Keywords: dead space, exercise, gas exchange inefficiency, HFpEF
Abstract
Heart failure with preserved ejection fraction (HFpEF) is associated with cardiopulmonary abnormalities that may increase physiological dead space to tidal volume (VD/VT) during exercise. However, studies have not corrected VD/VT for apparatus mechanical dead space (VDM), which may confound the accurate calculation of VD/VT. We evaluated whether calculating physiological dead space with (VD/VTVDM) and without (VD/VT) correcting for VDM impacts the interpretation of gas exchange efficiency during exercise in HFpEF. Fifteen HFpEF (age: 69 ± 6 yr; V̇o2peak: 1.34 ± 0.45 L/min) and 12 controls (70 ± 3 yr; V̇o2peak: 1.70 ± 0.51 L/min) were studied. Pulmonary gas exchange and arterial blood gases were analyzed at rest, submaximal (20 W for HFpEF and 40 W for controls), and peak exercise. VD/VT was calculated as – /. VD/VTVDM was calculated as – / – VDM/VT. VD/VT decreased from rest (HFpEF: 0.54 ± 0.07; controls: 0.32 ± 0.07) to submaximal exercise (HFpEF: 0.46 ± 0.07; controls: 0.25 ± 0.06) in both groups (P < 0.05), but remained stable (P > 0.05) thereafter to peak exercise (HFpEF: 0.46 ± 0.09; controls: 0.22 ± 0.05). In HFpEF, VD/VTVDM did not change (P = 0.58) from rest (0.29 ± 0.07) to submaximal exercise (0.29 ± 0.06), but increased (P = 0.02) thereafter to peak exercise (0.33 ± 0.06). In controls, VD/VTVDM remained stable such that no change was observed (P > 0.05) from rest (0.17 ± 0.06) to submaximal exercise (0.14 ± 0.06), or thereafter to peak exercise (0.14 ± 0.05). Calculating physiological dead space with and without a VDM correction yields quantitively and qualitatively different results, which could have impact on the interpretation of gas exchange efficiency in HFpEF. Further investigation is required to uncover the clinical consequences and the mechanism(s) explaining the increase in VD/VTVDM during exercise in HFpEF.
NEW & NOTEWORTHY Calculating VD/VT with and without correcting for VDM yields quantitively and qualitatively different results, which could have an important impact on the interpretation of V/Q mismatch in HFpEF. The finding that V/Q mismatch and gas exchange efficiency worsened, as reflected by an increase in VD/VTVDM during exercise, has not been previously demonstrated in HFpEF. Thus, further studies are needed to investigate the mechanisms explaining the increase in VD/VTVDM during exercise in patients with HFpEF.
INTRODUCTION
The physiological dead space (i.e., the sum of anatomic and alveolar dead space) to tidal volume ratio (VD/VT) is a valuable estimate of ventilation-perfusion (V/Q) matching (1). In healthy individuals, VD/VT is typically one-third of VT at rest and decreases to approximately one-fifth during exercise primarily due to a progressive increase in VT (2–4), as anatomic dead space remains relatively fixed and the contribution of alveolar dead space is expected to be negligible (5). In contrast, a VD/VT measurement that fails to decrease, or increases from rest to exercise, indicates worsening V/Q mismatch possibly due to increasing alveolar dead space, which can be ascribed to the presence of underlying cardiopulmonary abnormalities (1, 2, 6).
Heart failure with preserved ejection fraction (HFpEF) is associated with a number of cardiopulmonary abnormalities including elevated cardiac filling pressures (7–9), pulmonary edema (10, 11), impaired pulmonary vascular recruitment/distension (12–14), and right ventricle-pulmonary artery (RV-PA) uncoupling (15–19). These cardiopulmonary abnormalities could result in V/Q mismatch thereby increasing VD/VT (and the exercise ventilatory response), particularly during exercise when cardiopulmonary abnormalities are potentially exacerbated and the degree of V/Q mismatch is likely to worsen. However, in patients with HFpEF, studies have frequently reported a normal decrease in VD/VT from rest to exercise (11, 20–23), despite the presence of opposing (patho)physiological features (e.g., elevated cardiac filling pressures, pulmonary congestion, pulmonary vascular recruitment/distension, RV-PA uncoupling). In addition, the magnitude of decrease in VD/VT from rest to exercise in patients with HFpEF was even similar to that of age-matched healthy controls in some studies (20, 21). At present, the reason for these conflicting reports is unclear, but there is a possibility that it could be related to how the VD/VT measurement was calculated in these previous studies (11, 20–23).
The standard method of calculating VD/VT uses measurements of the partial pressure of arterial CO2 (), partial pressure of mixed expired CO2 (), and a correction for apparatus mechanical dead space volume (VDM) (1): VD/VT = – / – VDM/VT. Correcting for VDM is an important component of the VD/VT calculation, as it permits the measurement and quantification of changes in physiological dead space during exercise in studies of gas exchange (24). However, correcting VD/VT for VDM is seldom performed, even though studies have reported that assessing VD/VT, without correcting for VDM, may not be a sensitive means of detecting gas exchange inefficiencies arising from V/Q mismatch in patients with a range of cardiopulmonary abnormalities (25, 26). Notably, the aforementioned studies in patients with HFpEF also failed to account for VDM in their calculation of VD/VT at rest and during exercise (11, 20–23). As such, the VD/VT values reported in these previous studies may be misleading (11, 20–23), particularly when used to interpret gas exchange efficiency during exercise in patients with HFpEF.
Whether calculating physiological dead space with and without correcting for VDM yields quantitatively different results and impacts the interpretation of gas exchange efficiency during exercise in patients with HFpEF remains to be tested. Such information would be valuable for clinicians and physiologists since accurately assessing gas exchange efficiency is an important component for completing cardiopulmonary exercise testing (CPET). Therefore, the purpose of the present study was to calculate VD/VT using the Enghoff modification of the Bohr equation with (VD/VTVDM) and without (VD/VT) correcting for VDM, and to compare these responses at rest and during exercise in patients with HFpEF and age-matched healthy control participants. Given that patients with HFpEF exhibit many cardiopulmonary abnormalities that could result in V/Q mismatch, we hypothesized that VD/VTVDM would increase during exercise compared with VD/VT in patients with HFpEF, whereas VD/VTVDM and VD/VT would both decrease during exercise in controls.
