Keywords: feeding, energy expenditure, PACAP, PAC1R, rat
Abstract
Pituitary adenylate cyclase activating polypeptide (PACAP) exerts pleiotropic effects on ventromedial nuclei (VMN) of the hypothalamus and its control of feeding and energy expenditure through the type I PAC1 receptor (PAC1R). However, the endogenous role of PAC1Rs in the VMN and the downstream signaling responsible for PACAP’s effects on energy balance are unknown. Numerous studies have revealed that PAC1Rs are coupled to both Gαs/adenylyl cyclase/protein kinase A (Gαs/AC/PKA) and Gαq/phospholipase C/protein kinase C (Gαq/PLC/PKC), while also undergoing trafficking following stimulation. To determine the endogenous role of PAC1Rs and downstream signaling that may explain PACAP’s pleiotropic effects, we used RNA interference to knockdown VMN PAC1Rs and pharmacologically inhibited PKA, PKC, and PAC1R trafficking. Knocking down PAC1Rs increased meal sizes, reduced total number of meals, and induced body weight gain. Inhibition of either PKA or PKC alone in awake male Sprague–Dawley rats, attenuated PACAP’s hypophagic and anorectic effects during the dark phase. However, PKA or PKC inhibition potentiated PACAP’s thermogenic effects during the light phase. Analysis of locomotor activity revealed that PKA inhibition augmented PACAP’s locomotor effects, whereas PKC inhibition had no effect. Finally, PACAP administration in the VMN induces surface PAC1R trafficking into the cytosol which was blocked by endocytosis inhibitors. Subsequently, inhibition of PAC1R trafficking into the cytosol attenuated PACAP-induced hypophagia. These results revealed that endogenous PAC1Rs uniquely engage PKA, PKC, and receptor trafficking to mediate PACAP’s pleiotropic effects in VMN control of feeding and metabolism.
NEW & NOTEWORTHY Endogenous PAC1 receptors, integral to VMN management of feeding behavior and body weight regulation, uniquely engage PKA, PKC, and receptor trafficking to mediate the hypothalamic ventromedial nuclei control of feeding and metabolism. PACAP appears to use different signaling mechanisms to regulate feeding behavior from its effects on metabolism.
INTRODUCTION
Obesity is a consequence of homeostatic imbalances between energy intake and energy expenditure that can lead to health complications such as heart disease and diabetes. However, regulation of energy homeostasis involves an intricate neuroendocrine system involving numerous neuropeptides that can either suppress or promote feeding or alter energy expenditure. Various attempts to target neuropeptide systems have shown promise to mitigate obesity, however, none has succeeded, as reviewed in Boughton and Murphy (1). One emerging neuropeptide system that has demonstrated a capacity for regulating appetite and metabolism involves pituitary adenylate cyclase-activating polypeptide (PACAP). PACAP is a pleiotropic neuropeptide that has been implicated in a wide array of central nervous system functions including neural development, modulation of neurotransmitter release, and endocrine responsivity (2–7).
In the hypothalamus, PACAP is abundantly expressed and shown to be a potent regulator of energy homeostasis within several nuclei (8–12). Acute PACAP administration in hypothalamic ventromedial nuclei (VMN) decreases food intake with concomitant increases in core body temperature and spontaneous locomotor activity, which results in rapid reductions in body weight (10–12). Importantly, these effects in the VMN were confirmed to be mediated specifically by PAC1 receptors with no involvement of the VPAC receptors (10, 11). However, the intracellular signaling mechanisms that determine the comprehensive sensitivity of VMN cells to PACAP is poorly understood. Considering its clear and marked involvement in regulating energy homeostasis, it is essential to characterize downstream consequences of PACAP signaling to identify potential etiologies of obesity as well as exploring possible future therapies. Examination of the receptor-activated cascade may help to differentiate the mechanisms by which PACAP exerts a diverse array of actions on specific cellular and physiological processes that may ultimately impact disease states such as obesity.
PACAP is a 38-amino acid peptide from the vasoactive intestinal peptide glucagon secretin superfamily (13–17). PACAP has two receptor subtypes, type I and type II, that belong to the class B family of G protein-coupled receptors (GPCRs) (7, 18–21). Type II receptors include vasoactive intestinal polypeptide receptors VPAC1 and VPAC2, whereas the type I or PAC1 receptor (PAC1R) selectively binds PACAP. In the VMN, PACAP’s hypophagic and metabolic effects are mediated through the PAC1R (11). This raises the question, how does PAC1R signaling regulate disparate aspects of feeding and metabolism in the VMN. A recent analysis of PAC1R COOH-terminus revealed essential amino acid motifs that are necessary for Gs, Gq-protein coupling, and receptor trafficking. In addition, PAC1Rs exist in multiple isoforms that can generate diverse and complex signaling by coupling to multiple G-proteins to modulate channel function and cell responses in different tissues (3, 22–25). The isoforms found in the rat brain include PAC1null, PAC1hop1, PAC1hop2, PAC1hip, PAC1hip-hop, PAC1-short, and PAC1-very short (3, 18, 26). Although G-protein coupling has been the primary focus of PAC1R signaling, recent evidence in cardiac and dentate granule neurons have demonstrated that PAC1R endocytosis facilitates neuronal excitation further adding to the complex PACAP signaling repertoire (27–30). These characteristics of PAC1R signaling may explain PACAP’s pleiotropic effects in the VMN by fine-tuning PAC1R-dependent regulation of feeding and metabolism through G-protein coupling and PAC1R trafficking.
Identifying which specific PAC1R-mediated second messenger signaling is activated or whether PACAP-induced PAC1R endocytosis in the VMN affect feeding and metabolism is still unknown. Earlier studies have demonstrated that PKA, PKC, and receptor endocytosis influence both central and peripheral control of feeding and metabolism (31–35). As such, disruptions to PKA, PKC, and receptor endocytosis mechanisms could be a gateway leading to aberrant regulation of feeding and metabolism that ultimately results in overweight and obesity. Recent findings, for example, demonstrate that hypothalamic cAMP/PKA activity and regulation of scheduled feeding share an inverse relationship; cAMP/PKA activity levels are low during the dark cycle, when rats normally eat and are at peak during the light cycle, when rats eat little (35–37). Moreover, administration of a membrane permeable cAMP agonist (Sp-cAMP) in the perifornical hypothalamus results in a significant increase in VMN PKA activity and suppression of food intake (35). Taken together, hypothalamic circadian regulation of cAMP/PKA activity appears to be correlated with the regulation of feeding. Although PAC1Rs are known to couple with Gαq/PLC/PKC signaling, no study has directly assessed the potential for PKC signaling to influence feeding and metabolism in the VMN. Previous studies have shown that central and hypothalamic PKC is critical for food intake and energy metabolism control. As such, pharmacological or genetic suppression of PKC function leads to elevated food intake, poor glucose and lipid metabolism, and susceptibility to diet-induced obesity (34, 38–40). Although Gαs/cAMP/PKA and Gαq/PLC/PKC activity have been associated with aspects of feeding and metabolic regulation such as glucose and lipid metabolism, the functional consequences of cAMP/PKA and PLC/PKC activity on VMN locomotor activity, core body temperature, and body weight have not been examined.
More recently, we have shown that the activity of VMN neurons is regulated by cell surface PAC1Rs belonging to the Class B superfamily of GPCRs (41). Although G-proteins transduce second messenger signaling following ligand binding to surface GPCR receptors, the location of GPCRs following stimulation can be an important factor for diverse cell signaling. Canonically, GPCRs are endocytosed following stimulation, often considered as a means of signal termination. However, recent findings demonstrate that GPCR endocytosis facilitates non-classical signaling. In pig cardiac neurons, and dentate gyrus granule cells, blocking PAC1R endocytosis blocks PACAP-induced neuronal excitability (30, 42), demonstrating that PAC1R trafficking is a potential signaling mechanism regulating neuronal activation. Although PACAP-induced PAC1R endocytosis function has been examined in these two cell types, the functional consequence of PAC1R endocytosis in VMN regulation of feeding and metabolism has also not been examined.
To address these outstanding questions, the current study begins to delineate which signaling mechanisms are involved in the behavioral and metabolic responses of PACAP activation in the hypothalamic VMN. First, we characterized endogenous PAC1R signaling in the VMN regulation of food intake and body weight. Second, we examined the roles of PKA and PKC activity on PACAP-induced effects on food intake and body weight as well as core body temperature. Furthermore, since there is evidence that PAC1Rs undergo endocytosis following ligand binding, we explored the effects of transiently blocking endocytosis on feeding behavior following PACAP administration. These data suggest that PKA, PKC, and PAC1R endocytosis are critical signaling avenues for PACAP-dependent regulation of feeding, spontaneous locomotor activity, core body temperature, and body weight through the VMN.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Envigo, Indianapolis, IN) weighing 225–300 g were individually housed in either standard cages or BioDAQ feeding cages (Research Diets, Inc., New Brunswick, NJ) in a climate-controlled room under a 12-h light/dark cycle (lights on at 0200). Animals were weighed daily and provided ad libitum access to standard chow (Teklad global 18% protein diet 2018 formulation, Envigo, Indianapolis, IN) and water. On experiment days, animals were weighed and food intake measurements were collected using the BioDAQ Food Intake Monitor before and up to 24-h after the onset of the experiments. For BioDAQ meal pattern analysis, the data were analyzed over the first 12 h. Meals were defined as food intake of 0.2 g or more with less than 15 min elapsing between feeding bouts (10, 11, 43, 44). All procedures using animals were approved by the Marquette University Institutional Animal Care and Use Committee.
