Abstract
Objectives
COVID-19 patients affected by haematological malignancies have a more severe course of the disease and higher mortality, prompting for effective prophylaxis. The present study aims to evaluate the humoral response after mRNA vaccination as well as the impact of a third vaccine dose in patients with lymphoid malignancies.
Methods
We conducted a single-centre study, evaluating the serological responses of mRNA vaccination amongst a cohort of 200 patients affected by lymphoid malignancies after two or three doses using an industrial SARS-CoV-2 serology assay for anti-receptor binding domain (RBD) Spike IgG detection and quantification.
Results
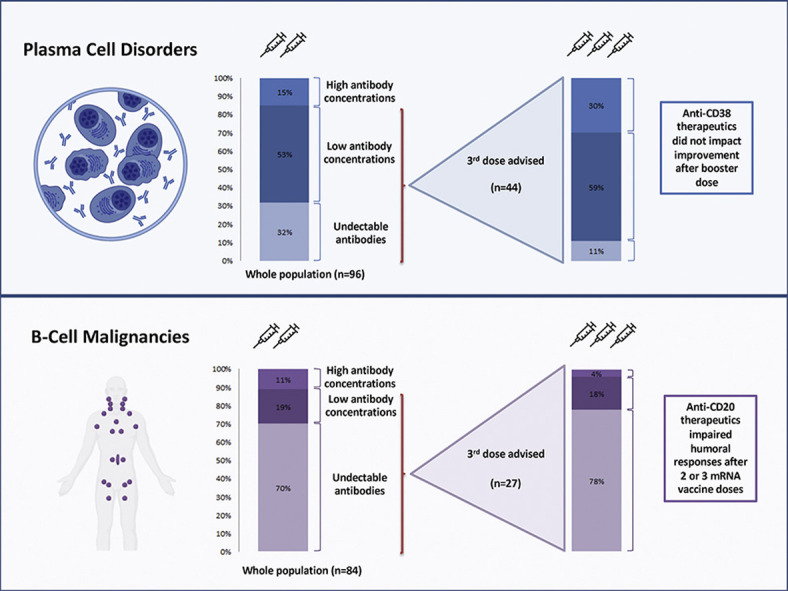
Among patients with plasma cell disorders, 59 of 96 (61%) had seroconversion (anti-RBD >50 AU/mL), and recent anti-CD38 therapies were associated with lower serological anti-RBD IgG concentrations (median IgG concentration 137 (IQR 0–512) AU/mL vs. 543 (IQR 35–3496) AU/mL; p < 0.001). Patients with B-cell malignancies had a lower seroconversion rate (20/84, 24%) mainly due to the broad usage of anti-CD20 monoclonal antibodies; only 2 of 53 (4%) patients treated by anti-CD20 antibodies during the last 12 months experienced a seroconversion. A total of 78 patients (44 with plasma cell disorders, 27 with B-cell malignancies, and 7 with other lymphomas) received a third dose of vaccine. The seroconversion rate and antibody concentrations increased significantly, especially in patients with plasma cell disorders, where an increment of anti-RBD IgG concentrations was observed in 31 of 44 (70%) patients, with an anti-RBD concentration median-fold increase of 10.6 (IQR 2.4–25.5). Its benefit in B-cell malignancies is uncertain, with only 2 of 25 (8%) patients having seroconverted after the vaccine booster, without increased median antibody concentration.
Discussion
A third mRNA vaccine dose significantly improved humoral responses among patients with plasma cell disorders, whereas the effect was limited among patients with B-cell malignancies.
Keywords: COVID-19, Haematologicalmalignancies, Immunocompromised, Third dose, Vaccine
Graphical abstract
Introduction
Early in the COVID-19 pandemic a more severe course of disease and higher mortality were reported among patients with malignant hemopathies [1]. Current French guidelines advise a third dose, with prioritization to immunocompromised recipients [2]. A third dose of vaccine in solid organ transplant recipients was able to significantly improve its immunogenicity [3], but available data among haematological patients remain scarce and incomplete. Previous reports showed particularly low response rates (especially with anti-CD20 therapy) among patients with lymphoid malignancies, and the effect of treatment such as anti-plasma cell drugs remains controversial [4]. Here, we report the anti-SARS-CoV-2 antibody (anti-receptor binding domain (RBD) IgG) response in vaccinated patients with lymphoid haematological malignancies in a tertiary centre and immunogenicity of a third dose as boost in a subgroup of this population.
