Visual Abstract
Key Words: H2, neutrophil extracellular traps, phorbol-12-myristate-13-acetate
Abbreviations and Acronyms: BAL, bronchoalveolar lavage; CitH3, citrullinated histone H3; CVD, cardiovascular disease; dsDNA, double-stranded DNA; HOCl, hypochlorous acid; LPS, lipopolysaccharide; MI, myocardial infarction; MPO, myeloperoxidase; NAC, N-acetyl-L-cysteine; NET, neutrophil extracellular trap; PA, pulmonary artery; PADI4, peptidyl arginine deiminase 4; PMA, phorbol-12-myristate-13-acetate; ROS, reactive oxygen species
Highlights
-
•
NETs have been implicated as therapeutic targets to address inflammation and thrombotic tissue damage in conditions such as sepsis, acute respiratory disease syndrome, COVID-19, and CVDs.
-
•
H2 has been clinically and experimentally proven to ameliorate inflammation; however, the underlying molecular mechanisms remain elusive.
-
•
Compared with control neutrophils, PMA-stimulated human neutrophils exposed to H2 exhibited reduced citrullination of histones and release of NET components; mechanistically, H2-mediated neutralization of HOCl produced during oxidative bursts suppresses DNA damage.
-
•
Inhalation of H2 inhibited the formation and release of NET components in the blood and BAL of the LPS-induced sepsis in mice and aged mini pigs.
-
•
H2 therapy is potentially a new therapeutic strategy for inflammatory diseases involving NETs associated with excessive neutrophil activation.
Summary
Neutrophil extracellular traps (NETs) contribute to inflammatory pathogenesis in numerous conditions, including infectious and cardiovascular diseases, and have attracted attention as potential therapeutic targets. H2 acts as an antioxidant and has been clinically and experimentally proven to ameliorate inflammation. This study was performed to investigate whether H2 could inhibit NET formation and excessive neutrophil activation. Neutrophils isolated from the blood of healthy volunteers were stimulated with phorbol-12-myristate-13-acetate (PMA) or the calcium ionophore A23187 in H2-exposed or control media. Compared with control neutrophils, PMA- or A23187-stimulated human neutrophils exposed to H2 exhibited reduced neutrophil aggregation, citrullination of histones, membrane disruption by chromatin complexes, and release of NET components. CXCR4high neutrophils are highly prone to NETs, and H2 suppressed Ser-139 phosphorylation in H2AX, a marker of DNA damage, thereby suppressing the induction of CXCR4 expression. H2 suppressed both myeloperoxidase chlorination activity and production of reactive oxygen species to the same degree as N-acetylcysteine and ascorbic acid, while showing a more potent ability to inhibit NET formation than these antioxidants do in PMA-stimulated neutrophils. Although A23187 formed NETs in a reactive oxygen species–independent manner, H2 inhibited A23187-induced NET formation, probably via direct inhibition of peptidyl arginine deiminase 4-mediated histone citrullination. Inhalation of H2 inhibited the formation and release of NET components in the blood and bronchoalveolar lavage fluid in animal models of lipopolysaccharide-induced sepsis (mice and aged mini pigs). Thus, H2 therapy can be a novel therapeutic strategy for NETs associated with excessive neutrophil activation.
Neutrophil extracellular traps (NETs) are innate immune responses that protect against infections by actively spreading neutrophil nuclear DNA in a spider web pattern around the periphery of the infection site. NETs trap pathogenic micro-organisms, including bacteria and viruses, and sterilize them with antimicrobial proteins that are attached to the DNA, such as histones, neutrophil elastase, and myeloperoxidase (MPO).1 Recent studies show that NETs are responsible for immunothrombosis, a process of thrombus formation involving immune cells that enhances the innate immune response to pathogens.2,3 In contrast, NETs also contribute to severe pathologies, such as acute respiratory distress syndrome associated with acute lung injury, and disseminated intravascular coagulation associated with sepsis.3, 4, 5 Dysregulation of NETs, either by overproduction or inadequate degradation, leads to tissue damage, hypercoagulability, and thrombosis.2,6 Recently, NETs have received much attention because of their involvement in the severity of COVID-19–related lung injury.3,7,8 In addition to infectious diseases, NETs are involved in the pathogenesis of several cardiovascular diseases (CVDs), including acute coronary syndrome, stable coronary artery disease, ischemia-reperfusion injury, pulmonary embolism, and atherosclerosis.9,10 Therefore, it is not surprising that NETs have been implicated as therapeutic targets to address inflammation and thrombotic tissue damage.
The nicotinamide adenine dinucleotide phosphate oxidase-mediated production of superoxide ions, a reactive oxygen species (ROS), plays a major role in the formation of NETs.11 Superoxide is converted to H2O2, which serves as a substrate for the MPO-catalyzed hypochlorous acid (HOCl) production.12 HOCl is a potent oxidant that acts as a disinfecting agent after it is released from the cell. However, intracellular HOCl plays a major role in NET formation.13
Hydrogen scavenges the hydroxyl radical (•OH), one of the most powerful oxidizing agents, and intervenes in lipid radical chain reactions in the cell membrane to modify lipid mediators.14,15 In addition, it is proven, both in clinical and animal studies, that it has therapeutic effects on acute lung injury, sepsis, and ischemia-reperfusion injury.16, 17, 18, 19, 20 It was also reported recently that H2 and O2 were used in combination during an outbreak of COVID-19 in Wuhan, China, to inhibit the severity of COVID-19–related lung injury.21 However, the potential link or direct correlation between H2 and NETs has not yet been established.
Therefore, in this study, we tested the hypothesis that hydrogen may inhibit NET formation associated with excessive neutrophil activation. We investigated the effect of H2 gas on NET formation using neutrophils from healthy volunteers. In addition, we studied the effects of H2 inhalation on NET formation in mice and micro mini pigs.
Methods
Animals and care
The present study was designed according to the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.22 The experiments were performed in accordance with our institutional guidelines and the Japanese law on the protection and management of animals. Ethical approval was granted by the Research Council and Animal Care and Use Committee of Keio University (approval nos: 12094-(8), 20008-(0)) and HAMRI Co, Ltd (approval no: 20-H055).
Male BALB/c mice (10 weeks old, weighing 25-30 g) were purchased from CLEA Japan Inc. The mice were housed in a temperature-controlled room with a 12-hour day/night cycle at 25 °C and had free access to food and water.
Female micro mini pigs (263-308 weeks old, weighing 23-37 kg) were purchased from Fuji Micra Inc. The pigs were housed in separate cages in a temperature-controlled room with a 12-hour day/night cycle at 25 °C and had free access to food and water. Before surgery, the pigs were fasted for 12 hours with free access to water.
H2 inhalation via nasal cannula by H2 generator
We adjusted the nostril inserts of the nasal cannula (Nakamura Medical Industry Co, Ltd) to fit the shape of the pig’s nose. This study used the H2JI1 H2 inhaler, manufactured by Doctors Man Co, Ltd. The inhaler uses a proton-exchange membrane water electrolysis system that can continuously generate high-purity (>99.999%) H2. The nasal cannula was inserted deep into the nasal cavity of the pig under spontaneous breathing, and H2 produced from the H2 generator was administered at a flow rate of 250 mL/min. A veterinary anesthesia mask (Shinano Manufacturing Co, Ltd) was placed over the nasal cannula, and an ADS 1000 (model 2000) veterinary anesthesia delivery system (Tokushima Iryoki Co, Ltd) was used to deliver a mixture of O2 and isoflurane. The flow rate of the O2/isoflurane gas mixture was maintained above 6 L/min to avoid reinhalation of exhaled CO2 that would remain in the mask.
