Abstract
Glypican-3 (GPC3) is a serological biomarker for the diagnosis of Hepatocellular carcinoma (HCC), but it is a challenging task to develop a bioassay for determination of the trace GPC3 in serum. In this study, Bioluminescense immunoassay based on bifunctional nanobody-nanoluciferase fusion was developed with the ultra-sensitive feature to achieve this goal. First, nanobodies special against GPC-3 binder as biological recognition element were generated by immunization and phage display technology. Second, The best clone GPN2 was fused with nanoluciferase as a dual-functional immunoreagent to establish an ultra-sensitive bioluminescence enzyme immunoassay (BLEIA), which is 30 and 5 times more sensitive than the traditional colorimetric assay and fluorescent assay, respectively. The cross-reactivity analysis of BLEIA showed that there was no cross-reactivity with HCC related tumor markers AFP, CEA, CA19–9 and GPC1/GPC2. The limit of detection (LOD) of developed BLEIA was 1.5 ng/mL, which assured its application in the diagnosis of GPC3 in 94 serum samples. This study indicates that BLEIA based on nanobody-nanoluciferase fusion could be used as a useful tool for the diagnosis of HCC patients.
Keywords: Anti-GPC3 binder, Nanobody/nanoluciferase fusion, Bioluminescence enzyme immunoassay, Diagnosis for HCC
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors and the second leading cause of cancer-related deaths worldwide [1].The development of diagnosis for HCC is essential for increasing the chances of effective treatment and reducing HCC-related mortalit [2]. As serum-based diagnostic methods are non-invasive and relatively inexpensive, to measure serum biomarkers by immunoassay for diagnosing HCC is a promising strategy, which has been applied widely in clinical diagnostic applications. Glypican-3 (GPC3) is a heparan sulfate proteoglycan molecule that is highly expressed in most HCC cell lines, but it is absent in normal liver cells [3–5]. Although GPC3 is a cell surface marker, it can be released into the serum by the lipase Notum, which cleaves the GPI anchor [6–8]. As an emerging biomarker for the diagnosis and treatment of HCC, GPC3 was used as a serological marker for HCC diagnosis, especially for the alpha fetal protein (AFP) negative HCC diagnosis [9–11].
GPC3 has been validated as a serum marker by developing methodologies such as immunoassays based on anti-GPC3 antibodies. To date, several monoclonal antibodies (mAb) against GPC3 had been produced. Most of the reported antibodies were raised against the entire GPC3 protein [12–13] or specific N/C terminal peptide [4,13–15].Up to now, many traditional antibodies [16–18] against GPC3 are widely used as immuno-reagents for the HCC serological detection or as imaging probes for early detection of HCC, including enzyme-linked immunosorbent assay (ELISA)[19], chemiluminescent immunoassay (CLIA) [20], radioimmunoassay[21], and immuno-PET [22–23]. However, some of the inherent limitations of monoclonal antibodies and polyclonal antibodies, such as poor specificity, high production costs, unreliable production, and consistency, have severely hampered the clinical transformation of antibody-based immunoassays [24–26].
Recently, a novel kind of nanobody attracted more attention due to its small size (15 kDa), high expression yields, and high thermostability[24]. As a replacement for traditional antibodies in immunoassay, nanobodies have great potential to be developed for protein biomarkers detection in the clinical diagnostic. Since nanobodies have a single domain structure, it is convenient to construct various nanobody fusion proteins by molecular biological engineering. For example, the fluorescent reporter protein like RFP and GFP [27], the reporter like alkaline phosphatase (AP) [28] and horseradish peroxidase (HRP) [29] has been fused with nanobodies as common reporters for one-step immunoassays. The one-step immunoassay based on nanobody-reporter fusion does not need secondary or tertiary-antibody conjugates, which resulting in more steps and long assay times. Compared with the conventional enzyme reporter like AP or HRP, the luciferase having bioluminescence properties produces higher signal intensity, more rapid measurement of signals, and has attracted more attention. Their small size and recombinant nature allow them to be anchored as a bacterial stab, a plasmid, a protein, even a sequence. So far, there are few reports about anti-GPC3 nanobodies [30], let alone the anti-GPC3 nanobody-reporter fusion. Therefore, nanobody-based immunoreagent is a promising biomaterial for developing a biosensor in the diagnosis of HCC.
In this study, we isolated anti-GPC3 nanobody from alpaca immunized phage display library, and then the selected nanobody was fused to the Nanoluciferase (NLuc) [31–32], the smallest luciferase. This Nanobody-Nluc fusion was served as dual-functional reagent for the development of a one-step ultra-sensitive bioluminescence enzyme immunoassay (BLEIA). As depicted in Scheme 1, this novel biosensor based on nanobody-nanoluciferase fusion was applied to measure the trace level of GPC3 in serum for clinical diagnosis of HCC patients.
Scheme 1.

The isolation of anti-GP3 nanobody and BLEIA for detection of GPC3 in serum.
2. Materials and methods
2.1. Serum Specimens
A total of 94 serum samples were collected from patients in the Liuzhou People’s Hospital and the second affiliated hospital of Guangxi University of Science and Technology between June 2019 and July 2020. Of these, 21 HCC patients including 12 males and 9 females (ages 47–79 years), 12 liver cirrhosis including 8 males and 4 females (ages 52–70 years), 21 hepatitis patients with HBV including 13 males and 8 females (ages 29–60 years), 40 healthy volunteers were enrolled as normal controls, including 23 males and 17 females (ages 31–70 years). All patients were diagnosed according to the pathological criteria. All serum specimens were aliquoted and stored at −80°C. All experiments methods were carried out in accordance with the relevant guidelines and regulations approved by the Ethical Committee of the Guangxi University of Science and Technology and Liuzhou People’s Hospital.
