Keywords: addiction, cocaine, nucleus accumbens, serotonin, voltammetry
Abstract
Although dopamine is the most implicated neurotransmitter in the mediation of the pathophysiology of addiction, animal studies show serotonin also plays a vital role. Cocaine is one of the most common illicit drugs globally, but the role of serotonin in its mechanism of action is insufficiently characterized. Consequently, we investigated the acute effects of the psychomotor stimulant cocaine on electrical stimulation-evoked serotonin (phasic) release in the nucleus accumbens core (NAcc) of urethane-anesthetized (1.5 g/kg ip) male Sprague–Dawley rats using N-shaped fast-scan cyclic voltammetry (N-FSCV). A single carbon fiber microelectrode was first implanted in the NAcc. Stimulation was applied to the medial forebrain bundle using 60 Hz, 2 ms, 0.2 mA, 2-s biphasic pulses before and after cocaine (2 mg/kg iv) was administered. Stimulation-evoked serotonin release significantly increased 5 min after cocaine injection compared with baseline (153 ± 21 nM vs. 257 ± 12 nM; P = 0.0042; n = 5) but was unaffected by saline injection (1 mL/kg iv; n = 5). N-FSCV’s selective measurement of serotonin release in vivo was confirmed pharmacologically via administration of the selective serotonin reuptake inhibitor escitalopram (10 mg/kg ip) that effectively increased the signal in a separate group of rats (n = 5). Selectivity to serotonin was further confirmed in vitro in which dopamine was minimally detected by N-FSCV with a serotonin to dopamine response ratio of 1:0.04 (200 nM of serotonin:1 µM dopamine ratio; P = 0.0048; n = 5 electrodes). This study demonstrates a noteworthy influence of cocaine on serotonin dynamics, and confirms that N-FSCV can effectively and selectively measure phasic serotonin release in the NAcc.
NEW & NOTEWORTHY Serotonin plays a vital role in drug addiction. Here, using N-shaped fast-scan cyclic voltammetry, we demonstrated the effect of cocaine on the phasic release of serotonin at the nucleus accumbens core. To the best of our knowledge, this has not previously been elucidated. Our results not only reinforce the role of serotonin in the mechanism of action of cocaine but also help to fill a gap in our knowledge and provide a baseline for future studies in cocaine addiction.
INTRODUCTION
Serotonin is a key neurotransmitter with important roles in physiological neuroplasticity and behavioral hedonic tone, motivational, and reinforcement processes, including cognitive functions such as learning and memory (1, 2). Addiction involves all of these processes and functions, thus serotonin signaling is increasingly implicated in substance use disorders (3–6). A meta-analysis showed that certain polymorphisms (5-HTTLPR) of the serotonin transporter (SERT) gene (SLC6A4) were associated with alcohol, heroin, cocaine, and methamphetamine dependence and abuse (5). Postmortem studies of human brains also showed 5-HT2A receptors in the prefrontal cortex are functionally altered in opiate addicts in comparison with control subjects (6).
It has also been suggested that changes in serotonin transmission may account for the emotional components of addiction, such as anhedonia and depression during drug withdrawal (2). In addition, animal studies have shown that serotonin receptors in the prefrontal cortex, nucleus accumbens, and ventral tegmental area modulate dopamine transmission, where activation of 5HT2A receptors increases dopamine release whereas 5-HT2C receptor activation does the opposite to mediate the effects of psychostimulants such as cocaine (1).
Given its emerging role in the pathophysiology of addictive disorders, measurements of serotonin release in the brain have been performed in a number of animal studies, with the majority of studies using in vivo microdialysis techniques (7–9). Although microdialysis is able to measure multiple analytes simultaneously, its utility is limited by its relatively low spatiotemporal resolution and the large dimension of the microdialysis probe (10–15). For this reason, electrochemical techniques, such as N-shaped fast-scan cyclic voltammetry (N-FSCV), have been developed to provide much more rapid measurements of changes in serotonin extracellular levels with higher spatiotemporal resolution when combined with carbon fiber microelectrodes (CFMs) compared with microdialysis (16).
Similar to dopamine release (17, 18), serotonin release is divided into tonic and phasic patterns. For example, in a study using fiber photometry and single-unit recording in mice, it was found that in the face of a delayed reward, most serotonin neurons fire tonically during the waiting part and then phasically on reward acquisition (19). Microdialysis is most commonly used to measure changes in tonic levels, whereas N-FSCV is able to measure phasic serotonin release evoked by electrical stimulation. Previous microdialysis studies have demonstrated that both acute systematic and intracerebral administration of cocaine dose-dependently increases extracellular concentrations of serotonin in the nucleus accumbens (8, 20). On that basis, we hypothesize that cocaine would also lead to increases in phasic serotonin release, which can be measured by N-FSCV.
N-FSCV has been used to study phasic (electrical stimulation-evoked) serotonin release in the substantia nigra pars reticulata (SNr), striatum, medial prefrontal cortex, and hippocampus (21–25). However, to date, this voltammetric technique has not been applied in the nucleus accumbens, nor has the effect of cocaine on serotonin levels been assessed using this method. Cocaine is one of the most common illicit drugs of abuse with a high prevalence of use and dependence (26), with an estimate of 5.5 million people in the USA using it in 2019 (27), and is therefore of immense theoretical and clinical interest to the healthcare system. Hence, our aim here is to use N-FSCV to characterize the acute effects of systemic (intravenous; iv) administration of cocaine and saline on phasic serotonin release in the nucleus accumbens core (NAcc) compared with baseline.
MATERIALS AND METHODS
Animals
Ten male Sprague–Dawley rats (Envigo) were used for this study (250–300 g). Rats were kept in social housing in an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) accredited vivarium following a standard 12-h light/dark cycle (lights on: 0600; lights off 1800) at constant temperature (21°C) and humidity (45%) with ad libitum food and water. The present study was approved by the Institutional Animal Care and Use Committee (IACUC), Mayo Clinic, Rochester, MN. The National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals guidelines (Department of Health and Human Services, NIH publication No. 86-23, revised 1985) were followed for all aspects of animal care.
Carbon Fiber Microelectrode Fabrication
Carbon fiber microelectrodes (CFMs) were fabricated using an established standardized design at Mayo Clinic (28, 29). Briefly, each microelectrode involved isolating and inserting a single carbon fiber (AS4, diameter = 7 μm; Hexcel, Dublin, CA) into a silica tube [20 µM inner diameter (ID), 90 µM outer diameter (OD), 10 µM coat with polyimide; Polymicro Technologies, Phoenix, AZ]. The connection between the carbon fiber extending out of the silica tubing was covered with epoxy resin. The carbon fiber in the silica tubing was then connected to a conductive wire composed of nitinol (Nitinol No. 1, an alloy of nickel and titanium; Fort Wayne Metals, IN) coated with a silver-based conductive paste (28). The carbon fiber-attached nitinol wire was then insulated with a polyimide tube [0.0089 in. ID, 0.0134 in. OD, 0.00225 in. wall thickness (WT); Vention Medical, Salem, NH] up to the carbon fiber sensing tip. The exposed carbon fiber was then trimmed under a dissecting microscope to a length of ∼50 µm. Teflon-coated silver (Ag) wire (A-M systems, Inc., Sequim, WA) was prepared as an Ag/AgCl counter-reference electrode by chlorinating the exposed tip in saline with a 9-V dry cell battery. CFMs were pretested in vitro in a TRIS buffer (15 mM Tris, 3.25 mM KCl, 140 mM NaCl, 1.2 mM CaCl2, 1.25 mM NaH2PO4, 1.2 mM MgCl2, and 2.0 mM Na2SO4, with the pH adjusted to 7.4) (11) before deposition of a Poly(3,4-ethylenedioxythiophene) PEDOT:Nafion coating to minimize the effects of biofouling in vivo (30).
