Abstract
Objectives
Post–COVID-19 symptoms experienced by many survivors have a further devastating effect. This study aimed to analyze the risk factors associated with long COVID-19 in a prospective cohort of hospitalized patients including those requiring intensive care unit (ICU) transfer, taking into account objective measures of COVID-19 severity.
Methods
Hospitalized patients with confirmed COVID-19 were enrolled. A structured follow-up visit was performed 4 months after hospital admission. Multivariable adjusted regression models were used to analyse the association between parameters at the acute phase and persistent symptoms.
Results
A follow-up visit was performed in 316 patients including 115 (36.4%) discharged from the ICU. Mean age was 64.1 years, and 201 patients (58.3%) were men. Female sex (odds ratio [OR], 1.94; 95% confidence interval [CI], 1.17-3.22; P =.01), hypertension (OR, 2.01; 95% CI, 1.22-3.31; P <.01), and the number of initial symptoms (NIS) (OR, 1.35; 95% CI, 1.17-1.54; P <.001) were significantly associated with long COVID-19. Number of persistent symptoms was significantly associated with NIS (adjusted incidence rate ratio [aIRR], 1.16; 95% CI, 1.11-1.22; P <.001), female sex (aIRR, 1.56; 95% CI 1.29-1.87; P <.001), hypertension (aIRR, 1.23; 95% CI, 1.02-1.50; P =.03), and length of stay in hospital (aIRR, 1.01; 95% CI, 1.005-1.017; P <.001).
Conclusion
Our study suggested that female sex, hypertension, and NIS had a significant impact on persistent symptoms in hospitalized patients in contrast to severity of acute COVID-19 infection.
Keywords: COVID-19, Persistent symptoms, Long COVID, Risk factor, Severity
Introduction
Post–COVID-19 symptoms experienced by many survivors after infection have a further devastating effect. Reports of risk factors of long COVID-19 are rising, but data including reliable assessment of persistent symptoms through structured face-to-face follow-up visits are scarce (Halpin et al., 2021). Here we report a study assessing risk factors associated with post–COVID-19 symptoms in hospitalized patients, including those requiring intensive care unit (ICU) transfer 4 months after admission. We also provide data on objective measures of COVID-19 severity, for example, oxygen requirement, inflammatory biomarkers, and radiologic findings.
Methods
We conducted a prospective cohort study of hospitalized patients with COVID-19, discharged from the Amiens-Picardie University Hospital, France, from 2nd February 2020 to 28th December 2020. SARS-CoV-2 infection was confirmed by PCR testing of nasopharyngeal swab. Clinical, biological, radiologic, and hospitalization data were collected from the hospital medical records. All patients were assessed by trained physicians during a face-to-face structured follow-up visit and they were asked about a list of post–COVID-19 symptoms. Clinical examination, blood analysis, and lung computed tomography (CT) scans were also performed during this dedicated visit. Long COVID-19 was suspected in patients who exhibited persistent post–COVID-19 symptoms 4 months after the hospital admission. Multivariable adjusted logistic regression models were constructed to identify clinical and hospitalization variables associated with post–COVID-19 syndrome in a stepwise manner, and Poisson regression was used to identify variables associated with the number of persistent symptoms. All tests were 2-sided, and a P value <.05 was considered statistically significant. For this study, we used data from the SEQCOV cohort, and the study protocol was approved by the institutional review board and ethics committee of the CHU Amiens-Picardie (PI2018_843_0049).
Results
From 586 discharged patients, a total of 316 patients attended a structured follow-up visit 4 months after hospital admission. Patients with follow-up evaluation conducted in other hospitals or those who declined (n = 138), died (n = 34), or had missing data (n = 98) were excluded. There were 201 (63.6%) patients who had at least 1 symptom at the follow-up.
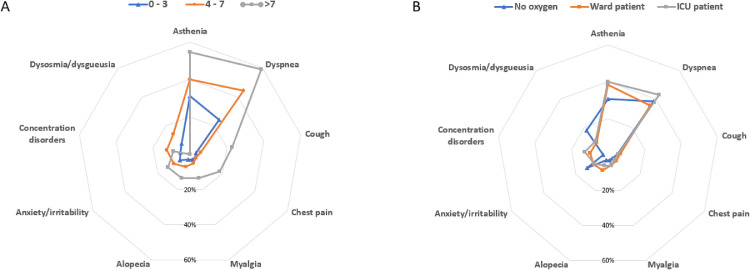
The demographic and clinical characteristics of participants are shown in the Table 1 . Overall, the mean age was 64 years, with 187 (59%) participants being men. The prevalence of hypertension was higher in patients with long COVID-19, but other comorbidities were not different between the 2 groups. Both patient groups had similar oxygen therapy requirement, admission to ICU, inflammatory markers, and CT-scan abnormalities. Patients reported 5 (interquartile range, 3-6) symptoms at the admission. Hyperthermia (79.1%), cough (70.6%), dyspnea (68%), and myalgia (50.6%) were mainly reported. Participants were assessed a median of 115 days after hospital discharge. The most frequent persistent symptoms were dyspnea (39.2%) and asthenia (37.1%). Distribution of symptoms is shown in the Figure 1 .