METHODS
Participants
We evaluated the first 15 patients with HFpEF that were enrolled in our larger ongoing study investigating mechanisms of exertional dyspnea and exercise intolerance in HFpEF (NCT04068844). We also retrospectively examined 12 controls that were part of a different study describing mechanical ventilatory constraints during exercise in older healthy sedentary individuals (27). Patients with HFpEF were eligible to participate if they had signs and symptoms of heart failure based on Framingham criteria (28), an ejection fraction ≥50%, and evidence of volume overload (e.g., pulmonary edema) confirmed by a pulmonary capillary wedge pressure (PCWP) of ≥20 mmHg at peak exercise or an increase in PCWP ≥15 mmHg from rest to peak exercise. Participants were excluded if they had severe valvular disease, congenital heart disease, known restrictive or infiltrative cardiomyopathy, acute myocarditis, NYHA Class IV chronic heart failure or chronic heart failure that cannot be stabilized on medical therapy, a prior ejection fraction <50%, manifest/provocable ischemic heart disease, or severe obstructive lung disease (FEV1 <40% predicted). Controls did not have a history of smoking >5 pack yr, asthma, cardiovascular disease, sleep disorders, or musculoskeletal abnormalities that would preclude exercise, or a BMI >30 kg/m2. Before all testing, the purpose and protocols of all testing procedures were disclosed in detail and written and informed consent was obtained. The experimental procedures were reviewed and approved by the UT Southwestern Institutional Review Board (Reference no: STU2019-0617).
Study Design
Patients with HFpEF visited the laboratory on two occasions separated by at least 48 h. During the first visit, patients with HFpEF underwent preparticipation health screening and performed pulmonary function testing according to the American Thoracic Society/European Respiratory Society guidelines (29) and a maximal CPET to determine participant eligibility. During the second visit, patients with HFpEF performed a 6-min submaximal constant-load cycling test and a maximal incremental cycling test with pulmonary artery and radial artery catheterizations. Controls visited the laboratory on two separate occasions separated by at least 48 h. During the first visit, controls underwent preparticipation health screening and performed pulmonary function testing according to the American Thoracic Society/European Respiratory Society guidelines (29) and a maximal CPET to determine participant eligibility. During the second visit, controls performed a maximal incremental cycling test with radial artery catheterization.
Submaximal Constant-Load Cycling Test for Patients with HFpEF
Following rest, patients with HFpEF cycled at 20 W for 6 min on a recumbent cycle ergometer (Lode BV, Groningen, The Netherlands). This exercise work rate (WR) was set to represent the hemodynamic demands of activities of daily living. Heart rate (HR) and rhythm were monitored continuously using a 12-lead electrocardiogram, and blood pressure was monitored via arterial waveform tracings. Invasive central hemodynamic measurements and mixed venous and radial arterial blood gases were measured (see Catheterization Protocol), and pulmonary gas exchange was measured using a customized breath-by-breath measurement system (Beck Integrative Physiological System, BIPS; KCBeck, Physiological Consulting, Liberty, UT) integrated with a mass spectrometer (Perkin-Elmer 1100). Calibration of the analyzer was performed by using reference gases before each test. Expired volume was measured at the mouth with a turbine flow device, which was calibrated before each test with the use of a 3-L calibration syringe.
Maximal Incremental Cycling Test for Patients with HFpEF
Patients with HFpEF performed a maximal incremental cycling test on the same recumbent cycle ergometer (Lode BV, Groningen, The Netherlands). After a brief rest period, patients with HFpEF started cycling at an initial WR equivalent to 90% of V̇o2peak that was determined during the CPET performed on the first visit. The WR increased by 5 or 10 W each minute until the participant reached volitional exhaustion or symptom limitation. HR and rhythm, and pulmonary gas exchange variables were measured as described for the submaximal constant-load cycling test. Invasive central hemodynamic measurements and mixed venous and radial arterial blood gases were also measured (see Catheterization Protocol).
Maximal Incremental Cycling Test for Controls
Controls performed a maximal incremental cycling test on an upright cycle ergometer (MedGraphics, model CPE 2000). After a brief rest period, exercise began at 10 W (women) or 20 W (men) and was incremented by 10 or 20 W each minute until the participant reached volitional exhaustion or symptom limitation. We chose to report submaximal exercise data collected at the 40 W stage because this yielded a comparable relative WR (expressed as a percentage of maximum WR) and ventilatory demand as that observed in the patients with HFpEF. HR and rhythm were monitored continuously using a 12-lead electrocardiogram, and blood pressure was monitored via an automated system. Radial arterial blood gases were also measured (see Catheterization Protocol). Pulmonary gas exchange was quantified using a customized breath-by-breath measurement system that was computerized (NEC 486DX) and integrated with a mass spectrometer (Marquette Electronics, model 1100). Calibration of the analyzer was performed by using reference gases before each test. Expired volume was measured at the mouth with a turbine flow device, which was calibrated before each test with the use of a 3-L calibration syringe.
Catheterization Protocol
Patients with HFpEF had a Swan–Ganz catheter placed in the pulmonary artery via brachial or antecubital vein access under fluoroscopic guidance, which was used to measure pulmonary artery pressure (PAP), PCWP, and central blood temperature. Controls did not have a Swan–Ganz catheter placed in the pulmonary artery. Both HFpEF and controls underwent radial artery catheterization using a modified Seldinger technique before testing during the second visit. Immediately following the elimination of sample-line dead space (i.e., 5 mL waste), 2 mL of arterial blood was withdrawn using a syringe with dry lithium heparin for gases and electrolytes for blood gas analysis. These blood gas samples were withdrawn over a 10-s period to reduce the fluctuations of blood gas tensions over a given respiratory cycle during the same timeframe in which pulmonary gas exchange determinations were made. For patients with HFpEF, arterial blood gases were measured at rest, during the final minute of constant-load exercise, and at peak exercise. For controls, arterial blood gases were measured at rest, during each WR increment, and at peak exercise. The blood samples were immediately placed in an ice bath and were sent to the institution’s biochemistry laboratory where data on , partial pressure of arterial O2 (), hemoglobin O2 saturation (HbO2%), lactate, pH, bicarbonate (HCO3), and base excess were measured. Reference gases and commercial standards were used to calibrate the blood-gas analyzer before all testing.
