Abstract
Hepatocellular carcinoma (HCC) is an aggressive human cancer with increasing incidence worldwide. Multiple efforts have been made to explore pharmaceutical therapies to treat HCC, such as targeted tyrosine kinase inhibitors, immune based therapies and combination of chemotherapy. However, limitations exist in current strategies including chemoresistance for instance. Tumor initiation and progression is driven by reprogramming of metabolism, in particular during HCC development. Recently, metabolic associated fatty liver disease (MAFLD), a reappraisal of new nomenclature for non-alcoholic fatty liver disease (NAFLD), indicates growing appreciation of metabolism in the pathogenesis of liver disease, including HCC, thereby suggesting new strategies by targeting abnormal metabolism for HCC treatment. In this review, we introduce directions by highlighting the metabolic targets in glucose, fatty acid, amino acid and glutamine metabolism, which are suitable for HCC pharmaceutical intervention. We also summarize and discuss current pharmaceutical agents and studies targeting deregulated metabolism during HCC treatment. Furthermore, opportunities and challenges in the discovery and development of HCC therapy targeting metabolism are discussed.
KEY WORDS: Metabolic dysregulation, Hepatocellular carcinoma, Glycolysis, Tricarboxylic acid cycle, Pentose phosphate pathway, Fatty acid β-oxidation, Glutamine metabolism, Cancer therapy
Abbreviations: 1,3-BPG, 1,3-bisphosphoglycerate; 2-DG, 2-deoxy-d-glucose; 3-BrPA, 3-bromopyruvic acid; ACC, acetyl-CoA carboxylase; ACLY, adenosine triphosphate (ATP) citrate lyase; ACS, acyl-CoA synthease; AKT, protein kinase B; AML, acute myeloblastic leukemia; AMPK, adenosine mono-phosphate-activated protein kinase; ASS1, argininosuccinate synthase 1; ATGL, adipose triacylglycerol lipase; CANA, canagliflozin; CPT, carnitine palmitoyl-transferase; CYP4, cytochrome P450s (CYPs) 4 family; DNL, de novo lipogenesis; EMT, epithelial-to-mesenchymal transition; ER, endoplasmic reticulum; ERK, extracellular-signal regulated kinase; FABP1, fatty acid binding protein 1; FASN, fatty acid synthase; FBP1, fructose-1,6-bisphosphatase 1; FFA, free fatty acid; G6PD, glucose-6-phosphate dehydrogenase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GLS1, renal-type glutaminase; GLS2, liver-type glutaminase; GLUT1, glucose transporter 1; GOT1, glutamate oxaloacetate transaminase 1; HCC, hepatocellular carcinoma; HIF-1α, hypoxia-inducible factor-1 alpha; HK, hexokinase; HMGCR, 3-hydroxy-3-methylglutaryl-CoA reductase; HSCs, hepatic stellate cells; IDH2, isocitrate dehydrogenase 2; LCAD, long-chain acyl-CoA dehydrogenase; LDH, lactate dehydrogenase; LPL, lipid lipase; LXR, liver X receptor; MAFLD, metabolic associated fatty liver disease; MAGL, monoacyglycerol lipase; MCAD, medium-chain acyl-CoA dehydrogenase; MEs, malic enzymes; mIDH, mutant IDH; MMP9, matrix metallopeptidase 9; mTOR, mammalian target of rapamycin; NADPH, nicotinamide adenine nucleotide phosphate; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; OTC, ornithine transcarbamylase; PCK1, phosphoenolpyruvate carboxykinase 1; PFK1, phosphofructokinase 1; PGAM1, phosphoglycerate mutase 1; PGK1, phosphoglycerate kinase 1; PI3K, phosphoinositide 3-kinase; PKM2, pyruvate kinase M2; PPARα, peroxisome proliferator-activated receptor alpha; PPP, pentose phosphate pathway; ROS, reactive oxygen species; SCD1, stearoyl-CoA-desaturase 1; SGLT2, sodium-glucose cotransporter 2; SLC1A5/ASCT2, solute carrier family 1 member 5/alanine serine cysteine preferring transporter 2; SLC7A5/LAT1, solute carrier family 7 member 5/L-type amino acid transporter 1; SREBP1, sterol regulatory element-binding protein 1; TAGs, triacylglycerols; TCA cycle, tricarboxylic acid cycle; TKIs, tyrosine kinase inhibitors; TKT, transketolase; VEGFR, vascular endothelial growth factor receptor; WD-fed MC4R-KO, Western diet (WD)-fed melanocortin 4 receptor-deficient (MC4R-KO); WNT, wingless-type MMTV integration site family
Graphical abstract
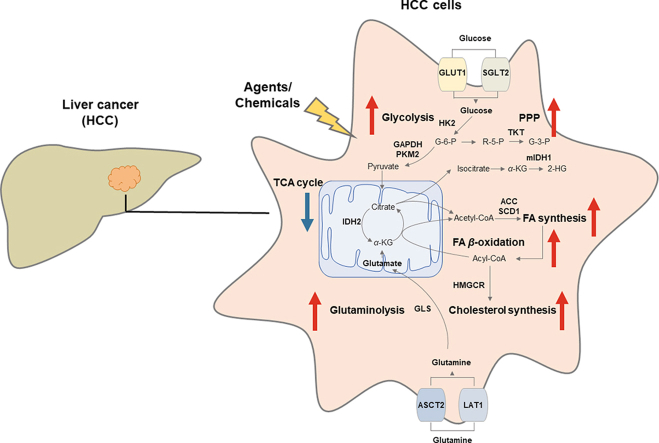
Metabolic dysregulation is emphasized in the pathogenesis of hepatocellular carcinoma (HCC), thus agents or chemicals are developed as potentials to treat HCC by targeting deregulated metabolism.
1. Introduction
Hepatocellular carcinoma (HCC) accounting for nearly 90% of primary liver cancer represents one of the most lethal and prevalent human cancers1,2. Most patients are diagnosed at an advanced stage due to heterogeneity and complexity of HCC; thereby systemic therapies are usually recommended as the standard of medical care3. Yet, conventional systemic chemotherapy yields negligible clinical benefits. Several phase III trials of doxorubicin alone are compared to doxorubicin plus sorafenib, or to FOLFOX4 regimen (fluorouracil, oxaliplatin and leucovorin (folinic acid)) for HCC treatment, however, all above have negative results, in several circumstances with considerable toxicity4,5.
Later, targeted small-molecule tyrosine kinase inhibitors (TKIs) or antibodies, primarily sorafenib6, lenvatinib7, regorafenib8, cabozantinib9, ramucirumab10 are approved by U.S. Food and Drug Administration (FDA) for the first/second-line choice for HCC treatment. Sorafenib shows survival benefits with a median survival of 10.7 months compared to placebo of 7.9 months6, and lenvatinib exerts a non-inferior median survival of 13.6 months to that of 12.3 months in sorafenib groups7, respectively. Even so, associated severe adverse events, such as hand-foot skin reaction and cardiac ischemia, need to be carefully managed, or else they could be an obstacle to these drugs’ clinical use11. Also, chemoresistance to TKIs developed during treatment limits their clinical use12. Although the clinical benefits of immune-based therapies for HCC are emerging, a phase III trial shows an overall survival of 67.2% with atezolizumab-bevacizumab and of 54.6% with sorafenib at 12 months13. It is still urgent to explore effective novel strategies to combat HCC.
Recently, metabolic (dysfunction) associated fatty liver disease (MAFLD)—a reappraisal of new nomenclature for non-alcoholic fatty liver disease (NAFLD), has attracted heated attention from biologists, pharmacologists, pharmaceutical companies and clinical researchers14. Inclusion of metabolic (dysfunction) in the nomenclature indicates growing appreciation of metabolism in the initiation and progression of liver disease, including HCC15. Notably, fast-rising NAFLD and non-alcoholic steatohepatitis (NASH) have made metabolic disorders a major risk factor for the development of HCC16,17, in parallel with other identified etiological factors as chronic hepatitis B and C viral infections18, as well as excessive chemical and alcohol exposure19. As earlier in 1956, Otto Warburg discovers that cancer cells prefer to consume large amounts of glucose through glycolysis rather than favor oxidative phosphorylation, even in a sufficient supply of oxygen. Now, it is clear that tumorigenesis is driven by the rearrangement of cellular metabolism resulting from direct and indirect consequences of oncogenic mutations20. And reprogramming of energy metabolism is characterized as a new hallmark to further rationalize the complexities of neoplastic disease21. However, whether and how the key enzymes or intermediates function in HCC initiation and progression remain to be deeply illustrated20. Here, we review the critical enzymes and intermediates in glucose, fatty acids, amino acids and glutamine metabolism during HCC pathogenesis, so as to provide metabolic targets suitable for pharmaceutical intervention. We also summarize and discuss current pharmaceutical agents and studies targeting deregulated metabolism for HCC treatment. Furthermore, the opportunities and challenges in the discovery and development of HCC therapy targeting metabolism are highlighted.
2. Metabolic rearrangements in HCC
2.1. Glucose metabolism
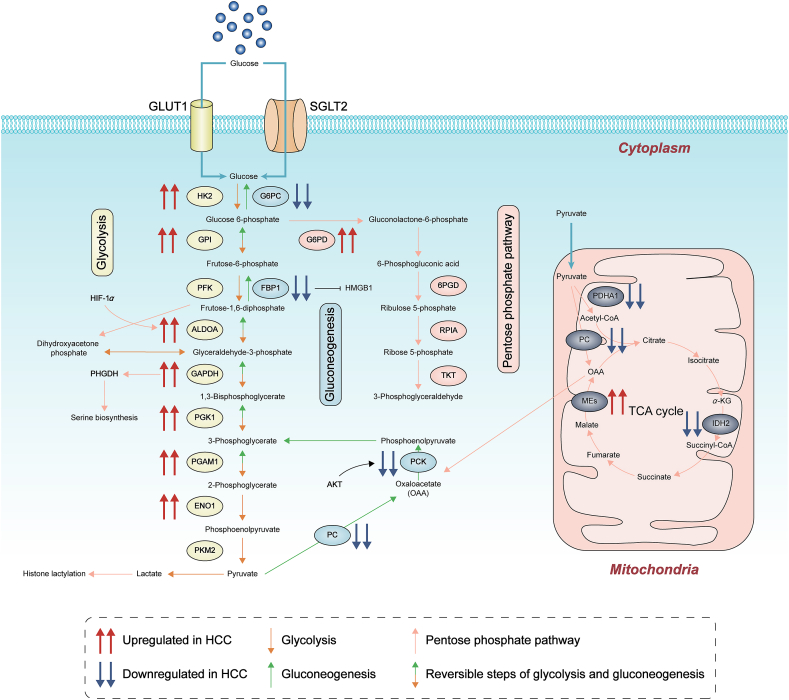
Carbohydrate metabolism is a complex biological process. Endless newly-found evidence reveals a well-recognized emphasis on the deregulation of glucose metabolism during the initiation and progression of HCC. HCC cells are metabolically distinct from normal hepatocytes and express different metabolic enzymes22. Glucose transporters sodium-glucose cotransporter 2 (SGLT2) and glucose transporter 1 (GLUT1) are highly expressed in HCC and functionally promote tumorigenicity. Enzymes and substrates in carbohydrate metabolism act as pathological fingerprints during the tumorigenesis of HCC, including the ones in glycolysis, pentose phosphate pathway (PPP), gluconeogenesis and tricarboxylic acid cycle (TCA cycle) (as shown in Fig. 1).
Figure 1.
Deregulated alterations of glucose metabolism in HCC. Deregulation of glucose metabolism during the initiation and progression of HCC has been early and well emphasized, including glucose transporters, enzymes and substrates in glycolysis, pentose phosphate pathway, gluconeogenesis and TCA cycle. Newly discovered functions of metabolic intermediates have also been referred, as histone lactylation by lactate. G6PC, glucose-6-phosphatase alpha; GPI, glucose-6-phosphate isomerase; HMGB1, high mobility group box 1; ALDOA, fructose-bisphosphate aldolase A; ENO1, enolase 1; PC, pyruvate carboxylase; PDHA1: pyruvate dehydrogenase A1; PHGDH, phosphoglycerate dehydrogenase; 6PGD, 6-phosphogluconate dehydrogenase; RPIA, ribose 5-phosphate isomerase A.
2.1.1. Glycolysis
Normal cells break down glucose or glycogen into lactate while produce a small amount of ATP under anaerobic or hypoxic conditions. However, due to the energy demand for rapid cell multiplication, even under aerobic conditions, tumor cells decompose glucose into lactate through glycolysis, known as Warburg effect23. This is one of the reasons why glycolysis is particularly important in tumor cell metabolism. Targeting an enzyme that is only over-activated in HCC cells but not in the corresponding normal liver tissue could be a selective therapeutic strategy. The enzymes included in glycolysis are highly expressed and closely related with poorer overall survival in HCC. Some enzymes have an isoform specificity during HCC tumorigenesis and progression. For example, a switch of hexokinase (HK) from low-affinity HK4 to high-affinity HK2 is made in hepatocytes, and a specific isoform of pyruvate kinase-pyruvate kinase M2 (PKM2) is highly expressed in all proliferating cells, including HCC24,25.
The diversion of glycolytic flux to other pathway arouses much attention recently. Glucose-6-phosphate isomerase diverges the glucose flow to the PPP to produce nicotinamide adenine nucleotide phosphate (NADPH) and pentose26. NADPH acts as a critical power-producer to fuel the protein-based antioxidant system and combat oxidative stress. Antioxidant machinery is increased in HCC to overcome reactive oxygen species (ROS)-induced cell death27. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is another knot to promote diversion from glycolysis to serine biosynthesis by elevating the transcription of phosphoglycerate dehydrogenase, a key enzyme for serine biosynthesis28. The requirement of de novo synthesis of serine from glucose is a characteristic of cancer cells to thrive the synthesis of cellular glycine and nucleotide, and also to support the folate cycle and amino acid transport29. Therefore, glycolytic enzymes fuel cell growth by tuning the HCC cells to cope with metabolic requirement for rapid proliferation.
Some enzymes have other functions apart from propelling glycolysis. Glucose-6-phosphate isomerase also acts as an extracellular cytokine to suppress apoptosis, promote tumor progression and invasiveness with the involvement of PI3K (phosphoinositide 3-kinase)/AKT (protein kinase B) activation30. GAPDH is proposed to contribute in nuclear tRNA export, DNA replication and repair, exocytosis, cytoskeletal organization, and cell death31. These above functions involved in other biological process boost HCC cell growth in a synergistic way.
The regulation of glycolytic enzymes is only partially understood. Hypoxia induced increases of glycolytic flux is exaggerated in cancer cells to promote cell survival and growth. Hypoxia induces nuclear GAPDH translocation in hepatic stellate cells (HSCs), which is associated with HCC patient prognosis and tumorigenesis32. Fructose-bisphosphate aldolase A is a target gene of hypoxia-inducible factor 1 alpha (HIF-1α)33,34, a critical transcription factor that controls hypoxia-induced gene expression and elicits metabolic events crucial for tumor development35. Besides, autophagy suppresses glycolysis with the autophagic degradation of HK236. However, the regulation of autophagy by PKM2 in tumor cells is intriguing but still contradictory. PKM2 knockdown inhibits autophagy vehicle formation37, as a participant in a positive feedback loop that promotes HIF-1α transactivation38. However, it is not consistent with the finding that AKT phosphorylation leads to mammalian target of rapamycin (mTOR) inhibition and the extrapolation of autophagy activation resulting from PKM2 knockdown39. Some upstream regulators affect tumorigenesis by regulating the expression of phosphoglycerate kinase (PGK1), as long noncoding RNA MSC-AS1 and microRNA-450b-3p target PGK1 post-transcriptionally and induce opposite effect to HCC cell proliferation40,41.
Lactate as the main metabolic product of glycolysis is actually the nutrient instead of waste, which is related to oxidative stress resistance and lipid biosynthesis in cancer cells. Lactate is not only a carbon source for membrane lipid biosynthesis, but also participates in the production of lipid substances. It is revealed that lactate enhances ferroptosis resistance in HCC cells and contributes to tumor growth42. A novel function of lactate, histone lysine lactylation, is newly proposed as a post-translational modification of histone proteins to regulate gene expression43. Histone lactylation is observed with M1 macrophage polarization, associated with the increased expression of inflammatory genes and activation of glycolysis. Further investigating the role of histone lactylation on modulating the activity of liver-specific macrophages and further HCC development will lead to intriguing findings.
Targeting glycolytic enzymes in HCC therapy is speculated to be a considerable approach, as some relevant drugs are now under investigation44,45. Fully addressing the favorable and unfavorable effects of those agents require further understanding the role of the enzymes in biological processes.
2.1.2. Pentose phosphate pathway
Through PPP, ribose-5-phosphate is synthesized besides NADPH. Therefore, PPP is essential for cancer cells to satisfy their demand for ribonucleotide synthesis and to maintain redox balance. The requirement of high proliferation rate and NADPH shortage is among the most important factors to affect PPP activity46. The enzymes representative for the oxidative or non-oxidative phase of PPP are glucose-6-phosphate dehydrogenase (G6PD) or transketolase (TKT) respectively. Both of the enzymes are robustly up-regulated and positively associated with poor prognosis and aggressive clinicopathological features of HCC47,48. As the rate-limiting enzyme in PPP, G6PD has been the most studied one in HCC. Besides well-established role in tumor growth49, G6PD also contributes to migration and invasion of HCC cells in vitro by inducing epithelial-to-mesenchymal transition (EMT) through the activation of signal transducer and activator of transcription 3 pathway50. TKT is a predominant form of the three human TKT genes (TKT, TKTL1, TKTL2) in the liver tissue. As uncovered by a cohort of 292 HCC patients with complete clinical data in The Cancer Genome Atlas database, TKT is an independent biomarker to predict the survival of HCC patients. The conclusion is based on the presence of vascular invasion in patients with HCC, cell viability under oxidative stress in vitro and the accelerated cell growth and metastasis in vivo48. TKT inhibitor oxythiamine significantly reverses the chemoresistance of human HCC cells to sorafenib treatment and suppresses tumor growth in vivo51. The elevated expression and exaggerated behavior of TKT is a specific feature to HCC, as TKT expression is not altered in urothelial and colorectal cancer52. It is suggested that the role of PPP enzymes in HCC development may be different from that in other cancer types and deserves further elucidation.