Methods
We conducted a single-centre cohort study evaluating immunological responses among patients treated for lymphoid malignancies in a tertiary centre. All patients with active follow-up in our centre were offered an on-site vaccination with the BNT162b2 mRNA vaccine beginning January 1, 2021 (two injections scheduled 21 days apart). Since March 2021, patients were advised to receive a third injection of vaccine. Humoral response was evaluated by SARS-CoV-2 serology using the IgG II Quant Assay (Abbot Laboratories, Wiesbaden, Germany) for anti-RBD Spike (S) IgG detection and quantification. A quantifiable anti-RBD IgG concentration above the manufacturer-defined threshold (50 AU/mL) was considered seroconversion. We stratified samples by anti-RBD IgG concentrations above or below 1000 AU/mL and 4160 AU/mL, associated with a high probability of virus neutralization against several variants of concern in in vitro plaque reduction assays [5,6]. The study was approved by local ethics committee and in accordance with the 1964 Helsinki Declaration. Categorical variables were expressed as percentages and quantitative variables as median (interquartile range, IQR). Statistical comparisons were performed using the Wilcoxon test and the Kruskal-Wallis nonparametric tests as appropriate. Additional details regarding immunological response evaluation, statistics, and ethics are available in the Appendix S1.
Results
The study included a total of 200 nonselected patients consecutively followed in our centre between January 1, 2021 and July 14, 2021. Baseline characteristics are shown in Table 1 . The seroconversion rate ranged from 3 of 20 (15%) patients with follicular lymphoma to 8 of 9 (89%) patients with Hodgkin lymphoma (Fig. 1 (a)). Aiming to assess factors affecting vaccine response, we clustered lymphoid diseases into groups based on common ontogeny and treatment type. Plasma cell disorders (PCDs) included multiple myeloma, plasma cell leukaemia, and light chain amyloidosis; B-cell malignancies (BCM) included aggressive, indolent B-cell non-Hodgkin lymphomas and chronic lymphocytic leukaemia; and other lymphomas included Hodgkin and T cell lymphomas.
