Abstract
Background
Inactivated vaccine (CoronaVac) and chimpanzee adenovirus-vector vaccine (ChAdOx1) have been widely used in resource-limited settings. However, the information on the reactogenicity and immunogenicity of these two vaccines in the same setting are limited.
Methods
Healthy health care workers (HCWs) aged 18 years or older were randomly assigned to receive either two doses of CoronaVac at 4 weeks interval or two doses of ChAdOx1 at 10 weeks interval. Self-reported adverse events (AEs) were collected for 7 days following each vaccination. Immunogenicity was determined by IgG antibodies levels against receptor binding domain (RBD) of the SARS-CoV-2 spike protein (S1 subunit) and the 50% plaque reduction neutralization titers against various strains.
Results
Of the 360 HCWs, 180 in each vaccine group, the median (interquartile range: IQR) age was 35 (29–44) years old and 84.2% were female. Participants who received ChAdOx1 reported higher frequency of AEs than those received CoronaVac after both the first dose (84.4% vs. 66.1%, P < 0.001) and second dose (75.6% vs. 60.6%, P = 0.002), with more AEs in those younger than 30 years of age for both vaccines. The seroconversion rates were 75.6% and 100% following the first dose of CoronaVac and ChAdOx1, respectively. All participants were seropositive at 2 weeks after the second dose. The anti-SARS-CoV-2 RBD IgG levels induced by CoronaVac was lower than ChAdOX1 with geometric means of 164.4 and 278.5 BAU/mL, respectively (P = 0.0066). Both vaccines induced similar levels of neutralizing antibodies against the Wuhan strain, with the titers of 337.4 and 331.2; however, CoronaVac induced significantly lower GMT against Alpha (23.1 vs. 92.5), Delta (21.2 vs. 69.7), and Beta (10.2 vs. 43.6) variants, respectively.
Conclusion
CoronaVac induces lower measurable antibodies against circulating variants but with lower frequency of AEs than ChAdOx1. An earlier boosting to prevent breakthrough infections may be needed.
Keywords: CoronVac, ChAdOx1, Heath care workers, Thailand, Immunogenicity, Variant of concern
Introduction
Coronavirus diseases-2019 (COVID-19) vaccination has been crucial to control the (COVID-19 pandemic. All vaccines that passed the WHO Emergency Use Listing Procedure (EUL) are safe and have been shown to prevent severity and mortality of COVID-19. However, continued surveillance of any rare adverse events and vaccine effectiveness are important [1], [2], [3], [4].
Most resource-limited settings had limited access to COVID-19 vaccines in early 2021. In most part of Asia, including Thailand, chimpanzee adenovirus-vector (ChAdOx1) vaccine (AZD1222, AstraZeneca/Oxford) and the whole-cell inactivated vaccines such as CoronaVac (Sinovac, Life Sciences) were the only vaccine available at that time. The phase III trials indicated 76% and 50.8% efficacy of ChAdOx1 and CoronaVac in preventing symptomatic infection, respectively, and that both vaccines reported 100% efficacy against severe COVID-19 [5], [6], [7]. However, these studies were largely based on the original Wuhan or Alpha variant and were conducted in the different settings. The efficacy in real world may differ from the trials due to heterogeneity of the population receiving the vaccines and the circulating variant of concern (VOCs).
The VOCs emerged in late 2020 has increased transmissibility and disease severity compared to the original Wuhan strain, posing the risk of breakthrough infections after natural infection or vaccination [8], [9]. A previous study found three to five folds lower neutralizing titers against the Alpha (B1.1.7) and Delta (B1.1617.2) variants compared with the original Wuhan strain [10]. In many settings, the Alpha variant was predominant circulating variant in April 2021, but has since been taken over by the Delta variant in August 2021. The Beta variant has also emerged in many areas, but has been replaced by the more infectious Delta variant. The appearance of these VOCs raised the concern of the effectiveness of the current available COVID-19 vaccines.
Healthcare workers (HCWs) are the priority population for COVID-19 vaccination due to an increased risk of exposure to SARS-CoV-2 [11]. In Thailand, most HCWs received two doses of either CoronaVac or ChAdOx1. Data on the immunogenicity and safety of these two vaccines is scarce in low- and middle-income countries (LMICs), particularly the immunogenicity against the VOCs. In this study, we investigated the reactogenicity and immunogenicity of CoronaVac and ChAdOx1 against the original Wuhan strain and circulating VOCs (Alpha, Delta and Beta variants) in HCWs of Thailand.
