Abstract
Drug repurposing or repositioning has been well-known to refer to the therapeutic applications of a drug for another indication other than it was originally approved for. Repurposing non-oncology small-molecule drugs has been increasingly becoming an attractive approach to improve cancer therapy, with potentially lower overall costs and shorter timelines. Several non-oncology drugs approved by FDA have been recently reported to treat different types of human cancers, with the aid of some new emerging technologies, such as omics sequencing and artificial intelligence to overcome the bottleneck of drug repurposing. Therefore, in this review, we focus on summarizing the therapeutic potential of non-oncology drugs, including cardiovascular drugs, microbiological drugs, small-molecule antibiotics, anti-viral drugs, anti-inflammatory drugs, anti-neurodegenerative drugs, antipsychotic drugs, antidepressants, and other drugs in human cancers. We also discuss their novel potential targets and relevant signaling pathways of these old non-oncology drugs in cancer therapies. Taken together, these inspiring findings will shed new light on repurposing more non-oncology small-molecule drugs with their intricate molecular mechanisms for future cancer drug discovery.
Key words: Drug repurposing, Non-oncology drug, Cancer therapy, Cardiovascular drug, Microbiological drug, Small-molecule antibiotics, Anti-viral drug, Anti-inflammatory drug
Graphical abstract
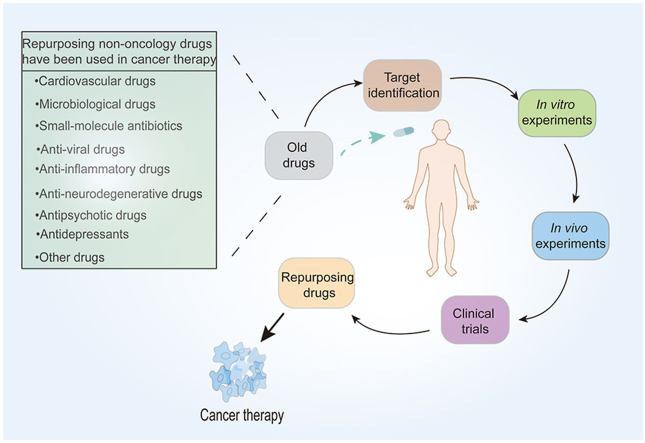
Repurposing non-oncology drugs have been used to improve cancer therapy with the processes of target identification, in vitro and in vivo experiments, and clinical trials from the current situation to future directions.
1. Introduction
Cancer is a major factor affecting global health and is now the second leading cause of death worldwide1. According to the global cancer statistics, there were an estimated 19.3 million new emerging cancer cases and almost 10.0 million cancer deaths occurred in 20202. With such high cancer-associated morbidity and mortality, more attention should be paid to the discovery of innovative treatments and effective drugs. Traditionally, discovery of de novo antitumor drugs involves extensive pre-clinical and clinical studies to determine the safety and efficacy of the drug. Thus, the median cost of cancer drug development was $648.0 million (range $157.3 million to $1950.8 million) and the average time was 10–17 years3. Despite such investment, less than 1% of compounds are expected to enter clinical trials, let alone eventually come to market4. Thus, there is an urgent need to promote the drug development process for cancer treatment.
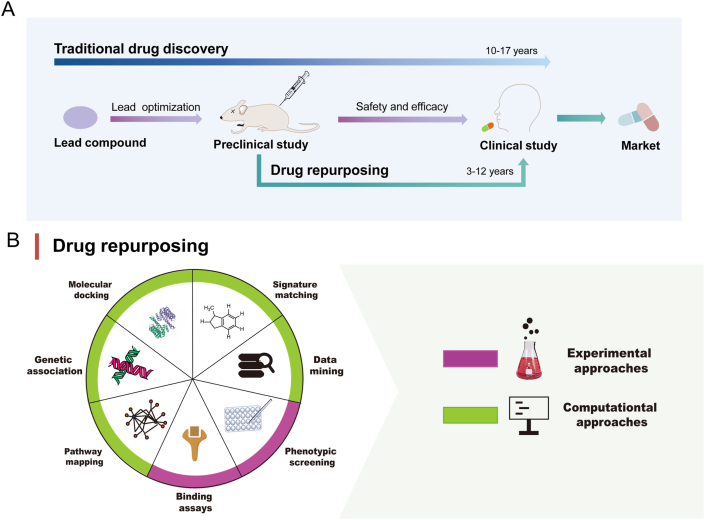
Drug repurposing (also called drug repositioning, reprofiling or redirecting), which applies an ‘old’ drug or drugs with structural modification to a new indication, has emerged as an alternative strategy for the development of antitumor drugs. Compared with de novo drug creation, there is already a great of well-defined data in terms of safety, toxicity, and pharmacological properties of repurposed drugs5. Thus, they can quickly pass preclinical and phase I clinical trials and transition rapidly to phase II and III clinical studies, and have higher probability of passing the clinical trial stage. Therefore, the time lag of research and development cycle can be reduced by 3–5 years and the costs by $0.3 billion5,6. Considering these advantages, drug repurposing could be an innovative strategy to identify effective agents for cancer treatment (Fig. 1A).
Figure 1.
(A) The estimated time and main steps in traditional drug discovery and drug repurposing for cancer therapy. Traditional drug discovery for cancer therapy takes 10–17 years and drug repurposing takes only 3–12 years as it bypasses discovery of lead compound. (B) Main approaches of drug repurposing. There are mainly computational approaches including molecular docking, genetic association, pathway mapping, data mining, and signature matching as well as experimental approaches including phenotypic screening and binding assays for drug repurposing.
There are several successful cases of non-oncology drug repurposing for cancer therapy. For example, arsenic trioxide, a highly toxic substance that can be used for external skin erosion to remove decay, was first used to treat leukemia in 19727. Now arsenic trioxide injection combined with tretinoin has been approved by the FDA as a first-line treatment for acute promyelocytic leukemia (APL)8. Thalidomide, a classic case of repurposing a drug, was used for treating morning sickness during pregnancy but eventually withdrawn from the market because of its teratogenicity. Now, it is repurposed for refractory multiple myeloma excluding in pregnant women9. Recently, metformin, a classic anti-diabetic II drug, is being intensively studied for its effects on many different cancers including prostate10, pancreatic11, breast, ovarian and endometrial cancer12. These drugs represent good demonstrations of non-oncology drug repurposing for cancer therapy and show that drug repurposing might be a way to develop new antitumor drugs.
2. New emerging technologies for drug repurposing
Given the increase of drug repurposing, several new technologies, especially bioinformatics and computer technology, have emerged and greatly improved the process of drug repurposing research. As we all know, the first step of drug repurposing strategy is to find effective targets of disease, and select compounds from the Drug Repurposing Hub (an interactive drug repurposing database which contains a comprehensive library of drugs that have reached the clinic, www.broadinstitute.org/repurposing) by certain methods13. Usually, the cancer genome database and other databases can be used to retrieve and screen the gene expression profile of the disease and proceed the enriched and the pathway analysis for differentially expressed genes to find their regulatory targets. Then, the optimal candidate compounds were calculated by Connectivity Map (CMap) ligatogram from numerous drug databases, such as PubChem, PharmGKB and DrugBank. Then, a pathway analysis and molecular docking can be conducted to evaluate the molecular mechanism of the candidate drug. Finally, the experiments in vitro and in vivo models are needed to validate their effects on new disease. With all these processes are finished, the drugs can carry out clinical trials to validate their pharmaceutical effects in human bodies14. At present, approaches to drug repurposing are mainly either computational approaches or experimental approaches, both of which are increasingly being used simultaneously in most situations to improve the efficiency of repurposing drug research. Computational approaches like signature matching, molecular docking, genetic association, pathway mapping and data mining are helping to find new targets of the drug by exploit known targets, drugs, disease biomarkers or pathways. They involve systematic analysis of big data (such as gene expression, chemical structure, genotype, or proteomic data) to match new indications of old drugs. For example, signature matching is based on the comparison of characteristics of one drug with those of another drug, disease, or clinical phenotype for its transcriptomic, structural, or adverse effect profiles to identify the same mechanism to treat other disease. It contains drug–disease and drug–drug similarity approaches for the same chemical structures expressing similar biological activity. Molecular docking can predict binding site complementarity between the ligand and the target, and this is an inexpensive, simple, and efficient way to find target drugs by high throughput screening. It may be interrogated against a particular target by drug libraries or drug libraries can be explored against an array of target receptors. For instance, mebendazole, an anti-parasitic drug, has potential to inhibit vascular endothelial growth factor receptor 2 (VEGFR2) by using high-throughput computational docking through molecular fit computations on plenty of FDA-approved drugs and protein structures. Another one is genetic association approaches to identify genes that are associated with a disease, and find potential drug targets of the disease. For instance, genome-wide association studies (GWAS) devote to identify genetic variants associated with common diseases and help identify novel targets for repurposing drug. However, these genes may not be ideal druggable targets. Therefore, pathway mapping could use upstream or downstream genetic, protein or disease data for identifying repurposing targets, and thus greatly improve the identification of drug targets. Recently, a technique termed network-wide association study (NetWAS) can identify disease gene associations much more accurately than GWAS and find more effective targets of anti-hypertensive drugs. To validate the effect of the old drug on cancer, a series of experiments need to be carried out to confirm their therapeutic effects in cancer. Therefore, experimental approaches, such as binding assays and phenotypic screening are indispensable, and can help to identify relevant target interactions in drug repurposing and to identify lead compounds from large compound libraries15. Binding assays can identify target interactions by interaction of ligand and receptor. For example, the cellular thermostability assay (CETSA) technique has been introduced as a way of mapping target engagement in cells using biophysical principles that predict thermal stabilization of target proteins. Phenotypic screening is to identify compounds that show disease-relevant effects in model systems without prior knowledge of the target affected. Typically, in vitro phenotypic screening can be carried out by a wide range of cell-based assays in a 96-well format. In the context of drug repurposing, it will screen numerous drugs in several cell lines. For instance, disulfiram, a drug used for alcohol abuse, is identified as a selective antineoplastic agent by high-throughput cell-based screening. With the assistance of these approaches, drug repurposing processes will improve greatly (Fig. 1B). Currently, there are many non-oncology drugs approved by FDA or in clinical trials including cardiovascular drug, antibiotics, antivirals, anti-inflammatory drugs, antipsychotics, and other non-oncology drugs which could be promising candidates for drug repurposing for cancer16. In this review, we focus on summarizing several repurposing non-oncology small-molecule drugs, as well as highlight their potential targets and molecular mechanisms in cancer therapy.
3. Molecular mechanisms repurposing non-oncology drugs in cancer therapy
There are several resources useful for screening non-oncology drug repurposing for cancer therapy. First, drugs that have been withdrawn due to cell toxicity when used for the given indication, but potentially exerting anticancer effects at lower doses. Second, drugs which are already on the market, especially the classic old drugs mentioned above that are used for drug repurposing. Because these drugs have been used in patients for many years, numerous new techniques could be used to find relationships between their original indications and cancer, and finally to identify antitumor effects of these drugs. In addition, compounds which have failed to pass the clinical trial phase could be restudied for their antitumor effects (Fig. 2). All these compounds might acquire or enhance their antitumor effects by structural modification, after which they can go to market for a new indication. All the above approaches provide abundant resources for discovering new anticancer drugs. Repurposing old drugs for antitumor therapy is an effective and economical way to develop new anticancer drugs. According to their original indications, we summarized these repurposing non-oncology small-molecule drugs for cancer therapy (Table 117, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114).
Figure 2.
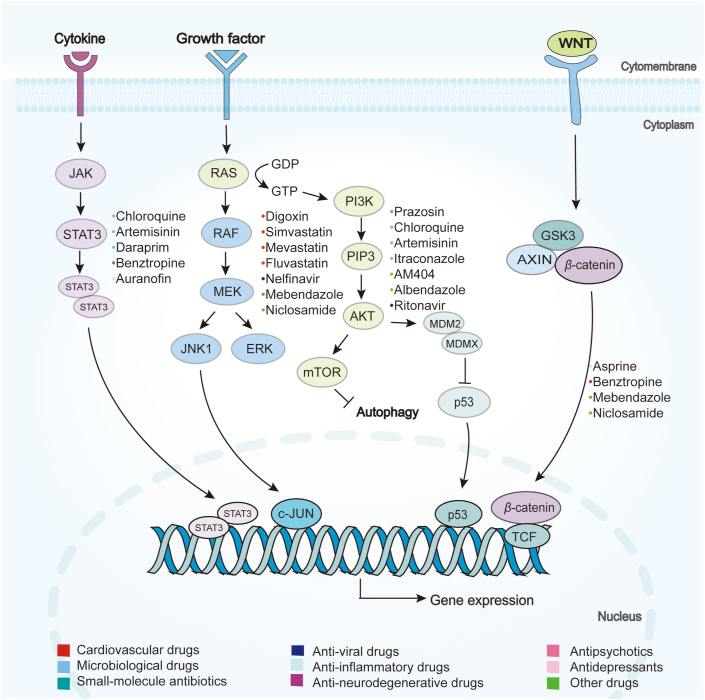
The relevant signaling pathways of repurposing drugs in cancer therapy. The first one is JAK/STAT3 pathway for chloroquine, artemisinin, daraprim, benztropine and auranofin; RAS/RAF/ERK pathway for Digoxin, simvastatin, mevastatin, fluvastatin, nelfinavir, mebendazole, niclosamide; PI3K/AKT/mTOR pathway for prazosin, chloroquine, artemisinin, itraconazole, AM404, albendazole, ritonavir; and WNT/β-catenin pathways for asprine, benztropine, mebendazole, niclosamide.
Table 1.
Repurposing non-oncology small-molecule drugs for cancer therapy.
