Abstract
Background
In Kazakhstan, cancer is the second leading cause of death with a major public health and economic burden. In the last decade, cancer care and cancer medicine costs have significantly increased. To improve the efficiency and efficacy of cancer care expenditure and planning, the Kazakhstan Ministry of Health requested assistance from the World Health Organization (WHO) and the European Society for Medical Oncology (ESMO) to review its systemic cancer treatment protocols and essential medicines list and identify high-impact, effective regimens.
Materials and methods
ESMO developed a four-phase approach to review Kazakhstan cancer treatment protocols: (i) perform a systematic analysis of the country’s cancer medicines and treatment protocols; (ii) cross-reference the country’s cancer protocols with the WHO Model List of Essential Medicines, the ESMO-Magnitude of Clinical Benefit Scale and the European Medicines Agency’s medicine availability and indications database; (iii) extract treatment recommendations from the ESMO Clinical Practice Guidelines; (iv) expert review for all cancer medicines not on the WHO Model List of Essential Medicines and the country treatment protocols.
Results
This ESMO four-phase approach led to the update of the Kazakhstan national essential cancer medicines list and the list of cancer treatment protocols. This review has led to the withdrawal of several low-value or non-evidence-based medicines and a budget increase for cancer care to include all essential and highly effective medicines and treatment options.
Conclusion
When applied effectively, this four-phase approach can improve access to medicines, efficiency of expenditure and sustainability of cancer systems. The WHO–ESMO collaboration illustrated how, by sharing best practices, tools and resources, we can address access to cancer medicines and positively impact patient care.
Key words: cancer care expenditure, cancer medicines, ESMO-MCBS, HTA, UHC, WHO
Highlights
-
•
In Kazakhstan, from 2009 to 2019, there was a significant increase in costs of cancer care and cancer medicines.
-
•
ESMO, under the guidance of WHO, reviewed the Kazakh cancer treatment protocols and national essential medicines list.
-
•
The ESMO four-phase approach helped Kazakhstan in prioritising the most effective and high-impact cancer medicines.
-
•
This approach can be applied in the evaluation of any national formulary as it uses open-access, evidence-based references.
-
•
This collaboration is an example of how we can improve access to cancer medicines and positively impact patient care.
Introduction
Kazakhstan is an upper-middle-income country in Central Asia where non-communicable diseases (NCDs) are responsible for 87% of all deaths, with cancers representing the second leading cause (∼17% of deaths), following cardiovascular diseases (47%).1
The Ministry of Health has adopted the overall national health plan, established for the period 2011-2015 and updated in 2016-2019, which brought important interventions especially on health promotion, screening and early diagnosis for the prevention and control of NCDs. This approach established health centres in all regions to carry out health promotion and early diagnosis services related to NCDs, including screening programmes for cancer, with the goal of reducing mortality, downstaging of newly diagnosed cases and increasing the 5-year survival from cancer.
Financing of cancer medicines in 2015-2016 was ∼18.1 billion tenges (US$97 million in January 2015), representing 56.1% of the total budget for all oncology services. From 2009 to 2019 there was a fivefold (7.5 to 35.3 billion tenges) and sevenfold (2.5 to 12.1 billion tenges) increase in costs of cancer care and cancer medicines respectively, which threatened the financial viability of including cancer services as part of the universal health coverage benefit package and the health care system’s broader stability.2
To continue offering patients quality cancer care, the Kazakhstan Ministry of Health requested an impact mission in 2016, led by the World Health Organization (WHO) along with the International Atomic Energy Agency and the International Agency for Research on Cancer.2 The objective was to assess national cancer services and to provide recommendations for how services can be maintained and access increased over time. The situational analysis revealed rapidly increasing budgetary expenditure on cancer treatment, particularly driven by increasing total amounts spent on systemic therapy.2
In the later part of 2017 and early 2018, the European Society for Medical Oncology (ESMO), under the guidance of WHO staff at their headquarters (Geneva) and the WHO Regional Office and Country Office (Kazakhstan), was asked to provide support and guidance to the Ministry of Health of Kazakhstan, to revise its systemic cancer treatment protocols and national essential medicines list.