Data and Statistical Analysis
VD/VT was calculated as: – /. VD/VTVDM was calculated as: – / – VDM/VT. VDM was estimated to be equal to 0.18 L and 0.14 L for patients with HFpEF and controls, respectively. Although the same breathing apparatus was used in both groups (i.e., a Hans Rudolph valve, model 2700), the reason as to why the VDM was larger in the HFpEF group compared with the control group was because there was an additional housing attached to the 2700 model to support the use of the acetylene rebreathe technique to measure cardiac output (Qc) in HFpEF (Qc data acquired from the acetylene rebreathe technique are not shown). was calculated as: 863 × V̇co2 (STPD)/V̇E (BTPS) in both HFpEF and controls, where STPD represents standard temperature and pressure dry and BTPS represents body temperature and pressure saturated. VD/VT and VD/VTVDM were calculated at rest, submaximal exercise (20 W for HFpEF and 40 W for controls), and peak exercise. In HFpEF, Qc was determined by the direct Fick method (Qc = V̇o2/arterial-venous O2 difference) and stroke volume (SV) was determined as the quotient of Qc and HR. Pulmonary vascular resistance (PVR) was also calculated as mean PAP–PCWP/Qc in HFpEF. All data were analyzed using SPSS 22.0 (SPSS, Chicago, IL). Differences in participant characteristics, cardiorespiratory parameters, and arterial blood gas data (rest, submaximal exercise, and peak exercise) were compared using independent t tests. Relationships between variables were assessed using Pearson’s Correlation Coefficient analyses. Differences in VD/VT measurements were compared between calculation (with and without correcting for VDM) and across conditions (rest, submaximal exercise, and peak exercise) using a two-way repeated-measures ANOVA in patients with HFpEF and controls, separately. Differences in VD/VTVDM measurements were also compared between groups (HFpEF vs. controls) across condition (rest, submaximal exercise, and peak exercise) using a two-way repeated-measures ANOVA. Where significant interactions were detected, post hoc comparisons using Bonferroni adjustments were performed. Statistical significance was defined as P < 0.05 and all data are presented as means ± SD.
RESULTS
Participant Characteristics
Participant characteristics and pulmonary function data including spirometric and pulmonary diffusing capacity variables are displayed in Table 1. All patients with HFpEF were not currently smoking at the time of the study. Fourteen out of 15 patients with HFpEF had never smoked, whereas the only patient with a history of smoking stopped 30 yr prior (∼29 pack yr history). Participants were similar in age and height, but patients with HFpEF overall were heavier and had a greater BMI compared with controls. Also, FVC and FEV1 were lower in patients with HFpEF compared with controls. Total lung capacity was lower in HFpEF compared with controls. Despite these differences, both HFpEF and controls had normal pulmonary function based on percentage of predicted values (using lower limits of normal), however, there was one patient with HFpEF who presented with moderate obstructive lung disease. Although the diffusing capacity of the lung for carbon monoxide (DLCO) was lower in HFpEF compared with controls, DLCO/VA was similar between groups. All cardiorespiratory responses are shown in Table 2.
Table 1.
Participant characteristics
| Controls | HFpEF | |
|---|---|---|
| Demographics | ||
| Age, yr | 70 ± 3 | 69 ± 6 |
| Sex (men/women) | 7/5 | 6/9 |
| Height, m | 1.72 ± 0.1 | 1.70 ± 0.1 |
| Weight, kg | 72.3 ± 11 | 109 ± 14** |
| BMI, kg/m2 | 27.4 ± 1.9 | 38.2 ± 7.3** |
| Pulmonary function | ||
| FVC, %predicted | 115 ± 17 | 85 ± 14** |
| FEV1, %predicted | 104 ± 13 | 83 ± 13** |
| FEV1/FVC, % | 71 ± 5 | 76 ± 11 |
| TLC, %predicted | 113 ± 13 | 96 ± 16** |
| DLCO, %predicted | 108 ± 14 | 69 ± 19** |
| DLCO/VA, %predicted | 109 ± 16 | 110 ± 28 |
Data are means ± SD. BMI, body mass index; DLCO, diffusing capacity of the lung for carbon monoxide; FVC, forced vital capacity; FEV1, forced expired volume in one second; HFpEF, heart failure with preserved ejection fraction; TLC, total lung capacity; VA, alveolar volume. **P < 0.01.
Table 2.
Cardiorespiratory parameters at rest, during submaximal exercise, and during peak exercise
| Controls | HFpEF | |
|---|---|---|
| Rest | ||
| V̇E, L/min | 11.56 ± 4.02 | 12.14 ± 2.32 |
| VT, L | 0.91 ± 0.26 | 0.73 ± 0.13* |
| Fb, breaths/min | 13.1 ± 3.9 | 17.2 ± 4.0* |
| V̇o2, L/min | 0.34 ± 0.08 | 0.33 ± 0.08 |
| V̇co2, L/min | 0.28 ± 0.06 | 0.26 ± 0.06 |
| V̇E/V̇co2 | 40.8 ± 9.6 | 48.3 ± 8.1* |
| RER | 0.83 ± 0.09 | 0.78 ± 0.06 |
| Submaximal exercise | ||
| WR, W | 40 | 20 |
| WR, %peak | 37 ± 14 | 28 ± 9* |
| V̇E, L/min | 27.79 ± 3.59 | 31.59 ± 3.95* |
| VT, L | 1.50 ± 0.39 | 1.12 ± 0.24** |
| Fb, breaths/min | 19.4 ± 3.7 | 29.3 ± 6.0** |
| V̇o2, L/min | 0.93 ± 0.10 | 0.92 ± 0.21 |
| V̇co2, L/min | 0.81 ± 0.14 | 0.80 ± 0.18 |
| V̇E/V̇co2 | 34.7 ± 4.5 | 40.5 ± 6.3* |
| RER | 0.87 ± 0.13 | 0.87 ± 0.05 |
| Peak exercise | ||
| WR, W | 120 ± 40 | 80 ± 29** |
| V̇E, L/min | 82.94 ± 24.42 | 61.14 ± 18.34** |
| VT, L | 2.15 ± 0.59 | 1.50 ± 0.55** |
| Fb, breaths/min | 39.0 ± 6.8 | 43.5 ± 10.7 |
| V̇o2, L/min | 1.70 ± 0.51 | 1.34 ± 0.45 |
| V̇o2, %pred | 106 ± 21 | 71 ± 17** |
| V̇o2, mL/min/kg | 23.35 ± 5.45 | 11.81 ± 3.47** |
| V̇co2, L/min | 2.1 ± 0.5 | 1.50 ± 0.63** |
| V̇E/V̇co2 | 39.6 ± 5.0 | 43.3 ± 8.8 |
| RER | 1.26 ± 0.09 | 1.10 ± 0.11** |
Data are means ± SD. HFpEF, heart failure with preserved ejection fraction; V̇E, minute ventilation; VT, tidal volume; Fb, breathing frequency; V̇o2, oxygen consumption; V̇co2, carbon dioxide elimination; RER, respiratory exchange ratio; WR, work rate. *P < 0.05; **P < 0.01.
Arterial Blood Gas and Hemodynamic Responses
All arterial blood gas and hemodynamic responses are shown in Table 3. While was lower and was higher in HFpEF compared with controls at each timepoint (rest, submaximal exercise, and peak exercise), lactate and pH were not different between groups at each timepoint (rest, submaximal exercise, and peak exercise). Base excess and HCO3 slightly decreased with exercise in HFpEF. Although HR was similar between groups at rest and submaximal exercise, peak HR was lower in HFpEF compared with controls. Furthermore, hemodynamic parameters including Qc, SV, mean PAP, and PCWP increased with exercise in HFpEF. PVR decreased from rest to throughout exercise, indicating pulmonary vasodilation or pulmonary vascular recruitment. Although HbO2% remained stable in both groups, HbO2% was consistently lower HFpEF compared with controls.
