Abstract
Movement initiation and control require the orchestrated activity of sensorimotor cortical and subcortical regions. However, the exact contribution of specific pathways and interactions to the final behavioral outcome are still under debate. Here, by combining structural lesions, pathway-specific optogenetic manipulations and freely moving electrophysiological recordings in rats, we studied cortico-striatal interactions in the context of forelimb bilaterally coordinated movements. We provide evidence indicating that bilateral actions are initiated by motor cortical regions where intratelencephalic bilateral cortico-striatal (bcs-IT) projections recruit the sensorimotor striatum to provide stability and duration to already commanded bilateral movements. Furthermore, striatal spiking activity was correlated with movement duration and kinematic parameters of the execution. bcs-IT stimulation affected only the representation of movement duration but spared that of kinematics. Our findings confirm the modular organization of information processing in the striatum and its involvement in moment-to-moment movement control but not initiation or selection.
Motor cortical regions recruit specific striatal subpopulations to provide movement stability and duration.
INTRODUCTION
The neural representation of actions within cortical and basal ganglia (BG) circuits has received constant and intensive attention. For example, we know that the sensorimotor striatum hosts a variety of behavior-related representations such as activations at the beginning/end of movements (1–4), speed, position and timing of movements or sequences of movements (5–9), and multisensory representations of contextual or movement-related information (5, 8, 10–12). Nevertheless, it is still debated whether these BG representations constitute commands for the beginning of actions, moment-to-moment movement modulatory signals, or passive portrayals of movements or sensory inputs that unavoidably accompany the execution of actions (13–16). The scenario becomes more complicated when analyzing the anatomical organization of the implicated neural networks. For instance, in rodents, the dorsolateral striatum (DLS) receives motor-related inputs from primary and secondary motor cortices (17, 18), sensory inputs from primary sensory cortices (19, 20), and specific (relay nuclei) and unspecific inputs from various thalamic nuclei such as the ventro postero lateral and medial (8, 21–24) or the centromedian-parafascicular complex (25, 26). The specific contribution of this heterogeneity of inputs is yet to be fully determined, but recent studies suggest a diversity of functions where modularly organized inputs would help to initiate, maintain, or time movements (8, 24, 27, 28). On the other hand, an often overlooked feature of many actions is the bilateral nature of its planning and execution. Driving a car or typing one’s name on a keyboard requires spatiotemporally coordinated bilateral movements that are thought to involve the orchestrated bilateral activity of cortical, BG, and thalamic networks (29, 30). However, the specific interactions and dynamics supporting these actions are yet to be revealed. Some hints arise from the basic anatomy of the communication between these networks. For example, sensorimotor cortical-striatal projections can be divided into unilateral pyramidal tract (PT) projections and bilateral intratelencephalic (IT) projections (20, 31–33), while thalamo-striatal projections are mainly unilateral (26, 34, 35). Another important feature is the absence of direct interhemispheric communication between the left and right striata. In this context, the DLS is anatomically relevant because it receives both bilateral and unilateral projections from sensorimotor cortices (M2, M1/S1). Together, the anatomical organization along with movement-related striatal representations suggest that the initiation of bilateral actions is most likely coordinated by cortical regions with bilateral projections, while the DLS may use bilateral or unilateral sensorimotor inputs to unilaterally modulate motor outputs.
To test these hypotheses, we implemented a novel behavioral protocol where rats execute bilaterally coordinated movements of the forelimbs to obtain a reward. In this task, movements can be further subdivided into a ballistic phase and a holding phase; the latter may be subjected to feedback control. We combined this behavioral strategy with structural lesions of cortical and striatal regions in different stages of learning, pathway-specific optogenetic manipulations, and high-density electrophysiological recordings in anesthetized and freely moving animals. Our results indicate that unilateral DLS lesions (DLS-les) produced persistent impairments in movement trajectories of the unilateral forelimb. These effects resulted in a general decrease of the bilaterally synchronized movement trajectories. Then, we observed that selective unilateral opto-activation of bilateral IT cortico-striatal projections produced an increase in bilateral movement duration and coordination. These pathway-specific manipulations also produced clear changes in neural activity for a subset of striatal neurons related to movement duration but largely spared typical striatal movement kinematic representations. Together, our data indicate that, during bilaterally coordinated actions, the DLS is recruited by upstream cortical regions, questioning its participation as an “action selector” but confirming its role in moment-to-moment control and kinematic modulation.
RESULTS
Unilateral lesions of the DLS impair the development of movement control but spare bilateral coordinated movement initiation
We previously described a novel task where rats are trained to perform bilaterally coordinated movements with the forelimbs to obtain rewards (Fig. 1A) (36). Briefly, animals were trained to simultaneously displace two levers vertically, one with each forepaw (Fig. 1A). Rats were asked to maintain the levers below a spatial threshold (2.6 cm) for at least 750 ms (in some experiments, we used a 50-ms threshold; see below). At the beginning of training, animals performed uncoordinated left-right movement trajectories (Fig. 1B, left), but with training, movement trajectories became less variable, and left-right movements tended to overlap (Fig. 1B, center and right). Using this behavioral protocol, multiple unilateral and bilateral execution parameters can be extracted from movement trajectories (fig. S1) and can be reliably followed throughout the learning curve. In a control group (n = 22), training improved interlimb correlations (Fig. 1C) and decreased bilateral movement onset synchrony variability [bilateral movement onset variability (BMOV); Fig. 1D]. BMOV is a good indicator of how well coordinated the beginning of the bilateral action is. Training also decreased movement overshoot (Fig. 1E), defined as the time that the animals maintain the levers in the holding position after a reward is given. Overshoot is a robust indicator of moment duration. The general effort, defined as the average amount of time that the animals pressed the levers to obtain single rewards, was also decreased with training (Fig. 1G). Movement speed remained stable throughout the learning curve (Fig. 1F). We were also able to estimate learning-related changes in individual forelimbs (unilateral variables, fig. S1C), for example, how stereotypical was the movement of each forelimb. The analysis of intralimb trajectory correlation (Fig. 1H) revealed that movement stereotypy developed differentially for each forepaw, and we were able to classify a forelimb that quickly developed higher correlation indexes (Fig. 1H, left; hereafter referred to as “best limb”), while the other expressed a slower learning curve and consistently presented lower correlation indexes (Fig. 1H, right; hereafter referred to as “worst limb”). Worst limbs consistently showed higher intralimb trajectory variabilities, a good indicator of how stable the movement of individual forelimbs in a trial-by-trial basis is (Fig. 1I), and higher unilateral movement onset variabilities (UMOVs; Fig. 1J).
Fig. 1. Learning curves in the bimanual coordination task.
(A) Schematic representation of the behavioral setup and protocol. (B) Representative lever’s trajectories for the left (green) and right (coral) forepaw movements in rewarded trials of three different sessions (time thresholds are indicated for each panel). Trajectories are aligned to reward onset indicated by zero (dashed line). Notice how training induces overlapping of left-right trajectories. (C to J) Learning curves for interlimb correlations (C), bilateral movement onset variability (BMOV) (D), overshoots (E), maximum speed (F), and effort (G). Learning curves for unilateral variables (H to J) are divided into best (left panels) and worst (right panels): intralimb correlation (H), intralimb trajectory variability (I), and unilateral movement onset variability (UMOV) (J). Data are presented as median (solid line) + 75th and 25th percentiles (shaded area). Red dashed lines are presented as visual reference.
In our behavioral protocol, we can evaluate multiple parameters attesting for both bilateral coordination and movement control in each forelimb, allowing us to analyze the contribution of bilateral structures in the representation of the general action (moving both paws together) and in the control individual forepaws. We reasoned that, if the DLS was implicated in both the initiation/selection of the bilateral action and the moment-to-moment control of the movement trajectory, then unilateral DLS-les would be sufficient to alter the initiation and would selectively impair the movement trajectory of the contralateral forepaw. On the contrary, if the DLS was exclusively implicated in only one of the processes, then we would expect specific impairments in either the initiation or the moment-to-moment control of movement trajectories. To test these possibilities, we designed an experiment where naïve rats were subjected to unilateral DLS-les (lesions were performed in the right hemisphere) before the beginning of training in the bilateral coordination task (Fig. 2, A and B). In this case, training started with a 50-session phase, where the temporal threshold was set at 50 ms. Hence, animals only needed to perform a quicker “bilateral ballistic” movement to obtain a reward. After 50 sessions in this configuration, the holding time threshold was increased to 750 ms (Fig. 2A), requiring, besides the ballistic phase, longer movements with potential feedback control. Both groups of animals (control, n = 17; and DLS-les, n = 8) performed the 50-ms version with slightly lower performance than in the 750-ms configuration (Fig. 2C). However, we found clear differences when we compared the performance of control versus lesion groups. In both phases of training, DLS-les animals presented significantly lower interlimb correlation values than the control group [Fig. 2C; Kruskal-Wallis (K-W), X2 = 163.752, P < 0.001; for this and the rest of the learning curve panels, Bonferroni post hoc test for multiple comparisons is in the figure legend], indicating a general uncoordinated bilateral movement. In our setup, the levers are located left to the waterpot (Fig. 1A), forcing the animals (control and lesioned) to approach the levers with a leftward movement. This approach naturally biased them to first touch the right lever and then reach with the left forepaw (contralateral to the lesion site) for the left lever (Fig. 2D; K-W, X2 = 84.385, P < 0.001). Consistent with contralateral effects, the analysis of the timing of the beginning of movements revealed that DLS-les presented problems reaching for the left lever, resulting in a persistent increase in the bilateral movement onset synchrony time [bilateral onset synchrony (BOS) Fig. 2E; K-W, X2 = 293.389, P < 0.001]. It also showed that DLS-les presented higher values of BMOV than the control group, but these differences were more noticeable at the beginning of learning or at the change from 50 to 750 ms (Fig. 2F; K-W, X2 = 162.904, P < 0.001). When we analyzed overshoots, DLS-les animals presented significantly higher values than the control group (Fig. 2G; K-W, X2 = 240.369, P < 0.001), suggesting that DLS is necessary for controlling the appropriate duration of movements. DLS-les animals also presented slightly but significantly higher forelimb movement speeds, especially in the 50-ms phase (Fig. 2H; K-W, X2 = 106.796). The effort to obtain rewards was also increased but only for the early phase of learning in the 50-ms configuration (Fig. 2I; K-W, X2 = 530.836, P < 0.001). Last, the amount of time required to obtain the first 100 rewards was similar in both groups throughout the entire learning curve (Fig. 2J; K-W, X2 = 163.752, P < 0.001). These two variables (effort and time to reach 100 trials) indicate that, despite lesions and differences in interlimb correlations, BOS, BMOV, and overshoot, DLS-les animals developed efficient strategies to obtain sufficient rewards.
Fig. 2. Unilateral DLS-les destabilize bilateral coordination learning.
(A) Schematic representation of the timeline for lesions, training phases, and sessions used for statistical comparisons. (B) Schematic representation (top) and representative magnetic resonance image (bottom) of the excitotoxic unilateral striatal lesions. (C to J) Learning curves (left panels) and boxplot comparisons for specific groups of sessions (right panels) for control (black code; n = 17), and DLS-lesioned (blue code; n = 8) animals for the following variables: interlimb correlations (C), first lever moved (D), BOS (E), BMOV (F), overshoots (movement duration) (G), maximum speed (H), effort (I), and time to reach 100 trials (J). Data for learning curves are presented as median (solid line) + 75th and 25th percentiles (shaded area). Box plots indicate median and 25th and 75th percentiles for groups of 10 sessions at different moments of the learning curves indicated in (A) and (C). Significant differences are indicated by asterisks and lines joining specific comparisons (Bonferroni post hoc test, P < 0.05). Red dotted lines aligned to the median of the first block of sessions are presented as visual reference.
