Summary
Background
In the prevention of cardiovascular disease, a WHO target is that at least 50% of eligible people use statins. Robust evidence is needed to monitor progress towards this target in low-income and middle-income countries (LMICs), where most cardiovascular disease deaths occur. The objectives of this study were to benchmark statin use in LMICs and to investigate country-level and individual-level characteristics associated with statin use.
Methods
We did a cross-sectional analysis of pooled, individual-level data from nationally representative health surveys done in 41 LMICs between 2013 and 2019. Our sample consisted of non-pregnant adults aged 40–69 years. We prioritised WHO Stepwise Approach to Surveillance (STEPS) surveys because these are WHO’s recommended method for population monitoring of non-communicable disease targets. For countries in which no STEPS survey was available, a systematic search was done to identify other surveys. We included surveys that were done in an LMIC as classified by the World Bank in the survey year; were done in 2013 or later; were nationally representative; had individual-level data available; and asked questions on statin use and previous history of cardiovascular disease. Primary outcomes were the proportion of eligible individuals self-reporting use of statins for the primary and secondary prevention of cardiovascular disease. Eligibility for statin therapy for primary prevention was defined among individuals with a history of diagnosed diabetes or a 10-year cardiovascular disease risk of at least 20%. Eligibility for statin therapy for secondary prevention was defined among individuals with a history of self-reported cardiovascular disease. At the country level, we estimated statin use by per-capita health spending, per-capita income, burden of cardiovascular diseases, and commitment to non-communicable disease policy. At the individual level, we used modified Poisson regression models to assess statin use alongside individual-level characteristics of age, sex, education, and rural versus urban residence. Countries were weighted in proportion to their population size in pooled analyses.
Findings
The final pooled sample included 116 449 non-pregnant individuals. 9229 individuals reported a previous history of cardiovascular disease (7.9% [95% CI 7.4–8.3] of the population-weighted sample). Among those without a previous history of cardiovascular disease, 8453 were eligible for a statin for primary prevention of cardiovascular disease (9.7% [95% CI 9.3–10.1] of the population-weighted sample). For primary prevention of cardiovascular disease, statin use was 8.0% (95% CI 6.9–9.3) and for secondary prevention statin use was 21.9% (20.0–24.0). The WHO target that at least 50% of eligible individuals receive statin therapy to prevent cardiovascular disease was achieved by no region or income group. Statin use was less common in countries with lower health spending. At the individual level, there was generally higher statin use among women (primary prevention only, risk ratio [RR] 1.83 [95% CI 1.22–2.76), and individuals who were older (primary prevention, 60–69 years, RR 1.86 [1.04–3.33]; secondary prevention, 50–59 years RR 1.71 [1.35–2.18]; and 60–69 years RR 2.09 [1.65–2.65]), more educated (primary prevention, RR 1.61 [1.09–2.37]; secondary prevention, RR 1.28 [0.97–1.69]), and lived in urban areas (secondary prevention only, RR 0.82 [0.66–1.00]).
Interpretation
In a diverse sample of LMICs, statins are used by about one in ten eligible people for the primary prevention of cardiovascular diseases and one in five eligible people for secondary prevention. There is an urgent need to scale up statin use in LMICs to achieve WHO targets. Policies and programmes that facilitate implementation of statins into primary health systems in these settings should be investigated for the future.
Funding
National Clinician Scholars Program at the University of Michigan Institute for Healthcare Policy and Innovation, and National Institute of Diabetes and Digestive and Kidney Diseases.
Introduction
Ischaemic heart disease and stroke are responsible for more than a fifth of all deaths worldwide.1 In low-income and middle-income countries (LMICs), where 80% of these deaths occur, improving outcomes for cardiovascular diseases (including heart disease and stroke) is necessary to achieve Sustainable Development Goal (SDG) target 3.4 outlined in 2015: a reduction of a third in premature mortality from non-communicable diseases by 2030.2 The use of statins to prevent cardiovascular diseases is an important strategy for health systems to reduce the population burden of cardiovascular diseases and to achieve this SDG target.3
Statins are a type of drug that reduce cholesterol concentration through inhibition of the HMG-CoA reductase enzyme. According to evidence from clinical trials demonstrating effectiveness and safety, statins are widely recommended for the primary and secondary prevention of cardiovascular diseases4 and have been included in WHO clinical practice guidelines for cardiovascular disease prevention and control since 2007.5 In individuals at high risk, statins are considered cost-effective for primary health systems and among a package of drugs considered the so-called best buys for non-communicable disease prevention and control.6 A key target in the WHO non-communicable disease Global Monitoring Framework is that by 2025, at least 50% of eligible people with existing cardiovascular diseases or at high risk of these diseases will receive effective drug therapies including statins.7 This high frequency of statin use in patients with cardiovascular diseases has been achieved in high-income countries.8–12
There is a need for rigorous monitoring of population-based estimates of statin use in LMICs. However, to our knowledge, there has been no comprehensive evaluation of statin use in LMICs using nationally representative samples. Important previous studies assessing statin use in LMICs used non-representative samples and included data collected before 20079,13–15 when statins were added to the WHO Essential Medicines List and became more affordable via increased generic production.16 The present study addresses a crucial evidence gap in the current understanding of global cardiovascular disease prevention by aiming to estimate statin use in LMICs to track progress towards the WHO target, and investigate the country-level and individual-level characteristics associated with statin use.
Methods
Study design and participants
In our cross-sectional study, we analysed individual-level data from national health surveys done between 2013 and 2019 in 41 LMICs. Our comprehensive methodology for pooling surveys has been previously described.17,18 We first identified all LMICs in which a WHO Stepwise Approach to Surveillance (STEPS) survey had been done. We prioritised STEPS surveys because they are WHO’s recommended method for population monitoring of non-communicable disease targets. To identify other surveys in countries in which no STEPS survey was available, we did a systematic internet search in April, 2020, for each country using search terms and other details described in appendix 2 (pp 3–4).
We included surveys that met the following criteria: (1) were done in an LMIC as classified by the World Bank in the survey year; (2) were done in 2013 or later; (3) were nationally representative; (4) had individual-level data available; and (5) asked questions on statin use and previous history of cardiovascular disease. We chose 2013 as the first year of survey eligibility because this was the year that STEPS surveys introduced questions on statin use and cardiovascular disease history. Additional details on the search process, data availability, and methodology of the underlying surveys are available in appendix 2 (pp 3–7).
Our sample consisted of non-pregnant respondents aged 40–69 years. We chose this age range to align with the WHO non-communicable diseases Global Monitoring target for drug therapy to prevent cardiovascular diseases (aged 40 years and older)7 and to encompass the upper age of 69 years in most surveys.
This study was judged to be exempt from institutional review board approval by the University of Michigan (HUM00199295), because the research involved survey data that could not be linked to a specific individual.
Outcomes and procedures
Our outcomes were the proportion of eligible individuals self-reporting use of statins for the primary and secondary prevention of cardiovascular diseases. We defined these outcomes to align with the monitoring indicator recommended in the WHO non-communicable diseases Global Monitoring Framework and WHO HEARTS Technical Package for cardiovascular diseases management in primary health care: “Proportion of eligible persons receiving drug therapy…to prevent heart attacks and strokes”.7 We defined statin use among respondents on the basis of the answer to the following question in STEPS surveys: “Are you currently taking statins regularly to prevent or treat heart disease?” We defined cardiovascular disease history on the basis of the answer to the following question in STEPS surveys: “Have you ever had a heart attack or chest pain from heart disease (angina) or a stroke (cerebrovascular accident or incident)?”
Eligibility for statin therapy for primary prevention was defined among individuals without a history of self-reported cardiovascular disease and with either: (1) a history of diagnosed diabetes or (2) a 10-year cardiovascular disease risk of more than 20% using the 2019 WHO laboratory-based risk equations.19,20 These equations use individual-level inputs of age, smoking status, systolic blood pressure, history of diabetes, and total cholesterol. The measurement of biological variables across surveys is summarised in appendix 2 (pp 30–33). Eligibility for statin therapy for secondary prevention was defined among individuals with a history of self-reported cardiovascular disease.