2.2. Chemicals and Reagents
T4 DNA ligase and Restriction enzymes Sfi I, and BamH I were purchased from New England Biolabs (Beverly, MA, USA). DNA Polymerase, B-PER™, HisPur™ Ni-NTA resin, Bolt™ 4–12% Bis-Tris Plus Gels, Sulfo-NHS-LC-Biotin, isopropyl-β-D-thiogalactopyranoside (IPTG) were purchased from Thermo Fisher Scientific Inc (Waltham, MA, US). Nano-Glo® Luciferase Assay System was purchased from Promega (Madison, WI 53711–5399 USA). GPC3 protein was purchased from ACRO Biosystems (Beijing, China). Phagemid vector pComb3X, M13KO7 helper phages, Escherichia coli (E. coli) ER2738, and Top 10 F’ cells were gifts from Prof. Bruce D. Hammock (University of California, Davis, US). Anti-M13 phage mAb-HRP, polyclonal antibody against GPC3 was purchased from the R&D system (Minneapolis, USA). Affinity analysis by biolayer interferometry (BLI) binding assays was performed using the Octet Qke system.
2.3. Immunization, Amplification of Nanobody genes, Construction of Nanobody Library and Biopanning
About 1.0×108 peripheral blood lymphocytes (PBLs) were isolated from 100 mL blood of immunized alpaca, which had been immunized with 7 injections of GPC3 (0.5 mg /dose) in incomplete adjuvant. The extracted total RNA was used to synthesize the cDNA, which was used as a template for PCR amplification of the nanobody genes using the primers (Table S1). The PCR products and pComb3X phagemid vector was digested with Sfi I and ligated using the T4 ligase overnight at 16°C. The ligations were electroporated into electrocompetent ER2738 cells. The transformed cells were cultured and rescued with helper phage M13KO7 to construct an immune nanobody phage library displaying the nanobodies.
Biotinylated GPC3 protein was prepared according to the routine protocol (Supporting Information 1.1). As shown in Fig. S 2A, bio-panning of anti-GPC3 nanobody clones was conducted by a unique procedure (Supporting Information 1.2). After biopanning, clones were picked from the output plate in last round to perform the phage ELISA (Supporting Information 1.3). The anti-GPC3 nanobody candidates were expressed and purified (Supporting Information 1.4), and their thermostability was analyzed by the binding ELISA (Supporting Information 1.5).
2.4. Flow Cytometry Analysis of Nanobody
The binding efficacy of the best nanobody was detected by the flow cytometry analysis. Liver cancer cell lines, such as HepG2, Huh-7, Hep3B, SMMC-7721, SK-Hep-1cells were incubated with nanobody individually in PBS (2% BSA) buffer for 30 min with gentle shaking. After washing with PBS, HA-Tag (C29F4) Rabbit mAb (PE Conjugate) was added to the cells for 30 min incubation at 4 °C. Cells were washed and then resuspended in PBS buffer for flow cytometry analysis. In addition, the western blot test was conducted to confirm the flow cytometry analysis using the liver cancer cell (Supporting Information 1.6).
2.5. Plasmid Construction, Expression, and Purification of GPN2-Nluc Fusion Protein
The GPN2-NLuc fusion gene was constructed as shown in Fig. 3. Briefly, the GPN2 nanobody gene was amplified by PCR with the primer GPN2-F and GPN2-R (Table S1), which were added Sfi I and BamH I and restriction enzyme sites flanking the 3’ and 5’ terminal of the GPN2 gene. The PCR products were purified and digested with Sfi I and BamH I enzyme. The GPN2 nanobody gene was ligated into the large fragment of digested pET22b using T4 DNA ligase, and the ligation product was transformed into the E. coli BL21(DE3), the positive clones were confirmed by sequencing, then the GPN2-Nluc fusion was expressed by the routine procedure according to our protocol (Supporting Information 1.7).
Figure 3.

(A), The construction and expression of GPN2-Nluc and GPN2-AP. (B), SDS–PAGE analysis of expression of the GPN2-NLuc fusion protein. Lane M, PageRuler unstained protein ladder and spectrum multicolor broad-range protein ladder. Lane 1: GPN2-Nluc was eluted with elution buffer (PBS containing 200 mM imidazole). Lane 2: GPN2-Nluc was eluted with elution buffer (PBS containing 100 mM imidazole). Lane 3: GPN2-Nluc was eluted with elution buffer (PBS containing 50 mM imidazole). Lane 4: the wash buffer from the Ni-NTA purification. Lane 5: whole cell extract under induced conditions.(C), SDS–PAGE analysis of expression of GPN2-AP fusion protein. Lane M, PageRuler unstained protein ladder and spectrum multicolor broad-range protein ladder. Lane 1, GPN2-AP was eluted with elution buffer (PBS containing 300 mM imidazole). Lane 2, the wash buffer from the Ni-NTA purification. Lane 3, Bacteria after induction. Lane 4, Bacteria before induction.
2.6. Plasmid Construction, Expression, and Purification of GPN2-AP Fusion Protein
For comparison, the GPN2 nanobody was also fused with another traditional reporter, named alkaline phosphatase (AP). The GPN2 gene was cloned in our general modified vector pecan45 containing the AP gene. The cloning was almost the same as the procedure above, except for the different primers listed in Table S1. The GPN2-AP fusion protein was expressed, purified, and applied in a one-step fluorescent immunoassay (Supporting Information 1.8). In parallel, a two-step ELISA was also developed for GPN2 and two GPN2 fusion proteins for comparison (Supporting Information 1.9).
