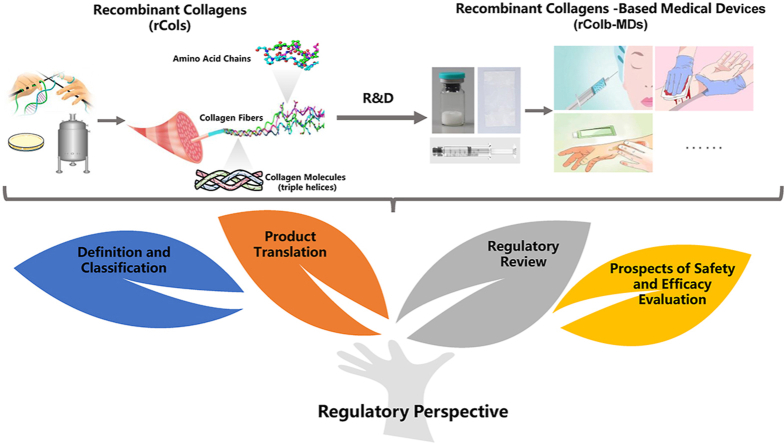
Abstract
As a class of novel biomaterials manufactured by synthetic biology technologies, recombinant collagens are candidates for a variety of medical applications. In this article, a regulatory scientific perspective on recombinant collagens and their medical devices is presented with a focus on the definition, translation, classification and technical review. Recombinant collagens are categorized as recombinant human collagen, recombinant humanized collagen and recombinant collagen-like protein, as differentiated by specific compositions and structures. Based on their intended uses and associated risks, recombinant collagen-based medical devices are generally classified as Class Ⅱ or Ⅲ in China. The regulatory review of recombinant collagen-based medical devices aims to assess their safety and efficacy demonstrated by scientific evidences generated from preclinical and clinical evaluations. Taken together, opportunities as well as challenges for their future clinical translation of recombinant collagen-based medical devices abound, which highlights the essential role of regulatory science to provide new tools, standards, guidelines and methods to evaluate the safety and efficacy of medical products.
Keywords: Collagen, Recombinant collagen, Product translation, Regulatory review, Safety and efficacy
Graphical abstract
Highlights
-
•
Recombinant collagens are novel biomaterials manufactured by biosynthesis methods.
-
•
The first regulatory article on recombinant collagen-based medical devices.
-
•
Recombinant collagen-based medical devices are defined and classified by NMPA.
-
•
Regulatory review assesses the safety and efficacy of medical devices.
-
•
Translation of recombinant collagens from bench to clinic needs regulatory science.
Collagen is the main structural protein of human tissues and organs. Currently, at least 28 types of collagen are reported, which account for about 30–40% of the total body proteins [1,2]. Collagen-based medical devices are widely used in a variety of clinical areas including general surgery [3,4], orthopedic [5,6], dental [7], and neurosurgery [8], as well as cosmetology [9]. At present, collagens used as raw materials for medical devices are mainly derived from animal tissues and allograft tissues [10]. The issues with animal- or allograft-sourced collagens include limited supply, immunogenicity risks as well as viral and other infectious agents. Such constraints restrict broader clinical applications of collagen-based medical devices. In addition, a major technical difficulty of animal-derived collagens is the purification of any specific type of collagen which generally contains heterogeneous subtypes.
Recombinant collagens (rCols) are protein products prepared with synthetic biology technologies [11,12]. Because mass production of naturally-derived biomaterials could be achieved through microbial fermentation technologies with high yield, low cost and less-limited raw material sources, the manufacture of some naturally-derived biomaterials including collagens are inclined to shift from animal tissue extraction to biosynthesis. For example, sodium hyaluronate was firstly found and extracted from rooster combs, but the majority of the commercial medical-grade sodium hyaluronate is now manufactured with synthetic biology [13,14]. With the advancement of technologies, it is expected that rCols may play an increasingly important role in the medical and healthcare field [15]. In recent years, several recombinant collagen-based medical devices (rColb-MDs) have been in clinical studies and/or approved by National Medical Products Administration of China (NMPA). In this article, a regulatory perspective on rCols and rColb-MDs is presented with a focus on the definition, translation, classification and technical review.