Statistical analysis
We calculated the proportion of individuals using statins for the primary and secondary prevention of cardiovascular disease in the overall pooled sample, by WHO region and World Bank income group, and by country. We compared these results with the WHO non-communicable diseases Global Monitoring Framework’s 2025 target that at least 50% of eligible individuals in the population receive statin therapy to prevent heart attacks and strokes.7
To investigate predictors of statin use across countries, we then plotted statin use for primary and secondary prevention of cardiovascular disease against four country-level characteristics: (1) per-capita health spending in the year the survey was done; (2) per-capita gross national income using World Bank estimates in the year the survey was done; (3) burden of atherosclerotic cardiovascular disease, as assessed by the sum of disability-adjusted life-years per 100 000 people for ischaemic heart disease and ischaemic stroke, as estimated by the Global Burden of Disease study;21 and (4) political commitment to non-communicable diseases, as assessed by a 2019 version of the non-communicable diseases policy implementation score. The non-communicable diseases implementation score ranges from 0–100%, with higher scores reflecting greater political commitment to these diseases. Country-specific external data included in our analysis are presented in appendix 2 (pp 34–35).
To investigate individual-level predictors of statin use within each country and across the pooled sample, we regressed statin use on age, sex, education as a marker of socioeconomic status, and rural versus urban residence. In the within-country models, we restricted the regressions to the secondary prevention outcome due to the low number of individuals using statins for primary prevention. We used Zou’s modified Poisson regression with robust error variance because it facilitates interpretation of model output as risk ratios (RRs) and is a valid approach for analysing binary outcomes.22 We also report the absolute difference in predicted probabilities using average marginal effects.
We did multiple sensitivity analyses. First, we assessed statin use for primary prevention only among individuals aged 40 years and older with no previous history of cardiovascular disease and estimated 10-year cardio vascular disease risk of at least 20% (ie, not adhering to the WHO recommendation for statin therapy among all people aged ≥40 years with diabetes). Second, because the 2019 WHO risk equations were published after most surveys were done, we reanalysed the primary prevention outcome using the 2007 WHO/International Society of Hypertension cardiovascular disease risk charts and a 10-year cardiovascular disease risk threshold of at least 30%.5–7 Third, we re-estimated the pooled regressions without the rural versus urban residence covariate, because this information was missing in about a third of the countries (n=14 surveys). Fourth, we rescaled individual survey weights such that each country was equally weighted in the pooled analyses.
In all analyses, we accounted for complex survey design by adjusting for stratification and clustering at the primary sampling unit using the Stata “svyset” command with subpopulation specification. Additionally, we applied sampling weights, which adjust for the probability of selection, non-response, and differences between the sample population and the target population. In the main pooled analyses, we rescaled survey weights using each country’s 2019 population of people aged 40–69 years and used country-level fixed-effects. Whenever survey weights were missing, the country-average weight was assigned to observations with missing weight values. For all other data, a complete case analysis was used. Analyses were done in Stata version 16.1 and R version 4.0.5. Additional methodological details are provided in appendix 2 (pp 3–7).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
The final pooled sample included 116 449 non-pregnant individuals, 50 383 men (49.6% [95% CI 49.0–50.2] of the population-weighted sample) and 66 066 women (50.4% [49.8–51.0] of the population-weighted sample). In the overall sample, 9229 reported a previous history of cardiovascular disease (7.9% [95% CI 7.4–8.3] of the population-weighted sample). Among those without a previous history of cardiovascular disease, 8453 were eligible for a statin for primary prevention of cardiovascular disease (9.7% [95% CI 9.3–10.1] of the population-weighted sample); table; appendix 2 pp 36–38).
Among the 41 included surveys, there were at least four countries in each WHO region. Nine surveys were done in low-income countries, 17 in lower-middle-income countries, and 15 in upper-middle-income countries. In the pooled sample, self-reported statin use and previous history of cardiovascular disease were missing in 0.4% of the sample (appendix 2 pp 39–40).
In the pooled sample across countries, statin use for primary prevention was 8.0% (95% CI 6.9–9.3) and for secondary prevention was 21.9% (20.0–24.0; figure 1). By region, statin use for both primary and secondary prevention was highest in the Eastern Mediterranean region (primary prevention, 13.7% [95% CI 12.2–15.2]; secondary prevention, 39.4% [35.7–43.3]) and lowest in Africa (primary prevention, 4.5% [2.7–7.5]; secondary prevention, 10.4% [8.4–12.7]. By World Bank income group, there was a positive gradient between statin use and country-level economic development, including a seven-fold greater use of statins for primary prevention (from 2.0% [95% CI 0.9–4.7] to 13.8% [12.5–15.3]) and four-fold greater use for secondary prevention (from 8.2% [6.1–11.0] to 31.6% [28.9–34.4]) within upper-middle-income countries than in low-income countries. No region or income group achieved the WHO target of 50% use of statins among eligible individuals in the population. At the country level, only Iran achieved the WHO target for secondary prevention, and no country achieved the target for primary prevention (appendix 2 pp 41–42).
Figure 1: Pooled estimates of self-reported use of statins for the primary and secondary prevention of cardiovascular disease in 41 low-income and middle-income countries.
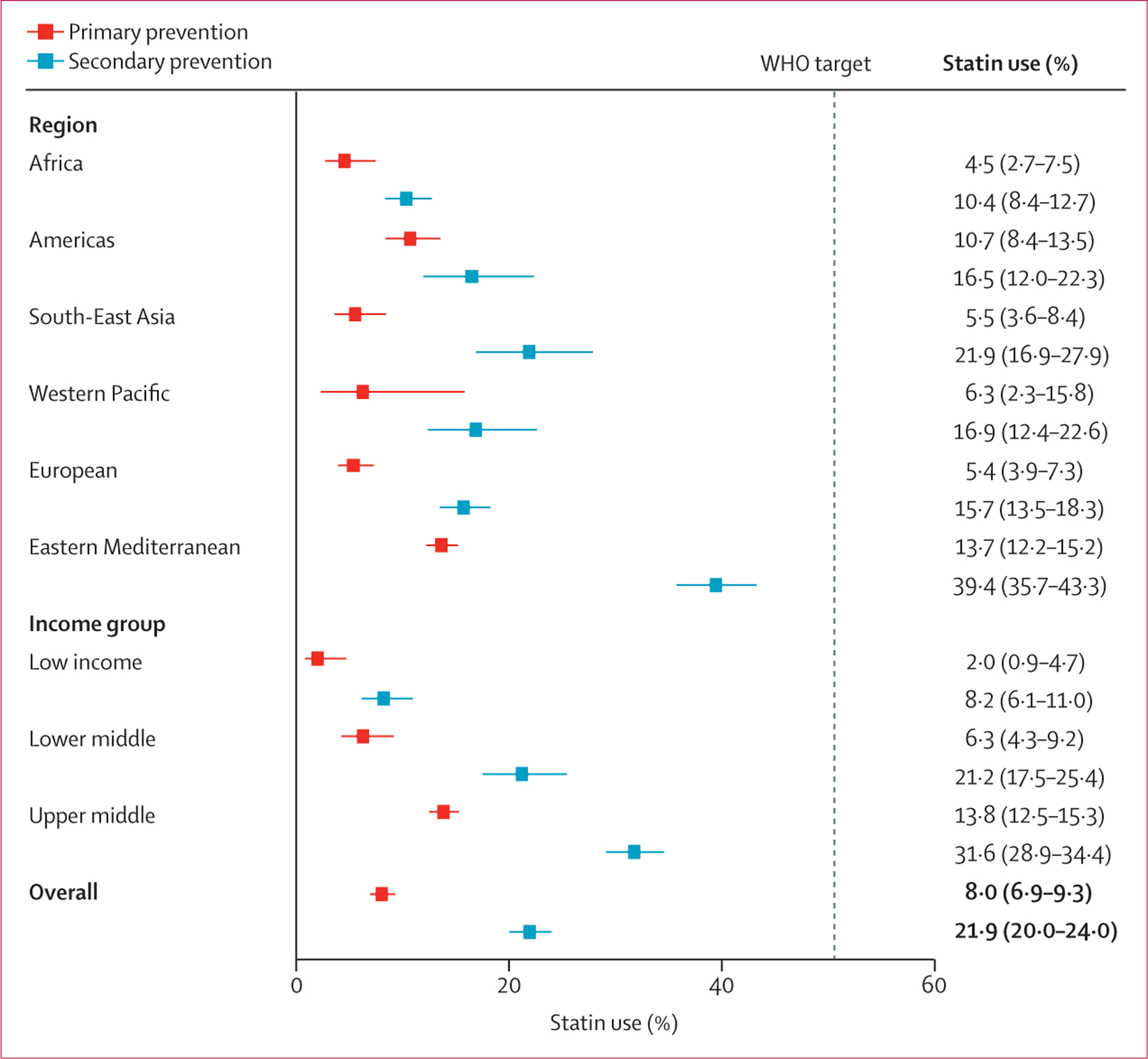
The sample includes non-pregnant individuals aged 40–69 years (age 40–64 years for Burkina Faso, Kyrgyzstan, Myanmar, and Tokelau). Estimates account for survey design and weighting by each country’s 2019 population of individuals who were aged 40–69 years. The error bars represent 95% CIs. The vertical dashed line represents the WHO target that at least 50% of eligible people use statins.