2.7. BLEIA For GPC3 Based on GPN2-Nluc Fusion Protein
For BLEIA, a sandwich format was performed, in which anti-GPC3 polyclonal antibody AF2119 was used as capture antibody and the GPN2-NLuc fusion protein as detect antibody. White 96-well microtiter plate was coated with polyclonal antibody (4 μg/mL) at 100 μL /well overnight at 4 °C. The wells were blocked with 5% skim milk in PBS for 1 h at 37 °C. After washing three times with 0.01 % PBST to remove the blocking solution, a diluted fusion protein (50μL/well) (0, 1.25, 2.5, 3.125, 5.0, 6.25, 10, 12.5, 20, 25, 50, 100, 200 and 400 ng/mL) was added into the well, along with 50μL of the GPN2-NLuc Fusion Protein(1μg/mL) in each wells, followed with 150 rpm shaking at room temperature. After washing five times with PBST, 100μL of Nano-Glo® Luciferase Assay Substrate dissolved in the corresponding buffer was added, and the bioluminescent signal was measured immediately. In a similar procedure, one-step FLEIA was also developed for GPN2-AP fusion proteins for comparison.
2.8. Validation Study in the Blank Serum Samples
The GPC3 antigen (10, 50, 250 ng/mL, respectively) was spiked into the healthy human serum, then the experimental values and the theoretical values were used to calculate the recovery rate. The cross-reactivity analysis was performed to detect the specificity of this BLEIA. Liver cancer-related markers, including AFP, CEA, CA19–9, and two related family proteins GPC1 and GPC2, were prepared as interferents and added into healthy human serum, respectively. The cross-reactivity (CR) was calculated using the following formula: CR (%) = [tested value of interferents / actual concentration of spiked protein] × 100%.
2.9. Analysis of Clinical Samples and Statistics
Serum samples of 21 HCC patients, 21 hepatitis, 12 liver cirrhosis, and 40 healthy controls were evaluated by the proposed BLEIA. The measured result was calculated and analyzed by GraphPad Prism 8.0, SPSS 21 (Chicago, USA), and Origin Pro8.5 (OriginLab, USA). Detected GPC3 in different groups were analyzed by t-test and Set significant difference P<0.05. The area under the receiver-operating curve (AUROC) was calculated to evaluate the diagnostic effectiveness by constructing receiver operating characteristics (ROC) curves. The cut-off value was determined by considering the balance of the best sensitivity and specificity according to the ROC curve [14].
3. RESULTS AND DISCUSSION
3.1. Immunized Library Construction
The serum titer after final immunization is more than 1:256000. Total RNA was reverse transcribed into cDNA. Using the cDNA as template, nanobody genes were amplified using two sets of primers (Table S1), among them the primers F and R1 were used to amplify the heavy-chain antibodies IgG3(short hinge regions), the primers F and R2 were used to amplify the heavy-chain antibodies IgG2(long hinge regions). 48 individual colonies from the nanobody library (>1.0 × 108 clones) were picked to perform the colony PCR; the insertion rate of the nanobody library was near 100% (Fig. S1). In addition, all these random-pick clones contained different nanobody sequences, which indicated that a high-quality phage display library with high diversity was obtained.
3.2. Bio-panning of Phage Display Library
Typically, bio-panning is performed by immobilizing the target antigen on a 96-well plate by physical adsorption, and the phage-displayed nanobody library interacted with the immobilized antigen directly. However, physical adsorption of the target antigen on the 96-well plate may lead to protein denaturation, especially the protein with high molecular weight. Moreover, part of the antigenic epitope of the target antigen cannot be exposed because of steric hindrance. These factors will result in a decrease in the effectiveness of the bio-panning. To avoid this situation, the phage library was bio-panning using the biotinylated GPC3 protein on the streptavidin-coated magnetic beads, which can expose the epitopes of GPC3 protein sufficiently and reduce GPC3 protein denaturation. In general, the biotinylated GPC3 protein was first mixed with the streptavidin-coated magnetic beads, and then the phage display nanobody library was added to the beads (Fig. S2A).
Five rounds of bio-panning were carried out in our study; the input titer of each round was similar to 1×1011 CFU. The concentrations of preincubated GPC3 protein was decreased from 1st round to 5th round (100 μg/mL, 20 μg/mL, 4μg/mL, 0.8 μg/mL, and 0.16 μg/mL, 100 μL per well) in order to capture the phage-displayed nanobody with high-affinity. The number of output phages after each round of panning is shown in Fig. S2B. It is obvious that the output of the second and third rounds was increased by more than 10 times, indicating that GPC3-specific displayed phage have been effectively enriched. The highest output was observed in the third round, even though the concentration of preincubated GPC3 was reduced 25-fold compared with that used in the first round. After two further rounds of biopanning against the low concentration of GPC3, 46 colonies in the 5th round were checked for their binding against GPC3 by phage-ELISA (Fig. S3A). 38 clones were identified as positive colonies as their OD450 in phage ELISA was 2 times higher than the negative control (Fig. S3B). After sequencing, 7 unique nanobodies were obtained, called GPN1, GPN2, GPN3, GPN4, GPN5, GPN6, GPN7 (Fig. 1).
Figure 1.

7-unique sequence alignment labeling the FR region and the CDR region. The stars represent the same amino acids of 7 clones, the introduction of dashed lines is used to improve the sequence alignment.
