Abstract
The etiologic diagnosis of infective endocarditis is easily made in the presence of continuous bacteremia with gram-positive cocci. However, the blood culture may contain a bacterium rarely associated with endocarditis, such as Lactobacillus spp., Klebsiella spp., or nontoxigenic Corynebacterium, Salmonella, Gemella, Campylobacter, Aeromonas, Yersinia, Nocardia, Pasteurella, Listeria, or Erysipelothrix spp., that requires further investigation to establish the relationship with endocarditis, or the blood culture may be uninformative despite a supportive clinical evaluation. In the latter case, the etiologic agents are either fastidious extracellular or intracellular bacteria. Fastidious extracellular bacteria such as Abiotrophia, HACEK group bacteria, Clostridium, Brucella, Legionella, Mycobacterium, and Bartonella spp. need supplemented media, prolonged incubation time, and special culture conditions. Intracellular bacteria such as Coxiella burnetii cannot be isolated routinely. The two most prevalent etiologic agents of culture-negative endocarditis are C. burnetti and Bartonella spp. Their diagnosis is usually carried out serologically. A systemic pathologic examination of excised heart valves including periodic acid-Schiff (PAS) staining and molecular methods has allowed the identification of Whipple's bacillus endocarditis. Pathologic examination of the valve using special staining, such as Warthin-Starry, Gimenez, and PAS, and broad-spectrum PCR should be performed systematically when no etiologic diagnosis is evident through routine laboratory evaluation.
Infective endocarditis (IE) is usually suspected in a patient with fever and a new or changing cardiac murmur and is diagnosed based on the presence of a vegetation on echocardiography and positive blood cultures. Diagnosis of endocarditis is usually easy in febrile patients with a continuous bacteremia and the presence of vegetation on echocardiography or on gross examination or histologic testing of the removed valve. However, although numerous clinical situations lead to a high degree of suspicion of endocarditis, culture or histologic examination does not confirm the diagnosis.
Although fever is the single most common finding in endocarditis, it may be absent in the elderly or in patients given previous antibiotic therapy before presentation or it may be intermittent or low-grade as for Q fever endocarditis 29. Cardiac murmur is the second most frequent finding in endocarditis. However, it is not usually present at the initial stage of right-sided endocarditis, and new or changing murmurs are detected in only 40% of patients with endocarditis 261; this rate is even lower in the elderly. Despite the fact that transesophageal echocardiography is more sensitive than transthoracic echocardiography, a vegetation is rarely detected in Q fever or Whipple's disease 29, 221. Sterile blood cultures have been noted for 2.5 to 31% of patients with endocarditis 286. Blood cultures are frequently sterile when antibiotic therapy was administered before sampling and in patients with subacute right-sided endocarditis, mural endocarditis, and endocarditis caused by slow-growing or fastidious organisms such as anaerobes, the HACEK group, Abiotrophia spp., Brucella spp., Bartonella spp., Legionella spp., and Mycoplasma spp. or when obligate intracellular organisms such as Coxiella burnetii are involved 3, 124. These bacteria require specific media and conditions such as l-cysteine-enriched medium for Abiotrophia spp., buffered charcoal yeast extract (BCYE) agar for Legionella spp., or special culture conditions favorable for anaerobes or intracellular bacteria. Moreover, in some cases, slow-growing bacteria require incubation times as long as 6 weeks 179. In such situations, infective endocarditis remains a diagnostic challenge.
To both assist physicians in establishing the final diagnosis of endocarditis and allow comparisons of published cases, diagnostic criteria have been defined 70, 122, 123, 290. For many years, the Beth Israel criteria 290 were the only recognized diagnostic criteria. In 1994, Durack et al. from the Duke Endocarditis Service 70 added echocardiographic findings and other clinical and laboratory data to the well-established clinical and microbiological criteria. More recently, additional criteria have been proposed to improve the list 147, such as including C. burnetii serology or culture as additional major criteria 86.
According to the Duke Endocarditis Service, the diagnosis of IE is definite (i) when a microorganism is demonstrated by culture or histologic testing in a vegetation, an embolism, or an intracardiac abscess; (ii) when active endocarditis is confirmed by histologic examination of the vegetation or intracardiac abscess; or (iii) in the presence of two major clinical criteria, one major and three minor criteria, or five minor criteria (the major and minor Duke criteria are listed in Table 1). The diagnosis of IE is rejected when a firm alternate diagnosis explains the manifestations of endocarditis, when the fever resolves with antibiotic therapy for 4 days or less, or when no pathologic evidence of infective endocarditis is found at surgery or autopsy after antibiotic therapy for 4 days or less 70.
TABLE 1.
Terminology used for diagnostic criteria for infective endocarditisa (Modified Duke's Endocarditis Service) 70, 86
| Major criteria |
| A positive blood culture for infective endocarditis as defined by the recovery of: |
| A typical microorganism from two separate blood cultures in the absence of a primary focus (viridans streptoccoccibS. bovis, HACEK group, or community-acquired S. aureus or enterococci) |
| or |
| A persistently positive blood culture defined as the recovery of a microorganism consistent with endocarditis from either blood cultures drawn more than 12 h apart or all three or a majority of four or more separate blood cultures with the first and last drawn at least 1 h apart. |
| Evidence of endocardial involvement: |
| Positive echocardiogram for infective endocarditis |
| (i) Oscillating intracardiac mass on valve or supporting structures, in the path of regurgitant jets, or on implanted material in the absence of an alternative anatomic explanation, or |
| (ii) Abscess, or |
| (iii) New partial dehiscence of prosthetic valve |
| or |
| New valvular regurgitation (increase or charge in preexisting murmur not sufficient) |
| Serology for Q fever by IFA showing phase 1 IgG antibodies at >800 |
| Minor criteria |
| Predisposition: predisposing heart condition or intravenous drug use |
| Fever: ≥38°C (100.4°F) |
| Vascular phenomena: major arterial emboli, septic pulmonary infarcts, mycotic aneurysm, intracranial hemorrhage, conjunctival hemorrhages, Janeway lesions |
| Immunologic phenomena: glomerulonephritis, Osler's nodes, Roth spots, rheumatoid factor |
| Microbiologic evidence: positive blood culture but not meeting major criterion as noted above,c or serologic evidence of an active infection with organism consistent with infective endocarditisd |
| Echocardiogram: consistent with infective endocarditis but not meeting major criterion as noted above |
Including Abiotrophia spp.
Excluding single positive blood cultures for coagulase-negative staphylococci and organisms that do not cause endocarditis.
Serologic test result positive for Brucella spp., Chlamydia spp., Legionella spp., and Bartonella spp.
Consequently, suspected cases of endocarditis should be investigated until all clinical, epidemiologic, echocardiographic, and laboratory data are compiled in order to be able to establish the diagnostic score.
New etiologic agents of endocarditis such as Bartonella spp. and the Whipple's disease bacillus 233 have been identified by PCR amplification and sequence analysis of the amplified DNA. These molecular tools have considerably improved the detection and the identification of noncultivable causative agents of endocarditis 88. This technique is particularly useful for microbial identification in excised infected valves, even after antibiotic therapy. PCR amplification and subsequent sequence analysis of amplified genes has also been used to better identify fastidious organisms such as Gemella spp. 152. New cultivation techniques, especially tissue cell culture, have also been successfully used for isolation of fastidious organism such as Bartonella 65 or strict intracellular pathogens such as C. burnetii and Chlamydia spp. 229, 253. Tissue cell culture has been greatly improved by using the shell vial technique 229. Culture of the removed valve has been shown to be much more efficient than blood culture in recovering the organisms in patients with culture-negative endocarditis 192, 193. The use of enriched media for specific microorganisms such as nutritionally deficient Streptococcus spp., Legionella spp., or Mycobacteria spp. has also been helpful in the etiologic diagnosis of endocarditis. The availability of these improved and new diagnostic tools has considerably enlarged the spectrum of bacteria involved in the etiologic diagnosis of endocarditis. Herein, we review the epidemiology, clinical presentation, and diagnosis of endocarditis caused by rare and fastidious organisms and then focus on the strategies used for the recovery and identification of the etiologic agents of IE. This review has been based on a MEDLINE search from 1966 to 1999 using Internet Grateful Med search V2.6.2 from the National Library of Medicine with the following key words: negative blood culture or culture-negative plus endocarditis or endocarditis plus (name of the bacteria and/or the disease). When a bacterium had different names, such as Abiotrophia spp., all known synonyms were used. The research was carried out without restriction, except that endocarditis or the name of the bacteria should be included in the title. Only papers written in English or French were selected. This research yielded 1,056 references, from which 304 have been selected.
APPROACH TO ETIOLOGIC DIAGNOSIS OF INFECTIVE ENDOCARDITIS
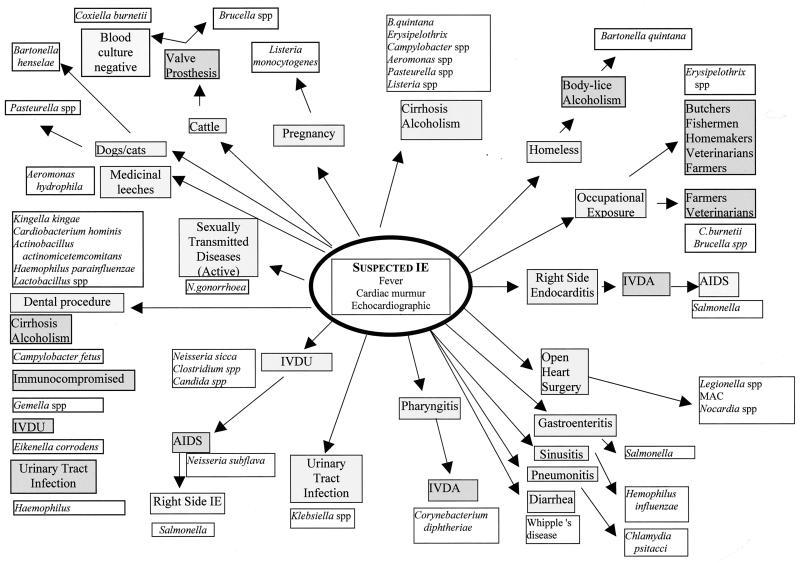
Once the diagnosis of IE is suggested or confirmed, the causative agent can be specifically investigated by evaluation of the medical history, clinical examination, and echographic findings (Fig. 1).
FIG. 1.
Probable bacterial etiology of endocarditis depending on the patient history and epidemiologic situation. IVDU/IVDA, intravenous drug user/abusers.
Medical History
The patient's medical history is an important clue for the etiological diagnosis of IE. Contact with animals is an important feature; for example, contact with a cat (including scratches or bites) suggests Bartonella henselae or Pasteurella spp., while contact with cattle should suggest a role for C. burnetii or Brucella spp. Parrots, pheasants, and pigeons have been reported to play a role in Chlamydia psittaci IE 253. Contact with domestic animals such as swine, fish, and poultry is frequently reported in the history of patients with Erysipelothrix endocarditis. Consequently, occupational exposure is an important finding. For example, farmers and veterinarians are exposed to both C. burnettii and Erysipelothrix. The latter is a causative agent of endocarditis in butchers, fishermen, and even in homemakers by contact with organic matter in which the organism is commonly found 230. Contact with arthropods such as lice and fleas should also be considered. The human body louse is involved in the transmission of Bartonella quintana 27, whereas cat fleas are involved in the epidemiology of B. henselae infections 143. Some pathogens are mostly nosocomial in origin. For example, IE following open heart surgery should suggest Legionella spp., Mycobacterium spp., Nocardia spp., or fastidious fungi, especially if routine blood cultures are negative 160, 281. Most reported cases of nosocomial endocarditis occur as small outbreaks, and all available epidemiologic information should be obtained for a diagnosis of postoperative IE. Since a large number of pathogens involved in IE are of oral origin, a history of a dental procedure is an important feature. HACEK group bacteria are often found to be pathogens in young adults after dental procedures, but Lactobacillus spp. and Gemella spp. have also been reported in such situations 152, 270. All information on underlying conditions should be obtained from the patient or from the patient's medical history. Patients with prosthetic valves are at particular risk for IE. Of note, patients with prosthetic valves for whom blood cultures are negative are likely to be infected by C. burnetii or B. henselae as etiologic agents, since more than 55% of C. burnetii endocarditis occurs in patients with prosthetic valves 266. Underlying immunocompromising conditions, including AIDS, cancer, and lymphoma, have been reported in 9% of C. burnetii endocarditis patients, especially in the absence of underlying valvular disease. A medical history of cancer, especially adenocarcinoma associated with disseminated intravascular coagulation, should also suggest the possibility of marantic or nonthrombotic endocarditis, a diagnosis of exclusion when all other investigations are nonrevealing 161. In a similar manner, IE can be suspected in patients with collagen vascular diseases such as systemic lupus erythematosus, particularly if associated with the presence of antiphopholipid antibodies such as in Libman-Sack endocarditis 238. Even if noninfective endocarditis is suspected, it is important to remember that the valvular lesions induced by inflammatory disease may facilitate the infection of the inflamed valve or graft and cause secondary IE. Patients should be questioned about their life habits, especially their sexual practices. A few decades ago, Neisseria gonorrhoeae IE was reported very frequently in sexually active patients; active surveillance and prompt treatment has now significantly reduced this rate. In a similar way, behaviors such as intravenous drug use and alcohol abuse should be evaluated. Intravenous drug users are at risk for Neisseria sicca, Clostridium spp., and Eikenella corrodens IE related to injection 8, 56, 118. E. corrodens has been reported in patients who clean needles with their saliva. For chronic alcoholics, the involved pathogens are more likely to be related to the underlying immunocompromising condition rather than to specific practices. B. quintana, Erysipelothrix rhusiopathiae, Campylobacter fetus, Aeromonas hydrophila, Pasteurella spp., and Listeria monocytogenes are most commonly found 38, 65, 77, 104. Portal hypertension and splenic shunt, which occurs with cirrhosis in these patients, may allow prolonged bacteremia and subsequent valvular infection. Social conditions may impart specific risks. For example, homeless persons are often chronic alcoholics and may harbor lice, allowing the transmission of B. quintana infection, the major fastidious etiologic agent of IE in the homeless 27. Finally, the relative frequency of the microorganism as a cause of IE should be considered in the laboratory investigation. For example, it is noteworthy that C. burnetii is as frequently recognized as an etiologic agent of IE as are all the HACEK group bacteria together (Table 2).
TABLE 2.
Relative frequency of bacteria as etiologic agents of IEa
| Bacterium | No. of cases reported | References |
|---|---|---|
| Coxiella burnetii | 359 | 29, 71, 207, 227, 228, 266, 280, 283 |
| Brucella spp. | 120 | 4, 58, 85, 129, 252; Micozzi et al., Letter; Sungur et al., Letter |
| Abiotrophia spp. | 100 | 21, 95, 235 |
| Actinobacillus actinomycetemcomitans | 93 | 11, 41, 106, 107, 139, 249 |
| 20, 100, 206 | ||
| Haemophilus aphrophilus | 78 | 52, 73, 99 |
| Cardiobacterium hominis | 76 | 155, 218, 273, 302 |
| Corynebacterium diphtheriae | 67 | 197, 278; Lortholary et al., Letter |
| Haemophilus parainfluenzae | 66 | 52, 99, 113, 167 |
| Listeria monocytogenes | 58 | 38, 93; Baddour, Letter |
Only infectious agents reported more than 50 times in the literature are listed.
Clinical Approach
Some clinical presentations are characteristic and should indicate possible IE. Febrile congestive heart failure is a frequent mode of presentation for patients with endocarditis caused by fastidious organisms, especially C. burnetii and Bartonella spp., but may also be present with Whipple's disease or HACEK group endocarditis. In fact, it is likely that this mode of presentation is related to a diagnostic delay. Emboli are reported in more than 50% of patients infected with microorganisms such as with Corynebacterium diphtheriae, N. sicca, and Haemophilus paraphrophilus 46, 278; R. Lopez-Velez, J. Fortun, C. de Pablo, and J. Martinez Beltran, Letter, Clin. Infect. Dis. 18:660–661, 1994). When these emboli involve the brain, they may mimic an acute cerebrovascular accident or stroke syndrome, but fever should suggest the diagnosis of endocarditis. Pulmonary manifestations such as repetitive bronchopneumonia are common in right-sided endocarditis. Such findings in intravenous drug users or in patients with intravenous catheters should suggest a diagnosis of right-sided endocarditis. Staphyloccocus spp., Candida spp., Eikenella corrodens, and Salmonella enterica serovar enteritidis in patients with AIDS and N. sicca and Clostridium spp. in other patients are potential etiologic agents. It is important to note that the absence of cardiac murmur does not rule out the diagnosis of IE and can suggest ride-side endocarditis. Genital organs should be examined, since most cases of gonoccocal endocarditis are associated with active sexually transmitted diseases. Concomitant gastroenteritis, sinusitis, or pneumonitis should suggest Salmonella spp., H. influenzae, or Chlamydia psittaci as etiologic agents, respectively, while upper respiratory tract infection and pharyngitis is frequently observed in patients with H. aphrophilus endocarditis and in intravenous drug users with C. diphtheriae endocarditis. In patients with Klebsiella pneumoniae endocarditis, urinary tract infection is common 9, and during pregnancy, Listeria monocytogenes is a possible etiologic agent. Finally, culture-negative endocarditis associated with chronic diarrhea should suggest Whipple's disease.
Echocardiography
Over the past 15 years, a number of investigations have confirmed the important role of transthoracic echocardiography in the diagnosis and management of IE. Echocardiography must be performed in all patients suspected of having IE. If transthoracic echocardiography is noncontributory but there is still a high suspicion of IE, transesophageal echocardiography should be performed. Compared to transthoracic echocardiography, transesophageal echocardiography has improved the sensitivity in defining both vegetative lesions and perivalvular infections, particularly in mural abscesses in patients with IE (sensitivity, 80 to 90%) 237, 247. Except in a few cases where vegetations are typically large, such as in Brucella endocarditis, echocardiographic findings are more effective in establishing a diagnosis of IE than in determining the microbial etiology. Recently, the diagnostic value of echocardiography was confirmed to improve the sensitivity of the Duke's criteria 111. Transesophageal echocardiography is particularly important in patients with negative blood cultures, but even under these conditions, 24% of patients with proven IE were misclassified as having “possible” IE 111.
STRATEGIES FOR IDENTIFICATION OF THE ETIOLOGIC AGENT OF INFECTIVE ENDOCARDITIS
Culture Methods
Blood cultures.
Quantitative culture techniques show that blood from patients with IE contain 1 to 10 bacteria per ml and that this quantity remains constant during the course of the disease 298. In 1947, Salazar Mallen et al. reported that 19 of 24 patients with a positive culture from at least one site had positive venous blood cultures, none with negative blood cultures had a positive arterial culture, and 5 with both negative venous and arterial cultures had positive bone marrow cultures 245. From these studies it appears that arterial cultures add nothing to the diagnosis of IE and that in rare cases with persistently negative venous blood cultures, bone marrow cultures may yield diagnostic information. Moreover, in a series of 82 patients with IE, 52 (63%) were diagnosed from the first culture, 78% from two blood cultures, 82% from three, and 91% from four 36. Most patients with culture-positive endocarditis are persistently culture positive even if only one, two, or three cultures are obtained 296. Culture-negative endocarditis is best defined when negative blood cultures (more that three venous blood culture of at least 5 ml each) are obtained repetitively. Therefore, the data support the concept that cultures (anaerobic and aerobic) of three sets of blood drawn within a 24- to 48-h period are sufficient to establish a diagnosis of culture-positive endocarditis 36. Because of the approximately linear relationship between the yield of bacteria from blood and the volume of blood, some authors recommend that at least 10 ml of blood be obtained for each culture and that as much as 30 ml of blood be obtained for each culture in an adult 184, 296.
When clinical and laboratory findings suggest endocarditis but blood cultures remain negative, attempts to elicit a history of prior antibiotic therapy should be made. Hampton and Harrison in 1967 studied 107 patients with IE 114 and reported that 59% had positive blood cultures and 41% had negative blood cultures. The 96 available records showed that 71 patients had been given antibiotics before blood culture, including 50% of the culture-positive group and 67% of the culture-negative group. In a New York series 298, prior administration of antibiotics reduced the incidence of positive blood cultures in patients with documented streptoccocal endocarditis from 97 to 91%. Another study showed that 62% of 52 patients with culture-negative endocarditis had previously received antibiotics compared with only 31% of 84 patients who had positive blood cultures 213. In 1982, Pazin et al. reported that 64% of 88 cultures from 17 patients who received antibiotics before hospitalization were positive compared with 100% from 15 patients who had not received antibiotics 210. The duration of prior antimicrobial therapy also appears to be an important factor. If antibiotics are given for only 2 to 3 days, blood cultures that were initially negative rapidly become positive. However, after longer noncurative courses of therapy, some blood cultures remained negative for weeks (A. R. Tunkel and D. Kaye, Editorial, N. Engl. J. Med. 326:1215–1217, 1992).
If prior antibiotic therapy has been documented, neutralization or diminution of the presence of antibiotics in blood may be accomplished by diluting the blood in broth in a ratio of 1:10 and by incorporating sodium polyanetholsulfonate, which inactivates aminoglycosides in the broth. Another approach to secure cultivation from the blood of previously treated patients is to process the blood in an antimicrobial agent removal device with cationic and polymeric adsorbent resins in saline with sodium polyanetholsulfonate 296. The resins have been pretreated to prevent bacterial retention; they remove up to 100 μg of antibiotics per ml as well as some bacterial inhibitors present in the blood 256, 296.
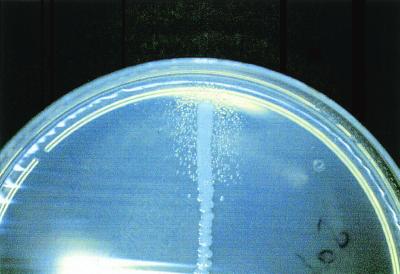
If previous antibiotic therapy has not been administered and the blood cultures are negative, fastidious organisms should be suspected, and special cultivation methods are needed. Although Abiotrophia spp. grow well in the presence of fresh human blood, they usually fail to grow in subcultures on conventional agar media. They do grow as satelliting colonies around a Staphylococcus aureus streak on blood agar (Fig. 2) and in medium supplemented with pyridoxal hydrochloride at 1 to 1,000 μg/ml 235. Therefore, the major problems with recognition of Abiotrophia spp. are the failure to grow when subcultured and the possibility that the sample may be regarded as containing only nonviable contaminants that are seen microscopically. The simplest approach to isolation is to inoculate the blood culture broth onto blood agar that is cross-streaked with S. aureus. Although uncommon, Legionella spp. should be considered in patients with prosthetic valve endocarditis and negative blood cultures; isolation requires special techniques such as BCYE agar and an incubation period of 15 days 281. Although most Mycobacterium spp. have been isolated in conventional blood culture systems including ISOSAT 92 and Bactec 254, the use of Bactec 13A bottles containing Middlebrook 7H13 broth should be considered, especially for Mycobacterium tuberculosis. Brucella spp. may be easily isolated using the Bactec NR blood culture system, but the use of lysis concentration (Isolator) system significantly shortens the time to isolation (3.5 versus 14 days) 145, 198. Bartonella spp. have been isolated from blood by prolonged incubation in the Bactec system, inoculation on rabbit blood agar, or tissue cell culture 277. In fact, they grow well in automated blood culture systems, but the small amount of CO2 produced fails to trigger the alert system 277. Consequently, both acridine orange and Gimenez staining of the blood with subculture on blood agar should be performed if cultures remain negative after 3 weeks 225.
FIG. 2.
Abiotrophia spp. (formerly known as nutritionally deficient streptococci) showing satellite growth with Staphylococcus aureus. Photo courtesy of B. La Scola (Unité des Rickettsies, Marseilles, France).
Culture of resected valves or biopsy specimens.
Pathogens can also be isolated from resected valves or biopsy specimens by inoculation onto agar or into tissue culture. This is particularly helpful and efficient, probably because of the large number of organisms in the tissue. C. burnetii, Mycobacterium spp., Brucella spp., Legionella spp., and numerous other fastidious bacteria have been identified as causative agents of endocarditis in this manner 159, 192, 225, 281. Thus, surgically resected materials should be cultured in appropriate medium when possible. In some instances, positive serologic test results for agents such as C. burnetii, Legionella spp., or Brucella spp. may be helpful in delineating the appropriate media to be employed.
Tissue cell culture.
Tissue cell culture is the only method for isolation of obligate intracellular pathogens. This procedure may be hazardous and should be attempted only in laboratories equipped for biosafety level 3 (BSL3) pathogens. The shell vial technique has been reported as the best method for isolation of C. burnetii from heparinized blood or resected heart valves 29, and it has also been successfully used for isolation of Bartonella spp. and Chlamydia psittaci isolation 63, 65, 253. Basically, different cell lines, chosen depending on the potential bacterial etiology, e.g., L929, Vero, and HEL cells for C. burnetii, L929 cells for Chlamydia psittaci, and ECV cells for Bartonella spp., are grown on coverslips in shell vials until a confluent monolayer is obtained. An inoculum of 200 μl of leukocyte-rich plasma from heparinized blood or a portion of the removed, infected valve is inoculated onto the tissue culture cells and centrifuged at 700 × g for 1 h, after which the remaining plasma is removed and replaced with fresh culture medium and the culture is incubated at 37°C in a CO2 incubator. On days 3, 6, and 15, coverslips are removed and growth is detected by indirect immunofluorescence either with antibodies that react with the bacteria suspected or with a 1:200 dilution of the patient's serum. When growth is observed, bacteria are subcultured and identified.