Table 3.
Hemodynamic, central pressures, and arterial blood gases at rest, during submaximal exercise, and peak exercise
| Controls | HFpEF | |
|---|---|---|
| Rest | ||
| HR, beats/min | 75 ± 11 | 75 ± 20 |
| Qc, L/min | 5.9 ± 1.3 | |
| SV, mL/beat | 81.8 ± 18.1 | |
| Mean PAP, mmHg | 15.7 ± 4.8 | |
| PCWP, mmHg | 6.1 ± 2.2 | |
| PVR | 1.8 ± 0.7 | |
| , mmHg | 99.2 ± 8.6 | 83.0 ± 5.9** |
| , mmHg | 36.2 ± 4.2 | 40.7 ± 3.8** |
| HbO2, % | 96 ± 1 | 94 ± 2** |
| Lactate, mmol/L | 0.8 ± 0.3 | 1.2 ± 0.6* |
| pH | 7.43 ± 0.02 | 7.42 ± 0.02 |
| HCO3, mmol/L | 26.5 ± 3.2 | |
| Base excess, mmol/L | 2.1 ± 3.4 | |
| Submaximal exercise | ||
| HR, beats/min | 109 ± 18 | 95 ± 17* |
| Qc, L/min | 9.7 ± 2.2 | |
| SV, mL/beat | 104.4 ± 22.4 | |
| Mean PAP, mmHg | 35.2 ± 7.1 | |
| PCWP, mmHg | 21.5 ± 6.8 | |
| PVR | 1.5 ± 0.5 | |
| , mmHg | 99.4 ± 9.1 | 85.3 ± 11.2** |
| , mmHg | 36.5 ± 4.1 | 40.6 ± 4.1* |
| HbO2, % | 97 ± 1 | 95 ± 2** |
| Lactate, mmol/L | 1.5 ± 0.7 | 2.1 ± 0.8 |
| pH | 7.42 ± 0.02 | 7.41 ± 0.01 |
| HCO3, mmol/L | 25.7 ± 2.7 | |
| Base excess, mmol/L | 1.1 ± 2.8 | |
| Peak exercise | ||
| HR, beats/min | 157 ± 9 | 124 ± 18** |
| Qc, L/min | 11.4 ± 3.4 | |
| SV, mL/beat | 91.4 ± 21.7 | |
| Mean PAP, mmHg | 44.7 ± 8.8 | |
| PCWP, mmHg | 33.3 ± 7.4 | |
| PVR | 1.0 ± 0.5 | |
| , mmHg | 108.5 ± 8.1 | 89.9 ± 13.9** |
| , mmHg | 31.6 ± 2.5 | 38.5 ± 4.7** |
| HbO2, % | 97 ± 1 | 95 ± 3** |
| Lactate, mmol/L | 6.5 ± 1.7 | 6.5 ± 2.4 |
| pH | 7.39 ± 0.08 | 7.39 ± 0.02 |
| HCO3, mmol/L | 23.4 ± 2.9 | |
| Base excess, mmol/L | −1.4 ± 3.1 |
Data are means ± SD. HbO2, hemoglobin O2 saturation; HCO3, bicarbonate; HFpEF, heart failure with preserved ejection fraction; HR, heart rate; Qc, cardiac output; SV, stroke volume; , partial pressure of arterial CO2; , partial pressure of arterial O2; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance. *P < 0.05; **P < 0.01.
VD/VT versus VD/VTVDM
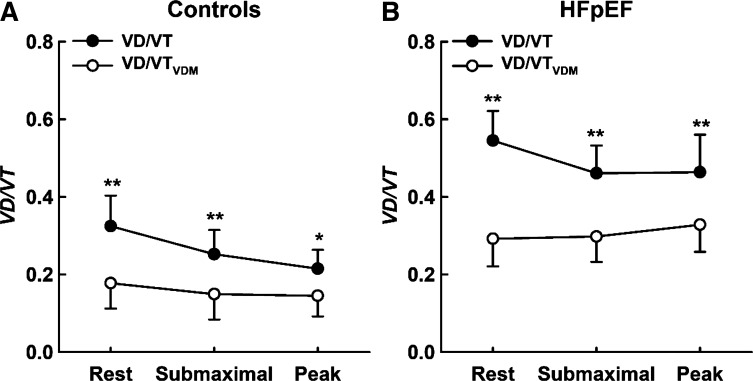
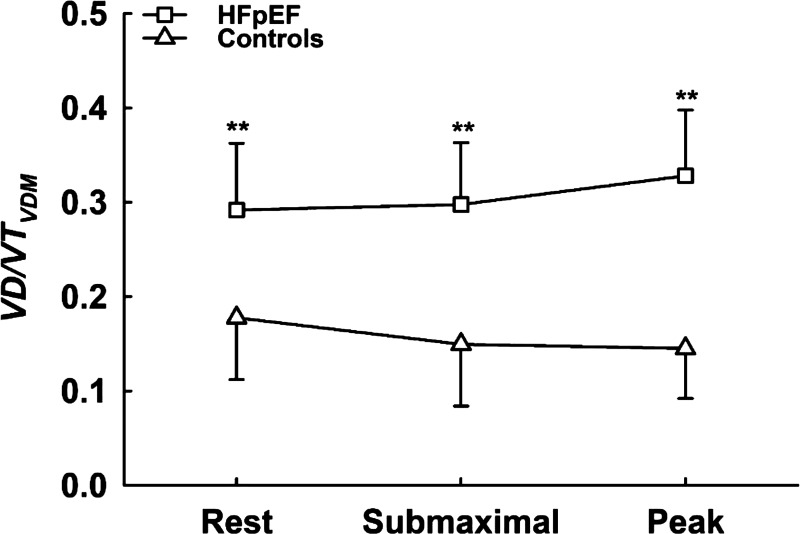
In HFpEF, VD/VT decreased from rest to submaximal exercise (Fig. 1; right, P < 0.01) but did not significantly change thereafter from submaximal exercise to peak exercise (P = 0.94); in contrast, VD/VTVDM did not significantly change from rest to submaximal exercise (Fig. 1A, P = 0.58), but significantly increased thereafter from submaximal exercise to peak exercise (P = 0.02). In addition, VD/VT was greater compared with VD/VTVDM at rest, submaximal exercise, and peak exercise (calculation by condition interaction, P = 0.007) in HFpEF. In controls, VD/VT decreased from rest to submaximal exercise (Fig. 1; left, P = 0.02), but did not significantly change thereafter from submaximal exercise to peak exercise (P = 0.10), whereas VD/VTVDM remained relatively stable such that no significant change was observed from rest to submaximal exercise (Fig. 1B, P = 0.42) or from submaximal exercise to peak exercise (P = 0.82). Furthermore, VD/VTVDM was greater in HFpEF compared with controls at rest, submaximal exercise, and peak exercise (Fig. 2; main effect for group, P < 0.001). Although we found a significant (albeit weak) relationship between VD/VT and PVR (r2 = 0.20; P = 0.004), VD/VT was not related to mean PAP (r2 = −0.07; P = 0.08) or PCWP (r2 = −0.05; P = 0.15), nor was VD/VTVDM related to PVR (r2 = 0.01; P = 0.43), mean PAP (r2 = 0.03; P = 0.28), or PCWP (r2 = 0.06; P = 0.11) in patients with HFpEF, likely reflecting heterogeneity in the processes causing increased VD/VTVDM. Lastly, VD/VTVDM was related to V̇o2peak in patients with HFpEF (both in L/min: r2 = 0.27; P = 0.02, and as a percent predicted: r2 = 0.30; P = 0.02), but VD/VTVDM was not related to V̇o2peak in controls (both in L/min: r2 = 0.09; P = 0.36, and as a percent predicted value: r2 = 0.003; P = 0.48), suggesting that V/Q mismatch may contribute to exercise intolerance in patients with HFpEF but not in controls.