These results suggest that the DLS is necessary for the duration and moment-to-moment control of the movement trajectories. However, this is not sufficient to clarify whether the behavioral effects are related to one or both forelimbs and perhaps to postural impairments potentially associated with these lesions. As we performed unilateral lesions, we expected that at least some of these effects would be related only to the forepaw contralateral to the lesion sites. To evaluate this possibility, we calculated the correlation coefficients between the movement trajectory across sessions from individual forepaws (intralimb correlation). We performed two types of comparisons between groups, best (worst) versus best (worst), and direct comparisons between left (right) versus left (right) forepaws for the following reasons: First, in the control animals, the best forelimb was not always the left forepaw, corresponding to the contralateral one in the lesioned group [from the 39 control animals, 23 (59%) presented lower variability values in the left forepaw]. Second, previous reports indicate that unilateral striatal lesions may produce both contralateral and ipsilateral movement impairments in forelimb reaching tasks (37–40). Third, in the DLS-les group, lesions were performed before training, and it was not possible to establish which forepaw would be the best. This would potentially generate artificial differences between groups, because we could be comparing the “lesioned forepaw” (left) in the lesion group with the one with highest performance in the control group. DLS-les induced no differences in the intralimb correlation values of the best forepaw (Fig. 3, A and B, top; K-W, X2 = 21.558, P = 0.003), but the intralimb correlation values for the worst forepaw were significantly lower than those of the control group (Fig. 3, A and B, bottom; K-W, X2 = 100.469, P < 0.001). These results suggest that the decreased interlimb correlation observed in Fig. 2A was related to impairments in the forepaw controlled by the lesioned striatum. In this context, if the DLS was implicated in the initiation of the bilateral coordinated action, then one could also expect that the lesion of a single DLS would be enough to impair the movement initiation in forelimbs both ipsi- and contralateral to the lesion site. On the contrary, if the coordination of the initiation was in upstream regions, then we would expect that the impairment in the initiation would occur exclusively in the forelimb affected by the lesions. To test this possibility, we calculated the intralimb correlation (Fig. 3B), intralimb trajectory variability (Fig. 3C), the UMOV (Fig. 3D), and overshoot (Fig. 3E) for individual forepaws. When we compared DLS-les with control animals, we found that neither the intralimb trajectory variability nor the UMOV was significantly different for the best forepaw (Fig. 3C, top; K-W, X2 = 291.580, P < 0.001; Fig. 3D, top; K-W, X2 = 51.951, P < 0.001). However, for the worst paw, trajectory variability was significantly higher in 50-ms time threshold sessions but not in the last phase of training (Fig. 3C, bottom; K-W, X2 = 143.760, P < 0.001), while UMOV was significantly higher in DLS-les animals in the second part of the training (Fig. 3D, bottom; K-W, X2 = 128.817, P < 0.001). When we made direct comparisons left (right) versus left (right) between groups, the results were similar (fig. S2), but given the postural bias and adjustments to approach the lever (Figs. 1A and 2, C and D), we found higher levels of variability in the right forepaw in the DLS-les animals (fig. S2, C and D), raising the question of the specific origin of unilateral impairments. Hence, to further ensure that unilateral DLS-les were mainly impairing unilateral forelimbs, we performed DLS bilateral lesions (n = 6; fig. S3, A and B). These lesions produced more devastating effects in all bilateral variables (fig. S3, C to J), but unilateral variables showed increased movement variability in both forelimbs (fig. S3, K to O). The effects of unilateral DLS-les were similar in animals lesioned after extensive training (fig. S4). Together, the data indicate that unilateral DLS-les affect bilateral execution by: (i) impairing the ability of the animals to reach for the left lever (Fig. 2, D and E), potentially related to postural changes; and (ii) affecting the initiation and moment-to-moment movement control of unilateral forelimbs (Fig. 3 and fig. S3), suggesting that the command for the coordinated bilateral movement must reach the BG from an upstream region.
Fig. 3. Unilateral DLS-les unilaterally impair execution parameters.
(A) Representative averaged trajectories (sessions 91 to 100) for the best (top) and the worst (bottom) forelimbs for control (black code; n = 17) and DLS-lesioned (blue code; n = 8) groups. (B to E) Learning curves (left panels) and boxplot comparisons between specific groups of sessions (right panels) are presented for the best (top panels) and worst (bottom panels) forelimbs, for control and DLS-lesioned groups: intralimb correlation (B), intralimb trajectory variability (C), UMOV (D), and unilateral overshoots (E). Data for learning curves are presented as median (solid line) + 75th and 25th percentiles (shaded area). Box plots indicate median and 25th and 75th percentiles for groups of 10 sessions at different moments of the learning curves indicated in Fig. 2A. Significant differences are indicated by asterisks and lines joining specific comparisons (Bonferroni post hoc test, P < 0.05). Red dotted lines aligned to the median of the first block of sessions are presented as visual reference.
Unilateral secondary motor cortex can orchestrate bilaterally coordinated movements
Primary (M1) and secondary (M2) motor cortical regions have been implicated in the initiation of voluntary, unilateral, and bilateral movements (17, 18, 41–44), and these functions arise from the activity of two main classes of principal neurons, PT and IT neurons. PT neurons project directly to the brainstem and spinal cord and unilaterally to the motor regions of the striatum and thalamus (32, 45). IT neurons project bilaterally to the striatum and other cortical areas (46–48). Even when the precise role of PT and IT is still under debate (18, 49–51), IT neurons are natural candidates to coordinate and initiate bilateral actions, given their bilateral organization. To explore this possibility, first, we performed unilateral lesions of M1 and M2 regions in naïve animals before (n = 10) and after (n = 5) training in the bilateral coordination task. Contrary to the outcome of unilateral striatal lesions, we found in cortically lesioned animals before training significant decreases in BMOV, overshoots, and time to reach 100 trials (fig. S5, A to K). Intralimb correlations and intralimb trajectory variability were not affected (fig. S5, L to N), and UMOV and unilateral movement overshoots were decreased for both forelimbs (fig. S5, O and P). These changes could be considered mild behavioral improvements. On the other hand, in cortically lesioned animals after training, the only noticeable effect was a reduction in overshoots (fig. S6).
These data suggest that the learning and expert execution of this bilateral movement can rely on the activity of unilateral motor-cortical regions. However, previous reports have demonstrated that, after extensive training, some forelimb motor skills in rats can be performed without the control of motor cortical regions (27). Hence, to further evaluate the potential contribution of bilateral motor cortico-striatal projections during the execution in our behavioral protocol, we designed a strategy to specifically manipulate bilateral IT projections originating in M2 (M2-IT). First, we injected the retrograde virus AAV-pgk-Cre [adeno-associated virus (AAV) retrograde] into one DLS. This virus induced the expression of the Cre-recombinase protein under the phosphoglycerate kinase (PGK) promoter in all neurons projecting to the DLS, including PT and IT (Fig. 4A, left). One month later, we injected the retrograde virus pAAV-EF1a–double floxed–hChR2 into the DLS contralateral to the first injection. This virus induced the expression of channelrhodopsin 2 (ChR2) only in Cre-positive neurons with bilateral projections (Fig. 4A, middle). At least 1 month after the second injection, animals were subjected to a third surgery, where we implanted two optical fibers directed to M2 and a 64-channel silicon-based microelectrode array directed to the DLS (Fig. 4A, right). Consistent with anatomical projections associated with IT cells, histological examination of these brains demonstrated the bilateral presence of enhanced yellow fluorescent protein (EYFP) in motor cortical regions (Fig. 4B). Conversely, thalamic regions such as the central medial complex or the ventral basal complex, known to unilaterally project to the DLS, did not express EYFP (Fig. 4B), confirming that our viral strategy was selective for bilateral projections to the DLS. To ensure the functionality of this strategy, we performed electrophysiological recordings in urethane anesthetized animals (Fig. 4C) and well-trained freely moving rats (Fig. 4D) subjected to the described infection protocol. Sixty-four–channel microelectrode arrays were directed to the forelimb region of the DLS, and we evaluated the responses to optical stimulation of M2. Optical stimulations were performed contralaterally in anesthetized recordings and ipsilaterally and contralaterally in freely moving recordings. In both cases, anesthetized and freely moving conditions, we observed clear short-latency striatal ipsilateral and contralateral responses to continue and train stimulations (Fig. 4, C to F). To estimate the number of M2 neurons affected by our stimulation, we simultaneously stimulated and recorded M2 neurons in three anesthetized animals (226 neurons; Fig. 4G). We found that 500-ms light pulses induced three types of responses: sustained or transient activations and sustained inhibitions (Fig. 4, H and I). Forty percent of the neurons presented significant increases in the firing rates, 41% presented significant decreases, and the remaining 19% presented no significant changes (Fig. 4J). The response latencies for the activations were significantly shorter than for the inhibitions (Fig. 4, J and K). These results indicate that our method produced robust changes in about 80% of M2 neurons. Together, these results indicate that, by using this viral strategy, we were able to specifically manipulate M2-IT to the DLS. However, a limitation of our strategy is that we cannot discard the possibility that unilateral stimulation could also activate the contralateral M2 neurons by antidromic activation of their callosal axon fibers.
Fig. 4. Targeting bilateral cortico-striatal projections from M2.
(A) Schematic representation of the infection strategy, optical fibers, and microelectrode implantation. (B) Histological confirmation of bilateral infections. Yellow fluorescence protein expression in the injection sites in both striata (i), cortical M1/M2 (ii and iii), and the corpus callosum (iv). No expression was found in thalamic regions (v). VPL, ventro postero lateral; VPM, ventro postero medial. CC, corpus callosum; CL, centrolateral thalamic nucleus; CM, central medial thalamic nucleus; PF, parafasicular nucleus; PO, posterior thalamic nuclear group. (C and D) Schematic (left) representation and representative spike rasters (top) and average perievent histograms (bottom) (right panels) for electrophysiological recordings and optical manipulations in anesthetized (C) and freely moving (D) rats. Activity in the raster plots is aligned to the optical stimulation (500-ms stimulus, left and center panels; 5 ms, five stimulus trains, right panels) delivered into the cortex ipsilateral or contralateral to the recording site. F. rate, firing rate. (E) M2-evoked response latencies for cells recorded in the DLS (ipsilateral or contralateral color-coded; normalized to the highest peak of the distribution; left). Comparison of the response latencies shorter than 30 ms (right). (F) Averaged firing rates evoked by ipsilateral (left) or contralateral (right) M2 stimulation, for cells recorded in DLS of freely moving animals expressed as z score (color-coded) and sorted according to the maximum averaged firing rate reached during the 500 ms of stimulation. Blue bars indicate the moments when optical stimulation was provided. (G) Stimulation and recording site in M2. (H) Representative spike rasters [as in (C)]. (I) Averaged firing rates evoked by optical stimulation in M2. (J) Response latencies for cells that increased (orange) or decreased (blue) their firing rate during stimulation. Inset displays the percentage of cells that significantly increased, decreased, or did not change (gray) their firing rate during stimulation. (K) Comparison of the response latencies. Box plots indicate median and 25th and 75th percentiles. Significant differences are indicated by asterisks (Bonferroni post hoc test, P < 0.05).
