Abstract
With an aging global population, identifying novel therapeutics are necessary to increase longevity and decrease the deterioration of essential end organs such as the vasculature. Secondary alcohol, 1,3-butanediol (1,3-BD), is commonly administered to stimulate the biosynthesis of the most abundant ketone body β-hydroxybutyrate (βHB), in lieu of nutrient deprivation. However, suprapharmacological concentrations of 1,3-BD are necessary to significantly increase systemic βHB, and 1,3-BD per se can cause vasodilation at nanomolar concentrations. Therefore, we hypothesized that 1,3-BD could be a novel antiaging therapeutic, independent of βHB biosynthesis. To test this hypothesis, we administered a low-dose (5%) 1,3-BD to young and old Wistar-Kyoto (WKY) rats via drinking water for 4 wk and measured indices of vascular function and metabolism posttreatment. We observed that low-dose 1,3-BD was sufficient to reverse age-associated endothelial-dependent and -independent dysfunction, and this was not associated with increased βHB bioavailability. Further analysis of the direct vasodilator mechanisms of 1,3-BD revealed that it is predominantly an endothelium-dependent vasodilator through activation of potassium channels and nitric oxide synthase. In summary, we report that 1,3-BD, at a concentration that does not stimulate βHB biosynthesis, could be a nutraceutical that can reverse the age-associated decline in vascular function. These results emphasize that 1,3-BD has multiple, concentration-dependent mechanisms of action. Therefore, we suggest alternative approaches to study the physiological and cardiovascular effects of βHB.
NEW & NOTEWORTHY 1,3-Butanediol (1,3-BD) is often administered to stimulate the biosynthesis of the most abundant ketone body, β-hydroxybutyrate (βHB), and its purported salubrious effects. Here, we report that a low dose of 1,3-BD (5%) is sufficient to reverse age-associated vascular dysfunction, independent of βHB. Therefore, low-dose 1,3-BD could be a novel therapeutic to increase blood flow and improve the quality of life in the elderly.
Keywords: aging, ketogenesis, metabolism, vascular function
INTRODUCTION
The aging process is associated with a progressive and natural deterioration of organ function. As a result, aging is the most important, nonmodifiable risk factor for many diseases, including cardiovascular disease (1). Generally, the first-line prophylactics to slow aging and promote longevity include regular exercise, abstinence from smoking, maintaining a healthy body mass, moderate alcohol consumption, and eating a balanced diet. Regarding the latter, ketogenic diets (i.e., diets high in fat and low in carbohydrate composition) and ketogenic interventions (i.e., caloric restriction) have long been promoted for improving cardiometabolic health (2, 3). In the absence of energy-dense nutrients, ketone bodies become the predominant energy source. β-Hydroxybutyrate (βHB) is the most abundant ketone body and is endogenously synthesized in the liver to be transported to the peripheral tissues (4). To study βHB, the secondary alcohol, 1,3-butanediol (1,3-BD) is commonly administered to raise βHB bioavailability (5). Experimentally, this has important application as exogenous 1,3-BD provides an alternative method to elevate βHB, in lieu of nutrient deprivation.
Supporting the notion that ketogenic diets are health enhancing, we have previously reported impressive antihypertensive effects when 20% 1,3-BD was administered in conjunction with a high-salt diet (6). However, 20% 1,3-BD treatment was also associated with adverse side effects including, stunted growth, metabolic acidosis, and hepatotoxicity (7). A recent dose-dependent analysis on 1,3-BD suggested that lower concentrations of 1,3-BD (i.e., 5%) were better tolerated than high concentrations (i.e., 20%) and avoided the undesirable side effects (8). However, the low concentration of 1,3-BD was not sufficient to significantly increase systemic βHB bioavailability (8). This observation, coupled with our original report that 1,3-BD can cause potent vasodilation of isolated resistance arteries (7), leads us to the hypothesis of the current investigation that 1,3-BD could reverse vascular aging independent of βHB biosynthesis. To test this hypothesis, we administered a low-dose 1,3-BD to young and old rats via drinking water for 4 wk and measured indices of vascular function and metabolism posttreatment.
MATERIALS AND METHODS
Experimental Animals
Inbred, male Wistar-Kyoto (WKY) rats were used. Because of our laboratory’s long-time interest in hypertension research, WKY rats are maintained in-house as a normotensive control for the spontaneously hypertensive rat (SHR). Our justification for using only male rats was because we wanted to determine whether the 5% concentration of 1,3-BD could avoid the deleterious side effects that have been specifically reported in male rats (7, 8), but not female rats (9), at the 20% concentration. Rats were bred and maintained on a low-salt diet (0.3% NaCl) composed of 26% protein, 17% fat, and 57% carbohydrate (Teklad diet 7034, Envigo). Rats were used on maturation to adulthood (15–19 wk old; 316–358 g) or allowed to age in our Department of Laboratory Animal Resources facility (64–85 wk old; 408–524 g).
