Abstract
Leishmaniae are obligatory intracellular protozoa in mononuclear phagocytes. They cause a spectrum of diseases, ranging in severity from spontaneously healing skin lesions to fatal visceral disease. Worldwide, there are 2 million new cases each year and 1/10 of the world's population is at risk of infection. To date, there are no vaccines against leishmaniasis and control measures rely on chemotherapy to alleviate disease and on vector control to reduce transmission. However, a major vaccine development program aimed initially at cutaneous leishmaniasis is under way. Studies in animal models and humans are evaluating the potential of genetically modified live attenuated vaccines, as well as a variety of recombinant antigens or the DNA encoding them. The program also focuses on new adjuvants, including cytokines, and delivery systems to target the T helper type 1 immune responses required for the elimination of this intracellular organism. The availability, in the near future, of the DNA sequences of the human and Leishmania genomes will extend the vaccine program. New vaccine candidates such as parasite virulence factors will be identified. Host susceptibility genes will be mapped to allow the vaccine to be targeted to the population most in need of protection.
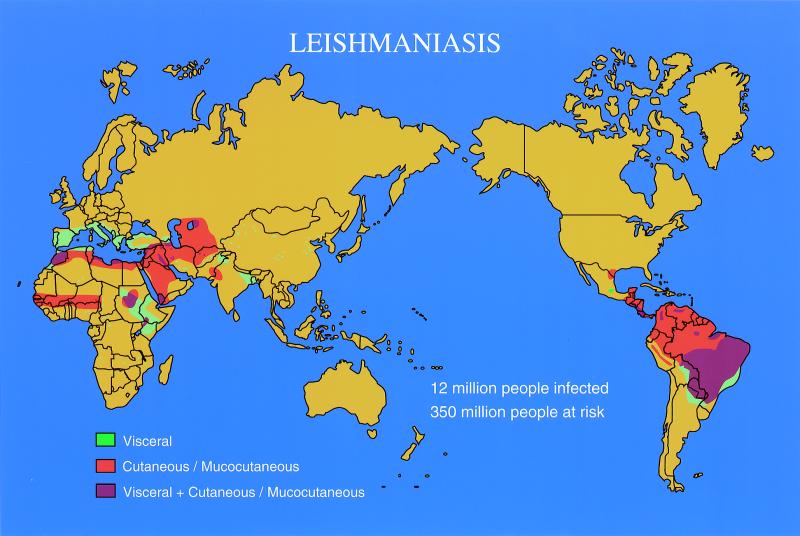
Although leishmaniasis is not a household name like malaria, the diseases caused by infection with Leishmania continue to have a major impact on much of the world's population. Worldwide, there are 2 million new cases each year and 1/10 of the world's population is at risk of infection (World Health Organization, Leishmaniasis Control home page: http://www.who.int/ctd/html/leis.html). The disease is endemic throughout parts of Africa, India, the Middle East, southern Europe, and Central and South America, and epidemics are also well recognized (Fig. 1). For example, more than 10% of the population died from visceral leishmaniasis over the last few years in Southern Sudan (85). With the advent of the human immunodeficiency virus (HIV) epidemic, leishmaniasis has surged as a reactivating infection in AIDS patients in many parts of the world (150; World Health Organization, Report Consult. Meet. Leishmania/HIV Coinfect., p. 6, 1994).
FIG. 1.
World map highlighting areas where cutaneous, visceral, and mucocutaneous leishmaniasis is endemic.
Current control measures rely on chemotherapy to alleviate disease and on vector control to reduce transmission. To date, there are no vaccines against leishmaniasis. However, there is consensus that in the longer term, vaccines ought to become a major tool in the control of this group of diseases. Unfortunately, the development of vaccines has been hampered by significant antigenic diversity and the fact that the parasites have a digenetic life cycle in at least two hosts (sandfly vector and human, but there is also an animal reservoir). An equally important consideration for the design and implementation of anti parasite vaccines in general is the contribution of the genetics of the target host population and their susceptibility to infection and disease, i.e. the severity of disease manifestations. The population most in need of protection may be the one which is normally unable to mount an appropriate innate or adaptive immune response and is therefore most susceptible to disease.
Clinical Leishmaniasis
The six species of Leishmania recognized to cause disease in humans (Table 1) are very similar morphologically but produce strikingly different pathological responses. The only feature common to all is the chronicity of disease manifestations. The infection may be predominantly visceral, as in visceral leishmaniasis or Indian kala-azar, or restricted to the skin, as with the chronic ulcer of Oriental sore, or spreading to the mucous membranes to produce the disfiguring South American espundia.
TABLE 1.
