Abstract
Objectives
Curcumin is a potential complementary treatment for ulcerative colitis (UC). This overview systematically summarizes and evaluates the existing evidence of curcumin in the treatment of UC.
Methods
Two researchers searched seven databases for systematic reviews (SRs)/meta-analyses (MAs) which are about randomized controlled trials (RCTs) on curcumin for UC. Two researchers use the Assessment of Multiple Systematic Reviews 2 (AMSTAR-2), the Risk of Bias in Systematic Reviews (ROBIS) scale, the list of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), and the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system to assess the included SRs/MAs.
Results
Seven published SRs/MAs were included in our study. According to the results of the AMSTAR-2 assessment, all SRs/MAs are considered to be of very low quality. According to the ROBIS evaluation results, no SR/MA has been assessed as a low risk of bias. According to the results of the PRISMA checklist assessment, no SR/MA has been fully reported on the PRISMA checklist. According to GRADE, a total of 19 outcome indicators extracted from the included SRs/MAs were evaluated. The quality of evidence was 10 moderate, 6 low, and 3 very low.
Conclusions
Curcumin may be an effective and safe complementary treatment for UC. However, further standard and comprehensive SRs/MAs and RCTs are needed to provide an evidence-based medical rationale for this.
1. Introduction
Ulcerative colitis (UC) is a type of inflammatory bowel disease characterized by recurrent abdominal pain, diarrhea, and bloody pus. The lesions involve the colonic mucosa and submucosa [1]. UC can cause significant disturbance of colon inflammation homeostasis and severe damage to the intestinal barrier function, affecting millions of people worldwide. It cannot be cured completely; it must be managed for life [2]. Aminosalicylic acid, hormones, and immunosuppressive agents are mainly used in the clinical treatment of UC, but there are obvious adverse reactions. For example, mesalazine is a first-line drug for the treatment of UC, but long-term use can damage the liver and kidney function of patients [3]. In addition, drug resistance, dependence, and adverse reactions further limit the clinical efficacy of UC [4]. Due to ineffective long-term treatment, UC is likely to develop into colon cancer, and as many as 15% of patients may require the surgical removal of the colon in the late stage of the disease [5]. Therefore, many UC patients as well as clinicians and researchers are increasingly considering complementary and alternative medicine options [6].
Curcumin is a yellow bioactive polyphenol compound extracted from the root of the turmeric plant (Curcuma longa). It has a wide range of physiological and pharmacological activities, including anti-inflammatory, antioxidant, anticancer, neuroprotective, and antidiabetic [7]. The drug is generally considered by the FDA to be safe and inexpensive [8], and it has been extensively studied in various diseases [9]. Curcumin has become quite common as a complementary therapy for UC [10]. Its anti-inflammatory effect is considered the most relevant mechanism by blocking IκB kinase to inhibit NF-κB, thereby inhibiting proinflammatory cytokines (IL-1, IL-6, and TNF-α) expression [11].
In the past few years, many systematic reviews/meta-analyses (SRs/MAs) have been conducted to evaluate the potential therapeutic benefits of curcumin for patients with UC. SRs/MAs are considered the gold standard for assessing the effects of clinical interventions; however, high-quality SRs/MAs can provide reliable evidence, whereas low-quality SRs/MAs may instead mislead clinical decisions [12]. Therefore, there may be a gap between the evidence-based clinical implementation of curcumin and its actual implementation in real-world dynamics. Clinical decision-making requires a comprehensive overview of the available evidence to identify potential benefits and harms of interventions [12]. Therefore, the aim of our study was to critically assess the scientific quality of relevant SRs/MAs regarding curcumin for the treatment of UC through a systematic overview.
2. Materials and Methods
2.1. Research Methods
The SR/MA overview is based on the guidelines specified in the Cochrane Handbook [13], the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [14], and some high-quality methodological overviews [15, 16]. Literature search, literature screening, data extraction, and quality evaluation of related evaluation tools are done independently by two researchers. If there is any inconsistency, disagreements are resolved through consensus or discussion with an experienced third researcher.
2.2. Development of Inclusion and Exclusion Criteria
2.2.1. Literature Inclusion Criteria
Study Design: This overview only includes SRs/MAs from randomized controlled trials (RCTs) of curcumin in the treatment of UC
Study Participants: This study includes subjects who have been clinically or radiologically diagnosed with UC according to national or international standards, regardless of age, race, or gender
Study Intervention: The experimental group was supplemented with curcumin on the basis of conventional treatment (CT), and the control group was treated with CT or CT supplemented with a placebo
Study Outcome: (1) The clinical remission is defined as follows: Clinical Activity Index (CAI) score ≤4; Ulcerative Colitis Disease Activity Index (UCDAI) ≤2 or<3; and Simple Clinical Colitis Activity Index (SCCAI) ≤2; (2) The clinical improvement, defined as a decrease in UCDAI by ≥3, decrease in partial Mayo score by ≥3, and SCCAI score by ≥3 points; (3) The endoscopic response, defined as Mayo score drop ≥1 point and mucosal appearance score drop ≥1 point; (4) The endoscopic remission, defined as baron endoscopic score 0/1, Mayo endoscopic score 0 or 1, and partial Mayo score ≤1; (5) Related inflammatory factors, including C-reactive protein (CRP) and Erythrocyte sedimentation rate (ESR); and (6) Safety profile, including adverse events
2.2.2. Exclusion Criteria
Repeated publications, other overviews, network meta-analysis, narrative reviews, and conference abstracts were excluded.