Of country characteristics examined, per-capita health spending accounted for most statistical variation in the observed statin use (R2=0.30 for primary prevention and R2=0.26 for secondary prevention; figure 2; appendix 2 43–45). Examples of countries that appeared to have greater than predicted statin use based on health spending included Iran, Jordan, Lebanon, and Sri Lanka. Per-capita income (primary prevention, R2=0.21; secondary prevention, R2=0.19), non-communicable diseases policy commitment (primary prevention, R2=0.05; secondary prevention, R2=0.11), and estimated burden of cardiovascular disease (primary prevention, R2<0.01; secondary prevention, R2=0.01) accounted for less statistical variation in observed statin use.
Figure 2: Self-reported statin use for primary prevention (A) and secondary prevention (B) by per-capita health spending.
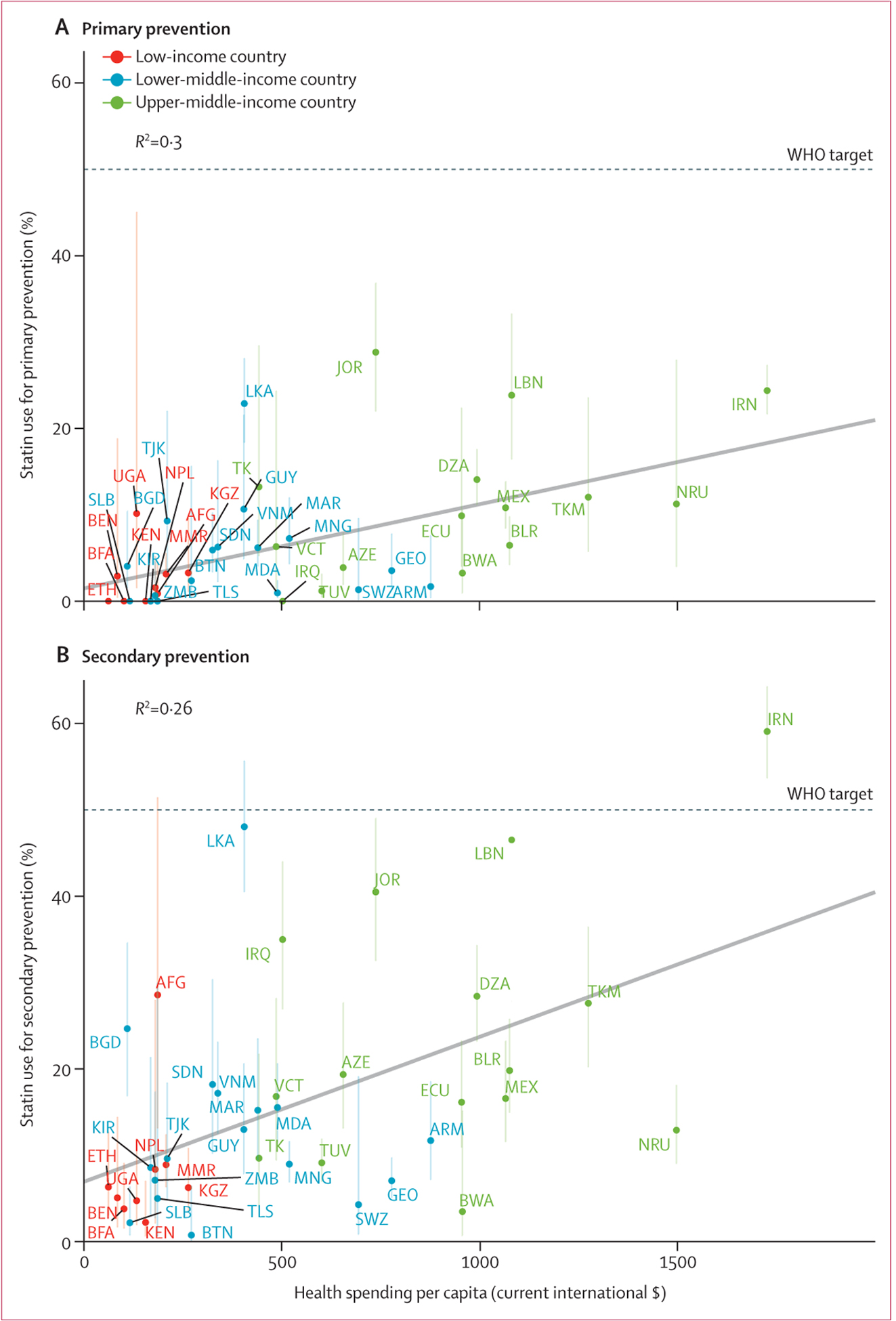
The sample includes non-pregnant individuals aged 40–69 years (aged 40–64 years for Burkina Faso, Kyrgyzstan, Myanmar, and Tokelau). Per-capita health spending is in current international dollars in the year the survey was done. Estimates account for survey design and weighting. The vertical error bars represent 95% CIs. The horizontal dashed line represents the WHO target that at least 50% of eligible people use statins. The diagonal line depicts an ordinary least-squares regression with each country having the same weight. The Iraq survey is excluded from the primary prevention analysis because use of statins was asked only among people self-reporting previous cardiovascular disease. The standardised regression coefficients were 0.55 (95% CI 0.28–0.82) for primary prevention and 0.51 (0.23–0.79) for secondary prevention. AZE=Azerbaijan. BEN=Benin. BFA=Burkina Faso. BGD=Bangladesh. BLR=Belarus. BTN=Bhutan. BWA=Botswana. DZA=Algeria. ECU=Ecuador. ETH=Ethiopia. GEO=Georgia. GUY=Guyana. IRN=Iran. IRQ=Iraq. JOR=Jordan. KEN=Kenya. KGZ=Kyrgyzstan. KIR=Kiribati. LBN=Lebanon. LKA=Sri Lanka. MAR=Morocco. MDA=Moldova. MEX=Mexico. MMR=Myanmar. MNG=Mongolia. NPL=Nepal. NRU=Nauru. SDN=Sudan. SLB=Solomon Islands. SWZ=Eswatini. TJK=Tajikistan. TK=Tokelau. TKM=Turkmenistan. TLS=Timor-Leste. TUV=Tuvalu. UGA=Uganda. VCT=St Vincent and the Grenadines. VNM=Vietnam. ZMB=Zambia.
