Keywords: balance, posture, reactive balance, stance, standing
Abstract
The aim of this study was to quantify balance impairments in standing in people with degenerative cervical myelopathy (PwDCM) in response to external perturbations. PwDCM have damage to their spinal cord due to degeneration of the cervical vertebral column, but little is known about balance. Balance was quantified by capturing kinetics, kinematic, and electromyographic data during standing in response to lateral waist pulls. Participants received pulls during predictable and unpredictable contexts in three stance widths at two magnitudes. In response to lateral waist pulls, PwDCM had larger center of mass excursion (P < 0.001) and delayed gluteus medius electromyography onset (P < 0.001) and peak (P < 0.001) timing. These main effects of history of myelopathy were consistent across predictability, stance width, and magnitude. A multilinear regression determined that gluteus medius peak timing + tibialis anterior peak timing most strongly predicted center of mass excursion (R2 = 0.50, P < 0.001). These data suggest that PwDCM have delays in generating voluntary and reactive motor commands, contributing to balance impairments. Future rehabilitation strategies should focus on generating rapid muscular contractions. Additionally, frontal plane postural control is regulated by the gluteus medius and the tibialis anterior, whereas other muscles (e.g. gluteus minimus, ankle invertors/evertors) not studied here may also contribute.
NEW & NOTEWORTHY Frontal plane reactive postural control is impaired in persons with degenerative cervical myelopathy because of delayed muscle responses. Additionally, postural control varies across stance width, predictability, and perturbation magnitude.
INTRODUCTION
The purpose of this study was to examine frontal plane (lateral) reactive balance in people with degenerative cervical myelopathy (PwDCM) across degree of predictability and pull magnitude while controlling for base of support (stance width). PwDCM experience increased risk of falls and impaired quiescent standing balance (1, 2). Frontal plane balance, likely important for sideways falls, has only been reported in a single study (3, 4). There is a higher incidence of falls and fall-related fractures in PwDCM than in healthy adults, which partially improves after spinal cord decompressive surgery (1, 5–7). Balance impairments, such as increased sway, are common in degenerative cervical myelopathy (DCM) (2). Standing balance is a complex process even in individuals without myelopathy, involving integration of sensory and motor information throughout the nervous system (2, 8–10). In PwDCM, balance impairments occur when degeneration of the cervical vertebral column causes symptomatic compression on the spinal cord that impacts white matter tracts (11). Moreover, residual sensorimotor impairments after surgical decompression are common (12). Therefore, understanding the characteristics of balance impairments in these patients will facilitate the development of effective balance rehabilitation strategies.
Although balance impairments are known to exist in PwDCM, substantial gaps in our understanding exist. What is known is that center of pressure (COP) or center of mass (COM) sway trajectory length, area, and velocity are increased compared with those without myelopathy (2, 4, 13–17). Of these, one study further observed that low-frequency COP sway, believed to be dependent on visual information, was elevated, suggesting an increased reliance on vision for balance (14). An additional two studies examined reactive balance responses to platform perturbations, finding that reaction time is delayed and associated with increased COP and COM sway (2, 15). Understanding balance is critical, as increased COP sway area was reportedly related to worse clinical measures of DCM severity, sensation, and power (2, 16). Therefore, standing sway is increased in PwDCM, but less is known about reactions to external perturbations, which may be more contextually appropriate for fall risk (18). Moreover, our knowledge of balance in PwDCM is largely in regard to center of pressure sway area or only sagittal plane sway (2, 13–16). Only one study (4) reported frontal plane center of pressure sway, which was collected with feet shoulder width apart, which is likely a highly stable posture. As falls occur sideways as well as forward and backward (3), characterizing frontal plane balance is critical to understanding overall balance impairments in this population. A final gap in the existing literature on balance in PwDCM is that motor deficits are more substantial in proximal muscles (19) but most reactive balance studies in DCM have examined distal muscles (2, 15). Responses to frontal plane perturbations are typically hip (gluteus medius) dominant (20–22).Therefore, research on balance in PwDCM should assess reactive responses in the frontal plane while assessing gluteus medius activity.
To test gaps in the literature on balance in PwDCM, there are various factors that can be experimentally manipulated. Three of these factors are predictability, magnitude of perturbation, and stance width. In healthy adults, predictability of perturbations (i.e., unpredictable vs. predictable) and magnitude of perturbations are known to impact balance responses (23–25). In these studies (23–25), participants stood on two forceplates and held a manipulandum that applied perturbations to the upper extremity forward, right, left, and backward. Specifically, reaction responses scale with the magnitude of the perturbation; however, the slope of this relationship is increased when perturbations are unpredictable (24). That is, in healthy adults responses to perturbations scale with magnitude because of differences in proprioceptive stimulus (23, 25). PwDCM, however, may not have appropriate scaling of responses because of altered proprioceptive input (19, 26–28). Furthermore, predictable perturbation responses are more reliant on feedforward responses than unpredictable perturbations (29–32). Again, PwDCM may have altered responses due to impairments in afferent/efferent signaling (26, 33). Finally, several modeling studies (34–37) have suggested that people with sensory delays may be less able to take advantage of increased leverage in wide stances. As PwDCM may have delayed sensory feedback (38), it is possible that they experience increased sway in both narrow and wide stance.
One factor that may impact postural control is the role of interlimb coordination. Interlimb coordination is known to be critical to maintaining standing balance in healthy individuals and is impaired in persons after stroke (39, 40). Similarly, interlimb coordination during walking is impaired in persons chronically after spinal cord injury (41). Yet, interlimb coordination has thus far been unassessed in standing in PwDCM. Specifically with respect to frontal plane balance, interlimb coordination of the abductor/adductor muscle groups should be assessed, as they play a dominant role in controlling frontal plane posture at the hip. Specifically, because of its size and anatomical ease of access for surface electromyography, the gluteus medius should be assessed for alterations in interlimb coordination.
Another factor is “off-axis” muscular responses (e.g., a tibialis anterior response to a lateral perturbation); several studies have reported responses of sagittal plane muscles to frontal plane postural disturbances (23, 25, 42–46). Importantly, the responses of the tibialis anterior to lateral perturbations are blunted in people with ataxia (46). One likely reason for action of muscles outside of their primary plane of action may be stabilization of the whole limb. For example, sagittal plane muscles (such as the tibialis anterior) are known to increase in amplitude from double-limb to single-limb support (47, 48).
To characterize balance responses in PwDCM in the frontal plane, we applied perturbations across two magnitudes, three stance widths, and degree of predictability of discrete, lateral waist pulls to standing balance. We hypothesized that in PwDCM COM excursion would be greatest in response to unpredictable large-magnitude perturbations in narrow standing compared to conditions with predictable, small-magnitude wide standing and to healthy control subjects. Secondarily, we hypothesized that COM sway would be related to delayed gluteus medius and tibialis anterior electromyography timing.
METHODS
Participants
For this study, we recruited 10 PwDCM and 10 nonmyelopathic control subjects. Inclusion criteria for PwDCM were previous diagnosis of DCM and previous surgical decompression of the spinal cord. Participants were excluded if they had any neurological injury other than DCM. This study was approved by the institutional review board at Marquette University and was in accordance with the Declaration of Helsinki. Participants were excluded if they had a diagnosis of an orthopedic or neurological disease that may impact balance or sensory function other than cervical myelopathy, if they were unable to stand and walk unassisted, or if they had an uncorrected vision or vestibular impairment. Sample size was based on a power analysis from the first 5 participants’ data suggesting that 10 people per group would provide us sufficient power to identify large effects.
Motion Capture Measures
Retroreflective markers were placed on participants with the modified plug-in-gait model. Markers were placed bilaterally on the anterior superior iliac spine, lateral thigh, lateral knee, lateral lower leg, lateral malleolus, heel, and 2nd metatarsal phalangeal joint. Additionally, markers were placed on the C7 spinous process, T10 spinous process, xiphoid notch, manubrium, right scapula, and sacrum, and four markers were placed on the head. Kinematics were sampled at 100 Hz with an eight-camera Vicon system (MX T-Series, Vicon, Oxford, UK) and were used to assess whole body center of mass and frontal plane joint angles.
Electromyography
Electromyography (EMG) data were collected bilaterally from the gluteus medius, rectus femoris, medial hamstring, tibialis anterior, and medial gastrocnemius muscles with a wired EMG system (Motion Lab Systems, Baton Rouge, LA). EMG data were amplified by 1,000 and sampled at 1,000 Hz. Although the muscle of greatest interest for this study was the gluteus medius as the primary stabilizer for lateral perturbations, numerous studies have identified contribution of sagittal plane muscles to frontal plane postural control, perturbation responses, and evoked torque in a variety of populations (23, 25, 42, 45, 46, 49–51). EMG data were visually inspected for cross talk, and sensors were relocated if necessary. Peroneus longus EMG collection was initially attempted but discontinued because of repeated issues with cross talk. EMGs were collected bilaterally, pooled, and analyzed according to side ipsilateral and contralateral to the direction of the pull.
Kinetic Measures
Participants stood on an instrumented treadmill (Bertec, Inc.) with a pair of servomotors (AKM-33H, AKD-0606; Kollmorgen, Radford, VA) on either side as previously done (52). Ground reaction forces were sampled from the respective forceplates at 1,000 Hz with Vicon Nexus (Vicon, Oxford, UK). Participants were connected to the servomotors via a cable pully system attached to a harness on their waist. An S-shaped linear force transducer was attached to each cable and sampled at 1,000 Hz in Vicon Nexus (Vicon, Oxford, UK). Participants also wore a fall arrest harness attached to an overhead frame.
Clinical and Strength Measures
Clinical measures of modified Japanese Orthopedic Association scale (mJOA) (19), Berg balance scale (Berg) (53), activity specific balance confidence scale (ABC) (54), and functional gait assessment (FGA) (55) scores were collected. Time since surgery was self-reported. mJOA is a six-item DCM specific scale that assesses motor and sensory function of the upper and lower extremities and trunk as well as sphincter function (19). The mJOA ranges from 0 (no function) to 17 (normal). Berg is a 14-item scale that assesses dynamic balance over a variety of tasks with a best possible score of 56. The ABC is a 16-item scale where participants self-report confidence in performing a variety of tasks without falling or losing balance, scored as a percentage. The best possible score is 100%. The FGA is a 10-item clinician-scored scale involving walking with various tasks including turning head, walking backward, walking with eyes closed, tandem gait, and stairs. The FGA ranges from 0 (worst) to 30 (best). Additional demographic information collected was weight and height.
Maximal Voluntary Isometric Contraction Measurements
Quadriceps isometric strength was assessed as a global measure of lower extremity strength. Maximal voluntary isometric contraction (MVC) strength was assessed while participants were seated in a Biodex system (Biodex Medical Systems, Shirley, NY). Participants were seated with hips and knees at 90°. A computer screen was placed at eye level 4 ft in front of the participant. The screen would display a “Go” indicator maintained for 4 s, during which participants were encouraged to kick their leg as hard as they could and maintain maximal output until the screen changed to read “Relax.” During this time, researchers strongly and loudly encouraged the participants to kick as hard as they could. Strength was assessed bilaterally as the maximum of five MVC contractions held for 4 s, with 2-min rest between trials performed by each leg. Testing was done with the knee in 90° flexion, and participants received strong verbal encouragement. MVC values were normalized to body mass in kilograms and averaged across limb and defined as the largest peak value extracted from five MVC trials. Clinical measures and strength testing were conducted in a session separate from balance testing.
Protocol
The response to waist-pull perturbations was measured under 12 conditions (2 magnitudes × 2 predictabilities × 3 stance widths), with eight trials per condition. Pulls by the servomotor system were controlled by a modified custom LabVIEW program (National Instruments) adapted from Walker et al. (52). Pulls consisted of a ramp and hold force trajectory with 300-ms ramp up, 200-ms hold at target force output, and 100-ms ramp down consistent with Sturnieks et al. (9, 56). Target pull forces were 12% and 6% body weight (BW). Baseline tension was maintained at 6 N to remove any slack in the cable system (52). Participants were instructed to stand in place and resist the pull of the cables as best as they could without taking a step, unless taking a step was necessary to prevent a fall.
Predictability was modulated by having verbally cued and noncued trials. Noncued trials involved randomized pull direction (left vs. right) at random intervals (range: 5–15 s) with no cuing on direction or timing. Cued trials involved randomized direction (left vs. right); however, verbal cuing of pull direction and 3-s countdown to pull start were provided.