2.3. Search Strategy
We searched 7 databases, including PubMed, Embase, Cochrane Library, CNKI, Wanfang Database, Chongqing VIP, and China Biomedical Literature Database from its establishment until December 15, 2021. The search strategy adopts a combination of MeSH terms and free words. We searched the above databases through the following key terms: curcumin, ulcerative colitis, systematic reviews, and meta-analysis. We also manually searched the references for related articles. The specific search strategy is modified according to different databases. Table 1 provides the search strategy for the PubMed database.
Table 1.
Search strategy for the PubMed database.
| #1 | Curcumin OR “Turmeric Yellow” OR “Yellow, Turmeric” OR Diferuloylmethane |
|---|---|
| #2 | “Colitis, Ulcerative”[MeSH] |
| #3 | “chronic ulcerative colitis” OR “colitis ulcerativa” OR “colitis ulcerosa” OR “colitis ulcerosa chronica” OR “colitis, mucosal” OR “colitis, ulcerative” OR “colitis, ulcerous” OR “colon, chronic ulceration” OR “histiocytic ulcerative colitis” OR “mucosal colitis” OR “ulcerative colorectitis” OR “ulcerative procto colitis” OR “ulcerative proctocolitis” OR “ulcerous colitis” OR “ulcerative colitis” |
| #4 | #2 AND #3 |
| #5 | Meta-Analysis as Topic [MeSH] |
| #6 | “Systematic review” OR “meta-analysis” OR “meta analysis” OR “meta-analyses” OR “Review, systematic” |
| #7 | #5 AND #6 |
| #8 | #1 AND #4 AND #7 |
2.4. Eligibility Assessment and Data Extraction
Document management software (Endnote X9, Clavirate Analytics, USA) is used to manage the retrieved articles. After deleting duplicates, researchers read the title and abstract to find potential SRs/MAs based on the inclusion and exclusion criteria then obtained full-text articles for further screening to determine eligibility. They then used the standardized data extraction form to extract the data independently. The following specific characteristics are extracted from each SR/MA: first author, year of publication, country, number of included studies, sample size, treatment intervention, control intervention, mode of administration, quality assessment methods, results, and main conclusions.
2.5. Quality Assessment
2.5.1. Assessment of Methodological Quality
The Assessment System for Evaluating Methodological Quality 2 (AMSTAR-2) [17] scale was used to assess the methodological quality of the included SRs/MAs. It consists of 16 items, 7 of which are critical areas (2, 4, 7, 9, 11, 13, and 15). Each item is assessed using three assessment options: yes, partial yes, or no.
2.5.2. Assessment of Risk of Bias
The risk of bias of the included SRs/MAs is assessed by the Risk of Bias in Systematic Reviews (ROBIS) [18]. The scale is completed in 3 stages to assess the overall risk of bias. The results are judged as “low,” “unclear,” or “high.”
2.5.3. Assessment of Reporting Quality
The list of PRISMA [14] is used to assess the quality of each SR/MA report based on the following areas: (a) title, (b) summary, (c) introduction, (d) method, (e) result, (f) discussion, and (g) funding. It consists of 27 projects, with a focus on the reporting methods and results in a meta-analysis. Based on the completeness of the project information report, each project is considered “yes” (full report), “partial yes” (partial report), or “no” (no report).
2.5.4. Assessment of Quality of Evidence
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) [19] system is used to assess the quality of the evidence of the included SRs/MAs, downgrading from five aspects: research limitations, inconsistencies, indirectness, imprecision, and publication bias.
2.6. Data Synthesis and Presentation
In this overview, an objective description is used. The characteristics and results of each SR/MA and the evaluation results of AMSTAR 2, ROBIS, PRISMA, and GRADE are reported in the form of a list.
3. Results
3.1. Results on Literature Search and Screening
A total of 61 articles were retrieved through these seven literature databases, and 28 duplicate articles were deleted. We filter by the title and abstract of the literature and finally obtained 7 studies for full-text screening. After evaluation according to the inclusion and exclusion criteria, we finally included these 7 studies from the literature (Figure 1).
Figure 1.
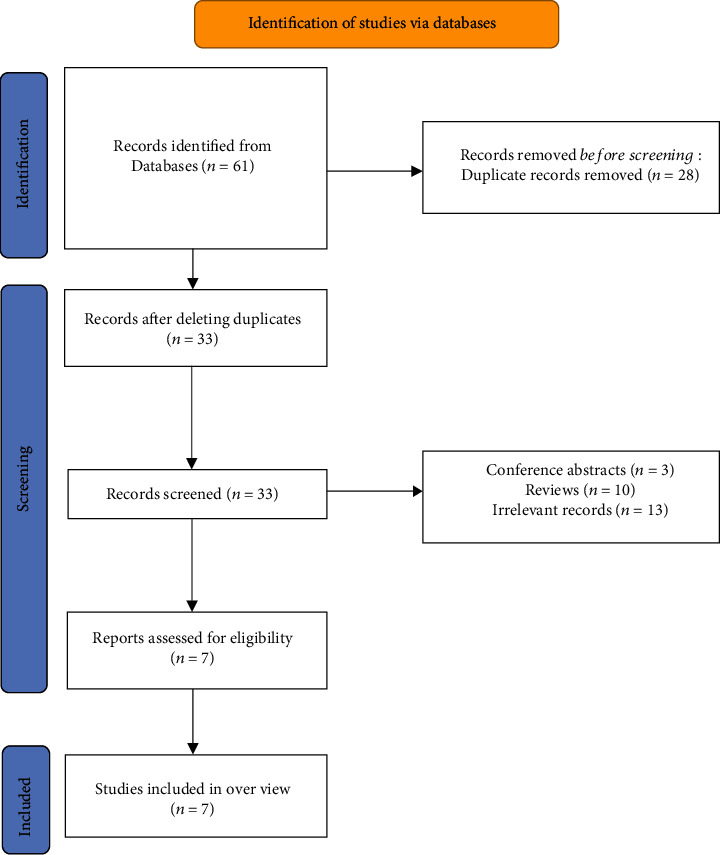
The flowchart of the screening process.
