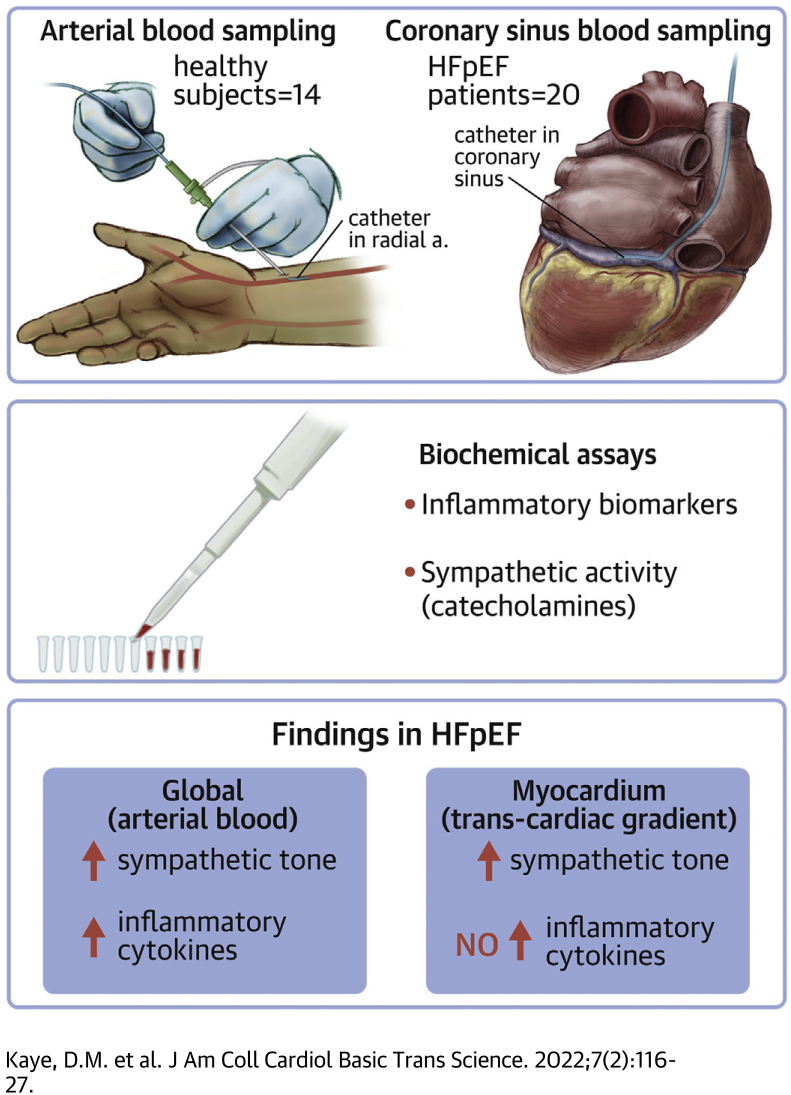
Visual Abstract
Key Words: cytokines, heart failure with preserved ejection fraction, sympathetic nervous system, transcardiac biomarker gradient
Abbreviations and Acronyms: BMI, body mass index; HFpEF, heart failure with preserved ejection fraction; NE, norepinephrine; PCWP, pulmonary capillary wedge pressure; SBP, systolic blood pressure; TCNE gradient, transcardiac NE gradient
Highlights
-
•
Although there is evidence for activation of the sympathetic nervous system and inflammatory pathways in peripheral blood samples, their relationship to myocardial activity is unknown.
-
•
Using arterial and coronary sinus blood sampling, we have shown the presence of cardiac and systemic sympathetic activation in HFpEF patients. However although systemic inflammatory activation was readily apparent, there was detectable myocardial release of inflammatory cytokines.
-
•
Key hemodynamic and demographic factors that typically cluster together in HFpEF appeared to drive cardiac sympathetic activation.
-
•
The data suggest that there may be a role for antiadrenergic therapies in selected HFpEF patients.
Summary
We have shown that systemic and cardiac sympathetic activation is present in heart failure with preserved ejection fraction (HFpEF) patients. Conversely, whereas systemic inflammatory activation was also detected in HFpEF, we did not detect local myocardial release of inflammatory cytokines. Activation of the sympathetic system correlated with both hemodynamic and demographic factors that characteristically cluster together in HFpEF. Together these data suggest that there may be a role for antiadrenergic therapies in certain HFpEF patients. The study does not implicate locally derived cytokines in the myocardial biology of HFpEF, although systemic sources may contribute to the global pathophysiology of HFpEF.
Heart failure with preserved ejection (HFpEF) continues to present one of the key contemporary diagnostic and therapeutic challenges in cardiovascular medicine. The growth in HFpEF prevalence is driven by several well known risk factors including hypertension, aging, and obesity acting in conjunction with a range of comorbidities including chronic kidney disease and chronic pulmonary disease. Various interventions proven to be effective in heart failure with reduced ejection fraction (HFrEF) have been conducted in HFpEF with limited or no impact on primary outcomes.1,2 For example, despite evidence for marked efficacy in HFrEF, neprilysin inhibition has been shown to benefit only a subset HFpEF patients with left ventricular ejection fraction (LVEF) at the lower range of normal.3,4 Recently, sodium-glucose transport protein 2 inhibitors were shown to exert favorable effects in HFpEF patients, principally due to an impact on heart failure (HF) hospitalization5; however, the mechanism responsible for this action remains uncertain.
The limited efficacy of many of the therapies tested in HFpEF could be explained by the possibility that the biological target of the relevant intervention may not be a key contributor to the HFpEF pathophysiology of HFpEF. In comparison with our understanding of the biology of HFrEF, current data regarding HFpEF is limited.2 In particular, data regarding the myocardial biology of human HFpEF is limited. Although some data regarding patterns of changes in key biomarkers are available in HFpEF patients, it is well known that direct correlations between peripheral plasma concentrations and myocardial release or myocardial pathophysiology cannot necessarily be drawn.6,7 A lack of therapeutic action for a particular drug class may also be explained, in part, by other factors including an inability to reverse an advanced HF phenotype or effectiveness in particular subphenotype.8 It is also likely that contributory comorbidities may also require concomitant attention. For example, it has been proposed that inflammation is a key contributor to the pathophysiology of HFpEF.9
Given the current uncertainty regarding aspects of the myocardial biology of HFpEF, we performed systemic and transcardiac blood sampling in HFpEF patients and healthy volunteers to investigate the relative activities of the sympathetic nervous system and inflammatory pathways. As such, our aim was to definitively characterize the role of these systems in HFpEF and to identify the key biological triggers for their activation.
Methods
Study population
The study included 20 patients with HFpEF and 14 healthy volunteers. HFpEF patients were referred to the Department of Cardiology, Alfred Hospital, for evaluation of exertional dyspnea with a LVEF >50% and in which HFpEF was suspected clinically. Exclusion criteria included significant coronary artery disease which had not been revascularized; moderate of greater aortic or mitral valve disease; infiltrative, restrictive, or hypertrophic myocardial disease; pericardial constriction; or significant right ventricular disease. Patients with significant pulmonary disease including chronic obstructive pulmonary disease were also excluded. The diagnosis of HFpEF was confirmed by the presence of a pulmonary capillary wedge pressure (PCWP) ≥15 mm Hg at rest or ≥25 mm Hg during symptom-limited exercise, according to published guidelines.10 Healthy volunteers were recruited from the general community and had no history of significant comorbidities including cardiovascular, pulmonary, other systemic diseases. The study was approved by the Alfred Hospital Research and Ethics Committee, and all participants provided written informed consent.