Figure 1.
Means ± SD values for VD/VT (closed circles) and VD/VTVDM (open circles) measured at rest and during exercise in controls (A, left) and patients with HFpEF (B, right). **Significant difference between VD/VT and VD/VTVDM at the P < 0.01 level. Differences in VD/VT measurements were compared between calculation (with and without correcting for VDM) and across conditions (rest, submaximal exercise, and peak exercise) using a two-way repeated-measures ANOVA in patients with HFpEF and controls, separately. HFpEF, heart failure with preserved ejection fraction; VDM, mechanical dead space; VD/VT, dead space to tidal volume.
Figure 2.
Means ± SD values for VD/VTVDM measured at rest and during exercise in controls (open triangles) and patients with HFpEF (open squares). **Significant difference between HFpEF and controls at the P < 0.01 level. Differences in VD/VTVDM measurements were compared between groups (controls vs. HFpEF) across condition (rest, submaximal exercise, and peak exercise) using a two-way repeated-measures ANOVA. HFpEF, heart failure with preserved ejection fraction; VDM, mechanical dead space; VD/VT, dead space to tidal volume.
DISCUSSION
Our findings show that calculating VD/VT with and without correcting for VDM yields quantitively and qualitatively different results in both HFpEF and controls. This was particularly evident when assessing the magnitude and direction of change in VD/VT measurements during exercise, whereby VD/VT decreased initially and remained low (relative to rest) throughout exercise in both groups, whereas VD/VTVDM increased and remained high (relative to rest) throughout exercise in HFpEF, but remained relatively stable in controls. These findings suggest that calculating VD/VT with and without correcting for VDM could have an important impact on the interpretation of pulmonary gas exchange (in)efficiency.
In the present study, we reported a decrease in VD/VT from rest to exercise in patients with HFpEF, the magnitude of which was similar to that observed in controls. These findings are consistent with previous studies that also observed a decrease in VD/VT from rest to exercise in patients with HFpEF (11, 20–23). Similar findings have also been reported in other conditions including heart failure with reduced ejection fraction (HFrEF) (30, 31) and in those with a range of conditions with which unexplained dyspnea was documented (6). From an interpretation perspective, the decrease in VD/VT reported in the present study, as well as in previous studies would suggest that V/Q mismatch and thus gas exchange efficiency improved during exercise in these patients. For clinicians and physiologists interested in evaluating gas exchange efficiency, these data may constitute an unexpected finding given that the presence of cardiopulmonary abnormalities should otherwise be expected to worsen V/Q mismatch and gas exchange efficiency, and manifest as an increase in VD/VT during exercise and subsequently contribute to exercise limitation. Importantly, we must emphasize that VD/VT measured in the present study, as well as that measured in previous studies (6, 11, 20–23, 30, 31), was calculated without accounting for the effects of VDM in the calculation, which is an important component in the calculation and if left unaccounted for, may confound the accurate calculation of VD/VT (24).
When VD/VT was corrected for VDM in the present study (VD/VTVDM), this yielded a VD/VTVDM measurement that increased during exercise in patients with HFpEF, the magnitude of which was greater compared with controls. In contrast to the results discussed regarding the measurement of VD/VT, these findings suggest that V/Q mismatch worsened during exercise in patients with HFpEF, and that the development of V/Q mismatch during exercise was more profound in HFpEF compared with controls. Our findings also agree with others (7, 8), who reported that hemodynamic derangements seem to only develop during the stress of exercise in patients with HFpEF. Notably, the increase in VD/VTVDM during exercise observed in HFpEF and controls in the present study is in keeping with earlier reports suggesting that a VD/VT ratio that increases, or fails to decrease from rest to exercise, is characteristic of V/Q mismatch and impaired gas exchange (1, 2, 6). In HFpEF, the increase in VD/VTVDM may be due to the fact that these patients exhibit various disease-related cardiopulmonary abnormalities in combination with age-related changes in pulmonary function, whereas controls only exhibit the latter. Thus, our findings support the contention that clinicians and physiologists should consider correcting VD/VT for VDM to obtain a measurement that may provide a better representation of the degree of V/Q mismatch and gas exchange inefficiency during exercise in patients with HFpEF, or any other cardiac or pulmonary diseases. Importantly, it may be argued that correcting VD/VT for VDM may increase the likelihood of identifying potential underlying pulmonary pathophysiology, which otherwise may not have been revealed if VDM was not accounted for in the VD/VT calculation.
The increase in VD/VTVDM has not been shown before in patients with HFpEF. Based on our data, the reason as to why VD/VTVDM increased in patients with HFpEF cannot be determined since our investigation was not designed to uncover the mechanism(s) responsible for the increase in VD/VTVDM. Nevertheless, it is well known that patients with HFpEF exhibit many cardiopulmonary abnormalities that could result in V/Q mismatch and manifest as an increase in VD/VTVDM, particularly during exercise when cardiopulmonary abnormalities are potentially exacerbated, and the degree of V/Q mismatch is likely to worsen. Therefore, we may only provide speculation on possible mechanisms responsible for the observed findings.