3.2. Description of Included SRs/MAs
Seven SRs/MAs [20–26] published from 2018 to 2021 were included. Of these published SRs/MAs, two are from China [25, 26], two from the United States [20, 23], and the remaining three from Brazil [22], Iran [21], and Greece [24]. Among them, 6 SRs/MAs were published in English [20–25], and one was published in Chinese [26]. The number of RCTs included in each SR/MA ranges from 2 to 7, and the sample size of a single study ranges from 104 to 380. The intervention of the treatment group was CT supplement curcumin, and the control group was treated with CT and a placebo. CT includes sulfasalazine and 5-aminosalicylic acid. The details of the SRs/MAs included are shown in Table 2.
Table 2.
Characteristics of the included SRs/MAs.
| Author, year (country) | Trials (subjects) | Intervention group | Control group | Mode of administration | Quality assessment | Main results |
|---|---|---|---|---|---|---|
| Saurabh Chandan, 2020 (USA) [20] | 7 (380) | Curcumin+mesalamine | Placebo+mesalamine | Oral administration, enema administration | Jadad | According to our research, compared with placebo, the clinical remission rate of mesalazine and curcumin combined treatment is about 3 times higher, and the side effects are minimal. This response is statistically significant, although there is heterogeneity, it may be due to the severity score index, the dose of curcumin, and the route of administration used. |
| Armin Ebrahimzadeh, 2021 (Iran) [21] | 2 (104) | Curcumin+CT | Placebo+CT | Oral administration | Cochrane | Curcumin supplementation is associated with a significant decrease in CRP and ESR levels in patients with ulcerative colitis. |
| Ricardo de Alvares Goulart, 2020 (Brazil) [22] | 4 (238) | Curcumin+CT | Placebo+CT | Oral administration | GRADE | Based on our results, we can say that the use of curcumin has a beneficial effect on the clinical remission of UC patients. However, when treating UC, we need to consider curcumin carefully, because more robust and well-designed studies are needed. |
| Umair Iqbal, 2018 (USA) [23] | 3 (142) | Curcumin+mesalamine | Placebo+mesalamine | Oral administration, enema administration | Jadad | This study shows that when curcumin is used in combination with mesalazine to achieve remission in UC patients, the clinical remission rate is higher. Because of its cost-effectiveness and safer side effects, curcumin can reduce the medical burden and morbidity associated with this recurrent and recurrent disease.. |
| Maria G. Grammatikopoulou, 2018 (Greece) [24] | 3 (194) | Curcumin+mesalamine | Placebo+mesalamine | Oral administration | Cochrane | Based on the currently available evidence, oral curcumin does not appear to be superior to placebo in alleviating the condition of UC patients. |
| Ting Zheng, 2020 (China) [25] | 6 (349) | Curcumin+CT | Placebo+CT | Oral administration, enema administration | Cochrane | In short, curcumin is an effective and safe drug that can be used in the treatment of UC together with standard treatments. |
| Liwei Zhu, 2019 (China) [26] | 5 (261) | Curcumin+CT | Placebo+CT | Oral administration, enema administration | Cochrane | Our systematic review and meta-analysis showed that curcumin combined with mesalazine as an adjuvant drug can significantly increase the clinical remission rate, DAI improvement rate, and mucosal healing rate of mesalamine. |
3.3. Results on SR/MA Quality Assessment
3.3.1. Methodological Quality Assessment
The AMSTAR-2 assessment breakdown for each review is shown in Table 3. Since more than one critical area is missing in the remaining SRs/MAs, the methodological quality of all SRs/MAs was assessed as very low. The method restriction comes from the following item: item 2 (only 1 SR/MA [24] has registered the protocol), item 7 (none of the SRs/MAs provides a research exclusion list), and item 15 (only 2 SRs/MAs [23, 25] conducted publication bias studies or discuss their impact on SR/MA).
Table 3.
Result of the AMSTAR-2 assessments.
| Author, year (country) | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Q15 | Q16 | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saurabh Chandan, 2020 (USA) [20] | Y | PY | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | Y | VL |
| Armin Ebrahimzadeh, 2021 (Iran) [21] | Y | PY | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | Y | VL |
| Ricardo de Alvares Goulart, 2020 (Brazil) [22] | Y | PY | Y | PY | Y | Y | N | Y | Y | Y | Y | Y | Y | N | N | Y | VL |
| Umair Iqbal, 2018 (USA) [23] | Y | PY | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | VL |
| Maria G. Grammatikopoulou, 2018 (Greece) [24] | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | Y | VL |
| Ting Zheng, 2020 (China) [25] | Y | PY | Y | PY | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | VL |
| Liwei Zhu, 2019 (China) [26] | Y | PY | Y | PY | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | Y | VL |
Note: Y, Yes; PY, partial Yes; N, No; VL, Very low; L, L. Note: Critical areas are marked in italic.