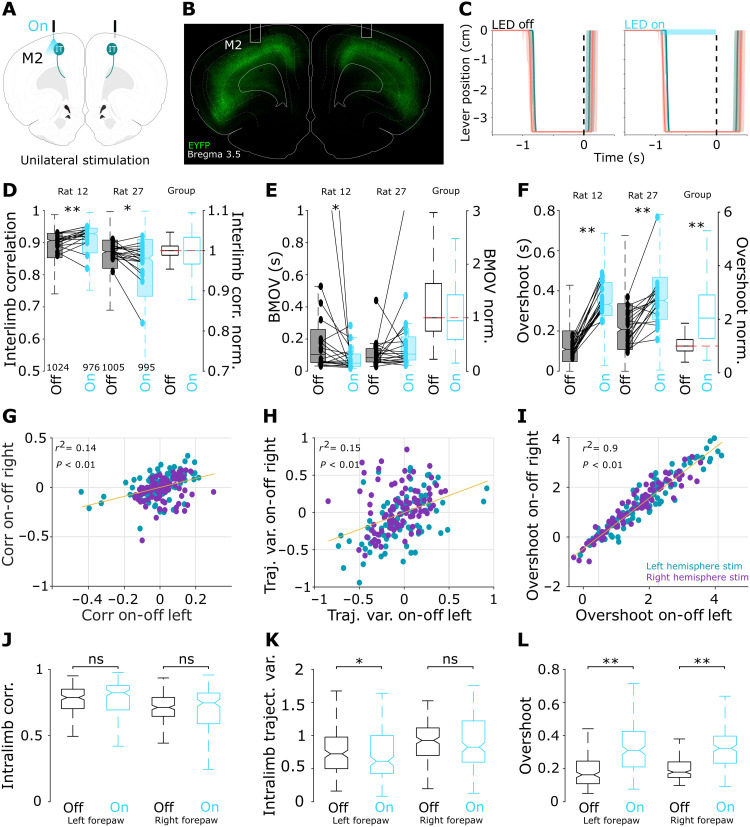
Then, we applied the same viral strategy to a group of well-trained animals in the bilateral coordination task (n = 9); four subjects were the same animals implanted for freely moving recordings from the previous section, and five subjects were only implanted with bilateral optical fibers directed to layer V of M2 (Fig. 5, A and B). We reasoned that, if M2 was involved in bilateral coordination, then unilateral stimulations would be sufficient to bilaterally affect execution. To test this possibility, in different sessions, we stimulated left or right M2. Optical stimulation was given in 50% of randomly selected trials and was triggered by minimum displacement (0.1 cm) of either lever. Stimulation lasted while either lever was pressed and stopped immediately when the reward was provided, even if the animal continued pressing the levers (Fig. 5C). We observed that, in most of the sessions, unilateral stimulation induced two main effects noticeable in the movement trajectories of both left and right forepaws: an increase (or decrease) of bilateral correlation coefficients and a very robust increase in overshoot (Fig. 5C). In Fig. 5 (D to F), we display two representative animals (left panels) and the cohort (right panels). Stimulation-related interlimb correlations (Fig. 5D) and BMOV (Fig. 5E) changes were variable, resulting in nonsignificant changes in the group. Stimulation produced longer overshoots, and these effects were robust and evident in all individuals of the group (Fig. 5F). It is important to highlight that optical stimulation stopped as soon as the reward was delivered; hence, the longer overshoots were the result of stimulation before reward delivery. It is also worth mentioning that these effects were bilateral; that is, animals presented longer overshoots in both forelimbs even when stimulation was provided unilaterally, independently of the stimulated hemisphere (see below).
Fig. 5. M2 cortico-striatal bilateral projections increase bilateral movement duration.
(A) Schematic representation of unilateral M2 stimulations. (B) Representative histological confirmation of optical fiber position and EYFP expression in M2. (C) Representative average trajectories (dashed area, 25th and 75th percentiles) for the left (green) and right (coral) levers aligned to reward delivery (time “0,” dashed line), for stimulated and nonstimulated trials. Optical stimulation (indicated by a horizontal blue bar) was triggered by minimum displacement of either lever and was stopped at reward delivery (black dashed line). Interlimb correlations (D), BMOV (E), and overshoot (F) for nonstimulated (black code) and stimulated trials (blue code) for two representative animals (filled box plots) and the complete group (open box plots). Box plots indicate the median and 25th and 75th percentiles for all trials; pairs of dots united by lines indicate medians of each session for each animal; the number of trials analyzed for each animal and condition is indicated at the bottom of (D). Data for each animal were normalized to the average value of the nonstimulated trials. Correlation plots between left and right forepaw values for intralimb correlation coefficients (G), unilateral trajectory variability (H), and unilateral overshoot (I) for trials where stimulation was provided in the left (blue) and right (purple) M2. Each dot represents the subtracted values from the stimulated minus nonstimulated trials. Unilateral intralimb correlation coefficients (J), trajectory variability (K), and overshoots (L) for the left and right forepaws during nonstimulated (black) and stimulated trials (blue). Box plots indicate the median (25th and 75th percentiles) of the stimulated versus nonstimulated trials. Significant differences were obtained by applying the Mann-Whitney test are indicated by asterisks (*P < 0.05 and **P < 0.001). ns, not significant.
To analyze the effects of M2-IT unilateral stimulations on individual forelimbs, for each experimental session and each forepaw, we calculated the average difference between stimulated and nonstimulated trials (stimulated minus nonstimulated) for the intralimb correlation values, trajectory variabilities, and overshoots. Figure 5 (G to L) shows that stimulation-related increases or decreases in these variables are reflected as positive or negative values, respectively. Then, we calculated the correlation coefficients between left and right forepaw values. If unilateral stimulations affected only the contralateral forelimb, then we could expect low correlation values between left and right forepaws. On the contrary, if unilateral stimulations affected both forelimbs, then we could expect high correlation values in the modulated variables. We found that intralimb correlations and trajectory variability showed moderate correlations (Fig. 5, G and H). However, overshoot correlations between left and right forepaws were very high (Fig. 5I). The bilateral effect on overshoot was further confirmed by simply comparing stimulated versus nonstimulated trials for each forepaw (Fig. 5, J to L). A significant decrease in trajectory variability was observed for the left forepaw (Fig. 5K). Together, these results confirm that the main effect of M2-IT cortico-striatal bilateral projection activations led to an increase in the duration of bilateral movements. This effect was reflected in a significant decrease in the number of attempts to obtain a reward (fig. S7).
Unilateral activation of M2-DLS projections spares the temporal structure of task-related striatal sequential activations
The previous results indicate that the unilateral activation of the cortico-striatal M2-IT projections is sufficient to bilaterally coordinate movements, while DLS would be recruited to implement movement modulations. To test this hypothesis and find a possible signature for bilateral cortico-striatal interactions, we performed the same stimulation experiments in well-trained animals implanted with microelectrode arrays directed to the right DLS (Fig. 4, A and D) and performed recordings during the execution in the bilateral coordination task. We analyzed the activity of 818 DLS neurons from three animals in 51 sessions. In each recording session, 50% of randomly selected trials were stimulated. Aligning spiking activity to reward delivery revealed that, during task execution, neurons in the DLS responded with different patterns of activity, and some of these patterns were affected by unilateral optical stimulation of M2 (Fig. 6A). Consistent with previous reports in different tasks (5, 7, 52), in nonstimulated trials, striatal neurons displayed continuous sequential activation covering the complete trial execution (Fig. 6B, left). When we aligned the neural activity from stimulated trials using the same index obtained from the nonstimulated trials, sequential activation seemed to maintain its temporal structure (Fig. 6B, right). To confirm this possibility, we calculated the distribution of correlation coefficients of the activity of individual cells between nonstimulated and stimulated trials for the full population, or the subpopulation of cells with peak activities during the stimulation period, and compared it with those obtained after shuffling the order of cells for nonstimulated and stimulated trials (1000 iterations; Fig. 6C). The fact that nonstimulated/stimulated coefficients were significantly higher than neuron-to-neuron coefficients confirmed that optical stimulation spared the temporal structure of the neural sequence. After this, we explored what proportion of the population was significantly affected by the stimulations. We subtracted the z-scored activity of the nonstimulated trials from that of the stimulated trials (Fig. 6D). Then, we calculated the absolute z-scored averaged differences between nonstimulated/stimulated trials in 500-ms sliding windows in steps of 50 ms until covering 8 s centered at reward delivery. Last, for each 500-ms window, we extracted the percentage of cells that expressed z-scored absolute differences higher than 2. As expected, the highest percentage of cells expressing differences between nonstimulated and stimulated trials occurred within the 750 ms before the reward, the moment when optical stimulation was triggered by pressing the levers (Fig. 6E). Together, these data confirmed the effectiveness of the optogenetic modulations during task performance. On the other hand, the fact that the sequential temporal structure was unaffected by the stimulation suggests that the behavioral effects associated with M2-IT stimulations were related to the activity of subpopulations of cells and not the entire population.
Fig. 6. Sequential activation of DLS activity during bilateral coordinated movements.
(A) Pairs of spike rasters (top) and average perievent histograms (bottom) depicting five representative cells with different response patterns recorded during the execution of bilateral coordinated movements during nonstimulated (top row, black code) and stimulated (bottom row, blue code) trials. Spiking activity was aligned to reward onset (red lines and arrowheads). The moment when the lever’s threshold was reached is indicated in orange lines and arrowheads, and the maximum firing rate (in hertz) for each perievent histogram is indicated. Trials are sorted according to the duration of the movement; longer trials or trials with more than one attempt before reward delivery are located in the upper regions. (B) Z-scored averaged activation of the firing rate of all recorded cells sorted to the moment of their highest peak firing rate between −2 and 2 s around reward delivery. (C) Correlation coefficients between firing patterns of individual cells during nonstimulated and stimulated trials. These correlations were compared with the distribution of correlation coefficients obtained from the shuffled order of cells (we ran 1000 iterations to create a confidence interval; indicated by gray dots and red dashed lines). (D) Color matrix of the z-scored differences between nonstimulated and stimulated trials, sorted according to the maximum differences during the immediate 700 ms before reward onset. (E) Percentage of cells that expressed z-scored absolute differences higher than 2. Differences were calculated in 500-ms sliding windows (50-ms steps). Red dashed line indicates reward onset.
Striatal kinematic-related representations are not affected by M2-IT-DLS projections activation
The previous section demonstrates that optical activation of M2-IT bilateral cortico-striatal projections does not alter the temporal structure of the DLS sequential activation accompanying the execution on the bilateral coordination task. However, it is well known that subpopulations of DLS neurons host kinematic representations of procedural execution (5, 6, 9, 53, 54). Hence, we explored whether these representations were affected by the stimulation of these projections. In this task, during bilateral movement execution, animals expressed stereotypical behavioral patterns, composed of movement transitions between the water port and the set of levers, head rotations, and bilateral movements of the forelimbs. To characterize these complex behavioral patterns, we recorded behavior by three means: First, with a video camera located above the setup, to monitor head/body displacements between the water port and the set of levers (fig. S8A). The position and speed trajectories of these movements were highly stereotyped, especially during the first ~120 trials of the sessions when animals were more engaged in obtaining rewards. Second, we used a three-axis accelerometer integrated into the recording head stages, producing a continuous analog representation of head rotations (fig. S8B). Last, as for all behavioral experiments reported above, the position of the levers was also continuously recorded (fig. S8C). For the following analyses, we excluded six sessions where behavioral quantifications were incomplete (not optimal video conditions); hence, we analyzed the activity of 780 DLS neurons from the same recording sessions as in the previous section. Consistent with previous reports (5, 9, 53), an important fraction of DLS neurons expressed evident positive and negative linear correlations with head/body position (Fig. 7, A to C) and velocity (Fig. 7, D to F). Partial correlation analysis, including time, lever position, lever speed, head/body position, head/body velocity, and the acceleration of the three head axes (Fig. 7, A to F), revealed that head velocity and position were the variables that best explained the variability in the firing rates (Fig. 7G). Multiple regression analysis confirmed that the spiking activity of DLS neurons integrates multiple execution variables [Fig. 7, C and F; (5)]. We did not find significant differences between the partial and multiple correlation values between stimulated and nonstimulated trials, indicating that these striatal representations are not directly related to or driven by M2-IT cortico-striatal bilateral projections (Fig. 7G). These results were further confirmed in sessions where optical stimulation was delivered continuously in 30-s periods (i.e., not triggered by lever pressing and alternated with 30-s off stimulation), covering the whole movement transition between the reward port and the levers (fig. S9). Then, to confirm the striatal specificity of these effects, we compared the partial and multiple correlation coefficients obtained from the DLS with the same coefficients obtained from recordings of the primary somatosensory cortex (S1) performed in two animals (196 units recorded in eight sessions). S1 recordings revealed very similar levels of representations for almost all variables (Fig. 7H) except for head/body velocity, which was significantly lower in S1, in both stimulated and nonstimulated trials (Fig. 7I). These experiments indicate that the animal’s general movement velocity to approach and move away from the reward port and the lever set is robustly encoded in the activity of DLS, and these representations are independent from bilateral projections arising from M2.