Surgery
Animals were anesthetized with 2% isoflurane using a SomnoSuite Low-Flow Anesthesia System (Kent Scientific, Torrington, CT) and placed in a stereotaxic apparatus (Stoelting Co, Wood Dale, IL). Stainless steel 26-gauge bilateral guide-cannulae (Plastics One, Roanoke, VA) were stereotaxically implanted 3 mm dorsal to the hypothalamic ventromedial nuclei (VMN) in all animals and secured to the surface of the skull with an acrylic resin. The stereotaxic coordinates for the VMN injection sites were anterior/posterior, −2.5 mm from bregma; medial/lateral, ±0.6 mm from midline; dorsal/ventral, −9.2 mm from the surface of the skull based on The Rat Brain in Stereotaxic Coordinates, 6th edition (45). Microinjectors extended 3 mm past the ventral tip of the cannulae reaching an injection site of −9.2 mm ventral from the surface of the skull. The upper incisor bar was positioned −3.3 mm below horizontal zero. Animals were given 2 mg/kg ketoprofen once postsurgery and allowed to recover from surgery for 1 wk before they were included in experiments, during which time the animals were handled daily to acclimate them to the necessary physical manipulations during experiments. Correct cannulae placements were confirmed when possible at the conclusion of each experiment by microscopic examination of fluorescent protein markers and Nissl-stained sections. Image capture was conducted on a confocal microscope using ×10 and ×20 magnification (Nikon-confocal; Nyquist sampling) with Nikon NIS Elements software (Nikon, Melville, NY). On average, the included studies produced 85% accurate stereotaxis placement. Only those with correct placement were included in the studies when experimental design allowed.
Microinjections
In all experiments, a microinjection pump was used to inject 0.25 µL/side of vehicle or treatment through bilateral guide-cannulae in awake animals over a 2-min period. After injection delivery was complete, an additional minute elapsed before removing injectors to minimize backflow. Control and treatment groups were reversed after a 2-day washout period and normal food intake levels were confirmed. Microinjections were completed ∼30 min before lights off for experiments assessing nocturnal food intake. For experiments assessing core body temperature and locomotor activity, microinjections were initiated 2 h after the onset of the lights-on and lasted approximately for 1 h.
Western Blot Analysis
Protein analysis using Western blot was conducted once for each animal in the study. However, each study involving signaling inhibitors were repeated twice and studies involving RNAi were repeated and analyzed four times. Bilateral dissections of the ventromedial hypothalamus (VMH; including the VMN and surrounding areas) were collected following rapid decapitation. VMH tissue was homogenized by hand (10 strokes) in ice-cold homogenization buffer (320 mM sucrose, 10 mM Tris·HCl, pH 7.4, 10 mM EDTA, 10 mM EGTA) containing Halt Protease and Phosphatase Inhibitor Cocktail (No. 78447; Thermo Fisher Scientific, Rockford, IL), followed by 3–4 s of sonication. Homogenates were centrifuged at 1,000 g for 2 min at 4°C and the resulting supernatant was further centrifuged at 10,000 g for 30 min at 4°C to remove crude membrane protein. The resulting supernatant rich in crude cytoplasmic protein was saved for further processing. Protein quantification of samples was determined using a bicinchoninic acid (BCA) assay (No. 23252; Pierce). Protein (30 µg) was run on an 8% gel by SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane (No. LC2002; Thermo Fisher Scientific, Rockford, IL). Membranes were blocked for 1 h at room temperature with 5% bovine serum albumin (BSA) or 5% nonfat milk in tris-buffered saline containing 0.1% Tween-20 (TBS-T). Blots were then probed with either rabbit anti-PAC1R antibody alone (No. AV-R003; 1:1,000; Alomone Labs, Jerusalem, Israel) or dually probed with β-actin using a mouse anti-β-actin (No. MA5-15452; 1:2,000; Thermo Fisher Scientific, Rockford, IL) overnight at 4°C, followed by washes with TBS-T and incubation with an horseradish peroxidase (HRP)-linked anti-rabbit secondary antibody (No. 7074P2; 1:3,000; Cell Signaling Technologies, Danvers, MA) and HRP-conjugated anti-mouse (No. 7076P2; 1:5,000; Cell Signaling Technologies, Danvers, MA) at room temperature for 2 h. Band intensities were developed using SuperSignal West Atto Chemiluminescent Substrate (No. A38556; Thermo Fisher Scientific, Rockford, IL) and visualized using the Odyssey Fc Dual-Mode Imaging System (LI-COR Biosciences, Lincoln, NE). Band densities were measured and quantified using Odyssey Fc Dual-Mode Imaging System software. PAC1R antibody specificity was validated by preincubating rabbit anti-PAC1R with PAC1R blocking peptide (No. BLP-VR003; Alomone Labs, Jerusalem, Israel). Following visualization of PAC1R signal alone, blots were stripped and reprobed in an identical fashion for β-actin using a mouse anti-β-actin (No. MA5-15452; 1:2,000; Thermo Fisher Scientific, Rockford, IL) and HRP-conjugated anti-mouse (No. 7076P2; 1:5,000; Cell Signaling Technologies, Danvers, MA).
Experiments
Design and construction of shRNA.
Hairpin RNA was designed to target specific regions of PAC1R mRNA ( CGGAATCCACTACACAGTATT) or a nonsilencing control (NSc; scrambled nucleotides) mRNA. The nucleotide sequences were individually inserted into an adeno-associated virus serotype 8 (AAV8) vector after the H1 promoter, followed by a fluorescent protein, reporter sequence for red fluorescent protein, tdTomato under the cytomegalovirus (CMV) enhancer. The resulting plasmids, AAV8-H1-shPAC1R-CMV-tdTomato and AAV8-H1-shNSC-CMV-tdTomto, were made into an infectious virus with concentrations of 1.05 × 1013 vg/mL and 5.25 × 1012 vg/mL, respectively.
Viral delivery.
Approximately 1 wk following cannulae surgery, rats (n = 8/group) were anesthetized and placed in a stereotaxic apparatus. Then, 0.25 µL of AAV8-H1-shPAC1R-CMV-tdTomato 1.05 × 1013 vg/mL or AAV8-H1-NSC-CMV-tdTomto 5.25 × 1013 vg/mL was microinjected through bilateral guide-cannulae over 10 min. Following each injection, an additional 20 min elapsed before removing injectors to minimize backflow of delivered material. Animals were allowed to recover from anesthesia and returned to their home cage. Food intake was measured using the BioDAQ Food Intake Monitor and body weight were collected manually. Food intake and body weight measures were collected for 21 days following virus injections.
AAV transduction and shRNA-mediated PAC1R knockdown.
Twenty-one days following AAV injection, rats were either euthanized and brains were collected for microscopic examination of fluorescent protein and Western blot analysis or they received microinjections of vehicle or pituitary adenylate cyclase-activating polypeptide (No. 350-35; PACAP; 50 pmol/0.25 µL/side; California Peptide Research; Napa, CA) followed by food intake measurements for 24 h and a final body weight measurement. To assess fluorescent markers, rat brains were sectioned coronally at 14 µm using a cryostat, thaw-mounted onto electrostatically clean sides, and stored at −80°C until postfixed. Before microscopic examination of fluorescent protein markers, brain sections were postfixed in 4% paraformaldehyde, rinsed in 0.1 M PBS (pH 7.4), and cover-slipped with mounting medium (Vector Labs, Burlingame, CA). Image capture was performed using a confocal microscope (Nikon Inc., Tokyo, Japan).
Cell signaling inhibitors.