LPS injection into micro mini pigs
A lipopolysaccharide (LPS) was administered as previously described,23 with modifications. The animals received continuous intravenous infusion of Escherichia coli LPS (serotype 055:B5, 20 μg/kg/h; Sigma-Aldrich) for 1 hour. LPS was dissolved in saline and delivered by a syringe pump (Supplemental Figure 1A). Hemodynamic and respiratory parameters were measured every 30 minutes during an experimental period of 180 minutes. The chest was opened through a midthoracic incision, the pulmonary artery was clamped, and pulmonary artery blood was collected from the distal part of the clamp with an 18-G needle (Supplemental Figure 1B). Plasma was collected from a container with 3.2% sodium citrate, and the cell pellets from a container with EDTA-2K were smeared onto glass slides using Smear Gell (Geno Staff) after centrifugation.
Isolation of neutrophils
Human neutrophils were isolated from the peripheral blood of healthy volunteers, who provided written informed consent, using Lympholyte-poly (CEDARLANE Laboratories) according to the manufacturer’s instructions, after approval from the Ethics Review Committee of Keio University (#20200183). Polynuclear cells containing neutrophils were collected, washed, and resuspended in 5 mL of red blood cell lysis buffer (BioLegend), then washed twice in Hank’s buffered salt solution without Ca2+/Mg2+ (Wako Pure Chemicals), and finally resuspended in the Roswell Park Memorial Institute 1640 medium (Wako).
Neutrophils from micro mini pigs were isolated using previously described methods,24 with modifications. Blood from the pulmonary artery of micro mini pigs was collected in a container coated with EDTA-2K and treated with red blood cell lysis buffer for 10 minutes at 4 °C. Neutrophils were separated from mononuclear cells by layering 5 mL of the cell suspension on 5 mL of Percoll 1.081 (GE Healthcare), which was layered under 5 mL of Percoll 1.087, followed by centrifugation at 1,000g for 20 minutes at 25 °C. The middle layer, enriched with neutrophils, was collected and washed twice in Hank’s buffered salt solution without Ca2+/Mg2+. The cells were then resuspended in red blood cell lysis buffer and washed twice with Hank’s buffered salt solution without Ca2+/Mg2+.
Induction of NETs ex vivo in H2-exposed cultures of human neutrophils
Cell cultures of neutrophils in Roswell Park Memorial Institute 1640 medium containing 15 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid were exposed to H2 using a hydrogen-filling device with a built-in hydrogen-absorbing alloy (Doctors Man Co, Ltd). The control medium was left untreated. Aliquots containing 1 × 107 isolated human neutrophils were stimulated with 100 nmol/L phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich) or A23187 (Sigma-Aldrich) in the control or H2-exposed medium at 37 °C for 3 hours in 15-mL Falcon tubes (Thermo Fisher Scientific). After incubation, the cells were centrifuged at 4 °C for 10 minutes at 500g to collect the supernatant. The cell pellets were then smeared to the glass slides using Smear Gell. The percentage of dead cells after stimulation is shown in Supplemental Figures 2A and 2B.
Quantification of HOCl production in neutrophils
The concentration of HOCl was analyzed using aliquots of human neutrophils with a colorimetric hypochlorite assay kit according to the manufacturer’s instructions (Cayman Chemical Company).
Enzyme-linked immunosorbent assay
Levels of citrullinated histone H3 (CitH3) (Clone 11D3) in culture supernatants or plasma were determined using enzyme-linked immunosorbent assay according to the manufacturer’s instructions (Cayman).
Quantification of NETs
Culture supernatants and plasma were collected and stored at −80 °C. DNA content was measured using the Quant-iT Picogreen double-stranded DNA (dsDNA) Assay Kit (Invitrogen), according to the manufacturer's instructions.
Statistical analysis
Differences between groups were analyzed by a paired or unpaired Student's t-test in case of normal distribution or by the Wilcoxon signed-rank or Mann-Whitney U test, as appropriate, in case of non-normal distribution. Differences among multiple groups were compared using analysis of variance followed by Tukey's post hoc test for multiple pairwise comparisons. Data are presented by dot plots with reference line at mean. A value of P < 0.05 was considered statistically significant. Statistical analysis was performed by IBM SPSS Statistics (version 26) software.
Results
H2 inhibits PMA-induced neutrophil aggregation and subsequent NET release
To determine the effect of H2 on NET formation, 1 × 107 neutrophils isolated from the peripheral blood of healthy volunteers (n = 5) were stimulated with 100 nmol/L PMA, a protein kinase C activator, in an H2-treated medium for 3 hours. Roswell Park Memorial Institute 1640 medium containing 15 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer (pH 7.2) was treated with H2 using a filling device with a built-in hydrogen-absorbing alloy; untreated medium was used as a control.
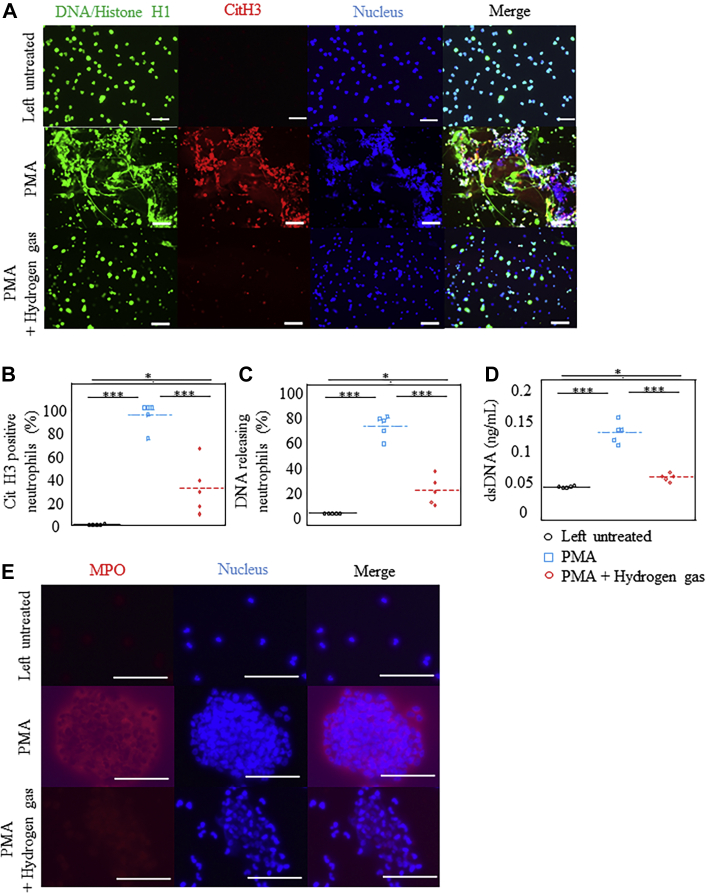
Immunostaining analysis revealed that citrullination of histones (CitH3), membrane disruption by chromatin complexes, and release of NET components (DNA/Histone H1, SYTOX Green) were suppressed in H2-treated cultures compared with those in control cultures (Figures 1A to 1C, Supplemental Figure 2C). Furthermore, the release of dsDNA from neutrophils was markedly inhibited in the H2-treated medium (Figure 1D).
Figure 1.
