Abstract
Ventricular septal rupture (VSR) is a rare but highly lethal (∼60%) mechanical complication of myocardial infarction (MI). Although surgical repair has been the gold standard to correct the structural anomaly, percutaneous closure of the defect may represent a valuable therapeutic alternative, with the advantage of immediate shunt reduction to prevent further hemodynamic deterioration in patients with prohibitive surgical risk. Nonetheless, catheter-based VSR closure has faced certain drawbacks that have hampered its application. We describe a clinical case of postinfarction VSR treated with a percutaneous closure device and discuss the procedure’s failure mechanism. (Level of Difficulty: Intermediate.)
Key Words: acute myocardial infarction, mechanical complication, percutaneous closure device, percutaneous septal defect closure, ventricular septal defect, ventricular septal rupture
Abbreviations and Acronyms: ASD, atrial septal defect; CMR, cardiac magnetic resonance; CT, computed tomography; IABP, intra-aortic balloon pump; LAD, left anterior descending; MI, myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment myocardial infarction; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography; VSD, ventricular septal defect; VSR, ventricular septal rupture
Central Illustration
A 57-year-old woman with no previous medical history presented to the emergency department because of a 7-hour history of chest pain and severe shortness of breath. On admission, her blood pressure was 70/44 mm Hg, her pulse was 128 beats/min with a regular rhythm, her respirations were 30 breaths/min, her oxygen saturation on room air was 92%, and her temperature 98.1 °F. Examination showed a patient in distress, mildly lethargic, with white, cold, and clammy extremities. There was jugular venous distention and capillary refill of 3 seconds. On auscultation, a harsh, loud pansystolic murmur was detected at the left parasternal margin. Bibasilar crackles were present. An electrocardiogram (ECG) demonstrated sinus tachycardia and 2- to 4-mm ST-segment elevation in leads V2 to V6 with prominent Q waves (Figure 1A). A chest radiograph demonstrated significant pulmonary vascular congestion (Figure 1B).
Learning Objectives
-
•
To recognize the presence of postinfarction VSR and its potential differentials in patients who present with STEMI and hemodynamic instability.
-
•
To know the different treatment strategies, their best timing and success rate, and when to select 1 modality over the other based on the available evidence.
-
•
To discuss the technical aspects, outcomes, and potential late complication (“failure mechanism”) of percutaneous closure of postinfarction VSR based on the pathophysiology of the infarcted myocardium.
Figure 1.
Initial Imaging
(A) Electrocardiogram and (B) chest radiograph on presentation.
Question 1: What is the differential diagnosis, and what test would you suggest should be performed first?
The patient is in cardiogenic shock as a result of a severe presentation of an ST-segment elevation myocardial infarction (STEMI) suggesting a large area of myocardial involvement leading to a critical decrease in cardiac output, an acute mechanical complication, or both. Considering the characteristics of the murmur, our first thought would be to rule out an acute mechanical complication of myocardial infarction (MI). Thus, the first test to obtain would be a transthoracic echocardiogram (TTE).
The development of hemodynamic compromise in the first hours following an anterior MI points toward a left main or proximal left anterior descending (LAD) artery occlusion with acute pump failure (Killip class IV) rather than a mechanical complication of MI. The latter usually takes place in the first days following an MI because during this period of the healing process, the infarcted myocardium is softest and most prone to rupture (1,2). However, the presence of deep Q waves on the ECG with persistent ST-segment elevation, together with a harsh pansystolic murmur, raises a strong suspicion of a delayed presentation of MI with ventricular septal rupture (VSR), although it is inconsistent with the patient’s history. Certain patients, however, may present with concomitant acute STEMI and a very early (<24 hours) mechanical complication (ie, type 1 in the Becker and Mantgem classification) caused by an abrupt tear in the wall without thinning (1,2). Becker type 1 ruptures are typically related to intramural hematomas dissecting through tissue planes in the setting of a relatively small inferior MI as a result of the shear stress generated by the adjacent hyperkinetic myocardium supplied by the nonoccluded LAD artery. This complication is extremely rare and pathophysiologically unlikely in the LAD artery distribution. The TTE revealed a left ventricular (LV) ejection fraction of 30% with anteroseptal wall akinesia and a single 12 × 22 mm midanterior ventricular septal defect (VSD) with irregular margins (Figure 2A, Supplemental Video 1A, Supplemental Video 1B, Supplemental Video 1C, Supplemental Video 1D, Supplemental Video 1E).