Implantation of CFM and Stimulating Electrode
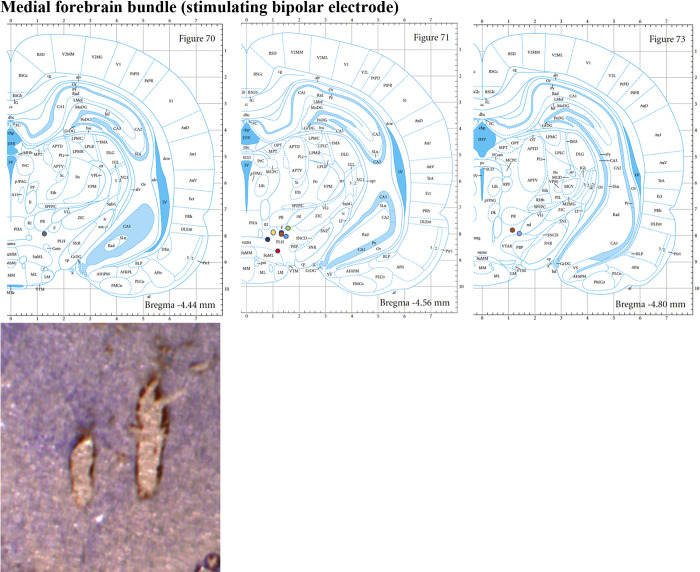
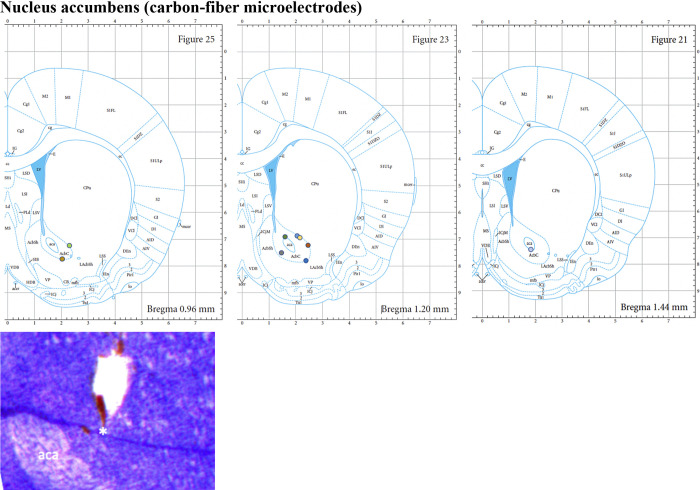
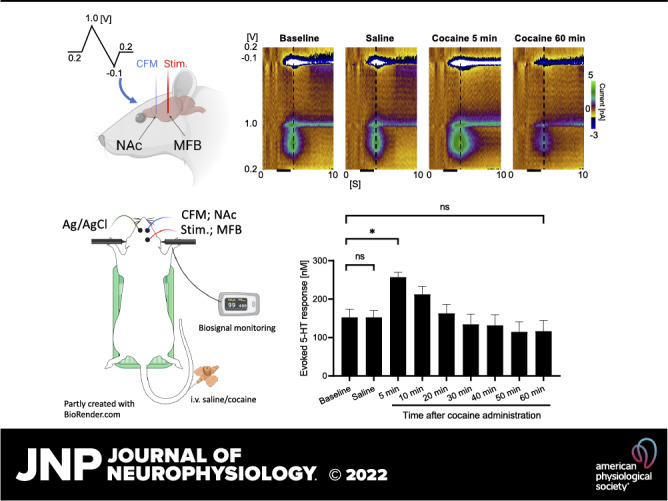
Each rat was anesthetized with urethane (1.5 g/kg ip; Sigma-Aldrich, St Louis, MO) and administered buprenorphine (0.05–0.1 mg/kg sc, Par Pharmaceutical, Chestnut Ridge, NY) for analgesia. Upon complete anesthesia, rats were placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA) with their body resting on a heating pad (41°C). Respiratory rate (RespiRAT, Intuitive Measurement Systems), hind-paw, and tail pinch were used to monitor their physiological state and depth of anesthesia over the entire course of the experiment. Using a standard rat atlas (31), three trephine holes were drilled, the first for placement of a CFM into the NAcc (AP 1.2 mm from bregma, ML 2.0 mm, and DV 6.5–7.5 mm from dura), the second for a stimulating electrode into the medial forebrain bundle (MFB) (twisted bipolar electrode—Plastics One, MS 303/2, Roanoke, VA, with the tips separated by 1 mm; AP −4.6 mm from bregma, ML 1.3 mm, and DV 8–9 mm from dura), and a third for an Ag/AgCl reference/counter electrode into the contralateral cortex (32) (Fig. 1A). The MFB is a well-known neural pathway through which serotoninergic axons from serotonin-containing cells in the dorsal raphe nucleus project to the NAcc (33, 34).
Figure 1.