Fig. 7. Kinematic representations in the DLS are not affected by M2-IT stimulation.
(A to F) Two representative units with firing rates correlated with head/body position (A to C) or head/body velocity (D to F) when transitioning between the water port and the levers. Trial-by-trial color-coded instantaneous head/body positions (A) and velocity (B) (top), spike rasters (middle), and average perievent histograms (bottom) aligned to reward onset (red lines and arrowheads). Trials were sorted according to the amplitude of head/body movements. Left and right columns depict activity and behavior collected in nonstimulated (black color code for the entire figure) and stimulated trials (blue color code for the entire figure), respectively. (B and E) Scatter plots with firing rates versus the best head/body position and speed (means ± SD) for nonstimulated and stimulated trials. (C and F) Partial and multiple correlation coefficients between firing rate and task variables. (G and H) Partial and multiple correlation coefficient (absolute values) for all recorded neurons and behavioral variables in the DLS (G) and S1 (H), during nonstimulated and stimulated trials. (I) Comparison between DLS and S1 for head/body position and velocity. Box plots indicate the median (25th and 75th percentiles) of the stimulated versus nonstimulated trials. Significant differences are indicated by asterisks and lines joining specific comparisons (Bonferroni post hoc test, P < 0.05).
Striatal overshoot/interlimb correlation representations are driven by M2-IT-DLS projections
The previous data demonstrate that optical activations of M2-IT bilateral cortico-striatal projections do not have a particular influence on kinematic parameters of the general body transitions (Fig. 7). However, the same optical activations produced clear behavioral effects on the bilateral forepaw coordination and overshoot (Fig. 5). Hence, we wondered whether the lever-related parameters could also be represented in the activity of DLS and potentially modulated by the pathway-specific stimulations. To test this possibility, for each cell and each trial, we calculated the average firing rate during the immediate 750 ms before the appearance of the reward and correlated this activity with the interlimb correlation coefficient or the overshoots for all trials of the session, the nonstimulated trials, or the stimulated trials (Fig. 8, A to C). For both variables, we found cells with clear correlation coefficients, but unlike kinematic representations, optical stimulation significantly changed these values. Then, we formally quantified whether kinematic or lever-related variables were differentially represented in neurons responsive or nonresponsive to M2-IT stimulation. To this aim, we first estimated the fraction of neurons from the entire population or from the M2-IT–modulated subpopulation (see Materials and Methods) that were also significantly modulated by either lever-related variables (bilateral coordination, overshoot, or effort) or kinematic variables (velocity or position). After, we randomly extracted an equal number of neurons from the nonoptically modulated cells and calculated the fraction significantly modulated by lever-related and kinematic variables. To create a confidence interval, we repeated this randomization 1000 times. We found that nearly 60% of the M2-IT–modulated neurons were also significantly modulated by level-related variables, a number that was above the confidence interval (Fig. 8D). For the rest of the population, this number decreased to nearly 30% and was below the confidence interval (Fig. 8D, left). These numbers were similar when we performed the analysis in stimulated or nonstimulated trials. In contrast, for the kinematic-related variables, both the full population and the M2-IT–modulated neurons presented similar percentages, and both were below the confidence intervals (Fig. 8E, left). Then, to estimate how lever-related or kinematic encoding was affected by light stimulation in the M2-IT–modulated subpopulation, we calculated the correlation coefficients of the correlation values between nonstimulated and stimulated trials but only in the M2-IT–modulated fraction of neurons. Kinematic variables displayed almost perfect correlations between nonstimulated and stimulated trials (correlation values above 0.9; Fig. 8E, right), confirming that stimulation did not affect these parameters. Conversely, these values dropped for the lever-related variables (correlation values below 0.45; Fig. 8D, right), indicating that these parameters were sensitive to the M2-IT stimulation. We complemented this analysis with Venn diagrams, showing that a large proportion of cells (> 50%) exhibited significant correlations with either lever-related or kinematic parameters (Fig. 8F). More than half of the cells classified as lever-related were exclusively modulated by this parameter, and the rest also presented kinematic modulations. On the other hand, almost half of the velocity-related cells were exclusively modulated by this parameter, and the rest presented shared modulations. Last, a third of position-related cells were modulated exclusively, with two-thirds showing shared modulations. These proportions were preserved in both nonstimulated and stimulated trials (Fig. 8F), suggesting that the main effects of the optical stimulation were related to a “gain” in the signal and not in the quality of it. To test this possibility, we calculated the average firing rates during the same time window (750 ms before reward delivery) for the subgroups of exclusively modulated cells (lever-related, velocity, or position) and found that, in general, lever-related cells presented significantly higher firing rates than speed- or position-modulated cells (Fig. 8G; K-W, X2 = 23.11, P < 0.001). Then, we calculated the absolute change in firing rate between nonstimulated and stimulated trials for each subgroup. Here again, the lever-related cells showed significantly higher values than the cells in the other subgroups (Fig. 8H; K-W, X2 = 12.58, P = 0.0019), confirming that this group was more sensitive to optical manipulation of the bilateral M2-IT projections.
Fig. 8. DLS movement duration representations are affected by M2-IT stimulation.
(A to C) Scatter plots for three representative units with firing rates correlated with interlimb correlation (A) or overshoot (B and C) values (means ± SD) for all trials (left panels) and nonstimulated (middle panels) and stimulated trials (right panels). (D and E) Left histograms display the fraction of cells significantly correlated with interlimb lever-related (D) or kinematic (E) variables for the entire population (all) or the subpopulation that was significantly modulated by M2-IT stimulation (sig.) during nonstimulated (gray) and stimulated trials (blue). Red dashed lines indicate the 99.5 confidence interval (see main text), and asterisks indicate values outside the confidence interval. Right scatter plots display correlation coefficients between nonstimulated and stimulated trials for lever-related (D) and kinematic variables (E). Significant correlations are indicated by asterisks associated to R values. (F) Venn diagrams indicating the proportion of cells expressing significant correlation coefficients with “lever-related” variables (interlimb correlations and overshoots pooled together), head/body position, and velocity (proportions during stimulated trials are indicated in blue). Percentage with respect to the total population of cells is indicated in parenthesis. (G) Baseline firing rates for each subpopulation of cells. (H) Absolute change in firing rate induced by optical stimulation of M2-IT for each subpopulation of cells. Significant differences are indicated by asterisks and lines joining specific comparisons (Bonferroni post hoc test, P < 0.05).
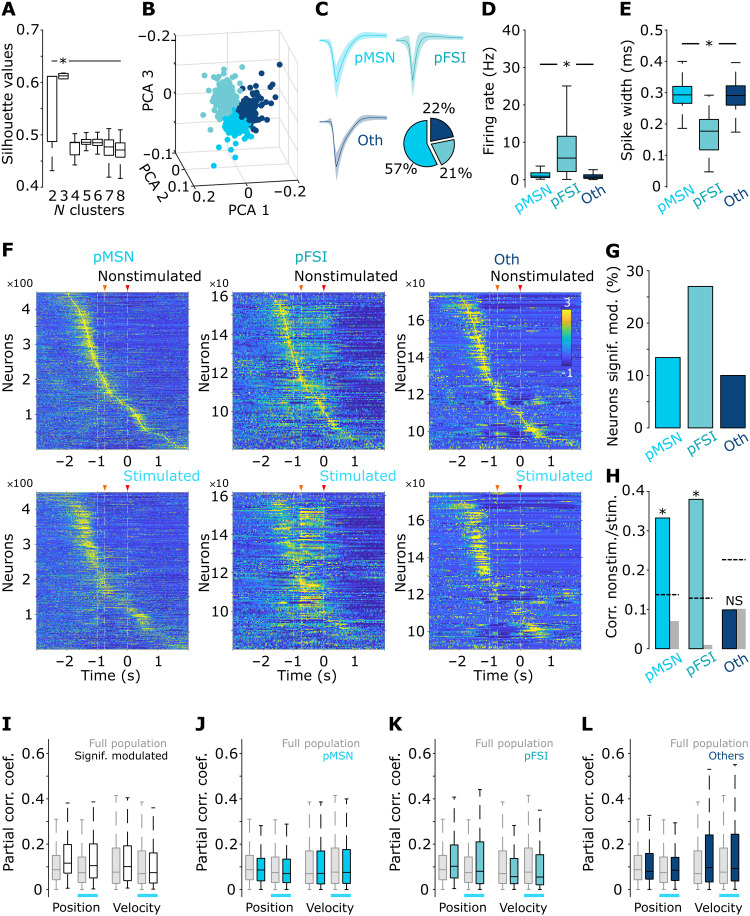
Last, previous reports have shown that, in the striatum, it is possible to classify subgroups of neurons into specific cell types (medium spiny neurons or fast-spiking interneurons), by analyzing the spike wave shapes (55, 56). Hence, we explored whether the subgroup of neurons responsive to M2-IT stimulation could be composed of a particular cell type. To this aim, we applied principal components analysis (PCA) to the average spike shapes of every neuron, then we projected the first three PCs and applied k-means to generate two to eight clusters with similar spike shapes (7000 iterations) (57, 58). In each iteration, we scored the projection with the silhouette method (53, 58). Last, we selected the projection with the highest silhouette score (Fig. 9A). This projection corresponded to three clusters (Fig. 9B) with characteristic spike shapes (Fig. 9C). The biggest cluster [putative medium spiny neurons (pMSNs)], with nearly 60% of the cells, presented low firing rates and longer spike widths (Fig. 9, C to E). A second cluster with 21% of the population [putative fast-spiking interneurons (pFSIs)] was composed of sharp spike shapes and the highest firing rates, consistent with previously reported FSI (55, 56). The remaining neurons were assigned to a third cluster named “other neurons.” Then, we applied our previous analyses (light modulation, sequential activation, and kinematic encoding) to each subpopulation separately. The three subpopulations displayed continuous sequential activation covering the complete trial execution (Fig. 9F), but we found that nearly 30% of pFSI were significantly modulated by optical stimulation of M2-IT, while this percentage was only about 10% in the other two subpopulations (Fig. 9G). We also found that, during the period of lever pressing (750 ms before reward delivery), optical stimulation significantly altered the temporal structure of the sequence for the subgroup “Others” but not for pMSN or pFSI (Fig. 9H). This was related to the fact that, in Others, optical stimulation induced a decrease in the firing rate (Fig. 9F, right). We did not find significant differences in kinematic encoding in either subpopulation (Fig. 9, I to L). Together, these analyses suggest that activation of M2-IT would recruit a subgroup of DLS neurons with a higher prevalence of FSI that, in turn, would modulate the striatal microcircuit to modify the duration of movement. However, this possibility must be thoroughly explored in future research.
Fig. 9. Bilateral coordination representation in subpopulations of striatal neurons.