Table 1.
Demographic and clinical characteristics of enrolled patients according to persistent COVID-19 symptoms.
| Overall (n = 316) | No persistent symptoms (n = 115) | Persistent symptoms (n = 201) | P value | |
|---|---|---|---|---|
| Characteristics | ||||
| Age (years) | 64.1 ± 14.3 | 64.7 ± 13.7 | 63.8 ± 14.7 | .62 |
| >65 | 156 (49.4) | 60 (52.2) | 96 (47.8) | .45 |
| Female | 129 (40.8) | 36 (31.3) | 93 (46.3) | .01 |
| Body mass index (kg/m²) | 30.27 ± 6.6 | 29.9 ± 7.0 | 30.4 ± 6.2 | .45 |
| Obesity >30 kg/m² | 147 (47) | 48 (42.5) | 99 (49.5) | .23 |
| Comorbidities | ||||
| Hypertension | 165 (52.2) | 51 (44.3) | 114 (56.7) | .03 |
| Diabetes | 85 (26.9) | 34 (29.6) | 51 (25.4) | .42 |
| Smoking | 119 (37.7) | 48 (41.7) | 71 (35.3) | .28 |
| Chronic cardiovascular disease | 97 (30.7) | 38 (30.0) | 59 (29.4) | .49 |
| Chronic lung disease | 68 (21.5) | 26 (22.6) | 42 (20.9) | .72 |
| Chronic kidney failure | 17 (5.4) | 7 (6.1) | 10 (5.0) | .67 |
| Neoplasia | 12 (3.8) | 5 (4.3) | 7 (3.5) | .70 |
| Other (pregnancy, hepatic or neurologic diseases, immunocompromised) | 57 (18.0) | 15 (13.0) | 42 (20.9) | .11 |
| Number of comorbidities | 2 (1-3) | 2 (0-3) | 2 (1-3) | .62 |
| 0 | 67 (21.2) | 30 (26.1) | 37 (18.4) | .27 |
| 1 or 2 | 134 (42.4) | 46 (40) | 88 (43.8) | - |
| 3 or more | 115 (36.4) | 39 (33.9) | 76 (37.8) | - |
| Chronic treatments | ||||
| Corticosteroids | 17 (5.4) | 6 (5.2) | 11 (5.5) | .92 |
| Immunosuppressive therapy | 21 (6.6) | 11 (9.6) | 10 (5.0) | .12 |
| ACE inhibitors | 68 (21.5) | 29 (25.2) | 39 (19.4) | .23 |
| ARB | 41 (13) | 11 (9.6) | 30 (14.9) | .17 |
| Beta-blocker | 66 (20.9) | 20 (17.4) | 46 (22.9) | .25 |
| Metformin | 50 (15.8) | 21 (18.3) | 29 (14.4) | .37 |
| Disease severity | ||||
| Number of initial symptoms | 5 (3-6) | 4 (3-5) | 5 (4-6) | .006 |
| 0-4 | 145 (45.9) | 69 (60) | 50 (24.9) | <.001 |
| 5 or more | 171 (54.1) | 46 (40) | 151 (75.1) | <.001 |
| ICU admission | 115 (36.4) | 41 (35.7) | 74 (36.8) | .84 |
| Oxygen requirement | ||||
| None | 39 (12.3) | 17 (14.8) | 22 (10.9) | .38 |
| O2<4l/min | 135 (42.7) | 47 (40.9) | 88 (43.8) | .57 |
| O2>4l/min | 50 (15.8) | 22 (19.0) | 28 (12.4) | .23 |
| HFNC or NIV | 38 (12.0) | 16 (13.7) | 22 (10.9) | .45 |
| IMV | 53 (16.8) | 13 (11.3) | 40 (19.9) | .05 |
| Laboratory results | ||||
| Nadir lymphocyte (/mm3) | 804 ± 385 | 795 ± 395 | 814 ± 379 | .68 |
| Lymphopenia <750/mm3 | 158 (50.9) | 63 (55.3) | 95 (48.5) | .25 |
| CRP max (mg/L) | 140 ± 94 | 139 ± 94 | 142 ± 96 | .76 |
| CRP > 150mg/L | 122 (39.1) | 44 (38.9) | 78 (39.2) | .96 |
| Lung parenchymal involvement at CT scan | .46 | |||
| No parenchymal abnormalities | 18 (7.1) | 10 (10.4) | 8 (5.1) | |
| <25% | 95 (37.5) | 34 (35.4) | 61 (38.9) | |
| 25%-50% | 81 (32.0) | 30 (31.3) | 51 (32.5) | |
| >50% | 59 (23.3) | 22 (22.9) | 37 (23.6) | |
| Clinical course | ||||