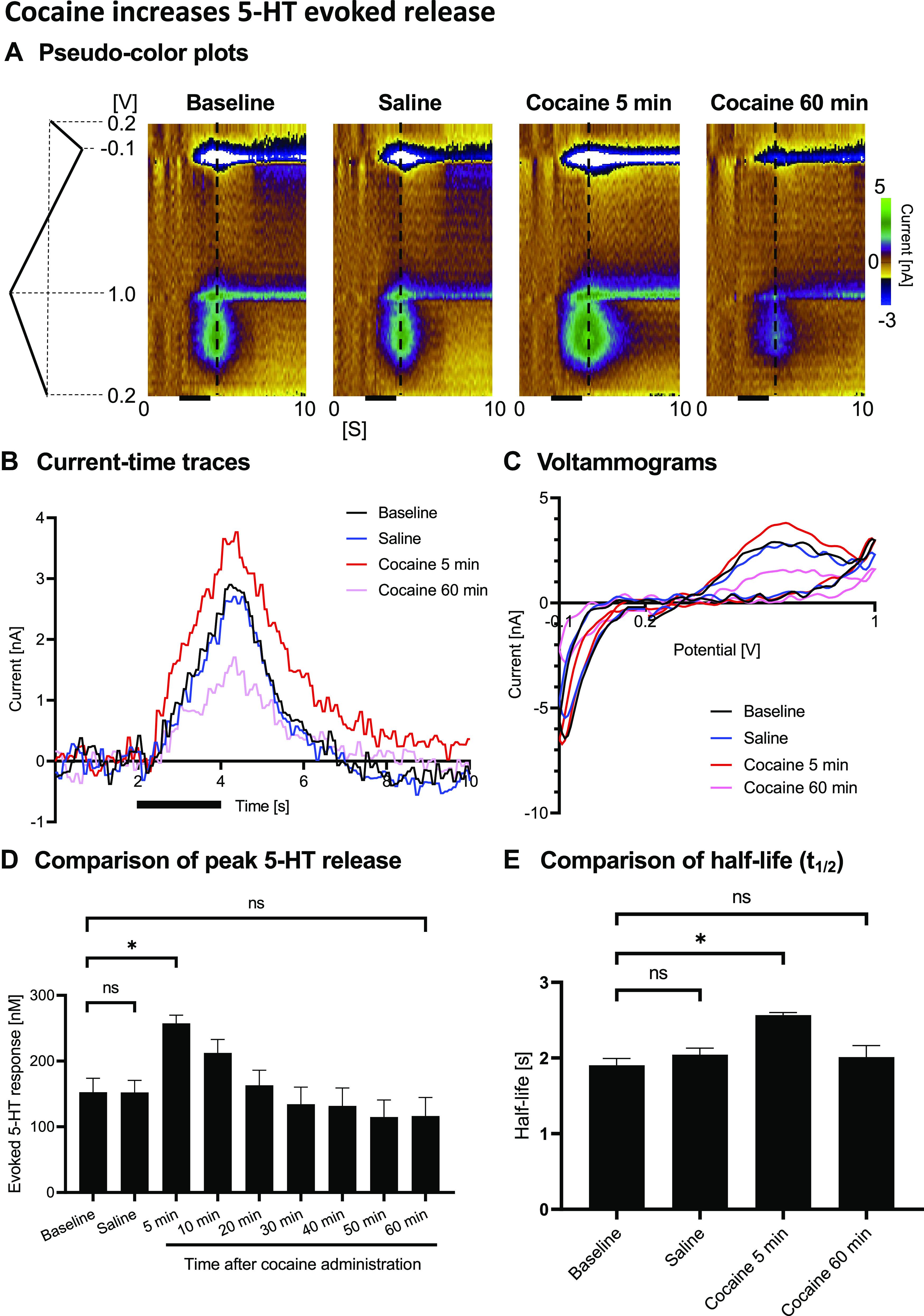
In vivo fast-scan cyclic voltammetry (FSCV) measurement in the nucleus accumbens core (Nacc) showing augmented serotonin release in response to cocaine injection (2 mg/kg iv). Responses were measured following medial forebrain bundle stimulation (60 Hz, 2 s, biphasic, 200 µA, 2 ms pulse width). A: representative pseudo-color plots before and after cocaine injection (black bars indicate time of stimulation), with the waveform depicted on the left and the dotted line indicating positions of shown voltammograms. Oxidation current-time traces (B), voltammograms (C), maximum change in serotonin concentration with medial forebrain bundle (MFB) stimulation at different time points (n = 5) (D), and half-lives from peak (E). Black bars represent electrical stimulation (2 s). *Statistical significance after Bonferroni correction (for three tests, the P value was reduced to 0.05/3 = 0.017).
Drug Administration, Voltammetric Recordings, and Processing
The depths of the stimulating electrode in the MFB and the CFM in the NAcc were first carefully adjusted to obtain a robust stimulation-evoked signal. N-FSCV (0.2 V, 1.0 V, −0.1 V; 1,000 V/s; 10 scans/s) was applied, as described in the literature (16). After stabilization of stimulation-evoked baseline responses, the tail vein was cannulated, and saline was injected (1 mL/kg iv). Stimulation (60 Hz, 2 ms, 0.2 mA, 2-s biphasic pulses) was applied three times (at 5, 10, and 20 min) to ensure consistent stable responses. At 10 min after the last stimulation, cocaine hydrochloride (Sigma-Aldrich, St. Louis, MO) was administered in a single intravenous (iv) bolus of 2 mg/kg (salt weight). Stimulation-evoked voltammetric responses were recorded at 5, 10, 20, 30, 40, 50, and 60 min postinjection. Voltammetric recordings and processing were performed using a wireless neurochemical sensing system developed by our laboratory, the WINCS (Wireless Instantaneous Neurotransmitter Concentration Sensing System) Harmoni system (35). In-house developed software (WINCSWare) was used for recording and for data analysis.
Calibration of Electrodes
In vitro analysis of N-FSCV was performed using a flow injection system (36). A CFM was placed at the center of an acrylic chamber that was connected with a flanged fluid line (BOLA, Germany) to a switching valve (Rheodyne MX series II, IDEX Health & Science). The flow stream was controlled and maintained at a rate of 2 mL/min by a syringe pump (Harvard Apparatus, Holliston, MA). During the constant injection of buffer solution, a known fixed concentration of analyte was injected as a bolus for quantification by N-FSCV.
In Vitro and In Vivo Confirmation
To confirm that the analyte recorded in vivo by N-FSCV was serotonin, both in vitro and in vivo tests were performed. The key analyte to distinguish from serotonin is dopamine; since not only is the nucleus accumbens known to have a high concentration of dopamine, but cocaine is both a dopamine and serotonin reuptake inhibitor (37). Also, previous studies showed that stimulation of MFB can lead to release of phasic dopamine (38). For in vitro confirmation, N-FSCV voltammograms were measured with serotonin concentrations of 200 nM and 500 nM, and dopamine concentrations of 200 nM, 500 nM, and 1 µM in the flow cell apparatus (n = 5 electrodes). Dopamine concentrations that are considered higher than physiological concentrations were chosen to amplify any possible interference. For in vivo confirmation, the selective serotonin reuptake inhibitor (SSRI), escitalopram (ESCIT, 10 mg/kg ip) was administered instead of cocaine to confirm enhancement of the evoked signal (MFB stimulation: 60 Hz, 2 ms, 0.2 mA, 2-s biphasic pulses applied every 10 min, 6 times). Intraperitoneal administration of escitalopram was chosen to confirm the presence of serotonin based on previous studies in the literature (21, 23). Here, the 5-min postinjection recording was omitted as intraperitoneal injection takes relatively longer for absorption compared with intravenous injections.
Histological Analysis
CFM and stimulation electrode trajectories were confirmed by histological analysis. Brains removed from euthanized animals were immersed in 4% paraformaldehyde overnight for fixation. After fixation, 60-μm coronal sections were cut on a freezing microtome. The sections were mounted on glass slides and stained with cresyl violet. The location of the stimulating and CFMs were identified under light microscopy.