Cardiac catheterization
Studies were conducted in the nonfasted state and background medications were continued. A 7-F balloon-tipped pulmonary artery catheter (Edwards Lifesciences) was inserted through an introducer sheath placed in the right internal jugular or a brachial vein for measurement of right atrial pressure, pulmonary artery pressure (PAP), and PCWP. The wedge position was confirmed by fluoroscopy and pressure waveform, and the mean PCWP was measured at end-expiration. Cardiac output was measured using thermodilution with measurements taken in triplicate or from five readings for patients in atrial fibrillation. A 3-F radial or brachial artery cannula was inserted for blood pressure recording and blood sampling. Following baseline hemodynamic measurements, a catheter was positioned within the coronary sinus at least 2 cm proximal to the coronary sinus ostium, as confirmed fluoroscopically. Subsequently, simultaneous arterial and coronary sinus blood samples were drawn. Subjects then performed graded supine exercise with evaluation of PAPs and cardiac output as previously described.11
Biochemical assays
Arterial and coronary sinus plasma catecholamine concentrations were determined by high-performance liquid chromatography with electrochemical detection as previously described, with an intra-assay coefficient of variation of 5%.12 Plasma arterial and coronary sinus concentrations of 92 inflammatory proteins were determined using the Olink Inflammation biomarker panel (Olink Proteomics). This assay, reported in arbitrary units, uses proximity extension assay technology in which oligonucleotide-antibody probe pairs bind to specific protein targets, with subsequent detection by polymerase chain reaction, as previously described, with an intra-assay variability of up to 12%.13,14
Statistical analysis
Continuous normally distributed data are presented as mean ± SEM, whereas non-normal data are presented as the median with 25th and 75th percentiles (Q1-Q3). Between-group comparisons were performed using the Student t-test or Mann-Whitney test. Pearson's correlation coefficients were determined to examine the association between normally distributed data. Where indicated, an analysis of covariance was conducted to examine the influence of key covariates including systolic blood pressure (SBP), PCWP, age, and body mass index (BMI) on between-group differences on relevant dependent variables. Given that the distribution of several cytokines was not normally distributed, the Mann-Whitney test was applied to the comparison of all cytokine data. To investigate for differences between healthy subjects and HFpEF patients in the expression of inflammatory markers in arterial blood and in their transmyocardial gradients, we used two complementary approaches. We compared inflammatory patterns between healthy subjects and HFpEF using principal component analysis (PCA). PCA provides an opportunity to reduce the complexity of the large number of inflammatory cytokines assayed and to explore whether within cohort patterns could be identified. Analysis of covariance was used to investigate for differences in the relationship between the first and second principal components. In addition, between-group comparisons of individual inflammatory markers were conducted using unpaired Student t-test with correction for multiple comparisons using the false discovery rate of Benjamini and Hochberg. A q value of <0.05 was considered to be statistically significant. Statistical analysis was performed using SPSS (version 26, SPSS Inc) and R version 3.6.1.
Results
Baseline characteristics and hemodynamic profiles
As shown in Table 1, HFpEF patients were aged 70 ± 2 years compared to 53 ± 2 years in healthy subjects (P < 0.001) and the proportion of women (n = 13 of 20) was greater in HFpEF than in the healthy cohort (n = 4 of 14) (P = 0.037). HFpEF patients were heavier than the healthy subjects (34 ± 2 kg/m2 vs 26 ± 1 kg/m2, P = 0.001). Consistent with a diagnosis of HFpEF, atrial fibrillation, treated hypertension, and diabetes were prevalent comorbidities. HFpEF patients had evidence of significantly increased PAP and PCWP, with a nonsignificant trend towards lower cardiac index as shown in Table 1. HFpEF patients had poorer exercise tolerance (peak workload capacity: 50 ± 8 W vs 122 ± 13 W, P < 0.001). The peak exercise PCWP was markedly elevated in comparison to the healthy subjects (31 ± 1 vs 16 ± 1 mm Hg, P < 0.001).
Table 1.
Clinical Profiles
| Healthy Subjects | HFpEF | |
|---|---|---|
| Demographics | ||
| Age, y | 53 ± 2 | 70 ± 2a |
| Sex, M/F | 10/4 | 7/13 |
| BMI, kg/m2 | 26 ± 1 | 34 ± 2b |
| Hypertension, % | — | 65 |
| Atrial fibrillation, % | — | 55 |
| Diabetes, % | — | 25 |
| Coronary disease, % | — | 20 |
| ACE/ARB, % | — | 60 |
| Beta blocker, % | — | 35 |
| Echocardiography | ||
| LVEDVI, mL/m2 | 57 ± 3 | 55 ± 3 |
| LVEF, % | 62 ± 1 | 63 ± 1 |
| LV mass index | 72 ± 4 | 95 ± 6b |
| LA volume index | 30 ± 2 | 44 ± 3b |
| Hemodynamics | ||
| HR, beats/min | 62 ± 3 | 68 ± 3 |
| MAP, mm Hg | 92 ± 3 | 99 ± 5 |
| SBP, mm Hg | 134 ± 4 | 144 ± 5 |
| DBP, mm Hg | 74 ± 2 | 75 ± 4 |
| RAP, mm Hg | 6 ± 1 | 7 ± 1 |
| mPAP, mm Hg | 14 ± 1 | 22 ± 2a |
| sPAP, mm Hg | 22 ± 1 | 35 ± 3a |
| dPAP, mm Hg | 9 ± 1 | 14 ± 1b |
| PCWP, mm Hg | 9 ± 1 | 13 ± 1c |
| Cardiac index, L/min/m2 | 2.8 ± 0.2 | 2.5 ± 0.1 |
| Ex PCWP, mm Hg | 16 ± 1 | 31 ± 1a |
| Ex cardiac index, L/min/m2 | 7.3 ± 0.1 | 4.5 ± 0.1a |
Values are mean ± SEM or n/n.
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; BMI = body mass index; DBP = diastolic blood pressure; dPAP = diastolic pulmonary artery pressure; Ex = exercise; HFpEF = heart failure with preserved ejection fraction; HR = heart rate; LA = left atrial; LV = left ventricular; LVEDVI = left ventricular end-diastolic index; LVEF = left ventricular ejection fraction; MAP = mean arterial pressure; mPAP = mean pulmonary artery pressure; PCWP = pulmonary capillary wedge pressure; RAP = right arterial pressure; SBP=systolic blood pressure; sPAP = systolic pulmonary artery pressure.
P < 0.001 vs healthy subjects.
P < 0.01.
P < 0.05.
Systemic and cardiac sympathetic activity in HFpEF
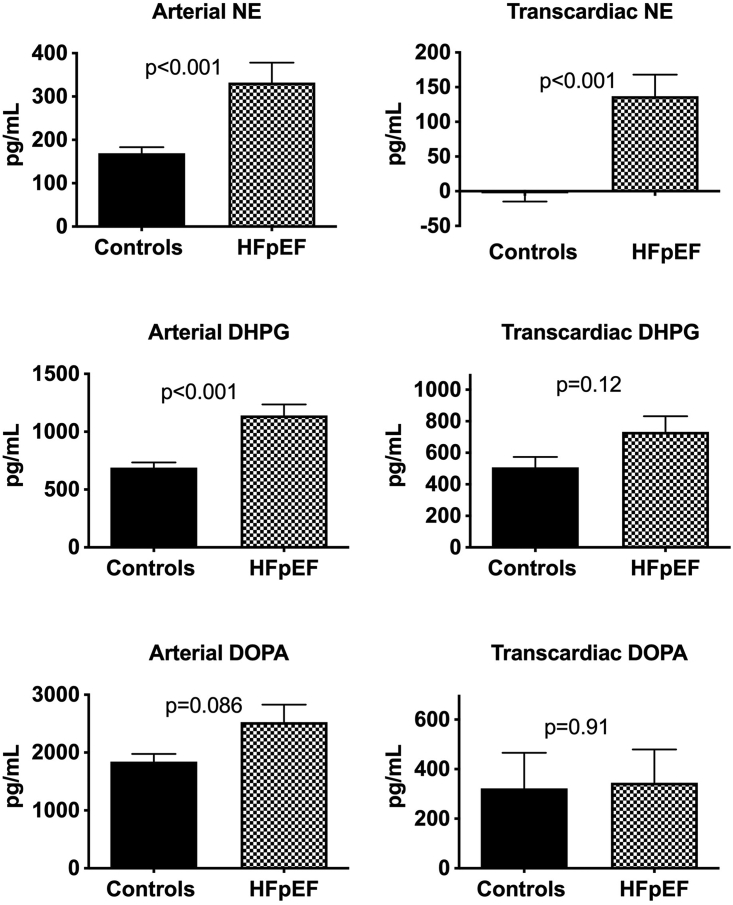
As shown in Figure 1, HFpEF patients displayed evidence of systemic sympathoexcitation as evidenced by an increase in the plasma arterial norepinephrine (NE) concentration compared to healthy subjects (331 ± 36 pg/mL vs 169 ± 14 pg/mL, P < 0.001). This finding was further supported by the presence of significantly greater plasma levels of dihydroxyphenylglycol (DHPG, a major intraneuronal metabolite of recaptured NE, P < 0.001) and a trend towards higher levels of dihydyroxyphenylalanine (DOPA, the neuronal NE precursor) (Figure 1). To evaluate the pattern of cardiac sympathetic activity in HFpEF we determined the transcardiac concentration gradients for NE (TCNE), DHPG, and DOPA. As shown in Figure 1, we identified evidence for significant activation of cardiac sympathetic nerves as reflected by a marked increase in the TCNE gradient. This finding was associated with a trend towards increased cardiac DHPG release, whereas the transcardiac DOPA gradient did not differ between groups.
