Abstract
Background:
Staple line leaks are a serious problem in bariatric surgery and a major cause of serious morbidity and mortality. Adverse events caused by medical devices are reported to the Food and Drug Administration which maintains the Manufacturer and User Facility Device Experience (MAUDE) database. We examined adverse stapler events reported to the MAUDE database, specifically with regards to bariatric surgery.
Methods:
The MAUDE database was queried for adverse events caused by staplers between January 1, 2018 – December 31, 2020; events reported by Intuitive, Ethicon, and Medtronic/Covidien; and limited our search to “gastric bypass”, “sleeve gastrectomy”, “stapler malfunction” combined with each company.
Results:
There were 883 adverse events reported for Medtronic, 353 for Ethicon, and 35 for Intuitive. Approximately 3.5 million staple reloads sold in the study period. The reported misfire rate for Medtronic was 0.04% and for Ethicon was 0.02%. Data for Intuitive was unavailable. The most common reported event for Medtronic was failure to fire (n = 349), followed by misfire (n = 186). For Ethicon, the most common event was failure to fire (n = 146), followed by mechanical problems (n = 27). The most common event with the Intuitive stapler was leak (n = 10) and bleeding from staple line (n = 8).
Conclusions:
Stapler malfunction is a very rare event in metabolic and bariatric surgery. All of the major stapler producers have transitioned to powered staplers with excellent safety profiles. Open and honest reporting about stapler malfunction is essential to determine the true safety of these ubiquitous devices.
Keywords: Bariatric surgery, Complications, MAUDE, Stapler malfunction, Stapler misfire
INTRODUCTION
Staple line leaks are a serious problem in bariatric surgery and a major cause of morbidity and mortality. Surgical staplers have been used to divide and approximate tissue for decades. In fact, the first surgical stapler was developed in 1908 by Victor Fischer and Hümér Hültl.1 Modern surgeons have come to rely on these devices, and they have continually undergone improvements since their introduction. Widespread use of surgical staplers occurred in the post World War II era when battlefield surgeons utilized staplers in emergency cases. For a primer on the history of surgical staplers, readers are directed to Baker et al., whose work entitled “The science of stapling and leaks” was published in Obesity Surgery in 2004.2 Surgical staplers were adapted for minimally invasive surgery in the 1990s.3 They have revolutionized bowel surgery and are one of the main technological advances that are responsible for the growth of minimally invasive bariatric and metabolic surgery. There have traditionally been two large producers of surgical staplers: Johnson and Johnson and Medtronic (formerly Covidien) have over 90% of the market, although smaller companies are trying to break into this competitive market. This includes robotic platforms and traditional laparoscopic staplers.
No matter how advanced these staplers become, there will still be failures as with any mechanical device. Surgical staplers have very low failure rates overall, but events include failure to fire, malformed staples, handles that lock up, and other complications. Many experienced surgeons have experienced events like this in their careers.4 Patients may ultimately pay the price for these malfunctions in the form of serious complications such as bleeding or leaks. One study using the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) evaluated which complications lead to worse outcomes in bariatric surgery and found that leak carried a relative risk (RR) for mortality of 19.7 and for bleeding was 9.4.5 Another study from the same database evaluating Roux-en-Y gastric bypass (RYGB) showed again poor outcomes with leak. There is no way to specifically evaluate if these leaks were from staple misfires, but they can be a contributing factor.6 The same group evaluated sleeve gastrectomy (SG) and found that bleeding increased mortality and morbidity. Staple misfire is not a collected variable in the MBSAQIP, but staple line bleeds are a primary cause of postoperative hemorrhage in SG. The authors also found that staple line oversewing or reinforcement was protective from bleeding.7
These events should be reported by the device representatives who should then report up the chain in their companies. Ideally, this leads to adverse events caused by medical devices being reported to the Food and Drug Administration (FDA), which maintains the Manufacturer and User Facility Device Experience (MAUDE) database.8 These reports are made voluntarily to the FDA by the manufacturers and are dependent on surgeons and industry reporting a problem in the first place.
