Abstract
Background
Cases of severe autoimmune blistering diseases (AIBDs) have recently been reported in association with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination.
Aims
To describe a report of oropharyngeal Pemphigus Vulgaris (OPV) triggered by the mRNABNT162b2 vaccine (Comirnaty®/ Pfizer/ BioNTech) and to analyze the clinical and immunological characteristics of the AIBDs cases reported following the SARS-CoV-2 vaccination.
Methods
The clinical and immunological features of our case of OPV were documented. A review of the literature was conducted and only cases of AIBDs arising after the SARS-CoV-2 vaccination were included.
Case report
A 60-year old female patients developed oropharyngeal and nasal bullous lesions seven days after the administration of a second dose of the mRNABNT162b2 vaccine (Comirnaty®/ Pfizer/BioNtech). According to the histology and direct immunofluorescence findings showing the presence of supra-basal blister and intercellular staining of IgG antibodies and the presence of a high level of anti-Dsg-3 antibodies (80 U/ml; normal < 7 U/ml) in the serum of the patients, a diagnosis of oropharyngeal Pemphigus Vulgaris was made.
Review
A total of 35 AIBDs cases triggered by the SARS-CoV-2 vaccination were found (including our report). 26 (74.3%) were diagnosed as Bullous Pemphigoid, 2 (5.7%) as Linear IgA Bullous Dermatosis, 6 (17.1%) as Pemphigus Vulgaris and 1 (2.9%) as Pemphigus Foliaceus. The mean age of the sample was 72.8 years and there was a predominance of males over females (F:M=1:1.7). In 22 (62.9%) cases, the disease developed after Pfizer vaccine administration, 6 (17.1%) after Moderna, 3 (8.6%) after AstraZeneca, 3 (8.6%) after CoronaVac (one was not specified). All patients were treated with topical and/or systemic corticosteroids, with or without the addition of immunosuppressive drugs, with a good clinical response in every case.
Conclusion
Clinicians should be aware of the potential, though rare, occurrence of AIBDs as a possible adverse event after the SARS-CoV-2 vaccination. However, notwithstanding, they should encourage their patients to obtain the vaccination in order to assist the public health systems to overcome the COVID-19 pandemic.
Keywords: Pemphigus, Bullous Pemphigoid, SARS-Cov-2, Covid-19 vaccine
1. Introduction
A number of vaccines have been developed to fight the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, which still represents the major global public health issue. The vaccination campaign against the COVID-19 pandemic is crucial for health care systems and the risk-benefit ratio continues to be remarkably favorable [1]. However, different vaccine-related side effects have been reported, predominantly mild-to-moderate in severity, the most common being fatigue, muscle pain, headache, chills, a redness/swelling at the injection site, joint pain and fever. On the contrary, the incidence of severe adverse events, such as allergic reactions or anaphylaxis, is rare and ranges between 0.2% and 0.3% [2]. Additionally, various dermatological manifestations have been correlated with the administration of SARS-CoV-2 vaccines, ranging from local reactions, such as local swelling, erythema and delayed local hypersensitivity, to distal and/or generalized reactions, such as pruritus, urticaria, erythema multiforme, vasculitis and bullous diseases [3]. Interestingly, recent data suggests that the SARS-CoV-2 vaccines may reactivate or even cause de novo autoimmune diseases, including hematological, neurological, rheumatic and dermatological diseases [3], [4], [5], [6]. In this regard, cases of autoimmune blistering diseases (AIBDs), triggered by the SARS-Cov-2 vaccination, have recently been reported [7], [8].
AIBDs are rare and potentially life-threatening diseases affecting the mucous membranes and skin, whose pathogenesis is mediated by an antibody-response against the structural proteins of the desmosome or basement membrane zone of the stratified epithelia, resulting in the formation of blisters. Based on the clinical, histological and immunological features, two principal subgroups of AIBDs have been recognized: the intra-epithelial group, which includes Pemphigus Vulgaris (PV), Pemphigus Foliaceus (PF), Pemphigus Vegetans, Pemphigus Herpetiformis, IgA Pemphigus and IgG/IgA Pemphigus; and the sub-epithelial group, which includes Bullous Pemphigoid (BP), Mucous Membrane Pemphigoid, Pemphigoid Gestationis, anti-p200 Pemphigoid, Lichen Planus Pemphigoides, Epidermolysis Bullosa Acquisita and Linear Immunoglobulin A Bullous Dermatosis (LABD) [9]. Several factors, including genetic susceptibility and certain drugs, have been reported to trigger AIBDs [9]. However, the onset of these diseases after antiviral/antibacterial vaccination has been exceptionally rare with only a few cases reported before the COVID-19 period [10]. Herein we present a new case report of PV after SARS-Cov-2 vaccination and a review of the literature of all the AIBDs cases developed after COVID-19 vaccine administration.
2. Methods
2.1. Case-report data collection
Demographic, clinical and immunological data were collected in relation to the case of the patient diagnosed with oropharyngeal Pemphigus Vulgaris (OPV) following an anti-SARSCov-2 vaccine at our department of Oral Medicine, University of Naples “Federico II”. Written informed consent was obtained from the patient.