Microinjections of vehicle, KT5720 (No. 1288/100 U; protein kinase A inhibitor; 10 nM/0.25 µL/side; Bio-Techne Corporation, Minneapolis, MN), GF109203X (No. 0741/1; protein kinase C inhibitor; 0.1 mM/0.25 µL/side; Bio-Techne Corporation, Minneapolis, MN) and endocytosis inhibitors, Pitstop 2 (No. ab120687; clathrin inhibitor; 6 mM/0.25 µL/side; Abcam, Cambridge, MA) and Hydroxy-Dynasore (No. HY-13863; dynamin inhibitor; Dyngo-4a; 0.38 mM/0.25 µL/side; MedChem Express LLC; Monmouth Junction, NJ) were administered 15 min before rats (n = 6/group) received a second bilateral injection of either saline containing 1% DMSO or PACAP (50 pmol/0.25 µL/side). Approximately 5 min after injections, animals were returned to their home cage and food intake was measured for the next 24 h followed by a final measurement of body weight. KT5720, GF109203X, Pitstop 2, and Dyngo-4a were prepared as DMSO stocks, diluted and injected in animals. To avoid potential VMN cell toxicity, low concentrations of Pitstop 2 and Dyngo-4a were combined and administered as an endocytosis inhibitor cocktail (46). The final concentration of DMSO was <1%. PACAP was prepared as a stock in 0.9% saline and diluted just before use.
Thermogenesis and spontaneous locomotor activity.
At the time of cannulation surgery, telemetry probes (Mini Mitter Co. Inc., Sunriver, OR) were implanted in the intraperitoneal cavity of rats to record core body temperature and spontaneous locomotor activity. On the experiment day, 2 h after the onset of the lights-on cycle, rats (n = 6/group) received bilateral injections of KT5720, GF109203X, or the endocytosis inhibitors Pitstop 2 and Dyngo-4a 10–15 min before an injection of either saline or PACAP. Animals were returned to their home cage, and telemetric data for core body temperature and spontaneous locomotor activity were collected remotely as previously described (10, 11). In brief, spontaneous locomotor activity was collected remotely every 5 min and then summed to give cumulative activity every hour. Core body temperature data were averaged by the hour, and spontaneous locomotor activity data were summated to give cumulative activity over a specified amount of time.
Data analysis.
Data are presented as means ± standard error and were analyzed statistically by analysis of variance (with repeated measures when appropriate). Fischer’s least significant difference (LSD), and Tukey’s honestly significant difference (HSD) analyses were used for post hoc group comparisons. Group sizes were designed to detect a 0.05 significance level with a power of 0.8 assuming a standard deviation of 10% of the mean. Statistical analyses were performed using SigmaPlot 11 (Systat Software, Inc., San Jose, CA). P values <0.05 were considered statistically significant.
RESULTS
AAV Transduction and shRNA-Mediated PAC1R Knockdown
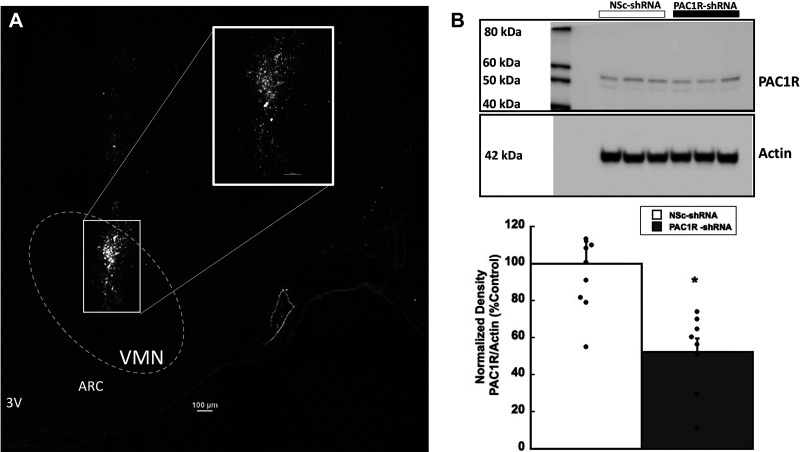
Confocal fluorescent microscopy analysis (×10 and ×20 Nikon-confocal; Nyquist sampling with Nikon NIS Elements software, Nikon, Melville, NY) validated rat brains injected with AAV8-shPAC1R-tdTomato resulted in transduction of a large population of VMN cells (Fig. 1A). tdTomato fluorescent images were converted to binary black and white images. Microinjections of virus were contained within the borders of the VMN and shown to be expressed in a large percentage of VMN cells. Western blot analysis of VMN homogenates (Fig. 1B) confirmed that AAV8-shPAC1R-tdTomato decreased VMN PAC1R expression by ∼50%–60% compared with the scrambled shRNA nonsilencing control (NSc; F1,14 = 11.276, P < 0.01).
Figure 1.
AAV8-mediated transduction of VMN cells with PAC1R shRNA decreases PAC1R protein expression. A: ×10 stitched confocal photomicrograph of VMN cells 21 days after transduction with AAV8 containing control or PAC1R-shRNA (scale bar = 100 µm). Inset is a ×20 magnification of the injection area (scale bar = 50 µm). B: representative Western blots and quantification of PAC1R protein expression in VMN tissue from Sprague-Dawley rats injected with AAV8 nonsilencing control (NSc) or PAC1R shRNA. Data are expressed as means ± SE, *P < 0.05 compared with control (n = 8 rats/group). ARC = arcuate nucleus, 3V = third ventricle; PAC1R, pituitary adenylate cyclase receptor; VMN, ventromedial nuclei.
PAC1R Knockdown: Food Intake and Body Weight
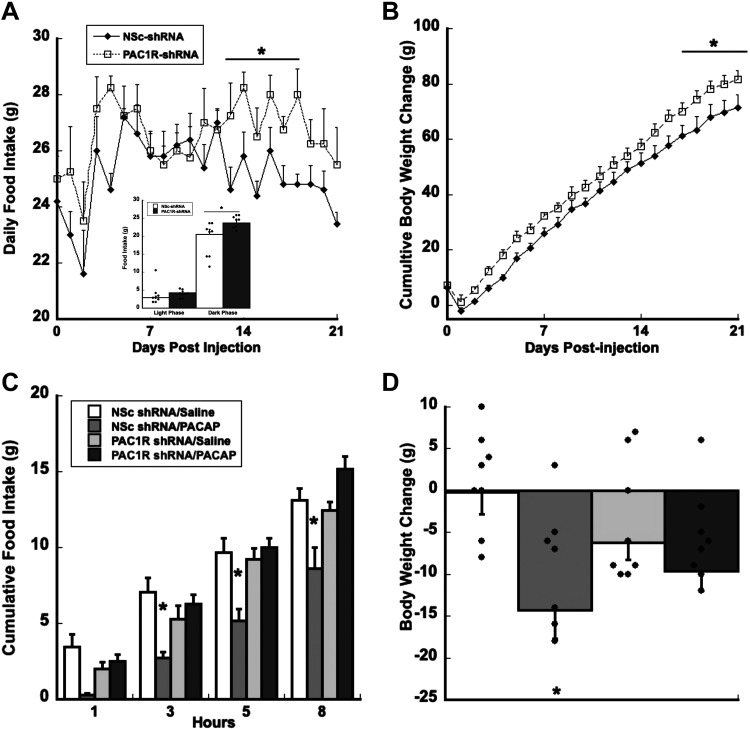
RNA interference of PAC1 receptors by AAV8-shPAC1R-tdTomato injections in the VMN increased both food intake and body weight compared with the scrambled shRNA nonsilencing control (Fig. 2, A and B). Specifically, decreasing PAC1R expression in the VMN reduced the number of meals consumed while increasing overall meal size (Table 1). In addition, significant increased food intake occurred only during the dark phase starting at day 4 and consistently between days 13–21. The inset graph in Fig 2A, illustrates significantly increased dark phase food intake averaged over the 21-day period but not light phase feeding. Consistent and significant differences were detected in feeding by day 13, whereas body weight changes were significant by day 18. Analysis of variance (ANOVA) showed a significant main effect of treatment for food intake F1,25 = 4.916, P < 0.04, and body weight F1,21 = 3.534, P < 0.001. All post hoc pairwise multiple-comparison procedures (Fisher’s LSD method) analysis showed that rats injected with AAV8-shPAC1R-tdTomato significantly increased body weight after day 18 (P < 0.03) compared with nonsilencing control shRNA (Fig. 2B)
Figure 2.
Knocking down PAC1Rs in the VMN increases daily food intake and cumulative body weight change. Knocking down VMN PAC1Rs increased daily food intake by day 12 (A) and increased body weight by day 18 (B). Inset in A shows a significant increase in dark phase food take but not light phase feeding averaged over the 21-day period. Knocking down VMN PAC1Rs is sufficient to block PACAP-induced decreases in food intake (C) and body weight (D). Data are expressed as means ± SE, *P < 0.05 compared with control (n = 8 rats/group). NSc, nonsilencing control; PAC1R, pituitary adenylate cyclase receptor; PACAP, pituitary adenylate cyclase-activating polypeptide; VMN, ventromedial nuclei.
Table 1.
Meal pattern analysis following PAC1R knockdown
| Nsc-shRNA | PAC1R-shRNA | |
|---|---|---|
| Average number of meals | 8.381 ± 0.257 | 7.333 ± 0.268* |
| Average meal size, g | 2.486 ± 0.08 | 2.883 ± 0.08* |
Data are means ± SE. Meal patterns were analyzed for 21 days postinjection. Nsc, non-silencing control shRNA; PAC1R, pituitary adenylate cyclase receptor.