All breeding and experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Toledo College of Medicine and Life Sciences. Pups were weaned between 28 and 30 days. All rats were maintained on a 12-h:12-h light/dark cycle and were allowed free access to chow, unless specifically fasted (please see treatments section below); water was continuously available regardless of nutrient availability. Euthanasia of rats was performed by thoracotomy and exsanguination via cardiac puncture under isoflurane anesthesia administered via nose cone (5% in 100% O2), consistent with the American Veterinary Medical Association Guidelines for the Euthanasia of Animals. All euthanasia and tissue harvesting were performed in the University of Toledo Department of Laboratory Animal Resources from 0530–0930 per experimental day.
Treatments
Stock 1,3-BD (Millipore-Sigma) was diluted to 5% (0.56 mol/L) concentration with drinking water for 28 days; drinking water served as the vehicle control. All rats had free access to food throughout the investigation. Some vehicle-treated rats were fasted for 16 h before study termination to serve as a positive control for βHB biosynthesis.
β-Hydroxybutyrate Measurement
Under isoflurane anesthesia, but before thoracotomy and exsanguination via cardiac puncture, arterial blood was collected from the abdominal aorta in silicone-coated collection tubes specified for serum (BD Vacutainer). Blood was left to clot at room temperature for ∼20 min. After clotting, blood was centrifuged at 2,000 g for 15 min at 4°C and the serum was separated, collected, flash frozen in liquid nitrogen, and stored at −80°C until the time of measurement. βHB measurement was performed using a colorimetric assay according to the manufacturer’s instructions (Cayman Chemical).
Vascular Function
Third- and fourth-order mesenteric resistance arteries (∼250–300 µm) were mounted onto Danish Myo Technology (DMT) wire myographs (Danish MyoTech) in 37°C Krebs buffer with 5% CO2 and 95% O2. Arteries were normalized to a lumen diameter optimal for tension development, as previously described (10, 11). To test the viability of mounted arteries, contraction to potassium chloride (KCl, 120 mmol/L) was measured. Subsequently, the integrity of the endothelium was tested with a phenylephrine (PE)-induced contraction (10 μmol/L) followed by endothelium-dependent vasodilation with acetylcholine (ACh, 10 μmol/L).
Cumulative concentration-response curves were performed to ACh (0.1 nmol/L to 100 μmol/L), sodium nitroprusside (SNP, 0.1 nmol/L to 100 μmol/L), PE (10 nmol/L to 100 μmol/L), and 1,3-BD (0.01 nmol/L to 100 μmol/L; all Millipore Sigma). Almost all relaxation concentration-response curves were performed after an initial contraction with phenylephrine (10 μmol/L), except for two experiments where KCl (60 mmol/L) was used instead.
To understand the contribution of the endothelium and endothelium-derived factors, some arteries were denuded (E−) with a hair shaft during mounting (denudation was considered successful if arteries relaxed less than 25% to ACh during the endothelium integrity test), or they were incubated with different pharmacological inhibitors for 30 min immediately before ACh and 1,3-BD concentration-response curves. Inhibitors of vasoactive factors included Nω-nitro-l-arginine methyl ester (100 μmol/L), indomethacin (10 μmol/L), tetraethylammonium (TEA, 10 mmol/L), iberiotoxin (100 nmol/L), UCL 1684 (100 nmol/L) and TRAM-34 (10 μmol/L) combined, and barium chloride (100 μmol/L; all Millipore Sigma). Receptor inhibitors included βHB (10 μmol/L; Cayman Chemical), GLPG 0974 (10 μmol/L; R&D Systems), mepenzolate bromide (MPN, 10 μmol/L; Millipore Sigma), picrotoxin (10 μmol/L; Tocris Bioscience), and CGP 55845 (10 μmol/L; Tocris Bioscience). All agonists and inhibitors were freshly prepared at the onset of the study and stored according to the manufacturer’s recommendations; the concentrations employed assumed specificity and efficacy against the respective targets based on literature. Relaxation concentration-response curves to ACh, SNP, and 1,3-BD are presented as a percentage (%) of the contractile response, and contraction concentration-response curves to PE and KCl are presented in units of tension (mN/mm). To determine whether a vasoactive factor elicited a significant effect, the cumulative area under the curve (AUC), with and without inhibitor, was analyzed. Therefore, the change (Δ) in AUC between ACh without inhibitor and the ACh with inhibitor was calculated and statistically tested. A similar approach was used to investigate the mechanisms of vasodilation for 1,3-BD. Specifically, concentration response curves to 1,3-BD were preformed first without inhibitor and then with inhibitor (30 min). Therefore, each artery served as its own control.
