Highlights
-
•
HF affects millions of patients every year, adding a significant financial burden to global health care systems.
-
•
This review discusses the role of novel transcatheter-based therapies for the management of HF.
-
•
Ongoing clinical trials will provide answers on the potential clinical benefits of these technologies in HF outcomes.
Key Words: catheter-based therapies, heart failure, interventional heart failure
Abbreviations and Acronyms: CI, confidence interval; COVID-19, coronavirus disease 2019; CS, coronary sinus; CVP, central venous pressure; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; LA, left atrium/atrial; LAP, left atrial pressure; LV, left ventricular; LVEF, left ventricular ejection fraction; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVESVi, left ventricular end-systolic volume index; NYHA, New York Heart Association; PCWP, pulmonary capillary wedge pressure; RA, right atrium/atrial; RAP, right atrial pressure; SVC, superior vena cava
Central Illustration
Summary
Chronic heart failure is one of the most debilitating chronic conditions affecting millions of people and adding a significant financial burden to health care systems worldwide. Despite the significant therapeutic advances achieved over the last decade, morbidity and mortality remain high. Multiple catheter-based interventional therapies targeting different physiological and anatomical targets are already under different stages of clinical investigation. The present paper provides a technical overview of the most relevant catheter-based interventional therapies under clinical investigation.
Heart failure (HF) is a major global health problem carrying significant morbidity and mortality despite the introduction of novel pharmacological and device-based therapies (1). Its prevalence has been estimated to increase to 2.97% of the U.S. population by 2030 (2), whereas the total costs associated with HF care will more than double. It is estimated that by 2030, the cost of care will amount to $69.8 billion, up from $30.7 billion in 2012 (1,2).
Over the last several years, the term interventional HF started to be incorporated in the cardiology community and to be considered a subspecialty in some academic centers. In this field, multiple catheter-based technologies targeting different physiological and anatomical targets are already in advanced clinical validation phases. Some of these technologies aim to alleviate symptoms and reduce rehospitalization, while others aim to improve quality of life and possibly impact mortality. In this review, we summarize the progress achieved to date in some of these rapidly emerging therapeutic areas intended to address a wide range of unmet needs experienced by patients with chronic HF (Central Illustration).
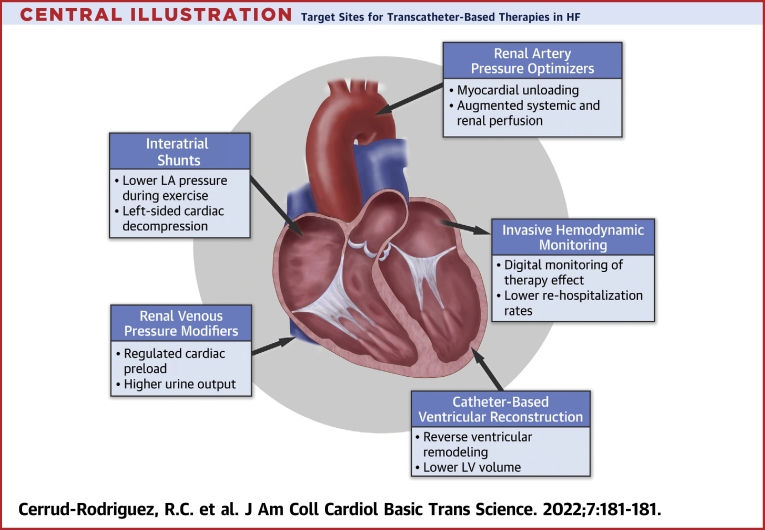
Central Illustration.
Target Sites for Transcatheter-Based Therapies in HF
Potential targets for transcatheter therapies in patients with advanced symptomatic heart failure (HF). LA = left atrial; LV = left ventricular.
Ventricular Remodeling and Myocardial Performance
Ventricular remodeling is the process by which a combination of myocyte hypertrophy, apoptosis, myofibroblast proliferation, and interstitial fibrosis result in progressive cavity dilation, distortion of the normal elliptical shape, and left ventricular (LV) contractile dysfunction (3). Alterations in myocyte length and width are the basis of the changes seen in LV end-systolic volume (LVESV) and LV end-diastolic volume (LVEDV) in LV remodeling (4). These pathological changes result in rightward shifts of the end-diastolic pressure-volume relationships toward larger volumes (5). Based on Laplace’s law, this shift of the end-diastolic pressure-volume relationships toward larger volumes results in a decreased slope (contractility) of the end-systolic pressure-volume relationship and increased systolic and diastolic mechanical stress on myocytes, which propagates the remodeling process (6). Specific to ischemic heart disease, necrotic myocardium undergoes fibrosis and scar formation (3), which also contribute to LV remodeling (7). Following this early stage, myocardial remodeling is predominantly driven by the hypertrophic myocyte elongation in the noninfarcted zone, as occurs in nonischemic dilated cardiomyopathy (8,9).
Thus, changes in LV mass ultimately represent a maladaptive response and represent one the most frequently seen features in myocardial remodeling (5), as this contributes to a worsened imbalance between energy supply and demand, as the degree of hypertrophy is generally unable to be compensated by capillary growth (10). All these mechanical changes result in inefficient oxygen delivery from capillaries to myocytes (11), resulting in subendocardial hypoperfusion and ischemia at times of high energy demand (10).
Surgical ventricular reconstruction aimed to reverse ventricular remodeling by excluding the infarcted akinetic region. However, in the overall study population, there were no changes in functional capacity, cardiac-related death, or rehospitalization rates (12). Specifically, in the STICH (Surgical Treatment of Ischemic Heart Failure) trial (13), 555 patients who underwent coronary artery bypass grafting plus surgical ventricular reconstruction and showed a postoperative LVESV index (LVESVi) ≤70 mL/m2 by echocardiography experienced a survival benefit compared with coronary artery bypass grafting alone (13). The benefit of this approach was less clear in patients with HF with reduced ejection fraction (HFrEF) and extensive ventricular dilation because it technically limits the ability to achieve sufficient reduction in LV volume (5). In addition, the highly invasive nature and risk associated with this surgical procedure have led most major surgical centers to abandon this procedure (5). The emergence of less invasive catheter-based techniques has revitalized this approach based on the premise of decreasing postoperative complications and mortality compared with open heart surgery (Figure 1).