2.2. Search strategy and case selection for the review
We conducted a case-based search in Medline (via PubMed), by combining Medical Subject Headings (MeSH) and free text-words from January 2021–28 th February 2022. The terms used for the PubMed search were as follow: ("COVID-19 Vaccines"[Mesh] OR "ChAdOx1 nCoV-19"[Mesh] OR "2019-nCoV Vaccine mRNA-1273"[Mesh] OR "BNT162 Vaccine"[Mesh] OR "Ad26COVS1"[Mesh] OR "COVID-19 vaccin*" OR "SARS-CoV-2 vaccin*" OR Pfizer OR Moderna OR AstraZeneca OR CoronaVac) AND ("Pemphigus"[Mesh] OR "Pemphigoid, Bullous"[Mesh] OR "Pemphigoid, Benign Mucous Membrane"[Mesh] OR "Pemphigoid Gestationis"[Mesh] OR "Epidermolysis Bullosa Acquisita"[Mesh] OR "Linear IgA Bullous Dermatosis"[Mesh] OR "Pemphigus Vulgaris" OR "Pemphigus Foliaceus" OR "Pemphigus Vegetans" OR "Pemphigus Herpetiformis" OR "IgA Pemphigus" OR "Bullous Pemphigoid" OR "Mucous Membrane Pemphigoid" OR Pemphigoid OR "Lichen Planus Pemphigoides" OR" Epidermolysis bullosa acquisita" OR "Linear Immunoglobulin A bullous dermatosis" OR "dermatological manifestation*" OR "dermatological complication*" OR "dermatological reaction*" OR "dermatological adverse event*" OR “cutaneous manifestation*” OR “cutaneous complication*" OR ”cutaneous reaction*" OR “cutaneous adverse event*” OR "cutaneous side effect*" OR "skin reaction*" OR "skin manifestation*" OR "skin complication*” OR “skin adverse event*”).
2.3. Criteria for considering studies for the review
The current literature was analyzed and all the case reports of AIBDs correlated to the SARS-CoV-2 vaccination were included, based on the following criteria: i) typical clinical findings of bullous and/or erosive lesions affecting the mucosal surfaces (oropharyngeal, genital, nasal etc.), and/or the skin; (ii) histopathological specimens exhibiting intra-epithelial or sub-epithelial detachment; and iii) at least one immunological evidence of autoantibody response, via direct immune-fluorescence microscopy (DIF) and/or serological detection of serum autoantibodies by indirect immune-fluorescence microscopy (IIF) and/or enzyme-linked immunosorbent assay (ELISA Test) [9], [11]. Cases of presumable AIBDs were excluded in case of negativity of DIF, IIF and ELISA Test, or if none of them was tested. The title, abstracts and the full texts of the case reports were independently screened by two authors (EC and FC) and a third reviewer (DA) resolved disagreements.
2.4. Data synthesis
Descriptive statistics were used to detail clinical characteristics of the patients. In case of normally distributed variables means, standard deviation and range were used, otherwise median and interquartile ranges (IQR). For categorical data, percentages were displayed.
3. Results
3.1. Case presentation
A female patient, 60 years old, was referred to the Oral Medicine Unit of the University of Naples “Federico II” on account of the occurrence of painful oropharyngeal and nasal lesions which had lasted for more than five months. Her past history revealed that the lesions had appeared seven days following the administration of a second dose of the mRNABNT162b2 vaccine (Comirnaty®/ Pfizer/BioNtech) ( Fig. 1). The oral lesions had been stable for three months before the diagnosis and the patient had not developed cutaneous lesions. A perilesional biopsy of the mandibular gingiva was taken. Histology showed a partially ulcerated mucosa covered with only one or more layers of keratinocytes aligned along the basement membrane. At one edge of the biopsy, the non-keratinizing squamous cell epithelium showed severe acantholysis, forming a suprabasal blister with a row of "gravestone" looking basal cells attached to the connective tissue. There was a moderate band-like lymphocytic infiltrate in the subepithelial chorion, with some eosinophils and several small vessels. ( Fig. 2 A-C). A diagnosis of bullous mucositis, as PV, was made. Direct immunofluorescence revealed intercellular staining of IgG antibodies, confirming the diagnosis of Pemphigus Vulgaris (Fig. 2 D). The patient’s serum presented a high level of anti-Dsg-3 antibodies (80 U/ml; normal < 7 U/ml) while the anti-Dsg-1 antibodies titer was within the limits (4.4 U/ml; normal <14 U/ml). Therefore, a diagnosis of OPV after SARS-CoV-2 vaccination was made, taking into account the timing of the onset of the bullous lesions. The patient was promptly treated with immunosuppressive therapy consisting in high dose corticosteroids (1 mg of prednisone per kg of body weight) for six weeks without achieving a satisfactory disease control. Indeed, due to the onset of dysphagia with a consequent difficulty in eating and drinking, further therapy with a monoclonal antibody anti-CD20, namely Rituximab, was scheduled (prescribed according to the rheumatoid arthritis protocol at a dose of 1000 mg twice at 2-week intervals [12]). This treatment resulted in an overall improvement in the patient’s condition within three weeks. She is currently in partial clinical remission and undergoing follow-up in our department.