Histologic Testing
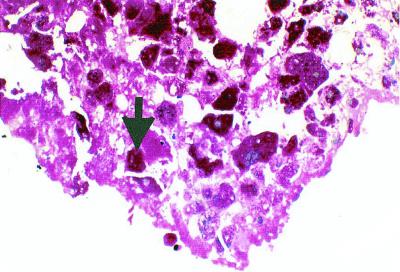
Histologic examination of the removed valve is critical. Standard stains such as hematoxylin and eosin can reveal whether the histologic findings from the valve are compatible with the diagnosis of IE. If no inflammation is observed in the valve, the diagnosis of IE should be reevaluated or excluded. Histologic parameters are included among the Duke diagnostic criteria for IE. Hematoxylin and eosin staining, the first step in the histologic examination, allows recognition of a consistent pattern of inflammation. Numerous special histologic stains are available, several of which are particularly appropriate for IE. Tissue Gram stains allow the differentiation of gram-positive and gram-negative microorganisms and, in the hands of a skilled observer, allow a rapid preliminary identification of the organisms based on their morphology 301. However, this technique has limitations since the causative bacteria may lack a cell wall (Mycoplasma spp.). The periodic acid-Schiff (PAS) stain is especially valuable for Whipple's disease and demonstrates PAS-positive foamy histiocytes variably surrounded by infiltrates of mixed neutrophils, lymphocytes, and mononuclear cells (Fig. 3) 301. This stain may also be used to detect fungi. The Giemsa stain not only allows the detection of bacteria, including Bartonella spp., but also stains white blood cells and therefore highlights the inflammatory infiltrate in vegetations 301. The Warthin-Starry technique, a silver impregnation method, is among the most sensitive methods for detection of bacteria, even those which stain weakly with a tissue Gram stain, such as Bartonella spp. (Fig. 4). Using this method, we have demonstrated massive vegetations on the valve surface with extensive destruction of the underlying valve tissue and the presence of numerous bacteria in Bartonella endocarditis 225. The Ziehl-Nielsen stain is used for detection of acid-fast bacteria, especially Mycobacterium spp. The Gimenez stain is a good method for the detection of C. burnetii and Legionella spp. In Q fever endocarditis, the vegetations, which are often smooth and nodular, are frequently infiltrated with mononuclear inflammatory cells containing many C. burnetii cells 25. Another stain of interest is the Grocott-Gomori methenamine silver stain, which provides the best contrast of the special fungal stains 301.
FIG. 3.
Whipple's disease endocarditis showing foamy PAS-positive macrophages (arrow). Magnification, ×1,000. Photo courtesy of H. Lepidi (Unité des Rickettsies, Marseilles, France).
FIG. 4.
Warthin-Starry silver stain of a Bartonella quintana-infected valve. Note the cluster of bacteria in black within the vegetation. Magnification, ×100. Photo courtesy of H. Lepidi.
Other stains are occasionally used. The acridine orange stain, a stain used in the nonspecific-fluorescence diagnostic technique, may also be used to detect any living microorganism in biopsy specimens. The Kinyoun stain or the Machiavello stain may be used to demonstrate large macrophages containing dark red granules in Chlamydia endocarditis 301.
Immunohistologic Testing
Immunohistologic methods have been used for the detection of C. burnetii, Bartonella spp., and Chlamydia spp. in valvular tissues 25, 75. Several techniques are available, including a capture enzyme-linked immunosorbent/immunofluorescent assay (ELISA/ELIFA) system, direct immunofluorescence using fluorescein-conjugated monoclonal antibodies, and immunoperoxidase staining (Fig. 5) 25.
FIG. 5.
Immunohistochemical demonstration of Coxiella burnetii in a heart valve of a patient with Q fever endocarditis. Magnification, ×600. Immumoalkaline phophatase staining was used.
Electron Microscopy
Although tedious and time-consuming, electron microscopy should be able to reveal microorganisms undetectable by molecular or immunological methods. IE use should be reserved for cases when all these other techniques fail.
PCR Amplification
The advent of DNA amplification methods for detecting microorganisms has raised great interest among infectious-disease physicians and clinical microbiologists. Such techniques, particularly PCR, have now been adapted for use with most types of clinical materials, including valvular specimens. For example, detection of a portion of the 16S rRNA gene has been used successful in the identification of the Whipple's disease bacterium as an etiologic agent of endocarditis (297; K. H. Wilson, R. B. Blitchington, R. Frothingham, and J. A. Wilson, Letter, N. Engl. J. Med. 328:62, 1993). This is also true for some cases of Bartonella endocarditis 225. The main advantages of these methods are that they are culture independent and that almost all bacteria can be detected in a single reaction through the incorporation of broad-spectrum primers. The organisms from which amplified DNA is derived can easily be identified by sequence analysis followed by comparison of the nucleotide sequence with a large database that contains sequences of the targeted gene from many other bacteria. Goldenberger et al. amplified bacterial DNA from 15 valvular samples and concluded that this technique was both easy and reliable when applied to surgically removed heart valves from patients with IE 103. However, a limitation of this system is the number and quality of DNA sequences available in databases. For instance, some of the reference sequences in the GenBank and the EMBL databases are too short or contain too many undetermined nucleotides for confident assignment of clinically derived sequences. Moreover, another likely limitation of molecular detection is the quality and nature of the infectious material collected (vegetation or piece of valve). Some clinical materials, especially blood, may contain substances which limit the sensitivity of PCR. Contamination may also occur, and therefore caution must be exercised in the interpretation of PCR-based sequence analyses when the organism has not been observed in stained valve tissues. Currently, specific primers are available for most bacterial genera, including Chlamydia, Brucella, Legionella, Mycobacterium, and Mycoplasma 87.
In contrast to its use directly on clinical samples, PCR with subsequent sequencing of the amplified gene can be used for identification of bacteria isolated by culture methods. This is particularly useful when the bacteria demonstrate unusual biochemical or staining characteristics. For example, molecular identification has successfully identified Gemella spp. in three cases of endocarditis that were reported to be due to small “gram-negative coccobacilli” 152. Such techniques for detection and molecular characterization should be used each time biochemical identification test fail.
Serologic Testing
In our laboratory, testing for culture-negative endocarditis includes the systematic determination of antibody titers against C. burnetii, Bartonella spp., Mycoplasma pneumoniae, Legionella pneumophila, Chlamydia spp., and Brucella melitensis, which are among the most common pathogens in culture-negative IE. These serologic tests are included as diagnostic criteria for IE in the Duke 70 and the modified Duke 86 criteria. However, in other instances, one must be cautious when relying solely on serologic methods to diagnose endocarditis because of cross-reactions. For example, currently available serologic tests for Bartonella-induced endocarditis may not reliably distinguish between antibody responses to B. henselae and B. quintana or between those to Bartonella spp. and Chlamydia spp. 176, 225. The most common serologic methods used are the tube agglutination test for Brucella melitensis infections, indirect immunofluorescence for Legionella pneumophila, ELISA for Mycoplasma pneumoniae, and complement fixation, ELISA, or indirect immunofluorescence for Chlamydia spp.
ETIOLOGY OF ENDOCARDITIS DUE TO LESS COMMON FASTIDIOUS BACTERIAL AGENTS
Agents of Blood Culture-Negative Endocarditis
The agents of blood culture-negative endocarditis are listed in Table 3 and discussed below.
TABLE 3.
Agents of blood culture-negative endocarditis
| Bacterium | Clinical presentation | Method of Diagnosis | Underlying disease or condition | Nosocomial infection | No. of cases | Reference(s) |
|---|---|---|---|---|---|---|
| Coxiella burnetii | Fever (68%); cardiac failure (67%); hepatomegaly (56%); splenomegaly (55%); exposure to risk factors (61%); negative blood culture; echocardiography not contributory | IFA: IgG phase 1 titer, >1/800; cultivation of blood and resected valve in tissue culture; PCR of the resected valve | Prosthetic valve; previous valve injury; rheumatic heart disease; immunocompromise (cancer, HIV, corticosteroid therapy, organ transplantation, renal dialysis) | No | 359 | 29, 71, 207, 227, 228, 266, 280, 283 |
| Mycoplasma spp. | Fever; prosthetic valve dysfunction; congestive heart failure; negative blood culture; no vegetations on echocardiography | Cultivation of a portion of the annulus of the resected valve and serologic testing | Lupus erythematosus; prednisone; prosthetic valve; rheumatic fever | Yes (1 patient) | 2 | 45, 217 |
| Bartonella henselae | Fever; cardiac murmur; acute cardiac failure; vegetation on echocardiography; cat owner | Serology: IFA | Previous valve injury | No | 8 | 10, 63, 112, 126, 225 |
| Bartonella spp. (not determined) | Fever; cardiac murmur; acute cardiac failure; vegetation on echocardiography | Blood culture | No | 20 | 75, 172, 225; unpublished data | |
| Bartonella quintana | Fever; cardiac murmur; acute cardiac failure; vegetation on echocardiography; body louse | Culture and PCR; sequence analysis of the resected valve | Alcoholism; homelessness | No | 20 | 30, 65, 131, 168, 225, 257, 258 |
| Bartonella vinsonii | No fever | Histologic testing with Warthin-Starry stain and immunoperoxidase | No | 1 | 239 | |
| Bartonella elisabethae | Fever; cardiac murmur; vegetation on echocardiography | Histologic testing with Warthin-Starry stain and immunoperoxidase | No | 1 | 51 | |
| Mycobacteria other than M. tuberculosis | Fever; acute cardiac failure | Blood culture; presence of acid-fast bacilli on valves; culture of valves | Late prosthetic valve infection; contaminated prosthesis, water or environment | Yes | 15 | 6, 43, 146, 149, 159, 160, 241, 254, 288; Butany, Letter |
| Mycobacterium tuberculosis | Fever; acute cardiac failure | Necropsy; presence of acid-fast bacilli on valves; culture of valves | Miliary tuberculosis; congenital heart defects | No | 16 | 47 |
| Chlamydia spp. | Fever; cardiac murmur | Serologic testing; culture of blood and throat swab; immunohistochemistry and monoclonal antibody | Pneumonia | No | 28 | 18, 24, 60, 61, 89, 132, 136, 156, 231, 253, 285, 291, 295; Dumont et al., Letter; Norton et al., Letter |
| Legionella spp. | Fever; mild congestive heart failure; vegetation on echocardiography | Serologic testing; valve cultures; blood culture | Valve prosthesis; surgery; rheumatic fever | Yes | 9 | 182, 208, 281 |
| Whipple's disease bacillus | Fever; diarrhea; acute cardiac failure; cardiac murmur; vegetation on echocardiography | PCR and sequence analysis from paraffin-embedded valve | Not known | Not known | Probably not rare | 84, 181, 297; Jeserich et al., Letter |
Bartonella spp.
Bartonella spp. are small facultative intracellular bacteria that stain gram negative and belong to the α2 division of the proteobacteria (Fig. 6). These organisms cause various clinical syndromes in immunocompetent and immunocompromised hosts. B. henselae causes cat scratch disease, meningoencephalitis, and prolonged fever in immunocompetent patients and bacillary angiomatosis and hepatic peliosis in human immunodeficiency virus HIV-infected patients 179. B. quintana causes trench fever, lymphadenopathy with fever, and bacillary angiomatosis. Endocarditis is caused by both B. henselae and B. quintana, and it has been found to be caused by B. elizabethae 51 and B. vinsonii 239 in only one reported case each.
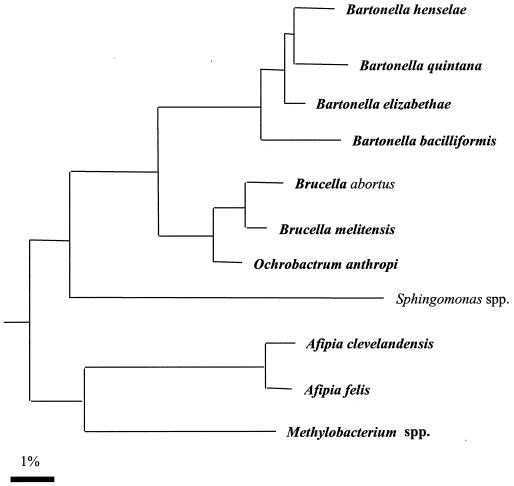
FIG. 6.
Phylogeny of the representative members of the α2 group of proteobacteria and location of Bartonella spp. based on analysis of the 16S rRNA sequence by the neighbor-joining method.
(i) Epidemiology. The epidemiology and clinical manifestations of Bartonella infections have been recently reviewed 175, 179. Bartonella infections were first reported from the United States and France, but many recent reports showed that Bartonella spp. are distributed worldwide. B. henselae is transmitted to humans by a cat scratch or bite or by cat fleas. B. quintana is transmitted by the human body louse. The reservoir of B. henselae is the cat, which is chronically bacteremic, and humans are likely to be the reservoir of B. quintana. Review of the literature and personal unpublished observations found a total of 54 cases of Bartonella endocarditis, 8 of which were caused by B. henselae 10, 63, 112, 126, 225, 20 were caused by B. quintana 30, 65, 131, 168, 225, 257, 258, 1 was caused by B. elizabethae 51, one was caused by B. vinsonii 239, and 20 were not characterized at the species level since they were diagnosed by serologic testing alone 75, 172, 225; unpublished data). Bartonella infection was diagnosed in 3 of 86 patients with endocarditis in Marseilles (3.5%), 3 of 123 patients with endocarditis in Halifax (2.4%), and 4 of 90 patients in Lyons (4.4%) 225. Bartonella endocarditis accounted for 10 of 299 cases of endocarditis (3%), being as frequent as C. burnetii (Q fever) endocarditis (unpublished data). Meta-analyses of these reported cases showed that B. henselae endocarditis occurs in 87% of patients with previous valve injury whereas only 30% of patients with B. quintana endocarditis have this underlying condition. Predisposing factors for B. quintana endocarditis are homelessness and alcoholism, a condition found in 70% of patients and associated with exposure to body lice 26. Consequently, epidemiology helps to distinguish between the two infections, one caused by B. quintana mainly in alcoholic homeless persons exposed to body lice and without previous valve injury and the other caused by B. henselae in patients with underlying valve injury who have had contact with a cat. B. quintana endocarditis has been reported in only one HIV-infected patient 257. The mean age of patients with Bartonella endocarditis is 48 years. These patients are significantly younger than control patients with IE caused by other microorganims (P. E. Fournier and D. Raoult, submitted for publication). The gender proportion is 85% male.
(ii) Signs and symptoms. Because Bartonella spp. cause a subacute insidious endocarditis, the diagnosis is usually considerably delayed, which may explain why most patients present with acute cardiac failure 75, 225. Fever higher than 38°C is found in most patients. Patients usually present with cardiac murmur, dyspnea on exertion, and bibasilar lung rales, suggesting global cardiac failure. Aortic valves are more often involved, and prosthetic valve endocarditis has been reported in only 3 of 32 patients. Embolic phenomena have been reported in 41% of patients and may initiate the clinical manifestations. Nonblanching purpura and petechiae are frequently observed on the lower extremities, palms, and conjunctiva. Laboratory data are not specific and a include normal white blood cell WBC count, thrombocytopenia (platelet count, <150 × 109/liter), and elevated blood creatinine levels in some patients with severe cardiac insufficiency.
(iii) Diagnosis. Pathologic diagnosis after valve surgery was performed in 86% of patients, and the remaining patients all had one major criterion (echocardiographic vegetations) and at least three minor criteria. In fact, the diagnosis of endocarditis is quite obvious in such patients, but blood cultures are usually negative. In such situations, especially in alcoholic homeless persons with body lice or in patients with previous valve injury who own cats, a diagnosis of Bartonella endocarditis is likely. Etiologic diagnosis is usually established by serologic testing, inoculation of the blood or resected valve into tissue culture and blood agar, PCR detection of the citrate synthase gene with identification of DNA by sequence analysis, or Warthin-Starry silver staining with immunohistochemical demonstration of the bacteria in valves. Serologic testing is carried out by an immunofluorescence assay (IFA). Low-level cross-reactions with C. burnetii may occur 151, and significant cross-reactions may be seen with Chlamydia pneumoniae 176. In one case, B. quintana endocarditis was diagnosed by detecting bacteria by PCR in the removed valve despite the fact that the patient did not demonstrate any antibodies to Bartonella spp. 225. For this reason, attempts to isolate the bacteria are necessary. The most reliable diagnostic tool, aside from serologic testing, is PCR of freshly removed valves. In our experience, a PCR amplicon was obtained in 72% of specimens even though 42% of valves were sampled during antibiotic therapy (P. E. Fournier, unpublished data). In fact, the lack of sensitivity of blood culture is probably related to the previous antibiotic therapy 153.
(iv) Echocardiography and pathology. The frequency of emboli is related to the large size of the vegetations in Bartonella endocarditis. this explains the efficiency of echocardiography, which identifies vegetations in 100% of B. henselae and 96% of B. quintana endocarditis patients 225; P. E. Fournier et al., unpublished data). Examination of removed valves showed that Bartonella spp. cause significant valvular tissue destruction. No well-formed or suppurative granulomas are observed. Typical lobular vascular proliferations, as found in cutaneous bacillary angiomatosis, are not observed. Gram staining, PAS staining, and Grocott-Gomori staining of the valve are not useful in detecting the bacteria, whereas Giemsa and, especially, Wharthin-Starry stains reveal many characteristic granular organisms in the valve vegetation or the valve (Fig. 4). Masses of bacteria occupy an extracellular location, mainly in the fibrin deposits. No organisms are found within areas of inflammation. Immunohistologic staining has been successfully used to demonstrate B. quintana or B. henselae in human cardiac valve tissues (H. Lepidi et al., unpublished data).
(v) Treatment and prognosis. In vitro antibiotic susceptibility of nine isolates of B. quintana, three isolates of B. henselae, one isolate of B. elizabethae, and one isolate of B. bacilliformis showed that all were highly susceptible to β-lactam agents, aminoglycosides, macrolides, tetracyclines, and rifampin. They were less susceptible to penicillin, narrow-spectrum cephalosporins, and clindamycin 177. Considerable variation was noted in their susceptibility to fluoroquinolones compounds. Cocultivation of Bartonella spp. with eukaryotic cells did not change the bacteriostatic activity of the antibiotics 194. However, it is interesting that only aminoglycosides (gentamicin, amikacin, and tobramycin) were bactericidal on either axenic medium or in cell cultures. Clinical data on effective treatment of Bartonella-induced endocarditis are scarce. Of 42 patients reviewed recently, 76% were treated with aminoglycosides, 74% were treated with a β-lactam compound, 35% were treated with a tetracycline, 20% were treated with vancomycin, 16% were treated with a fluoroquinolone, and 14% were treated with rifampin (Fournier and Raoult, submitted). Although a standard regimen for the antibiotic treatment of Bartonella endocarditis has not been established, based on the relevant clinical data and the in vitro activity of antimicrobial compounds, we suggest an aminoglycoside in combination with either doxycycline or ceftriaxone for a prolonged course 175. In addition to antibiotic treatment, surgery is often required owing to the extensive valve damage. In our series, valve replacement was performed in 80% of cases. It is likely that severe damage and delay in diagnosis contributed to the poor prognosis of Bartonella endocarditis. Of the 53 patients for whom the outcome was known, 42 (80%) survived and 11 died. The mortality rate observed among patients with B. quintana endocarditis was almost three times higher than that observed among the controls (Fournier and Raoult, submitted). Half of the patients who died and only one-third of survivors were homeless, suggesting that the lack of medical care for homeless people leads to delay in diagnosis and treatment and subsequently a poor outcome for Bartonella endocarditis.
Mycobacterium spp.
Mycobacteria are acid-alcohol-resistant bacteria that are visualized by the Ziehl-Nelsen stain. Tuberculous valvular endocarditis is exceptionally rare; only 16 cases have been reported in the literature 47. It usually presents in the context of miliary tuberculosis, and in all but one case the diagnoses were made at autopsy. The rare cases reported involved congenital defects of the aortic valves, prosthetic valves, and ventriculoatrial shunts. However, because of its rarity, there is still uncertainty whether true tuberculous endocarditis exists as a clinical entity 47.
Nonturberculous mycobacterial endocarditis involves mostly porcine or prosthetic valves. To our knowledge, only two cases involving damaged native valves have been reported 96, 254. In the literature, we found seven cases of prosthetic-valve endocarditis due to Mycobacterium chelonei 92, 160, 241, 288, six cases of prosthetic-valve endocarditis due to Mycobacterium fortuitum 6, 43, 146, 254, one case of prosthetic-valve endocarditis due to Mycobacterium gordonae 159, and one case of Mycobacterium avium-intracellulare-induced native-valve endocarditis (149; J. Butany, Letter, Con. J. Cardiol. 9:214, 252, 1993). Reported infections primarily involve atypical, rapidly growing strains that probably were inoculated at valve replacement 293. The ability of mycobacterial organisms to cause protracted disease is well known. Prosthetic-valve endocarditis due to mycobacteria is a rare, highly fatal infection that occur as small nosocomial outbreaks in which seasonal environmental conditions, contaminated bone wax, water baths, or surgical personnel are clearly epidemiologic factors. The disease occurs within 6 months of valve replacement and can be traced to valve contamination at surgery. In some cases, Mycobacterium spp. were found to contaminate porcine valves after inadequate sterilization during manufacturing and storage 241. Other cases have been associated with sternal wound infections following surgery 146. These patients presented with symptoms typical of endocarditis, such as fever, chills, night sweats, anorexia, and headaches 43, 146. The role of M. avium complex in causing endocarditis is still debated (117, 149; Butany, Letter), and misdiagnoses could result from laboratory contamination by water 117.
(i) Diagnosis. Mycobacterial endocarditis may be diagnosed by blood culture 92, 254, 288, 293; Middlebrook solid culture medium and the nonradiometric Bactec 9000 MB system have been shown to be equally effective in isolation of nontuberculous Mycobacterium spp. from blood 130. However, the diagnosis is frequently achieved by histologic examination of the resected valve or at autopsy by Ziehl-Neelsen staining of acid-fast bacilli. Mycobacterium spp. have been isolated from valve vegetations, bone marrow, urine, sputum 92, 159, and lung biopsy specimens 92.
(ii) Treatment. Combination therapy for infections due to Mycobacterium spp. seems advisable, since single-agent therapy has been shown to select for the emergence of resistant mutants 274. However, there are no controlled studies regarding therapy. Courses longer than 6 months may be needed for endocarditis. Consideration should be given to resection of the focus of infection and to removing foreign material in patients who are surgical candidates 288, 294.
Mycoplasma spp.
Despite the fact that endocarditis due to cell wall-deficient bacteria has been suggested since 1978 215, endocarditis due to Mycoplasma spp. has been reported in the literature only twice 45, 217. The first report described a patient with postrheumatic heart disease; the diagnosis was based on serologic test results alone, and no culture was reported. He was treated with 12 × 106 U of benzylpenicillin and 280 mg of gentamicin daily for 6 weeks and then given a 4-week course of oxytetracycline 217. The other case was reported in 1989 45 and involved a 25-year-old woman with a history of SLE, treated with corticosteroids, in whom Streptoccocus sanguis aortic and mitral valve endocarditis developed. Aortic and mitral prosthetic valve replacements were performed for acute cardiac failure. During penicillin treatment, she developed a sternal wound infection and fever. Swabs of the sternal wound and blood cultures were sterile. The prosthetic valves were replaced owing to significant valve dysfunction and renewed cardiac failure. At surgery there was no evidence of sternal or mediastinal infection, but extensive dehiscence of the aortic valve suture line was noted with multiple small abscesses and fistulous tracts extending from the aortic annulus. The mitral valves appeared grossly infected. Mycoplasma hominis was isolated from a portion of the mitral annulus. The presence of sodium polyanetholsulfonate in the blood culture media might be responsible for the lack of M. hominis growth 55. This patient was cured with clindamycin and rifampin administered for 6 weeks followed by a 4-week course of oral doxycycline 45. The role of Mycoplasma spp. in culture-negative endocarditis is unknown and very probably underestimated. This small organism cannot be detected by Gram stain, and growth is not detected in routine blood culture systems. Likewise, Mycoplasma spp. can be difficult to detect in paraffin-embedded valve tissues when using nonspecific staining techniques. Strategies for isolation of this pathogen in patients with culture-negative endocarditis should include systematic subculture of blood and excised valves. Inoculation should be made onto specific Mycoplasma agar media such as SP 4 glucose (pH 7.5), which can be used for both M. pneumoniae and M. hominis, provided that arginine is added for the latter organism. Mycoplasma serologic testing should be evaluated. PCR techniques will be probably very helpful in detecting Mycoplasma spp. in blood or in infected valves.
Whipple's disease bacterium.