Statistical Analysis
Statistical analysis was performed using two-tailed paired t tests in Prism 8 (GraphPad, CA). Significance was set at P < 0.05. Bonferroni correction was performed where appropriate. In the cocaine experiments, three planned paired t tests were performed (baseline vs. saline, response at 5 min after cocaine administration, or 60 min after cocaine administration; within-subject comparison) for both the peak serotonin releases and half-lives. The half-life is defined as the time interval between the two points where the serotonin level was half of the maximum at its peak. In the escitalopram experiments, baseline was compared against responses taken at 20-min postinjection (within-subject comparison). The half-lives at the said time intervals were also compared using two-tailed paired t tests. In vitro test results were compared using paired t tests (within-subject comparison). All concentration values are presented as the means ± SE.
RESULTS
The representative pseudo-color plots, voltammograms, and current-time traces of one representative rat experiment are shown in Fig. 1, A–C, which indicate an increased evoked serotonin response after cocaine administration. The overall result is summarized in Fig. 1D, where the increase in evoked release of serotonin in the NAcc occurred within 5 min of administration of cocaine. Serotonin-evoked release increased by 1.7-fold (257 ± 12 vs. 153 ± 21 nM; P = 0.0042, n = 5 rats) at 5 min after cocaine administration, compared with baseline values. In contrast, post-saline responses were not different from baseline-evoked responses (152 ± 18 nM; P = 0.983; n = 5 rats). There was a gradual decay after the response recorded at 5 min with the evoked responses falling below baseline at 60 min after cocaine administration (P = 0.0488). However, this was not statistically significant following a Bonferroni correction (for three tests, the P value was reduced to 0.05/3 = 0.017). Cocaine administration significantly (P = 0.0005; n = 5 rats) increased the half-life decay of the evoked response by 1.35-fold (1.90 ± 0.09 vs. 2.57 ± 0.04 s) at 5 min compared with saline control (2.04 ± 0.09 s; P = 0.06; Fig. 1E).
We confirmed the N-FSCV measurements to be specific to serotonin using the SSRI escitalopram (Fig. 2). Stimulation-evoked responses significantly increased 20 min after escitalopram administration and gradually reduced over time (baseline: 20 min after ESCIT administration, P = 0.0151, Fig. 2C). These changes are exemplified in the representative figures in Fig. 2, A and B. Escitalopram enhancement of the evoked responses was more delayed compared with intravenous cocaine administration, since escitalopram was administered intraperitoneally. Importantly, supra-physiological concentrations of dopamine in vitro caused minimal interference/detection (Fig. 3, A and B), validating that N-FSCV specifically measures serotonin. The response for freshly prepared CFMs was 100:4 for serotonin:dopamine (200 nM of serotonin:1 µM of dopamine ratio; P = 0.0048; n = 5 electrodes; Fig. 3C).
Figure 2.
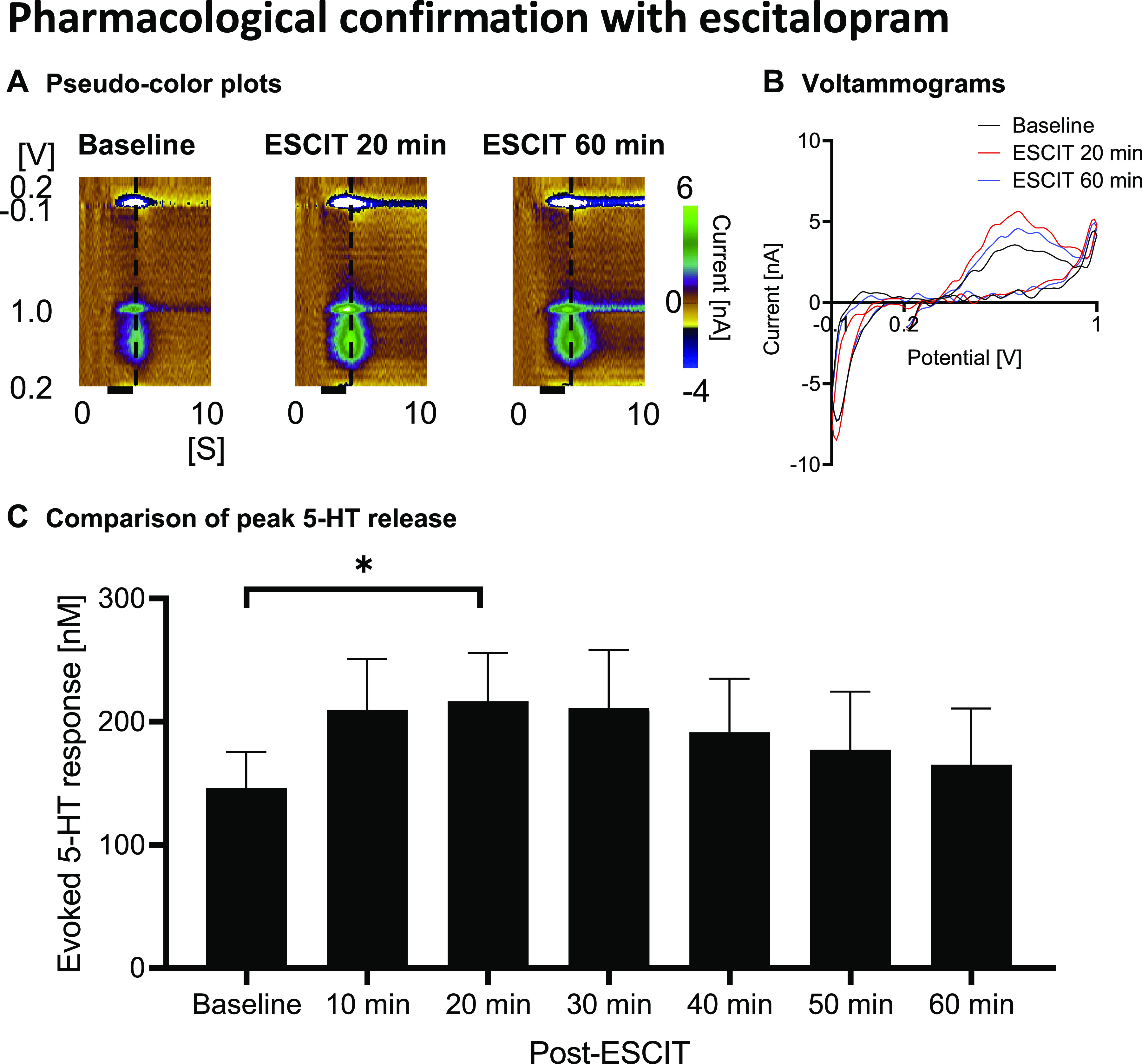
Pharmacological in vivo confirmation using escitalopram (ESCIT; 10 mg/kg ip). A: representative pseudo-color plots. B: voltammograms. C: comparison of peak evoked serotonin release showing the evoked response increased 10 min after escitalopram administration, remained augmented, and gradually decayed in a similar manner observed with cocaine administration but more slowly. *Statistical significance.
Figure 3.