Figure 2.
Ventricular Septal Defect Imaging
(A) Transthoracic echocardiography and (B) left ventriculography demonstrating a ventricular septal defect (arrows).
Question 2: What is the best next step in the management of this patient?
While ensuring supportive care, prompt restoration of the coronary blood flow with percutaneous coronary intervention (PCI) is the best step in the management of this patient because it would potentially limit further myocardial damage and reduce the risk of mortality.
Although coronary reperfusion of the culprit artery in patients presenting with an MI ≥24 to 48 hours from symptom onset has shown no clinical benefit (or harm), the patient was in cardiogenic shock, so PCI would be mandatory to potentially contribute to save the patient’s life (3,4). Coronary angiography demonstrated LAD artery thrombotic occlusion. Unfortunately, LAD artery reperfusion was unsuccessful (Supplemental Video 2A, Supplemental Video 2B, Supplemental Video 2C, Supplemental Video 2D, Supplemental Video 2E). Left ventriculography confirmed the shunt of contrast material from the left ventricle to the right ventricle (Figure 2B, Supplemental Video 2A, Supplemental Video 2B, Supplemental Video 2C, Supplemental Video 2D, Supplemental Video 2E). The patient’s hemodynamic condition worsened during the following 48 hours despite the insertion of an intra-aortic balloon pump (IABP).
Question 3: What is the best therapeutic option to treat the post-MI VSR, and what are the main factors to consider?
Delayed surgery remains the treatment of choice for patients with post-MI VSR. Percutaneous closure is a possible alternative in patients with a prohibitive surgical risk. An exhaustive evaluation of the anatomy of the defect is of the utmost significance to guide the procedure and to reduce the risk of procedural failure.
According to the GUSTO-I (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) trial, patients with post-MI VSR who were treated conservatively (ie, without closing the defect) faced a mortality rate >90% at 30 days (5). Thus, once post-MI VSR is diagnosed, a closure strategy should be promptly planned. If hemodynamic stability is achieved, delayed surgery (>21days after presentation) has a mortality rate of <20% compared with ∼60% within the first 24 hours (Table 1) (6). Although surgical repair has been the gold standard to correct the structural anomaly, percutaneous closure may represent a valuable therapeutic alternative, with the advantage of immediate shunt reduction to prevent further hemodynamic deterioration (7). Nonetheless, the overall published number of percutaneous VSR closures remains low, and most surviving patients underwent VSD closure either in the subacute or chronic phase or for residual shunting after surgery (8). When considering percutaneous closure, it is essential to analyze the structural characteristics of the defect (if possible, by computed tomography [CT] and cardiac magnetic resonance [CMR]) and its relationship with different heart structures (9). Considering the patient’s instability, the heart team considered that immediate surgery would have an unacceptable mortality risk and agreed to perform percutaneous closure.
Table 1.
Time to Intervention and Associated Mortality Rates in Post-MI VSR: Percutaneous Device Closure vs Surgical Repair vs Conservative Management
| Percutaneous Closurea | Surgical Repairb | Conservative Managementc | |
|---|---|---|---|
| Timing-associated mortality, d | — | — | 94% (n = 35) |
| 0-1 | — | 60% (n = 709) | — |
| 1-3 | 88% (n = 16) | — | — |
| 1-7 | — | 50% (n = 1,281) | — |
| 4-16 | 38% (n = 13) | — | — |
| 8-21 | — | 30% (n = 373) | — |
| >21 | — | 10% (n = 513) | — |
A selection bias could also explain the differences in mortality rates. Patients undergoing closure procedure were usually more critically ill, whereas those submitted to delayed procedure, were able to display hemodynamic stability during the waiting period.
Data from Thiele et al (8).