Methods
Study design and participants
This single-center prospective cohort study enrolled 360 healthy Thai HCWs aged 18 years or older at Siriraj Hospital, a university-based referral center located in Bangkok, Thailand from February to July 2021. The participants were excluded from the study if they had the following conditions: confirmed SARS-CoV-2 infection by reverse transcriptase polymerase chain reaction (RT-PCR), had received current prophylactic treatment or investigational agents against COVID-19 within 90 days, had unstable underlying diseases that may compromise the immune responses such as active cancer or hematologic malignancy, had a history of vaccine hypersensitivity, were pregnant, were immunocompromised or receiving immunosuppressive agent at any dose. The study protocol was approved by the Siriraj Institutional Review Board (COA no. Si 171/2021) before initiation.
Study procedures
The study was advertised in the form of poster in the hospital following ethics approval. All study participants were HCWs working at the hospital and their participation was entirely voluntary. After providing written informed consent, the participants medical records were reviewed to confirm the eligibility and were healthy. The participants were randomly assigned, using computerized randomization program, to receive intramuscular injections of either two doses of CoronaVac (lot 2021010041, manufactured by Sinovac Life Sciences, Co., Ltd., P.R. China) at 4 weeks interval or two doses of ChAdOx1 (lot CTMAV509, manufactured by SK Bioscience, Korea) at 10 weeks interval. The participants were informed about the type of vaccine they received. The study vaccines were provided by the Ministry of Public Health with cold-chain 2-8OC monitoring system. The HCWs aged 60 years or older were assigned to ChAdOx1 based on the local guidelines at that time. The participants were observed for at least 30 min following the vaccination for any immediate adverse events and were instructed to submit self-assessment report using an electronic diary (eDiary) in the Google Form for seven days after each dose of vaccination for any adverse events (AEs) both solicited local and systemic reactions. The solicited local AEs include pain, erythema, and swelling/induration at the injection site, and localized axillary swelling or tenderness ipsilateral to the injection arm. The systemic AEs include headache, fatigue, myalgia, arthralgia, diarrhea, dizziness, nausea/vomiting, rash, fever, and chills. The severity of solicited AEs was graded using a numerical scale from 1 to 4 based on the Common Terminology Criteria for Adverse Events - Version 5.0 guide by the United States National Cancer Institute (NCI/NIH). Unsolicited adverse events were also collected by spontaneous reporting from participants throughout the study period.
Chemiluminescent microparticle assay (CMIA) for anti-SARS-CoV-2 RBD IgG and anti-NP
Blood samples were collected at baseline before the first dose vaccination on the same day (pre-dose 1), on the day of the second dose (pre-dose 2), 4 weeks after the second dose and 8–12 weeks after the second dose. A subgroup of participants who received ChAdOx1 were randomly invited to test for anti-SARS-CoV-2 RBD IgG at 4 weeks after the first dose. The plasma samples were isolated from the blood collected in tubes with sodium citrate solution and stored at −80 °C. The level of antibody response (IgG) against receptor binding domain (RBD) of the SARS-CoV-2 spike protein (S1 subunit) was determined by a CMIA using the SARS-CoV-2 IgG II Quant (Abbott, List No. 06S60) on the ARCHITECT i System The anonymous convalescent sera of mildly symptomatic infection from outbreaks in Thailand caused by B.1.36.16 (D614G) strain in late December 2020 collected at 4 or 12 weeks of illness were tested as the reference. This assay linearly measures the level of antibody between 21.0 and 40,000.0 arbitrary unit (AU)/mL, which was converted later to WHO International Standard concentration as binding antibody unit per mL (BAU/mL) following the equation provided by the manufacturer (BAU/mL = 0.142 × AU/mL). The level greater or equal to the cutoff value of 50 AU/mL or 7.1 BAU/mL was defined as seropositive. Seroconversion was determined as becoming seropositive in those who had seronegative at baseline or an increase of 4-fold titers above the baseline seropositive levels.
Qualitative antibody response against the SARS-CoV-2 nucleocapsid protein (NP) in the plasma samples was determined by CMIA using the SARS-CoV-2 IgG (Abbott, List No. 06R86) on the ARCHITECT i System at baseline and 4 weeks after the second dose.