Although there was heterogeneity across countries, characteristics of older age, higher educational attainment, and urban residence were associated with greater use of statins for secondary prevention in the within-country models (figure 3; appendix 2 pp 46–53). In the multivariable regressions of statin use in the pooled sample, older age was associated with higher statin use for both primary prevention (60–69 years RR 1.86 [95% CI 1.04–3.33]) and secondary prevention (50–59 years RR 1.71 [1.35–2.18] and 60–69 years RR 2.09 [1.65–2.65]; figure 4). Women were more likely than were men to use statins for primary prevention (RR 1.83 [95% CI 1.22–2.76]) but not secondary prevention (RR 0.95 [0.80–1.13]). Individuals with secondary or higher education were more likely to use statins than were those with no schooling (primary prevention, RR 1.61 [95% CI 1.09–2.37]; secondary prevention, RR 1.28 [0.97–1.69]). Rural residence was associated with lower use of statins than was urban residence for secondary prevention (RR 0.82 [95% CI 0.66–1.00]) but not primary prevention (RR 0.81 [0.52–1.26]).
Figure 3: Relative and absolute differences in statin use for secondary prevention of cardiovascular disease by country using modified Poisson regressions for sex (A), age (B), education (C), and residence (D).
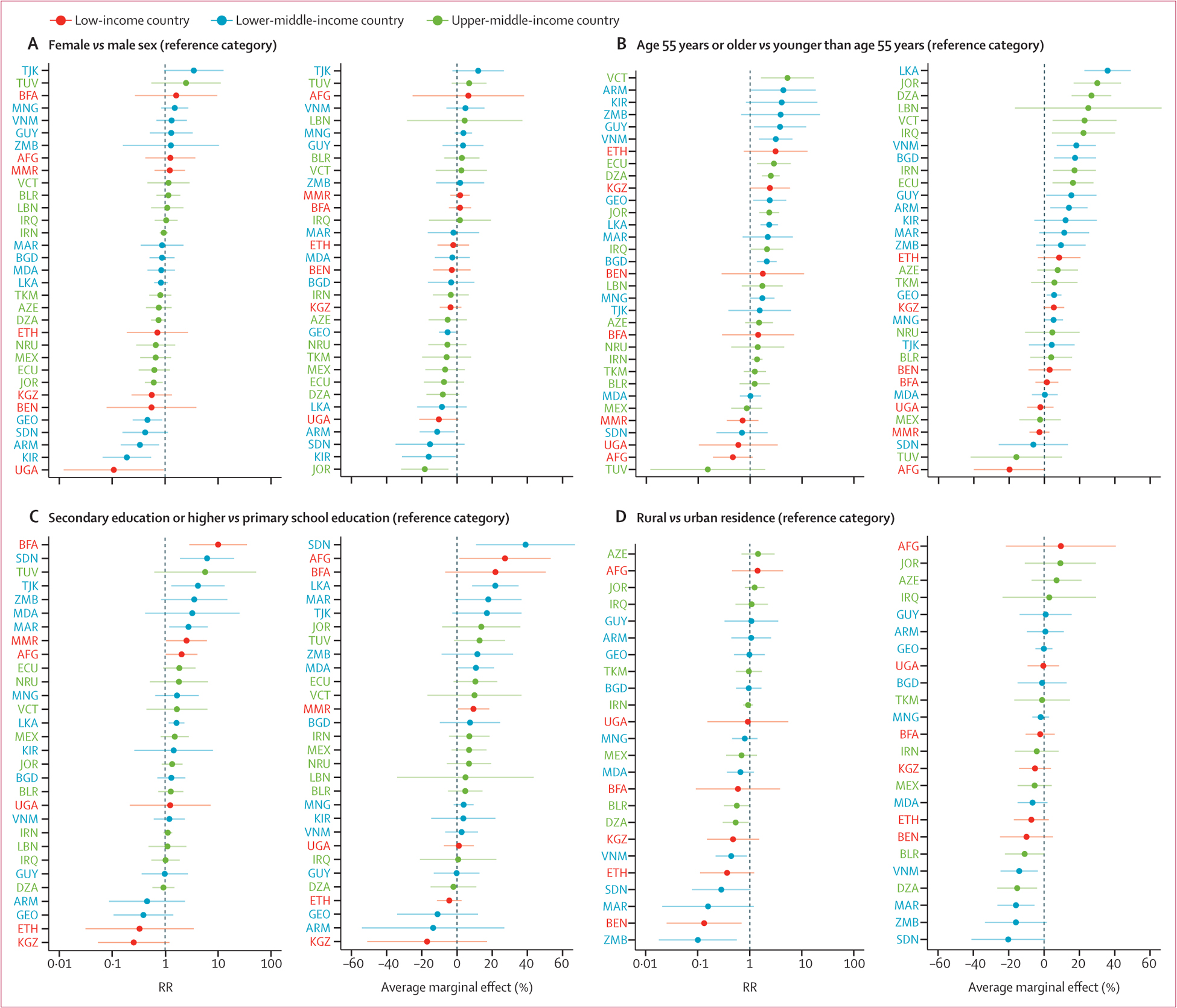
All regressions were adjusted for sex and age. Age was included in three categories (40–49 years, 50–59 years, and 60–69 years) for all the regressions except for in (B) age, in which it was dichotomised as age 55 years or older versus younger than age 55 years. The regressions account for sample weights, stratification in survey design, and clustering at the level of the primary sampling unit. Error bars show the 95% CIs. Education was not available in the survey from Tokelau. Rural versus urban residence was unavailable in 14 surveys (Botswana, Ecuador, Eswatini, Kiribati, Lebanon, Myanmar, Nauru, Solomon Islands, Sri Lanka, St Vincent and the Grenadines, Tajikistan, Timor-Leste, Tokelau, and Tuvalu). Differences in the ordering of countries by RRs versus average marginal effects is due to the difference in baseline statin use among countries. RR=risk ratio. AFG=Afghanistan. ARM=Armenia. AZE=Azerbaijan. BEN=Benin. BFA=Burkina Faso. BGD=Bangladesh. BLR=Belarus. BTN=Bhutan. BWA=Botswana. DZA=Algeria. ECU=Ecuador. ETH=Ethiopia. GEO=Georgia. GUY=Guyana. IRN=Iran. IRQ=Iraq. JOR=Jordan. KEN=Kenya. KGZ=Kyrgyzstan. KIR=Kiribati. LBN=Lebanon. LKA=Sri Lanka. MAR=Morocco. MDA=Moldova. MEX=Mexico. MMR=Myanmar. MNG=Mongolia. NPL=Nepal. NRU=Nauru. SDN=Sudan. SLB=Solomon Islands. SWZ=Eswatini. TJK=Tajikistan. TK=Tokelau. TKM=Turkmenistan. TLS=Timor-Leste. TUV=Tuvalu. UGA=Uganda. VCT=St Vincent and the Grenadines. VNM=Vietnam. ZMB=Zambia.
Figure 4: Relative and absolute differences in statin use across the pooled sample using modified Poisson regressions for primary prevention (A) and secondary prevention (B).
