Abstract
Accumulating evidence indicates a link between gut barrier dysfunction and hypertension. However, it is unclear whether hypertension causes gut barrier dysfunction or vice versa and whether the gut microbiome plays a role. To understand this relationship, we first cross-sectionally examined 153 nonhuman primates [NHPs; Chlorocebus aethiops sabaeus; mean age, 16 ± 0.4 yr; 129 (84.3%) females] for cardiometabolic risk factors and gut barrier function biomarkers. This analysis identified blood pressure and age as specific factors that independently associated with microbial translocation. We then longitudinally tracked male, age-matched spontaneously hypertensive NHPs (Macaca mulatta) to normotensives (n = 16), mean age of 5.8 ± 0.5 yr, to confirm hypertension-related gut barrier dysfunction and to explore the role of microbiome by comparing groups at baseline, 12, and 27 mo. Collectively, hypertensive animals in both studies showed evidence of gut barrier dysfunction (i.e., microbial translocation), as indicated by higher plasma levels of lipopolysaccharide-binding protein (LBP)-1, when compared with normotensive animals. Furthermore, plasma LBP-1 levels were correlated with diastolic blood pressure, independent of age and other health markers, suggesting specificity of the effect of hypertension on microbial translocation. In over 2 yr of longitudinal assessment, hypertensive animals had escalating plasma levels of LBP-1 and greater bacterial gene expression in mesenteric lymph nodes compared with normotensive animals, confirming microbes translocated across the intestinal barrier. Concomitantly, we identified distinct shifts in the gut microbial signature of hypertensive versus normotensive animals at 12 and 27 mo. These results suggest that hypertension contributes to microbial translocation in the gut and eventually unhealthy shifts in the gut microbiome, possibly contributing to poor health outcomes, providing further impetus for the management of hypertension.
NEW & NOTEWORTHY Hypertension specifically had detrimental effects on microbial translocation when age and metabolic syndrome criteria were evaluated as drivers of cardiovascular disease in a relevant nonhuman primate model. Intestinal barrier function exponentially decayed over time with chronic hypertension, and microbial translocation was confirmed by detection of more microbial genes in regional draining lymph nodes. Chronic hypertension resulted in fecal microbial dysbiosis and elevations of the biomarker NT-proBNP. This study provides insights on the barrier dysfunction, dysbiosis, and hypertension in controlled studies of nonhuman primates. Our study includes a longitudinal component comparing naturally occurring hypertensive to normotensive primates to confirm microbial translocation and dysbiotic microbiome development. Hypertension is an underappreciated driver of subclinical endotoxemia that can drive chronic inflammatory diseases.
Keywords: blood pressure, fecal microbiome, gut barrier dysfunction, hypertension, microbial translocation
INTRODUCTION
Gut barrier dysfunction and related diseases cost the US health care system over 100 billion dollars per year, yet the causes are mostly unknown (1, 2). A compromised gut barrier makes the gut mucosal lining “leaky,” enabling the translocation of microbes or microbial products into the systemic circulation from the gut lumen in a process known as microbial translocation (MT), leading to systemic low-grade endotoxemia and inflammation (3–5). Furthermore, studies have shown that a defective gut barrier alters the composition of the microbes residing in the gut (gut microbiome), resulting in dysbiosis, which worsens overall health (1, 3, 6–9). Therefore, it is essential to comprehend regulators of the gut barrier and gut microbiome-related changes to help prevent the onset of various chronic diseases.
Possible contributors to MT and dysbiosis are risk factors for cardiovascular disease, notably hypertension (6, 10, 11). Recently, a study on the spontaneous hypertensive rat model showed increased gut permeability and inflammation, suggesting a possible link between hypertension and gut barrier function (12, 13). However, it is unclear whether hypertension dictates gut barrier dysfunction and whether the gut microbiome play a role. Given the complex nature of these associations, larger cross-sectional studies and longitudinal studies are required to understand the nature of the associations. A further limitation of the existing studies in rats is that they only looked at the gut microbiome at one time point, but this is inadequate to assess the gut microbiome, because other research shows the gut microbiome can change dramatically from one time point to another. Changes in the microbiome result from factors such as aging, environment, diet, activity, and other influences, and so looking at the gut microbiome at several time points is imperative to capture the true effects (4, 5, 9). Because of rapid shifts in the gut microbiome composition and an assessment of whether the gut barrier and gut microbiota are linked to hypertension, more longitudinal studies are needed.
Animal models are valuable as they can be controlled and used in ways human studies cannot, in terms of age, diet, and other environmental factors (14). Although the rodent models are indisputably useful, they may not recapitulate the human conditions to the same degree as nonhuman primate (NHP) models (14, 15). In addition, unlike rodents, NHPs share >93% similarities with the human genome and faithfully recapitulate eating, sleeping, and aging patterns (4, 5, 15, 16). For this purpose, we selected a NHP model, which spontaneously displays several key features of human pathophysiology, including hypertension (14). Therefore, in the present study, we aimed to 1) cross-sectionally investigate the relationship between MT and cardiometabolic risk factors (study 1) and 2) longitudinally examine markers of health, hypertension, gut barrier function, and microbiome in a cohort of NHPs (study 2).
MATERIAL AND METHODS
Animals, Diet, and Study Design
For the cross-sectional study (study 1; Fig. 1), we assessed vervet monkeys (Chlorocebus aethiops sabaeus) of both sexes, residing in a breeding colony at Wake Forest School of Medicine, Winston‐Salem, NC. We used the definition of hypertension based on the American Heart Association’s blood pressure measures, systolic blood pressure (SBP) and diastolic blood pressure (DBP) threshold of 120/80 mmHg (17), and similarly confirmed in NHPs (18). For the longitudinal study (study 2; Fig. 1, rhesus monkeys (Macaca mulatta) were evaluated. These animals were classified as hypertensive and normotensives based on repeated assessments of blood pressure measures and grouped based on the definitions given above. All animals developed hypertension spontaneously and without intervention. Animals were housed either singly or socially in standard primate enclosures and were maintained at 21°C–26°C with ∼50%− 65% relative humidity with 12-h:12-h light/dark periods. Ten NHPs in this longitudinal study were exposed to 4-Gy total body irradiation (TB1) as previously described (19), which had no effects on study outcomes shown in Supplemental Table S1 and Fig. S1 (All Supplemental material is available at https://doi.org/10.6084/m9.figshare.16663375). Animals were fed a commercial laboratory primate chow (study 1, Laboratory Diet 5038; or study 2, 5LOP; LabDiet, St. Louis, MO) with daily supplemental fresh fruits and vegetables. All procedures were performed in accordance with the Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee (Nos. A18-185 and A18-062) at our Association for the Assessment and Accreditation of Laboratory Animal Care International accredited institution, which operates in compliance with the Animal Welfare Act.
Figure 1.
Overall study design. This schematic represents the overview of the 2 studies described in the methods section. BSL, baseline; n, sample size.