Data from Arnaoutakis et al (6).
Data from Crenshaw et al (5).
Question 4: What are the main steps of catheter-based VSR treatment?
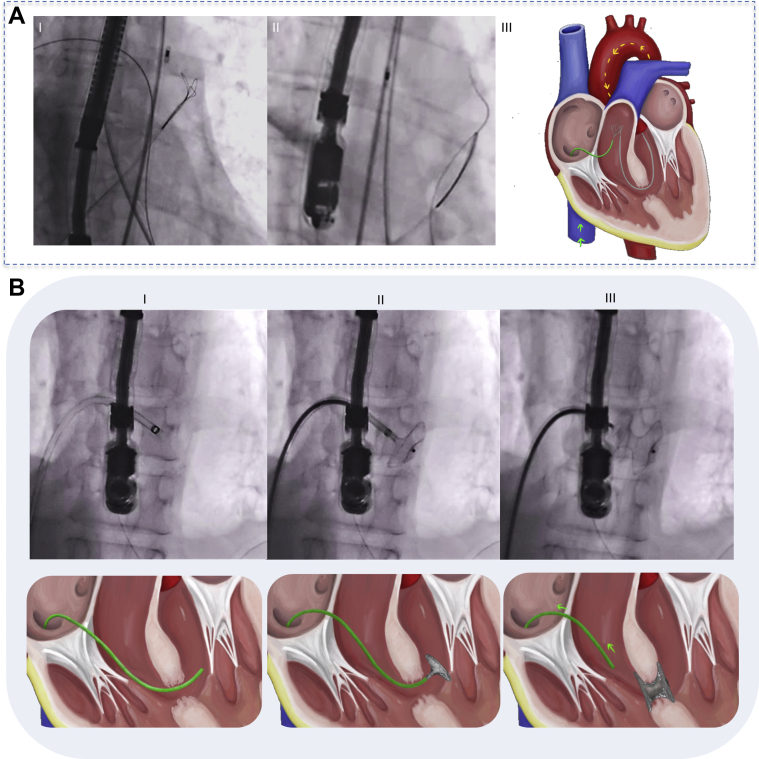
Catheter-based VSR closure starts by selecting the occluding device in accordance with the anatomic characteristics of the defect. The procedure is performed under fluoroscopic and transesophageal echocardiography (TEE) guidance. Both the right femoral artery and vein are accessed to obtain the final positioning of the sheath to release the device (Figures 3A and 3B).
Figure 3.
Catheter-Based Ventricular Septal Defect Closure Procedure
(A) (I) Guidewire positioning in the right pulmonary artery and the loop device in the pulmonary trunk. (II) The guidewire is captured and pulled back into the right atrium and inferior vena cava where it is exteriorized through the right femoral vein to create an arteriovenous loop. (III) Illustration of previous steps. (B) (I-III) Final positioning of the sheath where the device is deployed.
TEE is needed to determine the tract of the VSR, to select the size of the device, and to guide the closure procedure. CT and CMR may also be helpful in stable patients to define the anatomy of the defect more precisely (9). Unfortunately, our patient’s condition was too unstable to allow her to be transported for advanced imaging. Because there was no immediate availability of a dedicated VSD closure device in our institution, we decided to use a 14-mm atrial septal defect (ASD) Amplatzer (AGA Medical Corporation) occluder according to TEE measurements. Once both disks were deployed, TEE and ventriculography confirmed appropriate and stable device positioning and significant reduction of the left-to-right shunt (Videos 3, 4A, and 4B). There was a significant improvement in hemodynamics, thus allowing the removal of the IABP on the second day after the procedure. Pharmacologic support (dobutamine and norepinephrine) was completely discontinued by day 8. Extubation failed twice because of the patient’s severe agitation. The day before the third extubation attempt (12 days later), however, sudden hemodynamic deterioration occurred. The patient died a few hours later of refractory ventricular tachycardia and cardiogenic shock.
Question 5: What is the most likely cause of hemodynamic deterioration?