Figure 1.
Systemic and Cardiac Sympathetic Activity in HFpEF
Bar graphs showing the plasma arterial concentration of norepinephrine (NE) and the transcardiac NE plasma concentration gradient and related metabolites dihydroxyphenylglycol (DHPG) and dihydyroxyphenylalanine (DOPA) in healthy subjects and heart failure with preserved ejection fraction (HFpEF) patients.
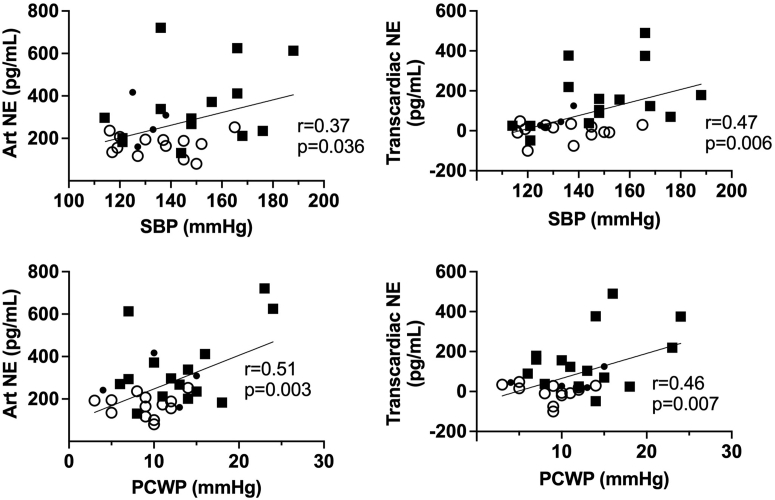
We next investigated the potential hemodynamic and demographic drivers for cardiac and sympathetic activation. As shown in Figure 2, the TCNE gradient was significantly correlated with the PCWP (r = 0.46, P = 0.007) and the arterial SBP (r = 0.47, P = 0.006). Given the close correlation between PCWP and mean PAP (r = 0.85, P < 0.001), we also identified a significant correlation between the TCNE gradient and mean PAP (r = 0.46, P = 0.009), although this was numerically less than that with the PCWP. We also found a stronger correlation between the TCNE gradient (measured at rest) and the exercise PCWP (r = 0.53, P = 0.002). The plasma arterial NE concentration was also significantly correlated with PCWP (r = 0.51, P = 0.003) and the SBP (r = 0.37, P = 0.036). In addition to the hemodynamic factors, the TCNE gradient and arterial NE concentration were significantly correlated with age (r = 0.51, P = 0.003 and r = 0.61, P < 0.001, respectively), whereas neither the TCNE gradient or arterial NE concentration were related to BMI (r = 0.29, P = 0.11 and r = 0.07, P = 0.70, respectively). Given the potential contribution of hemodynamic and demographic features that coexist with HFpEF, we conducted a multivariate analysis incorporating PCWP, SBP, age, BMI, and sex. Analysis of the determinants of the peripheral arterial NE plasma concentration identified only the PCWP as a significant contributor (F1,24 = 6.01, P = 0.022). In a similar analysis of the determinants of the TCNE gradient, we identified SBP (F1,24 = 5.55, P = 0.027), together with a borderline association with PCWP (F1,24 = 3.73, P = 0.065). There were no differences between males and females. Finally we investigated the effect of cardiac rhythm on sympathetic activity. HFpEF patients in atrial fibrillation (AF) compared to those in sinus rhythm (SR) had significantly elevated arterial NE levels (AF vs SR: 398 ± 56 pg/mL vs 238 ± 16 pg/mL, P = 0.019) and a significantly elevated TCNE gradient (AF vs SR: 192 ± 48 pg/mL vs 61 ± 23 pg/mL, P = 0.036). Furthermore, the arterial NE plasma levels and TCNE gradients of HFpEF patients in SR was also significantly greater than that in healthy subjects (P = 0.014 and P = 0.006, respectively).
Figure 2.
Physiologic Correlates of Sympathetic Activity in HFpEF
Scatterplots showing the correlations between hemodynamic parameters and plasma arterial concentration of NE and the transcardiac NE plasma concentration gradient healthy subjects and HFpEF patients. PCWP= pulmonary capillary wedge pressure; other abbreviations as in Figure 1.
Systemic and myocardial inflammation in HFpEF
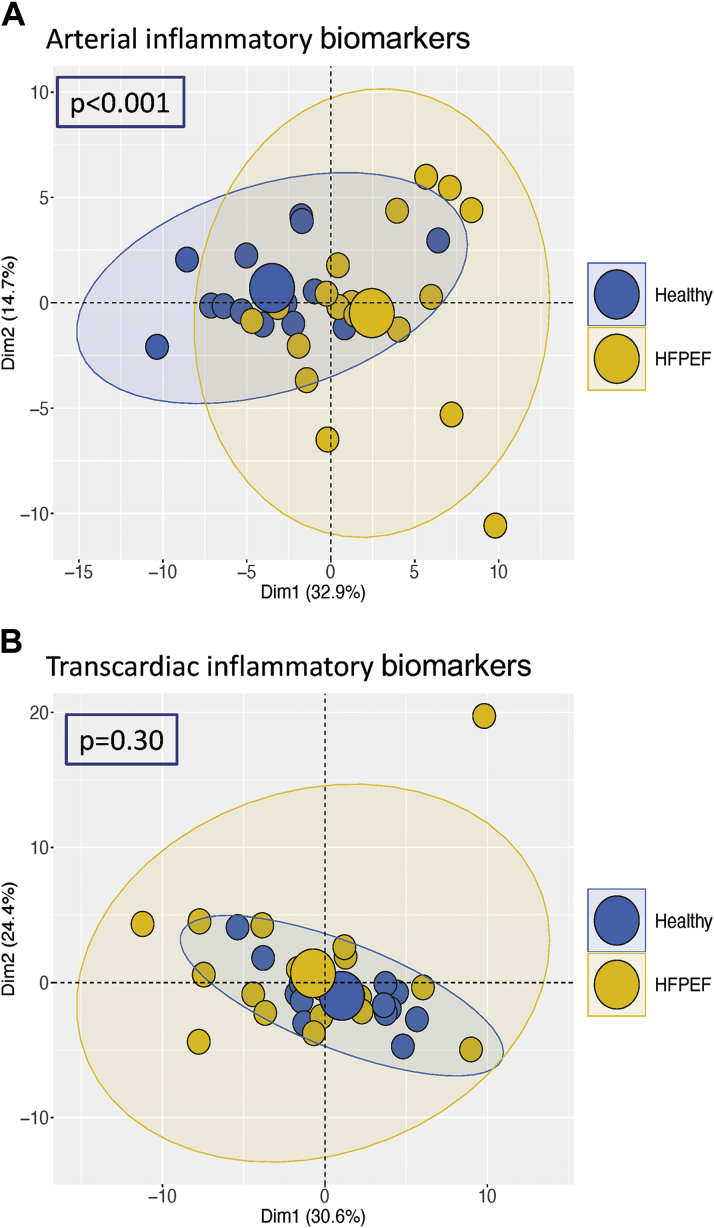
We measured the arterial and coronary sinus plasma concentrations of 92 biomarkers of inflammation using the Olink proteomic platform. For 16 biomarkers, plasma concentrations were below the level of detection of the assay in several patients; therefore, these were removed from the analysis, leaving a total of 76 biomarkers. As shown in Figure 3A, healthy subjects and HFpEF patients showed a significantly (P < 0.001) different pattern of inflammatory biomarker expression in arterial blood as determined by PCA. By contrast, the transcardiac gradient of the inflammatory biomarkers did not differ between healthy subjects and HFpEF (Figure 3B).
