage totals are more than 100%. XDR=extensively drug-resistant.
Local drug susceptibility testing results were based on the molecular Xpert MTB/RIF test system, line probe assays, and culture-based phenotypic tests, or a combination of these methods (table 2). Among the 582 isolates, 130 (22%) of 582 had discordant drug resistance results when comparing local drug susceptibility testing with WGS. 65 (11%) discordant drug resistance results potentially led to inappropriate treatment of patients with tuberculosis (table 2). We then looked at the regimens prescribed to patients. For six patients, we had no treatment information. Of 576 patients with known treatment, we observed that overall 86 (15%) of 576 patients received inappropriate treatment according to WGS results and WHO treatment guidelines: 67 (12%) of 576 patients were undertreated, and 19 (3%) were overtreated. Consequently, 490 (85%) patients were appropriately treated.
Table 2:
Drug resistance results from whole-genome sequencing and local testing by diagnosis concordance and potential consequences for treatment
| Drug resistance |
n (%) | Local drug susceptibility test diagnosis method |
||||
|---|---|---|---|---|---|---|
| Based on whole-genome sequencing | Based on local tests | Xpert MTB/RIF* | Culture | Line probe assay | Combination of tests | |
| Concordance between resistance patterns | ||||||
| Total | ·· | 452 (100%) | 242/452 (54%) | 195/452 (43%) | 60/452 (13%) | 102/452 (23%) |
| Pan-susceptible | Pan-susceptible | 293 (65%) | 196 | 139 | 49 | 53 |
| Monoresistant (3 isoniazid, 2 rifampicin) | 3 isoniazid, 2 rifampicin | 5 (1%) | 0 | 4 | 1 | 0 |
| MDR | MDR | 138 (31%) | 45 | 44 | 8 | 44 |
| Pre-XDR or XDR | Pre-XDR or XDR | 9 (2%) | 1 | 1 | 2 | 5 |
| Other (7 streptomycin) | 7 streptomycin | 7 (2%) | 0 | 7 | 0 | 0 |
| Discordance between resistance patterns | ||||||
| Total | ·· | 130 (100%) | 35/130 (27%) | 55/130 (42%) | 9/130 (7%) | 46/130 (35%) |
| Potentially leading to undertreatment | ·· | 34 (26%) | 17/130 (13%) | 12/130 (9%) | 1/130 (1%) | 1/130 (1%) |
| Pan-susceptible | ·· | 0 | 0 | 0 | 0 | 0 |
| Monoresistant (3 isoniazid) | 3 pan-susceptible | 3 (2%) | 2 | 1 | 0 | 0 |
| MDR | 3 pan-susceptible, 1 streptomycin-ethambutol | 4 (3%) | 2 | 1 | 0 | 1 |
| Pre-XDR or XDR | 15 MDR | 15 (12%) | 5 | 6 | 0 | 4 |
| Other (10 isoniazid-ethionamide, 1 isoniazid-streptomycin, 1 isoniazid-ethionamide-streptomycin) | 12 pan-susceptible | 12 (9%) | 8 | 4 | 0 | 0 |
| Potentially leading to overtreatment | ·· | 31 (24%) | 3/130 (2%) | 12/130 (9%) | 2/130 (2%) | 14/130 (11%) |
| Pan-susceptible | 1 isoniazid, 18 MDR, 4 rifampicin | 23 (18%) | 3 | 9 | 2 | 9 |
| Monoresistant (2 isoniazid) | 2 MDR | 2 (2%) | 0 | 2 | 0 | 0 |
| MDR | 3 Pre-XDR or XDR | 3 (2%) | 0 | 0 | 0 | 3 |
| Pre-XDR or XDR | ·· | 0 | 0 | 0 | 0 | 0 |
| Other (1 isoniazid-ethionamide, 1 isoniazid-pyrazinamide, 1 isoniazid-ethambutol-streptomycin) | 3 MDR | 3 (2%) | 0 | 1 | 0 | 2 |
| Other discordance | ·· | 65 (50%) | 15/130 (12%) | 31/130 (9%) | 6/130 (1%) | 27/130 (4%) |
| Pan-susceptible | 20 ethambutol, monoresistant†, streptomycin | 23 (18%) | 2 | 20 | 0 | 1 |
| Monoresistant (1 ethambutol, 2 pyrazinamide, 22 rifampicin) | 1 pan-susceptible, 2 pan-susceptible, 22 MDR | 25 (19%) | 6 | 1 | 1 | 20 |
| MDR | 1 rifampicin | 1 (1%) | 0 | 0 | 1 | 0 |
| Pre-XDR or XDR | ·· | 0 | 0 | 0 | 0 | 0 |
| Other‡ | 16 (12%) | 7 | 10 | 5 | 6 | |
| 1 cycloserine, 5 ethionamide 2 streptomycin |
1 pan-susceptible 5 pan-susceptible 1 pan-susceptible, 1 streptomycin-ethambutol |
|||||
| 3 isoniazid-ethionamide 1 isoniazid-levofloxacin-moxifloxacin-ofloxacin-para-aminosalicylic acid-ciprofloxacin |
3 isoniazid 1 isoniazid |
|||||
| 1 ethambutol-rifampicin-levofloxacin-moxifloxacinofloxacin-ciprofloxacin-streptomycin | 1 streptomycin | |||||
| 1 ethambutol-rifampicin 1 ethambutol-streptomycin 1 rifampicin-pyrazinamide-streptomycin-ethionamide |
1 MDR 1 MDR 1 MDR |
|||||
MDR=multidrug-resistant. XDR=extensively drug-resistant.