In this paper, we describe the evidence-based and value-driven four-phase approach developed by ESMO, working with WHO, to support this upper-middle-income country in prioritising the most effective and high-impact cancer medicines and ensure their inclusion in the Kazakhstan national benefit package.
Materials and methods
The ESMO approach for the review of anticancer medicines and treatment protocols for solid and haematological malignancies was developed across four phases (Figure 1), and included the use of the following reference tools: (i) WHO Model List of Essential Medicines (WHO EML),3 (ii) the European Medicines Agency (EMA) medicine availability and indications database,4 (iii) the ESMO Clinical Practice Guidelines (CPGs)5 and (iv) the ESMO-Magnitude of Clinical Benefit Scale (ESMO-MCBS) v1.1 scores.6
Figure 1.
ESMO four-phase approach to prioritise cancer systemic therapies.
Phase I—Systemic review of cancer medicines and treatment protocols
ESMO received from WHO a list of formulary anticancer medicines and a list of treatment protocols for solid and haematological malignancies used in Kazakhstan. The two lists were cross-checked to create a comprehensive list of all medicines used in the country. For each tumour type provided, lists were compiled for (i) all the anticancer medicines used in that setting (early/curable or advanced/metastatic) and (ii) all treatment protocols used in each line of treatment in metastatic disease (first, second or third line, etc.).
Phase II—Cross-reference with evidence-based tools and resources
This phase involved three steps: firstly, evaluation of compliance of the Kazakhstan list of medicines with the WHO EML in cancer treatments for each indication using the most recently updated version of the WHO EML (the 20th list served as the reference standard7) and to see if all medicines were included. Medicines that were on the Kazakh formulary but were not part of the WHO EML were evaluated for efficacy and relevance to contemporary practice standards by the expert reviewers.
Secondly, Kazakh formulary medicines were cross-referenced against the EMA database4 to understand if the medicines were EMA approved for the specific indications for which they were being utilised in the country.
The EMA was chosen as the reference regulatory agency for two reasons:
-
1.
ESMO scores cancer medicines approved by the EMA [since January 2020 ESMO has also been scoring medicines that have received Food and Drug Administration (FDA) approval].
-
2.
In Kazakhstan, the National Centre of Expertise on Drugs, Medical Supplies and Medical Equipment under the Ministry of Health, responsible for medicine approvals within the country, approves only evidence-based medicines previously approved by the EMA or the FDA and included in international guidelines such as the ESMO CPGs or the National Comprehensive Cancer Network guidelines.
In a third step, where available, ESMO-MCBS scores of the medications on formulary were assessed using the tables found in the supplementary file published in the Annals of Oncology.6 This process was restricted to solid tumours since ESMO-MCBS scores were not validated for haematological malignancies at the time of the review.
Phase III—Extraction of clinical recommendations
For each tumour type, the treatment protocols were listed and cross-correlated with recommendations from the ESMO CPGs.5 Sections of the ESMO CPGs mentioning the listed treatment protocols were extracted with references, and a link to the entire ESMO CPG was provided. ESMO CPGs that were available in 2017 served as the reference standard.
Phase IV—Expert review
An expert review was completed by ten expert clinicians, who evaluated all formulary medication in their field of expertise not included on the WHO EML but contained on the Kazakhstan formulary and protocols. This process integrated information from the previous steps, including ESMO-MCBS scores where available and cross-correlation with the ESMO CPGs.