Figure 3.
Systemic and Myocardial Cytokine Profiles in HFpEF
(A,B) Principal component analysis plots of the profile of plasma arterial concentrations of inflammatory cytokines and of their transcardiac concentration gradients in healthy subjects and HFpEF patients.
As shown in Table 2, a total of 28 biomarkers of inflammation were demonstrated to be significantly elevated in the arterial plasma of HFpEF patients. By contrast, none of the transcardiac gradients of the inflammatory biomarkers in HFpEF patients were significantly different from that in healthy subjects. The magnitude of the average percentage change in the transcardiac gradient for the inflammatory markers was small (healthy subjects vs HFpEF: -9 ± 10% vs -2 ± 2%, P = 0.41). In an exploratory analysis, we investigated potential demographic, autonomic, and hemodynamic drivers of elevated arterial cytokines in HFpEF. Specifically, we selected those cytokines in which the arterial plasma levels were at least 50% greater than in healthy subjects and included interleukin (IL)-17 (73%), fibroblast growth factor (FGF)-5 (71%), IL-6 (55%), and FGF-23 (53%). As shown in Table 3, we identified a significant correlation between age and the levels of IL-6, IL-17, FGF-23, and FGF-5. IL-6 levels were also correlated with PCWP and the arterial NE concentration. BMI was only correlated with IL-6 levels from the cytokines. FGF-5 levels were higher in females when combining the study groups (0.46 ± 0.15 vs 0.33 ± 0.13, P = 0.037); however, in multivariable analysis combining all factors, this difference no longer persisted. Given the multiple univariate correlates of plasma IL-6 levels, we further conducted a multivariable analysis. In this analysis, PCWP was the only significant determinant of plasma IL-6 levels (F1,24 = 6.28, P = 0.006).
Table 2.
Significantly Upregulated Cytokines in HFpEF
| Cytokines | Healthy Subjects | HFpEF | q Value |
|---|---|---|---|
| FGF-23 | 2.48 (2.36-2.64) | 3.56 (3.27-4.61) | <0.0001 |
| CCL-20 | 5.31 (4.95-5.87) | 6.36 (6.12-7.51) | <0.0001 |
| IL-6 | 2.80 (2.19-3.23) | 3.94 (3.62-4.60) | <0.0001 |
| CXCL-10 | 9.08 (8.68-9.67) | 10.37 (9.91-10.69) | <0.0001 |
| FGF-5 | 0.21 (0.19-0.35) | 0.45 (0.39-0.54) | 0.0001 |
| DNER | 9.01 (8.81-9.06) | 8.65 (8.43-8.80) | 0.0001 |
| VEGFA | 9.51 (9.31-9.66) | 9.95 (9.72-10.38) | 0.0001 |
| IL-17C | 0.62 (0.56-0.86) | 1.04 (0.94-1.48) | 0.0003 |
| CSF-1 | 9.82 (9.69-9.99) | 10.08 (9.99-10.24) | 0.0005 |
| OPG | 9.38 (9.09-9.57) | 9.77 (9.58-9.97) | 0.0005 |
| IL-8 | 5.25 (4.56-5.57) | 6.08 (5.51-6.32) | 0.0006 |
| CDCP1 | 3.50 (3.32-3.97) | 4.21 (3.92-4.65) | 0.0007 |
| Beta-NGF | 0.86 (0.78-0.96) | 1.05 (1.01-1.18) | 0.0007 |
| CXCL9 | 6.83 (6.05-7.31) | 7.59 (7.01-8.46) | 0.0010 |
| PD-L1 | 6.43 (6.15-6.59) | 6.97 (6.68-7.27) | 0.0011 |
| HGF | 8.45 (8.16-8.76) | 8.94 (8.65-9.23) | 0.0011 |
| MCP-3 | 1.01 (0.73-1.57) | 1.77 (1.32-2.09) | 0.0021 |
| CXCL-11 | 7.34 (7.15-8.07) | 8.27 (7.89-9.10) | 0.0023 |
| MCP-4 | 12.91 (12.68-13.33) | 13.61 (13.29-14.26) | 0.0026 |
| CCL-19 | 8.78 (8.67-9.24) | 9.37 (9.01-9.86) | 0.0037 |
| CCL-3 | 5.06 (4.77-5.53) | 5.88 (5.35-6.36) | 0.0063 |
| CD40 | 11.26 (10.88-11.49) | 12.06 (11.44-12.40) | 0.0078 |
| CCL-11 | 6.96 (6.72-7.09) | 7.35 (7.05-7.77) | 0.0087 |
| TNFRSF9 | 6.26 (6.09-6.55) | 6.63 (6.38-7.00) | 0.0096 |
| TGF-α | 3.84 (3.70-3.97) | 4.00 (3.94-4.26) | 0.0118 |
| IL-12B | 5.85 (5.25-6.21) | 6.37 (5.86-6.67) | 0.0130 |
| Flt3L | 8.79 (8.69-9.13) | 9.19 (8.87-9.55) | 0.0158 |
| LIF-R | 3.09 (2.92-3.23) | 3.29 (3.08-3.45) | 0.0173 |
Values are median with 25th and 75th percentiles (Q1-Q3).
Beta-NGF = beta nerve growth factor; CCL = C-C motif chemokine; CD40 = cluster of differentiation 40; CDCP1 = CUB domain containing protein 1; CSF = colony stimulating factor; CXCL = C-X-C motif chemokine; DNER = delta and notch-like epidermal growth factor related receptor; FGF = fibroblast growth factor; Flt3L = FMS-like tyrosine kinase 3 ligand; HGF = hepatocyte growth factor; IL = interleukin; LIF-R = leukemia inhibitory factor receptor; MCP = monocyte chemotactic protein; OPG = osteoprotegerin; PD-L1 = programmed cell death 1 ligand 1; TGF = transforming growth factor; TNFRSF9 = tumor necrosis factor receptor superfamily member 9; VEGFA = vascular endothelial growth factor A.
Table 3.
Hemodynamic and Demographic Correlates of Systemic Inflammation
| IL-17 |
FGF5 |
IL-6 |
FGF23 |
|||||
|---|---|---|---|---|---|---|---|---|
| r | P Value | r | P Value | r | P Value | r | P Value | |
| BMI | -0.01 | 0.98 | 0.22 | 0.22 | 0.45 | 0.007 | 0.25 | 0.15 |
| SBP | 0.27 | 0.12 | 0.22 | 0.22 | 0.18 | 0.92 | -0.06 | 0.74 |
| Age | 0.39 | 0.021 | 0.47 | 0.005 | 0.46 | 0.006 | 0.64 | <0.001 |
| PCWP | -0.03 | 0.86 | 0.16 | 0.35 | 0.53 | 0.001 | 0.42 | 0.012 |
| Arterial plasma NE | 0.28 | 0.13 | 0.32 | 0.08 | 0.50 | 0.004 | 0.24 | 0.20 |
Statistically significant correlations are in bold.