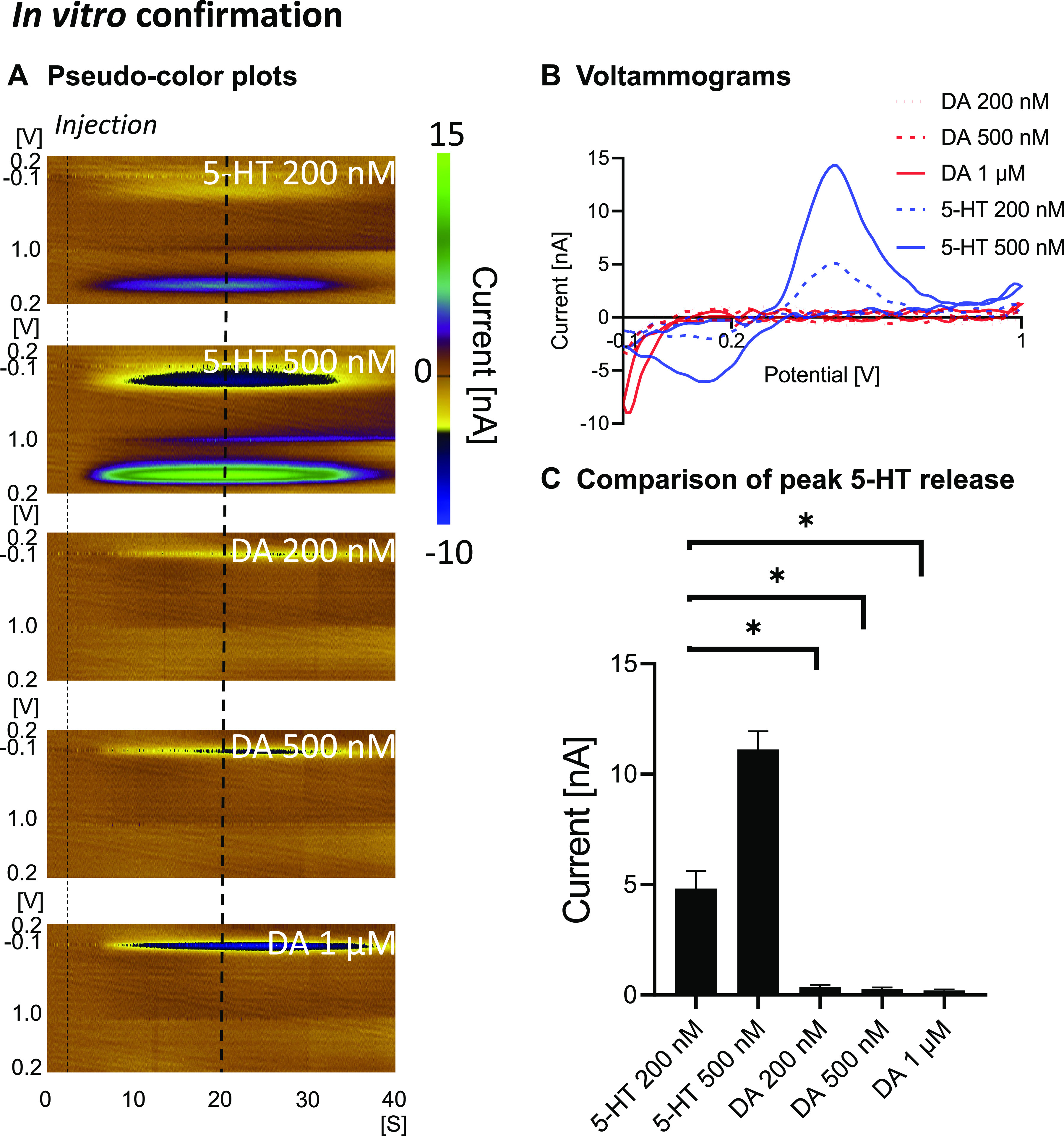
In vitro confirmation in a flow cell apparatus showing that dopamine does not result in a significant oxidation signal compared with serotonin. Pseudocolor plots (left dotted line denotes time of injection and right one denotes where the voltammograms were taken) (A), voltammograms (B), and summary of peak oxidation currents (C). n = 5 electrodes. *Statistical significance after Bonferroni correction (for 3 tests, the P value was reduced to 0.05/3 = 0.017).
Histological analysis (see appendix) verified that both the recording CFM and stimulating electrode were accurately implanted into the NAcc and MFB, respectively (n = 9 or 10).
DISCUSSION
We used N-FSCV and observed that systematic administration of cocaine led to an increase in evoked phasic serotonin release in the NAcc. The utility of N-FSCV in detecting serotonin rather than dopamine was confirmed by administration of escitalopram in vivo and in vitro application of dopamine using the same waveform. As recorded in various brain regions (Table 1), N-FSCV recordings of evoked serotonin release typically provides a much lower signal-to-noise ratio and is therefore technically more challenging to obtain a robust signal compared with dopamine recordings using FSCV. However, both in vitro and in vivo confirmation experiments evidenced that the cocaine-related changes detected by N-FSCV are most likely to be changes in evoked serotonin levels.
Table 1.
N-shaped fast-scan cyclic voltammetry study from the literature
| Species | Recording Site | Stimulating Target | Stimulation Parameters | Evoked Serotonin Response | |
|---|---|---|---|---|---|
| Jackson and Wightman (22) | Sprague–Dawley rats | Striatum (caudate-putamen) | MFB | 60 Hz 2 ms pulse width 300 µA Trains of 180 pulses (3 s) |
Up to 5.4 µM after pharmacological manipulation (including nomifensine) |
| Hashemi et al. (39) | Sprague–Dawley rats | SNr | DRN | 60 Hz 2 ms pulse width 300 µA 2 s in duration |
∼12.7 nM Increased to ∼50 nM with citalopram Half-life increased from 1.7 s to 5.2 s |
| Hashemi et al. (40) | Sprague–Dawley rats | SNr | MFB or DRN | 60 Hz 2 ms pulse width 350 µA 2 s in duration |
∼12.8 nM Half-life is ∼1.8 s |
| Wood and Hashemi (21) | C57BL/6J mice | SNr | MFB | 60 Hz 2 ms pulse width 250 µA 2 s in duration |
∼28 nM Increased to ∼98 nM with escitalopram but decreased to 71.5 nM at 120 min Half-life increased from 3.7 s to 5.7 s over 10 min and to 7.1 at 120 min |
| Saylor et al. (23) | C57BL/6J mice | Hippocampus | MFB | 60 Hz 2 ms pulse width 360 µA 2 s in duration |
∼32 to 35 nM Half-life 2.1 sIncreased post-drug by several fold depending on the dose of escitalopram |
| West et al. (24) | C57BL/6 mice | Medial prefrontal cortex | MFB | 60 Hz 4 ms pulse width 350 µA 2 s in duration |
∼35 nM Escitalopram affects release in a variable manner |
| Our study | Sprague–Dawley rats | Nucleus accumbens core | MFB | 60 Hz 2 ms pulse width 200 µA Trains of 120 pulses |
153 nM increased to 257 nM 5 min after iv cocaine 2 mg/kg; half-life increased from 1.9 s to 2.6 s In a separate group, at 10 min after ip escitalopram 10 mg/kg, it increased from 146 nM to 217 nM |
Comparison with Previous Literature
Comparing our findings with those of microdialysis studies makes little sense. As discussed in introduction, although N-FSCV has important advantages over microdialysis, it has its limitations. It cannot record tonic levels due to its need for subtraction of large capacitive currents. Also, stimulation is required to evoke the release of serotonin for measurement.