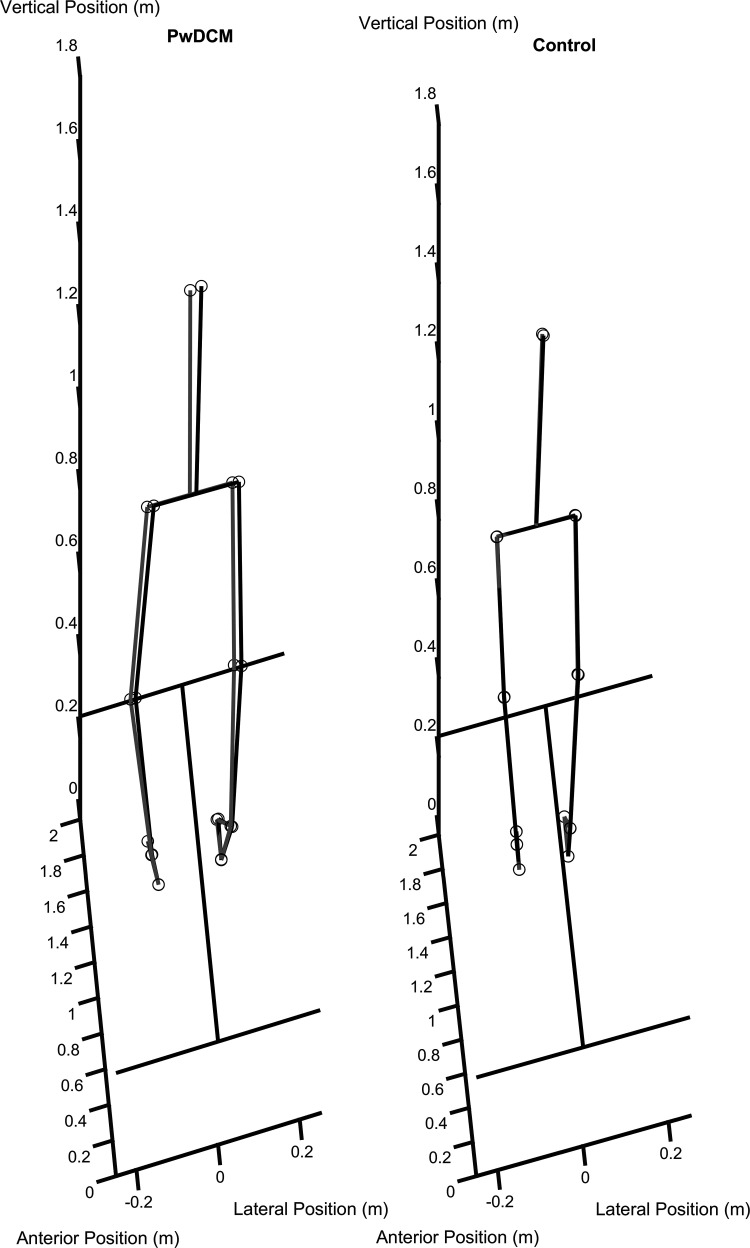
Stance width was varied across narrow, shoulder-width, and wide stances. Narrow standing involved standing with feet together but on separate treadmill belts. Interheel distance for narrow was 12 ± 2 cm. Shoulder width involved the participant standing at a self-selected distance and visibly verified by researchers to ensure approximately shoulder-width stance. Interheel distance in shoulder width was 24 ± 4 cm. Wide stance involved standing with the lateral boundary of the foot at the lateral margin of the treadmill belts, corresponding to approximately half the width of the respective force plates. Interheel distance in wide standing was 47 ± 4 cm. Still images of representative trials can be found in Fig. 1 and in Supplemental Video S1 (all Supplemental Material is available at https://doi.org/10.6084/m9.figshare.16777801).
Figure 1.
Series of still images of postural responses to right-side waist pulls. Left: a still image of peak postural displacement from a person with degenerative cervical myelopathy (PwDCM). Right: a response from a nonmyelopathic control subject. Gray lines, baseline; black lines, displaced.
Data Processing
Biomechanical data were collected in Vicon Nexus v. 1.8.5 (Vicon, Oxford, UK), with a modified plug-in-gait model as described above. Static and dynamic models were calculated in Vicon Nexus (Vicon, Oxford, UK). Data were exported to MATLAB v. 2019 (The MathWorks), where custom scripts were used to calculate the position of the center of mass (COM) using the formula in Winter (57). Kinematic data were low-pass filtered at 6 Hz. EMG data were band-pass filtered between 30 and 450 Hz and further notch filtered between 59 and 61 Hz. EMG data were then full-wave rectified and low-pass filtered at 6 Hz to obtain the linear envelope. All trials were then normalized by the maximal value obtained from the shoulder-width stance, unpredictable, 12% pull magnitude condition. Data were segmented from −2 s to +3 s after the onset of pull. Mediolateral (ML) COM excursion was determined by identifying the maximal position in the direction of the pull minus the mean of the 2-s baseline period. Because there was variability in swaying anterior versus posterior, the maximum of the absolute value of anteroposterior (AP) COM minus the mean of 2-s baseline was used for AP COM excursion. EMG onset timing was determined to be the point at which EMG values exceeded the mean + 3 × standard deviation of the 2-s baseline period. EMG peak amplitude and timing were additionally extracted from the period of onset of pull to onset of pull + 1 s. Furthermore, EMG amplitude asymmetry was quantified to assess changes to interlimb coordination. The formula for this was derived from formulas for step asymmetry of stepping previously described (58) and expressed as a percentage:
where EMGi and EMGc are the ipsilateral and contralateral muscle’s EMG amplitude, respectively.
Quadriceps torque traces were low-pass filtered at 20 Hz. The changes in center of pressure (both anteroposterior and mediolateral) were obtained by subtracting the prepull mean center of pressure from the peak center of pressure value within 1 s after pull onset. Center of pressure data were low-pass filtered at 6 Hz to be consistent with kinematics. All filters employed a fourth-order, zero-phase Butterworth filter. Frontal plane, eccentric phase joint angle excursions were processed from kinematic data in Vicon Nexus. Eccentric phase was operationally defined as the phase of the response when participants were moving in the direction of the pull, creating a joint angular change in the opposite direction (i.e., adduction) before any active ipsilateral joint angular active rotation toward the pull (abduction). Angles of interest were first peak trunk angular bend away from the pull, first peak of hip adduction, and first peak of ankle inversion. These angles were of interest to identify the magnitude of initial joint angular displacement as a direct result of the pull. Peak values were additionally obtained from the COM and respective EMG segments.
Statistical Analysis
Statistical analyses were performed in SPSS v. 26 (IBM, Inc., Armonk, NY). Linear mixed-effects models were constructed with fixed effects of myelopathy, predictability, stance width, and pull magnitude without random effects, and maximal-likelihood estimates were calculated. Sidak corrections were performed for estimated marginal means. Trials were subsequently split by whether or not a foot-off response occurred and reanalyzed with a linear mixed-effects model. For foot-off responses, convergence was not possible with all combinations of conditions; therefore, conditions were limited to narrow, 12% body mass pulls. Non-foot-off responses were sufficiently common across conditions to allow normal convergence with all combinations. These secondary analyses were performed for ML COM, AP COM, ML COP, AP COP, and ipsilateral gluteus medius onset and peak latencies. Pearson correlations between COM excursion with mJOA, Berg, FGA, and ABC were additionally performed. A forward linear regression model was used to assess all possible EMG timing (onset and peak) predictors to COM excursion. Entry into the forward regression model was set at P < 0.05 and exit was set at P > 0.10 based on an ANOVA test for change in R2. This model was thus self-limiting based on significance of increasing R2 and further limited to two total predictors to align with the 1-in-10 rule. Participant characteristics were assessed via independent-samples t test. Alpha was set a priori at 0.05.
RESULTS
Descriptive Data
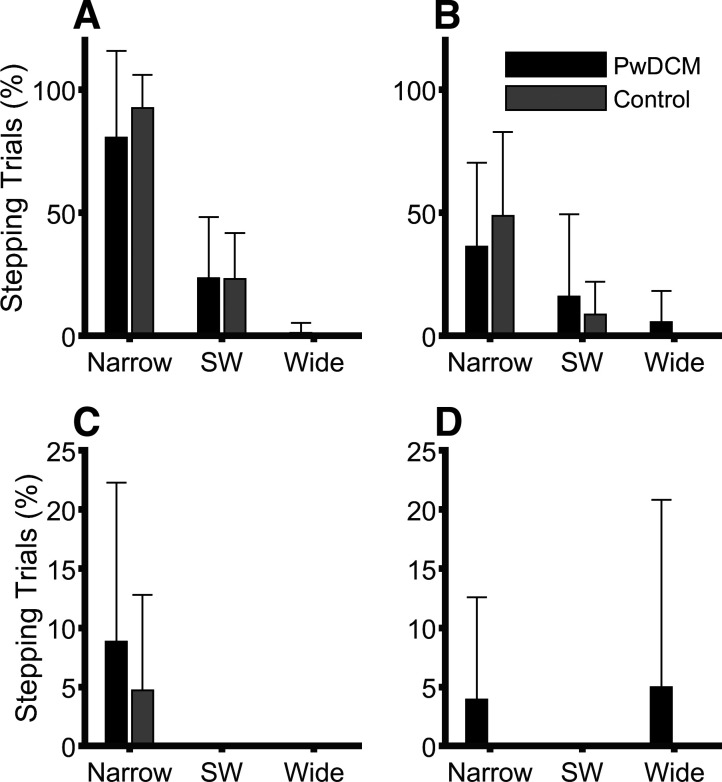
Participant characteristics can be found in Table 1. Participants were of similar age between groups and identical sex distribution. PwDCM were on average 3 yr post-surgical decompression with an average mJOA of 14.6 ± 0.81; thus, most were of mild myelopathy. Weight and Berg were statistically higher and lower in PwDCM, respectively. ABC tended to be lower in PwDCM, but this did not reach statistical significance. Quadriceps MVC was not different between groups, and there were no significant differences in MVC asymmetry (P > 0.05). In this study, 1,874 total trials were analyzed. When liftoff of one limb was necessary (301/1,874 trials), the dominant strategy was liftoff of the limb contralateral to the pull (260 trials; 86.4%). Multistep responses occurred in 14/301 (4.7%) liftoff trials, whereas liftoff of the limb ipsilateral to the pull occurred in 6/301 (2%) trials. Of these liftoff trials, nearly all occurred in 12% conditions (Fig. 2). Within only the 12% conditions, there was a Predictability × Stance interaction (F2,43.41 = 13.18, P < 0.001) whereby liftoff responses were more frequent as stance narrowed in Unpredictable 12% than in Predictable 12%. Additionally, there were main effects of Stance (F1,75.33 = 95.97, P < 0.001) and Predictability (F1,75.33 = 19.96, P < 0.001) whereby stepping frequency increases with narrower stances and in unpredictability. There was no main effect of or interactions with Myelopathy (P > 0.25). Thus clinical indicators of balance were generally impaired in PwDCM; however, strength and frequency of foot-off responses were not different between PwDCM and control subjects.
Table 1.
Participant characteristics
| PwDCM | Control | P | |
|---|---|---|---|
| Age, yr | 58.80 (12.37) | 55.78 (10.09) | 0.61 |
| Sample (female) | 10 (7) | 10 (7) | 0.62 |
| mJOA | 14.60 (0.81) | NA | |
| Post op, yr | 3.20 (3.96) | NA | |
| BMI | 31.57 (4.27) | 25.85 (2.22) | 0.01 |
| BBS | 51.30 (3.95) | 55.70 (0.41) | 0.01 |
| FGA | 23.20 (5.01) | 28.50 (1.84) | 0.01 |
| ABCs | 83.25 (5.91) | 95.00 (2.40) | 0.08 |
| Quadriceps MVC, Nm/kg | 1.43 (0.38) | 1.55 (0.51) | 0.57 |
Values are presented as mean (SD). ABCs, activities specific balance confidence scale; BBS, Berg balance scale; BMI, body mass index; FGA, functional gait assessment; mJOA, modified Japanese Orthopedic Association scale; MVC, maximal voluntary isometric contraction; NA, not applicable; PwDCM, people with degenerative cervical myelopathy.