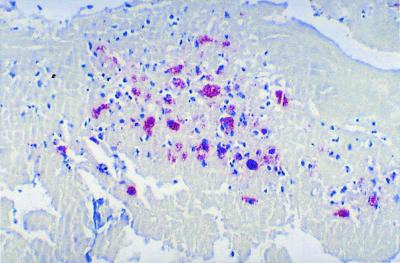
Whipple's disease is a rare systemic disorder presenting with arthralgia, diarrhea, abdominal pain, generalized lymphadenopathy, progressive weight loss, and central nervous system involvement. This disease is caused by a gram-positive bacterium that has been identified by sequence analysis of the 16S rRNA gene 234. The name Tropheryma whippelii has been suggested for cases when the bacteria have not successfully been established in culture 234. Recently, the Whipple's disease bacterium was propagated in an interleukin-4-treated macrophage cell culture but showed only limited propagation 251. In 2000, we reported the successful isolation and establishment of a strain of Whipple's disease bacterium obtained from the valve of a patient with blood culture-negative endocarditis 222. Up to one-third of patients with Whipple's disease present with concomitant cardiac symptoms, mostly attributed to endocarditis, myocarditis, pericarditis, or coronary lesions (125, 181, 188; M. Jeserich, C. Ihling, and C. Holubarsch, Letter, Ann. Intern. Med. 126:920, 1997). More recently, the relationship between endocarditis and the Whipple's disease bacillus was established in a patient who presented with diarrhea, fever, grand mal seizure, and a history of a 20-kg weight loss over 4 years 297. The diagnosis was based upon the presence of numerous PAS-positive macrophages in the duodenal biopsy specimen. After successful antibiotic therapy, he developed acute congestive heart failure due to aortic valve endocarditis, requiring urgent prosthetic valve replacement. Histologic examination revealed PAS-positive macrophages scattered throughout the entire valve (Fig. 3). DNA prepared from paraffin-embedded valve tissues identified T. whippelii by PCR and sequencing of the PCR product. Interestingly, PAS-positive granules have been demonstrated in the brains of patients with and without cerebral manifestation of the disease 84, and rheumatoid factor is found in 8% of these patients. It is likely that Whipple's disease endocarditis is much more frequent than currently reported. Recently, four cases were reported in patients without fever or evidence of systemic Whipple's disease 110. Before pathologic examination of the resected valves, which established the diagnosis, one patient fulfilled none of the Duke criteria, two patients fulfilled one major Duke criteria, and one patient fulfilled two Duke criteria (one major and one minor). Without examination of the excised valves, the diagnosis of IE could not have been made, indicating that Duke criteria for endocarditis are not pertinent for Whipple's disease endocarditis 110, 221. This underlined the fact that occurrence of the disease is probably underestimated and must depend on an active search for the cause by physician. In a recent, not yet published review on Whipple's disease endocarditis, we found that data for 34 patients had already been published with enough details to include the valvular lesion as a part of the disease and that those patients presented with aortic damage (14 of 34), mitral damage (5 of 34), tricuspid valve damage (2 of 34), mitral and aortic valve damage (5 of 34), mitral and tricuspid valve damage (3 of 34), and damage to three valves (5 of 34). The diagnosis is currently based on the histologic observation of PAS-positive macrophages in the surgically resected valve tissues and by PCR identification from either the valve or a duodenal biopsy specimen. Cultures on axenic media, using chocolate or Columbia sheep blood agar incubated at 32 or 37°C under 5% CO2, or in a microaerophilic or anaerobic atmosphere were unsuccessful. Similarly, cultures on cell culture medium and cell culture medium with lysates of HEL cells incubated at 32 or 37°C under 5% CO2 were not able to recover the bacilli. However, Whipple's bacilli can be cultured using human fibroblast cells lines (HEL and MRC5) which are grown in minimal essential medium with 10% fetal calf serum and 2 mM l-glutamine without antibiotics; a control strain is available for the scientific community (222). By observing the flask monolayer under an inverted microscope, a cytopathic effect could be observed after several weeks; small coarse dark inclusions and large coarse round structures were detected within cells. The cultured bacterium can been identified by PCR and sequencing of the 16S rRNA.
Legionella spp.
Legionella spp. are small, facultative, gram-negative intracellular bacteria that cause nosocomial pneumonia and, rarely, disease of extrapulmonary sites 165. Endocarditis due to Legionella spp. has been cited in only three publications. In 1984, McCabe reported the first case of Legionella pneumophila endocarditis in a patient with porcine aortic and mitral valve prostheses 182. In 1988, Tompkins et al. reported seven cases of nosocomial prosthetic-valve endocarditis at Stanford University Hospital Center. L. pneumophila was isolated in two cases, L. dumofii was isolated in three cases, both species were identified in one case, and an undefined Legionella sp. was serologically diagnosed in one case 281. In 1994, Park et al. reported one case of serologically diagnosed Legionella micdadei prosthetic-valve endocarditis 208.
(i) Epidemiology. The natural habitat for L. pneumophila appears to be water, and numerous outbreaks of nosocomial legionellosis due to contaminated water distribution systems have been reported. The most frequently implicated reservoirs for Legionella spp. are cooling towers and hot-water tanks 268. In the report of Tompkins et al., L. pneumophila was repeatedly isolated from drinking-water sites in the Medical Center, but these investigators were unable to clarify whether these bacteria were inoculated during or after surgery 281. All documented cases reported in the literature were nosocomial in origin.
(ii) Signs and symptoms. Patients with Legionella endocarditis often have chronic symptoms, including low-grade fever, night sweats, weight loss, malaise, and symptoms of congestive heart failure. Anemia was reported as a frequent feature, and its severity appeared to be correlated with the duration of the infection. Leukocyte counts were normal, but thrombocytopenia was observed. Unlike prosthetic-valve endocarditis caused by fungi, Legionella endocarditis has not been associated with embolic phenomena.
(iii) Echocardiography and pathology. Similar to the situation reported for Q fever, Legionella endocarditis vegetations are rarely reported on echocardiography, including transesophageal echocardiography. Only three of the nine patients reported in the literature were diagnosed by echocardiography 281. Small vegetations were observed on excised valves in five of six surgically treated patients 281. The small size of these vegetations may explain the lack of embolic manifestations and the low echocardiographic sensitivity.
(iv) Diagnosis. Legionella endocarditis should be suspected in febrile patients with prosthetic valves and negative blood cultures when serologic testing does not support a diagnosis of Q fever. Since most of reported cases are nosocomial in origin, Legionella endocarditis is likely to occur in small outbreaks. Diagnostic delays vary from 3 to 19 months after surgery. Definitive diagnosis may be made by cultivation of the excised valve, a method that was successful in seven of eight cases, and by blood culture, which was successful in three of eight cases 182, 281. In fact, when endocarditis caused by Legionella spp. is suspected, the clinical microbiology laboratory should be contacted. Legionella spp. will grow in most commercial automated blood culture systems, but the amount of growth may be inadequate to attain preestablished thresholds for detection. Consequently, periodic subcultures of incubating blood cultures from those systems to the preferred medium for Legionella spp.—buffered charcoal yeast extract (BCYE)—is recommended 15. Serum antibody titers are usually high in cases of Legionella endocarditis, but the role of serologic testing has not yet been evaluated and the positive predictive value is not known. PCR assays have been used to detect Legionella spp. in clinical samples 268. Despite the high specificity of PCR, it is no more sensitive than culture 268.
(v) Prognosis, prevention, and treatment. None of the nine reported patients with Legionella endocarditis died, but six patients required prosthetic valve replacement. Erythromycin at 2 g/day intravenously with rifampin at 600 to 1,200 mg/day or ciprofloxacin at 600 mg/day intravenously was used in all patients except for one, who was treated with doxycycline at 200 mg twice a day 208. The duration of therapy was at least 5 months, and no relapses were observed 281.
Chlamydia psittaci.
Chlamydia spp. are obligate intracellular organisms that can be grown only in tissue culture. The intracellular location explains the difficulty in isolation and identification and the difficulty in investigating its role as a causative agent of endocarditis. Chlamydia spp. have often been suggested to be causative agents of endocarditis (18, 24, 60, 61, 89, 132, 136, 156, 201, 231, 253, 285, 291, 295; R. Norton, S. Schepetiok, and T. W. Kok, Letter, Lancet 345:1376–1377, 1995. D. Dumont, D. Mathieu, M. Alemanni, F. Eb, and G. Manigand, Letter, Press Med. 19:1054, 1990). However, in a recent review of 10 patients reported to have chlamydial endocarditis, 8 were finally diagnosed with Bartonella endocarditis after their sera were tested by antibody cross-absorption and Western immunobloting 176, 225. Interestingly, epidemiologic data for three of these eight patients were comparable to those reported for Bartonella endocarditis patients, including homelessness and alcoholism 172, 176. Therefore, by using serologic testing only, it is difficult to know how many patients really have chlamydial endocarditis.
Culture-confirmed chlamydial endocarditis has been identified in only one patient 253, in whom Chlamydia psittaci was cultured from throat and blood. Even in this case, the diagnosis could not be totally accepted since the cultured microorganism was identified by immunologic techniques only, and still might have represented a Bartonella sp. or atherosclerosis-related Chlamydia pneumoniae 219.
The recent report of a serologic cross-reaction between Chlamydia and Bartonella suggest that serologic results should be carefully interpreted 176. In patients with suspected Chlamydia endocarditis, Bartonella spp. should be excluded by careful serologic analysis including cross-absorption assays and attempts at isolation of Bartonella spp. Direct identification of microorganisms by monoclonal antibody immunohistochemistry in resected valves may help, but the sensitivity and specificity of such techniques have not yet been evaluated 253. In the only reported case involving isolation of the organism, whole blood was collected in the absence of anticoagulant, the serum was removed, and the clot was liquefied in a vortex in the presence of glass beads before the suspension was centrifuged to remove debris 253. The suspension was inoculated onto L929 cells in a shell vial, and the organisms were detected with a rabbit polyclonal antibody directed against C. psittaci. However, it is again important to note that cross-reactions of Chlamydia spp. with Bartonella spp. using rabbit polyclonal antibody may preclude a definitive identification. Monoclonal antibodies, cross-absorption of sera with Western immunoblots, and/or molecular techniques such as PCR with hybridization, which is now commercially available, should be used to clearly identify Chlamydia spp. in patients with endocarditis.
Coxiella burnetii (Q fever agent).
Q fever endocarditis is caused by the obligate intracellular pathogen C. burnetii. This bacterium lives and multiplies in the phagolysosomes of infected cells at pH 4.8. In culture, it demonstrates phase variation (phase 1 to phase 2) that is equivalent to the lipopolysaccharide smooth-rough phase variation of members of the family Enterobactericeaceae, an extremely valuable property for diagnosis. Only C. burnetii cells expressing phase 1 lipopolysaccharide are infectious. Only patients with chronic Q fever present with high-level phase I immunoglobulin G (IgG) and IgA antibodies.
(i) Epidemiology. Q fever is prevalent in all countries where it has been studied 227. At the time of this writing, 359 cases of Q fever endocarditis have recorded throughout the world, with 249 being diagnosed in our laboratory 228. Q fever is a zoonosis that is widespread throughout the world and can present as an acute or chronic disease. Culture-negative endocarditis is the most prevalent chronic form and represents 78% of cases of chronic Q fever. Q fever endocarditis represents 8.7 to 11% of all reported Q fever cases 170, 207, causing 3 to 5% of all cases of endocarditis in France, Israel, and Great Britain 207. The incidence of the disease has been determined to be 1 case per million inhabitants per year in France. The reservoir of C. burnetii is only partially known. Although farm animals such as cattle, goats, and sheep are considered primary reservoirs, pets including cats and dogs have also been reported to be infected, explaining urban outbreaks 171. When infected, all of these mammals shed the desiccation-resistant organisms in urine, feces, milk, and birth products 12. The extreme resistance to physical agents allows C. burnetii to survive for a long period in the environment. Subsequently, efficient transmission and infection may be wind-borne over long distances owing to the ability of this organism to persist in an environment that lacks mammalian hosts 266, 276. In humans, infection usually results from inhalation of dust contaminated with parturient fluids of infected livestock. In France, 80.2% of patients with endocarditis had an identified risk exposure; 59.6% of patients reported animal exposures, and half of these reported exposures to sheep 29. Infection by consuming raw milk occurs in only 9.6% of patients 29. Interestingly, only 30% of endocarditis patients live in rural areas, indicating that contamination occurs mainly during short exposures such as farm visits or other recreational activities. Occupational exposure is found in 12.5% of endocarditis patients, of whom most are male farmers 29. Together, occupational risk, rural life, and raw milk are significantly associated with C. burnetii endocarditis compared with endocarditis of other etiologies (D. Raoult, unpublished data). Two-thirds of patients are male (gender ratio, 2.49). The relative risk of contracting Q fever endocarditis is five times higher in the 60- to 69-year age range, whereas patients younger than 40 years have only a minor risk. Underlying disease is found in 90% of patients 266. Most patients (88.5%) have a preexisting valvular injury.
Underlying heart diseases may be congenital, rheumatic, degenerative, or syphilitic. Aortic and mitral valves are equally involved, but tricuspid valve endocarditis has been reported only once, in a child with a congenital fistula 166. Valvular prostheses are present in 55.7% of patients. Immunocompromising conditions such as cancer, leukemia, and chronic renal failure with dialysis are found in 9% of Q fever endocarditis patients. Immunocompromise is especially frequent in endocarditis patients without preexisting valvular injury, being present in five of seven patients in one study 29, indicating that immunity plays a critical role in the clinical expression of Q fever 223.
(ii) Signs and symptoms. Q fever is a severe and often fatal disease that is often associated with a long diagnostic delay. Complaints are related either to the heart (cardiac failure, valve dysfunction) or to a general illness such as low-grade and intermittent fever, fatigue, and weight loss. Because these symptoms are not specific and vegetations are rarely detected by echocardiography, diagnosis is often made late, with a mean delay of 12 months. Fever and acute cardiac failure are the most frequent signs of Q fever endocarditis and are observed in 68 and 67% of patients, respectively. Cardiac failure is usually diagnosed with general manifestations of dyspnea, acute pulmonary edema, angina, and palpitation. Fever is often low-grade (38 to 38.5°C), remittent, and well tolerated, explaining why few patients directly seek medical intervention for this alone. The presence of splenomegaly can be very prominent and is correlated with diagnostic delays 259, sometimes leading to a misdiagnosis of hematologic malignancy 28. Hepatomegaly is also correlated with diagnostic delay, and when it is present, the liver is generally hard and considerably enlarged. Histologic test results for the liver in endocarditis are not specific; granulomas may be observed, but the fibrin ring surrounding the granulomas, with a lipid vacuole in the center giving the typical “doughnut” aspect frequently observed in acute Q fever, have never been found in the livers of patients with endocarditis 57, 283. Digital clubbing is seen in one-third of patients, a higher rate than is generally observed for other types of endocarditis 266. A purpuric rash is found in one-fifth of patients and generally occurs on the extremities and the mucosa 266. When a skin biopsy is performed, histologic examination of the specimen shows immune complex vasculitis. Renal involvement can occur, and proliferative glomerulonephritis has been reported 212, 280, 284, increasing the risk of renal insufficiency. Embolic manifestations occur in about 20% of patients and can involve cerebral and upper- or lower-extremity vessels. Embolectomy or amputation may be required. One typical manifestation of arterial embolism is stroke. Pulmonary and pleural manifestations can be observed as a complication of endocarditis 169.
Clues to the diagnosis of Q fever endocarditis include valvular heart disease, such as valvular dysfunction, in association with an unexplained infectious or inflammatory syndrome 227. This could be a purpuric rash, renal failure, stroke, or, more frequently, progressive heart failure. Some patients require several valve replacement surgeries before the diagnosis is established. Laboratory findings during Q fever endocarditis 29, 266 include circulating immune complexes (in 89% of patients), rheumatoid factor (in 83%), anaemia (<10 mg/100 ml) (in 60%), thrombocytopenia (<150,000/mm3) (in 50%), and microscopic hematuria (in 38%). Serum hepatic transaminase concentrations, particularly aspartate aminotransferase and alkaline phosphatase, are elevated in most patients 29. Lactate dehydrogenase and creatinine phosphokinase levels are also frequently increased, as are cryoglobulin levels 266. Hyperglobulinemia is observed in 94% of patients. This is reported to be helpful when the globulin fraction represents more than 50% of the total protein concentration. In this case, a polyclonal increase in the IgG and IgA levels is noted. The longer the diagnostic delay, the higher the globulin concentration becomes. Anti-smooth muscle antibodies, circulating anticoagulant antibodies, antimitochondrial antibodies, a positive Coombs test, and low titers of antinuclear antibodies may be observed (158; L. Elovaer-Blanc, C. Andre, E. S. Zafrani, M. F. Saint-Marc Girardin, M. Govault-Helmann, and D. Dhumeaux, Letter, Gastroenterol. Clin. Biol. 8:980, 1984).
(iii) Pathophysiology and echocardiography. Patients suffering from Q fever endocarditis have profound lymphocyte unresponsiveness to C. burnetii that results in a lack of macrophage activation 244. Peripheral blood mononuclear cells from patient with Q fever endocarditis produce large amounts of interleukin-10 and transforming growth factor β that may play a role in the survival of C. burnetii in macrophages 37. A very high level of specific antibodies is found in the humoral immune response in Q fever endocarditis. These antibodies are not protective and, in association with C. burnetii antigen, result in immune complexes that are responsible for many aspects of the disease 49. The general pathologic changes of valves in patients with Q fever endocarditis differs from those seen in patients with other causes of endocarditis in that Q fever vegetations often have a nodular appearance and a smooth surface. In some cases, the valves even appear normal on gross examination. By histologic testing, the valves demonstrate a mixture of acute and chronic inflammation with fibrin deposits, necrosis, and fibrosis; no well-formed granulomas are observed. C. burnetii can be detected in valves by immunohistochemistry, which shows that bacteria occur nearly exclusively in macrophages at sites of inflammation and valvular injury and only in the vegetations (Fig. 5) 25. Transthoracic echocardiography detects vegetations in only 12.5% of patients 29. In fact, Turck et al. reported that of 10 valves from patients with Q fever endocarditis examined either at autopsy or after surgery, small vegetations with insignificant valve damage were observed in 5 283. The use of transesophageal echocardiography appears to be promising 137.
(iv) Diagnosis. Q fever should be considered in all patients with culture-negative endocarditis. A specific diagnosis is easily made by serologic testing. Antibody to phase 1 and phase 2 antigens may be determined using a variety of tests. The reference technique is IFA, which should distinguish between titers of IgM, IgG, and IgA antibodies. Q fever endocarditis is characterized by a very high titer of antibody to both phase 1 and phase 2 antigens of C. burnetii. An anti-phase 1 IgG antibody titer of ≥800 plus IgA antibody ≥100 is highly predictive and sensitive 279. IgM titers may vary. In these situations, a single serum sample is sufficient for the diagnosis of Q fever endocarditis. C. burnetii may be isolated from the blood in 53% of untreated patient 193 and from the valves even in treated patients 192. Isolation of C. burnetii in cell culture should be restricted to laboratories equipped for isolation of dangerous pathogens. In our laboratory, culture of C. burnetii is obtained by inoculation of human fibroblasts grown in shell vials 229. After 6 days, infected tissue culture cells are revealed by IFA with rabbit polyclonal antibodies or mouse monoclonal antibodies. The diagnosis of Q fever endocarditis may also be established by demonstrating C. burnetii in heart valves by immunohistochemistry. In a recent immunohistologic study, C. burnetii was visualized in the valves of 10 of 14 culture-confirmed Q fever endocarditis patients 25. Detection of C. burnetii DNA by PCR is another diagnostic method 264 that can be used on blood or for paraffin-embedded infected heart valves 265.
(v) Prognosis and treatment. In untreated patients, the prognosis of Q fever endocarditis is poor. In older studies, death was reported in 60% of patients, most probably related to diagnosis delay 29. Due to better treatment, prognosis has considerably improved, but Q fever endocarditis is still a severe disease with numerous relapses unless properly treated. In vitro, C. burnetii is susceptible to doxycycline, rifampin, co-trimoxazole, and clarithromycin 178. Quinolones, chloramphenicol, ceftriaxone, and fucidic acid have variable efficacy 220. Until recently, a tetracycline in combination with a quinolone was recommended as first-line therapy for Q fever endocarditis. Although this approach was more effective than other combinations, relapses and positive valve cultures still occurred even after several years of therapy 157. Subsequently, prolonged treatment for at least 3 years was proposed 157. The ineffectiveness of this therapy was related to the fact that these antibiotics were only bacteriostatic in vitro. The lack of bactericidal activity was speculated to be due to antibiotic inactivation in the acidic phagolysosome in which C. burnetii lives and multiplies 224. The bactericidal activity of doxycycline was restored when a lysosomotropic alkalinizing agent was added 174. More recently, the combination of doxycycline with hydroxychloroquine, a lysosomotropic alkalinizing agent used for malaria, has proved more effective than therapy with doxycycline and a quinolone in human clinical trials 226; this treatment was prescribed for at least 18 months. No relapses were observed among 21 patients treated with hydroxychloroquine compared to relapses in 7 of the 14 patients treated with the doxycycline-quinolone regimen. Moreover, this treatment shortened the duration of therapy. Conditions for discontinuation of therapy were met after a mean of 31 months in the doxycycline-hydroxychloroquine group versus 55 months in the doxycycline-quinolone group 226. We conclude that the treatment for Q fever endocarditis should involve a combination of 200 mg of doxycycline per day plus 200 mg of hydroxychloroquine three times a day 226. The dosage of hydroxychloroquine should be determined by monitoring its levels in plasma to maintain a concentration between 0.8 and 1.2 μg/ml for at least 18 months 226. Surveillance of antibodies to phase 1 lipopolysaccharide by IFA is recommended six times a year, and treatment may be stopped when phase 1 IgG antibodies drop below a titer of 800 and phase 1 IgM and IgA antibodies drop below a titer of 50 226. Surgery for Q fever endocarditis has occasionally been reported as an effective therapy 19. In our opinion, surgery should be reserved for patients with hemodynamic instability only, since no studies have yet proved a beneficial effect. Although prevention of Q fever endocarditis has not yet been studied, patients with known underlying valve injury who acquire acute Q fever should probably be treated for longer than patients without valve damage since one-third will progress to Q fever endocarditis. Vaccination should be recommended for such patients who are specifically exposed, but currently an effective vaccine is available only in Australia.
Gram-Negative Bacilli
The gram-negative bacilli that cause endocarditis are listed in Table 4 and discussed below.
TABLE 4.