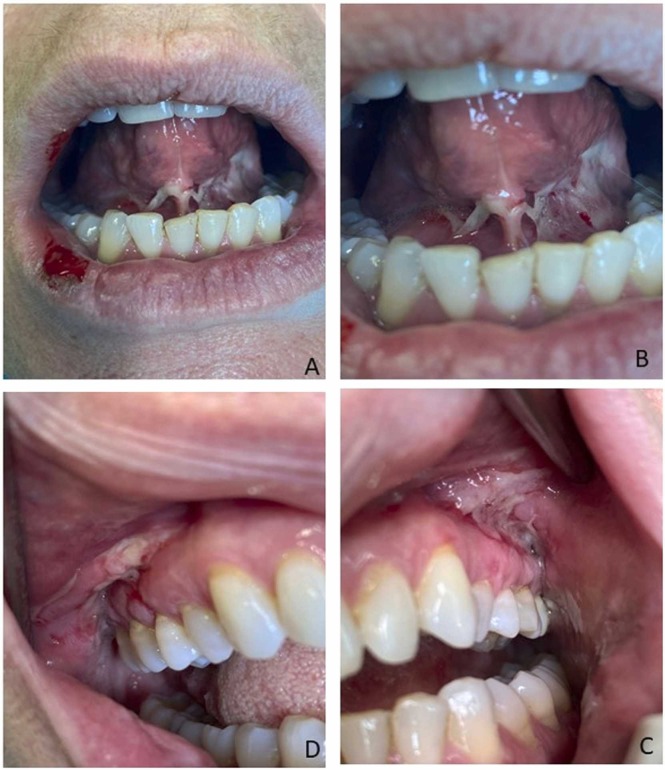
Fig. 1.
A) Extra-oral photograph showing blisters and erosions of the lower lip and upper vermillion border with right side localisation B) Intra-oral photograph showing extensive flaccid bullae present on the floor of mouth, also involving bilateral inferior surface mucosa of the tongue C) Intra-oral photograph showing multiple intact vesicles with irregular borders associated with erosive lesions involving left upper fornix and alveolar mucosae D) Intact and ruptured blisters on right fornix affected gingiva with mixed desquamative, ulcerative, vesicular lesions, extending to the attached and marginal gingiva with erosive features associated.
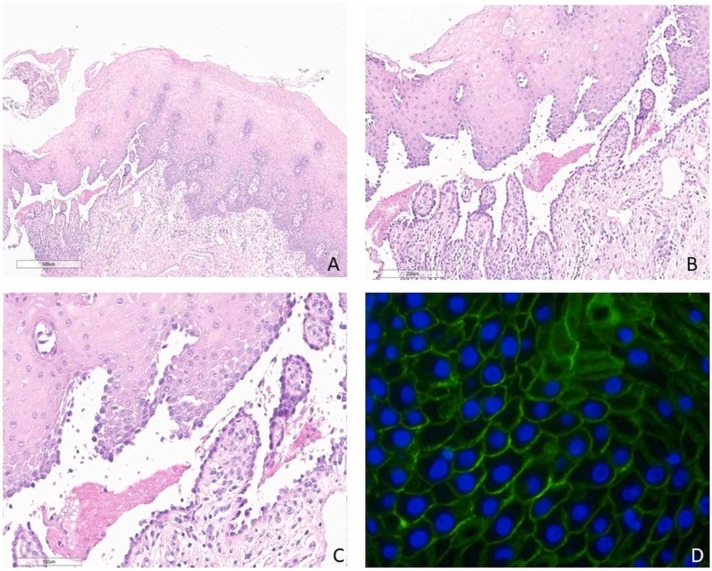
Fig. 2.
A) Lower magnification showed moderate subrabasal acantholysis with blister formation (haematoxylin and eosin, original magnification, x4) B, C) Higher magnification revealed a tombstone appearance of basal keratinocytes (haematoxylin and eosin, original magnification, Bx10 and Cx20) D) Direct immunofluorescence microscopy demonstrated intercellular staining of IgG antibodies (IgG antibody, original magnification, x10 or x20) (The slides were digitized with an Aperio AT2 scanner with 40x optics).
3.2. Literature review
A total of 195 articles was retrieved and after the screening, 20 articles were finally included for the review. A total of 35 AIBDs cases (including our case) were found, as shown in Table 1 [7], [8], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30]. The sub-epithelial diseases were the most frequent, accounting for 28 cases (80.0%), specifically 26 cases of BP (74.3%) and 2 case of LABD (5.7%). The intra-epithelial diseases were less common, accounting for 7 cases (20.0%), specifically 6 cases of PV (17.1%) (including our report), and 1 case of PF (2.9%). The median age of the whole sample was 77.5 years, (IQR: 64.5-84; mean 72.8 years range 38–97 years), specifically 60 years (IQR: 50-76) for the patients affected by the intra-epithelial subtypes and 80 years (IQR: 67.75-84.25) for the sub-epithelial subgroup. There was an overall predominance of males over females (13 females, 22 males, F:M=1:1.7). However, no gender predilection was observed for the pemphigus patients (4 females, 3 males. F:M=1.3:1), whereas males were the most frequently affected in the sub-epithelial group (9 females, 19 males, F:M=1:2.1). The majority of the cases, 22 (62.9%), developed after Pfizer vaccine administration, 6 (17.1%) after Moderna, 3 (8.6%) after AstraZeneca, 3 (8.6%) after CoronaVac (one was not specified). Moreover, 15 cases (42.9%) developed after the first administration, 18 (51.4%) after the second, and 2 (5.7%) after the third. Interestingly, the bullous lesions worsened or reactivated in 6/9 patients (62.5%) receiving the second dose of the vaccine and who had already developed bullous lesions after the first. The bullous lesions erupted after a mean of 9.8 days, range 1–35 days (median 7 days, IQR :3–14) developing within three weeks from the vaccination in 32 cases (91.4%). All the patients were treated with oral corticosteroids and/or immunosuppressive drugs, the majority showing a good clinical response.