Blood Pressure Measurement
The blood pressure was measured indirectly using a sphygmomanometer with digital high-density oscillometry readouts at 15–30 after ketamine anesthesia (10 mg/kg im) and was calculated as the mean of three measurements (18).
Circulating Biomarkers and Metabolic Health Markers
Animals were fasted overnight and anesthetized with intramuscular ketamine (10–15 mg/kg) to allow for sample and data collections for study 1, and for study 2, samples were collected at baseline, 12, and 27 mo. Each animal was weighed and waist circumference measured with a flexible tape measure at the level of the umbilicus. Blood samples were obtained by venipuncture of the femoral vein into ethylenediaminetetraacetic acid (EDTA) blood tubes. The EDTA anticoagulated blood was held on ice until it could be processed. After the plasma was processed, samples were stored at −80°C until analysis. General metabolic health and biochemistry panels including total plasma triglyceride (TG), high-density lipoprotein cholesterol (HDLC), and total plasma cholesterol (TPC) concentrations, were measured as previously described (5). Plasma was used to measure fasting blood glucoses, MT biomarkers, lipopolysaccharide-binding protein (LBP)-1 (Hycult Biotech, Inc., Plymouth Meeting, PA) and soluble (s) CD14, and cardia biomarker NT-proBNP (NH2-terminal pro-B-type natriuretic peptide; MyBiosource, San Diego, CA) via ELISA and following manufacturer’s instructions as previously described (4, 5).
Echocardiogram
Echocardiography was performed on each of the vervet monkeys in study 1 using a GE Logiq S8 ultrasound unit (GE Healthcare, Chicago, IL) using an approach that has been previously described (20). Cardiac morphometric and functional phenotypes include left ventricular diametric at end diastole (LVDd), left ventricular diameter at end systole (LVDs), left ventricular wall fractioning shortening percentage (LVDFS%) calculated as [(LVDd − LVDs)/LVDd] × 100, and ejection fraction percentage (EF%). The animal’s body weight (kg) was obtained at the time of echocardiography and used to calculate body surface area (BSA, m2) as BW2/3 × 0.0969. Echocardiographic analysis (hypertensive n = 10–12, normotensive n = 10) was performed using motion (M) mode, parasternal long axis, tissue doppler, two-, four-, and five-chamber views. TOMTEC software (TOMETEC, Chicago, IL) was used for echocardiographic analysis of motion (M) mode, parasternal long axis, tissue doppler, two-, four-, and five-chamber views.
Sampling and 16S rRNA Gene Sequencing and Data Analysis
Fecal samples and lymph node tissues from the 16 monkeys included in the longitudinal study (study 2) were collected for microbiome characterization and the DNA extracted as described previously (4, 5, 21). Fecal samples were collected from the rectum while the animal was sedated, and mesenteric lymph nodes and colon tissue were dissected out at the necropsy performed at study end. Tissues were collected from the colon and mesentery near the intersection of the transverse and descending colon. Tissues and fecal samples were immediately placed into liquid nitrogen for storage until DNA extraction. Briefly, DNA was extracted from these samples (Qiagen, Germantown, MD) and the quality of DNA determined by gel electrophoresis. Fecal DNA samples were amplified by PCR using barcoded primer pairs targeting the V3–V4 region of the bacterial 16S gene. Universal 16S gene PCR for bacterial abundance was performed as previously described (5). Fecal PCR amplicons were sequenced using the Mi-Seq Illumina sequencer. The resulting bacterial sequence fragments were clustered into Operational Taxonomic Units (OTUs) and aligned to Greengenes microbial gene database with 97% sequence similarity in QIIME (1.8.0). Bacterial taxonomy summarization, rarefaction analyses of microbial diversity, compositional differences (dissimilarity value indicated by Unweighted UniFrac Distance with the permutational multivariate analysis of variance [PERMANOVA] test) were calculated in QIIME as previously described using scripts (including pick_open_reference_otus.py, summarize_taxa.py, alpha_rarefaction.py, jackknifed_beta_diversity.py, and make_distance_boxplots.py). Principal coordinate of analysis (PCoA) plots were generated by QIIME script (make_2d_plots.py), and each point represents one animal represent the interquartile range during the rarefaction analyses (see http://www.wernerlab.org/teaching/qiime/overview for scripts details). To obtain functional predictive Gram-staining profiles of gut microbiome changes based on their BP status, we used METAGENassist (4).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 10 (San Diego, CA) and Statistica version 13 (StatSoft, Tulsa, OK). Data are presented as means ± SE for each group, and P values of <0.05 were considered significant. Variables were log transformed to achieve assumptions required for parametric statistical testing. Metabolic and phenotypic variables were analyzed for differences by analysis of covariance using baseline values, if available, and current body weight. Repeated measure of analysis of variance (ANOVA) was used for values measured during the 2 yr of longitudinal study (study 2). Fisher’s exact test was used to measure differences between proportions, and Pearson’s correlation coefficients were calculated to measure strength of the associations. Multiple regression modeling with backward stepwise removal of variables was used to find predictors of MT in study 1. TB1 exposure was included as a model factor for study 2 analyses but was not retained due to lack of significant effects.
RESULTS
Study 1: Cross-Sectional Study Identifies an Independent Relationship between Hypertension and MT
Detailed information on demographic and metabolic health biomarkers is shown in Table 1. A total of 153 vervet monkeys, with 15.6% males (n = 24) and 84.3% females (n = 129) were evaluated in this study. Expected sex differences in body size were present and male animals had 15%–16% higher blood pressures, which may be secondary to elevated stress responses to the capture process. To understand the role of hypertension on the gut barrier and cardiac failure risk, we measured MT via LBP-1 and sCD14, and cardiac biomarker via NT-proBNP in plasma samples. Sex differences in these parameters were not detected. Diastolic blood pressure was significantly associated with LBP-1 (r = 0.19, P = 0.02; Table 2 and Supplemental Fig. S3). Increasing age was also related to microbial translocation markers [LBP-1 (r = 0.17, P = 0.04) and sCD14 (r = 0.17, P = 0.04)]; however, aging did not have a relationship with blood pressure (SBP, P = 0.36 and DBP, P = 0.88). The association of pressure measures and LBP-1 became stronger and significant for both SBP and DPB after adjustment for age (Table 3 and Fig. 2A). We additionally performed a multivariate analysis to determine the predictors of MT. The significant variables in the final model were age (β = 0.033, P = 0.04) and DBP (β = 0.012, P = 0.02). This demonstrates the significant and independent effects of blood pressure regulation on MT.
Table 1.