Endocarditis caused by other fastidious and/or rare bacteriaa
| Bacterium | Clinical presentation | Epidemiology |
|---|---|---|
| HACEK group bacteria | ||
| H. paraphrophilus | Fever (95–100%); vegetation (70%); emboli (71%); Mitral valve (++); congestive heart failure (20%) | No clear evidence of a primary focus; dental infection in one case |
| H. aphrophilus | Fever (95–100%); vegetation (70%); emboli (50%); congestive heart failure (50%) | Upper respiratory tract infection (++); dental infections (++) |
| H. parainfluenzae | Fever (95–100%); vegetation (85%); emboli (66%); congestive heart failure (++) | Dental procedure (>1 mo) (+++); young middle-aged adults (+++) |
| H. influenzae | Fever; vegetation(+++) | Young middle-aged adults (+++); concomitant H. influenzae infections) (sinusitis, encephalitis) |
| Actinobacillus | Fever (95%); weight loss (44%); splenomegaly (50%); Hepatomegaly (30%); vegetation 60%; other echo (25%) | Dental procedure (>1 mo) (++); dental infections (40%); young middle-aged adults (+++) |
| Cardiobacterium | Low-grade fever (86%); splenomegaly (59%); emboli (44%); congestive heart failure (44%); aortic valve (+++) | Dental procedure (44%); middle-aged adults; gastrointestinal endoscopy |
| Eikenella | Fever | Dental procedure; IVDUb (+++) |
| Kingella kingae and other Kingella spp. | Fever; acute cardiac failure; septic shock; mitral valve (+++) | Oral surgery; poor dental hygiene; Young adults (40% <20 yrs old); viral pharyngitis, stomatitis, or oral ulcers |
| Other gram-negative bacilli | ||
| Campylobacter fetus | Not known | Alcoholism; cirrhosis |
| Pasteurella | Fever | Contact with cats or dogs (50%); Cirrhosis |
| Brucella spp. | Fever; cardiac murmur; hepatomegaly; splenomegaly; large vegetation on echocardiography | Blood culture positive in 80% of patients if incubated up to 6 wk; culture of valves; Serologic tests (IFA, ELISA, WB)b |
| Bordetella holmesii | Fever; vegetation on echocardiography | Grade IIA Hodgkin's lymphoma |
| Francisella tularensis | ||
| Aeromonas hydrophila | Not known | Cirrhosis; medicinal leeches |
| Yersinia | Fever; echocardiography (30%) | Underlying conditions (30%) |
| Salmonella | Fever (100%); heart murmur (100%); vegetations | AIDS (Salmonella non-Typhi); IVDU (tricuspid valve); concomitant Salmonella infection (gastroenteritidis) |
| Klebsiella spp. | Fever | Urinary tract infection; pacemaker |
| Serratia marcesens, Citrobacter freundii | IVDU (S. marcesens) | |
| Streptobacillus moniliformis | Fever; cardiac murmur | Previous valvulopathy |
| Gram-negative cocci | ||
| Neissaria gonorrhoeae | Fever (60%); congestive heart failure (30%); glomerulonephritis; echocardiographic vegetations (95%); renal failure (40%) | Young adults (mean, 27 yr); STDb (urethral, cervical, vaginal discharge) (35%); pharyngitis; sore throat |
| Neisseria sicca | Fever (100%); emboli (100%) | IVDU |
| Neisseria elongata subsp. nitroreducens | Fever (100%); vegetation (80%); emboli (20%) | Young middle-aged adults; dental procedure (20%) |
| Neisseria subflava | Fever (90%) | Middle-aged adults; IVDU (++); poor dental hygiene |
| Gram-Positive bacilli | ||
| Listeria | Fever (90%); cardiac murmur (46%); weakness (30%); congestive heart failure (50%) | Cancer; lymphoma; alcoholism; diabetes mellitus; steroid therapy; pregnancy; renal transplantation; hemodialysis; AIDS; septic abortion |
| Lactobacillus | Fever; emboli (42%) | Dental procedure (75%) |
| Nocardia | Fever after valve replacement | Nosocomial infection |
| Erysipelothrix rhusiopathiae | Fever; erysipeloid (40%); congestive heart failure (60%) | Occupational (89%) (butchers, 34%; fishermen, 14%; homemakers, 14%; farmers, 11%; veterinarians, 6%; alcohol abuse |
| Clostridium spp. | Fever; emboli (50%); intravascular hemolysis (30%) | IVDU; carcinoma of the colon or cervix |
| Corynebacterium diphtheriae | Fever; pharyngitis (++); septic arthritis; emboli (71%) | Young middle age; IVDU; epidemic or clustered cases |
| Gram-positive cocci | ||
| Gemella spp. | Intermittent fever; cardiac murmur; emboli; vegetations | Poor dental conditions (11/27); colorectal carcinoma or colorectal surgery (5/27) |
| Abiotrophia spp. | Fever (100%); vegetations (64%); cardiac murmur (70%); emboli (30%); congestive heart failure |
| Underlying disease or condition | Diagnosis | Outcomes | No. of cases reported | Reference(s) |
|---|---|---|---|---|
| Previous valve injury (+++); mitral valve prolapse (+++) | Blood culture and resected valve; 6 days and more with factor V and high CO2 concns | Valve replacement (24%) | 21 | 46, 52, 99 |
| Previous valve injury (+++); valve prosthesis | Blood culture and resected valve; 6 days and more with factor V and high CO2 concns | Death (++) | 78 | 52, 73, 99 |
| Previous heart disease (+++); valve prosthesis (+); mitral prolapse; previous valve damage (++); congenital heart disease(+) | Blood culture and resected valve; 6 days and up to 18 days with factor V and high CO2 concns; sometimes polymicrobial (10%) | Death (10–35%) | 66 | 52, 99, 113, 167 |
| No underlying heart disease | Blood culture and resected valve; 6 days and up to 18 days with factor V and high CO2 concns | Death (++) | 13 | 52, 99, 167 |
| Underlying herat disease (70%); valvulopathy (33%); valve prosthesis (28%); other (9%) | Blood culture and resected valve; 6 days and up to 30 days in high CO2 concns | Death (9–15%); valve replacement (28%) | 93 | 11, 20, 41, 100, 106, 107, 139, 206, 249 |
| Underlying heart disease (75%); valve prosthesis (13%) | Blood culture and resected valve; 6 days and up to 14 days in high CO2 concns | Death (13%); valve replacement (30%) | 76 | 97, 135, 155, 218, 248, 273, 302 |
| Previous valve injury; tricuspid valve; valve prosthesis; leukemia | Blood culture and resected valve; 6 days and up to 14 days in high CO2 concns; polymicrobial (50%) | Death (15%); valve replacement (1 case) | 19 | 56, 98, 115, 148, 203 |
| Normal valves (50%); prosthesis (30%) | Blood culture positive in 70% of cases if >4 but may take more than 5 days in conventional media | Death (16%); cerebral emboli (25%) | 28 5 | 79, 105, 116, 133, 183, 190, 202, 303 |
| Previous heart disease (30%); prosthetic valve (only 2 cases) | Grow in blood culture bottle at 37°C | Death (25%) | 21 | 77 |
| Native valve (90%); Prosthetic valve (only 1 case) | Blood culture always positive | Death (31%) | 20 | 200, 255, 287 |
| Prosthetic valve in 2/3 of patients | No | 120 | 4, 58, 85,129, 252; Micozzi et al., Letter; Sungur et al., Letter | |
| Bicuspid aortic valve | Blood culture and molecular typing | Alive | 1 | 272 |
| Detected in blood culture on day 9 after sampling | 1 | 271 | ||
| Not known | Blood culture positive | No deaths | 2 | 205 |
| Valve prosthesis (2/12 cases); rheumatic heart disease (3/12 cases) | Blood culture always positive | Death (25%) | 12 | 101 |
| Prosthetic valve (15%); congenital heart defect (5%); previous valve injury; rheumatic heart disease; mitral valve prolapse | Blood culture always positive | Myocardial abcess; death (10%) | 33 | 42, 80, 81, 236 |
| Native valve (60%); Prosthetic valve (30%) | Blood culture always positive | Death (49%); valve replacement (42%) | 50 | 9 |
| Blood culture always positive | ||||
| Rheumatic heart disease | Blood culture | No deaths | 16 | 243 |
| Previous valvular disease (15%) | Blood culture positive after 3 days (28%) blood cultures negative after 3 wk (7%) | Death (25%); surgery (50%) | 40 | 128, 138, 292 |
| Previous rheumatic valve injury; prosthetic valve | Blood culture always positive; Gram stain may appear positive | High mortality before 1941, no deaths since | 14 | 118, 240; Lopez-Velez et al., Letter |
| Previous valve injury (80%); Native-valve endocarditis (10%) | Blood culture always positive | Myocardial abcess (30%); Renal failure (30%); valve replacement (50%); no deaths | 27 | 62, 185 |
| HIV; asplenia; diabetes mellitus | Blood culture and culture of the removed valve positive | Death (40%) | 12 | 8 |
| Previous valve injury (50%); valve prosthesis (25%); no underlying heart disease (25%) | Blood culture always positive | Death (37%) | 58 | 38, 93; Baddour, Letter |
| Previous heart disease (83%); prosthesis (20%); rheumatic fever (20%); congenital heart disease (30%) | Grow well but identification difficult | Relapse (39%); death (25%) | 30 | 127, 270; Antony et al., Letter |
| Prosthetic valve in (3 patients) | Blood culture positive (1/3 patients); Nocardia isolated from the removed valve (2 patients) | 1 survival; 2 deaths | 3 | 74, 76, 289 |
| Preexisting heart disease ( 40%) | Blood culture always positive | Death (38%); valve replacement (1/3 of cases) | 44 | 17, 104, 108, 121, 230 |
| Prosthetic valves or rheumatic valvulopathy (50%); native valve (50%) | Anaerobic blood culture bottle; Sometimes reported as culture negative or only one positive culture; gram-positive bacilli on blood films | Death (30%) | 21 | 7, 50, 144, 187, 191 |
| Underlying heart disease; prothesis; rheumatic fever; congenital heart disease | Grow well but identification difficult and requires adequate laboratory or molecular analysis | Death (41% before 1950, 14% since 1950) | 67 | 164, 197, 278 |
| Previous valve injury (12/27); valve prosthesis (2/12) | Blood culture may appear negative; Gram stain; phenotypic test and possibly 16S rRNA gene sequence | Valve replacement (2/27); no deaths | 27 | 152, 275; M. J. Brack, P. G. Avery, P. J. Hubner, and R. A. Swann, Letter, Postgrad. Med. J. 67:210, 1991; |
| Preexisting heart disease (90%); prosthetic valve (10%) | Culture in automated system; subculture with blood agar supplemented with l-cysteine or with S. aureus as helper colony | Valve replacement (27%); death (17–20%) | >100 | 21, 95, 23 |
Frequencies, when reported, are given in parentheses. When not exactly documented, the following guide is used: +, one-third of cases; ++, two-thirds of cases; +++, most cases.
IVDU; intravenous drug use; STD, sexually transmitted diseases; WB, Western blotting.
HACEK group bacteria.
Fastidious, small gram-negative bacilli (primarily members of the oropharyngeal flora) have been long recognized as a cause of infective endocarditis. Collectively, these organisms are referred to as the HACEK group and include Haemophilus parainfluenzae, H. influenzae, H. aphrophilus, H. paraphrophilus, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella spp. Common features of the HACEK group of microorganisms are that they frequently colonize the oropharynx, they are slow growing, and their growth is enhanced by the presence of carbon dioxide 54. These organisms are not monophyletic based on their 16S rRNA sequence and do not represent a bacterial classification (Fig. 7).
FIG. 7.
Phylogeny of the HACEK group bacteria based on analysis of the 16S rRNA sequence by the neighbor-joining method.
HACEK microorganisms are reported to cause 3% of all endocarditis cases, with 399 cases having been reported in the literature, a number comparable to that of C. burnetii endocarditis cases (Table 2). Despite the common epidemiologic characteristics, which include previous dental procedures, infections in young and middle-aged adults, previous underlying heart disease, and a preference for mitral valves, every infectious agent demonstrates some unique characteristics that require description.
(i) Epidemiology. Only 21 cases of H. paraphrophilus endocarditis have been reported 46, 52, 99. Fever, cardiac murmur, acute congestive heart failure, and embolic manifestations are common clinical characteristics. H. paraphrophilus endocarditis usually occurs on previously abnormal valves, but it is interesting that mitral valve prolapse is a frequently reported predisposing condition, having been observed in 10 of 16 reported cases and potentially representing a clue to the etiological diagnosis 46. In one case, oral manipulation was reported as a possible source of infection. In other cases, there was no clear evidence of a primary focus. As in other HACEK group endocarditis cases, valve replacement has been performed in 24% of patients.
H. aphrophilus is frequently found in dental plaque and gingival scrapings. It is the most prevalent of the Haemophilus spp. involved in endocarditis 52. A total of 78 cases have been published 52, 73, 99. Dental manipulation as a source of infection is well documented, but upper respiratory tract infection is another site from which infection may be initiated 73. Previous valve injury is a common finding, and prosthetic valves are noted in 20% of patients 73. Echocardiography demonstrates vegetations in 70% of patients. Death following emboli or congestive heart failure in older reports was close to 50% 73. A shortened diagnostic delay has improved the prognosis, and no deaths were documented in the last study reported 52.
H. parainfluenzae has been reported in 66 cases of endocarditis 53, 99, 109, 113, 167. H. parainfluenzae endocarditis has an insidious onset, with the first symptoms developing more than 1 month following routine dental procedures 109. Endocarditis usually occurs in patients with previous heart disease, and prosthetic valves are involved in 10% of patients. The mitral valve is most often involved. When detected by echocardiography, emboli from large vegetations led to major occlusive disease in 85% of patients. H. parainfluenzae endocarditis is polymicrobial in 10% of patients, leading to potential misdiagnoses. The case fatality rate is reported to be between 10 to 35% 167.
H. influenzae endocarditis has been reported in 13 patients 52, 99, 167. The clinical presentation is unremarkable but is often associated with evidence of focal infection such as sinusitis or encephalitis; valve infection may occur following bacteremia. Underlying heart disease is rarely documented. The reservoir for H. influenzae is the oropharynx.
With 93 reported cases, Actinobacillus actinomycetemcomitans is the most frequent agent of endocarditis in the HACEK group 11, 20, 41, 100, 106, 107, 139, 206, 249. This bacterium was frequently found associated with Actinomyces infections, as suggested by its name, and is commonly found in human periodontal cultures 139. It may be transmissible among individuals since approximately 50% of family members of patients with localized juvenile periodontal disease harbor the organisms compared with less than 25% of the general population 41. Prior dental disease was found in 46% of patients with A. actinomycetemcomitans endocarditis, which occurs in young middle-aged patients. Underlying heart disease was noted in 70% of infected patients including those with previous cardiac valve damage (33%) and prosthetic valves (28%) 11, 106. The case fatality rate is between 9 and 15%, depending on the series. Valve replacement was required for 28% of patients after acute cardiac failure, persistent infection, or systemic emboli.
Cardiobacterium hominis endocarditis has been recorded in 76 patients 97, 135, 155, 218, 248, 273, 302. C. hominis is a member of the normal oral flora, and earlier dental procedures or oral infections were found in 44% of patients with endocarditis 302. Prior cardiac disease was reported in 75% of patients, including 13% with prosthetic valves that were infected as a late complication of cardiac surgery. In contrast to other HACEK organisms, C. hominis infects the aortic valve more frequently than it infects the other valves. Death occurs in 13% of patients, and valve replacement is required in 30% of patients.
Eikenella corrodens endocarditis has only been reported in 19 patients in the literature 56, 98, 115, 148, 203. E. corrodens has been found in dental and gingival scrapings and can be isolated from 16% of blood cultures taken 1 minute after tooth extraction 148. E. corrodens has been found with a particularly high frequency in intravenous drug users, perhaps due to contamination of syringe needles with saliva before injection. The infection is usually polymicrobial (50%) and is associated with Streptococcus spp. E. corrodens frequently infects the tricuspid valve. Other recognized predisposing conditions for endocarditis are previous valve damage, prosthetic heart valves, and immunocompromised states such as leukemia. E. corrodens endocarditis is an insidious disease that does not result in rapid clinical deterioration and is rarely fatal. The fatality rate in all studies since 1972 is 15%, and only 1 valve replacement was reported in these 19 patients.
Endocarditis due to Kingella spp. has been reported in 33 patients, 28 of whom were infected with Kingella kingae (79, 105, 183, 190, 202, 303; D. B. Jenny, P. W. Letendre, and G. Iverson, Letter, Rev. Infect. Dis. 10:1065–1066, 1988 [Erratum, 10:1065]) and 5 of whom were infected with Kingella denitrificans 116. K. kingae endocarditis occurred more frequently in very young children, and 40% of patients were younger than 20 years. Half of these patients had normal cardiac valves, while the others had congenital heart conditions such as tetralogy of Fallot or mitral valve prolapse. In older patients, the most prevalent underlying heart disease factor was prosthetic valve replacement, which was observed in one-third of patients. Poor dental hygiene or oral surgery have been reported to be associated with infection. However, it is interesting that Kingella spp. predominantly colonize the upper respiratory tract, in particular the tonsils. Rather than being a primary pathogen, this organism probably exploits mucosal impairment that results from viral infection. Thus, concomitant stomatitis, pharyngitis, or other varicella virus-induced buccal ulcers have been reported in children with Kingella bacteremia 303. A fatality rate of 16% has been reported in K. kingae endocarditis, and central nervous system emboli were detected in 25% of cases.
(ii) Diagnosis and echocardiography. Because of slow growth, HACEK group bacteria should be considered in the differential diagnosis of culture-negative endocarditis. Although the mean duration for incubation of blood cultures until detection of growth is 3 to 5 days, up to 30 days may be required, as reported with A. actinomycetemcomitans endocarditis. Nevertheless, blood cultures are often positive; for example, blood cultures are positive in 90% of cases of A. actinomycetemcomitans endocarditis when incubated for longer than 8 days 107. In fact, no significant difference in bacterial growth was found between the Septicheck bottle (Becton Dickinson, Cookeysville, Md.) containing 70 ml of tryptic soy broth and the Isolator tube (Wampole Laboratories, Cranbury, N.J.) 54, 190. Culture and identification of bacteria from the HACEK group has been reviewed elsewhere 35, 194, 242. Briefly, bacteria can be identified after subculture on 5 to 8% sheep blood and chocolate agar at 35 to 37°C for 48 to 72 h in an aerobic atmosphere containing 5 to 10% CO2. Most species of Haemophilus grow well on conventional chocolate agar but do not grow at all on standard 5% sheep blood agar. All species of Haemophilus require either exogenous hemin (X factor) or NAD (V factor). Haemophilus spp. can be grown on a nutrient-rich, nonselective medium lacking X and V factors (e.g., Mueller-Hinton agar) by applying a commercially available filter paper disk saturated with X, V, or X and V factors to the surface medium. This technique is useful for identification of Haemophilus at the level species. Several commercially available kits identify and biotype Haemophilus spp., such as the API 10E and API 20E systems (Biomérieux Vitek, St. Louis, Mo.) and the HNID system (Dade Behring, West Sacramento, Calif. 35. A. actinomycetemcomitans is positive for both the oxidase and alkaline phosphatase reactions. Nitrates are reduced to nitrite but not gas, no growth is observed on Simmons citrate medium, the arginine dihydrolase reaction is negative, and no acid is produced from adonitol and sorbose 195. Biochemical properties used to differentiate members of the HACEK group are listed in Table 5. However, under some circumstances, identification is difficult and molecular techniques using 16S rRNA gene analysis may be useful (Fig. 7) 53, 113. Valve vegetations are detected by echocardiography in 60 to 85% of patients and are characteristically thought to be large 15. No correlation between the size of the vegetations as determined by transthoracic echocardiography and the subsequent rate of emboli was found 54. Care must be taken when diagnosing polymicrobial endocarditis due to bacteria from the oropharynx because prolonged incubation may allow the recovery of an associated HACEK microorganism. This is important because the HACEK group organisms could be resistant to ampicillin and/or clindamycin, which may explain therapeutic failures.
TABLE 5.
Biochemical properties used to differentiate other member of the HACEK groupa
| Property | Reactionb of:
|
||||
|---|---|---|---|---|---|
| Actinobacillus | Eikenella | Kingella | Cardiobacterium | Streptobacillus | |
| Oxidase | + | + | + | + | − |
| Catalase | v | − | − | − | − |
| Nitrate reductase | + | + | v | − | − |
| Arginine dihydrolase | − | − | − | − | − |
| Urease | v | − | − | − | − |
| Indole production | − | − | − | + | − |
| Acid from: | |||||
| Mannitol | v | − | − | + | − |
| Glucose | + | − | + | + | + |
| Growth on MacConkey agar | v | − | − | − | − |
| Simmons citrate | − | − | − | − | − |
Adapted from reference 195.
+, shows property; −, does not show property; v, variable.
(iii) Treatment and outcome. Medical treatment alone or associated with surgical treatment can cure 82 to 87% of patients with endocarditis caused by HACEK group organisms 15. In the past, ampicillin plus gentamicin was the treatment of choice. However, β-lactamase-producing organisms in this group have prompted a change in treatment strategy, which should now include a β-lactamase-stable cephalosporin in place of ampicillin 109. The American Heart Association recommends that native-valve and prosthetic-valve endocarditis be treated for 4 and 6 weeks, respectively. The recommended prophylaxis for dental procedures is currently amoxicillin. For allergic patients, clindamycin or pristinamycin are alternatives, but it is important to note that 40% of A. actinomycetemcomitans strains are resistant to both antibiotics and E. corrodens is resistant to clindamycin, suggesting the use of tetracycline as yet another alternative 107.
Campylobacter fetus.
Campylobacter fetus is a small gram-negative bacterium. C. fetus endocarditis has been reported in 21 patients 77. Among these, males were more often involved, previous valve disease was noted in 30%, and prosthetic-valve endocarditis was reported only twice. One-third of the patients suffered from concurrent illness (hepatic cirrhosis, alcoholism, connective tissue disease, or tuberculosis) 77. Death occurred in 25% of patients. The current recommended treatment is imipenem, cilastatin, and gentamicin 77.
Pasteurella spp.
Pasteurella spp. are small, gram-negative, nonmotile coccobacilli that are distributed worldwide. These organisms are part of the normal flora of the nasopharynx or the gastrointestinal tract in wild animals and in 50% of cats and dogs; they are rarely present in the respiratory tracts of healthy humans. Endocarditis due to Pasteurella spp. has been reported in 21 cases in the literature 200, 255, 287. Common characteristics include close contact with a cat or a dog in 44% of reported cases and hepatic cirrhosis in 6%. Only one case involved a prosthetic valve. Other species of Pasteurella such as Pasteurella dagmatis, Pasteurella haemolytica, and Pasteurella gallinarum may also cause endocarditis 255. Death occurred in 31% of patients and was more likely to be related to diagnostic delay than to antibiotic susceptibility of the bacteria.
Brucella spp.
Brucella spp. are small, facultative, gram-negative, intracellular bacteria that cause abortion in domesticated animals: B. abortus in cattle, B. suis in swine, and B. melitensis in goats and sheep. Most cases of human brucellosis are caused by B. melitensis, a pathogen of sheep, which is the most virulent Brucella sp.
(i) Epidemiology. Brucellosis, primarily a worldwide zoonosis caused by Brucella spp., is transmitted to humans mainly through ingestion of contaminated milk or milk products or by close contact with infected livestock or their tissues or secretions. It is important to note that a similar epidemiologic situation is observed with Coxiella burnetii, the agent of Q fever. However, the degree of disease control in animals varies among countries, accounting for discrepancies in the number of reported cases, with most being reported from Spain and Italy. The disease, which is rarely fatal, may take a febrile acute, subacute, or chronic course. Endocarditis is a rare complication of brucellosis and occurs in 0.3 to 0.6% of patients but is the most frequent cause of death. Endocarditis due to Brucella spp. varies from less than 1 to 4% of all cases of bacterial endocarditis depending on the geographic area 82. We recorded 120 published cases of Brucella endocarditis 4, 58, 85, 129, 252; A. Micozzi, M. Venditti, G. Gentile, N. Alessandri, M. Santero, and P. Martino, Letter, Eur. J. Clin. Microbiol. Infect. Dis. 9:440–442, 1990; C. Sungur, A. Sungur, G. Gedikoglu, A. Usubutun, U. Yasavul, C. Turgan and S. Caglar, Letter, Nephron 67:234–235, 1994).
(ii) Signs and symptoms. Most patients with B. melitensis endocarditis follow a subacute course over a period of 2 to 10 weeks 83. Fever, generalized aches, cardiac murmur, and enlargement of the liver and spleen are the most prominent clinical findings. On rare occasions, Brucella endocarditis follows an afebrile course, complicated by disseminated intravascular coagulation 5. In patients with prosthetic valves, relapsing bacteremia after appropriate treatment for acute brucellosis is an important clue to the diagnosis of endocarditis. In a review on zoonotic endocarditis, Fernandez Guerrero reported that 70% of the 20 patients they monitored in Madrid developed cardiac failure 82. Thus, one should consider the diagnosis of Brucella endocarditis in an epidemiologically exposed patient who presents with febrile cardiac failure.
(iii) Pathology and echocardiography. Brucella endocarditis is a destructive process predominantly involving the aortic valve and perivascular tissues 129, 211. Evidence of underlying valvular disease, including prosthetic valves, is noted in two-thirds of patients 129. The valvular lesions have been described as bulky and ulcerative with gross abscesses of the myocardium, microabscesses within the cusps, destruction of the commissures, and calcification 211. Myocardial abscesses have been found in 43% of patients dying of Brucella endocarditis 211; aortic root abscess seems to be a common complication when the aortic valve is involved 4. Ring abscesses leading to valve detachment occur in most instances of prosthetic valve endocarditis caused by B. melitensis 83. These observations help to explain the high fatality rate for Brucella endocarditis.
(iv) Diagnosis. Diagnosis depends on the isolation of Brucella spp. from blood cultures or cardiac tissue. Isolation of Brucella spp. is hazardous, and the physician should inform the microbiologist of such a suspected diagnosis. Blood cultures are positive for Brucella in more than 80% of cases if the incubation time is prolonged to 4–6 weeks. Culture of vegetations commonly yields Brucella spp. even after prolonged antimicrobial chemotherapy 83. Historically, culture of blood for Brucella has been performed by broth methods that required prolonged incubation and the use of blind subculture. Brucella spp. grow on most standard laboratory media including blood, chocolate, and tryptic soy agar when incubated at 35°C in 5 to 10% CO2. Culture has also been performed with the biphasic Ruiz-Castaneda bottle. Currently available methods that have been advocated include both lysis-centrifugation (Isolator) blood cultures and the use of automated, continuous-monitoring blood culture instrumentation such as EPS, Bactec NR, Bactec NR660, or BacT/Alert. A shorter isolation time for Brucella spp. was obtained by the lysis-concentration technique than by using Bactec NR 198. These systems may yield positive cultures in 4 to 10 days, but prolonged incubation (4 to 6 weeks) may be needed, particularly in patients treated with antimicrobial agents 145. In laboratory practice, it is still recommended to perform both early and terminal subculture from the bottle in the ESP, Bactec NR, Bactec NR660, or BacT/Alert system when brucellosis is suspected and to hold these bottles for a minimum of 21 days. In the absence of bacteriologic confirmation, serologic tests are used to make a presumptive diagnosis 304. Although several methods have been developed to detect IgA, IgG, and IgM, such as IFA, ELISA, and Western blots analysis, the standard tube agglutination test is still the reference test. Most cases of active infection will be associated with titers of 1:160 or greater. The physician should be aware of the serologic cross-reactions that exist between Brucella, Yersinia, and Francisella spp. that may lead to confusion in etiologic diagnosis 64.
(v) Treatment. Although antimicrobial agent treatment can result in sterilization of valve vegetations 83, only a few patients with definite endocarditis caused by Brucella spp. have been cured by antibiotics alone (16, 44; Micozzi et al., Letter), and before the introduction of open-heart surgery, the mortality of Brucella endocarditis was greater than 80%. The current recommendation is combined medical and surgical treatment, especially with infected prosthetic valves 129, 252. Quinolones are very efficient in vitro but have been very disappointing in clinical trials, with failure rates of 16 to 66% in acute brucellosis; therefore, they should not be used 150. Most authors recommended treatment of Brucella endocarditis with a combination of doxycycline and either rifampin or streptomycin for several months. It is advisable to extend therapy for a minimum of 6 to 8 weeks postoperatively 82. A progressive drop in antibody titer is evidence that the patient is cured. Patients with a relapse or a failure have high levels of IgG remaining 31.
Bordetella spp.
Only one case of Bordetella holmesii endocarditis has been reported in the literature 272. It occurred in a patient with a bicuspid aortic valve who received radiation therapy for stage IIA Hodgkin's lymphoma. Diagnosis was confirmed by the visualization of a vegetation on the aortic valve at transeosophagal echocardiography. The organism was identified by molecular biology techniques after isolation from blood culture 272.
Francisella tularensis.
Only a single case of infective endocarditis due to F. tularensis has been reported. The organisms were detected on day 9 after sampling. The diagnosis would not be possible without extended incubation of blood in a radiometric blood culture system (Bactec) 271.
Aeromonas hydrophila.
Aeromonas hydrophila is a waterborne gram-negative bacillus associated with gastroenteritis and soft tissue infections. Only two cases of A. hydrophila endocarditis have been reported in the literature, both in cirrhotic patients. One patient had chronic renal failure treated by hemodialysis, and one patient had multiple malignancies including colon carcinoma 205. Sources of infection for A. hydrophila may include alligators, piranha, and the extensive use of medicinal leeches for the treatment of edema in burn patients 78.
Yersinia enterocolitica.