3.3. Property of Isolated Anti-GPC3 Nanobodies
The candidate clones were expressed to test their performance. The nanobody can be expressed without the pIII protein of phage in E.coil Top10 F’ cells, but with the HA and 6×His tags, which can be used for purification by Ni-NTA affinity column. 12% SDS-PAGE gel analysis demonstrated the size of purified nanobodies is about 17kDa (Fig. S4A). All nanobodies show the binding ability to antigen except GPN4, which shows a very weak binding signal. For example, the relative activity indicates that GPN2 remains approximately 100% active after being treated at 37 °C, even 63% activity after 80 °C for 1 hour, but the anti-GPC3-pAb AF2119 has lost its binding activity after being treated at 80 °C for 1 hour (Fig. S4B). The binding affinity was also analyzed by BioLayer interferometry (BLI) technology using the Octet system (Table S2). Among all nanobody candidates, GPN2 has the best affinity with equilibrium dissociation constants (KD) as 1.37 ×10−8 M. Therefore, the GPN2 nanobody was selected for the following research.
The cellular binding capacity of GPN2 was analyzed using flow cytometry. Expression of GPC3 in different liver cancer cell lines (HepG2, Hep3B, Huh-7, SK-Hep-1, SMMC-7721) was analyzed by western blot with anti-GPC3polyclonal antibody AF2119 (Fig. 2A). The expression level of GPC3 was high on Hep3B, HepG2, Huh-7, while it was weak on SMMC-7721 and barely observed on SK-Hep-1. In the flowcytometry study, GPN2 nanobody showed similar binding mode as AF2119, especially to GPC3-positive HCC cell lines (Hep3B, HepG2, Huh-7,) but not to GPC3-negative SK-Hep-1 cell (Fig. 2B).
Figure 2.

Binding properties of nanobody GPN2. (A), Western blot showed the expression of GPC3 in different liver cancer cell lines. β-actin is used as the internal reference. (B), Flow cytometry analysis of G2 antibody binding to SK-Hep1 (GPC3−), SMMC-7721(GPC3+), HepG2(GPC3+), Huh-7(GPC3+), Hep3B(GPC3+).
3.4. Generation of GPN2 Fusion Proteins
As shown in Fig. 3A, GPN2-Nluc fusion was generated successfully, which was determined by the SDS-PAGE with an expected band of approximately 35 kDa (Fig. 3B). For comparison, the GPN2-AP fusion was also generated and identified by SDS-PAGE (Fig. 3C). Usually, the sensitivity of immunoassay is highly related to its antigen-binding capacity. A conventional two-step ELISA was carried out to compare the binding of GPN2-NLuc/GPN2-AP fusion proteins with the parent GNP2 nanobody. It can be seen from the results that both GNP2 and two fusion proteins can bind to the antigen. Their EC50 in the two-step ELISA was similar, 1.48 μg/mL for GPN2, 1.52 μg/mL for GPN2-NLuc, 1.61 μg/mL for GPN2-AP, respectively (Fig. 4A). This means that the binding potency of GPN2 in the fusion protein is not reduced by the fusion partner, including Nluc and AP.
Figure 4.
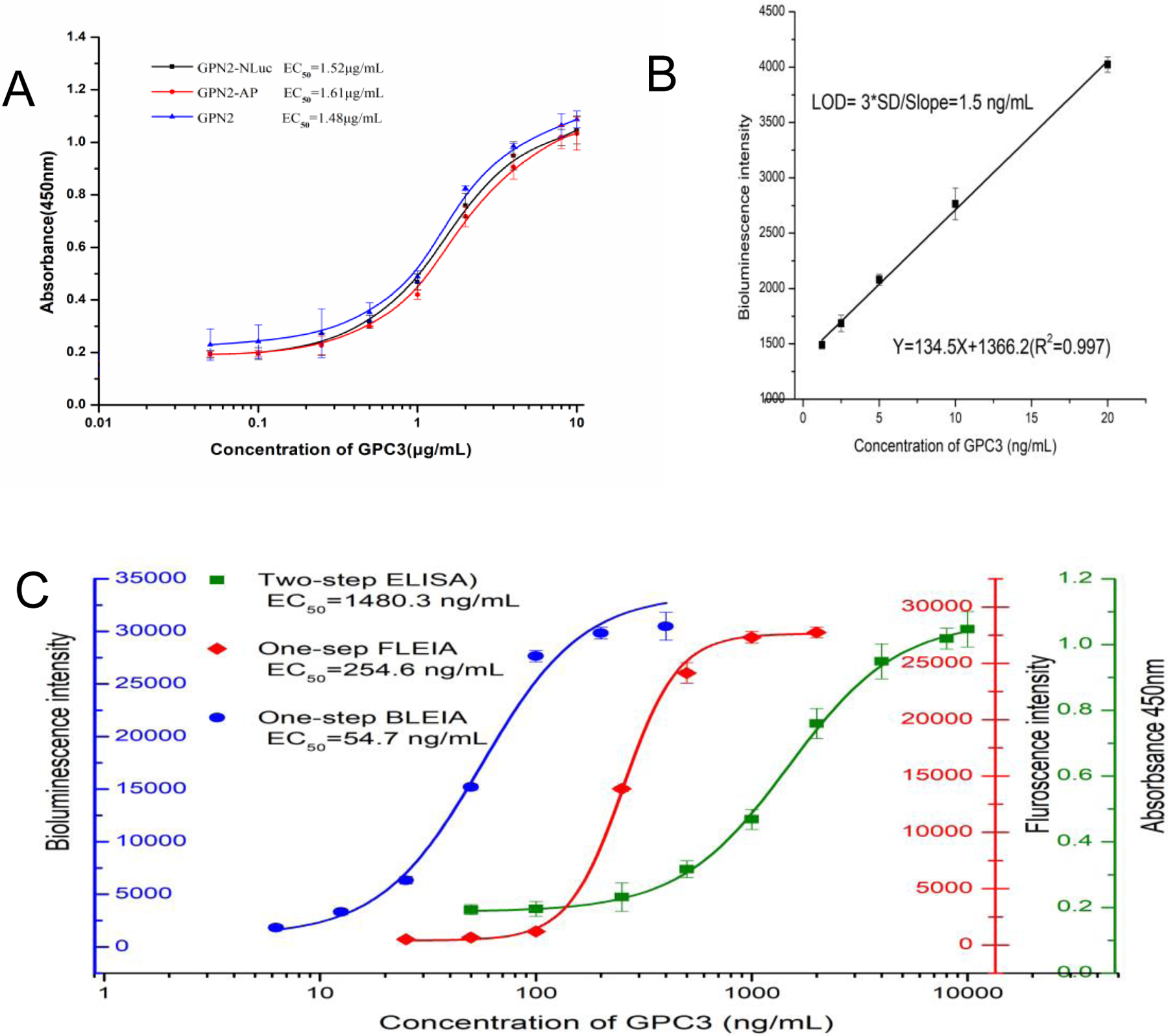
(A),Standard curves of GPN2-Nluc, GPN2-AP Fusion Protein and GPN2 parent nanobody in two-step ELISA. (B), Linear standard curve of one-step BLEIA for detection of GPC3, each point was tested in triplicate.(C), Standard curves of GPN2 parent nanobody in two-step ELISA, GPN2-AP in one-step FLEIA, GPN2-Nluc in one-step BLEIA.