1. Definition, classification and product translation
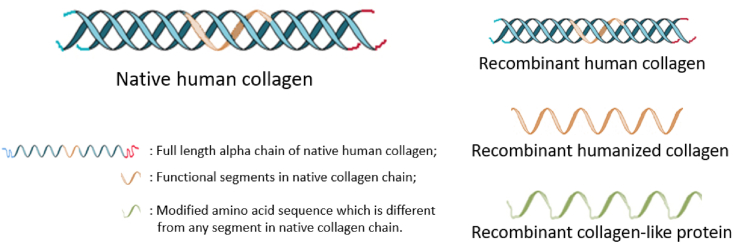
According to the different compositions and structures between rCols and native human collagens, rCols can be divided into one of the following three categories, schematically shown as Fig. 1 [16]. The first type is recombinant human collagen, which features a full-length amino acid sequence with a specific type of human collagen and a triple helix structure. The second type is recombinant humanized collagen, which contains a full-length or partial amino acid sequence fragment encoded by a specific type of human collagen gene, or a combination of functional fragments in native human collagen [17]. The third type is recombinant collagen-like protein, which is an amino acid sequence or fragment encoded by a specific designed or modified collagen gene, or a combination of such functional protein fragments. The gene coding sequence or amino acid sequence of recombinant collagen-like protein has low homology with that of native human collagen [18].
Fig. 1.
Schematic diagram of native human collagen and the classification of rCols.
Analogous to native collagen, rCols can be classified into different subtypes based on the corresponding collagen encoding genes. Specifically, recombinant type Ⅲ collagen is expressed based on full length or partial of the COL3A1 gene. In addition, as one of the merits of biosynthesis technology, the encoding gene could be optimized or combined from different types of collagens to express rCols which differ from any type of native collagen.
According to the risk degree of medical devices and the classification guidelines issued by NMPA, the recombinant collagen-based medical devices could be classified as follows [[19], [20], [21]]. A rColb-MD should be regulated as a Class III device if it is applied as an implant, or it would be absorbed when applied as a hemostatic or anti-adhesion device. A rColb-MD should be regulated as Class Ⅱ device if it is non-absorbable and applied only on body surface. For example, if a wound dressing product made of rCols could be partially or completely absorbed by the human body or such product is used for chronic wounds, it shall be regulated as Class III devices.
Furthermore, the amino acid sequence in rCols can be designed to achieve customized synthesis for specific requirements. Under this strategy, different types of native collagen including the subtypes with low abundance in human body could be manufactured. Moreover, after the identification and screening of functional segments in different types of collagen molecules, the customized and tailored combination of these segments may satisfy precise demands of regenerative medicine, which may create exciting future applications of rCols.
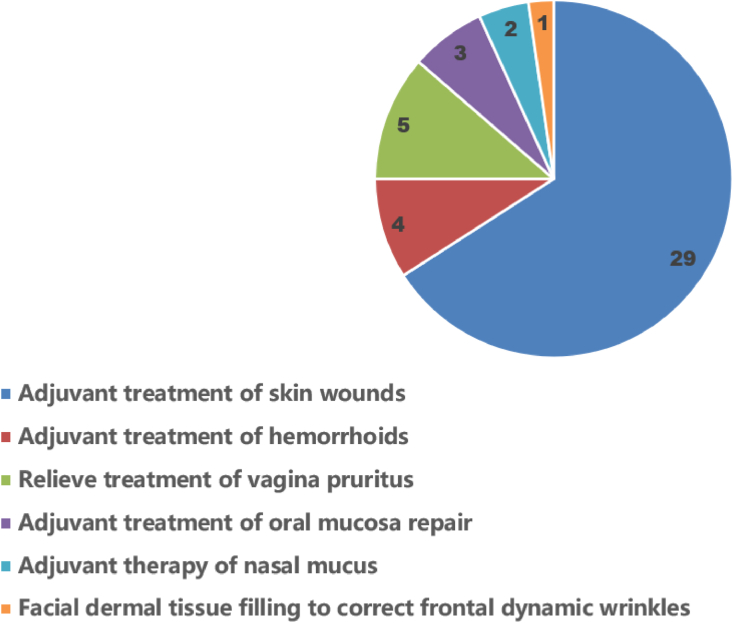
Currently, rCols have been investigated for clinical translations in many medical applications, including dermal filler [22], wound dressing [23], drug or cell carrier [24,25], bone void filler [26], and tissue engineering scaffolds [27,28]. After querying the NMPA device database [29], several rColb-MDs have been approved for market entry in China, and the statistic number of products with various expected uses is shown in Fig. 2. However, despite abundant opportunities as a class of novel biomaterials, rCols face several challenges for their future clinical translation, including but not limited to the developing of effective expression system with high yield and low cost, maintaining of collagen structure stabilization actuated by enzymatic processing, and implementing of efficacy validation.