*P < 0.05 vs. Nsc-shRNA.
To assess the functional degree to which PACAP receptors in the VMN were suppressed, exogenous PACAP was administered to assess hypophagia and changes in body weight in both PAC1R-shRNA and nonsilencing shRNA-treated animals. PAC1R-shRNA treatment prevented exogenous PACAP-induced hypophagia and ameliorated the decrease in body weight (Fig. 2, C and D). Thus, a 50%–60% PAC1R knockdown was sufficient to disrupt PAC1R regulation of feeding and body weight in the VMN. Analysis of food intake data show a significant effect of treatment (F3,37 = 6.499, P < 0.001), time (F11,33 = 361.500, P < 0.001), and treatment × time interaction (F33,460 = 2.460, P < 0.001; Fig. 2C), whereas body weight changes showed a significant main effect of treatment F3,37 = 2.964, P < 0.05 when compared with the control group (Fig. 2D).
Cell Signaling Inhibitor: PKA
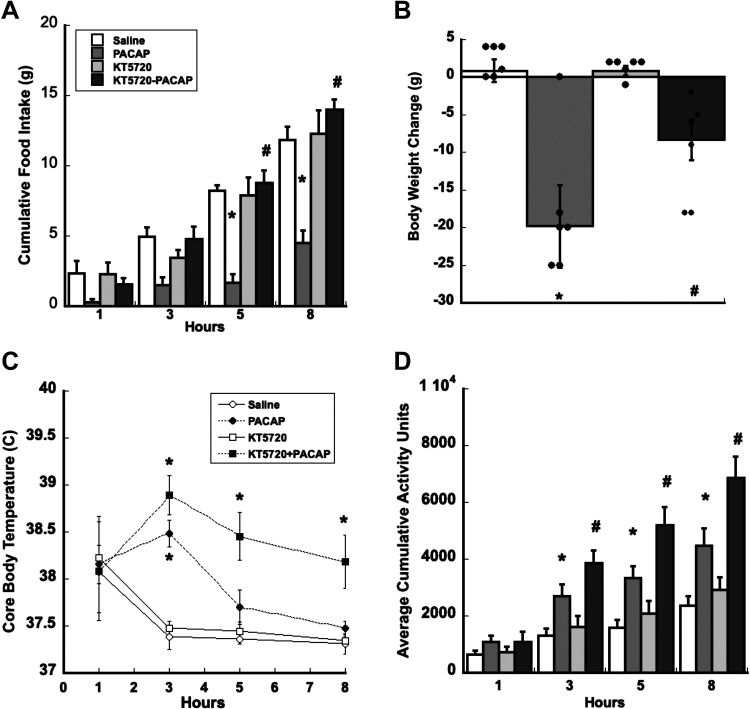
Bilateral VMN injections of KT5720, a PKA-specific inhibitor just before PACAP injections attenuates PACAP-induced decreases in nocturnal food intake and body weight compared with controls. Food intake analyses show a significant interaction of treatment × time (F33,264 = 9.820, P < 0.001) and a significant main effect of treatment (F3,24 = 20.536, P < 0.001) when compared with saline controls (Fig. 3A). Significant inhibition of PACAP-induced hypophagia by KT5720 is observed by 5 h and up to 8 h, P < 001(PACAP vs. KT5720 + PACAP), which was similar to feeding levels observed in animals that received saline or KT5720 alone (Fig. 3A). Analyses of body weight change (Fig. 3B) revealed that pretreatment of KT5720 significantly attenuated but did not completely block PACAP-induced decreases in body weight (saline controls vs. PACAP; F3,18 = 40.996, P < 0.001) and (PACAP vs.KT5720 + PACAP; P = 0.009).
Figure 3.
Blocking PKA signaling with KT5750 blocks PACAP-dependent decreases in food intake and attenuates PACAP-induced decrease in body weight. A: blocking PKA signaling with KT5720 reverses PACAP-induced suppression of food intake from 5 h postinjection lasting up to 8 h. B: KT5720 attenuated PACAP-induced reduction in body weight 24 h following injection. KT5720 potentiates PACAP’s thermogenic (C) and locomotor effects (D). Data are expressed as means ± SE, *P < 0.05 (treatment vs. control), #P < 0.05 (PACAP vs. PACAP + KT5720); n = 6 rats/group. PACAP, pituitary adenylate cyclase-activating polypeptide; PKA, protein kinase A.
Consequences of blocking PKA on PACAP-induced increases in core body temperature and spontaneous locomotor activity were assessed at the onset of the light phase when both are at their nadir. As previously shown (11), PACAP infusions in the VMN increase both core body temperature and locomotor activity. However, blocking PKA activity with KT5720 before PACAP infusions did not suppress PACAP-induced increases in core body temperature or locomotor activity. Interestingly, blocking PKA activity with KT5720 appeared to potentiate PACAP-induced increases in both core body temperature and locomotor activity (Fig. 3, C and D). ANOVA of core body temperature and locomotor activity show a significant treatment × time interaction (F24,120 = 13.527, P < 0.001; F24,127 = 10.152, P < 0.001), respectively, when compared with controls. Post hoc analysis of treatment on core body temperature and locomotor activity following a two-way repeated-measures assessment shows that blocking PKA before PACAP infusions potentiates both PACAP-induced increase in core body temperature and locomotor activity from 5 h up to 8 h, P < 0.01 (PACAP vs. KT5720 + PACAP; Fig. 3, C and D).
Cell Signaling Inhibitor: PKC
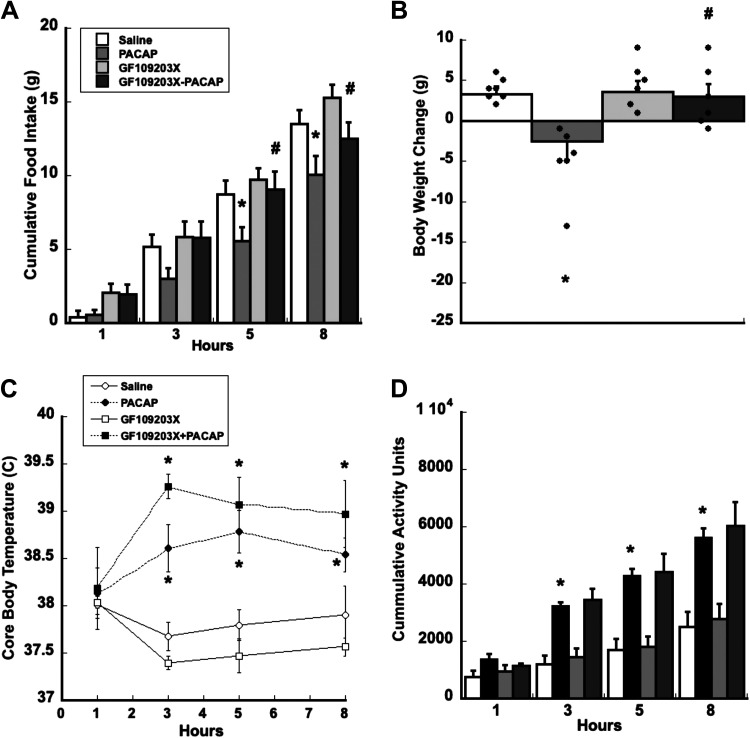
Activation of PKC is a downstream response of PAC1R activation through the Gαq/PLC pathway. Direct injections of GF109203X, a PKC-specific inhibitor in the VMN, preceding PACAP injections significantly reversed PACAP’s anorexigenic effects in rats during the first 8 h of the dark phase when rats normally eat. Food intake data show a significant interaction between treatment and time, F33,231 = 2.242, P < 0.001, whereas body weight data reveal a significant main effect of treatment, F3,21 = 3.821, P < 0.03 (Fig. 4, A and B). Post hoc analyses show that PACAP’s capacity to suppress food intake and decrease body weight is dependent on time. PACAP significantly inhibits food intake from 5 h (P = 0.028) up to 8 h (P = 0.016) and decreases body weight after 24 h (P = 0.019), respectively, when compared with saline controls (Fig. 4, A and B). PACAP’s hypophagic and anorexic effects are reversed by GF109203X at 5 h (P < 0.015), 8 h (P < 0.019), and 24 h (P = 0.023), respectively, when compared with the PACAP alone (PACAP vs. GF109203X + PACAP; Fig. 4, A and B). However, after 5 h PACAP’s hypophagic actions persisted and food intake decreased, although not significantly different from saline controls. This suggests that PACAP-dependent regulation of feeding and body weight in the VMN likely involves induction of PKC-related signaling.
Figure 4.