Results
Phase I—Systemic review of cancer medicines and treatment protocols
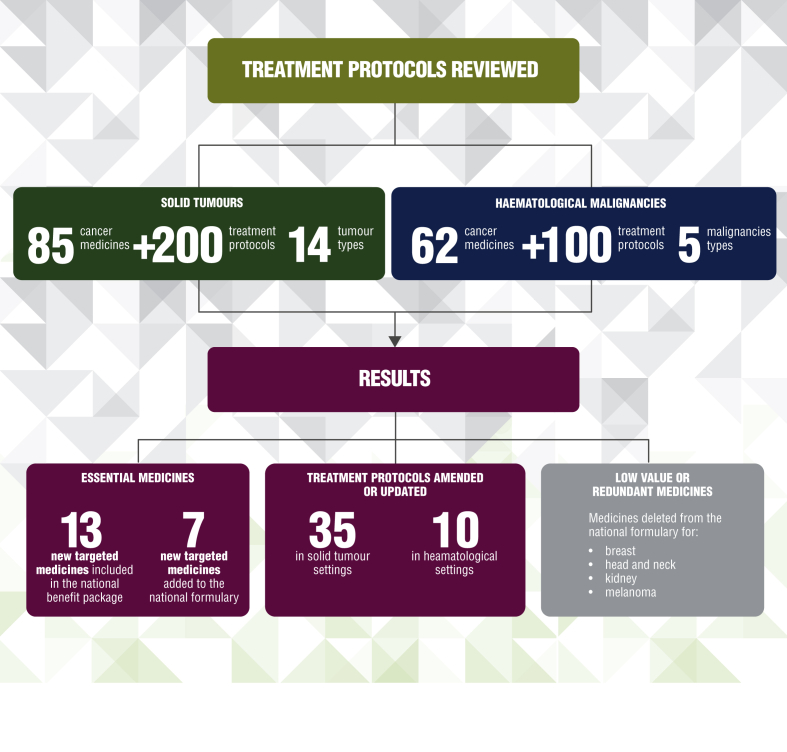
For solid tumours, 85 anticancer medications and over 200 treatment protocols across 14 tumour types were evaluated. For haematological malignancies, 62 cancer medicines and over 100 protocols in 5 different malignancies were reviewed. In Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100362, the tumour entities analysed are listed.
Phase II—Cross-reference with evidenced-based tools and resources
The analysis confirmed that the Kazakhstan formulary for the treatment of solid tumours included all relevant medicines on the WHO EML. For the haematological malignancies, only dasatinib and hydroxycarbamide were missing from the Kazakhstan formulary. The indications listed in the Kazakhstan formulary medicines were also cross-referenced against the EMA indications.4 An example from the Kazakhstan review is shown in Table 1.
Table 1.
Kazakhstan tumour settings compared against the WHO EML and the EMA listing
| Medicines | WHO EML tumour settinga | Kazakhstan tumour setting | EMA settinga |
|---|---|---|---|
| Abiraterone | Metastatic castration-resistant prostate cancer | Prostate | Prostate cancer (adult men when the cancer is metastatic) |
| Anastrozole |
|
Breast |
|
| Axitinib | Renal-cell carcinoma |
|
|
| Bevacizumab | Central nervous system tumour, colorectal, lung (NSCLC), renal-cell cancer, breast |
|
|
| Bicalutamide | Metastatic prostate cancer | Prostate | Advanced prostate cancer |
| Bleomycin |
|
Nasopharynx, oral, oesophageal, thyroid, skin (SCC, BCC), vulvar |
|
BCC, basal cell carcinoma; EMA, European Medicines Agency; EML, Essential Medicines List; NSCLC, non-small-cell lung cancer; SCC, squamous cell carcinoma; WHO, World Health Organization.
Data 2017.
The comparison of medications on formulary (only for solid tumours) with the ESMO-MCBS scores, where available at that time, showed that the Kazakhstan list of medicines included treatments that had been scored highly by the ESMO-MCBS (score ≥4 in the non-curative setting and ≥B in the curative setting). However, several treatments that were less highly scored (ESMO-MCBS ≤3 and C) were also included. An example of ESMO-MCBS scores for breast cancer from the Kazakhstan review is shown in Table 2.
Table 2.