2.1.3. Gluconeogenesis
Gluconeogenesis, a reverse process of glycolysis, generates glucose from small carbohydrate substrates such as pyruvate, lactate, glycerol, and gluconeogenic amino acids53. Generally, gluconeogenesis pathway is inhibited in cancers because it antagonizes glycolysis. Gluconeogenesis enzymes are therefore down-regulated in HCC tissues and cells, including fructose-1,6-bisphosphatase 1 (FBP1) and phosphoenolpyruvate carboxykinase 1 (PCK1), which are also associated with unfavorable prognosis in patients with HCC54,55. The loss of these enzymes is sufficient to induce HCC tumorigenesis. As shown, hepatic glucose-6-phosphatase alpha deficiency leads to autophagy impairment, mitochondrial dysfunction, enhanced glycolysis, and augmented PPP56, 57, 58, therefore contributes to hepatocarcinogenesis. Simultaneously, FBP1 loss elicits senescence and senescence-associated secretory phenotype in HSCs, partly because of the increase of high mobility group box 1 expression, to promote HCC growth54.
As the rate-limiting enzyme in gluconeogenesis, PCK1 is elevated in other cancer types as colon cancer while decreased in HCC. It indicates that HCC cells have unique metabolic property, especially in gluconeogenesis59. Forced PCK1 expression in glucose-starved HCC cells induces TCA cycle cataplerosis, leading to energy crisis and oxidative stress60. In the contrary, it is highlighted recently that following the phosphorylation by AKT, PCK1 is translocated to endoplasmic reticulum (ER) and then phosphorylates INSIG1/2, leading to the activation of sterol regulatory element-binding protein 1 (SREBP1) and lipogenesis, which results in accelerated development and deteriorated prognosis of HCC61. PCK1 plays as a linker between lipogenesis and carcinogenesis, and can be a potential treatment target for patients with HCC.
2.1.4. Tricarboxylic acid cycle
The TCA cycle is the final metabolic pathway of the three major nutrients and the hub of their metabolic connections. Lactate increases and provides energy as the primary carbon source for the TCA cycle in cancer cells62. Cataplerosis is a process in which intermediate products of the TCA cycle are transported away from the mitochondria to participate in biosynthesis, and anaplerosis replenishes these catalytic products to maintain the stability of the cycle63. Both anaplerosis and cataplerosis affect HCC development through the regulation of TCA substrates. For example, anaplerotic enzyme pyruvate carboxylase which catalyzes pyruvate carboxylation to support anaplerosis by supplying oxaloacetate to TCA cycle. Suppression of pyruvate carboxylase decreases the flux of TCA cycle intermediates, aspartate, glutamate and glucose derivatives, which in turn reduces the viability and proliferation of HCC HepG2 cells64.
The enzymes in TCA cycle could be major factors to regulate anaplerosis and cataplerosis, but will stay far from full illustration. As the main active subunit of pyruvate dehydrogenase complex, pyruvate dehydrogenase A1 has a lower expression in HCC tumor tissues compared with normal tissues and is positively correlated with survival rate of HCC patients. Pyruvate dehydrogenase A1 overexpression has a capability to inhibit glycolysis and boost oxidative phosphorylation, which increases cell apoptosis through a mitochondria-dependent pathway65. In consistence, isocitrate dehydrogenase 2 (IDH2) is also significantly decreased in HCC tissues. IDH2 has a strong negative correlation with matrix metallopeptidase 9 (MMP9), which might be the reason that decreased IDH2 promotes cell migration and HCC metastasis66. Furthermore, genetic variations in IDH genes (IDH1 and IDH2) act as prognosis predictors in HCC patients67. Malic enzymes (MEs) are important in the regulation of cellular energy balance and redox homeostasis by catalyzing the oxidation of TCA cycle intermediate malate to form pyruvate and CO2, with simultaneous reduction of NAD(P)+ to NAD(P)H68. MEs are considered vital for glycolysis, gluconeogenesis, fatty acid synthesis and glutamine metabolism. ME1 is known as a poor prognostic predictor of HCC, as silencing ME1 decreases HCC metastasis via inhibition of EMT through ROS-induced pathways69. Recently, our data also uncovers that ME1 integrates with mitochondrial IDH2 and therefore supports tumor growth and metastasis in a variety of cancer cell types, including breast cancer and non-small cell lung cancer70. Taken together, MEs, especially ME1, deserve deeper exploration in cancer research, including HCC.
It is noted that dysregulation of glucose metabolism acts as a definitive driving force in HCC development. Recent evidences also indicate that not only enzymes in glucose metabolism, but also many other intermediate substrates or interacting proteins intervene the pathological process of liver cancer cell through glucose metabolism. The investigation on glucose metabolism in HCC is far from over, and more in-depth exploration is expected in the future.
2.2. Fatty acid and cholesterol metabolism
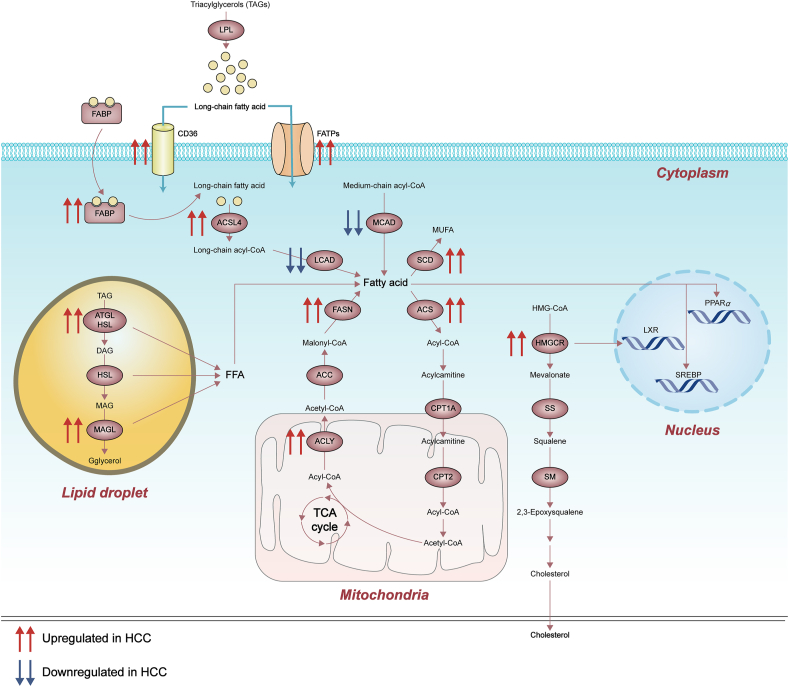
As a vital organ in lipid homeostasis, deregulated hepatic lipid metabolism has been considered as a driving force to HCC. Pathological conditions associated with lipid dysregulation are risks for HCC, including obesity, diabetes and NASH71. Fatty acids are used as signal precursors in the regulation of metabolism during HCC development as well as an energy source to support rapid cell proliferation, cell survival, invasion and angiogenesis72. Because of the poor nutrient supply in the center of tumor due to insufficient vascularization, fatty acid oxidation is another major catabolic pathway besides glycolysis, and therefore is up-regulated to provide energy for cell growth and metabolites for anabolic processes. Cholesterol appears more important for membrane integrity and fluidity and is required for highly proliferative cancer cells, including HCC73 (fatty acid and cholesterol metabolism deregulation in HCC is summarized in Fig. 2).
Figure 2.
Deregulated alterations of fatty acid and cholesterol metabolism in HCC. Fatty acid and cholesterol metabolism function as other major catabolic pathways in the regulation of metabolism during HCC development. FATPs, fatty acid transport proteins; ACSL4, acyl-CoA synthetase long chain family member 4; HSL, hormone-sensitive lipase; SS, squalene synthase; SM, squalene monooxygenase; MUFA, monounsaturated fatty acid; DAG, diacylglycerol; MAG, monoacyglycerol.
2.2.1. Fatty acid uptake and transport
Fatty acids are transported across cell membrane actively by several transmembrane transporters, such as fatty acid translocase/CD36, fatty acid transport proteins. Also, some specialized small proteins like fatty acid binding proteins which bind free fatty acids (FFAs) and facilitate FFAs intracellular transport74. To satisfy the metabolic need of HCC cells, these fatty acid transportation machineries are increased significantly in malignant tissues compared with adjacent tissues and normal liver cells. Apart from cell growth, other pathological properties of HCC cells are also involved. The up-regulation of CD36 is associated with the induction of EMT process75 by the potential activation of wingless-type MMTV integration site family (WNT) and transforming growth factor beta signaling pathways. Both fatty acid binding protein 1 (FABP1) and vascular endothelial growth factor receptor (VEGFR) expression are up-regulated in HCC, and positive correlation exists between the two. FABP1 induces HCC cell migration through VEGFR2/SRC proto-oncogene tyrosine-protein kinase signaling and focal adhesion kinase/cell division cycle 42 pathway76. The above findings support a role of FABP1 to promote angiogenesis, tumorigenesis and metastasis. FABP5 overexpression is also closely related to the occurrence, invasion and metastasis in HCC tumor tissues77. Treatment with oleate activates the FABP5/HIF-1α axis, thereby promoting lipid accumulation and cell proliferation in HCC cells78. The expression of FABP5 and HIF-1α are associated with poor prognosis in HCC. Patients with high expression of FABP5 deteriorate rapidly and have a high recurrence rate79.
Lipid lipase (LPL) hydrolyzes triacylglycerols (TAGs) into FFAs and represents another group of proteins that up-regulated in HCC samples to increase the absorption of lipoproteins by cells80. Inhibition of LPL restrains the uptake of exogenous lipids, resulting in the hindrance of HCC cell proliferation. In HCC patients, the expression level of LPL in stage III/IV is higher than that in stage I/II, positively related with poor prognosis81.
2.2.2. Fatty acid synthesis
Abnormally activated new fat formation is essential for the development and progression of HCC. Enhanced lipogenesis is characterized by increased activity and expression of various lipogenic enzymes, such as adenosine triphosphate (ATP) citrate lyase (ACLY), acetyl-CoA carboxylase (ACC), fatty acid synthase (FASN), acyl-CoA synthease (ACS) and stearoyl-CoA-desaturase 1 (SCD1). These enzymes are highly expressed in various cancers, including HCC.
Cancer cells use cytoplasmic acetyl-CoA as a substrate for fatty acid synthesis. Citrate is transported from the mitochondria to the cytoplasm, and then converted into acetyl-CoA and oxaloacetate through ACLY82. ACLY plays a key role in cancer metabolism by depriving cytosolic citrate to enhance glycolysis, as shown by the increased activity of phosphofructokinase1 (PFK1) and PFK2. Oncogenic drivers (such as PI3K/AKT) activate ACLY and Warburg effect in a feedback way82. As the rate-limiting enzyme for fatty acid synthesis, ACC is key to increase liver de novo lipogenesis (DNL) during HCC development. In the cytoplasm, ACC promotes the conversion of acetyl-CoA into the intermediate product malonyl-CoA. Phosphorylation of ACC decreases liver malonyl-CoA, suppresses fatty acid synthesis, alleviates early signs of NAFLD and fibrosis development in liver83. Liver-specific ACC inhibitor ND-654 inhibits liver DNL, inflammation and development of HCC84. Then, malonyl-CoA and acetyl-CoA are condensed by FASN to form palmitate and other fatty acid synthesis products. Elevated FASN heralds the poor prognosis of HCC patients. Artificial inhibition or suppression of FASN is very unfavorable for the growth of HCC cells. FASN also participates in the occurrence and development of HCC tumors. Targeted inhibition of FASN and its related lipogenesis is a potentially relevant therapy for the treatment of HCC85.
SCD catalyzes the conversion of saturated fatty acids into monounsaturated fatty acids86. SCD1 interfering effectively inhibits HCC progression, while its overexpression is related to the shortening of tumor-free survival. Multi-omics studies find that the increase in monounsaturated fatty acids contributes to the increased de novo synthesis of fatty acids in HCC cells. The effect of SCD1 in HCC is associated with the regulation of P5387, WNT/β-catenin88, epidermal growth factor receptor89 and autophagy90. Suppression of SCD1 sensitizes HCC cells to sorafenib by forcing the liver tumor-initiating cells to differentiate via the induction of ER stress. Agents targeting SCD1 in combination with sorafenib is a promising treatment strategy against HCC91.
ACS long chain family member 4 is one member of ACS family that convert fatty acid to fatty acyl-CoA esters with a substrate preference for arachidonic acid. Its knockout results in slower cell growth, while its overexpression promotes tumor formation in vivo and in vitro. ACS long chain family member 4 stabilizes c-MYC through extracellular-signal regulated kinase (ERK)/F-box and WD repeat domain-containing 7/c-MYC axis, and represents a valuable biomarker and potential therapeutic target for predicting the prognosis of liver cancer92.
The transcription factor SREBP1, a central player to boost DNL, is significantly higher in HCC tumor than adjacent tissues. Downregulation of SREBP1 inhibits proliferation of HCC HepG2 and MHCC97L cells and induces cell apoptosis. Knockout of SREBP1 robustly represses the migration and invasion of HCC cells. SREBP1 serves as a prognostic marker for HCC and aggravates tumor progression by promoting cell growth and metastasis93.
Liver X receptor (LXR) belongs to a member of ligand-activated nuclear receptor superfamily of transcription factors94. LXR (LXRα and LXRβ) functions to regulate cholesterol homeostasis and lipogenesis by transactivation of metabolic players as SREBP1 and FASN. Divergently, LXRα expression is down-regulated in HCC tumor tissues compared with normal liver tissues. LXR agonist TO901317 up-regulates LXRα, down-regulates GLUT1 and MMP9, and reduces intracellular glucose content, thereby inhibiting HCC progression95. In addition, LXR expression is correlated with liver fat deposition, liver inflammation and fibrosis in patients96. Treatment with long-term LXR agonist, oxidative stress, and high-fat diet together replicates liver conditions in mice similar to those patients with NASH and progression to HCC97. The above split findings indicate the complexity in the role of LXR in HCC development.
2.2.3. Fatty acid β-oxidation
Compatible with elevated efficiency of fatty acid β-oxidation pathway, enzymes involved in the oxidative decomposition of fatty acids have been dysregulated in HCC. Carnitine palmitoyl-transferase (CPT) is divided into two types: CPT1 and CPT2. As the rate-limiting enzyme, CPT1 locates in the outer mitochondrial membrane and transfers long-chain fatty acyl-CoA to carnitine for transport into mitochondria for further oxidation. It is worth noting that an increase in saturated long-chain acylcarnitine and a decrease in short-chain and medium-chain acylcarnitine are simultaneously observed in HCC samples98. Fatty acid β-oxidation and formation coordinate when ACC forms a complex with CPT1A to prevent its mitochondrial distribution under adequate glucose conditions. The formation of the complex between ACC and CPT1A is weakened with glucose starvation. The released free CPT1A molecules are relocated to the mitochondrial membrane, which enhances fatty acid β-oxidation. This provides a new mechanism for ACC and CPT1A to jointly protect HCC cells from metabolic stress99.
Medium-chain acyl-CoA dehydrogenase (MCAD) and long-chain acyl-CoA dehydrogenase (LCAD) are key enzymes by catalyzing the first step of mitochondrial fatty acid β-oxidation. They are downregulated by hypoxic stress, leading to the inhibition of fatty acid catabolism and promotion of HCC cells proliferation100. Consistently, forced expression of both MCAD and LCAD diminishes hypoxia-induced lipid accumulation in HCC cells. However, knockdown of LCAD, but not MCAD, facilitates tumor growth in vivo through the inhibition of tumor suppressor phosphatase and tensin homolog-mediated signaling pathway, which needs a further exploration101.
In addition, lipolysis also plays a role in fatty acid β-oxidation. Adipose triacylglycerol lipase (ATGL), hormone-sensitive lipase and monoacyglycerol lipase (MAGL) are three major enzymes involved in lipolysis and release of fatty acids102. MAGL catalyzes the conversion of monoacylglycerol esters into FFA and glycerol. Elevated expression of MAGL in cancer promotes the proliferation and invasion of HCC cells by generating signal lipids including monoacylglycerol, FFA and secondary lipid metabolites. NF-κB signaling pathway is involved in the MAGL-mediated EMT of HCC cells103. ATGL initiates the process of TAG hydrolysis into diacylglycerol and FFA. High levels of diacylglycerol and FFA are observed because of high expression of ATGL in HCC tissues, with an indication of poor prognosis. lncRNA-NEAT1 induces abnormal lipolysis in HCC cells through the up-regulation of ATGL, which is a driving force for the growth of HCC cells104.
2.2.4. Cholesterol metabolism
Dysregulated cholesterol biosynthesis is another metabolic event frequently observed in HCCs. Studies have revealed a new biochemical crosstalk between DNL and cholesterol biosynthetic pathways in the process of liver cancer105. 3-Hydroxy-3-methylglutaryl-CoA reductase (HMGCR) is a rate-limiting enzyme in mevalonate pathway for cholesterol synthesis that can be blocked by statins106. In fact, this inhibition has an anti-tumor effect in many tumor types. Up-regulation of HMGCR expression is reported previously in human HCC samples along with elevated mitochondrial cholesterol levels. Cholesterol depletion by inhibiting HMGCR or squalene synthase, another enzyme catalyzing the first step in cholesterol biosynthesis, enhances the sensitivity of HCC cells to chemotherapy via mitochondria107.
2.2.5. Others
Peroxisome proliferator-activated receptors are ligand-activated nuclear receptors of the steroid/thyroid hormone receptor superfamily. There are three isoforms, α, β/δ, and γ, related to lipid homeostasis108. Peroxisome proliferator-activated receptor α (PPARα) regulates the constitutive transcription of genes encoding enzymes involving in fatty acid transport and TAG homeostasis. The long-term administration of PPARα ligand in rodents causes accelerated hepatocyte proliferation, increased ROS production, and HCC development. Oncogene MYC directly acts as a transcription amplifier for specific PPARα target genes including KRT23 which promotes hepatocyte proliferation and potential HCC109.
The cytochrome P450s (CYPs) 4 family (CYP4) consists of a group of ω-hydroxylase that functions in the conversion of fatty acids that then transport to mitochondria to produce energy, thereby yielding elimination of excess FFA and synthesis of bioactive fatty acid molecules110. CYP4B, CYP4A and CYP4V, together with CYP4F metabolize short-chain fatty acids (approximately 7–10 carbon fatty acids), intermediate-chain fatty acids (C10 to 16), long-chain fatty acids (C16 to 26), respectively. Notably, decreased expression of CYP4 is associated with liver fat accumulation and NASH pathogenesis111. CYP4Z1 and its pseudogene CYP4Z2P, first identified by Rieger112, are highly expressed in breast cancer. CYP4Z1 promotes breast cancer angiogenesis and growth113. Our previous study discovers that CYP4Z2P 3′UTR also promotes breast cancer angiogenesis through the VEGF/VEGFR2 pathway114. Our later findings further explore that CYP4Z2P- and CYP4Z1-3′UTRs share miRNA-binding sites, including miR-211 and miR-197, thereby promoting tumor angiogenesis in breast cancer partly via miRNA-dependent activation of PI3K/AKT and ERK1/2 pathway115. However, whether the CYP4Z2P- and CYP4Z1-3′UTRs functions in fatty acid metabolism in cancer, especially HCC, still needs to be further revealed.