Figure 2.
Average percentage of trials requiring a liftoff of the contralateral limb. A: Unpredictable 12%. B: Predictable 12%. C: Unpredictable 6%. D: Predictable 6%. For the 12% Pull Magnitude condition, there was a significant (P < 0.001) Predictability × Stance interaction and main effects of Stance and Predictability. PwDCM, people with degenerative cervical myelopathy; SW shoulder width.
Foot Transport
The pull-related change in distance between feet was quantified to assess foot transport via swing or reposition while in stance. There were Magnitude × Stance (F2,26.47 = 8.01, P = 0.002), Magnitude × Predictability (F1,27.08 = 5.14, P = 0.032), and Predictability × Stance (F2,26.47 = 3.43, P = 0.047) interactions. These interactions indicated that foot motion was larger in narrower stance in 12% and unpredictable conditions. Additionally, there were main effects of Magnitude (F1,27.08 = 16.29, P < 0.001), Predictability (F1,27.08 = 6.21, P = 0.019), and Stance (F2,26.47 = 12.40, P < 0.001). There were no significant interactions or effects of Myelopathy (P > 0.111). Therefore, feet moved more with increasingly challenging conditions, especially those that required foot-off, but this was not consistently different between PwDCM and control subjects.
Mediolateral Center of Mass Excursion
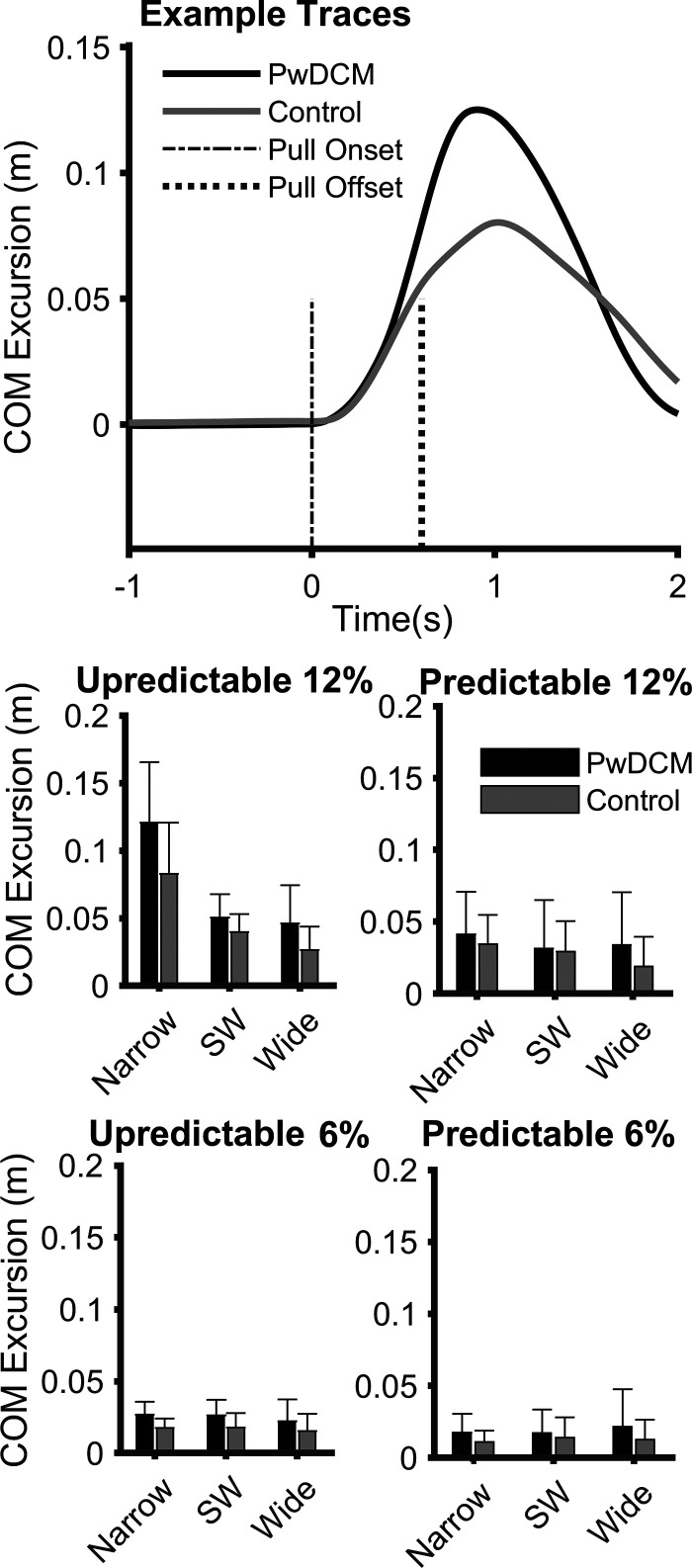
Center of mass excursion (Fig. 3) was higher in PwDCM regardless of Pull Magnitude, Predictability, or Stance width (F1,127.69 = 16.63, P < 0.001). There were additional main effects of Stance (F2,98.62 = 14.4, P < 0.001), Predictability (F1,127.69 = 37.68, P < 0.001), and Magnitude (F2,98.62 = 105.72, P < 0.001). COM excursion did not vary across stance width for noncued 6%, cued 6%, or cued 12% but did vary across stance width in noncued 12% with narrow stance, resulting in higher excursions. Noncued were higher than cued conditions, and 12% pulls resulted in larger excursions than 6% pulls. Narrow stance resulted in larger excursions than shoulder width and wide; however, this was likely driven by the narrow, 12%, noncued condition. Additionally, there were Magnitude × Predictability × Stance (F2,98.62 = 7.80, P = 0.001), Predictability × Stance (F2,98.62 = 9.60, P < 0.001), Magnitude × Stance (F1,127.69 = 15.41, P < 0.001), and Magnitude × Predictability (F1,127.69 = 18.37, P = 0.001) interactions. Therefore, regardless of history of DCM, participants experienced larger COM excursions in narrower stances following Unpredictable 12% pulls. A statistical map for ML COM excursion split by non-foot-off trials is presented in Supplemental Fig. S1. The above overall results are largely consistent with nonstepping trials. For trials with a foot-off response, there were main effects of Myelopathy (F1,29.67 = 4.27, P = 0.048) and Predictability (F1,29.67 = 4.27, P = 0.002) but no Predictability × Myelopathy interaction (P > 0.05) In summary, COM excursion was larger in PwDCM after waist pulls than in nonmyelopathic control subjects.
Figure 3.
Center of mass (COM) excursion in meters in response to waist pulls. Error bars are standard deviation. Top graph: single trial COM traces relative to baseline position for a representative PwDCM and control. Vertical dashed lines indicate start and stop of cable pull. There were significant interactions (P < 0.001) for Magnitude × Predictability × Stance, Predictability × Stance, Magnitude × Stance, and Magnitude × Predictability. Furthermore, there were significant main effects (P < 0.001) of Myelopathy, Stance, Predictability, and Magnitude. PwDCM, people with degenerative cervical myelopathy; SW shoulder width.
Mediolateral Center of Pressure Excursion
ML COP excursion was further quantified to identify the mediolateral weight shift response to waist pulls. There were no main effects or interactions involving Myelopathy (P > 0.24). There were, however, Magnitude × Stance (F2,92.05 = 6.76, P = 0.002) and Predictability × Magnitude (F1,121.00 = 5.30, P = 0.02) interactions. There were additional main effects of Predictability (F1,121.00 = 43.17, P < 0.001), Magnitude (F1,121.00 = 347.12, P < 0.001), and Stance (F2,92.05 = 23.58, P < 0.001). Thus PwDCM modulated COP similarly to nonmyelopathic control subjects (Supplemental Fig. S2).
Gluteus Medius EMG Onset Timing, Peak Timing, and Amplitude
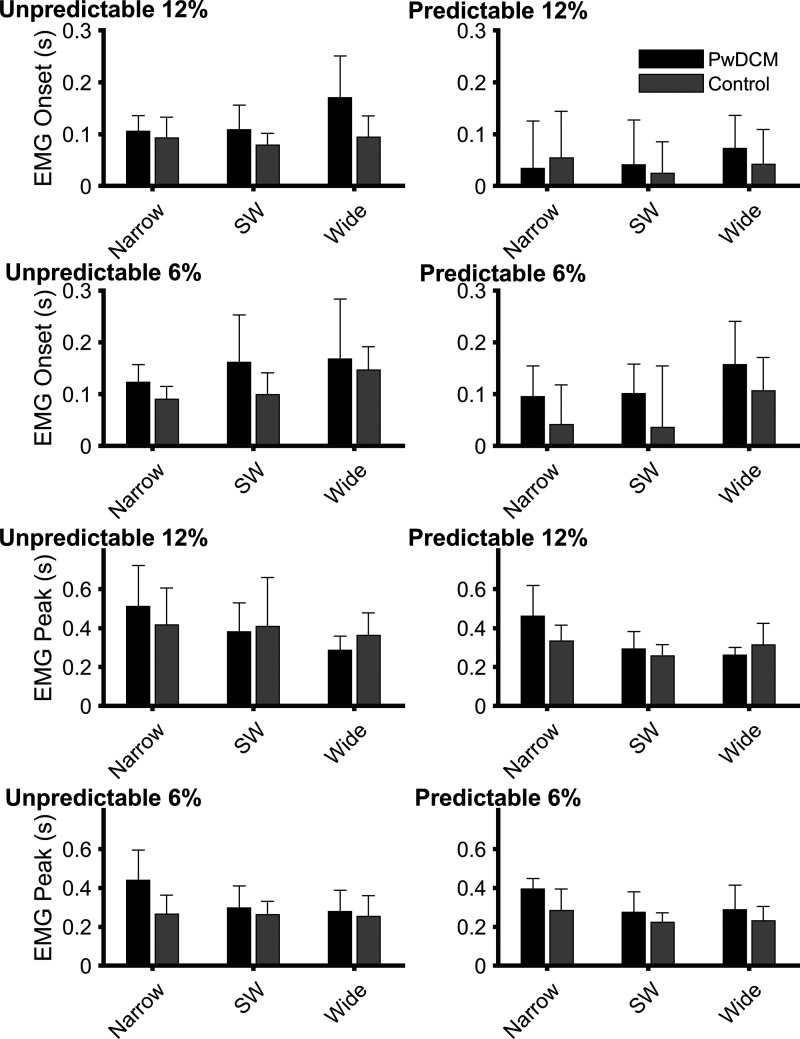
Figure 4 presents a single-trial trace of the ipsilateral gluteus medius EMG response to an Unpredictable 12%, narrow standing pull in a PwDCM and a control subject. Ipsilateral gluteus medius onset latency (Fig. 5) was later in PwDCM regardless of Pull Magnitude, Predictability, or Stance (F1,177.87 = 17.27, P < 0.001). There were additional main effects of Stance (F2,118.27 = 8.83, P < 0.001), Predictability (F1,177.87 = 37.25, P < 0.001), and Magnitude (F1,177.87 = 15.11, P < 0.001). There were no significant four-way, three-way, or two-way interactions (P > 0.05). Gluteus medius onset was delayed in noncued conditions, wide stance, and 6% pulls. Thus, PwDCM began to activate their gluteus medius later than control subjects regardless of the condition. Gluteus medius peak latency (Fig. 4) was similarly higher in PwDCM than in nonmyelopathic control subjects (F1,146.28 = 18.69, P < 0.001). There were also main effects of Stance (F2,132.14 = 8.27, P < 0.001), Predictability (F1,146.28 = 22.08, P < 0.001), and Magnitude (F1,146.28 = 10.321, P < 0.001). There were no significant four-way or three-way interactions (P > 0.05). There was a Magnitude × Stance width interaction (F2,132.14 = 9.50, P < 0.001). Gluteus medius peak timing was delayed in unpredictable conditions. For 12% pulls gluteus peak timing was higher in narrow stance, whereas in 6% pull conditions gluteus peak values occurred later than in shoulder-width and narrow stance. Therefore, PwDCM reached peak gluteus medius amplitude later than control subjects in addition to later EMG onsets.
Figure 4.