Yersinia enterocolitica, a member of the Enterobacteriaceae, causes bacteremia and septicemia that are usually associated with a predisposing underlying disease such as an iron-overloading state (hemoglobin disease, chronic hemolytic anemia, hemochromatosis, acute iron poisoning, deferoxamine therapy, alcoholism, and cirrhosis), immunosuppressive therapy, and immunocompromise such as that due to HIV infection. Twelve cases of Y. enterocolitica endocarditis have been reported in the literature, including two prosthetic-valve infections 101. The involved serotypes were O:3, O:8, and O:9, which are termed virulent serotypes and represent the most frequent causes of sporadic human disease 101. Rheumatic heart disease was reported in three patients. Etiologic diagnosis was achieved by blood culture in all cases. Of the 12 patients, 3 died, none of whom was treated appropriately. The patients who recovered were treated with a broad-spectrum cephalosporin and gentamicin 101.
Salmonella spp.
Salmonella spp. are causative agents of intravascular infections such as endarteritis or aneurysms, but endocarditis has been recognized since 1967, when the first cases were reported 40. Since that time, 33 cases have been reported in the literature, but a number of these reports do not contain enough information for review purposes 42, 66, 80, 81, 102, 236, 250. The most frequently observed species is Salmonella enterica serovar Enteritidis, with S. enterica serovars Choleraesuis Typhi being reported only rarely 102. Endocarditis occurs mostly during or after a concomitant Salmonella infection such as gastroenteritis in predisposed patients 42. Because patients with AIDS are more likely to have non-Typhi Salmonella bacteremia, they are at particular risk for endocarditis 80, 81. This is especially true if they have used intravenous drugs and have a previously damaged tricuspid valve 66. In patients without HIV infection, non-Typhi Salmonella endocarditis occurs in prosthetic valves 42 in 15% of patients, in patients with congenital heart defects in 5%, and less frequently in patients with other valve abnormalities including rheumatic disease and mitral valve prolapse 236. Echocardiography usually demonstrates large vegetations, and blood cultures are positive. A cardiac murmur is frequently reported but may be absent or missed in patients with right-sided endocarditis. Occasionally, blood cultures are negative in patients with myocardial abscess in whom the diagnosis is eventually made after surgery. Death occurs in 10% of reported cases, but treatment with a broad-spectrum cephalosporin or a fluoroquinolone is efficacious even in patients with prosthetic heart valve endocarditis 81.
Klebsiella spp. and other rarely isolated enterics.
The rarity of endocarditis caused by Klebsiella spp. limits its recognition and awareness of an often malignant course. Both K. pneumoniae and K. oxytoca have been reported to cause endocarditis, but the species of Klebsiella involved in endocarditis is rarely noted in published series. Klebsiella endocarditis has been reported in 50 patients 9. Characteristics of Klebsiella endocarditis include an antecedent Klebsiella urinary tract infection as a probable origin in 50% of patients and a high degree of virulence evidenced by native-valve endocarditis and prosthetic-valve involvement in 60 and 30% of patients, respectively 9. Infection of a pacemaker is also suspected to be a potential source by which endocarditis is established. Blood cultures are always positive, and continuous bacteremia with this organism suggests the diagnosis of endocarditis. When diagnosis is delayed, death occurs in 49% of patients despite efficient antibiotic therapy with broad-spectrum cephalosporins and aminoglycosides, and 42% of patients need valve replacement.
Serratia marcesens has been reported to cause endocarditis, but the number of published cases is difficult to evaluate precisely. From 1969 to 1974, 19 cases of S. marcesens endocarditis were observed in the San Francisco Bay Area (186). Seventeen of these patients were intravenous drug users, and S. marcesens caused 14% of all addict-associated endocarditis cases in San Francisco. Aortic or mitral valves were involved in 13 patients, and heart failure developed in 9 of these. Twelve patients had embolic episodes to the brain, iliofemoral arteries, or lungs. One of six patients with tricuspid valvulitis was cured by surgery combined with antibiotics, and the other five were cured by antibiotics alone. All 12 patients with aortic or mitral valvulitis treated medically died; 11 of these had unremitting sepsis. Aortic valve replacement and antibiotics were effective in one patient. There are only four reported cases of endocarditis due to Citrobacter freudii, with one being found in an intravenous drug abuser 216a.
Streptobacillus moniliformis.
Sixteen cases of Streptobacillus moniliformis endocarditis have been reported in the latest review of the literature 243. No other cases have been reported since that time. Endocarditis is a rare complication of septicemia after a rat bite. Endocarditis occurs most often in the setting of previously damaged heart valves, usually as a result of rheumatic heart disease 243. In two of four cases in which it was performed, echocardiography showed vegetations. Therapy with adequate doses of penicillin is usually curative.
Gram-Negative Cocci
The gram-negative cocci that cause endocarditis are listed in Table 4 and discussed below.
Neisseria gonorrhoeae.
N. gonorrheae is a gram-negative diplococcus which was first suggested as a potential cause of endocarditis in 1834 292. The incidence of this apparently frequent disease seems to have dropped sharply in incidence just after the beginning of the antibiotic era, and since that time only 40 proven cases of gonococcal endocarditis have been recorded in the English literature 128, 138, 292. The mean age of the patients is 27 years. The mean duration of symptoms before diagnosis is approximately 45 days, fever is recorded in 60% of patients, and acute congestive heart failure is found at presentation in one-third of patients. The most common presenting symptoms resemble those of other forms of endocarditis, except that renal failure with or without glomerulonephritis may complicate the course of disease; uremia is the cause of death in 40% of patients. A history of sexually transmitted disease including urethral, vaginal, or cervical discharge with proven active gonococcal disease is recorded in 35% of patients 292. Concurrent pharyngitis and/or sore throat is also found frequently. Vegetations are found by echocardiography in most patients (95%). Preexisting valvular disease is found in only 15% of patients. Valve replacement is required in 50% of patients, and 25% of patients die despite active antibiotic therapy 292.
Other Neisseria spp.
Endocarditis can also be caused by Neisseria elongata subsp. nitroreducens, N. elongata subsp. elongata, N. sicca, or N. subflava. In the literature, 27 cases of N. elongata subsp. nitroreducens have been reported 62, 185, 300. Endocarditis occurs in young middle-aged adults with a mean age of 39 years, preexisting valve conditions that predispose to infection include prosthetic valves (80% of patients) and previous dental procedures (20% of patients). Infection results in acute febrile endocarditis with very large vegetations and a destructive process that often causes severe cardiac and systemic complications including acute congestive heart failure, myocardial abscess (30% of patients), and renal failure (30% of patients); surgical valve replacement is required in half of the patients. The organisms are sensitive to a wide array of antibiotics including aminopenicillins and aminoglycosides, and despite the active destructive process, no deaths have been reported when appropriate medical and surgical therapy was administered. N. elongata subsp. elongata differs from N. elongata subsp. nitroreducens by its morphology (rod/coccobacillus) and by its lack of nitrate reduction. It is unclear if these two subspecies have different pathogenicity. A single case of endocarditis due to N. elongata subsp. elongata has been published 199. Fourteen cases of endocarditis due to N. sicca have been reported (94, 118, 240; Lopez-Velez et al., Letter), predominantly involving young patients; all but one cases were heralded by embolic manifestations (Lopez-Velez et al., Letter). This infection leads to an acute disease with high-grade fever. Intravenous drug use was documented in 3 of 14 patients. Preexisting heart disease was observed in four patients with rheumatic valvular damage, one with an aortic bicuspid valve, and one with a prosthetic valve. Before 1941, the fatality rate of N. sicca endocarditis was high, but no deaths have been noted in recent reports (Lopez-Velez et al., Letter). Twelve cases of endocarditis cause by Neisseria subflava have been reported; all but one patient presented with fever 8. This infection occured in middle-aged immunocompromised adults such as patients with AIDS, chronic alcoholism, asplenia, diabetes mellitus, and intravenous drug use. Sources of infection included dental abscesses in 3 patients, and the prognosis was severe since 5 of the 12 patients died (Lopez-Velez et al., Letter). Appropriate antibiotic therapy and valve replacements have improved the recovery, and no deaths have been reported since 1981.
Gram-Positive Bacilli
The gram-positive bacilli that cause endocarditis are listed in Table 4 and discussed below.
Listeria monocytogenes.
Listeria monocytogenes is a small gram-positive bacteria that is widely distributed in nature. A recent review of the world literature found 58 cases of endocarditis due to L. monocytogenes 260. Clinically, it appears as a subacute disease in which the most remarkable signs and symptoms are fever, weakness, dyspnea, and cardiac murmur 93. Acute congestive heart failure is found in 50% of patients. An underlying immunocompromised situation, such as cancer, lymphoma, prolonged corticosteroid therapy, kidney transplantation, chronic hemodialysis, HIV infection, diabetes mellitus, chronic alcoholism, or pregnancy, is recorded in most patients (38, 93, 260; L. M. Baddour, Letter, Rev. Infect. Dis. 11:669, 1989). A previous valvular disease, including rheumatic heart disease, prosthetic valve endocarditis, and mitral valve prolapse, is found in 50% of patients. Etiologic diagnosis is confirmed by blood cultures, which are always reported to be positive. Treatment includes ampicillin and aminoglycoside 93.
Lactobacillus spp.
Lactobacillus spp. are large, gram-positive, aerobic bacteria that are ubiquitous and generally nonpathogenic inhabitants of the oral cavity, gastrointestinal tract, and female genital tract. Although bacteremia without valvular involvement is frequently reported, only 30 cases of Lactobacillus endocarditis have been reported in the literature (127, 270; S. Antony, S. Dummer, and C. Stratton, Letter, Clin. Infect. Dis. 26:1483–1484, 1998). Common characteristics of Lactobacillus endocarditis are a high frequency of systemic emboli (42%) and dental manipulations (75%) as portals of entry. Underlying heart disease is reported in 83% of patients including 20% with valvular prostheses, 20% with rheumatic heart disease, and 30% with congenital heart disease such as tetralogy of Fallot, transposition of great vessels, or tricuspid atresia 270. Cultivation of Lactobacillus from blood is easy, but classical identification is obtained in only half of the isolates and molecular analysis of the 16S rRNA gene is useful for complete identification 209. The recommended treatment is high-dose penicillin and aminoglycoside. Relapse occurs in 39% of inadequately treated patients, and death occurs in 25%.
Nocardia spp.
To our knowledge, only three cases of endocarditis due to the gram-positive Nocardia spp. have been reported in the literature, all in patients with prosthetic valves 74, 76, 289. A blood culture was positive in one patient 74, and the organism was isolated from a surgically excised valve in the other two. Two of the three patients died, and the survivor was treated with intravenous imipenem and amikacin and valve replacement surgery, followed by co-trimoxazole 74. Care must be taken that the filamentous appearance of this microorganism at Gram staining does not lead to misidentification and a diagnosis of fungal endocarditis.
Erysipelothrix rhusiopathiae.
Erysipelothrix rhusiopathiae is a gram-positive bacterium that is easily decolorized by alcohol using the Gram stain protocol. These organisms are ubiquitous in nature and are found wherever nitrogenous substances decompose. They can be isolated from a variety of animals worldwide. The bacterium may be a commensal in swine. It is thought that the excretion of organisms by infected and colonized animals leads to contamination of the environment and subsequent acquisition by humans. E. rhusiopathiae infection in humans occurs mainly among those who have direct contact with animals or with organic matter in which the organism in commonly found—swine, fish, poultry, clams, geese, crabs, and fertilizer. The literature reports 44 cases of endocarditis (104, 121, 230; M. R. Bibler, Letter, Rev. Infect. Dis. 10:1062–1063 1988; W. R. Grandsen and S. J. Eykyn, Letter, Rev. Infect. Dis. 10:1228, 1988). In 89% of cases, an occupational exposure is found, including butchers (34%), fishermen (14%), homemakers (14%), farmers (11%), and veterinarians (6%) 104. Sources of contamination include pork (42%) and fish or shellfish (22%). Prior heart disease is found in 40% of patients, alcohol abuse is found in 33%, and other sources of immunocompromise are rarely reported. E. rhusiopathiae endocarditis is associated with the characteristic skin lesions (erysipeloid) at the site of inoculation in 40% of patients 121. Etiologic diagnosis is made on the basis of blood cultures, which are always positive. Overall mortality is 38%, and valve replacement is necessary in one-third of patients 230. Penicillin G at 12 × 106 U/day is the drug of choice. The duration of therapy is recommended to be 4 to 6 weeks. In penicillin-allergic patients, cephalosporins are the most appropriate alternatives since both clindamycin and erythromycin are only bacteriostatic 230.
Clostridium spp.
Clostridia are large, sporulated anaerobes. Most species stain gram positive during early stages of growth. However, some species, such as Clostridium ramosun and Clostridium clostridioforme, almost always stain gram negative. Endocarditis caused by anaerobes is uncommon. Despite claims in the older literature that these are responsible for 2 to 10% of all cases of endocarditis, the largest published series failed to report any cases. Anaerobic and microaerophilic streptococci and Bacteroides spp. are by far the most commonly isolated anaerobes (M. Montejo, G. Ruiz-Irastorza, K. Aguirrebengoa, E. Amutio, J. L. Hernandez, and C. Aguirre, Letter, Clin. Infect. Dis. 20:1431, 1995, R. Moyano, J. M. Gomez-Mateos, F. Lozano de Leon, C. Florez, C. Jimenez-Ocana, and F. Gamboa, Letter, Clin. Infect. Dis. 18:837, 1994). Among all anaerobes, endocarditis due to Clostridia spp. is exceedingly rare. A total of 21 cases of clostridial endocarditis have been reported in the literature, and the majority were due to Clostridium perfringens 7, 50, 144. The likely portals of entry include the oropharynx, skin, gastrointestinal tract, and genitourinary tract. Intravenous drug use was reported in two patients (144; Moyano et al., Letter). It is interesting that a study of street heroin and injection paraphernalia in Washington, D.C., revealed that bacterial contamination was common and that C. perfringens was frequently cultured 144. Other reported underlying conditions include carcinoma of the colon or cervix. Fever is reported frequently, and embolic manifestations are observed in 50% of patients. The most characteristic clinical sign is the occurrence of “clostridial sepsis,” which includes shock, jaundice, and intravascular hemolysis; although reported in only one-third of patients, these symptoms are very suggestive of the etiology. Preexisting valve disease, including prosthetic valves and rheumatic heart disease, is reported in 50% of patients. Anaerobic endocarditis is associated with culture-negative endocarditis. There is one report in which the diagnosis was made by visualization of gram-positive bacilli on a blood smear 7. Penicillin is the treatment of choice in clostridial endocarditis, but metronidazole should be considered in patients with prolonged bacteremia 144. The prognosis is poor, and 47% of patients die despite antibiotic treatment 50.
Nontoxigenic Corynebacterium diphtheriae.
Few laboratories perform tests for identification of the gram-positive Corynebacterium diphtheriae. A total of 67 cases of nontoxigenic C. diphtheriae endocarditis have been reported in the literature, and half were reported before 1950 (197, 278; O. Lortholary, A. Buu-Hoi, L. Gutmann, and J. Acar, Letter, Clin. Infect. Dis. 17:1072–1074, 1993). Sporadic cases may be observed, but most occur as small outbreaks or in clusters 278. Since the source of the organism is likely to be the upper respiratory tract or skin, a history of pharyngitis or intravenous drug use is frequent. Underlying heart diseases, including valve damage and prostheses, rheumatic heart disease, and congenital heart defects, have been reported. The recommended treatment is a combination of penicillin and aminoglycoside (Lortholary et al., Letter).
Gram-Positive Cocci
The gram-positive cocci that cause endocarditis are listed in Table 4 and discussed below.
Abiotrophia spp.
Endocarditis due to streptococcal L-forms that required metabolic products of other bacteria for growth were first reported in 1961; these organisms are now known as Abiotrophia defectiva and A. adjacens 90. Numerous synonyms were given to these fastidious organisms, such as satelliting Streptococcus, thiol-requiring Streptococcus, vitamin B6-dependant Streptococcus, pyridoxal-dependant Streptococcus, symbiotic Streptococcus, and nutritionally variant streptococci, before they were classified as a new genus, Abiotrophia 141 (Fig. 8).
FIG. 8.
Phylogeny of Abiotrophia spp. (formerly known as nutritionally deficient streptococci) based on analysis of the 16S rRNA sequence by the neighbor-joining method.
(i) Epidemiology. More than 100 cases of Abiotrophia endocarditis have been reported in the literature 21, 95, 235. Abiotrophia spp. cause 5 to 6% of all cases of streptococcal endocarditis 235. In our experience, 4.3% of cases of streptococcal endocarditis were caused by Abiotrophia spp. Because Abiotrophia spp. are fastidious organisms, it is likely that most cases are misdiagnosed as culture-negative endocarditis; therefore, their role in endocarditis may be underestimated. Preexisting heart disease is found in 90% of patients, in whom a known cardiac murmur is the most common finding, prosthetic heart valves being involved in only 10% of patients 267.
(ii) Signs and symptoms. A. adjacens and A. defectiva are part of the normal oral, genitourinary, and intestinal floras. Endocarditis usually occurs as a result of bacteremia in patients with an underlying valve injury. In most cases, Abiotrophia endocarditis is characterized by a slow and indolent course. With progression of the disease, complications such as septic arthritis are observed 21. The classical peripheral manifestations of endocarditis, including digital clubbing, petechiae, and Osler nodes, are not frequently observed, but embolization occurs in one-third of patients 267. Valve damage affects the aortic and mitral valves with similar frequency (13 and 11%, respectively). Congestive heart failure may be the first manifestation in some late-recognized cases.
(iii) Diagnosis. Following improvement of the culture media to include cysteine, A. adjacens and A. defectiva can be detected in routine blood cultures in 2 or 3 days 214. Fresh human blood enhances the recovery of these bacteria, probably by supplying nutrients required for growth. In contrast, subcultures usually require supplementation of blood agar with pyridoxal hydrochloride (10 to 100 mg/liter) 90 or L-cysteine (100 mg/liter) 214 under an aerobic or anaerobic atmosphere. Alternatively, a coagulase-positive Staphylococcus strain can be used as “helper” to induce satellite growth (Fig. 2) 21. Tiny alpha-hemolytic or nonhemolytic colonies appear after 18 h either alone or as satelliting colonies around a helper strain. Growth can also be obtained in liquid broth supplemented with pyridoxal hydrochloride or cysteine. Microscopic examination reveals both morphological pleiomorphism and variable Gram staining. Chains are formed by cocci, coccobacilli, rod-shaped bacteria, and globular (bulbous) bacteria. Current methods of identification rely on enzymatic and physiological properties, using commercially available identification systems such as Rapid ID 32 STREPT (BioMérieux, Marcy l'Etoile, France). Abiotrophia spp. are differentiated from viridans streptococci by production of pyrrolidonyl arylamidase.
(iv) Echocardiography and pathology. Two-dimensional echocardiography may be helpful for the diagnosis of Abiotrophia endocarditis. In a series reported by Steckelberg et al., 64% of patients had detectable vegetations by this method 263. Histologic examination of heart valves from humans and experimentally infected rabbits shows that bacteria are morphologically altered within the vegetation 21.
(v) Prognosis and treatment. Morbidity and mortality exceed those of the other forms of viridans streptococcal and enterococcal endocarditis 21. Therapy results in a bacteriological failure in 41% of patients in spite of the in vitro bactericidal effects of antibiotics. About 27% of patients require prosthetic valve replacement, and 17 to 20% of patients die due to uncontrolled congestive heart failure or major systemic emboli. More than 30% of strains of Abiotrophia are resistant to 0.12 mg of penicillin per liter 95. This in vitro resistance to penicillin is likely to be related to the abnormalities in division observed by electron microscopy 21. Using a rabbit model of endocarditis, the combination of penicillin and gentamicin therapy was more effective than penicillin alone 120. Interestingly, although vancomycin is not bactericidal in vitro, vancomycin alone is as effective as penicillin and gentamicin in vivo in the rabbit model 22. This observation has been confirmed in patients who have shown a poor response to penicillin-aminoglycoside combination therapy 267.
Gemella spp.
Gemella morbillorum and Gemella haemolysans are gram-positive cocci that are commensal organisms of the mouth, gastrointestinal tract, and genitourinary tract of humans and other warm-blood animals. These organisms are distantly related to streptococci and enterococci (Fig. 8). Gemella spp. are easily decolorized during Gram staining and usually appear as gram-negative cocci; as a result, organisms now classified as Gemella spp. were first described as Neisseria spp. The genus is probably a more important cause of clinical disease than is presently recognized, because misidentification as viridans group streptococci is frequent or isolates are not fully identified or are uncharacterized.
(i) Epidemiology. Among cases of Gemella infections, endocarditis is the most prevalent clinical presentation. A total of 27 cases of Gemella endocarditis have been reported in the literature, 14 due to G. haemolysans and 13 due to G. morbillorum (2, 34, 39, 48, 119, 140, 142, 152, 154, 162, 173, 180, 196, 275; E. Bell and A. C. McCartney, Letter, J. Infect. 25:110–112, 1992; O. Devuyst, P. Hainaut, J. Gigi, and G. Wauters, Letter, Acta. Clin. Belg. 48:52–53, 1993; A. Fresard, V. P. Michel, X. Rueda, G. Aubert, G. Dorche, and F. Lucht, Letter, Clin. Infect. Dis. 16:586–587, 1993; P. Morea, M. Toni, M. Bressan, and P. Stritoni, Letter, Infection 19:446, 1991; L. Samuel, P. Bloomfield, and P. Ross, Letter, Postgrad. Med. J. 71:188, 1995). In a study of 52 cases of “streptococcal” endocarditis, Gemella spp. represented 6% of misclassified viridans group streptococci and 5% of all isolates 69. In our hospital center, Gemella spp. were isolated in 2 of 70 cases of endocarditis diagnosed over a 3-year period 152. Endocarditis due to Gemella spp. is associated with poor dental hygiene and previous damaged cardiac valves. A review of the literature showed that previous valvular injury was found in 12 of 27 patients, of whom 2 had prosthetic valves (Morea et al., Letter; Samuel et al., Letter). Valve involvement included the mitral valve in eight patients, the aortic valve in six patients, and the tricuspid valve in two patients, one of whom was a known intravenous drug user and another of whom was known to be a chronic alcoholic with portal hypertension (196; Bell and McCartney, Letter). Poor dental status, dental manipulation, or dental surgery was reported in 11 of 27 patients, of whom 5 did not have any previously known valvular damage. Four cases were reported in patients who had undergone colorectal surgery 2, 119, 162, 180.
(ii) Signs and symptoms. A low-grade intermittent fever was reported in all patients, and a new or recently modified cardiac murmur was reported in two-thirds of patients. Embolic manifestations were observed, with 6 of 27 patients reported to have kidney abscesses 152, spondylodiscitis 39, pneumonia 2, 196, arthritis 204, or cerebral mycotic aneurysm 34. Echocardiography showed vegetations in two-thirds of patients or reports of valve abnormalities or dysfunction.
(iii) Diagnosis. The most frequent diagnostic situation was atypical endocarditis, which yielded “gram-negative” streptococci, bizarre viridans group streptococci, or an unidentified small fastidious gram-negative pathogen 152. G. haemolysans was first described as Neisseria haemolysans, but following the demonstration of biochemical differences from other Neisseria spp., it was transferred into the new Gemella genus. The 16S rRNA gene sequence of Streptococcus morbillorum was found to closely resemble that of G. haemolysans, and it was proposed that S. morbillorum be transferred to the genus Gemella as G. morbillorum comb. nov. (Fig. 8) 154.
Phenotypic characterization is still the standard approach to the identification of these pathogens; the Gram stain is the most important clue. A mistake at this step can lead to inappropriate testing, misidentification, or nonidentification of the organism. Therefore, it is important that great efforts be made to ensure correct interpretation of the Gram stains. To avoid confusing a gram-negative coccus with a gram-positive coccus, we add vancomycin and colimycin to our standard antibiotic susceptibility test medium 152; gram-positive bacteria are susceptible to vancomycin, whereas most gram-negative bacteria are susceptible to colimycin. However, since a vancomycin-resistant strain of G. haemolysans has been reported, the results of this test must be carefully interpreted 262. In some cases, phenotypic identification systems are unable to accurately identify all strains of these species, even though manufacturers have significantly improved their database over recent years. The use of DNA amplification and sequence analysis of the 16S rRNA gene has been reported to be an alternative method for definite identification (Fig. 8) 152.
(iv) Treatment and outcomes. In vitro antimicrobial susceptibility testing of Gemella spp. demonstrates that all isolates are highly sensitive to penicillin G and ampicillin, have low-level resistance to aminoglycosides, and show synergy between penicillin or vancomycin and gentamicin or streptomycin 33, 162. The authors suggest that penicillin and gentamicin be recommended for the treatment of Gemella endocarditis. The outcomes of Gemella endocarditis patients are usually good, since no fatalities occurred among the 27 reported patients and only 2 patients required surgery for valve replacement (162; Morea et al., Letter).
Stomatococcus mucilaginosus.
In the English literature, only five well-documented cases of Stomatococcus mucilaginosus endocarditis have been reported, three cases in intravenous drug abusers and two cases in patients with preexisting valvular heart disease 216, 232. Stomatococcus mucilaginosus, previously referred to as Staphyloccocus salivarius and Microccocus mucilaginosus, is a gram-positive, nonmotile, non-spore-forming coccus, considered to be part of the normal flora of the mouth and the upper respiratory tract 232. It is usually differentiated from the genera Micrococcus and Staphylococcus by an absent or weakly positive catalase reaction and by failure to grow in media containing 5% NaCl 216. The organisms are usually susceptible to penicillin and to cephalosporin, but a resistant strain has been found in a patient with endocarditis 216.