The dominant pathognomonic feature of HFpEF is that cardiac filling pressures markedly rise during exercise (i.e., evidenced by an increase in PCWP) (7–9). The increase in PCWP during exercise in HFpEF typically manifests from a passive backward transmission of elevated left-sided filling pressures due to the combined effects of impaired ventricular relaxation and an increase in venous return (during exercise) entering a stiff left ventricle (32, 33). These alterations significantly increase the propensity for elevated hydrostatic pressure gradients and a disruption to the microvascular permeability, which could result in transcapillary fluid movement and the development of pulmonary edema during exercise. In a study of 61 patients with compensated HFpEF, Reddy et al. (11) demonstrated that over half of the cohort studied (54% of total n) developed pulmonary edema during supine cycling exercise at 20 W. The mean PCWP reported in these patients during supine cycling was 38 ± 9 mmHg (11). Whether the development of pulmonary edema influences V/Q mismatch and gas exchange efficiency in patients with HFpEF remains to be tested. However, it is certainly possible that with pulmonary venous pressures reported in this range (11), as well as that reported in the present study, transcapillary fluid movement could be rapid enough to result in pulmonary edema sufficient to alter the distribution of ventilation and/or pulmonary blood flow (34, 35), and thus worsen V/Q mismatch during exercise in the patient with HFpEF. In addition, chronic elevations in pulmonary venous pressures can cause pulmonary vascular remodeling and decrease pulmonary vascular reserves, as evidenced by an impaired ability to recruit/distend the pulmonary vasculature to accommodate increases in pulmonary blood flow. Although studies have reported data indicating the presence of pulmonary vascular remodeling and reduced pulmonary vascular reserves in HFpEF (12–14), the influence of these pulmonary pathophysiological derangements on V/Q mismatch in HFpEF is unknown. Indeed, it is possible that these derangements played a role in the development of V/Q mismatch during exercise in present study, and addressing this question represents an important avenue for further investigation.
Moreover, recent studies have shown that some patients with HFpEF exhibit impaired RV-PA coupling (15–19), and that the presence of impaired RV-PA coupling could identify such patients who also exhibit considerable pulmonary perfusion abnormalities (19). Obokata et al. (20) recently demonstrated that exercise VD/VT was correlated with impaired RV systolic reserve, higher PVR, and lower PA compliance. These findings are similar to that reported in patients with HFrEF, whereby Lewis et al. (36) observed a link between VD/VT and pulmonary vasodilator and RV systolic reserve. In contrast to these previous observations (20, 36), we did not observe a relationship between VD/VTVDM and PVR. At present, a reason for the different findings between the present study and others is unclear. However, it should be noted that previous studies performed invasive measurements in the supine position, which augments venous return and potentially reduces pulmonary vasodilatory reserve (thereby increasing PVR), whereas we performed invasive measurements in the upright position. Given these postural differences, it may be that such differences in methodology could explain these discordant findings. Furthermore, because we did not measure RV function we are unable to provide insight into whether alterations in RV-PA coupling during exercise played a role in the development of V/Q mismatch during exercise in present study. Nevertheless, it is plausible that RV-PA uncoupling could limit pulmonary perfusion, and subsequently result in V/Q mismatch, particularly in those HFpEF patients who exhibit impairments in RV and/or PA function.
Although the results of the present study indicate that V/Q mismatch worsened during exercise in patients with HFpEF (evidenced by an increase in VD/VTVDM during exercise), we did not directly quantify how V/Q relationships were altered throughout exercise in these patients. Indeed, it should be noted that patients with HFpEF have not yet had their V/Q relationships analyzed by means of the multiple inert gas elimination technique (37), which remains the only way to quantify the distribution of V/Q relationships throughout the lung. During exercise, it is likely that patients with HFpEF (and perhaps to lesser degree in controls) developed lung units where the amount of ventilation is high relative to the amount of pulmonary blood flow, which would have resulted in a greater relative distribution of high V/Q lung units (and/or alveolar dead space) (38, 39). In keeping with this suggestion, the preservation of and HbO2% during exercise in HFpEF indicates that the influence of low V/Q lung units [i.e., where ventilation is low relative to the amount of pulmonary blood flow (and/or shunt)] were negligible (38, 39). Further evidence supporting the negligible influence of low V/Q units and/or shunt was provided from the observation that and HbO2% slightly increased following the inspiration of 45% O2 as part of the acetylene rebreathe technique (data not shown). Although we cannot fully exclude airway-related causes of V/Q mismatch, the lack of spirometric abnormities in the patients with HFpEF studied seem to point to abnormalities relating to the pulmonary circulation, rather than the airways as the primary origin of V/Q mismatch. The fact that the aforementioned cardiopulmonary abnormalities associated with HFpEF appear to primarily limit pulmonary perfusion supports this assumption.
Currently, the VD/VT ratio is widely used as an estimate of V/Q matching. Indeed, a VD/VT measurement that fails to decrease, or increases from rest to exercise (such as that observed in the present study based on VD/VTVDM), indicates worsening V/Q mismatch possibly due to increasing alveolar dead space. However, it must be noted that VT can also have a major effect on the VD/VT ratio, independent of the effects of alveolar dead space. Given that VT was in fact smaller in HFpEF compared with controls in the present study, one may argue that the smaller VT could have also at least, in part, contributed to the increased VD/VT observed in HFpEF compared with controls. Therefore, the extent to which the increase in VD/VTVDM is due to an increase in alveolar dead space (due to exacerbation of cardiopulmonary abnormalities) or a smaller VT (due to mechanical ventilatory constraints that limit VT expansion) in patients with HFpEF is unclear. Investigation into this question [i.e., the mechanism(s) governing the increase in V/Q mismatch and VD/VTVDM] is beyond the scope of the present study but represents an interesting avenue for further research, particularly since we observed a link between VD/VTVDM and exercise intolerance, and that exercise intolerance is a dominant symptom of HFpEF.
METHODOLOGICAL CONSIDERATIONS
We examined patients with HFpEF above 60 yr, which limits the generalization of our findings to only older individuals with HFpEF. However, 60 yr was selected as the lower age limit to reflect current HFpEF studies (40, 41), which characterize HFpEF as primarily a disease of seniors with the average age of 69 yr. Moreover, the submaximal exercise conditions in the present study were different between groups; that is, submaximal exercise responses were recorded during the final minute of a 6-min constant-load exercise test at 20 W in patients with HFpEF, whereas the submaximal exercise responses in controls were recorded at the 40 W stage during an incremental exercise test. Also noteworthy is that VDM was estimated in the present study as the effective dead space, rather than volumetric dead space. This is because previous studies have reported that VD/VT calculations that take into account the entire volumetric dead space can underestimate physiological dead space (42). As such, we estimated the effective dead space of the breathing apparatus using methods based on the study by Bradley and Younes (42). Although the effective dead space of the breathing apparatus was different between groups (i.e., 0.18 L for HFpEF and 0.14 L for controls), we must emphasize that differences in VDM cannot account for the differences observed in VD/VTVDM between groups. This is because correcting for VDM in the standard Enghoff Modification of the Bohr equation permits the calculation of the “physiological” dead space, which is isolated from the effects of VDM, irrespective of its volume. Therefore, the differences in VD/VTVDM observed in the present study must be governed by important (patho)physiological differences that exist between the two groups, rather than the independent effects (or different sizes) of VDM. Lastly, because controls did not undergo pulmonary artery catheterization, we were unable to make between-group comparisons of Qc, SV, mean PAP, PCWP, and PVR. Since it was not the intention of the present study, further investigations are warranted to determine whether potential between-group differences in these parameters may explain the between-groups differences in VD/VT measurements reported in the present study.