Table 1.
Demographic, clinical, histological and immunological characteristics of post SARS-Cov-2 vaccination AIBDs patients.
|
Pemphigus Vulgaris | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Patient | Age | Sex | Bullous Lesion localization | Vaccine | 1st, 2nd, 3rd dose | 2nd dose | Time-to-onset (days) | Histopathology | DIF/IFF | DSG1/DSG3 | Treatment | Outcome |
| Thongprasom K et al 2021 | 1 | 38 | F | Oral mucosa | AstraZeneca | 1st | NA | 7 | Histopathological features in keeping with a diagnosis of pemphigus (no better specified) | DIF in keeping with a diagnosis of pemphigus (no better specified) | NA | TC | Complete clinical resolution after 1 week |
| Solimani F et al 2021 | 2 | 40 | F | Oral mucosa, trunk and back | Pfizer | 1st | Given, lesions worsened | 5 | Subrabasal acantholysis | DIF: IgG intercellular deposition | +/+ | OC/AZ | Ongoing |
| Koutlas IJ 2021 | 3 | 60 | M | Oral mucosa | Moderna | 2nd | / | 7 | Suprabasal acantholysis | DIF: IgG/C3 intercellular deposition IIF: IgG intercellular pattern |
-/- | OC/RTX | Complete clinical resolution after 5.5 months |
| Knechtl GV et al 2021 | 4 | 89 | M | Oral mucosa, trunk, back, left arm | Pfizer | 2nd | / | 30 | Suprabasal acantholysis | DIF: IgG intercellular deposition | +/+ | OC/RTX | Control of disease after 10 weeks |
| Akoglu G et al 2022 | 5 | 69 | F | Oral mucosa, scalp, trunk, limbs | CoronaVac | 1st | NA | 7 | NA | NA | +/+ | MTX | Control of the diseases in 2 weeks, almost complete remission after 12 weeks |
| Our case | 6 | 60 | F | Oral mucosa, oropharynx mucosa | Pfizer | 2nd | / | 7 | Suprabasal acantholysis | DIF: IgG intercellular deposition | -/+ | OC/RTX | Improving at week 8 |
| Pemphigus Foliaceus | |||||||||||||
| Lua ACY et al 2021 | 1 | 83 | M | Face, scalp, trunk, limbs | Pfizer | 2nd | / | 2 | Subacute spongiotic dermatitis with dermal eosinophils and plasma cells | DIF: C3 at the DEJ and intercellular bridges within the epidermis. IIF: Intercellular pattern |
+/- | OC | Good clinical response (no better specified) |
| Bullous Pemphigoid | |||||||||||||
| Author | Patient | Age | Sex | Bullous Lesion localization | Vaccine | 1st, 2nd, 3rd dose | 2nd dose | Time-to-onset (days) | Histopathology | DIF/IIF | BP180/BP230 | Therapy | Outcome |
| Pauluzzi M et al 2021 | 1 | 46 | M | Trunk, arms | Pfizer | 1st | Not given | 15 | Subepidermal split | DIF: C3 at the BMZ | +/- | OC/AZ | Ongoing at week 7 |
| Agharbi FZ et al 2021 | 2 | 77 | M | Scalp, trunk, limbs | AstraZeneca | 1st | Not given | 1 | Subepidermal split | DIF: IgG at the BMZ IIF: IgG at the BMZ |
NA | TC/DC | Favorable outcome (no better specified) |
| Young J et al 2021 | 3 | 68 | M | Oral mucosa, trunk | Pfizer | 1st | Given, lesions worsened | 3 | Subepidermal split with infiltrate composed of eosinophils and hemosiderophages. | DIF: IgG/C3 at the BMZ | NA | TC | Resolution after 3 months |
| Gambichler T 2021 | 4 | 80 | M | Trunk, legs | Pfizer | 1st | Given, lesions worsened | 7 | Subepidermal split | DIF: IgG/C3 at the BMZ IIF: IgG at the BMZ |
+/+ | OC | NA |
| 5 | 89 | M | Entire integument | Pfizer | 1st | NA | 2 | Subepidermal split | DIF: IgG/C3 at the BMZ IIF: IgG at the BMZ |
+/+ | OC | NA | |
| Pérez-Lòpez I et al 2021 | 6 | 78 | F | Face, trunk, limbs | Pfizer | 1st | Given, lesions reactivated | 3 | Subepidermal split | DIF and IIF positive (no better specified). | NA | OC | Good clinical response (no better specified) |
| Nakamura K et al 2021 | 7 | 83 | F | All the body surfaces involved | Pfizer | 2nd | / | 3 | Subepidermal split, infiltrate with eosinophils | DIF: IgG at the BMZ | +/- | OC/IVIg | NA |
| Tomayko MM et al 2021 | 8 | 97 | F | NA | Pfizer | 2nd | / | 2 | Subepidermal split, infiltrate with eosinophils | DIF: IgG/C3/IgA | +/+ | TC/DC/NI | Improving at week 2 |
| 9 | 75 | M | NA | Pfizer | 2nd | / | 10 | Subepidermal split, infiltrate with eosinophils | DIF: C3 | +/NA | TC/OC/DC/NI | Improving at week 3 | |
| 10 | 64 | M | NA | Pfizer | 2nd | / | 14 | Subepidermal split, infiltrate with eosinophils | DIF: C3 | +/+ | TC | Improving at week 4 | |