(A) Silhouette values for 1000 iterations in two to eight k-means projections from the principal components analysis (PCA) on the average spike waves for all cells recorded during freely moving recordings. (B) PCA best projection corresponding to three clusters (color-coded). (C) Average spike waves from all cells classified as belonging to the best projection in (A) and (B). Spike waves were named as putative medium spiny neurons (pMSNs), putative fast-spiking interneurons (pFSIs), or others (Oth). The pie plot displays the percentage of each subpopulation. Average firing rates (D) and spike widths (E) for each subpopulation. (F) Z-scored averaged activation of the firing rate of all recorded cells belonging to each subpopulation and sorted to the moment of their highest peak firing rate between −2 and 2 s around reward delivery during nonstimulated (top row) and stimulated (bottom row) trials. (G) Percentage of significantly modulated neurons by M2-IT stimulation for the three subpopulations. (H) Correlation coefficients between firing patterns of individual cells during nonstimulated and stimulated trials for the three subpopulations. These correlations were compared with the distribution of correlation coefficients obtained from the shuffled order of cells (we ran 1000 iterations to create a confidence interval; indicated by gray bar and black dashed lines). (I to L) Position and velocity partial correlation coefficients (absolute values) for all significantly modulated cells (I), and the subpopulations of pMSN (J), pFSI (K), and others (L), during nonstimulated and stimulated trials. For comparative purposes, on each subpanel, the partial correlation values for the whole population are displayed. Box plots indicate the median (25th and 75th percentiles) of the stimulated versus nonstimulated trials. Significant differences are indicated by asterisks and lines joining specific comparisons (Bonferroni post hoc test, P < 0.05).
DISCUSSION
Bilaterally coordinated movements are a paramount feature of animal behavior (29, 30). Understanding the neural basis of these complex actions may help to disentangle the specific contribution of cortical and subcortical regions to the general process of motor control. In this work, we have explored the participation of cortico-striatal projections in the context of bilaterally coordinated movements in rats. Here, we provide robust evidence, indicating that the orchestration of bilateral movements is related to cortical regions, while the striatum is recruited for the implementation of the appropriate movement duration and trajectory. Our conclusions are based on various lines of evidence. First, unilateral lesions of the DLS produced lower behavioral indices in bilateral coordination–related variables (Fig. 2), yet these effects were explained by an increase in unilateral movement trajectory variability (Fig. 3) and not by an increase in the BMOV, suggesting that the final interlimb coordinated outcome was affected by mechanisms related to control of individual limbs and not by mechanisms related to motor coordination. This idea was further supported by the fact that bilateral DLS-les induced bilateral impairments in intralimb correlations and variability (fig. S3). The effect of unilateral DLS-les was consistent in naïve, apprentice, and expert animals (Fig. 3 and fig. S4), confirming its pivotal role in this function. These behavioral findings support previous studies suggesting that one of the main functions of the BG is to provide moment-to-moment control to motor commands arising in upstream regions (5, 8, 13). Second, our pathway-specific manipulations of M2-IT cortico-striatal projections provide strong support for this idea. Unilateral stimulation of M2 produced clear bilateral effects, affecting bilateral correlation coefficients but notably increasing bilateral movement duration (overshoot; Fig. 5, C, F, I, and L). Conversely, these manipulations did not impair the movement variability of the trajectories of individual forelimbs (Fig. 5, G, H, J, and K). Last, these behavioral effects were accompanied by changes in neural representations of bilateral execution–related variables but, interestingly, did not affect the striatal representations of kinematic variables (Figs. 7 to 9). Together, our data indicate that bilateral actions are orchestrated in cortical regions with high interhemispheric connectivity, recruiting subcortical regions, such as the DLS, to provide kinematic support to motor execution. These data also support the notion of a modular organization where specific cortico-striatal projections may be implicated in certain aspects of movement execution (28, 49). In this case, M2-IT bilateral projections to the DLS would be implicated in setting the duration of movements.
Actions are commonly defined as the implementation of movements to achieve a particular goal. While this operational definition has been very useful to advance popular concepts such as action selection, it may be limited when trying to decompose the complexity of goal-directed or habitual movements. In this context, several important observations have provided support for the notion of the dorsal striatum as an action selector (1–3). However, bilaterally coordinated movements are a good example of the complexity of action representations (59) and for when this definition falls short. For instance, there is no evidence for a direct interhemispheric projection between the two striata. Nevertheless, there is substantial evidence of bilateral cortico-striatal projections arising from multiple cortical regions, including the sensorimotor and premotor cortices (32, 46, 60). These anatomical features would theoretically disqualify the dorsal region of the striatum to coordinate or select bilateral movements. Our strategy to use unilateral lesions of the DLS supports this line of thinking: It produced clear unilateral increases in trajectory variability, but the UMOV of the best forelimb remained stable. While finding reliable indicators of action initiation is a complex task, movement onset synchrony variability (unilateral and bilateral) has been reported to be a reliable proxy of how stable the motor commands are on a trial-by-trial basis (41, 61, 62). In our behavioral protocol, we can evaluate two important phases of execution. In the first phase, the animals produce a ballistic movement, quickly displacing the levers downward. In this phase, movements are not subjected to feedback mechanisms and are thought to be subjected to feedforward control with corrections implemented on a trial-by-trial basis (63–65). This ballistic phase is followed by a holding phase where animals are required to maintain the position of the levers for a fixed period of 750 ms, long enough to be subjected to feedback control. The fact that DLS unilateral lesions produced robust effects in the second phase of the movement, that is, an increase in trajectory variability and movement duration (overshoot), is more consistent with a DLS modulatory function over already commanded movements from upstream regions. This possibility was later confirmed by our pathway-specific optogenetic manipulations. In the same context, previous examples from the literature have shown that unilateral striatal ablations produce mild or transitory behavioral effects in rats performing locomotor-related behaviors, such as rotarod or sequence execution on a running treadmill (8, 58, 66, 67). However, behavior was markedly impaired when the striatum was deprived from specific streams of information, for example, sensory (8) or dopaminergic pathways (58, 68–70), confirming its modulatory and not leading role in motor control. These antecedents may appear partially contrasting with our current observations. On the one hand, our unilateral striatal lesions produced no effects in effort or the time to reach 100 trials (Fig. 2), which would be consistent with the mild/transitory effects reported before. However, the trajectory-related variables were severely impaired, before and after training (Figs. 2 and 3 and figs. S3 and S4). This difference is most likely related to the fact that locomotion relies mainly on organized networks contained in the spinal cord, where multiple supraspinal nuclei modulate and time the changes in speed and locomotor patterns (71). On the contrary, in this task, forepaw bilateral movements directed to the levers are not sustained by rhythmic networks in the spinal cord, and similar movements have been previously shown to be more sensitive to motor cortical and subcortical lesions depending on the learning stage (27, 72).
Optogenetic manipulations of the bilateral M2-IT cortico-striatal projections resulted in robust increases in bilateral coordination indexes and movement duration (Fig. 5). A fundamental feature of this manipulation is that, even when stimulations were performed unilaterally, the effects on movement duration were bilateral (Fig. 5, F, I, and L). As mentioned before, the technical confound due to our viral strategy is that we cannot discard the possibility that unilateral stimulation could also activate the contralateral M2 neurons by antidromic activation of their callosal axon fibers. However, the fact that unilateral M2-IT stimulation produced similar response latencies in both right and left striata (Fig. 4E) strongly supports our interpretation. Together with M2 lesions in naïve (fig. S5P) and overtrained animals (fig. S6D) showing a decrease in movement duration, the data confirm the leading role of the motor cortex in orchestrating this action. This suggestion is consistent with recent experiments in rats, where unilateral subthreshold electrical stimulation of M2 strongly modulated bilateral M1-driven motor commands (73). Given our behavioral experimental design, we specifically intended to target cortico-striatal bilateral projections; hence, the role of PT cortico-striatal projections remains to be determined in this task. However, previous reports in nonhuman primates (33, 62) and recently in rodents (17, 49) strongly suggest that PT cells are related to setting the direction of movements. Our data are also consistent with a recent report in mice suggesting that forelimb movement duration can be shortened by inactivating IT neurons in M1 (49). On the other hand, the fact that bilateral activation of the M2-IT cortico-striatal projections induced such a specific effect on the “gross” duration of movement confirms and expands previous views highlighting the importance of cortico-striatal connectivity for specific components of skill reaching (28). It also provides further support for the idea of a cortico-striatal modular organization (28, 74). In the same line of thinking, our neural recordings of DLS not only confirmed the presence of subgroups of cells with clear kinematic representations (Fig. 7) (5, 9) but also revealed subgroups of cells modulated by bilateral execution variables such as interlimb correlation and movement duration (Fig. 8). Of particular relevance, kinematic-modulated neurons were not affected by the stimulation of bilateral M2-IT projections. By contrast, interlimb correlation– and movement duration–related cells were importantly modulated by these projections. This may be because we directed our stimulation to the forelimb region in M2 (17). It is important to mention that, unlike M2-IT optogenetic manipulations (Fig. 5), the behavioral effects of unilateral M2 lesions before learning were not restricted to movement duration (fig. S5). Two reasons may explain this apparent discrepancy. First, in the optogenetic experiments, we specifically targeted the M2-IT neurons projecting to the DLS, a significantly different manipulation than excitotoxic lesions. In the latter, we permanently remove all neural subpopulations of M2. This manipulation may affect not only a single parameter of movement (overshoot) but also others potentially related to this region, such as synchrony and interlimb correlation. Consistent with this interpretation, lesion experiments of M2 before and after learning also demonstrated a decrease in overshoot (figs. S5, H and P, and S6D), which is the opposite effect to the one observed by the optical manipulations. The second reason is that these effects may be different depending on the learning phase where manipulations were performed. It has been demonstrated that prelearning cortical lesions may result in the impossibility to develop stable movement sequences of the forelimbs, while postlearning lesions resulted in little or no behavioral changes (27). Consistent with this interpretation, M2 lesions before learning produced more diverse effects than lesions after learning (figs. S5 and S6). In our task, where bilateral coordination is required, unilateral lesions of M1/M2 after overtraining produced little behavioral changes, except for a significant decrease in overshoot (fig. S6D). Another apparent contradiction is that M2-IT optical stimulation (potentially producing an increased DLS output) and DLS-les (Fig. 2, decreased DLS output) both induced longer overshoots. However, as our electrophysiological results indicate, assuming a serial connectivity between M2 and DLS without considering the heterogeneity of the striatal microcircuit may be misleading. For instance, we found that M2-IT activation did not produce a generalized increased activity of DLS but rather the recruitment of a subpopulation of neurons (Fig. 6 and 8). Moreover, the exact effects of M2-IT activation in the cortical microcircuit are yet to be determined. For example, our manipulation produced a complex pattern of activation and inactivation (Fig. 4, G to K) that most likely included not only different cortico-striatal (PT and IT) but also cortico-thalamic, cortico-cortical, and inhibitory interneurons. Hence, sorting out the specific effects of the activation of these parallel cortico-striatal pathways would require additional experiments. Furthermore, after classifying striatal neurons on the basis of their spike shapes, we found evidence that this subpopulation contains a higher prevalence of pFSI neurons (Fig. 9). This is consistent with a recent report in vitro showing that FSI receives the strongest inputs from bilateral motor cortical regions (75), together suggesting a much more complex M2-DLS relationship to modulate movement and highlighting the importance to study the contribution of cortico-striatal projections originating in different cortical regions and subpopulations. Last, our observations suggest that cortico-striatal interactions could be organized into functional modules for specific components of movement control. In this context, it is important to notice that we did not address another fundamental level of organization: the direct- and indirect-pathway processing of the BG. For example, it has been suggested that primary motor regions preferentially target indirect-pathway neurons (76). On the other hand, it has been recently suggested that direct and indirect pathways might be involved in different motor aspects of postural adjustments during skill reaching in mice (77). Hence, to further decompose the participation of cortical and striatal circuits in the control of movements, it is necessary to create new strategies to target specific cortico-striatal projections that terminate in specific striatal subpopulations.