We examined adverse stapler events reported to the MAUDE database, specifically with regards to bariatric surgery. Our primary outcome of interest was reported stapler malfunctions and our secondary outcome of interest was malfunction by company.
METHODS
The MAUDE database was queried for adverse events caused by staplers between January 1, 2018 – December 31, 2020. We searched for events reported by Intuitive, Ethicon, and Medtronic. We also limited our search to “gastric bypass”, “sleeve gastrectomy”, “stapler malfunction” combined with each company. The MAUDE database reports events in a free form method, and we grouped them into categories such as “failure to fire”, “misfire”, “difficult to open”, “mechanical jam”, “firing problem”, etc. These descriptive terms are reported by the company representative and not the surgeon.
To determine the number of total staple loads used in the US, we used the ASMBS estimate of bariatric cases published in 2018.9 This included data from the MBSAQIP database and is broken down by procedures. We then created a model to calculate the number of staple loads used. Our model used the numbers for 2018 for SG and RYGB and makes some broad assumptions. The data for 2019 and 2020 is not available, so we assumed a similar number of cases for those years. In 2018, there were 154,976 SG and 42,945 RYGB procedures performed. We then multiplied this by 6, which is an average number of staple loads used for either case, giving us 1,187,526 staple loads. Assuming a roughly equal number of cases for 2019 and 2020 gives us a final number of 3,562,578 staple loads. The number for 2020 is likely overestimated because of cessation of elective cases secondary to the COVID-19 pandemic. However, even if we decrease the 2020 volume by 30%, there is still an overall number of 30.2 million staple loads. When we count the small number of reported malfunctions, this does not significantly change the percentage of malfunctions. We did not count duodenal switches or revisions because they may be more variable in terms of the number of staple loads used.
We contacted each of the major stapler companies to request the number of surgical staple loads sold for the study time period. Medtronic and Ethicon would not give exact sales numbers, but both companies felt they had about 50% market share. Intuitive ignored our request for information altogether. Since Ethicon and Medtronic have about an equal part of 95% of the market share, we estimated the total number of reloads sold by each company. It is more difficult to come up with Intuitive’s numbers as surgeons often use other stapling devices when using a robotic platform to perform bariatric surgery, despite Intuitive now offering a proprietary stapler. Our institutional review board reviewed these studies and deemed it exempt from review as it uses publicly available deidentified data. None of the authors have any financial relationships with Intuitive, Ethicon, or Medtronic.
RESULTS
We found that there were 883 adverse events reported for Medtronic, 353 for Ethicon, and 35 for Intuitive. According to our calculations, there were approximately 30.5 million staple reloads used for SG and RYGB from January 1, 2018 – December 31, 2020. Medtronic and Ethicon account for almost 100% of the market, divided equally, which puts each company at roughly 1.78 million staple loads used in that time period. The calculated misfire rate for Medtronic based on these numbers was 0.04% and 0.02% for Ethicon. Data to calculate the misfire rate for Intuitive was unavailable. Figure 1 shows the comparison of types of malfunctions between companies.
Figure 1.
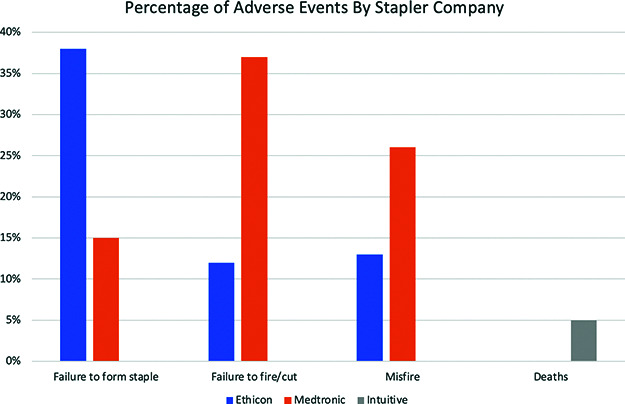
Bar graph of the most common adverse events by manufacturer.