In addition, it is difficult to perform a direct comparison with previous N-FSCV studies in the literature, as no prior studies of N-FSCV were performed in the nucleus accumbens. As a reference, results of some previous N-FSCV from the literature are given in Table 1. Two studies used Sprague–Dawley rats and MFB stimulation as with our study but focused on the SNr rather than the nucleus accumbens (39, 40). The stimulation parameters are similar except the authors used 300–350 µA current amplitude, which is around 50% higher than that in our study. The values obtained from these studies were ∼12.8 nM, which is 10 times smaller than what we observed even before pharmacological intervention. The discrepancy between values in our study is most likely attributed to the difference in recording locations. The SNr is traditionally thought of as one of the most densely innervated areas of the brain by serotonin neurons (41). Although the SNr may have a higher tonic (basal) serotonin extracellular level, our results suggest the nucleus accumbens has a higher phasic evoked response. The other possible explanation of the differences is that MFB stimulation may result in activation of the recurrent inhibitory collaterals of SNr projection cells onto serotonergic axonal terminals in the SNr, thus resulting in a lower level of serotonin release in the SNr, compared with the NAcc, following MFB stimulation (42). Further studies with more refined electrochemical methods may be able to verify this possibility. Also, our electrodes were calibrated individually at the end of the experiments but in the two cited studies they were calibrated with a standardized nM:nA conversion factor. This may also partly account for the differences between our values.
Another point of interest is the magnitude of increase following administration of SSRIs. Following escitalopram and citalopram administration, previous studies reported several-fold increase in evoked serotonin release (21, 23, 39). However, in the present study, there was only around a 50% increase following escitalopram injection, although it reached statistical significance. It appears that the serotonin release in the NAcc is less affected by SSRIs, compared with that in the SNr and hippocampus.
We previously stimulated MFB with the same parameters as the present study to assess accumbal phasic dopamine release (38). We found the evoked dopamine release to be 330 nM, which is around twice the evoked serotonin release found in this study. Also, cocaine increased MFB-stimulation evoked dopamine release by 2.6-fold in our previous study, whereas in the present study evoked serotonin release increased by 1.7-fold. This is consistent with the general understanding that the predominant monoamine in nucleus accumbens is dopamine, rather than serotonin. However, the amount of evoked serotonin release in nucleus accumbens, which has not been studied before, is significant and should therefore be studied further, including with other drugs.
Nucleus Accumbens Core and Serotonin
At the nucleus accumbens core, serotonin was found to be important in glutamate homeostasis as SERT knockout mice showed a reduction of glutamate signaling after prolonged self-administration of cocaine (43). This may partly explain the increased consumption of cocaine in such subjects. In addition, by applying serotonin receptor (5-HT2C) agonist (Ro 60-0175) to a rat experimenter-administered model, it was shown that 5-HT2C receptors modulate postsynaptic dopamine activity at the NAcc and inhibits cocaine-induced hyperlocomotion (44). Furthermore, intranucleus accumbens application of Ro 60-0175 led to dose-dependent excitation (0.1 µM) and inhibition (1 µM) of cocaine-induced dopamine outflow (45). These studies demonstrate the intricate relationship between 5-HT2C receptors, dopamine, and cocaine-induced hyperlocomotion.
Apart from 5-HT2C, 5-HT1A receptor at the nucleus accumbens is also shown to be important in cocaine-induced behavioral changes. Local application of 5-HT1A receptor agonist at the nucleus accumbens potentiated cocaine-induced hyperlocomotion, but attenuated rearing behavior in a dose-dependent fashion (46).
In addition, administration of the SSRI fluoxetine has been shown to decrease self-administration of cocaine in rodent studies (47, 48). Local injection of sertraline at the nucleus accumbens also abolished the preference of subchronically cocaine-treated, abstinent rats for a cocaine-associated environment in a conditioned place preference paradigm (49). This again shows the eminent role of serotonin in cocaine-induced behavior.
In summary, all these studies demonstrate a distinct role of accumbal serotonin in the mechanisms of action of cocaine, and our study shows that N-FSCV is another feasible way to study serotonin release at the nucleus accumbens, particularly the phasic pattern.
Although cocaine is a known dopamine and serotonin reuptake inhibitor (37), its effect on electrical stimulation-evoked release of serotonin in the nucleus accumbens core has not previously been elucidated. In this study, we used N-FSCV to demonstrate the magnitude of increase caused by acute systematic administration of cocaine. Our results reinforce a role of serotonin in the mechanism of action of cocaine. This may help to fill a gap in our knowledge and provide a baseline for future preclinical and treatment studies in cocaine addiction.
GRANTS
This research was supported by the National Institutes of Health (NIH) R01NS112176 award and Minnesota Partnership for Biotechnology and Medical Genomics Grant MNP No. 19.13. Training grant funding for A.E.R. was supported by NIH F31NS115202-01A1, NIH R25GM055252-23, NIH TL1TR002380-03, and NIH T32GM065841-17. M.B. is supported by a NHMRC Senior Principal Research Fellowship 1156072.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.H.L. and H.S. conceived and designed research; J.Y. performed experiments; J.Y., A.G., A.E.R., and H.S. analyzed data; J.Y., A.G., A.Z.K., M.B., J.H.K., S.J.T., C.D.B., K.E.B., K.H.L., Y.O., and H.S. interpreted results of experiments; J.Y. prepared figures; J.Y. drafted manuscript; J.Y., A.G., A.E.R., A.Z.K., M.B., J.H.K., S.J.T., C.D.B., K.E.B., K.H.L., Y.O., and H.S. edited and revised manuscript; J.Y., A.G., A.E.R., A.Z.K., M.B., J.H.K., S.J.T., C.D.B., K.E.B., K.H.L., Y.O., and H.S. approved final version of manuscript.
ACKNOWLEDGMENTS
Graphical Abstract Image created with BioRender and published with permission.
APPENDIX
Slides for histological analysis (Figs. A1 and A2) were prepared as described in the text and were compared with a standard atlas (31).
Figure A1.
The tracks of the stimulating electrode were also identified and plotted (n = 10), showing most targets are within the medial forebrain bundle. Images from the Paxinos and Watson Atlas (31) are reproduced by permission from Elsevier.
Figure A2.
Example of slide shown here where the track of carbon fiber microelectrode is seen next to the anterior commissure (aca). Most targets (n = 9; one slide cannot be reliably identified due to tissue destruction) are seen to be within the nucleus accumbens core (denoted as AcbC here). Images from the Paxinos and Watson Atlas (31) are reproduced by permission from Elsevier.