Metabolic Panel
Total cholesterol and triglycerides were measured in serum using colorimetric assays (Randox Laboratories). Glucose, alanine aminotransferase (ALT), sodium (Na+), chloride (Cl−), and total carbon dioxide (tCO2) were measured in serum using the VetScan VS2 Chemistry Analyzer and the Critical Care Plus reagent rotor (Zoetis). Metabolic acidosis was measured by calculating the anion gap [Na+ – (Cl− + (tCO2 – 1)].
Statistical Analysis
The sample size per experiment (see figure panels and legends) is the number of independent rats used, respective of age and treatment group. Previous work from our laboratory estimating a large effect size (Cohen’s d > 0.8), as well as power analysis (desired power of 0.80 to 0.85 with a probability of a Type I error of 0.05), has provided a basis for the projected number of rats required per experimental group.
The statistical procedures for concentration-response curves included repeated measures two-way analysis of variance (ANOVA) and nonlinear regression analysis [sigmoidal dose-response, half maximal (EC50), and maximal (Emax) effective concentrations]. In all other group comparisons, two-way ANOVA was used. The Sidak post hoc test was used in the case of a significant two-way ANOVA, and group differences were only reported when significant interactions were observed. All analyses were performed using data analysis software GraphPad Prism 9.2.0. Statistical significance was set at P < 0.05. Data are presented as means ± SE.
RESULTS
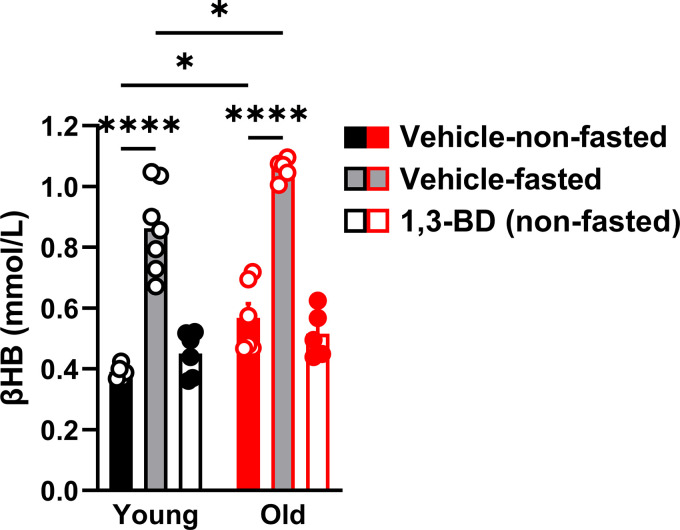
Evolutionarily, βHB is biosynthesized in time of nutrient scarcity. In support of this dogma, we observed that a 16-h fast was sufficient to significantly increase systemic βHB bioavailability to a similar magnitude, in both young and old rats. In contrast, and supporting our hypothesis, we observed that the low-dose 1,3-BD does not increase serum βHB in young or old rats (Fig. 1). Therefore, we can confirm that all the subsequent phenotypes measured after low-dose 1,3-BD treatment are independent of increased βHB bioavailability.
Figure 1.
Low-dose 1,3-butanediol (1,3-BD) treatment does not increase circulating β-hydroxybutyrate (βHB). βHB was measured in serum from young and old Wistar-Kyoto rats treated with either vehicle or 1,3-BD (5%) for 4 wk. Vehicle-treated rats were either nonfasted (free access to food) or fasted (16 h). Data are presented as means ± SE; n = 5–7. Two-way ANOVA: *P < 0.05, ****P < 0.0001.
Next, we tested the hypothesis that low-dose 1,3-BD could reverse age-associated vascular dysfunction. We observed that old rats had impaired endothelium-dependent and -independent vasodilation relative to the young. Confirming our hypothesis, old rats treated with 1,3-BD had improved endothelium-dependent and -independent vasodilation (Fig. 2, A and B), with no change in vascular contraction to PE (Fig. 2C) or KCl (120 mmol/L; Fig. 2D). On the other hand, and unexpectedly, we observed that young rats treated with 1,3-BD developed impaired endothelium-independent vasodilation (Fig. 2B). Investigation into the mechanisms underlying the endothelium-dependent improvements in old rats, we observed that old rats treated with 1,3-BD had increased potassium-dependent vasodilation and decreased cyclooxygenase-dependent activity, with no change in nitric oxide synthase (Fig. 2E). Analysis of the specific potassium channels contributing to the enhanced potassium-dependent vasodilation suggested that it was primarily mediated by small (SKCa)- and intermediate (IKCa)-conductance calcium-activated potassium channels, and not large (BKCa)-conductance calcium-activated potassium channels or inward rectifying potassium channels (Fig. 2F). Overall, these data support that low-dose 1,3-BD is an antivascular therapeutic, and its use in young and/or healthy individuals may be contraindicated.
Figure 2.