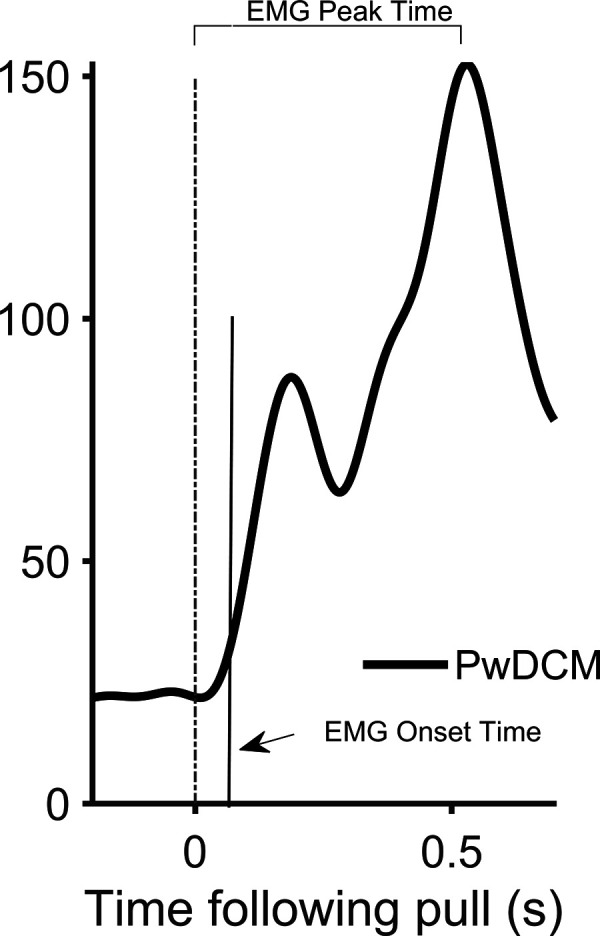
Gluteus medius electromyography (EMG) response to waist pulls. Single-participant trace of representative individual with degenerative cervical myelopathy (PwDCM).
Figure 5.
Group bar graphs of gluteus medius electromyography (EMG) onset and peak latency. For gluteus medius onset latency (top 4 graphs), there were significant (P < 0.001) main effects of Myelopathy, Pull Magnitude, Stance, and Predictability. For gluteus medius peak latency (bottom 4 graphs), there was a significant (P < 0.001) Pull Magnitude × Stance interaction and main effects (P ≤ 0.002) of Myelopathy, Magnitude, Stance, and Predictability. PwDCM, people with degenerative cervical myelopathy; SW, shoulder width.
Similarly, contralateral (to the pull) gluteus medius timing and amplitudes were analyzed. For gluteus medius onset timing, there was a main effect of Myelopathy (F1,153.64 = 8.95, P = 0.003) whereby PwDCM activated their contralateral gluteus medius 0.08 s slower than healthy control subjects. Furthermore, there was a main effect of Magnitude (F1,153.64 = 20.09, P < 0.001) whereby contralateral gluteus medius was activated 0.12 s slower in 6% pulls. For onset, there were no other interactions or main effects (P > 0.06). For contralateral peak timing, there was a Magnitude × Predictability × Myelopathy interaction (F1,199.40 = 4.59, P = 0.033) whereby PwDCM reached peak activity 0.12 s later than control subjects in Predictable 12% conditions and 0.11 s later than control subjects in Unpredictable 6% conditions. There was additionally a main effect of Myelopathy (F1,199.40 = 13.26, P < 0.001). Furthermore, there was a Magnitude × Stance interaction (F2,157.82 = 5.14, P = 0.007) and a main effect of Stance (F2,157.82 = 3.75, P = 0.026) whereby shoulder-width and wide stance induced smaller peak timings than narrow stance, which was dominated by 12% Magnitude conditions. For contralateral gluteus medius amplitude, there were main effects of Magnitude (F1,169.56 = 65.76, P < 0.001), Predictability (F1,69.56 = 4.62, P = 0.033), and Stance (F2,153.09 = 30.62, P < 0.001). In summary, PwDCM had delayed contralateral (in addition to ipsilateral) gluteus medius onset and peak EMG timings.
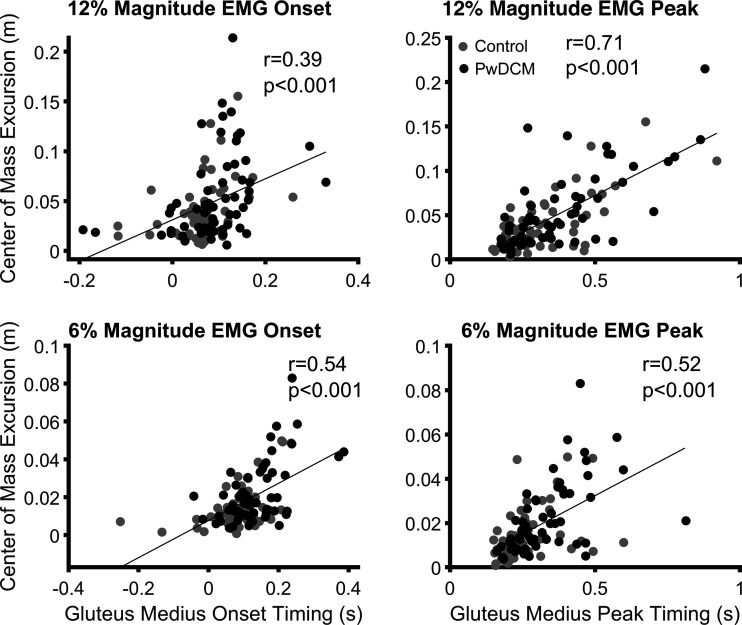
Lateral Center of Mass Excursion Is Related to Ipsilateral Gluteus Medius Timing
The relationship between gluteus medius EMG peak timing and center of mass excursion was stronger than EMG onset (Fig. 6). Furthermore, these relationships were not meaningfully different between PwDCM and control subjects but did differ based on pull magnitude when pooled across groups, predictability, and stance widths. Thus, the increased center of mass excursion in PwDCM was related to delayed EMG peak responses.
Figure 6.
Correlations between gluteus medius latency and center of mass excursion. Left: gluteus medius electromyography (EMG) onset is associated with center of mass excursion separately for 12% (top; r = 0.39, P < 0.001) and 6% (bottom; r = 0.54, P < 0.001) pulls. Right: gluteus medius EMG peak latency correlates with center of mass excursion separately for 12% (top; r = 0.71, P < 0.001) and 6% (bottom; r = 0.52, P < 0.001) pulls. PwDCM, people with degenerative cervical myelopathy.
Whereas gluteus medius timing was delayed in PwDCM, gluteus medius amplitudes (Table 2) normalized to peak EMG during shoulder width, 12%, noncued conditions were not different between PwDCM and nonmyelopathic control subjects (F1,80.06 = 3.58, P = 0.062). Gluteus medius amplitude did, however, vary across Predictability × Stance width (F2,107.91 = 5.06, P = 0.008), Magnitude × Stance width (F2,107.91 = 9.97, P < 0.001), and Magnitude × Predictability (F1,80.06 = 5.80, P = 0.018). Likewise, there were significant main effects of Stance width (F2,107.91 = 103.78, P < 0.001), Predictability (F1,80.06 = 13.08, P < 0.001), and Magnitude (F1,80.06 = 120.13, P < 0.001). Narrow unpredictable was higher than narrow predictable, whereas shoulder width and wide were similar between cued and noncued. Gluteus medius amplitude, regardless of Myelopathy, increased more as stance narrowed in unpredictable than in predictable conditions. Predictability had little effect at 6% pulls, but noncued 12% was larger than cued 12%. Asymmetry of gluteus medius amplitude, however, only varied across Stance (F2,140.12 = 24.88, P < 0.001). Wide stances were less asymmetric than shoulder-width and narrow stances. Gluteus medius amplitude normalized to shoulder-width responses, therefore, did not impact the elevated COM excursion.
Table 2.
Gluteus medius peak amplitude
| Noncued |
Cued |
||||||
|---|---|---|---|---|---|---|---|
| Group | Narrow | SW | Wide | Narrow | SW | Wide | |
| 12% | PwDCM | 139.00 | 83.45 | 46.94 | 49.91 | 62.17 | 42.18 |
| (52.08) | (7.05) | (16.67) | (25.51) | (25.46) | (21.08) | ||
| Control | 136.44 | 76.79 | 43.47 | 50.22 | 52.92 | 40.12 | |
| (54.66) | (10.65) | (20.69) | (23.54) | (21.40) | (25.56) | ||
| PwDCM | 66.88 | 88.15 | 61.47 | 47.41 | 58.21 | 37.03 | |
| 6% | (20.45) | (27.34) | (25.40) | (16.14) | (29.45) | (19.06) | |
| Control | 67.07 | 72.55 | 63.25 | 43.07 | 53.84 | 37.40 | |
| (40.22) | (16.57) | (32.16) | (23.62) | (18.17) | (24.40) | ||
Data are presented in mean (SD) format. Amplitudes are relative (%) to the peak amplitude of the 12% body weight, shoulder width (SW), noncued pull condition.
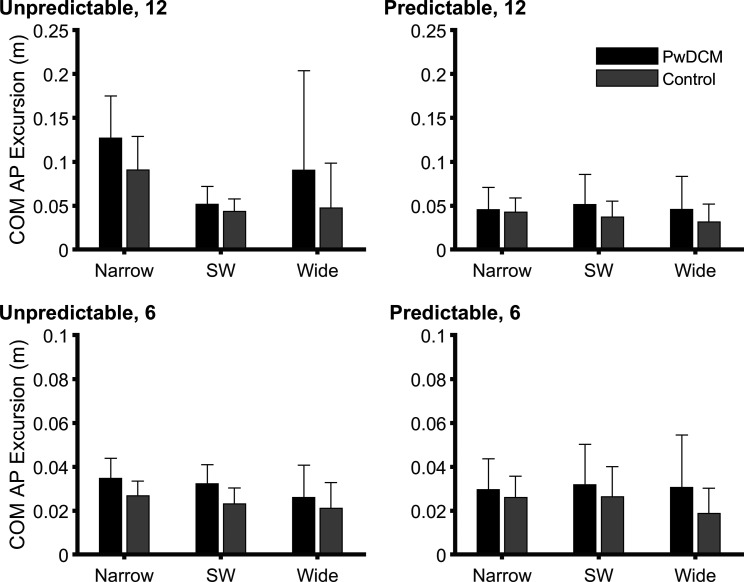
Anteroposterior Center of Mass Excursion
For AP COM (Fig. 7), PwDCM swayed on average 0.01 m further than healthy control subjects [main effect of Myelopathy (F1,53.86 = 10.90, P < 0.001)]. Additionally, there were Magnitude × Predictability × Stance (F2,46.87 = 8.99, P < 0.001), Predictability × Stance (F2,46.87 = 11.90, P < 0.001), Magnitude × Stance (F2,46.87 = 9.65, P < 0.001), and Magnitude × Predictability (F1,53.86 = 16.09, P < 0.001) interactions as well as main effects of Stance (F2,46.87 = 8.99, P < 0.001), Predictability (F1,53.86 = 16.37, P < 0.001), and Magnitude (F1,53.86 = 59.14, P < 0.001). Overall, narrow, unpredictable 12% BW conditions resulted in the greatest anteroposterior COM sway. When split by foot-off/-on responses, in foot-on conditions PwDCM and 12% BW conditions resulted in greater anteroposterior COM sway (Supplemental Fig. S1).
Figure 7.
Bar graphs of anteroposterior (AP) center of mass (COM) excursion. Data represent the absolute value of the maximal excursion from baseline. There were significant (P < 0.001) Pull Magnitude × Predictability × Stance, Predictability × Stance, Magnitude × Stance, and Magnitude × Predictability interactions. Additionally, there were significant (P ≤ 0.002) main effects of Myelopathy, Magnitude, Predictability, and Stance. PwDCM, people with degenerative cervical myelopathy; SW, shoulder width.