CONCLUSION AND PERSPECTIVES
When faced with the diagnosis of IE, the physician can use diagnostic scores such as the Duke criteria. However, before any conclusion about the diagnosis of IE can be made, the physician must be certain that all clinical and laboratory tests have been performed, including serologic testing for C. burnetii and Bartonella spp. Moreover, transesophageal echocardiography has been proven essential for diagnosis and should be performed by a well-trained physician. When the clinical diagnosis of infective endocarditis is strongly indicated and blood culture and serologic test results are negative for both C. burnetii and Bartonella spp., pathologic examination of the removed prosthesis or native valve using hematoxylin and eosin stains confirms the diagnosis of IE when an inflammatory response is observed. Special staining by the Gram, Giemsa, Gimenez, PAS, Warthin-Starry, and Grocott methods may guide the use of new diagnostic tools such as PCR and tissue culture for isolation and identification of the causative agent. By using this strategy, unusual clinical presentations of disease, such as the absence of fever and echocardiographic vegetations have been found in patients with negative blood cultures and Whipple's disease bacillus endocarditis. Broader investigation and application of such novel approaches should also lead to more comprehensive patient evaluation and the discovery of new etiologic agents of IE.
ACKNOWLEDGMENT
We thank J. S. Dumler (Johns Hopkins Medical Institution, Baltimore, Md.) for a review of English usage.
REFERENCES
- 1.Reference deleted.
- 2.Abboud R, Friart A. Deux cas d'endocardite tricuspidienne isolée après intervention colique. Acta Clin Belg. 1995;50:242–245. doi: 10.1080/17843286.1995.11718454. [DOI] [PubMed] [Google Scholar]
- 3.Ali A S, Trivedi V, Lesch M. Culture-negative endocarditis—a historical review and 1990s update. Prog Cardiovasc Dis. 1994;37:149–160. doi: 10.1016/s0033-0620(05)80040-4. [DOI] [PubMed] [Google Scholar]
- 4.al Kasab S, al Fagih M, al Rasheed A, Khan B, Bitar I, Shahed M, Sawyer W. Management of Brucella endocarditis with aortic root abscess. Chest. 1990;98:1532–1534. doi: 10.1378/chest.98.6.1532. [DOI] [PubMed] [Google Scholar]
- 5.al-Mudallal D S, Mousa A R, Marafie A A. Apyrexic Brucella melitensis aortic valve endocarditis. Trop Geogr Med. 1989;41:372–376. [PubMed] [Google Scholar]
- 6.Alvarez-Elcoro S, Mateos-Mora M, Zajarias A. Mycobacterium fortuitum endocarditis after mitral valve replacement with a bovine prosthesis. South Med J. 1985;78:865–866. doi: 10.1097/00007611-198507000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez-Elcoro S, Sifuentes-Osorio J. Clostridium perfringens bacteremia in prosthetic valve endocarditis. Diagnosis by peripheral blood smear. Arch Intern Med. 1984;144:849–850. [PubMed] [Google Scholar]
- 8.Amsel B J, Moulijn A C. Nonfebrile mitral valve endocarditis due to Neisseria subflava. Chest. 1996;109:280–282. doi: 10.1378/chest.109.1.280. [DOI] [PubMed] [Google Scholar]
- 9.Anderson M J, Janoff E N. Klebsiella endocarditis: report of two cases and review. Clin Infect Dis. 1998;26:468–474. doi: 10.1086/516330. [DOI] [PubMed] [Google Scholar]
- 10.Anonymous. Case records of the Massachussetts General Hospital. N Engl J Med. 1997;336:205–210. [Google Scholar]
- 11.Babinchak T J. Oral ciprofloxacin therapy for prosthetic valve endocarditis due to Actinobacillus actinomycetemcomitans. Clin Infect Dis. 1995;21:1517–1518. doi: 10.1093/clinids/21.6.1517. [DOI] [PubMed] [Google Scholar]
- 12.Babudieri B. Q fever: a zoonosis. Adv Vet Sci. 1959;5:82–182. [Google Scholar]
- 13.Reference deleted.
- 14.Reference deleted.
- 15.Berbari E F, Cockerill F R, Steckelberg J M. Infective endocarditis due to unusual or fastidious microorganisms. Mayo Clin Proc. 1997;72:532–542. doi: 10.4065/72.6.532. [DOI] [PubMed] [Google Scholar]
- 16.Bertrand A, Lepeu G, Jonquet O, Janbon F, Jourdan J. L'endocardite brucellienne. Aspects cliniques et immunologiques. Semin Hop. 1982;58:275–279. [PubMed] [Google Scholar]
- 17.Reference deleted.
- 18.Birkhead J S, Apostolov K. Endocarditis caused by a psittacosis agent. Br Heart J. 1974;36:728–731. doi: 10.1136/hrt.36.7.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanche C, Freimark D, Valenza M, Czer L S, Trento A. Heart transplantation for Q fever endocarditis. Ann Thorac Surg. 1994;58:1768–1769. doi: 10.1016/0003-4975(94)91688-8. [DOI] [PubMed] [Google Scholar]
- 20.Block P J, Yoran C, Fox A C, Kaltman A J. Actinobacillus actinomycetemcomitans endocarditis: report of a case and review of the literature. Am J Med Sci. 1973;266:387–392. doi: 10.1097/00000441-197311000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Bouvet A. Human endocarditis due to nutritionally variant streptococci: Streptococcus adjacens and Streptococcus defectivus. Eur Heart J. 1995;16(Suppl. B):24–27. doi: 10.1093/eurheartj/16.suppl_b.24. [DOI] [PubMed] [Google Scholar]
- 22.Bouvet A, Cremieux A C, Contrepois A, Vallois J M, Lamesch C, Carbon C. Comparison of penicillin and vancomycin, individually and in combination with gentamicin and amikacin, in the treatment of experimental endocarditis induced by nutritionally variant streptococci. Antimicrob Agents Chemother. 1985;28:607–611. doi: 10.1128/aac.28.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reference deleted.
- 24.Brearley B F, Hutchinson D N. Endocarditis associated with Chlamydia trachomatis infection. Br Heart J. 1981;46:220–221. doi: 10.1136/hrt.46.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brouqui P, Dumler J S, Raoult D. Immunohistologic demonstration of Coxiella burnetii in the valves of patients with Q fever endocarditis. Am J Med. 1994;97:451–458. doi: 10.1016/0002-9343(94)90325-5. [DOI] [PubMed] [Google Scholar]
- 26.Brouqui P, Houpikian P, Dupont H T, Toubiana P, Obadia Y, Lafay V, Raoult D. Survey of the seroprevalence of Bartonella quintana in homeless people. Clin Infect Dis. 1996;23:756–759. doi: 10.1093/clinids/23.4.756. [DOI] [PubMed] [Google Scholar]
- 27.Brouqui P, Lascola B, Roux V, Raoult D. Chronic Bartonella quintana bacteremia in homeless patients. N Engl J Med. 1999;340:184–189. doi: 10.1056/NEJM199901213400303. [DOI] [PubMed] [Google Scholar]
- 28.Brouqui P, Raoult D, Gabriel B. Chronic Coxiella burnetii infection mimicking malignant hematologic disease. Am J Hematol. 1992;39:309. doi: 10.1002/ajh.2830390415. [DOI] [PubMed] [Google Scholar]
- 29.Brouqui P, Tissot-Dupont H, Drancourt M, Berland Y, Etienne J, Leport C, Goldstein F, Massip P, Micoud M, Bertrand A, Raoult D. Chronic Q fever: ninety-two cases from France, including 27 without endocarditis. Arch Intern Med. 1993;153:642–648. doi: 10.1001/archinte.153.5.642. [DOI] [PubMed] [Google Scholar]
- 30.Bruneel F, D'estanque J, Fournier P E, Arlet G, Thuong M, Wolff M, Bedos J P, Lariven S, Regnier B. Isolated right-sided Bartonella quintana endocarditis in an immunocompetent adult. Scand J Infect Dis. 1998;30:424–425. doi: 10.1080/00365549850160783. [DOI] [PubMed] [Google Scholar]
- 31.Buchanan T M, Faber L C. 2-Mercaptoethanol Brucella agglutination test: usefuness for predicting recovery from brucellosis. J Clin Microbiol. 1980;11:691–693. doi: 10.1128/jcm.11.6.691-693.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reference deleted.
- 33.Buu-Hoi A, Sapoetra A, Branger C, Acar J F. Antimicrobial susceptibility of Gemella haemolysans isolated from patients with subacute endocarditis. Eur J Clin Microbiol. 1982;1:102–106. doi: 10.1007/BF02014200. [DOI] [PubMed] [Google Scholar]
- 34.Calopa M, Rubio F, Aguilar M, Peres J. Giant basilar aneurysm in the course of subacute bacterial endocarditis. Stroke. 1990;21:1625–1627. doi: 10.1161/01.str.21.11.1625. [DOI] [PubMed] [Google Scholar]
- 35.Campos J M. Haemophilus. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: ASM Press; 1999. pp. 604–611. [Google Scholar]
- 36.Cannady P B, Jr, Sanford J P. Negative blood cultures in infective endocarditis: a review. South Med J. 1976;69:1420–1424. doi: 10.1097/00007611-197611000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Capo C, Zaffran Y, Zugun F, Houpikian P, Raoult D, Mege J L. Production of interleukin-10 and transforming growth factor beta by peripheral blood mononuclear cells in Q fever endocarditis. Infect Immun. 1996;64:4143–4147. doi: 10.1128/iai.64.10.4143-4147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carvajal A, Frederiksen W. Fatal endocarditis due to Listeria monocytogenes. Rev Infect Dis. 1988;10:616–623. doi: 10.1093/clinids/10.3.616. [DOI] [PubMed] [Google Scholar]
- 39.Chatelain R, Croize J, Rouge P, Massot C, Dabernat H, Auvergnat J C, Buu-Hoi A, Stahl J P, Bimet F. Isolement de Gemella hemolysans dans trois cas d'endocardites bacteriennes. Med Mal Infect. 1982;1:25–30. [Google Scholar]
- 40.Chavanet P, Pechinot A, Lobjoy B, Portier H. Valeur comparée des réactions de fixation du complément et d'immuno-fluorescence indirecte pour le dépistage de la fièvre Q. Pathol Biol. 1984;32:53–55. [PubMed] [Google Scholar]
- 41.Chen Y C, Chang S C, Luh K T, Hsieh W C. Actinobacillus actinomycetemcomitans endocarditis: a report of four cases and review of the literature. Q J Med. 1991;81:871–878. [PubMed] [Google Scholar]
- 42.Choo P W, Gantz N M, Anderson C, Maguire J H. Salmonella prosthetic valve endocarditis. Diagn Microbiol Infect Dis. 1992;15:273–276. doi: 10.1016/0732-8893(92)90124-c. [DOI] [PubMed] [Google Scholar]
- 43.Chow W H, Leung W H, Tai Y T, Lee W T, Cheung K L. Echocardiographic diagnosis of an aortic root abscess after Mycobacterium fortuitum prosthetic valve endocarditis. Clin Cardiol. 1991;14:273–275. doi: 10.1002/clc.4960140319. [DOI] [PubMed] [Google Scholar]
- 44.Cisneros J M, Pachon J, Cuello J A, Martinez A. Brucella endocarditis cured by medical treatment. J Infect Dis. 1989;160:907. doi: 10.1093/infdis/160.5.907. [DOI] [PubMed] [Google Scholar]
- 45.Cohen J I, Sloss L J, Kundsin R, Golightly L. Prosthetic valve endocarditis caused by Mycoplasma hominis. Am J Med. 1989;86:819–821. doi: 10.1016/0002-9343(89)90479-8. [DOI] [PubMed] [Google Scholar]
- 46.Coll-Vinent B, Suris X, Lopez-Soto A, Miro J M, Coca A. Haemophilus paraphrophilus endocarditis: case report and review. Clin Infect Dis. 1995;20:1381–1383. doi: 10.1093/clinids/20.5.1381. [DOI] [PubMed] [Google Scholar]
- 47.Cope A P, Heber M, Wilkins E G. Valvular tuberculous endocarditis: a case report and review of the literature. J Infect. 1990;21:293–296. doi: 10.1016/0163-4453(90)94029-y. [DOI] [PubMed] [Google Scholar]
- 48.Coto H, Berk S L. Endocarditis caused by Streptococcus morbillorum. Am J Med Sci. 1984;287:54–58. doi: 10.1097/00000441-198405000-00019. [DOI] [PubMed] [Google Scholar]
- 49.Coyle P V, Thomson J, Adgey A A J, Rutter D A, Fay A, McNeill T A, Connolly J H. Change in circulating immune complex concentrations and antibody titers during treatment of Q fever endocarditis. J Clin Pathol. 1985;38:743–746. doi: 10.1136/jcp.38.7.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cutrona A F, Watanakunakorn C, Schaub C R, Jagetia A. Clostridium innocuum endocarditis. Clin Infect Dis. 1995;21:1306–1307. doi: 10.1093/clinids/21.5.1306. [DOI] [PubMed] [Google Scholar]
- 51.Daly J S, Worthington M G, Brenner D J, Moss C W, Hollis D G, Weyant R S, Steigerwalt A G, Weaver R E, Daneshvar M I, O'Connor S P. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J Clin Microbiol. 1993;31:872–881. doi: 10.1128/jcm.31.4.872-881.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Darras-Joly C, Lortholary O, Mainardi J L, Etienne J, Guillevin L, Acar J. Haemophilus endocarditis: report of 42 cases in adults and review. Haemophilus Endocarditis Study Group. Clin Infect Dis. 1997;24:1087–1094. doi: 10.1086/513624. [DOI] [PubMed] [Google Scholar]
- 53.Das I, DeGiovanni J V, Gray J. Endocarditis caused by Haemophilus parainfluenzae identified by 16S ribosomal RNA sequencing. J Clin Pathol. 1997;50:72–74. doi: 10.1136/jcp.50.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Das M, Badley A D, Cockerill F R, Steckelberg J M, Wilson W R. Infective endocarditis caused by HACEK microorganisms. Annu Rev Med. 1997;48:25–33. doi: 10.1146/annurev.med.48.1.25. [DOI] [PubMed] [Google Scholar]
- 55.Davies S, Spencer R C. Effect of blood culture media on the in vitro recovery of Mycoplasma hominis. J Infect. 1988;17:215–220. doi: 10.1016/s0163-4453(88)96474-2. [DOI] [PubMed] [Google Scholar]
- 56.Decker M D, Graham B S, Hunter E B, Liebowitz S M. Endocarditis and infections of intravascular devices due to Eikenella corrodens. Am J Med Sci. 1986;292:209–212. doi: 10.1097/00000441-198610000-00005. [DOI] [PubMed] [Google Scholar]
- 57.Delaney J C. Q fever endocarditis and chronic liver involvement. Practitioner. 1975;214:243–245. [PubMed] [Google Scholar]
- 58.Delvecchio G, Fracassetti O, Lorenzi N. Brucella endocarditis. Int J Cardiol. 1991;33:328–329. doi: 10.1016/0167-5273(91)90366-w. [DOI] [PubMed] [Google Scholar]
- 59.Reference deleted.
- 60.Dick D C, McGregor C G, Mitchell K G, Sommerville R G, Wheatley D J. Endocarditis as a manifestation of Chlamydia B infection (psittacosis) Br Heart J. 1977;39:914–916. doi: 10.1136/hrt.39.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dimmitt S B, Pearman J W, Woollard K V. Chlamydial endocarditis. Aust N Z J Med. 1985;15:340–342. doi: 10.1111/j.1445-5994.1985.tb04049.x. [DOI] [PubMed] [Google Scholar]
- 62.Dominguez E A, Smith T L. Endocarditis due to Neisseria elongata subspecies nitroreducens: case report and review. Clin Infect Dis. 1998;26:1471–1473. doi: 10.1086/517671. [DOI] [PubMed] [Google Scholar]
- 63.Drancourt M, Birtles R, Chaumentin G, Vandenesch F, Etienne J, Raoult D. New serotype of Bartonella henselae in endocarditis and cat-scratch disease. Lancet. 1996;347:441–443. doi: 10.1016/s0140-6736(96)90012-4. [DOI] [PubMed] [Google Scholar]
- 64.Drancourt M, Brouqui P, Raoult D. Afipia clevelandensis antibodies and cross-reactivity with Brucella spp. and Yersinia enterocolitica O:9. Clin Diagn Lab Immunol. 1997;4:748–752. doi: 10.1128/cdli.4.6.748-752.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Drancourt M, Mainardi J L, Brouqui P, Vandenesh F, Carta A, Lehnert F, Etienne J, Goldstein F, Acard J, Raoult D. Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N Engl J Med. 1995;332:419–423. doi: 10.1056/NEJM199502163320702. [DOI] [PubMed] [Google Scholar]
- 66.du Plessis J P, Govendrageloo K, Levin S E. Right-sided endocarditis due to Salmonella typhi. Pediatr Cardiol. 1997;18:443–444. doi: 10.1007/s002469900226. [DOI] [PubMed] [Google Scholar]
- 67.Reference deleted.
- 68.Dupuis G, Peter O, Mottiez M C, Vouilloz M. Séro-prévalence de la fièvre Q humaine en Suisse. Schweiz Med Wochenschr. 1986;116:494–498. [PubMed] [Google Scholar]
- 69.Durack D T, Kaplan E L, Bisno A L. Apparent failures of endocarditis prophylaxis. Analysis of 52 cases submitted to a national registry. JAMA. 1983;250:2318–2322. [PubMed] [Google Scholar]
- 70.Durack D T, Lukes A S, Bright D K. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96:200–209. doi: 10.1016/0002-9343(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 71.Ellis M E, Smith C C, Moffat M A. Chronic or fatal Q fever infection: a review of 16 patients seen in North East Scotland (1967–80) Q J Med. 1983;52:54–66. [PubMed] [Google Scholar]
- 72.Reference deleted.
- 73.Elster S K, Mattes L M, Meyers B R, Jurado R A. Hemophilus aphrophilus endocarditis: review of 23 cases. Am J Cardiol. 1975;35:72–79. doi: 10.1016/0002-9149(75)90561-5. [DOI] [PubMed] [Google Scholar]
- 74.Ertl G, Schaal K P, Kochsiek K. Nocardial endocarditis of an aortic valve prosthesis. Br Heart J. 1987;57:384–386. doi: 10.1136/hrt.57.4.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Etienne J, Ory D, Thouvenot D, Eb F, Raoult D, Loire R, Delahaye J P, Beaune J. Chlamydial endocarditis: a report on ten cases. Eur Heart J. 1992;13:1422–1426. doi: 10.1093/oxfordjournals.eurheartj.a060077. [DOI] [PubMed] [Google Scholar]
- 76.Falk R H, Dimock F R, Sharkey J. Prosthetic valve endocarditis resulting from Nocardia asteroides. Br Heart J. 1979;41:125–127. doi: 10.1136/hrt.41.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Farrugia D C, Eykyn S J, Smyth E G. Campylobacter fetus endocarditis: two case reports and review. Clin Infect Dis. 1994;18:443–446. doi: 10.1093/clinids/18.3.443. [DOI] [PubMed] [Google Scholar]
- 78.Fenollar F, Fournier P E, Legre R. Unusual case of Aeromonas sobria cellulitis associated with the use of leeches. Eur J Clin Microbiol Infect Dis. 1999;18:72–73. doi: 10.1007/s100960050232. [DOI] [PubMed] [Google Scholar]
- 79.Ferber B, Bruckheimer E, Schlesinger Y, Berger I, Glaser J, Olsha O, Branski D, Kerem E. Kingella kingae endocarditis in a child with hair-cartilage hypoplasia. Pediatr Cardiol. 1997;18:445–446. doi: 10.1007/s002469900227. [DOI] [PubMed] [Google Scholar]
- 80.Fernandez Guerrero M L, Ramos J M, Nunez A, de Gorgolas M. Focal infections due to non-typhi Salmonella in patients with AIDS: report of 10 cases and review. Clin Infect Dis. 1997;25:690–697. doi: 10.1086/513747. [DOI] [PubMed] [Google Scholar]
- 81.Fernandez Guerrero M L, Torres Perea R, Gomez Rodrigo J, Nunez Garcia A, Jusdado J J, Ramos Rincon J M. Infectious endocarditis due to non-typhi Salmonella in patients infected with human immunodeficiency virus: report of two cases and review. Clin Infect Dis. 1996;22:853–855. doi: 10.1093/clinids/22.5.853. [DOI] [PubMed] [Google Scholar]
- 82.Fernandez-Guerrero M L. Zoonotic endocarditis. Infect Dis Clin North Am. 1993;7:135–152. [PubMed] [Google Scholar]
- 83.Fernandez-Guerrero M L, Martinell J, Aguado J M, Ponte M C, Fraile J, de Rabago G. Prosthetic valve endocarditis caused by Brucella melitensis. A report of four cases successfully treated with tetracycline, streptomycin, and sulfamethoxazole and trimethoprim plus valve replacement. Arch Intern Med. 1987;147:1141–1143. [PubMed] [Google Scholar]
- 84.Fleming J L, Wiesner R H, Shorter R G. Whipple's disease: clinical, biochemical, and histopathologic features and assessment of treatment in 29 patients. Mayo Clin Proc. 1988;63:539–551. doi: 10.1016/s0025-6196(12)64884-8. [DOI] [PubMed] [Google Scholar]
- 85.Flugelman M Y, Galun E, Ben-Chetrit E, Caraco J, Rubinow A. Brucellosis in patients with heart disease: when should endocarditis be diagnosed? Cardiology. 1990;77:313–317. doi: 10.1159/000174614. [DOI] [PubMed] [Google Scholar]
- 86.Fournier P E, Casalta J P, Habib G, Messana T, Raoult D. Modification of the diagnostic criteria proposed by the Duke Endocarditis Service to permit improved diagnosis of Q fever endocarditis. Am J Med. 1996;100:629–633. doi: 10.1016/s0002-9343(96)00040-x. [DOI] [PubMed] [Google Scholar]
- 87.Fournier P E, Raoult D. Non-culture laboratory methods for the diagnosis of infectious endocarditis. Curr Infect Dis Rep. 1999;1:136–141. doi: 10.1007/s11908-996-0020-x. [DOI] [PubMed] [Google Scholar]
- 88.Fredericks D N, Relman D A. Sequence-based identification of microbial pathogens: a reconsideration of Koch's postulates. Clin Microbiol Rev. 1996;9:18–33. doi: 10.1128/cmr.9.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Freeman A P, Freeman Z S. Chlamydia endocarditis. Med J Aust. 1981;1:642. [PubMed] [Google Scholar]
- 90.Frenkel A, Hirsh W. Spontaneous development of L forms of Streptococci requiring secretions of other bacteria or sulfhydryl compounds for normal growth. Nature. 1961;191:728–730. doi: 10.1038/191728a0. [DOI] [PubMed] [Google Scholar]
- 91.Reference deleted.
- 92.Galil K, Thurer R, Glatter K, Barlam T. Disseminated Mycobacterium chelonae infection resulting in endocarditis. Clin Infect Dis. 1996;23:1322–1323. doi: 10.1093/clinids/23.6.1322. [DOI] [PubMed] [Google Scholar]
- 93.Gallagher P G, Watanakunakorn C. Listeria monocytogenes endocarditis: a review of the literature 1950–1986. Scand J Infect Dis. 1988;20:359–368. doi: 10.3109/00365548809032469. [DOI] [PubMed] [Google Scholar]
- 94.Gay R M, Sevier R E. Neisseria sicca endocarditis: report of a case and review of the literature. J Clin Microbiol. 1978;8:729–732. doi: 10.1128/jcm.8.6.729-732.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gephart J F, Washington J A. Antimicrobial susceptibilities of nutritionally variant streptococci. J Infect Dis. 1982;146:536–539. doi: 10.1093/infdis/146.4.536. [DOI] [PubMed] [Google Scholar]
- 96.Geraci J E, Anderson M, Karlson A G. Endocarditis due to a rapidly growing chromogenic Mycobacterium. Mayo Clin Proc. 1968;43:124–133. [PubMed] [Google Scholar]
- 97.Geraci J E, Greipp P R, Wilkowske C J, Wilson W R, Washington J A. Cardiobacterium hominis endocarditis. Four cases with clinical and laboratory observations. Mayo Clin Proc. 1978;53:49–53. [PubMed] [Google Scholar]
- 98.Geraci J E, Hermans P E, Washington J A. Eikenella corrodens endocarditis: report of cure in two cases. Mayo Clin Proc. 1974;49:950–953. [PubMed] [Google Scholar]
- 99.Geraci J E, Wilkowske C J, Wilson W R, Washington J A. Haemophilus endocarditis. Report of 14 patients. Mayo Clin Proc. 1977;52:209–215. [PubMed] [Google Scholar]
- 100.Geraci J E, Wilson W R, Washington J A. Infective endocarditis caused by Actinobacillus actinomycetemcomitans: report of four cases. Mayo Clin Proc. 1980;55:415–419. [PubMed] [Google Scholar]
- 101.Giamarellou H, Antoniadou A, Kanavos K, Papaioannou C, Kanatakis S, Papadaki K. Yersinia enterocolitica endocarditis: case report and literature review. Eur J Clin Microbiol Infect Dis. 1995;14:126–130. doi: 10.1007/BF02111871. [DOI] [PubMed] [Google Scholar]
- 102.Gill G V. Endocarditis caused by Salmonella enteritidis. Br Heart J. 1979;42:353–354. doi: 10.1136/hrt.42.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Goldenberger D, Kunzli A, Vogt P, Zbinden R, Altwegg M. Molecular diagnosis of bacterial endocarditis by broad-range PCR amplification and direct sequencing. J Clin Microbiol. 1997;35:2733–2739. doi: 10.1128/jcm.35.11.2733-2739.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gorby G L, Peacock J E., Jr Erysipelothrix rhusiopathiae endocarditis: microbiologic, epidemiologic, and clinical features of an occupational disease. Rev Infect Dis. 1988;10:317–325. doi: 10.1093/clinids/10.2.317. [DOI] [PubMed] [Google Scholar]
- 105.Goutzmanis J J, Gonis G, Gilbert G L. Kingella kingae infection in children: ten cases and a review of the literature. Pediatr Infect Dis J. 1991;10:677–683. [PubMed] [Google Scholar]
- 106.Grace C J, Levitz R E, Katz-Pollak H, Brettman L R. Actinobacillus actinomycetemcomitans prosthetic valve endocarditis. Rev Infect Dis. 1988;10:922–929. doi: 10.1093/clinids/10.5.922. [DOI] [PubMed] [Google Scholar]
- 107.Grand A, Laye J M, Etienne J, Pernot F, Durand de Gevigney G, Delahaye F, Touboul P, Froment A. Endocardite infectieuse a Actinobacillus actinomycetemcomitans. Huit nouvelles observations. Arch Mal Coeur Vaiss. 1994;87:1721–1729. [PubMed] [Google Scholar]
- 108.Reference deleted.