Fig. 2.
The statistics number of rColb-MDs approved by NMPA for various clinical applications.
2. Regulatory review
Concerns related to the characteristics of the new rCols' materials in rColb-MDs, including some key issues in safety and performance evaluation should be focused during the technical review. The development of novel biomaterials could improve the performance and clinical efficacy of medical devices. On the flip side, the new materials may also bring unknown risks. The safety and efficacy evaluation of rColb-MDs should focus on the scientific evidence of the rCols and rColb-MDs and identify key issues in safety and performance evaluation. In addition, a series of their regulatory science research projects should be carried out. Based on the current scientific understanding of rCols, the general safety and efficacy evaluation concerns related to the characteristics of rCols materials will be discussed in the following paragraphs.
2.1. Material's characterization and analysis
The measurement and analysis of material characteristics is the basis to ensure the quality control of medical devices, design requirement satisfaction of product performance and achievement of clinical application goals. At present, ASTM standards and Chinese industry standards on characterization of animal-derived collagen can be referred [[30], [31], [32]], while items include identification, composition and structural characterization should be required for rCols as well. In terms of compositions of rCols, the following analyses are recommended in order to confirm the consistence with the designed amino acid sequence: the peptide segments at C-terminal and/or N-terminal of rCols, the peptide coverage rate and Lysine protease proteolysate measured by peptide mapping and high performance liquid chromatography-mass spectrometry (HPLC-MS), and the amino acid content tested by amino acid analysis or Ultraviolet spectrophotometry. Collagen has the characteristics of quaternary advanced structures, including triple helix which endows collagen with specific biological functions or effects. In terms of protein structure analysis of rCols, the following characterizations are recommended: the secondary structure (triple helix structure) of the functional region that is confirmed by atomic structure analysis, the structural characteristics that is evaluated by various types of spectral analysis, micro-differential scanning calorimeter (μDSC) analysis,protease sensitivity analysis, and the microstructure that is imaged by scanning electron microscope (SEM).
2.2. Impurities and residues
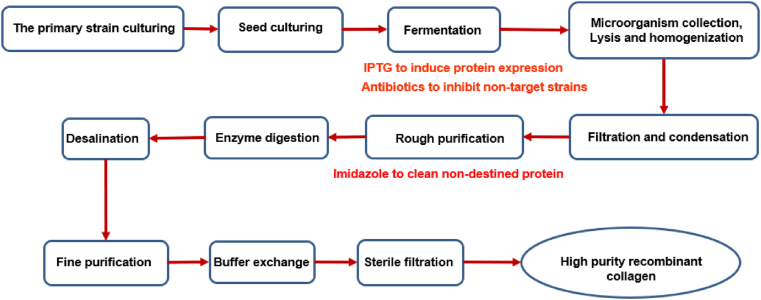
Impurities and residues in biological materials create major hidden risks to the safety of medical devices, which merits regulatory attention. If the rCol is prepared by fermentation of Escherichia coli and other microorganisms, there might be various microbial components at the initial stage of extraction. In addition, some additives may be used during the production and purification process. Fig. 3 presents a representative workflow of recombinant collagen manufacturing and the additives commonly used during this process. To ensure that the final product is a high-purity rCol, the followings are recommended to be tested and controlled: purity of rCol, contents of heavy metals and trace elements, residues of antibiotics, host cell protein (HCP), exogenous DNA, endotoxin, and residual additives such as isopropyl thiogalactoside (IPTG) and imidazole if applicable. Representative residues and impurities in recombinant collagen were exhibited in Table 1.
Fig. 3.
Representative manufacture workflow of recombinant collagen and additives used.
Table 1.