| Original indication | Name | Cancer type | Mechanism | Ref. |
|---|---|---|---|---|
| Cardiovascular drugs | Losartan | Glioblastoma/Ovarian cancer | Decompressing vessels by reducing solid stress/reducing cell proliferation and growth/apoptosis | 17,18 |
| Irbesartan | Prostate cancer | Targeting the proinflammatory cytokines, oxidative stress, apoptosis and TGF-β1/STAT-3 signaling | 19 | |
| Valsartan | Gastric cancer/Hepatic cancer | Targeting PI3K/AKT signaling | 19 | |
| Telmisartan | Lung cancer/Gastric cancer | WNT/β-catenin pathway | 20 | |
| Olmesartan | Breast cancer | Blocking RAS and NF-κB pathway leading to cytotoxicity and apoptosis | 20 | |
| Captopril | Colorectal cancer/Glioblastoma | Inhibiting angiogenesis and tumor invasion | 21 | |
| Enalapril | Colorectal cancer/Pancreatic cancer | Suppressing proliferation, angiogenesis, and NF-κB/STAT3-regulated proteins | 22,23 | |
| Verapamil | Colorectal cancer/Breast cancer/Liver cancer | P-gp inhibitor | 24 | |
| Benazepril | Esophageal carcinoma/Gastrointestinal cancers | Inhibiting the cell growth | 25 | |
| Carvedilol | Glioblastoma/Pancreatic cancer/Breast cancer | Inducing mitochondrial damage/inhibiting cell proliferation and migration | 26,27 | |
| Propranolol | Hemangioma/Melanoma/Prostate cancer | Inhibiting angiogenesis and migration of cancer cells/interfering SOX18 transcriptional activity | 28,29 | |
| Atenolol | Gastric cancer | Inhibiting apoptosis, proliferation, migration and inflammatory cell responses for cancer therapy | 30 | |
| Digoxin | Pulmonary tumor/Prostate cancer/Breast cancer/Glioblastoma/Leukemia | Inhibiting Na+/K+ ATPase/apoptosis/suppressing HIF-1α accumulation/reducing ERK pathway/inducing expression of P21Cip1 | 31, 32, 33, 34 | |
| Pitavastatin | Glioblastoma | Inhibiting mevalonate pathway/suppressing tumor cell MDR-1 protein and cell proliferation | 35,36 | |
| Simvastatin | Glioblastoma | Inhibiting cell proliferation and migration/apoptosis via increasing caspase-3 activity/downregulating the PI3K/AKT pathway | 36 | |
| Mevastatin | Glioblastoma | Inhibiting cell proliferation/apoptosis via suppressing RAS/ERK and PI3K/AKT pathways | 37 | |
| Fluvastatin | Breast cancer/Renal cancer | mTOR pathway/P21 | 38 | |
| Atorvastatin | Glioblastoma | Inhibiting proliferation, migration and invasion/downregulating MMP via P38 MAPK pathway/apoptosis via caspase-8 and caspase-3 pathway | 39 | |
| Lovastatin | Glioblastoma | Inhibiting cell proliferation and MAPK pathway to induce apoptosis/increasing G0/G1 cell arrest | 40 | |
| Cerivastatin | Glioblastoma | Downregulating FAK phosphorylation to inhibit cell adhesion and invasion | 39 | |
| Ticlopidine | Glioblastoma | Autophagy | 41 | |
| Prazosin | Glioblastoma | Apoptosis via activating caspase-3 | 42 | |
| Fenofibrate | Colon cancer | PPAR-α | 43 | |
| Verapamil | Glioblastoma | Inducing apoptosis by increasing the BAX/BCL-2 ratio | 42 | |
| Sildenafil | Glioblastoma | Increasing BBTB permeability via cGMP pathway | 43 | |
| Microbiological agents | Chloroquine | Glioblastoma/Pancreatic cancer | Autophagy/inhibiting MMP-2 activity, cell proliferation and invasion | 44 |
| Hydroxychloroquine | Cancer | Autophagy | 44 | |
| Mefloquine | Glioblastoma | Inhibiting cell proliferation via inducing cell cycle arrest in G2/M phase/autophagy | 44 | |
| Artemisinin | Breast cancer | Caspase-dependent apoptosis/STAT3 | 45,46 | |
| Dihydroartemisinin | Cancer | Inducing oxidative stress response by reactive oxygen species and nitric oxide, DNA damage | 45,46 | |
| Artesunate | Cancer | Inhibiting angiogenesis and tumor-related signal transduction pathways | 45,46 | |
| Artemether | Cancer | Apoptosis, autophagy and ferroptosis | 45,46 | |
| Arteether | Cancer | Targeting NF-κB, MYC/MAX, AP-1, CREBP | 45,46 | |
| Daraprim | Triple-negative breast cancer | STAT3 inhibitor | 47 | |
| Quinacrine | Glioblastoma | Apoptosis and autophagy | 48 | |
| Amodiaquine | Melanoma | Apoptosis and autophagy | 49 | |
| Artefenomel | Cancer | Regulating the PD-L1 to PD-1 axis for establishing CD4+ T cell immunity | 50 | |
| Itraconazole | Prostate cancer/Glioblastoma/Non-small cell lung cancer/Liver cancer/Gastric cancer | Inhibiting hedgehog pathway, AKT–mTOR pathway and angiogenesis | 51,52 | |
| Posaconazole | Acute myeloid leukemia/Glioblastoma cancer | Targeting HK2-expressing | 53 | |
| Ketoconazole | Glioblastoma/Colorectal cancer | Reducing glycolytic metabolism | 54 | |
| Tioconazole | Breast cancer | Inhibiting ATG4 to suppress autophagy | 55 | |
| Enilconazole | Colorectal cancer | Inhibiting WNT/β-catenin and PI3K/AKT pathways | 56 | |
| Ciprofloxacin | Colon cancer | Inhibiting the ABCB1 efflux function to reverse MDR | 57,58 | |
| Antibiotics | Bedaquiline | Triple-negative breast cancer | Leading mitochondrial dysfunction and ATP depletion | 59, 60, 61 |
| Doxycycline | Breast cancer | Inhibiting the cancer stem cell phenotype and epithelial-to-mesenchymal transition | 62 | |
| Tigecycline | Ovarian cancer/Myeloid leukemia | Targeting MYC, HIFs, PI3K/AKT or AMPK-mediated mTOR, cytoplasmic P21CIP1/Waf1, and WNT/β-catenin signaling | 62 | |
| Clofoctol | Prostate cancer | Inhibiting cancer cell growth through activating ER stress and three branches of the UPR pathway | 63 | |
| Anti-viral drugs | Ritonavir | Ovarian cancer/Melanoma | Blocking AKT/mTOR pathway/expressing G1 phase | 64 |
| Nelfinavir | Melanoma/Rectal cancer | Suppressing PAX3 and MITF expression | 65,66 | |
| Maraviroc | Triple-negative breast cancer | Blocking lymphangiogenesis and cancer metastasis | 67,68 | |
| L-870810 | Cancer | Cytotoxicity in cancer cell and inhibiting select oncogenic kinases | 69 | |
| Anti-inflammatory drugs | Aspirin | Colon cancer/Breast cancer | Inhibiting COX enzymes/downregulating WNT/β-catenin pathway | 70,71 |
| Auranofin | Glioblastoma | Inducing cell ferroptosis | 72 | |
| Celecoxib | Glioblastoma/Lung cancer | P53-dependent G1 cell cycle arrest and autophagy/downregulating NF-κB, caspase-9, BAX and BCL-XL | 73,74 | |
| Ibuprofen | Prostate cancer/Melanoma/Lung cancer/Adenocarcinoma/Gastric cancer | Inhibiting COX enzymes/reducing angiogenesis and cell proliferation/apoptosis | 75 | |
| Anti-neurodegenerative drugs | Benztropine | Breast cancer/Pancreatic cancer | Acting on SLC6A3/DAT and STAT3 pathways | 76 |
| Benserazide | Colon cancer/Melanoma | Inhibiting HK2 pathway | 77 | |
| Donepezil | Glioblastoma | Increasing cell mitosis duration and inducing cell mitotic arrest | 78 | |
| Memantine | Glioblastoma | Inhibiting proliferation/autophagy | 79 | |
| Riluzole | Hepatocellular carcinoma | Inducing caspase-dependent apoptosis and G2/M cell cycle arrest | 80 | |
| Antipsychotic drugs | Chlorpromazine | Glioblastoma/Colorectal cancer | Inhibiting AKT/mTOR pathway | 81 |
| Fluphenazine | Triple-negative breast cancer | Inducing G0/G1 cell cycle arrest/apoptosis | 82 | |
| Penfluridol | Glioblastoma/Triple-negative breast cancer | Inhibiting transcription factor GLI1 and integrin pathway | 83 | |
| Olanzapine | Glioblastoma | Inhibiting proliferation, migration and anchorage-independent growth/inducing apoptosis, necrosis and cytostasis | 84 | |
| Risperidone | Adenocarcinoma | Decreasing proliferation | 85 | |
| Clomipramine | Glioblastoma | Activating JNK/JUN pathway/increasing cytochrome c release and apoptosis activated by caspase-3 | 86 | |
| KRICT-9 | Cancer | Inhibiting STAT3 | 47 | |
| Antidepressants | Imipramine | Glioblastoma/Small cell lung cancer | Inducing high level of autophagy and cell death | 87 |
| Trimipramine | Prostate cancer | Increasing plasma levels of CYP2D6 substrates | 88 | |
| Amitriptyline | Prostate cancer | Increasing plasma levels of CYP2D6 substrates | 88 | |
| All-trans retinoic acid (ATRA) | Colorectal cancer/Acute myeloid leukemia | Enhancing the effects of EVI1 | 89 | |
| Tranylcypromine | Breast cancer | LSD1/HDACs dual inhibitor | 90 | |
| Fluoxetine | Hepatocellular cancer/Lung cancer | Blocking AKT/NF-κB or ERK/NF-κB activation and inducing apoptosis | 91 | |
| Citalopram | Burkitt lymphoma | Inhibiting proliferation/inducing apoptosis in cancer cell/downregulating AKT pathway | 92 | |
| Paroxetine | Breast cancer | Inducing apoptosis through Ca2+ and P38 MAP kinase-dependent ROS generation | 93 | |
| Sertraline | Liver cancer/Breast cancer/Prostate cancer | Dual activation of apoptosis and autophagy signaling by deregulating redox balance | 94 | |
| Proscillaridin A | Glioblastoma | Activating GSK3β/alternating microtubule dynamics | 95 | |
| Valproic acid | Prostate cancer/Gastric cancer | Inhibiting histone deacetylase to reduce cancer cell proliferation/apoptosis/inhibiting angiogenesis | 96 | |
| Other drugs | Metformin | Glioblastoma/Non-small cell lung cancer/Rectal cancer/Prostate cancer/Ovarian cancer | Autophagy/apoptosis/inducing G1 cell arrest | 97 |
| Pioglitazone | Lung cancer/Glioblastoma | PPARγ/inhibiting WNT/β-catenin pathway/apoptosis | 98,99 | |
| Repaglinide | Glioblastoma | Inhibiting proliferation and migration of tumor cell/reducing BCL-2, Beclin1 and PD-L1 expression/blocking immune checkpoint signaling | 95 | |
| Rosiglitazone | Glioblastoma | Inhibiting cell proliferation by causing G2/M arrest and apoptosis | 100 | |
| Buformin | Breast cancer | Inhibiting receptor tyrosine kinase (RTK) and mTOR pathway/reducing cell proliferation | 97 | |
| Ciglitazone | Glioblastoma | Lossing mitochondrial membrane potential along with increasing ROS | 98 | |
| Phenformin | Breast cancer/Rectal cancer | Targeting signal transducer and activator of transcription 3 and transforming growth factor-β/Smad signaling | 101 | |
| Mebendazole | Glioblastoma | Inhibiting proliferation and angiogenesis/BRAF–MEK–ERK pathway/upregulating expression of pro-inflammatory genes | 102 | |
| Albendazole | Lung cancer | Inhibiting HIF-1α-dependent glycolysis and VEGF | 103 | |
| Flubendazole | Breast cancer | Inhibiting STAT3 and activating autophagy/targeting P53 and promoting ferroptosis | 104 | |
| Niclosamide | Hepatocellular carcinoma/Adrenocortical carcinoma/Leukemia/Lung cancer/Osteosarcoma | Reversing cancer gene expression/inhibiting tumor cell proliferation | 105, 106, 107 | |
| Disulfiram | Glioblastoma | Activating JNK and P38 pathways/inhibiting NF-κB and MGMT/inducing ROS/DNA damage | 108 | |
| AM404 | Colorectal cancer/Glioblastoma | Induce cell differentiation and impede neoplastic cell growth/suppressing the oncogenic FBXL5 | 109 | |
| Salbutamol | Breast cancer | Inhibiting migration, invasion and metastasis | 110 | |
| Raloxifene | Breast cancer | Inhibiting IL-6/GP130 interaction | 111 | |
| Bazedoxifene | Breast cancer | Inhibiting cell viability, cell migration, colony formation, and tumor growth and inducing apoptosis | 112 | |
| Thiabendazole | Prostate cancer/Breast cancer | Cytotoxicity | 113 | |
| Azelnidipine | Cancer | Blocking CD47/SIRPα and TIGIT/PVR pathways through co-targeting SIRPα and PVR | 114 |
3.1. Cardiovascular drugs repurposing for cancer therapy
Several experimental studies have demonstrated that drugs approved by FDA, which are commonly prescribed for the treatment or prevention of cardiovascular diseases, have promising anticancer properties115. Some analyses of retrospective clinical data suggest that the use of cardiovascular drugs to treat hypertension in cancer patients is likely to extend survival of patients with pancreatic and other cancers. Some of these drugs have advanced to randomized clinical trials (RCTs) to confirm their antitumor effects (Table 2, sourced by http://clinicaltrials.gov). For example, losartan (Fig. 3, 1) is a cardiovascular agent affecting the renin-angiotensin system (RAS), which is also a potential target for modulating tumor-infiltrating immune cells. In tumors, it could act on RAS to decompress vessels by reducing solid stress to improve vessel density and decrease vessel diameter and eventually normalize the tumor microenvironment17. Subsequently, it was found that losartan depletes the matrix via inducing antifibrotic miRNAs including miR-133 and miR-29, which can reduce collagen I levels in cancer cells to modulate solid stress17,18. Additionally, losartan reduced cell proliferation and tumor growth as well as inducing apoptosis. In a phase II clinical trial of losartan, patients with previously untreated unresectable pancreatic tumor showed downstaging of locally advanced pancreatic ductal adenocarcinoma and a higher success rate on subsequent surgical resection116. This suggests that losartan may be worth studying as a drug active for pancreatic cancer. Similarly, irbesartan (Fig. 3, 2), valsartan (Fig. 3, 3), telmisartan (Fig. 3, 4) and olmesartan (Fig. 3, 5) also mediate anticancer effects via a mechanism like that of lorsartan19,20. Captopril (Fig. 3, 6) also exerts effect on RAS and shows efficacy for inhibiting colorectal liver metastases by significantly decreasing tumor viability and impairing metastatic growth. It modulates the spatial and temporal infiltration of CD3+ CD4+ lymphocytes into the tumor, and the activation of T cell subtypes in the tumor and liver tissues21. Enalapril (Fig. 3, 7), verapamil (Fig. 3, 8) and benazepril (Fig. 3, 9) are also reported to show effects on cancer22, 23, 24, 25.
Table 2.
Repurposing non-oncology drugs under clinical trials.
| Drug name | Cancer type | Clinical trial identifier |
|---|---|---|
| Losartan | Pancreatic cancer | NCT03563248 (phase 2) |
| Pancreatic cancer | NCT01821729 (phase 2) | |
| Pancreatic cancer | NCT04106856 (phase 1) | |
| Osteosarcoma | NCT03900793 (phase 1) | |
| Glioblastoma | NCT03951142 (phase 2) | |
| Captopril | Lung cancer | NCT00077064 (phase 2) |
| Infantile hemangioma | NCT04288700 (phase 4) | |
| Verapamil | Brain cancer | NCT00706810 (phase 2) |
| Hodgkin lymphoma | NCT03013933 (phase 1) | |
| Carvedilol | Breast cancer | NCT02993198 (phase 2) |
| Glioblastoma | NCT03980249 (early phase 1) | |
| Glioblastoma | NCT03861598 (early phase 1) | |
| Propranolol | Gastric cancer | NCT04005365 (phase 2) |
| Breast cancer | NCT02596867 (phase 2) | |
| Bladder cancer | NCT04493489 (phase 2) | |
| Pancreatic neoplasms | NCT03838029 (phase 2) | |
| Breast cancer | NCT02596867 (phase 2) | |
| Breast cancer | NCT02013492 (early phase 1) | |
| Prostate carcinoma | NCT03152786 (phase 2) | |
| Skin melanoma | NCT01988831 (phase 2) | |
| Esophageal adenocarcinoma | NCT04682158 (phase 2) | |
| Infantile hemangioma | NCT01056341 (phase 2/3) | |
| Cavernous malformations | NCT03523650 (phase 1) | |
| Atenolol | Hemangioma | NCT02342275 (phase 3) |
| Infantile hemangioma | NCT03237637 (phase 3) | |
| Digoxin | Head and neck cancer | NCT02906800 (phase 1/2) |
| Prostate cancer | NCT01162135 (phase 2) | |
| Malignant melanoma | NCT01765569 (phase 1) | |
| Melanoma | NCT02138292 (phase 1) | |
| Pitavastatin | Breast cancer | NCT04705909 (phase 2/3) |
| Acute myeloid leukemia | NCT04512105 (phase 1) | |
| Simvastatin | Breast cancer | NCT03324425 (phase 2) |
| Breast cancer | NCT00334542 (phase 2) | |
| Pancreatic cancer | NCT00944463 (phase 2) | |
| Breast cancer | NCT00807950 (phase 2) | |
| Colorectal cancer | NCT01110785 (phase 2) | |
| Adenocarcinoma of rectum | NCT02161822 (phase 2) | |
| Colorectal cancer | NCT00313859 (phase 2) | |
| Myeloma | NCT01332617 (phase 2) | |
| Multiple myeloma | NCT00281476 (phase 1/2) | |
| Chronic lymphocytic leukemia | NCT00828282 (phase 1) | |
| Atorvastatin | Prostate cancer | NCT04026230 (phase 3) |
| Prostatic neoplasms | NCT01821404 (phase 2) | |
| Endometrial cancer | NCT02767362 (early phase 1) | |
| Malignant disease | NCT03560882 (phase 1) | |
| Kidney cancer | NCT00490698 (phase 2) | |
| Acute myelogenous leukemia | NCT01491958 (phase 2) | |
| Myeloma | NCT00164086 (phase 1) | |
| Glioblastoma multiforme | NCT02029573 (phase 2) | |
| Hepatocellular carcinoma | NCT02819869 (phase 2) | |
| Lovastatin | Breast cancer | NCT00584012 (phase 1) |
| Ovarian cancer | NCT00585052 (phase 2) | |
| Breast cancer | NCT00285857 (phase 2) | |
| Acute myeloid leukemia | NCT00583102 (phase 1/2) | |
| Melanoma | NCT00963664 (phase 2) | |
| Melanoma | NCT00462280 (phase 2) | |
| Neurofibromatosis type 1 | NCT00853580 (phase 2) | |