Table 1.
Baseline characteristics
| All (n = 200) | Plasma cell dyscrasias (n = 96) | B-cell malignancies (n = 84) | Other haematological malignancies (n = 20) | |
|---|---|---|---|---|
| Age (y), median (interquartile range) | 70 (59–76) | 70 (61.5–76) | 71 (59–76) | 56.5 (46.25–70.75) |
| BMI (kg/m2), median (interquartile range) | 24.00 (22.00–27.00) | 23.50 (22.00–27.00) | 24.00 (22.00–26.25) | 24.00 (20.50–26.00) |
| Female gender, n (%) | 83 (42) | 43 (45) | 33 (39) | 7 (35) |
| Disease response status, n (%) | ||||
| Partial/complete responses | 136 (68) | 70 (76) | 53 (63) | 13 (65) |
| Stable disease | 8 (4) | 6 (6) | 2 (2) | 0 (0) |
| Progressive disease | 36 (18) | 16 (17) | 16 (19) | 7 (35) |
| Treatment status, n (%) | ||||
| Naïve | 16 (8) | 4 (4) | 10 (12) | 0 (0) |
| On therapy | 151 (76) | 83 (87) | 53 (63) | 18 (90) |
| Off therapy— complete/partial response | 22 (11) | 8 (8) | 12 (14) | 1 (5) |
| Off therapy—relapse | 11 (6) | 1 (1) | 9 (11) | 1 (5) |
| Number of prior lines of therapy, n (interquartile range) | 5 (3–5) | 5 (3–6) | 3 (3–5) | 1 (1–2) |
| Previous autologous haematopoietic stem cell transplantation, n (%) | 39 (20) | 29 (30) | 6 (7) | 4 (20) |
| <12 mo before vaccination, n (%) | 5 (3) | 4 (4) | 0 | 1 (5) |
| ≥12 mo before vaccination, n (%) | 34 (17) | 25 (26) | 6 (7) | 3 (15) |
| Malignancy type, n (%) | — | Multiple myeloma 68 (71) Amyloidosis 28 (29) |
CLL 12 (14) FL 20 (24) MCL 9 (11) MZL 12 (14) DLBCL 31 (37) |
Hodgkin lymphoma 9 (45) T cell lymphoma 10 (50) Other 1 (5) |
| Receiving specific immunological therapy, n (%) | — | Anti-CD38 therapy: 66 (69) | Anti-CD20 therapy:70 (83) | Anti-CD20 therapy: 4 (20) |
| Previous vaccination in the last 6 mo | 52 (54) | 46 (55) | 1 (5) | |
| 6–12 mo before vaccination | 2 (2) | 7 (8) | 1 (5) | |
| >12 mo before vaccination | 12 (13) | 16 (19) | 2 (10) | |
| Third vaccine injection, n (%) | 78 (39) | 44 (46) | 27 (32) | 7 (35) |
BMI, body mass index; CLL, chronic lymphoid leukaemia; DLBC, diffuse large B cell lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma and lymphoplasmacytic lymphoma/Waldenström macroglobulinemia.
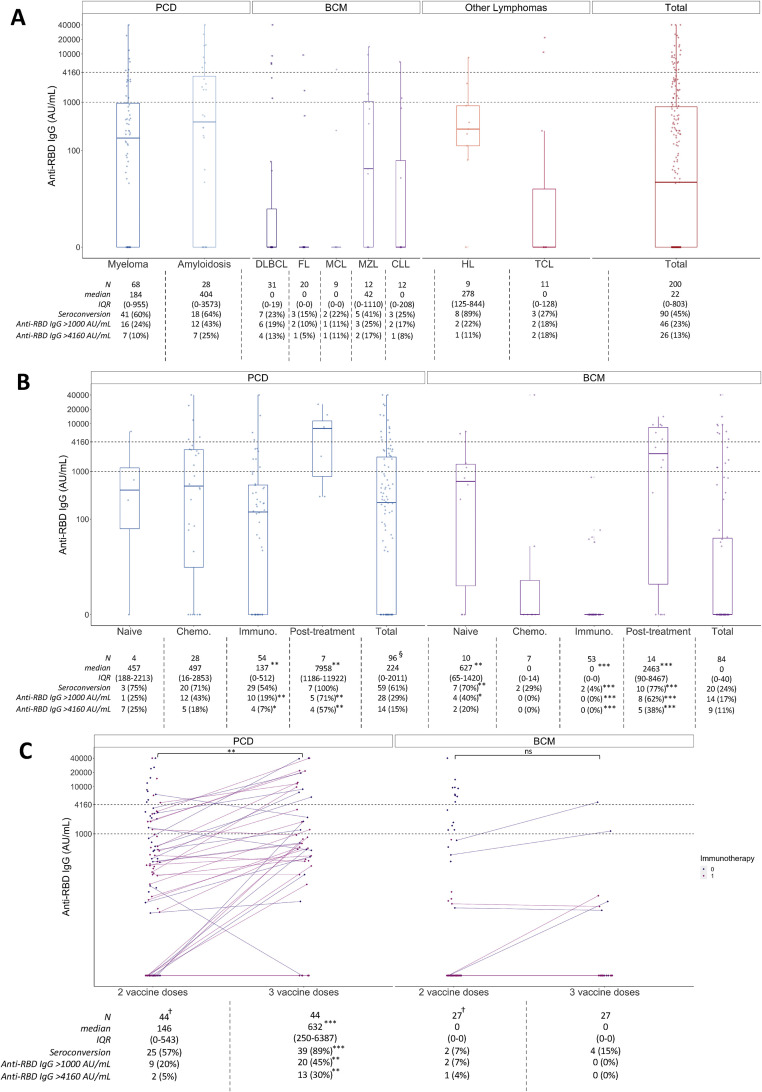
Fig. 1.