Breast cancer anticancer medicines scored with ESMO-MCBSa
| Medicines | Setting | ESMO-MCBS score | References |
|---|---|---|---|
| Chemotherapy + trastuzumab | Adjuvant or neoadjuvant HER2-positive tumours | A | 9 |
| Trastuzumab and docetaxel + pertuzumab | Neoadjuvant HER2-overexpressed invasive ductal breast | Cb | 10,11 |
| Trastuzumab + docetaxel + pertuzumab | First line metastatic | 4 | 12, 13, 14, 15 |
| Lapatinib + trastuzumab | Third line metastatic | 4 | 16,17 |
| Fulvestrant + palbociclib | Metastatic HR-positive, HER2-negative second line | 4 | 18 |
| T-DM1 | Second line metastatic after trastuzumab failure | 4 | 19,20 |
| Letrozole + palbociclib | Metastatic HR-positive, HER2-negative first line | 3 | 21 |
| Letrozole + palbociclib | Metastatic HR-positive, HER2-negative first line | 3b | 22 |
| Letrozole + ribociclib | First line metastatic post-menopause ER/PR positive | 3 | 23 |
| Capecitabine + lapatinib | Second line metastatic after trastuzumab failure | 3 | 24 |
| Paclitaxel + bevacizumab | First line metastatic, no crossover | 2 | 25 |
| Exemestane + everolimus | Metastatic after failure of aromatase inhibitor (with PFS >6 months), no crossover | 2 | 26 |
| Eribulin | Third line metastatic after anthracycline and taxane | 2 | 27 |
| Eribulin | Third line metastatic after anthracycline and taxane in patients with HER2-negative tumours | 1 | 28 |
Included in the Kazakhstan’s list of medicines (Bolded).
ESMO-MCBS, ESMO-Magnitude of Clinical Benefit Scale; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; PFS, progression-free survival; PR, progesterone receptor; T-DM1, trastuzumab emtansine.
Data 2017.
Randomised phase II study.
Phase III—Extraction of clinical recommendations
All of the Kazakhstan treatment protocols were cross-checked with the ESMO CPGs. All identified discrepancies were reviewed by an expert clinician for annotation and feedback. An example for gastric cancer from the Kazakhstan review is shown in Table 3.
Table 3.
Treatment protocols and ESMO CPGsa for gastric cancer
| Kazakh treatment regimens | Expert review |
|---|---|
| Docetaxel + cisplatin + 5-FU | No clear advantage |
| Cisplatin + 5-FU | Important option |
| Epirubicin + cisplatin + 5-FU | Epirubicin probably not needed |
| Epirubicin + oxaliplatin + 5-FU | Epirubicin probably not needed |
| Epirubicin/cisplatin/capecitabine | Epirubicin probably not needed |
| Etoposide + calcium folinate + 5-FU | Not needed |
| Irinotecan + cisplatin | Second line option |
| 5-FU+ doxorubicin + cisplatin | Not needed |
| Docetaxel + cisplatin | Not in common use |
| Trastuzumab + capecitabine + cisplatin | First line option if HER2 overexpressed |
| 5-FU | Single agent has minimal activity |
| Monotherapy protocols (5-FU, docetaxel) | Second-line therapy |
| ESMO Clinical Practice Guidelines |
|---|
First-line treatment
|
5-FU, 5-fluorouracil; CPGs, Clinical Practice Guidelines; DCF, docetaxel, cisplatin, 5-day infusion of 5-FU; HER2, human epidermal growth factor receptor 2; OS, overall survival; PFS, progression-free survival; PS, performance status; RT, radiotherapy; S1, S-1 is a novel oral fluoropyrimidine derivative, widely used for treating gastric, pancreatic, lung, head, neck and breast carcinomas. It is designed to enhance the clinical utility of an oral fluoropyrimidine and is associated with low gastrointestinal toxicity.
Data 2017.
This analysis identified protocols that were not recommended or of low and/or marginal benefit. In some situations, medicines recommended for later lines of therapy in ESMO CPGs were listed as first-line therapies in national protocols.
Phase IV—expert review
The expert review of the Kazakhstan treatment protocols for solid tumours identified instances where the protocols were not consistent with acknowledged standards of care regarding indications, medicine combinations and lines of treatment. In the haematological formulary, a small subset of medicines was used for indications outside of ESMO CPG recommendations. An example from this review is shown in Table 4.
Table 4.