Representative residues and impurities in recombinant collagen.
| Residues and impurities | Necessity for manufacturing | Procedures or sources | Purposes |
|---|---|---|---|
| Imidazole | Yes | Rough purification | Cleaning solution for affinity chromatography |
| Isopropyl Thiogalactoside, IPTG | Yes | Fermentation | Induce protein expression |
| Antibiotics | Yes | Seed culturing and fermentation | Inhibit non-target strains |
| Exogenous DNA | No | Originate from exogenous microorganisms | NA |
| Host cell protein | No | Secreted by microorganisms | NA |
| Endotoxin | No | Originate from microorganisms | NA |
| Heavy metals and trace elements | No | Originate from raw materials and containers | NA |
2.3. Immunogenicity evaluation
Evaluation and verification of immunogenicity risks should be conducted for biological materials prepared through microbial fermentation methods. On one hand, the immunogenicity-related items such as exogenous DNA content and HCP residue can be specified in product specifications of rCols for quality control purposes. On the other hand, the immunogenicity risk of the rCols product can be evaluated through immunotoxicology testing. Compared with animal-derived collagens, the composition and structure of rCols are determined and analyzed more easily at molecular level. At the same time, the composition of recombinant collagen is simpler than that of animal-derived collagen, which facilitates purification and removal of various impurities, and these impurities are often the inducement of immunogenicity. Therefore, in theory, the risk of immunogenicity of this type of material is lower than that of comparable products.
2.4. Degradation and metabolism mechanism
The degradation and metabolism mechanisms of bio-absorbable materials have an important impact on long-term safety and efficacy. If the degradation and metabolism mechanisms of rCols are the same as those of native human collagen, the normal degradation and metabolism of rCols will not bring unknown risks. For various new rCols, it is recommended to evaluate their degradation and metabolism mechanisms, including the absorption and metabolism of degradation products.
2.5. Post-market clinical follow-up
Post-market clinical follow-up is necessary for durable implantable medical devices made of new materials. The process helps track long-term safety issues after implantation in the human body. The follow-up in pre-market clinical research of medical devices is often limited in duration. Therefore, long-term safety and efficacy evidence should be collected and reported by the manufacturers after the device has been marketed. In such cases, the specific clinical follow-up requirements will be indicated in the remark column of the device registration certificate. In case of a serious long-term adverse event of a device, the regulatory authority can take corresponding regulatory measures, including improving and standardizing product instructions, recalling defective products, and revoking registration certificates. Because rColb-MDs are devices composed of a new class of biomaterials, post-approval clinical follow-ups and post-market surveillance are recommended. Any adverse event related to rColb-MDs should be recorded, analyzed and reported. Special attentions should be paid to any abnormal immune response and metabolic function.
3. Prospects of safety and efficacy evaluation
The establishment of the evaluation system and updating of evaluation methods for the safety and efficacy tests of medical devices have accelerated the translation of new biomaterial into the industry. The NMPA initiated Chinese Drug Regulatory Science Action Plan (i.e., Action Plan), which is an implementation plan for regulatory scientific research on medical products, has promoted the establishment of the safety and efficacy evaluation system. NMPA launched its first and second Action Plan in April 2019 and March 2021, respectively [33,34]. In April 2021, Biomaterials Innovation and Cooperation Platform, led by NMPA was also established. The advancement of regulatory science has promoted medical product innovation and industry development. For example, Evaluation of the Performance, Safety and Efficacy for Innovative Biomaterials-based Medical Devices, which include rColb-MDs, is among one of the key projects of the Action Plan. In addition, a device master file of a recombinant collagen has also been successfully submitted to NMPA.
At present, methods used by regulatory agencies to evaluate the safety and efficacy of collagen-based devices are mainly aimed at native collagens, which are different from rCols. For example, native collagens are biomacromolecules generally insoluble in neutral solution and cannot be filtered to sterilize, while most of the rCols are relative smaller molecule than the native ones, can be dissolved in water and further filtered to sterilize. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) is used to measure the purity of animal-derived collagen by using collagenase which has specificity on native collagen but may not suitable for rCols. Thus, it is necessary to establish scientific evaluation methods for rColb-MDs. Characterization methods of material, structure, property and performance of rCols and rColb-MDs need to be scientifically evaluated and standardized. As a part of regulatory science, standards and guidance documents are very important for the effective translation of rColb-MDs from bench to clinic. The research on rColb-MDs is expected to provide new regulatory tools and methods for the evaluation of their safety and efficacy. The considerations on future research projects which may provide scientific evidence for the regulatory review of rColb-MDs are following.