50% plaque reduction neutralization test (PRNT50) against SARS-CoV-2 strains
Subgroup of subjects in each vaccine group were randomly invited for additional blood collection at two weeks after the second dose for determining the level of anti-SARS-CoV-2 RBG IgG and the neutralizing antibody titers against original (Wuhan) strain and VOCs, which were Alpha (B1.1.7), Delta (B1.1617.2), and Beta (B.1.351) strains by 50% plaque reduction neutralization test (PRNT50). The convalescent sera of the mildly symptomatic patients recovered from D614G strain infections in late December 2020 and Delta strain in June 2021, collected by the Ministry of Public Health (MOPH) at 2 weeks of illness, were tested for PRNT50 against Delta variant as reference. Any titers below the detection limit of PRNT50, which is 1:10 will be indicated as an average of 1:5 for statistical analyses. Briefly, Vero cells were seeded at 2x105 cells/well/3 mL and placed in 37 °C, 5% CO2 incubator for 1 day. Sera were serially diluted at 1:10, 1:40, 1:160 and 1:640, respectively. The SARS-CoV-2 virus was diluted in culture medium to yield 40–120 plaques/well and mixed in equal volume of the diluted serum at 37˚C in water bath for 1 h. Convalescent and uninfected sera were used as assay controls. The virus-serum mixture (200 µL) was inoculated into Vero-cell monolayer in triplicate and the culture plates were rocked every 15 min for 1 h. Three mL of the overlay semisolid medium containing 1% of carboxymethylcellulose (Sigma Aldrich, USA), 1% of 10,000 units/mL Penicillin with 10,000 ug/mL Streptomycin (Sigma, USA) and 10% fetal bovine serum (FBS) were added to the cells after removing the virus-serum mixture. Cells were incubated at 37˚C, 5% CO2 for 7 days, then fixed with 10% (v/v) formaldehyde and stained with 0.5% crystal violet in phosphate-buffered saline (PBS). The number of plaques was counted and the percentage of PRNT50 was calculated. The titer of each sample was defined as the reciprocal of the highest test serum dilution, which the virus infectivity was reduced by 50% of an average plaque counts in the virus control wells and was calculated by using a four-point linear regression method.
Statistical analysis
The AEs endpoints were presented as frequencies and the Chi-square test was used to test for statistical difference. The immunological endpoints including the level of anti-SARS-CoV-2 RBD IgG and PRNT50 titer were reported as geometric mean (GM) with 95% confidence interval (CI). The PRNT50 titer below 10 were arbitrarily assigned a value of 5. GraphPad Prism 9 version 9.2.0 (2 8 3) (GraphPad Software, CA, USA) was used for unpaired t-test analyses to compare GM of the IgG concentrations between groups and Pearson’s correlation coefficient to assess the correlation between log10 of anti-SARS-CoV-2 RBD IgG and log10 of PRNT50. As the participants who were 60 years or older received non-randomized ChAdOx1, an additional analysis that included only participants aged 18 to lower than 60 years was performed. The ANOVA was performed to examine the geometric mean of anti-SARS-CoV-2 RBD IgG among different age groups using STATA version 17 (StataCorp, LP, College Station, TX, USA).
Results
Of the 360 HCWs enrolled (Fig. 1), 180 were in each vaccine group with the median (IQR) age of 35 (29–44) years old, and 303 (84.2%) were female. There were 14 participants (3.9%) aged 60 years or older and all received ChAdOx1 vaccine. The participants who received ChAdOx1 were older than those who received CoronaVac with the median of 40 (32–48) years old and 31 (26–39) years old, respectively. Participants who received ChAdOx1 had higher frequencies of hypertension (10% vs. 2.2%, P = 0.003) and dyslipidemia (10.6% vs. 2.8%) than those received CoronaVac (Table 1).
Fig. 1.
Flow diagram of enrollment and vaccination of healthcare worker participants.
Table 1.