3.3.2. Risk of Bias of the Included SRs/MAs
The risk of bias for all SRs/MAs in the first stage (assessment of relevance) and Domain 1 (research eligibility criteria) of the ROBIS scale evaluation was assessed as low risk. Domain 2 evaluated the identification and selection of research, and 4 of the SRs/MAs [20, 21, 23, 24] were assessed as low risk. In Domain 3 (data collection and research evaluation), 3 SRs/MAs [20, 21, 23] were assessed as low risk of bias and none of the SRs/MAs is assessed as low risk in Domain 4 (synthesis and discovery). Phase 3 assessed the risk of bias in the review, and the 7 SRs/MAs had a low risk of bias. The evaluation details of the included SRs/MAs on the ROBIS scale are shown in Table 4.
Table 4.
Results of the ROBIS assessments.
| Author, year (country) | Phase 1 | Phase 2 | Phase 3 | |||
|---|---|---|---|---|---|---|
| Assessing relevance |
Domain 1: study eligibility criteria |
Domain 2: identification and selection of studies |
Domain 3: collection and study appraisal |
Domain 4: synthesis and findings |
Risk of bias in the review |
|
| Saurabh Chandan, 2020 (USA) [20] | √ | √ | √ | √ | × | √ |
| Armin Ebrahimzadeh, 2021 (Iran) [21] | √ | √ | √ | √ | × | √ |
| Ricardo de Alvares Goulart, 2020 (Brazil) [22] | √ | √ | × | × | × | √ |
| Umair Iqbal, 2018 (USA) [23] | √ | √ | √ | √ | × | √ |
| Maria G. Grammatikopoulou, 2018 (Greece) [24] | √ | √ | √ | × | × | √ |
| Ting Zheng, 2020 (China) [25] | √ | √ | × | × | × | √ |
| Liwei Zhu, 2019 (China) [26] | √ | √ | × | × | × | √ |
Note: √, low risk; ×, high risk.
3.3.3. Report Quality
The results of the PRISMA inventory evaluation are shown in Table 5. 20 out of 27 items have a “yes” answer rate of more than 70%, and this shows that the report is relatively complete. However, there are some reporting deficiencies in other projects. Items 5 (protocol and registration), Items 15 (methods: risk of bias across studies), Items 16 (methods: additional analyses), and Items 22 (results risk of bias across studies) are inadequately reported (“yes” response rate is less than 50%).
Table 5.
Results of the PRISMA checklist.
| Section/topic | Items | Saurabh Chandan, 2020 (USA) [20] | Armin Ebrahimzadeh, 2021 (Iran) [21] | Ricardo de Alvares Goulart, 2020 (Brazil) [22] | Umair Iqbal, 2018 (USA) [23] | Maria G. Grammatikopoulou, 2018 (Greece) [24] | Ting Zheng, 2020 (China) [25] | Liwei Zhu, 2019 (China) [26] | Number of yes (%) |
|---|---|---|---|---|---|---|---|---|---|
| Title | Q1.Title | Y | Y | Y | Y | Y | Y | Y | 100% |
| Abstract | Q2. Structured summary | Y | Y | Y | Y | Y | Y | Y | 100% |
| Introduction | Q3. Rationale | Y | Y | Y | Y | Y | Y | Y | 100% |
| Q4. Objectives | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Methods | Q5. Protocol and registration | N | N | N | N | Y | N | N | 14.30% |
| Q6. Eligibility criteria | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Q7. Information sources | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Q8. Search | N | Y | Y | N | Y | Y | N | 57.10% | |
| Q9. Study selection | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Q10. Data collection process | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Q11. Data items | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Q12. Risk of bias in individual studies | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Q13. Summary measures | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Q14. Synthesis of results | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Q15. Risk of bias across studies | N | N | N | Y | N | Y | N | 28.60% | |
| Q16. Additional analyses | Y | Y | N | N | N | N | N | 28.60% | |
| Results | Q17. Study selection | Y | Y | Y | Y | Y | Y | Y | 100% |
| Q18. Study characteristics | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Q19. Risk of bias within studies | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Q20. Results of individual studies | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Q21. Synthesis of results | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Q22. Risk of bias across studies | N | N | N | Y | N | Y | N | 28.60% | |
| Q23. Additional analysis | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Discussion | Q24. Summary of evidence | Y | Y | Y | Y | Y | Y | Y | 100% |
| Q25. Limitations | Y | Y | N | Y | Y | Y | N | 71.40% | |
| Q26. Conclusions | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Funding | Q27. Funding | Y | N | Y | Y | N | Y | N | 57.10% |
Note: Y, yes; N, no.