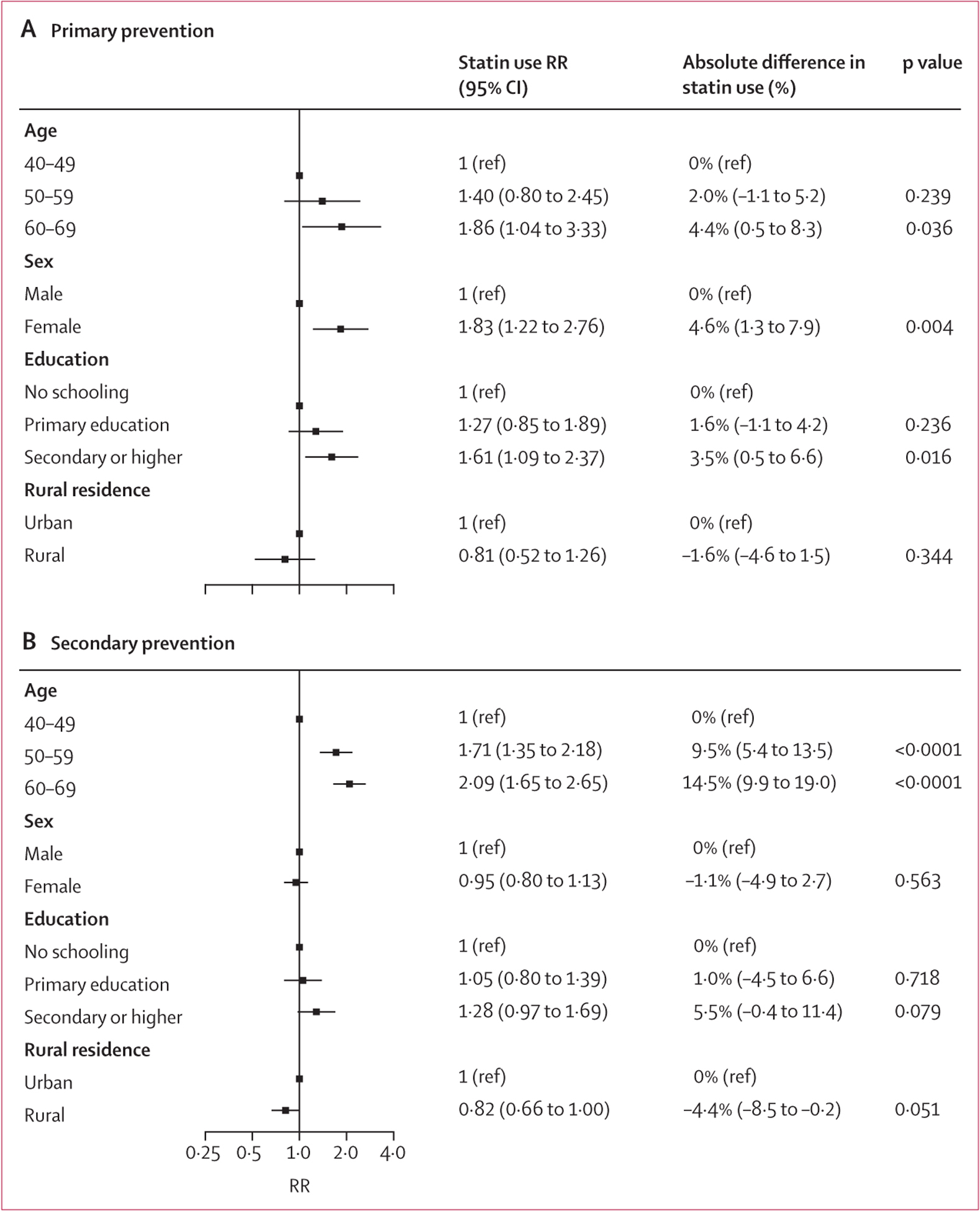
Results are presented as RRs (95% CIs) and average marginal effects weighting each country by its 2019 population of individuals aged 40–69 years. The models include each of the covariates listed in the plot, country-level fixed effects, and account for clustering at the level of the primary sampling unit. Due to incomplete data, the surveys from Botswana, Ecuador, Eswatini, Kiribati, Lebanon, Myanmar, Nauru, Solomon Islands, Sri Lanka, St Vincent and the Grenadines, Tajikistan, Timor-Leste, Tokelau, and Tuvalu were excluded from the pooled regression analysis. The Iraq survey only contributes to the analysis of secondary prevention of cardiovascular disease. p values refer to the output from the modified Poisson regression model rather than p value of the average marginal effect. RR=risk ratio.
The sensitivity analyses assessing statin use for primary prevention among individuals aged 40 years and older with 10-year cardiovascular disease risk of more than 20% (ie, removing the universal indication for statins among people with diabetes), using the 2007 WHO/International Society of Hypertension cardiovascular disease risk charts with 10-year cardiovascular disease risk threshold of at least 30%, and excluding the rural versus residence variable, were consistent with the main analyses. The sensitivity analysis using equal country weights showed slightly lower estimates for overall statin use for primary (6.7% [95% CI 5.8–7.7]) and secondary (15.9% [14.7–17.2]) prevention of cardiovascular disease. The remainder of the results from this sensitivity analysis mirrored the patterns of individual-level associations with statin use that were observed in the main analyses. Full results from these sensitivity analyses are provided in appendix 2 (pp 54–60).
Discussion
In a geographically and economically diverse sample of nationally representative surveys from 41 low-income and middle-income countries, we found that statins were used by approximately one in ten eligible people for the primary prevention of cardiovascular disease and one in five eligible people for the secondary prevention of cardiovascular disease. The WHO target that at least 50% of eligible individuals receive statin therapy to prevent cardiovascular disease was achieved by no region or income group and by just a single country (and only for secondary prevention) in this set of LMICs. At the country level, statin use was lower in countries with lower health spending. At the individual level, there was generally lower statin use among men (primary prevention only) and individuals who were younger, less educated, or lived in rural areas. These estimates provide the first nationally representative and most geographically expansive evidence about the patterns of statin use in many LMICs. Our findings can serve to evaluate progress towards global non-communicable disease targets and to guide health systems’ responses to the large and rising cardiovascular disease burden in LMICs.
Statins are widely recommended in clinical practice guidelines and were added to the WHO Essential Medicine List in 2007.5,16 Nevertheless, we find that statin use for cardiovascular disease has remained very low.9 By contrast, in surveys done in the USA and other high-income countries, statin use is 60–70% for secondary cardiovascular disease prevention8,9 and approximately 50% for primary cardiovascular disease prevention in people with diabetes or a 10-year cardiovascular disease risk of at least 20%.10–12 Statin use is much higher in the upper-middle-income countries than in the lower-middle-income or low-income countries included in our study. Previous work using pooled surveys from LMICs has demonstrated that about three quarters of people with diagnosed hypertension take antihypertensive medications,17 and 85% of people with diagnosed diabetes take glucose-lowering medications.23 Given the disproportionate burden of cardiovascular disease in LMICs and the strong clinical evidence supporting statin therapy, our findings emphasise the urgent need to scale up statin use relative to other medicines to prevent and control non-communicable diseases.
Important previous studies assessing the use of statins in multiple LMICs include the WHO study on Prevention of REcurrences of Myocardial Infarction and StrokE (WHO-PREMISE)15 and the Prospective Urban Rural Epidemiology (PURE) study.9,13,14 WHO-PREMISE was a cross-sectional study at health facilities in ten LMICs from 2002 to 2003 in which 20% of patients with a previous history of cardiovascular disease reported using statins.15 PURE is a prospective cohort study done in more than 20 high-income, middle-income, and low-income countries. In baseline data collected between 2003 and 2009, statin use for secondary prevention was reported by 3.3% of individuals in low-income countries, 4.3% in lower-middle-income countries, 17.6% in upper-middle-income countries, and 66.5% in high-income countries.9 Most of the variation in statin use was explained by between-country differences,9 but there were differences observed by individual-level characteristics such as socioeconomic position.13
Our study adds to the evidence previously provided by WHO-PREMISE and PURE to substantially advance the understanding of statin use globally for many LMICs. First, previous studies used sampling frames that were not strictly representative compared with nationally representative data used in the current study. Such nationally representative estimates are preferred by WHO to monitor progress in meeting non-communicable disease targets.7 Second, we compiled the most recently available survey data (2013 or later) on statin use in LMICs. By contrast to previous studies, all surveys included in our study were done after simvastatin was added to the WHO Essential Medicine List in 2007 and after statin patents expired in the USA (2006–12), which was associated with large decreases in international prices for statins.16,24 Third, we include a much larger sample of countries than did WHO-PREMISE or PURE. Fourth, a novel aspect of our study is that we estimate statin use not only for secondary prevention of cardiovascular disease, but also for primary prevention by applying eligibility criteria from recently updated WHO clinical guidelines and risk equations.19,20 Finally, our study uses the most up-to-date data available to track progress towards the stated target for statin therapy in the WHO Global Monitoring Framework for non-communicable diseases.7
An important finding in our study was the substantial variation in statin use between countries. We found that country-level characteristics explained only a modest amount of the observed between-country differences in statin use. For example, the variation in per-capita health spending explained approximately a quarter of the variance in statin use for secondary prevention (R2=0.26) in our study, which is substantially lower than in the PURE study (R2=0.77), although PURE also included data from high-income countries.9 Our comparisons also allowed us to identify countries where statins were more commonly used than what would be predicted on the basis of health spending or other country characteristics alone. Examples included several countries in the Eastern Mediterranean WHO region, including Iraq, Iran, Jordan, and Lebanon. The results from Iran are notable because it was the only country in our sample that has already achieved the 2025 WHO non-communicable disease target of 50% statin use for secondary prevention of cardiovascular disease, although this was not the case for primary prevention. Potential explanations for these observations in Iran include the country’s political commitment to non-communicable diseases, establishment of a multisectoral national non-communicable diseases committee, and prioritisation of interventions classified by WHO as so-called best buys.25 Our findings can inform subsequent health system research investigating the underlying reasons why some health systems—including those in countries with low per-capita health spending—are more likely to offer statin therapy to eligible individuals.