Immunogenicity of two and three mRNA vaccines doses among patients with lymphoid malignancies. Graphics display a representation of the median anti-RBD IgG concentration value (IQR) through boxplot representation. To appreciate the data dispersion, interquartile range was included in the attached tables. (a) Anti-RBD IgG concentration (AU/mL) according to underlying haematological pathology, after two mRNA vaccine doses. (b) Anti-RBD IgG concentration (AU/mL) according to main pathology groups and treatment characteristics, after two mRNA vaccine doses. “Naïve” condition represents patients who received at least one vaccine dose before initiation any treatment; “Chemotherapy” condition represents patients who only received in the previous year standard cytotoxic chemotherapy as treatment; “Immunotherapy” condition represents patients who received in the previous year either anti-CD20 or anti-CD38 therapy (accordingly to the underlying disease); “Post-treatment” condition represents patients who had their last treatment at least 12 months before vaccination. Statistical analyses were made by comparing each group to the other three. (c) IgG anti-RBD IgG concentrations (AU/mL) after two and three mRNA vaccine doses, according to main pathology groups and stratified into subgroups based on the use of immunotherapy in last 12 months (anti-CD20 or anti-CD38 accordingly to the underlying disease). CLL, chronic lymphoid leukaemia; DLBC, diffuse large B cell lymphoma; FL, follicular lymphoma; HL, Hodgkin lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma and lymphoplasmacytic lymphoma/Waldenström macroglobulinemia; TCL, T cell lymphoma. †Graphic displays the whole population vaccinated with two doses to identify the patients selected for a third vaccine dose (44 and 27 patients in PCD and BCM groups, respectively); attached table describes the population who received three vaccine doses. §Three patients affected by plasma cell disorders did not have clear or available treatment information. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ns: not statistically significant.
In the 96 patients with PCD, 59 of 96 (61%) seroconverted, and the median anti-RBD concentration was 223.5 AU/mL (IQR 0–2011) (Fig. 1(b)). Being actively treated in the previous 6 months (83/96) was associated with a lower anti-RBD IgG concentration (147 (IQR 0–978) vs. 5245 (IQR 297–8534) AU/mL; p 0.001). More specifically, use of anti-CD38 antibodies in the last 12 months (54/96 (56%) patients, including 52 in the last 6 months) was not associated with lower seroconversion rates (seroconversion observed in 29/54 (54%) vs. 31/42 (74%) among patients with or without such treatment respectively (p = 0.07)), but fewer patients reached the thresholds of 1000 (10/54 (19%) vs. 18/48 (38%), p = 0.005) and 4160 AU/mL (4/54 (7%) vs. 11/42 (26%), p = 0.008).
Among the 84 patients with BCM, 20 (24%) seroconverted and the median anti-RBD concentration was 0 AU/mL (IQR 0–40) (Fig. 1(b)). We observed seroconversion in 18 of 31 (58%) patients who did not receive anti-CD20 treatment in the 12 months prior to vaccination, but only in 1 of 46 (2%) patients who received anti-CD20 antibodies in the last 6 months (with anti-RBD concentration <1000 AU/mL). Although limited by a small number of patients, a similar magnitude of seroconversion was observed when anti-CD20 was administered 6 to 12 months before vaccination (1/7 (14%); p = 0.7). On the other hand, seven of ten (70%) patients who received at least one vaccine dose before starting any treatment (n = 10) had a seroconversion, the median time before treatment being 16 days (IQR 5–29).
In a multivariate analysis, factors affecting vaccine humoral response in patients with lymphoid malignancies were age (p < 0.001), number of prior lines of therapy (p = 0.01), and recent treatment by anti-CD20/CD38 antibodies (<12 months) (p = 0.007) (Table S1).
A total of 78 patients received a third BNT162b2 dose. Forty-four PCD patients (46%) received a third dose of mRNA vaccine (median time from second dose: 70 days (IQR 47–87); median anti-RBD concentration after 2 doses 146 AU/mL (IQR 0–543)). An increment of IgG anti-RBD concentration was observed in 31 patients (70%), with a median-fold increase of 10.6 (IQR 2.4–25.4; p < 0.001), whereas 14 patients (32%) who had undetectable levels of anti-RBD antibodies after two doses seroconverted (Fig. 1(c)). Treatment with anti-CD38 antibody did not alter the response improvement (median increase of 450 (IQR 0–2204) vs. 466 (IQR 77–5791) AU/mL; p = 0.8).