The study of bimanually coordinated movements can provide invaluable inputs to unravel the mechanism and neural computations implemented by motor systems to organize multiple command streams (30). In this study, by using a bimanual approach, we were able to disentangle the contribution of specific cortico-striatal projections to the final action. Our data confirm the existence of modular and parallel processing of information in the sensorimotor striatum (8, 9). The present results highlight the importance of future investigations in dissecting the exact origin of kinematic representations in this region. In addition, the pathway-specific manipulations and recordings support previous proposals (13, 16), indicating that BG circuitries and, specifically, the DLS are recruited by cortical regions to provide stability and duration to already commanded movements.
MATERIALS AND METHODS
All experimental procedures were approved by the Animal Ethics Committee of the Institute of Neurobiology, National Autonomous University of Mexico (UNAM) and conformed to the principles outlined in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health). All efforts were made to minimize the number of animals used and their suffering. All stereotaxic coordinates were calculated on the basis of the Paxinos and Watson brain atlas or brain maps by Swanson (78) and are reported in millimeters with respect to bregma.
Animals
A total of 75 Long-Evans rats were used in this study. Animals were housed in the satellite bioterium of our laboratory in home cages with stable temperature (23°C) and humidity (66%) and under a constant 12-hour:12-hour light-dark cycle (lights on at 8 a.m.). Animals had free access to food and water and were constantly supervised by specialized personnel from the general facilities of our institute. All experimental procedures were conducted during the light phase of the cycle. Seventy-six male rats (300 to 650 g) were trained in the bimanual coordination task; they were water-restricted and consumed all the water requirements during the training session (20 to 30 ml in 40 to 60 min per session, one session per day). Animal weight was monitored daily and maintained over 85% of the weight expected by age. If the animals did not consume their daily water proportion, then water was provided for short periods after the training. Animals were trained for 6 days every week with 24-hour free access to water on the seventh day. Animals were divided into the following groups: control short training (n = 22), control long training protocol (n = 17), unilateral DLS-les (n = 8), bilateral DLS-les (n = 6), and unilateral cortex lesion on M1 (n = 4) or M2 (n = 6) before training. Six animals from the long training protocol were lesioned on the DLS after training, and five animals from the short protocol were lesioned on M2 after training. Sixteen well-trained animals were used for optogenetic experiments (4 of them were recorded under freely moving conditions). Seven rats were used for anesthetized recordings (three females and four males).
Behavioral apparatus and training
Apparatus
Animals were trained in customized behavioral boxes (50 cm by 50 cm by 50 cm; Fig. 1A) equipped with two sets of levers, a water port, a green light-emitting diode (LED) to indicate correct trials, and two white LEDs to indicate the availability of a set of levers. Each set of levers consists of two levers that protrude 5 cm from the wall (Fig. 1A). The levers can move vertically and horizontally and are connected to a voltage transducer (3.5 cm = 2.5 V). All training and experimental procedures reported in this work were performed on the left set of levers. Voltage signals from the levers were digitized and stored at 250 Hz through National Instruments cards (NI PXIe-6363) and LabView custom-made routines. Animals were rewarded with water delivered with a solenoid valve through the center water port.
Task
We used a bimanual coordination task as previously described (36). Animals were trained to hold the two levers, one with each paw, and to vertically displace them at least 2.6 cm (vertical threshold) for a fixed time threshold (50, 500, and 750 ms) (Fig. 1B). The time counter started as soon as both levers crossed the spatial threshold and was restarted when either lever was above the threshold. Trials were self-initiated; there was no time limit to start a trial or to get a reward. A white light over the levers indicates the set of levers available; correct trials and availability of the reward were indicated with the green light (1 s on) over the water port. During this time, the white light over the levers was off (800 ms), and the animal could not get another reward; after that time, the white light indicated the pair of levers available. The animal performed an unlimited trial number (~250 trials) in the lapse of 40 min to 1 hour.
Training
Rats were exposed to the training boxes for one session (40 min) before the beginning of training and the water restriction. During the next session, the rats were trained to obtain rewards (drops of water, 60 μl) when approaching the water port. In subsequent sessions, animals were trained to obtain rewards by touching any lever and slightly moving it down (> 0.1 cm). In general, all animals quickly learned the rule of touch and displace any lever in less than two sessions. Then, animals learned to displace both levers simultaneously. We progressively increased the spatial threshold to get a reward until the final target position was reached for at least 50 ms. This pretraining phase lasted, in average, 4.5 days for all animals, including control and lesioned groups (the learning curves displayed in Figs. 1 to 3 do not include the pretraining phase). Formal training started immediately after. For the long training protocol, the animals were trained for 100 sessions. The temporal threshold was changed as follows: 45 sessions of 50 ms, 10 sessions of 500 ms, and 45 sessions of 750 ms (Fig. 2A). For the short training protocol, the animals were trained for 100 sessions. During the first eight sessions, the temporal threshold was established at 50 ms and then increased to 750 ms (Fig. 1).
Movement trajectory analysis
All behavioral measurements described below were calculated from raw lever positions using custom-made software in MATLAB. The interlimb correlation was calculated as the Pearson correlation coefficient between the left and right lever trajectories (4 s before and 2 s after reward). The first limb to touch the lever was calculated as the difference in time (milliseconds) between the movement onset of left and right levers. Positive or negative values indicate that the first lever to be touched was the left or the right, respectively. BOS was defined as the absolute time difference between the beginning of the movement of each forepaw, but only for the rewarded movements. Bilateral movement onset synchrony variability was defined as the variance of BOS. Overshoot was defined as the time that the levers remained pressed under the spatial threshold after the reward delivery (left and right levers were averaged for this parameter, except when explicitly stated, e.g., Fig. 5). The effort was calculated as the total time that levers were pressed for a single reward (left and right levers’ times were averaged together). Speed was calculated as the instantaneous difference in position in 4-ms time bins, and the maximum speed value for each trial was used for the analyses. Intralimb correlations (Pearson), intralimb trajectory variability (variance of trajectories on a trial-by-trial basis), and effort were calculated over all the possible pairs of left (or right) trajectories for single sessions.
Pharmacological lesions and lesion evaluation
All surgical processes (except for anesthetized recordings; see below) were performed under deep anesthesia induced by ketamine/xylazine (85/5 mg/kg) and, if necessary, maintained with sevoflurane (0.5 to 2%). Pharmacological lesions were performed by infusing N-methyl-d-aspartate (Sigma-Aldrich, 200 mM in sterilized saline; 1 ml per site of injection) unilaterally into the DLS [anterior-posterior (AP), 0.6; mediolateral (ML), ±3.5; dorsoventral (DV), −4.4], M2 (AP, 3.5; ML, 1.9; DV, 1.3), or M1 (AP, 1; ML, 2.4; DV, 1.4). Magnetic resonance imaging: Lesions were evaluated with magnetic resonance imaging (MRI) under deep anesthesia with the same dose of ketamine/xylazine and acquired with a Bruker Pharmascan 70/16Us, 7T MR scan (Bruker, Ettlingen, Germany). High-resolution anatomical T2 volumes were obtained using a spin-echo rapid acquisition with refocused echo sequences (Turbo RARE) with the following parameters: repetition time, 1800 ms; echo time, 38 ms; rapid imaging with refocused echoes (RARE) factor, 16; number of averages, 2; field of view, 18 mm by 20 mm; matrix dimension, 144 × 160; slice thickness, 0.5 mm; with a voxel resolution of 0.8 mm by 0.8 mm by 0.5 mm. MRI T2 processing: To quantify the lesion size, T2 images were processed using a custom pipeline to improve contrast and quantitative analysis (source code, https://github.com/akpimentel/first-step/blob/master/mrat_preproc). T2 MRI volumes were reoriented to standard space. An optimized three-dimensional nonlocal means filter was applied (minc-toolkit) (79, 80) followed by nonuniform intensity normalization for the bias field correction [N4BiasFieldCorrection by Advanced normalization tools (ANTs)]. Subsequently, manual segmentation of individual lesions was performed with Insight toolkit (ITK) snap, and binary masks were obtained for each region of interest (DLS, M1, and M2). The lesion area limits were identified by the voxel intensity change compared with the contralateral nonlesioned area.
Viruses
All viruses were obtained from Addgene. For M2-IT stimulation experiments, the animals received 1 μl of injections of the viruses into the DLS (coordinates: AP, −0.6; ML, ±3.5; DV, −4.4). Injections were separated by at least 1 month to ensure protein expression (for further details, see “Unilateral secondary motor cortex can orchestrate bilaterally coordinated movements” section and Fig. 4). AAV-pgk-Cre was a gift from P. Aebischer (Addgene, plasmid no. 24593; http://n2t.net/addgene:24593; RRID: Addgene_24593). pAAV-EF1a–double floxed–hChR2(H134R)-EYFP-WPRE-HGHpA was a gift from K. Deisseroth (Addgene, plasmid no. 20298; http://n2t.net/addgene:20298; RRID: Addgene_20298).
Optogenetic manipulations
Stimulations were given unilaterally. For anesthetized experiments (Fig. 4), we used two types of stimulation: five stimulus trains (5 ms each stimulus, 3.3 Hz delivered every 5 s) and a 500-ms single stimulus delivered every 5 s. For behavioral experiments, in all animals, we used minimum stimulation intensities between 0.2 and 0.6 mW. Animals would not work properly with intensities higher than 1.2 mW, but none of the intensities used produced visible movements of the forelimbs. For triggered stimulation experiments (Figs. 5 to 8), stimulation was initiated and maintained as soon as the animal would touch and displace either lever for more than 0.1 mm. Stimulation was stopped by reward delivery (i.e., as soon as the animal would press both levers simultaneously for at least 750 ms). For continuous stimulation sessions (fig. S5), 15-s periods of continuous stimulation were alternated with 15-s periods of nonstimulation. Trials falling into each type of period were classified as stimulated or nonstimulated accordingly. All hardware was acquired from Plexon including PlexBright LD-1 single-channel LED drivers, PlexBright LED modules (blue, 465 nm), PlexBright Dual LED commutators, Optical Patch cables, and Fiber Stub implants.
Anesthetized experiments
Animals were anesthetized with urethane (1 g/kg) and mounted on the stereotaxic frame (Fig. 4); supplementary doses (0.15 g/kg) were given during the recording when necessary. Silicon probe–based recordings were performed through a craniotomy (1.4 mm by 2.5 mm) centered at 0.6 mm anterior and 3.7 mm lateral to bregma. All the stereotaxic coordinates were selected on the basis of previous reports focused on the DLS forelimb region (8). Histological confirmation of silicon probe position in the brain was achieved by applying DiI lipophilic carbocyanine dye (1%; Sigma-Aldrich) to the back of the probes before insertion. For optogenetic experiments, an optical fiber (200 mm) was directed to M2 (coordinates: AP, 3.5; ML, 1.9; DV, 1.3) or S1 (coordinates: AP, 0.6; ML, 3.6; DV, 1.3), contralaterally to the recording site. The silicon probe was slowly lowered to record neuronal activity from the S1 forelimb region (0.8- to 1.6-mm depth) and until the DLS (3.5- to 4.5-mm depth). For each animal, we performed recordings in 2 to 3 and 3 to 5 depths (at least 250 μm between each depth) in the S1 and DLS, respectively. Immediately after the experiments, animals were injected with a lethal dose of pentobarbital and transcardially perfused. The brains were extracted and processed for histology.