Anterior Center of Pressure Excursion
Like ML COP, there were no interactions or main effects involving Myelopathy for AP COP excursion (Supplemental Fig. S3). There were, however, Predictability × Magnitude × Stance (F2,63.76 = 3.84, P = 0.027), Magnitude × Stance (F2,63.76 = 6.50, P = 0.003), Predictability × Stance (F2,63.76 = 4.10, P = 0.021), and Predictability × Magnitude (F1,48.14 = 12.61, P < 0.001) interactions. Likewise, there were main effects of Predictability (F1,48.14 = 26.54, P < 0.001), Magnitude (F1,48.14 = 103.74, P < 0.001), and Stance (F2,63.76 = 10.04, P < 0.001). AP COP was consistent across stance width and predictability at 6% BW but increased in 12% BW conditions. This was especially true in Unpredictable 12%, narrow stance. AP COP significance results split by foot-off responses are presented in Supplemental Fig. S1. The foot-on responses largely mirrored the above results.
Sagittal Plane Electromyography Onset Timing
In addition to the gluteus medius, we collected EMG from the rectus femoris, medial hamstring, medial gastrocnemius, and tibialis anterior muscles. EMG onset, peak timing, and peak amplitude data from these muscles are reported in Supplemental Tables S1–S3, respectively. For brevity, only interactions and effects involving DCM are discussed. For rectus femoris onset, there was a Myelopathy × Stance width interaction (F2,80.93 = 3.49, P = 0.035). There was a smaller change in EMG across stance width in PwDCM than in nonmyelopathic control subjects. Tibialis anterior onset was affected by Myelopathy × Stance width (F2,77.68 = 3.34, P = 0.041). Tibialis anterior onset was later in PwDCM than in control subjects at narrow stances but earlier than in control subjects at wider stances. In summary, EMG onset was delayed for both rectus femoris and tibialis anterior in narrower standing conditions in PwDCM.
Sagittal Plane Electromyography Peak Timing
For peak EMG timing, there was a significant Predictability × Myelopathy interaction for rectus femoris (F1,194.99 = 7.51, P = 0.007). Rectus femoris peak timing was later in cued conditions for control subjects but did not vary across cuing in PwDCM. For medial hamstring peak timing there was a Magnitude × Myelopathy × Stance width interaction for EMG peak timing (F2,164.80 = 4.17, P = 0.017). Hamstring EMG peak timing was higher in PwDCM, but this was most strongly observed in wider stances with 12% pulls. Therefore, nonmyelopathic control subjects modulate EMG peak timing across predictability and PwDCM have delayed hamstring timing in wider stances with higher pull magnitudes.
Sagittal Plane Electromyography Peak Amplitude
EMG amplitudes were scaled to peak amplitude from the shoulder width, Unpredictable 12% pull magnitude condition. There were two separate two-way interactions for rectus femoris peak amplitude involving Myelopathy. There was a Myelopathy × Stance width interaction (F2,80.96 = 5.02, P = 0.009) and a Magnitude × Myelopathy interaction (F1,58.45 = 5.88, P = 0.018). Rectus femoris peak amplitude EMG was only lower in PwDCM than in control subjects at narrow stance width and 12% pull magnitude but not at wider stance widths and 6% pulls. Tibialis anterior EMG peak amplitude was higher in PwDCM than in nonmyelopathic control subjects (F1,95.83 = 14.27, P < 0.001). Overall, there was a shift in peak amplitude, scaled to shoulder-width responses, responses from proximal (rectus femoris) to distal (tibialis anterior) in PwDCM.
Sagittal Plane Electromyography Asymmetry
In addition to peak amplitudes, peak amplitude asymmetry was quantified to assess interlimb differences in muscular responses. For the rectus femoris, there were significant Magnitude × Myelopathy (F1,199.92 = 9.89, P = 0.002), Predictability × Myelopathy (F1,199.92 = 5.91, P = 0.016), and Magnitude × Stance (F2,135.41 = 6.11, P = 0.003) interactions. There were also main effects of Stance (F2,135.41 = 6.56, P = 0.002) and Magnitude (F1,199.92 = 36.97, P < 0.001). Overall, PwDCM scaled rectus femoris asymmetry less across Magnitude and Predictability than did control subjects. For hamstring asymmetry, there was only a main effect of Stance (F2,123.50 = 6.33, P = 0.002). Activity of the contralateral hamstring favored the contralateral limb and was greatest in narrow stance. Additionally, there was a Magnitude × Stance (F2,131.46 = 4.48, P = 0.013) interaction and main effects of Magnitude (F1,181.51 = 5.29, P = 0.023) and Stance (F2,131.46 = 44.17, P < 0.001) for the tibialis anterior. Conversely, there was only a Magnitude × Stance (F2,114.70 = 4.23, P = 0.017) interaction and a main effect of Stance (F2,114.70 = 37.79, P < 0.001) for the gastrocnemius. For both the tibialis anterior and gastrocnemius, asymmetry was higher in shoulder width 12% than shoulder width 6%. Therefore, overall, except for the hamstring, participants controlled responses by increasing EMG in the ipsilateral leg more than the contralateral leg.
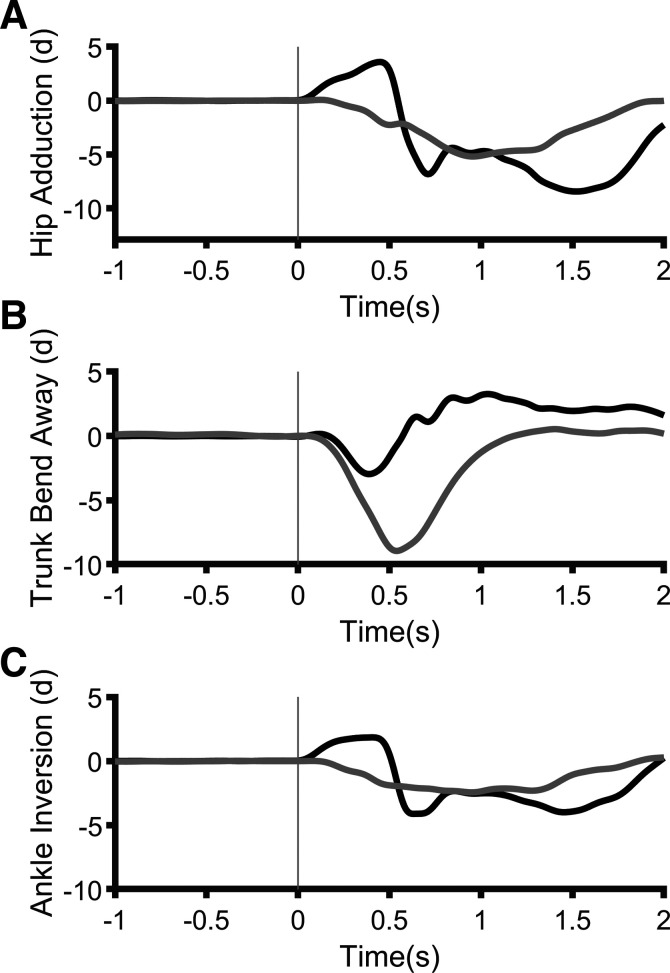
Mediolateral Joint Kinematics
Single-participant joint angle data can be found in Fig. 8. For hip adduction, there were main effects of Myelopathy (F1,104.04 = 22.89, P < 0.001), Predictability (F1,104.04 = 11.25, P = 0.001), Stance (F2,81.21 = 20.10, P < 0.001), and Pull Magnitude (F1,104.04 = 9.21, P = 0.003). Hip adduction was smallest in narrow standing but larger in noncued trials and 12% trials and in PwDCM. There were no significant effects for trunk lateral bend away (P > 0.05). Ankle inversion was similar to hip adduction, with significant main effects of Myelopathy (F1,123.23 = 23.02, P < 0.001), Predictability (F1,123.23 = 4.23, P= 0.032), Stance (F2,86.22 = 12.74, P < 0.001), and Pull Magnitude (F1,23.23 = 10.50, P = 0.002). Again, ankle inversion was higher in 12%, wider stances, and unpredictable and among PwDCM. There were no interactions or main effects for onset time of hip frontal angle (P ≥ 0.05). Overall, the mean kinematic onset time was 0.2 s. Therefore, coinciding with increased COM excursion, there were increased frontal plane joint angular excursions before onset of muscular responses.
Figure 8.
Single-participant traces of hip (A), trunk (B), and ankle (C) angles from a 12%, noncued, narrow trial. Black, person with degenerative cervical myelopathy (PwDCM); gray, control.
Anteroposterior Joint Kinematics
For sagittal plane joint kinematics, peak hip flexion angles were higher in PwDCM than in control subjects (F1,95.18 = 5.82, P = 0.018) and there was a Magnitude × Stance interaction along with main effects of Magnitude (F1,95.18 = 5.82, P = 0.028) and Stance (F2,95.63 = 6.34, P = 0.003). Additionally, PwDCM extended their ipsilateral hips more than control subjects (F1,153.16 = 4.19, P = 0.042) and peak hip extension was greater in wide stance than in shoulder-width stance (F2,136.63 = 5.20, P = 0.007). Furthermore, PwDCM extended their ankles more than control subjects (F1,66.48 = 5.68, P = 0.02). Additionally, there was a Predictability × Stance interaction (F2,71.20 = 3.21, P = 0.046) and main effects of Predictability (F1,66.48 = 4.56, P = 0.037), Stance (F2,71.20 = 15.08, P < 0.001), and Magnitude (F1,66.48 = 7.15, P = 0.009). Ankle extension was greatest in unpredictable narrow and shoulder width conditions and in 12% pull conditions. There were no Myelopathy-related interactions or effects for trunk flexion or extension or ankle flexion (P > 0.05). Overall, PwDCM flexed their hips more than control subjects in response to lateral perturbations, and peak ipsilateral peak hip flexion and ankle extension were greater in narrower stances whereas hip extension was larger in wide stance.
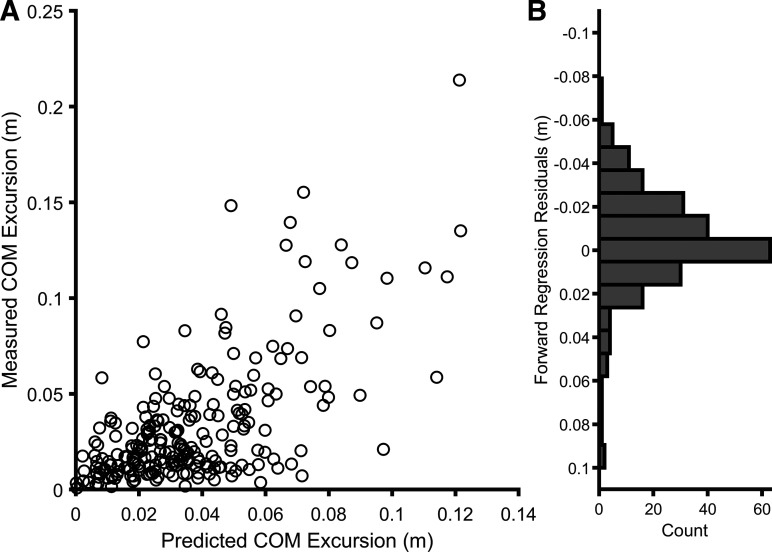
Forward Regression Predictors of Frontal Plane Center of Mass Excursion
Subsequent to the univariate correlation analysis between gluteus medius and center of mass described above, we entered onset and peak timing EMG variables (ipsilateral gluteus medius, rectus femoris, medial hamstring, tibialis anterior, and medial gastrocnemius) into a forward linear regression model pooled across conditions (59, 60). Model 1 identified gluteus medius peak as the strongest single predictor of COM excursion (R2 = 0.43, F1,145 = 110.44, P < 0.001). Model 2 added tibialis anterior peak timing (R2 = 0.50, ΔR2 = 0.07, ΔF1,144 = 19.74, P < 0.001) (Fig. 9). The final equation [95% confidence interval (CI)] was COM = 0.13 (0.11–0.16) × gluteus peak timing + 0.07 (0.04–0.11) × tibialis anterior peak timing − 0.04. Therefore, lateral COM excursions following waist pulls were largely related to gluteus medius and tibialis anterior peak latency.
Figure 9.
Results of the final forward regression model predicting center of mass (COM) excursion. A: scatterplot of predicted COM excursion from the final model (horizontal axis) vs. recorded COM excursion (vertical axis) excursion pooled across Myelopathy, Magnitude, Predictability, and Stance. B: histogram showing distribution of residuals.