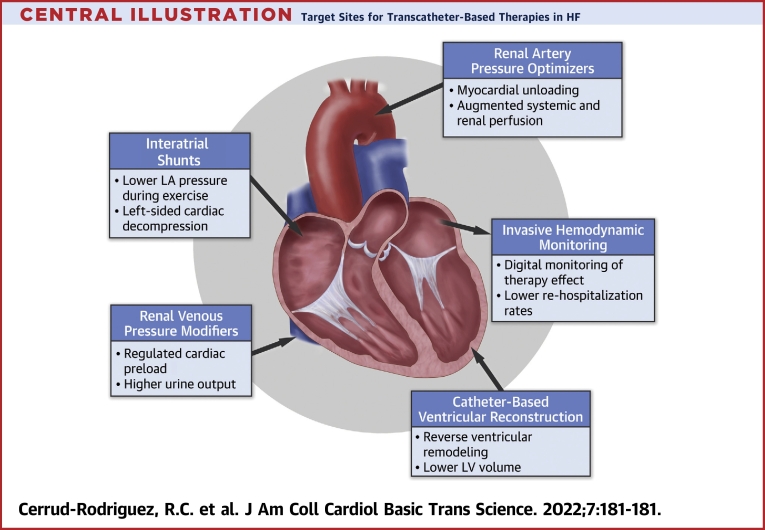
Figure 1.
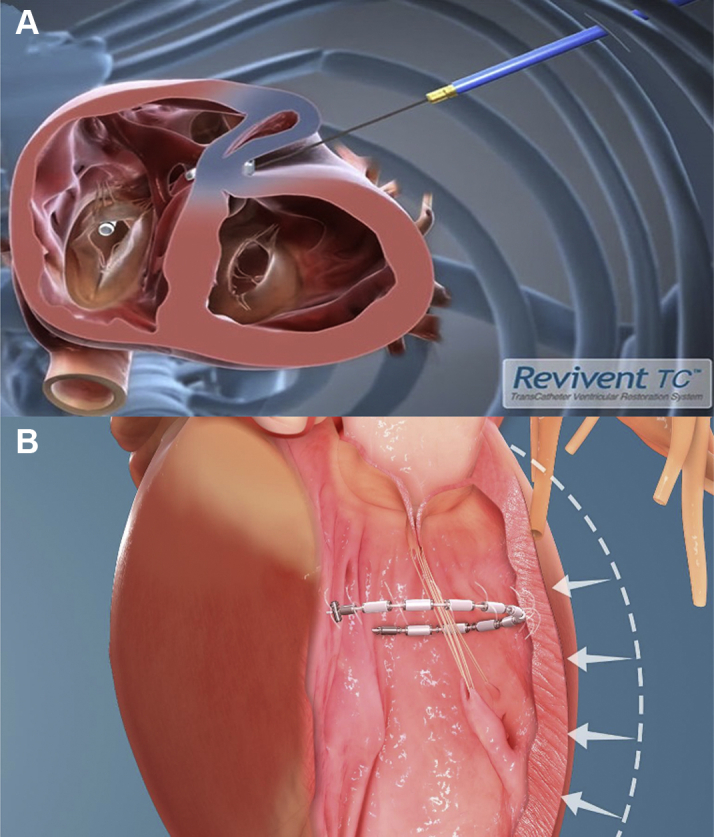
Transcatheter-Based Ventricular Reconstruction
Catheter-based reconstruction devices seeking to reverse ventricular remodeling: (A) Revivent TC (BioVentrix) and (B) AccuCinch (Ancora Heart).
Catheter-based ventricular reconstruction.
The Revivent TC Transcatheter Ventricular Enhancement System (BioVentrix) achieves LV volume reduction by using micro-anchors designed to exclude anterior scarred myocardium from viable tissue, reducing volume, radius, and wall tension while restoring the conical morphology to the LV. The system is being studied in patients with ischemic cardiomyopathy and New York Heart Association (NYHA) functional class III or ambulatory IV who had a previous transmural myocardial infarction (at least 90 days before implantation) leaving a residual scar in the anterolateral LV wall. LV dilatation or aneurysm must be present and viable myocardium must exist at the LV base as a requirement for device implantation.
The system is implanted using the LIVE procedure, performed by both an interventional cardiologist and a cardiac surgeon (14). A 5-cm to 8-cm mini-thoracotomy is made in the left fifth intercostal space to provide access to the lateral wall of the heart. Meanwhile, the interventionalist introduces a multipurpose catheter into the right internal jugular vein containing a trilobed snare that is opened in the RV chamber. The surgeon then passes a straight needle from a predetermined site on the anterolateral LV wall at the edge of the scarred area, through the LV chamber and across the anterior septum into the RV. A guidewire is passed through the needle, which is snared in the RV and externalized from the right neck. The interventionalist then introduces a polyether ether ketone tether over the guidewire through the RV and out the LV. This tether has in incorporated hinged anchor (25 × 5 mm) that is deployed against the RV septal wall. The surgeon then advances a paired, external (locking) anchor of identical size over the tether, and the 2 walls are brought into apposition with controlled compression. This excludes the scarred region spanned by these 2 anchors. This process is repeated as many times as needed at progressively more basilar levels so as to exclude as much of the infarcted, dyskinetic myocardium as possible.
The first-generation system, used in 51 patients, required a median sternotomy. The current, second-generation system uses the less invasive LIVE procedure described previously and has been used in 35 patients. No significant differences in terms of major or minor adverse events have been reported between the 2 procedures. At 12 months, significant improvements were reported in echocardiographic variables (LVEDV index declined from 106 ± 33 mL/m2 to 80 ± 26 mL/m2 and LVESVi declined from 74 ± 28 mL/m2 to 54 ± 23 mL/m2), functional NYHA functional class, 6-minute walk distance, and Minnesota Living with Heart Failure Questionnaire (14). Major adverse events included tricuspid valve insufficiency increase, mitral valve insufficiency increase, pulmonary valve insufficiency increase, ventricular septal defect, bleeding, respiratory failure, stroke, and late cardiac arrest, without significant difference between the sternotomy and the hybrid approach group (14). The ongoing ALIVE (The Azimilide Post-Infarct Survival Evaluation) trial (NCT02931240) is designed to test the safety and efficacy of the second-generation system.
The AccuCinch Ventricular Restoration System (Ancora Heart) was initially developed with the objective of improving the myocardial distortion responsible for the development of functional mitral regurgitation in HFrEF patients at high risk for surgical treatment. During device implantation, the LV is accessed using a retrograde approach via the aortic valve. A guidewire is placed behind the mitral chordae providing access for the TracCath (Ancora Heart), which serves as a template for anchor placement. Using this rail system, a series of anchors and spacers connected with a cable are used to cinch and lock the system into the LV wall 1 to 2 cm below the mitral valve. Between 12 and 16 nitinol anchors are implanted over a 220° arc in the subannular space and cinched together with a polyethylene cable, with the goal to reduce the basal-to-mid-LV circumference and wall tension and increase mitral leaflet opposition (5). A multicenter, prospective, single-arm Early Feasibility Study (NCT02806570) evaluated the safety and performance of the system at 6 months in 21 patients enrolled in 8 U.S. sites. Device success was 90% (n = 19 of 21) with an average procedure duration of 150 minutes. No major adverse events were reported (15). At 6 months, in 8 patients who had complete imaging follow up there was an improvement of +10% in LVEF, a decrease of -19 mL/m2 in LVESVi, and reduction of the effective mitral orifice area by -0.16 cm2. Improvements of NYHA functional class and quality of life as measured by the Kansas City Cardiomyopathy Questionnaire were also reported (15). A pivotal study aiming to determine the benefit of this device in patients with HFrEF (LVEF ≤40% and ≥20%) and NYHA functional class III and IVa without significant mitral regurgitation has recently been initiated (NCT04331769).