2.3. Amino acid metabolism and glutamine metabolism
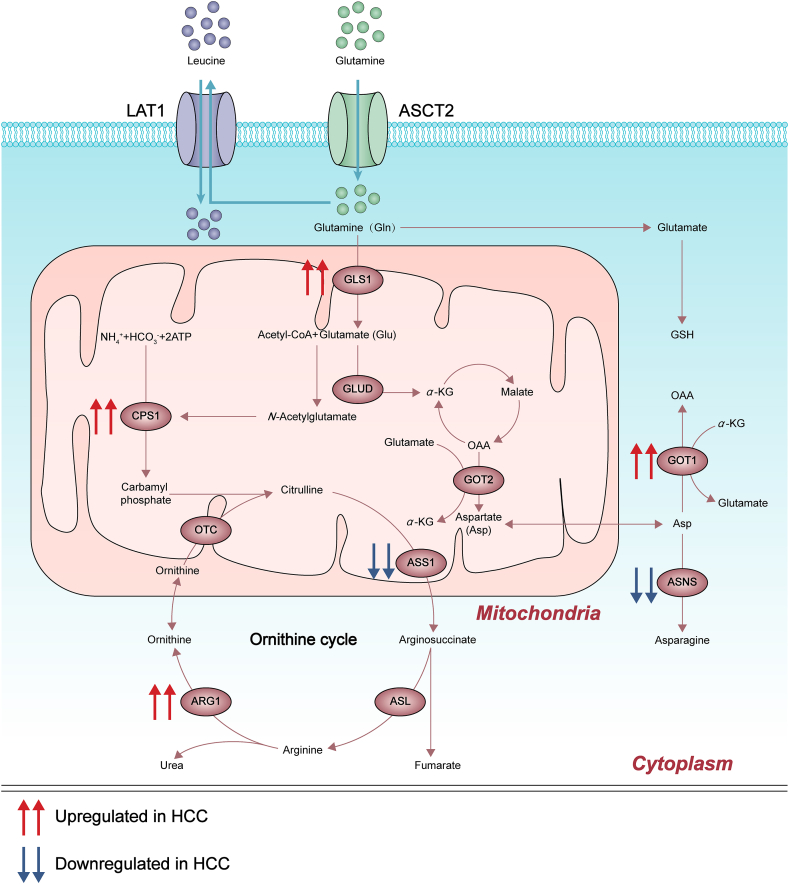
Liver is responsible for more than 80% of protein synthesis such as albumin, growth factors, and many other functionally important peptides. The carbon skeleton formed by hepatic protein decomposition is oxidized into CO2 and H2O to produce ATP, and also provides a carbon framework for the synthesis of new proteins, sugars and fatty acids. Furthermore, urea cycle unique in liver deals with nitrogenous wastes produced by amino acid metabolism that impair cellular activity. This part focuses on the understanding of targets related to amino acid and glutamine metabolism in HCC (Fig. 3).
Figure 3.
Deregulated alterations of amino acid metabolism and glutamine metabolism in HCC. Genes and metabolic intermediates of the amino acid and glutamine metabolism are dysregulated in HCC, some of which can be regulated by aberrantly activated oncogenes and loss of tumor suppressors, such as mutant Kirsten rat sarcoma 2 viral oncogene homolog, aflatoxin B1 as well as noncoding RNAs. CPS1, carbamyl phosphate synthase 1; ARG1, arginase-1; GLUD, glutamate dehydrogenase; GSH, glutathione; ASNS, asparagine synthetase; ASL, argininosuccinate lyase.
2.3.1. Glutamine metabolism and transportation
Glutamine is a nitrogen and carbon donor as its skeleton is incorporated versatilely when synthesizing proteins for different cell compartments116. Since proliferative tumor cells have increased glutamine demand, its catabolism, anabolism and transportation are essential for the survival and development of HCC117. Increased glutamine catabolism, also known as glutaminolysis, is one of the critical metabolic features for cancer cells. Glutamine-based therapy has been proposed as a potential strategy for cancer treatment118. Mammalian cells contain two distinct but related genes encoding glutaminase (enzyme for glutaminolysis), one of which is found in the kidney and a number of other tissues, including many cancer cells (called renal-type glutaminase or GLS1). Another is exclusively expressed in the liver (called liver-type glutaminase or GLS2). GLS1 overexpression boosts colony formation and cell proliferation of HCC cells with the involvement of AKT/glycogen synthase kinase 3 beta/cyclin D1 axis119. Its upregulation is positively correlated with late-stage clinicopathological features and stem cell phenotype. GLS1 regulates the identity of cancer stem cells through ROS/WNT/β-catenin pathway. Knockout of GLS1 inhibits tumorigenicity in vivo, suggesting that it act as a therapeutic target by eliminating cancer stem cells120. In the contrast, GLS2 negatively regulates PI3K/AKT signaling which is often activated in HCC. Blocking the PI3K/AKT signaling pathway destroys the inhibitory effect of GLS2 on anchor dependent cell growth and xenograft growth of HCC cells121. In fibrotic livers, GLS2 level is decreased and glutamine synthase is absent, but solute carrier family 1 member 5 (SLC1A5) and GLS1 are up-regulated. The latter is accumulated in fibrotic septa and does not compensate for the loss of GLS2 in hepatocytes. The restriction of glutamine inhibits the growth and fibrogenic activity of HSCs during NASH pathogenesis122.
Due to the increased request of glutamine, cancer cells absorb more from extracellular area than normal cells. Solute carrier transporters are highly expressed in the liver and act as metabolic gateways of cells, mediating the transport of a variety of essential nutrients and metabolites, such as glucose, amino acids, vitamins, neurotransmitters and inorganic/metal ions123. Solute carrier transporters play an important role in the etiology of many metabolic diseases, as well as cancer. SLC1A5, known as alanine serine cysteine-preferring transporter 2 (ASCT2), transports glutamine in a Na+-dependent manner124. SLC1A5 expression in tumor tissues is significantly up-regulated compared with adjacent non-tumor tissues and positively correlated with tumor size, suggesting that SLC1A5 could be a promising prognostic indicator for HCC patients125. SLC1A5 promoter activity and protein expression are both dependent on the presence of glutamine126.
SLC7A5, also known as L-Type amino acid transporter 1 (LAT1), belongs to the amino acid–polyamine–organocation superfamily and forms a heterodimer amino acid transporter through the conserved disulfide bond interaction with 4F2 cell-surface antigen heavy chain (4F2hc; also known as SLC3A2 or CD98)—a type II membrane glycoprotein that is indispensable for the protein stability of LAT1 and for its localization to the plasma membrane127. SLC7A5 protein in HCC tumor lesions is significantly higher than that in non-tumor areas, indicating the association of SLC7A5 expression with tumor growth128. Downstream oncogenic effector Yes-associated protein 1 and transcriptional coactivator with PDZ-binding motif of Hippo pathway increases the uptake of amino acids, activates cell growth regulator mammalian target of rapamycin complex 1 and promotes cell proliferation by up-regulating the expression of SLC7A5129. Patients with higher SLC7A5 expression have significantly shortened survival rate, suggesting that SLC7A5 is a potential therapeutic target for HCC130,131. Notably, the structure of human LAT1-4F2hc heteromeric amino acid transporter complex has been clearly resolved in recent years132, thereby providing deep insights into the function and mechanisms by which they influence human diseases, including HCC.
The coordination between the use of carbohydrates and amino acids to meet nutritional requirements is essential for physiological maintenance as well as cell growth, as the enzyme glutamate oxaloacetate transaminase 1 (GOT1) is one of the typical examples to regulate cell metabolism133. Kirsten rat sarcoma 2 viral oncogene homolog is the most frequently mutated RAS isoform in cancer cells, its mutation has recently been shown to rely on GOT1 to support long-term cell proliferation134. lncRNA TMPO-AS1 accelerates the progression of HCC by targeting the miR-429/GOT1 axis, which provides a new way of thinking for the treatment of HCC133.
2.3.2. Ornithine cycle
The toxic final product of amino acid metabolism, ammonia, is synthesized into urea through the ornithine cycle and excreted in urine as a detoxification pathway. Carbamyl phosphate synthase 1 is the first rate-limiting enzyme in the ornithine cycle and has been reported as an HCC-hypermethylated gene135,136. It is down-regulated by aflatoxin B1 and thus inhibits cell proliferation and induces cell apoptosis in HCC137. Argininosuccinate synthase 1 (ASS1) is another rate-limiting enzyme in the ornithine cycle, and tumor ASS1 deficiency is both a biomarker of prognosis and a predictor of susceptibility to arginine deprivation therapy138. The expression of ASS1 in HCC patients is significantly reduced due to DNA methylation. Stable silencing of ASS1 promotes HCC cell migration and invasion. In addition, ASS1 knockdown increases phosphorylation of signal transducer and activator of transcription 3 in Ser727 and boosts HCC metastasis by upregulating differentiation inhibitor 1. It is suggested that ASS1 inhibits HCC metastasis and is a potential target for HCC diagnosis and therapy139. Arginase-1, expressed in the liver cytoplasm, plays an important role in the ornithine cycle and is involved in anti-inflammatory effects, tumor immunity and immunosuppression-related diseases. Arginase-1 overexpression enhances HCC Huh7 cell viability, migration, and invasion, leading to a significant increase in protein and mRNA expression of key factors during EMT, such as vimentin, N-cadherin, and β-catenin140. Ornithine transcarbamylase (OTC) localizes in mitochondrial matrix and catalyzes the conversion of ornithine and carbamylphosphate to citrulline. OTC deficiency disrupts the metabolic pathways of urea, resulting in the accumulation of ammonia in the blood which is life-threatening in severe cases141. OTC silencing promotes proliferation of HCC SK-HEP-1 and Huh-7 cells. Clinical data indicate that patients with low level of OTC have a shorter overall survival time142. Above targets for metabolic dysfunction in HCC have been summarized in Table 1.
Table 1.
Targets for metabolic dysfunction in HCC.
| Metabolism | Target |
|---|---|
| Targets in glucose metabolism in HCC | |
| Glycolysis | HK224, PKM225,37,38, GAPDH28,31,32, GPI30, ALDOA33,34, PGK140,41 |
| Pentose phosphate pathway | G6PD47,49,50, TKT48,51 |
| Gluconeogenesis | FBP154, PCK155,59, 60, 61, G6PC56, 57, 58 |
| Tricarboxylic acid cycle | PC64, PDHA165, IDH266,67, ME169,70 |
| Targets in fatty acid and cholesterol metabolism in HCC | |
| Fatty acid uptake and transport | CD3675, FABPs76, 77, 78, 79, LPL80,81 |
| Fatty acid synthesis | ACLY82, ACC83, FASN85, SCD187, 88, 89, 90, 91, ACSL492, SREBP193, LXR95 |
| Fatty acid β-oxidation | CPT1A99, MCAD100,101, LCAD100,101, MAGL103, ATGL104 |
| Cholesterol metabolism | HMGCR107, SS107 |
| Others | PPARα109 |
| Targets in amino acid metabolism and glutamine metabolism in HCC | |
| Glutamine metabolism and transportation | GLS1119,120, GLS2121, ASCT2125, LAT1128, 129, 130, 131, GOT1133,134 |
| Ornithine cycle | CPS1136,137, ASS1138,139, ARG1140, OTC141,142 |
3. Emerging therapeutical strategies for the treatment of HCC
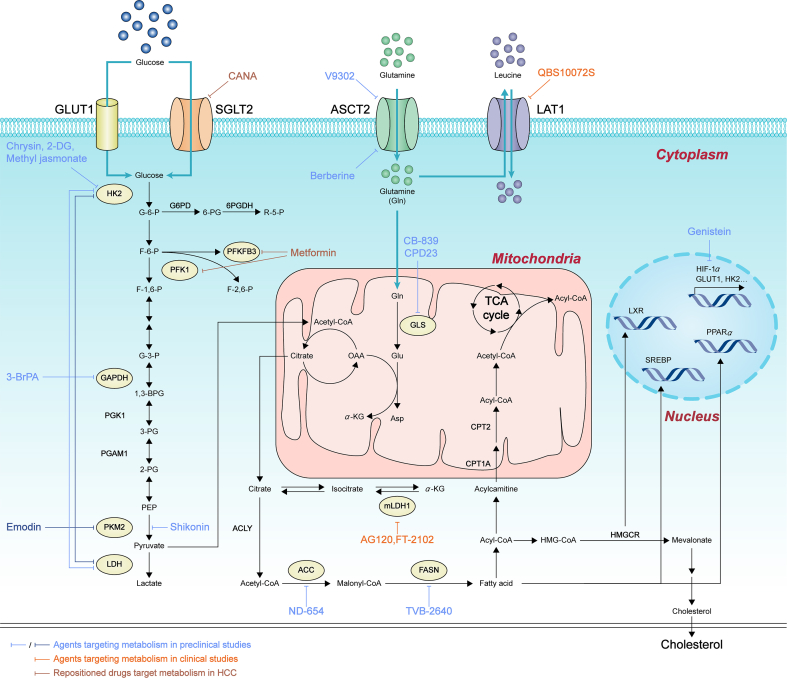
Given that metabolic rearrangements exist in HCC, the key or rate-limiting metabolic enzymes, metabolites, even drivers among metabolic changes, have attracted much attention. So far, a variety of drugs or chemicals have been developed as potentials to treat HCC. Whereas many agents are still in preclinical studies, some drugs have approached to clinical trials. Besides, some old drugs are repurposed to combat HCC by targeting metabolism (Fig. 4).
Figure 4.
Agents targeting metabolism in HCC. This figure lists all drugs or chemicals developed as potentials to treat HCC by targeting metabolism. Light blue and dark blue lines depict agents targeting metabolism in preclinical studies. Orange lines depict agents targeting metabolism in clinical studies. Brown lines depict repositioned drugs targeting metabolism in HCC.
3.1. Preclinical studies
3.1.1. Glycolysis inhibitors
As one of the hallmarks in cancer, glucose metabolism has been targeted to combat against cancer challenges with the agents mainly inhibiting glycolysis in HCC therapy. 2-Deoxy-d-glucose (2-DG), an analog of glucose, is catalyzed by HK2 into 2-deoxy-d-glucose-6-phosphate, which is distinct from glucose 6-phosphate to noncompetitively inhibit the activity of HK2143. 2-DG alone suppresses cell growth, metastasis and invasion in HCC cells, while enhances the anti-cancer effects synergistically with sorafenib144,145. These results provide some insights into the design of combined strategies of 2-DG and other chemotherapeutic drugs for HCC treatment.
3-Bromopyruvic acid (3-BrPA) functions as a potential clinical chemosensitizer to optimize the therapeutic index of chloroethylnitrosoureas, a bifunctional antitumor alkylating agent146. Besides the inhibition of HK2, the inhibition of GAPDH, PGK1, lactate dehydrogenase (LDH) and succinate dehydrogenase are also reported to be a part of anti-cancer activities of 3-BrPA147,148. In all, GAPDH is characterized to be the primary intracellular target of 3-BrPA via mass spectrometry, immunoprecipitation techniques and in vitro enzyme kinetic studies149. 3-BrPA could be a promising agent targeting glycolysis, or made into a fine combination strategy with other chemotherapeutic drugs for HCC treatment. Using a quantitative approach of metabolic control analysis, the central role of GAPDH in Warburg effect is established. A docking analysis indicates that koningic acid, a natural product, binds to the active site of GAPDH as a specific GAPDH inhibitor. The response to koningic acid covers NCI60 cell lines (a collection of 60 cancer cell lines from diverse tissue and genetic origins) in vitro shows broad but heterogeneous effects. Notably, only glucose uptake and lactate excretion are predictive of koningic acid response, independent of the extent of enzyme inhibition and tissue type150. However, whether koningic acid functions in HCC needs to be fully explored.
Inhibitors of some other glycolytic enzymes exert anti-cancer effects on cancer types other than HCC in preclinical studies, including PGK1 and phosphoglycerate mutase 1 (PGAM1). PGK1 catalyzes the conversion of 1,3-diphosphoglycerate (1,3-BPG) and ADP to 3-phosphoglycerate and ATP, which requires the binding of PGK1 to its substrates (1,3-BPG and ADP). Therefore, the design of PGK1 inhibitors has commonly focused on analogues of its natural substrate 1,3-BPG. Although several PGK1 inhibitors (CBR-470-1, bisphosphonates, terazosin or their derivatives) have been designed, the effects on cancer cells have not been reported yet151. GQQ-792, a thiodiketopiperazine derivative from the marine nature products, is characterized as a non-ATP-competitive PGK1 inhibitor, which leads to decreased glucose uptake and lactate production, and subsequent apoptosis of glioblastoma cells152. Moreover, a known yeast cell cycle-regulating kinase inhibitor, NG52, dose-dependently suppresses the proliferation of glioma cells in vitro and alleviates glioma tumor in vivo through inhibiting kinase activity of PGK1153. A potent PGAM1 allosterical inhibitor, PGMI-004A, decreases 2-phosphoglycerate and increases 3-phosphoglycerate levels in lung cancer cells. Treatment of PGMI-004A also results in decreased lactate production, suppressed oxidative PPP flux and NADPH/NADP+ ratio, reduced biosynthesis of lipids and RNA, and attenuated cell proliferation, which are rescued by methyl-2-PG treatment. PGMI-004A treatment also suppresses cell proliferation in diverse human cancer and leukemia cells, but not in human dermal fibroblasts, human foreskin fibroblasts, human keratinocyte HaCaT cells and human melanocyte PIG1 cells, suggesting specific toxicity of PGMI-004A to the malignantly proliferating human cells154. Given these findings, PGAM1 inhibitor PGMI-004A is implied to be a hopeful compound in cancer treatment. Targeting PGK1 and PGAM1 kinase activity could be a potential strategy for some types of cancer including glioma and lung cancer; however, how these inhibitors function in HCC needs further exploration.