3.3.4. Evidence Quality
Table 6 shows the results of GRADE evaluation including SR/MA-related outcome indicators. The 7 SRs/MAs included 19 outcome indicators related to the efficacy and safety of curcumin on UC. In the evaluation results based on the outcome indicators, the quality of evidence was 10 moderate, 6 low, and 3 very low. Imprecision (n = 15) was the most common downgrade factor, followed by publication bias (n = 12), inconsistency (n = 5), risk of bias (n = 0), and imprecision (n = 0).
Table 6.
Results of evidence quality.
| Author, year (country) | Outcomes | Studies (participants) | Limitations | Inconsistency | Indirectness | Imprecision | Publication bias | Relative effect (95% CI) | Heterogeneity | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Saurabh Chandan, 2020(USA) [20] | Clinical remission rate | 5 (282) | 0 | 0 | 0 | 0 | -1④ | OR: 2.9 (95% CI: 1.5, 5.5)∗ | I2 = 45% | Moderate |
| Clinical improvement rate | 5 (255) | 0 | 0 | 0 | 0 | -1④ | OR: 2.6 (95% CI: 1.5, 4.5)∗ | I2 = 74% | Moderate | |
| Summary rate of endoscopic improvement and remission | 5 (235) | 0 | 0 | 0 | 0 | -1④ | OR: 2.3 (95% CI: 1.2, 4.6)∗ | I2 = 35.5% | Moderate | |
| Armin Ebrahimzadeh, 2021(Iran) [21] | CRP | 1 (63) | 0 | -1② | 0 | -1③ | -1④ | WMD: -0.15 (95% CI: -0.28, -0.02)∗ | NA | Very low |
| ESR | 2 (104) | 0 | 0 | 0 | -1③ | -1④ | WMD: -6.92 (95% CI: -11.83, -2)∗ | I2 = 59.3% | Low | |
| Ricardo de Alvares Goulart, 2020(Brazil) [22] | Clinical remission rate | 3 (182) | 0 | -1② | 0 | -1③ | -1④ | RD: 0.31 (95% CI: 0.02, 0.60)∗ | I2 = 82% | Very low |
| Clinical improvement rate | 3 (182) | 0 | -1② | 0 | -1③ | -1④ | RD: 0.24 (95% CI: -0.15, 0.63) | I2 = 90% | Very low | |
| Umair Iqbal, 2018(USA) [23] | Clinical remission rate | 2 (95) | 0 | -1② | 0 | -1③ | 0 | OR: 6.78 (95% CI: 2.39, 19.23)∗ | I2 = 75.9% | Low |
| Clinical improvement rate | 3 (142) | 0 | 0 | 0 | -1③ | 0 | OR: 4.65 (95% CI: 2.18, 9.92)∗ | I2 = 40.7% | Moderate | |
| Endoscopic improvement rate | 2 (95) | 0 | 0 | 0 | -1③ | 0 | OR: 3.82 (95% CI: 1.40, 10.40)∗ | I2 = 63.7% | Moderate | |
| Endoscopic remission rate | 2 (102) | 0 | 0 | 0 | -1③ | 0 | OR: 12.74 (95% CI: 1.56, 104.07)∗ | I2 = 0% | Moderate | |
| Maria G. Grammatikopoulou, 2018(Greece) [24] | Clinical remission rate | 3 (201) | 0 | 0 | 0 | 0 | -1⑤ | OR: 3.80 (95% CI: 0.55,26.28) | I2 = 63.7% | Moderate |
| Ting Zheng, 2020(China) [25] | Clinical remission rate | 4 (198) | 0 | 0 | 0 | -1③ | 0 | OR:5.18 (95% CI: 1.84, 14.56)∗ | I2 = 33% | Moderate |
| Endoscopic remission rate | 3 (121) | 0 | 0 | 0 | -1③ | -1⑤ | OR: 5.69 (95% CI:1.28, 25.27)∗ | I2 = 28% | Low | |
| Clinical improvement rate | 4 (158) | 0 | 0 | 0 | -1③ | 0 | OR:4.79 (95% CI: 1.02, 22.43)∗ | I2 = 75% | Moderate | |
| Endoscopic improvement rate | 2 (71) | 0 | 0 | 0 | -1③ | 0 | OR:17.05 (95% CI:: 1.30, 233.00)∗ | I2 = 57% | Moderate | |
| Liwei Zhu, 2019(China) [26] | Clinical remission rate | 3 (181) | 0 | 0 | 0 | -1③ | -1④ | OR: 4.78 (95% CI:1.24, 18.47)∗ | I2 = 52% | Low |
| Clinical improvement rate | 3 (142) | 0 | 0 | 0 | -1③ | -1④ | OR: 4.61 (95% CI:2.22, 9.57)∗ | I2 = 28% | Low | |
| Endoscopic remission rate | 3 (142) | 0 | 0 | 0 | -1③ | -1④ | OR: 4.58 (95% CI:1.79, 11.73)∗ | I2 = 49% | Low |
Note: ①The included studies have a large bias in methodology such as randomization, allocation concealment, and blinding. ②The confidence interval overlaps less or the I2 value of the combined results was larger. ③The sample size from the included studies does not meet the optimal sample size or the 95% confidence interval crosses the invalid line. ④The funnel chart is asymmetry. ⑤Few studies were included, and their results were all positive, which may result in a large publication bias; ∗The 95% confidence interval does not cross the invalid line.
3.4. Summary of Results of the Included Studies
The result indicators extracted from the included studies are listed in Table 6.