Blocking PKC with GF109203X transiently reduces PACAP’s hypophagic and anorectic effects. A: blocking PKC with GF109203X attenuates PACAP-induced decrease in food intake for 3 h postinjection. B: GF109203X blocked PACAP-induced decrease in body weight 24 h following injection. GFX109203X potentiates PACAP-dependent increase in core body temperature (C) but not spontaneous locomotor activity (D). Data are expressed as means ± SE, *P < 0.05 (PACAP vs. control), #P < 0.05 (PACAP vs. PACAP + GF109203X); n = 6 rats/group. PACAP, pituitary adenylate cyclase-activating polypeptide; PKC, protein kinase A.
During the light phase, however, when rodent core body temperature and locomotor activity are at their nadir, GF109203X injections in the VMN before PACAP injections were not sufficient to prevent PACAP-induced increases in temperature and locomotor activity. In fact, GF109203X-mediated inhibition of PKC enhanced PACAP-induced increase in core body temperature. Core body temperature showed a significant interaction between treatment and time, F24,96 = 3.405, P < 0.001. Post hoc pairwise comparisons show that PACAP increased both core body temperature and locomotor activity from 3 h lasting up to 8 h post injection, P < 0.03, when compared with saline controls. GF109203X enhanced PACAP’s potentiation of core body temperature at 3 h but not locomotor activity, P < 0.25, when compared with PACAP treatment alone (Fig. 4, C and D).
Endocytosis Inhibition
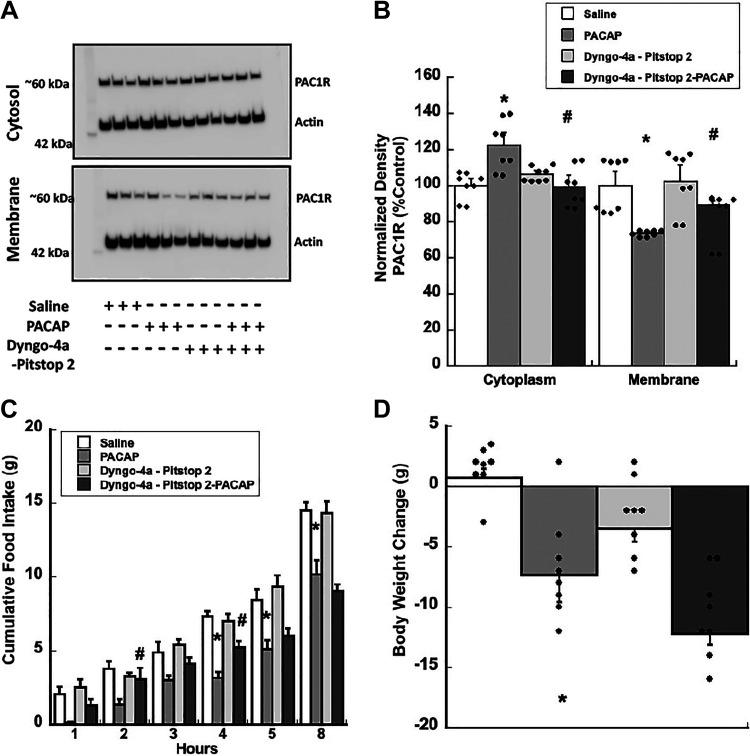
PAC1Rs undergo endocytosis following stimulation by their cognate ligand, PACAP. We measured changes in PAC1R subcellular expression from rat VMN tissue injected with PACAP. Western blot analysis of VMN tissue homogenates confirmed that PACAP promotes PAC1R trafficking from the membrane to the cytosol. Crude subcellular fractionation analyses show that PACAP significantly increases cytosolic PAC1Rs and decreases membrane PAC1Rs, F3,12 = 4.084, P = 0.03, F3,12 = 4.619, P < 0.03, respectively (Fig. 5, A and B). This PACAP-induced shift in PAC1R subcellular localization was blocked using a cocktail of dynamin and clathrin inhibitors, Dyngo-4a and Pitstop, 2 P = 0.025 (membrane) and P = 0.023 (cytosolic), respectively, when compared with PACAP treatment alone (Fig. 5, A and B).
Figure 5.
Inhibiting surface PAC1R trafficking attenuates PACAP-induced decrease in food intake and exaggerates PACAP-induced decrease in body weight. A and B: Western blot analysis showing PACAP promotes PAC1R trafficking from the membrane to the cytosol, which can be blocked by Dyngo-4a/Pitstop 2 and measured at 30 mins following PACAP injection. C: Dyngo-4a/Pitstop 2, dynamin and clathrin-dependent endocytosis inhibitors, briefly attenuate PACAP-induced suppression of food intake for 5 h postinjection. D: Dyngo-4a/Pitstop 2 does not prevent PACAP-induced reduction in body weight at 24 h. Data are expressed as means ± SE, *P < 0.05 (PACAP vs. control), #P < 0.05 (PACAP vs. PACAP+Dyngo-4a/Pitstop 2); n = 8 rats/group. PACAP, pituitary adenylate cyclase-activating polypeptide; PAC1R, pituitary adenylate cyclase receptor.
We further assessed whether PACAP-induced PAC1R endocytosis was required for PACAP’s anorexigenic effects in the VMN. In rats, combined Dyngo-4a and Pitstop 2 infusions in the VMN before PACAP administration briefly blocked PACAP-induced decreases in food intake but did not block body weight change (Fig. 5, C and D). Analyses of food intake data show that blocking endocytosis transiently reversed PACAP-induced hypophagia at 2 h and 4 h, P < 0.03 and P = 0.023, respectively, when compared with PACAP treatment alone (Fig. 5C). There is a significant interaction between treatment and time, F12,80 = 2.320, P < 0.02. Comparably, blocking endocytosis before PACAP injection did not prevent PACAP-induced decreases in body weight; it appears that PACAP continued to suppress body weight at 24 h following injections. There is a significant main effect of treatment, F3,20 = 7.267, P < 0.003 (Fig. 5D).
DISCUSSION
In these experiments, we demonstrate that PACAP’s type I receptor (PAC1R) signaling in the VMN is endogenously relevant for feeding and body weight control. shRNA-mediated PAC1R knockdown in the VMN, resulting in a 50%–60% reduction in PAC1R protein expression, functionally increased daily food consumption and body weight gain. Specifically, diminishing PAC1R expression decreased the overall number of meals while increasing the size of their meals (Table 1). Moreover, the ability for exogenous PACAP administration to potently induce hypophagia and body weight loss was blocked following VMN PAC1R knockdown, indicating that this magnitude of PAC1R knockdown was sufficient to disrupt endogenous VMN PACAP signaling. Thus, there is a clear role for endogenous PACAP signaling in the VMN for the control of feeding behavior and body weight regulation. The changes in food consumption and body weight gain following VMN-specific PAC1R knockdown are similar to that observed in postnatal steroidogenic factor-1 (SF1) knockout mice (47). Interestingly, VMN SF1-positive neurons coexpress both PACAP and PAC1Rs (41, 47, 48). Although VMN SF1 knockout animals also exhibit dysregulated temperature regulation and locomotion, we did not evaluate the consequences of shRNA-induced PAC1R knockdown on these measures. However, we would predict that animals lacking VMN PAC1Rs would exhibit decreased spontaneous locomotion and core body temperature since the PAC1R antagonist in the VMN reverses PACAP’s thermogenic and locomotor effects in addition to preventing hypophagia (11, 41). Furthermore, there are salient similarities between our findings and those in mice with genetic mutations in PAC1R. These PAC1R mutations result in phenotypes that are replicated by local genetic or pharmacological PAC1R inhibition such as increased feeding, suggesting that the hypothalamic VMN could be a primary control site (49).
With the clarity of the PAC1R site-specific knockdown demonstrating an endogenous role for PACAP in feeding and metabolism, it becomes critical to begin to understand the subsequent signaling cascades following PAC1R activation. We and others have previously reported that PACAP in the VMN reduces nocturnal food consumption and subsequent decreases in body weight (8–12). In addition, VMN PACAP increases core body temperature and spontaneous locomotor activity during the light phase (10, 50). It is now well established that PAC1Rs are dually coupled GPCRs that stimulate both Gs/cAMP/PKA and Gq/PLC/PKC-related signaling that can influence various physiological systems (3, 25, 51, 52). This might explain PACAP’s pleiotropic effects in the VMN as well as in the hypothalamus since PKA and PKC activity are implicated in neuronal regulation of feeding and metabolism (34, 35, 38, 53, 54).