A total of 27 patients with BCM received a third dose of BNT162b2 vaccine (median time from second dose: 58 days (IQR 43–88); median anti-RBD concentration after two doses: 0 (IQR 0–0) AU/mL). The seroconversion rate increased modestly (4/27 (15%) to 6/27 (22%)), and only 2 of 27 patients (without anti-CD20 treatment in the last year) reached the 1000 AU/mL threshold.
Discussion
The results of the present study show a large heterogeneity in anti-RBD IgG concentration levels among patients with lymphoid malignancies, reflecting the diversity of malignancies and treatments [7,8]. Anti-CD20 immunotherapies were associated with negligible serological responses. The extent of anti-CD38 treatments impairment on vaccine immunogenicity is unclear [9,10]. Our study showed a significantly reduced proportion of patients with anti-RBD concentrations above predefined thresholds compared to patients receiving only cytotoxic chemotherapy.
To our knowledge, this is the first available study evaluating the effect of a third vaccine dose in a significant number of patients affected by lymphoid malignancies. We showed a relevant humoral response improvement in patients affected by PCD, including patients treated by anti-CD38 therapies. In contrast, the effect of the third dose in BCM patients appeared limited [11]. It remains crucial to determine the timing of the booster dose from last administration of anti-CD20, which could be guided by monitoring B cell restoration [12].
Our study has some limitations. First, some analyses were limited by a small number of patients. Second, while persistence of specific antibodies in COVID-19 patients with haematologic malignancies has been reported [13], the dynamic of vaccine-induced antibodies across time has yet to be explored. Third, predefined antibody concentration thresholds have been established among healthy people after natural infection, and no clear correlation with clinical protection has been demonstrated. The thresholds used in this study encompass a rather large range of antibody concentrations and should be evaluated through neutralization capacity of the antibodies obtained in this population, as well as the incident infection rate. Fourth, our study did not evaluate T cell responses, which could play an important role in controlling infection [14,15].
As new variants of concern continue to emerge worldwide, updated data regarding the efficacy of the available vaccine in one of the most vulnerable populations is critical. Our study suggests that a third vaccine dose could increase the seroconversion rate and antibody concentrations, especially in patients with PCD, whereas its benefit among patients receiving anti-CD20 therapy is uncertain, prompting alternative protective strategies.
Transparency declaration
L. Roulin received travel grants from Janssen. F. Lemonnier received research funding from Institut Roche and a travel grant from Gilead. F. Le Bras received honoraria and research funding from Takeda, honoraria from Kite Gilead, honoraria from Novartis, and research funding from Celgene BMS. K. Belhadj received nonfinancial support from Abbvie. C. Haioun received honoraria and research funding from Amgen, Celgen, Gilead, Janssen, and Novartis. F. Hoffmann-La Roche Ltd, Servier, Takeda. and Miltenyi. G. Melica received nonfinancial support from MSD and research grants (Pfizer ID WS2165564 - Drevac Study) and nonfinancial support from Pfizer. No specific funding was received for this study.
Author contributions
S.B. Gressens: conceptualization, formal analysis, investigation, data curation, writing – original draft, visualization. S. Fourati: conceptualization, methodology, investigation, writing – review and editing, supervision. A. Le Bouter, F. Le Bras, J. Dupuis, M. Hammoud, T. El Gnaoui, R. Gounot, L. Roulin, K. Belhadj: investigation, resources, writing – review and editing. C. Haioun: investigation, resources, project administration, writing – review and editing. S. Gallien: investigation, resources, project administration, supervision, writing – review and editing. G. Melica: conceptualization, investigation, resources, supervision, writing – review and editing, project administration. F. Lemonnier: conceptualization, investigation, resources, supervision, writing – review and editing, project administration.
Acknowledgements
The Graphical abstract was created with BioRender.com.