Renal Perfusion in Decompensated HF
Acute decompensated HF is the cause for a majority of hospitalizations for patients with chronic HF. Factors considered to contribute to a decline in renal perfusion include decreased cardiac output, increased central venous pressure (CVP), and renal arterial vasoconstriction due to activation of the renin-angiotensin-aldosterone system, leading to decreased urine output and diuretic resistance (16,17). Based on current mechanistic understanding of determinants of urine output and fluid balance in HF, multiple new catheter-based technologies have been developed to treat volume overload not responding adequately to intravenous diuretics. In general, these technologies fall into several categories, including devices that induce venodilation, remove fluid from the vasculature or interstitial space, increase renal artery pressure, decrease renal vein pressure, or selectively dilate the renal arteries (18, 19, 20). Only the devices relevant to catheter-based interventions, those that enhance renal artery pressure, and those that decrease renal vein pressure are summarized subsequently (Figure 2).
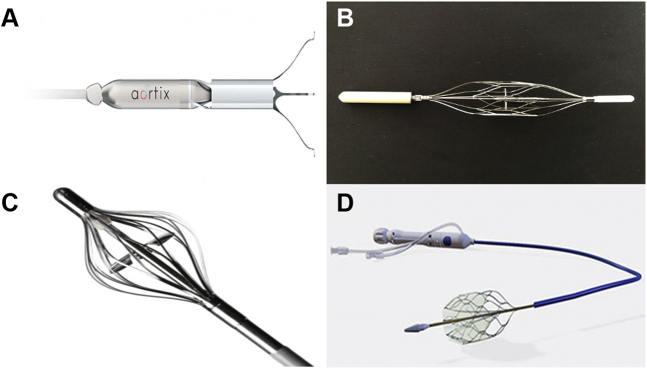
Figure 2.
Transcatheter Renal Hemodynamics Optimizers
Devices in development aiming to modify renal hemodynamics in heart failure: (A) Aortix pump (Procyrion), (B) Second Heart Assist Device (Second Heart Assist), (C) Reitan Catheter Pump (Cardiobridge), and (D) Doraya Renal Flow Regulator (Revamp Medical).
Renal artery pressure optimizers
The Aortix percutaneous mechanical circulatory support system (Procyrion) is a 6-mm axial flow pump placed via a transfemoral approach in the descending aorta, proximal to the renal arteries. The device is intended to provide cardiac unloading and augmentation of renal and systemic perfusion (21). Aortix reduces afterload and increases cardiac output at low speeds; higher speeds seem to increase the mean PA pressure, possibly owing to increased venous return (16,22). The system provides up to 5 L/min of flow at 30,000 rpm, with a mean flow of 3.5 L/min at 25,000 rpm. The device was tested in a single-center, first-in-human safety and feasibility study enrolling 6 patients with ischemic cardiomyopathy and LVEF between 20% and 28% who were scheduled to undergo complex coronary intervention. It was successfully delivered in all patients within a mean of 5.8 minutes after access was obtained, and it was removed after a successful percutaneous coronary intervention in all patients (21). No major complications or significant hemolysis occurred, and urine output had a temporary 10-fold increase in the 4 patients in whom urine output was recorded (21). A larger (n = 45) multicenter, nonrandomized feasibility study is being planned to test the safety and performance of Aortix system in the management of patients hospitalized with acute decompensated HF and cardiorenal syndrome (NCT04145635). The impact of this or other devices that increase renal artery pressure on CVP has not yet been investigated, and in the upcoming pilot clinical study the device will be studied for up to 7 days.
The Second Heart Assist Device (Second Heart Assist) is an investigational, catheter-based device also being considered for management of cardiorenal syndrome. A wirelessly powered chronic device is being developed for longer-term use for patients with chronic HF. At the core of both platforms is a pulsatile-conforming aortic radial collapsible stent pump design allowing for a relatively easy femoral insertion and aortic positioning. Once properly positioned in the descending aorta proximal to the renal arteries, the stent distends the aortic wall (∼1-2 mm) to provide fixation without need for invasive anchoring. A 22-mm nitinol stent cage protects the aortic wall from the pump impellers. Because the stent fills the entire inner diameter of the aorta and possesses a 20- to 23-mm impeller, the pump needs lower revolutions per minute to achieve 4 to 6 L/min of flow. The pump increases blood flow to the kidneys, improving renal perfusion and urine output, potentially reducing the need for diuretics and possibly decreasing HF readmissions. However, formal published data regarding this device are lacking at this time.
The Reitan Catheter Pump (Cardiobridge) is another percutaneous ventricular assist device to help treat patients with acute decompensated HF and cardiorenal syndrome. It consists of a catheter-mounted distal pump head with a foldable propeller and surrounding cage, an interface unit, and an external drive unit with user console. The collapsed 10-F pump-head is delivered via a 14-F sheath placed in the femoral artery and is advanced to the proximal descending aorta about 5 to 10 cm distal to the left subclavian artery. Once in position, the pump head is deployed, and the propeller blades are extended and set to rotate at 8,000 to 11,000 rpm with the goal to achieve a radiofemoral mean arterial gradient of 10 mm Hg. This is thought to help unload the LV and increase the perfusion pressure distally. A first-in-human study confirmed the device safety and feasibility during high-risk percutaneous coronary intervention (23), and a subsequent study by Keeble et al (24) studied the hemodynamic and renal effects of this device in 20 patients (2 were excluded due to anatomic incompatibility with the device) with advanced HF (LVEF <30%) in acute decompensation requiring inotropic or mechanical circulatory support. Patients received support for a mean of 18.3 hours, and the device increased cardiac index (1.8 ± 0.3 L/min/m2 to 2.4 ± 0.5 L/min/m2; P = 0.04) and urine output (71 ± 65 mL/h to 227 ± 179 mL/h; P = 0.006) while decreasing serum creatinine (188 ± 87 μmol/L to 161 ± 78 μmol/L; P = 0.0007) without clinically significant hemolysis, vascular injury, or thromboembolic complications (24).