Medicines not on the WHO EML with ESMO expert review
| Kazakhstan medicines | Kazakhstan tumour setting | ESMO expert review |
|---|---|---|
| Bortezomib | No protocola | Use in multiple myeloma |
| Brentuximab | No protocola | Use to treat relapsed or refractory Hodgkin’s lymphoma and systemic anaplastic large-cell lymphoma |
| Cyproterone | Prostate | Old medicine for prostate cancer. Do not use |
| Darbepoetin alfa | No protocola | Supportive care medication |
| Decitabine | No protocola | Use for myelodysplastic syndromes |
| Degarelix | Prostate | Monthly LHRH antagonist for prostate cancer |
| Epirubicin | Multiple (breast, oesophageal, gastric, uterine) | ESMO-MCBS score A. Use perioperative epirubicin, cisplatin, 5-FU, gastric or distal oesophagus stage II-III setting |
| Flutamide | Prostate | Old androgen blocker. No longer in wide use |
| Fotemustine | Melanoma | Old medicine for melanoma. Do not use |
| Goserelin | Prostate | LHRH agonist use for prostate cancer |
| Lenalidomide | No protocola | Use for multiple myeloma |
| Lenograstim | Multiple | G-CSF as supportive care |
| Mifamurtide | Sarcoma | Mifamurtide for adjuvant treatment of high-grade, non-metastasizing, resectable osteosarcoma following complete surgical removal in children, adolescents and young adults, aged 2-30 years |
| Nilutamide | Prostate | Anti-androgen not recommended because of severe adverse effects |
| Octreotide | GI neuroendocrine | Use for low-grade neuroendocrine tumours |
| Pegaspargase | Acute lymphoblastic leukaemia | PEGylation L-asparaginase used in paediatric acute lymphoblastic leukaemia |
| Temozolomide | Multiple (central nervous system) tumour, lung (SCLC), melanoma, Uterine) | Temozolomide is mainly used for high-grade brain tumours. Now has minimal use in melanoma. Not recommended in lung cancer |
| The BCG | Bladder | BCG important treatment for superficial non-invasive transitional cell carcinoma bladder |
5-FU, 5-fluorouracil; EML, Essential Medicines List; G-CSF, granulocyte-colony stimulating factor; GI, gastrointestinal; LHRH, luteinising hormone-releasing hormone; SCLC, small-cell lung cancer.
Medicines not registered for use in Kazakhstan at the time of the ESMO review. After the ESMO expert review, the medicines have been included in the Kazakhstan treatment protocols.
Summary report
The analysis concluded with an evidence-based final report and recommendations that included results of the above-mentioned four phases and provided the country with information on:
-
1.
Essential medicines to be added to the national formulary.
-
2.
Treatment protocols that were redundant or low value that could be updated or removed.
-
3.
Low-value or redundant medicines to be deleted from the national formulary.
ESMO shared the evidence-based report with WHO Europe Office to enable further dialogue with the Ministry of Health of Kazakhstan. Following, the Ministry of Health implemented the recommendations.
Outcome of the review process in Kazakhstan
The information provided in the ESMO summary report had a positive impact on the Kazakhstan National Cancer Control Plan 2018-2022 and has led to increases in the budget allocated to cancer care focusing all essential and highly effective medicines and treatment options, and withdrawal of low-value or non-evidence-based therapies (Figure 2). It is important to note that many of the treatment reviewed included anticancer medicines and treatment protocols used for indications which are no longer recommended by ESMO. After the ESMO review, these medicines were removed from national protocols and additional new medicines have been included in the national medicine list.
Figure 2.
Outcome of the review process in Kazakhstan.
Specifically, this has led to:
-
•
The updating of the national essential cancer medicines list with an increase from 6 to 19 of new targeted medicines included in the national benefit package.
-
•
The amendment or combination of treatment protocols (from 35 to 30 after the ESMO review) across 14 solid tumour settings and 10 treatment protocols for haematological malignancies that were updated to reflect the best current scientific evidence.