Implantation of silicon probes for unit recording in behaving animals
Four well-trained animals were used for these experiments. All animals were transfected with two viruses in the DLS to specifically stimulate M2-IT cortico-striatal neurons as explained in the section “Unilateral secondary motor cortex can orchestrate bilaterally coordinated movements” and Fig. 4. Under deep sevoflurane anesthesia, 64-channel silicon probes were implanted in forelimb S1 above the striatum (coordinates: AP, −0.6; ML, ±3.5). Two miniature screws were implanted above the cerebellum and served as ground and reference. In the same surgery, two optical fibers directed to M2 were also implanted (coordinates: AP, 3.5; ML, 1.9; DV, 1.3). The microdrive, the connector, and the optical fibers were secured to the skull with miniature screws, adhesive cement (C&B Metabond), and dental acrylic (Meliodent). After surgery, a prophylactic analgesic treatment was administered (meloxicam, 0.5 mg/kg). After the recovery period (at least 6 days), probes were slowly lowered toward the recording sites (75 to 150 mm/day). Recordings in S1 were performed in two animals (rats 24 and 28) between 1.4 and 1.6 mm below the surface of the brain. Recordings in DLS were performed in three animals (rats 23, 24, and 27) between 3.1 and 5 mm below the surface of the brain. A total of 196 units were recorded in eight sessions in S1 (Fig. 7). A total of 998 units were recorded in 56 sessions in the DLS (Figs. 4 to 8). In 51 behavioral sessions (886 units), stimulation was triggered by lever pressing (Figs. 6 to 8). At the end of each behavioral session, animals were maintained for 15 to 20 min in the behavioral boxes and were passively stimulated with train protocols as described in the section “Unilateral secondary motor cortex can orchestrate bilaterally coordinated movements” (Fig. 4). In five behavioral sessions (112 units), animals were subjected to alternating 15-s on-off stimulation periods (fig. S6).
Electrophysiological data acquisition and processing
For both anesthetized and freely moving recordings, wide-band (0.1 to 8000 Hz) neurophysiological signals from silicon probes (NeuroNexus, Buzsaki-64) were amplified 1000 times via Intan RHD2000 series Amplifier System and continuously acquired at 20 kHz. Data visualization and processing were performed from raw data using Neuroscope and NDManager (http://neurosuite.sourceforge.net). Spike sorting was performed semiautomatically using the clustering software KlustaKwik (http://klustakwik.sourceforge.net) (81) and the graphical spike-sorting application Klusters (http://klusters.sourceforge.net) (82).
Spiking activity in anesthetized and freely moving recordings
Latencies
To determine the response latencies to optical stimulation in Fig. 4, the neuronal data were binarized to a resolution of 1 ms. Perievent histograms were constructed (−1 to +2 s with respect to stimulus onset), and a confidence limit of 99% based on baseline activity (−1 s before each stimulation) was calculated for each cell. Responses to stimulation were considered significant if they exceeded the confidence limit by at least 1 ms, and the time of the first bin was defined as the response latency.
Spiking activity during bilateral coordination task execution
For the activity in raster plots and color-coded matrices in Fig. 6, first, we extracted spiking activity from each trial in 1-ms bins over an 8-s window centered on reward onset (± 4 s). Spike trains from stimulated and nonstimulated trials were smoothed with a Gaussian kernel filter with an SD of 50 ms. For each neuron, the average perievent histogram was transformed into z scores.
M2-IT modulated neurons
To estimate the number of striatal cells that were significantly modulated by optical stimulation of M2-IT neurons, for each cell, we calculated the average firing rate during the 750 ms immediately before reward delivery (lever pressing) and compared nonstimulated versus stimulated trials (Mann-Whitney rank sum test, P < 0.05). For the calculation of kinematic and lever press–related correlations (Fig. 7), trials (± 4 s centered on reward onset) were divided into nonoverlapping windows of 100 ms. The firing rate was calculated for each window (spike count divided by 0.1) and smoothed with a Gaussian kernel filter with an SD of 200 ms. The position of the head of the animal was automatically extracted from a video (30 fps) with a custom-made program (Vision, National Instruments) and averaged in 100-ms-long sliding windows. The acceleration (three axes) of the head movements was extracted directly (20 kHz) from the accelerometers integrated to recording head stages (Intan Technologies RHD2132). The movement trajectories from the levers were obtained as described in the previous sections. Pearson’s partial and multiple correlation coefficients between spiking activity and the position and velocity and acceleration of the head and levers were calculated for each cell.
Statistics
The electrophysiological and behavioral data are presented as median + 25th and 75th percentiles. For all groups, normality was estimated with the Kolmogorov-Smirnov test. Statistical significance for group comparison was calculated using the Mann-Whitney or K-W test for electrophysiological and behavioral data. Multiple comparison analysis was performed with the Bonferroni post hoc test. Statistical analysis was performed using MATLAB software (The MathWorks Inc.). Differences were considered statistically significant if the P value was ≤0.05.
Acknowledgments
We thank A. Inácio, H. Merchant, L. Tellez, and V. de Lafuente for critical reading and discussions on this manuscript. We thank the support provided by C. I. Pérez Díaz and all the members of Laboratory A-02 from the Institute of Neurobiology, UNAM; C. Pacheco and M. García for providing invaluable support in animal maintenance and care; O. Prospéro for donations of indispensable equipment; R. Rodríguez-Cruces, J. Ortiz, and the “Laboratorio Nacional de Imagenología por Resonancia Magnética” for support in MRI experiments; A. Antaramian and A. González from Unidad de Proteogenómica, INB; and J. Gonzalez-Norris for proofreading. A. K. Pimentel-Farfan is a doctoral student from Programa de Doctorado en Ciencias Biomédicas, UNAM, and supported by fellowship 463747 from CONACyT-México.
Funding: This work was funded by grants: UNAM-DGAPA-PAPIIT: IA201918 and IA201020 (P.E.R.-O.), and CONACyT: FDC_1702 (P.E.R.-O.).
Author contributions: Conceptualization: A.K.P.-F. and P.E.R.-O. Methodology: A.K.P.-F. and P.E.R.-O. Investigation: A.K.P.-F., A.S.B.-C., T.M.P.-R., and P.E.R.-O. Data curation: A.K.P.-F. and P.E.R.-O. Formal analysis: A.K.P.-F. and P.E.R.-O. Writing—original draft: A.K.P.-F. and P.E.R.-O. Writing—review and editing: A.S.B.-C. and T.M.P.-R. Supervision: P.E.R.-O. Project administration: P.E.R.-O. Funding acquisition: P.E.R.-O.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S9
REFERENCES AND NOTES
- 1.Jog M. S., Kubota Y., Connolly C. I., Hillegaart V., Graybiel A. M., Building neural representations of habits. Science 286, 1745–1749 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Barnes T. D., Kubota Y., Hu D., Jin D. Z., Graybiel A. M., Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature 437, 1158–1161 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Jin X., Costa R. M., Start/stop signals emerge in nigrostriatal circuits during sequence learning. Nature 466, 457–462 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martiros N., Burgess A. A., Graybiel A. M., Inversely active striatal projection neurons and interneurons selectively delimit useful behavioral sequences. Curr. Biol. 28, 560–573.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rueda-Orozco P. E., Robbe D., The striatum multiplexes contextual and kinematic information to constrain motor habits execution. Nat. Neurosci. 18, 435–460 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panigrahi B., Martin K. A., Li Y., Graves A. R., Vollmer A., Olson L., Mensh B. D., Karpova A. Y., Dudman J. T., Dopamine is required for the neural representation and control of movement vigor. Cell 162, 1418–1430 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Sales-Carbonell C., Taouali W., Khalki L., Pasquet M. O., Petit L. F., Moreau T., Rueda-Orozco P. E., Robbe D., No discrete start/stop signals in the dorsal striatum of mice performing a learned action. Curr. Biol. 28, 3044–3055.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hidalgo-Balbuena A. E., Luma A. Y., Pimentel-Farfan A. K., Peña-Rangel T., Rueda-Orozco P. E., Sensory representations in the striatum provide a temporal reference for learning and executing motor habits. Nat. Commun. 10, 4074 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim N., Li H. E., Hughes R. N., Watson G. D. R., Gallegos D., West A. E., Kim I. H., Yin H. H., A striatal interneuron circuit for continuous target pursuit. Nat. Commun. 10, 2715 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.West M. O., Carelli R. M., Pomerantz M., Cohen S. M., Gardner J. P., Chapin J. K., Woodward D. J., A region in the dorsolateral striatum of the rat exhibiting single-unit correlations with specific locomotor limb movements. J. Neurophysiol. 64, 1233–1246 (1990). [DOI] [PubMed] [Google Scholar]
- 11.Carelli R. M., West M. O., Representation of the body by single neurons in the dorsolateral striatum of the awake, unrestrained rat. J. Comp. Neurol. 309, 231–249 (1991). [DOI] [PubMed] [Google Scholar]
- 12.Shi L. H., Luo F., Woodward D. J., Chang J. Y., Neural responses in multiple basal ganglia regions during spontaneous and treadmill locomotion tasks in rats. Exp. Brain Res. 157, 303–314 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Dudman J. T., Krakauer J. W., The basal ganglia: From motor commands to the control of vigor. Curr. Opin. Neurobiol. 37, 158–166 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Robbe D., To move or to sense? Incorporating somatosensory representation into striatal functions. Curr. Opin. Neurobiol. 52, 123–130 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Yin H. H., The basal ganglia in action. Neuroscience 23, 299–313 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner R. S., Desmurget M., Basal ganglia contributions to motor control: A vigorous tutor. Curr. Opin. Neurobiol. 20, 704–716 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soma S., Saiki A., Yoshida J., Ríos A., Kawabata M., Sakai Y., Isomura Y., Distinct laterality in forelimb-movement representations of rat primary and secondary motor cortical neurons with intratelencephalic and pyramidal tract projections. J. Neurosci. 37, 10904–10916 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isomura Y., Harukuni R., Takekawa T., Aizawa H., Fukai T., Microcircuitry coordination of cortical motor information in self-initiation of voluntary movements. Nat. Neurosci. 12, 1586–1593 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Hoover J. E., Hoffer Z. S., Alloway K. D., Projections from primary somatosensory cortex to the neostriatum: The role of somatotopic continuity in corticostriatal convergence. J. Neurophysiol. 89, 1576–1587 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Hooks B. M., Papale A. E., Paletzki R. F., Feroze M. W., Eastwood B. S., Couey J. J., Winnubst J., Chandrashekar J., Gerfen C. R., Topographic precision in sensory and motor corticostriatal projections varies across cell type and cortical area. Nat. Commun. 9, 3549 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erro M. E., Lanciego J. L., Giménez-Amaya J. M., Re-examination of the thalamostriatal projections in the rat with retrograde tracers. Neurosci. Res. 42, 45–55 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Erro M. E., Lanciego J. L., Arribas J., Gimenez-Amaya J. M., Striatal input from the ventrobasal complex of the rat thalamus. Histochem. Cell Biol. 115, 447–454 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Smith J. B., Mowery T. M., Alloway K. D., Thalamic POm projections to the dorsolateral striatum of rats: Potential pathway for mediating stimulus-response associations for sensorimotor habits. J. Neurophysiol. 108, 160–174 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Díaz-Hernández E., Contreras-López R., Sánchez-Fuentes A., Rodríguez-Sibrían L., Ramírez-Jarquín J. O., Tecuapetla F., The thalamostriatal projections contribute to the initiation and execution of a sequence of movements. Neuron 100, 739–752.e5 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Smith Y., Galvan A., Ellender T. J., Doig N., Villalba R. M., Huerta-Ocampo I., Wichmann T., Bolam J. P., The thalamostriatal system in normal and diseased states. Front. Syst. Neurosci. 8, 5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berendse H. W., Groenewegen H. J., Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J. Comp. Neurol. 299, 187–228 (1990). [DOI] [PubMed] [Google Scholar]