Baseline characteristics of enrolled 360 health care worker* participants by vaccine type.
| Total (n = 360) |
Vaccine Group |
|||
|---|---|---|---|---|
| ChAdOx1 (n = 180) | CoronaVac (n = 180) | |||
| Male, n (%) | 57 (15.8) | 30 (16.6) | 27 (15.0) | |
| Age: year (median, IQR) | 35.00 (29.00, 44.00) | 40.00 (32.00, 48.00) | 31.00 (26.00, 39.00) | |
| Body mass index: kg/m2 (median, IQR) | 23.12 (20.50, 26.36) | 23.26 (20.70, 26.29) | 22.87 (20.37, 26.36) | |
| Direct COVID-19 patient care, n (%) | 117 (32.50) | 55 (30.56) | 62 (34.44) | |
| Comorbidity, n (%) | ||||
| Hypertension | 22 (6.1) | 18 (10.0) | 4 (2.2) | |
| Dyslipidemia | 24 (6.7) | 19 (10.6) | 5 (2.8) | |
| Diabetes mellitus | 9 (2.5) | 6 (3.4) | 3 (1.7) | |
| Obesity | 7 (1.9) | 4 (2.2) | 3 (1.7) | |
*There were 19 subjected excluded after screening and were not enrolled due to receiving systemic steroid treatment (10), and methotrexate treatment (9).
Adverse events
Subjects who received ChAdOx1 reported more frequent AEs than CoronaVac recipients following the first and second dose vaccinations (84.4% vs. 66.1%, P < 0.001 and 75.6% vs. 60.6%, P = 0.002, respectively) (Fig. 2A, B). All AEs were mild (grade 1) or moderate (grade 2) in severity and recovered within 2–3 days. Compared with the first dose, the ChAdOx1 vaccine group reported less frequent and milder AEs following the second dose, while the CoronaVac vaccine group reported overall similar frequency of systemic AEs between the first and second dose, but with more local AEs after the second dose. There was no serious AEs reported in any participant. In both vaccine groups, the subjects younger than 30 years of age reported more systemic AEs, particularly myalgia and fever (Fig. 2 C, D). There was no difference between gender for overall AEs after the CoronaVac and after the first dose of ChAdOx1; however, female reported overall AEs more than male (70.3% vs. 56.1%, P = 0.035) after the second dose of ChAdOx1.
Fig. 2.
Adverse events following CoronaVac or ChAdOx1 vaccination. The stacked bars showed the percentage of participants who had indicated mild and moderate adverse events after the first and second dose of CoronaVac (A) and ChAdOx1 (B). The stacked bars showed the percentage of participants aged lower (light color bar) or above thirty years old (dark color bar), who had indicated adverse events after the first and second dose of CoronaVac (C) and ChAdOx1 (D). Chi-square was used for statistical analyses and the p values were shown on the graphs.
Immunogenicity
All participants were negative for anti-SARS-CoV-2 NP IgG at baseline and none had history of COVID-19. Five participants (2 in CoronaVac and 3 in ChAdOx1 group) had positive anti-RBD at baseline with low titers varying between 7.3 BAU/mL and 30.25 BAU/mL. At 4 weeks after the first dose, CoronaVac induced a significantly lower anti-SARS-CoV-2 RBD IgG seroconversion rate (75.6% (136/180) vs. 100% (180/180), P < 0.0001), and antibody levels (geometric mean (GM) 12.7 BAU/mL vs. 69.8 BAU/mL, P < 0.0001) than ChAdOx1 recipients. For the ChAdOx1 recipients, the level of anti-SARS-CoV-2 RBD IgG decreased to a GM of 37.1 BAU/mL prior to the second dose vaccination. After the second dose, both vaccines induced 100% seroconversion within 2 weeks. ChAdOx1 induced higher levels of anti-SARS-CoV-2 RBD IgG than CoronaVac at all follow-up time points after the second dose: 2 weeks, GM 278.5 BAU/mL vs. 164.4 BAU/mL (P = 0.0066); 4 weeks, GM 178.2 BAU/mL vs. 94.8 BAU/mL (P < 0.0001); and 8–12 weeks, GM 92.5 BAU/mL vs. 34.7 BAU/mL (P < 0.0001). In comparison with the D614G convalescent sera at 4 and 12 weeks, CoronaVac induced significantly lower levels of anti-SARS-CoV-2 RBD IgG while ChAdOx1 induced higher levels (Fig. 3A). Among those who received ChAdOx1 vaccine, the anti-SARS-CoV-2 RBD IgG levels at 4 weeks after the second dose varied by age group (P < 0.0001 by ANOVA) with a trend towards higher levels in younger age groups. This age-related response was not seen in CoronaVac group (P = 0.058, Fig. 3B).
Fig. 3.