3.5. BLEIA for GPC3 Based on GPN2-Nluc Fusion Protein
Usually, the immunoassay for protein like GPC3 was established on a sandwich format, in which the capture and a detect antibody should recognize different epitopes on target. In our study, the isolated nanobodies were likely to recognize any possible epitope of GPC3 since the whole protein of GPC3 was used as the immunogen. It is not feasible to form the sandwich immune-complex if the “detect” nanobodies recognized the same epitope as the “capture” antibody. To avoid this situation, we chose an anti-GPC3 polyclonal antibody AF2119 as the “capture” antibody, which recognized many epitopes of GPC3 with no conflict when paired with the GPN2-NLuc fusion protein recognizing a single epitope.
The optimal concentrations of the capture antibody, the GPN2-NLuc fusion protein, and GPC3 protein were determined by checkerboard titration. A standard curve of the BLEIA was established with EC50 as 54.7 ng/mL (Fig. 4C). It was almost 30 times lower than that of the traditional two-step ELISA based on the parent GPN2 (EC50=1480.3 ng/mL), and it is also about 5 times lower than fluorescence enzyme immunoassay (FLEIA) based on GPN2-AP (EC50=254.6 ng/mL). As shown in Fig. 4B, the linear equation (Y=134.5X+1366.2) from 1.25 ng/mL to 20 ng/mL was obtained in our study, with a limit of detection (LOD) as 1.5 ng/mL, which was defined by using 3×SD of the blank divided the slope.
To evaluate the accuracy of BLEIA, different concentrations of GPC3 protein were spiked into the serum samples. As shown in Table S4, the recovery range is from 93.0% to 105.7%, and dilution of the serum sample also has no impact on the final recovery rate. The results indicated that the proposed BLEIA could be used for serum samples without matrix effect.
Biomarkers related to HCC, such as AFP, CEA, and CA19–9, were spiked into serum samples to evaluate the specificity of the BLEIA for diagnosis of HCC. The cross-reactivity percentage against the interferent was less than 1% (Table 1). The BLEIA has low cross-reactivity with GPC1 and GPC3, which are GPC3 related family proteins. Additionally, only low cross-reactivity (less than 5%) was observed even in high spiked concentrations. That indicated the BLEIA based on the GPN2-NLuc has high specificity to detect GPC3 protein in human serum samples.
Table 1.
Cross-reactivity rates analyzed by the BLEIA assay
| Interferents | Spiked concentration (ng/mL) | Measured value Mean ±SD (ng/mL) | Cross-reactivity (%) |
|---|---|---|---|
| AFP | 1000 | 1.52±0.127 | 0.152 |
| CEA | 1000 | 2.02±0.203 | 0.202 |
| CA19–9 | 1000 | 1.27±0.068 | 0.127 |
| GPC1 | 1000 | 31.8±0.153 | 3.18 |
| GPC2 | 1000 | 38.2±0.128 | 3.82 |
AFP, Alpha-fetoprotein. CEA,Carcinoembryonic Antigen. CA19–9, carbohydrate antigen 19–9
3.6. Clinical Diagnosis of BLEIA in Human Samples
The GPC3 detected results are shown in Fig. 5A, 91.41±64.27 ng/mL for HCC, 20.28±9.22 ng/mL for liver cirrhosis, 26.38±19.18 ng/mL for hepatitis, and 12.29±8.807 ng/mL for healthy controls. The detected GPC3 in HCC patients is significantly higher than that of liver cirrhosis patients, hepatitis patients, and healthy controls.
Figure 5.