Some natural products also exhibit promising anti-HCC effects, at least partly through inhibiting glycolysis genes or proteins expression, including GLUT1, HK2, PKM2 and LDHA. Inhibition of HK2 expression by chrysin, a natural flavone found in plant extracts which are widely used in traditional medicine in China, results in decreased glucose uptake and lactate production in HCC cells. The reduced expression of HK2 combines with voltage dependent anion channel-1 on mitochondria, which results in the translocation of BAX from the cytosol to the mitochondria and induction of cell apoptosis. And these effects of chrysin are markedly impaired in HK2 exogenously overexpressed HCC cells. Moreover, chrysin treatment restrains tumor growth in a HCCLM3 xenograft model and significantly decreases HK2 expression in chrysin-treated tumor tissue155. Methyl jasmonate, a plant stress hormone, detaches HK2 from voltage dependent anion channel to cause a reduction in mitochondrial transmembrane potential. It leads to the release of cytochrome c and apoptosis inducing factor, thereby promoting cell apoptosis and exerting a prominent inhibitory effect on the growth of HCC LM3 and BEL-7402 cells, with little effect on normal liver cells156. Shikonin, a natural naphthoquinone isolated from Lithospermum erythrorhizon, has therapeutic effects on various diseases, including cancer157,158. Shikonin, suppresses cell proliferation and glycolysis, and thus leads to cell apoptosis in HCCLM3 and SMMC-7721 cells. Artificial manipulation of PKM2 abolishes the effect of shikonin on cell proliferation, apoptosis, and glycolysis. Moreover, shikonin enhances the drug sensitivity of HCC cells to sorafenib159. Genistein, a natural isoflavone with many therapeutic effects, including anti-tumor effects. Genistein inhibits glycolysis and induces mitochondrial apoptosis in HCC cells by directly down-regulating HIF-1α, therefore inactivating GLUT1 and HK2. Genistein also reverses chemoresistance in sorafenib-resistant HCC cells and HCC-bearing mice160. Emodin, another natural product, decreases the expression of glycolytic enzymes (HK2, PKM2, and LDHA), thus inhibits glycolysis, limits energy supply and attenuates the growth of HCC HepG2 cells161. Moreover, our previous study also showed that saponin monomer 13 of dwarf lilyturf tuber (DT-13), the main steroidal saponin from Liriopes Radix, inhibits glucose uptake and ATP generation, reduces lactate production of colorectal cancer cells. DT-13 also remarkably inhibits GLUT1 expression in both mRNA and protein levels. Knockdown of GLUT1 decreases inhibitory ratio of DT-13 on colorectal cancer cell growth in vitro and in an orthotopic implantation mouse model162. However, whether and how DT-13 functions in HCC still need to be illustrated. Taken together, natural products serve as a rich resource to show potential to be applied in future HCC clinical treatment as glycolysis inhibitors.
3.1.2. Pentose phosphate pathway inhibitors
In addition to glycolysis, blocking PPP in HCC therapy has been illustrated. TKT inhibitor oxythiamine, a thiamine antagonist, synergizes with sorafenib to halt HCC cell growth both in vitro and in vivo, mechanically by raising the ROS levels51. These findings suggest that oxythiamine could be used in a combination therapy together with sorafenib.
3.1.3. Chemical inhibitors targeting gluconeogenesis
FBP1 loss is frequently displayed in HCC, as epigenetic regulation163,164 and ubiquitin-mediated degradation165 lead to the suppression of FBP1. Thus, chemical inhibitors targeting histone deacetylase, promoter methylation, and other upstream regulators have been explored to restore the expression of FBP1 for HCC treatment. Treatment with histone deacetylase inhibitors sodium butyrate, SAHA or LBH589 upregulates FBP1 expression in HCC, leading to a switch from glycolysis to gluconeogenesis. The therapy inhibits HCC HepG2 and SK-HEP-1 cells growth in vitro and restrains tumor growth in a SK-HEP-1 xenograft model in vivo163. Besides, treatment with lysine specific histone demethylase 1 inhibitor tranylcypromine robustly increases FBP1 expression in HCC HepG2 cells166. However, these epigenetic drugs for HCC treatment are still far away from clinical application, in particular, due to their low specificity and associated pleiotropic effects167. In HCC cells, tripartite motif-containing protein 28 directly binds to FBP1 and leads to its ubiquitination and degradation. Meanwhile, 26S proteasome inhibitor bortezomib abolishes FBP1 loss, thus inhibits cell growth, decreases glucose consumption and lactate production165. Significant activity of bortezomib monotherapy in HCC patients is still absent, but a combination therapy is suggested168. Furthermore, dexamethasone, a synthesized glucocorticoid, is reported to restore the expression of gluconeogenesis genes, including FBP1, thereby antagonizing the Warburg effect and showing efficacy in HCC treatment169. Nevertheless, clinical relevance is limited due to adverse effects caused by poor specificity.
3.1.4. Agents targeting fatty acid metabolism
Given that ACC functions as rate-limiting enzymes in de novo fatty acid synthesis, it is of special interest to establish ACC as a therapeutic target in multiple cancer types, including HCC170. While ACC inhibition has been profiled in preclinical analysis of various other cancer types, little evidence exists in HCC models exclusively171. ND-654, a hepatoselective (about 3000:1 liver to muscle exposure), allosteric inhibitor of ACC1 and ACC2, is explored for efficacy in rat models of liver cirrhosis and HCC. Interestingly, dual inhibition of ACC1 and ACC2 by ND-654 significantly reduces HCC incidence by 41%, which is comparable to sorafenib alone (57% reduction). The combination of ND-654 and sorafenib further decreases HCC incidence by 81%172. Numerous FASN inhibitors, such as C75, C93, orlistat, GSK2194069 and GSK837149A have been examined for preclinical anti-tumor activities173,174. Unfortunately, agents above have shown unfavorable toxicity, preventing them from moving into the clinic as viable treatment options for patients. To date, another small molecule TVB-2640 is the only FASN inhibitor moving into the phase I human clinical trial. It is initiated to investigate safety and to determine the recommended phase II dose of TVB-2640 as monotherapy and in combination with paclitaxel or docetaxel in patients with advanced or metastatic solid tumor cancers175. Although no objective responses are observed, an interesting trend of longer median time to progression is observed among patients with Kirsten rat sarcoma 2 viral oncogene homolog-mutant non-small cell lung cancer compared to wild-type. TVB-2640 is also being studied for the treatment of NASH, as well as other cancer types, including ovarian, and breast cancer176. ACLY inhibitors have been reported as cholesterol-lowering drugs in human clinical trials177,178. Several natural and synthetic ACLY inhibitors alone or in combination with other chemotherapeutics have demonstrated anti-proliferative effects on several cancer cell lines in vitro and in vivo, including lung cancer, ovarian cancer, and leukemia179,180. However, further human clinical trials dedicated to evaluate ACLY inhibitors as anti-tumor therapeutics and in the treatment of HCC are still needed. A939572, an SCD1 inhibitor, suppresses the number and size of spheres formation, migration and invasion, in both SCD1-high expressing HCC Huh7 and PLC/PRF/5 cells. Moreover, A939572 sensitizes those cells to sorafenib via induction of ER stress-induced differentiation91. Thus, the deep exploration for A939572 in HCC therapy deserves to be conducted.
In addition to target the enzymes (ACC, FASN, ACLY, and SCD1) of fatty acid synthesis for HCC therapy, transcriptional factors like LXR has also been emphasized. TO901317, a specific LXR agonist, inhibits HCC progression by upregulating LXRα, downregulating GLUT1 and MMP9 expression, and decreasing glucose uptake in HCC SMMC-7721 and HepG2 cells95.
YM-53601, a squalene synthase inhibitor of cholesterol biosynthesis pathway, synergizes doxorubicin-mediated HCC growth arrest and cell death in vivo107. It is suggested that targeting enzymes in cholesterol synthesis pathways in combination with other drugs could be an approach to HCC therapy.
3.1.5. Agents targeting amino acid and glutamine metabolism
Targeting GLS1 is expected to selectively halt the growth of tumor cells but not normal cells181. Various GLS1 inhibitors have been developed for cancer; however, some of which show good anti-tumor activity but serious toxicity in vivo, i.e., acivicin and 6-diazo-5-oxo-l-norleucine, perhaps due to the lack of selectivity as GLS2 is important for liver function182. Bis-2-(5-phenylacetamido-1,2,4-thiadiazol) ethyl sulfide (BPTES) is a GLS1 allosteric inhibitor but with poor solubility (0.01 μmol/L)183, thus a series of BPTES derivatives including CB-839 have been explored to improve solubility184. As another potent GLS1 allosteric inhibitor, CB-839 inhibits triple-negative breast cancer cells growth in vitro, but results only 61% tumor inhibition in patient-derived triple-negative breast cancer xenograft model and 54% tumor inhibition in JIMT-1 xenograft model184. Later, CPD23 (a selenium analogue of CB-839) is designed to improve the anti-cancer activity in vivo, but still shows partial tumor growth inhibition in a glutamine-dependent mouse H22 liver cancer xenograft model. Despite of this, CPD23 causes tumor tissue damage and prolongs survival in this model182. After all, effective and selective GLS1 inhibitors for HCC treatment need deep exploration. V-9302 is a competitive small molecule antagonist of transmembrane glutamine flux which selectively and potently inhibits SLC1A5 (ASCT2)-mediated glutamine uptake in a concentration-dependent manner. It exhibits a 100-fold improvement in potency over gamma-l-glutamyl-p-nitroanilide, and results in attenuated cancer cell growth and proliferation, increased cell death, and increased oxidative stress. V-9302 prevents tumor growth in xenograft mouse models in vivo185 and sensitizes glutamine-dependent HCC cells to glutaminase inhibitor CB-839 treatment by inducing ROS and promoting apoptosis186. Dual inhibition of glutamine metabolism by targeting both glutaminase and SLC1A5 represents a potential novel treatment strategy for glutamine addicted HCC. Our recent data reveal a novel anti-cancer mechanism of a Topo I inhibitor topotecan as inhibition of glutamine uptake via downregulation of SLC1A5 in gastric cancer cells187. Some natural products also exhibit promising anti-cancer effects via affecting genes or proteins related to amino acid and glutamine metabolism. For example, berberine, a natural compound isolated from herbal medicines, suppresses glutamine intake and the growth of HCC tumor xenografts, as well as the proliferation of HCC cells in vitro188. Agents targeting deregulated metabolism for the treatment of HCC in preclinical studies has been summarized in Table 2.
Table 2.
Agents targeting deregulated metabolism for the treatment of HCC in preclinical studies.
| Compd. | Highest phase | Mechanism of action | Functions in HCC | Ref. |
|---|---|---|---|---|
| Agents targeted glucose metabolism | ||||
|
Glycolysis inhibitors | ||||
| 2-DG | Pre-clinical | An analog of glucose to inhibit HK2 activity | Suppresses cell growth, metastasis and invasion in HCC HLF and PLC/PRF/5 cells; synergistically enhances the effect of sorafenib; | 144,145 |
| 2-DG and sorafenib increase HCC Hep3B and Huh7 persister cells apoptosis and inhibit colony formation, significantly inhibit tumor growth in a Hep3B persister cell xenograft model | ||||
| 3-BrPA | Pre-clinical | Targets GAPDH, decreases GAPDH, LDH and SDH expression at protein levels; | — | 146, 147, 148 |
| Targets HK2 and dissociates HK2 from mitochondrial complex | A potential clinical chemosensitizer to optimize the therapeutic index of CENUs | |||
| Chrysin | Pre-clinical | Inhibits HK2 | Decreases glucose uptake and lactate production in HCC cells, reduces HK2 which combined with VDAC1 on mitochondria, thus induction of cell apoptosis; Restrains tumor growth in a HCCLM3 xenograft model and significantly decreases HK2 expression | 155 |
| Methyl jasmonate | Pre-clinical | A plant stress hormone to detach HK2 from VDAC | Causes a reduction in mitochondrial transmembrane potential that leads to the release of cytochrome c and apoptosis inducing factor, results in intrinsic apoptosis, thereby exerting a prominent inhibitory effect on the growth of HCC cells | 156 |
| Shikonin | Pre-clinical | Inhibits PKM2 | Suppresses cell proliferation and glycolysis and thus leads to cell apoptosis in HCC LM3 and SMMC-7721 cells, enhances the drug sensitivity of HCC cells to sorafenib | 159 |
| Genistein | Pre-clinical | Directly downregulates HIF-1α, therefore inactivating GLUT1 and HK2 | Inhibits glycolysis and induces mitochondrial apoptosis in HCC cells, and enhances the antitumor effect of sorafenib in sorafenib-resistant HCC cells and HCC-bearing mice | 160 |
| Emodin |
Pre-clinical |
Decreases glycolytic enzymes expression (HK2, PKM2, and LDHA) |
Inhibits glycolysis, limits energy supply and attenuates the growth of HCC HepG2 cells |
161 |
|
Pentose phosphate pathway inhibitors | ||||
| Oxythiamine |
Pre-clinical |
A TKT inhibitor |
Synergizes sorafenib to halt HCC cell growth both in vitro and in vivo |
51 |
|
Chemical inhibitors targeting gluconeogenesis | ||||
| Sodium butyrate, SAHA, LBH589 | Pre-clinical | HDAC inhibitors | Upregulates FBP1 expression in HCC, leading to a switch from glycolysis to gluconeogenesis and inhibits HCC HepG2 and SK-HEP-1 cells growth in vitro and restrains tumor growth in a SK-HEP-1 xenograft model in vivo | 163 |
| Tranylcypromine | Pre-clinical | An LSD1 inhibitor | Increases FBP1 expression in HCC HepG2 cells | 166 |
| Bortezomib | Pre-clinical | A 26S proteasome inhibitor | Abolishes FBP1 loss, thus inhibits cell growth, decreases glucose consumption and lactate production | 165 |
| Dexamethasone |
Pre-clinical |
A synthesized glucocorticoid |
Restores the expression of gluconeogenesis genes, including FBP1, thereby antagonizing the Warburg effect and showing efficacy in HCC treatment |
169 |
| Agents targeted for fatty acid metabolism | ||||
| ND-654 | Pre-clinical | An allosteric inhibitor of ACC1 and ACC2 | Reduces HCC incidence by 41%, which is comparable to the results with sorafenib alone (57%); ND-654 and sorafenib significantly reduce HCC incidence by 81% | 172 |
| A939572 | Pre-clinical | An SCD1 inhibitor | Suppresses the number and size of spheres formation, migration and invasion, in both SCD1-high expressing HCC Huh7 and PLC/PRF/5 cells; sensitizes those cells to sorafenib via induction of ER stress-induced differentiation | 91 |
| TO901317 | Pre-clinical | A specific LXR agonist | Inhibits HCC progression by upregulating LXRα, downregulating GLUT1 and MMP9 expression, and decreasing glucose uptake in HCC SMMC-7721 and HepG2 cells | 95 |
| YM-53601 |
Pre-clinical |
An SS inhibitor |
Synergizes doxorubicin-mediated HCC growth arrest and cell death in vivo |
107 |
| Agents targeted for amino acid and glutamine metabolism | ||||
| CPD23 | Pre-clinical | A selenium analogue of CB839 that targets GLS1 | Shows partial tumor growth inhibition in a GD mouse H22 liver cancer xenograft model; Causes tumor tissue damage and prolongs survival | 182 |
| V-9302 | Pre-clinical | Selectively and potently targets ASCT2 (SLC1A5) | Inhibits ASCT2-mediated glutamine uptake in human cells in a concentration-dependent manner, attenuates cancer cell growth and proliferation, increases cell death, and increases oxidative stress | 185,186 |
| Sensitizes GD HCC cells to glutaminase inhibitor CB-839, by inducing ROS, thus results in apoptosis of GD cells; Shows tumor inhibition in HCC xenograft mouse models in vivo | ||||
–, not applicable. CENUs, chloroethylnitrosoureas; GD, glutamine-dependent; HDAC, histone deacetylase; LSD1, lysine specific histone demethylase 1; SDH, succinate dehydrogenase; VDAC, voltage dependent anion channel.
3.2. Clinical trials
3.2.1. Mutant IDH inhibitors
A number of mutant IDH (mIDH) inhibitors have been developed to directly inhibit the neomorphic activity of mIDH enzymes in an effort to reduce oncometabolite D-2-hydroxyglutarate production. The inhibitors exert anti-cancer effects especially in IDH-mutant acute myeloblastic leukemia (AML), gliomas and other cancers. Small molecule ivosidenib (AG-120) is an orally available inhibitor of mIDH1 that has been developed for the treatment of cancer in patients with IDH1 mutations189. Meanwhile, relevant clinical trials for AML, cholangiocarcinoma, glioma, myelodysplastic syndromes and solid tumors are ongoing worldwide (NCT04056910 and NCT02073994). Olutasidenib (FT-2102) is another highly potent, orally active, brain penetrant and selective inhibitor of mIDH1, with IC50 values of 21.2 and 114 nmol/L for IDH1- R132H and IDH1- R132C, respectively. FT-2102 is under study in the treatment of AML or myelodysplastic syndrome190. Another phase Ib/II study of FT-2102 in participants with advanced solid tumors (including HCC, bile duct carcinoma, intrahepatic cholangiocarcinoma, other hepatobiliary carcinomas), and gliomas with an IDH1 mutation is ongoing (NCT03684811). To sum up, although pharmaceutical agents targeting mIDH have been approved for cancers with IDH mutation, efficacy of these agents in HCC patients with mIDH is still under expectation in above trials.
3.2.2. Agents targeting fatty acid metabolism
In terms of therapeutically targeting dysregulated fatty acid metabolism in cancer, FASN has arguably received the most widespread interest. It is not surprising considering its multifaceted roles in supporting both anabolic metabolism and oncogenic signaling. However, the transition of FASN inhibitors from bench to bedside has largely been elusive, and marked with several challenges and shortcomings. The first-generation FASN-targeting drugs, such as C75, orlistat and cerulenin, initially show obvious anti-cancer effects in preclinical studies191, 192, 193. Systemic side effects as drastic weight loss and anorexia are found in clinical study194, which highlights the involvement of fatty acid metabolic enzymes in both disease pathology and normal whole-body metabolic homoeostasis. More recently, next-generation FASN inhibitors, TVB-3166 (an orally-available, reversible, and selective FASN inhibitor) and TVB-2640 (the first inhibitor of FASN to enter the clinic), have shown tremendous anti-tumor effects in preclinical breast and colorectal cancer models, as well as excellent tolerability and limited systemic toxicity in early-phase clinical trials195,196. One explanation for the improved tolerance of the next-generation inhibitors is that TVB-3166 and TVB-2640 do not contribute to the indirect activation of CPT1 in peripheral tissues, in contrast with C75 and cerulenin195. The induction of β-oxidation peripherally following C75 or cerulenin treatment contributes to increased energy expenditure, loss of adipose tissue and significant weight loss, whereas next-generation inhibitors display higher specificity for FASN with limited off-target effects195,197. Although there is not any FASN inhibitors applied in clinical trials for now for HCC treatment, the application of FASN inhibitors in other cancer types provides guidance to HCC treatment.