A likely course of a large, nonreperfused MI consists of severe progressive heart failure and intractable ventricular arrhythmias. However, because the patient’s initial clinical condition improved significantly after closing the VSD, an acute complication of the septal device closure should be considered. Despite the initial procedural success, the prognosis in this type of patient is still ominous, mainly given the occurrence of new defects in the weakened surrounding myocardium. A TTE demonstrated a new left-to-right shunt located between the device and the LV apex (Figures 4A to 4C).
Figure 4.
Transthoracic Echocardiogram and Illustration of Ventricular Septal Defect
(A and B) Transthoracic echocardiogram demonstrating a new (“secondary”) ventricular septal defect (arrows). (C) Illustration of A.
Despite great advances in device technology and high rates (∼90%) of successful device implantation, VSR still remains a highly lethal (∼60%) complication of MI (5). In this case, the occurrence of a new VSD (“secondary VSD”) precluded late technical success. An area of extensive coagulative necrosis surrounding the VSD may have led to further extension or weakening of the myocardium, thereby creating more areas of VSR. This process was certainly aggravated by the impossibility of restoring the LAD artery circulation. Thus, the tissue viability or ischemic condition of the marginal myocardium (ie, where the device will finally appose) was critical in the late patient’s prognosis (Figures 4A to 4C). Whether the use of a VSD closing device (with larger waist) or oversizing the ASD occluder would have prevented this outcome remains uncertain.
Question 6: Would serial TTEs and early repeat surgical evaluation after the initial procedure and stabilization have decreased the risk of death?
On the basis of the expected course of the histopathologic process, serial TTEs should be considered to monitor the macroscopic healing process closely. An early repeat surgical evaluation must be considered after catheter-based closure once hemodynamic values stabilize, although the optimal timing of surgery is still a matter of debate.
According to the joint guidelines of the American College of Cardiology and the American Heart Association, emergency surgical repair is indicated regardless of hemodynamic status (4). Undoubtedly, better outcomes are achieved in hemodynamically stable patients, with favorable anatomy and no signs of organ dysfunction. However, even in these cases, the optimal timing for definitive surgery remains unknown. For instance, the European Society of Cardiology guidelines recommend delayed elective repair in patients initially responding to aggressive conservative management (3), which could apply to this patient after stabilization. Percutaneous closure may offer precious time to facilitate successful surgical repair by allowing friable tissue to organize and strengthen. Jones et al (2) proposed a therapeutic approach for VSR on the basis of hemodynamics and defect anatomy (Figure 5).
Figure 5.
Proposed Management of Post-Myocardial Infarction Ventricular Septal Defect
∗Consider delaying surgery for 3 weeks if hemodynamic values allow. †The ischemic condition of surrounding myocardium and the morphology and number of ventricular septal defects need to be considered before closure attempt. Adapted from Jones et al (2).
Question 7: What are the learning objectives of this case?
The main therapeutic challenge in VSR is to identify the precise timing to attempt closure of the defect. In view of the possible benefits of delaying surgery, expectant management should be adopted whenever hemodynamics will allow it. In case of hemodynamic deterioration, closure should be attempted assuming a high mortality risk, although it offers patients a small but real possibility of survival. Late procedural success of percutaneous management may be limited by the occurrence of secondary VSDs. Hopefully, technology improvements, with more efficacious and available devices, will make this complication more forgiving.
Funding Support and Author Disclosures
Dr Biondi-Zoccai has reported consulting for Abbott Vascular and Bayer. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors would like to thank Dr Luis Guzmán for the careful review of the manuscript. In addition, the authors are indebted to Paula Lojek and Florencia Domínguez for assistance in figure editing.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
VSR Imaging. (A) Parasternal short-axis view (transthoracic echocardiography) showing the ventricular septal rupture (VSR). (B) Parasternal short-axis view with color Doppler demonstrating the ventricular septal rupture. (C) Apical 4-chamber view showing the ventricular septal rupture. (D) Apical chamber view (foreshortened) depicting the ventricular septal rupture. (E). Apical chamber view (foreshortened) with color Doppler demonstrating the ventricular septal rupture.