- 109.Greene J N, Sandin R L, Villanueva L, Sinnott J T. Haemophilus parainfluenzae endocarditis in a patient with mitral valve prolapse. Ann Clin Lab Sci. 1993;23:203–206. [PubMed] [Google Scholar]
- 110.Gubler J G, Kuster M, Dutly F, Bannwart F, Krause M, Vôgelin H P. Whipple endocarditis without overt gastrointestinal disease: report of four cases. Ann Intern Med. 1999;131:112–116. doi: 10.7326/0003-4819-131-2-199907200-00007. [DOI] [PubMed] [Google Scholar]
- 111.Habib G, Derumeaux G, Avierinos J F, Casalta J P, Jamal F, Volot F, Garcia M, Lefevre J, Biou F, Maximovitch-Rodaminoff A, Fournier P E, Ambrosi P, Velut J G, Cribier A, Harle J R, Weiller P J, Raoult D, Luccioni R. Value and limitations of the Duke criteria for the diagnosis of infective endocarditis. J Am Coll Cardiol. 1999;33:2023–2029. doi: 10.1016/s0735-1097(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 112.Hadfield T L, Warren R, Kass M, Brun E, Levy C. Endocarditis caused by Rochalimaea henseale. Hum Pathol. 1993;24:1140–1141. doi: 10.1016/0046-8177(93)90196-n. [DOI] [PubMed] [Google Scholar]
- 113.Hamed K A, Dormitzer P R, Su C K, Relman D A. Haemophilus parainfluenzae endocarditis: application of a molecular approach for identification of pathogenic bacterial species. Clin Infect Dis. 1994;19:677–683. doi: 10.1093/clinids/19.4.677. [DOI] [PubMed] [Google Scholar]
- 114.Hampton J R, Harrison M J. Sterile blood cultures in bacterial endocarditis. Q J Med. 1967;36:167–174. [PubMed] [Google Scholar]
- 115.Hansen L A, Salem A G, Edson R S. Eikenella corrodens: an unusual cause of endocarditis in a patient with silent mitral valve prolapse. S D J Med. 1989;42:5–8. [PubMed] [Google Scholar]
- 116.Hassan I J, Hayek L. Endocarditis caused by Kingella denitrificans. J Infect. 1993;27:291–295. doi: 10.1016/0163-4453(93)92213-g. [DOI] [PubMed] [Google Scholar]
- 117.Hedderwick S A, Bonilla H F, Barg N L, Arbeit R D, Kauffman C A. Mycobacterium avium complex endocarditis: spurious diagnosis resulting from laboratory cross contamination. Diagn Microbiol Infect Dis. 1997;27:147–150. doi: 10.1016/s0732-8893(97)00030-8. [DOI] [PubMed] [Google Scholar]
- 118.Heiddal S, Sverrisson J T, Yngvason F E, Cariglia N, Kristinsson K G. Native-valve endocarditis due to Neisseria sicca: case report and review. Clin Infect Dis. 1993;16:667–670. doi: 10.1093/clind/16.5.667. [DOI] [PubMed] [Google Scholar]
- 119.Helft G, Tabone X, Metzger J P, Vacheron A. Gemella haemolysans endocarditis with colonic carcinoma. Eur J Med. 1993;2:369–370. [PubMed] [Google Scholar]
- 120.Henry N K, Wilson W R, Roberts R B, Acar J F, Geraci J E. Antimicrobial therapy of experimental endocarditis caused by nutritionally variant viridans group streptococci. Antimicrob Agents Chemother. 1986;30:465–467. doi: 10.1128/aac.30.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hill D C, Ghassemian J N. Erysipelothrix rhusiopathiae endocarditis: clinical features of an occupational disease. South Med J. 1997;90:1147–1148. [PubMed] [Google Scholar]
- 122.Hoen B, Beguinot I, Rabaud C, Jaussand R, Selton-Suty C, May T, Canton P. The Duke criteria for diagnosing infective endocarditis are specific: analysis of 100 patients with acute fever or fever of unknown origin. Clin Infect Dis. 1996;23:298–302. doi: 10.1093/clinids/23.2.298. [DOI] [PubMed] [Google Scholar]
- 123.Hoen B, Selton-Suty C, Danchin N, Weber M, Villemot J P, Mathieu P, Floquet J, Canton P. Evaluation of the Duke criteria versus the Beth Israel criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 1995;21:905–909. doi: 10.1093/clinids/21.4.905. [DOI] [PubMed] [Google Scholar]
- 124.Hoen B, Selton-Suty C, Lacassin F, Etienne J, Briancon S, Leport C, Canton P. Infective endocarditis in patients with negative blood cultures: analysis of 88 cases from a one-year nationwide survey in France. Clin Infect Dis. 1995;20:501–506. doi: 10.1093/clinids/20.3.501. [DOI] [PubMed] [Google Scholar]
- 125.Hollerbach S, Holstege A, Muscholl M, Mohr V, Ruschoff J, Geissler A, Scholmerich J. Maskierter Verlauf eines Morbus Whipple mit Uveitis, Sepsis, Endokardbeteiligung und abdominellen Lymphomen—Fallbericht und Ubersicht. Z Gastroenterol. 1995;33:362–367. [PubMed] [Google Scholar]
- 126.Holmes A H, Greenough T C, Balady G J, Regnery R L, Anderson B E, Conor O'Keane J, Fonger J D, McCrone E L. Bartonella henselae endocarditis in an immunocompetent adult. Clin Infect Dis. 1995;21:1004–1007. doi: 10.1093/clinids/21.4.1004. [DOI] [PubMed] [Google Scholar]
- 127.Husni R N, Gordon S M, Washington J A, Longworth D L. Lactobacillus bacteremia and endocarditis: review of 45 cases. Clin Infect Dis. 1997;25:1048–1055. doi: 10.1086/516109. [DOI] [PubMed] [Google Scholar]
- 128.Jackman J D, Jr, Glamann D B. Gonococcal endocarditis: twenty-five year experience. Am J Med Sci. 1991;301:221–230. doi: 10.1097/00000441-199103000-00012. [DOI] [PubMed] [Google Scholar]
- 129.Jacobs F, Abramowicz D, Vereerstraeten P, Le Clerc J L, Zech F, Thys J P. Brucella endocarditis: the role of combined medical and surgical treatment. Rev Infect Dis. 1990;12:740–744. doi: 10.1093/clinids/12.5.740. [DOI] [PubMed] [Google Scholar]
- 130.Jacomo V, Musso D, Gevaudan M J, Drancourt M. Isolation of blood-borne Mycobacterium avium by using the nonradioactive BACTEC 9000 MB system and comparison with a solid-culture system. J Clin Microbiol. 1998;36:3703–3706. doi: 10.1128/jcm.36.12.3703-3706.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jalava J, Kotilainen P, Nikkari S, Skurnik M, Vanttinen E, Lehtonen O P, Eerola E, Toivanen P. Use of the polymerase chain reaction and DNA sequencing for detection of Bartonella quintana in the aortic valve of a patient with culture- negative infective endocarditis. Clin Infect Dis. 1995;21:891–896. doi: 10.1093/clinids/21.4.891. [DOI] [PubMed] [Google Scholar]
- 132.Jariwalla A G, Davies B H, White J. Infective endocarditis complicating psittacosis: response to rifampicin. Br Med J. 1980;280:155. doi: 10.1136/bmj.280.6208.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reference deleted.
- 134.Reference deleted.
- 135.Jobanputra R S, Moysey J. Endocarditis due to Cardiobacterium hominis. J Clin Pathol. 1977;30:1033–1036. doi: 10.1136/jcp.30.11.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jones R B, Priest J B, Kuo C. Subacute chlamydial endocarditis. JAMA. 1982;247:655–658. [PubMed] [Google Scholar]
- 137.Jortner R, Demopoulos L A, Bernstein N E, Tunick P A, Shapira Y, Shaked Y, Kronzon I. Transesophageal echocardiography in the diagnosis of Q-fever endocarditis. Am Heart J. 1994;128:827–831. doi: 10.1016/0002-8703(94)90284-4. [DOI] [PubMed] [Google Scholar]
- 138.Jurica J V, Bomzer C A, England A C. Gonococcal endocarditis: a case report and review of the literature. Sex Transm Dis. 1987;14:231–233. [PubMed] [Google Scholar]
- 139.Kaplan A H, Weber D J, Oddone E Z, Perfect J R. Infection due to Actinobacillus actinomycetemcomitans: 15 cases and review. Rev Infect Dis. 1989;11:46–63. doi: 10.1093/clinids/11.1.46. [DOI] [PubMed] [Google Scholar]
- 140.Kaufhold A, Franzen D, Lutticken R. Endocarditis caused by Gemella haemolysans. Infection. 1989;17:385–387. doi: 10.1007/BF01645552. [DOI] [PubMed] [Google Scholar]
- 141.Kawamura Y, Hou X G, Sultana F, Liu S, Yamamoto H, Ezaki T. Transfer of Streptococcus adjacens and Streptococcus defectivus to Abiotrophia gen. nov. as Abiotrophia adiacens comb. nov. and Abiotrophia defectiva comb. nov., respectively. Int J Syst Bacteriol. 1995;45:798–803. doi: 10.1099/00207713-45-4-798. [DOI] [PubMed] [Google Scholar]
- 142.Kerr J R, Webb C H, McGimpsey J G, Campbell N P. Infective endocarditis due to Gemella morbillorum complicating hypertrophic obstructive cardiomyopathy. Ulster Med J. 1994;63:108–110. [PMC free article] [PubMed] [Google Scholar]
- 143.Koehler J E, Glaser C A, Tappero J W. Rochalimaea henselae infection. A new zoonosis with the domestic cat as reservoir. JAMA. 1995;271:531–535. doi: 10.1001/jama.271.7.531. [DOI] [PubMed] [Google Scholar]
- 144.Kolander S A, Cosgrove E M, Molavi A. Clostridial endocarditis. Report of a case caused by Clostridium bifermentans and review of the literature. Arch Intern Med. 1989;149:455–456. doi: 10.1001/archinte.149.2.455. [DOI] [PubMed] [Google Scholar]
- 145.Kolman S, Maayan M C, Gotesman G, Rozenszajn L A, Wolach B, Lang R. Comparison of the Bactec and lysis concentration methods for recovery of Brucella species from clinical specimens. Eur J Clin Microbiol Infect Dis. 1991;10:647–648. doi: 10.1007/BF01975817. [DOI] [PubMed] [Google Scholar]
- 146.Kuritsky J N, Bullen M G, Broome C V, Silcox V A, Good R C, Wallace R J., Jr Sternal wound infections and endocarditis due to organisms of the Mycobacterium fortuitum complex. Ann Intern Med. 1983;98:938–939. doi: 10.7326/0003-4819-98-6-938. [DOI] [PubMed] [Google Scholar]
- 147.Lamas C C, Eykyn S J. Suggested modifications to the Duke criteria for the clinical diagnosis of native valve and prosthetic valve endocarditis: analysis of 118 pathologically proven cases. Clin Infect Dis. 1997;25:713–719. doi: 10.1086/513765. [DOI] [PubMed] [Google Scholar]
- 148.Landis S J, Korver J. Eikenella corrodens endocarditis: case report and review of the literature. Can Med Assoc J. 1983;128:822–824. [PMC free article] [PubMed] [Google Scholar]
- 149.Landymore R W, Murphy D A, Marrie T J, Johnston B L. Mycobacterium avium-intracellulare endocarditis causing rupture: replacement and repair with aortic homograft Can. J Cardiol. 1992;8:729–732. . (Erratum, 9:214–215, 1993.) [PubMed] [Google Scholar]
- 150.Lang R, Rubinstein E. Quinolones for the treatment of brucellosis. J Antimicrob Chemother. 1992;29:357–363. doi: 10.1093/jac/29.4.357. [DOI] [PubMed] [Google Scholar]
- 151.La Scola B, Raoult D. Serological cross-reactions between Bartonella quintana, Bartonella henselae, and Coxiella burnetii. J Clin Microbiol. 1996;34:2270–2274. doi: 10.1128/jcm.34.9.2270-2274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.La Scola B, Raoult D. Molecular identification of Gemella species from three patients with endocarditis. J Clin Microbiol. 1998;36:866–871. doi: 10.1128/jcm.36.4.866-871.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.La Scola B, Raoult D. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998) J Clin Microbiol. 1999;37:1899–905. doi: 10.1128/jcm.37.6.1899-1905.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Laudat P, Cosnay P, Icole B, Raoult A, Raynaud P, Brochier M. Endocardite a Gemella hemolysans: une nouvelle observation. Med Mal Infect. 1984;14:159–161. [Google Scholar]
- 155.Lecluse E, Scanu P, Saloux E, Vergnaud M, Valette B, Agostini D, Grard Y, Braud J, Grollier G, Potier J C. Endocarditis a Cardiobacterium hominis. Presse Med. 1994;23:325–328. [PubMed] [Google Scholar]
- 156.Levison D A, Guthrie W, Ward C, Green D M, Robertson P G. Infective endocarditis as part of psittacosis. Lancet. 1971;ii:844–847. doi: 10.1016/s0140-6736(71)90221-2. [DOI] [PubMed] [Google Scholar]
- 157.Levy P Y, Drancourt M, Etienne J, Auvergnat J C, Beytout J, Sainty J M, Goldstein F, Raoult D. Comparison of different antibiotic regiments for therapy of 32 cases of Q fever endocarditis. Antimicrob Agents Chemother. 1991;35:533–537. doi: 10.1128/aac.35.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Levy P Y, Raoult D, Razongles J J. Q fever and autoimmunity. Eur J Epidemiol. 1989;5:447–453. doi: 10.1007/BF00140139. [DOI] [PubMed] [Google Scholar]
- 159.Lohr D C, Goeken J A, Doty D B, Donta S T. Mycobacterium gordonae infection of a prosthetic aortic valve. JAMA. 1978;239:1528–1530. [PubMed] [Google Scholar]
- 160.Lombardo J A, O'Rourke M F, Shanahan M X, Harkness J L. Endocarditis due to an atypical mycobacterium following porcine heart valve replacement. Aust N Z J Med. 1980;10:432–434. doi: 10.1111/j.1445-5994.1980.tb04096.x. [DOI] [PubMed] [Google Scholar]
- 161.Lopez J A, Ross R S, Fishbein M C, Siegel R J. Nonbacterial thrombotic endocarditis: a review. Am Heart J. 1987;113:773–784. doi: 10.1016/0002-8703(87)90719-8. [DOI] [PubMed] [Google Scholar]
- 162.Lopez-Dupla M, Creus C, Navarro O, Raga X. Association of Gemella morbillorum endocarditis with adenomatous polyps and carcinoma of the colon: case report and review. Clin Infect Dis. 1996;22:379–380. doi: 10.1093/clinids/22.2.379. [DOI] [PubMed] [Google Scholar]
- 163.Reference deleted.
- 164.Reference deleted.
- 165.Lowry P W, Tompkins L S. Nosocomial legionellosis: a review of pulmonary and extrapulmonary syndromes. Am J Infect Control. 1993;21:21–27. doi: 10.1016/0196-6553(93)90203-g. [DOI] [PubMed] [Google Scholar]
- 166.Lupoglazoff J M, Brouqui P, Magnier S, Hvass U, Casasoprana A. Q fever tricuspid valve endocarditis. Arch Dis Child. 1997;77:448–449. doi: 10.1136/adc.77.5.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lynn D J, Kane J G, Parker R H. Haemophilus parainfluenzae and influenzae endocarditis: a review of forty cases. Medicine (Baltimore) 1977;56:115–128. doi: 10.1097/00005792-197703000-00003. [DOI] [PubMed] [Google Scholar]
- 168.Mainardi J L, Drancourt M, Roland J M, Gestin J L, Raoult D, Acar J F, Goldstein F W. Bartonella (Rochalimaea) quintana endocarditis in an Algerian farmer. Clin Microbiol Infect. 1996;1:275–276. doi: 10.1016/s1198-743x(15)60288-9. [DOI] [PubMed] [Google Scholar]
- 169.Marmion B P, Higgins F E, Bridges J B, Edwards A T. A case of subacute rickettsial endocarditis; with a survey of cardiac patients for this infection. Br Med J. 1960;2:1264–1267. doi: 10.1136/bmj.2.5208.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Marrie T J. A comparison of Q fever endocarditis with native valve endocarditis. Ann N Y Acad Sci. 1990;590:61–67. doi: 10.1111/j.1749-6632.1990.tb42207.x. [DOI] [PubMed] [Google Scholar]
- 171.Marrie T J, Durant H, Williams C J, Mintz E, Waag D M. Exposure to parturient cats: a risk factor for acquisition of Q fever in Maritime Canada. J Infect Dis. 1988;158:101–108. doi: 10.1093/infdis/158.1.101. [DOI] [PubMed] [Google Scholar]
- 172.Marrie T J, Harczy M, Mann O E, Landymore R W, Raza A, Wang S P, Grayston J T. Culture-negative endocarditis probably due to Chlamydia pneumoniae. J Infect Dis. 1990;161:127–129. doi: 10.1093/infdis/161.1.127. [DOI] [PubMed] [Google Scholar]
- 173.Matsis P P, Easthope R N. Gemella haemolysans endocarditis. Aust N Z J Med. 1994;24:417–418. doi: 10.1111/j.1445-5994.1994.tb01481.x. [DOI] [PubMed] [Google Scholar]
- 174.Maurin M, Benoliel A M, Bongrand P, Raoult D. Phagolysosomal alkalinization and the bactericidal effect of antibiotics: the Coxiella burnetii paradigm. J Infect Dis. 1992;166:1097–1102. doi: 10.1093/infdis/166.5.1097. [DOI] [PubMed] [Google Scholar]
- 175.Maurin M, Birtles R, Raoult D. Current knowledge of Bartonella species. Eur J Clin Microbiol Infect Dis. 1997;16:487–506. doi: 10.1007/BF01708232. [DOI] [PubMed] [Google Scholar]
- 176.Maurin M, Eb F, Etienne J, Raoult D. Serological cross-reactions between Bartonella and Chlamydia species: implications for diagnosis. J Clin Microbiol. 1997;35:2283–2287. doi: 10.1128/jcm.35.9.2283-2287.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Maurin M, Gasquet S, Ducco C, Raoult D. MICs of 28 antibiotic compounds for 14 Bartonella (formerly Rochalimaea) isolates. Antimicrob Agents Chemother. 1995;39:2387–2391. doi: 10.1128/aac.39.11.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Maurin M, Raoult D. In vitro susceptibilities of spotted fever group rickettsiae and Coxiella burnetti to clarithromycin. Antimicrob Agents Chemother. 1993;37:2633–2637. doi: 10.1128/aac.37.12.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Maurin M, Raoult D. Bartonella (Rochalimaea) quintana infections. Clin Microbiol Rev. 1996;9:273–292. doi: 10.1128/cmr.9.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Maxwell S. Endocarditis due to Streptococcus morbillorum. J Infect. 1989;18:67–72. doi: 10.1016/s0163-4453(89)93717-1. [DOI] [PubMed] [Google Scholar]
- 181.McAllister H A, Jr, Fenoglio J J., Jr Cardiac involvement in Whipple's disease. Circulation. 1975;52:152–156. doi: 10.1161/01.cir.52.1.152. [DOI] [PubMed] [Google Scholar]
- 182.McCabe R E, Baldwin J C, McGregor C A, Miller D C, Vosti K L. Prosthetic valve endocarditis caused by Legionella pneumophila. Ann Intern Med. 1984;100:525–527. doi: 10.7326/0003-4819-100-4-525. [DOI] [PubMed] [Google Scholar]
- 183.Meis J F, Sauerwein R W, Gyssens I C, Horrevorts A M, van Kampen A. Kingella kingae intervertebral diskitis in an adult. Clin Infect Dis. 1992;15:530–532. doi: 10.1093/clind/15.3.530. [DOI] [PubMed] [Google Scholar]
- 184.Mermel L A, Maki D G. Detection of bacteremia in adults: consequence of culturing an inadequate volume of blood. Arch Intern Med. 1993;119:270–272. doi: 10.7326/0003-4819-119-4-199308150-00003. [DOI] [PubMed] [Google Scholar]
- 185.Meuleman P, Erard K, Herregods M C, Peetermans W E, Verhaegen J. Bioprosthetic valve endocarditis caused by Neisseria elongata subspecies nitroreducens. Infection. 1996;24:258–260. doi: 10.1007/BF01781107. [DOI] [PubMed] [Google Scholar]
- 186.Mills J, Dew D. Serratia marcesens endocarditis: a regional illness associated with intravenous drug abuse. Ann Intern Med. 1976;84:29–35. doi: 10.7326/0003-4819-84-1-29. [DOI] [PubMed] [Google Scholar]
- 187.Reference deleted.
- 188.Mooney E E, Kenan D J, Sweeney E C, Gaede J T. Myocarditis in Whipple's disease: an unsuspected cause of symptoms and sudden death. Mod Pathol. 1997;10:524–529. [PubMed] [Google Scholar]
- 189.Reference deleted.
- 190.Morrison V A, Wagner K F. Clinical manifestations of Kingella kingae infections: case report and review. Rev Infect Dis. 1989;11:776–782. doi: 10.1093/clinids/11.5.776. [DOI] [PubMed] [Google Scholar]
- 191.Reference deleted.
- 192.Mühlemann K, Matter L, Meyer B, Shopfer K. Isolation of Coxiella burnetii from heart valves of patients treated for Q fever endocarditis. J Clin Microbiol. 1995;33:428–431. doi: 10.1128/jcm.33.2.428-431.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Musso D, Raoult D. Coxiella burnetii blood cultures from acute and chronic Q fever patients. J Clin Microbiol. 1995;33:3129–3132. doi: 10.1128/jcm.33.12.3129-3132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Musso D, Raoult D. In vitro susceptibility of Bartonella quintana cultivated on cell culture. J Antimicrob Chemother. 1995;36:101–108. doi: 10.1093/jac/36.1.101. [DOI] [PubMed] [Google Scholar]
- 195.Mutters R. Actinobacillus, Capnocytophaga, Eikenella, Kingella, and other fastidious or rarely encountered gram-negative rods. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: ASM Press; 1999. pp. 561–569. [Google Scholar]
- 196.Nadakumar R, Raju G. Isolated tricuspid valve endocarditis in nonaddicted patients: a diagnostic challenge. Am J Med Sci. 1997;314:207–212. doi: 10.1097/00000441-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 197.Namnyak S S, Bhat R P, Al-Jama A, Fathalla S E. Prosthetic valve endocarditis caused by Corynebacterium diphtheriae in a patient with pemphigus vulgaris. J Clin Microbiol. 1987;25:1330–1332. doi: 10.1128/jcm.25.7.1330-1332.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Navas E, Guerrero A, Cobo J, Loza E. Faster isolation of Brucella spp. from blood by isolator compared with BACTEC NR. Diagn Microbiol Infect Dis. 1993;16:79–81. doi: 10.1016/0732-8893(93)90135-t. [DOI] [PubMed] [Google Scholar]
- 199.Nawaz T, Hardy D J, Bonnez W. Neisseria elongata subsp. elongata, a case of human endocarditis complicated by pseudoaneurysm. J Clin Microbiol. 1996;34:756–758. doi: 10.1128/jcm.34.3.756-758.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nettles R E, Sexton D J. Pasteurella multocida prosthetic valve endocarditis: case report and review. Clin Infect Dis. 1997;25:920–921. doi: 10.1086/597637. [DOI] [PubMed] [Google Scholar]
- 201.Reference deleted.