At the individual level, although there was heterogeneity among surveys, we found greater statin use among individuals who were older, had greater educational attainment, and lived in urban rather than rural areas, which was generally consistent with previous studies.9,15 In previous research on hypertension and diabetes care in LMICs, older age and higher education have emerged as strong predictors of diagnosis, treatment, and control of these conditions.17,18 The greater use of statins for primary prevention of cardiovascular disease among women has also been reported in the PURE study.26 However, unlike in the PURE study, women with a previous history of cardiovascular disease had similar rates of statin use to those of men in our study.
Several reasons might explain the lower use of statins relative to other cardiovascular disease medicines in LMICs. Cholesterol measurements are typically more costly than measurements of other risk factors such as blood pressure or blood glucose. Previous clinical guidelines focused on cholesterol target concentrations for statin initiation and monitoring, so these higher measurement costs might have led clinicians and policy makers to focus less on statins. Additionally, the burden of cardiovascular disease attributable to elevated cholesterol has been lower than that attributable to elevated blood pressure in cohort studies.27 As a result, national policies might have prioritised blood pressure medications over statins even though the relative risk reduction for statin therapy is similar to that of anti-hypertensive therapy.28 An example demonstrating this dynamic is that statins were added to the WHO Essential Medicine List in 2007,16 yet statins are included in national essential medicines lists in only two thirds of LMICs—a lower proportion than other essential cardiovascular disease medicines.3,29 Finally, statins are less affordable in LMICs than are other medicines used to prevent and control cardiovascular disease, as documented by PURE’s finding that statins cost 17% of discretionary household income in urban areas and 49% in rural areas of low-income countries.30 International prices for statins are similar to those of these other medicines,31 suggesting that procurement prices alone are probably insufficient to explain the low statin use observed in our study.
The WHO HEARTS Technical Package provides a template for implementing multilevel strategies to scale up statin use in LMICs.32 Along with structural enabling factors such as health system capacity for point-of-care lipid testing and political buy-in to harness necessary investments, relevant HEARTS package components include simplified clinical protocols, secure procurement of quality-assured medications and measurement devices, task-sharing among clinical teams, community-based delivery of care, and strengthened information systems.32 In settings with limited laboratory capacity, greater use of non-laboratory risk scores could support a risk-based approach to cardiovascular disease prevention, as recommended in HEARTS. Finally, fixed-dose combination medications (ie, so-called polypills), which are effective in reducing cardiovascular disease,33 also have the potential to increase appropriate use of both statins and blood pressure medications.
Our study has several limitations. First, we rely on self-reported measures of a previous history of cardiovascular disease and statin use. We justify using these self-reported measures as they are the recommended methodology in the WHO non-communicable disease Monitoring Framework.7 To our knowledge, there is no research validating self-reported medical history or medication use in STEPS surveys. Previous studies support the reliability of self-reports of cardiovascular disease history, including accuracy of 89% in the PURE study.9 Self-reports for cardiovascular disease medications have also been found to have high levels of accuracy in previous studies.34,35 Second, we were unable to capture important details of medication use such as the specific statin agent or dose, whether the statin was generic or branded, and whether the respondent had taken statins in the past but stopped them due to side-effects. Although these details would not have affected our estimates of statin eligibility as defined by WHO, it would have allowed us to comment on the appropriateness of statin intensity, cost, and other factors. Third, our findings are mainly generalisable to the countries in which surveys were done, and we were unable to include surveys from some large LMICs, such as China and India. Results at the country level should be interpreted with caution. However, our study is unique in its use of nationally representative, individual-level data from surveys done in a diverse set of countries that collectively represent a total population of more than 1 billion people. In future research, we hope to assess statin use using harmonised data from countries of all income levels. Fourth, we did not assess statin use by target lipid concentrations—an approach recommended in previous guidelines and applied in our group’s previous work36—because low-density lipoprotein cholesterol data were unavailable in approximately two-thirds of surveys. Finally, our analyses of statin use for primary prevention rely on cardiovascular risk scores developed by WHO that might not be accurately calibrated to all countries in our analysis.
In conclusion, our results emphasise the urgent need to scale up statin use in LMICs, where most of the global cardiovascular risk burden occurs. Policies and programmes that facilitate the successful implementation of statins into primary health systems in these settings must be investigated in future research and advocacy.