VSR Imaging. (A) Parasternal short-axis view (transthoracic echocardiography) showing the ventricular septal rupture (VSR). (B) Parasternal short-axis view with color Doppler demonstrating the ventricular septal rupture. (C) Apical 4-chamber view showing the ventricular septal rupture. (D) Apical chamber view (foreshortened) depicting the ventricular septal rupture. (E). Apical chamber view (foreshortened) with color Doppler demonstrating the ventricular septal rupture.
VSR Imaging. (A) Parasternal short-axis view (transthoracic echocardiography) showing the ventricular septal rupture (VSR). (B) Parasternal short-axis view with color Doppler demonstrating the ventricular septal rupture. (C) Apical 4-chamber view showing the ventricular septal rupture. (D) Apical chamber view (foreshortened) depicting the ventricular septal rupture. (E). Apical chamber view (foreshortened) with color Doppler demonstrating the ventricular septal rupture.
VSR Imaging. (A) Parasternal short-axis view (transthoracic echocardiography) showing the ventricular septal rupture (VSR). (B) Parasternal short-axis view with color Doppler demonstrating the ventricular septal rupture. (C) Apical 4-chamber view showing the ventricular septal rupture. (D) Apical chamber view (foreshortened) depicting the ventricular septal rupture. (E). Apical chamber view (foreshortened) with color Doppler demonstrating the ventricular septal rupture.
VSR Imaging. (A) Parasternal short-axis view (transthoracic echocardiography) showing the ventricular septal rupture (VSR). (B) Parasternal short-axis view with color Doppler demonstrating the ventricular septal rupture. (C) Apical 4-chamber view showing the ventricular septal rupture. (D) Apical chamber view (foreshortened) depicting the ventricular septal rupture. (E). Apical chamber view (foreshortened) with color Doppler demonstrating the ventricular septal rupture.
Imaging of LAD Occlusion and VSR. (A) Coronary angiography showing the left anterior descending artery (LAD) thrombotic occlusion. (B) First balloon angioplasty attempt of the left anterior descending artery. (C) Second balloon angioplasty attempt of the left anterior descending artery. (D) Left coronary angiography demonstrating failed left anterior descending artery percutaneous coronary intervention. (E) Left ventricular ventriculography demonstrating the ventricular septal rupture (VSR).
Imaging of LAD Occlusion and VSR. (A) Coronary angiography showing the left anterior descending artery (LAD) thrombotic occlusion. (B) First balloon angioplasty attempt of the left anterior descending artery. (C) Second balloon angioplasty attempt of the left anterior descending artery. (D) Left coronary angiography demonstrating failed left anterior descending artery percutaneous coronary intervention. (E) Left ventricular ventriculography demonstrating the ventricular septal rupture (VSR).
Imaging of LAD Occlusion and VSR. (A) Coronary angiography showing the left anterior descending artery (LAD) thrombotic occlusion. (B) First balloon angioplasty attempt of the left anterior descending artery. (C) Second balloon angioplasty attempt of the left anterior descending artery. (D) Left coronary angiography demonstrating failed left anterior descending artery percutaneous coronary intervention. (E) Left ventricular ventriculography demonstrating the ventricular septal rupture (VSR).
Imaging of LAD Occlusion and VSR. (A) Coronary angiography showing the left anterior descending artery (LAD) thrombotic occlusion. (B) First balloon angioplasty attempt of the left anterior descending artery. (C) Second balloon angioplasty attempt of the left anterior descending artery. (D) Left coronary angiography demonstrating failed left anterior descending artery percutaneous coronary intervention. (E) Left ventricular ventriculography demonstrating the ventricular septal rupture (VSR).
Imaging of LAD Occlusion and VSR. (A) Coronary angiography showing the left anterior descending artery (LAD) thrombotic occlusion. (B) First balloon angioplasty attempt of the left anterior descending artery. (C) Second balloon angioplasty attempt of the left anterior descending artery. (D) Left coronary angiography demonstrating failed left anterior descending artery percutaneous coronary intervention. (E) Left ventricular ventriculography demonstrating the ventricular septal rupture (VSR).