-
•
The withdrawal of eight low-value or redundant medicines to treat breast, head and neck, kidney cancers and melanoma from the Kazakhstan national formulary.
-
•
The updating of the cancer treatment protocols list with an increase from 11 to 26 new targeted medicines included in treatment protocols.
-
•
A plan to add seven new targeted medicines to the national formulary.
Discussion
Providing comprehensive, effective, accessible, high-quality and sustainable cancer care is a major challenge to health care systems globally. It is particularly taxing for countries that experience rapid rises in incidence of cases or that have expedited uptake of more advanced, and often more costly, cancer medicines and technologies.
Accordingly, focusing on value for money linked to universal health coverage has emerged as the predominant public health principle to ensure optimal cancer care for entire populations.8 By extension, countries must optimise the availability of affordable quality cancer care through appropriate selection and effective pricing approaches. Any failure can push families into poverty because of high out-of-pocket costs, while threatening the health system’s sustainability.
In Kazakhstan, cancer care is covered by the government and services are provided without user fees. This includes all diagnostics and treatment procedures which are part of national guidelines and are considered standard of care. While cancer care in the country is covered by the universal health coverage package, budgetary considerations must account for the rising number of cancer cases and increasing cost of cancer care. The experience of the Kazakhstan Ministry of Health to improve access to quality cancer care as part of universal health coverage highlights the value of collaboration across sectors, namely with WHO and ESMO, to deliver results. This major formulary review and overhaul illustrates a model approach to this vital challenge. The evidence-based prioritisation of cancer medicines and protocols resulted in the Kazakhstan Government’s increase in and efficiency of the budget for cancer care to include all essential and highly effective medicines and treatment options and the withdrawal of low-value or non-evidence-based therapies. Thus, this four-phase approach contributed to the country’s ability to offer patients access to the best possible cancer care.
We contend that this approach is generalisable and can be applied in the evaluation of any national formulary. It has the advantage of using open-access, evidence-based references such as:
-
•
the WHO EML,3 which serves as a model for countries to develop their own national lists and to facilitate sustainable, equitable access to medicines and diagnostics tests and to promote their appropriate use.
-
•
the ESMO-MCBS,6 a validated and reproducible tool to assess the magnitude of clinical benefit that can be anticipated from anticancer therapies.
-
•
the EMA-approved medicines and indications database,4 which provide necessary information on medicines to enable their rational use.
-
•
the ESMO CPGs5 developed and reviewed by leading experts, helping to provide patients with the best evidence-based standard of care.
This collaboration is an example of how, by sharing best practices, tools and resources, we can address the issue of access to cancer medicines and positively impact clinicians, their daily practice and, vitally so, patient care and outcomes. These tools and this four-phase approach are available for any government or stakeholder seeking to set priorities maximising the impact of their cancer programmes, to invest strategically based on value for money and to provide cancer care for all.
Acknowledgements
The authors wish to acknowledge Lars Bullinger, Josep Carreras, Nicola Gökbuget, Mats Jerkeman, Ulrich Mey, Gert J. Ossenkoppele and Josep-Maria Ribera for the review of the Kazakhstan haematological formulary and treatment protocols and Ukolova Yelena from the Kazakh Research Institute of Oncology/Radiology for the review of the Kazakhstan treatment protocols. The authors also wish to thank Rosa Giuliani, ESMO Director of Public Policy, George Pentheroudakis, ESMO Chief Medical Officer, Dario Trapani, member of the ESMO-MCBS Extended Working Group, and Marilys Corbex, senior technical officer of WHO Europe Office, for their invaluable contributions, comments and input on this manuscript. The authors sincerely thanks the ESMO leadership for their invaluable support.
Funding
ESMO received funding from WHO, Switzerland for performance of the work.
Disclosure
EGEdV declares institutional financial support for advisory board/consultancy from Sanofi, Daiichi, Sankyo, NSABP, Pfizer and Merck, and institutional support for clinical trials or contracted research from Amgen, Genentech, Crescendo, Roche, AstraZeneca, Synthon, Nordic Nanovector, G1 Therapeutics, Bayer, Chugai Pharma, CytomX Therapeutics, Servier and Radius Health. AI is a staff member of WHO and AI alone is responsible for the views expressed in this manuscript and they do not necessarily represent the decisions, policy or views of WHO. The remaining authors have declared no conflicts of interest.