H2 Inhibited the Formation of NETs by Human Neutrophils
Neutrophils purified from the blood of healthy volunteers were left untreated or stimulated with phorbol-12-myristate-13-acetate (PMA) in either a H2-exposed medium or control medium for 1 hour. (A) Microscopic images depicting the fluorescence of DNA/histone H1 (Alexa Fluor 488, green), citrullination of histone 3 (CitH3) (citrulline R2 + R8 + R17; Alexa Fluor 594, red), and 4′,6-diamidino-2-phenylindole (blue) in cultured neutrophils. Original magnification ×20. Bars = 100 μm. (B,C) Percentage of CitH3-positive or DNA-releasing neutrophils (n = 5). (D) Quantification of double-stranded DNA (dsDNA) released by activated neutrophils into the culture medium (n = 6). (E) Microscopic images depicting the fluorescence of myeloperoxidase (MPO) (Alexa Fluor 594, red) and 4′,6-diamidino-2-phenylindole (blue) in cultured neutrophils. Original magnification ×120. Bars = 50 μm). ∗P < 0.05; ∗∗∗P < 0.001. NET = neutrophil extracellular trap.
After 1 hour of PMA stimulation, preceding NET release, aggregation of neutrophils was observed in the control medium, which was significantly suppressed in the H2-treated medium (Figure 1E, Supplemental Figure 2D, Video 1). Aggregation of neutrophils promotes an oxidative burst and the formation of NETs.25 These results indicate that H2 inhibits neutrophil aggregation and, possibly, the subsequent release of NETs.
H2 had a minor effect on gene expression in PMA-stimulated neutrophils
To the best of our knowledge, this is the first study to demonstrate the bioactive effects of H2 at the cellular level. To elucidate the mechanism of the inhibitory effect of H2 on neutrophil priming leading to NET formation and release, neutrophils isolated from 3 healthy volunteers were either left unstimulated or stimulated with PMA in either the control or H2-treated medium for 1 hour, and global transcriptome changes were compared by RNA sequencing.
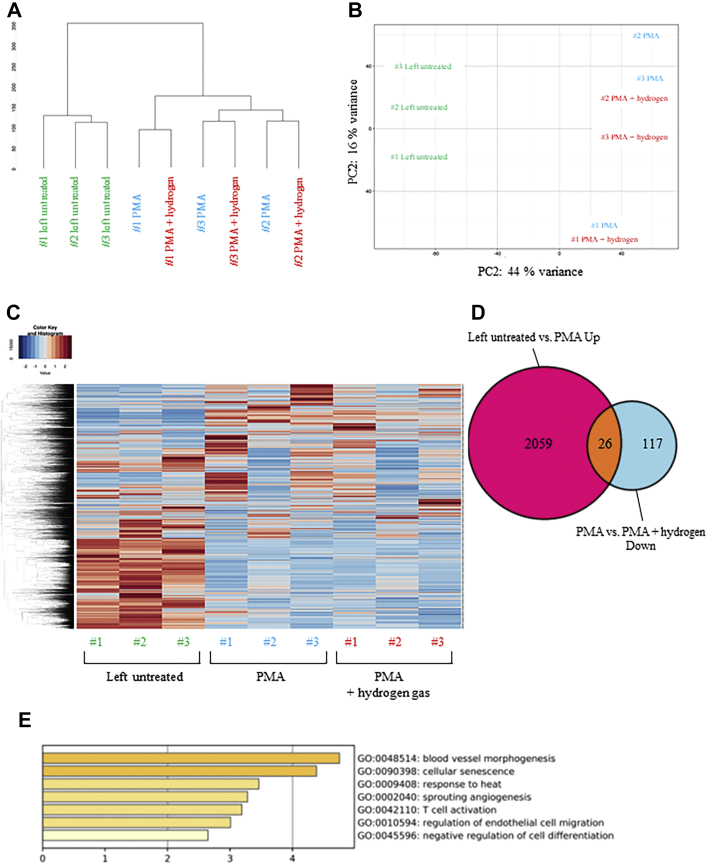
Hierarchical clustering of the RNA sequencing data showed that the untreated population and the PMA-stimulated population formed separate clusters; however, the presence or absence of H2 had no effect on cluster formation (Figure 2A). Principal component analysis showed similar results (Figure 2B). Furthermore, the heat maps showed that H2 administration did not cause drastic changes in the gene expression of PMA-stimulated human neutrophils (Figure 2C). These results suggest that the variations in gene expression that are induced by PMA stimulation alone were greater than those induced by the effect of H2 after PMA stimulation in human neutrophils.
Figure 2.
Bioinformatic Analysis of RNA-Seq Data From Human Neutrophils
Neutrophils purified from the blood of healthy volunteers were stimulated with phorbol-12-myristate-13-acetate (PMA) in either a H2-exposed medium or control medium for 1 hour. (A) The dendrogram depicting hierarchical clustering of all RNA-sequencing (RNA-seq) samples. (B) Principal component (PC) analysis plot of all RNA-seq samples. Replicates of the same cell type are indicated by the same color, as shown in the legend. (C) Heat map of gene expression profiles shown with gene-wise (row) and sample-wise (column) clustering dendrograms. The color corresponds to the square root of standardized transcripts per million values by row, as shown in the legends on the upper left. (D) Venn diagram showing the overlap between genes up-regulated on PMA treatment (untreated vs PMA) with those down-regulated on subsequent H2 administration (PMA vs PMA + H2). (E) Gene Ontology (GO) enrichment analysis of biological processes for the 26 overlapping genes.
The genes whose expression was increased by PMA and repressed by H2 administration were investigated. Our results showed that, compared with the group left untreated, 2,059 differentially expressed genes were up-regulated in the PMA-stimulated group. PMA-stimulation with H2-exposed medium down-regulated 117 differentially expressed genes compared with those in the control medium. To further examine the differences in gene expression in these groups, Venn diagrams were plotted for the overlap of the genes. Among the 2.059 genes up-regulated on PMA stimulation, 26 genes were down-regulated by H2 pretreatment (Figure 2D). Furthermore, gene ontology analysis of these 26 genes showed that H2 administration repressed the genes involved in cellular senescence among the genes induced by PMA (Figure 2E, Table 1). These results indicate that although H2 has a drastic effect on PMA-induced neutrophil aggregation and NET formation and release, its effect on the gene expression profile of neutrophils during the first hour of PMA stimulation is very limited. The few changes in gene expression that were observed suggest that H2 may affect the process of neutrophil aging.
Table 1.