Basic demographic and metabolic details in the cross-sectional study (study 1)
| Variables | n | Mean | Median | Minimum | Maximum | SD | SE |
|---|---|---|---|---|---|---|---|
| Age, yr | 153 | 16 | 17 | 6.4 | 28 | 5 | 0.4 |
| Male | 24 | ||||||
| Female | 129 | ||||||
| Body wt, kg | 153 | 6 | 6 | 3.5 | 11 | 1 | 0.1 |
| Waist, cm | 153 | 36 | 36 | 25 | 51 | 5 | 0.4 |
| FBG, mg/dL | 153 | 82 | 71 | 47 | 429 | 43 | 3.5 |
| Hemoglobin, HbA1c | 153 | 4 | 4 | 3.9 | 10 | 1 | 0.1 |
| SBP, mmHg | 153 | 120 | 115 | 74.3 | 208 | 25 | 2 |
| DBP, mmHg | 153 | 70 | 67 | 33 | 128 | 18 | 1.5 |
| HDLC, mg/dL | 153 | 113 | 120 | 9.7 | 264 | 54 | 4.3 |
| TPC, mg/dL | 153 | 64 | 58 | 25.1 | 279 | 31 | 2.5 |
| LBP-1, ng/mL | 147 | 155 | 59 | 9.8 | 1,276 | 222 | 18.3 |
| sCD14, ng/L | 148 | 4,109 | 3,747 | 617.6 | 10,137 | 20,14 | 165.6 |
DBP, diastolic blood pressure; FBG, fasting blood glucose; HDLC, high-density lipoprotein cholesterol; LBP-1, lipopolysaccharide-binding protein-1; SBP, systolic blood pressure; sCD14, soluble CD14; TPC, total plasma cholesterol; Waist, waist circumference.
Table 2.
Correlation analysis between plasma concentrations of LBP-1 in a cross-sectional study (study 1)
| Variables | Age | Body Wt | Waist | FBG | TG | HDLC | SBP | DBP |
|---|---|---|---|---|---|---|---|---|
| LBP-1 | ||||||||
| R | 0.17 | 0.03 | 0.02 | 0.12 | −0.07 | 0.04 | 0.15 | 0.19 |
| n | 147 | 147 | 147 | 147 | 147 | 147 | 147 | 147 |
| P value | 0.04* | 0.69 | 0.79 | 0.16 | 0.39 | 0.51 | 0.08 | 0.02* |
*P < 0.05. DBP, diastolic blood pressure; FBG, fasting blood glucose; HDLC, high-density lipoprotein cholesterol; LBP-1, lipopolysaccharide-binding protein; n, sample size; R, correlation coefficient; SBP, systolic blood pressure; TG, total plasma triglycerides; waist, waist circumference.
Table 3.
Age-adjusted correlation between plasma concentrations of LBP-1 in a cross-sectional study (study 1)
| Variables | SBP | DBP |
|---|---|---|
| LBP-1 | ||
| R | 0.16 | 0.20 |
| n | 147 | 147 |
| P value | 0.05* | 0.02* |
Age-adjusted correlation between plasma concentrations of lipopolysaccharide-binding protein (LBP)-1, an indicator of microbial translocation and systolic blood pressure (SBP) and diastolic blood pressure (DBP). *P < 0.05. n, sample size; R, correlation coefficient.
Figure 2.
Study 1. Microbial translocation biomarker LBP-1 was independently and positively associated with diastolic blood pressure (DBP) and age, with a 3-D interactive surface plot shown in A. Partial correlation coefficient between LBP-1 and DBP was R = 0.20, P = 0.02. No relationship was found between blood pressure measures and age. When classified as hypertensive (n = 61) or normotensive (n = 86) animals had significantly higher systolic blood pressure (SBP; B), DBP (C), lipopolysaccharide-binding protein (LBP-1; P = 0.06; D), plasma concentrations of NH2-terminal pro-B-type natriuretic peptide (NT-proBNP; E), ratio of the early E to late A ventricular filling velocities (F).Values are derived from data collected in study 1 and shown as means ± SE. *P < 0.05.
When the cohort was classified by AHA criteria, the mean SBP (Fig. 2B) and DBP (Fig. 2C) of the hypertensive animals (143 ± 2.11 and 83 ± 2.11, respectively) were significantly higher than those of the normotensive animals (102 ± 1.26 and 60 ± 1.16, respectively; P < 0.0001). Animals classified by AHA criteria as hypertensive (>130/80 mmHg at repeated assessments) had higher levels of LBP-1 (P = 0.06) compared with the normotensive animals (Fig. 2D).
We observed significantly higher plasma concentrations of NT-proBNP in hypertensive animals (Fig. 2E), which is an indicator of strain on cardiomyocytes and of high interest as a prognosticator of heart failure. Consistently, echocardiogram data showed significantly higher E/A (Fig. 2F) compared with normotensive animals. This data is an important phenotype as it relates to diastolic dysfunction and risk of heart failure particularly that described as heart failure with preserved ejection fraction. These changes in hypertensive animals showed dramatic remodeling, and almost no left ventricular also noticed in the example image. Full echocardiographic results are shown in Supplemental Table S2 and Fig. S2.
Study 2: Longitudinal Study of Spontaneous Hypertension, MT, and Microbiome
Sixteen adult male rhesus macaques (mean age 5.8 ± 0.5 yr) were included in this study. These animals were divided into hypertensive (n = 8) and normotensive (n = 8) groups based on their natural presentation, and their metabolic health characteristics at baseline and 27-mo time points are given in Table 4. The SBP and DBP in normotensive monkeys consistently showed low levels, whereas in hypertensive monkeys, the systolic pressure values were sustained at higher values (repeated measures ANOVA group effect P = 0.008, time and time × group effects P > 0.05; Fig. 3A). Diastolic blood pressures were also trending higher in the hypertensive group (repeated-measures ANOVA group effect P = 0.06; Fig. 3B) and in all animals increased over the course of study (time effect P < 0.05, time × group effect P = 0.82). We observed significantly higher plasma concentrations of NT-proBNP in hypertensive animals at baseline and these also stayed higher at study end (P = 0.04). Overall, the corrected means for the hypertensive group from repeated measures of ANOVA showed a significant group effect (P = 0.04) for plasma concentrations of NT-proBNP being elevated with hypertension (Fig. 3C).
Table 4.
Basic demographic and metabolic details of the longitudinal study (study 2) at baseline and end of study
| Variables | Baseline |
Study End at 27 mo |
||
|---|---|---|---|---|
| Normotensive | Hypertensive | Normotensive | Hypertensive | |
| n | 8 | 8 | 8 | 8 |
| Age, yr | 5.02 ± 0.16 | 5.04 ± 0.16 | ||
| Body wt, kg | 9.08 ± 0.62 | 10.4 ± 0.82 | 15.7 ± 1.08 | 16.7 ± 1.28 |
| Body fat, % | 15.4 ± 1.27 | 18.8 ± 1.94 | 24.6 ± 3.00 | 30.0 ± 2.32 |
| Waist, cm | 36.4 ± 1.71 | 38.8 ± 2.95 | 47.3 ± 3.69 | 51.7 ± 2.56 |
| FBG, mg/dL | 64.5 ± 3.17 | 67.5 ± 2.96 | 76.1 ± 23.8 | 87.5 ± 17.5 |
| A1c, % | 4.71 ± 0.08 | 4.79 ± 0.11 | 5.83 ± 0.75 | 5.40 ± 0.44 |
| TPC, mg/dL | 161 ± 15.6 | 173 ± 7.67 | 204 ± 18.9 | 179 ± 18.4 |
| HDLC, mg/dL | 98.4 ± 9.58 | 115 ± 12.3 | 120 ± 9.74 | 113 ± 7.95 |
| TG, mg/dL | 37.0 ± 11.9 | 39.8 ± 5.55 | 39.9 ± 9.24 | 57.4 ± 18.4 |
Values are means ± SE. No significant differences were observed at baseline or at study end (ANCOVA, P > 0.05). A1c, glycosylated hemoglobin; FBG, fasting glucose; HDLC, high-density lipoprotein cholesterol; TG, total plasma triglycerides; TPC, total plasma cholesterol; waist, waist circumference.