Conclusions
In summary, our findings demonstrate that calculating VD/VT with and without correcting for VDM yields quantitively and qualitatively different results, which could have an important impact on the interpretation of V/Q mismatch and gas exchange (in)efficiency in patients with HFpEF. Furthermore, the finding that V/Q mismatch and gas exchange efficiency worsened, as reflected by an increase in VD/VTVDM during exercise, has not been previously demonstrated in patients with HFpEF; however, it is clear that further studies are needed to carefully investigate the mechanisms that may explain the increase in VD/VTVDM in patients with HFpEF. Lastly, since we demonstrated a link between VD/VTVDM and exercise intolerance in patients with HFpEF, the rise in VD/VTVDM (or V/Q mismatch) during exercise could represent a viable target for new therapeutic interventions designed to improve symptoms and outcomes in patients with HFpEF.
GRANTS
B. N. Balmain is supported by an American Heart Association Postdoctoral Fellowship. This research was also supported by the National Institutes of Health Grant 1P01HL137630 and Texas Health Presbyterian Hospital Dallas.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.N.B. conceived and designed research; B.N.B. performed experiments; B.N.B. analyzed data; B.N.B., A.R.T., J.P.M., S.S., B.D.L., L.S.H., and T.G.B. interpreted results of experiments; B.N.B. prepared figures; B.N.B., A.R.T., J.P.M., S.S., B.D.L., L.S.H., and T.G.B. drafted manuscript; B.N.B., A.R.T., J.P.M., S.S., B.D.L., L.S.H., and T.G.B. edited and revised manuscript; B.N.B., A.R.T., J.P.M., S.S., B.D.L., L.S.H., and T.G.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Quinn M. Halverson, Raksa B. Moran, Sheryl Livingston, Margot Morris, Cindi Foulk, Marcus Payne, Mitchell Samels, Teverick Boyd, Erika Silva, David Lee, and Stephanie Baird for help with data collection and processing for this project. We would also like to extend our sincere gratitude to Dr. Peter D. Wagner M.D. for assistance with the calculation and interpretation of these data.
REFERENCES
- 1.Lewis DA, Sietsema KE, Casaburi R, Sue DY. Inaccuracy of noninvasive estimates of VD/VT in clinical exercise testing. Chest 106: 1476–1480, 1994. doi: 10.1378/chest.106.5.1476. [DOI] [PubMed] [Google Scholar]
- 2.Wasserman K, Hanse JE, Sue DY, Stringer WW, Whipp BJ (Editors). Principles of Exercise Testing and Interpretation (4th ed.). Philadelphia, PA: Lippincott Williams & Wilkins, 2005. [Google Scholar]
- 3.Wasserman K, Van Kessel AL, Burton GG. Interaction of physiological mechanisms during exercise. J Appl Physiol 22: 71–85, 1967. doi: 10.1152/jappl.1967.22.1.71. [DOI] [PubMed] [Google Scholar]
- 4.Jones NL. Normal values for pulmonary gas exchange during exercise. Am Rev Respir Dis 129: S44–S46, 1984. doi: 10.1164/arrd.1984.129.2P2.S44. [DOI] [PubMed] [Google Scholar]
- 5.Sinha P, Flower O, Soni N. Deadspace ventilation: a waste of breath!. Intensive Care Med 37: 735–746, 2011. doi: 10.1007/s00134-011-2194-4. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman MI, Miller A, Brown LK, Bhuptani A, Sloane MF, Teirstein AS. Estimated vs actual values for dead space/tidal volume ratios during incremental exercise in patients evaluated for dyspnea. Chest 106: 131–136, 1994. doi: 10.1378/chest.106.1.131. [DOI] [PubMed] [Google Scholar]
- 7.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 3: 588–595, 2010. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorfs S, Zeh W, Hochholzer W, Jander N, Kienzle RP, Pieske B, Neumann FJ. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J 35: 3103–3112, 2014. doi: 10.1093/eurheartj/ehu315. [DOI] [PubMed] [Google Scholar]
- 9.Eisman AS, Shah RV, Dhakal BP, Pappagianopoulos PP, Wooster L, Bailey C, Cunningham TF, Hardin KM, Baggish AL, Ho JE, Malhotra R, Lewis GD. Pulmonary capillary wedge pressure patterns during exercise predict exercise capacity and incident heart failure. Circ Heart Fail 11: e004750, 2018. doi: 10.1161/CIRCHEARTFAILURE.117.004750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fermoyle CC, Stewart GM, Borlaug BA, Johnson BD. Effects of exercise on thoracic blood volumes, lung fluid accumulation, and pulmonary diffusing capacity in heart failure with preserved ejection fraction. Am J Physiol Regul Integr Comp Physiol 319: R602–R609, 2020. doi: 10.1152/ajpregu.00192.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy YNV, Obokata M, Wiley B, Koepp KE, Jorgenson CC, Egbe A, Melenovsky V, Carter RE, Borlaug BA. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur Heart J 40: 3721–3730, 2019. doi: 10.1093/eurheartj/ehz713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fayyaz AU, Edwards WD, Maleszewski JJ, Konik EA, DuBrock HM, Borlaug BA, Frantz RP, Jenkins SM, Redfield MM. Global pulmonary vascular remodeling in pulmonary hypertension associated with heart failure and preserved or reduced ejection fraction. Circulation 137: 1796–1810, 2018. doi: 10.1161/CIRCULATIONAHA.117.031608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olson TP, Johnson BD, Borlaug BA. Impaired pulmonary diffusion in heart failure with preserved ejection fraction. JACC Heart Fail 4: 490–498, 2016. doi: 10.1016/j.jchf.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis GD. The role of the pulmonary vasculature in heart failure with preserved ejection fraction. J Am Coll Cardiol 53: 1127–1129, 2009. doi: 10.1016/j.jacc.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J 37: 3293–3302, 2016. doi: 10.1093/eurheartj/ehw241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guazzi M, Dixon D, Labate V, Beussink-Nelson L, Bandera F, Cuttica MJ, Shah SJ. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging 10: 1211–1221, 2017. doi: 10.1016/j.jcmg.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 35: 3452–3462, 2014. doi: 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen MJ, Hwang SJ, Kane GC, Melenovsky V, Olson TP, Fetterly K, Borlaug BA. Enhanced pulmonary vasodilator reserve and abnormal right ventricular: pulmonary artery coupling in heart failure with preserved ejection fraction. Circ Heart Fail 8: 542–550, 2015. doi: 10.1161/CIRCHEARTFAILURE.114.002114. [DOI] [PubMed] [Google Scholar]