Editor: L. Kaiser
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.02.029.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Duléry R., Lamure S., Delord M., Di Blasi R., Chauchet A., Hueso T., et al. Prolonged in-hospital stay and higher mortality after Covid-19 among patients with non-Hodgkin lymphoma treated with B-cell depleting immunotherapy. Am J Hematol. 2021;96:934–944. doi: 10.1002/ajh.26209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.France recommandations vaccins - COVID-19. Haute Autorité de Santé. Date of access : August 23rd 2021. Available from: https://www.has-sante.fr/upload/docs/application/pdf/2021-08/avis_n2021.0061.ac.seesp_du_23_aout_2021_du_college_de_la_has_sur_la_campagne_de_rappel_vaccinal_contre_la_covid_19.pdf
- 3.Kamar N., Abravanel F., Marion O., Couat C., Izopet J., Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Oekelen O., Gleason C.R., Agte S., Srivastava K., Beach K.F., Aleman A., et al. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell. 2021;39:1028–1030. doi: 10.1016/j.ccell.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebinger J.E., Fert-Bober J., Printsev I., Wu M., Sun N., Prostko J.C., et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021;27:981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monin-Aldama L., Laing A.G., Muñoz-Ruiz M., McKenzie D.R., del Molino del Barrio I., Alaguthurai T., et al. Oncology; 2021. Interim results of the safety and immune-efficacy of 1 versus 2 doses of COVID-19 vaccine BNT162b2 for cancer patients in the context of the UK vaccine priority guidelines.http://medrxiv.org/lookup/doi/10.1101/2021.03.17.21253131 [Internet]Mar [cited 2021 Jul 9]. Available from: [Google Scholar]
- 7.Peeters M., Verbruggen L., Teuwen L., Vanhoutte G., Vande Kerckhove S., Peeters B., et al. Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO Open. 2021;6:100274. doi: 10.1016/j.esmoop.2021.100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moor M.B., Suter-Riniker F., Horn M.P., Aeberli D., Amsler J., Möller B., et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021;3:e789–e797. doi: 10.1016/S2665-9913(21)00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pimpinelli F., Marchesi F., Piaggio G., Giannarelli D., Papa E., Falcucci P., et al. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: preliminary data from a single institution. J Hematol Oncol. 2021;14:81. doi: 10.1186/s13045-021-01090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghandili S., Schönlein M., Lütgehetmann M., Schulze zur Wiesch J., Becher H., Bokemeyer C., et al. Post-vaccination anti-SARS-CoV-2-antibody response in patients with multiple myeloma correlates with low CD19+ B-lymphocyte count and anti-CD38 treatment. Cancers. 2021;13:3800. doi: 10.3390/cancers13153800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberger L.M., Saltzman L.A., Senefeld J.W., Johnson P.W., DeGennaro L.J., Nichols G.L. Anti-spike antibody response to SARS-CoV-2 booster vaccination in patients with B cell-derived hematologic malignancies. Cancer Cell. 2021;39:1297–1299. doi: 10.1016/j.ccell.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mrak D., Tobudic S., Koblischke M., Graninger M., Radner H., Sieghart D., et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann Rheum Dis. 2021;80:1345–1350. doi: 10.1136/annrheumdis-2021-220781. [DOI] [PubMed] [Google Scholar]
- 13.Cattaneo C., Cancelli V., Imberti L., Dobbs K., Sottini A., Pagani C., et al. Production and persistence of specific antibodies in COVID-19 patients with hematologic malignancies: role of rituximab. Blood Cancer J. 2021;11:151. doi: 10.1038/s41408-021-00546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bange E.M., Han N.A., Wileyto P., Kim J.Y., Gouma S., Robinson J., et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat Med. 2021;27:1280–1289. doi: 10.1038/s41591-021-01386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felten R., Gallais F., Schleiss C., Chatelus E., Javier R.-M., Pijnenburg L., et al. Cellular and humoral immunity after the third dose of SARS-CoV-2 vaccine in patients treated with rituximab. Lancet Rheumatol. 2022;4:e13–e16. doi: 10.1016/S2665-9913(21)00351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.