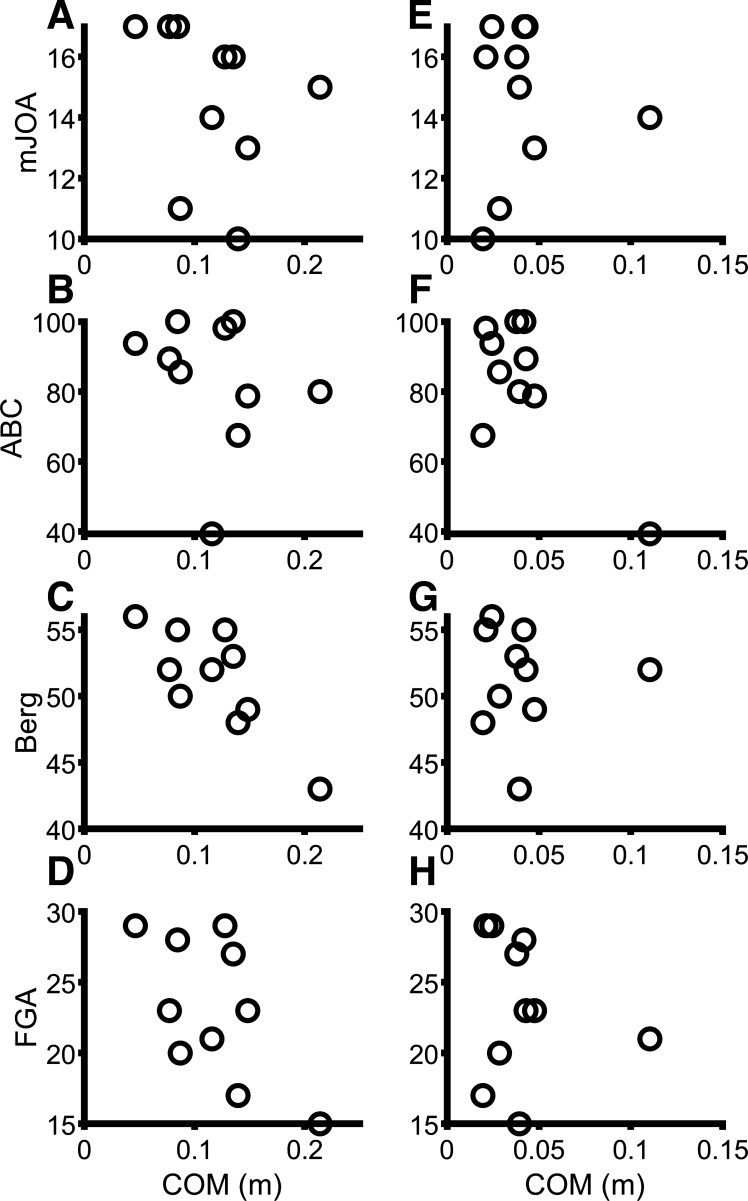
Clinical Correlations
Correlational analyses were performed between narrow, shoulder-width, and wide stance center of mass responses from the 12%, noncued conditions and the mJOA, Berg balance scale, and ABC. Scatterplots are presented in Fig. 10. Center of mass excursion in narrow stance correlated with Berg balance scale scores (r = −0.85, P = 0.002), but center of mass in shoulder width and wide did not (P > 0.11). Center of mass in narrow was not associated with ABC scores (P = 0.26); however, shoulder width (r = −0.91, P < 0.001) and wide (r = −0.77, P = 0.009) responses were. Narrow and wide center of mass responses were both associated with FGA scores (r = −0.71, P = 0.022 and r = −0.79, P = 0.007, respectively), but shoulder width was not (P = 0.055). Center of mass excursion was not significantly associated with mJOA scores (P > 0.10) or time since surgery (P > 0.05) at any stance width. For associations between body mass index (BMI) and COM excursion across all stance widths, cuing, and magnitudes while pooled across group, there was only a significant correlation at wide, Unpredictable 12% (r = 0.61, P = 0.004) and narrow, Unpredictable 6% (r = 0.50, P = 0.035). BMI was not correlated with COM in any other condition (P > 0.11). Quadriceps MVC strength pooled across groups was not associated with COM excursion for any condition (P > 0.62). In summary, COM excursions were associated with clinical measures of balance function but not myelopathic severity.
Figure 10.
Scatterplots for correlations between center of mass (COM) excursion and modified Japanese Orthopedic Association (mJOA; A and E), activity specific balance confidence (ABC; B and F), Berg (C and G), and functional gait assessment (FGA; D and H) scales. A–D: narrow stance, Unpredictable 12%. E–H: shoulder-width stance, Unpredictable 12%.
DISCUSSION
While accounting for variability across pull magnitude, predictability, and stance width, this study examined the center of mass excursions of PwDCM relative to nonmyelopathic control subjects following frontal plane perturbations. Our primary hypothesis that PwDCM would have larger COM excursions with larger pull magnitudes, reductions in predictability, and narrower standing was partially supported. PwDCM did have larger COM excursions than nonmyelopathic control subjects, but there were no interactions with Predictability, Stance width, or Magnitude. There were interactions, however, between Predictability, Stance width, and Magnitude regardless of history of myelopathy. Furthermore, the data supported our secondary hypothesis that delayed gluteus medius activity would be associated with increased COM excursions. As PwDCM have greater impairments of hip muscles versus more distal leg muscles (19), the gluteus medius is of primary importance for determining mechanisms of frontal plane balance impairments in this population and, additionally, may be of greater importance for regulating postural control in this condition because of its proximity to the pull (22). COM excursions were also typically associated with clinical and self-reported measures of balance function. Overall, the primary finding was that PwDCM have residual reactive balance impairments even in predictable conditions. Potential mechanisms and implications are discussed below.
Balance and Degenerative Cervical Myelopathy
In the present study, PwDCM were observed to have worse balance, evidenced by increased COM excursions. PwDCM are known to have impaired quiet standing balance and increased risk of falls; however, the data on postural responses to external perturbations is more limited (1, 2, 4, 7, 14–16). In static balance, PwDCM are known to have increased postural sway (2, 14, 16, 17). For reactive balance, PwDCM continue to have impaired postural responses to platform tilts and surface translations even after surgery (2, 15); however, neither of these studies examined postural responses to frontal plane perturbations or across predictability, base of support, or perturbation magnitude. The results of the present study coincide with these anteroposterior perturbation studies (2, 15), observing that PwDCM have increased reactive postural excursions. Importantly, one of these studies (15) further reported that reactive perturbation training improves balance in PwDCM. The results from the present data as well as previous findings in PwDCM (2, 15) imply that therapies to improve balance should focus on improving EMG reaction time and/or power development.
Mechanisms of these balance impairments in DCM are frequently conceived as impairments of white matter conduction through the cervical spinal cord (2). Namely, both sensory and motor signals may be delayed and white matter integrity may decline because of demyelination and/or axonal loss (27, 33, 38, 61–63). Classically, balance impairments in PwDCM have been considered largely sensory related (2). Our data on responses to predictable perturbations suggest that motor delays or reductions in power generation may also contribute to these balance impairments. Subsequent research correlating sensory/motor delays and/or sensory/motor white matter integrity with balance impairments are needed to further elucidate the relative contribution of each system to balance impairments.
Electromyography Latency and Balance
Our findings indicate that PwDCM have delayed EMG onset and peak. Gluteus medius EMG amplitude and MVC strength values, however, were not impaired in our cohort. As mentioned above, PwDCM have delayed EMG responses to surface tilts and translations (2, 15). These studies, however, reported only sagittal plane muscle EMG responses to anteroposterior perturbations, i.e., delayed tibialis anterior and gastrocnemius muscles (2, 15). We here further describe prolonged reaction times of the gluteus medius muscle in response to lateral waist pulls. Our data, however, further imply that timing of peak EMG responses is more strongly associated with COM excursion than EMG onset per se. Additionally, interactions between DCM and Stance width, Magnitude, and/or Predictability were observed. Notably, we were unable to collect reliable peroneus longus EMG. It is possible that PwDCM may have been able to compensate for limitations at the gluteus medius through activity at the peroneal muscles.
We postulated that the presence of EMG in these sagittal plane muscles would be related to maintaining upright stance during perturbation responses (i.e., stabilizing the lower limb to allow the gluteus a stable base upon which to act). This would then make these muscles important for maintaining postural control regardless of specific condition that may also be impaired in DCM. Notably, tibialis anterior and medial gastrocnemius activity has been previously demonstrated with left/right arm perturbations (23, 24) associated with ankle dorsiflexion and an anteroposterior translation of the center of pressure and center of mass. In these studies, however, anteroposterior motion of the center of mass did not scale with stance width, whereas tibialis anterior and gastrocnemius EMG did (23), suggesting that EMG was not coupled with anteroposterior motion. As delayed tibialis anterior peaks were associated with a significant, albeit small, amount of lateral COM excursion beyond variance accounted for by the gluteus medius in our present study, it is likely that sagittal muscles (such as the tibialis anterior) are used to ensure a stable lower extremity, which may enhance the effectiveness of the gluteus medius to abut lateral motion. This appears to especially be true in conditions in which participants required a contralateral foot-off response. It is also possible that sagittal plane muscles were recruited because of anatomically crossing both sagittal and frontal planes or through recruitment of muscle synergies,(64, 65), but we were unable to assess morphological or synergistic variations in the present study. It is also possible that stepping responses directly impacted anteroposterior motion in those trials in which participants lifted their foot. Interestingly, gastrocnemius responses were not impaired in PwDCM. As gastrocnemii are more reliant on reflex responses (66), this might indicate that below lesion triceps surae reflexes are preserved in people with mild DCM. Additionally, the similarities of muscle response asymmetries suggests that interlimb coordination of responses was not altered in this cohort. Scaling of asymmetries does, however, appear to change across stance and perturbation magnitude to control the postural response. Therefore, proper control of balance responses across pull magnitudes, predictability, and stance width involves asymmetric recruitment of sagittal plane muscles scaling with task difficulty.
Stance Width and Balance Function
In our results, we observed that stance width impacted COM excursions more in higher-magnitude pulls. Stance width is known to impact responses to lateral motion in both computational models and neurologically impaired individuals and impacts the mechanical conditions affecting the relative utilization of hip versus ankle muscles (23, 34–37, 67, 68). Therefore, our study may have been unable to detect a relative shift in hip versus ankle strategy. The impact of stance width on responses to lateral perturbations is believed to be due to a combination of sensory reweighting across stance widths as well as changes in inertia and leverage (34–37, 67). In our present study, COM excursion varied with Stance width, but a Stance width × Myelopathy interaction was not observed. Thus it appears that PwDCM after decompression are able to scale sensory weighting across stance width even though some sensory weighting impairments remain (14).
Predictability and Balance Responses
The present findings indicated that in predictable conditions muscles became active earlier and COM excursions were smaller. Predictability of postural perturbations is known to play an important role in shaping both the response itself and the neural correlates of those responses (29–32). Specifically, in predictable perturbations, cortical activity begins before the onset of the perturbation localized over the sensorimotor and supplementary motor areas when the timing of the perturbation was known (69), as it was known in the present study. In unpredictable conditions, however, cortical activity, e.g., sensorimotor beta power, does not increase until ∼100 ms after the onset of the perturbation (70). In these cases, cortical activity likely begins to shape the later postural responses following the longer-latency spinal-cortico-spinal loop (29). In our study, gluteus medius EMG onset and peak occurred earlier when direction and magnitude were predictable having received verbal cuing. However, EMG responses were delayed in PwDCM. The delayed EMG onset in PwDCM suggests a spinal cord- or brain stem-level delay, whereas the delayed peak EMG, which would occur after receiving input from supraspinal structures, suggests a delay in afferent-efferent signaling. Specifically, as these delays were still present in the predictable conditions, when the cortex can begin to generate the response before onset, this is likely to represent an efferent delay. This suggests that functional impairments in PwDCM are more in line with delayed motor evoked potentials and corticospinal demyelination than with a pure dorsal column dysfunction, or are at least of mixed origin (27, 33).