Statins, known as HMGCR inhibitors, exert an anti-tumor effect in many cancer types, including breast cancer, AML, and have a high therapeutic index to target tumors in vivo, despite the ubiquitous expression of the mevalonate pathway106. However, how statins function in HCC is obscure. A combined treatment of pravastatin and chemoembolization improves survival of patients with advanced HCC compared to patients receiving chemoembolization alone198. Still, deep exploration for statins in HCC therapy needs to be conducted in clinical trials.
3.2.3. SLC7A5 inhibitors
Since SLC7A5 is overexpressed in a variety of cancers, efforts are undertaken in order to synthesize and characterize potent inhibitors of SLC7A5-mediated amino-acids transport for cancer intervention. JPH203 is recently evaluated in a first phase I clinical trial as a SLC7A5 selective inhibitor. Six out of 17 patients diagnosed with advanced solid tumors achieves partial response or stable stage. Four out of the 6 responders are diagnosed with biliary tract cancer, in which plasma levels of SLC7A5 substrates remains high199. High plasma levels of SLC7A5 substrate amino acids could be an important factor that determines the efficacy of JPH203 in biliary tract cancer. However, since only 17 patients are enrolled in this phase I clinical trial, these findings need to be validated in a larger cohort of patients. Importantly, based on the promising results of the phase I clinical trial, JPH203 is currently being evaluated in a phase II clinical trial in patients with advanced biliary tract cancers (UMIN Clinical Trials Registry UMIN000034080). Besides, QBS10072S, another novel small-molecule inhibitor targeting SLC7A5, is now under a dose escalation phase I study to assess its safety, tolerability, pharmacokinetics, and pharmacodynamics (NCT04430842), and the applicable cancer types includes HCC. The response of the test is still expecting. The agents used to target metabolism for HCC treatment in clinical trials are summarized in Table 3.
Table 3.
Agents targeting metabolism for the treatment of HCC in clinical trials.
| Compd. | Highest phase | Mechanism of action | Types of cancer | Ref./NCT No. |
|---|---|---|---|---|
| AG-120 (Ivosidenib) | I | Targets IDH1 and prevents production of d-2-hydroxyglutarate | IDH1-mutant advanced cholangiocarcinoma; | NCT04056910, NCT02073994 |
| II | IDH1-mutant advanced solid tumors | |||
| FT-2102 (Olutasidenib) | Ib/II | Targets IDH1 and reduces production of d-2-hydroxyglutarate | Advanced solid tumors (including HCC, bile duct carcinoma, intrahepatic cholangiocarcinoma, other hepatobiliary carcinomas), and gliomas with an IDH1 mutation | NCT03684811 |
| Pravastatin | – | A HMGCR inhibitor | Pravastatin and chemoembolization improve survival of patients with advanced HCC compared to patients receiving chemoembolization alone | 198 |
| QBS10072S | I | Inhibits LAT1 (SLC7A5) | Patients with advanced or metastatic cancers with high LAT1 (SLC7A5) expression including liver cancer | NCT04430842 |
–Not applicable.
3.3. Repositioned drugs target metabolism
Drug repositioning, also known as drug rediscovery or drug repurposing, is well defined to explore new anti-cancer indications for existing drugs due to their long clinical history and established safety records200,201. For instance, Repurposing Drugs in Oncology project (ReDO project) (http://www.redo-project.org) has identified more than 270 licensed non-cancer drugs with evidence of anticancer activity. Among them, some drugs are explored to exert anti-cancer properties at least in part by targeting metabolism (Table 4).
Table 4.
Repositioned drugs targeting metabolism in HCC treatment.
| Drug | Highest phase | Mechanism of action | Functions in cancer/types of cancer | Ref./NCT No. |
|---|---|---|---|---|
| Metformin | [1] [2] Pre-clinical | [1] Mitochondrial complex I inhibitor which diminishes the increase in respiration upon HK2 depletion | [1] Induces HCC Huh7 and HepG2 cell death in vitro and inhibits Huh7 subcutaneous tumor growth in vivo upon HK2 depletion | [1]202 |
| [2] Decreases glycolytic flux through the HIF-1α/PFKFB3/PFK1 pathway | [2] Alleviates HCC Huh7 and HepG2 cell proliferation | [2]203 | ||
| [3] [4] Phase 2 | [3] & [4] Needs to be monitored and illustrated | [3] Evaluates the efficacy and safety of high-dose vitamin C combined with metformin in the treatment of malignant tumors, including HCC | [3] NCT04033107 | |
| [4] Compares the role of celebrex alone, metformin alone, and celebrex plus metformin in preventing HCC recurrence after hepatic resection | [4] NCT03184493 | |||
| Canagliflozin (CANA) | [1] [2] Pre-clinical | [1] Inhibits glucose uptake presumably in a SGLT2-dependent way, induces G2/M arrest and facilitates apoptosis with inhibited phosphorylation of ERK, AKT, P38 and cleavage of caspase3 | [1] Suppresses SGLT2-expressing HCC Huh7 and HepG2 cells proliferation and xenograft tumors growth both in vitro and in vivo, but not SGLT2-null HCC HLE cells, or PHH and LX-2 cells | [1]204 |
| [2] Inhibits ectopic fat accumulation in the liver and suppresses the ratio of GSSG/GSH to reduce oxidative stress in adipose tissue | [2] One year CANA treatment significantly reduces the number of liver tumors in WD-fed MC4R-KO mice compared to vehicle-treated groups | [2]205 |
GSSG/GSH, oxidized/reduced glutathione; PFKFB3, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3.
3.3.1. Metformin
Metformin, a first-line therapy for the treatment of type 2 diabetes, is found to have potentials as an anti-cancer agent or an adjuvant chemotherapy sensitizer in vitro and in vivo206,207. Mechanistically, metformin affects mitochondrial metabolism in ovarian cancer cells208 through the liver kinase B1/adenosine mono-phosphate-activated protein kinase (AMPK) signaling pathway209,210 or AMPK-independent signaling211. Besides, metformin also induces breast cancer cell apoptosis and inhibits cell proliferation in nutrient-poor conditions through downregulating PKM2 expression, which is mediated by AMPK activation212. In a recent study, metformin alleviates HCC cell proliferation by decreasing glycolytic flux through inhibition of PFK1 in vitro. Besides, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3, a potent allosteric activator of PFK1, is also significantly inhibited by decreased HIF-1α accumulation induced by metformin203. Moreover, metformin diminishes the increase in respiration upon HK2 depletion in HCC cells, and thus induces cell death both in vitro and in vivo202. It is partly because that loss of HK2 causes a reduction in glycolysis and thus a compensatory upregulation of oxidative phosphorylation. Collectively, these results provide new insights into the mechanisms of metformin in combatting HCC.
Since the side effects and safety records have been well-established for metformin, its anti-cancer effect is promisingly applicable in a variety of cancer types mentioned above. Till now, about 361 clinical trials using metformin with/without other drugs as treatment conducted in cancer patients are ongoing (https://clinicaltrials.gov)213. Although there is no clinical trial conducted using metformin as a single treatment for HCC for now, its combination with other drugs for HCC treatment are included. A phase II clinical trial is aimed to evaluate the efficacy and safety of high-dose vitamin C combined with metformin in the treatment of malignant tumors, including HCC (NCT04033107). Besides, a phase III clinical trial is conducted to compare the role of celebrex (a selective cyclic oxidase-2 inhibitor) alone, metformin alone, and celebrex plus metformin in preventing HCC recurrence after hepatic resection (NCT03184493), since some observational studies with small sample size have found that metformin plus celebrex reduce the recurrence rate of HCC after surgery214. Yet, the clinical efficacy needs to be monitored and underlying mechanisms need to be illustrated thoroughly.
3.3.2. Canagliflozin
Canagliflozin (CANA) is a SGLT2 inhibitor, which decreases circulating glucose levels in type 2 diabetes patients by inhibiting SGLT2-dependent reabsorption of glucose in the kidney. A newly research has found that CANA exerts anti-cancer effects in pancreatic cancer and prostate cancer in vitro and in vivo, potentially through inhibition of SGLT2-mediated glucose uptake215. Besides, several other studies about CANA actions in HCC are also carried out.
CANA has an inhibitory effect on the glucose uptake of SGLT2-expressing HCC Huh7 and HepG2 cells, but not SGLT2-null HCC HLE cells, or primary human hepatocytes and HSCs (LX-2). As three above HCC cell lines all overexpresses GLUT1216, it suggests that the glucose uptake inhibitory effect of CANA on HCC cells is presumably SGLT2-dependent. Similarly, CANA inhibits cell proliferation and subcutaneous xenograft tumors growth in HCC Huh7 and HepG2 cells, but not SGLT2-null HCC HLE cells. In addition, CANA also induces G2/M arrest and cell apoptosis with suppressed phosphorylation of ERK, AKT, P38 and cleavage of caspase3 in Huh7 and HepG2 cells204. Inconsistently, HCC cell growth is not directly inhibited by tofogliflozin, another SGLT2 inhibitor, even under insulin resistance-mimicking and hyperglycemic conditions217. One possible explanation for this discrepancy is at least partially the differential effect on SGLT1 and SGLT2 between CANA and tofogliflozin218. Above all, CANA is a potential new strategy for the treatment of HCC.
In a study investigating the effect of CANA on NASH and NASH-associated HCC, a mouse model of human NASH is established using Western diet (WD)-fed melanocortin 4 receptor-deficient (MC4R-KO) mice. CANA treatment for 8 or 20 weeks alleviates hepatic steatosis and hepatic fibrosis in WD-fed MC4R-KO mice, separately. Not surprisingly, CANA-treatment for 1 year significantly reduces the number of liver tumors in WD-fed MC4R-KO mice compared to vehicle-treated groups. Mechanically, CANA induces adipose expansion without deteriorating inflammation or fibrosis, and markedly suppresses the ratio of oxidized/reduced glutathione to reduce oxidative stress in adipose tissue. It implies an inhibitory effect of CANA on ectopic fat accumulation in the liver and therefore NASH and NASH-associated HCC via promoting healthy adipose expansion205. Nevertheless, clinical trials to investigate CANA as monotherapy or in combination with other drugs in cancer treatment is still rare. For now, there is only one project conducted to check whether controlling the glucose/insulin feedback enhances the efficacy of serabelisib, a PI3K alpha isoform inhibitor, in treating solid tumors without the inclusion of HCC patients though (NCT04073680). Thus, CANA for HCC treatment needs further exploration.
4. Challenges and opportunities
Currently, primarily two targeted small-molecule TKIs, sorafenib6 and lenvatinib7, are approved by FDA as the first line choice for HCC treatment. Although their targets are mainly cancer-related protein kinases (i.e., RAF/MEK/ERK, VEGFR, PDGFR), it is recently revealed that sorafenib disrupts lipogenesis and triggers liver cancer cell death by targeting SCD1 through the ATP/AMPK/mTOR/SREBP1 pathway219. Moreover, it uncovers that decreased mitochondrial biogenesis is found in sorafenib-resistant cells under sorafenib treatment, mechanically by accelerated degradation of peroxisome proliferator-activated receptor γ coactivator 1β220. The above studies reveal cell metabolism-associated signaling pathways mediated by sorafenib, which potentially allow strategies to augment the efficacy and specificity of sorafenib in HCC cells. Taken together, metabolic targeting therapy could also be a supplemental choice in combination with sorafenib or lenvatinib to treat HCC in the future.
Whether metabolic dysregulation is the causative factor of HCC or a consequence of HCC development still remains inconclusive; however, sufficient evidence suggests that metabolic dysregulation acts as a critical contributor in the initiation and progression of HCC. For instance, although overexpression of GAPDH fails to develop HCC without carcinogen presence in mouse models, it does aggravate tumor development by using GAPDH transgenic mouse in a diethylnitrosamine-induced HCC mouse model. Mechanically, overexpression of GAPDH promotes diversion from glycolysis to serine biosynthesis, by elevating phosphoglycerate dehydrogenase and promoting histone methylation independent of its catalytic function, thereby accelerates cell proliferation and liver tumorigenesis28. Moreover, hepatocyte-specific loss of the gluconeogenic enzyme FBP1 disrupts liver metabolic homeostasis which induces steatosis with the activation and senescence of HSCs, and therefore aggravates tumor progression54. Moreover, preclinical mouse models for studying NASH-driven HCC221 also supports the causative role of metabolic dysregulation in HCC. A recent review article also echoes that increasing evidence points to the involvement of metabolic stress in the initiation of primary liver cancers, over 90% among which are HCC222. Also, we are not able to deny that the consequence of HCC development results in metabolic alterations. The rapid cell growth surely requires more metabolic supply. As shown, genomic deviations in HCC such as mutations in TP53 (in 31% of patients), MYC (in 15%), fuels from pentose phosphate pathway as well as serine metabolism223,224. Although a range of preclinical studies targeting metabolism have shown tremendous anti-tumor effects in HCC, only AG120 and FT2102 (both target mIDH1) [NCT02073994] or QBS10072S (targets SLC7A5) [NCT04430842] are now tested in phase I/II clinical trials for HCC treatment. Thus, mIDH1 or SLC7A5 are possibly most promising targets to intervene metabolic dysfunction in HCC to date, but still needs further supportive evidence.
In the past decade, the application of integrative high-throughput omics approaches, including genomics, transcriptomics, proteomics and metabolomics, in parallel with informatics and computational analysis studies, has uncovered many novel genes or deregulated metabolites and therefore built up a metabolic network during HCC development. This information contributes greatly to the discovery and development of new agents targeting metabolism. However, the use of HCC cell lines in the in vitro screening studies has significant limitations, since these cells have at least partially different metabolic properties and therefore fails to recapitulate the key features of HCC cells in cancer tissues in vivo. Mouse models and patient-derived xenograft models with full pictures of metabolic dysregulation instead have a seat at the table of drug discovery in HCC therapy. Even though some genetically engineered murine models are validated to investigate the potential as a drug target of a certain gene or abnormally altered metabolic pathway, the limitations as high-cost and time-consuming set an obstacle as an efficient tool for in vivo drug screening against this life-threatening malignancy. In addition, due to the genetic heterogeneity of HCC in clinic, the available genetically modified animal models are still far from full coverage. Alternatively, long-term organoid culture derived from tumor biopsies of HCC patients is a good choice to screen the emerging drug agents before clinical phase and to develop tailored therapies individually225. The approach to recapitulate the histological properties of the original HCC tumors will at least partially cover the shortage of current models; however, new strategies to copy the metabolic properties in vivo is still a challenge in the field.
5. Conclusions
A recent nomenclatural alteration from NAFLD to MAFLD highlights the importance of deregulated metabolism during HCC tumorigenesis and development. As one of the hallmarks of cancer, metabolic reprogramming in HCC could be of significance to combat the existing limitations in current systemic therapies of HCC. Herein, a comprehensive summary of key enzymes, and intermediates in nutrients metabolism contributes to deep understanding of potential targets for HCC treatment (Table 1). A range of preclinical and clinical studies targeting dysregulated metabolic enzymes in HCC are carried out, requiring fully and carefully monitoring and evaluation though (Table 2, Table 3). Besides, drug repositioning provides a new direction for the treatment of neoplastic diseases, including HCC (Table 4). Fully utilizing the unique metabolic variations in HCC therapy remains a huge challenge for biologists, pharmacologists, and clinicians.
Acknowledgments
The study was supported by the National Natural Science Foundation of China (No. 82070883), and Scientific Research Foundation for high-level faculty, China Pharmaceutical University (Nanjing, China).
Author contributions
Jing Xiong contributed to the conception and preparation of the manuscript; Danyu Du, Chan Liu, Mengyao Qin and Xiao Zhang jointly drafted the manuscript via an intense literature survey and made the figures; Danyu Du made the tables; Tao Xi and Shengtao Yuan reviewed and edited the manuscript; Haiping Hao and Jing Xiong supervised the manuscript.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Haiping Hao, Email: haipinghao@cpu.edu.cn.