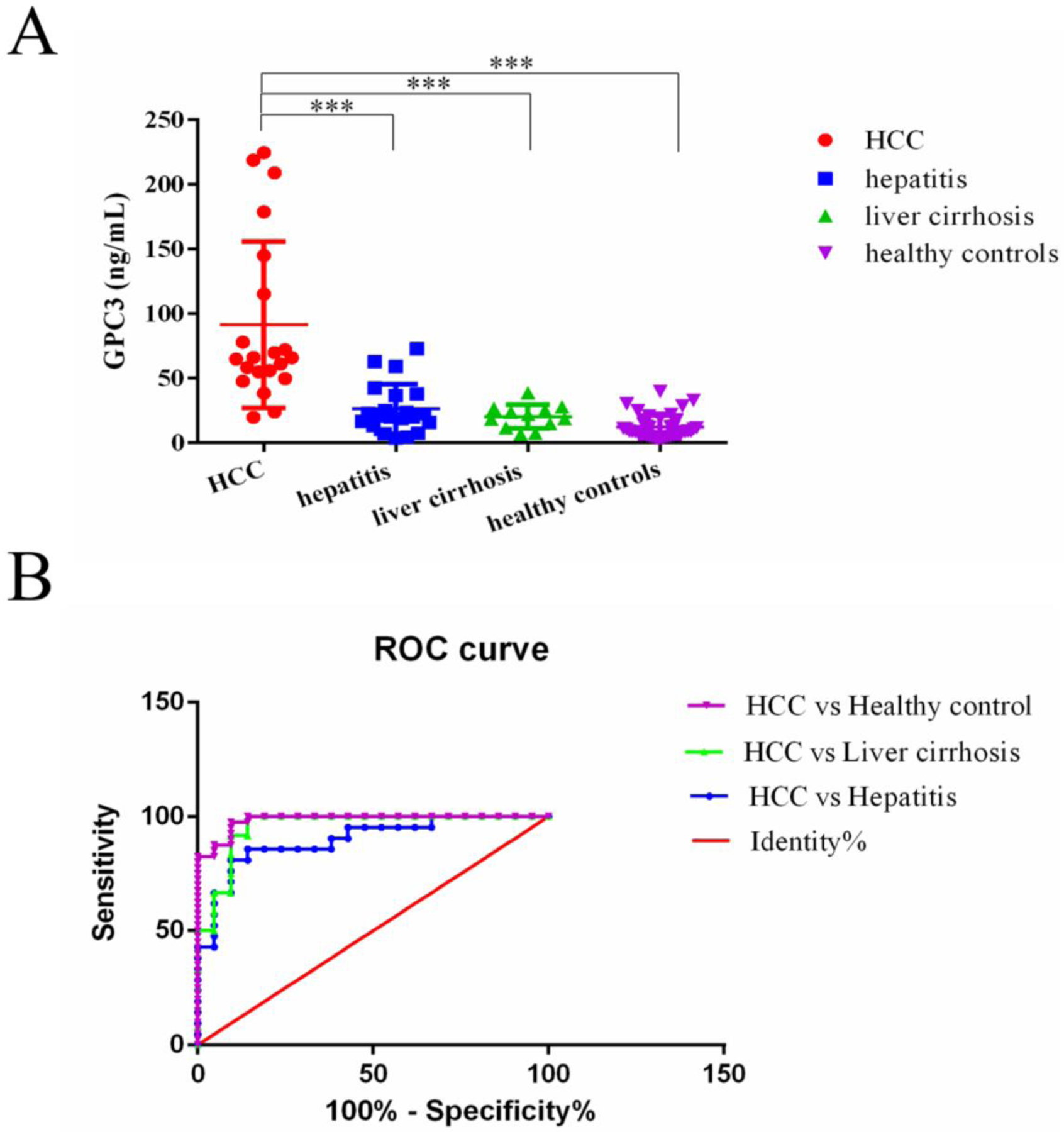
(A), Concentration of serum GPC3 in HCC cases, hepatitis, liver cirrhosis and healthy controls. ***P<0.001(A). (B), The ROC curve for serum GPC3 detected based on the BLEIA for the differentiation of HCC from healthy controls, liver cirrhosis and hepatitis.
To set the cut-off value, the receiver operating characteristic (ROC) curve of the BLEIA was constructed by pairwise comparing HCC with liver cirrhosis, hepatitis, and healthy controls. The area under the ROC of three groups was 0.9563, 0.898, 0.9845, respectively (Fig. 5B). If the cut-off value was set at 24.5 ng/mL, the sensitivity of GPC3 for HCC diagnosis was 87.5%, and the specificity was 90.48% in healthy controls. For the group of HCC versus liver cirrhosis, the cut-off is 33.2 ng/ml with a sensitivity of 91.67% and a specificity of 90.48%. For the group of HCC versus hepatitis, the cut-off is 45.19 ng/ml with a sensitivity of 85.71% and a specificity of 85.71%. It indicated this BLEIA assay could distinguish HCC from healthy people and other liver disease patients.
4. CONCLUSION
Small recombinant binders like nanobodies are promising biomaterial for immune biosensor development since they are available for engineering by molecular biology to create versatile immunoreagents. In this study, six nanobodies were isolated by a stringent bio-panning procedure based on GPC3 coated magnetic beads. This approach offers an efficient method for bio-panning the libraries to obtain nanobodies against GPC3, with great affinity and high specificity. In addition, the best nanobody clone GPN2 was used to generate the nanobody-Nluc and nanobody-AP fusion to reduce the analysis time and enhance the signal amplification in the immune biosensor. These dual-functional immune-reagents have great potential in biosensors developing for diagnosis application. An ultra-sensitive BLEIA based on the GPN2-Nluc fusion was successfully developed to measure the trace level of GPC3 in serum for clinical diagnosis of HCC, which exhibited good sensitivity, recovery, stability, and specificity. These findings provide the applicability of nanobody-based biosensor in the field of serological diagnosis.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Scientific Foundation of China (No. 81760551, 82003250, 32000660, 21766003), Guangxi Natural Science Foundation Project (2018GXNSFAA294117), Guangxi Medical and Health Appropriate Technology Development and Application Project (S2020005), Partial support was provided by the NIH - NIEHS (RIVER Award) R35 ES030443–01, the NIEHS Superfund Research Program P42 ES004699.