List of Genes Common Between Those Up-Regulated on PMA Treatment and Those Down-Regulated on Subsequent H2 Administration
| Gene Symbol | Log2 Fold Change | P Value |
|---|---|---|
| CA13 | −4.18 | 0.019 |
| LY6E | −3.77 | 0.016 |
| DLL4 | −2.63 | 0.020 |
| MIR6514 | −2.47 | 0.008 |
| TEAD2 | −2.23 | 0.025 |
| RNH1 | −2.08 | 0.030 |
| NDC1 | −1.62 | 0.005 |
| NRARP | −1.48 | 0.007 |
| DNAJA4 | −1.48 | 3.00 × 10−5 |
| CD160 | −1.36 | 0.033 |
| EIF3C | −1.35 | 0.039 |
| RASD1 | −1.33 | 0.040 |
| GPA33 | −1.33 | 0.038 |
| MCUR1 | −1.29 | 0.035 |
| RRAD | −1.27 | 0.036 |
| WDR61 | −1.22 | 0.001 |
| HAVCR2 | −1.17 | 0.015 |
| H2AFX | −1.13 | 2.10 × 10−5 |
| ID2 | −1.10 | 6.23 × 10−5 |
| TECR | −1.10 | 0.009 |
| SNHG1 | −1.07 | 3.42 × 10−6 |
| BAG3 | −1.07 | 0.009 |
| POU1F1 | −1.06 | 0.026 |
| PLK2 | −1.06 | 0.001 |
| CCDC85B | −1.06 | 0.012 |
H2 suppressed PMA-induced DNA damage in neutrophils
H2AX is a variant of H2A, one of the core histones that make up nucleosomes. Formation of γ-H2AX via phosphorylation of the Ser-139 residue of H2AX is an early cellular response to the induction of DNA double-strand breaks and a molecular marker of DNA damage.26,27 The expression of γ-H2AX was markedly increased in neutrophils stimulated with PMA for 1 hour in the control medium. In contrast, this change was significantly suppressed in hydrogen-containing medium (Figure 3A). Flow cytometry analysis demonstrated that SYTOX+ NET-forming neutrophils increased 1 hour after PMA stimulation in the control medium. However, subsequent H2 administration decreased the SYTOX+ neutrophils to a level comparable to that of the control neutrophils (Figure 3B). CXCR4hi aged neutrophils are known to be prone to NET formation.28,29 Interestingly, the subset of neutrophils expressing CXCR4 on their cell surface increased 1 hour after PMA stimulation (Figure 3C). Furthermore, CXCR4hi neutrophils were also detected to be SYTOX+ (Figures 3D and 3E). In the H2-treated medium, the increase in the population of CXCR4hi neutrophils seen in the control medium was suppressed, and the population of SYTOX+ CXCR4hi neutrophils was also lower than that in the control medium (Figures 3C to 3E). The ability of neutrophils to form NETs is also regulated by the content of their intracellular granules.28,30 We hypothesized that CXCR4hi neutrophils are prone to NET formation because CXCR2-mediated homeostatic degranulation is impaired and granules containing NET-forming proteins accumulate in the cells. Interestingly, the intracellular MPO content in the H2-treated medium was lower than that in the control medium, which is consistent with the suppression of CXCR4 expression on the cell surface (Figures 3F and 3G). These results suggest that H2 may inhibit PMA-stimulated CXCR4 expression in neutrophils and maintain homeostatic degranulation, thereby suppressing NET formation.
Figure 3.
H2 Ameliorated the DNA Damage in Human Neutrophils
Neutrophils purified from the blood of healthy volunteers were stimulated with PMA in either a H2-exposed medium or control medium for 1 hour. (A) Representative flow-cytometric analysis of phospho-histone H2A.X (Ser-139) indicating neutrophils. (B) Percentage of SYTOX+ neutrophils (n = 3). (C) Flow cytometric analysis of CXCR4+ cells in neutrophils (n = 3). (D,E) Flow cytometric analysis of SYTOX+ CXCR4+ cells in neutrophils (n = 3). (F) Microscopic images depicting the fluorescence of MPO (Alexa Fluor 594, red), DNA/histone H1 (Alexa Fluor 488, green), and 4′,6-diamidino-2-phenylindole (blue) in cultured neutrophils. Original magnification ×20. Bars = 100 μm. (G) Mean fluorescence intensity of MPO in neutrophils (n = 5) measured using the particle analysis function of the BZ-H1C software (KEYENCE). (H) Quantification of dsDNA released by activated neutrophils into the culture medium at the indicated time points (n = 6). Flow cytometric analysis was performed in n ≥ 3 independent experiments. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001. AU = arbitrary unit; other abbreviations as in Figure 1.
From midnight to early morning, CXCR4 expression in neutrophils is high and they are prone to form NETs. In the daytime, neutrophils have low CXCR4 expression and low intracellular granule content, suggesting that their NET-forming capacity is reduced.28 The release of dsDNA from neutrophils was significantly higher in neutrophils collected at 8:00 am than in those collected at 2:00 pm (Supplemental Figure 3H). The inhibitory effect of H2 on the release of dsDNA was confirmed regardless of the time of day, but it was more pronounced in neutrophils collected at 8 am (Supplemental Figure 3H). These results indicate that the expression level of CXCR4 regulates NET-forming capacity in humans as well as in mice. In addition, in relation to the diurnal variation of homeostatic degranulation, it was suggested that the inhibitory effect of H2 on NET formation may also vary depending on the time of day.
H2 suppressed HOCl generation
MPO catalyzes the reaction between H2O2 and chloride anion (Cl−), leading to the production of HOCl, which has strong oxidizing abilities and mediates inflammatory cellular damage.12,31,32 We tested whether H2 inhibits the ability of PMA-stimulated neutrophils to generate HOCl. The HOCl generation ability of isolated human neutrophils, evaluated by MPO chlorination activity, was notably suppressed in the hydrogen-exposed medium compared with that in the control medium (Figures 4A and 4B). However, H2 did not suppress phagocytosis or chemotaxis (Figures 4C and 4D). These results indicate that the quenching of HOCl by H2 is the key mechanism for the suppression of NET formation.
Figure 4.
H2 Reduced the Production of Hydroxy Radicals and HOCl in PMA-Stimulated Human Neutrophils
Neutrophils purified from the blood of healthy volunteers were stimulated with PMA in either a H2-exposed medium or control medium for 1 hour. (A) Time course of MPO chlorination activity (n = 6). (B) Hypochlorous acid (HOCl) generation, as assessed by MPO chlorination activity (n = 6). (C) Latex beads were added to cultured human neutrophils for 1 hour and were analyzed by flow cytometry. Representative flow cytometric analysis of latex bead–positive cells in human neutrophils among different groups is depicted, indicating phagocytosis. (D) Chemotaxis assay of cultured human neutrophils. N-formylmethionyl-leucyl-phenylalanine–treated neutrophils were allowed to migrate through a porous membrane filter for 3 hours. The mean fluorescence intensity of migrated neutrophils among different groups is depicted (n = 3). ∗∗∗P < 0.001. NS = not significant; RFU = relative fluorescence unit; other abbreviations as in Figures 1 and 3.
H2 suppressed A23187-induced NETs formation
We examined whether hydrogen has an inhibitory effect on NET formation induced by other than PMA. The calcium ionophore A23187 is known to form NETs in a reactive oxygen species (ROS)-independent manner by increasing the intracellular Ca2+ concentration.33 A 23187-induced CitH3, membrane disruption by chromatin complexes, release of NET components (DNA/histone H1, SYTOX Green), and the release of dsDNA from neutrophils were suppressed in H2-treated cultures compared with those in control cultures (Supplemental Figures 3A and 3B). However, A23187 stimulation, compared with PMA stimulation, resulted in lower ROS production in neutrophils, and H2 had little effect on ROS production (Supplemental Figure 3C). There was also no significant difference in MPO chlorination activity between the control medium and the H2-treated medium (Supplemental Figure 3D). Nevertheless, the expression of γ-H2AX was markedly increased in neutrophils stimulated with A23187 in the control medium. In contrast, this change was significantly suppressed in H2-treated medium (Supplemental Figure 3E). Flow cytometric analysis demonstrated that CXCR4 and SYTOX expression was significantly suppressed in the H2-treated medium compared with those in the control medium (Supplemental Figures 3F and 3G).
It has been reported that A23184-induced NETs formation is more dependent on the activity of peptidyl arginine deiminase 4 (PADI4) than PMA-induced NETs formation is.33 Indeed, the PADI4-specific inhibitor GSK484 was able to completely inhibit A23187-induced NETs formation, whereas it only partially inhibited PMA-induced NET formation (Supplemental Figures 4A to 4C). In neutrophils stimulated with A23187, the release of dsDNA from stimulated neutrophils with GSK484 and H2 was comparable, and there was no additive effect of GSK484 and H2 (Supplemental Figure 4C). These results suggest that in A23184-induced NETs formation, H2 suppresses NET formation by inhibiting Ca2+-dependent PADI4 activation, not by suppressing ROS.