The most common reported event for Medtronic was failure to fire with 390 events, followed by misfire with 201 events (Figure 2). The data for Medtronic included 16 events for the EEA™ circular stapler, most of which were the anvil becoming detached prematurely. There were 464 events for the Endo GIA™ stapler. When considering the Endo GIA™ alone, the most commonly reported event was failure to fire with 232 events, followed by 75 misfires (defined as partial firing of staple line). The iDriveTM was reported 20 times, with failure to fire and misfire being the most commonly reported events. The SigniaTM stapler was reported 128 times, again misfire and failure to fire being the most common events. Also reported for the SigniaTM was “device difficult to set-up or prepare” which was usually described as difficulty with the powered handle.
Figure 2.
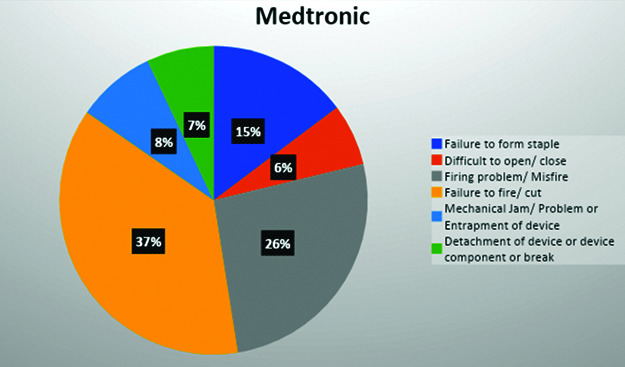
Type of malfunction by category for Medtronic.
For Ethicon, the most common event was “failure to form staple” with 110 events, followed by “mechanical problems/jams” with 29 events. The other commonly reported events were “failure to cut”, “difficulty opening/closing”, “failure to form staples”, and “breakage” (Figure 3). The most common stapler reported was the powered 60 mm EchelonTM followed by the Echelon FlexTM.
Figure 3.
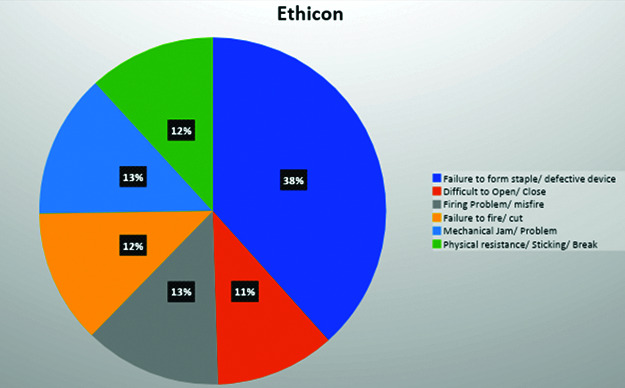
Type of malfunction by category for Ethicon.
There were 35 of events reported by Intuitive. The most common events with the Intuitive stapler were leak (n = 10) and bleeding from staple line (n = 8). The only 2 reported deaths in the MAUDE database were in cases performed with Intuitive staplers. Both deaths were associated with postoperative sepsis due to a staple line leak, which were not noted intraoperatively.