LV Ventriculography After Device Deployment Showing Stable Positioning and Significant Reduction of the Left-to-Right Shunt
TEE of Device Positioning and Shunt Reduction. (A) Parasternal short-axis view (transesophageal echocardiography [TEE]) confirming appropriate device positioning. (B) Parasternal short-axis view with color Doppler (transesophageal echocardiography) showing significant reduction of the left-to-right shunt.
TEE of Device Positioning and Shunt Reduction. (A) Parasternal short-axis view (transesophageal echocardiography [TEE]) confirming appropriate device positioning. (B) Parasternal short-axis view with color Doppler (transesophageal echocardiography) showing significant reduction of the left-to-right shunt.
References
- 1.Becker A.E., van Mantgem J.P. Cardiac tamponade. A study of 50 hearts. Eur J Cardiol. 1975;3:349–358. [PubMed] [Google Scholar]
- 2.Jones B.M., Kapadia S.R., Smedira N.G., et al. Ventricular septal rupture complicating acute myocardial infarction: a contemporary review. Eur Heart J. 2014;35:2060–2068. doi: 10.1093/eurheartj/ehu248. [DOI] [PubMed] [Google Scholar]
- 3.Ibanez B., James S., Agewall S., et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the Management of Acute Myocardial Infarction in Patients Presenting With ST-segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 4.O’Gara P.T., Kushner F.G., Ascheim D.D., et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Crenshaw B.S., Granger C.B., Birnbaum Y., et al. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. GUSTO-I (Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries) trial investigators. Circulation. 2000;101:27–32. doi: 10.1161/01.cir.101.1.27. [DOI] [PubMed] [Google Scholar]
- 6.Arnaoutakis G.J., Zhao Y., George T.J., Sciortino C.M., McCarthy P.M., Conte J.V. Surgical repair of ventricular septal defect after myocardial infarction: outcomes from the Society of Thoracic Surgeons National Database. Ann Thorac Surg. 2012;94:436–443. doi: 10.1016/j.athoracsur.2012.04.020. discussion 443-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlotter F., de Waha S., Eitel I., Desch S., Fuernau G., Thiele H. Interventional post-myocardial infarction ventricular septal defect closure: a systematic review of current evidence. EuroIntervention. 2016;12:94–102. doi: 10.4244/EIJV12I1A17. [DOI] [PubMed] [Google Scholar]
- 8.Thiele H., Kaulfersch C., Daehnert I., et al. Immediate primary transcatheter closure of postinfarction ventricular septal defects. Eur Heart J. 2009;30:81–88. doi: 10.1093/eurheartj/ehn524. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton M.C.K., Rodrigues J.C.L., Martin R.P., Manghat N.E., Turner M.S. The in vivo morphology of post-infarct ventricular septal defect and the implications for closure. J Am Coll Cardiol Intv. 2017;10:1233–1243. doi: 10.1016/j.jcin.2017.03.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
VSR Imaging. (A) Parasternal short-axis view (transthoracic echocardiography) showing the ventricular septal rupture (VSR). (B) Parasternal short-axis view with color Doppler demonstrating the ventricular septal rupture. (C) Apical 4-chamber view showing the ventricular septal rupture. (D) Apical chamber view (foreshortened) depicting the ventricular septal rupture. (E). Apical chamber view (foreshortened) with color Doppler demonstrating the ventricular septal rupture.
VSR Imaging. (A) Parasternal short-axis view (transthoracic echocardiography) showing the ventricular septal rupture (VSR). (B) Parasternal short-axis view with color Doppler demonstrating the ventricular septal rupture. (C) Apical 4-chamber view showing the ventricular septal rupture. (D) Apical chamber view (foreshortened) depicting the ventricular septal rupture. (E). Apical chamber view (foreshortened) with color Doppler demonstrating the ventricular septal rupture.
VSR Imaging. (A) Parasternal short-axis view (transthoracic echocardiography) showing the ventricular septal rupture (VSR). (B) Parasternal short-axis view with color Doppler demonstrating the ventricular septal rupture. (C) Apical 4-chamber view showing the ventricular septal rupture. (D) Apical chamber view (foreshortened) depicting the ventricular septal rupture. (E). Apical chamber view (foreshortened) with color Doppler demonstrating the ventricular septal rupture.