In the hypothalamus, cAMP/PKA is a second messenger target of several neuropeptides and hormones such as neuropeptide Y (NPY) and has been shown to influence leptin signaling, both of which have been implicated in the neuronal regulation of feeding and metabolism (35, 54). Earlier reports find that cAMP and PKA activity increase in the hypothalamus including the VMN when animals are fasting and asleep whereas, cAMP and PKA levels diminish during nocturnal feeding when rats are active (35–37). In VMN cells, genetic ablation of cAMP-response element binding protein (CREB)-binding protein, a downstream target of PKA-binding protein causes hyperphagia, and thermogenic dysregulation with concomitant decreases in brain-derived neurotrophic factor (BDNF) and proopiomelanocortin (POMC) mRNA (54). Not surprising, both BDNF and POMC mRNA levels increase following PACAP administration (9, 10, 41). Taken together, these findings position PKA as a key signaling cascade in the VMN regulation of energy balance. In the current study, we found that directly inhibiting PKA exerted opposing effects on PACAP signaling. Administering a PKA inhibitor just before PACAP completely blocks PACAP-induced hypophagia, yet facilitates PACAP-induced thermogenesis and locomotor activity, suggesting that the involvement of PKA in PACAP signaling is behavior specific. This combination of both positive and negative influences of PKA on energy regulation is reflected in the partial reversal of PACAP-induced body weight loss. The selectivity of PKA involvement suggests that other signaling mechanisms may be primary for the metabolic features of PACAP signaling that contribute to its overall effects on body weight. This raises important future questions of how downstream PAC1R signaling differentially regulates indices of homeostasis. In the arcuate, for example, impaired PKA signaling exaggerates leptin’s catabolic and molecular effects (54). In the absence of functional PKA, low-leptin doses potently decrease feeding with concomitant increases in energy expenditure, suggesting there exists a PKA-regulating signaling system that may dictate the magnitude of leptin’s influence on metabolism (54). Considering that accumulating evidence in the VMN is establishing a relationship between PAC1 and leptin receptor signaling, it is possible that VMN PKA may be involved in the gating of leptin signaling although this remains to be tested (9, 41).
It is also well recognized that PAC1Rs couple to Gq/PLC/PKC second messenger signaling, however, the direct consequences of this signaling on VMN regulation of food intake and metabolism remains to be thoroughly investigated. Previous studies have shown that VMN PKC is involved in regulating other physiological and behavioral outcomes in rodents including sexual behaviors and glucose metabolism (34, 39, 55, 56). In addition to PKA, it cannot be overlooked that PAC1R signaling may also use PKC activity to regulate energy balance. One recent study showed that isoforms of PKC are highly expressed in hypothalamic nuclei including the VMN, where they have been shown to influence feeding and metabolism in response to leptin and insulin (39). Interestingly, a recent study demonstrated that POMC-neuron-specific deletion of PKCλ, a PKC isoform disrupts leptin signaling and renders high-fat diet-fed mice susceptible to obesity (38). VMN neurons also regulate glucose and lipid metabolism and are significant targets of leptin signaling. In addition, emerging evidence suggest a dependent relationship between PAC1 and leptin receptor signaling, positioning PKC as a possible link. We found that PKC is required for PACAP signaling in the VMN since PKC inhibition abrogated PACAP-induced hypophagia whereas, it augmented PACAP’s thermogenic effects and had no effect on PACAP-induced locomotor activity. The failure of PKA inhibition to only attenuate PACAP-induced body weight loss may suggest that signaling mechanisms independent of PKA inhibition continue to engage metabolic systems and reduce body weight following PACAP administration. Taken together, however, these findings suggest a putative role for VMN PACAP receptors to engage both PKA- and PKC-related signaling to regulate different aspects of energy homeostasis.
In addition to PKA and PKC signaling mechanisms, we explored whether endocytosis of PAC1R was essential to PACAP’s behavioral and physiological effects. Canonically, cell surface GPCRs are endocytosed following stimulation, in part, as a desensitization mechanism. However, earlier reports show that ligand-activated PAC1R endocytosis is important for neuronal action potential firing for PAC1R in hippocampal and cardiac neurons (30, 57, 58). Considering that our laboratory recently reported that PACAP increases action potential firing frequency in ex vivo VMN slices, it is possible that blocking VMN PAC1R endocytosis would alter action potential generation and subsequently food intake or metabolic indices such as thermogenesis and/or locomotor activity. In the VMN, PACAP promotes PAC1R endocytosis from the membrane to the cytosol, which was subsequently blocked by the inhibitors of endocytosis, clathrin, and dynamin. Inhibition of PAC1R trafficking temporarily attenuated PACAP-induced hypophagia but did not prevent its anorexic effects after 5 h. The short-lived effects of endocytosis inhibitors are in line with data demonstrating that endogenous endocytosis can be restored within ∼1 h following washout (59). This experiment demonstrates that PACAP’s hypophagic effects in the VMN appear to be mediated, in part, by PAC1R endocytosis. Conversely, temporal blockade of PAC1R trafficking and hypophagia produced a more pronounced body weight loss, suggesting that transient inhibition of trafficking is not sufficient to attenuate PACAP’s decrease in body weight. The fact that we observe partial potentiation of PACAP’s effects on body weight may indicate a delayed effect of PAC1R endocytosis on other metabolic factors that may contribute to body weight change. Future studies will need to assess whether blocking PAC1R trafficking delays the onset of thermogenesis and increase in locomotor activity as well as the need to determine the consequences of blocking PAC1R endocytosis on PACAP-induced increase in firing frequency.
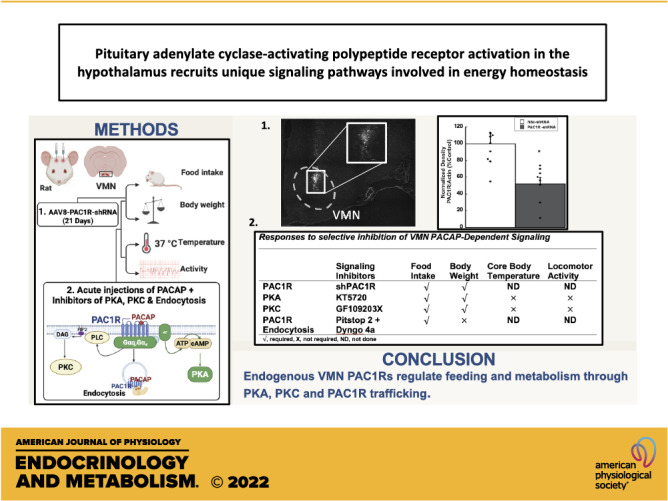
Collectively, these studies suggest that PACAP signaling in the VMN is clearly a regulating factor of both feeding behavior and metabolic outputs such as thermogenesis and locomotor activity. The approximate 16% increase in feeding and 20% increase in body weight following PAC1R knockdown demonstrate that PACAP signaling in the VMN still has a wide influence over energy balance. Moreover, these studies reveal that PACAP’s effects are elicited by its actions at the membrane and that PAC1R uses both PKA- and PKC-dependent signaling to differentially regulate feeding behavior and metabolism. The findings and conclusions from these studies are summarized in Table 2.
Table 2.
Responses to selective inhibition of VMN PACAP-dependent signaling
| Signaling Inhibitors | Food Intake | Body Weight | Core Body Temperature | Locomotor Activity | |
|---|---|---|---|---|---|
| PAC1R | PACAP 6-38 | √ | √ | √ | √ |
| PAC1R | shPAC1R | √ | √ | ND | ND |
| PKA | KT5720 | √ | √ | × | × |
| PKC | GF109203X | √ | √ | × | × |
| PAC1R trafficking | Pitstop 2 + Dyngo 4a | √ | × | ND | ND |
√, required; ×, not required; ND, not done; PACAP, pituitary adenylate cyclase-activating polypeptide; PAC1R, pituitary adenylate cyclase receptor; PKA, protein kinase A; PKC, protein kinase C; VMN, ventromedial nuclei.
GRANTS
This work was supported by National Institute on Drug Abuse Grant No. NIH DA050180 and National Institute of Diabetes and Digestive and Kidney Diseases Grant No. NIH R15DK113608.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.M. and S.C. conceived and designed research; B.M., K.W.B., N.N.D., C.C., and F.C. performed experiments; B.M., K.W.B., N.N.D., F.C., D.B., and S.C. analyzed data; B.M. and S.C. interpreted results of experiments; B.M. and S.C. prepared figures; B.M. and S.C. drafted manuscript; B.M. and S.C. edited and revised manuscript; B.M. and S.C. approved final version of manuscript.