Anti-SARS-CoV-2 RBD IgG levels following CoronaVac or ChAdOx1 vaccination. (A) Anti-SARS-CoV-2 RBD IgG levels in the plasma of study subjects before and various time points after vaccination with CoronaVac (blue) or ChAdOx1 (red). Convalescent sera at 4 weeks and 12 weeks after illness from the patients who recovered from the COVID-19 during Dec 2020 and early 2021 were included as reference level (orange). (B) Anti-SARS-CoV-2 RBD IgG levels in the plasma of study subjects among different age groups at 4 weeks after the second dose vaccination of CoronaVac (blue) or ChAdOx1 (red). The numbers in the graph represent geometric mean and the error bars represent 95% confidence interval. The number of tested samples were indicated below each time point. Two-tailed unpair t test was used to compared the IgG level between two conditions with indicated p value. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
CoronaVac induced anti-SARS-CoV-2 NP IgG seroconversion in 53% of vaccinees at 4 weeks following the second dose whereas only one subject who received ChAdOx1 had anti-SARS-CoV-2 NP IgG seroconversion due to mild symptomatic SARS-CoV-2 infection approximately at 4 weeks after the first dose. CoronaVac recipients who were seropositive for SARS-CoV-2 NP IgG had significantly higher level of anti-SARS-CoV-2 RBD IgG compared to those seronegative for SARS-CoV-2 NP IgG with the GM of 121.7 BAU/mL and 71.3 BAU/mL, respectively (P < 0.001), (Supplementary Figure S1).
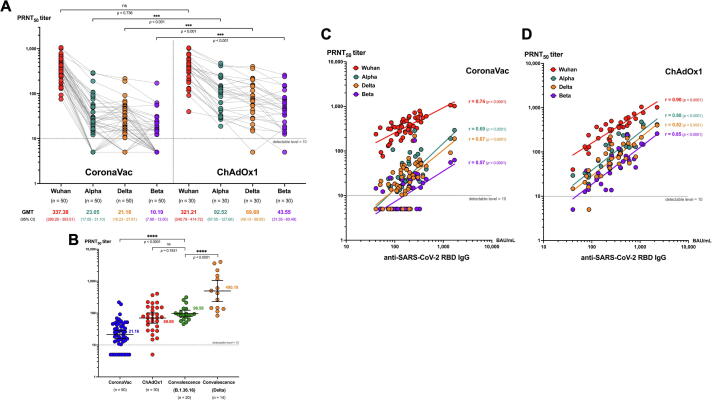
The PRNT50 against the original Wuhan strain and VOCs at 2 weeks following the second dose vaccination was shown in Fig. 4A. Both CoronaVac and ChAdOx1 induced similar PRNT50 against the original Wuhan strain (337.4 vs 321.2, P = 0.736). Compared with CoronaVac, the ChAdOx1 induced significantly higher PRNT50 against all VOCs, (Alpha 92.5 vs. 23.0, Delta 69.7 vs. 21.2, and Beta 43.5 vs. 10.2, P < 0.001 for all comparisons) (Fig. 4A). The PRNT50 GMTs against Delta variant induced by CoronaVac were significantly lower than convalescent sera of individuals infected with D614G strain (21.2 vs. 96.6, P < 0.0001), while ChAdOx1 induced similar level (69.7 vs. 96.6, P = 0.183). The convalescent sera of individuals infected with the Delta variant had significantly higher PRNT50 than those induced by both vaccine groups (Fig. 4B).
Fig. 4.
Plaque reduction neutralization test (PRNT50) titers for different SARS-CoV-2 strains. (A) Dot plots demonstrates PRNT50 titer against Wuhan (red), Alpha (teal), Delta (orange) and Beta (purple) strains in the plasma of study subjects at 2 weeks after two doses of CoronaVac or ChAdOx1. (B) Scatter dot plots demonstrates PRNT50 titer against Delta strain at 2 weeks after 2-dose vaccination with CoronaVac (blue) and ChAdOx1 (red) compared with the convalescent sera at 2 weeks after illness of the patients infected with B.1.36.16 strain (green) and Delta strain (orange). The PRNT50 titer of 1:5 was used for all that were below the detectable level (<1:10). The geometric mean titer (GMT) and lower and upper 95% confidence interval (CI) are indicated. (C) Correlation between the level of anti-SARS-CoV-2 RBD IgG and plaque reduction neutralization test (PRNT50) titers for wildtype and VOC. Dot plots shows the correlation between the level of anti-SARS-CoV-2 RBD IgG and PRNT50 titer against Wuhan (red), Alpha (teal), Delta (orange) and Beta (purple) strains in the plasma of study subjects at 2 weeks after two doses of CoronaVac (n = 50) or (D) ChAdOx1 (n = 30). The PRNT50 titer of 1:5 was used for all that were below the detectable level (<1:10). Pearson’s correlation coefficient (r) with p value for each strain was indicated. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The anti-SARS-CoV-2 RBD IgG levels and the PRNT50 titers against all four strains were strongly correlated in the ChAdOx1 group (r = 0.82 to 0.9, P < 0.0001). In contrast there was only moderate correlation in the CoronaVac group (r = 0.57–0.74, P < 0.0001) (Fig. 4C, D).