Low-dose 1,3-butanediol (1,3-BD) treatment reverses age-associated endothelium-dependent and -independent vascular dysfunction. Concentration-response curves to acetylcholine (ACh; A), sodium nitroprusside (SNP; B), and phenylephrine (PE; C), as well as contraction to potassium chloride (120 mmol/L administered in bolus; D) in mesenteric resistance arteries from young and old Wistar-Kyoto rats treated with either vehicle or 1,3-BD (5%) for 4 wk. Some concentration-response curves to ACh were incubated with Nω-nitro-l-arginine methyl ester (l-NAME, 100 μmol/L), indomethacin (10 μmol/L), tetraethylammonium (TEA, 10 mmol/L), or contracted to potassium chloride (KCl, 60 mmol/L), as opposed to PE (10 μmol/L; E). Additional concentration-response curves to ACh were incubated with iberiotoxin (100 nmol/L), UCL 1684 (100 nmol/L) and TRAM-34 (10 μmol/L) combined, or barium chloride (100 μmol/L; F). Data are presented as means ± SE; n = 4–6. Two-way ANOVA: *P < 0.05, **P < 0.01.
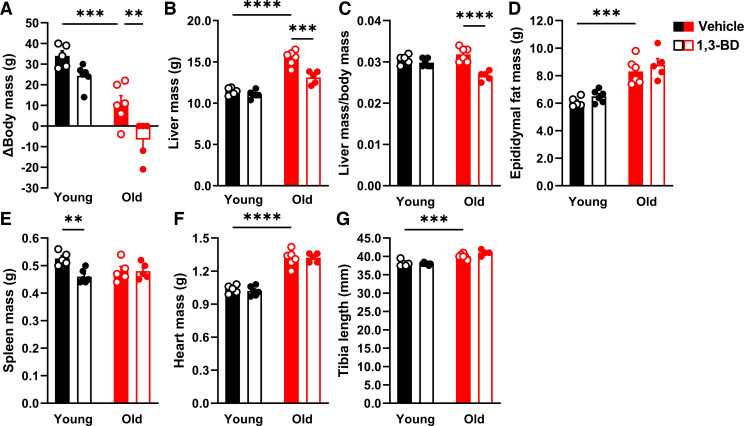
Associated with these improvements in vascular function in old rats, we observed that low-dose 1,3-BD decreased body mass (Fig. 3A) and liver mass (Fig. 3, B and C), without impacting other tissues, including epididymal fat mass (Fig. 3D), spleen mass (Fig. 3E), heart mass (Fig. 3F), or tibia length (Fig. 3G). Despite the decreased body and liver masses, no changes were observed for blood glucose or total cholesterol (Fig. 4, A and B). On the other hand, we observed that low-dose 1,3-BD increased triglycerides in both young and old rats (Fig. 4C). Finally, low-dose 1,3-BD was able to reserve age-associated liver dysfunction, as indicated by ALT (Fig. 4D), and did not cause metabolic acidosis, as measured by the anion gap (Fig. 4E). Overall, these data suggest that low-dose 1,3-BD reverses age-associated vascular dysfunction independent of changes in glucose and cholesterol homeostasis, despite elevated triglycerides.
Figure 3.
Low-dose 1,3-butanediol (1,3-BD) treatment decreases body mass and liver mass in old rats. Change in (Δ) body mass (A), liver mass (B), liver mass normalized for Δ body mass (C), epididymal fat mass (D), spleen mass (E), total heart mass (F), and tibia length (G) were measured from young and old Wistar-Kyoto rats treated with either vehicle or 1,3-BD (5%) for 4 wk. Data are presented as means ± SE; n = 4–6. Two-way ANOVA: **P < 0.01, ***P < 0.001, ****P < 0.0001.
Figure 4.
Low-dose 1,3-butanediol (1,3-BD) treatment reverses age-associated vascular dysfunction independent of changes in glucose and cholesterol, and despite elevated triglycerides. Blood glucose (A), triglycerides (B), total cholesterol (C), alanine aminotransferase (ALT; D), and the anion gap (E) were measured in serum from young and old Wistar-Kyoto rats treated with either vehicle or 1,3-BD (5%) for 4 wk. Data are presented as means ± SE; n = 4–6. Two-way ANOVA: **P < 0.01, ****P < 0.0001.