DISCUSSION
To our knowledge, this is the first publication evaluating the MAUDE database in regard to surgical staplers in metabolic and bariatric surgery (MBS). Surgical staplers are a common tool used by bariatric surgeons. These devices have increased in ease-of-use, reliability, and accessibility. Fortunately, the results demonstrated in this paper show that staple malfunction is very rare and that most stapler misfires do not lead to serious adverse events for the patient. All major bariatric operations rely on surgical stapling technology at this time as the adjustable gastric band has been essentially abandoned.10 This heavy reliance of metabolic and bariatric surgeons on stapling technology can make failures of these devices seem glaring and unforgivable. When a stapler misfire leads to a leak or a serious bleeding event this can cause angst as well as distrust of the device on the part of the surgeon. In our results, we discovered that most of the adverse events reported to the database are in fact misfires where the stapler either will not fire properly or will not open but has not cut any tissue. There were a limited number of reported events leading to death. The market has been dominated by two large companies: Ethicon (a subsidiary of Johnson and Johnson) and Medtronic (formerly known as Covidien). There are smaller stapler companies trying to break into this market, but it is very difficult as surgeons become comfortable with one device and know that these devices have an outstanding safety record.
The MAUDE database has been used to evaluate stapler malfunction before. Kwazneski et al. evaluated all surgical stapler reported events in 2013 and found a lower rate of misfire with 84 documented misfires giving a reported incidence of malfunction of 0.00003. They also surveyed 124 minimally invasive surgeons about their experience and 66% of them had experienced a stapler malfunction.11 Eight years later, the number of reported incidents has increased dramatically. This may reflect better reporting by users and the companies that produce staplers.
The reported rate of stapler malfunction does not indicate the severity of the complication for the patient. Most of the reported events are simple mechanical issues, but more serious complications such as bleeding, leak, or even death can occur. The great majority of the adverse events did not have clinical significance and the device was usually replaced and the procedure continued. The amount of detail reported in the remarks section varied from a few words to a descriptive paragraph. The MAUDE database reports in a descriptive manner as described in the methods section. The terminology used to report events is vague and may not accurately reflect what happened. For example, a “misfire” may mean that the stapler did not form an appropriate staple line, but it also may mean that the stapler did not fire because of safety mechanisms built in to keep it from deploying in tissue that is too thick, such as the Intuitive or Signia staples are designed to do. Our study was limited to bariatric cases and only listed 2 deaths, but a review of the literature of other surgical specialties demonstrates more severe complications and mortality. Gopal et al. recently reviewed the MAUDE database for stapler malfunctions in nephrectomies.12 They found 383 reported events in the last 10 years. Ethicon accounted for 63% of complications (240 cases); 28% of complications were due to the GIA™ stapler (107 cases); and 9% of complications were due to the TA™ stapler (36 cases). There were also 22 deaths (5.7% of total complications) attributed to endovascular staplers which was generally due to stapler misfiring during transection of the renal artery. There is a difference in reported events by company, with Ethicon having a calculated failure rate of 0.02%, which is exactly what we found in bariatric surgery.
Surgeons come to trust in these staple device companies over time due to their successful operations. One of the worst feelings as a surgeon is when a device you consistently use with good outcomes is recalled. In April 2019, two Ethicon circular staplers (total of 92,496) were recalled due to insufficient firing which led to malformed staples compromising the integrity of the staple line.13 We did not find any reported EEA™ events for the Ethicon data but did find 16 events in the Medtronic data. We cannot explain why the EEA™ stapler data was not reported for Ethicon. Design defects can be a cause of malfunction but that is not always the case. Surgeons who have a malfunction of a stapler often send the device back to the company for testing. Similar to our findings, a previous study that utilized the MAUDE database found that despite the perception that staplers were intrinsically flawed, 32.5% of properly functioning devices were sent back and 65% of malfunctions were due to human misuse of the product. The detailed notes on each event in the MAUDE database usually states that the device functioned normally when retested in the lab. Training of the operating staff on the proper use of the devices is critical to minimize potentially significant complications from occurring.12 Also, user error is not reported per se in the MAUDE database. For example, Ethicon claims in their package insert that holding compression will decrease bleeding from the staple line. There is no way to tell from the MAUDE database if surgeons did this. This may have affected outcomes. The MAUDE database also does not specify if the appropriate staple height was chosen by the surgeon. Bleeding rates can be increased if an inappropriate staple height is used.