Outcome variables to assess balance in laboratory and clinical environments in PwDCM are poorly understood. In our cohort, reactive balance COM responses were unrelated to mJOA, a common scale of severity of DCM, but were related to balance confidence (ABC) as well as clinical measures of balance (Berg and FGA). To our knowledge, only one other study has reported associations between the (unmodified) Japanese Orthopedic Association scale and standing balance (16); however, this study only reported on mild versus severe myelopathy. Conversely, most of our participants were of mild severity at the time of testing. This likely obscured our ability to observe an association due to a ceiling effect. Furthermore, Yoshikawa et al. (16) examined static posturography, whereas we examined postural responses to external perturbations. It is possible that the (m)JOA is more sensitive to quiescent standing than perturbation responses; however, substantially more research is needed to understand the relationship between postural control and myelopathic severity. Presently, little is known regarding Berg, (53) FGA, and ABC in PwDCM; however, our data suggest that these would be meaningful outcome measures in this population. Moreover, these are sufficiently validated in other populations to warrant translation to DCM.
Clinical Implications
Because postural responses were still impaired in PwDCM after decompressive surgery associated with delayed EMG responses, balance therapies should focus on appropriate timing of muscle responses. Perturbation-based balance therapies during standing and walking have demonstrated potential for improving EMG reaction time in PwDCM (15). In healthy older adults and people with other neurological diseases such as stroke, multiple sclerosis, etc., reactive balance training has shown benefits in improving balance function (72–75). Moreover, this study examined relationships between COM responses and clinical/self-reported measures of balance and DCM severity. Based on these results, mJOA may not be sufficient to identify deficits in postural responses; therefore balance assessments should be included in assessments of PwDCM.
Limitations
This study was limited by being unable to assess motor and somatosensory evoked potential latencies or white matter diffusion measures. Instead, the purpose of this study was to experimentally assess the impact of stance width and predictability on postural responses, as these are known to impact sensory weighting and cortical control of balance. Additionally, we were unable to longitudinally examine the change of balance responses from pre- to postdecompression to observe how much recovery occurs. Nevertheless, this allowed us to observe the residual balance impairments that occur. Additionally, our sample was of individuals who were relatively high functioning; thus the ability to generalize our results may be limited. Likewise, the sample size in this study was small; however, this was a pilot study to examine the effects of magnitude, stance width, and predictability. Larger studies based on our findings should be conducted before and after surgery to allow greater generalization about the recovery course. Finally, groups were not perfectly matched for BMI; however, BMI was only correlated in 2 of 12 combinations of conditions. Since these conditions were less challenging (wide stance Unpredictable 12% and narrow stance Unpredictable 6%), BMI was unlikely to have substantially impacted our findings.
Conclusions
Balance impairments persist chronically after decompression of the spinal cord in PwDCM. These impairments are associated with delayed rather than reduced gluteus medius muscle responses but do not depend upon perturbation predictability, magnitude, or stance width. Laboratory balance responses are associated with Berg balance scale, functional gait assessment scale, and activities specific balance confidence scale scores.
SUPPLEMENTAL DATA
Supplemental Video S1, Supplemental Figs. S1–S3, and Supplemental Tables S1–S3: https://doi.org/10.6084/m9.figshare.16777801.
GRANTS
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number TL1TR001437.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.F.B., M.C.W., B.D.S., and A.S.H. conceived and designed research; T.F.B. and L.M. performed experiments; T.F.B. and L.M. analyzed data; T.F.B. and A.S.H. interpreted results of experiments; T.F.B. prepared figures; T.F.B. drafted manuscript; T.F.B., L.M., M.C.W., B.D.S., and A.S.H. edited and revised manuscript; T.F.B., L.M., M.C.W., B.D.S., and A.S.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the assistance of Megan Bellman, who assisted us with recruitment, and Marissa Clare, who assisted us with data collection.
REFERENCES
- 1.Horowitz JA, Puvanesarajah V, Jain A, Raad M, Gjolaj JP, Shen FH, Hassanzadeh H. Fragility fracture risk in elderly patients with cervical myelopathy. Spine 44: 96–102, 2019. doi: 10.1097/BRS.0000000000002762. [DOI] [PubMed] [Google Scholar]
- 2.Nardone A, Galante M, Grasso M, Schieppati M. Stance ataxia and delayed leg muscle responses to postural perturbations in cervical spondylotic myelopathy. J Rehabil Med 40: 539–547, 2008. doi: 10.2340/16501977-0214. [DOI] [PubMed] [Google Scholar]
- 3.Morikawa M, Urabe Y, Maeda N, Suzuki Y, Junpei S, Kobayashi T, Shirakawa T. Association between falling direction and age in older patients with hip fractures. Z Gerontol Geriatr 54: 547–554, 2021. doi: 10.1007/s00391-020-01824-0. [DOI] [PubMed] [Google Scholar]
- 4.Cheng CH, Lai DM, Lau PY, Wang SF, Chien A, Wang JL, Hsu WL. Upright balance control in individuals with cervical myelopathy following cervical decompression surgery: a prospective cohort study. Sci Rep 10: 10357–10357, 2020. doi: 10.1038/s41598-020-66057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radcliff KE, Curry EP, Trimba R, Walker JB, Purtill JJ, Austin MS, Parvizi J, Vaccaro AR, Hilibrand AS, Albert TJ. High incidence of undiagnosed cervical myelopathy in patients with hip fracture compared with controls. J Orthop Trauma 30: 189–193, 2016. doi: 10.1097/BOT.0000000000000485. [DOI] [PubMed] [Google Scholar]
- 6.Kimura A, Seichi A, Takeshita K, Inoue H, Kato T, Yoshii T, Furuya T, Koda M, Takeuchi K, Matsunaga S, Seki S, Ishikawa Y, Imagama S, Yamazaki M, Mori K, Kawasaki Y, Fujita K, Endo K, Sato K, Okawa A. Fall-related deterioration of subjective symptoms in patients with cervical myelopathy. Spine (Phila Pa 1976) 42: E398–E403, 2017. doi: 10.1097/BRS.0000000000001798. [DOI] [PubMed] [Google Scholar]
- 7.Kimura A, Takeshita K, Shiraishi Y, Inose H, Yoshii T, Maekawa A, Endo K, Miyamoto T, Furuya T, Nakamura A, Mori K, Seki S, Kanbara S, Imagama S, Matsunaga S, Okawa A. Effectiveness of surgical treatment for degenerative cervical myelopathy in preventing falls and fall-related neurological deterioration: a prospective multi-institutional study. Spine (Phila Pa 1976) 45: E631–E638, 2020. doi: 10.1097/BRS.0000000000003355. [DOI] [PubMed] [Google Scholar]
- 8.Lee SW, Cho KH, Lee WH. Effect of a local vibration stimulus training programme on postural sway and gait in chronic stroke patients: a randomized controlled trial. Clin Rehabil 27: 921–931, 2013. doi: 10.1177/0269215513485100. [DOI] [PubMed] [Google Scholar]
- 9.Sturnieks DL, Menant J, Vanrenterghem J, Delbaere K, Fitzpatrick RC, Lord SR. Sensorimotor and neuropsychological correlates of force perturbations that induce stepping in older adults. Gait Posture 36: 356–360, 2012. doi: 10.1016/j.gaitpost.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Mochizuki G, Sibley KM, Esposito JG, Camilleri JM, McIlroy WE. Cortical responses associated with the preparation and reaction to full-body perturbations to upright stability. Clin Neurophysiol 119: 1626–1637, 2008. doi: 10.1016/j.clinph.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine (Phila Pa 1976) 40: E675–E693, 2015. doi: 10.1097/BRS.0000000000000913. [DOI] [PubMed] [Google Scholar]
- 12.Kawaguchi Y, Nakano M, Yasuda T, Seki S, Hori T, Suzuki K, Makino H, Kanamori M, Kimura T. More than 20 years follow-up after en bloc cervical laminoplasty. Spine (Phila Pa 1976) 41: 1570–1579, 2016. doi: 10.1097/BRS.0000000000001579. [DOI] [PubMed] [Google Scholar]
- 13.Tanishima S, Nagashima H, Ishii H, Fukata S, Dokai T, Murakami T, Morio Y. Significance of stabilometry for assessing postoperative body sway in patients with cervical myelopathy. Asian Spine J 11: 763–769, 2017. doi: 10.4184/asj.2017.11.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin IS, Lai DM, Ding JJ, Chien A, Cheng CH, Wang SF, Wang JL, Kuo CL, Hsu WL. Reweighting of the sensory inputs for postural control in patients with cervical spondylotic myelopathy after surgery. J Neuroeng Rehabil 16: 96, 2019. doi: 10.1186/s12984-019-0564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng Y, Chien A, Lai D, Lee Y, Cheng C, Wang S, Chang Y, Wang J, Hsu W. Perturbation-based balance training in postoperative individuals with degenerative cervical myelopathy. Front Bioeng Biotechnol 8: 108, 2020. doi: 10.3389/fbioe.2020.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshikawa M, Doita M, Okamoto K, Manabe M, Sha N, Kurosaka M. Impaired postural stability in patients with cervical myelopathy: evaluation by computerized static stabilometry. Spine (Phila Pa 1976) 33: E460–E464, 2008. doi: 10.1097/BRS.0b013e318178e666. [DOI] [PubMed] [Google Scholar]
- 17.Haddas R, Lieberman I, Boah A, Arakal R, Belanger T, Ju KL. Functional balance testing in cervical spondylotic myelopathy patients. Spine (Phila Pa 1976) 44: 103–109, 2019. doi: 10.1097/BRS.0000000000002768. [DOI] [PubMed] [Google Scholar]
- 18.Maneeprom N, Taneepanichskul S, Panza A. Falls among physically active elderly in senior housings, Bangkok, Thailand: situations and perceptions. Clin Interv Aging 13: 2149–2159, 2018. doi: 10.2147/CIA.S175896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiles BW 3rd, Leonard MA, Choudhri HF, Cooper PR. Cervical spondylotic myelopathy: patterns of neurological deficit and recovery after anterior cervical decompression. Neurosurgery 44: 762–769, 1999. doi: 10.1097/00006123-199904000-00041. [DOI] [PubMed] [Google Scholar]
- 20.Meyer PF, Oddsson LI, De Luca CJ. Reduced plantar sensitivity alters postural responses to lateral perturbations of balance. Exp Brain Res 157: 526–536, 2004. doi: 10.1007/s00221-004-1868-3. [DOI] [PubMed] [Google Scholar]
- 21.Lin YH, Tang PF, Wang YH, Eng JJ, Lin KC, Lu L, Jeng JS, Chen SC. Reactive postural control deficits in patients with posterior parietal cortex lesions after stroke and the influence of auditory cueing. Am J Phys Med Rehabil 93: 849–859, 2014. doi: 10.1097/PHM.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 22.Winter DA. Human balance and posture control during standing and walking. Gait Posture 3: 193–214, 1995. doi: 10.1016/0966-6362(96)82849-9. [DOI] [Google Scholar]
- 23.Forghani A, Preuss R, Milner T. Short-latency muscle response patterns to multi-directional, unpredictable perturbations to balance applied to the arm are context dependent. Neuroscience 352: 170–179, 2017. doi: 10.1016/j.neuroscience.2017.03.062. [DOI] [PubMed] [Google Scholar]
- 24.Forghani A, Preuss R, Milner T. Effects of amplitude and predictability of perturbations to the arm on anticipatory and reactionary muscle responses to maintain balance. J Electromyogr Kinesiol 35: 30–39, 2017. doi: 10.1016/j.jelekin.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Forghani A, Milner T. Origin of directionally tuned responses in lower limb muscles to unpredictable upper limb disturbances. PloS One 12: e0187006, 2017. doi: 10.1371/journal.pone.0187006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi Q, Huang S, Ling Z, Chen Y, Hu H, Zhan P, Zhang B, Zou X, Peng X. A new diagnostic medium for cervical spondylotic myelopathy: dynamic somatosensory evoked potentials. World Neurosurg 133: e225–e232, 2020. doi: 10.1016/j.wneu.2019.08.205. [DOI] [PubMed] [Google Scholar]
- 27.Wen CY, Cui JL, Mak KC, Luk KD, Hu Y. Diffusion tensor imaging of somatosensory tract in cervical spondylotic myelopathy and its link with electrophysiological evaluation. Spine J 14: 1493–1500, 2014. doi: 10.1016/j.spinee.2013.08.052. [DOI] [PubMed] [Google Scholar]