| Length of stay in hospital in days (IQR) | 10 (6-19) | 11 (6-16) | 10 (6-21) | .84 |
| 1-6 | 88 (27.8) | 35 (30.4) | 53 (26.4) | .44 |
| 7 or more | 228 (72.2) | 80 (69.6) | 148 (73.3) | - |
| Infectious complications | 57 (18) | 17 (14.8) | 40 (19.9) | .26 |
| Thrombotic complications | 23 (7.3) | 8 (7.0) | 15 (7.5) | .87 |
| Follow-up | ||||
| Time from symptom onset to follow-up (days) | 121 (109-139) | 121 (110-137) | 120 (109-140) | .78 |
| Time from hospital admission to follow-up (days) | 115 (103-130) | 115 (104-130) | 114 (102-131) | .48 |
| Persisting symptoms: | ||||
| Asthenia | 121 (38.3) | 121 (60.2) | ||
| Myalgia | 16 (5.1) | 16 (8) | ||
| Chest pain | 14 (4.4) | 14 (7.0) | ||
| Cough | 19 (6.0) | 19 (9.5) | ||
| Dyspnea | 124 (39.2) | 124 (61.7) | ||
| Anosmia/dysosmia | 20 (6.3) | 20 (10.0) | ||
| Ageusia/dysgeusia | 15 (4.7) | 15 (7.5) | ||
| Headache | 7 (2.2) | 7 (3.5) | ||
| Concentration disorder | 32 (10.1) | 32 (15.9) | ||
| Anxiety/irritability | 29 (9.2) | 29 (14.4) | ||
| Alopecia | 20 (6.3) | 20 (10.0) | ||
| Lung parenchymal involvement at CTscana | .33 | |||
| No parenchymal abnormalities | 122 (40.7) | 48 (43.2) | 74 (39.2) | |
| <25% | 127 (42.4) | 47 (42.3) | 80 (42.3 | |
| 25%-50% | 42 (14.0) | 15 (13.5) | 27 (14.3) | |
| >50% | 9 (3.0) | 1 (0.9) | 8 (4.2) | |
| HAD-A score | 6.7 ± 4.3 | 4.7 ± 3.4 | 7.5 ± 4.3 | <.001 |
| HAD-D score | 5.2 ± 5.1 | 2.8 ± 3.5 | 6.4 ± 5.4 | <.001 |
Quantitative variables are presented as mean ± standard deviation or median (interquartile range); categorical variables are presented as absolute numbers (percentages).
Abbreviations: ACE: angiotensin convertase enzyme; ARB: angiotensin II receptor blocker; CRP: C-reactive protein; CT: computed tomography; HAD: Hospital Anxiety and Depression Scale (a subscale score [A or D] ≥11 denotes anxiety or depression); HNFC: high-flow nasal cannula for oxygen therapy; ICU: intensive care unit; IQR: InterQuartile Range; IMV: invasive mechanical ventilation; NIV: noninvasive ventilation.
A total of 300 patients.
Figure 1.
Prevalence of persistent symptoms.
A: Distribution of persistent symptoms according to the number of initial symptoms (0-3: n = 96; 4-7: n = 198; >7: n = 22)
B: Distribution of persistent symptoms according to the severity of acute COVID-19 (no oxygen: n = 39; ward patients: n = 135 patients; ICU patients: n = 142).
Abbreviations: ICU: intensive care unit.
In univariate analysis, women had significantly higher risk (odds ratio [OR], 1.89; 95% confidence interval [CI], 1.16-3.03; P =.01) than men. Patients with hypertension experienced persistent symptoms more frequently (OR, 1.64; 95% CI, 1.04-2.61; P =.04). Higher numbers of initial symptoms (OR, 1.27; 95% CI, 1.12-1.45; P <.001), especially having 5 or more initial symptoms (OR, 2.47; 95% CI, 1.12-3.03), were associated with greater risk of long COVID-19.