3.4.1. Clinical Remission and Improvement Rate
6 SRs/MAs [20, 22–26] reported the clinical remission rate, of which 5 SRs/MAs [20, 22, 23, 25, 26] showed that curcumin can significantly increase the clinical remission rate of UC patients. 5 SRs/MAs [20, 22, 23, 25, 26] reported the clinical improvement rate, of which 4 SRs/MAs [20, 23, 25, 26] showed that curcumin can significantly improve the clinical improvement rate of UC patients.
3.4.2. Endoscopic Remission and Improvement Rate
One SR/MA [20] showed that curcumin can significantly increase the summary rate of endoscopic improvement and remission, and 2 SRs/MAs [23, 25] reported that curcumin can significantly increase the endoscopic improvement rate; in addition, 3 SRs/MAs [23, 25, 26] reported curcumin can significantly improve the endoscopic remission rate.
3.4.3. Inflammatory Factors
One SR/MA [21] showed that curcumin can significantly reduce CRP and ESR levels.
3.4.4. Adverse Events
Although none of the SR/MA provides a quantitative comparison of adverse events between curcumin and the control group, there are 3 SRs/MAs [20, 22, 25] that narratively report that there is no significant difference in the occurrence of adverse events between the curcumin group and the control group, and no serious adverse events occurred.
4. Discussion
UC is a chronic disease characterized by local tissue damage, intestinal flora imbalance, and colon inflammation. With the exception of corticosteroids, conventional treatments for UC are very expensive. Therefore, supplementary treatments are needed [27]. At the same time, more and more related SRs/MAs have been carried out. However, the quality of these publications has not been evaluated so far. In this case, we integrated the published results of SRs/MAs to provide a comprehensive evidence-based summary of the results of the clinical application of curcumin to UC.
4.1. Summary of the Main Findings
This is the first overview of SR/MA to study the effectiveness and safety of curcumin in the therapy of UC, including 7 SRs/MAs published between 2018 and 2021. This indicates more and more attention is paid to the effectiveness and safety of curcumin treatment of UC.
According to the results of the AMSTAR-2 evaluation, the methodological quality of all SRs/MAs was assessed as very low, especially in projects 2 (protocol registration), 7 (exclusion list), and 15 (publication bias). Only 1 SR/MA [24] was registered for the meta-analysis protocol. When conducting SR/MA, protocol registration is very important, and protocol registration should be done when determining the topic selection, which helps reduce the possibility of selective reporting bias and ensures SR/MA is carried out in an orderly manner [28]. None of the SRs/MAs provided an exclusion of the reasons for each study, which may affect the reliability of the results. Providing a list of exclusion research can strongly demonstrate the rigor of the literature screening process. In addition, the lack of publication bias assessment may reduce the accuracy of the final results, which is also related to the insufficient number of RCTs included in the SRs/MAs. ROBIS is used to assess the risk of bias in the included SRs/MAs. Among them, the lack of sensitivity analysis and insufficient evaluation of publication bias are the main factors leading to a high risk of bias, which may affect the reliability of the final results. Similar to the results of the AMSTAR-2 and ROBIS assessments, the PRISMA assessment results indicate a lack of registration of programs and a lack of publication bias risk assessment. All of the above reporting deficiencies may affect the clarity and transparency of the SR/MA implementation method.
The quality of the evidence is assessed by the GRADE system. Among the 19 outcome indicators, the quality of evidence was 10 moderate, 6 low, and 3 very low. Imprecision is the most common degrading factor, followed by publication bias and inconsistency. Through further analysis, the total research population sample size of the RCTs included in the meta-analysis of the related outcome indicators is insufficient. This is an important reason for the insufficient quality of evidence. In addition, the publication bias analysis of the outcome indicators included in the SRs/MAs is not comprehensive, or there is a risk of publication bias, which can also lead to the unreliability of results.
Descriptive analysis shows that curcumin is an effective treatment for UC and may be safer than CT. However, there is a SR/MA that indicates that curcumin may be ineffective for UC patients. The reason may be that the SR/MA was published relatively early, and the inclusion of RCTs was relatively limited. Another possible explanation may stem from the highly heterogeneous oral curcumin doses observed in the retrieved RCTs. In addition, the administration of curcumin in SRs/Mas published later is not limited to oral administration. Due to the low methodological quality of the included studies, the conclusions of the SRs/MAs may be different from the real results, and caution should be taken when recommending curcumin as a supplementary intervention for UC.
4.2. Implications for Future Research
The AMSTAR-2, PRISMA, and ROBIS assessments can be used to evaluate all aspects of the included SRs/Mas, determine the outlook for the future SR/MA, and make it more standardized. Researchers should register or publish the research plan in advance when conducting SRs/Mas to avoid risk of bias and ensure the recognition of the SR/MA results. A list of excluded literature should be provided with explanations to ensure transparency and avoid publication bias. For literature with a high risk of bias, researchers should conduct a separate analysis and give a reasonable explanation to ensure the quality of the evidence. In addition, a complete evaluation of publication bias will also increase the accuracy of the meta-analysis results. The bioavailability of curcumin is low, resulting in lower plasma and tissue levels of curcumin. Therefore, in the next RCTs, researchers will explore better curcumin bioavailability by changing dosage forms, such as enema administration [29] and a self-microemulsifying drug delivery system [30]. With the development of evidence-based medicine, it is hoped that in the future, researchers will continue to promote the standardization of related single RCTs. Well-designed and strictly implemented RCTs can minimize or avoid bias. This is the gold standard for evaluating interventions [31].