REFERENCES
- 1.Howell LL, Cunningham KA. Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev 67: 176–197, 2015. doi: 10.1124/pr.114.009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Müller CP, Homberg JR. The role of serotonin in drug use and addiction. Behav Brain Res 277: 146–192, 2015. doi: 10.1016/j.bbr.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Guerin AA, Nestler EJ, Berk M, Lawrence AJ, Rossell SL, Kim JH. Genetics of methamphetamine use disorder: a systematic review and meta-analyses of gene association studies. Neurosci Biobehav Rev 120: 48–74, 2021. doi: 10.1016/j.neubiorev.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones JA, Zuhlsdorff K, Dalley JW. Neurochemical substrates linked to impulsive and compulsive phenotypes in addiction: a preclinical perspective. J Neurochem 157: 1525–1546, 2021. doi: 10.1111/jnc.15380. [DOI] [PubMed] [Google Scholar]
- 5.Cao J, Hudziak JJ, Li D. Multi-cultural association of the serotonin transporter gene (SLC6A4) with substance use disorder. Neuropsychopharmacology 38: 1737–1747, 2013. doi: 10.1038/npp.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odagaki Y, Kinoshita M, Meana JJ, Callado LF, García-Sevilla JA. 5-HT2A receptor- and M1 muscarinic acetylcholine receptor-mediated activation of Gαq/11 in postmortem dorsolateral prefrontal cortex of opiate addicts. Pharmacol Rep 73: 1155–1163, 2021. doi: 10.1007/s43440-021-00248-w. [DOI] [PubMed] [Google Scholar]
- 7.Bradberry CW, Nobiletti JB, Elsworth JD, Murphy B, Jatlow P, Roth RH. Cocaine and cocaethylene: microdialysis comparison of brain drug levels and effects on dopamine and serotonin. J Neurochem 60: 1429–1435, 1993. doi: 10.1111/j.1471-4159.1993.tb03305.x. [DOI] [PubMed] [Google Scholar]
- 8.Teneud LM, Baptista T, Murzi E, Hoebel BG, Hernandez L. Systemic and local cocaine increase extracellular serotonin in the nucleus accumbens. Pharmacol Biochem Behav 53: 747–752, 1996. doi: 10.1016/0091-3057(95)02087-x. [DOI] [PubMed] [Google Scholar]
- 9.Parsons LH, Justice JB Jr.. Serotonin and dopamine sensitization in the nucleus accumbens, ventral tegmental area, and dorsal raphe nucleus following repeated cocaine administration. J Neurochem 61: 1611–1619, 1993. doi: 10.1111/j.1471-4159.1993.tb09794.x. [DOI] [PubMed] [Google Scholar]
- 10.Chefer VI, Thompson AC, Zapata A, Shippenberg TS. Overview of brain microdialysis. Curr Protoc Neurosci 47: 7.1.1–7.1.28, 2009. doi: 10.1002/0471142301.ns0701s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh Y, Heien ML, Park C, Kang YM, Kim J, Boschen SL, Shin H, Cho HU, Blaha CD, Bennet KE, Lee HK, Jung SJ, Kim IY, Lee KH, Jang DP. Tracking tonic dopamine levels in vivo using multiple cyclic square wave voltammetry. Biosens Bioelectron 121: 174–182, 2018. doi: 10.1016/j.bios.2018.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Chiara G, Carboni E, Morelli M, Cozzolino A, Tanda GL, Pinna A, Russi G, Consolo S. Stimulation of dopamine transmission in the dorsal caudate nucleus by pargyline as demonstrated by dopamine and acetylcholine microdialysis and Fos immunohistochemistry. Neuroscience 55: 451–456, 1993. doi: 10.1016/0306-4522(93)90514-g. [DOI] [PubMed] [Google Scholar]
- 13.Morelli M, Carboni E, Cozzolino A, Tanda GL, Pinna A, Di Chiara G. Combined microdialysis and Fos immunohistochemistry for the estimation of dopamine neurotransmission in the rat caudate-putamen. J Neurochem 59: 1158–1160, 1992. doi: 10.1111/j.1471-4159.1992.tb08359.x. [DOI] [PubMed] [Google Scholar]
- 14.Blaha CD, Coury A, Phillips AG. Does monoamine oxidase inhibition by pargyline increase extracellular dopamine concentrations in the striatum? Neuroscience 75: 543–550, 1996. doi: 10.1016/0306-4522(96)00289-8. [DOI] [PubMed] [Google Scholar]
- 15.Rusheen AE, Gee TA, Jang DP, Blaha CD, Bennet KE, Lee KH, Heien ML, Oh Y. Evaluation of electrochemical methods for tonic dopamine detection in vivo. Trends Analyt Chem 132: 116049, 2020. doi: 10.1016/j.trac.2020.116049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson BP, Dietz SM, Wightman RM. Fast-scan cyclic voltammetry of 5-hydroxytryptamine. Anal Chem 67: 1115–1120, 1995. doi: 10.1021/ac00102a015. [DOI] [PubMed] [Google Scholar]
- 17.Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience 41: 1–24, 1991. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- 18.Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci 17: 524–532, 2016. doi: 10.1038/nrn.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Zhong W, Wang D, Feng Q, Liu Z, Zhou J, Jia C, Hu F, Zeng J, Guo Q, Fu L, Luo M. Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat Commun 7: 10503, 2016. doi: 10.1038/ncomms10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews CM, Lucki I. Effects of cocaine on extracellular dopamine and serotonin levels in the nucleus accumbens. Psychopharmacology (Berl) 155: 221–229, 2001. doi: 10.1007/s002130100704. [DOI] [PubMed] [Google Scholar]
- 21.Wood KM, Hashemi P. Fast-scan cyclic voltammetry analysis of dynamic serotonin responses to acute escitalopram. ACS Chem Neurosci 4: 715–720, 2013. doi: 10.1021/cn4000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson BP, Wightman RM. Dynamics of 5-hydroxytryptamine released from dopamine neurons in the caudate putamen of the rat. Brain Res 674: 163–166, 1995. doi: 10.1016/0006-8993(95)00019-m. [DOI] [PubMed] [Google Scholar]
- 23.Saylor RA, Hersey M, West A, Buchanan AM, Berger SN, Nijhout HF, Reed MC, Best J, Hashemi P. In vivo hippocampal serotonin dynamics in male and female mice: determining effects of acute escitalopram using fast scan cyclic voltammetry. Front Neurosci 13: 362, 2019. [Erratum in Front Neurosci 13: 726, 2019]. doi: 10.3389/fnins.2019.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.West A, Best J, Abdalla A, Nijhout HF, Reed M, Hashemi P. Voltammetric evidence for discrete serotonin circuits, linked to specific reuptake domains, in the mouse medial prefrontal cortex. Neurochem Int 123: 50–58, 2019. doi: 10.1016/j.neuint.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdalla A, West A, Jin Y, Saylor RA, Qiang B, Pena E, Linden DJ, Nijhout HF, Reed MC, Best J, Hashemi P. Fast serotonin voltammetry as a versatile tool for mapping dynamic tissue architecture. I. Responses at carbon fibers describe local tissue physiology. J Neurochem 153: 33–50, 2020. doi: 10.1111/jnc.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.John WS, Wu LT. Trends and correlates of cocaine use and cocaine use disorder in the United States from 2011 to 2015. Drug Alcohol Depend 180: 376–384, 2017. doi: 10.1016/j.drugalcdep.2017.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2019 National Survey on Drug Use and Health. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, 2020. [Google Scholar]