Supplementary Material
Table:
Survey characteristics
| ISO code | Income group* | Year† | Response rate‡ | Sample size§ (n) | Proportion of female participants | Median age, years (IQR) | |
|---|---|---|---|---|---|---|---|
| Africa | |||||||
| Algeria | DZA | UMIC | 2016–17 | 93.8 | 3648 | 54% | 50 (44–58) |
| Benin | BEN | LIC | 2015 | 98.5 | 2010 | 48% | 50 (44–56) |
| Botswana | BWA | UMIC | 2014 | 64.0 | 1511 | 70% | 51 (45–58) |
| Burkina Faso | BFA | LIC | 2013 | 97.2 | 1936 | 48% | 49 (44–55) |
| Eswatini | SWZ | L-MIC | 2014 | 70.0 | 1360 | 69% | 52 (45–60) |
| Ethiopia | ETH | LIC | 2015 | 95.5 | 3236 | 54% | 50 (42–56) |
| Kenya | KEN | LIC | 2015 | 93.0 | 1750 | 59% | 51 (44–59) |
| Uganda | UGA | LIC | 2014 | 92.2 | 1337 | 60% | 50 (44–57) |
| Zambia | ZMB | L-MIC | 2017 | 65.0 | 1597 | 62% | 50 (44–59) |
| Americas | |||||||
| Ecuador | ECU | UMIC | 2018 | 69.4 | 2352 | 57% | 52 (45–60) |
| Guyana | GUY | L-MIC | 2016 | 77.0 | 1370 | 57% | 52 (46–60) |
| Mexico | MEX | UMIC | 2018–19 | 98.0 | 20 287 | 55% | 51 (45–59) |
| St Vincent and the Grenadines | VCT | UMIC | 2013 | 67.8 | 1965 | 52% | 52 (46–58) |
| Eastern Mediterranean | |||||||
| Afghanistan | AFG | LIC | 2018 | NA | 1621 | 42% | 50 (45–60) |
| Iran | IRN | UMIC | 2016 | 98.4 | 14 378 | 52% | 52 (45–59) |
| Iraq | IRQ | UMIC | 2015 | 93.5 | 1839 | 58% | 50 (44–59) |
| Jordan | JOR | UMIC | 2019 | 63.0 | 2595 | 59% | 51 (45–59) |
| Lebanon | LBN | UMIC | 2017 | 65.9 | 1301 | 58% | 53 (47–59) |
| Morocco | MAR | L-MIC | 2017 | 89.0 | 2782 | 63% | 52 (45–59) |
| Sudan | SDN | L-MIC | 2016 | 88.0 | 3267 | 57% | 50 (45–58) |
| Europe | |||||||
| Armenia | ARM | L-MIC | 2016 | 42.0 | 1399 | 70% | 55 (48–61) |
| Azerbaijan | AZE | UMIC | 2017 | 97.3 | 1783 | 60% | 55 (48–60) |
| Belarus | BLR | UMIC | 2016 | 87.0 | 3453 | 60% | 54 (47–61) |
| Georgia | GEO | L-MIC | 2016 | 75.7 | 2938 | 71% | 56 (49–62) |
| Kyrgyzstan | KGZ | LIC | 2013 | 100.0 | 1602 | 63% | 51 (46–57) |
| Moldova | MDA | L-MIC | 2013 | 83.5 | 3133 | 62% | 55 (49–61) |
| Tajikistan | TJK | L-MIC | 2016 | 94.0 | 1330 | 57% | 50 (45–57) |
| Turkmenistan | TKM | UMIC | 2018 | 93.8 | 2005 | 58% | 50 (44–58) |
| South-East Asia | |||||||
| Bangladesh | BGD | L-MIC | 2018 | 83.8 | 3666 | 47% | 49 (44–56) |
| Bhutan | BTN | L-MIC | 2014 | 89.9 | 1343 | 57% | 50 (45–57) |
| Myanmar | MMR | LIC | 2014 | 90.0 | 5506 | 65% | 51 (45–57) |
| Nepal | NPL | LIC | 2019 | 86.4 | 2662 | 58% | 51 (45–60) |
| Sri Lanka | LKA | L-MIC | 2014 | 72.0 | 3148 | 59% | 53 (46–60) |
| Timor-Leste | TLS | L-MIC | 2014 | 96.3 | 1334 | 53% | 51 (44–61) |
| Western Pacific | |||||||
| Kiribati | KIR | L-MIC | 2015 | 55.0 | 943 | 54% | 50 (44–57) |
| Mongolia | MNG | L-MIC | 2019 | 98.0 | 3415 | 56% | 52 (45–59) |
| Nauru | NRU | UMIC | 2015–16 | 74.5 | 461 | 55% | 50 (44–56) |
| Solomon Islands | SLB | L-MIC | 2015 | 58.4 | 1155 | 50% | 50 (44–57) |
| Tokelau | TK | UMIC | 2014 | 70.0 | 266 | 53% | 51 (46–57) |
| Tuvalu | TUV | UMIC | 2015 | 76.0 | 615 | 55% | 53 (47–59) |
| Vietnam | VNM | L-MIC | 2015 | 79.8 | 2150 | 55% | 52 (45–59) |
| Total | .. | .. | .. | 86.7 (70.0–93.9)¶ | 116 449 | 57% (54–60)¶ | 51 (50–52)¶ |
World regions are defined by WHO. ISO=International Organization for Standardization. UMIC=Upper-middle-income country. LIC=low-income country. L-MIC=lower-middle-income country. NA=not applicable.
Income groups are defined by the World Bank fiscal year categories in the year the survey was done.
Year reflects the year(s) of survey data collection.
Values are the response rate for biochemical measurements, if available, as reported by the survey.
The sample includes non-pregnant individuals aged 40–69 years of age (40–64 years of age for Burkina Faso, Kyrgyzstan, Myanmar, and Tokelau).
Median value and IQR with an equal weighting for each country.
Research in context.
Evidence before this study
We searched PubMed on July 5, 2021, without language or date restrictions using the search terms “statin*” and (“developing countries” or “low-and middle-income countries” or “low-income countries” or “middle-income countries”) in the title and abstract. We identified two large important studies assessing the use of statins in multiple low-income and middle-income countries (LMICs). The first was the WHO study on Prevention of REcurrences of Myocardial Infarction and StrokE (WHO-PREMISE)—a cross-sectional study done at health facilities in ten LMICs from 2002 to 2003, in which 20% of patients with a previous history of cardiovascular disease reported using statins. The second was the Prospective Urban Rural Epidemiology (PURE) cohort study done in more than 20 high-income, middle-income, and low-income countries. In baseline data for secondary prevention collected between 2003 and 2009, the PURE study reported statin use of 3.3% for individuals in low-income countries, 4.3% in lower-middle-income countries, 17.6% in upper-middle-income countries, and 66.5% in high-income countries. However, these previous studies used sampling frames that were not nationally representative, included data collected before statins were added to the WHO Essential Medicine List in 2007, and did not estimate statin use for primary prevention using recommended WHO eligibility criteria.
Added value of this study
To our knowledge, our study provides the most up-to-date and geographically expansive evidence to track progress towards the target in the WHO Global Monitoring Framework for non-communicable diseases that, by 2025, at least 50% of eligible people with existing cardiovascular disease or at high risk of cardiovascular disease receive statins. Our study adds value through three crucial findings. First, in a diverse sample of nationally representative health surveys from 41 LMICs, we found that statins were used by about one in ten eligible people for the primary prevention of cardiovascular disease and by about one in five eligible people for secondary prevention. Second, the WHO target that at least 50% of eligible individuals receive statin therapy to prevent cardiovascular disease was achieved by no region or income group and by just a single country (and only for the secondary prevention outcome) in this set of LMICs. Third, in assessing country-level and individual-level factors associated with statin use, we observed less statin use in less wealthy countries, in the African region, and among traditionally marginalised groups such as those with less education or living in rural settings.
Implications of all the available evidence
Although there were variations between and within countries, our study emphasises the crucial need to scale up statin therapy in LMICs, even relative to other common medicines used to prevent and control cardiovascular disease such as anti-hypertensive and glucose-lowering therapies. Policies and programmes that facilitate implementation of statins into primary health systems in these settings should be investigated. A potential approach to scaling up statin use in LMICs would build on the successful experiences of countries implementing hypertension care programmes using the WHO HEARTS framework, which emphasises simplified clinical protocols, secure procurement of quality-assured medications and measurement devices, task-sharing among clinical teams, community-based delivery of care, and strengthened information systems.
Acknowledgments
DF was supported by the National Clinician Scholars Program at the University of Michigan Institute for Healthcare Policy & Innovation. JM-G was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grant number, K23 DK125162). PG was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (award number, KL2TR003143). MH was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grant number P30DK092926). This study was supported in part by the Open Access Publication Funds of the University of Goettingen. The contents of this research are solely the responsibility of the authors and do not necessarily represent the official views of the US National Institutes of Health.
Declaration of interests
JML reports graduate research funding from the German Academic Scholarship Foundation. RA reports contracts with Novo Nordisk, Union for International Cancer Control’s, National Institute for Health Research, and Sloan Memorial Kettering Hospital, outside of the submitted work. RA also reports payments or honoraria from Merck, Novartis, and F Hoffmann-La Roche, outside of the submitted work. TWB reports support from the Alexander von Humboldt Foundation. MH reports grants from the National Institutes of Health and receives salary support from Physician for Human Rights. MDH received funding in the past 3 years from the World Heart Federation to serve as its senior programme advisor for the Emerging Leaders programme, which has been supported by Boehringer Ingelheim, Novartis, Bupa, and AstraZeneca. MDH also received support from the American Heart Association, Verily, AstraZeneca, and American Medical Association for work unrelated to this research. MDH plans to submit patents for heart failure polypill. MDH has received meeting or travel support from the American Heart Association and World Heart Federation. MDH has an appointment at The George Institute for Global Health, which has a patent, licence, and has received investment funding with intent to commercialise fixed-dose combination therapy through its social enterprise business, George Medicines. DF reports grant funding within the past 3 years from a Pilot and Feasibility Grant funded by the Michigan Center for Diabetes Translational Research (NIH Grant P30-DK092926) and a grant from the Swinmurn Foundation to implement a sustainable diabetes clinic in Guatemala. DF also reports volunteer affiliations with Wuqu’ Kawoq and GlucoSalud, outside of the submitted work. During the course of this study, DF has received research fellowship funding from National Clinician Scholars Program at the University of Michigan Institute for Healthcare Policy & Innovation. All other authors declare no competing interests.