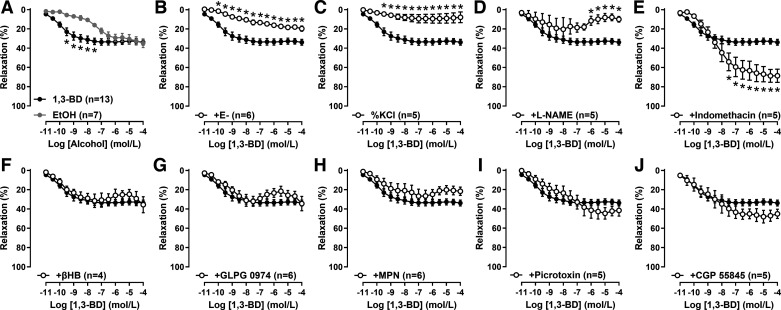
To understand the mechanism by which 1,3-BD reverses age-associated vascular dysfunction, we further investigated the mechanisms of our original observation that 1,3-BD is a direct vasodilator in mesenteric resistance arteries from Dahl salt-sensitive and salt-resistant rats (7). Using isolated arteries from young vehicle-treated WKY rats, we confirmed that 1,3-BD is a weak vasodilator (Emax: 32.7 ± 1.0%) irrespective of genetic background, with a similar magnitude, but greater potency, than ethanol (EtOH; Fig. 5A). Denudation (E−) of some arteries indicated that the vasodilation to 1,3-BD was predominantly endothelium-dependent (Fig. 5B). Incubation with inhibitors of different endothelium-dependent factors suggested that 1,3-BD-dependent vasodilation was mediated by potassium (Fig. 5C) and nitric oxide (Fig. 5D), but not cyclooxygenase-derived products (e.g., prostacyclin; Fig. 5E). Finally, we investigated whether 1,3-BD could cause vasodilation via activation of a G protein-coupled receptor (GPCR). Due to structural similarities with βHB, we hypothesized that 1,3-BD may activate a free fatty acid receptor or peripheral γ-aminobutyric acid (GABA) receptors (12). However, in opposition to this hypothesis, inhibition of Gpr41 (Fig. 5F), Gpr43 (Fig. 5G), or Gpr109a (Fig. 5H) did not prevent vasodilation, and nor did antagonism of GABA A (Fig. 5I) or GABA B (Fig. 5J) receptors. Therefore, although we have confirmed the endothelium-dependency of 1,3-BD-induced vasodilation, it was not confirmed whether this was due to direct activation of a potassium channel and nitric oxide synthase, or via a GPCR.
Figure 5.
1,3-Butanediol (1,3-BD) mediates vasodilation via potassium channels and nitric oxide synthase, but not cyclooxygenase-derived products, free fatty acid receptors, or γ-aminobutyric acid (GABA) receptors. Concentration-response curves to 1,3-BD and ethanol (EtOH) in mesenteric resistance arteries from Wistar-Kyoto rats (A). Some concentration-response curves to 1,3-BD were performed in arteries with the endothelium denuded (E−; B), contracted to potassium chloride (KCl, 60 mmol/L), as opposed to phenylephrine (10 μmol/L; C), incubated with Nω-nitro-L-arginine methyl ester (L-NAME; 100 μmol/L; D), indomethacin (10 μmol/L; E), β-hydroxybutyrate (βHB, 10 μmol/L; F), GLPG 0974 (10 μmol/L; G), mepenzolate bromide (MPN, 10 μmol/L; H), picrotoxin (10 μmol/L; I), or CGP 55845 (10 μmol/L; J). Data are presented as means ± SE; n = 4–13. Two-way ANOVA: *P < 0.05.
DISCUSSION
To summarize our major findings, we observed that low-dose 1,3-BD was sufficient to reverse age-associated endothelial-dependent and -independent dysfunction, and this was not associated with increased βHB bioavailability. Further analysis of the direct vasodilator actions of 1,3-BD revealed that it is predominantly an endothelium-dependent vasodilator through activation of potassium channels and nitric oxide synthase. Overall, these data support the concept that low-dose 1,3-BD could be promoted as an antivascular aging therapeutic.
Although we acknowledge that a systemic treatment of 1,3-BD could have several contributing mechanisms of action to reverse age-associated vascular dysfunction, it is well established that vasodilators are efficacious antivascular aging therapeutics by reducing resistance and enhancing compliance (13, 14). This can subsequently lower blood pressure and arterial stiffening, which are well established to increase with advancing age (15). Nitric oxide is the most commonly cited endothelium-derived factor that diminishes with age (16), which is a logical application of Harman’s free radical theory of aging (17, 18). In contrast, our investigation using mesenteric resistance arteries from WKY rats observed that low-dose 1,3-BD enhanced nitric oxide-independent vasodilation via increased potassium-dependent relaxation (also referred to as endothelium-derived hyperpolarization factor or EDH) and decreased cyclooxygenase-dependent activity. These observations are consistent with what we and others have previously reported for this vascular bed in healthy and diseased conditions (7, 19, 20). Importantly, 1,3-BD offers a nitric oxide-independent therapeutic, which is pertinent to elderly individuals who do not respond to pharmacologic agents that stimulate the nitric oxide pathway (21).