4.3. Strengths and Limitations
As far as we know, our study is the first overview of SRs/Mas on the use of curcumin in the treatment of UC, which can provide a comprehensive evidence reference for clinical practice. Second, the evaluation process of AMSTAR-2, PRISMA, ROBIS, and GRADE revealed the obvious limitations of SRs/MAs and RCT, which may help guide high-quality research in the future. However, we must also acknowledge the limitations of this overview due to the generally low quality of SRs/MAs and outcome measures to draw firm conclusions. Caution is required in recommending curcumin as a complementary treatment for UC, and we only included SRs/MAs published in English and Chinese, so a small subset of studies in other languages may be missed.
5. Conclusion
According to the currently published evidence, curcumin may be effective and safe for the treatment of UC, especially in increasing clinical remission and improvement rate. However, due to the generally low quality of methodologies, reports, and evidence for the inclusion of SRs/MAs, caution should be exercised when recommending curcumin as a complementary treatment for UC. More standardized and comprehensive RCTs and SRs/MAs are needed to provide stronger evidence. In addition, it is also necessary to explore the multidosage form administration of curcumin in the application of UC.
Acknowledgments
The study was supported by the Natural Science Foundation of Shandong Province (ZR2021LZY013).
Abbreviations
- UC:
Ulcerative colitis
- SR:
Systematic review
- MA:
Meta-analysis
- RCT:
Randomized controlled trial
- CT:
Conventional treatment
- AMSTAR-2:
Assessment System for Evaluating Methodological Quality 2
- ROBIS:
Risk of Bias in Systematic reviews
- PRISMA:
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- GRADE:
Grading of Recommendations Assessment, Development, and Evaluation
- CAI:
Clinical Activity Index
- UCDAI:
Ulcerative Colitis Disease Activity Index
- SCCAI:
Simple Clinical Colitis Activity Index
- CRP:
C-reactive protein
- ESR:
Erythrocyte sedimentation rate.
Data Availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Disclosure
Hongshuo Shi and Dan Wang are the co-first authors.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
YTT and SGM participated in the research design. SHS, LYH, and WD conducted a literature search and screened data extraction. WD and LYH analysed the data, did a statistical analysis, and wrote a manuscript. YTT and SHS participated in the correction of the manuscript. All authors reviewed the manuscript. All authors read and approved the final version of the manuscript.
References
- 1.Ungaro R., Mehandru S., Allen P. B., Peyrin-Biroulet L., Colombel J. F. Ulcerative colitis. Lancet . 2017;389(10080):1756–1770. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng S. C., Shi H. Y., Hamidi N., et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet . 2017;390(10114):2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 3.Sehgal P., Colombel J. F., Aboubakr A., Narula N. Systematic review: safety of mesalazine in ulcerative colitis. Alimentary Pharmacology & Therapeutics . 2018;47(12):1597–1609. doi: 10.1111/apt.14688. [DOI] [PubMed] [Google Scholar]
- 4.Sinopoulou V., Gordon M., Dovey T. M., Akobeng A. K. Interventions for the management of abdominal pain in ulcerative colitis. Cochrane Database of Systematic Reviews . 2021;7(7, article CD013589) doi: 10.1002/14651858.CD013589.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feuerstein J. D., Moss A. C., Farraye F. A. Ulcerative colitis. Mayo Clinic Proceedings . 2019;94(7):1357–1373. doi: 10.1016/j.mayocp.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Xiong T., Zheng X., Zhang K., et al. Ganluyin ameliorates DSS-induced ulcerative colitis by inhibiting the enteric-origin LPS/TLR4/NF-κB pathway. Journal of Ethnopharmacology . 2022;(article 115001) doi: 10.1016/j.jep.2022.115001. [DOI] [PubMed] [Google Scholar]
- 7.Yue W., Liu Y., Li X., Lv L., Huang J., Liu J. Curcumin ameliorates dextran sulfate sodium-induced colitis in mice via regulation of autophagy and intestinal immunity. The Turkish Journal of Gastroenterology . 2019;30(3):290–298. doi: 10.5152/tjg.2019.18342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang C., Chen Z., He Y., et al. Oral colon-targeting core-shell microparticles loading curcumin for enhanced ulcerative colitis alleviating efficacy. Chinese Medicine . 2021;16(1):p. 92. doi: 10.1186/s13020-021-00449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chainoglou E., Hadjipavlou-Litina D. Hadjipavlou-Litina D., editor. Curcumin in health and diseases: Alzheimer's disease and curcumin analogues, derivatives, and hybrids. International Journal of Molecular Sciences . 2020;21(6):p. 1975. doi: 10.3390/ijms21061975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banerjee R., Pal P., Penmetsa A., et al. Novel bioenhanced curcumin with mesalamine for induction of clinical and endoscopic remission in mild-to-moderate ulcerative colitis: a randomized double-blind placebo-controlled pilot study. Journal of Clinical Gastroenterology . 2021;55(8):702–708. doi: 10.1097/MCG.0000000000001416. [DOI] [PubMed] [Google Scholar]