H2 inhibits PMA-induced NETs formation more effectively than antioxidants
It is known that ROS is deeply involved in PMA-induced NETs formation. N-acetyl-L-cysteine (NAC) inhibited PMA-induced ROS and HOCl production to the same level as H2 did (Supplemental Figures 5A and 5B). The antioxidant capacity of L-ascorbic acid was weaker than that of NAC (Supplemental Figures 5A and 5B). Interestingly, in PMA-stimulated neutrophils, the inhibitory effects of hydrogen on H2AX phosphorylation and CXCR4 expression and on neutrophil aggregation were stronger than those of NAC (Supplemental Figures 5C to 5G). As a result, PMA-induced CitH3, membrane disruption by chromatin complexes, release of NET components (DNA/histone H1, SYTOX Green), and the release of dsDNA from neutrophils were inhibited more strongly by hydrogen than by antioxidants such as NAC and L-ascorbic acid (Supplemental Figures 6A and 6B). These results suggest that not only removal of ROS, but also other mechanisms are involved in the inhibition of neutrophil aggregation and protection from DNA damage by hydrogen.
Inhalation of H2 inhibited release of NETs in mice and aged micro mini pigs
Next, we tested the inhibitory effect of H2 inhalation on NET formation in mice and micro mini pigs. An acute lung injury model was generated in 10-week-old male BALB/c mice by intratracheal administration of 8 mg/kg LPS. The mice were reared and exposed to H2 as previously described34 and sacrificed 24 hours later (n = 5). H2 inhalation inhibited the aggregation of neutrophils and formation of NETs (Figures 5A and 5B) in bronchoalveolar lavage (BAL). The concentrations of CitH3 and dsDNA in BAL were also lower in the H2-exposed group than in the control group (Figures 5C and 5D). Similarly, in neutrophils isolated from mouse blood, inhalation of H2 suppressed NET formation (Figures 5E to 5H). CitH3+ cells appeared in the pulmonary arteries (PAs) of the LPS-induced acute lung injury model mice (Supplemental Figures 7A and 7B); however, inhalation of H2, compared with control gas, significantly suppressed this change (Supplemental Figures 7C and 7D).
Figure 5.
Inhalation of H2 Inhibited NET Formation in BAL Fluid of Mice
(A) Microscopic images depicting DNA/histone H1 (Alexa Fluor 488, green), MPO (Alexa Fluor 594, red), and nuclei (4′,6-diamidino-2-phenylindole, blue) in the bronchoalveolar lavage (BAL) fluid from mice that were intratracheally administered with lipopolysaccharide (E coli serotype O55:B5) and kept in cages perfused with either H2 or control gas. Original magnification ×20. Bar = 100 μm. (B) Quantification of the percentage of DNA-releasing neutrophils in the BAL fluid of each group (n = 5). (C) Quantification of CitH3 in the BAL fluid of each group (n = 5). (D) Quantification of dsDNA released in the BAL fluid of each group (n = 5). (E) Microscopic images depicting DNA/histone H1 (Alexa Fluor 488, green), MPO (Alexa Fluor 594, red), and nuclei (4′,6-diamidino-2-phenylindole, blue) in the blood of mice that were intratracheally administered with lipopolysaccharide and kept in cages perfused with either H2or control gas. Original magnification ×20. Bar = 100 μm. (F) Quantification of the percentage of DNA-releasing neutrophils in the blood of each group (n = 5). (G) Quantification of CitH3 in the plasma of each group (n = 5). (H) Quantification of dsDNA released in the plasma of each group (n = 5). ∗P < 0.05; ∗∗P < 0.01, ∗∗∗P < 0.001. Abbreviations as in Figure 1.
Next, 5-year-old mini pigs were randomly assigned to the H2 or control groups, and LPS (20 μg/kg) was administered through the internal jugular vein continuously for 1 hour using an infusion pump (Supplemental Figure 1A). In the H2-exposed group, 100% hydrogen was administered via nasal cannula at a rate of 250 mL/min with spontaneous breathing, as previously reported.35 We confirmed that the H2 concentration in the carotid blood during H2 inhalation was maintained at approximately 5%. After 3 hours of LPS administration, the main trunk of the PAs was exposed through a midthoracic incision, and blood was collected from the distal part after clamping the proximal part of the PAs (Supplemental Figure 1B). In the PAs, LPS administration resulted in a high degree of NET formation in the control group (Figure 6A), whereas H2 inhalation significantly inhibited NET formation and reduced plasma dsDNA levels (Figures 6B and 6C). H2 inhalation also reduced CitH3+ cells in the lung compared with the number of cells in the control gas group (Figures 6D and 6E). These results indicate that inhalation of hydrogen can inhibit the formation and release of NETs in the blood, BAL, and lung in sepsis models of mice and aged pigs.
Figure 6.
Inhalation of H2 Inhibited NET Formation in the PAs of Micro Mini Pigs
(A) Microscopic images depicting DNA/histone H1 (Alexa Fluor 488, green) and nuclei (4′,6-diamidino-2-phenylindole, blue) in purified neutrophils isolated from the pulmonary artery (PA) of micro mini pigs administered with 20 μg/kg lipopolysaccharide intravenously with the inhalation of H2 or control gas. Original magnification ×20. Bars = 100 μm. (B) Quantification of the percentage of DNA-releasing neutrophils (n = 3). (C) Quantification of dsDNA released in the blood from the PA of each group (n = 3). (D) Representative immunofluorescence images of lung sections from micro mini pigs administered with 20 μg/kg lipopolysaccharide intravenously with the inhalation of H2 or control gas. Microscopic images depicting the fluorescence of CitH3 (Alexa Fluor 594, red), DNA/histone H1 (Alexa Fluor 488, green), and 4′,6-diamidino-2-phenylindole (blue). Original magnifications ×40 and ×120, respectively. Bars = 100 μm. (E) Number of CitH3-positive cells in low power field (LPF) (n = 5). ∗∗P < 0.01; ∗∗∗P < 0.001. Abbreviations as in Figure 1.
Discussion
In the present study, we found that H2 inhibited the PMA- and A23187-induced NET formation and release in human neutrophils. Furthermore, we confirmed that H2 inhalation therapy suppressed NET formation and release in mice and aged micro mini pig models of LPS-induced sepsis.
PMA directly activates protein kinase C, resulting in the potent induction of both extracellular and intracellular ROS and triggers the formation of NETs in a ROS-dependent manner.33 The nicotinamide adenine dinucleotide phosphate oxidase activation can convert oxygen to superoxide radicals (O2−), and superoxide can be converted to H2O2 spontaneously or by superoxide dismutase.36 In neutrophils, MPO can catalyze the production of HOCl from H2O2 and chloride ions.30 This process is called oxidative burst.34 Oxidative bursts induce hypercitrullination of histones, decrease nucleosome binding, and cause overall chromatin decondensation.33 Following intracellular NET formation, the cell envelope is broken, and NETs are released into the extracellular space.
Ca2+ ionophore A23187 increases intracellular Ca2+ concentration by directly facilitating the transport of Ca2+ across the plasma membrane,37 which induces a rapid citrullination of multiple cellular proteins.38,39 Therefore, PADI4-mediated histone citrullination contributes greatly to NET formation in A23187-stimulated human neutrophils.33 It has been reported that A23187-induced NETs are not suppressed by administration of protein kinase C inhibitor,40 A23187 leads to a lower level of ROS compared with PMA or suppresses oxidative bursts in human neutrophils.41 In fact, ROS scavenger or MPO inhibitor has no inhibitory effect on NET formation.40 Thus, PMA and A23187 have different mechanisms leading to NET formation.