Renal venous pressure modifiers
Elevated CVP, with subsequent renal venous congestion, impairs renal function in the setting of decompensated HF, causing decreased kidney function and neurohormonal changes (25). Several studies (18, 19, 20) have found a correlation between elevated CVP and progressive decline in renal function. The classical study by Mullens et al (26) demonstrated that, in patients with decompensated advanced HF (baseline characteristics: mean age 54 ± 14 years, mean LVEF 20 ± 8%, cardiac index 1.9 ± 0.6 L/min/m2), 40% developed worsening renal function (“congestive kidney failure”) within the first 5 days of hospitalization. These subjects were more likely to have worse kidney function at baseline (serum creatinine 1.9 ± 0.9 mg/dL vs 1.5 ± 0.8 mg/dL; P = 0.007), and more importantly, the mean baseline CVP was significantly higher in the group that developed worsening kidney failure versus those who did not (18 ± 7 mm Hg vs 12 ± 6 mm Hg; P < 0.001) (26). Unexpectedly, the mean cardiac index was significantly higher in the group that developed worsening kidney failure. There are several investigational devices aiming to reduce renal afterload to increase renal blood flow, increase urine output and sodium excretion, and improve responsiveness to diuretics.
The preCARDIA system (preCARDIA) consists of a pump console and a catheter that is introduced through the internal jugular vein and positioned into the pulmonary artery (PA) (16). A proprietary balloon located on the shaft of the catheter is positioned in the superior vena cava (SVC) above the junction of the right atrium (RA). This proprietary balloon is used to create intermittent mechanical occlusion as a therapeutic approach in significantly congested patients with acute decompensated HF (16). In contrast to inferior vena cava occlusion, SVC occlusion reduces preload and unloads the heart without reducing systemic blood pressure or cardiac output; no neurological or vascular injuries were reported after 12 to 18 hours of intermittent SVC occlusion (27). The net effect of the preCARDIA catheter is a reduction in CVP and sustained mean arterial pressure, thus creating improving hemodynamics and improving renal perfusion. An early open-label feasibility study is currently enrolling HF patients with NYHA functional class III to IV with inadequate diuresis with the objective of determining freedom from major adverse events throughout 30 days (NCT03836079). Preliminary data suggest a beneficial effect on cardiac output, diuresis, right atrial pressure (RAP), and pulmonary capillary wedge pressure (PCWP). The system has recently obtained the Breakthrough Device Designation by the Food and Drug Administration.
The Doraya Renal Flow Regulator (Revamp Medical) is a temporary intravenous flow regulator that is percutaneously positioned in the inferior vena cava distal to the renal veins to partially restricts venous flow and reduce renal vein pressure (16). The device is introduced via the femoral vein using a 12-F sheath and consists of a 25-mm nitinol frame mounted on the distal portion of the catheter. Adjusting the opening of the frame allows for a partial obstruction of venous flow, decreasing preload and congestion and creating an iliac-to-CVP gradient of about 5 mm Hg. By decreasing congestion, the system is expected to achieve unloading of the renal venous system and improve perfusion pressure to the kidneys. A second effect is unloading of the LV, which results in reduced filling pressures and wall stress (28). Currently, an ongoing first-in-human study is evaluating the safety and performance of this device (NCT03234647). Early preliminary results from 5 enrolled patients have been promising, showing improvement in urine output and CVP; no serious adverse events associated with device placement or distal venous congestion were seen (29). The system was also granted Breakthrough Device Designation by the Food and Drug Administration in July 2020.
Interatrial Shunts for Reducing PCWP During Exercise
In healthy individuals, cardiac output augments during exercise by increasing stroke volume (SV) and heart rate without an excessive increase in filling pressures. SV increases up to 40% as a result of both an increase in the LVEDV and a decrease in the LVESV, yielding a 15% absolute increase in LVEF (4,30). These mechanics depends on a compliant ventricle with normal early diastolic relaxation to avoid a significant increase in pressure at higher heart rate. Furthermore, in healthy individuals, the PCWP at peak supine exercise is <20 mm Hg and the LV end-diastolic pressure is almost invariably <25 mm Hg (31). In contrast, patients with HF (both HF with preserved ejection fraction [HFpEF] and HFrEF) experience significantly elevated PCWP and LV end-diastolic pressure just by raising their legs and placing their feet in the pedals of a supine cycle, and these pressures raise even further after exercise is initiated (4,32,33). In HFpEF, in which exercise hemodynamics have been most extensively studied, up to 88% of the patients reach a mean PCWP of 35 mm Hg or greater at peak exercise (4,32). This increase in the wedge is associated with development of B-lines in thoracic ultrasound and a decrease in aerobic capacity (4,34).
Some theoretical models explored the concept of an interatrial shunt for reducing this exercise-induced rise of PCWP to improve exercise tolerance (31). In one study, the contractile properties of each cardiac chamber were modeled by time-varying elastances and systemic and pulmonary vascular beds modeled by series of resistance and capacitance elements, based on data from 2 previously published papers of patients with HFpEF (4,32,35). An interatrial shunt was introduced in the model by allowing flow between the left atrium (LA) and RA, calculating the results that were obtained by varying the shunt diameter up to 12 mm (4). By creating a conduit in the interatrial septum, the pressure gradient between the LA and RA would potentially allow decompression of the left heart when the PCWP increases exaggeratedly during exercise. It was determined that the pressure gradient between chambers decreases as the shunt diameter increases, with the effect plateauing at around 10 mm (4). At peak exercise, the interatrial shunt model produced a decrease in LA pressure (LAP)–RAP gradient from 17 to 5 mm Hg, mainly owing to an 8-mm Hg decrease in LAP and a 3-mm Hg increase in RAP (4). At rest, the mean shunt flow was 1.4 L/min, corresponding to a Qp/Qs of 1.3. At peak exercise, the Qp/Qs was 1.4 with an average flow of 2.8 L/min. Moreover, the peak and mean PA pressures did not increase with the increased flow, owing to the reduction in LAP (4). Importantly, according to the 2019 American College of Cardiology/American Heart Association congenital heart disease guidelines, congenital shunts of these sizes and Qp/Qs values in this range are unlikely to have deleterious effects on RV size, function, or pulmonary pressures (36). These guidelines have been used to rationalize the likely safety profile of this approach, an assumption that will need to be proved in long-term follow-up studies. Several devices are under development based on these data, aiming to improve clinical outcomes in HF and to improve symptoms by altering the LA and pulmonary hemodynamics via implantable devices (Figure 3).
Figure 3.