Footnotes
The authors declare no competing financial interest.
Supporting Information
Supplementary experimental section, Biotinylation of GPC3 protein, Bio-panning of anti-GPC3 nanobody clones by phage display, Identification of anti-GPC3 nanobody clones by phage ELISA, Expression and purification of anti-GPC3 Nanobody, Nanobody thermostability analysis, Western Blot of GNP2, Expression, and purification of GPN2-NLuc and GPN2-AP Fusion Protein, Two-step ELISA based on GPN2 nanobody and GPN2 fusion proteins, One-step FLISA based on GPN2-AP fusion protein. Tables, Primers were used in this article, KD determination, the Protein sequence of GPN2-Nluc and GPN2-AP fusion, recovery test. Figures, Construction of nanobody library, bio-panning work-flow, Scheme of sandwich Phage- ELISA, Nanobody expression and their thermostability analysis, the construction of GPN-AP, Assay format two-step nanobody based ELISA.
References
- [1].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA Cancer J Clin 68 (2018) 394–424. [DOI] [PubMed] [Google Scholar]
- [2].Feng MQ, Gao W, Wang R, Chen W, Man YG, Figg WD, Wang XW, Dimitrov DS, Mitchell H, Therapeutically targeting glypican-3 via a conformation-specific single-domain antibody in hepatocellular carcinoma, Proc Natl Acad Sci U S A 110 (2013) E1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wu Y, Liu H, Ding H, GPC-3 in hepatocellular carcinoma: current perspectives, J Hepatocell Carcinoma 3 (2016) 63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nakatsura T, Yoshitake Y, Senju S, Monji M, Komori H, Motomura Y, Hosaka S, Beppu T, Ishiko T, Kamohara H, Ashihara H, Katagiri T, Furukawa Y, Fujiyama S, Ogawa M, Nakamura Y, Nishimura Y, Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker, Biochem Biophys Res Commun 306 (2013) 16–25. [DOI] [PubMed] [Google Scholar]
- [5].Hanaoka H, Nagaya T, Sato K, Nakamura Y, Watanabe R, Harada T, Gao W, Feng M, Phung Y, Kim I, Paik CH, Choyke PL, Ho M, Kobayashi HH, Glypican-3 targeted human heavy chain antibody as a drug carrier for hepatocellular carcinoma therapy, Mol Pharm 12 (2015) 2151–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Haruyama Y, Kataoka H, Glypican-3 is a prognostic factor and an immunotherapeutic target in hepatocellular carcinoma, World J Gastroenterol 22 (2016) 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Phung Y, Gao W, Man YG, Nagata S, Ho M, High-affinity monoclonal antibodies to cell surface tumor antigen glypican-3 generated through a combination of peptide immunization and flow cytometry screening, MAbs 4 (2012) 592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Traister A, Shi W, Filmus J, Mammalian Notum induces the release of glypicans and other GPI-anchored proteins from the cell surface, Biochem J 410 (2018) 503–511. [DOI] [PubMed] [Google Scholar]
- [9].Mu W, Jiang D, Mu S, Liang S, Liu Y, Zhang N, Promoting Early Diagnosis and Precise Therapy of Hepatocellular Carcinoma by Glypican-3-Targeted Synergistic Chemo-Photothermal Theranostics, ACS Appl Mater Interfaces 11 (2019) 23591–23604. [DOI] [PubMed] [Google Scholar]
- [10].Xu D, Su C, Sun L,Gao Y, Li Y, Performance of Serum Glypican 3 in Diagnosis of Hepatocellular Carcinoma: A meta-analysis, Ann Hepatol 18 (2019) 58–67. [DOI] [PubMed] [Google Scholar]
- [11].Zhao S, Long M, Zhang X, Lei S, Dou W, Hu J, Du X, Liu L, The diagnostic value of the combination of Golgi protein 73, glypican-3 and alpha-fetoprotein in hepatocellular carcinoma: a diagnostic meta-analysis, Ann Transl Med 8 (2020) 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ma RJ, Wang SH, Qin SN, Wang XB, Li GF, Li M, Xu W, Preparation and characterization of monoclonal antibody against glypican-3, Hybridoma (Larchmt) 31(2012) 455–461. [DOI] [PubMed] [Google Scholar]
- [13].Hippo Y, Watanabe k., Watanabe A, Midorikawa Y, Yamamoto S, Ihara S, Tokita S, Iwanari H, Ito Y, Nakano K, Nezu J, Tsunoda H, Yoshino T, Ohizumi I, Tsuchiya M, Ohnishi S, Makuuchi M, Hamakubo T, Kodama T, Aburatani H, Identification of soluble NH2-terminal fragment of glypican-3 as a serological marker for early-stage hepatocellular carcinoma, Cancer Res 64 (2004) 2418–2423. [DOI] [PubMed] [Google Scholar]
- [14].Chen M, Li G, Yan J, Lu X, Cui J, Ni Z, Cheng W, Qian G, Zhang J, Tu H, Reevaluation of glypican-3 as a serological marker for hepatocellular carcinoma, Clin Chim Acta 423 (2013)105–111. [DOI] [PubMed] [Google Scholar]
- [15].Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, Filmus J, Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma, Gastroenterology 125 (2003) 89–97. [DOI] [PubMed] [Google Scholar]