Supplementary data
References
- 1.World Health Organization Global Health Estimates: Leading Causes of Death. https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death Available at.
- 2.World Health Organization. International Atomic Energy Agency. International Agency for Research on Cancer . 2017. Integrated Missions of PACT (imPACT). Cancer Control Capacity and Needs Assessment Report. [Google Scholar]
- 3.World Health Organization WHO Model Lists of Essential Medicines. https://www.who.int/groups/expert-committee-on-selection-and-use-of-essential-medicines/essential-medicines-lists Available at.
- 4.European Medicines Agency Medicines. https://www.ema.europa.eu/en/medicines Available at.
- 5.ESMO ESMO Clinical Practice Guidelines. https://www.esmo.org/Guidelines Available at.
- 6.Cherny N.I., Dafni U., Bogaerts J., et al. ESMO-Magnitude of Clinical Benefit Scale version 1.1. Ann Oncol. 2017;28:2340–2366. doi: 10.1093/annonc/mdx310. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization 20th WHO Model List of Essential Medicines. https://apps.who.int/iris/bitstream/handle/10665/273826/EML-20-eng.pdf?ua=1 Available at.
- 8.World Health Organization . World Health Organization; 2020. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All.https://apps.who.int/iris/handle/10665/330745 Available at. [Google Scholar]
- 9.Piccart-Gebhart M.J., Procter M., Leyland-Jones B., et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 10.Gianni L., Pienkowski T., Im Y.-H., et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 11.Gianni L., Pienkowski T., Im Y.H., et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17:791–800. doi: 10.1016/S1470-2045(16)00163-7. [DOI] [PubMed] [Google Scholar]
- 12.Swain S.M., Kim S.-B., Cortés J., et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baselga J., Cortés J., Kim S.-B., et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swain S.M., Baselga J., Kim S.-B., et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortes J., Baselga J., Im Y.H., et al. Health-related quality-of-life assessment in CLEOPATRA, a phase III study combining pertuzumab with trastuzumab and docetaxel in metastatic breast cancer. Ann Oncol. 2013;24:2630–2635. doi: 10.1093/annonc/mdt274. [DOI] [PubMed] [Google Scholar]
- 16.Blackwell K.L., Burstein H.J., Storniolo A.M., et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2–positive metastatic breast cancer: final results from the EGF104900 study. J Clin Oncol. 2012;30:2585–2592. doi: 10.1200/JCO.2011.35.6725. [DOI] [PubMed] [Google Scholar]
- 17.Blackwell K.L., Burstein H.J., Storniolo A.M., et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 18.Cristofanilli M., Turner N.C., Bondarenko I., et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 19.Verma S., Miles D., Gianni L., et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welslau M., Dieras V., Sohn J.H., et al. Patient-reported outcomes from EMILIA, a randomized phase 3 study of trastuzumab emtansine (T-DM1) versus capecitabine and lapatinib in human epidermal growth factor receptor 2-positive locally advanced or metastatic breast cancer. Cancer. 2014;120:642–651. doi: 10.1002/cncr.28465. [DOI] [PubMed] [Google Scholar]
- 21.Finn R.S., Martin M., Rugo H.S., et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 22.Finn R.S., Crown J.P., Lang I., et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 23.Hortobagyi G.N., Stemmer S.M., Burris H.A., et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 24.Geyer C.E., Forster J., Lindquist D., et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;55:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 25.Miller K., Wang M., Gralow J., et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 26.Baselga J., Campone M., Piccart M., et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cortes J., O'Shaughnessy J., Loesch D., et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377:914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 28.Pivot X., Marme F., Koenigsberg R., et al. Pooled analyses of eribulin in metastatic breast cancer patients with at least one prior chemotherapy. Ann Oncol. 2016;27:1525–1531. doi: 10.1093/annonc/mdw203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.