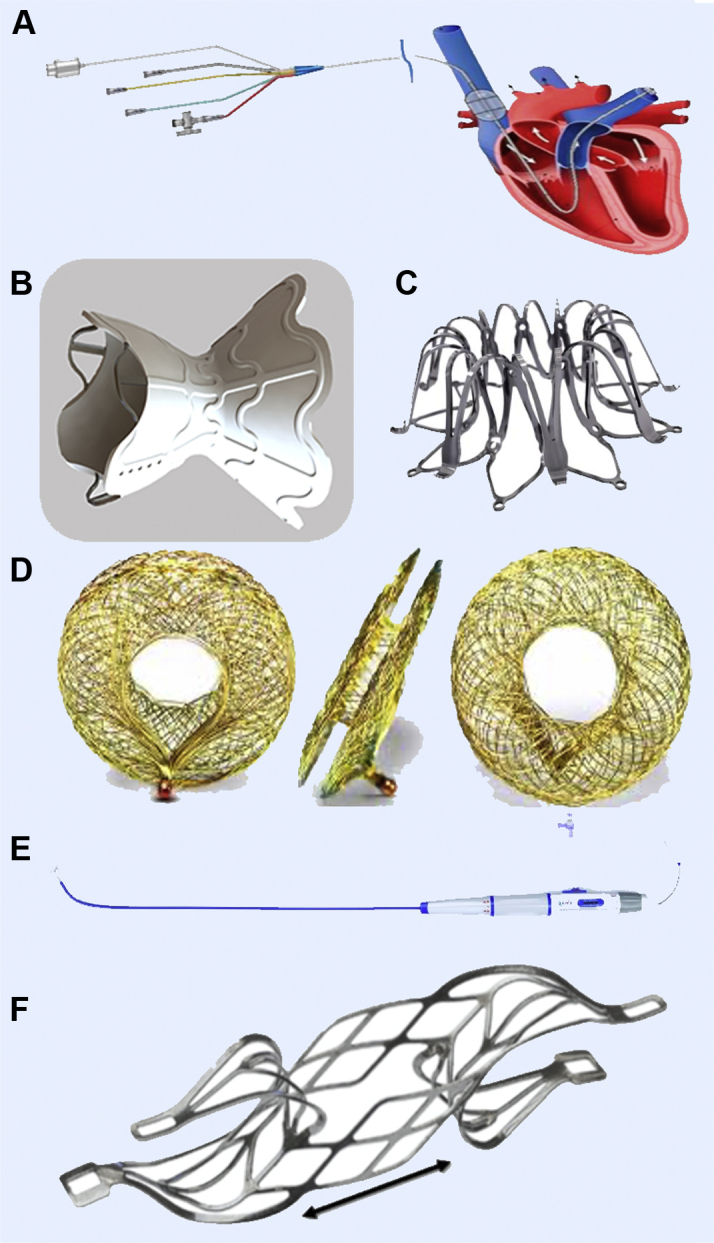
Transcatheter Interatrial Shunt Devices
Interatrial shunt devices aiming to improve clinical outcomes in heart failure altering the left atrial and pulmonary hemodynamics: (A) preCARDIA system (preCARDIA), (B) V-Wave Shunt system (V-Wave), (C) IASD system (Corvia Medical), (D) Atrial Flow Regulator (Occlutech), (E) NoYa system (DiNoVa), and (F) Transcatheter Atrial Shunt System (Edwards Lifesciences).
The V-Wave Shunt System (V-Wave) is a prosthesis built as an hourglass-shaped, self-expanding nitinol stent frame with an internal “neck,” which is placed across the fossa ovalis. The device is secured in place with the wide entry funnel located in the LA and the wide exit funnel in the RA. The entry funnel and the neck are encapsulated with polytetrafluoroethylene to limit tissue growth and to facilitate laminar flow (37). The device is implanted percutaneously under transesophageal echocardiography guidance via the right femoral vein. The initial version of the device had a valve, which is no longer present in the current version of the V-Wave. A first-in-human trial showed improvement in NYHA functional class, quality-of-life measures, 6-minute walk test distance, and PCWP (37). However, a longer-term follow-up revealed a 14% (n = 5 of 36) rate of occlusion and 36% (n = 13 of 36) rate of stenosis at 12 months (38). An ongoing randomized trial of the nonvalved device (RELIEVE-HF [REducing Lung congestIon Symptoms Using the v-wavE Shunt in adVancEd Heart Failure] trial [NCT03499236]) is actively recruiting patients to test if the device improves clinical outcomes in patients with NYHA functional class III and IVa symptoms, and is including patients with preserved and reduced LVEF; the only exclusion criterion is an LV end-diastolic dimension >8.0 cm.
The IASD system (Corvia Medical) consists of a nitinol frame with a 19-mm outer diameter that creates a permanent 8-mm atrial septal shunt. As noted previously, the mathematical model described predicted that an 8-mm shunt diameter yields a Qp/Qs of 1.3. The device is implanted using a proprietary delivery catheter via the right femoral vein. The REDUCE LAP-HFI (Reduce Elevated Left Atrial Pressure in Patients With Heart Failure I) I trial was a small (n = 44) randomized, sham-controlled trial that enrolled HFpEF patients showing significant benefit (P = 0.028) in decreasing peak PCWP during exercise (20, 40, and 60 W) at 1-month follow-up when compared with the sham control group, without any major adverse cardiac or cerebrovascular events (39). That same population was subsequently followed for a longer term (median follow-up 739 days) and had a significantly better survival when compared with the predicted mortality as calculated using the MAGGIC (Meta-analysis Global Group in Chronic Heart Failure) (40,41) prognostic score (3.4 per 100 patient-years vs 10.2 per 100 patient-years; P = 0.014) (42). A multicenter, randomized, triple-blinded (participant, care provider and outcomes assessor) controlled trial, the REDUCE LAP-HF II trial (NCT03088033), is ongoing. All of the intended 608 patients have been recruited, and patients are now in the follow up period, which will define the potential clinical benefits of the IASD system.
The Atrial Flow Regulator (Occlutech) is another interatrial shunt device designed with the goal of trying to decrease LAP. It consists of a nitinol mesh device composed of 2 flat discs and a 1- to 2-mm connecting neck with a central fenestration allowing bidirectional flow. The device is manufactured with different fenestration sizes (6, 8, or 10 mm), and it is delivered following balloon atrial septostomy, as the Atrial Flow Regulator has less radial force than similar devices (43). The AFR-PRELIEVE (Pilot Study to Assess Safety and Efficacy of a Novel Atrial Flow Regulator [AFR] in Heart Failure Patients) trial was a small (n = 36) prospective, nonrandomized, open-label study enrolling NYHA functional class III or IV HFrEF and HFpEF patients with a 3-month follow-up that showed that the implant was safe and had few (n = 5 of 36) device-related complications. A single patient in the HFrEF group died 30 days after implant due to causes unrelated to the procedure (pneumonia and sepsis), and 3 of 36 patients were hospitalized due to worsening HF (43). The PROLONGER (Pomeranian atRial flOw reguLatOr iN conGestive hEart failuRe) trial (NCT04334694) is an ongoing nonrandomized, open-label trial that is currently enrolling patients. The study is designed to ascertain improvement in certain clinical (6-minute walk test, NYHA functional class) and hemodynamic outcomes (PCWP at rest and during handgrip test) after a 12-month follow-up.
