Abstract
The purpose of this study is to explore the clinicopathological features of Kikuchi–Fujimoto disease (KFD) following vaccination against coronavirus disease 2019 (COVID-19). One case of KFD following vaccination against COVID-19 was examined clinically, histologically, and immunohistochemically. The patient was a 36-year-old Chinese man who suffered from fever and cervical lymph node swelling following simultaneous administration of the COVID-19 vaccine. The patient was diagnosed with KFD based on the histopathological findings of a lymph node core needle biopsy, and his fever and swelling resolved 2 months later without therapy. Although the exact pathogenesis of the development of KFD following immunization remains unknown, this information should be added to the list of potential triggers or factors associated with the development of KFD.
Keywords: KFD, Histiocytic necrotizing lymphadenitis, COVID-19, Vaccine
Introduction
Kikuchi–Fujimoto disease (KFD), known as histiocytic necrotizing lymphadenitis, is a rare disease seen mostly in Asian populations. KFD is a benign and self-limiting disease characterized by lymphadenopathy, mild fever, fatigue, and leukopenia [1]. The characteristic histopathologic features include extensive coagulative necrosis and apoptosis associated with prominent nuclear karyorrhexis and phagocytic activity [2]. Although the pathogenesis of KFD remains unclear, infectious agents, autoimmune causes, and physicochemical factors have been suggested as triggers [3]. However, KFD following vaccination has rarely been reported. We present herein a patient with KFD following simultaneous administration of vaccines against coronavirus disease 2019 (COVID-19).
Case presentation
A 36-year-old Chinese man presented to our local ear, nose, and throat (ENT) neck lump clinic with a new nonpainful left cervical lump. Upon clinical examination, it was revealed that he had received the first dose of the Sinopharm-inactivated COVID-19 vaccine (Vero Cell) into the right deltoid muscle 34 days earlier with no obvious side effects. Then, he received the second dose into the left deltoid muscle 28 days later. Six days after the second injection, he suffered from fatigue, mild fever, and a nonpainful left cervical lump. The patient was febrile with a body temperature of 38.2 °C but otherwise appeared well. Physical examination revealed left cervical lymph node swelling with tenderness.
Laboratory studies showed a white blood cell count of 2.3×109/l (normal, 4.0–9.0×109/l). The erythrocyte sedimentation rate (ESR) was elevated at 48 mm/h, while other serologic tests were unremarkable. Serology testing for SARS-CoV-2 RNA by real-time reverse transcription polymerase chain reaction was negative.
An ultrasound scan (USS) was subsequently requested. USS of the neck showed a left posterior triangle node measuring 1.2 cm in diameter. The node had an echogenic center and color flow centrally, suggesting a reactive lymph node.
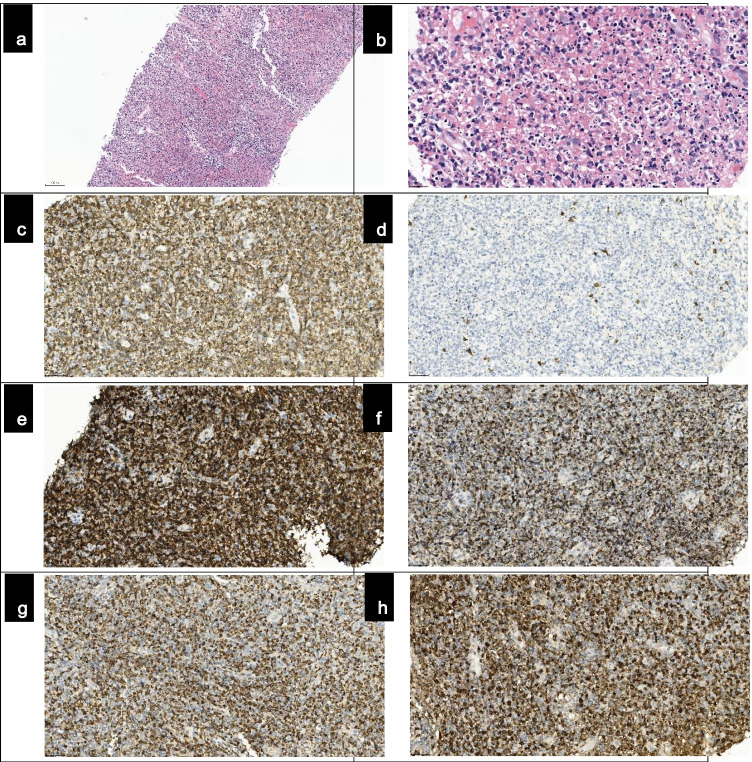