- 19.Ghio S, Guazzi M, Scardovi AB, Klersy C, Clemenza F, Carluccio E, Temporelli PL, Rossi A, Faggiano P, Traversi E, Vriz O, Dini FL; all investigators. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur J Heart Fail 19: 873–879, 2017. doi: 10.1002/ejhf.664. [DOI] [PubMed] [Google Scholar]
- 20.Obokata M, Olson TP, Reddy YNV, Melenovsky V, Kane GC, Borlaug BA. Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Heart J 39: 2810–2821, 2018. doi: 10.1093/eurheartj/ehy268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Iterson EH, Johnson BD, Borlaug BA, Olson TP. Physiological dead space and arterial carbon dioxide contributions to exercise ventilatory inefficiency in patients with reduced or preserved ejection fraction heart failure. Eur J Heart Fail 19: 1675–1685, 2017. doi: 10.1002/ejhf.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddy YNV, Stewart GM, Obokata M, Koepp KE, Borlaug BA. Peripheral and pulmonary effects of inorganic-nitrite during exercise in heart failure with preserved ejection fraction. Eur J Heart Fail 23: 814–823, 2021. doi: 10.1002/ejhf.2093. [DOI] [PubMed] [Google Scholar]
- 23.Fermoyle CC, Stewart GM, Borlaug BA, Johnson BD. Simultaneous measurement of lung diffusing capacity and pulmonary hemodynamics reveals exertional alveolar-capillary dysfunction in heart failure with preserved ejection fraction. J Am Heart Assoc 10: e019950, 2021. doi: 10.1161/JAHA.120.019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones NL. Clinical Exercise Testing (4th ed.). Philadelphia, PA: WB Saunders, 1997. [Google Scholar]
- 25.Lifshay A, Fast CW, Glazier JB. Effects of changes in respiratory pattern on physiological dead space. J Appl Physiol 31: 478–483, 1971. doi: 10.1152/jappl.1971.31.3.478. [DOI] [PubMed] [Google Scholar]
- 26.Mohsenifar Z, Tashkin DP, Levy SE, Bjerke RD, Clements PJ, Furst D. Lack of sensitivity of measurements of VD/VT at rest and during exercise in detection of hemodynamically significant pulmonary vascular abnormalities in collagen vascular disease. Am Rev Respir Dis 123: 508–512, 1981. doi: 10.1164/arrd.1981.123.5.508. [DOI] [PubMed] [Google Scholar]
- 27.Babb TG. Ventilatory response to exercise in subjects breathing CO2 or HeO2. J Appl Physiol (1985) 82: 746–754, 1997. doi: 10.1152/jappl.1997.82.3.746. [DOI] [PubMed] [Google Scholar]
- 28.Löfström U, Hage C, Savarese G, Donal E, Daubert JC, Lund LH, Linde C. Prognostic impact of Framingham heart failure criteria in heart failure with preserved ejection fraction. ESC Heart Fail 6: 830–839, 2019. doi: 10.1002/ehf2.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J; ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J 26: 319–338, 2005. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 30.Clark AL, Volterrani M, Swan JW, Coats AJS. Ventilation-perfusion matching in chronic heart failure. Int J Cardiol 48: 259–270, 1995. doi: 10.1016/0167-5273(94)02267-M. [DOI] [PubMed] [Google Scholar]
- 31.Banning AP, Lewis NP, Northridge DB, Elborn JS, Hendersen AH. Perfusion/ventilation mismatch during exercise in chronic heart failure: an investigation of circulatory determinant. Br Heart J 74: 27–33, 1995. doi: 10.1136/hrt.74.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, Dwivedi G, Patel K, Steendijk P, Ashrafian H, Henning A, Frenneaux M. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol 54: 402–409, 2009. doi: 10.1016/j.jacc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 33.Prasad A, Hastings JL, Shibata S, Popovic ZB, Arbab-Zadeh A, Bhella PS, Okazaki K, Fu Q, Berk M, Palmer D, Greenberg NL, Garcia MJ, Thomas JD, Levine BD. Characterization of static and dynamic left ventricular diastolic function in patients with heart failure with a preserved ejection fraction. Circ Heart Fail 3: 617–626, 2010. doi: 10.1161/CIRCHEARTFAILURE.109.867044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagner PD. Ventilation-perfusion matching during exercise. Chest 101: 192S–198S, 1992. doi: 10.1378/chest.101.5_supplement.192s. [DOI] [PubMed] [Google Scholar]
- 35.Wagner PD, Gale GE, Moon RE, Torre-Bueno JR, Stolp BW, Saltzman HA. Pulmonary gas exchange in humans exercising at sea level and simulated altitude. J Appl Physiol (1985) 61: 260–270, 1986. doi: 10.1152/jappl.1986.61.1.260. [DOI] [PubMed] [Google Scholar]
- 36.Lewis GD, Shah RV, Pappagianopolas PP, Systrom DM, Semigran MJ. Determinants of ventilatory efficiency in heart failure: the role of right ventricular performance and pulmonary vascular tone. Circ Heart Fail 1: 227–233, 2008. doi: 10.1161/CIRCHEARTFAILURE.108.785501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hopkins SR, Wagner PD. The Multiple Inert Gas Elimination Technique (MIGET). New York: Springer, 2017. [DOI] [PubMed] [Google Scholar]
- 38.Petersson J, Glenny RW. Gas exchange and ventilation-perfusion relationships in the lung. Eur Respir J 44: 1023–1041, 2014. doi: 10.1183/09031936.00037014. [DOI] [PubMed] [Google Scholar]
- 39.Robertson HT. Dead space: the physiology of wasted ventilation. Eur Respir J 45: 1704–1716, 2015. [Erratum in Eur Respir J 46: 1226, 2015]. doi: 10.1183/09031936.00137614. [DOI] [PubMed] [Google Scholar]
- 40.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E; RELAX Trial. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 309: 1268–1277, 2013. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 370: 1383–1392, 2014. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 42.Bradley PW, Younes M. Relation between respiratory valve dead space and tidal volume. J Appl Physiol Respir Environ Exerc Physiol 49: 528–532, 1980. doi: 10.1152/jappl.1980.49.3.528. [DOI] [PubMed] [Google Scholar]