Figure 3.
Study 2. Hypertensive animals (n = 8) had significantly higher levels of systolic blood pressure (SBP; A), diastolic blood pressure (DBP; B) by design, and we found plasma concentrations of NH2-terminal pro-B-type natriuretic peptide (NT-proBNP; C) was also elevated. Microbial translocation biomarkers lipopolysaccharide-binding protein (LBP)-1 (D) and universal 16S bacterial gene expression (E) in mesenteric lymph nodes were both higher when compared with normotensive animals (n = 8) in longitudinal study (study 2). For bacterial gene expression, Ct values that are lower have higher gene expression. Values are shown as means ± SE. *P < 0.05.
Higher MT Biomarkers in Hypertensive Individuals
At baseline, the individual MT biomarker (LBP-1 concentrations) was higher in hypertensive animals, with >14-fold difference between these two groups (46 vs. 668 ng/mL, respectively) and these levels progressively and dramatically increased over 2 yr, compared with the normotensive animals (Fig. 3D). This result is consistent with results of the larger cross-sectional study 1, where hypertension was associated with higher MT biomarkers and independently predicted this outcome. We took additional steps to confirm these MT biomarkers did indicate actual movement of microbes across the intestinal barrier to extraintestinal tissues. To this end, we observed a higher amplification of universal bacterial 16S gene in draining mesenteric lymph node tissue from the large intestine, which confirms higher MT and barrier dysfunction in hypertensive animals compared with normotensive counterparts (Fig. 3E and Supplemental Fig. S4).
Fecal Microbiome Shifts in the Hypertensive Group
For the fecal microbiome samples, there was no difference in the number of microbial sequences per sample (Supplemental Fig. S5A), or the α and β diversity profiles at baseline (Fig. 4, A and C) and 12 mo (Fig. 4, B and D) between the groups despite preexisting disparate blood pressures. However, at 27 mo, both α (Fig. 4C; P = 0.04) and β diversity profiles showed significant (R2 = 0.21, P < 0.004) differences between the two groups (Fig. 4F).
Figure 4.
Fecal microbial diversity profiles in normotensive and hypertensive monkeys. No significant changes in α diversity profiles by Shannon’s index at baseline (A), 12 mo (B) but significantly (P = 0.03) different at 27 mo (C). Principal coordinate analysis (PCoA; unweighted UniFrac) show no divergence at baseline (D; R2 =0.01, P < 0.09) and 12 mo (E; R2 =0.11, P < 0.07), but show clear divergence at 27 mo (F; R2 = 0.21, P < 0.004). Data shown as means ± SE. *P < 0.05. ns, not significant.
The most dominant phyla were Firmicutes, Bacteroidetes, and Actinobacteria in both the groups (Fig. 5A). However, the proportions of each taxon changed between groups and across time points, with the largest changes detected at 27 mo. In normotensives, Firmicutes proportions increased by 11.8% from baseline to 12 mo and remained the same at 27 mo. In the hypertensive group, Firmicutes proportions increased by 6% from baseline to 12 mo and decreased by 35% at 27 mo. We observed an overall Firmicutes decrease of 37% at 27 mo in hypertensive animals, compared with normotensive animals. Although, we observed changes in proportions of Bacteroidetes and Actinobacteria at baseline and 12 mo, these changes remained constant at 27 mo in both groups. When compared with normotensives, Proteobacteria proportions increased at 27 mo only. Firmicutes-to-Bacteroidetes (F/B) ratio were found to be higher in hypertensive individuals (Fig. 5B; P = 0.05 for effect of hypertension) and became increasingly higher over time such that the overall F/B ratio was significantly different in hypertensive animals at 27 mo (P = 0.025).
Figure 5.
Development of fecal microbiome shifts in hypertensive animals in longitudinal study (study 2). A: the phylum-level taxonomic composition of fecal samples in normotensive and hypertensive monkeys. B: overall, the Firmicutes to Bacteroidetes ratio was higher in hypertensive monkeys (P = 0.05 for effect of group) and was significantly different at 27 mo (repeated measures ANCOVA, P = 0.025). C: the percentage counts of families Lactobacillaceae. D: Ruminococcaceae from baseline to 27 mo. E: the genus-level taxonomic composition of fecal samples in normotensive and hypertensive monkeys. Values are shown as means ± SE. * P < 0.05, ****P < 0.0001. HTN, hypertensive; ns, not significant; NTN, normotensive.
During the follow-up period, the percentage counts of the beneficial bacterial family, Lactobacillaceae (Fig. 5C), were decreased and the percentage counts of the opportunistic families, such as Ruminococcaceae (Fig. 5D), were increased in the hypertensive group compared with normotensive counterparts. At genus level, Lactobacillus, Bifidobacterium, and Corynebacterium were reduced, whereas Ruminococcus and Lachnospira were shown to be more abundant in hypertensive groups, with most of these changes noticeable visible at 12 and 27 mo (Fig. 5E). Furthermore, our overall taxonomic-to-phenotype mapping revealed phenotypic changes with Gram staining showing a small increase in Gram-negative bacteria hypertensive monkeys when compared with normotensive monkeys (39.6 vs. 34%; Supplemental Fig. 5B). As these changes developed slowly under environmentally controlled conditions, the progressive decline of the intestinal barrier function in the face of consistent hypertension, blood pressure is considered a possible causative factor in shaping the microbiome.