-
●
rCol-cell interactions at molecular and cellular levels. As the representation of their bioactivity and bio-function, the effects of rColb-MDs on cell behaviors including adhesion, migration, proliferation and differentiation could provide evidence for the safety and efficacy evaluation of the products.
-
●
Secretion and construction of extracellular matrix (ECM). While the secretion of ECM including collagen and polysaccharide is an important cell behavior, the effects of rCols on newly formed ECM may provide evidence for their efficacy.
-
●
Efficacy of tissue repair and regeneration. Because some rCols are water-soluble, evaluation methods for a series of in vivo properties may need further studies. Such properties include, but not limited to the microscopic stability of the rColb-MDs, the microstructure and microenvironment of the rColb-MDs, and the synergistic compatibility between degradation of rColb-MDs and tissue regeneration.
-
●
Long-term safety and performance in the body. At the current stage, the long-term safety and efficacy of rColb-MDs are predicted through the comparative analyses between rCols and the human native collagens in terms of their structures, physical and chemical properties, as well as preclinical animal studies and clinical evaluations. As a new type of biomaterial, the long-term safety and performance of rCols as well the metabolic outcome after implantation in the body awaits comprehensive research. It is necessary to develop novel and effective tools and methods that can predict the long-term safety and performance of rColb-MDs in human body.
4. Conclusion
This is the first regulatory perspective on recombinant collagen-based medical devices. Recombinant collagens manufactured by biosynthesis methods are novel biomaterials that have potential to compete with and replace native collagens for many medical applications. With the advancement of regulatory science programs, NMPA in China has been promoting the research and development of innovative medical products including those made of novel biomaterials, such as recombinant collagens. NMPA has issued documents on the definition of recombinant collagens and classification of recombinant collagen-based medical devices. Based on the scientific evidence provided by both pre-clinical and clinical evaluations, regulatory agencies conduct regulatory reviews to assess the safety and efficacy of medical devices including those based on recombinant collagens. With more science-based approval of recombinant collagen-based devices by NMPA, the future bench-to-clinic translation of more recombinant collagen-based devices are expected.
Declaration of interest
The authors declare no competing financial interests in this work.
CRediT authorship contribution statement
Wenbo Liu: Conceptualization, Investigation, Writing – original draft. Hai Lin: Conceptualization, Investigation, Writing – original draft. Peng Zhao: Writing – review & editing, Writing – original draft. Lina Xing: Writing – review & editing. Jie Li: Writing – review & editing. Zehua Wang: Writing – review & editing. Shan Ju: Writing – review & editing. XinLi Shi: Investigation. Yinghui Liu: Investigation. Gang Deng: Supervision. Guobiao Gao: Supervision. Lei Sun: Supervision. Xindong Zhang: Supervision.
Acknowledgements
The authors thank the Center for Medical Device Standardization Administration of NMPA for its contributions in the definition of rCols and classification of rColb-MDs. This study was supported by the first batch of Chinese Drug Regulatory Science Action Plan (Regulatory science research on new materials for medical device) and the second batch of Chinese Drug Regulatory Science Action Plan (Research on safety and effectiveness evaluation of novel biomaterials).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Wenbo Liu, Email: liuwb@cmde.org.cn.
Hai Lin, Email: linhai028@scu.edu.cn.