In multivariate logistic regression, female sex (OR, 1.94; 95% CI, 1.17-3.22; P =.01), hypertension (OR, 2.01; 95% CI, 1.22-3.31; P <.01), and the number of initial symptoms (OR, 1.35; 95% CI, 1.17-1.54; P <.001) remained significantly associated with persistent symptoms (Supplementary Table 1). Similarly, in multivariate Poisson regression, the number of persistent symptoms was significantly associated with the number of initial symptoms (adjusted incidence rate ratio [aIRR], 1.16; 95% CI, 1.11-1.22; P <.001), female sex (aIRR, 1.56; 95% CI, 1.29-1.87; P <.001), hypertension (aIRR, 1.23; 95% CI, 1.02-1.50; P =.03), and length of stay in hospital (aIRR, 1.01; 95% CI, 1.005-1.017; P <.001).
Discussion
In our prospective cohort study, female sex, hypertension, and high number of initial symptoms increased the risk of long COVID-19 and the number of persistent symptoms, independently of acute disease severity and clinical course in hospitalized patients.
Our study is in line with reports suggesting that the prevalence of long COVID-19 is higher in women than in men (Bai et al., 2021; Blomberg et al., 2021; Carvalho et al., 2021; Huang et al., 2021; Munblit et al., 2021; Wynberg et al., 2021). After detailed analysis of comorbidities, we showed that hypertension was the most significant risk factor for long COVID-19. Number of initial symptoms had already been described as a risk factor for persistent symptoms in ambulatory (Sudre et al., 2021) and hospitalized patients (Fernández-de-Las-Peñas et al., 2021; Peghin et al., 2021), which is confirmed in this large prospective cohort. Interestingly, severity of acute COVID-19 infection evaluated by oxygen requirement, inflammatory response, or CT-scan findings was not associated with persistent symptoms. Our study suggests that long-term burden of COVID-19 involves multiple nonrespiratory symptoms and these were not associated with acute severity or related post–intensive care syndrome. Strengths of this study are the completeness of data including objective markers of disease severity and reliable symptom collection by face-to-face interview during medical assessment. Our study included sufficient severe forms of COVID-19 with more than a third of ICU admission cases, and infectious or thrombotic complications. This study has several limitations: first, only hospitalized patient alive at follow-up were analysed, and we might not have captured enough discharged patients; second, we performed follow-up visits in a single university center, thus the results might not reflect all hospitalized patients.
Identification of risk factors associated with long COVID-19 could be used to target early intervention and developments to support rehabilitation.
Disclosures
None.
Funding
No funding to declare.
Ethical approval
This research was approved by the ethics review committee of the CHU Amiens-Picardie.
Appendix B. Supplementary materials
Declaration of Competing Interest
The authors declare no conflict of interests.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.03.006.
References
- Bai F, Tomasoni D, Falcinella C, Barbanotti D, Castoldi R, Mulè G, et al. Female gender is associated with “long COVID” syndrome: a prospective cohort study. Clinical Microbiology and Infection. 2021 doi: 10.1016/j.cmi.2021.11.002. Nov 9 [Online ahead of print] doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg B, Mohn KG-I, Brokstad KA, Zhou F, Linchausen DW, Hansen B-A, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27(9):1607–1613. doi: 10.1038/s41591-021-01433-3. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Schneider C, Laurent E, Lemaignen A, Beaufils E, Bourbao-Tournois C, Laribi S, et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27(2):258–263. doi: 10.1016/j.cmi.2020.09.052. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, et al. Long-term post-COVID symptoms and associated risk factors in previously hospitalized patients: A multicenter study. J Infect. 2021;83(2):237–279. doi: 10.1016/j.jinf.2021.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J Med Virol. 2021;93:1013–1022. doi: 10.1002/jmv.26368. doi.org/10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. The Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. Jan 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munblit D, Bobkova P, Spiridonova E, Shikhaleva A, Gamirova A, Blyuss O, et al. Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID-19. Clin Exp Allergy. 2021;51(9):1107–1120. doi: 10.1111/cea.13997. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peghin M, Palese A, Venturini M, De Martino M, Gerussi V, Graziano E, et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect. 2021;27(10):1507–1513. doi: 10.1016/j.cmi.2021.05.033. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–631. doi: 10.1038/s41591-021-01292-y. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynberg E, van Willigen HDG, Dijkstra M, Boyd A, Kootstra NA, van den Aardweg JG, et al. Evolution of COVID-19 symptoms during the first 12 months after illness onset. Clinical Infectious Diseases. 2021;27(2):258–263. doi: 10.1016/j.cmi.2020.09.052. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.