DISCUSSION
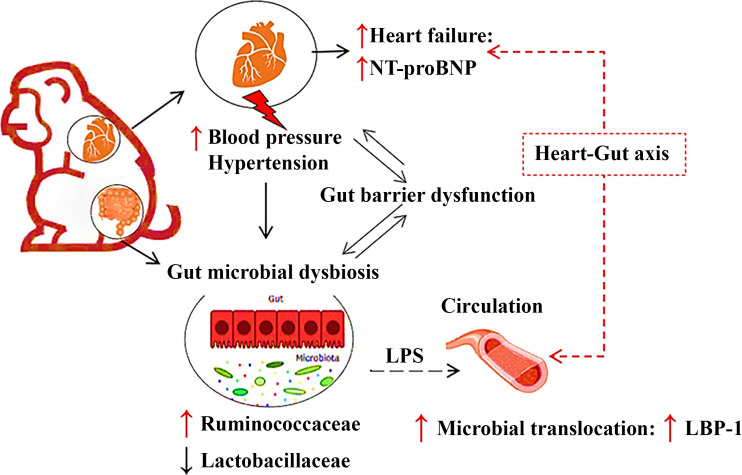
To the best of our knowledge, this is the first study to examine the associations between gut barrier dysfunction and microbiome shifts with hypertension in a nonhuman or human primate model that includes a longitudinal component to the study design, and thus has high translational relevance to human health. In the present study, we showed that hypertensive animals had higher concentrations of plasma LBP-1 (a MT biomarker) and over time develop unhealthy shifts in the gut microbiome compared with normotensive animals, suggesting that hypertension leads to MT and eventually imbalances in the gut microbial community. Specifically, our major findings are that 1) hypertensive animals had higher MT, measured by plasma LBP-1 concentration and extraintestinal bacteria abundance when compared with normotensive animals; 2) increases in plasma concentration of LBP-1 are positively correlated with DBP, independent of age and other health markers, suggesting specificity of the independent effect of hypertension; and 3) in longitudinal analyses of environmentally controlled individuals, hypertensive animals had remodeling of their microbiome toward a less healthy profile (Fig. 6). These findings suggest that hypertension is a contributor to MT and nonbeneficial microbiome shifts. Together these factors may contribute to poor health in ways not considered before, and points potentially to another important reason for an early diagnosis and treatment of hypertension. As MT and perturbed microbiome drive inflammatory processes along with hypertension-related changes in tissue perfusion and function, we describe a novel connection between the gut and heart.
Figure 6.
Proposed overview of the interactions between hypertension, gut barrier function, microbiome, and acceleration of cardiac disease.
The findings of our cross-sectional study (study 1) are consistent with a few animal studies and recent clinical studies, which showed the associations between hypertension and gut barrier dysfunction (7, 10, 12, 19). Nonetheless, the aforementioned studies were cross-sectional and were designed to examine associations and not to determine cause-and-effect relationships. Besides, the gut microbiota is considered as a regulator of the gut barrier function, and studies have shown dysbiotic changes in the gut microbial composition with hypertension (6, 22). However, no study has investigated whether microbial alterations precede, accompany, or result from hypertension. To address these gaps, we performed a longitudinal study (study 2) to identify the gut microbiota changes in hypertension.
We have previously demonstrated LBP-1 as a reliable marker for MT and its positive association with age-related metabolic health decline in NHPs and humans (3–5, 15). More recently, a Japanese-based population longitudinal study showed that higher LBP-1 levels are associated with an increased risk of the cardiovascular disease (CVD) development (10). However, no studies have investigated the role of MT in hypertension, which is a major contributor to CVD. Our cross-sectional study (study 1) found the evidence to support our hypothesis, as mentioned in the introduction section, that MT is associated with hypertension, an indicator of gut barrier dysfunction. A dysfunctional gut barrier promotes lipopolysaccharide (LPS measured by LBP-1 concentrations) of the gut microbiota to the host, thereby inducing host inflammation and, finally, impacting cardiovascular health (9, 10, 12).
The combination of hypertension and MT was also observed alongside higher NT-proBNP and impaired diastolic function measured by echocardiogram, which relates to the risk of heart failure (23, 24). NT-proBNP has recently attracted a lot of attention as a biomarker for heart function. The parent BNP peptide is synthesized by atrial and ventricular cardiomyocytes and exerts potent diuretic, natriuretic, vasorelaxant and favorable metabolic effects, which are mediated via guanyl cyclase-A. BNP suppresses the renin-angiotensin pathway and sympathetic activity (25, 26). Collectively, the parent protein should exert positive effects; however, it has been found in clinical trials to be useful both diagnostically and prognostically within the range of concentrations that we report here measured from at-risk monkeys (27).
Our observed association between hypertension and MT was independent of age. Although aging is associated with worsening gut barrier function, our observed association between hypertension and age occurred even when age was accounted for. Previously, we showed that age-related increases in MT and Western diet, either alone or in combination, led to a decline in metabolic health (3, 5, 15). However, in the present study, LBP-1 levels were not associated with any components of metabolic syndrome, which could be related to the healthy diet consumed by these animals. These findings demonstrate that the association between hypertension and MT is partially driven by aging, but hypertension is an independent effect that worsens the overall health.
To confirm the involvement of the gut microbiome, we collected fecal samples over a 2-yr period (baseline, 12-, and 27-mo time points) and investigated microbiome changes using 16S rRNA gene sequencing technology to identify gut microbiota changes between hypertensive versus normotensive animals. Consistent with our hypothesis, the results from our longitudinal study (study 2) demonstrated that the hypertension drives echocardiographic markers that suggest higher risk of heart failure and drove gut barrier dysfunction, which led to the fecal microbiome perturbations in hypertensive animal. Moreover, for the first time, we found that microbiome shifts did not manifest at baseline but was evidently discerned at 12-mo and remained perturbed at the 27-mo time point in hypertensive animals compared with normotensive animals. Most of the previous studies examining these relationships relied on a single time point only and detailed information on the causal/functional mechanisms are thus limited. The current study builds on this earlier evidence using microbiome sequencing, appropriate NHP model, and longitudinal sampling.
Human gut microbiome studies have reported an association between a higher abundance of Gram-negative bacteria and higher BP (28–30). Consistently, we observed overall increases in Gram-negative, along with MT, in hypertensive animals, suggesting the presence of higher inflammation via microbe-immune cross talk. Most published microbiome studies in humans identified reduced α diversity in patients with hypertension or patients with higher BP (22, 29). In our study, we found similar trends in at baseline and 12 mo, but α diversity by Shannon’s index was significantly higher at 27 mo in hypertensive animals. Alongside, our β diversity analysis shown revealed distinct clustering and dissimilar between the two groups at 27 mo. Although the α diversity findings are not in line with the limited existing research, our findings are novel and need to be further investigated.
We found several alterations in microbial taxonomic compositions between normotensive and hypertensive animals specific to the sample type. Consistently, we found the gut microbiome was dominated by Bacteroidetes and Firmicutes phyla. The F/B ratio is widely accepted to have an important influence in maintaining normal intestinal homeostasis (22). Increased or decreased F/B ratio is regarded as dysbiosis, often observed in metabolic and inflammatory diseases (31). Our study showed numerical increases in F/B ratio in line with previous studies, reaching significance at 27 mo, along with Proteobacteria abundance increases (31). When normotensive rats were gavaged with microbiota from hypertensive rats, there was an increase in the F/B ratio and systolic BP consistent with our findings (32). Thus, maintaining the right F/B balance in the gut is important, especially when it comes to managing obesity and other metabolic conditions.