Abbreviations as in Table 1.
Discussion
In the present study, we have shown that HFpEF is characterized by both cardiac and systemic sympathetic activation, together with peripheral but not myocardial inflammatory activation. Our cohort of HFpEF patients were characterized by invasive exercise hemodynamics, which is considered to be the gold standard.10 These data highlight the fact that peripheral measures of a particular biological process cannot necessarily be construed as representing the activity of their respective pathways within the myocardium. It is possible that very low levels of cytokine release from the myocardium below the limits of detection occurred, although the biological relevance of this would be uncertain. As such, our data provide new insights into the mechanisms that contribute to the myocardial pathogenesis of HFpEF. The relative paucity of studies that have directly characterized the myocardial biology of HFpEF by tissue or coronary sinus blood sampling was recently highlighted in an HFpEF taskforce report.2
The clinical features of HFpEF represent the net influences a complex set of physiologic abnormalities, both cardiac and extracardiac. Of paramount importance, measures of increased left atrial pressure, particularly during physical activity, are directly correlated with symptoms and outcomes, including cardiovascular mortality.15,16 The mechanisms responsible for elevated filling pressures include increases in passive ventricular and atrial stiffness, impaired active ventricular relaxation that are further accentuated by the impact of increased arterial stiffness, and a failure of exercise-mediated vasodilatation.1,11,17,18 To date, clinical trials of a multitude of interventions have not proven to significantly alter outcomes in HFpEF, suggesting that these treatments are not directed towards relevant cellular or physiologic targets. For example, it has been shown that the plasma levels of aldosterone, brain natriuretic peptide, and plasma renin activity are commonly within the normal range in patients with HFpEF, potentially explaining the limited impact of interventions directed towards these pathways.19,20
Our study contributes important new data regarding cardiac sympathetic activity in HFpEF patients, and its potential contribution to disease pathophysiology. Sympathetic neural drive shows substantial regional heterogeneity, limiting the ability of peripheral measures of sympathetic activity to reflect organ sympathetic tone.21 This underscores the importance of our assessment of cardiac sympathetic drive. Activation of the sympathetic nervous system has been well demonstrated in HFrEF and hypertension, and in both cases the magnitude of sympathoexcitation has been correlated with clinical outcomes.22, 23, 24 In this study we measured, for the first time, the net TCNE in HFpEF patients and demonstrated it to be significantly greater than that in healthy subjects, signifying the presence of cardiac sympathetic activation. It is important to appreciate that the net TCNE reflects the balance between local release from the sympathetic synaptic cleft to plasma, local uptake–mediated NE reuptake and flow.21 Although in the current study we did not measure coronary sinus blood flow, it has been previously shown that resting myocardial blood flow is greater in HFpEF.25 As such, it is unlikely that differences in myocardial blood flow account for the increased TCNE in HFpEF patients. Similarly, our results cannot be explained by impaired reuptake of NE from sympathetic nerve endings. The current study revealed somewhat higher levels of DHPG, the intraneuronal metabolite of NE. It has been previously shown by our group that impaired reuptake of NE is associated with a decreased transcardiac DHPG gradient.26 In the present study, transcardiac DOPA levels did not differ between healthy subjects and HFpEF. Although previous studies in HFrEF patients have indicated net release of DOPA from the heart, the net release of DOPA from sympathetic neurons is estimated to be small, suggesting that net cardiac DOPA release is only likely to be detected at high levels of neuronal NE synthesis.26,27
Our finding of elevated cardiac sympathetic drive raises 2 key questions. 1) What is the trigger for elevated cardiac sympathetic drive in HFpEF patients? 2) What are the consequences? In univariate analysis we found significant correlations between the TCNE gradient and PCWP, age, and SBP. In a multivariable analysis, the association with SBP remained significant, with a borderline relationship with PCWP, although this analysis was likely influenced by the limited sample size. Of importance, in this analysis the diagnosis of HFpEF per se was not an independent determinant of cardiac sympathetic tone, suggesting that relevant hemodynamic factors are key determinants of cardiac adrenergic drive in HFpEF. This relationship between the PCWP and cardiac NE gradient may be mechanistically explained by the presence of left atrial (LA) and pulmonary venous stretch receptors which are known to reflexively activate cardiac sympathetic nerves.28 Consistent with this hypothesis, we have previously shown that cardiopulmonary baroreceptor unloading reduces cardiac adrenergic drive in HFrEF patients.29 In this context, a pathophysiologic hallmark of HFpEF is the rapid increase in LA pressure during physical activity.11,30 We also observed increased cardiac and systemic adrenergic drive in HFpEF patients in AF compared to those in SR; however, HFpEF patients in SR also exhibited greater sympathetic activity than the healthy volunteers. Whether the relationship between LA pressure and cardiac adrenergic activity drives a more marked exercise-mediated increase in cardiac sympathetic tone in HFpEF patients is not known. Of clinical relevance, increased cardiac sympathetic tone might be expected to contribute to the development of atrial arrhythmias.31 In a similar context, the role of excess cardiac sympathetic drive as a trigger for ventricular arrhythmias is also well known, and it is recognized that sudden cardiac death contributes to a significant proportion of the cardiovascular deaths in HFpEF patients.32,33 The complex interrelationship between SBP and cardiac sympathetic activity is consistent with previous studies in which we showed that cardiac sympathetic drive was increased in hypertensives with left ventricular hypertrophy (LVH) but not in those without LVH despite similar blood pressure.34 We also found that the correlation between peak exercise PCWP and the TCNE gradient was numerically greater than that at rest. This suggests that the level of cardiac sympathetic tone might be set by an integrated hemodynamic input in conjunction with acute hemodynamic stimuli.
In the present study we have also shown that the peripheral plasma NE concentration was increased in HFpEF patients compared with healthy subjects. Previous studies have generally shown that plasma NE levels are modestly elevated in HFpEF, although this finding has not been uniform possibly due variation in subject inclusion definitions, as reviewed by Badrov et al.35 In univariate analysis we found that SBP, PCWP, and age were all significantly associated with the arterial NE concentration; whereas, in multivariate analysis only, PCWP was the only significant correlate and, consistent with the analysis for TCNE release, we did not find HFpEF diagnosis per se to be an independent driver of the plasma NE concentration. Keir et al35 recently examined the influence of age and sex on muscle sympathetic nerve activity (MSNA). These investigators found that MSNA increased with age in a nonlinear manner, with marked differences when comparing subjects aged 30 years versus 70 years. Similarly, our previous studies using radiotracer methodology showed that age may influence cardiac sympathetic tone when comparing a wide range of ages.23 Keir et al35 also evaluated the influence of sex, finding no difference in MSNA for those older than the age of 50 years, whereas significant differences were observed particularly in subjects aged 20 to 30 years.36 Taken together, our data are consistent with the notion that many of the factors that are associated with HFpEF, including hemodynamic and demographic, lead to the autonomic phenotype observed in HFpEF. A much larger study with fully matched comorbidities in the absence of HFpEF symptoms would be required to fully elucidate the contribution of each potential factor. Given the invasive nature of our study, this would be particularly challenging.
The use of antiadrenergic therapies in HFpEF remains controversial, and, in particular, the role of beta blockade is uncertain. Although observational studies have suggested potential benefit, this has not been supported by randomized trials.37 The relative importance of myocardial beta receptors in the pathophysiology of HFpEF is unclear. By contrast, alpha receptors have been implicated in that pathogenesis of cardiac fibrosis in the context of excess sympathetic drive.38 The utility of centrally acting sympathoinhibitory drugs has not been investigated in hypertensive HFpEF patients.
Elevated peripheral levels of several inflammatory cytokines, including members of the IL, tumor necrosis factor, and chemokine families are well-described in HFpEF, consistent with the present findings.6,39 A key finding of our study is that the myocardium does not appear to release cytokines in HFpEF. As such, these data provide further insights into origins of inflammation in HFpEF and into its potential role in the pathophysiology of HFpEF.