Another notable advance in surgical stapling technology has been the development of powered staplers. The Ethicon Echelon stapler was released in 2011 and the Medtronic SigniaTM followed suit with their “tri staple” powered device in 2017.14 Both staplers increased the ease of use for the surgeon by automating some of the functions of the stapler, especially the firing process. Our current study focused mainly on powered staplers as these were widely available after the years of 2018. We demonstrated a low rate of misfires in both the Ethicon and Medtronic products. Adverse events were reported at a rate of 1 every 2500 uses to 1 every 5000 staple loads and a large majority of these events did not cause any harm to the patient. The powered staplers from Ethicon and Medtronic have been compared before using the Premier Healthcare Database in 2020. Ethicon was reported to have a lower bleeding rate, but the authors used a payer database which only reports the diagnostic codes for bleeding. Some confounding factors were lack of specificity about the cause of bleeding, no reporting of staple line reinforcement (SLR) and surgeon experience. The authors also are paid consultants for Johnson and Johnson.15 There are other examples of Johnson and Johnson funding papers studying their staplers.16–18
Intuitive has joined the stapling market with a stapler specifically designed for their robotic platform. They have been making inroads into the MBS market and released their surgical stapler for the da Vinci SI™ in 2012 and the da Vinci XI™ in 2014.19 In 2014 the SI stapler was recalled by the FDA due to reports of “inability to remove the stapler from tissue”.13 However, this was withdrawn soon afterwards, and the company redesigned the device, releasing it in 2016. There are 49 documented recalls for Intuitive products on the FDA website from January 1, 2005 – December 31, 2020 and 29 of them involve the EndoWrist™ stapler. Despite this, there are many robotic surgeons who still use a different companies’ stapling device rather than the robotic stapler. In a study by Hagen et al. in 2017, they looked at the use of the robotic stapler versus a linear stapling device during robotic bariatric surgery.20 The study followed 49 patients undergoing robotic RYGB with the Intuitive stapler and matched them with 49 patients undergoing robotic RYGB with an Ethicon Echelon™ stapler. They found that patients undergoing surgery with the robotic stapler on average took an additional 22 minutes. The robotic stapler had unsuccessful clamping in 19.0% of all recorded attempts. Two intraoperative complications that were unrelated to stapling and one complication due to stapling were observed in the robotic stapler cohort, while none were observed for the Echelon group. They also found that the robotic stapler required more reloads than the linear stapler to complete the gastric pouch. As of now, there is no way to determine the number of surgeons who use a laparoscopic stapler in their robotic MBS cases.
Although there were isolated mentions of SLR reported in the database, it was not a collected variable. SLR has been definitively shown to reduce bleeding rates in SG by up to 30%.21 Gagner et al. recently published a systematic review and demonstrated reductions in leak rates using SLR.22 Although SLR is mentioned in some of the comments in the MAUDE database, there is no evidence that the misfires were caused by SLR.
The chief limitation of this paper is the nature of the MAUDE database. The data sometimes overlaps, and the eports can be incomplete as the reporting onus is on the companies and the company equipment representatives that are in the field. There is probably a gross underestimation of misfires and adverse events that are not reported to the database, as reporting in the responsibility of industry and not the surgeon. Another limitation of this study is the fact that neither of the large stapler companies would give more than a rough estimate of the number of staple loads that they sold for bariatric surgeries. The data that we used to calculate the number of total staple loads used in the country for the RYGB and SG was from the ASMBS estimate and not provided by either company, although both Johnson and Johnson and Medtronic were asked to share this information with the authors. We also did not have access to the 2020 numbers for the MBSAQIP since they had not been released at the time of our writing this article. Case volume may vary as much as 20,000 cases a year. But given the fact that we are estimating over 30.5 million staple loads, a difference of 20,000 a year would be insignificant mathematically. Therefore, our numbers are a rough estimate but are probably as accurate as we can make them given the lack of access to sales data. If one company has a more dominant share of the market, they may have more or less staple line failures which could change our best guess of calculated failure rates. It is impossible to calculate these rates with certainty, thanks to the manufacture’s refusal to provide data.