- 27.Kawai R., Markman T., Poddar R., Ko R., Fantana A. L., Dhawale A. K., Kampff A. R., Ölveczky B. P., Motor Cortex Is Required for Learning but Not for Executing a Motor Skill. Neuron 86, 800–812 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemke S. M., Ramanathan D. S., Guo L., Won S. J., Ganguly K., Emergent modular neural control drives coordinated motor actions. Nat. Neurosci. 22, 1122–1131 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rueda-Delgado L. M., Solesio-Jofre E., Serrien D. J., Mantini D., Daffertshofer A., Swinnen S. P., Understanding bimanual coordination across small time scales from an electrophysiological perspective. Neurosci. Biobehav. Rev. 47, 614–635 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Swinnen S. P., Wenderoth N., Two hands, one brain: Cognitive neuroscience of bimanual skill. Trends Cogn. Sci. 8, 18–25 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Reiner A., Jiao Y., Del Mar N., Laverghetta A. V., Lei W. L., Differential morphology of pyramidal tract-type and intratelencephalically projecting-type corticostriatal neurons and their intrastriatal terminals in rats. J. Comp. Neurol. 457, 420–440 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Shepherd G. M. G., Corticostriatal connectivity and its role in disease. Nat. Rev. Neurosci. 14, 278–291 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evarts E. V., Pyramidal tract activity associated with a conditioned hand movement in the monkey. J. Neurophysiol. 29, 1011–1027 (1966). [DOI] [PubMed] [Google Scholar]
- 34.Smith Y., Raju D. V., Pare J.-F., Sidibe M., The thalamostriatal system: A highly specific network of the basal ganglia circuitry. Trends Neurosci. 27, 520–527 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Takada M., Fishell G., Li Z. K., Van der Kooy D., Hattori T., The development of laterality in the forebrain projections of midline thalamic cell groups in the rat. Brain Res. 432, 275–282 (1987). [DOI] [PubMed] [Google Scholar]
- 36.Báez-Cordero A. S., Pimentel-Farfan A. K., Peña-Rangel T., Rueda-Orozco P. E., Unbalanced inhibitory/excitatory responses in the substantia nigra pars reticulata underlie cannabinoid-related slowness of movements. J. Neurosci. 40, 5769–5784 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fricker R. A., Annett L. E., Torres E. M., Dunnett S. B., The placement of a striatal ibotenic acid lesion affects skilled forelimb use and the direction of drug-induced rotation. Brain Res. Bull. 41, 409–416 (1996). [DOI] [PubMed] [Google Scholar]
- 38.Jeyasingham R. A., Baird A. L., Meldrum A., Dunnett S. B., Differential effects of unilateral striatal and nigrostriatal lesions on grip strength, skilled paw reaching and drug-induced rotation in the rat. Brain Res. Bull. 55, 541–548 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Vergara-Aragon P., Gonzalez C. L. R., Whishaw I. Q., A novel skilled-reaching impairment in paw supination on the “Good” side of the hemi-parkinson rat improved with rehabilitation. J. Neurosci. 23, 579–586 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miklyaeva E. I., Whishaw I. Q., HemiParkinson analogue rats display active support in good limbs versus passive support in bad limbs on a skilled reaching task of variable height. Behav. Neurosci. 110, 117–125 (1996). [DOI] [PubMed] [Google Scholar]
- 41.Churchland M. M., Afshar A., Shenoy K. V., A central source of movement variability. Neuron 52, 1085–1096 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cisek P., Crammond D. J., Kalaska J. F., Neural activity in primary motor and dorsal premotor cortex in reaching tasks with the contralateral versus ipsilateral arm. J. Neurophysiol. 89, 922–942 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Ames K. C., Churchland M. M., Motor cortex signals for each arm are mixed across hemispheres and neurons yet partitioned within the population response. eLife 8, e46159 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donchin O., Gribova A., Steinberg O., Bergman H., Vaadia E., Primary motor cortex is involved in bimanual coordination. Nature 395, 274–278 (1998). [DOI] [PubMed] [Google Scholar]
- 45.Levesque M., Charara A., Gagnon S., Parent A., Deschenes M., Corticostriatal projections from layer V cells in rat are collaterals of long-range corticofugal axons. Brain Res. 709, 311–315 (1996). [DOI] [PubMed] [Google Scholar]
- 46.Carman J. B., Cowan W. M., Powell T. P. S., Webster K. E., A bilateral cortico-striate projection. J. Neurol. Neurosurg. Psychiatry 28, 71–77 (1965). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferino F., Thierry A. M., Saffroy M., Glowinski J., Interhemispheric and subcortical collaterals of medial prefrontal cortical neurons in the rat. Brain Res. 417, 257–266 (1987). [DOI] [PubMed] [Google Scholar]
- 48.Wilson C. J., Morphology and synaptic connections of crossed corticostriatal neurons in the rat. J. Comp. Neurol. 263, 567–580 (1987). [DOI] [PubMed] [Google Scholar]
- 49.J. Park, J. W. Phillips, K. A. Martin, A. W. Hantman, J. T. Dudman, Descending neocortical output critical for skilled forelimb movements is distributed across projection cell classes. bioRxiv 772517 [Preprint]. 22 February 2021. https://doi.org/10.1101/772517.
- 50.Miri A., Warriner C. L., Seely J. S., Elsayed G. F., Cunningham J. P., Churchland M. M., Jessell T. M., Behaviorally selective engagement of short-latency effector pathways by motor cortex. Neuron 95, 683–696.e11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turner R. S., DeLong M. R., Corticostriatal activity in primary motor cortex of the macaque. J. Neurosci. 20, 7096–7108 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gouvêa T. S., Monteiro T., Motiwala A., Soares S., Machens C., Paton J. J., Striatal dynamics explain duration judgments. eLife 4, e11386 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.W. Taouali, P. E. Rueda-Orozco, D. Robbe, A minority-ruled population coding of kinematics in the striatum. bioRxiv 130237 [Preprint]. 26 April 2017. 10.1101/130237. [DOI]
- 54.Bartholomew R. A., Li H., Gaidis E. J., Stackmann M., Shoemaker C. T., Rossi M. A., Yin H. H., Striatonigral control of movement velocity in mice. Eur. J. Neurosci. 43, 1097–1110 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Berke J. D., Okatan M., Skurski J., Eichenbaum H. B., Oscillatory entrainment of striatal neurons in freely moving rats. Neuron 43, 883–896 (2004). [DOI] [PubMed] [Google Scholar]
- 56.Berke J. D., Uncoordinated firing rate changes of striatal fast-spiking interneurons during behavioral task performance. J. Neurosci. 28, 10075–10080 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barter J. W., Li S., Lu D., Bartholomew R. A., Rossi M. A., Shoemaker C. T., Salas-Meza D., Gaidis E., Yin H. H., Beyond reward prediction errors: The role of dopamine in movement kinematics. Front. Integr. Neurosci. 9, 39 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peña-Rangel T. M., Lugo-Picos P. I., Báez-Cordero A. S., Hidalgo-Balbuena A. E., Luma A. Y., Pimentel-Farfan A. K., Rueda-Orozco P. E., Altered sensory representations in parkinsonian cortical and basal ganglia networks. Neuroscience 466, 10–25 (2021). [DOI] [PubMed] [Google Scholar]
- 59.Oliveira F. T. P., Ivry R. B., The representation of action. Curr. Dir. Psychol. Sci. 17, 130–135 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGeorge A. J., Faull R. L. M., The organization and collateralization of corticostriate neurones in the motor and sensory cortex of the rat brain. Brain Res. 423, 318–324 (1987). [DOI] [PubMed] [Google Scholar]
- 61.Afshar A., Santhanam G., Yu B. M., Ryu S. I., Sahani M., Shenoy K. V., Single-trial neural correlates of arm movement preparation. Neuron 71, 555–564 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Evarts E. V., Relation of pyramidal tract activity to force exerted during voluntary movement. J. Neurophysiol. 31, 14–27 (1968). [DOI] [PubMed] [Google Scholar]
- 63.Sauerbrei B. A., Guo J.-Z., Cohen J. D., Mischiati M., Guo W., Kabra M., Verma N., Mensh B., Branson K., Hantman A. W., Cortical pattern generation during dexterous movement is input-driven. Nature 577, 386–391 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo Z. V., Inagaki H. K., Daie K., Druckmann S., Gerfen C. R., Svoboda K., Maintenance of persistent activity in a frontal thalamocortical loop. Nature 545, 181–186 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dhawale A. K., Miyamoto Y. R., Smith M. A., Ölveczky B. P., Adaptive regulation of motor variability. Curr. Biol. 29, 3551–3562.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang L., Chen J., Li Y., Zhang Z. G., Chopp M., Quantitative measurement of motor and somatosensory impairments after mild (30 min) and severe (2 h) transient middle cerebral artery occlusion in rats. J. Neurol. Sci. 174, 141–146 (2000). [DOI] [PubMed] [Google Scholar]
- 67.Haelewyn B., Freret T., Pcacary E., Shumannbard P., Boulouard M., Bernaudin M., Bouet V., Long-term evaluation of sensorimotor and mnesic behaviour following striatal NMDA-induced unilateral excitotoxic lesion in the mouse. Behav. Brain Res. 178, 235–243 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Iancu R., Mohapel P., Brundin P., Paul G., Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson’s disease in mice. Behav. Brain Res. 162, 1–10 (2005). [DOI] [PubMed] [Google Scholar]
- 69.Campos F. L., Carvalho M. M., Cristovão A. C., Je G., Baltazar G., Salgado A. J., Kim Y.-S., Sousa N., Rodent models of Parkinson’s disease: Beyond the motor symptomatology. Front. Behav. Neurosci. 7, 175 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ogura T., Ogata M., Akita H., Jitsuki S., Akiba L., Noda K., Hoka S., Saji M., Impaired acquisition of skilled behavior in rotarod task by moderate depletion of striatal dopamine in a pre-symptomatic stage model of Parkinson’s disease. Neurosci. Res. 51, 299–308 (2005). [DOI] [PubMed] [Google Scholar]
- 71.Takakusaki K., Neurophysiology of gait: From the spinal cord to the frontal lobe. Mov. Disord. 28, 1483–1491 (2013). [DOI] [PubMed] [Google Scholar]
- 72.A. K. Dhawale, S. B. E. Wolff, R. Ko, B. P. Ölveczky, The basal ganglia can control learned motor sequences independently of motor cortex. bioRxiv 827261 [Preprint]. 1 November 2019. 10.1101/827261. [DOI]
- 73.Touvykine B., Elgbeili G., Quessy S., Dancause N., Interhemispheric modulations of motor outputs by the rostral and caudal forelimb areas in rats. J. Neurophysiol. 123, 1355–1368 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sjöbom J., Tamtè M., Halje P., Brys I., Petersson P., Cortical and striatal circuits together encode transitions in natural behavior. Sci. Adv. 6, eabc1173 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johansson Y., Silberberg G., The functional organization of cortical and thalamic inputs onto five types of striatal neurons is determined by source and target cell identities. Cell Rep. 30, 1178–1194.e3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wall N. R., De La Parra M., Callaway E. M., Kreitzer A. C., Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron 79, 347–360 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lopez-Huerta V. G., Denton J. A., Nakano Y., Jaidar O., Garcia-Munoz M., Arbuthnott G. W., Striatal bilateral control of skilled forelimb movement. Cell Rep. 34, 108651 (2021). [DOI] [PubMed] [Google Scholar]
- 78.Swanson L. W., Brain maps 4.0-Structure of the rat brain: An open access atlas with global nervous system nomenclature ontology and flatmaps. J. Comp. Neurol. 526, 935–943 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Coupe P., Yger P., Prima S., Hellier P., Kervrann C., Barillot C., An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans. Med. Imaging 27, 425–441 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tustison N. J., Avants B. B., Cook P. A., Zheng Y., Egan A., Yushkevich P. A., Gee J. C., N4ITK: Improved N3 bias correction. IEEE Trans. Med. Imaging 29, 1310–1320 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harris K. D., Henze D. A., Csicsvari J., Hirase H., Buzsáki G., Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J. Neurophysiol. 84, 401–414 (2000). [DOI] [PubMed] [Google Scholar]
- 82.Hazan L., Zugaro M., Buzsáki G., Klusters, NeuroScope, NDManager: A free software suite for neurophysiological data processing and visualization. J. Neurosci. Methods 155, 207–216 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S9