In the present study we showed a positive correlation between age with several peripheral biomarkers of inflammation including IL-6, IL-17, FGF-5, and FGF-23. Our finding of a 54% increase in plasma IL-6 levels in HFpEF patients is consistent with prior studies in HFpEF patients.39,40 Although the healthy subjects were significantly younger than HFpEF patients, the difference in IL-6 levels could not be explained on the basis of age alone in a multivariable analysis. The relationship between aging and inflammation is well recognized; however, the underpinning mechanism(s) remain speculative.41 Proposed contributing mechanisms for the process of “inflammageing” include visceral obesity, gut dysbiosis, chronic infection, and altered immune regulation.41 In addition to age per se, many of the putative contributors cluster in patients with HFpEF. For example, we have recently shown the presence of gut dysbiosis in HFpEF patients and epidemiologic data clearly indicate the close association between obesity the prevalence of HFpEF.42,43 In the current study, we found a close relationship between BMI and IL-6 levels, and expression of IL-6 in adipose tissue has been shown to be increased at the mRNA and protein level in obesity.44,45 It has been shown that the abundance of intra-abdominal visceral adipose tissue is strongly predictive of HFpEF risk, leading to speculation that activation of inflammatory pathways contributes to the pathogenesis of HFpEF rather than as an epiphenomenon.46, 47, 48 Consistent with this, patients with nonmetabolic, noncardiovascular causes of inflammation such as rheumatologic disorders also have evidence of diastolic dysfunction consistent with the notion that inflammatory cytokines may, in part, drive the development of HFpEF.48 Interestingly, visceral adiposity is also associated with sympathetic activation, as we also observed in HFpEF patients.49
From a mechanistic perspective, IL-6 is a cytokine with a pleiotropic repertoire of actions, including stimulation of fibroblast proliferation and the biosynthesis of extracellular matrix proteins.50,51 Genetic deletion of IL-6 attenuated myocardial fibrosis in hypertensive mice secondary to angiotensin II/high salt.52 IL-6 signaling is complex and involves its binding to a membrane signaling complex that comprises an IL-6 receptor and the gp130 transmembrane protein. This mechanism is negatively regulated by a circulating complex formed by IL-6 together soluble fractions of gp130 and IL-6R.53 Whether the increased circulating levels of IL-6 are definitively pathogenic in HFpEF is unknown. However, the plasma concentrations observed in HFpEF patients have been shown to increase expression of matrix metalloproteinase (MMP)-2 in cell culture studies.54
In addition to aging and obesity as likely drivers of inflammation, we observed significant correlations between the peripheral plasma levels of IL-6 and FGF-23 with PCWP. This observation raises the possibility that the lung may release cytokines in HFpEF under the influence of elevated filling pressures. This observation is consistent with recent studies of pulmonary cytokine expression in experimental HF.55 It is also possible that a more complex balance between hemodynamics, obesity, and inflammation is present as suggested recently by Sorimachi et al.56 We observed elevated levels of FGF-23 in HFpEF patients, and the plasma levels of FGF-23 were found to correlate significantly with PCWP. FGF-23 has previously been shown to be associated with increased risk of cardiovascular disease, pulmonary hypertension, LVH, AF, and mortality.57, 58, 59 This finding suggests the concept that the lung itself might be an important source of FGF-23 under the influence of elevated pulmonary pressures.
In the current study, we did not detect any evidence of inflammatory cytokine release from the myocardium in HFpEF patients. Recently it has been proposed that epicardial adipose tissue (EAT) may play a role in the pathophysiology of HFpEF either by a direct mechanical effect or by the release of inflammatory cytokines in a manner similar to visceral adipose tissue.60 Previously, it has been shown that levels of expression of inflammatory cytokines including IL1-b, IL-6, and tumor necrosis factor (TNF)-α at the mRNA and protein level are elevated in EAT compared to peripheral subcutaneous fat.61 However, these studies did not investigate the levels of inflammatory cytokines within the myocardium per se. Although the presence of metabolically active EAT in apposition with epicardial coronary arteries acting as an amplifier of vascular inflammation is widely accepted, the influence of EAT as a direct paracrine source of cytokines which drive myocardial remodeling in HFpEF is more speculative. Our findings are consistent with prior studies performed in patients with coronary disease in which no transcardiac gradient of IL-6 and TNF-α was detectable.62, 63, 64 Given that the myocardium and EAT share a common microcirculation, we would have predicted that a significant transmyocardial gradient would be detectable in the presence of significant release of cytokines by the EAT.65 We did not assess the volume of EAT in this study; however, our HFpEF patient profiles are consistent with other studies in which increased EAT volumes have been measured in HFpEF patients.66 Nevertheless, we cannot exclude the presence of a small concentration of gradients that lie beneath the sensitivity of the aptamer assay, although previous studies used an enzyme-linked immunosorbent assay–based assay. Similarly, we did not obtain myocardial biopsy specimens. Although these studies have shown the presence of inflammatory cells such as CD68 cells, the level of increase above that seen in healthy subjects is only modest.67 We cannot exclude the possibility that cytokines released by EAT are drained by myocardial and pericardial lymphatics, although the myocardial lymph flow rate is relatively small.68
Study limitations
The sample size in this study was relatively small; however, we conducted a detailed invasive study which included healthy normal volunteers. Despite the small sample size, we were able to detect evidence of cardiac sympathetic activation and systemic inflammation compared to the healthy volunteers. We assayed a large number of cytokines and used a PCA-based approach to reduce the dimensionality of the data. Our between-group comparisons were appropriately statistically corrected for the number of between-group comparisons. As observed in the current study, HFpEF patients are characterized by key hemodynamic features in conjunction with a cluster of comorbidities including aging, hypertension, obesity, AF, diabetes, and female sex which may all drive both systemic and cardiac sympathetic activity and systemic inflammation. It is likely that the pathophysiology of HFpEF results from the combined influence of all of these factors to a certain extent. Because of the challenging nature of recruiting healthy volunteers for invasive studies, we were not able to match subjects appropriately with HFpEF patients to elucidate the contribution of each contributing comorbidity individually.
Conclusions
The current study provides new insights into the complex nature of HFpEF pathophysiology. In particular, we showed the presence of elevated cardiac sympathetic activity in HFpEF patients and extend prior evidence supporting the presence of systemic sympathetic and inflammatory activation. By contrast, we did not find evidence in support of inflammatory activation in the myocardium of HFpEF patients as evaluated by transcardiac cytokine gradients. Our findings may offer potential insights into mechanisms that might underpin the recently reported positive effects of sodium-glucose transport protein 2 inhibition in HFpEF patients.5 The current findings are likely to reflect the integrated effects of the typical cluster of comorbidities that associate with HFpEF, acting together with the characteristic hemodynamic profile. Finally, the finding of cardiac sympathetic activation in HFpEF patients requires further investigation as a potential contributor to cardiovascular outcomes and therefore it may be a therapeutic target.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Advances in the management of HF, particularly HFrEF, are the result of detailed mechanistic studies. Although HFpEF accounts for approximately 50% of all HF cases, limited human data is available regarding local myocardial pathophysiology. The current study shows that HFpEF is characterized by activation of the cardiac sympathetic nervous system; however, we did not detect evidence for local myocardial inflammation. Our findings suggest that a re-evaluation of the role of antiadrenergic therapies may be warranted in HFpEF patients.
TRANSLATIONAL OUTLOOK: Left ventricular remodeling is a fundamental feature of HF. The local pathophysiological inputs that drive this process in HFpEF have not been investigated in detail. Coordinated investigation of the autonomic, inflammatory, and metabolic provide of the myocardium in HFpEF patients will allow the identification and manipulation of therapeutic targets that can subsequently be translated into clinical trials.