The NoYa system (DiNoVa Medtech) is a stentless shunt system using a self-expanding nitinol stent that ablates the contacting tissue of the atrial septum using radiofrequency energy. The stent possesses adjustable strings that are used to adjust the diameter of the waist from 4 to 10 mm. The system creates an atrial septal defect without the need of an implantable component allowing future interventional procedures. Preliminary data from a first-in-human trial presented at TCT (44) showed significant improvement in N-terminal pro–B-type natriuretic peptide levels and in 6-minute walk test distance.
Finally, the Transcatheter Atrial Shunt System (Edwards Lifesciences) is a bare-nitinol implant flanked by 4 arms with an internal shunting diameter of 7 mm. Two arms situated on the LA wall, while the other 2 arms lie within the coronary sinus (CS). Intraprocedural CS multimodality imaging (angiography, fluoroscopy, transesophageal echocardiography, and hemodynamics) are done to verify appropriate device positioning and LA-to-CS shunting. A first-in-human trial was recently published by Simard et al (45), showing successful implant in 8 of 11 participants. After a median follow-up of 201 days, no major adverse periprocedural events were recorded, NYHA functional class improved to I or II in 87.5%, PCWP was reduced by a mean of -9 mm Hg, and shunt was sustained (ΔQp/Qs = 0.25) (45).
Invasive Hemodynamic Monitoring
PCWP provides invasive hemodynamic information reflecting LAP. Remote invasive monitoring of PA pressure with the goal of achieving early detection of impending volume overload and modifying therapy or patient behavior in HF patients was shown to be associated with fewer HF hospitalizations than traditional management based on symptoms and clinical signs alone (46). The seminal CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients trial) study showed that during the entire follow-up (mean 15 ± 7 months), there was a 37% reduction in HF-related hospitalization compared with the control group (hazard ratio [HR]: 0.63; 95% confidence interval [CI]: 0.52-0.77; P < 0.0001) (46). A 1-year follow-up post-approval, multicenter, prospective, open-label, observational, single-arm trial of 1,200 patients with NYHA functional class III HF and a prior HF hospitalization within 12 months showed a reduction of HF hospitalization rates from 1.25 events per patient-year the year prior to enrollment compared with 0.54 events per patient-year the year following device implantation. This amounted to a risk reduction of 57% (HR: 0.43; 95% CI, 0.39-0.47; P < 0.0001) (47). Similar findings were noted in a registry study conducted in the European Union, with a 62% reduction of HF hospitalization the year following implantation compared with the year prior to implantation (HR: 0.38; 95% CI: 0.31-0.48; P < 0.0001). All of these beneficial findings have stimulated industry to invest into the development of new monitoring technology (Figure 4), which will be reviewed subsequently. However, most recently the results of the prospective, randomized, single blind GUIDE-HF (Hemodynamic-GUIDEd Management of Heart Failure) study that included 1,022 primarily NYHA functional class II and III patients comparing HF management with and without knowledge of CardioMEMS readings reported no significant difference between groups (HR: 0.88; 95% CI: 0.74-1.05; P = 0.16) (48). Unfortunately, the study conduct was complicated by the coronavirus disease 2019 (COVID-19) pandemic; a prespecified subgroup analysis assessing the result in the pre-COVID era showed a significant reduction of HF events in the active management group (HR: 0.81; 95% CI: 0.66-1.00; P = 0.049). During the COVID-19 era of the study, there was no treatment effect, and the rate of HF events decreased in the control group compared the active group (∼0.55 events per patient-year), which did not change significantly from the pre- to post-COVID-19 eras. The implications of these findings to the adoption of pressure monitoring for HF patients remain to be seen. Nevertheless, as noted, there are several invasive monitoring technologies that remain under development.
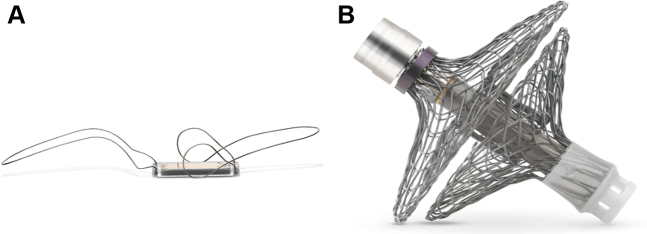
Figure 4.
Transcatheter Remote Invasive Hemodynamic Monitoring
Remote invasive monitoring devices: (A) Cordella PA Pressure Sensor System (Endotronix) and (B) V-LAP left atrial pressure monitoring system (Vectorious Medical).
The Cordella PA Pressure Sensor System (Endotronix) is intended to measure, record, and transmit PA pressure from congestive HF NYHA functional class III patients to clinicians for evaluation and management, with the goal to improve clinical outcomes—mainly all-cause mortality and HF hospitalizations or emergency department visits. The device is implanted via a right heart catheterization, using the femoral vein as access site, and positioning the sensor at the inferoposterior inflection of the right PA. A first-in-human trial demonstrated that the Cordella system enables the safe and accurate measurement of PA pressures in HF NYHA functional class III patients (49). No device system–related complications, defined as invasive treatment, device explantation, or death, occurred (49). Currently there are 2 ongoing trials to try to secure regulatory clearance in the United States (PROACTIVE HF IDE [PROACTIVE-HF IDE Trial Heart Failure NYHA Class III] trial [NCT04089059]) and in the European Union (SIRONA II CE-Mark [SIRONA 2 Trial Heart Failure NYHA Class III] trial).
The V-LAP LAP monitoring system (Vectorious Medical Technologies) is implanted across the interatrial septum, under angiographic and echocardiographic guidance, and transmits hemodynamic data to a secure cloud-based platform that allows for remote monitoring. The system consists of the leadless implant, a proprietary delivery system, and an external unit that powers the implant, collects data using radiofrequency, and transmits these data to the cloud. A first-in-human multicenter, single-arm, open-label trial (VECTOR-HF [adaptatiVe Endovascular Strategy to the CloT MRI in Large Intracranial Vessel Occlusion-Heart Failure] trial [NCT03775161]) is currently enrolling NYHA functional class III patients to assess the safety, performance, and usability of the V-LAP. Coincidentally, the first patient was enrolled immediately before the COVID-19 pandemic began, which allowed the researchers to remotely monitor his rising LAP using the implanted device, illustrating an impossible-to-foresee benefit of this technology (50). We are eagerly awaiting for the results of this promising trial.