Our results suggest that the central mechanism that H2 suppresses NET formation is the strong reduction of ROS production like MPO-induced HOCl in PMA-stimulated human neutrophils; however, H2 mainly targets other than ROS in A23187-stimulated human neutrophils.
In fact, H2 significantly suppressed histone citrullination and NET formation despite little effect on ROS production and MPO chlorination activity in A23187-stimulated human neutrophils. GSK484, a PADI4-specific inhibitor, showed no additional effect of suppressing A23187-induced NET formation compared to H2 alone. These results suggest that H2 may have an inhibitory effect of Ca2+-dependent PADI4 activation. Furthermore, PMA-induced NET formation was also suppressed by antioxidants such as NAC and ascorbic acid, which is consistent with previous reports.42,43 NAC suppressed ROS and MPO chlorination activity to the same degree as H2 did; nevertheless, the effect of suppressing NET formation was stronger with H2 than with NAC. These results also support that H2 suppresses NET formation by its inhibitory effect of not only ROS but also other mechanisms.
Neutrophils from patients with chronic granulomatous disease without functional nicotinamide adenine dinucleotide phosphate oxidase lack the ability to release NETs in response to classical stimuli.44 To promote NET formation, ROS must be processed internally by MPO.30,45 Neutrophils derived from patients deficient in MPO fail to form NETs when exposed to PMA.45 These lines of evidence confirm the ability of HOCl to stimulate NET release. Recently, it has been reported that CXCR2-CXCL2 signaling induces degranulation of neutrophils and mitigates their potential histotoxicity through a “disarming” mechanism.28 In other words, CXCR2-CXCL2 signaling reduces the intracellular granule content, thereby reducing NET formation capacity. As neutrophils age, CXCR4, a negative regulator of CXCR2 signaling,46 on the cell surface is up-regulated.47,48 This change facilitates the clearance of senescent neutrophils in the bone marrow,47,48 and CXCR4hi neutrophils are known to be prone to NET formation.28,29 Furthermore, it has been shown that NET formation in response to PMA was strongly reduced in MRP8CRE Cxcr4fl/fl mice, which lacked CXCR4 specifically in neutrophils.49
Oxidative burst also induces DNA damage.50 DNA damage is the primary cause of cellular aging,51 and extensive DNA damage and the subsequent DNA repair pathway induce NETosis, leading to chromatin decondensation.52 We detected oxidative burst–induced phosphorylation of the Ser-139 residue of H2AX, a marker of double-strand breaks in DNA,26,27 and up-regulation of CXCR4 in PMA- or A23187-stimulated human neutrophils. This up-regulation of CXCR4 is also a response to DNA damage.53 In fact, NETs forming neutrophils expressed CXCR4. The process of NET release is thought to be driven by the amount of HOCl; as HOCl production exceeds a certain threshold, NETs are released. These results suggest that H2 reduces DNA damage associated with oxidative burst and suppresses CXCR4 expression, thereby keeping intracellular MPO content low and preventing intracellular production of HOCl from reaching the threshold where the process of NET release begins.
One hour after PMA stimulation, neutrophils exhibited oxidative burst and aggregation, and H2 strongly inhibited these changes. However, RNA-sequencing analysis showed that its effect on gene expression was insignificant. This means that H2 does not directly affect signaling pathways and gene expression involved in PMA-induced neutrophil priming. In fact, H2 had no effect on phagocytosis or chemotaxis in PMA-stimulated neutrophils. HOCl is an order of magnitude more reactive with biomolecules than peroxynitrite (ONOO−) and H2O2 are and causes damage to biomacromolecules such as nucleic acids, proteins, and lipids.12,31 H2 is thought to mitigate DNA damage by reducing HOCl, a potent oxidant.
A recent multicenter randomized clinical trial showed that H2 inhalation improved the severity and symptoms in patients with COVID-19 pneumonia.21 Neutrophilia in patients with COVID-19 pneumonia is indicative of a poor prognosis,54 and NET hyperplasia has been observed in the blood, BAL fluid, and PAs of patients with severe COVID-19 pneumonia.3,55,56 The inhibitory effect of H2 on excessive neutrophil activation and NET formation may lead to improved prognosis in patients with COVID-19 pneumonia. Furthermore, HOCl has been shown to play a pivotal role in the pathogenesis of various inflammatory diseases and tissue injuries through protein modification.31,32 Modification of low-density lipoprotein by HOCl has been implicated in human atherosclerosis. HOCl-modified low-density lipoprotein leads to unregulated uptake of oxidized low-density lipoprotein into macrophages, followed by their differentiation into foamy macrophages.57 HOCl oxidizes cardiac myoglobin, which may cause cardiac inflammation and dysfunction in mice after myocardial infarction (MI).58 HOCl is also shown to have a profound adverse effect on left ventricular remodeling and function after MI.59
Study limitations
First, we indirectly demonstrated the possibility that H2 suppresses PADI4 activity by using GSK484, a PADI4-specific inhibitor, but the exact target of H2 remains unclear because of its difficulty of depletion of PADI4 by using small, interfering RNA in terminally differentiated human neutrophils.
Next, we showed that H2 suppresses NET formation more effectively than other antioxidants, such as NAC or L-ascorbic acid, do in vitro, but we did not directly compare H2 with other antioxidants in vivo. However, in the present study, it was confirmed that inhalation of low concentrations of H2 effectively suppressed NET formation.
Finally, it has not been directly proven that H2 inhibits NET formation in patients with MI or COVID-19. H2 has been already shown to be safe for inhalation and effective in patients with MI and COVID-19. Thus, elucidating the inhibitory mechanism of NET formation in humans would provide a mechanistic framework for its use in treatment of sepsis, acute respiratory disease syndrome, COVID-19, and CVDs.
Conclusions
In this study, we show for the first time that hydrogen inhibits the formation and release of NETs in human neutrophils induced by PMA stimulation in vitro and in mouse and aged mini-pig models of LPS-induced sepsis in vivo. The key mechanism is thought to be that H2 neutralizes HOCl produced during oxidative bursts and suppresses DNA damage. In addition, H2 may suppress NET formation even by a mechanism independent of ROS scavenging. Hydrogen therapy targeting NETs or extracellular HOCl is expected to be effective in acute conditions such as excessive neutrophil activation and NET formation associated with sepsis, as well as in chronic conditions such as atherosclerosis and myocardial remodeling after MI.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: NETs contribute to severe pathologies, such as acute respiratory distress syndrome associated with and disseminated intravascular coagulation associated with sepsis or COVID-19. NETs are also involved in the pathogenesis of several CVDs, including acute coronary syndrome, stable coronary artery disease, ischemia-reperfusion injury, pulmonary embolism, and atherosclerosis. H2 has been clinically and experimentally proven to ameliorate inflammation in these disorders; however, the underlying molecular mechanisms remain elusive. Our study shows that inhalation of H2 may offer an attractive option for ameliorating inflammation by inhibiting the formation and release of NETs and excessive neutrophil activation.
TRANSLATIONAL OUTLOOK: Our findings revealed the inhibitory effect of H2 on NET formation in human neutrophils, mice, and micro mini-pigs. H2 has been clinically proven to be safe and to ameliorate inflammation in patients with MI. A multicenter randomized clinical trial demonstrated that H2 inhalation improved severity and symptoms in patients with COVID-19 pneumonia. These findings support clinical translation of hydrogen therapy as ameliorating inflammatory diseases involving NETs associated with excessive neutrophil activation.