| Verapamil | Brain cancer | NCT00706810 (phase 2) |
| Recurrent hodgkin lymphoma | NCT03013933 (phase 1) | |
| Sildenafil | Pancreatic cancer | NCT02106871 (phase 1) |
| Lung cancer | NCT00752115 (phase 2/3) | |
| Solid tumor | NCT02466802 (phase 1) | |
| Lymphatic malformations | NCT02335242 (phase 2) | |
| Glioblastoma | NCT01817751 (phase 2) | |
| Lymphangioma | NCT01290484 (phase 1/2) | |
| Waldenstrom's macroglobulinemia | NCT00165295 (phase 2) | |
| Chloroquine | Lung cancer | NCT01575782 (phase 1) |
| Breast cancer | NCT02333890 (phase 2) | |
| Lung cancer | NCT00969306 (phase 1) | |
| Breast cancer | NCT01446016 (phase 2) | |
| Malignant neoplasm | NCT02071537 (phase 1) | |
| Carcinoma | NCT01023477 (phase 1/2) | |
| Glioma | NCT02496741 (phase 1/2) | |
| Brain metastasis | NCT01894633 (phase 2) | |
| Glioblastoma multiforme | NCT02378532 (phase 1) | |
| Glioblastoma | NCT02432417 (phase 2) | |
| Glioblastoma | NCT04772846 (phase 1/2) | |
| Glioblastoma multiforme | NCT00224978 (phase 3) | |
| Artemisinin | Ovarian cancer | NCT04805333 (phase 1) |
| Quinacrine | Prostatic cancer | NCT00417274 (phase 2) |
| Lung cancer | NCT01839955 (phase 1) | |
| Colorectal adenocarcinoma | NCT01844076 (phase 1/2) | |
| Renal cell carcinoma | NCT00574483 (phase 2) | |
| Itraconazole | Prostate cancer | NCT03513211 (phase 1/2) |
| Prostate cancer | NCT00887458 (phase 2) | |
| Prostate cancer | NCT02054793 (phase 1/2) | |
| Lung cancer | NCT03664115 (phase 2) | |
| Esophageal cancer | NCT02749513 (early phase 1) | |
| Lung cancer | NCT02357836 (early phase 1) | |
| Ovarian cancer | NCT03081702 (phase 1/2) | |
| Lung cancer | NCT00769600 (phase 2) | |
| Lung cancer | NCT02157883 (phase 1) | |
| Solid tumours | NCT01900028 (phase 1) | |
| Prostate cancer | NCT01450683 (phase 2) | |
| Lung cancer | NCT01752023 (phase 2) | |
| Esophageal neoplasm | NCT04481100 (phase 2) | |
| Prostate adenocarcinoma | NCT01787331 (phase 2) | |
| Advanced solid tumors | NCT02259010 (phase 1) | |
| Ketoconazole | Prostate cancer | NCT00895310 (phase 2) |
| Breast cancer | NCT00212095 (phase 2) | |
| Prostate cancer | NCT01036594 (phase 2) | |
| Prostate cancer | NCT00953576 (phase 1/2) | |
| Prostate cancer | NCT00003084 (phase 2) | |
| Granulosa cell tumour of the ovary | NCT01584297 (phase 2) | |
| Advanced cancer | NCT00708591 (phase 1) | |
| Prostatic neoplasms | NCT00032825 (phase 1) | |
| Breast cancer | NCT03796273 (early phase 1) | |
| Solid tumors | NCT00697437 (phase 2) | |
| Ciprofloxacin | Prostate cancer | NCT02252978 (phase 2) |
| Bladder cancer | NCT00003824 (phase 3) | |
| Leukemia | NCT02773732 (phase 1/2) | |
| Pancreatic ductal adenocarcinoma | NCT04523987 (phase 1) | |
| Doxycycline | Pancreatic cancer | NCT02775695 (phase 2) |
| Breast carcinoma | NCT02874430 (phase 2) | |
| Breast cancer | NCT01847976 (phase 2) | |
| Head and neck squamous cell carcinoma | NCT03076281 (phase 2) | |
| Lymphangioleiomyomatosis | NCT00989742 (phase 4) | |
| Cutaneous T-cell lymphoma | NCT02341209 (phase 2) | |
| Melanoma | NCT01590082 (phase 1/2) | |
| Ritonavir | Breast cancer | NCT01009437 (phase 1) |
| Nelfinavir | Non-hodgkin lymphoma/Hodgkin lymphoma/Gastric cancer/Nasopharyngeal cancer | NCT02080416 (early phase 1) |
| Colorectal cancer | NCT00704600 (phase 1/2) | |
| Head and neck neoplasms | NCT01065844 (phase 2) | |
| Non-small cell lung cancer | NCT00791336 (phase 2) | |
| Non-small cell lung cancer | NCT01108666 (phase 2) | |
| Glioblastoma | NCT00694837 (phase 1) | |
| Glioma | NCT01020292 (phase 1) | |
| Maraviroc | Hematologic malignancy | NCT01785810 (phase 2) |
| Metastatic colorectal cancer | NCT03274804 (phase 1) | |
| Aspirin | Colorectal cancer | NCT00578721 (phase 2) |
| Colorectal cancer | NCT02607072 (phase 3) | |
| Colorectal cancer | NCT00565708 (phase 3) | |
| Colorectal cancer | NCT02647099 (phase 3) | |
| Colorectal cancer | NCT02945033 (phase 3) | |
| Colorectal cancer | NCT03957902 (phase 2) | |
| Colorectal cancer | NCT00002527 (phase 3) | |
| Colorectal cancer | NCT02125409 (phase 3) | |
| Colorectal cancer | NCT02965703 (phase 2) | |
| Lynch syndrome | NCT02813824 (phase 3) | |
| Lynch syndrome I | NCT02497820 (phase 3) | |
| Colon cancer/Rectal cancer | NCT00468910 (phase 2) | |
| Colon cancer | NCT02467582 (phase 3) | |
| Colon cancer | NCT02301286 (phase 3) | |
| Colon cancer | NCT03464305 (phase 3) | |
| Colon cancer | NCT00224679 (phase 3) | |
| Rectal cancer | NCT03170115 (phase 2) | |
| Gastric cancer | NCT04214990 (phase 3) | |
| Breast cancer | NCT03491410 (phase 2/3) | |
| Breast cancer | NCT00727948 (early phase 1) | |
| Node positive HER2 negative breast cancer | NCT02927249 (phase 3) | |
| Fallopian tube cancer | NCT03771651 (early phase 1) | |
| Non-small cell lung cancer | NCT01058902 (phase 3) | |
| Non-small cell lung cancer | NCT01707823 (early phase 1) | |
| Prostate cancer/Gastro-oesophageal cancer/Colorectal cancer/Breast cancer | NCT02804815 (phase 3) | |
| Urinary bladder neoplasms | NCT02350543 (phase 4) | |
| Esophageal squamous cell carcinoma | NCT03900871 (early phase 1) | |
| Cutaneous melanoma | NCT03396952 (phase 2) | |
| Nasopharyngeal carcinoma | NCT03290820 (phase 2) | |
| Glioblastoma | NCT00790452 (phase 2) | |
| Melanoma | NCT04062032 (phase 2) | |
| Melanoma | NCT04066725 (phase 2) | |
| Celecoxib | Endometrium cancer | NCT03896113 (phase 2) |
| Breast cancer | NCT00328432 (phase 1) | |
| Breast cancer | NCT01695226 (phase 2) | |
| Breast cancer | NCT00056082 (phase 2) | |
| Breast cancer | NCT00045591 (phase 2) | |
| Breast cancer | NCT00291694 (phase 2) | |
| Breast cancer | NCT02429427 (phase 3) | |
| Metastatic cancer | NCT03864575 (phase 2) | |
| Oral squamous cell carcinoma | NCT02739204 (phase 2) | |
| Lung cancer | NCT00104767 (phase 1) | |
| Lung cancer | NCT01503385 (phase 2) | |
| Lung cancer | NCT00020878 (phase 2) | |
| Prostate cancer | NCT02840162 (phase 2) | |
| Prostate cancer | NCT00022399 (phase 2) | |
| Prostate cancer | NCT00073970 (phase 2) | |
| Prostate cancer | NCT00136487 (phase 2/3) | |
| Recurrent respiratory papillomatosis | NCT00571701 (phase 2) | |
| Cervix neoplasms | NCT00152828 (phase 1/2) | |
| Head and neck cancer/Lung cancer | NCT00527982 (phase 2) | |
| Non muscle invasive bladder cancer | NCT02343614 (phase 2) | |
| Head and neck cancer | NCT00061906 (phase 2) | |
| Head and neck cancer | NCT00014404 (phase 2) | |
| Smoldering multiple myeloma | NCT00099047 (phase 2) | |
| Recurrent bladder cancer | NCT00006124 (phase 2/3) | |
| Uterine cancer | NCT00231829 (phase 2) | |
| Colorectal carcinoma | NCT00582660 (phase 2) | |
| Cervical carcinoma | NCT00081263 (phase 2) | |
| Non-small cell lung cancer | NCT00108186 (phase 1) | |
| Lymphangioleiomyomatosis | NCT02484664 (phase 2) | |
| Memantine | Glioblastoma | NCT01260467 (phase 2) |
| Chlorpromazine | Glioblastoma multiforme/MGMT-unmethylated glioblastoma | NCT04224441 (phase 2) |
| Fluphenazine | Multiple myeloma and plasma cell neoplasm | NCT00335647 (phase 1/2) |
| Multiple myeloma | NCT00821301 (phase 1) | |
| Imipramine | Breast cancer | NCT03122444 (early phase 1) |
| Metformin | Breast cancer | NCT01302002 (early phase 1) |
| Breast cancer | NCT04387630 (phase 2/3) | |
| Breast cancer | NCT01266486 (phase 2) | |
| Breast cancer | NCT01310231 (phase 2) | |
| Breast cancer | NCT01589367 (phase 2) | |
| Breast cancer | NCT01566799 (phase 2) | |
| Breast cancer | NCT00930579 (phase 2) | |
| Breast cancer | NCT00909506 (phase 2) | |
| Prostate cancer | NCT03137186 (phase 2) | |
| Prostate cancer | NCT01620593 (phase 2) | |
| Prostate cancer | NCT01243385 (phase 2) | |
| Prostate cancer | NCT01215032 (phase 2) | |
| Prostate cancer | NCT01677897 (phase 2) | |
| Prostate cancer | NCT01733836 (phase 2) | |
| Prostate cancer | NCT04925063 (phase 2) | |
| Prostate cancer | NCT00881725 (phase 2) | |
| Prostate cancer | NCT01864096 (phase 3) | |
| Prostate cancer | NCT03031821 (phase 3) | |
| Endometrial cancer | NCT01205672 (early phase 1) | |
| Endometrial cancer | NCT01911247 (early phase 1) | |
| Endometrial cancer | NCT02990728 (phase 2) | |
| Endometrial cancer | NCT02042495 (phase 2) | |
| Endometrial cancer | NCT04792749 (phase 3) | |
| Colorectal cancer | NCT01632020 (phase 2) | |
| Colorectal cancer | NCT01926769 (phase 2) | |
| Colon cancer | NCT01440127 (phase 1) | |
| Rectal cancer | NCT02437656 (phase 2) | |
| Bladder cancer | NCT03379909 (phase 2) | |
| Head and neck cancer | NCT02402348 (early phase 1) | |
| Head and neck squamous cell cancer | NCT02083692 (early phase 1) | |
| Non-small cell lung cancer | NCT01997775 (phase 2) | |
| Non-small cell lung cancer/Lung cancer | NCT03086733 (phase 2) | |
| Non-small cell lung cancer/Lung cancer | NCT01717482 (phase 2) | |
| Well-differentiated neuroendocrine tumors | NCT02279758 (phase 2) | |
| Hepatocellular carcinoma | NCT02319200 (phase 3) | |
| Chronic lymphocytic leukemia | NCT01750567 (phase 2) | |
| Pioglitazone | Lung cancer | NCT00780234 (phase 2) |
| Thyroid cancer | NCT01655719 (phase 2) | |
| Non-small cell lung cancer | NCT00923949 (phase 2) | |
| Non-small cell lung cancer | NCT01342770 (phase 2) | |
| Pancreatic cancer | NCT01838317 (phase 2) | |
| Pancreatic cancer | NCT00867126 (early phase 1) | |
| Prostate cancer | NCT04658849 (early phase 1) | |
| Skin squamous cell cancer | NCT02347813 (phase 2) | |
| Mebendazole | Medulloblastoma/Astrocytoma/Glioblastoma/Anaplastic astrocytoma/Brain stem neoplasms/Oligodendroblastoma/Anaplastic oligodendroglioma/Malignant glioma | NCT02644291 (phase 1) |
| High-grade glioma | NCT01729260 (phase 1) | |
| Niclosamide | Colon cancer | NCT02687009 (phase 1) |
| Colorectal cancer | NCT02519582 (phase 2) | |
| Disulfiram | Metastatic breast cancer | NCT03323346 (phase 2) |
| Melanoma | NCT00256230 (phase 1/2) | |
| Raloxifene | Endometrial cancer | NCT00004915 (phase 2) |
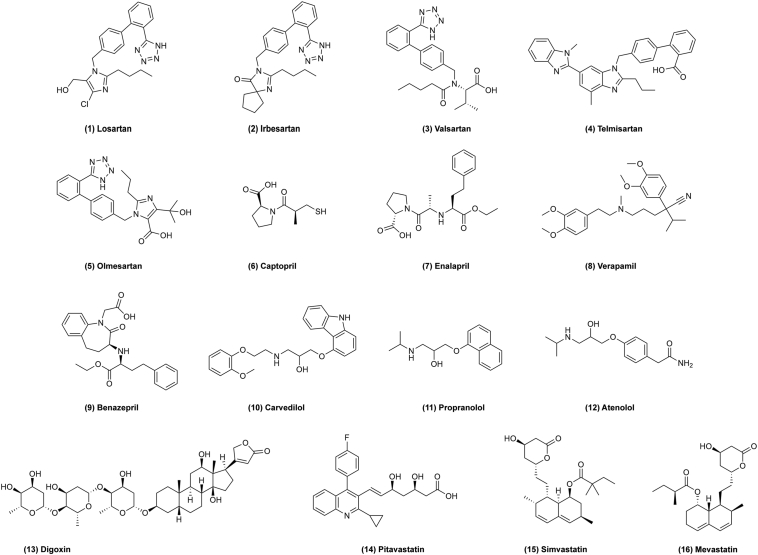
Figure 3.
Chemical structures of 1–16 as cardiovascular drugs for repurposing in cancer therapy.
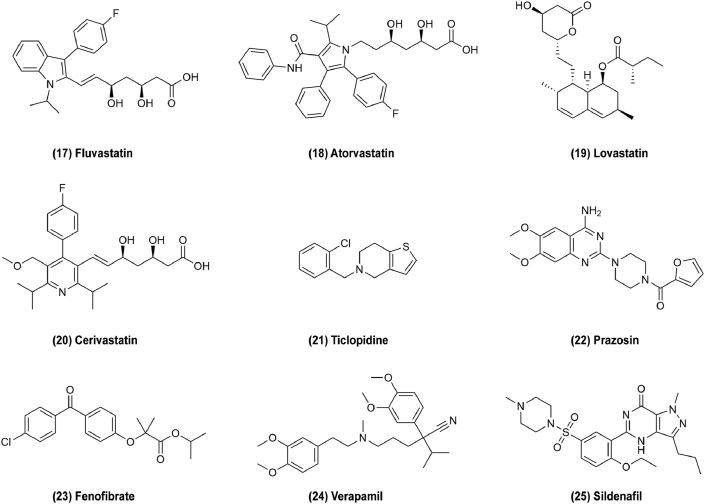
Another cardiovascular drug carvedilol (Fig. 3, 10) affects β-adrenoreceptor for treatment of hypertension, and its potential ability to resistance to skin cancer caused by sun exposure was serendipitously revealed as a result of an experimental error, a new finding first published in 2017117,118. However, it is little known about how carvedilol affects cancer-related outcomes. To explain this, it was discovered that carvedilol blocks the effects of sympathetic nervous system activation through β-adrenoreceptor signaling and metastasis and invasion of breast cancer were blocked in a mouse model26. Additionally, carvedilol induced mitochondrial damage including mitochondrial swelling, crista damage and formation of myelin figures, and inhibited growth factor receptors to mediate anti-proliferative and anti-migratory effects in cancer cells and increase survival in experimental animal models of pancreas cancer27. In clinical trials, carvedilol is often combined with imatinib to enhance in vitro anticancer activity in glioma or with trastuzumab to prevent and alleviate drug-related cardiotoxicity in breast cancer119,120. These results suggest that β-blockers could represent novel drugs to treat cancer progression. Propranolol (Fig. 3, 11) is a non-selective β-adrenoreceptor blocker used to treat arrhythmia, but it also exerts effect on melanoma by inhibiting angiogenesis and disrupting migration of cancer cells via inhibition of noradrenaline-dependent responses28. Propranolol is the first line therapy for infantile hemangioma, acting by interference with SOX18 transcriptional activity to inhibit angiogenesis29. Atenolol (Fig. 3, 12) acts in the same manner as propranolol to inhibit apoptosis, proliferation, migration, and inflammatory cell responses for cancer therapy30. These drugs are new attractive, inexpensive, and relatively safe therapeutics for cancers121. Cardiac glycosides (CGs) such as digoxin (Fig. 3, 13) and digitoxin are compounds found in natural sources that are used to treat different cardiac conditions. The antitumor effects of CG were suggested in 1979 in a study of chemotherapy for breast cancer treated in combination with digitalis122. It was used to treat cardiovascular disease by inhibiting the Na+/K+-ATPase which results in an elevation of the intracellular Na+ level in cardiomyocytes, such that the Na+/Ca2+ exchanger mechanism is stimulated. This results in increased intracellular Ca2+ concentration, prompting myocardial contractibility31,32. However, CGs can also bind to Na+/K+-ATPase in cancer cells and disrupt it, causing apoptosis115. In addition, CGs could treat cancer via other targets. The eukaryotic translation initiation factor 4F complex (eIF4F) which regulates the translational initiation has been recognized as a potential chemotherapeutic target in triple-negative breast cancer (TNBC). CGs could treat TNBC as an inhibitor of eIF4F which is modulated by c-MYC levels32. c-MYC can be phosphorylated through the RAS–ERK pathway, and CGs may also be related to the reduction of hypoxia-induced activation of the ERK pathway33. CGs also induce the expression of P21Cip1, an inhibitor of cyclin-dependent kinases, resulting in growth arrest16. In addition, CGs can affect TRAIL-mediated apoptosis and suppress HIF-1a accumulation for cancer therapy34. Therefore, CGs are a broad category of compounds derived from natural products which show potential anticancer effects by targeting multiple pathways123. Statins are common cholesterol-lowering prescription drugs which inhibit 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA), a rate-limiting enzyme of the mevalonate pathway, thus decreasing cholesterol biosynthesis. Recently, it was discovered that the mevalonate pathway coincides with some cancer pathway, and thus, that cholesterol-lowering drugs may also be used in cancer therapy35. For example, pitavastatin (Fig. 3, 14), which is the most promising of these for repurposing as an antitumor drug, can induce death in almost half of neoplastic cells but with much lower mortality in normal cells. Its antitumor mechanism is related to the mevalonate pathway and macropinocytosis. Mevalonate pathway provides geranylgeranyl pyrophosphate (GGPP) which cancer cells depend for macropinocytosis nutrient uptake. Statins selectively inhibit the mevalonate pathway, which eventually leads to amino acid starvation and subsequent cell death due to the depletion of GGPP36. Pitavastatin also shows anticancer activity through inducing autophagic death by inhibiting NF-κB, suppressing tumor cell MDR-1 protein and inhibiting cell proliferation. In addition, other statins like simvastatin (Fig. 3, 15), mevastatin (Fig. 3, 16) and fluvastatin (Fig. 4, 17) can suppress the PI3K/AKT and RAS/ERK pathways to inhibit cancer37,38,124. Atorvastatin (Fig. 4, 18) downregulates expression of membrane type 1 MMP via the P38 MAPK pathway39. Lovastatin (Fig. 4, 19) may inhibit G0/G1 cell cycle transition to prevent cell proliferation and activate LKB1–AMPK–P38MAPK–P53–survivin cascade to cause MCF-7 cell death40. Cerivastatin (Fig. 4, 20) downregulates FAK phosphorylation to inhibit cell adhesion and invasion6,125. Statins were found to regulate cell proliferation, apoptosis, and tumor progression in cancer patients, and could reduce cancer induction and improve survival126.
Figure 4.
Chemical structures of 17–25 as cardiovascular drugs for repurposing in cancer therapy.
In addition, ticlopidine (Fig. 4, 21) also shows antitumor efficacy via inducing cell autophagy by coordinately elevating the level of cAMP41. Prazosin (Fig. 4, 22) has been found to have off-target activity to inhibit glioblastoma growth via the PKCδ-dependent inhibition of the AKT pathway, which results in caspase-3 activation42. Fenofibrate (Fig. 4, 23) can be developed for chemoprevention or treatment of colon cancer by activating peroxisome proliferators-activated receptors-α (PPAR-α) which regulates the occurrence of colon cancer associated with DNMT1-mediated methylation of P21 and PRMT6-mediated methylation of P2743. Verapamil (Fig. 4, 24) and sildenafil (Fig. 4, 25) are also reported to effect on cancer127,128.