Rifampicin resistance diagnosed with Xpert MTB/RIF was classified as MDR.
Exact monoresistance is not known.
The agreement between local drug susceptibility testing and WGS was 80% for pan-susceptible, 8% for monoresistant, 66% for MDR, and 33% for pre-XDR or XDR tuberculosis (figure 1). Agreement of local drug susceptibility testing and WGS for rifampicin resistance was 86% and it was 65% for isoniazid resistance. Rifampicin resistance was, in contrast to other drug resistance, more frequently diagnosed with local drug susceptibility testing than with WGS (figure 1). Only three sites tested for drugs other than rifampicin and isoniazid. Two sites tested for streptomycin, two for fluoroquinolones, and two for injectable drugs. One site tested for pyrazinamide and one site for ethambutol. Resistance to pyrazinamide, cycloserine, ethambutol, linezolid, bedaquiline, clofazimine, and delamanid was not tested at any site. WGS did not identify any resistance to bedaquiline, clofazimine, or delamanid (appendix p 8).
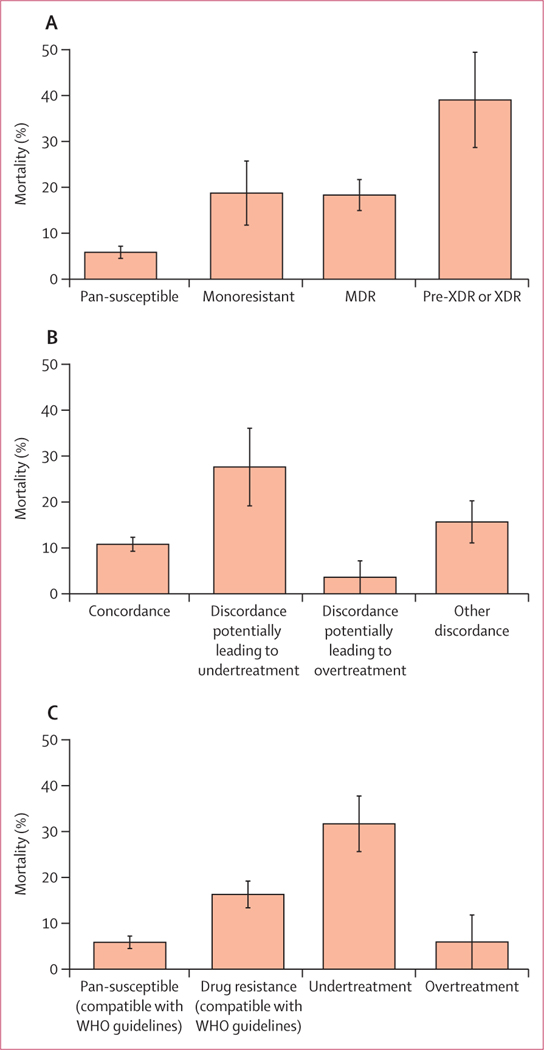
We excluded 52 (9%) of 582 patients from the mortality analyses due to missing data (appendix p 2). Based on WGS, the isolates of 310 (58%) of 530 patients were pan-susceptible, 32 (6%) monoresistant, 131 (25%) MDR, 23 (4%) pre-XDR or XDR, and 34 (6%) other polyresistances. Among the 530 patients, 121 (23%) had discordant drug susceptibility testing results. For 29 (66%) of 44 patients, underdiagnosis of drug resistance potentially led to undertreatment, and for 28 (36%) of 77, overdiagnosis potentially led to overtreatment. During treatment, 63 (12%) of 530 patients died (table 3). Mortality was 6% (18 of 310) in patients with pan-susceptible tuberculosis, 19% (six of 32) in patients with monoresistant tuberculosis, and 18% (24 of 131) in patients with MDR tuberculosis. Patients with pre-XDR or XDR tuberculosis had a mortality of 39% (nine of 23; figure 2A). Overall, mortality ranged from 6% (16 of 267) among patients with pan-susceptible strains and concordant diagnosis to 47% (seven of 15) among patients with pre-XDR or XDR tuberculosis and a discordant diagnosis potentially leading to undertreatment (table 3). In patients with a discordant diagnosis potentially leading to undert- reatment, mortality was 28% (eight of 29), and in patients with a discordant diagnosis potentially leading to overtreatment, it was 4% (one of 28; figure 2B). Mortality ranged from 6% (17 of 293) in patients with pan-susceptible tuberculosis treated according to WHO guidelines to 32% (19 of 60) in undertreated patients and 6% (one of 17) in patients who were overtreated (figure 2C).