- [16].Ishiguro T, Sugimoto M, Kinoshita Y, Miyazaki Y, Nakano K, Tsunoda H, Sugo I, Ohizumi I, Aburatani H, Hamakubo T, Kodama T, Tsuchiya M, Yamada-Okabe H, Anti-glypican 3 antibody as a potential antitumor agent for human liver cancer, Cancer Res 68 (2008) 9832–9838. [DOI] [PubMed] [Google Scholar]
- [17].Zhu AX, Gold PJ, El-Khoueiry AB, Abrams TA, Morikawa H, Ohishi N, Ohtomo T, Philip PA, First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma, Clin Cancer Res 19 (2013) 920–928. [DOI] [PubMed] [Google Scholar]
- [18].Xie C, Tiede C, Zhang X, Wang C, Li Z, Xu X, McPherson MJ, Tomlinson DC, Xu W, Development of an Affimer-antibody combined immunological diagnosis kit for glypican-3, Scientific reports 7 (2017) 9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yasuda E, Kumada T, Toyoda H, Kaneoka Y, Maeda A, Okuda S, Yoshimi N, Kozawa O, Evaluation for clinical utility of GPC3, measured by a commercially available ELISA kit with Glypican-3 (GPC3) antibody, as a serological and histological marker for hepatocellular carcinoma Hepatol Res 40 (2010) 477–485. [DOI] [PubMed] [Google Scholar]
- [20].Yu JP, Xu XG, Ma RJ, Qin SN, Wang CR, Wang XB, Li M, Li MS, Ma Q, Xu WW, Development of a clinical chemiluminescent immunoassay for serum GPC3 and simultaneous measurements alone with AFP and CK19 in diagnosis of hepatocellular carcinoma, J Clin Lab Anal 29 (2015) 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang Q, Xiao Q, Lin Z, Ying X, Li Z, Lin JM. Development of a competitive radioimmunoassay for glypican-3 and the clinical application in diagnosis of hepatocellular carcinoma, Clin Biochem 43 (2010) 1003–1008. [DOI] [PubMed] [Google Scholar]
- [22].Yang X, Liu H, Sun CK, Natarajan A, Hu X, Wang X, Allegretta M, Guttmann RD, Gambhir SS, Chua MS, Cheng Z, So SK,. Imaging of hepatocellular carcinoma patient-derived xenografts using 8 9 Zr-labeled anti-glypican-3 monoclonal antibody, Biomaterials 35 (2014) 6964–6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sham JG, Kievit FM, Grierson JR, Miyaoka RS, Yeh MM, Zhang M, Yeung RS, Minoshima S, Park JO, Glypican-3-targeted 89Zr PET imaging of hepatocellular carcinoma. Journal of nuclear medicine: official publication, Society of Nuclear Medicine 55 (2014) 799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bever CS, Dong JX, Vasylieva N, Barnych B, Cui Y, Xu ZL, Hammock BD, Gee SJ, VHH antibodies: emerging reagents for the analysis of environmental chemicals, Anal Bioanal Chem 408 (2016) 5985–6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ubah OC, Buschhaus MJ, Ferguson L, Kovaleva M, Steven J, Porter AJ, Barelle CJ, Next-generation flexible formats of VNAR domains expand the drug platform’s utility and developability, Biochem Soc Trans 46 (2018) 1559–1565. [DOI] [PubMed] [Google Scholar]
- [26].Kim HJ, McCoy MR, Majkova Z, Dechant JE, Gee SJ, Tabares-da Rosa S, González-Sapienza GG, Hammock BD, Isolation of alpaca anti-hapten heavy chain single domain antibodies for development of sensitive immunoassay, Anal Chem 84 (2012) 1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lu Q, Li X, Zhao J, Zhu J, Luo Y, Duan H, Ji PP, Wang K, Liu B, Wang X, Fan W, Sun Y, Zhou E, Zhao Q, Nanobody-horseradish peroxidase and -EGFP fusions as reagents to detect porcine parvovirus in the immunoassays, J Nanobiotechnology 18 (2020) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Huo JQ, Li ZF, Wan DB, Li DY, Qi M, Barnych B, Vasylieva N, Zhang JL, Hammock BD, Development of a Highly Sensitive Direct Competitive Fluorescence Enzyme Immunoassay Based on a Nanobody-Alkaline Phosphatase Fusion Protein for Detection of 3-Phenoxybenzoic Acid in Urine, J Agric Food Chem 66 (2018) 11284–11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li DY, Cui YL, Morisseau C, Gee SJ, Bever CS, Liu XJ, Jian W, Hammock BD, Nanobody Based Immunoassay for Human Soluble Epoxide Hydrolase Detection Using Polymeric Horseradish Peroxidase (PolyHRP) for Signal Enhancement: The Rediscovery of PolyHRP? Anal Chem 89 (2017) 6248–6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Xia L, Teng Q, Chen Q, Zhang F. Preparation and Characterization of Anti-GPC3 Nanobody Against Hepatocellular Carcinoma, Int J Nanomedicine 15 (2020) 2197–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li ZF, Wang Y, Vasylieva N, Wan DB, Yin Z, Dong JX, Hammock BD, An Ultrasensitive Bioluminescent Enzyme Immunoassay Based on Nanobody/Nanoluciferase Heptamer Fusion for the Detection of Tetrabromobisphenol A in Sediment, Anal Chem 92 (2020) 10083–10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chen JJ, Xie CM, Wang CR, Wan Y, Dong ZN, Li M, Xu WW. Development of a Time-Resolved Fluorescence Immunoassay for the Diagnosis of Hepatocellular Carcinoma Based on the Detection of Glypican-3, J Fluoresc 27 (2017) 1479–1485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