In the sub-analysis including only the participants aged 18 to lower than 60 years the baseline characteristics and immunogenicity results were unchanged (Supplementary Table 1 and 2).
Discussion
Our study found that CoronaVac was less reactogenic and induced lower anti- SARS-CoV-2 RBD IgG than ChAdOx1. Although neutralizing antibody against Wuhan strain was similar between the two vaccine groups, CoronaVac induced a significantly lower neutralising antibodies against the VOCs than ChAdOx1. This study is relevant to many settings, particularly in LMICs where these two vaccines are widely used.
We found both vaccines to be well tolerated with more AEs following ChAdOx1 than CoronaVac. Any AEs following the first dose of ChAdOx1 were reported at a lower rate (66.1%) in our study than those reported in the phase III trial (86% in participants age 18–55 years) [12], but closer to the rate of 59% reported in the United Kingdom (UK) where a self-reporting application was used [13]. Systemic AEs for both vaccines were significantly higher in the vaccinees younger than 30 years of age with more AEs reported by females following the second dose. This was consistent with the self-report application in the UK. For CoronaVac, the AEs rates were similar to those reported in a phase II trial in China and a phase III trial in Brazil [6], [7], but the higher AE rate in younger individuals has not been reported.
Overall, the anti-SARS-CoV-2 RBG IgG induced by the ChAdOx1 was significantly higher than that the levels induced by the CoronaVac at all time points up to 8–12 weeks following the second dose of vaccination. The PRNT50 GMT induced by the CoronaVac and the ChAdOx1 were similar against the Wuhan strain, but the ChAdOx1 induced 3–4 times higher GMTs against each VOCs than CoronaVac. Compared with those of the Wuhan strain, the PRNT50 GMTs against the VOCs were 16–30 times lower in the CoronaVac group and 5–7 times lower in the ChAdOx1 group. The PRNT50 GMTs against the Alpha and Delta variants were not different in both vaccine groups and were much higher than those against the Beta variants. These data concurred with the previous study and suggested more distance genetic variability of the Beta variant [14]. This suggest that both vaccines are less effective against the Beta variant.
The neutralizing antibodies induced by both vaccines against the Delta variant were lower than convalescence sera from post infection with Delta variant, with lower levels in the CoronaVac group as seen in a previous study [15]. Although protective levels of neutralizing antibodies have not yet been established, Khoury et al. suggested the protective threshold against symptomatic infections to be at 28.6% (95% CI = 19.2–29.2%) of the mean convalescent level [16]. Using this assumption, based on our data, both vaccines may have reduced protection against symptomatic infection by the Delta variant.
The presence of anti-SARS-CoV-2 NP antibody is used as the marker for natural infection and is expected to be positive following whole virus inactivated vaccine. However, we found positive anti-SARS CoV-2 NP antibody in only about half of the CoronaVac recipients, consistent with a previous report [17]. This suggest that CoronaVac may be less immunogenic than natural infection.
After completing 2 doses of both vaccines, the level of anti-SARS CoV-2 RBD IgG in the blood peaks at 2 weeks before declining by 1.5 to 1.7 times at 4 weeks and 3 to 5 times at 8–12 weeks afterward. This half-life is shorter than that suggested for the half-life of neutralizing antibodies of 108 days [16]. Our data also suggests that the peak anti-SARS-CoV-2 RBD IgG antibody response is around 2 weeks after completing the 2-dose schedule. Consistent with the previous study [18], we found a strong positive correlation of neutralizing antibodies and anti-SARS-CoV-2 RBD IgG, supporting the use of anti-SARS-CoV-2 RBD IgG as a proxy marker for neutralizing antibodies.