These drugs were initially found to have antitumor effect via their original target, but some of them have multiple targets for cancer treatment. Therefore, other new targets are likely to be found by studying and seeking the repurposing of cardiovascular drugs. Thus, cardiovascular drugs represent a broad category of drugs for repurposing for cancer therapy by several pathways. The first step of drug repurposing is to find its effective target for cancer, whereby some drugs have the same antitumor target as in their original indications. In fact, the potential of cardiovascular drugs in oncology remains clinically untapped because most available clinical data originate from retrospective studies and small prospective studies that are potentially biased. Therefore, more effort could be exerted in this area of repurposing drugs for tumor treatment.
3.2. Microbiological agents repurposing for cancer therapy
Microbiological agents are mainly used for treating malaria, fungal and bacterial infections. Some of these drugs have shown promising anticancer activity and entered phase II/III clinical trials.
3.2.1. Anti-malaria drugs
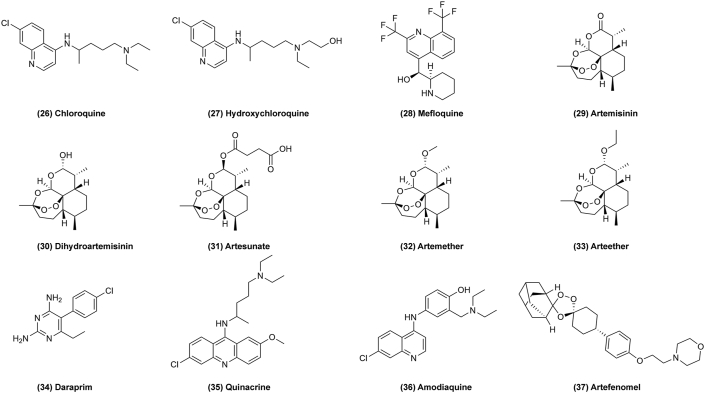
Chloroquine (Fig. 5, 26) and its derivatives hydroxychloroquine (Fig. 5, 27) and mefloquine (Fig. 5, 28) are used for the prevention and treatment of malaria and have shown promising results in combination with other anticancer drugs. A key target is the inhibition of autophagy by preventing lysosomal acidification or lysosome fusion through targeting AMPK pathway. NF-κB and P53 also inhibit tumor cell proliferation and growth by autophagy in cancer cells44. In a phase I/II trial of everolimus and hydroxychloroquine in patients, decreased cytotoxicity when mTOR inhibitors are combined with an autophagy inhibitor was observed129. For autophagy in stromal cells, chloroquine or their derivatives could be promising antineoplastic agents in the next decades.
Figure 5.
Chemical structures of 26–37 as microbiological agents for repurposing in cancer therapy.
Artemisinin (Fig. 5, 29) and its derivatives dihydroartemisinin (Fig. 5, 30), artesunate (Fig. 5, 31), artemether (Fig. 5, 32), arteether (Fig. 5, 33) have also shown selective anticancer properties in phase I/II trials via their cytotoxic effects. It was found that artemisinin could induce caspase-dependent apoptosis by the intrinsic mitochondrial pathway, regulated by antiapoptotic proteins like BCL-2, and proapoptotic factors like BID and BAK in cancer cells45. Additionally, artemisinin exhibits immunomodulation in cancer by inhibiting T-cell activation and proliferation and inhibiting signal transducer and activator of transcription (STAT3), cancer metabolism and cancer stem cells as a novel and promising approach to treat tumors46. However, the use of artemisinin has unexpected resulted in hepatotoxicity, thus, its repurposing for cancer therapy must be approached with caution130. Daraprim (Fig. 5, 34) is another anti-malaria drug which also targets STAT3 to treat cancer47. Quinacrine (Fig. 5, 35) and amodiaquine (Fig. 5, 36) have been found to kill tumors by induction of apoptosis and autophagy48,49. These drugs similarly target cell autophagy or apoptosis via several pathways to treat cancer. Some of these drugs like artemisinin, which is derived from the extracted ingredients of traditional Chinese medicine to treat malaria worldwide, show effects on cancer therapy. Artemisinin derivatives with structure modification could have enhanced anticancer effects. For example, one synthetic derivative, OZ439 (artefenomel) (Fig. 5, 37), shows effective and promising alternative cancer therapeutic effects by stabilizing the peroxide pharmacophore50. Therefore, natural products of traditional Chinese medicine are also a new way to explore effective anticancer drugs.
3.2.2. Anti-fungal drugs
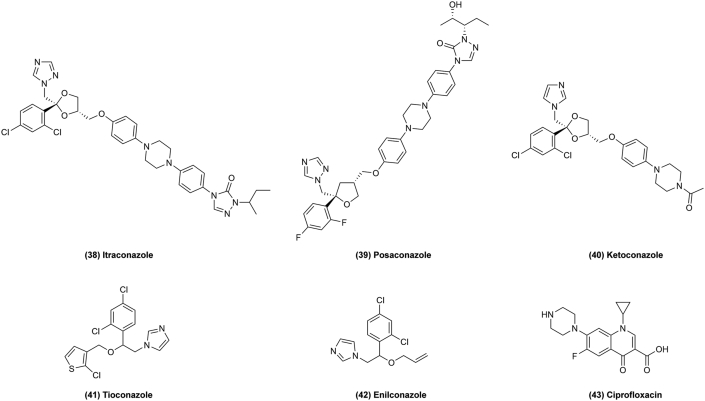
Some azole anti-fungal agents approved by the FDA also show antitumor effects, for example, itraconazole was recently identified as a promising anticancer chemotherapeutic through high-throughput screens. Itraconazole (Fig. 6, 38) exerts its antifungal effects through potent inhibition of CYP51, while its side chain region may inhibit the hedgehog (Hh) signaling pathway which is a signaling cascade responsible for cell proliferation, differentiation, and tissue growth in embryogenesis51. In addition, itraconazole can induce cholesterol redistribution to trigger autophagy via inhibition of the AKT-mTOR signaling pathway, finally inhibiting cell proliferation52. Posaconazole (Fig. 6, 39) is a derivative of itraconazole that exerts antitumor effects by targeting hexokinase2 (HK2) to regulate tumor growth, invasion, and angiogenesis53. Ketoconazole (Fig. 6, 40) was the first oral active azole antifungal agent synthesized (in 1976) but it was withdrawn from the European market in 2013 because of its severe hepatotoxic adverse reactions. It also has anticancer effects by targeting the HK2 pathway and exacerbating mitophagy to induce apoptosis by downregulating cyclooxygenase-2 (COX2) in hepatocellular carcinoma54. Tioconazole (Fig. 6, 41) shows anticancer effect by enhancing chemotherapeutic drug-induced cytotoxicity in cancer cells and suppressing autophagy by inhibiting ATG4 to regulate tumor growth, metastasis and resistance to chemotherapy and sensitize cancer cells to chemotherapy55. Enilconazole (Fig. 6, 42) can target the WNT/β-catenin/TCF signaling pathway to inhibit cancer cell migration and growth56. These anti-fungal agents originally targeted fungal pathogens, but are also found to treat cancer in vivo. Their therapeutic targets in humans are also like other drugs like those targeting the WNT, PI3K/AKT/mTOR and ROS pathways.
Figure 6.
Chemical structures of 38–43 as microbiological agents for repurposing in cancer therapy.
3.2.3. Synthetic anti-bacterial drugs
Ciprofloxacin (Fig. 6, 43) belongs to the second generation of synthetic quinolone anti-bacterial agent with a broad spectrum of anti-bacterial activity. ATP-binding cassette subfamily B member 1 (ABCB1) is one of the major drug efflux transporters that is known to cause multidrug resistance (MDR) in cancer patients, ciprofloxacin can reverse MDR by inhibiting the ABCB1 efflux function57. In addition, ciprofloxacin can promote cell apoptosis and regulate the function of immune cells for cancer treatment. According to research, ciprofloxacin is able to promote the production of interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and the polarization of CD86+CD206– macrophages, while inhibiting the polarization of CD86+CD206– macrophages which plays an important role in tumor regulation. Ciprofloxacin may inhibit liver cancer by upregulating the expression of CD86+CD206– macrophages58. Thus, ciprofloxacin has a potential to be developed as a combination anticancer therapy. Microbiological agents are promising candidates for drug repurposing for cancer therapy, but further clinical development and research are required to translate these findings into clinical applications.
3.3. Antibiotics repurposing for cancer therapy
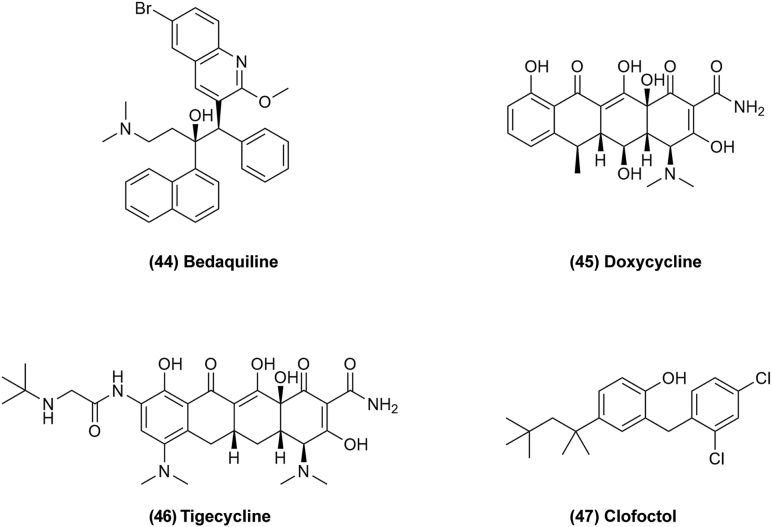
Antibiotics are used to treat bacterial infection, but they are also being studied for their ability to target cancer cells. Those exhibiting antitumor activity including β-lactams, tetracyclines and anti-tuberculosis drugs. Bedaquiline (Fig. 7, 44) is an FDA-approved antibiotic that is used to treat multi-drug resistant pulmonary tuberculosis (TB) by inhibiting the bacterial ATP-synthase59. Due to eukaryotic mitochondria originally evolving from engulfed aerobic bacteria, it is reasonable to hypothesize that bedaquiline might target the mitochondrial ATP-synthase and lead to mitochondrial dysfunction and ATP depletion in cancer cells60. In fact, bedaquiline was found to inhibit mitochondrial oxygen consumption, glycolysis in MCF7 breast cancer cells and block the propagation and expansion in MCF7-derived stem-like cancer cells (CSCs). Mitochondria play an important role in cancer treatment and offer potential applications for mtDNA modifications as novel anticancer targets61. Therefore, bedaquiline could be a promising chemotherapeutic for cancer, but further pre-clinical studies and human clinical trials in cancer patients are needed to confirm this.
Figure 7.
Chemical structures of 44–47 as antibiotics for repurposing in cancer therapy.
Tetracyclines drugs like doxycycline (Fig. 7, 45) and tigecycline (Fig. 7, 46) could target oxidative mitochondrial metabolism to prevent the propagation of cancer. They could induce cell cycle arrest, apoptosis, autophagy, and oxidative stress in cancer, and inhibit cell proliferation, migration, invasion, and angiogenesis through targeting PI3K/AKT, AMPK-mediated mTOR, cytoplasmic P21, and WNT/β-catenin signaling62.
Clofoctol (Fig. 7, 47) was used for the treatment of upper respiratory tract infections in France and Italy by alteration of bacterial membrane permeability owing to its hydrophobic nature131. Now, it has been reported that clofoctol can inhibit protein translation in mammalian cells, so it could serve as a potential anticancer drug132. Clofoctol could inhibit cancer cell growth through activation of the unfolded protein response (UPR) pathway in the endoplasmic reticulum (ER), which is a major cellular organelle for folding and maturation of secretory proteins. If misfolded proteins accumulate over the limit of the capacity of ER-associated degradation, the UPR pathway will be activated by one of three sensing proteins, either IRE1, PERK or ATF6, to improve the protein folding and down-regulate protein translation in the ER to reduce the ER folding load. There is already evidence suggesting that many types of cancer cells have higher levels of ER stress, so the UPR pathways are already active to maintain cellular homeostasis. Therefore, further increasing the ER stress could result in overload of the stress responses in cancer cells and in turn induce cell cycle arrest or cell death. Clofoctol induces ER stress and activates all three UPR pathways in PC3 cells, thus inhibiting protein translation and inducing G1 phase cell cycle arrest63. Antibiotics are attractive compounds for their ability to target cancer cells and inhibit bacterial infection. The repurposing of antibiotics to treat human tumors is based on a reasonable hypothesis that they target a new mechanism, namely, eukaryotic mitochondria originally evolved from engulfed aerobic bacteria. In these studies, antibiotics show promising antitumor effect and will be worthwhile to make expand efforts.
3.4. Antiviral drugs repurposing for cancer therapy
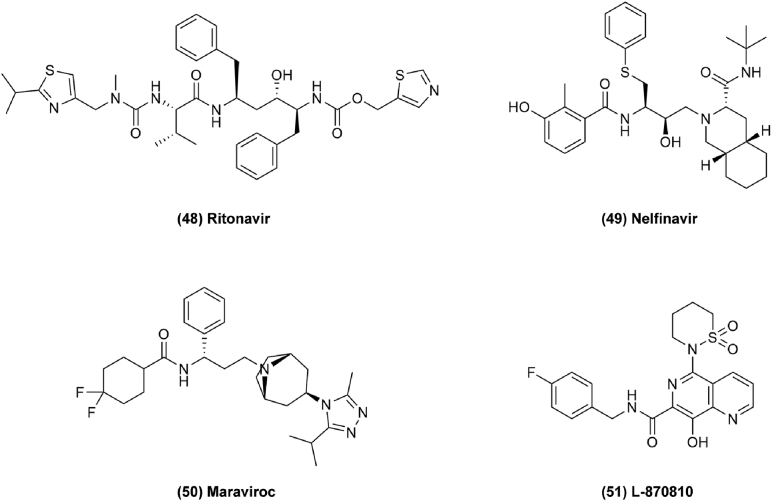
Antiviral drugs are used to treat a group of viruses inducing serious diseases. Apart from the human immune deficiency virus (HIV), most viral diseases are self-limiting illnesses. For example, ritonavir (Fig. 8, 48) shows a potential use for ovarian cancer, glioblastoma, and lung cancer with additive effects in conjunction with conventional chemotherapeutic regimens133,134. Ritonavir was found to exert antitumor effects by inducing cell cycle arrest with decreasing expression of G1 phase cyclins and cyclin dependent kinases (CDKs), and by promoting apoptosis by increasing the expression of BAK and inhibiting BCL-2 in ovarian cells in a dose dependent manner. Inhibition of AKT pathway also leads to cell apoptosis and inhibits cell migration and invasiveness for cancer therapy64. Nelfinavir (Fig. 8, 49) can improve current approaches of targeted melanoma therapy. MAPK-pathway inhibitors (MAPKi) are usually used for treating melanoma, pancreatic cancer, and rectal cancer, but long-term BRAF and MEK inhibition will induce drug resistance in melanoma. It has been found that the acquired resistance is induced by the upregulation of the melanoma survival oncogene MITF which is regulated by PAX3. Nelfinavir can decrease MAPKi-induced drug tolerance by suppressing PAX3 and MITF expression in BRAF mutant melanomas. The PAX3–MITF axis is a good target for MITF-driven drug tolerance and the expression of PAX3 is tightly regulated by BRAF-initiated MAPK signaling through SMAD2/4 and the SKI complex which is controlled by transforming growth factor b (TGF-b). Therefore, targeting SMAD2/4 and the SKI complex to keep them in a steady-state condition is correlated with a reduction in PAX3 and MITF expression65. In clinical research, combination therapy of nelfinavir with short course hypofractionated radiotherapy (SCHRT) increases tumor perfusion and radiosensitizes tumors in rectal cancer66.
Figure 8.
Chemical structures of 48–51 as antiviral drugs for repurposing in cancer therapy.
Maraviroc (Fig. 8, 50) is another antiviral drug repurposed for triple-negative breast cancer (TNBC) therapy which acts as a C-C chemokine receptor 5 (CCR5) inhibitor to block lymphangiogenesis and lung metastasis. It has been shown that interleukin-6 (IL-6) and C-C chemokine ligand 5 (CCL5) play important roles in TNBC tumor growth and metastasis. Therefore, CCR5, which is widely expressed on tumors, is a new therapeutic target for metastatic cancers like breast and colon cancer in clinical trials. Thus, maraviroc could be repurposed for cancer treatment67. In addition, maraviroc combined with other anticancer drugs may exert unexpected effects. In one study, a combination of tocilizumab and maraviroc decreased cellular viability, and a combination of maraviroc and cMR16-1 resulted in greater inhibition of tumor growth compared to each single agent68. Compounds with structure modifications might have enhanced antitumor effects as well. For example, 8-hydroxy-[1,6]naphthyridines (L-870810) (Fig. 8, 51) is one of a promising class of antiretroviral drugs developed by Merck Laboratories. Optimization of L-870810 shows significant cytotoxicity in a panel of cancer cell lines and effectively inhibits select oncogenic kinases69. Therefore, it is possible to apply it to cancer therapy, but this requires more verification. Antiviral drugs may reveal antitumor activity by examining clinical data retrospectively and finding decreased incidence rates, for example, of HIV-related cancers after taking antiviral drugs. These clinical data suggest that antiviral drugs have potential to treat cancer.