Table 3:
Mortality by concordance of local diagnosis and whole-genome sequencing
| Total | Concordant with diagnosis at sites | Discordant with diagnosis at sites |
||||
|---|---|---|---|---|---|---|
| Any discordance | Potentially leading to undertreatment | Potentially leading to overtreatment | Other discordance | |||
| Resistance based on whole-genome sequencing | 63/530 (12%) | 44/409 (11%) | 19/121 (16%) | 8/30 (27%) | 1/28 (4%) | 10/63 (16%) |
| Pan-susceptible | 18/310 (6%) | 16/267 (6%) | 2/43 (5%) | 0/0 | 0/20 | 2/23 (9%) |
| Any resistance | 45/220 (20%) | 28/142 (20%) | 17/78 (22%) | 8/30 (27%) | 1/8 (13%) | 8/40 (20%) |
| Monoresistance | 6/32 (19%) | 2/4 (50%) | 4/28 (14%) | 0/1 | 0/2 | 4/25 (16%) |
| Isoniazid | 1/6 (17%) | 1/3 (33%) | 0/3 | 0/1 | 0/2 | 0/0 |
| Pyrazinamide | 1/2 (50%) | 0/0 | 1/2 (50%) | 0/0 | 0/0 | 1/2 (50%) |
| Ethambutol | 0/1 | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Rifampicin | 4/23 (17%) | 1/1 (100%) | 3/22 (14%) | 0/0 | 0/0 | 3/22 (14%) |
| Polyresistance | 39/188 (21%) | 26/138 (19%) | 13/50 (26%) | 8/29 (28%) | 1/6 (17%) | 4/15 (27%) |
| Multidrug resistance | 24/131 (18%) | 23/123 (19%) | 1/8 (13%) | 1/4 (25%) | 0/3 | 0/1 |
| Pre-XDR or XDR | 9/23 (39%) | 2/8 (25%) | 7/15 (47%) | 7/15 (47%) | 0/0 | 0/0 |
| Other | 6/34 (18%) | 1/7 (14%) | 5/27 (19%) | 0/9 | 1/3 (33%) | 4/15 (27%) |
Analysis based on 530 patients with complete data. The category other discordance includes the following drug resistances: cycloserine (n=1); ethionamide (n=5); streptomycin (n=9); ethambutol and rifampicin (n=1); isoniazid and ethionamide (n=12); isoniazid and pyrazinamide (n=1); ethambutol, isoniazid, and streptomycin (n=1); ethambutol, isoniazid, and streptomycin (n=1); rifampicin, pyrazinamide, streptomycin, and ethionamide (n=1); isoniazid, levofloxacin, moxifloxacin, ofloxacin, para-aminosalicylic acid, and ciprofloxacin (n=1); and ethambutol, rifampicin, levofloxacin, moxifloxacin, ofloxacin, ciprofloxacin, and streptomycin (n=1). XDR=extensively drug-resistant.
Figure 2: Mortality according to drug resistance, concordance of diagnosis, and treatment appropriateness.
Mortality data are shown based on drug resistance (A), concordance of diagnosis (B), and treatment appropriateness (C). Appropriateness was considered according to WHO guidelines (appendix pp 6–7). Error bars are SEs. Analysis based on 530 patients with complete data. Mortality was calculated by dividing deaths by the number of patients in the respective category.
MDR=multidrug-resistant. XDR=extensively drug-resistant.
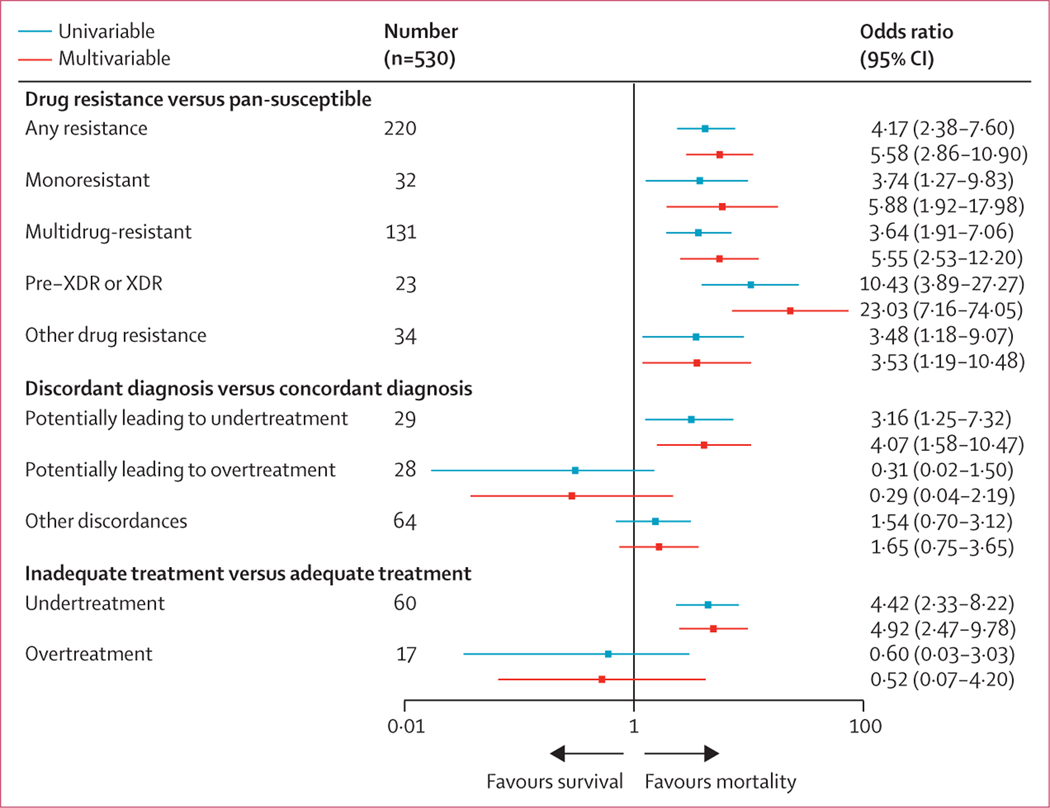
In the multivariable logistic regression, resistance to any of the anti-tuberculosis drugs was associated with higher mortality (figure 3). The adjusted odds ratio (OR) was 5·58 (95% CI 2·86–10·90). The association with mortality became stronger with a higher degree of drug resistance. Compared with pan-susceptible tuberculosis, the adjusted OR for monoresistant was 5·88 (95% CI 1·92–17·98), for MDR was 5·55 (2·53–12·20), and for pre-XDR or XDR tuberculosis was 23·03 (7·16–74·05; figure 3). The adjusted OR for mortality during tuberculosis treatment was 4·07 (95% CI 1·58–10·47) in patients with a diagnosis potentially leading to undertreatment, and 0·29 (0·04–2·19) in the case of a diagnosis potentially leading to overtreatment, compared with patients with appropriate treatment (figure 3). Overall, 77 (15%) of 530 patients received inappropriate treatment based on WGS drug resistance results and WHO guidelines (appendix p 7). 60 (11%) of 530 patients were undertreated, and 17 (3%) of 530 were overtreated. The OR for mortality for undertreatment was 4·92 (95% CI 2·47–9·78), and for overtreatment was 0·52 (0·07–4·20), compared with patients receiving appropriate treatment (figure 3). In a sensitivity analysis, we showed that mortality among undertreated patients remained higher than among appropriately treated patients after excluding patients with pre-XDR or XDR tuberculosis (adjusted OR 5·97 [95% CI 2·58–13·80]). The unadjusted covariate ORs for mortality during tuberculosis treatment are shown in the appendix (p 13). The sensitivity analysis of the logistic regression models using different variant frequency cutoffs (≥0% and 100%) produced similar results (appendix pp 3–4). When restricting the analysis to drug resistances that could be diagnosed at sites, again similar results were obtained (appendix p 5).
Figure 3: Logistic regression models to assess the effect of any drug resistance, drug resistance categories, diagnosis discordance, and treatment appropriateness on mortality.
The models were adjusted for sex, age, HIV status, history of tuberculosis, and sputum microscopy, and country of participating site was included as random effect on the intercept. Appropriateness was considered according to WHO guidelines (appendix pp 6–7). XDR=extensively drug-resistant.
Discussion
In this multicentre cohort study, we compared drug resistance predicted by WGS with the results from local drug susceptibility testing in seven countries with a high burden of tuberculosis. We examined mortality by drug resistance predicted by WGS, and by concordance or discordance with local diagnosis and the appropriateness of treatment. We found that the diagnosis was discordant between local drug resistance results and WGS in about one in five patients. The agreement between local and centralised WGS was the highest for rifampicin and isoniazid, but low for other drugs. Of note, resistance to streptomycin, para-aminosalicylic acid, pyrazinamide, cycloserine, ethionamide, ethambutol, fluoroquinolones, and injectable drugs was rarely investigated locally. Mortality during treatment ranged from 6% among patients with pan-susceptible strains and concordant results between WGS and local drug resistance testing to 47% among patients with pre-XDR or XDR tuberculosis and discordant results.
To our knowledge, this is the first study to compare the results from drug susceptibility testing in real-world settings in high-burden countries with WGS and to examine the effect of discordant resistance results on mortality. In a previous analysis of this cohort, we compared the results from local drug susceptibility testing with those obtained at the Swiss National Center for Mycobacteria for six drugs.9 In the present study, we used a well established bioinformatics pipeline and its corresponding database to analyse the WGS data.10 The analysis covered 19 anti-tuberculosis drugs, including streptomycin, kanamycin, pyrazinamide, ethionamide, ethambutol, and levofloxacin, as well as newer drugs. Specifically, we were able to detect more single-drug resistance with WGS than with phenotypic drug susceptibility testing.
Rapid and accurate diagnosis, prompt and appropriate treatment, and the control of airborne infection are key strategies to prevent drug-resistant tuberculosis 23 Routine testing at sites focused mainly on the identification of rifampicin and isoniazid resistance used to diagnose MDR tuberculosis and did not address the efficacy of other drugs. Also, isoniazid monoresistance would typically be missed if drug susceptibility testing relies on the Xpert MTB/RIF system, which could lead to the undertreatment of some patients. Furthermore, culture-based drug susceptibility testing is challenging for several drugs—eg, pyrazinamide, ethionamide, and ethambutol— due to poor drug solubility.11,24 Yet, pyrazinamide is essential for shortening tuberculosis therapy, and resistance to pyrazinamide is associated with worse outcomes.23 However, pyrazinamide resistance testing is often unavailable. Only one site could test pyrazinamide resistance in our study.
WGS has the potential to predict resistance profiles for most anti-tuberculosis drugs without the need for time- consuming phenotypic drug susceptibility testing.10,12–19 WGS provides simultaneous and comprehensive information on relevant mutations conferring resistance to first-line and second-line drugs, anywhere in the genome. By contrast, targeted sequencing only identifies mutations in a priori defined regions covered by the amplifications. WGS allows effective individualised treatment, and thus reduces the risk of propagating drug resistance. Ineffective treatment could lead to the acquisition of additional drug resistance and increases the risk of transmitting drug-resistant strains.23 These considerations support the use of WGS to replace the current drug susceptibility testing methods, which cover only a limited number of drugs.
The broader range of drug resistance captured by WGS explains some of the discordant results found in this study; however, restricting the analysis of discordances between drug resistance diagnosed locally and by WGS to the most clinically relevant WHO categories of drug resistance will have minimised this effect.13 Thus, discordant results potentially leading to inappropriate treatment were mainly due to important drug resistance not captured with the available local tests at sites, rather than to a wider range of drug resistances captured by WGS. The detection of drug resistance is also influenced by the type of sample collected, and the methods used for culturing, DNA extraction and sequencing, and the pipeline used to analyse the sequences.25 The pipeline used to analyse the sequences was determined by a 90% or greater variant frequency cutoff, the robustness of the TBprofiler pipeline, and its coverage of all relevant resistance-conferring mutations. Our sensitivity analysis showed that the cutoff for variant frequency had little effect on results.
For new drugs, most resistance-conferring mutations are unknown at the time of introduction, and relevant drug resistance mechanisms become apparent only when the mutation becomes established in the population. The TBprofiler database is continuously updated with newly identified resistance-conferring mutations, such as bedaquiline in 2013 and dalamanid in 2014. Yet, the accuracy of the prediction of phenotypic resistance by molecular markers varies by drugs, depending on the molecular mechanisms involved and the evidence generated so far. We showed that the identification of drug-resistance-conferring mutations predicted phenotypic resistance to rifampicin better than to ethambutol.11 Discrepancies in results between local drug susceptibility testing and WGS might also be explained by mixed infections, heteroresistance, minority resistant populations, or methodological differences,25–27 which can lead to uncertainties in treatment decisions.28 Of note, overtreatment did not increase mortality, but the analysis was based on few patients (n=28) and should be interpreted with caution. Anti-tuberculosis drugs, especially second-line drugs, can cause serious side-effects, which can lead to treatment interruption, and failure, or acquired drug resistance, and should therefore only be used when needed.29
Our study has several limitations. We sampled eligible patients within strata defined by drug resistance and HIV infection, and therefore, could not estimate the incidence or prevalence of drug-resistant tuberculosis in patients who were HIV-coinfected or HIV-negative. Also, we could not evaluate differences in drug resistance between M tuberculosis lineages because the sample size was small for several lineages. Our analysis is mainly based on L2 and L4 strains, as expected from the geographical distribution of these lineages.30 Further, we sequenced strains before treatment and thus could not diagnose potentially acquired drug resistance, which might influence treatment outcomes. Finally, this study reflects the years 2013–16. Since then, the availability of drug resistance tests has increased (appendix p 14). For example, the MTBDRsl assay (Hain Lifescience, Nehren, Germany), a line probe assay for the detection of pre-XDR or XDR, is now available at four sites. However, three of the seven sites still have no access to rapid molecular tests to diagnose resistance to second-line drugs. In general, there were only a few changes in the drug resistances that are tested routinely between the study period and 2020 (appendix p 14).
Treatment guidelines also changed over the study period. In 2013, WHO published an interim policy guideline on bedaquiline, and in 2014 on delamanid in the treatment of MDR tuberculosis.31,32 In our study, patients were rarely given newer drugs such as bedaquiline or delamanid. In 2020, only South Africa included bedaquiline in their short and long MDR tuberculosis regimens. By contrast, the other sites are still using the so-called Bangladesh regimen (ie, a standardised short course MDR tuberculosis treatment regimen of 9–12 months), although guidelines will probably change in the near future. Identifying the emergence of resistance to recently introduced drugs will be crucial alongside the roll-out of new regimens.33
Our study shows that treatment strategies guided by comprehensive drug resistance data are likely to save lives. Our results thus support WHO’s call for an accurate point- of-care test based on WGS that can be done directly from sputum samples.34 Such tests would allow rapid diagnosis and efficient, individual-based treatment of drug-resistant tuberculosis.35 Test systems performing WGS on sputum samples, using new laboratory and bioinformatics pipelines are in development. High-burden countries should consider building central, high-throughput sequencing capacities.36 The establishment of a trustworthy, widely accepted drug resistance database similar to the Stanford HIV drug resistance database will be essential in this context.37 Finally, we support the call for clinical trials evaluating the safety, efficacy, and tolerability of new drugs and drug susceptibility testing strategies for drug-resistant tuberculosis.23,29 The role of new drugs like bedaquiline, delamanid, and pretomanid in regimens with fewer, more effective, and safer drugs needs to be evaluated.23 Future studies should also examine treatment duration and adherence.23 The duration of the intensive and continuation phases of tuberculosis treatment and treatment adherence are crucial for efficient therapy.
In conclusion, our study shows that both the accuracy of drug susceptibility testing in routine care, and the access to testing for resistance for several essential drugs is limited in high-burden tuberculosis countries, which leads to inappropriate treatment, and contributes to higher mortality. Our results support the role of WGS to improve the management of drug-resistant tuberculosis in high-burden settings.
Supplementary Material
Research in context.
Evidence before this study
Drug-resistant tuberculosis, in particular multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis, is threatening the control of tuberculosis worldwide. WHO has highlighted the need to improve drug susceptibility testing and treatment of drug-resistant tuberculosis, particularly in countries with a high burden of tuberculosis. Whole-genome sequencing (WGS) has the potential to provide resistance profiles for all first-line and second-line anti-tuberculosis drugs and is increasingly replacing other drug resistance testing methods. Yet, the potential of WGS in routine clinical care has not been shown in low-income and middle-income countries, where the burden of drug-resistant tuberculosis is high. We searched PubMed for systematic reviews and original research articles published in any language up to June 29, 2020. We combined terms for “tuberculosis”, “whole-genome sequencing”, and “mortality”. Several validation studies showed that WGS could accurately predict drug resistance; however, we could not find any study showing the potential benefit of WGS-based drug resistance testing on survival.
Added value of this study
In this study, we compared drug resistance profiles from WGS with routine drug susceptibility test results in seven countries across three continents with a high tuberculosis burden and assessed the effect of undiagnosed drug resistance on mortality. Results from WGS and routine drug susceptibility testing were discordant in 22% of patients. Resistance to isoniazid and rifampicin was accurately identified at local clinics, whereas resistance to ethambutol, pyrazinamide, and second-line drugs was rarely tested locally. Mortality ranged from 6% in patients with pan-susceptible tuberculosis who were appropriately treated to 32% in patients with drug-resistant tuberculosis who were undertreated.
Implications of all the available evidence
Routine drug susceptibility testing in resource-limited settings with a high tuberculosis burden is often insufficient to inform the prescription of the most effective treatment regimen, which in turn contributes to higher mortality. Our results support the implementation of point-of-care protocols for WGS, ideally directly from sputum to obtain comprehensive drug resistance profiles and facilitate the initiation of personalised and effective treatment regimens.
Acknowledgments
We thank all sites and patients who participated in this study. We are also grateful to the Tuberculosis Working Group of International epidemiology Databases to Evaluate AIDS (IeDEA) for helpful discussions. Calculations were done on UBELIX, the HPC cluster at the University of Bern. The IeDEA is supported by the US National Institutes of Health, National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute of Mental Health, the National Institute on Drug Abuse, the National Heart, Lung, and Blood Institute, the National Institute on Alcohol Abuse and Alcoholism, the National Institute of Diabetes and Digestive and Kidney Diseases, the Fogarty International Center, and the National Library of Medicine: Asia-Pacific, U01AI069907; CCASAnet, U01AI069923; Central Africa, U01AI096299; East Africa, U01AI069911; NA-ACCORD, U01AI069918; Southern Africa, U01AI069924; West Africa, U01AI069919. ME was supported by special project funding from the Swiss National Science Foundation (grant number 17481). KZ, MB, CL, LF and ME were supported by the Swiss National Science Foundation (grant number 320030_153442). SG, CL, SB, and MR were supported by the Swiss National Science Foundation (grant numbers 153442, 310030_188888, IZRJZ3_164171, IZLSZ3_170834 and CRSII5_177163) and by the European Research Council (grants 309540 and 883582). RJW received support from the Francis Crick Institute, which is funded by UK Research and Innovation, Cancer Research UK (grant number FC0010218), and Wellcome (grant numbers 104803, 203135). This work is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above.
Footnotes
Declaration of interests
RJW reports grants from Wellcome, European and Developing Countries Clinical Trials Partnership, UK Research and Innovation, Cancer Research UK, and National Institutes of Health, during the conduct of the study. ECB reports personal fees from AID Diagnostika and COPAN, outside the submitted work. MY reports grants from US National Institutes of Health, during the conduct of the study. All other authors declare no competing interests.
Data sharing
Whole-genome sequencing data from the strains included in this analysis have been submitted to the National Center for Biotechnology Information (PRJNA300846; appendix p 15).
References
- 1.WHO. Global tuberculosis report 2020. Geneva: World Health Organization, 2020. https://www.who.int/publications/i/item/9789240013131 (accessed April 28, 2020). [Google Scholar]
- 2.Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS 2015; 29: 1987–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mesfin YM, Hailemariam D, Biadgilign S, Kibret KT. Association between HIV/AIDS and multi-drug resistance tuberculosis: a systematic review and meta-analysis. PLoS One 2014; 9: e82235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gandhi NR, Nunn P, Dheda K, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet 2010; 375: 1830–43. [DOI] [PubMed] [Google Scholar]
- 5.Müller AM, Osório CS, Silva DR, Sbruzzi G, de Tarso P, Dalcin R. Interventions to improve adherence to tuberculosis treatment: systematic review and meta-analysis. Int J Tuberc Lung Dis 2018; 22: 731–40. [DOI] [PubMed] [Google Scholar]
- 6.Alsultan A, Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs 2014; 74: 839–54. [DOI] [PubMed] [Google Scholar]
- 7.Samuels JP, Sood A, Campbell JR, Ahmad Khan F, Johnston JC. Comorbidities and treatment outcomes in multidrug resistant tuberculosis: a systematic review and meta-analysis. Sci Rep 2018; 8: 4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinkhof MWG, Egger M, Boulle A, et al. Tuberculosis after initiation of antiretroviral therapy in low-income and high- income countries. Clin Infect Dis 2007; 45: 1518–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zürcher K, Ballif M, Fenner L, et al. Drug susceptibility testing and mortality in patients treated for tuberculosis in high-burden countries: a multicentre cohort study. Lancet Infect Dis 2019; 19: 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phelan JE, O’Sullivan DM, Machado D, et al. Integrating informatics tools and portable sequencing technology for rapid detection of resistance to anti-tuberculous drugs. Genome Med 2019; 11: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gygli SM, Keller PM, Ballif M, et al. Whole-genome sequencing for drug resistance profile prediction in Mycobacterium tuberculosis. Antimicrob Agents Chemother 2019; 63: e02175–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allix-Béguec C, Arandjelovic I, Bi L, et al. Prediction of susceptibility to first-line tuberculosis drugs by DNA sequencing. N Engl J Med 2018; 379: 1403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Consolidated guidelines on drug-resistant tuberculosis treatment. Geneva: World Health Organization, 2019. https://www.who.int/tb/publications/2019/consolidated-guidelines-drug-resistant-TB-treatment/en/ (accessed April 28, 2020). [PubMed] [Google Scholar]