Another limitation or confounding factor is the widespread use of SLR. Over half of SG reported in the MBSAQIP used SLR.23 Medtronic does have its own SLR but there are 2 large independent providers of SLR. Their products are specifically designed to work with the larger stapler manufacturers, but there is a slight chance that the SLR interfered in the function of the stapler, and it is impossible to determine this given the current state of the MAUDE database.
CONCLUSION
Stapler malfunction is a very rare event in metabolic and bariatric surgery. All of the major stapler producers have transitioned to powered staplers with excellent safety profiles. The robotic stapler is still in its infancy and its safety profile is more difficult to determine given the lack of data about it. Open and honest reporting about stapler malfunction is essential to determine the true safety of these ubiquitous devices.
Footnotes
Disclosure: none.
Funding sources: none.
Conflict of interests: none.
Informed consent: Dr. Benjamin Clapp declares that written informed consent was obtained from the patient/s for publication of this study/report and any accompanying images.
Contributor Information
Benjamin Clapp, Department of Surgery, Texas Tech HSC Paul Foster School of Medicine, El Paso, TX, USA..
Alexander Schrodt, Department of Surgery, Texas Tech HSC Paul Foster School of Medicine, El Paso, TX, USA..
Maria Ahmad, Department of Surgery, Texas Tech HSC Paul Foster School of Medicine, El Paso, TX, USA..
Ellen Wicker, Department of Surgery, Texas Tech HSC Paul Foster School of Medicine, El Paso, TX, USA..
Nishtha Sharma, Department of Surgery, Texas Tech HSC Paul Foster School of Medicine, El Paso, TX, USA..
Andres Vivar, Universidad Autonoma Guadalajara, Guadalajara, Mexico..
Brian Davis, Department of Surgery, Texas Tech HSC Paul Foster School of Medicine, El Paso, TX, USA..
References:
- 1.Robicsek F. The birth of the surgical stapler. J Am Coll Surg. 1980;150(4):579–583. [PubMed] [Google Scholar]
- 2.Baker RS, Foote J, Kemmeter P, Brady R, Vroegop T, Serveld M. The science of stapling and leaks. Obes Surg. 2004;14(10):1290–1298. [DOI] [PubMed] [Google Scholar]
- 3.Lee-Kong S, Feingold DL. The history of minimally invasive surgery. Semin Colon Rectal Surg. 2013;24(1):3–6. 2013 [Google Scholar]
- 4.Makanyengo SO, Thiruchelvam D. Literature review on the incidence of primary stapler malfunction. Surg Innov. 2020;27(2):229–234. [DOI] [PubMed] [Google Scholar]
- 5.Daigle CR, Brethauer SA, Tu C, et al. Which postoperative complications matter most after bariatric surgery? Prioritizing quality improvement efforts to improve national outcomes. Surg Obes Relat Dis. 2018;14(5):652–657. [DOI] [PubMed] [Google Scholar]
- 6.Mocanu V, Dang J, Ladak F, Switzer N, Birch DW, Karmali S. Predictors and outcomes of leak after Roux-en-Y gastric bypass: an analysis of the MBSAQIP data registry. Surg Obes Relat Dis. 2019;15(3):396–403. [DOI] [PubMed] [Google Scholar]
- 7.Mocanu V, Dang J, Ladak F, Switzer N, Birch DW, Karmali S. Predictors and outcomes of bleed after sleeve gastrectomy: an analysis of the MBSAQIP data registry. Surg Obes Relat Dis. 2019;15(10):1675–1681. [DOI] [PubMed] [Google Scholar]
- 8.MAUDE - Manufacturer and User Facility Device Experience. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm. Accessed December 15, 2020.