1,3-BD is by no means the first alcohol to have direct vasoactive effects. It has been well established that EtOH can exert concentration-dependent vasodilation in arteries and veins (22–24), independent of its metabolism into acetaldehyde and acetate (25), and via endothelium-dependent and -independent mechanisms (26). In fact, metabolites of EtOH have been attributed to the biphasic vasoactive response post-EtOH consumption (25, 27). Furthermore, 1,3-BD adds to the growing list of butyl-group compounds that can cause vasodilation including βHB (7) and butyrate (28). Although the precise mechanism(s) of action for these compounds remain(s) elusive, in the case of 1,3-BD, we have ruled out some of the most well-known free fatty acid receptors, and peripheral GABA receptors, as potential candidates.
Throughout the literature, 1,3-BD has been reported to have a contrasting mix of physiological effects that before now have been largely attributed to its ketogenic properties (8). However, as we have suggested before (7, 8), and now have supporting data, the βHB-independent effects of 1,3-BD are significant, and they need to be taken into consideration when designing studies that aim to study the physiological and cardiovascular effects of βHB. Given this dual, and confounding action of 1,3-BD, we suggest alternative approaches to study βHB such as treatment with pure βHB or a ketone monoester. However, it should also be acknowledged that ketogenic diets and interventions are sometimes questioned for their long-term adherence (29) and pathophysiological effects (30, 31).
Limitations of the current study include the investigation of only male rats, the omission of blood pressure and vascular stiffening measurements, and the use of WKY rats as our strain of choice. Although we believe the use of only male rats was justified based on the current literature (7–9), given that aging is a ubiquitous physiological phenomenon irrespective of sex, investigating whether low-dose 1,3-BD is similarly efficacious in female animals is certainly warranted. The inclusion of blood pressure and vascular stiffening measurements would be valuable in future studies given that 1) it is well known that vascular stiffening (e.g., aortic stiffening) and blood pressure generally rise as we age and, subsequently, increase the risk of terminal cardiovascular diseases (15); 2) we have previously reported that 20% 1,3-BD can cause impressive decreases in blood pressure in salt-sensitive rats (6); and 3) 1,3-BD can cause vasodilation of resistance arteries at nanomolar concentrations (current study). Finally, although WKY rats are commonly used as a control strain for many hypertension studies, it presents an abnormal phenotype relative to its parental Wistar stock (32). Therefore, its application as a control strain has been questioned (33, 34). For example, the WKY strain is hyperreactive to stress and may react to subtle environmental stimuli. As a result, WKY rats present hormonal, behavioral, and physiological measures that emulate those found in symptom-presenting depressive patients (35, 36). These abnormal phenotypes have been postulated to have occurred because breeding stocks were distributed before the strain was fully inbred (∼F6 generation) (33, 34). Despite these concerns, this potential stain effect was controlled for in our study by using in-house WKY rats that have been selectively inbred for our own genetic studies. Given that aging is ubiquitous phenomenon and it occurs in all biological organisms, even use of a strain that is less than optimal for certain parameters has translational relevance. Despite these limitations, we still think this study makes a significant contribution to the antivascular aging therapy literature and will be of interest to geriatricians and the elderly alike.
In summary, our desire to reverse (or at least delay) the aging process has long been the focus of biomedical research and homeopathic medicine. With the increasing life expectancy of the global population (37), developing and identifying novel therapeutics to reverse age-associated vascular dysfunction and cardiovascular disease is of the utmost importance (16). We have revealed for the first time that 1,3-BD, at a concentration that does not stimulate βHB biosynthesis, could be a nutraceutical that assists in this reversal. By way of its vasodilatory properties, low-dose 1,3-BD could be an over the counter, and easily tolerated therapeutic, to increase blood flow and improve the quality of life in the elderly.
GRANTS
This work was supported by grants from the American Heart Association (18POST34060003) and National Institutes of Health (K99HL151889, R01CA219144, R00GM118885, R01HL149762, and R01HL143082).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.G.M. conceived and designed research; C.G.M., E.W.W., B.S.Y., and B.M. performed experiments; C.G.M. analyzed data; C.G.M. interpreted results of experiments; C.G.M. prepared figures; C.G.M. drafted manuscript; C.G.M., E.W.W., B.S.Y., B.M., M.V-K., C.F.W., and B.J. edited and revised manuscript; C.G.M., E.W.W., B.S.Y., B.M., M.V-K., C.F.W., and B.J. approved final version of manuscript.
ACKNOWLEDGMENTS
C. G. McCarthy acknowledges support from the Dean’s Postdoctoral to Faculty Fellowship from the University of Toledo College of Medicine and Life Sciences (2018–2021).
Present address of C. G. McCarthy, E. W. Waigi, and C. F. Wenceslau: Dept. of Cell Biology and Anatomy, Cardiovascular Translational Research Center, School of Medicine, University of South Carolina, Columbia, SC.