We have previously shown in NHPs that increased abundance of Proteobacteria cooccurred with higher MT, alterations in F/B, and higher proinflammatory biomarkers (4). Inflammation due to the immune response triggered by LPS is the key feature of Gram-negative bacteria pathogenesis, such as Proteobateria, and has been identified as contributor to the pathogenesis of hypertension. In the current study, we observed higher abundances of Proteobacteria in chronically hypertensive animals compared with normotensive animals. Particularly, hypertension can elevate proinflammatory effector memory T cells and T-helper cell subtypes, T-helper 17 cells [Th17; producing interleukin (IL)-17] and type 1 helper cells, which promote both hypertension and cardiovascular target organ damage. Whereas regulatory T cells (Tregs) typically produce high amounts of anti-inflammatory IL-10 and can ameliorate vascular, cardiac, and renal damage (33–36). At the 12- and 27-mo time points, at the family level, we found increases in Ruminococcaceae and decreases in Lactobacillaceae families in the hypertensive group compared with the normotensive group. At the genus level, consistent with family level taxonomic changes, we observed decreases in Lactobacillus spp. at 12 and 27 mo in hypertensive animals relative to normotensive animals. Furthermore, we observed decline in short-chained fatty acids (SCFAs) producing genera such as Corynebacterium and Veillonella in the hypertensive group. A decline in SCFA producers represents another mechanism that might relate to their capacity to decrease the production of proinflammatory cytokines (e.g., tumor necrosis factor-α and interferon-γ) and increase the production of anti-inflammatory cytokines (e.g., IL-4 and -10) (37).
Several mechanisms might explain why hypertension can induce gut barrier dysfunction and dysbiosis. First, animal studies have shown that hypertension triggered changes in arterioles (a part of microcirculatory system), which decreased intestinal blood flow (12, 13). In addition, from recent human studies, we know that hypertension induces vascular pathologies such as arterial stiffness, which reduce intestinal perfusion (22, 38). Decreased blood flow and intestinal perfusion dampen the nutrient supply and absorption in the gut, causing gut mucosal damage and barrier dysfunction leading to dysbiosis (22, 39). Second, SCFAs are known to energize the gut epithelium, reduce gut permeability and inflammation, and regulate blood pressure (9, 22, 40). Particularly, SCFAs may regulate blood pressure by acting on the G protein-coupled and olfactory receptors, expressed in the kidney and smooth muscle cells of blood vessels respectively (41). Although a few members of the Ruminococcaceae family are recognized as SCFA producers in healthy conditions, they are inversely correlated with gut permeability and overabundant in some gut inflammatory disorders (42). In contrast, Lactobacillaceae family members, particularly the taxa Lactobacillus, are known for their beneficial effects via an increase in SCFA production (40, 42). A recent population-based study by Palmu et al. (43) demonstrated strong negative associations of certain Lactobacillus species with both dietary sodium intake and BP, where SCFA may play an important role in regulating BP. Thus, microbiome-induced SCFA-led alterations could be one of the main mechanisms of the hypertension condition, as observed in our study. Although there is evidence regarding the significant role of gut microbiome-produced SCFA in modulating blood pressure, the role of SCFA in hypertension warrants further research.
The strengths of our study include a larger sample size used for screening risk factors, and then use of a prospective cohort study design with ideal experimental conditions for follow-up over 2 yr, coupled with an accurate diagnosis of hypertension and MT based on available clinical information. Moreover, this could be the first longitudinal study to show the gut microbiome shifts are resulting from hypertension. We also address repeatability and generalizability of the relationship between hypertension and gut barrier dysfunction via the use of two separate nonhuman primate cohorts and species, which show consistent results. However, there are a few limitations of the present study. A subset of animals from the study 2 received TB1; however, we found no significant differences in their LBP, BP measures, and microbiome (Supplemental Fig. S1). Plasma biomarkers only indirectly reflect gut barrier dysfunction. However, these biomarkers act as potential predictors for screening and diagnosis of the disease condition and we confirmed barrier dysfunction by detection of higher bacterial genome levels in lymph nodes draining the colon (44). While providing novel information about microbial signatures of hypertension, possibly more robust work is needed at the species level to understand the alterations in microbial metabolism and inflammation and their contributions to hypertension. Understanding the host-microbiome-metabolic axis will enable us to gain insights into diagnosis and develop a microbiome-related therapeutic intervention to manage blood pressure in patients with hypertension. To this end, probiotics are natural and safe modulators of the gut microbiome (9, 42). Although bacterial probiotics are attractive options, it is still unclear if they would benefit patients with hypertension (45). Instead, engineered bacteria probiotics, such as Lactobacillus spp., to target specific tissues and cells rather than the whole body might be new modalities to develop a therapeutic approach on a clinical scale.
Perspectives
The present study indicates that hypertension is associated with MT and gut barrier dysfunction and the risk of heart failure in spontaneous hypertensive primates (study 1). Furthermore, hypertension and gut barrier dysfunction, either alone or in combination, triggered the gut microbial changes consistently over 2 yr (study 2). Our data indicated support for the role of the novel “heart gut” axis by showing a high risk of heart failure, elevated NT-proBNP concentrations as well as cardiac pathology (echocardiography) during hypertension, which led to gut barrier dysfunction and imbalances in the gut microbiome composition (46). Thus, the interaction between gut microbiota and host can be applied as a potential therapeutic option to help overcome the global burden of hypertension and CVD. Our research will be expanded to determine the functional role of gut microbiota by combining taxonomic profiling with simultaneous determination of leakiness and related metabolites, such as SCFA, in the gut. Additional studies are required to determine the detailed microbial metabolism and inflammatory mechanisms of the gut barrier dysfunction and dysbiosis linked to hypertension along with shifts that cooccur with antihypertensive therapy.
SUPPLEMENTAL DATA
Supplemental Figs. S1–S5 and Tables S1 and S2: https://doi.org/10.6084/m9.figshare.16663375.
GRANT
This work was supported by National Institutes of Health Grants R01 HL142930, T35 OD010946, P40 OD010965, and UL1TR001420 and Department of Defense Grants W81XWH-15-1-0574 and NIAID U19-A1067798.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M.C. and K.K. conceived and designed research; R.V., A.R., J.M.W., M.R.B., H.G., and K.K. performed experiments; R.V., G.O.D., J.M.C., M.R.B., H.G., and K.K. analyzed data; A.R., J.M.W., J.M.C., H.G., and K.K. interpreted results of experiments; R.V. and K.K. prepared figures; R.V. and K.K. drafted manuscript; G.O.D., H.G., and K.K. edited and revised manuscript; R.V., A.R., J.M.W., G.O.D., J.M.C., M.R.B., H.G., and K.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the animal care, veterinary, and research staff at the Wake Forest School of Medicine Clarkson Campus, UNC Microbiome Core Facility (the Center for Gastrointestinal Biology and Disease (CGIBD P30 DK034987) and the UNC Nutrition Obesity Research Center (NORC P30 DK056350) as well as the NHPs themselves.