3.5. Anti-inflammatory drugs repurposing for cancer therapy
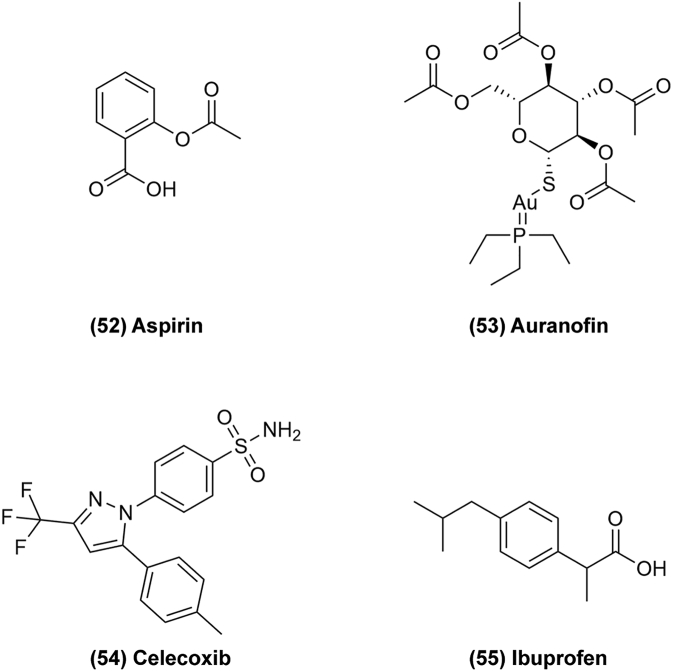
Anti-inflammatory drugs have anticancer effects because inflammation can also induce cancer. They mainly exhibit anticancer activity through inhibition of cyclooxygenase (COX) enzymes135. For example, aspirin (Fig. 9, 52) is commonly used for the treatment and prevention of atherosclerotic disease. In 1988, it was first shown to reduce the risk of colorectal cancer in a clinical trial. Since then, several studies have been demonstrated that aspirin can reduce the incidence of several different cancers. The anti-neoplastic effects of aspirin have been attributed to its inhibition of COX-1 and COX-2 enzymes which promote carcinogenesis through synthesis of PGE270. PGE2 effects are mediated by E-prostanoid (EP) receptor signaling impacting on G-protein coupled receptors to promote proliferation, invasion, secretion of angiogenic factors and inhibition of apoptosis. In one study, knocking out the gene for COX-2 or any of several PGE2 receptor genes resulted in decreased intestinal tumor incidence in mouse models of colorectal cancer (CRC). In addition, phosphatidylinositol 3-kinase (PI3K) and WNT/β-catenin/TCF signaling pathways play a major role for aspirin inhibition of carcinogenesis71. The WNT/β-catenin/TCF signaling pathway is activated by extracellular WNT ligand binding to surface receptors, followed by β-catenin translocating to the nucleus and binding to TCF protein, finally affecting cell proliferation and migration. TCF activity is related to the transcription of a TCF-responsive reporter gene and the WNT/TCF transcriptional target CYCLIN D1. Aspirin can attenuate TCF-responsive reporter gene activity and downregulate CYCLIN D1 by modulating TCF activity. In clinical trials, aspirin could enhance the anticancer effects in non-small cell lung cancer by targeting GRP78 activity, and therefore it could be a potential drug for novel anticancer treatments.
Figure 9.
Chemical structures of 52–55 as anti-inflammatory drugs for repurposing in cancer therapy.
Auranofin (AUR) (Fig. 9, 53) is an FDA-approved anti-rheumatoid arthritis (anti-RA) drug. Recently, it was reported that AUR potently upregulates hepcidin expression and induces ferroptosis both in vitro and in vivo, which is related to some diseases72. Its canonical signaling pathways to regulate iron, including the BMP/SMAD and IL-6/JAK/STAT3 pathways, play indispensable roles in mediating AUR's effects. Interestingly, AUR also exerts an effect on glioblastoma multiforme by inhibiting cathepsin B expression, which may inhibit glioblastoma growth, and by inhibiting thioredoxin reductase (TXNRD) activity, thus increasing intracellular ROS and resulting in cell death. However, AUR shows only moderate activity as a single agent in clinical applications in cancer treatment. Celecoxib (CE) (Fig. 9, 54) was unexpectedly identified as potently enhancing the anticancer activity of AUR in vitro and in vivo through a high-throughput screening of the FDA-approved drug library. The AF/CE combination induces severe oxidative stress that causes ROS-mediated inhibition of hexokinase (HK) and a disturbance of mitochondrial redox homeostasis, resulting in a significant decrease of ATP generation in tumors73. According to one study, CE promotes ROS generation by disrupting the mitochondrial respiration chain, but the exact targets of celecoxib in the mitochondria remain unclear. It was reported that CE could reduce cell viability by induction of DNA damage, leading to P53-dependent G1 cell cycle arrest and P53-dependent autophagy by downregulation of NF-κB, caspase-9, BAX and BCL-XL74.
Ibuprofen (Fig. 9, 55) has a long history in research seeking new anticancer agents. Previously, S ibuprofen was found have anti-inflammatory effects, while the R form is inactive, but it could mediate anticancer effects in humans by converting R ibuprofen into S ibuprofen. A recent study by the National Institutes of Health (INSA) found that ibuprofen has an anticancer effect on colon cancer and inhibits malignant cell growth. In their research, ibuprofen's anticancer mechanism is to prevent cancer cells from producing tumorigenic variants of certain proteins. Additionally, a combination of ibuprofen and celecoxib showed effects on cancer in the liver of Walker-256 tumor-bearing rats by increasing the expression of genes associated with fatty acid oxidation (PPARα and CPT1) and consequently the production of ATP to normalize the energy status75. Tumors are caused by repeated stimulation of inflammation, and therefore chronic inflammation is often followed by neoplasia and inflammatory cells are also present in tumor tissues as well. Some inflammatory factors can increase the invasive ability of malignant tumor cells. Thus, inflammation is inextricably linked to tumorigenesis, and anti-inflammatory drugs could represent a resource for discovering antitumor drugs.
3.6. Anti-neurodegenerative drugs repurposing for cancer therapy
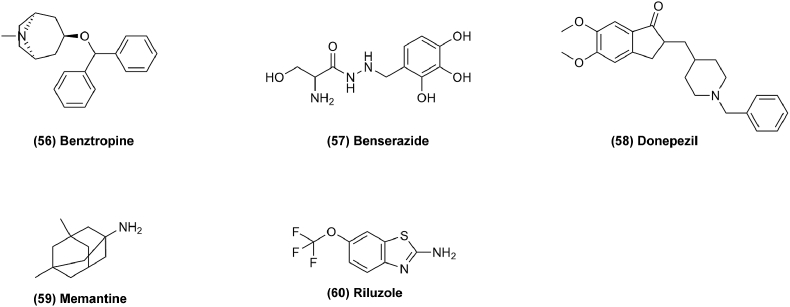
Neurodegenerative diseases result from the loss of neurons or their myelin sheaths. Commonly seen disease mainly including Parkinson's disease, Alzheimer's disease and amyotrophic lateral sclerosis (ALS)136. The endogenous cholinergic system plays a key role in neuronal cells by suppressing neurite outgrowth and myelination and is found to favor tumor growth in some cancer cells. Drugs for treating neurodegenerative disease have been found to have antitumor effects as well137. For example, benztropine (Fig. 10, 56) is currently a second-line drug for the treatment of Parkinson's disease, but has been discovered to possess anticancer activity by a tumor spheroid-based multiplex phenotypic screening system. It was found to be an effective compound for inhibition of in vitro tumor spheroid formation and cancer cell survival. Benztropine could suppress tumor growth, reduce circulating tumor cells and metastasis by acting on SLC6A3/DAT and reducing STAT376. In addition, matrix metalloproteinases (MMP), especially MMP9, are often highly expressed in human cancers and are related to the poor prognosis of patients. In further studies, MMPs were found to enhance tumor progression. The promoters of MMPs contain binding sites for oncogenic transcription factors like STAT, NF-κB and the TCF/β-catenin complex. Benztropine can suppress MMP expression by regulating these transcription factors to inhibit cancer cell invasiveness. Additionally, benserazide (Fig. 10, 57) was reported to suppress tumor growth by inhibiting HK2 which plays a vital role in tumor initiation and maintenance. In addition, benserazide could reduce glucose uptake, lactate production and intracellular ATP levels, and cause cell apoptosis and increased loss of mitochondrial membrane potential as well77.
Figure 10.
Chemical structures of 56–60 as anti-neurodegenerative drugs for repurposing in cancer therapy.
Donepezil (Fig. 10, 58) is used for Alzheimer's disease, but it also shows antitumor activity by increasing cell mitosis duration or inducing cell mitosis arrest78. Memantine (Fig. 10, 59) also shows to be effective on cancer through inhibition of proliferation and induction of autophagy mediated by NMDAR179. Riluzole (Fig. 10, 60) is an amyotrophic lateral sclerosis (ALS) drug, the action of which was also investigated on hepatocellular carcinoma, where it was found to suppress cell proliferation in liver cancer and to induce the caspase-dependent apoptosis pathway and G2/M cell cycle arrest80. In addition, a new antitumor target pathway for riluzole is to inhibit the release of cellular glutamate, which decreases the production of cellular GSH and increases the production of ROS, which in turn results in cell death as well. Neurodegenerative diseases are characterized by deposits of misfolded proteins in the brain, and one treatment approach is to regulate them by inhibiting poly ADP-ribose polymerase (PARP), which is also a target of cancer treatment. Therefore, anti-neurodegenerative drugs could be studied for treating cancer as well. However, more detailed research is necessary for drug repurposing to utilize anti-neurodegenerative drugs to treat cancer.
3.7. Antipsychotic drugs repurposing for cancer therapy
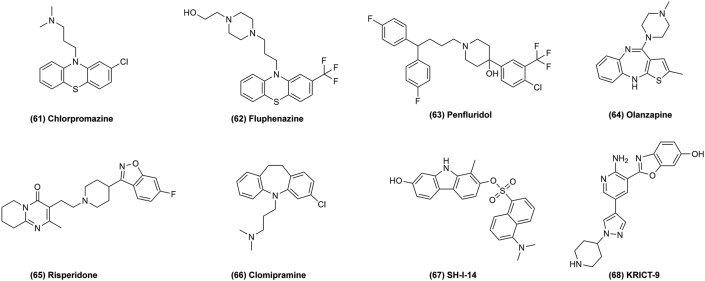
Antipsychotic drugs are commonly prescribed for the treatment of psychosis, schizophrenia, bipolar disorder, and other diseases, but many studies have indicated that some of these drugs may also mediate antitumor activity. In the 1950's, chlorpromazine (CPZ) (Fig. 11, 61), the first drug to treat psychosis, was found by accident in clinical trials, which has opened a new era of modern psychotropic drug therapy. According to the structure and pharmacological mechanisms of action, antipsychotics can be divided into first and second generation drugs16. CPZ, belonging to the phenothiazine class of anti-psychotic drugs, was a first-generation anti-psychotic used in the late sixties and early seventies as the most important drug for schizophrenia and psychotic disorders. In the meantime, CPZ has been identified as a potential candidate for drug repurposing in cancer. It was found that it exerted antitumor effects linked to the inhibition of cancer cell growth and proliferation by regulating the expression of related proteins and interfering with mitochondrial processes. In glioma cell lines, CPZ exerts its anti-proliferative effect by inducing autophagic cell death through inhibition of the PI3K/AKT/mTOR pathway. In addition, CPZ can increase the expression of the cyclin-dependent kinase (CDK) inhibitor P21, which thus causes cell cycle arrest in the G2/M-phase. In colorectal cancer cells, CPZ induces apoptosis via up-regulation of P53. Additionally, CPZ inhibits the mitotic kinesin KSP/Eg-5, resulting in mitotic arrest and cell cycle inhibition81.
Figure 11.
Chemical structures of 61–68 as antipsychotics for repurposing in cancer therapy.
Fluphenazine hydrochloride (Flu; Fig. 11, 62) is another commonly prescribed antipsychotic drug. In one study, Flu showed potential for the treatment of TNBC and its brain metastases by inducing G0/G1 cell cycle arrest and promoting mitochondria-mediated intrinsic apoptosis in vitro. Moreover, Flu could suppress the growth and proliferation of breast cancer cells by decreasing the expression of p44/42 ERK and phosphorylated AKT, which are two key molecules in RAS/RAF/MEK/ERK and PI3K/AKT/mTOR pathways in both TNBC cell lines. In addition, Flu could induce G0/G1 phase arrest in TNBC cells through downregulation of expression of CDK2, CDK4 and CYCLIN D1 and up-regulation of P21 and P2782. According to one study, Flu might induce apoptosis via the mitochondria-mediated intrinsic apoptotic pathway and suppress TNBC cell migration and invasion in vitro. Penfluridol (PF) (Fig. 11, 63) also shows to exert antitumor effects in vivo by suppressing tumor growth via AKT-mediated inhibition of the transcription factor GLI1. In glioma cells, PF treatment could reduce cancer cell migration and invasion by decreasing integrin α6 and uPAR levels, which was mediated by downstream activation of FAK, paxillin, RAC, and ROCK proteins. In breast cancer cell lines, PF treatment inhibited the expression and activation of these downstream proteins. Integrins are an important therapeutic target in various cancer types related to cancer cell metastasis. Hence, reducing integrin expression might suppress the expression of the epithelial-to-mesenchymal transition (EMT) factors, vimentin and Zeb1 to inhibit the cancer metastasis. Furthermore, a combination of PF and temozolomide (TMZ) significantly suppressed tumor growth and prolonged survival in vivo83.
Olanzapine (Fig. 11, 64) is a second-generation anti-psychotic drug mainly used in clinical treatment. It can inhibit cancer cell proliferation, migration and anchorage-independent growth and induce apoptosis, necrosis and cytostasis in tumor cells. It also disrupts cholesterol homeostasis to kill cancer cells84. Similarly, risperidone (Fig. 11, 65) also shows promise as a potential adenocarcinoma treatment by slowing proliferation of PC3 prostate cancer cells85. Clomipramine (Fig. 11, 66) is repurposed for cancer therapy by activating phosphorylation of c-JUN and increasing cytochrome c release and caspase-3-like activation, thus leading to apoptosis of cancer cells86. SH-I-14 (Fig. 11, 67) and KRICT-9 (Fig. 11, 68) are used to treat dementia, but also have new indications for cancer by inhibiting STAT347. Antipsychotic drugs are an important group for repurposing and show promising anticancer effects. Hence, more research is justified to identify their mechanism of action.
3.8. Antidepressants repurposing for cancer therapy
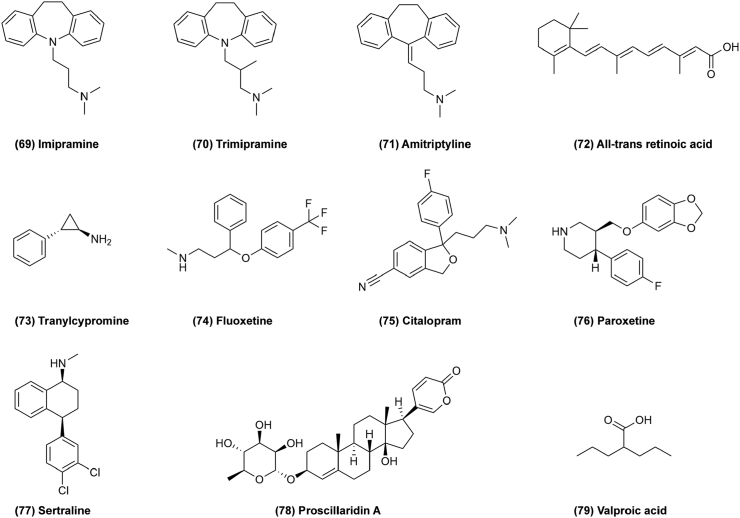
Antidepressants are a group of psychotropic drugs used primarily for the treatment of psychiatric disorders characterized by mood depression. Antidepressants were first introduced in the 1950s, and mainly used to treat depression and various depressive states. Notably, several studies have reported that antidepressants may have antitumor activity. For example, tricyclic antidepressants (TCAs) are the most common first-generation antidepressants containing three fused rings in their structure. Imipramine (Fig. 12, 69) exerts effects on cancers such as glioma, small cell lung cancer (SCLC) and others87. Imipramine induces cell autophagy and cell death by inhibiting P2RY1241. Trimipramine (Fig. 12, 70) and amitriptyline (Fig. 12, 71) are also used to treat cancer88. Recently, it has been reported that combining an antidepressant and a drug derived from vitamin A is a promising way to treat leukemia. All-trans retinoic acid (ATRA) (Fig. 12, 72) has been successfully used to treat acute myeloid leukemia (AML), but it is not effective for other common types of AMLs89. Tranylcypromine (Fig. 12, 73) could also exert effects on AMLs by inducing the leukemic cells to mature and die naturally. Selective serotonin reuptake inhibitors (SSRI) are a new class of antidepressants which were first tested in clinical trials in the 1980s. They reduce cell proliferation and induce apoptosis in cancer cells and downregulate pAKT to mediate the synergistic anti-proliferative interactions with other chemotherapeutic drugs90. For example, fluoxetine (Fig. 12, 74) induces autophagic cell death via eEF2K–AMPK–mTOR–ULK pathway in triple negative breast cancer91. Citalopram (Fig. 12, 75), paroxetine (Fig. 12, 76) and sertraline (Fig. 12, 77) are all SSRIs and show anticancer activity as well92, 93, 94,138. Proscillaridin A (ProA) (Fig. 12, 78) exerts antitumor effects through GSK3β activation in WNT pathway and alteration of microtubule dynamics in glioblastoma95. Valproic acid (VPA; Fig. 12, 79) inhibits histone deacetylase to reduce cancer cell proliferation and induce apoptosis, as well as inducing differentiation and inhibiting angiogenesis in cancer96. Antidepressants mainly inhibit reuptake of amine neurotransmitters, but their antitumor mechanism are more complicated, and more effort should be required for drug repurposing.
Figure 12.
Chemical structures of 69–79 as antidepressants for repurposing in cancer therapy.