- 9.English WJ, DeMaria EJ, Hutter MM, et al. American Society for Metabolic and Bariatric Surgery 2018 estimate of metabolic and bariatric procedures performed in the United States. Surg Obes Relat Dis. 2020;16(4):457–463. [DOI] [PubMed] [Google Scholar]
- 10.Clapp B, Harper B, Dodoo C, et al. Trends in revisional bariatric surgery using the MBSAQIP database 2015-2017. Surg Obes Relat Dis. 2020;16(7):908–915. [DOI] [PubMed] [Google Scholar]
- 11.Kwazneski II, D, Six C, Stahlfeld K. The unacknowledged incidence of laparoscopic stapler malfunction. Surg Endosc. 2013;27(1):86–89. [DOI] [PubMed] [Google Scholar]
- 12.Gopal N, Long B, Phillips J, Eshghi M. Endovascular stapler complications during minimally invasive nephrectomy: an updated review of the FDA MAUDE Database from 2009-2019. Urology. 2021;153:181–184. [DOI] [PubMed] [Google Scholar]
- 13.FDA. Ethicon Recalls Circular Staplers for Insufficient Firing and Failure to Completely Form Staples. Available at: https://www.fda.gov/medical-devices/medical-device-recalls/ethicon-recalls-circular-staplers-insufficient-firing-and-failure-completely-form-staples. Accessed February 1, 2021.
- 14.Medtronic website. Available at: https://www.medtronic.com/covidien/en-us/products/surgical-stapling/signia-stapling-system.html. Accessed February 1, 2021.
- 15.Rawlins L, Johnson BH, Johnston SS, et al. Comparative effectiveness assessment of two powered surgical stapling platforms in laparoscopic sleeve gastrectomy: a retrospective matched study. Med Devices (Auckl)). 2020;13:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller DL, Roy S, Kassis ES, Yadalam S, Ramisetti S, Johnston SS. Impact of powered and tissue-specific endoscopic stapling technology on clinical and economic outcomes of video-assisted thoracic surgery lobectomy procedures: a retrospective, observational study. Adv Ther. 2018;35(5):707–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roy S, Yoo A, Yadalam S, Fegelman EJ, Kalsekar I, Johnston SS. Comparison of economic and clinical outcomes between patients undergoing laparoscopic bariatric surgery with powered versus manual endoscopic surgical staplers. J Med Econ. 2017;20(4):423–433. [DOI] [PubMed] [Google Scholar]
- 18.Fegelman E, Knippenberg S, Schwiers M, et al. Evaluation of a powered stapler system with gripping surface technology on surgical interventions required during laparoscopic sleeve gastrectomy. J Laparoendosc Adv Surg Tech A. 2017;27(5):489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Intuitive Surgical. Intuitive Surgical Brings Stapler Instrumentation to da Vinci Robotic-Assisted Surgical Systems in the U.S., Europe & Asia. Available at: (https://isrg.gcs-web.com/news-releases/news-release-details/intuitive-surgical-brings-stapler-instrumentation-da-vinci). Accessed February 1, 2021.
- 20.Hagen ME, Jung MK, Fakhro J, et al. Robotic versus laparoscopic stapling during robotic Roux-en-Y gastric bypass surgery: a case-matched analysis of costs and clinical outcomes. Surg Endosc. 2018;32(1):472–477. [DOI] [PubMed] [Google Scholar]
- 21.Zafar SN, Felton J, Miller K, Wise ES, Kligman M. Staple line treatment and bleeding after laparoscopic sleeve gastrectomy. JSLS. 2018;22(4):e2018.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gagner M, Kemmeter P. Comparison of laparoscopic sleeve gastrectomy leak rates in five staple-line reinforcement options: a systematic review. Surg Endosc. 2020;34(1):396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]