VSR Imaging. (A) Parasternal short-axis view (transthoracic echocardiography) showing the ventricular septal rupture (VSR). (B) Parasternal short-axis view with color Doppler demonstrating the ventricular septal rupture. (C) Apical 4-chamber view showing the ventricular septal rupture. (D) Apical chamber view (foreshortened) depicting the ventricular septal rupture. (E). Apical chamber view (foreshortened) with color Doppler demonstrating the ventricular septal rupture.
VSR Imaging. (A) Parasternal short-axis view (transthoracic echocardiography) showing the ventricular septal rupture (VSR). (B) Parasternal short-axis view with color Doppler demonstrating the ventricular septal rupture. (C) Apical 4-chamber view showing the ventricular septal rupture. (D) Apical chamber view (foreshortened) depicting the ventricular septal rupture. (E). Apical chamber view (foreshortened) with color Doppler demonstrating the ventricular septal rupture.
Imaging of LAD Occlusion and VSR. (A) Coronary angiography showing the left anterior descending artery (LAD) thrombotic occlusion. (B) First balloon angioplasty attempt of the left anterior descending artery. (C) Second balloon angioplasty attempt of the left anterior descending artery. (D) Left coronary angiography demonstrating failed left anterior descending artery percutaneous coronary intervention. (E) Left ventricular ventriculography demonstrating the ventricular septal rupture (VSR).
Imaging of LAD Occlusion and VSR. (A) Coronary angiography showing the left anterior descending artery (LAD) thrombotic occlusion. (B) First balloon angioplasty attempt of the left anterior descending artery. (C) Second balloon angioplasty attempt of the left anterior descending artery. (D) Left coronary angiography demonstrating failed left anterior descending artery percutaneous coronary intervention. (E) Left ventricular ventriculography demonstrating the ventricular septal rupture (VSR).
Imaging of LAD Occlusion and VSR. (A) Coronary angiography showing the left anterior descending artery (LAD) thrombotic occlusion. (B) First balloon angioplasty attempt of the left anterior descending artery. (C) Second balloon angioplasty attempt of the left anterior descending artery. (D) Left coronary angiography demonstrating failed left anterior descending artery percutaneous coronary intervention. (E) Left ventricular ventriculography demonstrating the ventricular septal rupture (VSR).
Imaging of LAD Occlusion and VSR. (A) Coronary angiography showing the left anterior descending artery (LAD) thrombotic occlusion. (B) First balloon angioplasty attempt of the left anterior descending artery. (C) Second balloon angioplasty attempt of the left anterior descending artery. (D) Left coronary angiography demonstrating failed left anterior descending artery percutaneous coronary intervention. (E) Left ventricular ventriculography demonstrating the ventricular septal rupture (VSR).
Imaging of LAD Occlusion and VSR. (A) Coronary angiography showing the left anterior descending artery (LAD) thrombotic occlusion. (B) First balloon angioplasty attempt of the left anterior descending artery. (C) Second balloon angioplasty attempt of the left anterior descending artery. (D) Left coronary angiography demonstrating failed left anterior descending artery percutaneous coronary intervention. (E) Left ventricular ventriculography demonstrating the ventricular septal rupture (VSR).
LV Ventriculography After Device Deployment Showing Stable Positioning and Significant Reduction of the Left-to-Right Shunt
TEE of Device Positioning and Shunt Reduction. (A) Parasternal short-axis view (transesophageal echocardiography [TEE]) confirming appropriate device positioning. (B) Parasternal short-axis view with color Doppler (transesophageal echocardiography) showing significant reduction of the left-to-right shunt.
TEE of Device Positioning and Shunt Reduction. (A) Parasternal short-axis view (transesophageal echocardiography [TEE]) confirming appropriate device positioning. (B) Parasternal short-axis view with color Doppler (transesophageal echocardiography) showing significant reduction of the left-to-right shunt.