3.9. Other drugs repurposing for cancer therapy
3.9.1. Antidiabetics
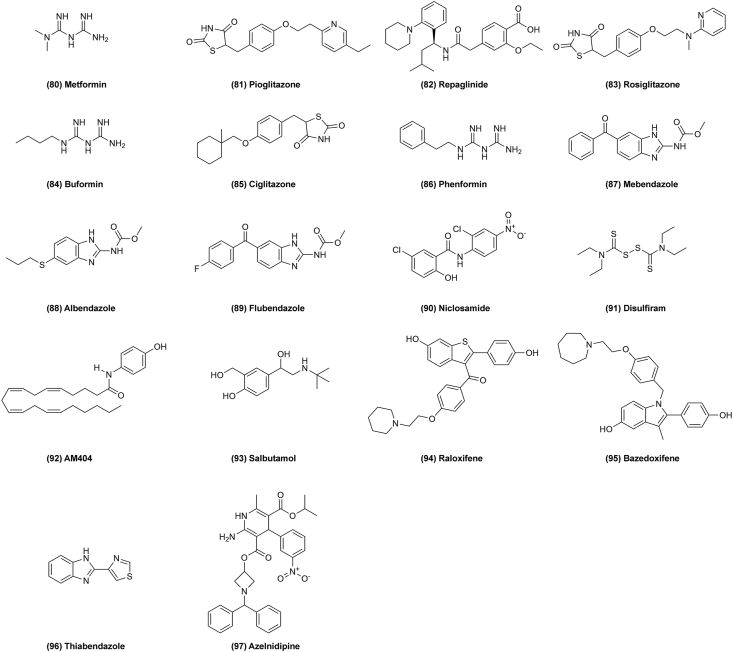
In addition to the above drugs, there are many others that could be repurposed for cancer as well. For example, some of the drugs for type II diabetes show anti-neoplastic effects. Metformin (Fig. 13, 80) is a classic drug for diabetes, and shows effects on several cancers like glioblastoma multiforme, non-small cell lung cancer, rectal cancer, prostate cancer, and ovarian cancer. It might be able to treat cancer by inducing autophagy, apoptosis, and cell death via activating AMPK. In addition, it downregulates the AKT–mTOR signaling pathway and inhibits CLIC1 activity to induce G1 cell arrest97. Pioglitazone (Fig. 13, 81) is also an anti-diabetes drug that might be used to treat lung cancer by its highly selectivity for peroxisome proliferator-activated receptor gamma (PPARγ). It can inhibit β-catenin expression to block the WNT signal pathway and reduce cell viability and proliferation, and induce apoptosis98,99. Repaglinide (Fig 13, 82), rosiglitazone (Fig. 13, 83), buformin (Fig. 13, 84), ciglitazone (Fig. 13, 85) and phenformin (Fig. 13, 86) all have anti-hyperglycemic effects and show anticancer activity100,101,139,140.
Figure 13.
Chemical structures of 80–97 as other drugs for repurposing in cancer therapy.
3.9.2. Anthelminthic drugs
Anthelminthic drugs are also a class of repurposed drugs for cancer treatment. For example, mebendazole (Fig. 13, 87) inhibits proliferation of cancer cell by inducing tubulin depolymerization resulting in mitotic arrest. It leads to P53 accumulation and cell cycle arrest in the G2/M phase, consequently inducing cell apoptosis mediated by cytochrome c, caspase-9, and caspase-3. Additionally, it inhibits TNIK, which is an activator of the WNT/TCF/β-catenin transcriptional program. Inhibition of the BRAF–MEK–ERK signal pathway, which is related to cell growth, proliferation, and survival, is another target of this drug. Mebendazole also upregulates the expression of pro-inflammatory genes to reduce levels of VEGF and VCAM-1 and eventually inhibit tumor growth102. Albendazole (Fig. 13, 88) is also repurposed for antitumor treatment to target the altered redox status of cancer cells, which is an interesting approach for new cancer therapy. It promotes the generation of ROS, thereby oxidizing DNA at C4 of deoxyribose causing DNA breakage and triggering apoptosis as well as inducing cell death by increasing P53 and BAX expression, at the same time as diminishing BCL-XL expression. Albendazole activity appears to be related to tubulin polymerization inhibition which is responsible for separation of duplicated chromosomes during cell division, thereby promoting cell cycle arrest at the G2/M phase103. Flubendazole (Fig. 13, 89) can target ATG4B in breast cancer to induce cell autophagy104. Additionally, it was found to up-regulate the expression of EVA1A, which is related to cell autophagy and apoptosis in triple-negative breast cancer141. Niclosamide (Fig. 13, 90) is an anti-parasitic drug showing antitumor activity in hepatocellular carcinoma through reversing the HCC gene expression signature, based on computational modeling of public data105. In this way, niclosamide and its ethanolamine salt was discovered to bind to the cell division cycle 37 (CDC37) protein and disrupt its interaction with heat shock protein 90 (HSP90) to inhibit tumor cell proliferation. Simultaneously, it also reduces the expression of proteins in the WNT/β-catenin, STAT3, AKT–mTOR, EGFR–RAS–RAF signaling pathways, which are fundamental to HCC development106,107.
3.9.3. Other drugs
Disulfiram (Fig. 13, 91) is used for treating alcoholism but may be repurposed to treat glioma, NSCLC, CRC, and esophageal squamous cell carcinoma. The molecular anticancer target of disulfiram is an adaptor of the human ubiquitin selective protein segregase P97, which plays a role in the turnover of proteins orchestrating stress-related signaling pathways108. AM404 (Fig. 13, 92) is a metabolite of acetaminophen with antibacterial activity, and has been validated as an inducer of cell differentiation and inhibitor of neoplastic cell growth in both in vitro and ex vivo models. It exerts anticancer activity by suppressing oncogenic FBXL5 in colorectal cancer cells to regulate the PTEN/PI3K/AKT signal pathway and HIF-1 transcriptional activity which then results in colon cancer death. To further confirm this result, AM404 is sensitive to FBXL5-knockout cells and causes further additive effects by inhibiting cell migration109. Salbutamol (Fig. 13, 93) is used for bronchial asthma, but is found also to affect cancer by activating the β2 adrenoreceptor110. Raloxifene (Fig. 13, 94) and bazedoxifene (Fig. 13, 95) are also used to treat osteoporosis with potential usefulness for cancer therapy by inhibiting IL-6/GP130 interactions in redirection studies111. Cationic amphiphilic antihistamines have been studied for their anticancer actions as well; they were found to induce lysosomal membrane permeabilization and cell death preferentially in cancer cells112. Thiabendazole (Fig. 13, 96) might be a potential new chemotherapeutic for cancer by disrupting vascular networks113.
More recently, immunotherapy has been the hot topic of cancer treatment and some old drugs have also been repurposed for potential cancer immunotherapy by targeting PD-1/PD-L1, CD47, and TIGIT/PVR. For instance, niclosamide can enhance the cancer cell lysis mediated by T cells in the presence of PD-L1 blockade and delayed the tumor growth and increase the survival which is associated with the increase of tumor infiltrating T cells and granzyme B release. It suggests that the combination therapy of niclosamide and PD-1/PD-L1 blockade is worth to be further studied in clinic107. In addition, azelnidipine (Fig. 13, 97), an anti-hypertensive drug approved by the FDA, can be repositioned for cancer immunotherapy by dual blocking CD47/SIRPα and TIGIT/PVR pathways by co-targeting SIRPα and PVR. In vivo, azelnidipine alone or combined with irradiation can significantly inhibits the growth of MC38 tumors and CT26 tumors by enhancing the infiltration and function of CD8+ T cell in tumor114. As mentioned above, an increasing number of drugs have been repurposed for cancer immunotherapy, indicating a promising potential on cancer therapy.
4. Concluding remarks and future perspectives
Drug repurposing is becoming an attractive therapeutic strategy for exploiting more approved non-oncology drugs to improve potential cancer therapy, which, in turn provides a new avenue for discovery of candidate small-molecule compounds for cancer drug development. It is well-known that discovery of novel antitumor agents is costly and time-consuming; therefore, new strategies for drug repurposing may drastically reduce the research and development cycle because of their safety, toxicity, and pharmacological properties. Accordingly, many FDA-approved drugs or experimental drugs used for treating other diseases can be reused for exploring their potential antitumor effects142. Repurposed drugs can be further investigated according to their off-target effects, which might be inversely related to their therapeutic target in cancer110. Based on the retrospective clinical data, prior knowledge of the side effects of old drugs may have a huge therapeutic potential on different types of human cancers. Interestingly, some of these old drugs have the same target that is related to the of occurrence two or more different diseases; therefore, a drug which retains its original indication can also be used to treat human cancers143.
Presently, a set of new technologies has been emerging for drug repurposing, which would help identifying novel druggable targets for old therapeutic agents. For instance, large-scale multi-omics sequencing containing genomics, proteomics, transcriptomics and phenomics can help understand disease mechanism, elucidate drug mechanism of actions and identify new use of old drugs. Usually, multi-omics is an approach to identify the drug–disease relationships by comparing drug gene expression profiles and disease gene expression profiles with the help of CMap, a key source of data to construct a detailed map for functional associations among diseases, genetic perturbations, and drug actions. For example, antidepressant drugs have been identified for the treatment of small cell lung cancer by using multi-omics sequencing. Another approach is to discover small-molecule drugs that provoke similar transcriptional responses for they can share similar mode of action in one disease. In addition, multi-omics sequencing analyzes gene expression profiles of cancer cells and identify a few of mutated cancer genes that are associated with drug sensitivity may serve as potential biomarkers of drug response by using hundreds of clinical or preclinical drugs for large-scale screening of human cancer cell lines. In addition, genome-wide positioning systems network (GPSnet) algorithms can be used to identify the similarity between drug targets and disease proteins in human protein–protein interaction (PPI) groups, which may be conducive to determining repurposed drug action targets144. With the increasing focus on drug repurposing, there have accumulated a plenty of information about these researches than ever before, therefore it is difficult for scientists to process data and find useful information by themselves. But data processing advances have made a rapid progress in artificial intelligence (AI), which has been also used in drug repurposing by outsource our reasoning to a machine intelligence when it comes to the analysis of multisource and multidimensional data145. Applying a particular AI algorithm in drug repurposing is a sequential process that requires the proper definition of the problem domain. Thus, the first step is to constructing a model which ensures the choice of the machine learning method appropriate for the problem under study. Once the model and the data sets have been established, one may proceed to model training and evaluation. For example, machine learning models implementing AI methods have been successfully applied in several aspects of the virtual screening including structure-based virtual screening (SBVS) and ligand-based virtual screening (LBVS). These approaches greatly improve the drug repurposing process and decrease the cost of drug discovery. Additionally, combinations of multi-omics and AI approaches can be used to clarify the key signaling pathways of cancer progression and metastasis, and further identify druggable targets and relevant mechanisms. They can be applied for modeling biological processes to identify new disease-relevant targets and drug phenotype associations. For example, structure-based virtual screening methods have greatly decreased the costs of drug redirecting processes. Also, molecular docking and ligand-based chemo-informatics can be used to elucidate new drug–target interactions14. More importantly, CRISPR/Cas9 genome editing technology has been developing into a robust tool for identifying drug targets for future cancer therapy by knock-down and knock-out specific genes in cancer cells95,146. High-throughput screening on cell phenotype can be used to discover systematic target–phenotype relationship by screening thousands of compounds in numerous types of cancer cells.
In conclusion, we summarized about 100 small-molecule non-oncology drugs with therapeutic potential, including cardiovascular drugs, microbiological drugs, small-molecule antibiotics, anti-viral drugs, anti-inflammatory drugs, anti-neurodegenerative drugs, antipsychotic drugs, antidepressants, and other drugs in cancer. Moreover, we discussed their potential targets and relevant key pathways for cancer treatment. Although the current success rate of drug reuse is low, it is a promising avenue for discovery of small-molecule drugs for fighting human cancers. With the development of newer biological, chemical, and computational technologies, the bottleneck of drug repurposing should be ultimately overcome; thereby providing a new perspective from old non-oncology drugs to new anticancer agents in the future.
Acknowledgments
This work was supported by Natural Science Foundation of China (Grant Nos. 82172649, 81873089, 31970374, 81803365, and 81873939), The National Key Research and Development Program of China (2021YFE0203100), Key R&D Program of Sichuan Province (Grant No. 2021YFS0046, China), and Applied Basic Research Programs of Science and Technology Department of Sichuan Province (Grant No. 2020YJ0285, China). And, we also thank Wuhan Edisprings Technology & Culture Co., Ltd. (P-202104271435368589) for polishing English language in this manuscript.
Author contributions
Lan Zhang, Bo Liu and Haiyang Yu revised, and submitted the manuscript. Leilei Fu, Wenke Jin, Jiahui Zhang and Lingjuan Zhu wrote the manuscript. Leilei Fu, Wenke Jin, Jia Lu, Yongqi Zhen and Liang Ouyang collected the information. All the authors approved the final manuscript.
Conflicts of interest
The authors declare that they have no conflict of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Lan Zhang, Email: zhanglanx_9@126.com.
Bo Liu, Email: liubo2400@163.com.
Haiyang Yu, Email: hyyu@tjutcm.edu.cn.
References
- 1.Mattiuzzi C., Lippi G. Current cancer epidemiology. J Epidemiol Glob Health. 2019;9:217–222. doi: 10.2991/jegh.k.191008.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Prasad V., Mailankody S. Research and development spending to bring a single cancer drug to market and revenues after approval. JAMA Intern Med. 2017;177:1569–1575. doi: 10.1001/jamainternmed.2017.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaushik I., Ramachandran S., Prasad S., Srivastava S.K. Drug rechanneling: a novel paradigm for cancer treatment. Semin Cancer Biol. 2021;68:279–290. doi: 10.1016/j.semcancer.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal S., Verma S.S., Aggarwal S., Gupta S.C. Drug repurposing for breast cancer therapy: old weapon for new battle. Semin Cancer Biol. 2021;68:8–20. doi: 10.1016/j.semcancer.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basso J., Miranda A., Sousa J., Pais A., Vitorino C. Repurposing drugs for glioblastoma: from bench to bedside. Cancer Lett. 2018;428:173–183. doi: 10.1016/j.canlet.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 7.Sanz M.A., Fenaux P., Tallman M.S., Estey E.H., Lowenberg B., Naoe T., et al. Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European LeukemiaNet. Blood. 2019;133:1630–1643. doi: 10.1182/blood-2019-01-894980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abaza Y., Kantarjian H., Garcia-Manero G., Estey E., Borthakur G., Jabbour E., et al. Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab. Blood. 2017;129:1275–1283. doi: 10.1182/blood-2016-09-736686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimopoulos M.A., Kastritis E. Thalidomide for myeloma: still here?. Lancet Haematol. 2018;5:e439–e440. doi: 10.1016/S2352-3026(18)30154-6. [DOI] [PubMed] [Google Scholar]
- 10.Liu Q., Tong D., Liu G., Gao J., Wang L.A., Xu J., et al. Metformin inhibits prostate cancer progression by targeting tumor-associated inflammatory infiltration. Clin Cancer Res. 2018;24:5622–5634. doi: 10.1158/1078-0432.CCR-18-0420. [DOI] [PubMed] [Google Scholar]
- 11.Wang C., Zhang T., Liao Q., Dai M., Guo J., Yang X., et al. Metformin inhibits pancreatic cancer metastasis caused by SMAD4 deficiency and consequent HNF4G upregulation. Protein Cell. 2021;12:128–144. doi: 10.1007/s13238-020-00760-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quinn B.J., Kitagawa H., Memmott R.M., Gills J.J., Dennis P.A. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol Metabol. 2013;24:469–480. doi: 10.1016/j.tem.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Corsello S.M., Bittker J.A., Liu Z., Gould J., McCarren P., Hirschman J.E., et al. The drug repurposing hub: a next-generation drug library and information resource. Nat Med. 2017;23:405–408. doi: 10.1038/nm.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Issa N.T., Stathias V., Schurer S., Dakshanamurthy S. Machine and deep learning approaches for cancer drug repurposing. Semin Cancer Biol. 2021;68:132–142. doi: 10.1016/j.semcancer.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pushpakom S., Iorio F., Eyers P.A., Escott K.J., Hopper S., Wells A., et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 16.Sleire L., Forde H.E., Netland I.A., Leiss L., Skeie B.S., Enger P.O. Drug repurposing in cancer. Pharmacol Res. 2017;124:74–91. doi: 10.1016/j.phrs.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y., Cao J., Melamed A., Worley M., Gockley A., Jones D., et al. Losartan treatment enhances chemotherapy efficacy and reduces ascites in ovarian cancer models by normalizing the tumor stroma. Proc Natl Acad Sci U S A. 2019;116:2210–2219. doi: 10.1073/pnas.1818357116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chauhan V.P., Martin J.D., Liu H., Lacorre D.A., Jain S.R., Kozin S.V., et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4:2516. doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones M.R., Schrader K.A., Shen Y., Pleasance E., Ch'ng C., Dar N., et al. Response to angiotensin blockade with irbesartan in a patient with metastatic colorectal cancer. Ann Oncol. 2016;27:801–806. doi: 10.1093/annonc/mdw060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin Y.T., Wang H.C., Tsai M.H., Su Y.Y., Yang M.Y., Chien C.Y. Angiotensin II receptor blockers valsartan and losartan improve survival rate clinically and suppress tumor growth via apoptosis related to PI3K/AKT signaling in nasopharyngeal carcinoma. Cancer. 2021;127:1606–1619. doi: 10.1002/cncr.33391. [DOI] [PubMed] [Google Scholar]