Funding Support and Author Disclosures
This research was funded by Japanese Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (B) grant 18H02812 (2018-2020) and grants from Doctors Man Co, Ltd (to MS); and JSPS Grant-in Aid for Young Scientists grant JP18K15197 (2018–2020), Grant in-Aid for JSPS Fellows grant JP19J00583H (2018-2020). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. Drs Kobayashi and Sano have received advisory fees and research fees from Doctors Man Co, Ltd. Dr Sano has received advisory fees and research fees from Taiyo Nippon Sanso; is the registered inventor of the following patents jointly filed by Keio University and Taiyo Nippon Sanso: hydrogen mixed gas supply device for medical purposes (patent number: 5631524), medicinal composition for improving prognosis after restart of patient’s own heartbeat, and medicinal composition for improving and/or stabilizing circulatory dynamics after onset of hemorrhagic shock; and 3 other patents (whose names are translated into English): pharmaceutical compositions for reducing weight loss after organ harvesting (joint application with Keio University and Taiyo Nippon Sanso), method for generating organ preservation solution containing hydrogen and organ preservation solution containing hydrogen (joint application with Keio University and Doctors Man, application number PCT/JP2019/045790). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors are grateful to Nagahiro Minato and Yoko Hamazaki (Kyoto University, Japan) for technical assistance. The authors are also grateful to Dr R. Nakaki, Dr Y. Kondo, and M. Kawamura (Rhelixa, Inc) for analyzing the RNA-sequencing data.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental methods, figures, video, and references, please see the online version of this paper.
Appendix
H2 Inhibited the Formation of NETs by Human Neutrophils. Neutrophils purified from the blood of healthy volunteers were primed with or without phorbol-12-myristate-13-acetate (PMA) in either a H2-exposed medium or control medium for 1 hour. Neutrophil aggregate formation in the hydrogen-exposed medium with PMA (left), the control medium without PMA (middle), and the control medium with PMA (right).
References
- 1.Brinkmann V., Reichard U., Goosmann C., et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 2.Grover S.P., Mackman N. Neutrophils, NETs, and immunothrombosis. Blood. 2018;132(13):1360–1361. doi: 10.1182/blood-2018-08-868067. [DOI] [PubMed] [Google Scholar]
- 3.Middleton E.A., He X.Y., Denorme F., et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song C., Li H., Li Y., et al. NETs promote ALI/ARDS inflammation by regulating alveolar macrophage polarization. Exp Cell Res. 2019;382(2):111486. doi: 10.1016/j.yexcr.2019.06.031. [DOI] [PubMed] [Google Scholar]
- 5.Stiel L., Mayeur-Rousse C., Helms J., Meziani F., Mauvieux L. First visualization of circulating neutrophil extracellular traps using cell fluorescence during human septic shock-induced disseminated intravascular coagulation. Thromb Res. 2019;183:153–158. doi: 10.1016/j.thromres.2019.09.036. [DOI] [PubMed] [Google Scholar]
- 6.Yang J., Wu Z., Long Q., et al. Insights into immunothrombosis: the interplay among neutrophil extracellular trap, von Willebrand Factor, and ADAMTS13. Front Immunol. 2020;11:610696. doi: 10.3389/fimmu.2020.610696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leppkes M., Knopf J., Naschberger E., et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 2020;58:102925. doi: 10.1016/j.ebiom.2020.102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med. 2020;217(6) doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Döring Y., Libby P., Soehnlein O. Neutrophil extracellular traps participate in cardiovascular diseases: recent experimental and clinical insights. Circ Res. 2020;126(9):1228–1241. doi: 10.1161/CIRCRESAHA.120.315931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laridan E., Martinod K., De Meyer S.F. Neutrophil extracellular traps in arterial and venous thrombosis. Semin Thromb Hemost. 2019;45(1):86–93. doi: 10.1055/s-0038-1677040. [DOI] [PubMed] [Google Scholar]
- 11.Ravindran M., Khan M.A., Palaniyar N. Neutrophil extracellular trap formation: physiology, pathology, and pharmacology. Biomolecules. 2019;9(8):365. doi: 10.3390/biom9080365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ulfig A., Leichert L.I. The effects of neutrophil-generated hypochlorous acid and other hypohalous acids on host and pathogens. Cell Mol Life Sci. 2021;78(2):385–414. doi: 10.1007/s00018-020-03591-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer L.J., Cooper P.R., Ling M.R., Wright H.J., Huissoon A., Chapple I.L. Hypochlorous acid regulates neutrophil extracellular trap release in humans. Clin Exp Immunol. 2012;167(2):261–268. doi: 10.1111/j.1365-2249.2011.04518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iuchi K., Imoto A., Kamimura N., et al. Molecular hydrogen regulates gene expression by modifying the free radical chain reaction-dependent generation of oxidized phospholipid mediators. Sci Rep. 2016;6:18971. doi: 10.1038/srep18971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohsawa I., Ishikawa M., Takahashi K., et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13(6):688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 16.Moon D.H., Kang D.Y., Haam S.J., et al. Hydrogen gas inhalation ameliorates lung injury after hemorrhagic shock and resuscitation. J Thorac Dis. 2019;11(4):1519–1527. doi: 10.21037/jtd.2019.03.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie K., Liu L., Yu Y., Wang G. Hydrogen gas presents a promising therapeutic strategy for sepsis. Biomed Res Int. 2014;2014:807635. doi: 10.1155/2014/807635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sano M., Suzuki M., Homma K., et al. Promising novel therapy with hydrogen gas for emergency and critical care medicine. Acute Med Surg. 2017;5(2):113–118. doi: 10.1002/ams2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashida K., Sano M., Ohsawa I., et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun. 2008;373(1):30–35. doi: 10.1016/j.bbrc.2008.05.165. [DOI] [PubMed] [Google Scholar]
- 20.Katsumata Y., Sano F., Abe T., et al. The effects of hydrogen gas inhalation on adverse left ventricular remodeling after percutaneous coronary intervention for ST-elevated myocardial infarction—first pilot study in humans. Circ J. 2017;81(7):940–947. doi: 10.1253/circj.CJ-17-0105. [DOI] [PubMed] [Google Scholar]
- 21.Guan W.J., Wei C.H., Chen A.L., et al. Hydrogen/oxygen mixed gas inhalation improves disease severity and dyspnea in patients with Coronavirus disease 2019 in a recent multicenter, open-label clinical trial. J Thorac Dis. 2020;12(6):3448–3452. doi: 10.21037/jtd-2020-057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilkenny C., Browne W.J., Cuthi I., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Vet Clin Pathol. 2012;41(1):27–31. doi: 10.1111/j.1939-165X.2012.00418.x. [DOI] [PubMed] [Google Scholar]
- 23.Albertini M., Clement M.G., Lafortuna C.L., et al. Role of poly-(ADP-ribose) synthetase in lipopolysaccharide-induced vascular failure and acute lung injury in pigs. J Crit Care. 2000;15(2):73–83. doi: 10.1053/jcrc.2000.7903. [DOI] [PubMed] [Google Scholar]
- 24.Bréa D., Meurens F., Dubois A.V., et al. The pig as a model for investigating the role of neutrophil serine proteases in human inflammatory lung diseases. Biochem J. 2012;447(3):363–370. doi: 10.1042/BJ20120818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hahn J., Schauer C., Czegley C., et al. Aggregated neutrophil extracellular traps resolve inflammation by proteolysis of cytokines and chemokines and protection from antiproteases. FASEB J. 2019;33(1):1401–1414. doi: 10.1096/fj.201800752R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mah L.J., El-Osta A., Karagiannis T.C. gammaH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia. 2010;24:679–686. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- 27.Sharma A., Singh K., Almasan A. Histone H2AX phosphorylation: a marker for DNA damage. Methods Mol Biol. 2012;920:613–626. doi: 10.1007/978-1-61779-998-3_40. [DOI] [PubMed] [Google Scholar]