Funding Support and Author Disclosures
This work was supported by a National Health & Medical Research Council (NHMRC) of Australia Fellowship support to Dr Kaye. Dr Marques is supported by a National Heart Foundation Future Leader Fellowship and National Heart Foundation Grants. The Baker Heart & Diabetes Institute is supported in part by the Victorian Government's Operational Infrastructure Support Program. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Pfeffer M.A., Shah A.M., Borlaug B.A. Heart failure with preserved ejection fraction in perspective. Circ Res. 2019;124:1598–1617. doi: 10.1161/CIRCRESAHA.119.313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah S.J., Borlaug B.A., Kitzman D.W., et al. Research priorities for heart failure with preserved ejection fraction: National Heart, Lung, and Blood Institute Working Group Summary. Circulation. 2020;141:1001–1026. doi: 10.1161/CIRCULATIONAHA.119.041886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMurray J.J., Packer M., Desai A.S., et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 4.Solomon S.D., McMurray J.J.V., Anand I.S., et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]
- 5.Anker S.D., Butler J., Filippatos G., et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 6.Tromp J., Khan M.A., Klip I.T., et al. Biomarker profiles in heart failure patients with preserved and reduced ejection fraction. J Am Heart Assoc. 2017;6(4) doi: 10.1161/JAHA.116.003989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaye D.M., Mariani J.A., van Empel V., Maeder M.T. Determinants and implications of elevated soluble ST2 levels in heart failure. Int J Cardiol. 2014;176:1242–1243. doi: 10.1016/j.ijcard.2014.07.206. [DOI] [PubMed] [Google Scholar]
- 8.Shah S.J., Katz D.H., Selvaraj S., et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–279. doi: 10.1161/CIRCULATIONAHA.114.010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paulus W.J., Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 10.McDonagh T.A., Metra M., Adamo M., et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 11.Maeder M.T., Thompson B.R., Brunner-La Rocca H.P., Kaye D.M. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56:855–863. doi: 10.1016/j.jacc.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 12.Lambert G.W., Jonsdottir I.H. Influence of voluntary exercise on hypothalamic norepinephrine. J Appl Physiol (1985) 1998;85:962–966. doi: 10.1152/jappl.1998.85.3.962. [DOI] [PubMed] [Google Scholar]
- 13.Lundberg M., Eriksson A., Tran B., Assarsson E., Fredriksson S. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res. 2011;39:e102. doi: 10.1093/nar/gkr424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assarsson E., Lundberg M., Holmquist G., et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014;9 doi: 10.1371/journal.pone.0095192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolsk E., Kaye D., Borlaug B.A., et al. Resting and exercise haemodynamics in relation to six-minute walk test in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2018;20:715–722. doi: 10.1002/ejhf.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorfs S., Zeh W., Hochholzer W., et al. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3103–3112. doi: 10.1093/eurheartj/ehu315. [DOI] [PubMed] [Google Scholar]
- 17.Telles F., Nanayakkara S., Evans S., et al. Impaired left atrial strain predicts abnormal exercise haemodynamics in heart failure with preserved ejection fraction. Eur J Heart Fail. 2019;21(4):495–505. doi: 10.1002/ejhf.1399. [DOI] [PubMed] [Google Scholar]
- 18.Nanayakkara S., Telles F., Beale A.L., et al. Relationship of degree of systolic dysfunction to variations in exercise capacity and hemodynamic status in HFpEF. J Am Coll Cardiol Img. 2019;13(2 Pt 1):528–530. doi: 10.1016/j.jcmg.2019.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Vergaro G., Aimo A., Prontera C., et al. Sympathetic and renin-angiotensin-aldosterone system activation in heart failure with preserved, mid-range and reduced ejection fraction. Int J Cardiol. 2019;296:91–97. doi: 10.1016/j.ijcard.2019.08.040. [DOI] [PubMed] [Google Scholar]
- 20.Van Veldhuisen D.J., Linssen G.C.M., Jaarsma T., et al. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61:1498–1506. doi: 10.1016/j.jacc.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 21.Esler M., Jennings G., Lambert G., Meredith I., Horne M., Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiol Rev. 1990;70:963–985. doi: 10.1152/physrev.1990.70.4.963. [DOI] [PubMed] [Google Scholar]
- 22.Kaye D.M., Lefkovits J., Jennings G.L., Bergin P., Broughton A., Esler M.D. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol. 1995;26:1257–1263. doi: 10.1016/0735-1097(95)00332-0. [DOI] [PubMed] [Google Scholar]
- 23.Kaye D., Esler M. Sympathetic neuronal regulation of the heart in aging and heart failure. Cardiovasc Res. 2005;66:256–264. doi: 10.1016/j.cardiores.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Esler M., Rumantir M., Kaye D., Lambert G. The sympathetic neurobiology of essential hypertension: disparate influences of obesity, stress, and noradrenaline transporter dysfunction? Am J Hypertens. 2001;14:139S–146S. doi: 10.1016/s0895-7061(01)02081-7. [DOI] [PubMed] [Google Scholar]
- 25.AbouEzzeddine O.F., Kemp B.J., Borlaug B.A., et al. Myocardial energetics in heart failure with preserved ejection fraction. Circ Heart Fail. 2019;12 doi: 10.1161/CIRCHEARTFAILURE.119.006240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisenhofer G., Esler M.D., Meredith I.T., et al. Sympathetic nervous function in human heart as assessed by cardiac spillovers of dihydroxyphenylglycol and norepinephrine. Circulation. 1992;85:1775–1785. doi: 10.1161/01.cir.85.5.1775. [DOI] [PubMed] [Google Scholar]
- 27.Kaye D.M., Lambert G.W., Lefkovits J., Morris M., Jennings G., Esler M.D. Neurochemical evidence of cardiac sympathetic activation and increased central nervous system norepinephrine turnover in severe congestive heart failure. J Am Coll Cardiol. 1994;23:570–578. doi: 10.1016/0735-1097(94)90738-2. [DOI] [PubMed] [Google Scholar]
- 28.Kurz M.A., Wead W.B., Roberts A.M. Reflex inotropic responses to distension of left atrium or pulmonary veins. Am J Physiol. 1990;258:H121–H126. doi: 10.1152/ajpheart.1990.258.1.H121. [DOI] [PubMed] [Google Scholar]
- 29.Kaye D.M., Jennings G.L., Dart A.M., Esler M.D. Differential effect of acute baroreceptor unloading on cardiac and systemic sympathetic tone in congestive heart failure. J Am Coll Cardiol. 1998;31:583–587. doi: 10.1016/s0735-1097(97)00525-1. [DOI] [PubMed] [Google Scholar]
- 30.Borlaug B.A., Nishimura R.A., Sorajja P., Lam C.S.P., Redfield M.M. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen P.S., Chen L.S., Fishbein M.C., Lin S.F., Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014;114:1500–1515. doi: 10.1161/CIRCRESAHA.114.303772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen M.J., Zipes D.P. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. 2014;114:1004–1021. doi: 10.1161/CIRCRESAHA.113.302549. [DOI] [PubMed] [Google Scholar]
- 33.Vaduganathan M., Patel R.B., Michel A., et al. Mode of death in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2017;69:556–569. doi: 10.1016/j.jacc.2016.10.078. [DOI] [PubMed] [Google Scholar]
- 34.Schlaich M.P., Kaye D.M., Lambert E., Sommerville M., Socratous F., Esler M.D. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation. 2003;108:560–565. doi: 10.1161/01.CIR.0000081775.72651.B6. [DOI] [PubMed] [Google Scholar]
- 35.Badrov M.B., Mak S., Floras J.S. Cardiovascular autonomic disturbances in heart failure with preserved ejection fraction. Can J Cardiol. 2021;37:609–620. doi: 10.1016/j.cjca.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Keir D.A., Badrov M.B., Tomlinson G., et al. Influence of sex and age on muscle sympathetic nerve activity of healthy normotensive adults. Hypertension. 2020;76:997–1005. doi: 10.1161/HYPERTENSIONAHA.120.15208. [DOI] [PubMed] [Google Scholar]