Jing Xiong, Email: jxiong@cpu.edu.cn.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Chen W., Zheng R., Baade P.D., Zhang S., Zeng H., Bray F., et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Forner A., Reig M., Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 3.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 4.Abou-Alfa G.K., Johnson P., Knox J.J., Capanu M., Davidenko I., Lacava J., et al. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA. 2010;304:2154–2160. doi: 10.1001/jama.2010.1672. [DOI] [PubMed] [Google Scholar]
- 5.Qin S., Bai Y., Lim H.Y., Thongprasert S., Chao Y., Fan J., et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501–3508. doi: 10.1200/JCO.2012.44.5643. [DOI] [PubMed] [Google Scholar]
- 6.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 7.Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J., Qin S., Merle P., Granito A., Huang Y.H., Bodoky G., et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 9.Abou-Alfa G.K., Meyer T., Cheng A.L., El-Khoueiry A.B., Rimassa L., Ryoo B.Y., et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu A.X., Park J.O., Ryoo B.Y., Yen C.J., Poon R., Pastorelli D., et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859–870. doi: 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- 11.Rimassa L., Danesi R., Pressiani T., Merle P. Management of adverse events associated with tyrosine kinase inhibitors: improving outcomes for patients with hepatocellular carcinoma. Cancer Treat Rev. 2019;77:20–28. doi: 10.1016/j.ctrv.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Zhai B., Sun X.Y. Mechanisms of resistance to sorafenib and the corresponding strategies in hepatocellular carcinoma. World J Hepatol. 2013;5:345–352. doi: 10.4254/wjh.v5.i7.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.Y., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 14.Eslam M., Sanyal A.J., George J., International Consensus Panel MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 15.Xiong J., Liu T., Mi L., Kuang H., Xiong X., Chen Z., et al. hnRNPU/TrkB defines a chromatin accessibility checkpoint for liver injury and nonalcoholic steatohepatitis pathogenesis. Hepatology. 2020;71:1228–1246. doi: 10.1002/hep.30921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Degasperi E., Colombo M. Distinctive features of hepatocellular carcinoma in non-alcoholic fatty liver disease. Lancet Gastroenterol Hepatol. 2016;1:156–164. doi: 10.1016/S2468-1253(16)30018-8. [DOI] [PubMed] [Google Scholar]
- 17.Kanwal F., Kramer J.R., Duan Z., Yu X., White D., El-Serag H.B. Trends in the burden of nonalcoholic fatty liver disease in a United States Cohort of Veterans. Clin Gastroenterol Hepatol. 2016;14:301–308. doi: 10.1016/j.cgh.2015.08.010. e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Serag H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan T.R., Mandayam S., Jamal M.M. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127:S87–S96. doi: 10.1053/j.gastro.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Gingold J.A., Zhu D., Lee D.F., Kaseb A., Chen J. Genomic profiling and metabolic homeostasis in primary liver cancers. Trends Mol Med. 2018;24:395–411. doi: 10.1016/j.molmed.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Pavlova N.N., Thompson C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hay N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy?. Nat Rev Cancer. 2016;16:635–649. doi: 10.1038/nrc.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guzman G., Chennuri R., Chan A., Rea B., Quintana A., Patel R., et al. Evidence for heightened hexokinase II immunoexpression in hepatocyte dysplasia and hepatocellular carcinoma. Dig Dis Sci. 2015;60:420–426. doi: 10.1007/s10620-014-3364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazurek S., Boschek C.B., Hugo F., Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300–308. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Tong X., Zhao F., Thompson C.B. The molecular determinants of de novo nucleotide biosynthesis in cancer cells. Curr Opin Genet Dev. 2009;19:32–37. doi: 10.1016/j.gde.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mossenta M., Busato D., Dal Bo M., Toffoli G. Glucose metabolism and oxidative stress in hepatocellular carcinoma: role and possible implications in novel therapeutic strategies. Cancers (Basel) 2020;12:1668. doi: 10.3390/cancers12061668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu S., Sun Y., Jiang M., Li Y., Tian Y., Xue W., et al. Glyceraldehyde-3-phosphate dehydrogenase promotes liver tumorigenesis by modulating phosphoglycerate dehydrogenase. Hepatology. 2017;66:631–645. doi: 10.1002/hep.29202. [DOI] [PubMed] [Google Scholar]
- 29.Guo H., Xu J., Zheng Q., He J., Zhou W., Wang K., et al. NRF2 SUMOylation promotes de novo serine synthesis and maintains HCC tumorigenesis. Cancer Lett. 2019;466:39–48. doi: 10.1016/j.canlet.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Fruman D.A., Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colell A., Green D.R., Ricci J.E. Novel roles for GAPDH in cell death and carcinogenesis. Cell Death Differ. 2009;16:1573–1581. doi: 10.1038/cdd.2009.137. [DOI] [PubMed] [Google Scholar]
- 32.Gong Y.H., Zou B.J., Peng S.Q., Li P.P., Zhu G.L., Chen J.F., et al. Nuclear GAPDH is vital for hypoxia-induced hepatic stellate cell apoptosis and is indicative of aggressive hepatocellular carcinoma behavior. Cancer Manag Res. 2019;11:4947–4956. doi: 10.2147/CMAR.S202268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semenza G.L., Roth P.H., Fang H.M., Wang G.L. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 34.Sharp F.R., Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci. 2004;5:437–448. doi: 10.1038/nrn1408. [DOI] [PubMed] [Google Scholar]
- 35.Kawai K., Uemura M., Munakata K., Takahashi H., Haraguchi N., Nishimura J., et al. Fructose-bisphosphate aldolase A is a key regulator of hypoxic adaptation in colorectal cancer cells and involved in treatment resistance and poor prognosis. Int J Oncol. 2017;50:525–534. doi: 10.3892/ijo.2016.3814. [DOI] [PubMed] [Google Scholar]
- 36.Jiao L., Zhang H.L., Li D.D., Yang K.L., Tang J., Li X., et al. Regulation of glycolytic metabolism by autophagy in liver cancer involves selective autophagic degradation of HK2 (hexokinase 2) Autophagy. 2018;14:671–684. doi: 10.1080/15548627.2017.1381804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu W.R., Tian M.X., Yang L.X., Lin Y.L., Jin L., Ding Z.B., et al. PKM2 promotes metastasis by recruiting myeloid-derived suppressor cells and indicates poor prognosis for hepatocellular carcinoma. Oncotarget. 2015;6:846–861. doi: 10.18632/oncotarget.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo W., Hu H., Chang R., Zhong J., Knabel M., O'Meally R., et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dey P., Kundu A., Sachan R., Park J.H., Ahn M.Y., Yoon K., et al. PKM2 knockdown induces autophagic cell death via AKT/mTOR pathway in human prostate cancer cells. Cell Physiol Biochem. 2019;52:1535–1552. doi: 10.33594/000000107. [DOI] [PubMed] [Google Scholar]
- 40.Cao C., Zhong Q., Lu L., Huang B., Li J., Meng L., et al. Long noncoding RNA MSC-AS1 promotes hepatocellular carcinoma oncogenesis via inducing the expression of phosphoglycerate kinase 1. Cancer Med. 2020;9:5174–5184. doi: 10.1002/cam4.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Z., Zhuang W., Wang Z., Xiao W., Don W., Li X., et al. MicroRNA-450b-3p inhibits cell growth by targeting phosphoglycerate kinase 1 in hepatocellular carcinoma. J Cell Biochem. 2019;120:18805–18815. doi: 10.1002/jcb.29196. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Y., Li M., Yao X., Fei Y., Lin Z., Li Z., et al. HCAR1/MCT1 regulates tumor ferroptosis through the lactate-mediated AMPK–SCD1 activity and its therapeutic implications. Cell Rep. 2020;33:108487. doi: 10.1016/j.celrep.2020.108487. [DOI] [PubMed] [Google Scholar]
- 43.Zhang D., Tang Z., Huang H., Zhou G., Cui C., Weng Y., et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574:575–580. doi: 10.1038/s41586-019-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu H., Pan L., Gao C., Xu H., Li Y., Zhang L., et al. Quercetin inhibits the proliferation of glycolysis-addicted HCC cells by reducing hexokinase 2 and Akt–mTOR pathway. Molecules. 2019;24:1993. doi: 10.3390/molecules24101993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li M., Shao J., Guo Z., Jin C., Wang L., Wang F., et al. Novel mitochondrion-targeting copper(II) complex induces HK2 malfunction and inhibits glycolysis via Drp1-mediating mitophagy in HCC. J Cell Mol Med. 2020;24:3091–3107. doi: 10.1111/jcmm.14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riganti C., Gazzano E., Polimeni M., Aldieri E., Ghigo D. The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radic Biol Med. 2012;53:421–436. doi: 10.1016/j.freeradbiomed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Hong X., Song R., Song H., Zheng T., Wang J., Liang Y., et al. PTEN antagonises Tcl1/hnRNPK-mediated G6PD pre-mRNA splicing which contributes to hepatocarcinogenesis. Gut. 2014;63:1635–1647. doi: 10.1136/gutjnl-2013-305302. [DOI] [PubMed] [Google Scholar]
- 48.Qin Z., Xiang C., Zhong F., Liu Y., Dong Q., Li K., et al. Transketolase (TKT) activity and nuclear localization promote hepatocellular carcinoma in a metabolic and a non-metabolic manner. J Exp Clin Cancer Res. 2019;38:154. doi: 10.1186/s13046-019-1131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sukhatme V.P., Chan B. Glycolytic cancer cells lacking 6-phosphogluconate dehydrogenase metabolize glucose to induce senescence. FEBS Lett. 2012;586:2389–2395. doi: 10.1016/j.febslet.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 50.Lu M., Lu L., Dong Q., Yu G., Chen J., Qin L., et al. Elevated G6PD expression contributes to migration and invasion of hepatocellular carcinoma cells by inducing epithelial–mesenchymal transition. Acta Biochim Biophys Sin. 2018;50:370–380. doi: 10.1093/abbs/gmy009. (Shanghai) [DOI] [PubMed] [Google Scholar]
- 51.Xu I.M., Lai R.K., Lin S.H., Tse A.P., Chiu D.K., Koh H.Y., et al. Transketolase counteracts oxidative stress to drive cancer development. Proc Natl Acad Sci U S A. 2016;113:E725–E734. doi: 10.1073/pnas.1508779113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Langbein S., Zerilli M., Zur Hausen A., Staiger W., Rensch-Boschert K., Lukan N., et al. Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br J Cancer. 2006;94:578–585. doi: 10.1038/sj.bjc.6602962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X.P., Yang S.S., Chen J.L., Su Z.G. Unraveling the regulation of hepatic gluconeogenesis. Front Endocrinol (Lausanne) 2018;9:802. doi: 10.3389/fendo.2018.00802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li F., Huangyang P., Burrows M., Guo K., Riscal R., Godfrey J., et al. FBP1 loss disrupts liver metabolism and promotes tumorigenesis through a hepatic stellate cell senescence secretome. Nat Cell Biol. 2020;22:728–739. doi: 10.1038/s41556-020-0511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tuo L., Xiang J., Pan X.M., Gao Q.Z., Zhang G.J., Yang Y., et al. PCK1 downregulation promotes TXNRD1 expression and hepatoma cell growth via the Nrf2/Keap1 pathway. Front Oncol. 2018;8:611. doi: 10.3389/fonc.2018.00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cho J.H., Kim G.Y., Pan C.J., Anduaga J., Choi E.J., Mansfield B.C., et al. Downregulation of SIRT1 signaling underlies hepatic autophagy impairment in glycogen storage disease type Ia. PLoS Genet. 2017;13 doi: 10.1371/journal.pgen.1006819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho J.H., Kim G.Y., Mansfield B.C., Chou J.Y. Sirtuin signaling controls mitochondrial function in glycogen storage disease type Ia. J Inherit Metab Dis. 2018;41:997–1006. doi: 10.1007/s10545-018-0192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho J.H., Kim G.Y., Mansfield B.C., Chou J.Y. Hepatic glucose-6-phosphatase-α deficiency leads to metabolic reprogramming in glycogen storage disease type Ia. Biochem Biophys Res Commun. 2018;498:925–931. doi: 10.1016/j.bbrc.2018.03.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Montal E.D., Dewi R., Bhalla K., Ou L., Hwang B.J., Ropell A.E., et al. PEPCK coordinates the regulation of central carbon metabolism to promote cancer cell growth. Mol Cell. 2015;60:571–583. doi: 10.1016/j.molcel.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu M.X., Jin L., Sun S.J., Liu P., Feng X., Cheng Z.L., et al. Metabolic reprogramming by PCK1 promotes TCA cataplerosis, oxidative stress and apoptosis in liver cancer cells and suppresses hepatocellular carcinoma. Oncogene. 2018;37:1637–1653. doi: 10.1038/s41388-017-0070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu D., Wang Z., Xia Y., Shao F., Xia W., Wei Y., et al. The gluconeogenic enzyme PCK1 phosphorylates INSIG1/2 for lipogenesis. Nature. 2020;580:530–535. doi: 10.1038/s41586-020-2183-2. [DOI] [PubMed] [Google Scholar]
- 62.Hui S., Ghergurovich J.M., Morscher R.J., Jang C., Teng X., Lu W., et al. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551:115–118. doi: 10.1038/nature24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Owen O.E., Kalhan S.C., Hanson R.W. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem. 2002;277:30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- 64.Wattanavanitchakorn S., Ansari I.H., El Azzouny M., Longacre M.J., Stoker S.W., MacDonald M.J., et al. Differential contribution of pyruvate carboxylation to anaplerosis and cataplerosis during non-gluconeogenic and gluconeogenic conditions in HepG2 cells. Arch Biochem Biophys. 2019;676:108124. doi: 10.1016/j.abb.2019.108124. [DOI] [PubMed] [Google Scholar]
- 65.Sun J., Li J., Guo Z., Sun L., Juan C., Zhou Y., et al. Overexpression of pyruvate dehydrogenase E1α subunit inhibits Warburg effect and induces cell apoptosis through mitochondria-mediated pathway in hepatocellular carcinoma. Oncol Res. 2019;27:407–414. doi: 10.3727/096504018X15180451872087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tian G.Y., Zang S.F., Wang L., Luo Y., Shi J.P., Lou G.Q. Isocitrate dehydrogenase 2 suppresses the invasion of hepatocellular carcinoma cells via matrix metalloproteinase 9. Cell Physiol Biochem. 2015;37:2405–2414. doi: 10.1159/000438593. [DOI] [PubMed] [Google Scholar]
- 67.Zhang H., Guo X., Dai J., Wu Y., Ge N., Yang Y., et al. Genetic variations in IDH gene as prognosis predictors in TACE-treated hepatocellular carcinoma patients. Med Oncol. 2014;31:278. doi: 10.1007/s12032-014-0278-z. [DOI] [PubMed] [Google Scholar]
- 68.Su T.F., Gao H.W. Expression of malic enzymes in sebaceous lesions. Am J Dermatopath. 2016;38:580–585. doi: 10.1097/DAD.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 69.Wen D., Liu D., Tang J., Dong L., Liu Y., Tao Z., et al. Malic enzyme 1 induces epithelial–mesenchymal transition and indicates poor prognosis in hepatocellular carcinoma. Tumour Biol. 2015;36:6211–6221. doi: 10.1007/s13277-015-3306-5. [DOI] [PubMed] [Google Scholar]
- 70.Shao C., Lu W.J., Du Y., Yan W.C., Bao Q.Y., Tian Y., et al. Cytosolic ME1 integrated with mitochondrial IDH2 supports tumor growth and metastasis. Redox Biol. 2020;36:101685. doi: 10.1016/j.redox.2020.101685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grohmann M., Wiede F., Dodd G.T., Gurzov E.N., Ooi G.J., Butt T., et al. Obesity drives STAT-1-dependent NASH and STAT-3-dependent HCC. Cell. 2018;175:1289–1306.e20. doi: 10.1016/j.cell.2018.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang M., Han J., Xing H., Zhang H., Li Z., Liang L., et al. Dysregulated fatty acid metabolism in hepatocellular carcinoma. Hepat Oncol. 2016;3:241–251. doi: 10.2217/hep-2016-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Riscal R., Skuli N., Simon M.C. Even cancer cells watch their cholesterol!. Mol Cell. 2019;76:220–231. doi: 10.1016/j.molcel.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Attane C., Muller C. Drilling for oil: tumor-surrounding adipocytes fueling cancer. Trends Cancer. 2020;6:593–604. doi: 10.1016/j.trecan.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 75.Nath A., Li I., Roberts L.R., Chan C. Elevated free fatty acid uptake via CD36 promotes epithelial–mesenchymal transition in hepatocellular carcinoma. Sci Rep. 2015;5:14752. doi: 10.1038/srep14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ku C.Y., Liu Y.H., Lin H.Y., Lu S.C., Lin J.Y. Liver fatty acid-binding protein (l-FABP) promotes cellular angiogenesis and migration in hepatocellular carcinoma. Oncotarget. 2016;7:18229–18246. doi: 10.18632/oncotarget.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ohata T., Yokoo H., Kamiyama T., Fukai M., Aiyama T., Hatanaka Y., et al. Fatty acid-binding protein 5 function in hepatocellular carcinoma through induction of epithelial–mesenchymal transition. Cancer Med. 2017;6:1049–1061. doi: 10.1002/cam4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seo J., Jeong D.W., Park J.W., Lee K.W., Fukuda J., Chun Y.S. Fatty-acid-induced FABP5/HIF-1 reprograms lipid metabolism and enhances the proliferation of liver cancer cells. Commun Biol. 2020;3:638. doi: 10.1038/s42003-020-01367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jeong C.Y., Hah Y.S., Cho B.I., Lee S.M., Joo Y.T., Jung E.J., et al. Fatty acid-binding protein 5 promotes cell proliferation and invasion in human intrahepatic cholangiocarcinoma. Oncol Rep. 2012;28:1283–1292. doi: 10.3892/or.2012.1922. [DOI] [PubMed] [Google Scholar]
- 80.Mead J.R., Irvine S.A., Ramji D.P. Lipoprotein lipase: structure, function, regulation, and role in disease. J Mol Med (Berl) 2002;80:753–769. doi: 10.1007/s00109-002-0384-9. [DOI] [PubMed] [Google Scholar]