References
- 1.Salvatore L., Gallo N., Natali M.L., Terzi A., Sannino A., Madaghiele M. Mimicking the hierarchical organization of natural collagen: toward the development of ideal scaffolding material for tissue regeneration. Frontiers in Bioengineering and Biotechnology. 2021;9:644595. doi: 10.3389/fbioe.2021.644595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bielajew B.J., Hu J.C., Athanasiou K.A. Collagen: quantification, biomechanics and role of minor subtypes in cartilage. Nature Reviews Materials. 2020;5(10):730–747. doi: 10.1038/s41578-020-0213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oosterlaken B.M., Vena M.P., de With G. In vitro mineralization of collagen. Adv. Mater. 2021;33(16):2004418. doi: 10.1002/adma.202004418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeung D.A., Kelly N.H. The role of collagen-based biomaterials in chronic wound healing and sports medicine applications. Bioengineering. 2021;8(1):8. doi: 10.3390/bioengineering8010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu L., Wei M. Biomineralization of collagen-based materials for hard tissue repair. Int. J. Mol. Sci. 2021;22(2):944. doi: 10.3390/ijms22020944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z., Du T., Ruan C., Niu X. Bioinspired mineralized collagen scaffolds for bone tissue engineering. Bioactive Materials. 2021;6(5):1491–1511. doi: 10.1016/j.bioactmat.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vallecillo C., Toledano-Osorio M., Vallecillo-Rivas M., Toledano M., Rodriguez-Archilla A., Osorio R. Collagen matrix vs. Autogenous connective tissue graft for soft tissue augmentation: a systematic review and meta-analysis. Polym. Bull. 2021;13(11) doi: 10.3390/Polym13111810. Artn 1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ucar B. Natural biomaterials in brain repair: a focus on collagen. Neurochem. Int. 2021;146:105033. doi: 10.1016/j.neuint.2021.105033. [DOI] [PubMed] [Google Scholar]
- 9.Sionkowska A., Adamiak K., Musial K., Gadomska M. Collagen based materials in cosmetic applications: a review. Materials. 2020;13(19):4217. doi: 10.3390/ma13194217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rezvani Ghomi E., Nourbakhsh N., Akbari Kenari M., Zare M., Ramakrishna S. Collagen-based biomaterials for biomedical applications. J. Biomed. Mater. Res. B Appl. Biomater. 2021 doi: 10.1002/jbm.b.34881. [DOI] [PubMed] [Google Scholar]
- 11.Fertala A. Three decades of research on recombinant collagens: reinventing the wheel or developing new biomedical products? Bioengineering. 2020;7(4):155. doi: 10.3390/bioengineering7040155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramshaw J.A.M., Werkmeister J.A., Glattauer V. Recent progress with recombinant collagens produced in Escherichia coli. Curr Opin Biomed Eng. 2019;10:149–155. doi: 10.1016/j.cobme.2019.06.001. [DOI] [Google Scholar]
- 13.Huang W.-C., Chen S.-J., Chen T.-L. Production of hyaluronic acid by repeated batch fermentation. Biochem. Eng. J. 2008;40(3):460–464. doi: 10.1016/j.bej.2008.01.021. [DOI] [Google Scholar]
- 14.Yao Z.-Y., Qin J., Gong J.-S., Ye Y.-H., Qian J.-Y., Li H., Xu Z.-H., Shi J.-S. Versatile strategies for bioproduction of hyaluronic acid driven by synthetic biology. Carbohydr. Polym. 2021;264:118015. doi: 10.1016/j.carbpol.2021.118015. [DOI] [PubMed] [Google Scholar]
- 15.Wang T., Lew J., Premkumar J., Poh C.L., Win Naing M. Production of recombinant collagen: state of the art and challenges. Eng. Biol. 2017;1(1):18–23. doi: 10.1049/enb.2017.0003. [DOI] [Google Scholar]
- 16.NMPA Guidelines for the naming and definition of recombinant collagen biomaterials. https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20210315175109170.html NMPA 2021 No. 21 Available at:
- 17.Hua C., Zhu Y., Xu W., Ye S., Zhang R.G., Lu L., Jiang S.B. Characterization by high-resolution crystal structure analysis of a triple-helix region of human collagen type III with potent cell adhesion activity. Biochem. Biophys. Res. Commun. 2019;508(4):1018–1023. doi: 10.1016/j.bbrc.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Z.Y., Fan D.D., Shang L.J. Exploring the potential of the recombinant human collagens for biomedical and clinical applications: a short review. Biomed. Mater. 2021;16(1):19. doi: 10.1088/1748-605X/aba6fa. [DOI] [PubMed] [Google Scholar]