Future Perspectives
Multiple catheter-based technologies targeting different anatomical and physiological variables are under clinical investigation. It is clear that there will be a growing demand for physicians specifically trained in the implantation of these devices that can be attentive to the needs of the HF population. Some of these devices require more advanced skills (eg, the structural remodeling devices and interatrial shunts), while others require little more than the skills required to perform a right heart catheterization. The overall need is fostering training physicians specializing in “interventional HF.” Several of the technologies in development offer potential solutions to some of the specific unmet clinical needs of HF patients, and early clinical studies are providing encouraging results. These advances, along with advances in tissue engineering, pharmacology, and cardiac genetics may provide more comprehensive solutions to this syndrome affecting millions of patients worldwide.
Funding Support and Author Disclosures
Dr Latib has served as a consultant and/or on the advisory board of Medtronic, Abbott, Boston Scientific, Edwards Lifesciences, Neochord, Nuvera, Supira, and Coramaze. Dr Burkhoff has received institutional research grant support from Ancora Heart; and received consultant fees from preCARDIA, Revamp Medical, and Corvia Medical. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
William Abraham, MD, served as Guest Associate Editor for this paper.
Michael Bristow, MD, PhD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Virani S.S., Alonso A., Benjamin E.J., et al. Heart Disease and Stroke Statistics - 2020 Update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich P.A., Albert N.M., Allen L.A., et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konstam M.A., Kramer D.G., Patel A.R., Maron M.S., Udelson J.E. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. J Am Coll Cardiol Img. 2011;4:98–108. doi: 10.1016/j.jcmg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Griffin J.M., Borlaug B.A., Komtebedde J., et al. Impact of interatrial shunts on invasive hemodynamics and exercise tolerance in patients with heart failure. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brener M.I., Uriel N., Burkhoff D. Left ventricular volume reduction and reshaping as a treatment option for heart failure. Struct Heart. 2020;4:264–283. [Google Scholar]
- 6.Fletcher P.J., Pfeffer J.M., Pfeffer M.A., Braunwald E. Left ventricular diastolic pressure-volume relations in rats with healed myocardial infarction. Effects on systolic function. Circ Res. 1981;49:618–626. doi: 10.1161/01.res.49.3.618. [DOI] [PubMed] [Google Scholar]
- 7.Cohen M.V., Yang X.M., Neumann T., Heusch G., Downey J.M. Favorable remodeling enhances recovery of regional myocardial function in the weeks after infarction in ischemically preconditioned hearts. Circulation. 2000;102:579–583. doi: 10.1161/01.cir.102.5.579. [DOI] [PubMed] [Google Scholar]
- 8.McKay R.G., Pfeffer M.A., Pasternak R.C., et al. Left ventricular remodeling after myocardial infarction: a corollary to infarct expansion. Circulation. 1986;74:693–702. doi: 10.1161/01.cir.74.4.693. [DOI] [PubMed] [Google Scholar]
- 9.Rumberger J.A., Behrenbeck T., Breen J.R., Reed J.E., Gersh B.J. Nonparallel changes in global left ventricular chamber volume and muscle mass during the first year after transmural myocardial infarction in humans. J Am Coll Cardiol. 1993;21:673–682. doi: 10.1016/0735-1097(93)90100-f. [DOI] [PubMed] [Google Scholar]
- 10.Anversa P., Capasso J.M., Sonnenblick E.H., Olivetti G. Mechanisms of myocyte and capillary growth in the infarcted heart. Eur Heart J. 1990;11:123–132. doi: 10.1093/eurheartj/11.suppl_b.123. [DOI] [PubMed] [Google Scholar]
- 11.Sharov V.G., Todor A.V., Sabbah H.N. Left ventricular histomorphometric findings in dogs with heart failure treated with the Acorn cardiac support device. Heart Fail Rev. 2005;10:141–147. doi: 10.1007/s10741-005-4641-1. [DOI] [PubMed] [Google Scholar]
- 12.Jones R.H., Velazquez E.J., Michler R.E., et al. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009;360:1705–1717. doi: 10.1056/NEJMoa0900559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michler R.E., Rouleau J.L., Al-Khalidi H.R., et al. Insights from the STICH trial: change in left ventricular size after coronary artery bypass grafting with and without surgical ventricular reconstruction. J Thorac Cardiovasc Surg. 2013;146:1139–1145.e6. doi: 10.1016/j.jtcvs.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein P., Anker S.D., Wechsler A., et al. Less invasive ventricular reconstruction for ischaemic heart failure. Eur J Heart Fail. 2019;21:1638–1650. doi: 10.1002/ejhf.1669. [DOI] [PubMed] [Google Scholar]
- 15.Reisman M., Wudel J., Martin S., et al. TCT-88 6-month outcomes of an early feasibility study of the AccuCinch Left Ventricular Repair system in patients with heart failure and functional mitral regurgitation. J Am Coll Cardiol. 2019;74:B88. [Google Scholar]
- 16.Rosenblum H., Kapur N.K., Abraham W.T., et al. Conceptual considerations for device-based therapy in acute decompensated heart failure: DRI(2)P(2)S. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.119.006731. [DOI] [PubMed] [Google Scholar]
- 17.Boorsma E.M., Ter Maaten J.M., Damman K., et al. Congestion in heart failure: a contemporary look at physiology, diagnosis and treatment. Nat Rev Cardiol. 2020;17:641–655. doi: 10.1038/s41569-020-0379-7. [DOI] [PubMed] [Google Scholar]
- 18.Nohria A., Hasselblad V., Stebbins A., et al. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51:1268–1274. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 19.Jessup M., Costanzo M.R. The cardiorenal syndrome: do we need a change of strategy or a change of tactics? J Am Coll Cardiol. 2009;53:597–599. doi: 10.1016/j.jacc.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Damman K., van Deursen V.M., Navis G., Voors A.A., van Veldhuisen D.J., Hillege H.L. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 21.Vora A.N., Schuyler Jones W., DeVore A.D., Ebner A., Clifton W., Patel M.R. First-in-human experience with Aortix intraaortic pump. Catheter Cardiovasc Interv. 2019;93:428–433. doi: 10.1002/ccd.27857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Annamalai S.K., Esposito M.L., Reyelt L.A., et al. Abdominal positioning of the next-generation intra-aortic fluid entrainment pump (Aortix) improves cardiac output in a swine model of heart failure. Circ Heart Fail. 2018;11 doi: 10.1161/CIRCHEARTFAILURE.118.005115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith E.J., Reitan O., Keeble T., Dixon K., Rothman M.T. A first-in-man study of the Reitan catheter pump for circulatory support in patients undergoing high-risk percutaneous coronary intervention. Catheter Cardiovasc Interv. 2009;73:859–865. doi: 10.1002/ccd.21865. [DOI] [PubMed] [Google Scholar]
- 24.Keeble T.R., Karamasis G.V., Rothman M.T., et al. Percutaneous haemodynamic and renal support in patients presenting with decompensated heart failure: A multi-centre efficacy study using the Reitan Catheter Pump (RCP) Int J Cardiol. 2019;275:53–58. doi: 10.1016/j.ijcard.2018.09.085. [DOI] [PubMed] [Google Scholar]
- 25.Bock J.S., Gottlieb S.S. Cardiorenal syndrome: new perspectives. Circulation. 2010;121:2592–2600. doi: 10.1161/CIRCULATIONAHA.109.886473. [DOI] [PubMed] [Google Scholar]
- 26.Mullens W., Abrahams Z., Francis G.S., et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapur N.K., Reyelt L., Crowley P., et al. Intermittent occlusion of the superior vena cava reduces cardiac filling pressures in preclinical models of heart failure. J Cardiovasc Transl Res. 2020;13:151–157. doi: 10.1007/s12265-019-09916-y. [DOI] [PubMed] [Google Scholar]
- 28.Dierckx R., Vanderheyden M., Heggermont W., Goethals M., Verstreken S., Bartunek J. Treatment of diuretic resistance with a novel percutaneous blood flow regulator: concept and initial experience. J Card Fail. 2019;25:932–934. doi: 10.1016/j.cardfail.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 29.Jorde U. Reducing renal venous pressure with the Doraya Renal Flow Regulator - early FIM experience. Paper presented at: TCT 2019; September 28, 2019; San Francisco, CA, USA.