- 202.Odum L, Jensen K T, Slotsbjerg T D. Endocarditis due to Kingella kingae. Eur J Clin Microbiol. 1984;3:263–266. doi: 10.1007/BF02014899. [DOI] [PubMed] [Google Scholar]
- 203.Olopoenia L A, Mody V, Reynolds M. Eikenella corrodens endocarditis in an intravenous drug user: case report and literature review. J Nat Med Assoc. 1994;86:313–315. [PMC free article] [PubMed] [Google Scholar]
- 204.Omran Y, Wood C A. Endovascular infection and septic arthritis caused by Gemella morbillorum. Diagn Microbiol Infect Dis. 1993;16:131–134. doi: 10.1016/0732-8893(93)90007-t. [DOI] [PubMed] [Google Scholar]
- 205.Ong K R, Sordillo E, Frankel E. Unusual case of Aeromonas hydrophila endocarditis. J Clin Microbiol. 1991;29:1056–1057. doi: 10.1128/jcm.29.5.1056-1057.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Overholt B F. Actinobacillus actinomycetemcomitans endocarditis. Arch Intern Med. 1966;117:99–102. [PubMed] [Google Scholar]
- 207.Palmer S R, Young S E. Q fever endocarditis in England and Wales 1975–1981. Lancet. 1982;ii:1448–1449. doi: 10.1016/s0140-6736(82)91341-1. [DOI] [PubMed] [Google Scholar]
- 208.Park D, Pugliese A, Cunha B A. Legionella micdadei prosthetic valve endocarditis. Infection. 1994;22:213–215. doi: 10.1007/BF01716708. [DOI] [PubMed] [Google Scholar]
- 209.Parola P, Maurin M, Alimi Y, Juhan C, Brouqui P. Use of 16S rRNA gene sequencing to identify Lactobacillus casei in septicaemia secondary to a paraprosthetic enteric fistula. Eur J Clin Microbiol Infect Dis. 1998;17:203–205. doi: 10.1007/BF01691119. [DOI] [PubMed] [Google Scholar]
- 210.Pazin G J, Saul S, Thompson M E. Blood culture positivity: suppression by outpatient antibiotic therapy in patients with bacterial endocarditis. Arch Intern Med. 1982;142:263–268. [PubMed] [Google Scholar]
- 211.Peery T M, Belter L F. Brucellosis and heart disease. II Fatal brucellosis: a review of the literature and report of new cases. Am J Pathol. 1960;36:673–696. [PMC free article] [PubMed] [Google Scholar]
- 212.Perez-Fontan M, Huarte E, Tellez A, Rodriguez-Carmona A, Picazo M L, Martinez-Ara J. Glomerular nephropathy associated with chronic Q fever. Am J Kidney Dis. 1988;11:298–306. doi: 10.1016/s0272-6386(88)80134-3. [DOI] [PubMed] [Google Scholar]
- 213.Pesanti E L, Smith I M. Infective endocarditis with negative blood cultures. An analysis of 52 cases. Am J Med. 1979;66:43–50. doi: 10.1016/0002-9343(79)90480-7. [DOI] [PubMed] [Google Scholar]
- 214.Peterson C E. Media-dependent subculture of nutritionally variant streptococci. Am J Clin Pathol. 1981;75:634–66. doi: 10.1093/ajcp/75.4.634. [DOI] [PubMed] [Google Scholar]
- 215.Piepkorn M W, Reichenbach D D. Infective endocarditis associated with cell wall-deficient bacteria. Electron microscopic findings in four cases. Hum Pathol. 1978;9:163–173. doi: 10.1016/s0046-8177(78)80107-5. [DOI] [PubMed] [Google Scholar]
- 216.Pinsky R L, Piscitelli V, Patterson J E. Endocarditis caused by relatively penicillin-resistant Stomatococcus mucilaginosus. J Clin Microbiol. 1989;27:215–216. doi: 10.1128/jcm.27.1.215-216.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216a.Plantholt S J, Trofa A F. Citrobacter freudii endocarditis in an intravenous drug abuser. South Med J. 1987;80:1439–1441. doi: 10.1097/00007611-198711000-00026. [DOI] [PubMed] [Google Scholar]
- 217.Popat K, Barnardo D, Webb-Peploe M. Mycoplasma pneumoniae endocarditis. Br Heart J. 1980;44:111–112. doi: 10.1136/hrt.44.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Pritchard T M, Foust R T, Cantely J R, Leman R B. Prosthetic valve endocarditis due to Cardiobacterium hominis occurring after upper gastrointestinal endoscopy. Am J Med. 1991;90:516–518. [PubMed] [Google Scholar]
- 219.Ramirez J A. Isolation of Chlamydia pneumoniae from the coronary artery of a patient with coronary atherosclerosis. The Chlamydia pneumoniae/Atherosclerosis Study Group. Ann Intern Med. 1996;125:979–982. doi: 10.7326/0003-4819-125-12-199612150-00008. [DOI] [PubMed] [Google Scholar]
- 220.Raoult D. Treatment of Q fever. Antimicrob Agents Chemother. 1993;37:1733–1736. doi: 10.1128/aac.37.9.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Raoult D. Afebrile blood culture-negative endocarditis. Arch Intern Med. 1999;131:144–146. doi: 10.7326/0003-4819-131-2-199907200-00012. [DOI] [PubMed] [Google Scholar]
- 222.Raoult D, Birg M L, La Scola B, Fournier P E, Enea M, Lepidi H, Roux V, Piette J C, Vandenesch F, Vital-Durand D, Marrie T. Cultivation of the bacillus of Whipple's disease. N Engl J Med. 2000;342:620–625. doi: 10.1056/NEJM200003023420903. [DOI] [PubMed] [Google Scholar]
- 223.Raoult D, Brouqui P, Marchou M, Gastaut J A. Acute and chronic Q fever in patients with cancer. Clin Infect Dis. 1992;14:127–130. doi: 10.1093/clinids/14.1.127. [DOI] [PubMed] [Google Scholar]
- 224.Raoult D, Drancourt M, Vestris G. Bactericidal effect of doxycycline associated with lysosomotropic agents on Coxiella burnetii in P 388 D1 cells. Antimicrob Agents Chemother. 1990;34:1512–1514. doi: 10.1128/aac.34.8.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Raoult D, Fournier P E, Drancourt M, Marrie T J, Etienne J, Cosserat J, Cacoub P, Poinsignon Y, Leclercq P, Sefton A M. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med. 1996;125:646–652. doi: 10.7326/0003-4819-125-8-199610150-00004. . (Erratum, 127:249, 1997.) [DOI] [PubMed] [Google Scholar]
- 226.Raoult D, Houpikian P, Tissot Dupont H, Riss J M, Arditi-Djiane J, Brouqui P. Treatment of Q fever endocarditis: comparison of 2 regimens containing doxycycline and ofloxacin or hydroxychloroquine. Arch Intern Med. 1999;159:167–173. doi: 10.1001/archinte.159.2.167. [DOI] [PubMed] [Google Scholar]
- 227.Raoult D, Marrie T. Q fever. Clin Infect Dis. 1995;20:489–496. doi: 10.1093/clinids/20.3.489. [DOI] [PubMed] [Google Scholar]
- 228.Raoult D, Tissot-Dupont H, Foucault C, Gouvernet J, Fournier P E, Bernit E, Stein A, Nesri M, Harle J R, Weiller P J. Q fever 1985–1998: clinical and epidemiological features of 1383 infections. Medicine (Baltimore) 2000;79:109–123. doi: 10.1097/00005792-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 229.Raoult D, Vestris G, Enea M. Isolation of 16 strains of Coxiella burnetii from patients by using a sensitive centrifugation cell culture system and establishment of strains in HEL cells. J Clin Microbiol. 1990;28:2482–2484. doi: 10.1128/jcm.28.11.2482-2484.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Reboli A C, Farrar W E. Erysipelothrix rhusiopathiae: an occupational pathogen. Clin Microbiol Rev. 1989;2:354–359. doi: 10.1128/cmr.2.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Regan R J, Dathan J R, Treharne J D. Infective endocarditis with glomerulonephritis associated with cat chlamydia (C. psittaci) infection. Br Heart J. 1979;42:349–352. doi: 10.1136/hrt.42.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Relman D A, Ruokk K, Ferraro M J. Stomatococcus mucilaginosus endocarditis in an intravenous drug abuser. J Infect Dis. 1987;155:1080–1081. doi: 10.1093/infdis/155.5.1080. [DOI] [PubMed] [Google Scholar]
- 233.Relman D A. The identification of uncultured microbial pathogens. J Infect Dis. 1993;168:1–8. doi: 10.1093/infdis/168.1.1. [DOI] [PubMed] [Google Scholar]
- 234.Relman D A, Schmidt T M, MacDermott R P, Falkow S. Identification of the uncultured bacillus of Whipple's disease. N Engl J Med. 1992;327:293–301. doi: 10.1056/NEJM199207303270501. [DOI] [PubMed] [Google Scholar]
- 235.Roberts R B, Krieger A G, Schiller N L, Gross K C. Viridans streptococcal endocarditis: the role of various species, including pyridoxal-dependent streptococci. Rev Infect Dis. 1979;1:955–966. doi: 10.1093/clinids/1.6.955. [DOI] [PubMed] [Google Scholar]
- 236.Rodriguez C, Olcoz M T, Izquierdo G, Moreno S. Endocarditis due to ampicillin-resistant nontyphoid Salmonella: cure with a third-generation cephalosporin. Rev Infect Dis. 1990;12:817–819. doi: 10.1093/clinids/12.5.817. [DOI] [PubMed] [Google Scholar]
- 237.Rohmann S, Erbel R, Mohr-Kahaly S, Meyer J. Use of transoesophageal echocardiography in the diagnosis of abscess in infective endocarditis. Eur Heart J. 1995;16(Suppl. B):54–62. doi: 10.1093/eurheartj/16.suppl_b.54. [DOI] [PubMed] [Google Scholar]
- 238.Roldan C A. Valvular disease associated with systemic illness. Cardiol Clin. 1998;16:531–550. doi: 10.1016/s0733-8651(05)70030-8. [DOI] [PubMed] [Google Scholar]
- 239.Roux V, Eykyn S J, Wyllie S, Raoult D. Bartonella vinsonii subsp. berkhoffii as an agent of afebrile blood culture-negative endocarditis in a human. J Clin Microbiol. 2000;38:1698–1700. doi: 10.1128/jcm.38.4.1698-1700.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rozenberg V, Chauveau P, Gryman R, Clavier H. Endocardite a Neisseria sicca revelée par un syndrome de defaillance multiviscerale. Arch Mal Coeur Vaiss. 1996;89:1689–1693. [PubMed] [Google Scholar]
- 241.Rumisek J D, Albus R A, Clarke J S. Late Mycobacterium chelonei bioprosthetic valve endocarditis: activation of implanted contaminant? Ann Thorac Surg. 1985;39:277–279. doi: 10.1016/s0003-4975(10)62596-9. [DOI] [PubMed] [Google Scholar]
- 242.Ruoff K L. Leuconostoc, Pediococcus, Stomatococcus, and micellaneous gram-positive cocci that grow aerobically. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: ASM Press; 1999. pp. 306–315. [Google Scholar]
- 243.Rupp M E. Streptobacillus moniliformis endocarditis: case report and review. Clin Infect Dis. 1992;14:769–772. doi: 10.1093/clinids/14.3.769. [DOI] [PubMed] [Google Scholar]
- 244.Sabatier F, Dignat-George F, Mege J L, Brunet C, Raoult D, Sampol J. CD4+ T-cell lymphopenia in Q fever endocarditis. Clin Diagn Lab Immunol. 1997;4:89–92. doi: 10.1128/cdli.4.1.89-92.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Salazar Mallen M, Lozano Hube E, Brenes M. Comparative study of blood cultures made from artery, vein, and bone marrow in patients with subacute bacterial endocarditis. Am Heart J. 1947;33:692–695. doi: 10.1016/0002-8703(47)90086-0. [DOI] [PubMed] [Google Scholar]
- 246.Reference deleted.
- 247.San Roman J A, Vilacosta I, Zamorano J L, Almeria C, Sanchez-Harguindey L. Transesophageal echocardiography in right-sided endocarditis. J Am Coll Cardiol. 1993;21:1226–1230. doi: 10.1016/0735-1097(93)90250-5. [DOI] [PubMed] [Google Scholar]
- 248.Savage D D, Kagan R L, Young N A, Horvath A E. Cardiobacterium hominis endocarditis: description of two patients and characterization of the organism. J Clin Microbiol. 1977;5:75–80. doi: 10.1128/jcm.5.1.75-80.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Schack S H, Smith P W, Penn R G, Rapoport J M. Endocarditis caused by Actinobacillus actinomycetemcomitans. J Clin Microbiol. 1984;20:579–581. doi: 10.1128/jcm.20.3.579-581.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Schneider P J, Nernoff J, Gold J A. Acute Salmonella endocarditis. Report of a case and review. Arch Intern Med. 1967;120:478–486. doi: 10.1001/archinte.120.4.478. [DOI] [PubMed] [Google Scholar]
- 251.Schoedon G, Goldenberger D, Forrer R, Gunz A, Dutly F, Hochli M, Altwegg M, Schaffner A. Deactivation of macrophages with interleukin-4 is the key to the isolation of Tropheryma whippelii. J Infect Dis. 1997;176:672–677. doi: 10.1086/514089. [DOI] [PubMed] [Google Scholar]
- 252.Schvarcz R, Svedenhag J, Radegran K. A case of Brucella melitensis endocarditis successfully treated by a combination of surgical resection and antibiotics. Scand J Infect Dis. 1995;27:641–642. doi: 10.3109/00365549509047083. [DOI] [PubMed] [Google Scholar]
- 253.Shapiro D S, Kenney S C, Johnson M, Davis C H, Knight S T, Wyrick P B. Brief report: Chlamydia psittaci endocarditis diagnosed by blood culture. N Engl J Med. 1992;326:1192–1195. doi: 10.1056/NEJM199204303261805. [DOI] [PubMed] [Google Scholar]
- 254.Singh M, Bofinger A, Cave G, Boyle P. Mycobacterium fortuitum endocarditis in a patient with chronic renal failure on hemodialysis. Pathology. 1992;24:197–200. doi: 10.3109/00313029209063173. [DOI] [PubMed] [Google Scholar]
- 255.Sorbello A F, O'Donnell J, Kaiser-Smith J, Fitzharris J, Shinkarow J, Doneson S. Infective endocarditis due to Pasteurella dagmatis: case report and review. Clin Infect Dis. 1994;18:336–338. doi: 10.1093/clinids/18.3.336. [DOI] [PubMed] [Google Scholar]
- 256.Spaargaren J, van Boven C P, Voor G P. Effectiveness of resins in neutralizing antibiotic activities in Bactec plus Aerobic/F culture medium. J Clin Microbiol. 1998;36:3731–3733. doi: 10.1128/jcm.36.12.3731-3733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Spach D H, Callis K P, Paauw D S, Houze Y B, Shoenknecht F D, Welch D F. Endocarditis caused by Rochalimaea quintana in a patient infected with human inmmunodeficiency virus. J Clin Microbiol. 1993;31:692–694. doi: 10.1128/jcm.31.3.692-694.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Spach D H, Kanter A S, Daniels N A, Nowowiejski D J, Larson A M, Schmidt R A, Swaminathan B, Brenner D J. Bartonella (Rochalimaea) species as a cause of apparent “culture-negative” endocarditis. Clin Infect Dis. 1995;20:1044–1047. doi: 10.1093/clinids/20.4.1044. [DOI] [PubMed] [Google Scholar]
- 259.Spring W J, Hampson J. Chronic Q fever endocarditis causing massive splenomegaly and hypersplenism. Br Med J (Clin ResEd) 1982;285:1244. doi: 10.1136/bmj.285.6350.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Spyrou N, Anderson M, Foale R. Listeria endocarditis: current management and patient outcome—world literature review. Heart. 1997;77:380–383. doi: 10.1136/hrt.77.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Stamboulian D, Carbone E. Recognition, management and prophylaxis of endocarditis. Drugs. 1997;54:730–744. doi: 10.2165/00003495-199754050-00005. [DOI] [PubMed] [Google Scholar]
- 262.Steckelberg J M, Melton L J, Ilstrup D M, Rouse M S, Wilson W R. Influence of referral bias on the apparent clinical spectrum of infective endocarditis. Am J Med. 1990;88:582–588. doi: 10.1016/0002-9343(90)90521-e. [DOI] [PubMed] [Google Scholar]
- 263.Steckelberg J M, Murphy J G, Ballard D, Bailey K, Tajik A J, Taliercio C P, Giuliani E R, Wilson W R. Emboli in infective endocarditis: the prognostic value of echocardiography. Ann Intern Med. 1991;114:635–640. doi: 10.7326/0003-4819-114-8-635. [DOI] [PubMed] [Google Scholar]
- 264.Stein A, Raoult D. Detection of Coxiella burnetti by DNA amplification using polymerase chain reaction. J Clin Microbiol. 1992;30:2462–2466. doi: 10.1128/jcm.30.9.2462-2466.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Stein A, Raoult D. A simple method for amplification of DNA from paraffin-embedded tissues. Nucleic Acids Res. 1992;20:5237–5238. doi: 10.1093/nar/20.19.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Stein A, Raoult D. Q fever endocarditis. Eur Heart J. 1995;16:19–23. doi: 10.1093/eurheartj/16.suppl_b.19. [DOI] [PubMed] [Google Scholar]
- 267.Stein D S, Nelson K E. Endocarditis due to nutritionally deficient streptococci: therapeutic dilemma. Rev Infect Dis. 1987;9:908–916. doi: 10.1093/clinids/9.5.908. [DOI] [PubMed] [Google Scholar]
- 268.Stout J E, Yu V L. Legionellosis. N Engl J Med. 1997;337:682–687. doi: 10.1056/NEJM199709043371006. [DOI] [PubMed] [Google Scholar]
- 269.Reference deleted.
- 270.Sussman J I, Baron E J, Goldberg S M, Kaplan M H, Pizzarello R A. Clinical manifestations and therapy of Lactobacillus endocarditis: report of a case and review of the literature. Rev Infect Dis. 1986;8:771–776. doi: 10.1093/clinids/8.5.771. [DOI] [PubMed] [Google Scholar]
- 271.Tancik C A, Dillaha J A. Francisella tularensis endocarditis. Clin Infect Dis. 2000;30:399–400. doi: 10.1086/313678. [DOI] [PubMed] [Google Scholar]
- 272.Tang Y W, Hopkins M K, Kolbert C P, Hartley P A, Severance P J, Persing D H. Bordetella holmesii-like organims associated with septicemia, endocarditis, and respiratory failure. Clin Infect Dis. 1998;26:389–392. doi: 10.1086/516323. [DOI] [PubMed] [Google Scholar]
- 273.Taveras J M, Campo R, Segal N, Urena P E, Lacayo L. Apparent culture-negative endocarditis of the prosthetic valve caused by Cardiobacterium hominis. South Med J. 1993;86:1439–1440. doi: 10.1097/00007611-199312000-00030. [DOI] [PubMed] [Google Scholar]
- 274.Tebas P, Sultan F, Wallace R J, Jr, Fraser V. Rapid development of resistance to clarithromycin following monotherapy for disseminated Mycobacterium chelonae infection in a heart transplant patient. Clin Infect Dis. 1995;20:443–444. doi: 10.1093/clinids/20.2.443. [DOI] [PubMed] [Google Scholar]
- 275.Terada H, Miyahara K, Sohara H, Sonoda M, Uenomachi H, Sanada J, Arima T. Infective endocarditis caused by an indigenous bacterium (Gemella morbillorum) Intern Med. 1994;33:628–631. doi: 10.2169/internalmedicine.33.628. [DOI] [PubMed] [Google Scholar]
- 276.Thomas D R, Treweek L, Kench S M, Coleman T J, Maedows D, Morgan-Capner P, Caul E O. The risk of acquiring Q fever on farms: a seroepidemiological study. Occup Environ Med. 1995;52:644–647. doi: 10.1136/oem.52.10.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Tierno P M, Jr, Inglima K, Parisi M T. Detection of Bartonella (Rochalimaea) henselae bacteremia using BacT/Alert blood culture system. Am J Clin Pathol. 1995;104:530–536. doi: 10.1093/ajcp/104.5.530. [DOI] [PubMed] [Google Scholar]
- 278.Tiley S M, Kociuba K R, Heron L G, Munro R. Infective endocarditis due to nontoxigenic Corynebacterium diphtheriae: report of seven cases and review. Clin Infect Dis. 1993;16:271–275. doi: 10.1093/clind/16.2.271. [DOI] [PubMed] [Google Scholar]
- 279.Tissot-Dupont H, Thirion X, Raoult D. Q fever serology: cutoff determination for microimunofluorescence. Clin Diagn Lab Immunol. 1994;1:189–196. doi: 10.1128/cdli.1.2.189-196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Tobin M J, Cahill N, Gearty G, Maurer B, Blake S, Daly K, Hone R. Q fever endocarditis. Am J Med. 1982;72:396–400. doi: 10.1016/0002-9343(82)90495-8. [DOI] [PubMed] [Google Scholar]
- 281.Tompkins L S, Roessler B J, Redd S C, Markowitz L E, Cohen M L. Legionella prosthetic-valve endocarditis. N Engl J Med. 1988;318:530–535. doi: 10.1056/NEJM198803033180902. [DOI] [PubMed] [Google Scholar]
- 282.Reference deleted.
- 283.Turck W P, Howitt G, Turnbert L A, Fox G, Longson M, Matthews M B, Das Gupta R. Chronic Q fever. Q J Med. 1976;178:193–217. [PubMed] [Google Scholar]
- 284.Vacher-Coponat H, Dussol B, Raoult D, Casanova P, Berland Y. Proliferative glomerulonephritis revealing chronic Q fever. Am J Nephrol. 1996;16:159–161. doi: 10.1159/000168990. [DOI] [PubMed] [Google Scholar]
- 285.van der Bel-Kahn J M, Watanakunakorn C, Menefee M G, Long H D, Dicter R. Chlamydia trachomatis endocarditis. Am Heart J. 1978;95:627–636. doi: 10.1016/0002-8703(78)90305-8. [DOI] [PubMed] [Google Scholar]
- 286.Van Scoy R E. Culture-negative endocarditis. Mayo Clin Proc. 1982;57:149–154. [PubMed] [Google Scholar]
- 287.Vasquez J E, Ferguson D A, Jr, Bin-Sagheer S, Myers J W, Ramsak A, Wilson M A, Sarubbi F A. Pasteurella multocida endocarditis: a molecular epidemiological study. Clin Infect Dis. 1998;26:518–520. doi: 10.1086/517105. [DOI] [PubMed] [Google Scholar]
- 288.Viscidi R, Geller A, Caplan W, Natsios G A, Gleckman R A. Prosthetic valve endocarditis caused by Mycobacterium chelonei: case report and literature review. Heart Lung. 1982;11:555–559. [PubMed] [Google Scholar]
- 289.Vlachakis N D, Gazes P C, Hairston P. Nocardial endocarditis following mitral valve replacement. Chest. 1973;63:276–278. doi: 10.1378/chest.63.2.276. [DOI] [PubMed] [Google Scholar]
- 290.Von Reyn C F, Levy B S, Arbeit R D, Friedland G, Crumpacker C S. Infective endocarditis: an analysis based on strict case definitions. Ann Intern Med. 1981;94:505–518. doi: 10.7326/0003-4819-94-4-505. [DOI] [PubMed] [Google Scholar]
- 291.Walker L J, Adgey A A. Successful treatment by doxycycline of endocarditis caused by ornithosis. Br Heart J. 1987;57:58–60. doi: 10.1136/hrt.57.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Wall T C, Peyton R B, Corey G R. Gonococcal endocarditis: a new look at an old disease. Medicine (Baltimore) 1989;68:375–380. [PubMed] [Google Scholar]
- 293.Wallace R J, Jr, Musser J M, Hull S I, Silcox V A, Steele L C, Forrester G D, Labidi A, Selander R K. Diversity and sources of rapidly growing mycobacteria associated with infections following cardiac surgery. J Infect Dis. 1989;159:708–716. doi: 10.1093/infdis/159.4.708. [DOI] [PubMed] [Google Scholar]
- 294.Wallace R J, Jr, Swenson J M, Silcox V A, Bullen M G. Treatment of nonpulmonary infections due to Mycobacterium fortuitum and Mycobacterium chelonei on the basis of in vitro susceptibilities. J Infect Dis. 1985;152:500–514. doi: 10.1093/infdis/152.3.500. [DOI] [PubMed] [Google Scholar]
- 295.Ward C, Sagar H J, Cooper D, Ward A M. Insidious endocarditis caused by Chlamydia psittaci. Br Med J. 1975;4:734–735. doi: 10.1136/bmj.4.5999.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Washington J A. The role of the microbiology laboratory in the diagnosis and antimicrobial treatment of infective endocarditis. Mayo Clin Proc. 1982;57:22–32. [PubMed] [Google Scholar]
- 297.Wendler D, Mendoza E, Schleiffer T, Zander M, Maier M. Tropheryma whippelii endocarditis confirmed by polymerase chain reaction. Eur Heart J. 1995;16:424–425. doi: 10.1093/oxfordjournals.eurheartj.a060928. [DOI] [PubMed] [Google Scholar]
- 298.Werner A S, Cobbs C G, Kaye D, Hook E W. Studies on the bacteremia of bacterial endocarditis. JAMA. 1967;202:199–203. [PubMed] [Google Scholar]
- 299.Reference deleted.
- 300.Wong J D, Janda J M. Association of an important Neisseria species, Neisseria elongata subsp. nitroreducens, with bacteremia, endocarditis, and osteomyelitis. J Clin Microbiol. 1992;30:719–720. doi: 10.1128/jcm.30.3.719-720.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Woods G L, Walker D H. Detection of infection or infectious agents by use of cytologic and histologic stains. Clin Microbiol Rev. 1996;9:382–404. doi: 10.1128/cmr.9.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Wormser G P, Bottone E J, Tudy J, Hirschman S Z. Case report. Cardiobacterium hominis: review of prior infections and report of endocarditis on a fascia lata prosthetic heart valve. Am J Med Sci. 1978;276:117–126. [PubMed] [Google Scholar]
- 303.Yagupsky P, Dagan R. Kingella kingae: an emerging cause of invasive infections in young children. Clin Infect Dis. 1997;24:860–866. doi: 10.1093/clinids/24.5.860. [DOI] [PubMed] [Google Scholar]
- 304.Young E J. Serologic diagnosis of human brucellosis: analysis of 214 cases by agglutination tests and review of the literature. Rev Infect Dis. 1991;13:359–372. doi: 10.1093/clinids/13.3.359. [DOI] [PubMed] [Google Scholar]