- 37.Bavishi C., Chatterjee S., Ather S., Patel D., Messerli F.H. Beta-blockers in heart failure with preserved ejection fraction: a meta-analysis. Heart Fail Rev. 2015;20:193–201. doi: 10.1007/s10741-014-9453-8. [DOI] [PubMed] [Google Scholar]
- 38.Perlini S., Palladini G., Ferrero I., et al. Sympathectomy or doxazosin, but not propranolol, blunt myocardial interstitial fibrosis in pressure-overload hypertrophy. Hypertension. 2005;46:1213–1218. doi: 10.1161/01.HYP.0000185689.65045.4c. [DOI] [PubMed] [Google Scholar]
- 39.Abernethy A., Raza S., Sun J.L., et al. Pro-inflammatory biomarkers in stable versus acutely decompensated heart failure with preserved ejection fraction. J Am Heart Assoc. 2018;7(8) doi: 10.1161/JAHA.117.007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanders-van Wijk S., van Empel V., Davarzani N., et al. Circulating biomarkers of distinct pathophysiological pathways in heart failure with preserved vs. reduced left ventricular ejection fraction. Eur J Heart Fail. 2015;17:1006–1014. doi: 10.1002/ejhf.414. [DOI] [PubMed] [Google Scholar]
- 41.Ferrucci L., Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beale A.L., O'Donnell J.A., Nakai M.E., et al. The gut microbiome of heart failure with preserved ejection fraction. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.020654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Owan T.E., Hodge D.O., Herges R.M., Jacobsen S.J., Roger V.L., Redfield M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 44.Berg A.H., Scherer P.E. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 45.Carey A.L., Bruce C.R., Sacchetti M., et al. Interleukin-6 and tumor necrosis factor-alpha are not increased in patients with Type 2 diabetes: evidence that plasma interleukin-6 is related to fat mass and not insulin responsiveness. Diabetologia. 2004;47:1029–1037. doi: 10.1007/s00125-004-1403-x. [DOI] [PubMed] [Google Scholar]
- 46.Rao V.N., Zhao D., Allison M.A., et al. Adiposity and incident heart failure and its subtypes: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol HF. 2018;6:999–1007. doi: 10.1016/j.jchf.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kitzman D.W., Nicklas B.J. Pivotal role of excess intra-abdominal adipose in the pathogenesis of metabolic/obese HFpEF. J Am Coll Cardiol HF. 2018;6:1008–1010. doi: 10.1016/j.jchf.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Packer M., Lam C.S.P., Lund L.H., Maurer M.S., Borlaug B.A. Characterization of the inflammatory-metabolic phenotype of heart failure with a preserved ejection fraction: a hypothesis to explain influence of sex on the evolution and potential treatment of the disease. Eur J Heart Fail. 2020;22:1551–1567. doi: 10.1002/ejhf.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alvarez G.E., Beske S.D., Ballard T.P., Davy K.P. Sympathetic neural activation in visceral obesity. Circulation. 2002;106:2533–2536. doi: 10.1161/01.cir.0000041244.79165.25. [DOI] [PubMed] [Google Scholar]
- 50.Chou C.H., Hung C.S., Liao C.W., et al. IL-6 trans-signalling contributes to aldosterone-induced cardiac fibrosis. Cardiovasc Res. 2018;114:690–702. doi: 10.1093/cvr/cvy013. [DOI] [PubMed] [Google Scholar]
- 51.Leicht M., Briest W., Zimmer H.G. Regulation of norepinephrine-induced proliferation in cardiac fibroblasts by interleukin-6 and p42/p44 mitogen activated protein kinase. Mol Cell Biochem. 2003;243:65–72. doi: 10.1023/a:1021655023870. [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez G.E., Rhaleb N.E., D'Ambrosio M.A., et al. Deletion of interleukin-6 prevents cardiac inflammation, fibrosis and dysfunction without affecting blood pressure in angiotensin II-high salt-induced hypertension. J Hypertens. 2015;33:144–152. doi: 10.1097/HJH.0000000000000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones S.A., Jenkins B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018;18:773–789. doi: 10.1038/s41577-018-0066-7. [DOI] [PubMed] [Google Scholar]
- 54.Sun W., Liu D.B., Li W.W., et al. Interleukin-6 promotes the migration and invasion of nasopharyngeal carcinoma cell lines and upregulates the expression of MMP-2 and MMP-9. Int J Oncol. 2014;44:1551–1560. doi: 10.3892/ijo.2014.2323. [DOI] [PubMed] [Google Scholar]
- 55.Uray K.S., Peng Z., Cattano D., Eltzschig H.K., Doursout M.F. Development of pulmonary fibrosis after heart failure induced by elevated left atrial pressure. Am J Transl Res. 2020;12:4639–4647. [PMC free article] [PubMed] [Google Scholar]
- 56.Sorimachi H., Obokata M., Takahashi N., et al. Pathophysiologic importance of visceral adipose tissue in women with heart failure and preserved ejection fraction. Eur Heart J. 2021;42:1595–1605. doi: 10.1093/eurheartj/ehaa823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stohr R., Schuh A., Heine G.H., Brandenburg V. FGF23 in cardiovascular disease: innocent bystander or active mediator? Front Endocrinol (Lausanne) 2018;9:351. doi: 10.3389/fendo.2018.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mehta R., Cai X., Lee J., et al. Association of fibroblast growth factor 23 with atrial fibrillation in chronic kidney disease, from the Chronic Renal Insufficiency Cohort Study. JAMA Cardiol. 2016;1:548–556. doi: 10.1001/jamacardio.2016.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bouzina H., Hesselstrand R., Radegran G. Higher plasma fibroblast growth factor 23 levels are associated with a higher risk profile in pulmonary arterial hypertension. Pulm Circ. 2019;9 doi: 10.1177/2045894019895446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borlaug B.A., Reddy Y.N.V. The role of the pericardium in heart failure: implications for pathophysiology and treatment. J Am Coll Cardiol HF. 2019;7:574–585. doi: 10.1016/j.jchf.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mazurek T., Zhang L., Zalewski A., et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 62.Shimabukuro M., Hirata Y., Tabata M., et al. Epicardial adipose tissue volume and adipocytokine imbalance are strongly linked to human coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2013;33:1077–1084. doi: 10.1161/ATVBAHA.112.300829. [DOI] [PubMed] [Google Scholar]
- 63.Shibasaki I., Nishikimi T., Mochizuki Y., et al. Greater expression of inflammatory cytokines, adrenomedullin, and natriuretic peptide receptor-C in epicardial adipose tissue in coronary artery disease. Regul Pept. 2010;165:210–217. doi: 10.1016/j.regpep.2010.07.169. [DOI] [PubMed] [Google Scholar]
- 64.Vrselja Z., Sram M., Andrijevic D., et al. Transcardial gradient of adiponectin, interleukin-6 and tumor necrosis factor-alpha in overweight coronary artery disease patients. Cytokine. 2015;76:321–327. doi: 10.1016/j.cyto.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 65.Corradi D., Maestri R., Callegari S., et al. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc Pathol. 2004;13:313–316. doi: 10.1016/j.carpath.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Tromp J., Bryant J.A., Jin X., et al. Epicardial fat in heart failure with reduced versus preserved ejection fraction. Eur J Heart Fail. 2021;23:835–838. doi: 10.1002/ejhf.2156. [DOI] [PubMed] [Google Scholar]
- 67.Hahn V.S., Yanek L.R., Vaishnav J., et al. Endomyocardial biopsy characterization of heart failure with preserved ejection fraction and prevalence of cardiac amyloidosis. J Am Coll Cardiol HF. 2020;8:712–724. doi: 10.1016/j.jchf.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brakenhielm E., Alitalo K. Cardiac lymphatics in health and disease. Nat Rev Cardiol. 2019;16:56–68. doi: 10.1038/s41569-018-0087-8. [DOI] [PubMed] [Google Scholar]