REFERENCES
- 1.Boughton CK, Murphy KG. Can neuropeptides treat obesity? A review of neuropeptides and their potential role in the treatment of obesity. Br J Pharmacol 170: 1333–1348, 2013. doi: 10.1111/bph.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahrén B. Role of pituitary adenylate cyclase-activating polypeptide in the pancreatic endocrine system. Ann N Y Acad Sci 1144: 28–35, 2008. doi: 10.1196/annals.1418.003. [DOI] [PubMed] [Google Scholar]
- 3.Blechman J, Levkowitz G. Alternative splicing of the pituitary adenylate cyclase-activating polypeptide receptor PAC1: mechanisms of fine tuning of brain activity. Front Endocrinol (Lausanne) 4: 55, 2013. doi: 10.3389/fendo.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denes V, Geck P, Mester A, Gabriel R. Pituitary adenylate cyclase-activating polypeptide: 30 years in research spotlight and 600 million years in service. J Clin Med 8: 1488, 2019. doi: 10.3390/jcm8091488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong L, Albano R, Madayag A, Raddatz N, Mantsch JR, Choi S, Lobner D, Baker DA. Pituitary adenylate cyclase-activating polypeptide orchestrates neuronal regulation of the astrocytic glutamate-releasing mechanism system xc (.). J Neurochem 137: 384–393, 2016. doi: 10.1111/jnc.13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao C, de Molliens MP, Schneebeli ST, Brewer M, Song G, Chatenet D, Braas KM, May V, Li J. Targeting the PAC1 receptor for neurological and metabolic disorders. Curr Top Med Chem 19: 1399–1417, 2019. doi: 10.2174/1568026619666190709092647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BKC, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 61: 283–357, 2009. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- 8.Chang R, Hernandez J, Gastelum C, Guadagno K, Perez L, Wagner EJ. Pituitary Adenylate cyclase-activating polypeptide excites proopiomelanocortin neurons: implications for the regulation of energy homeostasis. Neuroendocrinology 111: 45–69, 2021. doi: 10.1159/000506367. [DOI] [PubMed] [Google Scholar]
- 9.Hawke Z, Ivanov TR, Bechtold DA, Dhillon H, Lowell BB, Luckman SM. Behavioral/systems/cognitive PACAP neurons in the hypothalamic ventromedial nucleus are targets of central leptin signaling. J Neurosci 29: 14828–14835, 2009. doi: 10.1523/JNEUROSCI.1526-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Resch JM, Boisvert JP, Hourigan AE, Mueller CR, Yi SS, Choi S. Stimulation of the hypothalamic ventromedial nuclei by pituitary adenylate cyclase-activating polypeptide induces hypophagia and thermogenesis. Am J Physiol Regul Integr Comp Physiol 301: R1625–R1634, 2011. doi: 10.1152/ajpregu.00334.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Resch JM, Maunze B, Gerhardt AK, Magnuson SK, Phillips KA, Choi S. Intrahypothalamic pituitary adenylate cyclase-activating polypeptide regulates energy balance via site-specific actions on feeding and metabolism. Am J Physiol Endocrinol Metab 305: E1452–E1463, 2013. doi: 10.1152/ajpendo.00293.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Resch JM, Maunze B, Phillips KA, Choi S. Inhibition of food intake by PACAP in the hypothalamic ventromedial nuclei is mediated by NMDA receptors. Physiol Behav 133: 230–235, 2014. doi: 10.1016/J.PHYSBEH.2014.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickson L, Finlayson K. VPAC and PAC receptors: from ligands to function. Pharmacol Ther 121: 294–316, 2009. doi: 10.1016/j.pharmthera.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Gottschall PE, Tatsuno I, Miyata A, Arimura A. Characterization and distribution of binding sites for the hypothalamic peptide, pituitary adenylate cyclase-activating polypeptide. Endocrinology 127: 272–277, 1990. doi: 10.1210/endo-127-1-272. [DOI] [PubMed] [Google Scholar]
- 15.Lam H-C, Takahashi K, Ghatei MA, Kanse SM, Polak JM, Bloom SR. Binding sites of a novel neuropeptide pituitary-adenylate-cyclase-activating polypeptide in the rat brain and lung. Eur J Biochem 193: 725–729, 1990. doi: 10.1111/j.1432-1033.1990.tb19392.x. [DOI] [PubMed] [Google Scholar]
- 16.Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun 164: 567–574, 1989. doi: 10.1016/0006-291X(89)91757-9. [DOI] [PubMed] [Google Scholar]
- 17.Miyata A, Jiang L, Dahl RD, Kitada C, Kubo K, Fujino M, Minamino N, Arimura A. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochem Biophys Res Commun 170: 643–648, 1990. doi: 10.1016/0006-291X(90)92140-U. [DOI] [PubMed] [Google Scholar]
- 18.Apostolakis EM, Riherd DN, O'Malley BW. PAC1 receptors mediate pituitary adenylate cyclase-activating polypeptide- and progesterone-facilitated receptivity in female rats. Mol Endocrinol 19: 2798–2811, 2005. doi: 10.1210/me.2004-0387. [DOI] [PubMed] [Google Scholar]
- 19.Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol 48: 301–331, 1998. doi: 10.2170/jjphysiol.48.301. [DOI] [PubMed] [Google Scholar]
- 20.Jozwiak-Bebenista M, Dejda A, Nowak JZ. Effects of PACAP, VIP and Related Peptides on Cyclic AMP Formation in Rat Neuronal and Astrocyte Cultures and Cerebral Cortical Slices (Online). http://www.if-pan.krakow.pl/pjp/pdf/2007/4_414.pdf [2018 Dec 21]. [PubMed]
- 21.Warfvinge K, Edvinsson L. Cellular distribution of PACAP-38 and PACAP receptors in the rat brain: relation to migraine activated regions. Cephalalgia 40: 527–542, 2020. doi: 10.1177/0333102419893962. [DOI] [PubMed] [Google Scholar]
- 22.Fukuchi M, Tabuchi A, Kuwana Y, Watanabe S, Inoue M, Takasaki I, Izumi H, Tanaka A, Inoue R, Mori H, Komatsu H, Takemori H, Okuno H, Bito H, Tsuda M. Neuromodulatory effect of Gαs- or Gαq-coupled G-protein-coupled receptor on NMDA receptor selectively activates the NMDA receptor/Ca2+/calcineurin/cAMP response element-binding protein-regulated transcriptional coactivator 1 pathway to effectively induce brain-derived neurotrophic factor expression in neurons. J Neurosci 35: 5606–5624, 2015. doi: 10.1523/JNEUROSCI.3650-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holighaus Y, Mustafa T, Eiden LE. PAC1hop, null and hip receptors mediate differential signaling through cyclic AMP and calcium leading to splice variant-specific gene induction in neural cells. Peptides 32: 1647–1655, 2011. doi: 10.1016/J.PEPTIDES.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyu R-M, Germano PM, Choi K, Le SV, Pisegna JR. Identification of an essential amino acid motif within the C terminus of the pituitary adenylate cyclase-activating polypeptide type I receptor that is critical for signal transduction but not for receptor internalization. J Biol Chem 275: 36134–36142, 2000. doi: 10.1074/jbc.M004612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou C-J, Yada T, Kohno D, Kikuyama S, Suzuki R, Mizushima H, Shioda S. PACAP activates PKA, PKC and Ca2+ signaling cascades in rat neuroepithelial cells. Peptides 22: 1111–1117, 2001. doi: 10.1016/S0196-9781(01)00437-5. [DOI] [PubMed] [Google Scholar]
- 26.Amir-Zilberstein L, Blechman J, Sztainberg Y, Norton WHJ, Reuveny A, Borodovsky N, Tahor M, Bonkowsky JL, Bally-Cuif L, Chen A, Levkowitz G. Homeodomain protein otp and activity-dependent splicing modulate neuronal adaptation to stress. Neuron 73: 279–291, 2012. doi: 10.1016/J.NEURON.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson GC, Parsons RL, May V, Hammack SE. Pituitary adenylate cyclase-activating polypeptide-induced PAC1 receptor internalization and recruitment of MEK/ERK signaling enhance excitability of dentate gyrus granule cells. Am J Physiol Cell Physiol 318: C870–C878, 2020. doi: 10.1152/ajpcell.00065.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.May V, Lutz E, MacKenzie C, Schutz KC, Dozark K, Braas KM. Pituitary adenylate cyclase-activating polypeptide (PACAP)/PAC1HOP1 receptor activation coordinates multiple neurotrophic signaling pathways: Akt activation through phosphatidylinositol 3-kinase gamma and vesicle endocytosis for neuronal survival. J Biol Chem 285: 9749–9761, 2010. doi: 10.1074/jbc.M109.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.May V, Parsons RL. G protein-coupled receptor endosomal signaling and regulation of neuronal excitability and stress responses: signaling options and lessons from the PAC1 receptor. J Cell Physiol 232: 698–706, 2017. doi: 10.1002/jcp.25615. [DOI] [PubMed] [Google Scholar]