- 81.Wu Z., Ma H., Wang L., Song X., Zhang J., Liu W., et al. Tumor suppressor ZHX2 inhibits NAFLD-HCC progression via blocking LPL-mediated lipid uptake. Cell Death Differ. 2020;27:1693–1708. doi: 10.1038/s41418-019-0453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Icard P., Wu Z., Fournel L., Coquerel A., Lincet H., Alifano M. ATP citrate lyase: a central metabolic enzyme in cancer. Cancer Lett. 2020;471:125–134. doi: 10.1016/j.canlet.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 83.Fullerton M.D., Galic S., Marcinko K., Sikkema S., Pulinilkunnil T., Chen Z.P., et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013;19:1649–1654. doi: 10.1038/nm.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lally J.S.V., Ghoshal S., DePeralta D.K., Moaven O., Wei L., Masia R., et al. Inhibition of acetyl-CoA carboxylase by phosphorylation or the inhibitor ND-654 suppresses lipogenesis and hepatocellular carcinoma. Cell Metab. 2019;29:174–182.e5. doi: 10.1016/j.cmet.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Che L., Paliogiannis P., Cigliano A., Pilo M.G., Chen X., Calvisi D.F. Pathogenetic, prognostic, and therapeutic role of fatty acid synthase in human hepatocellular carcinoma. Front Oncol. 2019;9:1412. doi: 10.3389/fonc.2019.01412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ntambi J.M., Miyazaki M. Recent insights into stearoyl-CoA desaturase-1. Curr Opin Lipidol. 2003;14:255–261. doi: 10.1097/00041433-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 87.Qiang L., Kon N., Zhao W., Jiang L., Knight C.M., Welch C., et al. Hepatic SirT1-dependent gain of function of stearoyl-CoA desaturase-1 conveys dysmetabolic and tumor progression functions. Cell Rep. 2015;11:1797–1808. doi: 10.1016/j.celrep.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lai K.K.Y., Kweon S.M., Chi F., Hwang E., Kabe Y., Higashiyama R., et al. Stearoyl-CoA desaturase promotes liver fibrosis and tumor development in mice via a Wnt positive-signaling loop by stabilization of low-density lipoprotein-receptor-related proteins 5 and 6. Gastroenterology. 2017;152:1477–1491. doi: 10.1053/j.gastro.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bhushan B., Banerjee S., Paranjpe S., Koral K., Mars W.M., Stoops J.W., et al. Pharmacologic inhibition of epidermal growth factor receptor suppresses nonalcoholic fatty liver disease in a murine fast-food diet model. Hepatology. 2019;70:1546–1563. doi: 10.1002/hep.30696. [DOI] [PubMed] [Google Scholar]
- 90.Huang G.M., Jiang Q.H., Cai C., Qu M., Shen W. SCD1 negatively regulates autophagy-induced cell death in human hepatocellular carcinoma through inactivation of the AMPK signaling pathway. Cancer Lett. 2015;358:180–190. doi: 10.1016/j.canlet.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 91.Ma M.K.F., Lau E.Y.T., Leung D.H.W., Lo J., Ho N.P.Y., Cheng L.K.W., et al. Stearoyl-CoA desaturase regulates sorafenib resistance via modulation of ER stress-induced differentiation. J Hepatol. 2017;67:979–990. doi: 10.1016/j.jhep.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 92.Chen J., Ding C., Chen Y., Hu W., Lu Y., Wu W., et al. ACSL4 promotes hepatocellular carcinoma progression via c-Myc stability mediated by ERK/FBW7/c-Myc axis. Oncogenesis. 2020;9:42. doi: 10.1038/s41389-020-0226-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li C., Yang W., Zhang J., Zheng X., Yao Y., Tu K., et al. SREBP-1 has a prognostic role and contributes to invasion and metastasis in human hepatocellular carcinoma. Int J Mol Sci. 2014;15:7124–7138. doi: 10.3390/ijms15057124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Surugiu-Wärnmark I., Wärnmark A., Toresson G., Gustafsson J., ABülow L. Selection of DNA aptamers against rat liver X receptors. Biochem Biophys Res Commun. 2005;332:512–517. doi: 10.1016/j.bbrc.2005.04.147. [DOI] [PubMed] [Google Scholar]
- 95.Xiong T., Li Z., Huang X., Lu K., Xie W., Zhou Z., et al. TO901317 inhibits the development of hepatocellular carcinoma by LXRα/Glut1 decreasing glycometabolism. Am J Physiol Gastrointest Liver Physiol. 2019;316:G598–G607. doi: 10.1152/ajpgi.00061.2018. [DOI] [PubMed] [Google Scholar]
- 96.Ahn S.B., Jang K., Jun D.W., Lee B.H., Shin K.J. Expression of liver X receptor correlates with intrahepatic inflammation and fibrosis in patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2014;59:2975–2982. doi: 10.1007/s10620-014-3289-x. [DOI] [PubMed] [Google Scholar]
- 97.Shimizu Y., Tamura T., Kemmochi A., Owada Y., Ozawa Y., Hisakura K., et al. Oxidative stress and liver X receptor agonist induce hepatocellular carcinoma in non-alcoholic steatohepatitis model. J Gastroenterol Hepatol. 2020;36:800–810. doi: 10.1111/jgh.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lu X., Zhang X., Zhang Y., Zhang K., Zhan C., Shi X., et al. Metabolic profiling analysis upon acylcarnitines in tissues of hepatocellular carcinoma revealed the inhibited carnitine shuttle system caused by the downregulated carnitine palmitoyltransferase 2. Mol Carcinog. 2019;58:749–759. doi: 10.1002/mc.22967. [DOI] [PubMed] [Google Scholar]
- 99.Wang M.D., Wu H., Fu G.B., Zhang H.L., Zhou X., Tang L., et al. Acetyl-coenzyme A carboxylase alpha promotion of glucose-mediated fatty acid synthesis enhances survival of hepatocellular carcinoma in mice and patients. Hepatology. 2016;63:1272–1286. doi: 10.1002/hep.28415. [DOI] [PubMed] [Google Scholar]
- 100.Zhang H. HIF-1 suppresses lipid catabolism to promote cancer progression. Mol Cell Oncol. 2015;2 doi: 10.4161/23723556.2014.980184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang D., Li T.T., Li X.H., Zhang L., Sun L.C., He X.P., et al. HIF-1-mediated suppression of acyl-CoA dehydrogenases and fatty acid oxidation is critical for cancer progression. Cell Rep. 2014;8:1930–1942. doi: 10.1016/j.celrep.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 102.Bolsoni-Lopes A., Alonso-Vale M.I. Lipolysis and lipases in white adipose tissue—an update. Arch Endocrinol Metab. 2015;59:335–342. doi: 10.1590/2359-3997000000067. [DOI] [PubMed] [Google Scholar]
- 103.Zhu W., Zhao Y., Zhou J., Wang X., Pan Q., Zhang N., et al. Monoacylglycerol lipase promotes progression of hepatocellular carcinoma via NF-κB-mediated epithelial–mesenchymal transition. J Hematol Oncol. 2016;9:127. doi: 10.1186/s13045-016-0361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu X., Liang Y., Song R., Yang G., Han J., Lan Y., et al. Long non-coding RNA NEAT1-modulated abnormal lipolysis via ATGL drives hepatocellular carcinoma proliferation. Mol Cancer. 2018;17:90. doi: 10.1186/s12943-018-0838-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Che L., Chi W., Qiao Y., Zhang J., Song X., Liu Y., et al. Cholesterol biosynthesis supports the growth of hepatocarcinoma lesions depleted of fatty acid synthase in mice and humans. Gut. 2020;69:177–186. doi: 10.1136/gutjnl-2018-317581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mullen P.J., Yu R., Longo J., Archer M.C., Penn L.Z. The interplay between cell signalling and the mevalonate pathway in cancer. Nat Rev Cancer. 2016;16:718–731. doi: 10.1038/nrc.2016.76. [DOI] [PubMed] [Google Scholar]
- 107.Montero J., Morales A., Llacuna L., Lluis J.M., Terrones O., Basanez G., et al. Mitochondrial cholesterol contributes to chemotherapy resistance in hepatocellular carcinoma. Cancer Res. 2008;68:5246–5256. doi: 10.1158/0008-5472.CAN-07-6161. [DOI] [PubMed] [Google Scholar]
- 108.Desvergne B., Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 109.Kim D., Brocker C.N., Takahashi S., Yagai T., Kim T., Xie G.M., et al. Keratin 23 is a peroxisome proliferator-activated receptor alpha-dependent, MYC-amplified oncogene that promotes hepatocyte proliferation. Hepatology. 2019;70:154–167. doi: 10.1002/hep.30530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jarrar Y.B., Lee S.J. Molecular functionality of cytochrome P450 4 (CYP4) genetic polymorphisms and their clinical implications. Int J Mol Sci. 2019;20:4274. doi: 10.3390/ijms20174274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hardwick J.P. Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases. Biochem Pharmacol. 2008;75:2263–2275. doi: 10.1016/j.bcp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 112.Rieger M.A., Ebner R., Bell D.R., Kiessling A., Rohayem J., Schmitz M., et al. Identification of a novel mammary-restricted cytochrome P450, CYP4Z1, with overexpression in breast carcinoma. Cancer Res. 2004;64:2357–2364. doi: 10.1158/0008-5472.can-03-0849. [DOI] [PubMed] [Google Scholar]
- 113.Yu W., Chai H., Li Y., Zhao H., Xie X., Zheng H., et al. Increased expression of CYP4Z1 promotes tumor angiogenesis and growth in human breast cancer. Toxicol Appl Pharmacol. 2012;264:73–83. doi: 10.1016/j.taap.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zheng L., Li X., Gu Y., Ma Y., Xi T. Pseudogene CYP4Z2P 3′UTR promotes angiogenesis in breast cancer. Biochem Biophys Res Commun. 2014;453:545–551. doi: 10.1016/j.bbrc.2014.09.112. [DOI] [PubMed] [Google Scholar]
- 115.Zheng L., Guo Q., Xiang C., Liu S., Jiang Y., Gao L., et al. Transcriptional factor six2 promotes the competitive endogenous RNA network between CYP4Z1 and pseudogene CYP4Z2P responsible for maintaining the stemness of breast cancer cells. J Hematol Oncol. 2019;12:23. doi: 10.1186/s13045-019-0697-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang J., Pavlova N.N., Thompson C.B. Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine. EMBO J. 2017;36:1302–1315. doi: 10.15252/embj.201696151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bhutia Y.D., Ganapathy V. Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim Biophys Acta. 2016;1863:2531–2539. doi: 10.1016/j.bbamcr.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang L., Venneti S., Nagrath D. Glutaminolysis: a hallmark of cancer metabolism. Annu Rev Biomed Eng. 2017;19:163–194. doi: 10.1146/annurev-bioeng-071516-044546. [DOI] [PubMed] [Google Scholar]
- 119.Xi J., Sun Y., Zhang M., Fa Z., Wan Y., Min Z., et al. GLS1 promotes proliferation in hepatocellular carcinoma cells via AKT/GSK3beta/Cyclin D1 pathway. Exp Cell Res. 2019;381:1–9. doi: 10.1016/j.yexcr.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 120.Li B., Cao Y., Meng G., Qian L., Xu T., Yan C., et al. Targeting glutaminase 1 attenuates stemness properties in hepatocellular carcinoma by increasing reactive oxygen species and suppressing Wnt/beta-catenin pathway. EBioMedicine. 2019;39:239–254. doi: 10.1016/j.ebiom.2018.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Suzuki S., Tanaka T., Poyurovsky M.V., Nagano H., Mayama T., Ohkubo S., et al. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc Natl Acad Sci U S A. 2010;107:7461–7466. doi: 10.1073/pnas.1002459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Du K., Chitneni S.K., Suzuki A., Wang Y., Henao R., Hyun J., et al. Increased glutaminolysis marks active scarring in nonalcoholic steatohepatitis progression. Cell Mol Gastroenterol Hepatol. 2020;10:1–21. doi: 10.1016/j.jcmgh.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang Y., Zhang Y., Sun K., Meng Z., Chen L. The SLC transporter in nutrient and metabolic sensing, regulation, and drug development. J Mol Cell Biol. 2019;11:1–13. doi: 10.1093/jmcb/mjy052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Scalise M., Pochini L., Console L., Losso M.A., Indiveri C. The human SLC1A5 (ASCT2) amino acid transporter: from function to structure and role in cell biology. Front Cell Dev Biol. 2018;6:96. doi: 10.3389/fcell.2018.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun H.W., Yu X.J., Wu W.C., Chen J., Shi M., Zheng L.M., et al. GLUT1 and ASCT2 as predictors for prognosis of hepatocellular carcinoma. PLoS One. 2016;11 doi: 10.1371/journal.pone.0168907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bungard C.I., McGivan J.D. Glutamine availability up-regulates expression of the amino acid transporter protein ASCT2 in HepG2 cells and stimulates the ASCT2 promoter. Biochem J. 2004;382:27–32. doi: 10.1042/BJ20040487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Scalise M., Galluccio M., Console L., Pochini L., Indiveri C. The Human SLC7A5 (LAT1): the intriguing histidine/large neutral amino acid transporter and its relevance to human health. Front Chem. 2018;6:243. doi: 10.3389/fchem.2018.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ohkame H., Masuda H., Ishii Y., Kanai Y. Expression of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (4F2hc) in liver tumor lesions of rat models. J Surg Oncol. 2001;78:265–271. doi: 10.1002/jso.1165. discussion 71-2. [DOI] [PubMed] [Google Scholar]
- 129.Park Y.Y., Sohn B.H., Johnson R.L., Kang M.H., Kim S.B., Shim J.J., et al. Yes-associated protein 1 and transcriptional coactivator with PDZ-binding motif activate the mammalian target of rapamycin complex 1 pathway by regulating amino acid transporters in hepatocellular carcinoma. Hepatology. 2016;63:159–172. doi: 10.1002/hep.28223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Janpipatkul K., Suksen K., Borwornpinyo S., Jearawiriyapaisarn N., Hongeng S., Piyachaturawat P., et al. Downregulation of LAT1 expression suppresses cholangiocarcinoma cell invasion and migration. Cell Signal. 2014;26:1668–1679. doi: 10.1016/j.cellsig.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 131.Li J., Qiang J., Chen S.F., Wang X., Fu J., Chen Y. The impact of L-type amino acid transporter 1 (LAT1) in human hepatocellular carcinoma. Tumour Biol. 2013;34:2977–2981. doi: 10.1007/s13277-013-0861-5. [DOI] [PubMed] [Google Scholar]
- 132.Yan R., Zhao X., Lei J., Zhou Q. Structure of the human LAT1-4F2hc heteromeric amino acid transporter complex. Nature. 2019;568:127–130. doi: 10.1038/s41586-019-1011-z. [DOI] [PubMed] [Google Scholar]
- 133.Liu X., Shen Z. LncRNA TMPO-AS1 aggravates the development of hepatocellular carcinoma via miR-429/GOT1 axis. Am J Med Sci. 2020;360:711–720. doi: 10.1016/j.amjms.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 134.Liu P., Wang Y., Li X. Targeting the untargetable KRAS in cancer therapy. Acta Pharm Sin B. 2019;9:871–879. doi: 10.1016/j.apsb.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Martinez A.I., Perez-Arellano I., Pekkala S., Barcelona B., Cervera J. Genetic, structural and biochemical basis of carbamoyl phosphate synthetase 1 deficiency. Mol Genet Metab. 2010;101:311–323. doi: 10.1016/j.ymgme.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 136.Cancer Genome Atlas Research Network Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169:1327–1341. doi: 10.1016/j.cell.2017.05.046. e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang C., Fu R., Zhuang Z., Wang S. Studies on the biological functions of CPS1 in AFB1 induced hepatocarcinogenesis. Gene. 2016;591:255–261. doi: 10.1016/j.gene.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 138.Phillips M.M., Sheaff M.T., Szlosarek P.W. Targeting arginine-dependent cancers with arginine-degrading enzymes: opportunities and challenges. Cancer Res Treat. 2013;45:251–262. doi: 10.4143/crt.2013.45.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tao X., Zuo Q., Ruan H., Wang H., Jin H., Cheng Z., et al. Argininosuccinate synthase 1 suppresses cancer cell invasion by inhibiting STAT3 pathway in hepatocellular carcinoma. Acta Biochim Biophys Sin (Shanghai) 2019;51:263–276. doi: 10.1093/abbs/gmz005. [DOI] [PubMed] [Google Scholar]
- 140.You J., Chen W., Chen J., Zheng Q., Dong J., Zhu Y. The oncogenic role of ARG1 in progression and metastasis of hepatocellular carcinoma. Biomed Res Int. 2018;2018:2109865. doi: 10.1155/2018/2109865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bartlett A.L., Leslie N.D., Gupta A., Geller J.I. Acquired ornithine transcarbamylase deficiency in pediatric and adolescent patients with fibrolamellar hepatocellular carcinoma. Pediatr Blood Cancer. 2018;65 doi: 10.1002/pbc.27392. [DOI] [PubMed] [Google Scholar]
- 142.He L., Cai X.F., Cheng S.T., Zhou H.Z., Zhang Z.Z., Ren J.H., et al. Ornithine transcarbamylase downregulation is associated with poor prognosis in hepatocellular carcinoma. Oncol Lett. 2019;17:5030–5038. doi: 10.3892/ol.2019.10174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee M., Ko H., Yun M.J. Cancer metabolism as a mechanism of treatment resistance and potential therapeutic target in hepatocellular carcinoma. Yonsei Med J. 2018;59:1143–1149. doi: 10.3349/ymj.2018.59.10.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tomizawa M., Shinozaki F., Motoyoshi Y., Sugiyama T., Yamamoto S., Ishige N. 2-Deoxyglucose and sorafenib synergistically suppress the proliferation and motility of hepatocellular carcinoma cells. Oncol Lett. 2017;13:800–804. doi: 10.3892/ol.2016.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang L., Yang Q., Peng S.Y., Liu X.X. The combination of the glycolysis inhibitor 2-DG and sorafenib can be effective against sorafenib-tolerant persister cancer cells. Onco Targets Ther. 2019;12:5359–5373. doi: 10.2147/OTT.S212465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sun X., Sun G., Huang Y., Hao Y., Tang X., Zhang N., et al. 3-Bromopyruvate regulates the status of glycolysis and BCNU sensitivity in human hepatocellular carcinoma cells. Biochem Pharmacol. 2020;177:113988. doi: 10.1016/j.bcp.2020.113988. [DOI] [PubMed] [Google Scholar]
- 147.Pereira da Silva A.P., El-Bacha T., Kyaw N., dos Santos R.S., da-Silva W.S., Almeida F.C., et al. Inhibition of energy-producing pathways of HepG2 cells by 3-bromopyruvate. Biochem J. 2009;417:717–726. doi: 10.1042/BJ20080805. [DOI] [PubMed] [Google Scholar]
- 148.Yadav S., Pandey S.K., Kumar A., Kujur P.K., Singh R.P., Singh S.M. Antitumor and chemosensitizing action of 3-bromopyruvate: implication of deregulated metabolism. Chem Biol Interact. 2017;270:73–89. doi: 10.1016/j.cbi.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 149.Ganapathy-Kanniappan S., Geschwind J.F.H., Kunjithapatham R., Buijs M., Vossen J.A., Tchernyshyov I., et al. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is pyruvylated during 3-bromopyruvate mediated cancer cell death. Anticancer Res. 2009;29:4909–4918. [PMC free article] [PubMed] [Google Scholar]
- 150.Liberti M.V., Dai Z., Wardell S.E., Baccile J.A., Liu X., Gao X., et al. A predictive model for selective targeting of the Warburg effect through GAPDH inhibition with a natural product. Cell Metab. 2017;26:648–659.e8. doi: 10.1016/j.cmet.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.He Y., Luo Y., Zhang D., Wang X., Zhang P., Li H., et al. PGK1-mediated cancer progression and drug resistance. Am J Cancer Res. 2019;9:2280–2302. [PMC free article] [PubMed] [Google Scholar]
- 152.Wang Y., Sun L., Yu G., Qi X., Zhang A., Lu Z., et al. Identification of a novel non-ATP-competitive protein kinase inhibitor of PGK1 from marine nature products. Biochem Pharmacol. 2020;183:114343. doi: 10.1016/j.bcp.2020.114343. [DOI] [PubMed] [Google Scholar]