- 19.NMPA . 2015. Principles for the Classification of Medical Devices.http://www.gov.cn/gongbao/content/2015/content_2961719.htm No.15, 2015) Available at: [Google Scholar]
- 20.NMPA . 2017. Classification Catalogue of Medical Devices.https://www.nmpa.gov.cn/directory/web/nmpa/xxgk/ggtg/qtggtg/20170904150301406.html No.104, 2017) Available at: [Google Scholar]
- 21.NMPA Principles for the classification of recombinant collagen medical products. https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20210415160940113.html NMPA 2021 No. 27 Available at:
- 22.Tytgat L., Markovic M., Qazi T.H., Vagenende M., Bray F., Martins J.C., Rolando C., Thienpont H., Ottevaere H., Ovsianikov A., Dubruel P., Van Vlierberghe S. Photo-crosslinkable recombinant collagen mimics for tissue engineering applications. J. Mater. Chem. B. 2019;7(19):3100–3108. doi: 10.1039/c8tb03308k. [DOI] [PubMed] [Google Scholar]
- 23.Ben C., Liu X., Shen T., Song Y., Li H., Pan B., Hou W., Liu T., Luo P., Ma B., Sun Y., Xiao S., Xia Z., Cheng D., Zhu S. A recombinant human collagen hydrogel for the treatment of partial-thickness burns: a prospective, self-controlled clinical study. Burns. 2021;47(3):634–642. doi: 10.1016/j.burns.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Munoz M.S., Confalonieri D., Walles H., van Dongen E.M.W.M., Dandekar G. Recombinant collagen I peptide microcarriers for cell expansion and their potential use as cell delivery system in a bioreactor model. Jove-Journal of Visualized Experiments. 2018;132 doi: 10.3791/57363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mashiko T., Takada H., Wu S.-H., Kanayama K., Feng J., Tashiro K., Asahi R., Sunaga A., Hoshi K., Kurisaki A., Takato T., Yoshimura K. Therapeutic effects of a recombinant human collagen peptide bioscaffold with human adipose-derived stem cells on impaired wound healing after radiotherapy. J. Tissue Eng. Regenerative Med. 2018;12(5):1186–1194. doi: 10.1002/term.2647. [DOI] [PubMed] [Google Scholar]
- 26.Furihata T., Miyaji H., Nishida E., Kato A., Miyata S., Shitomi K., Mayumi K., Kanemoto Y., Sugaya T., Akasaka T. Bone forming ability of recombinant human collagen peptide granules applied with beta-tricalcium phosphate fine particles. J. Biomed. Mater. Res. B Appl. Biomater. 2020;108(7):3033–3044. doi: 10.1002/jbm.b.34632. [DOI] [PubMed] [Google Scholar]
- 27.Tytgat L., Dobos A., Markovic M., Van Damme L., Van Hoorick J., Bray F., Thienpont H., Ottevaere H., Dubruel P., Ovsianikov A., Van Vlierberghe S. High-Resolution 3D bioprinting of photo-cross-linkable recombinant collagen to serve tissue engineering applications. Biomacromolecules. 2020;21(10):3997–4007. doi: 10.1021/acs.biomac.0c00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLaughlin S., McNeill B., Podrebarac J., Hosoyama K., Sedlakova V., Cron G., Smyth D., Seymour R., Goel K., Liang W., Rayner K.J., Ruel M., Suuronen E.J., Alarcon E.I. Injectable human recombinant collagen matrices limit adverse remodeling and improve cardiac function after myocardial infarction. Nat. Commun. 2019;10:4866. doi: 10.1038/s41467-019-12748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.NMPA National medical products administration data query. http://app1.nmpa.gov.cn/data_nmpa/face3/dir.html?CbSlDlH0=qGq2kAkAM91.Z8qGCFp1sLSfpjDx428M9BgF8hGZZ19qqD3 Available at.
- 30.ASTMF 2212 – 02 Standard Guide for Characterization of Type I Collagen as Starting Material for Surgical Implants and Substrates for Tissue Engineered Medical Products (TEMPs).
- 31.YY 0954-2015 Nonactive Surgical Implants- Type I Collagen Implants- Specific Requirements.
- 32.YY/T1453-2016 Tissue engineering medical device products- Methods for determination of type I collagen.
- 33.NMPA The second batch of key projects of China's drug regulatory scientific action plan is released. https://www.nmpa.gov.cn/yaowen/ypjgyw/20210628171415103.html Available at:
- 34.NMPA NMPA launched the regulatory science action plan for medical products in China. https://www.nmpa.gov.cn/directory/web/nmpa/yaowen/ypjgyw/20190430213401392.html Available at: