Abstract
Purpose
To investigate urothelial cell proliferation, cytoskeleton, inflammation, and barrier function protein expressions in patients with interstitial cystitis/bladder pain syndrome (IC/BPS) after intravesical platelet-rich plasma (PRP) injections
Methods
A total of 19 patients with IC/BPS underwent 4 monthly intravesical PRP injections. Bladder biopsies were taken at the first and fourth PRP treatment. The bladder specimens were analyzed using the Western blot and immunochemical staining for progenitor cell markers for sonic hedgehog (Shh), CD34, and cytoskeleton proteins cytokeratin 5 (CK5), CK14, CK20; barrier function markers for zonula occludens-1 (ZO-1), E-cadherin, and intercellular adhesive molecule-1, tryptase and transforming growth factor-β (TGF-β). Global response assessment (GRA) was used to evaluate treatment outcomes.
Results
The mean age of patients was 55.6 years. After PRP injections, the functional bladder capacity and maximum flow rate increased, and the visual analogue scale (VAS) of pain, interstitial cystitis (IC) symptom index, IC problem index, O’Leary-Sant symptom score, and GRA improved in all patients. Urothelium Shh, CK5, ZO-1, E-cadherin, and TGF-β expressions increased significantly after repeated PRP injections. By subgrouping, according to PRP treatment outcomes, significant increases in Shh, E-cadherin, and ZO-1 expressions were noted only in patients with GRA ≥1 or improved VAS, but not in patients with GRA=0 and no improvement in VAS.
Conclusions
The level of urothelial barrier function protein and cell proliferation protein expression in the patients with IC/BPS was increased after repeat intravesical PRP injections. Intravesical repeat PRP injections may have potential to improve urothelial health and result in symptoms improvement in the patients with IC/BPS.
Keywords: Urothelium, Pathogenesis, Human, Platelet, Bladder
INTRODUCTION
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic inflammatory bladder disorder. Previous histopathological studies revealed bladder inflammation in some patients with IC/BPS, and suggested that the bladder inflammation might play central role in the pathogenesis of IC/BPS [1]. Our previous study also showed that the chronic inflammation in IC/BPS might be associated with increased urothelial cell apoptosis and deficits in the urothelial barrier function [2,3].
Chronic inflammation in IC/BPS could decrease urothelial basal cell proliferation, affecting the maturation from basal cells to apical umbrella cells and resulting in defective barrier function [4]. Abnormal cell differentiation of the urothelial cells in IC/BPS causes abnormal expression of the differentiation-related proteins and proteoglycan core proteins in the apical cells in the urothelium [5]. Aberrant cell differentiation, altered synthesis of proteoglycans, deficit of tight junction proteins, and lack of cell adhesive proteins are components of the fundamental pathophysiology of IC/BPS [6].
The human bladder urothelium is composed of different cell layers, including umbrella cells, intermediate cells, and basal cells, which have different immunohistochemical expressions of molecular markers. Previous studies have shown that the mature umbrella cells expressed cytokeratin CK20 and uroplakins (Upks), but not CK5, tumor protein TP63, or sonic hedgehog protein (Shh). Intermediate cells expressed TP63, Shh, Upks, but not CK20. Basal cells expressed CK5, Shh, and TP63, but not Upks or CK20 [7,8]. Laguna et al. [9], using immunochemical staining, showed weak expression of CK5 and CK20 in urothelium from patients with IC/BPS, whereas CK 7, CK9, and CK 13 expression was similar to that of healthy control subjects.
Because the defective urothelial barrier is caused by altered proliferation and differentiation of the basal cells in IC/BPS, restoration of the regenerative function is crucial in the treatment of IC/BPS. Our previous study showed that repeated intravesical autologous platelet-rich plasma (PRP) injections could improve clinical symptoms in patients with IC/BPS [10]. The purpose of current study is to investigate the protein expressions involving urothelial cell proliferation, cytoskeleton, apoptosis, inflammation, and barrier function in IC/BPS before and after repeated PRP intravesical injections.
MATERIALS AND METHODS
We randomly enrolled 17 patients with IC/BPS without Hunner’s lesion (NHIC) from our previous PRP injection study who had provided bladder specimens [10] and also 2 newly enrolled patients with IC/BPS with Hunner’s lesion (HIC). Significant symptom improvement was achieved in both the currently enrolled 17 patients with NHIC, and the total 40 NHIC patients in previous cohorts [10]. All patients with IC/BPS who had undergone 4 monthly intravesical PRP injections (with platelet concentration of approximately 5 times that of the peripheral blood) were recruited into this study and provided bladder specimens. The diagnosis of refractory IC/BPS was established based on characteristic IC symptoms and cystoscopic findings of glomerulations, petechia, or mucosal fissures after hydrodistension. All patients had been treated previously with at least 2 modalities including oral pentosane polysulfate, intravesical heparin instillation, hyaluronic acid, or onabotulinumtoxinA injection for at least 6 months, but symptoms remained or the patient relapsed. Before intravesical PRP injection, at least 6 months without previous treatment were required for patient inclusion in the study.
Patients were investigated thoroughly on enrollment and were excluded if they did not meet inclusion criteria for IC/BPS according to the National Institute of Diabetes and Digestive and Kidney Diseases. Global response assessment (GRA) was used to evaluate treatment outcome (1=slight improvement; 2=moderate improvement; and 3=marked improvement) [11]. In 7–10 days after fourth PRP injection, the patients were evaluated at baseline and after PRP injections for changes in the O’Leary-Sant symptom score (OSS) including IC symptom index (ICSI) and IC problem index (ICPI), visual analogue scale (VAS) for pain, maximum flow rate, video volume, postvoid residual, functional bladder capacity (FBC) recorded as the largest volume voided in a 3-day voiding diary, and GRA. Urodynamic study was routinely performed at baseline and after PRP treatment. The potassium chloride (KCl) test was performed after the urodynamic study, and a painful bladder sensation after instillation of 0.4 M KCl solution was considered a positive response.
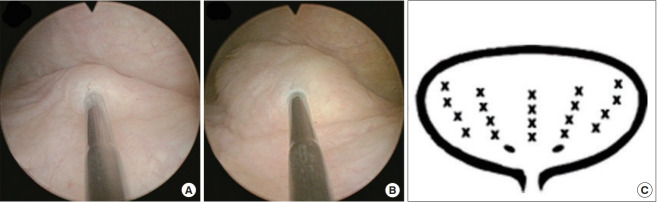
The patients were admitted for intravesical PRP injections every month for 4 months. The preparation of PRP and injection technique has been reported previously [10]. Briefly, a total of 50 mL whole blood was withdrawn and was centrifuged with a first soft spin (190 g, 20 minutes, <20°C). Then the supernatant plasma containing platelets was transferred into another sterile tube without disturbing buffy coat, and was further centrifuged by a hard spin (2,000 g, 20 minutes, <20°C). The platelet pellets were added to the plasma by gently shaking the tube to form 10 mL sterile PRP. Then the patients underwent intravesical PRP injection into bladder submucosa under general anesthesia (Fig. 1A, B). The injection volume was 0.5 mL in each site, and a total of 20 sites were injected in bladder posterior lateral wall except trigone (Fig. 1C). The concentration of PRP was about 5 times of the peripheral blood. Patients were not treated with another medicine for IC/BPS during the treatment course.
Fig. 1.
The intravesical PRP injection for the patients with IC/BPS. (A) The endoscopic injection needle was inserted to the bladder submucosal space. (B) 0.5-mL PRP was pushed into the submucosal pace, with the bladder mucosa bulging up. (C) A total of 20 sites was injected in bladder posterior and lateral walls except trigone. PRP, platelet-rich plasma; IC/BPS, interstitial cystitis/bladder pain syndrome.
Bladder specimens were obtained by cold-cup endoscopic biopsy at the first (baseline) and at fourth PRP injection (at 1 month after third PRP injection). Four bladder tissues approximately 2 mm×2 mm were obtained from each patient during bladder biopsies at 2 cm above the ureteral orifice at the lateral wall and posterior wall. Only bladder mucosa was obtained. For the HIC, the bladder biopsy site was also the posterior wall and near the HIC with grossly intact bladder mucosa.
The bladder biopsy specimens were homogenized with liquid nitrogen and lysis buffer (ROC-05892970001, Roche Diagnostics, Mannheim, Germany). The primary urothelial tissues were washed 2 times in ice-cold phosphate-buffered saline and then lysed for 10 minutes on ice with PRO-PREP Protein Extraction solution (iNtRON Biotechnology, Seongnam, Korea) supplemented by a protease inhibitor cocktail and a phosphatase inhibitor cocktail (Roche Diagnostics). The proteins were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the proteins investigated, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) enzymes were evaluated using Western blotting and GAPDH as a loading control. The procedures for tissue preparation were reported in with our previous study [12].
The bladder specimens were investigated using Western blotting for progenital cell marker by Shh and stem cell marker CD34; urothelial cytoskeleton markers by CK5, CK14, CK20; barrier function markers by intercellular adhesive molecule (ICAM-1), zonula occludens-1 (ZO-1), and E-cadherin; tryptase; and transforming growth factor beta (TGF-β) at baseline and after PRP treatment. We used GAPDH as a normalizing protein for the quantification. The methodology followed the procedures reported in our previous studies [2,12]. The suppliers, series numbers, and concentrations of antibodies used for the targets are shown in Table 1.
Table 1.
The antibody sources and concentrations used for Western blotting and immunochemical staining
| Target proteins | Antibody brand (series number) | Concentration for Western blotting | IHC concentration for immunochemical staining |
|---|---|---|---|
| E-cadherin | BD transduction Laboratories (610182) | 1:5,000 | 1:200 |
| CK5 | Abcam (ab52635) | 0.388888889 | 1:100 |
| CK14 | Abcam (ab9220) | 1:1,000 | 1:25 |
| CK20 | Abcam (ab76126) | 1:5,000 | 1:100 |
| Shh | Abcam (ab53281) | 1:10,000 | 1:300 |
| ZO-1 | GeneTex (GTX108627) | 1:1,000 | - |
| CD34 | Abcam (ab81289) | 1:10,000 | - |
| ICAM-1 | Abcam (ab53013) | 1:1,000 | - |
| tryptase | Sigma-Aldrich (MAB1222) | 1:1,000 | - |
| TGF-β | CellSignaling (56E4) | 1:1,000 | - |
| GAPDH | GeneTex (GTX100118) | 1:100,000 | - |
IHC, immunohistochemistry; CK, cytokeratin; Shh, sonic hedgehog; ZO-1, zonula occludens-1; ICAM-1, intercellular adhesive molecule-1; TGF-β, transforming growth factor-β; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Immunohistochemistry staining also was performed for characteristic proteins in the bladder urothelium. The bladder specimens used for immunochemical staining was embedded in optimal cutting temperature medium and stored at -80°C. Four sections per specimen were cut using a cryostat at a thickness of 8μm and collected on new silane III-coated slides. The sections were post-fixed in acetone at -20°C and blocked with rabbit serum. The sections were incubated overnight at 4°C with primary antibodies to Shh, CK5, CK14, CK20, and antihuman E-cadherin. After rinsing the sections with 0.1% Tween-20 in phosphate-buffered saline, anti-rabbit conjugated fluorescein isothiocyanate secondary antibodies were applied to the sections and incubated for 1 hour. Finally, the sections were counterstained with DAPI. Image capture, quantitation, and processing followed methodology reported previously [13]. Immunofluorescence stained images were assessed using fluorescence microscopy and processed using a digital imaging system. The distribution and fluorescence intensity were obtained using a confocal microscope.
Statistical analysis was performed using the P-value determined using the paired t-test for baseline and posttreatment data, and the chi-square test was used for categorical data. The correlation between the changes of bladder proteins expression and changes of clinical symptoms was analyzed with Pearson correlation. All calculations were performed using SPSS ver. 10.0 (SPSS Inc., Chicago, IL, USA). A P-value <0.05 was considered statistically significant.
RESULTS
A total of 19 patients (3 males) with a mean age of 55.6±15.8 years were enrolled in this study. After PRP injections, the FBC and maximum flow rate increased, and VAS, ICSI, ICPI, OSS, and GRA improved in patients overall. A positive KCl test result was noted in 16 patients (84.2%) at baseline and decreased to 6 patients (31.6%) after PRP treatment (Table 2). However, subjective improvement using GRA ≥2 was found in only 5 patients (26.3%); 11 patients (57.9%) had a GRA=1 and 3 patients (15.8%) did not show improvement in GRA after PRP treatment. A total of 13 patients (68.4%) showed improved VAS after PRP injection, whereas 6 patients (31.6%) did not. An increase in FBC by 25% from baseline was noted in 12 patients (63.2%). In the 2 patients with HIC enrolled in this study, VAS improved (from 10 and 4, to 2 and 0, respectively), and GRA was 1 and 2, respectively.
Table 2.
The changes of measured parameters of IC/BPS from baseline to post-PRP treatment in 19 patients
| Variable | Baseline | After PRP | P-value |
|---|---|---|---|
| OSS | 21.00 ± 8.37 | 14.70 ± 6.37 | 0.009 |
| ICSI | 11.50 ± 4.86 | 7.95 ± 3.36 | 0.006 |
| ICPI | 9.47 ± 4.17 | 6.58 ± 3.50 | 0.015 |
| VAS | 4.05 ± 3.27 | 1.95 ± 2.34 | 0.003 |
| Functional capacity | 217.6 ± 116.9 | 290.3 ± 124.0 | 0.001 |
| Qmax (mL/sec) in UFM | 9.55 ± 4.59 | 20.20 ± 6.96 | 0.000 |
| PVR (mL) in UFM | 37.3 ± 92.1 | 11.8 ± 21.1 | 0.395 |
| VUDS parameters | |||
| FSF (mL) | 138.0 ± 65.5 | 151.0 ± 43.0 | 0.444 |
| FS (mL) | 207.0 ± 89.2 | 220.0 ± 48.4 | 0.591 |
| CBC (mL) | 271.0 ± 97.2 | 251.0 ± 144 0 | 0.719 |
| Pdet (cm H2O) | 15.0 ± 7.1 | 17.6 ± 6.8 | 0.400 |
| Qmax (mL/sec) | 10.2 ± 7.1 | 11.8 ± 5.9 | 0.551 |
| Volume voided (mL) | 194 ± 124 | 246 ± 121 | 0.191 |
| PVR (mL) | 86.4 ± 150.0 | 26.4 ± 31.0 | 0.240 |
| Positive KCl test | 16/19 (84.2) | 6/19 (31.6) | 0.001 |
| Glomerulation | 1.84 ± 1.21 | - | - |
| Maximum capacity | 579.0 ± 212.3 | - | - |
| GRA | - | 1.11 ± 0.66 |
Values are presented as mean±standard deviation or number (%).
IC/BPS, interstitial cystitis/bladder pain syndrome; PRP, platelet-rich plasma; OSS, O’Leary-Sant symptom score; ICSI, interstitial cystitis symptom index; ICPI, interstitial cystitis problem index; VAS, visual analogue scale; UFM, uroflowmetry; Qmax, maximum flow rate; PVR, postvoid residual; VUDS, videourodynamic study; FSF, first sensation of filling; FS, fullness sensation; CBC, cystometric bladder capacity; Pdet, detrusor pressure; KCl, potassium chloride; GRA, global response assessment.
Immunohistochemistry study of the expressions of Shh, CK5, CK14, CK20, E-cadherin, and ZO-1 from single patient are shown in Fig. 2. The immunoactivity of Shh, CK5, CK14, CK20 and E-cadherin both expressed on the uroepithelium, which were compatible previous study [13]. The immunochemical staining figures suggested the antibodies we used was specific for the target protein. The Western blot bands from all the 19 IC/BPS patients were shown in Fig. 3. The quantification results of changes in urothelial proteins expression in the total patients after PRP injections are shown in Table 3. The data for all patients (n=19), HIC (n=2), and NHIC (n=17) were presented separately. Urothelium Shh, CK5, ZO-1, and E-cadherin expressions in the total patients with IC/BPS significantly increased after repeated PRP injections. Significant increases in Shh, E-cadherin, ZO-1, and TGF-β expressions were noted in patients with GRA ≥1, improved VAS, and increased FBC by ≥25%, but no changes occurred in VAS improvement or FBA change <25% in patients with GRA =0. However, tryptase, ICAM-1, and CD34 all showed no significant change after PRP injections (Table 4). The change in urothelial E-cadherin expression (from baseline to after PRP) was correlated with the improvement in ICPI, ICSI, OSS, and FBC in the all patients with IC/BPS (P=0.026, P=0.049, P=0.043, and P=0.016, respectively). The change in CK5 also correlated with improvement in OSS (P=0.038).
Fig. 2.
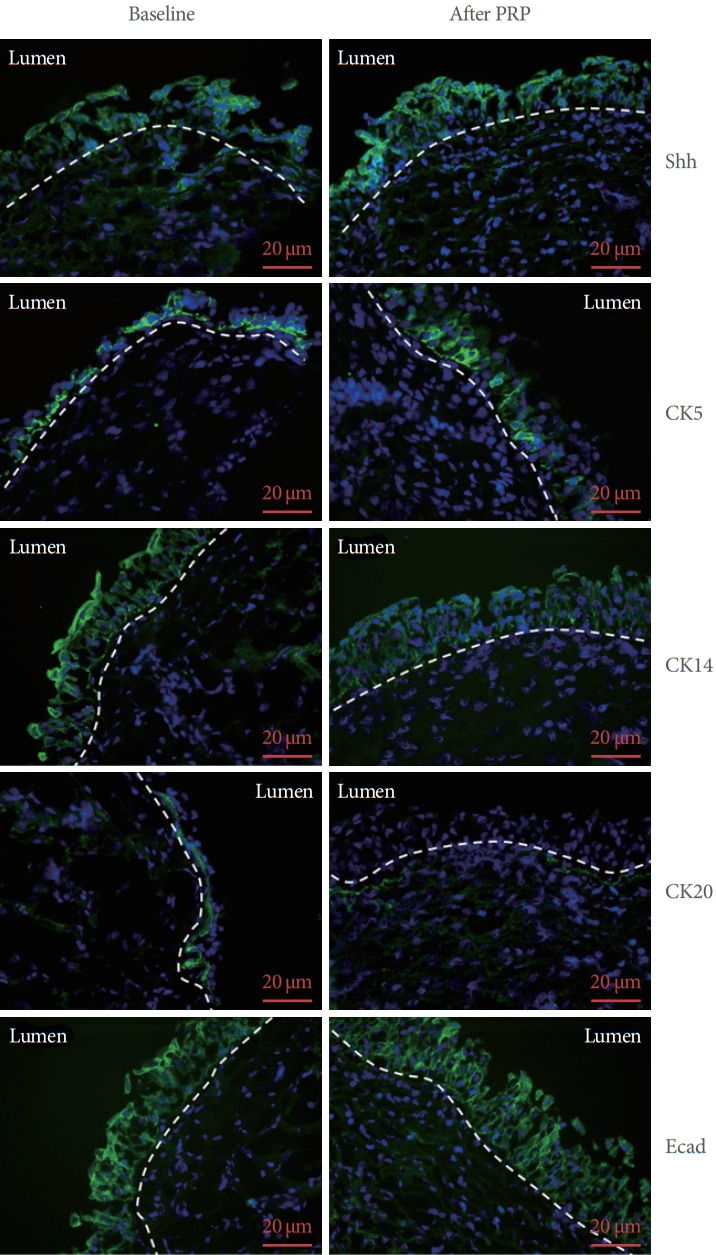
The immunochemical staining expressions of sonic hedgehog protein (Shh), cytokeratin 5 (CK5), cytokeratin 14 (CK14), cytokeratin 20 (CK20), and E-cadherin (Ecad) at baseline (left), and after fourth PRP treatment (right) in single IC/BPS patient. The cell nuclear was counterstained with DAPI. Both of Shh, CK5, CK14, CK20, and Ecad expressed on the uroepithelium, which were compatible previous study. The immunochemical staining figures suggested the antibodies we used were specific for the target protein. PRP, platelet-rich plasma; IC/BPS, interstitial cystitis/bladder pain syndrome; DAPI, 4’-6-diamidino-2-phenylindole.
Fig. 3.
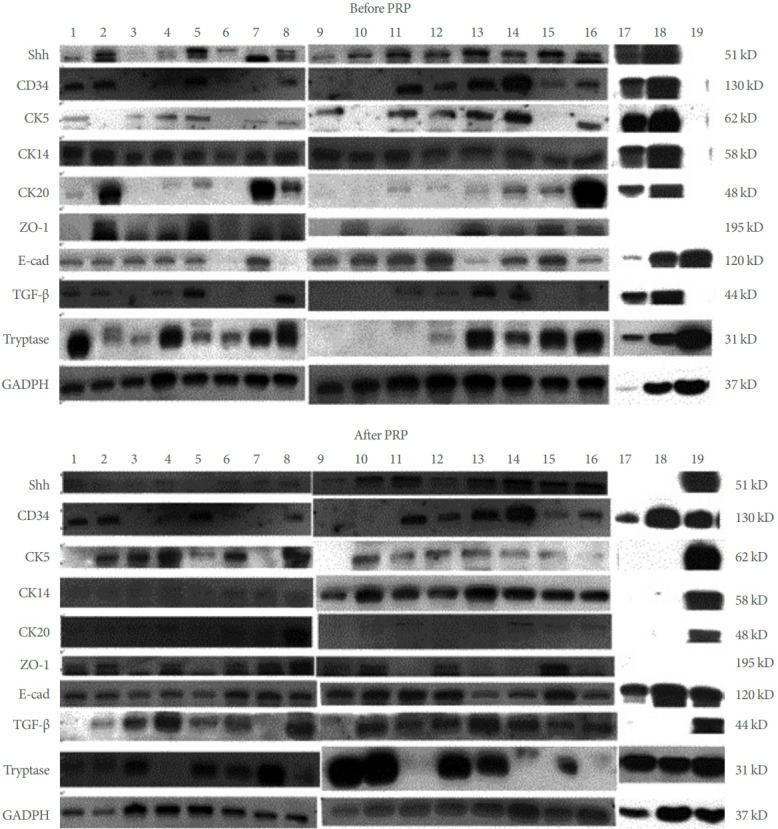
The bands of Western blots of the expressions of sonic hedgehog protein (Shh), CD34, cytokeratin 5 (CK5), cytokeratin 14 (CK14), cytokeratin 20 (CK20), zonula occludens-1 (ZO-1), E-cadherin (E-cad), and transforming growth factor beta (TGF-β) at baseline and after PRP treatment from all of the 19 patients with IC/BPS. PRP, platelet-rich plasma; IC/BPS, interstitial cystitis/bladder pain syndrome; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Table 3.
The Western blot quantification results of changes of urothelial cell proliferation, cytoskeleton, inflammation, and barrier function protein expression after repeat PRP injections
| Biomarker | Patient | Baseline | After PRP | P-value |
|---|---|---|---|---|
| Shh | Total patients | 0.12 ± 0.05 | 0.29 ± 0.20 | 0.002 |
| HIC | 0.06 ± 0.02 | 0.13 ± 0.05 | 0.156 | |
| NHIC | 0.21 ± 0.26 | 0.36 ± 0.25 | 0.004 | |
| CD34 | Total patients | 0.70 ± 0.46 | 0.60 ± 0.33 | 0.467 |
| HIC | 0.27 ± 0.16 | 0.38 ± 0.20 | 0.602 | |
| NHIC | 0.74 ± 0.42 | 0.62 ± 0.32 | 0.391 | |
| CK5 | Total patients | 0.33 ± 0.10 | 0.68 ± 0.34 | 0.027 |
| HIC | 0.26 ± 0.16 | 1.06 ± 0.06 | 0.054 | |
| NHIC | 0.74 ± 1.09 | 0.8 ± 0.55 | 0.135 | |
| CK14 | Total patients | 0.25 ± 0.10 | 0.39 ± 0.36 | 0.136 |
| HIC | 0.18 ± 0.05 | 0.02 ± 0.01 | 0.118 | |
| NHIC | 0.37 ± 0.30 | 0.48 ± 0.37 | 0.080 | |
| NHIC | 0.27 ± 0.10 | 0.44 ± 0.36 | 0.082 | |
| CK20 | Total patients | 0.19 ± 0.19 | 0.10 ± 0.13 | 0.095 |
| HIC | 0.07 ± 0.01 | 0.11 ± 0.05 | 0.453 | |
| NHIC | 0.25 ± 0.27 | 0.17 ± 0.29 | 0.071 | |
| ZO-1 | Total patients | 0.17 ± 0.08 | 0.47 ± 0.28 | 0.002 |
| HIC | 0.33 ± 0.01 | 0.27 ± 0.07 | 0.383 | |
| NHIC | 0.14 ± 0.06 | 0.5 ± 0.29 | 0.001 | |
| E-cadherin | Total patients | 0.46 ± 0.44 | 0.68 ± 0.54 | 0.001 |
| HIC | 0.33 ± 0.12 | 0.31 ± 0.05 | 0.877 | |
| NHIC | 0.48 ± 0.46 | 0.72 ± 0.55 | < 0.001 | |
| TGF-β | Total patients | 0.31 ± 0.46 | 0.80 ± 0.28 | 0.000 |
| HIC | 0.16 ± 0.18 | 0.94 ± 0.39 | 0.072 | |
| NHIC | 0.38 ± 0.46 | 0.75 ± 0.25 | 0.003 | |
| ICAM-1 | Total patients | 2.26 ± 2.63 | 0.74 ± 0.71 | 0.054 |
| HIC | 4.23 ± 0.96 | 1.33 ± 0.39 | 0.204 | |
| NHIC | 1.54 ± 2.24 | 0.76 ± 0.73 | 0.201 | |
| Mast cell activity (tryptase) | Total patients | 1.58 ± 4.14 | 1.19 ± 1.44 | 0.560 |
| HIC | 0.72 ± 0.51 | 0.50 ± 0.55 | 0.820 | |
| NHIC | 1.68 ± 4.38 | 1.27 ± 1.50 | 0.584 |
Values are presented as mean±standard deviation.
The data of Western blot quantification results presented as the ratio of different targets/GAPDH. The results in total patients (n=19), HIC (IC/BPS with Hunner’s lesion, n=2) and NHIC (IC/BPS without Hunner’s lesion, n=17) were presented separately.
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PRP, platelet-rich plasma; Shh, sonic hedgehog; IC/BPS, interstitial cystitis/bladder pain syndrome; CK, cytokeratin; ZO-1, zonula occludens-1; TGF-β, transforming growth factor-β; ICAM-1, intercellular adhesive molecule-1.
Table 4.
The Western blot quantification results in IC/BPS patients with different treatment outcome groups
| Biomarker | Time point | GRA=0 (n=3) | GRA≥1 (n=16) | P-value† | ΔVAS <1 (n=6) | ΔVAS ≥ 1 (n=13) | P-value† | ΔFBC< 25% (n=7) | ΔFBC≥25% (n=12) | P-value† |
|---|---|---|---|---|---|---|---|---|---|---|
| Shh | Baseline | 0.29 ± 0.29 | 0.18 ± 0.25 | 0.716 | 0.24 ± 0.22 | 0.18 ± 0.27 | 0.611 | 0.26 ± 0.39 | 0.16 ± 0.16 | 0.345 |
| After PRP | 0.34 ± 0.22 | 0.33 ± 0.26* | 0.40 ± 0.34 | 0.30 ± 0.21* | 0.45 ± 0.31 | 0.26 ± 0.19* | ||||
| CD34 | Baseline | 1.09 ± 0.68 | 0.58 ± 0.32 | 0.342 | 0.90 ± 0.57 | 0.54 ± 0.26 | 0.688 | 0.89 ± 0.52 | 0.58 ± 0.36 | 0.158 |
| After PRP | 0.75 ± 0.40 | 0.56 ± 0.30 | 0.84 ± 0.34 | 0.45 ± 0.19 | 0.57 ± 0.27 | 0.63 ± 0.37 | ||||
| CK5 | Baseline | 0.78 ± 0.85 | 0.64 ± 1.09 | 0.793 | 0.68 ± 0.73 | 0.67 ± 1.15 | 0.585 | 1.22 ± 1.80 | 0.46 ± 0.47 | 0.642 |
| After PRP | 0.71 ± 0.63 | 0.85 ± 0.53 | 1.05 ± 0.84 | 0.75 ± 0.35 | 1.02 ± 0.63 | 0.69 ± 0.40 | ||||
| CK14 | Baseline | 0.52 ± 0.36 | 0.31 ± 0.28 | 0.490 | 0.31 ± 0.29 | 0.43 ± 0.31 | 0.962 | 0.44 ± 0.41 | 0.30 ± 0.22 | 0.781 |
| After PRP | 0.28 ± 0.30 | 0.44 ± 0.39 | 0.38 ± 0.35 | 0.54 ± 0.46 | 0.54 ± 0.38 | 0.36 ± 0.38 | ||||
| CK20 | Baseline | 0.57 ± 0.36 | 0.16 ± 0.19 | 0.925 | 0.19 ± 0.21 | 0.34 ± 0.37 | 0.380 | 0.11 ± 0.11 | 0.29 ± 0.30 | 0.043 |
| After PRP | 0.29 ± 0.34 | 0.14 ± 0.27 | 0.08 ± 0.06 | 0.35 ± 0.47 | 0.31 ± 0.43 | 0.08 ± 0.05* | ||||
| ZO-1 | Baseline | 0.12 ± 0.02 | 0.17 ± 0.09 | 0.768 | 0.12 ± 0.05 | 0.18 ± 0.09 | 0.347 | 0.11 ± 0.05 | 0.19 ± 0.09 | 0.233 |
| After PRP | 0.36 ± 0.09 | 0.49 ± 0.30* | 0.57 ± 0.39 | 0.44 ± 0.25* | 0.57 ± 0.33* | 0.43 ± 0.26* | ||||
| E-cad | Baseline | 0.35 ± 0.42 | 0.48 ± 0.45 | 0.439 | 0.50 ± 0.31 | 0.44 ± 0.50 | 0.815 | 0.60 ± 0.68 | 0.38 ± 0.20 | 0.071 |
| After PRP | 0.66 ± 0.46 | 0.68 ± 0.56* | 0.74 ± 0.38 | 0.65 ± 0.61* | 0.94 ± 0.74* | 0.52 ± 0.32* | ||||
| TGF-β | Baseline | 0.82 ± 0.83 | 0.25 ± 0.25 | 0.098 | 0.59 ± 0.69 | 0.25 ± 0.25 | 0.045 | 0.58 ± 0.71 | 0.25 ± 0.24 | 0.749 |
| After PRP | 0.97 ± 0.57 | 0.75 ± 0.21* | 0.81 ± 0.38 | 0.77 ± 0.23* | 0.84 ± 0.28 | 0.73 ± 0.26* | ||||
| ICAM-1 | Baseline | 2.73 ± 3.93 | 1.70 ± 1.87 | 0.048 | 2.92 ± 3.19 | 1.52 ± 1.89 | 0.037 | 1.84 ± 3.06 | 1.97 ± 1.96 | 0.818 |
| After PRP | 2.15 ± 0.00 | 0.73 ± 0.61 | 1.26 ± 0.83 | 0.72 ± 0.66 | 0.96 ± 0.92 | 0.75 ± 0.49 | ||||
| Mast cell | Baseline | 1.06 ± 0.32 | 1.68 ± 4.53 | 0.995 | 0.82 ± 0.59 | 1.93 ± 5.01 | 0.399 | 3.12 ± 6.83 | 0.68 ± 0.40 | 0.355 |
| After PRP | 0.68 ± 0.59 | 1.28 ± 1.54 | 1.28 ± 0.79 | 1.14 ± 1.69 | 1.90 ± 2.18 | 0.77 ± 0.54 |
Values are presented as mean±standard deviation.
IC/BPS, interstitial cystitis/bladder pain syndrome; GRA, global response assessment; VAS, visual analogue scale; FBC, functional bladder capacity; Shh, sonic hedgehog; PRP, platelet-rich plasma; CK, cytokeratin; ZO-1, zonula occludens-1; E-cad, E-cadherin; TGF-β, transforming growth factor-β; ICAM-1, intercellular adhesive molecule-1.
P<0.05 in paired t-test. Comparing the urothelial protein expression in baseline and after PRP treatment within each group.
P-value analyzed by comparing the changes of urothelial protein expression between different treatment outcome groups (independent t-test).
DISCUSSION
The results of this study showed that the expressions of urothelial cell proliferation marker Shh and cytoskeleton marker CK5, and the adhesive protein expressions of E-cadherin and ZO-1 increased in patients with IC/BPS after repeated PRP injections. These results indicate that PRP injections can increase progenital cell proliferative activity and increase barrier protein expression. These protein changes may be associated with improved IC symptoms and pain reduction after PRP injections. However, not all urothelial proteins show similar changes after PRP injection. The small number of patients studied may be a limitation of this study.
From the histopathological and electron microscopic evidence, the bladder urothelium in patients with IC/BPS is usually defective or absent [3,14]. The underlying pathophysiology could be chronic suburothelial inflammation, which impairs the basal cell proliferation and differentiation of urothelium upon bladder injury. Therefore, in the bladders of patients with IC/BPS, the apical urothelial cells are not hexagonally arranged umbrella cells, but are unevenly matched with immature intermediate cells that suggest that tight cell junction and adhesive proteins may be defective [14]. The expressions of E-cadherin and ZO-1 in the bladder urothelium are significantly lower in patients with IC/BPS than expressions in the healthy control subjects. After repeated PRP injections as carried out in this study, we observed that the increased expressions of E-cadherin and ZO-1 were significant in the bladders of patients with IC/BPS, typically in patient groups who had GRA ≥1 and VAS improvement by ≥1, together with the high rate of negative KCI test results after PRP treatment, suggesting that increases in these barrier proteins are associated with symptomatic improvement of patients with IC/BPS. In addition, this study revealed the baseline Shh, E-cadherin, CD34, CK20, and CK14 expression in the bladder of HIC was lower than that in NHIC, though statistical significance was not confirmed. This finding suggested the urothelial defect might be more severe in the HIC bladders than the NHIC.
The urothelial basal cells marked by the expression of the secreted protein Shh are the progenitor cells, which are considered to act as the primary source of urothelial regeneration in urothelial injury [7]. The bladder urothelial cells have a slow replacement time, but will eventually initiate the regeneration process upon bladder insult by the rapid proliferation of progenitor cells in the basal layers and adequate differentiation into the mature umbrella cells in the superficial mucosal layer [15]. In this study, we found that Shh and CK5 increased expressions after PRP injections, but CK14 and CK20 did not, suggesting the 3 PRP injections may be insufficient to adequately improve the maturation of apical cells compared with those in the healthy bladders. Nevertheless, the differentiation makers E-cadherin and ZO-1 all showed significantly increased expressions after PRP injections, which provide better barrier function to the urine solutes and, hence, change the positive KCl to negative and improve IC symptoms.
The autologous PRP has been found to promote wound healing and facilitate tissue regeneration and has been used as wound healing therapy in dermatology, plastic surgery, orthopedics, and ophthalmology [16]. Several regeneration-acquired growth factors and anti-inflammation related cytokines and chemokines have been found in PRP, such as epidermal growth factor, platelet-derived growth factor, transforming growth factor, and interleukins. These cytokines and growth factors can reduce tissue inflammation and facilitate wound healing processes by promoting epithelial cell proliferation and differentiation [17]. Furthermore, the PRP-secreted proinflammatory and anti-inflammatory cytokines may induce the enhancement of tissue inflammation and end in complete resolution of inflammation, together with tissue remodeling and axon regeneration, resulting in elimination of neuropathic pain [18].
The rationale for using autologous PRP in the treatment of IC/BPS is the ease of obtaining plasma and concentration of the platelets, economic advantage, and delivery of the bioactive factors into the bladder wall by injection [16,19]. Platelet-released factors can promote revascularization of the damaged tissue and facilitate proliferation and differentiation of the mesenchymal cells to the specific cells, resulting in improve recovery of damaged tissue [16]. Although our result revealed improvement of urothelial barrier function protein expression after PRP injection, bladder tryptase and ICAM-1 expression was not changed, suggesting inflammation did not improve significantly. This result indicates that intravesical injection of PRP for 3 times might not be adequate to eradicate the inflammation. Further study with different PRP preparation technique, concentration and various repeat injections is necessary to explore the potential anti-inflammatory effect in IC/BPS.
We have recently conducted a preliminary clinical study using PRP injections to treat patients with IC/BPS. The results show IC symptoms can be significantly improved and the pain VAS also decreased after 4 injections [10]. We also investigated the changes in urinary cytokines, growth factors, and functional proteins in these patients at baseline and after PRP injections. The results showed that the urinary levels of nerve growth factor, matrix metalloproteinase-13, and vascular endothelial growth factor all significantly decreased after repeated PRP injections, whereas the level of platelet-derived growth factor-AB significantly increased at 12 weeks after the first PRP treatment compared with baseline. The bladder pain VAS score, frequency, and nocturia episodes decreased significantly, and GRA improved (all P<0.05). The pilot clinical trial has shown that repeated intravesical PRP injections can significantly improve IC symptoms and bladder conditions. Moreover, they also appear to be safe [20]. Although PRP injection might be effective for patient with IC/BPS, its efficacy for the HIC is still uncertain. The HIC patients have more obvious bladder inflammation and urothelium defect, hence using PRP to promote urothelial health and improve bladder symptoms should be reasonable. In current study, although the pain reduction after PRP injection was obvious in the 2 HIC patients, the bladder protein expression was not statistical changed. Further study enrolled more HIC patients is necessary to prove the efficacy of intravesical PRP.
In this study, we also found TGF-β expression increases after PRP injections. TGF-β is rich in human platelets and is involved in lower urinary tract neuroplasticity after urinary bladder inflammation [21]. TGF-β is a secreted protein that has been found to play a role in the control of cell growth, proliferation, differentiation, and apoptosis. Together with the increased expressions of proliferative and regenerative marker Shh and basal cell markers CK5, and the junction protein ZO-1 and adhesive protein E-cadherin, it is suggested that PRP can promote urothelial reconstruction by improving cellular proliferation and growth. These findings indicate that PRP injections can provide stimulation for basal cell proliferation in the bladders of patients with IC/BPS, facilitate urothelial cell differentiation, and improve the defective urothelial barrier function.
The limitations of this study are the small patient sample, lack of control arm, and that the bladder tissues were harvested only after 3 PRP injections. In addition, a smaller bladder biopsy specimen may not be enough to be represented to bladder pathological changes. However, the remarkable changes in proliferation, cytoskeleton, and barrier protein expressions have indicated the potential of repeated PRP injections in the treatment of IC/BPS refractory to conventional therapies. There has been no known clinical study in this field; therefore, we do not know how many PRP injections are optimal and which patients are appropriate candidates for this treatment. Without a control arm, we cannot know the increased progenital cell proliferative activity and increased barrier protein expression are truly due to PRP injection but not through natural healing or effect of hydrodistention. A comparative study is necessary to clarify this issue.
In conclusion, our results revealed that repeated intravesical PRP injections could improve clinical symptoms in patients with IC/BPS. The urothelial barrier function protein and cell proliferation protein expression were also increased after repeat PRP injections. Intravesical repeat PRP injections may have potential to improve urothelial health and result in symptoms improvement in the patients with IC/BPS.
Footnotes
Research Ethics
This study was approved by the Institutional Review Board and Ethics Committee of the Hualien Tzu Chi Hospital (IRB No. 106-173-A). Each patient was informed about the study rationale and procedures, and written informed consent was obtained before the procedures.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
·Conceptualization: LB, TL, HK
·Data curation: JJ, YJ, YH, HH
·Formal analysis: YJ, YH
·Methodology: HH, TL, HK
·Project administration: HK
·Writing-original draft: JJ, YJ
·Writing-review & editing: JJ, YH, HH, LB, TL, HK
REFERENCES
- 1.Keay S. Cell signaling in interstitial cystitis/painful bladder syndrome. Cell Signal. 2008;20:2174–9. doi: 10.1016/j.cellsig.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Shie JH, Kuo HC. Higher levels of cell apoptosis and abnormal E-cadherin expression in the urothelium are associated with inflammation in patients with interstitial cystitis/painful bladder syndrome. BJU Int. 2011;108(2 Pt 2):E136–41. doi: 10.1111/j.1464-410X.2010.09911.x. [DOI] [PubMed] [Google Scholar]
- 3.Shie JH, Liu HT, Kuo HC. Increased cell apoptosis of urothelium mediated by inflammation in interstitial cystitis/painful bladder syndrome. Urology. 2012;79:484.e7–13. doi: 10.1016/j.urology.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 4.Tomaszewski JE, Landis JR, Russack V, Williams TM, Wang LP, Hardy C, et al. Biopsy features are associated with primary symptoms in interstitial cystitis: results from the interstitial cystitis database study. Urology. 2001;57(6 Suppl 1):67–81. doi: 10.1016/s0090-4295(01)01166-9. [DOI] [PubMed] [Google Scholar]
- 5.Hauser PJ, Dozmorov MG, Bane BL, Slobodov G, Culkin DJ, Hurst RE. Abnormal expression of differentiation related proteins and proteoglycan core proteins in the urothelium of patients with interstitial cystitis. J Urol. 2008;179:764–9. doi: 10.1016/j.juro.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slobodov G, Feloney M, Gran C, Kyker KD, Hurst RE, Culkin DJ. Abnormal expression of molecular markers for bladder impermeability and differentiation in the urothelium of patients with interstitial cystitis. J Urol. 2004;171:1554–8. doi: 10.1097/01.ju.0000118938.09119.a5. [DOI] [PubMed] [Google Scholar]
- 7.Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, et al. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472:110–4. doi: 10.1038/nature09851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pignon JC, Grisanzio C, Geng Y, Song J, Shivdasani RA, Signoretti S. p63-expressing cells are the stem cells of developing prostate, bladder, and colorectal epithelia. Proc Natl Acad Sci U S A. 2013;110:8105–10. doi: 10.1073/pnas.1221216110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laguna P, Smedts F, Nordling J, Horn T, Bouchelouche K, Hopman A, et al. Keratin expression profiling of transitional epithelium in the painful bladder syndrome/interstitial cystitis. Am J Clin Pathol. 2006;125:105–10. [PubMed] [Google Scholar]
- 10.Jhang JF, Lin TY, Kuo HC. Intravesical injections of platelet-rich plasma is effective and safe in treatment of interstitial cystitis refractory to conventional treatment-A prospective clinical trial. Neurourol Urodyn. 2019;38:703–9. doi: 10.1002/nau.23898. [DOI] [PubMed] [Google Scholar]
- 11.Propert KJ, Mayer RD, Wang Y, Sant GR, Hanno PM, Peters KM, et al. Responsiveness of symptom scales for interstitial cystitis. Urology. 2006;67:55–9. doi: 10.1016/j.urology.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Chuang FC, Kuo HC. Increased urothelial cell apoptosis and chronic inflammation are associated with recurrent urinary tract infection in women. PLoS One. 2013;8:e63760. doi: 10.1371/journal.pone.0063760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kullmann FA, Clayton DR, Ruiz WG, Wolf-Johnston A, Gauthier C, Kanai A, et al. Urothelial proliferation and regeneration after spinal cord injury. Am J Physiol Renal Physiol. 2017;313:F85–102. doi: 10.1152/ajprenal.00592.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jhang JF, Ho HC, Jiang YH, Lee CL, Hsu YH, Kuo HC. Electron microscopic characteristics of interstitial cystitis/bladder pain syndrome and their association with clinical condition. PLoS One. 2018;13:e0198816. doi: 10.1371/journal.pone.0198816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreft ME, Sterle M, Veranic P, Jezernik K. Urothelial injuries and the early wound healing response: tight junctions and urothelial cytodifferentiation. Histochem Cell Biol. 2005;123:529–39. doi: 10.1007/s00418-005-0770-9. [DOI] [PubMed] [Google Scholar]
- 16.Etulain J. Platelets in wound healing and regenerative medicine. Platelets. 2018;29:556–68. doi: 10.1080/09537104.2018.1430357. [DOI] [PubMed] [Google Scholar]
- 17.Mussano F, Genova T, Munaron L, Petrillo S, Erovigni F, Carossa S. Cytokine, chemokine, and growth factor profile of platelet-rich plasma. Platelets. 2016;27:467–71. doi: 10.3109/09537104.2016.1143922. [DOI] [PubMed] [Google Scholar]
- 18.Kuffler DP. Platelet-rich plasma and the elimination of neuropathic pain. Mol Neurobiol. 2013;48:315–32. doi: 10.1007/s12035-013-8494-7. [DOI] [PubMed] [Google Scholar]
- 19.Furuta A, Yamamoto T, Igarashi T, Suzuki Y, Egawa S, Yoshimura N. Bladder wall injection of mesenchymal stem cells ameliorates bladder inflammation, overactivity, and nociception in a chemically induced interstitial cystitis-like rat model. Int Urogynecol J. 2018;29:1615–22. doi: 10.1007/s00192-018-3592-8. [DOI] [PubMed] [Google Scholar]
- 20.Jiang YH, Kuo YC, Jhang JF, Lee CL, Hsu YH, Ho HC, et al. Repeated intravesical injections of platelet-rich plasma improve symptoms and alter urinary functional proteins in patients with refractory interstitial cystitis. Sci Rep. 2020;10:15218. doi: 10.1038/s41598-020-72292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S, Gu X, Yi S. The regulatory effects of transforming growth factor-beta on nerve regeneration. Cell Transplant. 2017;26:381–94. doi: 10.3727/096368916X693824. [DOI] [PMC free article] [PubMed] [Google Scholar]