| 11 | 82 | M | NA | Pfizer | 2nd | / | 1 | Subepidermal split, infiltrate with eosinophils | DIF: IgG/C3/week IgA at the BMZ | -/- | TC | Resolved at week 2 | |
| 12 | 95 | F | NA | Pfizer | 1st | Given, no lesion reactivation | 5 | Subepidermal split, infiltrate with eosinophils | DIF: IgG/C3/week IgA at the BMZ | -/- | TC/DC/NI | Resolved at week 8 | |
| 13 | 87 | M | NA | Moderna | 2nd | / | 21 | Subepidermal split, infiltrate with eosinophils | DIF: C3 at the BMZ | +/+ | OC/DC/NI | Ongoing after 105 days | |
| 14 | 42 | F | NA | Moderna | 2nd | / | 3 | Subepidermal split, infiltrate with eosinophils | DIF: IgG/C3/weak granular IgM at the BMZ | +/+ | IMC/TC/IVC | Ongoing at day 23 | |
| 15 | 85 | M | NA | Pfizer | 1st | Not given | 5 | Subepidermal split, infiltrate with eosinophils | DIF: IgG/C3 at the BMZ | NA | OC | Ongoing at day 59 | |
| Bostan E et al 2021 | 16 | 67 | M | Oral mucosa, trunk, arms | Inactivated Covid-19 vaccine (no better specified) | 1st | Given, no lesion reactivation | 35 | Subepidermal split, mixed infiltrate rich in eosinophils | DIF: IgG/C3 at the BMZ | NA | OC/OM | Considerable response but without full recovery after 8 months from the second vaccine dose |
| Schmidt V et al 2021 | 17 | 84 | F | Trunk, back, arms, legs | Moderna | 1st | Given, lesions worsened | Few days (no better specified) | Subepidermal split, spongiosis and infiltrate with eosinophils | NA | +/+ | NA | NA |
| Coto-Segura P et al 2021 | 18 | 85 | M | Trunk, arms | Pfizer | 2nd | / | 8 | Subepidermal split, infiltrate with eosinophils | DIF: IgG/C3 at the BMZ | NA | TC/OC | In resolution |
| 19 | 84 | M | Trunk arms | Pfizer | 2nd | / | 7 | Subepidermal split, infiltrate with eosinophils | DIF: IgG/IgM/C3 at the BMZ | NA | TC/OC | In resolution | |
| Larson V et al 2021 | 20 | 76 | M | Legs | Pfizer | 1st | Given, lesions worsened | 21 | Subepidermal split, infiltrate with eosinophils | DIF: IgG/C3 at the BMZ | NA | TC/OC/DC/NI | Improvement |
| 21 | 84 | M | Legs | Moderna | 2nd | / | 14 | Intraepidermal spongiotic vesicles and eosinophilic spongiosis | DIF: IgG/C3 at the BMZ | NA | TC/OC | Improvement | |
| Hung WK et al 2022 | 22 | 39 | M | Trunk, hands, feet | Moderna | 1st | Not specified | 30 | Subepidermal split, infiltrate with eosinophils | DIF: IgG/C3 at the BMZ IIF: positive titer of 1: 40 for anti-basement membrane zone antibodies. |
NA | IVC/OC/DC | Resolution |
| Afacan E et al 2022 | 23 | 88 | F | NA | CoronaVac | 2nd | / | 30 | Subepidermal split | DIF positive (no better specified) | NA | TC/OC/MTX | NA |
| 24 | 82 | F | NA | Pfizer | 3rd | / | 14 | Subepidermal split | DIF positive (no better specified) | NA | TC/OC/DA | Improvement | |
| 25 | 65 | M | NA | Pfizer | 3rd | / | 14 | Subepidermal split | DIF positive (no better specified) | NA | TC/DC | Improvement | |
| 26 | 82 | F | NA | CoronaVac | 2nd | / | 14 | Subepidermal split | DIF positive (no better specified) | NA | TC/DC | Improvement | |
| Linear IgA disease | |||||||||||||
| Author | Patient | Age | Sex | Bullous Lesion localization | Vaccine | 1st, 2nd, 3rd dose | 2nd dose | Time-to-onset (days) | Histopathology | DIF/IFF | BP180/BP230 DSG1/DSG3 | Therapy | Outcome |
| Hali et al 2021 | 1 | 61 | M | Oral mucosa, genital mucosa, trunk, legs | AstraZeneca | 2nd | / | 3 | Subepidermal split with an inflammatory infiltrate composed of lymphocytes, histiocytes and some eosinophilic polynuclear lymphocytes | DIF: IgA at the BMZ IIF: IgA at the BMZ |
-/- -/- |
OC | Clinical improvement (no better specified) |
| Coto-Segura P et al 2021 | 2 | 71 | M | Legs | Pfizer | 2nd | / | 3 | Subepidermal split, infiltrate with eosinophils | DIF: IgA at the BMZ | NA | TC | In resolution |
AIBDs= autoimmune blistering diseases; AZ= Azathioprine; BMZ: basement membrane zone; DA: dapsone; DC= Doxycycline; DIF= direct immunofluorescence; DSG1= antibody anti-desmogleiin 1; DSG3= antibody anti-desmoglein 3; IIF= indirect immunofluorescence; IMC= intramuscular corticosteroids; IVC= intravenous corticosteroids; IVIg= intravenous immunoglobulins; MO=mupirocin ointment; MTX: methotrexate; NA= Not available; NI=nicotinamide; OC= oral corticosteroids, OM= omalizumab; RTX= rituximab; TC= topical corticosteroids.