- 30.Parsons RL, May V. PACAP-induced PAC1 receptor internalization and recruitment of endosomal signaling regulate cardiac neuron excitability. J Mol Neurosci 68: 340–347, 2019. doi: 10.1007/s12031-018-1127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Conde K, Zhang P, Lilascharoen V, Xu Z, Lim BK, Seeley RJ, Zhu JJ, Scott MM, Pang ZP. Enhanced AMPA receptor trafficking mediates the anorexigenic effect of endogenous glucagon-like peptide-1 in the paraventricular hypothalamus. Neuron 96: 897–909.e5, 2017. doi: 10.1016/J.NEURON.2017.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.London E, Bloyd M, Stratakis CA. PKA functions in metabolism and resistance to obesity: lessons from mouse and human studies. J Endocrinol 246: R51–R64, 2020. doi: 10.1530/JOE-20-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nijhawan P, Behl T, Arora S. Role of protein kinase C in obesity. Obes Med 18: 100207, 2020. doi: 10.1016/J.OBMED.2020.100207. [DOI] [Google Scholar]
- 34.Ross R, Wang PYT, Chari M, Lam CKL, Caspi L, Ono H, Muse ED, Li X, Gutierrez-Juarez R, Light PE, Schwartz GJ, Rossetti L, Lam TKT. Hypothalamic protein kinase C regulates glucose production. Diabetes 57: 2061–2065, 2008. doi: 10.2337/db08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheriff S, Chance WT, Iqbal S, Rizvi TA, Xiao C, Kasckow JW, Balasubramaniam A. Hypothalamic administration of cAMP agonist/PKA activator inhibits both schedule feeding and NPY-induced feeding in rats. Peptides 24: 245–254, 2003. doi: 10.1016/S0196-9781(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 36.Murakami N, Takahashi K. Circadian rhythm of adenosine-3′,5′-monophosphate content in suprachiasmatic nucleus (SCN) and ventromedial hypothalamus (VMH) in the rat. Brain Res 276: 297–304, 1983. doi: 10.1016/0006-8993(83)90737-0. [DOI] [PubMed] [Google Scholar]
- 37.Valases C, Wright SJ, Catravas GN. Diurnal changes in cyclic nucleotide levels in the hypothalamus of the rat. Exp Brain Res 40: 261–264, 1980. doi: 10.1007/BF00237790. [DOI] [PubMed] [Google Scholar]
- 38.Dorfman MD, Krull JE, Scarlett JM, Guyenet SJ, Sajan MP, Damian V, Nguyen HT, Leitges M, Morton GJ, Farese RV, Schwartz MW, Thaler JP. Deletion of protein kinase C λ in POMC neurons predisposes to diet-induced obesity. Diabetes 66: 920–934, 2017. doi: 10.2337/db16-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haley JA. Atypical protein kinase C activity in the hypothalamus is required for lipopolysaccharide-mediated sickness responses. Endocrinology 150: 5362–5372, 2009. doi: 10.1210/en.2009-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolczynska K, Loza-Valdes A, Hawro I, Sumara G. Diacylglycerol-evoked activation of PKC and PKD isoforms in regulation of glucose and lipid metabolism: a review. Lipids Health Dis 19: 113, 2020. doi: 10.1186/s12944-020-01286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hurley MM, Anderson EM, Chen C, Maunze B, Hess EM, Block ME, Patel N, Cooper Z, McCoy R, Dabra T, Conley W, Reilly MJ, Hearing M, Choi S. Acute blockade of PACAP-dependent activity in the ventromedial nucleus of the hypothalamus disrupts leptin-induced behavioral and molecular changes in rats. Neuroendocrinology 110: 271–281, 2020. doi: 10.1159/000501337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.May V, Johnson GC, Hammack SE, Braas KM, Parsons RL, May V. PAC1 receptor internalization and endosomal MEK/ERK activation is essential for PACAP-mediated neuronal excitability. J Mol Neurosci 71: 1536–1542, 2021. doi: 10.1007/s12031-021-01821-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunn-Meynell AA, Sanders NM, Compton D, Becker TC, Eiki JI, Zhang BB, Levin BE. Relationship among brain and blood glucose levels and spontaneous and glucoprivic feeding. J Neurosci 29: 7015–7022, 2009. doi: 10.1523/JNEUROSCI.0334-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farley C, Cook JA, Spar BD, Austin TM, Kowalski TJ. Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obes Res 11: 845–851, 2003. doi: 10.1038/oby.2003.116. [DOI] [PubMed] [Google Scholar]
- 45.Paxinos G, Watson C. The Rat Brain In Stereotaxic Coordinates. San Diego, CA: Elsevier, 2007. [Google Scholar]
- 46.Kuriyan J, Pinilla-Macua I, Grassart A, Duvvuri U, Watkins SC, Sorkin A. EGF receptor signaling, phosphorylation, ubiquitylation and endocytosis in tumors in vivo. eLife 6: e31993, 2017. doi: 10.7554/eLife.31993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim KW, Zhao L, Donato J, Kohno D, Xu Y, Elias CF, Lee C, Parker KL, Elmquist JK. Steroidogenic factor 1 directs programs regulating diet-induced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proc Natl Acad Sci USA 108: 10673–10678, 2011. doi: 10.1073/pnas.1102364108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gonçalves GHM, Tristão SM, Volpi RE, Almeida-Pereira G, de Carvalho Borges B, Donato J, de Castro M, Antunes-Rodrigues J, Elias LLK. STAT3 but Not ERK2 Is a Crucial Mediator Against Diet-Induced Obesity via VMH Neurons. Diabetes 70: 1498–1507, 2021. doi: 10.2337/db20-0658. [DOI] [PubMed] [Google Scholar]
- 49.Hashimoto H, Shintani N, Nishino A, Okabe M, Ikawa M, Matsuyama S, Itoh K, Yamamoto K, Tomimoto S, Fujita T, Hagihara N, Mori W, Koyama Y, Matsuda T, Nagata S, Baba A. Mice with markedly reduced PACAP (PAC 1) receptor expression by targeted deletion of the signal peptide. J Neurochem 75: 1810–1817, 2000. doi: 10.1046/j.1471-4159.2000.0751810.x. [DOI] [PubMed] [Google Scholar]
- 50.Adams BA, Gray SL, Isaac ER, Bianco AC, Vidal-Puig AJ, Sherwood NM. Feeding and metabolism in mice lacking pituitary adenylate cyclase-activating polypeptide. Endocrinology 149: 1571–1580, 2008. doi: 10.1210/en.2007-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hardwick JC, Clason TA, Tompkins JD, Girard BM, Baran CN, Merriam LA, May V, Parsons RL. Recruitment of endosomal signaling mediates the forskolin modulation of guinea pig cardiac neuron excitability. Am J Physiol Cell Physiol 313: C219–C227, 2017. doi: 10.1152/ajpcell.00094.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macdonald DS, Weerapura M, Beazely MA, Martin L, Czerwinski W, Roder JC, Orser BA, MacDonald JF. Modulation of NMDA Receptors by Pituitary Adenylate Cyclase Activating Peptide in CA1 Neurons Requires Gαq, Protein Kinase C, and Activation of Src. J Neurosci 25: 11374–11384, 2005. [Erratum in J Neurosci 25: table of contents, 2005]. doi: 10.1523/jneurosci.3871-05.2005.[16339032] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.London E, Nesterova M, Stratakis CA. Acute vs chronic exposure to high fat diet leads to distinct regulation of PKA. J Mol Endocrinol 59: 1–12, 2017. doi: 10.1530/JME-16-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang L, McKnight GS. Hypothalamic PKA regulates leptin sensitivity and adiposity. Nat Commun 6: 8237, 2015. doi: 10.1038/ncomms9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dewing P, Christensen A, Bondar G, Micevych P. Protein kinase C signaling in the hypothalamic arcuate nucleus regulates sexual receptivity in female rats. Endocrinology 149: 5934–5942, 2008. doi: 10.1210/en.2008-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Irani BG, Donato J, Olson DP, Lowell BB, Sacktor TC, Reyland ME, Tolson KP, Zinn AR, Ueta Y, Sakata I, Zigman JM, Elias CF, Clegg DJ, Clegg DJ. Distribution and neurochemical characterization of protein kinase C-theta and -delta in the rodent hypothalamus. Neuroscience 170: 1065–1079, 2010. doi: 10.1016/j.neuroscience.2010.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liao C, Remington JM, May V, Li J. Molecular basis of class B GPCR selectivity for the neuropeptides PACAP and VIP. Front Mol Biosci 8: 131, 2021. doi: 10.3389/fmolb.2021.644644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tompkins JD, Clason TA, Buttolph TR, Girard BM, Linden AK, Hardwick JC, Merriam LA, May V, Parsons RL. Src family kinase inhibitors blunt PACAP-induced PAC1 receptor endocytosis, phosphorylation of ERK, and the increase in cardiac neuron excitability. Am J Physiol Cell Physiol 314: C233–C241, 2018. doi: 10.1152/ajpcell.00223.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCluskey A, Daniel JA, Hadzic G, Chau N, Clayton EL, Mariana A, Whiting A, Gorgani NN, Lloyd J, Quan A, Moshkanbaryans L, Krishnan S, Perera S, Chircop M, von Kleist L, McGeachie AB, Howes MT, Parton RG, Campbell M, Sakoff JA, Wang X, Sun J-Y, Robertson MJ, Deane FM, Nguyen TH, Meunier FA, Cousin MA, Robinson PJ. Building a better dynasore: the dyngo compounds potently inhibit dynamin and endocytosis. Traffic 14: 1272–1289, 2013. doi: 10.1111/tra.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]