- 11.Sreedhar R., Arumugam S., Thandavarayan R. A., Karuppagounder V., Watanabe K. Curcumin as a therapeutic agent in the chemoprevention of inflammatory bowel disease. Drug Discovery Today . 2016;21(5):843–849. doi: 10.1016/j.drudis.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Bougioukas K. I., Vounzoulaki E., Mantsiou C. D., et al. Methods for depicting overlap in overviews of systematic reviews: an introduction to static tabular and graphical displays. Journal of Clinical Epidemiology . 2021;132:34–45. doi: 10.1016/j.jclinepi.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Higgins J. P., Thomas J., Chandler J., et al. Cochrane Handbook for Systematic Reviews of Interventions . 2nd Edition. Chichester (UK): John Wiley & Sons; 2019. version 6.0(updated July 2019) [DOI] [Google Scholar]
- 14.Moher D., Liberati A., Tetzlaff J., Altman D. G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ . 2009;339(jul21 1):p. b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu N., Zhang T., Sun J., et al. An overview of systematic reviews of Chinese herbal medicine for Alzheimer's disease. Frontiers in Pharmacology . 2021;12 doi: 10.3389/fphar.2021.761661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen M., Huang J., Qiu T., Qiu T. Quality of the evidence supporting the role of acupuncture for stable angina pectoris: an umbrella review of systematic reviews. Front Cardiovasc Med . 2021;8 doi: 10.3389/fcvm.2021.732144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shea B. J., Reeves B. C., Wells G., et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ . 2017;358:p. j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whiting P., Savović J., Higgins J. P., et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. Journal of Clinical Epidemiology . 2016;69:225–234. doi: 10.1016/j.jclinepi.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atkins D., Best D., Briss P. A., et al. Grading quality of evidence and strength of recommendations. BMJ . 2004;328(7454):p. 1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandan S., Mohan B. P., Chandan O. C., et al. Curcumin use in ulcerative colitis: is it ready for prime time? A systematic review and meta-analysis of clinical trials. Annals of Gastroenterology . 2019;33(1):53–58. doi: 10.20524/aog.2019.0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebrahimzadeh A., Abbasi F., Ebrahimzadeh A., Jibril A. T., Milajerdi A. Effects of curcumin supplementation on inflammatory biomarkers in patients with rheumatoid arthritis and ulcerative colitis: a systematic review and meta-analysis. Complementary Therapies in Medicine . 2021;61, article ??? doi: 10.1016/j.ctim.2021.102773. [DOI] [PubMed] [Google Scholar]
- 22.Goulart R. A., Barbalho S. M., Rubira C. J., et al. Curcumin therapy for ulcerative colitis remission: systematic review and meta-analysis. Expert Review of Gastroenterology & Hepatology . 2020;14(12):1171–1179. doi: 10.1080/17474124.2020.1808460. [DOI] [PubMed] [Google Scholar]
- 23.Iqbal U., Anwar H., Quadri A. A. Use of curcumin in achieving clinical and endoscopic remission in ulcerative colitis: a systematic review and meta-analysis. The American Journal of the Medical Sciences . 2018;356(4):350–356. doi: 10.1016/j.amjms.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 24.Grammatikopoulou M. G., Gkiouras K., Theodoridis X., Asteriou E., Forbes A., Bogdanos D. P. Oral adjuvant curcumin therapy for attaining clinical remission in ulcerative colitis: a systematic review and meta-analysis of randomized controlled trials. Nutrients . 2018;10(11) doi: 10.3390/nu10111737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng T., Wang X., Chen Z., He A., Zheng Z., Liu G. Efficacy of adjuvant curcumin therapy in ulcerative colitis: a meta-analysis of randomized controlled trials. Journal of Gastroenterology and Hepatology . 2020;35(5):722–729. doi: 10.1111/jgh.14911. [DOI] [PubMed] [Google Scholar]
- 26.Liwei Z. Clinical Evaluation of Curcumin Combined with Mesalazine in the Treatment of Mild to Moderate Ulcerative Colitis . Southern Medical University; 2019. [Google Scholar]
- 27.Liu Y., Zhou M., Yang M., et al. Pulsatilla chinensis saponins ameliorate inflammation and DSS-induced ulcerative colitis in rats by regulating the composition and diversity of intestinal flora. Frontiers in Cellular and Infection Microbiology . 2021;11, article 728929 doi: 10.3389/fcimb.2021.728929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart L., Moher D., Shekelle P. Why prospective registration of systematic reviews makes sense. Systematic Reviews . 2012;1(1):p. 7. doi: 10.1186/2046-4053-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banerjee R., Medaboina K., Boramma G. G., Amsrala S., Reddy D. N. Novel bio-enhanced curcumin with mesalamine for induction of remission in mild to moderate ulcerative colitis. Gastroenterology . 2017;152(5):p. S587. doi: 10.1016/S0016-5085(17)32111-X. [DOI] [Google Scholar]
- 30.Banerjee R., Chaudhari H., Shah N., Saravanan A., Tandan M., Reddy D. N. Addition of Lubiprostone to polyethylene glycol(PEG) enhances the quality & efficacy of colonoscopy preparation: a randomized, double-blind, placebo controlled trial. Gastroenterology . 16(1):p. 133. doi: 10.1186/s12876-016-0542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moher D., Hopewell S., Schulz K. F., et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. International Journal of Surgery . 2012;10(1):28–55. doi: 10.1016/j.ijsu.2011.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.