4. Discussion
The current literature review summarizes the cases of AIBDs following an anti-SARS-Cov-2 vaccination published so far, reporting demographic, clinical and immunological characteristics of the patients. To the best of our knowledge, this is the fifth case of PV developing after SARS-Cov-2 vaccination. During our research, we found a total of 35 case-reports of patients with clinical and immunological diagnosis of AIBDs, however, the number may be even higher as there are, in fact, few case-reports with a diagnosis of AIBDs based on clinical findings. In this case-series, the sub-epithelial diseases represented the majority of the cases (80.0%), especially the BP type, followed by the intraepithelial disease (20.0%). The median ages of AIBDs onset in these patients did not differ from those reported for the spontaneous forms [6]. Interestingly, males were more affected than females (F:M=1:1.7) especially in the sub-epithelial group (F:M=1:2.1). Conversely, no gender difference has been reported in respect of any of pemphigoid diseases occurring spontaneously [6]. Notably, almost the 62.5% of the AIBDs cases developed after Pfizer vaccine administration. This figure may possibly be explained in terms of the more frequent use of the Pfizer vaccine compared to the others, as it has been administered to 28% of the population compared to the Moderna vaccine (18%) and the AstraZeneca (12%) [31]. AIBDs developed after either the first, the second and third administration of the vaccine, and, in some cases, the bullous lesions either worsened or reactivated in patients receiving the second dose. Altogether, these findings suggest the potential association between new-onset AIBDs and COVID-19 vaccine, which may enhance or even trigger the immunological response, as also reported in other autoimmune diseases [32], [33]. Virus- or vaccine-associated autoimmunity is a well-known phenomenon as many viruses have been proposed to trigger a variety of autoimmune responses [34], as well as vaccines due to either the cross-reactivity between antigens or the effect of adjuvant [35]. One of the most accredited hypotheses is based on the cross-reaction between antibodies anti-SARS-CoV-2 spike glycoproteins with structurally similar host peptide protein sequences due to a molecular mimicry mechanism [36]. It may be speculated also that susceptible individuals with a pre-existing predisposition to autoimmune/autoinflammatory dysregulation may present a higher risk of immunological side effects after the administration of such vaccines, some of which contains nucleic acids [37]. Nonetheless, a cause-effect relationship cannot be established, although the presence of a temporal correlation may be suggestive of this event.
It is of upmost importance to increase awareness of this potential adverse effect related to the SARS-CoV-2 vaccination and, therefore, to promote the report of other cases for a better understanding of the phenomenon. In this regard, clinicians should carefully weigh the potential side effects of the vaccine against the well described severe complications of the SARS-CoV-2 infection. Indeed, although diseases flares of already diagnosed AIBDs have been documented, the occurrence of AIBDs post-vaccination is overall a rare event and, according to this review’s data, the disease can be safely controlled with immune-suppressive therapies. Therefore, clinicians should encourage patients to obtain the vaccination in order to assist the public health systems to overcome the COVID-19 pandemic.
Funding information
This research has not been supported by any private or corporate financial institutions nor has any grant been received for this study.
References
- 1.Marcellusi A., Fabiano G., Sciattella P., Andreoni M., Mennini F.S. The impact of COVID-19 vaccination on the italian healthcare system: a scenario analysis. Clin. Drug Investig. 2022 doi: 10.1007/s40261-022-01127-9. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beatty A.L., Peyser N.D., Butcher X.E., Cocohoba J.M., Lin F., Olgin J.E., Pletcher M.J., Marcus G.M. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw. Open. 2021;4(12) doi: 10.1001/jamanetworkopen.2021.40364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman E.E., Sun Q., McMahon D.E., Singh R., Fathy R., Tyagi A., Blumenthal K., Hruza G.J., French L.E., Fox L.P. Skin reactions to COVID-19 vaccines: an American Academy of Dermatology/International League of Dermatological Societies registry update on reaction location and COVID vaccine type. J. Am. Acad. Dermatol. 2021;(21):02841–02843. doi: 10.1016/j.jaad.2021.11.016. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Báez-Negrón L., Vilá L.M. New-onset systemic lupus erythematosus after mRNA SARS-CoV-2 vaccination. Case Rep. Rheumatol. 2022;11(2022) doi: 10.1155/2022/6436839. PMID: 35186342; PMCID: PMC8856802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prabhahar A., Naidu G.S.R.S.N.K., Chauhan P., Sekar A., Sharma A., Sharma A., Kumar A., Nada R., Rathi M., Kohli H.S., Ramachandran R. ANCA-associated vasculitis following ChAdOx1 nCoV19 vaccination: case-based review. Rheumatol. Int. 2022;(Feb 5):1–10. doi: 10.1007/s00296-021-05069-x. Epub ahead of print. PMID: 35124725; PMCID: PMC8817770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y., Xu Z., Wang P., Li X.M., Shuai Z.W., Ye D.Q., Pan H.F. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2021 doi: 10.1111/imm.13443. Epub ahead of print. PMID: 34957554. [DOI] [PubMed] [Google Scholar]