- 28.Lee JH, Lee SH, Seo IS. The characteristics of gait disturbance and its relationship with posterior tibial somatosensory evoked potentials in patients with cervical myelopathy. Spine (Phila Pa 1976) 36: E524–E530, 2011. doi: 10.1097/BRS.0b013e3181f412d9. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm (Vienna) 114: 1339–1348, 2007. doi: 10.1007/s00702-007-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coelho DB, Teixeira LA. Disambiguating the cognitive and adaptive effects of contextual cues of an impending balance perturbation. Hum Mov Sci 61: 90–98, 2018. doi: 10.1016/j.humov.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Coelho DB, Teixeira LA. Cognition and balance control: does processing of explicit contextual cues of impending perturbations modulate automatic postural responses? Exp Brain Res 235: 2375–2390, 2017. doi: 10.1007/s00221-017-4980-x. [DOI] [PubMed] [Google Scholar]
- 32.Kaewmanee T, Liang H, Aruin A. Effect of predictability of the magnitude of a perturbation on anticipatory and compensatory postural adjustments. Exp Brain Res 238: 2207–2219, 2020. doi: 10.1007/s00221-020-05883-y. [DOI] [PubMed] [Google Scholar]
- 33.Lanza G, Puglisi V, Vinciguerra L, Fisicaro F, Vagli C, Cantone M, Pennisi G, Pennisi M, Bella R. TMS correlates of pyramidal tract signs and clinical motor status in patients with cervical spondylotic myelopathy. Brain Sci 10: 806, 2020. doi: 10.3390/brainsci10110806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bingham JT, Choi JT, Ting LH. Stability in a frontal plane model of balance requires coupled changes to postural configuration and neural feedback control. J Neurophysiol 106: 437–448, 2011. doi: 10.1152/jn.00010.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodworth AD, Peterka RJ. Influence of stance width on frontal plane postural dynamics and coordination in human balance control. J Neurophysiol 104: 1103–1118, 2010. doi: 10.1152/jn.00916.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodworth AD, Peterka RJ. Sensorimotor integration for multisegmental frontal plane balance control in humans. J Neurophysiol 107: 12–28, 2012. [Erratum in J Neurophysiol 108: 2338, 2012]. doi: 10.1152/jn.00670.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodworth AD, Mellodge P, Peterka RJ. Stance width changes how sensory feedback is used for multisegmental balance control. J Neurophysiol 112: 525–542, 2014. doi: 10.1152/jn.00490.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu H, MacMillian EL, Jutzeler CR, Ljungberg E, MacKay AL, Kolind SH, Mädler B, Li DK, Dvorak MF, Curt A, Laule C, Kramer JL. Assessing structure and function of myelin in cervical spondylotic myelopathy: evidence of demyelination. Neurology 89: 602–610, 2017. doi: 10.1212/WNL.0000000000004197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dietz V, Horstmann G, Berger W. Interlimb coordination of leg-muscle activation during perturbation of stance in humans. J Neurophysiol 62: 680–693, 1989. doi: 10.1152/jn.1989.62.3.680. [DOI] [PubMed] [Google Scholar]
- 40.Dietz V, Berger W. Interlimb coordination of posture in patients with spastic paresis. Impaired function of spinal reflexes. Brain 107: 965–978, 1984. doi: 10.1093/brain/107.3.965. [DOI] [PubMed] [Google Scholar]
- 41.Thibaudier Y, Tan AQ, Peters DM, Trumbower RD. Differential deficits in spatial and temporal interlimb coordination during walking in persons with incomplete spinal cord injury. Gait Posture 75: 121–128, 2020. doi: 10.1016/j.gaitpost.2019.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sozzi S, Honeine JL, Do MC, Schieppati M. Leg muscle activity during tandem stance and the control of body balance in the frontal plane. Clin Neurophysiol 124: 1175–1186, 2013. doi: 10.1016/j.clinph.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Carpenter MG, Allum JH, Honegger F. Directional sensitivity of stretch reflexes and balance corrections for normal subjects in the roll and pitch planes. Exp Brain Res 129: 93–113, 1999. doi: 10.1007/s002210050940. [DOI] [PubMed] [Google Scholar]
- 44.Carpenter MG, Allum JH, Honegger F. Vestibular influences on human postural control in combinations of pitch and roll planes reveal differences in spatiotemporal processing. Exp Brain Res 140: 95–111, 2001. doi: 10.1007/s002210100802. [DOI] [PubMed] [Google Scholar]
- 45.Gage WH, Frank JS, Prentice SD, Stevenson P. Postural responses following a rotational support surface perturbation, following knee joint replacement: frontal plane rotations. Gait Posture 27: 286–293, 2008. doi: 10.1016/j.gaitpost.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Bakker M, Allum JH, Visser JE, Grüneberg C, van de Warrenburg BP, Kremer BH, Bloem BR. Postural responses to multidirectional stance perturbations in cerebellar ataxia. Exp Neurol 202: 21–35, 2006. doi: 10.1016/j.expneurol.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Koshino Y, Samukawa M, Chida S, Okada S, Tanaka H, Watanabe K, Chijimatsu M, Yamanaka M, Tohyama H. Postural stability and muscle activation onset during double- to single-leg stance transition in flat-footed individuals. J Sports Sci Med 19: 662–669, 2020. [PMC free article] [PubMed] [Google Scholar]
- 48.Dingenen B, Staes FF, Janssens L. A new method to analyze postural stability during a transition task from double-leg stance to single-leg stance. J Biomech 46: 2213–2219, 2013. doi: 10.1016/j.jbiomech.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 49.Carpenter MG, Allum JH, Honegger F. Directional sensitivity of stretch reflexes and balance corrections for normal subjects in the roll and pitch planes. Exp Brain Res 129: 93–113, 1999. doi: 10.1007/s002210050940. [DOI] [PubMed] [Google Scholar]
- 50.Carpenter MG, Allum JH, Honegger F. Vestibular influences on human postural control in combinations of pitch and roll planes reveal differences in spatiotemporal processing. Exp Brain Res 140: 95–111, 2001. doi: 10.1007/s002210100802. [DOI] [PubMed] [Google Scholar]
- 51.Vieira TM, Minetto MA, Hodson-Tole EF, Botter A. How much does the human medial gastrocnemius muscle contribute to ankle torques outside the sagittal plane? Hum Mov Sci 32: 753–767, 2013. doi: 10.1016/j.humov.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walker ER, Hyngstrom AS, Onushko T, Schmit BD. Locomotor adaptations to prolonged step-by-step frontal plane trunk perturbations in young adults. PLoS One 13: e0203776, 2018. doi: 10.1371/journal.pone.0203776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalsi-Ryan S, Singh A, Massicotte EM, Arnold PM, Brodke DS, Norvell DC, Hermsmeyer JT, Fehlings MG. Ancillary outcome measures for assessment of individuals with cervical spondylotic myelopathy. Spine (Phila Pa 1976) 38: S111–S122, 2013. doi: 10.1097/BRS.0b013e3182a7f499. [DOI] [PubMed] [Google Scholar]
- 54.Schinkel-Ivy A, Wong JS, Mansfield A. Balance confidence is related to features of balance and gait in individuals with chronic stroke. J Stroke Cerebrovasc Dis 26: 237–245, 2017. doi: 10.1016/j.jstrokecerebrovasdis.2016.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Bloemendaal M, Bout W, Bus SA, Nollet F, Geurts AC, Beelen A. Validity and reproducibility of the Functional Gait Assessment in persons after stroke. Clin Rehabil 33: 94–103, 2019. doi: 10.1177/0269215518791000. [DOI] [PubMed] [Google Scholar]
- 56.Sturnieks DL, Menant J, Delbaere K, Vanrenterghem J, Rogers MW, Fitzpatrick RC, Lord SR. Force-controlled balance perturbations associated with falls in older people: a prospective cohort study. PLoS One 8: e70981, 2013. doi: 10.1371/journal.pone.0070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winter DA. Biomechanics and Motor Control of Human Movement. Hoboken, NJ: Wiley, 2005. [Google Scholar]
- 58.Musselman KE, Patrick SK, Vasudevan EV, Bastian AJ, Yang JF. Unique characteristics of motor adaptation during walking in young children. J Neurophysiol 105: 2195–2203, 2011. doi: 10.1152/jn.01002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schieppati M, Giordano A, Nardone A. Variability in a dynamic postural task attests ample flexibility in balance control mechanisms. Exp Brain Res 144: 200–210, 2002. doi: 10.1007/s00221-002-1028-6. [DOI] [PubMed] [Google Scholar]
- 60.Gebel A, Lüder B, Granacher U. Effects of increasing balance task difficulty on postural sway and muscle activity in healthy adolescents. Front Physiol 10: 1135, 2019. doi: 10.3389/fphys.2019.01135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nicotra A, King NK, Catley M, Mendoza N, McGregor AH, Strutton PH. Evaluation of corticospinal excitability in cervical myelopathy, before and after surgery, with transcranial magnetic stimulation: a pilot study. Eur Spine J 22: 189–196, 2013. doi: 10.1007/s00586-012-2554-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ellingson BM, Salamon N, Grinstead JW, Holly LT. Diffusion tensor imaging (DTI) predicts functional impairment in mild to moderate cervical spondylotic myelopathy. Spine J 14: 2589–2597, 2014. doi: 10.1016/j.spinee.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grabher P, Mohammadi S, Trachsler A, Friedl S, David G, Sutter R, Weiskopf N, Thompson AJ, Curt A, Freund P. Voxel-based analysis of grey and white matter degeneration in cervical spondylotic myelopathy. Sci Rep 6: 24636, 2016. doi: 10.1038/srep24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torres-Oviedo G, Macpherson JM, Ting LH. Muscle synergy organization is robust across a variety of postural perturbations. J Neurophysiol 96: 1530–1546, 2006. doi: 10.1152/jn.00810.2005. [DOI] [PubMed] [Google Scholar]
- 65.Lee SS, Piazza SJ. Inversion-eversion moment arms of gastrocnemius and tibialis anterior measured in vivo. J Biomech 41: 3366–3370, 2008. doi: 10.1016/j.jbiomech.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 66.Miranda Z, Pham A, Elgbeili G, Barthélemy D. H-reflex modulation preceding changes in soleus EMG activity during balance perturbation. Exp Brain Res 237: 777–791, 2019. doi: 10.1007/s00221-018-5459-0. [DOI] [PubMed] [Google Scholar]
- 67.Goodworth A, Chandan A, Chase H, Foster E, Francoeur H, Michaud J, Terry K. Stance width influences frontal plane balance responses to centripetal accelerations. Gait Posture 37: 98–102, 2013. doi: 10.1016/j.gaitpost.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 68.Horak FB, Dimitrova D, Nutt JG. Direction-specific postural instability in subjects with Parkinson’s disease. Exp Neurol 193: 504–521, 2005. doi: 10.1016/j.expneurol.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 69.Jacobs JV, Fujiwara K, Tomita H, Furune N, Kunita K, Horak FB. Changes in the activity of the cerebral cortex relate to postural response modification when warned of a perturbation. Clin Neurophysiol 119: 1431–1442, 2008. doi: 10.1016/j.clinph.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghosn NJ, Palmer JA, Borich MR, Ting LH, Payne AM. Cortical beta oscillatory activity evoked during reactive balance recovery scales with perturbation difficulty and individual balance ability. Brain Sci 10: 860, 2020. doi: 10.3390/brainsci10110860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCrum C, Gerards MH, Karamanidis K, Zijlstra W, Meijer K. A systematic review of gait perturbation paradigms for improving reactive stepping responses and falls risk among healthy older adults. Eur Rev Aging Phys Act 14: 3, 2017. doi: 10.1186/s11556-017-0173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chien JE, Hsu WL. Effects of dynamic perturbation-based training on balance control of community-dwelling older adults. Sci Rep 8: 17231, 2018. doi: 10.1038/s41598-018-35644-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mansfield A, Aqui A, Danells CJ, Knorr S, Centen A, DePaul VG, Schinkel-Ivy A, Brooks D, Inness EL, Mochizuki G. Does perturbation-based balance training prevent falls among individuals with chronic stroke? A randomised controlled trial. BMJ Open 8: e021510, 2018. doi: 10.1136/bmjopen-2018-021510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mohamed Suhaimy M, Okubo Y, Hoang PD, Lord SR. Reactive balance adaptability and retention in people with multiple sclerosis: a systematic review and meta-analysis. Neurorehabil Neural Repair 34: 675–685, 2020. doi: 10.1177/1545968320929681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video S1, Supplemental Figs. S1–S3, and Supplemental Tables S1–S3: https://doi.org/10.6084/m9.figshare.16777801.