Footnotes
For the Spanish translation of the abstract see Online for appendix 1
See Online for appendix 2
Data sharing
Data included in this study are publicly available for 38 of the 41 included country surveys. A complete list of web addresses and contacts regarding data access is presented in appendix 2 (p 29). The replication code is available at the Harvard Dataverse (https://doi.org/10.7910/DVN/BTSHNR).
References
- 1.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol 2020; 76: 2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NCD. Countdown collaborators. NCD Countdown 2030: pathways to achieving Sustainable Development Goal target 3.4. Lancet 2020; 396: 918–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wirtz VJ, Kaplan WA, Kwan GF, Laing RO. Access to medications for cardiovascular diseases in low-and middle-income countries. Circulation 2016; 133: 2076–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cholesterol Treatment Trialists. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet 2010; 376: 1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Prevention of cardiovascular disease: guidelines for assessment and management of cardiovascular risk. Geneva: World Health Organization, 2007. [Google Scholar]
- 6.WHO. Tackling NCDs: “Best buys” and other recommended interventions for the prevention and control of noncommunicable diseases. Geneva: World Health Organization, 2017. [Google Scholar]
- 7.WHO. Noncommunicable Diseases Global Monitoring Framework: indicator definitions and specifications. 2014. https://www.who.int/nmh/ncd-tools/indicators/GMF_Indicator_Definitions_Version_NOV2014.pdf (accessed Sept 29, 2021).
- 8.Shah NS, Huffman MD, Ning H, Lloyd-Jones DM. Trends in myocardial infarction secondary prevention: the National Health and Nutrition Examination Surveys (NHANES), 1999–2012. J Am Heart Assoc 2015; 4: e001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yusuf S, Islam S, Chow CK, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): a prospective epidemiological survey. Lancet 2011; 378: 1231–43. [DOI] [PubMed] [Google Scholar]
- 10.Patel N, Bhargava A, Kalra R, et al. Trends in lipid, lipoproteins, and statin use among US adults: impact of 2013 cholesterol guidelines. J Am Coll Cardiol 2019; 74: 2525–28. [DOI] [PubMed] [Google Scholar]
- 11.Leino AD, Dorsch MP, Lester CA. Changes in statin use among US adults with diabetes: a population-based analysis of NHANES 2011–2018. Diabetes Care 2020; 43: 3110–12. [DOI] [PubMed] [Google Scholar]
- 12.Ueda P, Lung TW, Lu Y, et al. Treatment gaps and potential cardiovascular risk reduction from expanded statin use in the US and England. PLoS One 2018; 13: e0190688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy A, Palafox B, O’Donnell O, et al. Inequalities in the use of secondary prevention of cardiovascular disease by socioeconomic status: evidence from the PURE observational study. Lancet Glob Health 2018; 6: e292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow CK, Nguyen TN, Marschner S, et al. Availability and affordability of medicines and cardiovascular outcomes in 21 high-income, middle-income and low-income countries. BMJ Glob Health 2020; 5: e002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendis S, Abegunde D, Yusuf S, et al. WHO study on prevention of REcurrences of Myocardial Infarction and StrokE (WHO-PREMISE). Bull World Health Organ 2005; 83: 820–29. [PMC free article] [PubMed] [Google Scholar]
- 16.Kishore SP, Blank E, Heller DJ, et al. Modernizing the World Health Organization list of essential medicines for preventing and controlling cardiovascular diseases. J Am Coll Cardiol 2018; 71: 564–74. [DOI] [PubMed] [Google Scholar]
- 17.Geldsetzer P, Manne-Goehler J, Marcus ME, et al. The state of hypertension care in 44 low-income and middle-income countries: a cross-sectional study of nationally representative individual-level data from 1.1 million adults. Lancet 2019; 394: 652–62. [DOI] [PubMed] [Google Scholar]
- 18.Manne-Goehler J, Geldsetzer P, Agoudavi K, et al. Health system performance for people with diabetes in 28 low-and middle-income countries: a cross-sectional study of nationally representative surveys. PLoS Med 2019; 16: e1002751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. WHO package of essential noncommunicable (PEN) disease interventions for primary health care. Geneva: World Health Organization, 2020. [Google Scholar]
- 20.Kaptoge S, Pennells L, De Bacquer D, et al. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health 2019;7: e1332–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396: 1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159: 702–06. [DOI] [PubMed] [Google Scholar]
- 23.Flood D, Seiglie JA, Dunn M, et al. The state of diabetes treatment coverage in 55 low-income and middle-income countries: a cross-sectional study of nationally representative, individual-level data in 680 102 adults. Lancet Healthy Longevity 2021; 2: e340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kidd J Life after statin patent expiries. Nat Rev Drug Discov 2006; 5: 813–14. [DOI] [PubMed] [Google Scholar]
- 25.Bakhtiari A, Takian A, Majdzadeh R, Haghdoost AA. Assessment and prioritization of the WHO “best buys” and other recommended interventions for the prevention and control of non-communicable diseases in Iran. BMC Public Health 2020; 20: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walli-Attaei M, Joseph P, Rosengren A, et al. Variations between women and men in risk factors, treatments, cardiovascular disease incidence, and death in 27 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet 2020; 396: 97–109. [DOI] [PubMed] [Google Scholar]
- 27.Yusuf S, Joseph P, Rangarajan S, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet 2020; 395: 795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karmali KN, Lloyd-Jones DM, Berendsen MA, et al. Drugs for primary prevention of atherosclerotic cardiovascular disease: an overview of systematic reviews. JAMA Cardiol 2016; 1: 341–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Husain MJ, Datta BK, Kostova D, et al. Access to cardiovascular disease and hypertension medicines in developing countries: an analysis of essential medicine lists, price, availability, and affordability. J Am Heart Assoc 2020; 9: e015302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khatib R, McKee M, Shannon H, et al. Availability and affordability of cardiovascular disease medicines and their effect on use in high-income, middle-income, and low-income countries: an analysis of the PURE study data. Lancet 2016; 387: 61–69. [DOI] [PubMed] [Google Scholar]
- 31.Management Sciences for Health. International medical products price guide, 2015 edn. Medford, MA: Management Sciences for Health, 2016. [Google Scholar]
- 32.WHO. Hearts: technical package for cardiovascular disease management in primary health care. Geneva: World Health Organization, 2016. [Google Scholar]
- 33.Joseph P, Roshandel G, Gao P, et al. Fixed-dose combination therapies with and without aspirin for primary prevention of cardiovascular disease: an individual participant data meta-analysis. Lancet 2021; 398: 1133–46. [DOI] [PubMed] [Google Scholar]
- 34.Hafferty JD, Campbell AI, Navrady LB, et al. Self-reported medication use validated through record linkage to national prescribing data. J Clin Epidemiol 2018; 94: 132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richardson K, Kenny RA, Peklar J, Bennett K. Agreement between patient interview data on prescription medication use and pharmacy records in those aged older than 50 years varied by therapeutic group and reporting of indicated health conditions. J Clin Epidemiol 2013; 66: 1308–16. [DOI] [PubMed] [Google Scholar]
- 36.Marcus ME, Ebert C, Geldsetzer P, et al. Unmet need for hypercholesterolemia care in 35 low- and middle-income countries: a cross-sectional study of nationally representative surveys. PLoS Med 2021; 18: e1003841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in this study are publicly available for 38 of the 41 included country surveys. A complete list of web addresses and contacts regarding data access is presented in appendix 2 (p 29). The replication code is available at the Harvard Dataverse (https://doi.org/10.7910/DVN/BTSHNR).