- 7.Tomayko M.M., Damsky W., Fathy R., McMahon D.E., Turner N., Valentin M.N., Rallis T., Aivaz O., Fox L.P., Freeman E.E. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID-19 vaccination. J. Allergy Clin. Immunol. 2021;148(3):750–751. doi: 10.1016/j.jaci.2021.06.026. Epub 2021 Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thongprasom K., Pengpis N., Phattarataratip E., Samaranayake L. Oral pemphigus after COVID-19 vaccination. Oral. Dis. 2021 doi: 10.1111/odi.14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egami S., Yamagami J., Amagai M. Autoimmune bullous skin diseases, pemphigus and pemphigoid. J. Allergy Clin. Immunol. 2020;145(4):1031–1047. doi: 10.1016/j.jaci.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Bell H., Kamal N., Wong U. Blistering autoimmune skin reaction following SHINGRIX vaccination in an ulcerative colitis patient: case report and literature review. Vaccine. 2020;38(47):7455–7457. doi: 10.1016/j.vaccine.2020.09.073. Epub 2020 Oct 13. [DOI] [PubMed] [Google Scholar]
- 11.Murrell D.F., Peña S., Joly P., Marinovic B., Hashimoto T., Diaz L.A., Sinha A.A., Payne A.S., Daneshpazhooh M., Eming R., Jonkman M.F., Mimouni D., Borradori L., Kim S.C., Yamagami J., Lehman J.S., Saleh M.A., Culton D.A., Czernik A., Zone J.J., Fivenson D., Ujiie H., Wozniak K., Akman-Karakaş A., Bernard P., Korman N.J., Caux F., Drenovska K., Prost-Squarcioni C., Vassileva S., Feldman R.J., Cardones A.R., Bauer J., Ioannides D., Jedlickova H., Palisson F., Patsatsi A., Uzun S., Yayli S., Zillikens D., Amagai M., Hertl M., Schmidt E., Aoki V., Grando S.A., Shimizu H., Baum S., Cianchini G., Feliciani C., Iranzo P., Mascaró J.M., Jr., Kowalewski C., Hall R., Groves R., Harman K.E., Marinkovich M.P., Maverakis E., Werth V.P. Diagnosis and management of pemphigus: Recommendations of an international panel of experts. J. Am. Acad. Dermatol. 2020;82(3):575–585. doi: 10.1016/j.jaad.2018.02.021. Epub 2018 Feb 10. PMID: 29438767; PMCID: PMC7313440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Food and Drug Administration Rituxan label . 〈https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103705s5311lbl.pdf〉. (Accessed 17 January 2022).
- 13.Solimani F., Mansour Y., Didona D., Dilling A., Ghoreschi K., Meier K. Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2. J. Eur. Acad. Dermatol. Venereol. 2021;35(10):e649–e651. doi: 10.1111/jdv.17480. Epub 2021 Jul 12. PMID: 34169588; PMCID: PMC8447452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koutlas I.G., Camara R., Argyris P.P., Davis M.D.P., Miller D.D. Development of pemphigus vulgaris after the second dose of the mRNA-1273 SARS-Cov-2 vaccine. Oral. Dis. 2021 doi: 10.1111/odi.14089. Epub ahead of print. PMID: 34825752. [DOI] [PubMed] [Google Scholar]
- 15.Knechtl G.V., Seyed Jafari S.M., Berger T., Rammlmair A., Feldmeyer L., Borradori L. Development of pemphigus vulgaris following mRNA SARS-CoV-19 BNT162b2 vaccination in an 89-year-old patient. J. Eur. Acad. Dermatol. Venereol. 2021 doi: 10.1111/jdv.17868. Epub ahead of print. PMID: 34897817. [DOI] [PubMed] [Google Scholar]
- 16.Lua A.C.Y., Ong F.L.L., Choo K.J.L., Yeo Y.W., Oh C.C. An unusual presentation of pemphigus foliaceus following COVID-19 vaccination. Austral J. Dermatol. 2021 doi: 10.1111/ajd.13755. Epub ahead of print. PMID: 34817063. [DOI] [PubMed] [Google Scholar]
- 17.Pauluzzi M., Stinco G., Errichetti E. Bullous pemphigoid in a young male after COVID-19 mRNA vaccine: a report and brief literature review. J. Eur. Acad. Dermatol. Venereol. 2021 doi: 10.1111/jdv.17891. Epub ahead of print. PMID: 34928518. [DOI] [PubMed] [Google Scholar]