This study has several limitations. Although the strength of this study is the homogeneity of the population, the population included only healthy HCWs, which may not be able to generalize to other population. In addition, only a subset of participants with small sample size were tested for the PRNT50 against the VOCs; however, significant difference was demonstrated. This study also lacks the data of cell-mediated immune responses, which could be an important factor to control the disease severity. This study adds to the limited data available for CoronaVac and ChAdOx1 in the Asian population and provides direct comparison of these two vaccines particularly against the VOCs by using the gold-standard measurement of neutralizing antibody.
CoronaVac and ChAdOx1 are well tolerated vaccines and equally immunogenic for original Wuhan strain. However, the ChAdOx1 induced more AEs (mild to moderate) but provide higher level of anti-SARS-CoV-2 RBD IgG and the PRNT50 against all VOCs compared to the CoronaVac. Both vaccines induced neutralizing antibodies against the Delta variant but at a significantly lower level than titers detected in the Delta convalescent sera. These results support the need for booster vaccination to prevent breakthrough infections from the VOCs, particularly among those who received the CoronaVac.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors gratefully acknowledge the Siriraj Institute of Clinical Research (SICRES) team, Abbott Laboratories Ltd. for technical supports and all health care workers who took part and enabled this study to be possible. We thank Professor Kim Mulholland and Zheng Quan Toh at Murdoch Children’s Research Institute for their critical data review and suggestion.
Funding statement
This study was supported by the National Research Council of Thailand (grant contract number 66/2564) and Abbott Laboratories Ltd. supported the reagents for the anti-SARS-CoV-2 RBD IgG and anti-SARS-CoV-2 NP IgG in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jvacx.2022.100153.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary figure 1.

References
- 1.Mullard A. COVID-19 vaccine development pipeline gears up. Lancet. 2020;395(10239):1751–1752. doi: 10.1016/S0140-6736(20)31252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.So A.D., Woo J. Reserving coronavirus disease 2019 vaccines for global access: cross sectional analysis. BMJ. 2020;371 doi: 10.1136/bmj.m4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M., Costa Clemens S.A., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palacios R., Patiño E.G., de Oliveira Piorelli R., Conde M.T.R.P., Batista A.P., Zeng G., et al. Double-Blind, Randomized, Placebo-Controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of treating Healthcare Professionals with the Adsorbed COVID-19 (Inactivated) Vaccine Manufactured by Sinovac - PROFISCOV: A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1) doi: 10.1186/s13063-020-04775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hacisuleyman E., Hale C., Saito Y., Blachere N.E., Bergh M., Conlon E.G., et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N Engl J Med. 2021;384(23):2212–2218. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabino E.C., Buss L.F., Carvalho M.P.S., Prete C.A., Crispim M.A.E., Fraiji N.A., et al. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet. 2021;397(10273):452–455. doi: 10.1016/S0140-6736(21)00183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Planas D., Veyer D., Baidaliuk A., Staropoli I., Guivel-Benhassine F., Rajah M.M., et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596(7871):276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 11.WHO SAGE values framework for the allocation and prioritization of COVID-19 vaccination. Available: https://www.nitag-resource.org/media-center/who-sage-values-framework-allocation-and-prioritization-covid-19-vaccination.
- 12.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu C., Ginn H.M., Dejnirattisai W., Supasa P., Wang B., Tuekprakhon A., et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184(16):4220–36 e13. doi: 10.1016/j.cell.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vacharathit V., Aiewsakun P., Manopwisedjaroen S., Srisaowakarn C., Laopanupong T., Ludowyke N., et al. CoronaVac induces lower neutralising activity against variants of concern than natural infection. Lancet Infect Dis. 2021;21(10):1352–1354. doi: 10.1016/S1473-3099(21)00568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 17.Bochnia-Bueno L, De Almeida SM, Raboni SM, Adamoski D, Amadeu LLM, Carstensen S, et al. Dynamic of humoral response to SARS-CoV-2 anti Nucleocapsid and Spike proteins after CoronaVac vaccination. Diagn Microbiol Infect Dis 2022;102(3):115597. [DOI] [PMC free article] [PubMed]
- 18.Salazar E, Kuchipudi SV, Christensen PA, Eagar T, Yi X, Zhao P, et al. Convalescent plasma anti-SARS-CoV-2 spike protein ectodomain and receptor-binding domain IgG correlate with virus neutralization. J Clin Invest. 2020;130(12):6728–6738. doi: 10.1172/JCI141206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.