REFERENCES
- 1.Genua F, Raghunathan V, Jenab M, Gallagher WM, Hughes DJ. The role of gut barrier dysfunction and microbiome dysbiosis in colorectal cancer development. Front Oncol 11: 626349–626349, 2021. doi: 10.3389/fonc.2021.626349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, Jensen ET, Shaheen NJ, Barritt AS, Lieber SR, Kochar B, Barnes EL, Fan YC, Pate V, Galanko J, Baron TH, Sandler RS. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology 156: 254–272.e11, 2019. doi: 10.1053/j.gastro.2018.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kavanagh K, Hsu F-C, Davis AT, Kritchevsky SB, Rejeski WJ, Kim S. Biomarkers of leaky gut are related to inflammation and reduced physical function in older adults with cardiometabolic disease and mobility limitations. Geroscience 41: 923–933, 2019. doi: 10.1007/s11357-019-00112-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vemuri R, Sherrill C, Davis MA, Kavanagh K. Age-related colonic mucosal microbiome community shifts in monkeys. J Gerontol A Biol Sci Med Sci 76: 1906–1914, 2020. doi: 10.1093/gerona/glaa256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson QN, Wells M, Davis AT, Sherrill C, Tsilimigras MCB, Jones RB, Fodor AA, Kavanagh K. Greater microbial translocation and vulnerability to metabolic disease in healthy aged female monkeys. Sci Rep 8: 1–10, 2018. doi: 10.1038/s41598-018-29473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim S, Goel R, Kumar A, Qi Y, Lobaton G, Hosaka K, Mohammed M, Handberg EM, Richards EM, Pepine CJ, Raizada MK. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci 132: 701–718, 2018. doi: 10.1042/CS20180087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drolia R, Bhunia AK. Crossing the intestinal barrier via Listeria adhesion protein and internalin A. Trends Microbiol 27: 408–425, 2019. doi: 10.1016/j.tim.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Yu LC-H. Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: exploring a common ground hypothesis. J Biomed Sci 25: 1–14, 2018. doi: 10.1186/s12929-018-0483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vemuri R, Gundamaraju R, Shastri MD, Shukla SD, Kalpurath K, Ball M, Tristram S, Shankar EM, Ahuja K, Eri R. Gut microbial changes, interactions, and their implications on human lifecycle: an ageing perspective. Biomed Res Int 2018: 4178607, 2018. doi: 10.1155/2018/4178607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asada M, Oishi E, Sakata S, Hata J, Yoshida D, Honda T, Furuta Y, Shibata M, Suzuki K, Watanabe H. Serum lipopolysaccharide‐binding protein levels and the incidence of cardiovascular disease in a general Japanese population: the Hisayama Study. J Am Heart Assoc 8: e013628, 2019. doi: 10.1161/JAHA.119.013628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lepper PM, Schumann C, Triantafilou K, Rasche FM, Schuster T, Frank H, Schneider EM, Triantafilou M, von Eynatten M. Association of lipopolysaccharide-binding protein and coronary artery disease in men. J Am Coll Cardiol 50: 25–31, 2007. doi: 10.1016/j.jacc.2007.02.070. [DOI] [PubMed] [Google Scholar]
- 12.Jaworska K, Huc T, Samborowska E, Dobrowolski L, Bielinska K, Gawlak M, Ufnal M. Hypertension in rats is associated with an increased permeability of the colon to TMA, a gut bacteria metabolite. PLoS One 12: e0189310, 2017. doi: 10.1371/journal.pone.0189310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, Cole-Jeffrey CT, Lobaton GO, Stewart DC, Rubiano A, Simmons CS, Garcia-Pereira F, Johnson RD, Pepine CJ, Raizada MK. Hypertension-linked pathophysiological alterations in the gut. Circ Res 120: 312–323, 2017. doi: 10.1161/CIRCRESAHA.116.309006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lerman LO, Kurtz TW, Touyz RM, Ellison DH, Chade AR, Crowley SD, Mattson DL, Mullins JJ, Osborn J, Eirin A, Reckelhoff JF, Iadecola C, Coffman TM. Animal models of hypertension: a scientific statement from the American Heart Association. Hypertension 73: e87–e120, 2019. doi: 10.1161/HYP.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell EL, Davis AT, Brass K, Dendinger M, Barner R, Gharaibeh R, Fodor AA, Kavanagh K. Reduced intestinal motility, mucosal barrier function, and inflammation in aged monkeys. J Nutr Health Aging 21: 354–361, 2017. doi: 10.1007/s12603-016-0725-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kavanagh K, Jones KL, Sawyer J, Kelley K, Carr JJ, Wagner JD, Rudel LL. Trans fat diet induces abdominal obesity and changes in insulin sensitivity in monkeys. Obesity (Silver Spring) 15: 1675–1684, 2007. doi: 10.1038/oby.2007.200. [DOI] [PubMed] [Google Scholar]
- 17.Kaneko H, Itoh H, Yotsumoto H, Kiriyama H, Kamon T, Fujiu K, Morita K, Michihata N, Jo T, Takeda N. Association of isolated diastolic hypertension based on the cutoff value in the 2017 American College of Cardiology/American Heart Association Blood Pressure Guidelines with subsequent cardiovascular events in the general population. J Am Heart Assoc 9: e017963, 2020. doi: 10.1161/JAHA.120.017963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brownlee RD, Kass PH, Sammak RL. Blood pressure reference intervals for ketamine-sedated rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 59: 24–29, 2020. doi: 10.30802/AALAS-JAALAS-19-000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bacarella N, Ruggiero A, Davis AT, Uberseder B, Davis MA, Bracy DP, Wasserman DH, Cline JM, Sherrill C, Kavanagh K. Whole body irradiation induces diabetes and adipose insulin resistance in nonhuman primates. Int J Radiat Oncol Biol Phys 106: 878–886, 2020. doi: 10.1016/j.ijrobp.2019.11.034. [DOI] [PubMed] [Google Scholar]
- 20.DeBo RJ, Lees CJ, Dugan GO, Caudell DL, Michalson KT, Hanbury DB, Kavanagh K, Cline JM, Register TC. Late effects of total-body gamma irradiation on cardiac structure and function in male rhesus macaques. Radiat Res 186: 55–64, 2016. doi: 10.1667/RR14357.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo H, Chou W-C, Lai Y, Liang K, Tam JW, Brickey WJ, Chen L, Montgomery ND, Li X, Bohannon LM, Sung AD, Chao NJ, Peled JU, Gomes ALC, van den Brink MRM, French MJ, Macintyre AN, Sempowski GD, Tan X, Sartor RB, Lu K, Ting JPY. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 370: eaay9097, 2020. doi: 10.1126/science.aay9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silveira-Nunes G, Durso DF, Cunha EHM, Maioli TU, Vieira AT, Speziali E, Corrêa-Oliveira R, Martins-Filho OA, Teixeira-Carvalho A, Franceschi C. Hypertension is associated with intestinal microbiota dysbiosis and inflammation in a Brazilian population. Front Pharmacol 11: 258, 2020. doi: 10.3389/fphar.2020.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rørth R, Jhund PS, Yilmaz MB, Kristensen SL, Welsh P, Desai AS, Køber L, Prescott MF, Rouleau JL, Solomon SD, Swedberg K, Zile MR, Packer M, McMurray JJV. Comparison of BNP and NT-proBNP in patients with heart failure and reduced ejection fraction. Circ Heart Fail 13: e006541, 2020. doi: 10.1161/CIRCHEARTFAILURE.119.006541. [DOI] [PubMed] [Google Scholar]