- 21.Vallejo Ardila D.L., Walsh K.A., Fifis T., Paolini R., Kastrappis G., Christophi C., et al. Immunomodulatory effects of renin–angiotensin system inhibitors on T lymphocytes in mice with colorectal liver metastases. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y., Ma L., Xu Y., Liu Y., Li W., Cai J., et al. Enalapril overcomes chemoresistance and potentiates antitumor efficacy of 5-FU in colorectal cancer by suppressing proliferation, angiogenesis, and NF-κB/STAT3-regulated proteins. Cell Death Dis. 2020;11:477. doi: 10.1038/s41419-020-2675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fendrich V., Chen N.M., Neef M., Waldmann J., Buchholz M., Feldmann G., et al. The angiotensin-I-converting enzyme inhibitor enalapril and aspirin delay progression of pancreatic intraepithelial neoplasia and cancer formation in a genetically engineered mouse model of pancreatic cancer. Gut. 2010;59:630–637. doi: 10.1136/gut.2009.188961. [DOI] [PubMed] [Google Scholar]
- 24.Zhao L., Zhao Y., Schwarz B., Mysliwietz J., Hartig R., Camaj P., et al. Verapamil inhibits tumor progression of chemotherapy-resistant pancreatic cancer side population cells. Int J Oncol. 2016;49:99–110. doi: 10.3892/ijo.2016.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao H., Jiang L., Lin X., Liang Z.G. Fluvastatin combined with benazepril may contribute to the favorable prognosis of patients with atrial fibrillation. Biomed Pharmacother. 2016;83:687–692. doi: 10.1016/j.biopha.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 26.Gillis R.D., Botteri E., Chang A., Ziegler A.I., Chung N.C., Pon C.K., et al. Carvedilol blocks neural regulation of breast cancer progression in vivo and is associated with reduced breast cancer mortality in patients. Eur J Cancer. 2021;147:106–116. doi: 10.1016/j.ejca.2021.01.029. [DOI] [PubMed] [Google Scholar]
- 27.Partecke L.I., Speerforck S., Kading A., Seubert F., Kuhn S., Lorenz E., et al. Chronic stress increases experimental pancreatic cancer growth, reduces survival and can be antagonised by beta-adrenergic receptor blockade. Pancreatology. 2016;16:423–433. doi: 10.1016/j.pan.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 28.De Giorgi V., Grazzini M., Benemei S., Marchionni N., Botteri E., Pennacchioli E., et al. Propranolol for off-label treatment of patients with melanoma: results from a cohort study. JAMA Oncol. 2018;4 doi: 10.1001/jamaoncol.2017.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Overman J., Fontaine F., Wylie-Sears J., Moustaqil M., Huang L., Meurer M., et al. R-Propranolol is a small molecule inhibitor of the SOX18 transcription factor in a rare vascular syndrome and hemangioma. Elife. 2019;8 doi: 10.7554/eLife.43026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Regulska K., Regulski M., Karolak B., Murias M., Stanisz B. Can cardiovascular drugs support cancer treatment? The rationale for drug repurposing. Drug Discov Today. 2019;24:1059–1065. doi: 10.1016/j.drudis.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Platz E.A., Yegnasubramanian S., Liu J.O., Chong C.R., Shim J.S., Kenfield S.A., et al. A novel two-stage, transdisciplinary study identifies digoxin as a possible drug for prostate cancer treatment. Cancer Discov. 2011;1:68–77. doi: 10.1158/2159-8274.CD-10-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howard C.M., Estrada M., Terrero D., Tiwari A.K., Raman D. Identification of cardiac glycosides as novel inhibitors of eIF4A1-mediated translation in triple-negative breast cancer cells. Cancers (Basel) 2020;12:2169. doi: 10.3390/cancers12082169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma L., Meng Y., Tu C., Cao X., Wang H., Li Y., et al. A cardiac glycoside HTF-1 isolated from Helleborus thibetanus Franch displays potent in vitro anti-cancer activity via caspase-9, MAPK and PI3K-Akt-mTOR pathways. Eur J Med Chem. 2018;158:743–752. doi: 10.1016/j.ejmech.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Rasheduzzaman M., Yin H., Park S.Y. Cardiac glycoside sensitized hepatocellular carcinoma cells to TRAIL via ROS generation, p38MAPK, mitochondrial transition, and autophagy mediation. Mol Carcinog. 2019;58:2040–2051. doi: 10.1002/mc.23096. [DOI] [PubMed] [Google Scholar]
- 35.Pisanti S., Picardi P., Ciaglia E., D'Alessandro A., Bifulco M. Novel prospects of statins as therapeutic agents in cancer. Pharmacol Res. 2014;88:84–98. doi: 10.1016/j.phrs.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 36.Jiao Z., Cai H., Long Y., Sirka O.K., Padmanaban V., Ewald A.J., et al. Statin-induced GGPP depletion blocks macropinocytosis and starves cells with oncogenic defects. Proc Natl Acad Sci U S A. 2020;117:4158–4168. doi: 10.1073/pnas.1917938117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin L., He Z., Yi B., Xue L., Sun J. Simvastatin suppresses human breast cancer cell invasion by decreasing the expression of pituitary tumor-transforming Gene 1. Front Pharmacol. 2020;11:574068. doi: 10.3389/fphar.2020.574068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okubo K., Isono M., Miyai K., Asano T., Sato A. Fluvastatin potentiates anticancer activity of vorinostat in renal cancer cells. Cancer Sci. 2020;111:112–126. doi: 10.1111/cas.14225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones H.M., Fang Z., Sun W., Clark L.H., Stine J.E., Tran A.Q., et al. Atorvastatin exhibits anti-tumorigenic and anti-metastatic effects in ovarian cancer in vitro. Am J Cancer Res. 2017;7:2478–2490. [PMC free article] [PubMed] [Google Scholar]
- 40.Huang S.W., Chyuan I.T., Shiue C., Yu M.C., Hsu Y.F., Hsu M.J. Lovastatin-mediated MCF-7 cancer cell death involves LKB1–AMPK–p38MAPK–p53–survivin signalling cascade. J Cell Mol Med. 2020;24:1822–1836. doi: 10.1111/jcmm.14879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shchors K., Massaras A., Hanahan D. Dual targeting of the autophagic regulatory circuitry in gliomas with repurposed drugs elicits cell-lethal autophagy and therapeutic benefit. Cancer Cell. 2015;28:456–471. doi: 10.1016/j.ccell.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Assad Kahn S., Costa S.L., Gholamin S., Nitta R.T., Dubois L.G., Feve M., et al. The anti-hypertensive drug prazosin inhibits glioblastoma growth via the PKCdelta-dependent inhibition of the AKT pathway. EMBO Mol Med. 2016;8:511–526. doi: 10.15252/emmm.201505421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo Y., Xie C., Brocker C.N., Fan J., Wu X., Feng L., et al. Intestinal PPARalpha protects against colon carcinogenesis via regulation of methyltransferases DNMT1 and PRMT6. Gastroenterology. 2019;157:744–759 e4. doi: 10.1053/j.gastro.2019.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varisli L., Cen O., Vlahopoulos S. Dissecting pharmacological effects of chloroquine in cancer treatment: interference with inflammatory signaling pathways. Immunology. 2020;159:257–278. doi: 10.1111/imm.13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin G., Zhao C., Zhang L., Liu H., Quan Y., Chai L., et al. Dihydroartemisinin induces apoptosis preferentially via a Bim-mediated intrinsic pathway in hepatocarcinoma cells. Apoptosis. 2015;20:1072–1086. doi: 10.1007/s10495-015-1132-2. [DOI] [PubMed] [Google Scholar]
- 46.Wong Y.K., Xu C., Kalesh K.A., He Y., Lin Q., Wong W.S.F., et al. Artemisinin as an anticancer drug: recent advances in target profiling and mechanisms of action. Med Res Rev. 2017;37:1492–1517. doi: 10.1002/med.21446. [DOI] [PubMed] [Google Scholar]
- 47.Thilakasiri P.S., Dmello R.S., Nero T.L., Parker M.W., Ernst M., Chand A.L. Repurposing of drugs as STAT3 inhibitors for cancer therapy. Semin Cancer Biol. 2021;68:31–46. doi: 10.1016/j.semcancer.2019.09.022. [DOI] [PubMed] [Google Scholar]
- 48.Oien D.B., Pathoulas C.L., Ray U., Thirusangu P., Kalogera E., Shridhar V. Repurposing quinacrine for treatment-refractory cancer. Semin Cancer Biol. 2021;68:21–30. doi: 10.1016/j.semcancer.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 49.Qiao S., Tao S., Rojo de la Vega M., Park S.L., Vonderfecht A.A., Jacobs S.L., et al. The antimalarial amodiaquine causes autophagic-lysosomal and proliferative blockade sensitizing human melanoma cells to starvation- and chemotherapy-induced cell death. Autophagy. 2013;9:2087–2102. doi: 10.4161/auto.26506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gozari M., Alborz M., El-Seedi H.R., Jassbi A.R. Chemistry, biosynthesis and biological activity of terpenoids and meroterpenoids in bacteria and fungi isolated from different marine habitats. Eur J Med Chem. 2021;210:112957. doi: 10.1016/j.ejmech.2020.112957. [DOI] [PubMed] [Google Scholar]
- 51.Antonarakis E.S., Heath E.I., Smith D.C., Rathkopf D., Blackford A.L., Danila D.C., et al. Repurposing itraconazole as a treatment for advanced prostate cancer: a noncomparative randomized phase II trial in men with metastatic castration-resistant prostate cancer. Oncol. 2013;18:163–173. doi: 10.1634/theoncologist.2012-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu R., Li J., Zhang T., Zou L., Chen Y., Wang K., et al. Itraconazole suppresses the growth of glioblastoma through induction of autophagy: involvement of abnormal cholesterol trafficking. Autophagy. 2014;10:1241–1255. doi: 10.4161/auto.28912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agnihotri S., Mansouri S., Burrell K., Li M., Mamatjan Y., Liu J., et al. Ketoconazole and posaconazole selectively target HK2-expressing glioblastoma cells. Clin Cancer Res. 2019;25:844–855. doi: 10.1158/1078-0432.CCR-18-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Y., Chen H.N., Wang K., Zhang L., Huang Z., Liu J., et al. Ketoconazole exacerbates mitophagy to induce apoptosis by downregulating cyclooxygenase-2 in hepatocellular carcinoma. J Hepatol. 2019;70:66–77. doi: 10.1016/j.jhep.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 55.Liu P.F., Tsai K.L., Hsu C.J., Tsai W.L., Cheng J.S., Chang H.W., et al. Drug repurposing screening identifies tioconazole as an ATG4 inhibitor that suppresses autophagy and sensitizes cancer cells to chemotherapy. Theranostics. 2018;8:830–845. doi: 10.7150/thno.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Noort V., Scholch S., Iskar M., Zeller G., Ostertag K., Schweitzer C., et al. Novel drug candidates for the treatment of metastatic colorectal cancer through global inverse gene-expression profiling. Cancer Res. 2014;74:5690–5699. doi: 10.1158/0008-5472.CAN-13-3540. [DOI] [PubMed] [Google Scholar]
- 57.Gupta P., Gao H.L., Ashar Y.V., Karadkhelkar N.M., Yoganathan S., Chen Z.S. Ciprofloxacin enhances the chemosensitivity of cancer cells to ABCB1 substrates. Int J Mol Sci. 2019;20:268. doi: 10.3390/ijms20020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fan M., Chen S., Weng Y., Li X., Jiang Y., Wang X., et al. Ciprofloxacin promotes polarization of CD86+CD206– macrophages to suppress liver cancer. Oncol Rep. 2020;44:91–102. doi: 10.3892/or.2020.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tiberi S., du Plessis N., Walzl G., Vjecha M.J., Rao M., Ntoumi F., et al. Tuberculosis: progress and advances in development of new drugs, treatment regimens, and host-directed therapies. Lancet Infect Dis. 2018;18:e183–e198. doi: 10.1016/S1473-3099(18)30110-5. [DOI] [PubMed] [Google Scholar]
- 60.Fiorillo M., Lamb R., Tanowitz H.B., Cappello A.R., Martinez-Outschoorn U.E., Sotgia F., et al. Bedaquiline, an FDA-approved antibiotic, inhibits mitochondrial function and potently blocks the proliferative expansion of stem-like cancer cells (CSCs) Aging (Albany NY) 2016;8:1593–1607. doi: 10.18632/aging.100983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen K., Lu P., Beeraka N.M., Sukocheva O.A., Madhunapantula S.V., Liu J., et al. Mitochondrial mutations and mitoepigenetics: focus on regulation of oxidative stress-induced responses in breast cancers. Semin Cancer Biol. 2020 doi: 10.1016/j.semcancer.2020.09.012. Available from: [DOI] [PubMed] [Google Scholar]
- 62.Dong Z., Abbas M.N., Kausar S., Yang J., Li L., Tan L., et al. Biological functions and molecular mechanisms of antibiotic tigecycline in the treatment of cancers. Int J Mol Sci. 2019;20:3577. doi: 10.3390/ijms20143577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang M., Shim J.S., Li R.J., Dang Y., He Q., Das M., et al. Identification of an old antibiotic clofoctol as a novel activator of unfolded protein response pathways and an inhibitor of prostate cancer. Br J Pharmacol. 2014;171:4478–4489. doi: 10.1111/bph.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar S., Bryant C.S., Chamala S., Qazi A., Seward S., Pal J., et al. Ritonavir blocks AKT signaling, activates apoptosis and inhibits migration and invasion in ovarian cancer cells. Mol Cancer. 2009;8:26. doi: 10.1186/1476-4598-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith M.P., Brunton H., Rowling E.J., Ferguson J., Arozarena I., Miskolczi Z., et al. Inhibiting drivers of non-mutational drug tolerance is a salvage strategy for targeted melanoma therapy. Cancer Cell. 2016;29:270–284. doi: 10.1016/j.ccell.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meyn R.E., Krishnan S., Skinner H.D. Everything old is new again: using nelfinavir to radiosensitize rectal cancer. Clin Cancer Res. 2016;22:1834–1836. doi: 10.1158/1078-0432.CCR-16-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiao X., Nawab O., Patel T., Kossenkov A.V., Halama N., Jaeger D., et al. Recent advances targeting CCR5 for cancer and its role in immuno-oncology. Cancer Res. 2019;79:4801–4807. doi: 10.1158/0008-5472.CAN-19-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jin K., Pandey N.B., Popel A.S. Simultaneous blockade of IL-6 and CCL5 signaling for synergistic inhibition of triple-negative breast cancer growth and metastasis. Breast Cancer Res. 2018;20:54. doi: 10.1186/s13058-018-0981-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zeng L.F., Wang Y., Kazemi R., Xu S., Xu Z.L., Sanchez T.W., et al. Repositioning HIV-1 integrase inhibitors for cancer therapeutics: 1,6-naphthyridine-7-carboxamide as a promising scaffold with drug-like properties. J Med Chem. 2012;55:9492–9509. doi: 10.1021/jm300667v. [DOI] [PubMed] [Google Scholar]
- 70.Drew D.A., Cao Y., Chan A.T. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer. 2016;16:173–186. doi: 10.1038/nrc.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kmietowicz Z. Study finds possible role for aspirin as treatment for colon cancer. BMJ. 2012;344 doi: 10.1136/bmj.e2988. [DOI] [PubMed] [Google Scholar]
- 72.Han Y., Chen P., Zhang Y., Lu W., Ding W., Luo Y., et al. Synergy between auranofin and celecoxib against colon cancer in vitro and in vivo through a novel redox-mediated mechanism. Cancers (Basel) 2019;11:931. doi: 10.3390/cancers11070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang L., Wang H., Yang X., Wu Q., An P., Jin X., et al. Auranofin mitigates systemic iron overload and induces ferroptosis via distinct mechanisms. Signal Transduct Target Ther. 2020;5:138. doi: 10.1038/s41392-020-00253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zuo C., Hong Y., Qiu X., Yang D., Liu N., Sheng X., et al. Celecoxib suppresses proliferation and metastasis of pancreatic cancer cells by down-regulating STAT3/NF-κB and L1CAM activities. Pancreatology. 2018;18:328–333. doi: 10.1016/j.pan.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 75.de Souza C.O., Kurauti M.A., Silva Fde F., de Morais H., Curi R., Hirabara S.M., et al. Celecoxib and ibuprofen restore the ATP content and the gluconeogenesis activity in the liver of walker-256 tumor-bearing rats. Cell Physiol Biochem. 2015;36:1659–1669. doi: 10.1159/000430326. [DOI] [PubMed] [Google Scholar]
- 76.Sogawa C., Eguchi T., Tran M.T., Ishige M., Trin K., Okusha Y., et al. Antiparkinson drug benztropine suppresses tumor growth, circulating tumor cells, and metastasis by acting on SLC6A3/DAT and reducing STAT3. Cancers (Basel) 2020;12:523. doi: 10.3390/cancers12020523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li W., Zheng M., Wu S., Gao S., Yang M., Li Z., et al. Benserazide, a dopadecarboxylase inhibitor, suppresses tumor growth by targeting hexokinase 2. J Exp Clin Cancer Res. 2017;36:58. doi: 10.1186/s13046-017-0530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Priyanka H.P., Singh R.V., Mishra M., ThyagaRajan S. Diverse age-related effects of Bacopa monnieri and donepezil in vitro on cytokine production, antioxidant enzyme activities, and intracellular targets in splenocytes of F344 male rats. Int Immunopharm. 2013;15:260–274. doi: 10.1016/j.intimp.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 79.Kreutzwiser D., Tawfic Q.A. Expanding role of NMDA receptor antagonists in the management of pain. CNS Drugs. 2019;33:347–374. doi: 10.1007/s40263-019-00618-2. [DOI] [PubMed] [Google Scholar]
- 80.Seol H.S., Lee S.E., Song J.S., Lee H.Y., Park S., Kim I., et al. Glutamate release inhibitor, riluzole, inhibited proliferation of human hepatocellular carcinoma cells by elevated ROS production. Cancer Lett. 2016;382:157–165. doi: 10.1016/j.canlet.2016.08.028. [DOI] [PubMed] [Google Scholar]
- 81.Abbruzzese C., Matteoni S., Persico M., Villani V., Paggi M.G. Repurposing chlorpromazine in the treatment of glioblastoma multiforme: analysis of literature and forthcoming steps. J Exp Clin Cancer Res. 2020;39:26. doi: 10.1186/s13046-020-1534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu F., Xia Y., Feng Z., Lin W., Xue Q., Jiang J., et al. Repositioning antipsychotic fluphenazine hydrochloride for treating triple negative breast cancer with brain metastases and lung metastases. Am J Cancer Res. 2019;9:459–478. [PMC free article] [PubMed] [Google Scholar]