- 28.Adrover J.M., Aroca-Crevillén A., Crainiciuc G., et al. Programmed “disarming” of the neutrophil proteome reduces the magnitude of inflammation. Nat Immunol. 2020;21(2):135–144. doi: 10.1038/s41590-019-0571-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radermecker C., Sabatel C., Vanwinge C., et al. Locally instructed CXCR4(hi) neutrophils trigger environment-driven allergic asthma through the release of neutrophil extracellular traps. Nat Immunol. 2019;20(11):1444–1455. doi: 10.1038/s41590-019-0496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Björnsdottir H., Welin A., Michaëlsson E., et al. Neutrophil NET formation is regulated from the inside by myeloperoxidase-processed reactive oxygen species. Free Radic Biol Med. 2015;89:1024–1035. doi: 10.1016/j.freeradbiomed.2015.10.398. [DOI] [PubMed] [Google Scholar]
- 31.Biedroń R., Konopiński M.K., Marcinkiewicz J., Józefowski S. Oxidation by neutrophils-derived HOCl increases immunogenicity of proteins by converting them into ligands of several endocytic receptors involved in antigen uptake by dendritic cells and macrophages. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawkins C.L. Hypochlorous acid-mediated modification of proteins and its consequences. Essays Biochem. 2020;64(1):75–86. doi: 10.1042/EBC20190045. [DOI] [PubMed] [Google Scholar]
- 33.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18(2):134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 34.Sugai K., Tamura T., Sano M., et al. Daily inhalation of hydrogen gas has a blood pressure-lowering effect in a rat model of hypertension. Sci Rep. 2020;10(1):20173. doi: 10.1038/s41598-020-77349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sano M., Shirakawa K., Katsumata Y., Ichihara G., Kobayashi E. Low-flow nasal cannula hydrogen therapy. J Clin Med Res. 2020;12(10):674–680. doi: 10.14740/jocmr4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen G.T., Green E.R., Mecsas J. Neutrophils to the ROScue: mechanisms of NADPH oxidase activation and bacterial resistance. Front Cell Infect Microbiol. 2017;7:373. doi: 10.3389/fcimb.2017.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dedkova E.N., Sigova A.A., Zinchenko V.P. Mechanism of action of calcium ionophores on intact cells: ionophore-resistant cells. Membr Cell Biol. 2000;13(3):357–368. [PubMed] [Google Scholar]
- 38.Konig M.F., Andrade F. A critical reappraisal of neutrophil extracellular traps and NETosis mimics based on differential requirements for protein citrullination. Front Immunol. 2016;7:461. doi: 10.3389/fimmu.2016.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y., Di Pucchio T., Sims G.P., Mittereder N., Mustelin T. Characterization of the hypercitrullination reaction in human neutrophils and other leukocytes. Mediators Inflamm. 2015;2015:236451. doi: 10.1155/2015/236451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kenny E.F., Herzig A., Krüger R., et al. Diverse stimuli engage different neutrophil extracellular trap pathways. eLife. 2017;6 doi: 10.7554/eLife.24437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y., An L.L., Chaerkady R., et al. Evidence for a direct link between PAD4-mediated citrullination and the oxidative burst in human neutrophils. Sci Rep. 2018;8(1):15228. doi: 10.1038/s41598-018-33385-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirchner T., Hermann E., Möller S., et al. Flavonoids and 5-aminosalicylic acid inhibit the formation of neutrophil extracellular traps. Mediators Inflamm. 2013;2013:710239. doi: 10.1155/2013/710239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohammed B.M., Fisher B.J., Kraskauskas D., et al. Vitamin C: a novel regulator of neutrophil extracellular trap formation. Nutrients. 2013;5(8):3131–3151. doi: 10.3390/nu5083131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Röhm M., Grimm M.J., D'Auria A.C., Almyroudis N.G., Segal B.H., Urban C.F. NADPH oxidase promotes neutrophil extracellular trap formation in pulmonary aspergillosis. Infect Immun. 2014;82(5):1766–1777. doi: 10.1128/IAI.00096-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Metzler K.D., Fuchs T.A., Nauseef W.M., et al. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117(3):953–959. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eash K.J., Greenbaum A.M., Gopalan P.K., Link D.C. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. 2010;120(7):2423–2431. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Németh T., Sperandio M., Mócsai A. Neutrophils as emerging therapeutic targets. Nat Rev Drug Discov. 2020;19(4):253–275. doi: 10.1038/s41573-019-0054-z. [DOI] [PubMed] [Google Scholar]
- 48.Jaillon S., Ponzetta A., Di Mitri D., Santoni A., Bonecchi R., Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. 2020;20(9):485–503. doi: 10.1038/s41568-020-0281-y. [DOI] [PubMed] [Google Scholar]
- 49.Adrover J.M., Del Fresno C., Crainiciuc G., et al. A neutrophil timer coordinates immune defense and vascular protection. Immunity. 2019;50(2):390–402.e10. doi: 10.1016/j.immuni.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Barzilai A., Yamamoto K. DNA damage responses to oxidative stress. DNA Repair. 2004;3(8-9):1109–1115. doi: 10.1016/j.dnarep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Schumacher B., Pothof J., Vijg J., Hoeijmakers J.H.J. The central role of DNA damage in the ageing process. Nature. 2021;592(7856):695–703. doi: 10.1038/s41586-021-03307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azzouz D., Khan M.A., Palaniyar N. ROS induces NETosis by oxidizing DNA and initiating DNA repair. Cell Death Discov. 2021;7(1):113. doi: 10.1038/s41420-021-00491-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nair R.R., Madiwale S.V., Saini D.K. Clampdown of inflammation in aging and anticancer therapies by limiting upregulation and activation of GPCR, CXCR4. NPJ Aging Mech Dis. 2018;4:9. doi: 10.1038/s41514-018-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guan W.J., Ni Z.Y., Hu Y., et al. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zuo Y., Yalavarthi S., Shi H., et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5(11) doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Radhika A., Sudhakaran P.R. Upregulation of macrophage-specific functions by oxidized LDL: lysosomal degradation-dependent and -independent pathways. Mol Cell Biochem. 2013;372(1-2):181–190. doi: 10.1007/s11010-012-1459-8. [DOI] [PubMed] [Google Scholar]
- 58.Wang X.S., Kim H.B., Szuchman-Sapir A., McMahon A., Dennis J.M., Witting P.K. Neutrophils recruited to the myocardium after acute experimental myocardial infarct generate hypochlorous acid that oxidizes cardiac myoglobin. Arch Biochem Biophys. 2016;612:103–114. doi: 10.1016/j.abb.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 59.Vasilyev N., Williams T., Brennan M.L., et al. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation. 2005;112(18):2812–2820. doi: 10.1161/CIRCULATIONAHA.105.542340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
H2 Inhibited the Formation of NETs by Human Neutrophils. Neutrophils purified from the blood of healthy volunteers were primed with or without phorbol-12-myristate-13-acetate (PMA) in either a H2-exposed medium or control medium for 1 hour. Neutrophil aggregate formation in the hydrogen-exposed medium with PMA (left), the control medium without PMA (middle), and the control medium with PMA (right).