- 14.van Embden JD, Cave MD, Crawford JT, et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol 1993; 31: 406–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coll F, Phelan J, Hill-Cawthorne GA, et al. Genome-wide analysis of multi- and extensively drug-resistant Mycobacterium tuberculosis. Nat Genet 2018; 50: 307–16. [DOI] [PubMed] [Google Scholar]
- 16.Cole ST, Brosch R, Parkhill J, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998; 393: 537–44. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009; 25: 1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Handsaker B, Wysoker A, et al. The sequence alignment/map format and SAMtools. Bioinformatics 2009; 25: 2078–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coll F, McNerney R, Preston MD, et al. Rapid determination of anti- tuberculosis drug resistance from whole-genome sequences. Genome Med 2015; 7: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw 2015; 67: 1–48. [Google Scholar]
- 21.R Core Team. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2019. [Google Scholar]
- 22.Python Software Foundation. Python: a dynamic, open source programming language. Python Software Foundation, 2019. [Google Scholar]
- 23.Nahid P, Mase SR, Migliori GB, et al. Treatment of drug-resistant tuberculosis. An official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med 2019; 200: e93–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Domínguez J, Boettger EC, Cirillo D, et al. Clinical implications of molecular drug resistance testing for Mycobacterium tuberculosis: a TBNET/RESIST-TB consensus statement. Int J Tuberc Lung Dis 2016; 20: 24–42. [DOI] [PubMed] [Google Scholar]
- 25.Metcalfe JZ, Streicher E, Theron G, et al. Mycobacterium tuberculosis subculture results in loss of potentially clinically relevant heteroresistance. Antimicrob Agents Chemother 2017; 61: e00888–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen T, van Helden PD, Wilson D, et al. Mixed-strain Mycobacterium tuberculosis infections and the implications for tuberculosis treatment and control. Clin Microbiol Rev 2012; 25: 708–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Streicher EM, Bergval I, Dheda K, et al. Mycobacterium tuberculosis population structure determines the outcome of genetics-based second-line drug resistance testing. Antimicrob Agents Chemother 2012; 56: 2420–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofmann-Thiel S, Hoffmann H, Hillemann D, Rigouts L, Van Deun A, Kranzer K. How should discordance between molecular and growth-based assays for rifampicin resistance be investigated? Int J Tuberc Lung Dis 2017; 21: 721–26. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad N, Ahuja SD, Akkerman OW, et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet 2018; 392: 821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gagneux S, Small PM. Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect Dis 2007; 7: 328–37. [DOI] [PubMed] [Google Scholar]
- 31.WHO. The use of bedaquiline in the treatment of multidrug-resistant tuberculosis: interim policy guidance. Geneva: World Health Organization, 2013. https://www.who.int/tb/publications/mdrtb-treatment-guideline/en/ (accessed April 28, 2020). [PubMed] [Google Scholar]
- 32.WHO. The use of delamanid in the treatment of multidrug-resistant tuberculosis. Geneva: World Health Organization, 2014. https://www.who.int/tb/publications/delamanid-in-mdr-tb-treatment/en/ (accessed April 28, 2020). [Google Scholar]
- 33.Nimmo C, Millard J, van Dorp L, et al. Population-level emergence of bedaquiline and clofazimine resistance-associated variants among patients with drug-resistant tuberculosis in southern Africa: a phenotypic and phylogenetic analysis. Lancet Microbe 2020; 1: e165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO. The use of next-generation sequencing technologies for the detection of mutations associated with drug resistance in Mycobacterium tuberculosis complex: technical guide. Geneva: World Health Organization, 2018. https://apps.who.int/iris/handle/10665/274443 (accessed April 28, 2020). [Google Scholar]
- 35.Goig GA, Cancino-Muñoz I, Torres-Puente M, et al. Whole-genome sequencing of Mycobacterium tuberculosis directly from clinical samples for high-resolution genomic epidemiology and drug resistance surveillance: an observational study. Lancet Microbe 2020; 1: e175–83. [DOI] [PubMed] [Google Scholar]
- 36.Africa’s Makoni M. $100-million Pathogen Genomics Initiative. Lancet Microbe 2020; 1: e318. [DOI] [PubMed] [Google Scholar]
- 37.Shafer RW. Rationale and uses of a public HIV drug-resistance database. J Infect Dis 2006; 194 (suppl 1): S51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.