- 83.Kim H., Chong K., Ryu B.K., Park K.J., Yu M.O., Lee J., et al. Repurposing penfluridol in combination with temozolomide for the treatment of glioblastoma. Cancers (Basel) 2019;11:1310. doi: 10.3390/cancers11091310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wiklund E.D., Catts V.S., Catts S.V., Ng T.F., Whitaker N.J., Brown A.J., et al. Cytotoxic effects of antipsychotic drugs implicate cholesterol homeostasis as a novel chemotherapeutic target. Int J Cancer. 2010;126:28–40. doi: 10.1002/ijc.24813. [DOI] [PubMed] [Google Scholar]
- 85.Dilly S.J., Clark A.J., Marsh A., Mitchell D.A., Cain R., Fishwick C.W.G., et al. A chemical genomics approach to drug reprofiling in oncology: antipsychotic drug risperidone as a potential adenocarcinoma treatment. Cancer Lett. 2017;393:16–21. doi: 10.1016/j.canlet.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 86.Hicks J.K., Sangkuhl K., Swen J.J., Ellingrod V.L., Muller D.J., Shimoda K., et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin Pharmacol Ther. 2017;102:37–44. doi: 10.1002/cpt.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang J., Byers L.A. Teaching an old dog new tricks: drug repositioning in small cell lung cancer. Cancer Discov. 2013;3:1333–1335. doi: 10.1158/2159-8290.CD-13-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jahchan N.S., Dudley J.T., Mazur P.K., Flores N., Yang D., Palmerton A., et al. A drug repositioning approach identifies tricyclic antidepressants as inhibitors of small cell lung cancer and other neuroendocrine tumors. Cancer Discov. 2013;3:1364–1377. doi: 10.1158/2159-8290.CD-13-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nguyen C.H., Bauer K., Hackl H., Schlerka A., Koller E., Hladik A., et al. All-trans retinoic acid enhances, and a pan-RAR antagonist counteracts, the stem cell promoting activity of EVI1 in acute myeloid leukemia. Cell Death Dis. 2019;10:944. doi: 10.1038/s41419-019-2172-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang J., Zhao D., Liu Z., Liu F. Repurposing psychiatric drugs as anti-cancer agents. Cancer Lett. 2018;419:257–265. doi: 10.1016/j.canlet.2018.01.058. [DOI] [PubMed] [Google Scholar]
- 91.Sun D., Zhu L., Zhao Y., Jiang Y., Chen L., Yu Y., et al. Fluoxetine induces autophagic cell death via eEF2K–AMPK–mTOR–ULK complex axis in triple negative breast cancer. Cell Prolif. 2018;51 doi: 10.1111/cpr.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Antoszczak M., Markowska A., Markowska J., Huczynski A. Antidepressants and antipsychotic agents as repurposable oncological drug candidates. Curr Med Chem. 2021;28:2137–2174. doi: 10.2174/0929867327666200907141452. [DOI] [PubMed] [Google Scholar]
- 93.Cho Y.W., Kim E.J., Nyiramana M.M., Shin E.J., Jin H., Ryu J.H., et al. Paroxetine induces apoptosis of human breast cancer MCF-7 cells through Ca2+-and p38 MAP kinase-dependent ROS generation. Cancers (Basel) 2019;11:64. doi: 10.3390/cancers11010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chinnapaka S., Bakthavachalam V., Munirathinam G. Repurposing antidepressant sertraline as a pharmacological drug to target prostate cancer stem cells: dual activation of apoptosis and autophagy signaling by deregulating redox balance. Am J Cancer Res. 2020;10:2043–2065. [PMC free article] [PubMed] [Google Scholar]
- 95.Berges R., Denicolai E., Tchoghandjian A., Baeza-Kallee N., Honore S., Figarella-Branger D., et al. Proscillaridin A exerts anti-tumor effects through GSK3beta activation and alteration of microtubule dynamics in glioblastoma. Cell Death Dis. 2018;9:984. doi: 10.1038/s41419-018-1018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park H.K., Han B.R., Park W.H. Combination of arsenic trioxide and valproic acid efficiently inhibits growth of lung cancer cells via G2/M-phase arrest and apoptotic cell death. Int J Mol Sci. 2020;21:2649. doi: 10.3390/ijms21072649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee W.H., Loo C.Y., Ghadiri M., Leong C.R., Young P.M., Traini D. The potential to treat lung cancer via inhalation of repurposed drugs. Adv Drug Deliv Rev. 2018;133:107–130. doi: 10.1016/j.addr.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 98.Cilibrasi C., Butta V., Riva G., Bentivegna A. Pioglitazone effect on glioma stem cell lines: really a promising drug therapy for glioblastoma?. PPAR Res. 2016;2016 doi: 10.1155/2016/7175067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wan Z., Shi W., Shao B., Shi J., Shen A., Ma Y., et al. Peroxisome proliferator-activated receptor gamma agonist pioglitazone inhibits beta-catenin-mediated glioma cell growth and invasion. Mol Cell Biochem. 2011;349:1–10. doi: 10.1007/s11010-010-0637-9. [DOI] [PubMed] [Google Scholar]
- 100.Ishay-Ronen D., Diepenbruck M., Kalathur R.K.R., Sugiyama N., Tiede S., Ivanek R., et al. Gain fat-lose metastasis: converting invasive breast cancer cells into adipocytes inhibits cancer metastasis. Cancer Cell. 2019;35 doi: 10.1016/j.ccell.2018.12.002. 17-32.e6. [DOI] [PubMed] [Google Scholar]
- 101.Parris A.B., Zhao Q., Howard E.W., Zhao M., Ma Z., Yang X. Buformin inhibits the stemness of ErbB-2-overexpressing breast cancer cells and premalignant mammary tissues of MMTV-ErbB-2 transgenic mice. J Exp Clin Cancer Res. 2017;36:28. doi: 10.1186/s13046-017-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guerini A.E., Triggiani L., Maddalo M., Bonù M.L., Frassine F., Baiguini A., et al. Mebendazole as a candidate for drug repurposing in oncology: an extensive review of current literature. Cancers (Basel) 2019;11:1284. doi: 10.3390/cancers11091284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Castro L.S., Kviecinski M.R., Ourique F., Parisotto E.B., Grinevicius V.M., Correia J.F., et al. Albendazole as a promising molecule for tumor control. Redox Biol. 2016;10:90–99. doi: 10.1016/j.redox.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang L., Guo M., Li J., Zheng Y., Zhang S., Xie T., et al. Systems biology-based discovery of a potential Atg4B agonist (flubendazole) that induces autophagy in breast cancer. Mol Biosyst. 2015;11:2860–2866. doi: 10.1039/c5mb00466g. [DOI] [PubMed] [Google Scholar]
- 105.Chen B., Wei W., Ma L., Yang B., Gill R.M., Chua M.S., et al. Computational discovery of niclosamide ethanolamine, a repurposed drug candidate that reduces growth of hepatocellular carcinoma cells in vitro and in mice by inhibiting cell division cycle 37 signaling. Gastroenterology. 2017;152:2022–2036. doi: 10.1053/j.gastro.2017.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lu L., Dong J., Wang L., Xia Q., Zhang D., Kim H., et al. Activation of STAT3 and Bcl-2 and reduction of reactive oxygen species (ROS) promote radioresistance in breast cancer and overcome of radioresistance with niclosamide. Oncogene. 2018;37:5292–5304. doi: 10.1038/s41388-018-0340-y. [DOI] [PubMed] [Google Scholar]
- 107.Luo F., Luo M., Rong Q.X., Zhang H., Chen Z., Wang F., et al. Niclosamide, an antihelmintic drug, enhances efficacy of PD-1/PD-L1 immune checkpoint blockade in non-small cell lung cancer. J Immunother Cancer. 2019;7:245. doi: 10.1186/s40425-019-0733-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Siegelin M.D., Schneider E., Westhoff M.A., Wirtz C.R., Karpel-Massler G. Current state and future perspective of drug repurposing in malignant glioma. Semin Cancer Biol. 2021;68:92–104. doi: 10.1016/j.semcancer.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 109.Ahmed M., Jinks N., Babaei-Jadidi R., Kashfi H., Castellanos-Uribe M., May S.T., et al. Repurposing antibacterial AM404 as a potential anticancer drug for targeting colorectal cancer stem-like cells. Cancers (Basel) 2019;12:106. doi: 10.3390/cancers12010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim J.H., Lee J., Park K.S., Hong S.W., Gho Y.S. Drug repositioning to alleviate systemic inflammatory response syndrome caused by Gram-negative bacterial outer membrane vesicles. Adv Healthc Mater. 2018;7 doi: 10.1002/adhm.201701476. [DOI] [PubMed] [Google Scholar]
- 111.Li H., Xiao H., Lin L., Jou D., Kumari V., Lin J., et al. Drug design targeting protein-protein interactions (PPIs) using multiple ligand simultaneous docking (MLSD) and drug repositioning: discovery of raloxifene and bazedoxifene as novel inhibitors of IL-6/GP130 interface. J Med Chem. 2014;57:632–641. doi: 10.1021/jm401144z. [DOI] [PubMed] [Google Scholar]
- 112.Ellegaard A.M., Dehlendorff C., Vind A.C., Anand A., Cederkvist L., Petersen N.H.T., et al. Repurposing cationic amphiphilic antihistamines for cancer treatment. EBioMedicine. 2016;9:130–139. doi: 10.1016/j.ebiom.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cha H.J., Byrom M., Mead P.E., Ellington A.D., Wallingford J.B., Marcotte E.M. Evolutionarily repurposed networks reveal the well-known antifungal drug thiabendazole to be a novel vascular disrupting agent. PLoS Biol. 2012;10 doi: 10.1371/journal.pbio.1001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhou X., Jiao L., Qian Y., Dong Q., Sun Y., Zheng W.V., et al. Repositioning azelnidipine as a dual inhibitor targeting CD47/SIRPalpha and TIGIT/PVR pathways for cancer immuno-therapy. Biomolecules. 2021;11:706. doi: 10.3390/biom11050706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ishida J., Konishi M., Ebner N., Springer J. Repurposing of approved cardiovascular drugs. J Transl Med. 2016;14:269. doi: 10.1186/s12967-016-1031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Murphy J.E., Wo J.Y., Ryan D.P., Clark J.W., Jiang W. Yeap BY, et al. Total neoadjuvant therapy with FOLFIRINOX in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5:1020–1027. doi: 10.1001/jamaoncol.2019.0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huang K.M., Liang S., Yeung S., Oiyemhonlan E., Cleveland K.H., Parsa C., et al. Topically applied carvedilol attenuates solar ultraviolet radiation induced skin carcinogenesis. Cancer Prev Res (Phila) 2017;10:598–606. doi: 10.1158/1940-6207.CAPR-17-0132. [DOI] [PubMed] [Google Scholar]
- 118.Kim J., Grotegut C.A., Wisler J.W., Mao L., Rosenberg P.B., Rockman H.A., et al. The beta-arrestin-biased beta-adrenergic receptor blocker carvedilol enhances skeletal muscle contractility. Proc Natl Acad Sci U S A. 2020;117:12435–12443. doi: 10.1073/pnas.1920310117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Erguven M., Yazihan N., Aktas E., Sabanci A., Li C.J., Oktem G., et al. Carvedilol in glioma treatment alone and with imatinib in vitro. Int J Oncol. 2010;36:857–866. doi: 10.3892/ijo_00000563. [DOI] [PubMed] [Google Scholar]
- 120.Guglin M., Krischer J., Tamura R., Fink A., Bello-Matricaria L., McCaskill-Stevens W., et al. Randomized trial of lisinopril versus carvedilol to prevent trastuzumab cardiotoxicity in patients with breast cancer. J Am Coll Cardiol. 2019;73:2859–2868. doi: 10.1016/j.jacc.2019.03.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fumagalli C., Maurizi N., Marchionni N., Fornasari D. Beta-blockers: their new life from hypertension to cancer and migraine. Pharmacol Res. 2020;151:104587. doi: 10.1016/j.phrs.2019.104587. [DOI] [PubMed] [Google Scholar]
- 122.Karasneh R.A., Murray L.J., Cardwell C.R. Cardiac glycosides and breast cancer risk: a systematic review and meta-analysis of observational studies. Int J Cancer. 2017;140:1035–1041. doi: 10.1002/ijc.30520. [DOI] [PubMed] [Google Scholar]
- 123.Kaushik V., Azad N., Yakisich J.S., Iyer A.K. Antitumor effects of naturally occurring cardiac glycosides convallatoxin and peruvoside on human ER+ and triple-negative breast cancers. Cell Death Dis. 2017;3:17009. doi: 10.1038/cddiscovery.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bytautaite M., Petrikaite V. Comparative study of lipophilic statin activity in 2D and 3D in vitro models of human breast cancer cell lines MDA-MB-231 and MCF-7. OncoTargets Ther. 2020;13:13201–13209. doi: 10.2147/OTT.S283033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mezquita B., Mezquita P., Pau M., Gasa L., Navarro L., Samitier M., et al. All-trans-retinoic acid activates the pro-invasive Src-YAP-interleukin 6 axis in triple-negative MDA-MB-231 breast cancer cells while cerivastatin reverses this action. Sci Rep. 2018;8:7047. doi: 10.1038/s41598-018-25526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mei Z., Liang M., Li L., Zhang Y., Wang Q., Yang W. Effects of statins on cancer mortality and progression: a systematic review and meta-analysis of 95 cohorts including 1,111,407 individuals. Int J Cancer. 2017;140:1068–1081. doi: 10.1002/ijc.30526. [DOI] [PubMed] [Google Scholar]
- 127.Wang L., Sun Y. Efflux mechanism and pathway of verapamil pumping by human P-glycoprotein. Arch Biochem Biophys. 2020;696:108675. doi: 10.1016/j.abb.2020.108675. [DOI] [PubMed] [Google Scholar]
- 128.Muniyan S., Rachagani S., Parte S., Halder S., Seshacharyulu P., Kshirsagar P., et al. Sildenafil potentiates the therapeutic efficacy of docetaxel in advanced prostate cancer by stimulating NO–cGMP signaling. Clin Cancer Res. 2020;26:5720–5734. doi: 10.1158/1078-0432.CCR-20-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Haas N.B., Appleman L.J., Stein M., Redlinger M., Wilks M., Xu X., et al. Autophagy inhibition to augment mTOR inhibition: a phase I/II trial of everolimus and hydroxychloroquine in patients with previously treated renal cell carcinoma. Clin Cancer Res. 2019;25:2080–2087. doi: 10.1158/1078-0432.CCR-18-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Efferth T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin Cancer Biol. 2017;46:65–83. doi: 10.1016/j.semcancer.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 131.Bailly C., Vergoten G. A new horizon for the old antibacterial drug clofoctol. Drug Discov Today. 2021;26:1302–1310. doi: 10.1016/j.drudis.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 132.Hu Y., Zhang M., Tian N., Li D., Wu F., Hu P., et al. The antibiotic clofoctol suppresses glioma stem cell proliferation by activating KLF13. J Clin Invest. 2019;129:3072–3085. doi: 10.1172/JCI124979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rauschenbach L., Wieland A., Reinartz R., Kebir S., Till A., Darkwah Oppong M., et al. Drug repositioning of antiretroviral ritonavir for combinatorial therapy in glioblastoma. Eur J Cancer. 2020;140:130–139. doi: 10.1016/j.ejca.2020.09.017. [DOI] [PubMed] [Google Scholar]
- 134.Marima R., Hull R., Dlamini Z., Penny C. Efavirenz and lopinavir/ritonavir alter cell cycle regulation in lung cancer. Front Oncol. 2020;10:1693. doi: 10.3389/fonc.2020.01693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brasky T.M., Felix A.S., Cohn D.E., McMeekin D.S., Mutch D.G., Creasman W.T., et al. Nonsteroidal anti-inflammatory drugs and endometrial carcinoma mortality and recurrence. J Natl Cancer Inst. 2017;109:1–10. doi: 10.1093/jnci/djw251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dugger B.N., Dickson D.W. Pathology of neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2017;9:a028035. doi: 10.1101/cshperspect.a028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cerles O., Goncalves T.C., Chouzenoux S., Benoit E., Schmitt A., Bennett Saidu N.E., et al. Preventive action of benztropine on platinum-induced peripheral neuropathies and tumor growth. Acta Neuropathol Commun. 2019;7:9. doi: 10.1186/s40478-019-0657-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ray W.A., Chung C.P., Murray K.T., Hall K., Stein C.M. High-dose citalopram and escitalopram and the risk of out-of-hospital death. J Clin Psychiatr. 2017;78:190–195. doi: 10.4088/JCP.15m10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim T.W., Hong D.W., Hong S.H. CB13, a novel PPARgamma ligand, overcomes radio-resistance via ROS generation and ER stress in human non-small cell lung cancer. Cell Death Dis. 2020;11:848. doi: 10.1038/s41419-020-03065-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Salcher S., Spoden G., Huber J.M., Golderer G., Lindner H., Ausserlechner M.J., et al. Repaglinide silences the FOXO3/Lumican axis and represses the associated metastatic potential of neuronal cancer cells. Cells. 2019;9:1. doi: 10.3390/cells9010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhen Y., Zhao R., Wang M., Jiang X., Gao F., Fu L., et al. Flubendazole elicits anti-cancer effects via targeting EVA1A-modulated autophagy and apoptosis in triple-negative breast cancer. Theranostics. 2020;10:8080–8097. doi: 10.7150/thno.43473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nowak-Sliwinska P., Scapozza L., Ruiz i Altaba A. Drug repurposing in oncology: compounds, pathways, phenotypes and computational approaches for colorectal cancer. Biochim Biophys Acta Rev Cancer. 2019;1871:434–454. doi: 10.1016/j.bbcan.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dinic J., Efferth T., Garcia-Sosa A.T., Grahovac J., Padron J.M., Pajeva I., et al. Repurposing old drugs to fight multidrug resistant cancers. Drug Resist Updates. 2020;52:100713. doi: 10.1016/j.drup.2020.100713. [DOI] [PubMed] [Google Scholar]
- 144.Cheng F., Lu W., Liu C., Fang J., Hou Y., Handy D.E., et al. A genome-wide positioning systems network algorithm for in silico drug repurposing. Nat Commun. 2019;10:3476. doi: 10.1038/s41467-019-10744-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang X., Wang Y., Byrne R., Schneider G., Yang S. Concepts of artificial intelligence for computer-assisted drug discovery. Chem Rev. 2019;119:10520–10594. doi: 10.1021/acs.chemrev.8b00728. [DOI] [PubMed] [Google Scholar]
- 146.Wang H., Xu X., Nguyen C.M., Liu Y., Gao Y., Lin X., et al. Crispr-mediated programmable 3D genome positioning and nuclear organization. Cell. 2018;175 doi: 10.1016/j.cell.2018.09.013. 1405-1417.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]