Clinical follow-up was advised. However, the swelled lymph node was still present after 2 weeks. Then, an ultrasound-guided core needle biopsy of the node was performed to rule out nasopharyngeal carcinoma or tuberculous lymphadenitis. The biopsy revealed an effaced architecture with numerous phagocytic histiocytes, prominent nuclear karyorrhexis, lymphoid cells, and some immunoblasts (Fig. 1a–b). No plasma cells or neutrophils were present. Immunohistochemically, we found CD68+ histiocytes in necrotic areas admixed with numerous CD3+ T cells. CD8+ T cells significantly outnumbered CD4+ T cells. Most cells were cytotoxic T cells that expressed GrB, TIA-1, and perforin. CD20+ B cells were rare. CD68+ histiocytes coexpressed myeloperoxidase (MPO) (Fig. 1c–h), and EBER in situ hybridization was negative. These findings were compatible with KFD. No therapy was given, and his fever and swelling resolved 2 months later.
Fig. 1.
Histopathological and immunohistochemical findings from the biopsy specimen. Lymph node core needle biopsy showed effaced architecture (a, H&E, ×10 objective, ×100 magnification), with numerous phagocytic histiocytes, prominent nuclear karyorrhexis, lymphoid cells, and some immunoblasts (b, H&E, ×40 objective, ×400 magnification). There were numerous CD3+ T cells and few CD20+ B cells (c and d, ×20 objective, ×200 magnification). Most T cells expressed CD8 and GrB (e and f, ×20 objective, ×200 magnification). These histiocytes coexpressed CD68 and MPO (g and h, ×20 objective, ×200 magnification).
Discussion
KFD, known as histiocytic necrotizing lymphadenitis, is a rare disease seen mostly in Asian populations. It is typically characterized by cervical lymphadenopathy with tenderness, mild fever, and fatigue and is usually a benign and self-limiting disease [1].
The diagnosis of KFD is made on histopathologic examination of the affected lymph node. The most striking histologic features of KFD include the presence of coagulative necrosis, apoptosis associated with prominent nuclear karyorrhexis, and phagocytic activity. Neutrophils and eosinophils are not found [1, 2]. The differential diagnosis of KFD includes systemic erythematosus (SLE), infectious lymphadenitis, non-Hodgkin lymphoma, leukemia, and Kawasaki disease [3].
There is no specific treatment for KFD, and only symptomatic treatment should be used to relieve distressing local and systemic complaints [3]. However, some patients with severe or persisting symptoms have been treated with corticosteroids [3].
Although the exact etiology of KFD remains unknown, the clinical presentation, course, and histologic changes suggest a T cell-mediated hyperimmune reaction induced by diverse antigens, such as infectious agents or physicochemical factors [4]. Some viral infections, such as human herpes virus-6, Epstein-Barr virus, cytomegalovirus, parvovirus B19, and human immunodeficiency virus, have also been proposed as possible triggering factors for KFD [4]. Several physicochemical factors have been identified as triggers of KFD, including pacemaker implantation, and ruptured silicone breast implantation [4].