- 24.Salah K, Stienen S, Pinto YM, Eurlings LW, Metra M, Bayes-Genis A, Verdiani V, Tijssen JGP, Kok WE. Prognosis and NT-proBNP in heart failure patients with preserved versus reduced ejection fraction. Heart 105: 1182–1189, 2019. doi: 10.1136/heartjnl-2018-314173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buglioni A, Cannone V, Cataliotti A, Sangaralingham SJ, Heublein DM, Scott CG, Bailey KR, Rodeheffer RJ, Dessi-Fulgheri P, Sarzani R, Burnett JC Jr.. Circulating aldosterone and natriuretic peptides in the general community: relationship to cardiorenal and metabolic disease. Hypertension 65: 45–53, 2015. doi: 10.1161/HYPERTENSIONAHA.114.03936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 117: e510–e526, 2008. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 27.Maisel AS, Duran JM, Wettersten N. Natriuretic peptides in heart failure: atrial and B-type natriuretic peptides. Heart Fail Clin 14: 13–25, 2018. doi: 10.1016/j.hfc.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Dan X, Mushi Z, Baili W, Han L, Enqi W, Huanhu Z, Shuchun L. Differential analysis of hypertension-associated intestinal microbiota. Int J Med Sci 16: 872–881, 2019. [Erratum in Int J Med Sci 18: 3748, 2021]. doi: 10.7150/ijms.29322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, Zhang W, Weldon R, Auguste K, Yang L, Liu X, Chen L, Yang X, Zhu B, Cai J. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5: 14, 2017. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan Q, Gu Y, Li X, Yang W, Jia L, Chen C, Han X, Huang Y, Zhao L, Li P, Fang Z, Zhou J, Guan X, Ding Y, Wang S, Khan M, Xin Y, Li S, Ma Y. Alterations of the gut microbiome in hypertension. Front Cell Infect Microbiol 7: 381, 2017. doi: 10.3389/fcimb.2017.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L, Zhong S-J, Hu S-y, Cheng B, Qiu H, Hu Z-X. Changes of gut microbiome composition and metabolites associated with hypertensive heart failure rats. BMC Microbiol 21: 1–14, 2021. doi: 10.1186/s12866-021-02202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM Jr, Durgan DJ. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics 49: 96–104, 2017. doi: 10.1152/physiolgenomics.00081.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Itani HA, Harrison DG. Memories that last in hypertension. Am J Physiol Renal Physiol 308: F1197–F1199, 2015. doi: 10.1152/ajprenal.00633.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itani HA, Xiao L, Saleh MA, Wu J, Pilkinton MA, Dale BL, Barbaro NR, Foss JD, Kirabo A, Montaniel KR, Norlander AE, Chen W, Sato R, Navar LG, Mallal SA, Madhur MS, Bernstein KE, Harrison DG. CD70 exacerbates blood pressure elevation and renal damage in response to repeated hypertensive stimuli. Circ Res 118: 1233–1243, 2016. doi: 10.1161/CIRCRESAHA.115.308111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markó L, Kvakan H, Park J-K, Qadri F, Spallek B, Binger KJ, Bowman EP, Kleinewietfeld M, Fokuhl V, Dechend R, Müller DN. Interferon-γ signaling inhibition ameliorates angiotensin II–induced cardiac damage. Hypertension 60: 1430–1436, 2012. doi: 10.1161/HYPERTENSIONAHA.112.199265. [DOI] [PubMed] [Google Scholar]
- 36.Didion SP, Kinzenbaw DA, Schrader LI, Chu Y, Faraci FM. Endogenous interleukin-10 inhibits angiotensin II–induced vascular dysfunction. Hypertension 54: 619–624, 2009. doi: 10.1161/HYPERTENSIONAHA.109.137158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarkar A, Mandal S. Bifidobacteria—insight into clinical outcomes and mechanisms of its probiotic action. Microbiol Res 192: 159–171, 2016. doi: 10.1016/j.micres.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Nakanishi R, Baskaran L, Gransar H, Budoff MJ, Achenbach S, Al-Mallah M, Cademartiri F, Callister TQ, Chang HJ, Chinnaiyan K, Chow BJW, DeLago A, Hadamitzky M, Hausleiter J, Cury R, Feuchtner G, Kim YJ, Leipsic J, Kaufmann PA, Maffei E, Raff G, Shaw LJ, Villines TC, Dunning A, Marques H, Pontone G, Andreini D, Rubinshtein R, Bax J, Jones E, Hindoyan N, Gomez M, Lin FY, Min JK, Berman DS. Relationship of hypertension to coronary atherosclerosis and cardiac events in patients with coronary computed tomographic angiography. Hypertension 70: 293–299, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Granger DN, Holm L, Kvietys P. The gastrointestinal circulation: physiology and pathophysiology. Compr Physiol 5: 1541–1583, 2015. doi: 10.1002/cphy.c150007. [DOI] [PubMed] [Google Scholar]
- 40.Vemuri R, Gundamaraju R, Shinde T, Perera AP, Basheer W, Southam B, Gondalia SV, Karpe AV, Beale DJ, Tristram S, Ahuja KDK, Ball M, Martoni CJ, Eri R. Lactobacillus acidophilus DDS-1 modulates intestinal-specific microbiota, short-chain fatty acid and immunological profiles in aging mice. Nutrients 11: 1297, 2019. doi: 10.3390/nu11061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes 5: 202–207, 2014. doi: 10.4161/gmic.27492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vemuri RC, Gundamaraju R, Shinde T, Eri R. Therapeutic interventions for gut dysbiosis and related disorders in the elderly: antibiotics, probiotics or faecal microbiota transplantation? Benef Microbes 8: 179–192, 2017. doi: 10.3920/BM2016.0115. [DOI] [PubMed] [Google Scholar]
- 43.Palmu J, Salosensaari A, Havulinna AS, Cheng S, Inouye M, Jain M, Salido RA, Sanders K, Brennan C, Humphrey GC. Association between the gut microbiota and blood pressure in a population cohort of 6953 individuals. J Am Heart Assoc 9: e016641, 2020. doi: 10.1161/JAHA.120.016641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ellison S, Abdulrahim JW, Kwee LC, Bihlmeyer NA, Pagidipati N, McGarrah R, Bain JR, Kraus WE, Shah SH. Novel plasma biomarkers improve discrimination of metabolic health independent of weight. Sci Rep 10: 1–9, 2020. doi: 10.1038/s41598-020-78478-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jama HA, Kaye DM, Marques FZ. The gut microbiota and blood pressure in experimental models. Curr Opin Nephrol Hypertens 28: 97–104, 2019. doi: 10.1097/MNH.0000000000000476. [DOI] [PubMed] [Google Scholar]
- 46.Forkosh E, Ilan Y. The heart-gut axis: new target for atherosclerosis and congestive heart failure therapy. Open Heart 6: e000993, 2019. doi: 10.1136/openhrt-2018-000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs. S1–S5 and Tables S1 and S2: https://doi.org/10.6084/m9.figshare.16663375.