- 153.Wang W.L., Jiang Z.R., Hu C., Chen C., Hu Z.Q., Wang A.L., et al. Pharmacologically inhibiting phosphoglycerate kinase 1 for glioma with NG52. Acta Pharmacol Sin. 2020;42:633–640. doi: 10.1038/s41401-020-0465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hitosugi T., Zhou L., Elf S., Fan J., Kang H.B., Seo J.H., et al. Phosphoglycerate mutase 1 coordinates glycolysis and biosynthesis to promote tumor growth. Cancer Cell. 2012;22:585–600. doi: 10.1016/j.ccr.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xu D., Jin J., Yu H., Zhao Z., Ma D., Zhang C., et al. Chrysin inhibited tumor glycolysis and induced apoptosis in hepatocellular carcinoma by targeting hexokinase-2. J Exp Clin Cancer Res. 2017;36:44. doi: 10.1186/s13046-017-0514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li J., Chen K., Wang F., Dai W., Li S., Feng J., et al. Methyl jasmonate leads to necrosis and apoptosis in hepatocellular carcinoma cells via inhibition of glycolysis and represses tumor growth in mice. Oncotarget. 2017;8:45965–45980. doi: 10.18632/oncotarget.17469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim H.J., Hwang K.E., Park D.S., Oh S.H., Jun H.Y., Yoon K.H., et al. Shikonin-induced necroptosis is enhanced by the inhibition of autophagy in non-small cell lung cancer cells. J Transl Med. 2017;15:123. doi: 10.1186/s12967-017-1223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tang J.C., Ren Y.G., Zhao J., Long F., Chen J.Y., Jiang Z. Shikonin enhances sensitization of gefitinib against wild-type EGFR non-small cell lung cancer via inhibition PKM2/STAT3/cyclin D1 signal pathway. Life Sci. 2018;204:71–77. doi: 10.1016/j.lfs.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 159.Liu T., Li S.N., Wu L.W., Yu Q., Li J.J., Feng J., et al. Experimental study of hepatocellular carcinoma treatment by shikonin through regulating PKM2. J Hepatocell Carcino. 2020;7:19–31. doi: 10.2147/JHC.S237614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li S., Li J., Dai W., Zhang Q., Feng J., Wu L., et al. Genistein suppresses aerobic glycolysis and induces hepatocellular carcinoma cell death. Br J Cancer. 2017;117:1518–1528. doi: 10.1038/bjc.2017.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xing Y.X., Li M.H., Tao L., Ruan L.Y., Hong W., Chen C., et al. Anti-cancer effects of emodin on HepG2 cells as revealed by 1H NMR based metabolic profiling. J Proteome Res. 2018;17:1943–1952. doi: 10.1021/acs.jproteome.8b00029. [DOI] [PubMed] [Google Scholar]
- 162.Wei X., Mao T., Li S., He J., Hou X., Li H., et al. DT-13 inhibited the proliferation of colorectal cancer via glycolytic metabolism and AMPK/mTOR signaling pathway. Phytomedicine. 2019;54:120–131. doi: 10.1016/j.phymed.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 163.Yang J., Jin X., Yan Y., Shao Y., Pan Y., Roberts L.R., et al. Inhibiting histone deacetylases suppresses glucose metabolism and hepatocellular carcinoma growth by restoring FBP1 expression. Sci Rep. 2017;7:43864. doi: 10.1038/srep43864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pan D.N., Mao C.X., Wang Y.X. Suppression of gluconeogenic gene expression by LSD1-mediated histone demethylation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jin X., Pan Y., Wang L., Zhang L., Ravichandran R., Potts P.R., et al. MAGE–TRIM28 complex promotes the Warburg effect and hepatocellular carcinoma progression by targeting FBP1 for degradation. Oncogenesis. 2017;6:312. doi: 10.1038/oncsis.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schmidt Dawn M.Z., McCafferty D.G. trans-2-Phenylcyclopropylamine is a mechanism-based inactivator of the histone demethylase LSD1. Biochemistry. 2007;46:4408–4416. doi: 10.1021/bi0618621. [DOI] [PubMed] [Google Scholar]
- 167.Roberti A., Valdes A.F., Torrecillas R., Fraga M.F., Fernandez A.F. Epigenetics in cancer therapy and nanomedicine. Clin Epigenetics. 2019;11:81. doi: 10.1186/s13148-019-0675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kim G.P., Mahoney M.R., Szydlo D., Mok T.S.K., Marshke R., Holen K., et al. An international, multicenter phase II trial of bortezomib in patients with hepatocellular carcinoma. Invest New Drugs. 2012;30:387–394. doi: 10.1007/s10637-010-9532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ma R.H., Zhang W.G., Tang K., Zhang H.F., Zhang Y., Li D.P., et al. Switch of glycolysis to gluconeogenesis by dexamethasone for treatment of hepatocarcinoma. Nat Commun. 2013;4:2508. doi: 10.1038/ncomms3508. [DOI] [PubMed] [Google Scholar]
- 170.Abramson H.N. The lipogenesis pathway as a cancer target. J Med Chem. 2011;54:5615–5638. doi: 10.1021/jm2005805. [DOI] [PubMed] [Google Scholar]
- 171.Wei Q.Q., Mei L.K., Yang Y.F., Ma H., Chen H.Y., Zhang H.B., et al. Design, synthesis and biological evaluation of novel spiro-pentacylamides as acetyl-CoA carboxylase inhibitors. Bioorgan Med Chem. 2018;26:3866–3874. doi: 10.1016/j.bmc.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 172.Wei L., Harriman G., Ghoshal S., Moaven O., Greenwood J., Bhat S., et al. Combination therapy with a liver selective acetyl-CoA carboxylase inhibitor ND-654 and sorafenib improves efficacy in the treatment of cirrhotic rats with hepatocellular carcinoma. Cancer Res. 2016;76:3781. [Google Scholar]
- 173.Zhou W., Simpson P.J., McFadden J.M., Townsend C.A., Medghalchi S.M., Vadlamudi A., et al. Fatty acid synthase inhibition triggers apoptosis during S phase in human cancer cells. Cancer Res. 2003;63:7330–7337. [PubMed] [Google Scholar]
- 174.Flavin R., Peluso S., Nguyen P.L., Loda M. Fatty acid synthase as a potential therapeutic target in cancer. Future Oncol. 2010;6:551–562. doi: 10.2217/fon.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Falchook G., Patel M., Infante J., Arkenau H.T., Dean E., Brenner A., et al. Abstract CT153: first in human study of the first-in-class fatty acid synthase (FASN) inhibitor TVB-2640. Cancer Res. 2017;77:CT153. doi: 10.1016/j.eclinm.2021.100797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Duke G., Wagman A.S., Buckley D., McCulloch W., Kemble G. Establishing the foundation for a novel, first-in-class, fatty acid synthase inhibitor, TVB-2640, for the treatment of NASH. J Hepatol. 2017;66:S99–S100. [Google Scholar]
- 177.Gutierrez M.J., Rosenberg N.L., Macdougall D.E., Hanselman J.C., Margulies J.R., Strange P., et al. Efficacy and safety of ETC-1002, a novel investigational low-density lipoprotein-cholesterol-lowering therapy for the treatment of patients with hypercholesterolemia and type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2014;34:676–683. doi: 10.1161/ATVBAHA.113.302677. [DOI] [PubMed] [Google Scholar]
- 178.Ray K.K., Bays H.E., Catapano A.L., Lalwani N.D., Bloedon L.T., Sterling L.R., et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 2019;380:1022–1032. doi: 10.1056/NEJMoa1803917. [DOI] [PubMed] [Google Scholar]
- 179.Hatzivassiliou G., Zhao F.P., Bauer D.E., Andreadis C., Shaw A.N., Dhanak D., et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 180.Gao Y., Islam M.S., Tian J., Lui V.W., Xiao D. Inactivation of ATP citrate lyase by cucurbitacin B: a bioactive compound from cucumber, inhibits prostate cancer growth. Cancer Lett. 2014;349:15–25. doi: 10.1016/j.canlet.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 181.Choi Y.K., Park K.G. Targeting glutamine metabolism for cancer treatment. Biomol Ther (Seoul) 2018;26:19–28. doi: 10.4062/biomolther.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen Z., Li D., Xu N., Fang J.Z., Yu Y., Hou W., et al. Novel 1,3,4-selenadiazole-containing kidney-type glutaminase inhibitors showed improved cellular uptake and antitumor activity. J Med Chem. 2019;62:589–603. doi: 10.1021/acs.jmedchem.8b01198. [DOI] [PubMed] [Google Scholar]
- 183.Shukla K., Ferraris D.V., Thomas A.G., Stathis M., Duvall B., Delahanty G., et al. Design, synthesis, and pharmacological evaluation of bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide 3 (BPTES) analogs as glutaminase inhibitors. J Med Chem. 2012;55:10551–10563. doi: 10.1021/jm301191p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gross M.I., Demo S.D., Dennison J.B., Chen L., Chernov-Rogan T., Goyal B., et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther. 2014;13:890–901. doi: 10.1158/1535-7163.MCT-13-0870. [DOI] [PubMed] [Google Scholar]
- 185.Schulte M.L., Fu A., Zhao P., Li J., Geng L., Smith S.T., et al. Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat Med. 2018;24:194–202. doi: 10.1038/nm.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jin H., Wang S., Zaal E.A., Wang C., Wu H., Bosma A., et al. A powerful drug combination strategy targeting glutamine addiction for the treatment of human liver cancer. Elife. 2020;9 doi: 10.7554/eLife.56749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang L., Liu Y., Zhao T.L., Li Z.Z., He J.Y., Zhang B.J., et al. Topotecan induces apoptosis via ASCT2 mediated oxidative stress in gastric cancer. Phytomedicine. 2019;57:117–128. doi: 10.1016/j.phymed.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 188.Zhang P., Wang Q., Lin Z., Yang P., Dou K., Zhang R. Berberine inhibits growth of liver cancer cells by suppressing glutamine uptake. Onco Targets Ther. 2019;12:11751–11763. doi: 10.2147/OTT.S235667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dhillon S. Ivosidenib: first global approval. Drugs. 2018;78:1509–1516. doi: 10.1007/s40265-018-0978-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Caravella J.A., Lin J., Diebold R.B., Campbell A.M., Ericsson A., Gustafson G., et al. Structure-based design and identification of FT-2102 (olutasidenib), a potent mutant-selective IDH1 inhibitor. J Med Chem. 2020;63:1612–1623. doi: 10.1021/acs.jmedchem.9b01423. [DOI] [PubMed] [Google Scholar]
- 191.Guri Y., Colombi M., Dazert E., Hindupur S.K., Roszik J., Moes S., et al. mTORC2 promotes tumorigenesis via lipid synthesis. Cancer Cell. 2017;32:807–823.e12. doi: 10.1016/j.ccell.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 192.Liu H., Liu Y., Zhang J.T. A new mechanism of drug resistance in breast cancer cells: fatty acid synthase overexpression-mediated palmitate overproduction. Mol Cancer Ther. 2008;7:263–270. doi: 10.1158/1535-7163.MCT-07-0445. [DOI] [PubMed] [Google Scholar]
- 193.Menendez J.A., Vellon L., Lupu R. Antitumoral actions of the anti-obesity drug orlistat (XenicalTM) in breast cancer cells: blockade of cell cycle progression, promotion of apoptotic cell death and PEA3-mediated transcriptional repression of Her2/neu (erbB-2) oncogene. Ann Oncol. 2005;16:1253–1267. doi: 10.1093/annonc/mdi239. [DOI] [PubMed] [Google Scholar]
- 194.Buckley D., Duke G., Heuer T.S., O'Farrell M., Wagman A.S., McCulloch W., et al. Fatty acid synthase—modern tumor cell biology insights into a classical oncology target. Pharmacol Therapeut. 2017;177:23–31. doi: 10.1016/j.pharmthera.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 195.Menendez J.A., Lupu R. Fatty acid synthase (FASN) as a therapeutic target in breast cancer. Expert Opin Ther Tar. 2017;21:1001–1016. doi: 10.1080/14728222.2017.1381087. [DOI] [PubMed] [Google Scholar]
- 196.Zaytseva Y.Y., Rychahou P.G., Le A.T., Scott T.L., Flight R.M., Kim J.T., et al. Preclinical evaluation of novel fatty acid synthase inhibitors in primary colorectal cancer cells and a patient-derived xenograft model of colorectal cancer. Oncotarget. 2018;9:24787–24800. doi: 10.18632/oncotarget.25361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Thupari J.N., Landree L.E., Ronnett G.V., Kuhajda F.P. C75 increases peripheral energy utilization and fatty acid oxidation in diet-induced obesity. Proc Natl Acad Sci U S A. 2002;99:9498–9502. doi: 10.1073/pnas.132128899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Graf H., Jungst C., Straub G., Dogan S., Hoffmann R.T., Jakobs T., et al. Chemoembolization combined with pravastatin improves survival in patients with hepatocellular carcinoma. Digestion. 2008;78:34–38. doi: 10.1159/000156702. [DOI] [PubMed] [Google Scholar]
- 199.Okano N., Naruge D., Kawai K., Kobayashi T., Nagashima F., Endou H., et al. First-in-human phase I study of JPH203, an L-type amino acid transporter 1 inhibitor, in patients with advanced solid tumors. Invest New Drugs. 2020;38:1495–1506. doi: 10.1007/s10637-020-00924-3. [DOI] [PubMed] [Google Scholar]
- 200.Sachs R.E., Ginsburg P.B., Goldman D.P. Encouraging new uses for old drugs. JAMA. 2017;318:2421–2422. doi: 10.1001/jama.2017.17535. [DOI] [PubMed] [Google Scholar]
- 201.Pantziarka P., Pirmohamed M., Mirza N. New uses for old drugs. BMJ. 2018;361 doi: 10.1136/bmj.k2701. [DOI] [PubMed] [Google Scholar]
- 202.DeWaal D., Nogueira V., Terry A.R., Patra K.C., Jeon S.M., Guzman G., et al. Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat Commun. 2018;9:446. doi: 10.1038/s41467-017-02733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hu Zeng Z., Xia Q., Liu Z., Feng X., Chen J., et al. Metformin attenuates hepatoma cell proliferation by decreasing glycolytic flux through the HIF-1alpha/PFKFB3/PFK1 pathway. Life Sci. 2019;239:116966. doi: 10.1016/j.lfs.2019.116966. [DOI] [PubMed] [Google Scholar]
- 204.Kaji K., Nishimura N., Seki K., Sato S., Saikawa S., Nakanishi K., et al. Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int J Cancer. 2018;142:1712–1722. doi: 10.1002/ijc.31193. [DOI] [PubMed] [Google Scholar]
- 205.Shiba K., Tsuchiya K., Komiya C., Miyachi Y., Mori K., Shimazu N., et al. Canagliflozin, an SGLT2 inhibitor, attenuates the development of hepatocellular carcinoma in a mouse model of human NASH. Sci Rep. 2018;8:2362. doi: 10.1038/s41598-018-19658-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Chen K., Qian W., Jiang Z., Cheng L., Li J., Sun L., et al. Metformin suppresses cancer initiation and progression in genetic mouse models of pancreatic cancer. Mol Cancer. 2017;16:131. doi: 10.1186/s12943-017-0701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bai M.Z., Yang L.L., Liao H., Liang X.Y., Xie B.Y., Xiong J., et al. Metformin sensitizes endometrial cancer cells to chemotherapy through IDH1-induced Nrf2 expression via an epigenetic mechanism. Oncogene. 2018;37:5666–5681. doi: 10.1038/s41388-018-0360-7. [DOI] [PubMed] [Google Scholar]
- 208.Liu X.J., Romero I.L., Litchfield L.M., Lengyel E., Locasale J.W. Metformin targets central carbon metabolism and reveals mitochondrial requirements in human cancers. Cell Metab. 2016;24:728–739. doi: 10.1016/j.cmet.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Dowling R.J., Zakikhani M., Fantus I.G., Pollak M., Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 210.Shaw R.J., Kosmatka M., Bardeesy N., Hurley R.L., Witters L.A., DePinho R.A., et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Watson J.D. Type 2 diabetes as a redox disease. Lancet. 2014;383:841–843. doi: 10.1016/S0140-6736(13)62365-X. [DOI] [PubMed] [Google Scholar]
- 212.Silvestri A., Palumbo F., Rasi I., Posca D., Pavlidou T., Paoluzi S., et al. Metformin induces apoptosis and downregulates pyruvate kinase M2 in breast cancer cells only when grown in nutrient-poor conditions. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Camacho L., Dasgupta A., Jiralerspong S. Metformin in breast cancer—an evolving mystery. Breast Cancer Res. 2015;17:88. doi: 10.1186/s13058-015-0598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chan K.M., Kuo C.F., Hsu J.T., Chiou M.J., Wang Y.C., Wu T.H., et al. Metformin confers risk reduction for developing hepatocellular carcinoma recurrence after liver resection. Liver Int. 2017;37:434–441. doi: 10.1111/liv.13280. [DOI] [PubMed] [Google Scholar]
- 215.Scafoglio C., Hirayama B.A., Kepe V., Liu J., Ghezzi C., Satyamurthy N., et al. Functional expression of sodium–glucose transporters in cancer. Proc Natl Acad Sci U S A. 2015;112:E4111–E4119. doi: 10.1073/pnas.1511698112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Amann T., Maegdefrau U., Hartmann A., Agaimy A., Marienhagen J., Weiss T.S., et al. GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis. Am J Pathol. 2009;174:1544–1552. doi: 10.2353/ajpath.2009.080596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Obara K., Shirakami Y., Maruta A., Ideta T., Miyazaki T., Kochi T., et al. Preventive effects of the sodium glucose cotransporter 2 inhibitor tofogliflozin on diethylnitrosamine-induced liver tumorigenesis in obese and diabetic mice. Oncotarget. 2017;8:58353–58363. doi: 10.18632/oncotarget.16874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lehmann A., Hornby P.J. Intestinal SGLT1 in metabolic health and disease. Am J Physiol Gastrointest Liver Physiol. 2016;310:G887–G898. doi: 10.1152/ajpgi.00068.2016. [DOI] [PubMed] [Google Scholar]
- 219.Liu G., Kuang S., Cao R., Wang J., Peng Q., Sun C. Sorafenib kills liver cancer cells by disrupting SCD1-mediated synthesis of monounsaturated fatty acids via the ATP–AMPK–mTOR–SREBP1 signaling pathway. FASEB J. 2019;33:10089–10103. doi: 10.1096/fj.201802619RR. [DOI] [PubMed] [Google Scholar]
- 220.Xu J., Ji L., Ruan Y., Wan Z., Lin Z., Xia S., et al. UBQLN1 mediates sorafenib resistance through regulating mitochondrial biogenesis and ROS homeostasis by targeting PGC1beta in hepatocellular carcinoma. Signal Transduct Target Ther. 2021;6:190. doi: 10.1038/s41392-021-00594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Febbraio M.A., Reibe S., Shalapour S., Ooi G.J., Watt M.J., Karin M. Preclinical models for studying NASH-driven HCC: how useful are they?. Cell Metab. 2019;29:18–26. doi: 10.1016/j.cmet.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Satriano L., Lewinska M., Rodrigues P.M., Banales J.M., Andersen J.B. Metabolic rearrangements in primary liver cancers: cause and consequences. Nat Rev Gastroenterol Hepatol. 2019;16:748–766. doi: 10.1038/s41575-019-0217-8. [DOI] [PubMed] [Google Scholar]
- 223.Jiang P., Du W., Wang X., Mancuso A., Gao X., Wu M., et al. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat Cell Biol. 2011;13:310–316. doi: 10.1038/ncb2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sun L.C., Song L.B., Wan Q.F., Wu G.W., Li X.H., Wang Y.H., et al. cMyc-mediated activation of serine biosynthesis pathway is critical for cancer progression under nutrient deprivation conditions. Cell Res. 2015;25:429–444. doi: 10.1038/cr.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Nuciforo S., Fofana I., Matter M.S., Blumer T., Calabrese D., Boldanova T., et al. Organoid models of human liver cancers derived from tumor needle biopsies. Cell Rep. 2018;24:1363–1376. doi: 10.1016/j.celrep.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]