KFD following vaccination has rarely been reported, and Toru Watanabe et al. reported a patient with KFD after HPV vaccination [5]. Viral vaccines might induce KFD because they have some viral or other antigens, which could lead to an aberrant immune response in vaccine recipients. Our patient developed KFD following simultaneous administration of COVID-19 vaccines.
COVID-19 is an emerging respiratory infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which has crippled human health worldwide. The public health emergency required urgent efforts to develop and test the efficacy and safety of vaccines to combat the COVID-19 pandemic. As of May 9, 2021, 183 vaccines were in preclinical development, and 97 were in clinical trials [6]. Trials have demonstrated that these vaccines are safe with no serious side effects. The commonly reported local adverse events were pain at the site of injection, swelling, and redness. Systemic reactions included fever, fatigue, myalgia, and headache. All reactions resolved within 3–4 days [7–9]. Some trials have also reported laboratory derangements, such as decreased hemoglobin, increased bilirubin, and altered SGOT and SGPT. None of these derangements were clinically manifested, and all were self-limiting [10]. KFD following vaccination against COVID-19 has never been reported. Our patient developed KFD following simultaneous administration of COVID-19 vaccines. Because the patient had no discomfort before the vaccines were administered, the COVID-19 vaccine was the more likely cause of KFD in our patient.
In summary, we describe a patient with KFD following vaccination against COVID-19. Although the precise pathogenesis of the development of KFD following vaccination remains unknown, vaccination should be added to the list of potential triggers or factors associated with the development of KFD.
Author contribution
Study conception and design, Yingying Guan and Huadong Lu; data collection, Xiao Xia; draft manuscript preparation, Yingying Guan and Huadong Lu; all authors reviewed the results and approved the final version of the manuscript.
Funding
This study was supported in part by grants from Xiamen Municipal Bureau of Science and Technology. Guiding project of Xiamen Science and Technology, 3502z20199181
Data availability
All data included.
Code availability
Not applicable
Declarations
Ethics approval
Compliance with ethical standards.
Consent to participate
Written informed consent for participation of their details was obtained from the patient.
Consent for publication
Written informed consent for publication of their details was obtained from the patient.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yingying Guan, Email: guan.yingying@zsxmhospital.com.
Xiao Xia, Email: 54119539@qq.com.
Huadong Lu, Email: 13606913781@139.com.
References
- 1.Kucukardali Y, Solmazgul E, Kunter E, et al. Kikuchi-Fujimoto disease: analysis of 244 cases. Clin Rheumatol. 2007;26(1):50–54. doi: 10.1007/s10067-006-0230-5. [DOI] [PubMed] [Google Scholar]
- 2.Perry AM, Choi SM. Kikuchi-Fujimoto disease: a review. Arch Pathol Lab Med. 2018;142(11):1341–1346. doi: 10.5858/arpa.2018-0219-RA. [DOI] [PubMed] [Google Scholar]
- 3.Shenjie Xu, Sun Weilian, Liu Jiamei. Kikuchi-Fujimoto disease: a case report and the evaluation of diagnostic procedures. BMC Oral Health. 2019;19(1):223–227. doi: 10.1186/s12903-019-0920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosch X, Guilabert A, Miquel R, Campo E. Enigmatic Kikuchi-Fujimoto disease: a comprehensive review. Am J Clin Pathol. 2004;122(1):141–152. doi: 10.1309/YF081L4TKYWVYVPQ. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe Toru, Hashidate Hideki, Hirayama Yutaka, Iinuma Yasushi. Kikuchi-Fujimoto disease following vaccination against human papilloma virus infection and Japanese encephalitis. Eur J Pediatr. 2012;171(9):1409–1411. doi: 10.1007/s00431-012-1729-1. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization: Draft landscape and tracker of COVID-19 candidate vaccines. (2021). Accessed May 10th, 2021
- 7.Xia S, Duan K, Zhang Y, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ella R, Vadrevu KM, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis. 2021;21(5):637–646. doi: 10.1016/S1473-3099(20)30942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included.
Not applicable