REFERENCES
- 1.Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol 22: R741–R52, 2012. doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Bueno NB, de Melo IS, de Oliveira SL, da Rocha Ataide T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr 110: 1178–1187, 2013. doi: 10.1017/S0007114513000548. [DOI] [PubMed] [Google Scholar]
- 3.Varady KA, Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Haus JM, Hoddy KK, Calvo Y. Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr J 12: 146, 2013. doi: 10.1186/1475-2891-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab 25: 42–52, 2014. doi: 10.1016/j.tem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tate RL, Mehlman MA, Tobin RB. Metabolic fate of 1,3-butanediol in the rat: conversion to -hydroxybutyrate. J Nutr 101: 1719–1726, 1971. doi: 10.1093/jn/101.12.1719. [DOI] [PubMed] [Google Scholar]
- 6.Chakraborty S, Galla S, Cheng X, Yeo JY, Mell B, Singh V, Yeoh B, Saha P, Mathew AV, Vijay-Kumar M, Joe B. Salt-responsive metabolite, β-hydroxybutyrate, attenuates hypertension. Cell Rep 25: 677–689.e4, 2018. doi: 10.1016/j.celrep.2018.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarthy CG, Chakraborty S, Singh G, Yeoh BS, Schreckenberger ZJ, Singh A, Mell B, Bearss NR, Yang T, Cheng X, Vijay-Kumar M, Wenceslau CF, Joe B. Ketone body beta-hydroxybutyrate is an autophagy-dependent vasodilator. JCI Insight 6: e149037, 2021. doi: 10.1172/jci.insight.149037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCarthy CG, Waigi EW, Singh G, Castaneda TR, Mell B, Chakraborty S, Wenceslau CF, Joe B. Physiologic, metabolic, and toxicologic profile of 1,3-butanediol. J Pharmacol Exp Ther 379: 245–252, 2021. doi: 10.1124/jpet.121.000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishimwe JA, Garrett MR, Sasser JM. 1,3-Butanediol attenuates hypertension and suppresses kidney injury in female rats. Am J Physiol Renal Physiol 319: F106–F114, 2020. doi: 10.1152/ajprenal.00141.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulvany MJ, Halpern W. Mechanical properties of vascular smooth muscle cells in situ. Nature 260: 617–619, 1976. doi: 10.1038/260617a0. [DOI] [PubMed] [Google Scholar]
- 11.Wenceslau CF, McCarthy CG, Earley S, England SK, Filosa JA, Goulopoulou S, Gutterman DD, Isakson BE, Kanagy NL, Martinez-Lemus LA, Sonkusare SK, Thakore P, Trask AJ, Watts SW, Webb RC. Guidelines for the measurement of vascular function and structure in isolated arteries and veins. Am J Physiol Heart Circ Physiol 321: H77–H111, 2021. doi: 10.1152/ajpheart.01021.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman JC, Verdin E. β-Hydroxybutyrate: a signaling metabolite. Annu Rev Nutr 37: 51–76, 2017. doi: 10.1146/annurev-nutr-071816-064916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finkelstein SM, Collins VR, Cohn JN. Arterial vascular compliance response to vasodilators by Fourier and pulse contour analysis. Hypertension 12: 380–387, 1988. doi: 10.1161/01.hyp.12.4.380. [DOI] [PubMed] [Google Scholar]
- 14.O'Rourke MF, Adji A, Namasivayam M, Mok J. Arterial aging: a review of the pathophysiology and potential for pharmacological intervention. Drugs Aging 28: 779–795, 2011. doi: 10.2165/11592730-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation 143: e254–e743, 2021. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 16.Seals DR, Kaplon RE, Gioscia-Ryan RA, LaRocca TJ. You're only as old as your arteries: translational strategies for preserving vascular endothelial function with aging. Physiology (Bethesda) 29: 250–264, 2014. doi: 10.1152/physiol.00059.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 320: 454–456, 1986. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 18.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol 11: 298–300, 1956. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy CG, Wenceslau CF, Calmasini FB, Klee NS, Brands MW, Joe B, Webb RC. Reconstitution of autophagy ameliorates vascular function and arterial stiffening in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 317: H1013–H1027, 2019. doi: 10.1152/ajpheart.00227.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang J, Zheng JP, Li Y, Gan Z, Jiang Y, Huang D, Li H, Liu Z, Ke Y. Differential contribution of endothelium-derived relaxing factors to vascular reactivity in conduit and resistance arteries from normotensive and hypertensive rats. Clin Exp Hypertens 38: 393–398, 2016. doi: 10.3109/10641963.2016.1148155. [DOI] [PubMed] [Google Scholar]