- 28.Chang SY, Kimble CJ, Kim I, Paek SB, Kressin KR, Boesche JB, Whitlock SV, Eaker DR, Kasasbeh A, Horne AE, Blaha CD, Bennet KE, Lee KH. Development of the Mayo Investigational Neuromodulation Control System: toward a closed-loop electrochemical feedback system for deep brain stimulation. J Neurosurg 119: 1556–1565, 2013. doi: 10.3171/2013.8.JNS122142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh Y, Park C, Kim DH, Shin H, Kang YM, DeWaele M, Lee J, Min HK, Blaha CD, Bennet KE, Kim IY, Lee KH, Jang DP. Monitoring in vivo changes in tonic extracellular dopamine level by charge-balancing multiple waveform fast-scan cyclic voltammetry. Anal Chem 88: 10962–10970, 2016. doi: 10.1021/acs.analchem.6b02605. [DOI] [PubMed] [Google Scholar]
- 30.Vreeland RF, Atcherley CW, Russell WS, Xie JY, Lu D, Laude ND, Porreca F, Heien ML. Biocompatible PEDOT:Nafion composite electrode coatings for selective detection of neurotransmitters in vivo. Anal Chem 87: 2600–2607, 2015. doi: 10.1021/ac502165f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Amsterdam: Academic Press/Elsevier, 2007. [Google Scholar]
- 32.Clark JJ, Sandberg SG, Wanat MJ, Gan JO, Horne EA, Hart AS, Akers CA, Parker JG, Willuhn I, Martinez V, Evans SB, Stella N, Phillips PE. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat Methods 7: 126–129, 2010. doi: 10.1038/nmeth.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakahara D, Ozaki N, Miura Y, Miura H, Nagatsu T. Increased dopamine and serotonin metabolism in rat nucleus accumbens produced by intracranial self-stimulation of medial forebrain bundle as measured by in vivo microdialysis. Brain Res 495: 178–181, 1989. doi: 10.1016/0006-8993(89)91234-1. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura K. The role of the dorsal raphe nucleus in reward-seeking behavior. Front Integr Neurosci 7: 60, 2013. doi: 10.3389/fnint.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee KH, Lujan JL, Trevathan JK, Ross EK, Bartoletta JJ, Park HO, Paek SB, Nicolai EN, Lee JH, Min HK, Kimble CJ, Blaha CD, Bennet KE. WINCS Harmoni: closed-loop dynamic neurochemical control of therapeutic interventions. Sci Rep 7: 46675, 2017. doi: 10.1038/srep46675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin H, Oh Y, Park C, Kang Y, Cho HU, Blaha CD, Bennet KE, Heien ML, Kim IY, Lee KH, Jang DP. Sensitive and selective measurement of serotonin in vivo using fast cyclic square-wave voltammetry. Anal Chem 92: 774–781, 2020. doi: 10.1021/acs.analchem.9b03164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci USA 98: 5300–5305, 2001. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuen J, Goyal A, Rusheen AE, Kouzani AZ, Berk M, Kim JH, Tye SJ, Blaha CD, Bennet KE, Jang D-P, Lee KH, Shin H, Oh Y. Cocaine-induced changes in tonic dopamine concentrations measured using multiple-cyclic square wave voltammetry in vivo. Front Pharmacol 12: 705254, 2021. doi: 10.3389/fphar.2021.705254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hashemi P, Dankoski EC, Petrovic J, Keithley RB, Wightman RM. Voltammetric detection of 5-hydroxytryptamine release in the rat brain. Anal Chem 81: 9462–9471, 2009. doi: 10.1021/ac9018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashemi P, Dankoski EC, Wood KM, Ambrose RE, Wightman RM. In vivo electrochemical evidence for simultaneous 5-HT and histamine release in the rat substantia nigra pars reticulata following medial forebrain bundle stimulation. J Neurochem 118: 749–759, 2011. doi: 10.1111/j.1471-4159.2011.07352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallman MJ, Gagnon D, Parent M. Serotonin innervation of human basal ganglia. Eur J Neurosci 33: 1519–1532, 2011. doi: 10.1111/j.1460-9568.2011.07621.x. [DOI] [PubMed] [Google Scholar]
- 42.Deniau JM, Kitai ST, Donoghue JP, Grofova I. Neuronal interactions in the substantia nigra pars reticulata through axon collaterals of the projection neurons. An electrophysiological and morphological study. Exp Brain Res 47: 105–113, 1982. doi: 10.1007/BF00235891. [DOI] [PubMed] [Google Scholar]
- 43.Caffino L, Mottarlini F, Targa G, Verheij MMM, Homberg J, Fumagalli F. Long access to cocaine self-administration dysregulates the glutamate synapse in the nucleus accumbens core of serotonin transporter knockout rats. Br J Pharmacol. In press. doi: 10.1111/bph.15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cathala A, Devroye C, Maitre M, Piazza PV, Abrous DN, Revest JM, Spampinato U. Serotonin2C receptors modulate dopamine transmission in the nucleus accumbens independently of dopamine release: behavioral, neurochemical and molecular studies with cocaine. Addict Biol 20: 445–457, 2015. doi: 10.1111/adb.12137. [DOI] [PubMed] [Google Scholar]
- 45.Navailles S, Moison D, Cunningham KA, Spampinato U. Differential regulation of the mesoaccumbens dopamine circuit by serotonin2C receptors in the ventral tegmental area and the nucleus accumbens: an in vivo microdialysis study with cocaine. Neuropsychopharmacology 33: 237–246, 2008. doi: 10.1038/sj.npp.1301414. [DOI] [PubMed] [Google Scholar]
- 46.Müller CP, Thönnessen H, De Souza Silva MA, Fink H, Bert B, Carey RJ, Huston JP. Nucleus accumbens serotonin1A receptors control cocaine-induced hyperactivity but not local serotonin increase: an in vivo microdialysis study. Neuropharmacology 47: 205–215, 2004. doi: 10.1016/j.neuropharm.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 47.Carroll ME, Lac ST, Asencio M, Kragh R. Fluoxetine reduces intravenous cocaine self-administration in rats. Pharmacol Biochem Behav 35: 237–244, 1990. doi: 10.1016/0091-3057(90)90232-7. [DOI] [PubMed] [Google Scholar]
- 48.Peltier R, Schenk S. Effects of serotonergic manipulations on cocaine self-administration in rats. Psychopharmacology (Berl) 110: 390–394, 1993. doi: 10.1007/BF02244643. [DOI] [PubMed] [Google Scholar]
- 49.Harris GC, Altomare K, Aston-Jones G. Preference for a cocaine-associated environment is attenuated by augmented accumbal serotonin in cocaine withdrawn rats. Psychopharmacology (Berl) 156: 14–22, 2001. doi: 10.1007/s002130100693. [DOI] [PubMed] [Google Scholar]