- 18.Agharbi F.Z., Eljazouly M., Basri G., Faik M., Benkirane A., Albouzidi A., Chiheb S. Bullous pemphigoid induced by the AstraZeneca COVID-19 vaccine. Ann. Dermatol. Venereol. 2021;30(21) doi: 10.1016/j.annder.2021.07.008. Epub ahead of print. PMID: 34686374; PMCID: PMC8481089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young J., Mercieca L., Ceci M., Pisani D., Betts A., Boffa M.J. A case of bullous pemphigoid after the SARS-CoV-2 mRNA vaccine. J. Eur. Acad. Dermatol. Venereol. 2022;36(1):e13–e16. doi: 10.1111/jdv.17676. Epub 2021 Oct 5. PMID: 34547137; PMCID: PMC8661451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gambichler T., Hamdani N., Budde H., Sieme M., Skrygan M., Scholl L., Dickel H., Behle B., Ganjuur N., Scheel C., Abu Rached N., Ocker L., Stranzenbach R., Doerler M., Pfeiffer L., Becker J.C. Bullous pemphigoid after SARS-CoV-2 vaccination: Spike protein-directed immunofluorescence confocal microscopy and T cell receptor studies. Br. J. Dermatol. 2021 doi: 10.1111/bjd.20890. doi: 10.1111/bjd.20890. Epub ahead of print. PMID: 34773638; PMCID: PMC8653321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pérez-López I., Moyano-Bueno D., Ruiz-Villaverde R. Bullous pemphigoid and COVID-19 vaccine. Med. Clin. 2021;157:e333–e334. doi: 10.1016/j.medcli.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura K., Kosano M., Sakai Y., Saito N., Takazawa Y., Omodaka T., Kiniwa Y., Okuyama R. Case of bullous pemphigoid following coronavirus disease 2019 vaccination. J. Dermatol. 2021;48(12):e606–e607. doi: 10.1111/1346-8138.16170. Epub 2021 Sep 21. PMID: 34545973; PMCID: PMC8652433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bostan E., Yel B., Akdogan N., Gokoz O. New-onset bullous pemphigoid after inactivated Covid-19 vaccine: synergistic effect of the Covid-19 vaccine and vildagliptin. Dermatol. Ther. 2021 doi: 10.1111/dth.15241. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt V., Blum R., Möhrenschlager M. Biphasic bullous pemphigoid starting after first dose and boosted by second dose of mRNA-1273 vaccine in an 84-year-old female with polymorbidity and polypharmacy. J. Eur. Acad. Dermatol. Venereol. 2021 doi: 10.1111/jdv.17722. [DOI] [PubMed] [Google Scholar]
- 25.Hali F., Kerouach A., Alatawna H., Chiheb S., Lakhdar H. Linear IgA bullous dermatosis following Oxford AstraZeneca COVID-19 vaccine. Clin. Exp. Dermatol. 2021 doi: 10.1111/ced.15007. doi: 10.1111/ced.15007. Epub ahead of print. PMID: 34762342; PMCID: PMC8652795.Fine modulo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coto-Segura P., Fernández-Prada M., Mir-Bonafé M., García-García B., González-Iglesias I., Alonso-Penanes P., González-Guerrero M., Gutiérrez-Palacios A., Miranda-Martínez E., Martinón-Torres F. Vesiculobullous skin reactions induced by COVID-19 mRNA vaccine: report of four cases and review of the literature. Clin. Exp. Dermatol. 2022;47(1):141–143. doi: 10.1111/ced.14835. Epub 2021 Sep 2. PMID: 34236711; PMCID: PMC8444733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larson V., Seidenberg R., Caplan A., Brinster N.K., Meehan S.A., Kim R.H. Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID-19 vaccination. J. Cutan. Pathol. 2022;49(1):34–41. doi: 10.1111/cup.14104. Epub 2021 Aug 8. PMID: 34292611; PMCID: PMC8444807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hung W.K., Chi C.C. Incident bullous pemphigoid in a psoriatic patient following mRNA-1273 SARS-CoV-2 vaccination. J. Eur. Acad. Dermatol. Venereol. 2022 doi: 10.1111/jdv.17955. Epub ahead of print. PMID: 35073431. [DOI] [PubMed] [Google Scholar]
- 29.Afacan E., Edek Y.C., İlter N., Gülekon A. Can Covid-19 vaccines cause or exacerbate bullous pemphigoid? A report of seven cases from one center. Int. J. Dermatol. 2022 doi: 10.1111/ijd.16086. Epub ahead of print. PMID: 35080255. [DOI] [PubMed] [Google Scholar]
- 30.Akoglu G. Pemphigus vulgaris after SARS-CoV-2 vaccination: a case with new-onset and two cases with severe aggravation. Dermatol. Ther. 2022 doi: 10.1111/dth.15396. Epub ahead of print. PMID: 35187768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO Coronavirus (COVID-19) Dashboard . 〈https://covid19.who.int〉. (Accessed 17 January 2022).
- 32.Damiani G., Pacifico A., Pelloni F., Iorizzo M. The first dose of COVID-19 vaccine may trigger pemphigus and bullous pemphigoid flares: is the second dose therefore contraindicated? J. Eur. Acad. Dermatol. Venereol. 2021;35(10):e645–e647. doi: 10.1111/jdv.17472. Epub 2021 Jul 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Portuguese A.J., Sunga C., Kruse-Jarres R., Gernsheimer T., Abkowitz J. Autoimmune- and complement-mediated hematologic condition recrudescence following SARS-CoV-2 vaccination. Blood Adv. 2021;5(13):2794–2798. doi: 10.1182/bloodadvances.2021004957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barzilai O., Ram M., Shoenfeld Y. Viral infection can induce the production of autoantibodies. Curr Opin Rheumatol. 2007;19(6):636–643. doi: 10.1097/BOR.0b013e3282f0ad25. [DOI] [PubMed] [Google Scholar]
- 35.Goriely S., Goldman M. From tolerance to autoimmunity: is there a risk in early life vaccination? J Comp Pathol. 2007;137(1):57–61. doi: 10.1016/j.jcpa.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 36.Vojdani A., Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020;217 doi: 10.1016/j.clim.2020.108480. Epub 2020 May 24. PMID: 32461193; PMCID: PMC7246018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases”. Clin. Immunol. 2021;224 doi: 10.1016/j.clim.2021.108665. Epub 2021 Jan 8. PMID: 33429060; PMCID: PMC7833091. [DOI] [PMC free article] [PubMed] [Google Scholar]