- 30.Higginbotham M.B., Morris K.G., Williams R.S., McHale P.A., Coleman R.E., Cobb F.R. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. 1986;58:281–291. doi: 10.1161/01.res.58.2.281. [DOI] [PubMed] [Google Scholar]
- 31.Kaye D., Shah S.J., Borlaug B.A., et al. Effects of an interatrial shunt on rest and exercise hemodynamics: results of a computer simulation in heart failure. J Card Fail. 2014;20:212–221. doi: 10.1016/j.cardfail.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Borlaug B.A., Nishimura R.A., Sorajja P., Lam C.S., Redfield M.M. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fudim M., Boortz-Marx R.L., Ganesh A., et al. Splanchnic nerve block for chronic heart failure. J Am Coll Cardiol HF. 2020;8:742–752. doi: 10.1016/j.jchf.2020.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Abudiab M.M., Redfield M.M., Melenovsky V., et al. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15:776–785. doi: 10.1093/eurjhf/hft026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maeder M.T., Thompson B.R., Brunner-La Rocca H.P., Kaye D.M. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56:855–863. doi: 10.1016/j.jacc.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 36.Stout K.K., Daniels C.J., Aboulhosn J.A., et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:1494–1563. doi: 10.1016/j.jacc.2018.08.1028. [DOI] [PubMed] [Google Scholar]
- 37.Del Trigo M., Bergeron S., Bernier M., et al. Unidirectional left-to-right interatrial shunting for treatment of patients with heart failure with reduced ejection fraction: a safety and proof-of-principle cohort study. Lancet. 2016;387:1290–1297. doi: 10.1016/S0140-6736(16)00585-7. [DOI] [PubMed] [Google Scholar]
- 38.Rodés-Cabau J., Bernier M., Amat-Santos I.J., et al. Interatrial shunting for heart failure: early and late results from the first-in-human experience with the V-Wave system. J Am Coll Cardiol Intv. 2018;11:2300–2310. doi: 10.1016/j.jcin.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Feldman T., Mauri L., Kahwash R., et al. Transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction (REDUCE LAP-HF I [Reduce Elevated Left Atrial Pressure in Patients With Heart Failure]) Circulation. 2018;137:364–375. doi: 10.1161/CIRCULATIONAHA.117.032094. [DOI] [PubMed] [Google Scholar]
- 40.Rich J.D., Burns J., Freed B.H., Maurer M.S., Burkhoff D., Shah S.J. Meta-Analysis Global Group in Chronic (MAGGIC) Heart Failure Risk Score: validation of a simple tool for the prediction of morbidity and mortality in heart failure with preserved ejection fraction. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.009594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2012;33:1750–1757. doi: 10.1093/eurheartj/ehr254. [DOI] [PubMed] [Google Scholar]
- 42.Kaye D.M., Petrie M.C., McKenzie S., et al. Impact of an interatrial shunt device on survival and heart failure hospitalization in patients with preserved ejection fraction. ESC Heart Fail. 2019;6:62–69. doi: 10.1002/ehf2.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paitazoglou C., Özdemir R., Pfister R., et al. The AFR-PRELIEVE trial: a prospective, non-randomised, pilot study to assess the Atrial Flow Regulator (AFR) in heart failure patients with either preserved or reduced ejection fraction. EuroIntervention. 2019;15:403–410. doi: 10.4244/EIJ-D-19-00342. [DOI] [PubMed] [Google Scholar]
- 44.Lotan C. A Novel, Stentless RF-based shunt solution - the NoYa system. Paper presented at TCT 2019; September 26, 2019; San Francisco, CA, USA.
- 45.Simard T., Labinaz M., Zahr F., et al. Percutaneous atriotomy for levoatrial-to-coronary sinus shunting in symptomatic heart failure: first-in-human experience. J Am Coll Cardiol Intv. 2020;13:1236–1247. doi: 10.1016/j.jcin.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 46.Abraham W.T., Adamson P.B., Bourge R.C., et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 47.Shavelle D.M., Desai A.S., Abraham W.T., et al. Lower rates of heart failure and all-cause hospitalizations during pulmonary artery pressure-guided therapy for ambulatory heart failure. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.119.006863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindenfeld J., Zile M.R., Desai A.S., et al. Haemodynamic-guided management of heart failure (GUIDE-HF): a randomised controlled trial. Lancet. 2021;398:991–1001. doi: 10.1016/S0140-6736(21)01754-2. [DOI] [PubMed] [Google Scholar]
- 49.Mullens W., Sharif F., Dupont M., Rothman A.M.K., Wijns W. Digital health care solution for proactive heart failure management with the Cordella Heart Failure System: results of the SIRONA first-in-human study. Eur J Heart Fail. 2020;22:1912–1919. doi: 10.1002/ejhf.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perl L., Ben Avraham B., Vaknin-Assa H., Ben Gal T., Kornowski R. A rise in left atrial pressure detected by the V-LAP™ system for patients with heart failure during the coronavirus disease 2019 pandemic. ESC Heart Fail. 2020;7:4361–4366. doi: 10.1002/ehf2.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]