- 21.Huang ST, Hsieh ML. Different hemodynamic responses by color Doppler ultrasonography studies between sildenafil non-responders and responders. Asian J Andrology 9: 129–133, 2007. doi: 10.1111/j.1745-7262.2007.00227.x. [DOI] [PubMed] [Google Scholar]
- 22.Altura BM. Pharmacology of venular smooth muscle: new insights. Microvasc Res 16: 91–117, 1978. doi: 10.1016/0026-2862(78)90047-x. [DOI] [PubMed] [Google Scholar]
- 23.Altura BM, Ogunkoya A, Gebrewold A, Altura BT. Effects of ethanol on terminal arterioles and muscular venules: direct observations on the microcirculation. J Cardiovasc Pharmacol 1: 97–113, 1979. doi: 10.1097/00005344-197901000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Horvath SM, Willard PW. Effect of ethyl alcohol upon splanchnic hemodynamics. Proc Soc Exp Biol Med 111: 295–298, 1962. doi: 10.3181/00379727-111-27772. [DOI] [PubMed] [Google Scholar]
- 25.Altura BM, Gebrewold A. Failure of acetaldehyde or acetate to mimic the splanchnic arteriolar or venular dilator actions of ethanol: direct in situ studies on the microcirculation. Br J Pharmacol 73: 580–582, 1981. doi: 10.1111/j.1476-5381.1981.tb16789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tawakol A, Omland T, Creager MA. Direct effect of ethanol on human vascular function. Am J Physiol Heart Circ Physiol 286: H2468–H2473, 2004. doi: 10.1152/ajpheart.01207.2003. [DOI] [PubMed] [Google Scholar]
- 27.Criscione L, Powell JR, Burdet R, Engesser S, Schlager F, Schoepfer A. Alcohol suppresses endothelium-dependent relaxation in rat mesenteric vascular beds. Hypertension 13: 964–967, 1989. doi: 10.1161/01.hyp.13.6.964. [DOI] [PubMed] [Google Scholar]
- 28.Aaronson PI, McKinnon W, Poston L. Mechanism of butyrate-induced vasorelaxation of rat mesenteric resistance artery. Br J Pharmacol 117: 365–371, 1996. doi: 10.1111/j.1476-5381.1996.tb15200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Neill B, Raggi P. The ketogenic diet: pros and cons. Atherosclerosis 292: 119–126, 2020. doi: 10.1016/j.atherosclerosis.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 30.Jia P, Huang B, You Y, Su H, Gao L. Ketogenic diet aggravates kidney dysfunction by exacerbating metabolic disorders and inhibiting autophagy in spontaneously hypertensive rats. Biochem Biophys Res Commun 573: 13–18, 2021. doi: 10.1016/j.bbrc.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Guo Y, Wang X, Jia P, You Y, Cheng Y, Deng H, Luo S, Huang B. Ketogenic diet aggravates hypertension via NF-κB-mediated endothelial dysfunction in spontaneously hypertensive rats. Life Sci 258: 118124, 2020. doi: 10.1016/j.lfs.2020.118124. [DOI] [PubMed] [Google Scholar]
- 32.Aiello EA, Villa-Abrille MC, Escudero EM, Portiansky EL, Pérez NG, de Hurtado MC, Cingolani HE. Myocardial hypertrophy of normotensive Wistar-Kyoto rats. Am J Physiol Heart Circ Physiol 286: H1229–H12235, 2004. doi: 10.1152/ajpheart.00779.2003. [DOI] [PubMed] [Google Scholar]
- 33.Kurtz TW, Morris RC, Jr. Biological variability in Wistar-Kyoto rats. Implications for research with the spontaneously hypertensive rat. Hypertension 10: 127–131, 1987. doi: 10.1161/01.hyp.10.1.127. [DOI] [PubMed] [Google Scholar]
- 34.Kurtz TW, Montano M, Chan L, Kabra P. Molecular evidence of genetic heterogeneity in Wistar-Kyoto rats: implications for research with the spontaneously hypertensive rat. Hypertension 13: 188–192, 1989. doi: 10.1161/01.hyp.13.2.188. [DOI] [PubMed] [Google Scholar]
- 35.Will CC, Aird F, Redei EE. Selectively bred Wistar-Kyoto rats: an animal model of depression and hyper-responsiveness to antidepressants. Mol Psychiatry 8: 925–932, 2003. doi: 10.1038/sj.mp.4001345. [DOI] [PubMed] [Google Scholar]
- 36.Solberg LC, Olson SL, Turek FW, Redei E. Altered hormone levels and circadian rhythm of activity in the WKY rat, a putative animal model of depression. Am J Physiol Regul Integr Comp Physiol 281: R786–R794, 2001. doi: 10.1152/ajpregu.2001.281.3.R786. [DOI] [PubMed] [Google Scholar]
- 37.Kochanek KD, Xu J, Arias E. Mortality in the United States, 2019. NCHS Data Brief (395): 1–8, 2020. https://pubmed.ncbi.nlm.nih.gov/33395387/. [PubMed] [Google Scholar]