Leishmania species pathogenic for humans, their vectors, host range and disease manifestations
| Species | Host range | Main vector | Disease manifestations |
|---|---|---|---|
| L. donovani | Dogs, savannah rodents, humans | P. argentipes, L. longipalpis | Visceral leishmaniasis (kala azar), PKDL |
| L. major | Desert and savannah rodents; Rhombomys, Psammomys, Arvicanthis | P. papatasi | Cutaneous leishmaniasis, (rural, wet Oriental sore) |
| L. tropica | Humans | P. sergenti | Cutaneous leishmaniasis (urban, dry Oriental sore), visceral leishmaniasis |
| L. aethiopica | Rock hyrax | P. longipes | Cutaneous leishmaniasis, diffuse cutaneous leishmaniasis |
| L. braziliensis complex | Sloth, dog | L. umbratilis and many others | Cutaneous leishmaniasis, mucocutaneous leishmaniasis |
| L. mexicana complex | Forest rodents | L. flaviscutellata, L. olmeca | Cutaneous leishmaniasis, diffuse cutaneous leishmaniasis |
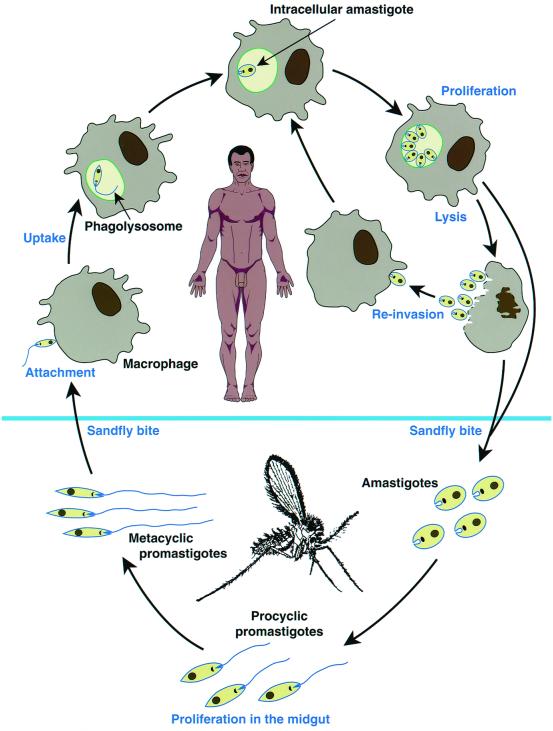
In the vertebrate host, Leishmania parasites survive and multiply intracellularly in mononuclear phagocytes as nonmotile amastigotes, about 2 to 4 μm in diameter (Fig. 2). However, recent evidence indicates that cells other than mononuclear phagocytes, for example fibroblasts, may also harbor parasites (129). Transmission to the vertebrate host is from Phlebotomus sandflies, in which parasites develop and replicate as 20-μm flagellated promastigotes. In the fly, the parasites undergo a developmental program starting with the amastigote in the blood meal, continuing through several stages of promastigote maturation, and culminating with the infectious metacyclic form. The environmental cues which trigger this program are not well understood, but temperature and pH appear to play a role.
FIG. 2.
Schematic diagram of the Leishmania digenetic life cycle.
Leishmaniasis is considered a zoonosis, and humans are generally accidental hosts. An important exception to the zoonotic character of leishmaniasis is that the reservoir for cutaneous disease caused by Leishmania tropica in the Middle East and visceral disease in India is probably made up of other infected humans. The animal reservoir shows geographic variation and includes rodents, dogs, and other mammals.
Self-limiting cutaneous and visceral leishmaniasis.
It is often assumed that the type of disease is determined by the species of the parasite, but this may be an oversimplification. The genetics and immunocompetence of the host may be equally important for some parasite species. Self-healing skin ulcers are caused by L. major, L. tropica, L. aethiopica, and subspecies of L. mexicana. However, strains which are normally dermotropic may migrate to the draining lymph nodes and may even visceralize (136, 105).
In general, skin lesions caused by the dermotropic Leishmania species may be mild or severe ulcers which eventually heal, provoking solid immunity and leaving the individual resistant to reinfection. Cell-mediated immunity underpins this process, as reflected by strong delayed-type hypersensitivity reactions or by in vitro T-cell assays. Histologically, a granuloma composed of a prominent infiltration of lymphocytes, epithelioid cells, and parasites is the hallmark of the syndrome. An interesting feature of leishmaniasis is that despite the disappearance of the lesion and resistance to reinfection, residual parasites remain in the host, probably for a very long time, if not forever. They can be reactivated by trauma or immunosuppression, although visceral rather than cutaneous leishmaniasis is the common reactivating syndrome in AIDS patients (10, 149).
Several related species of Leishmania, i.e., L. donovani (India and Africa), L. infantum (Mediterranean region, the Middle East, and Asia) and L. chagasi (South America), appear to home to visceral organs and lead to marked alterations in the function of the spleen, liver, and bone marrow. In these instances, the initial site of infection is rarely observed and skin lesions at the presumed site of the sandfly bite are rare. However, parasites are sometimes isolated from the skin. The skin becomes a major focus of infection in the syndrome known as post-kala-azar dermal leishmaniasis (PKDL) in some patients treated with chemotherapy.
Infection does not necessarily lead to disease, and a significant proportion of the population in areas of endemic infection may harbor subclinical infection, which may only declare itself upon immunosuppression such as in AIDS patients (9, 12, 149).
The factors determining susceptibility or resistance to visceral leishmaniasis remain unclear, but the genetics of the host may play a major role. In diseased individuals, visceral leishmaniasis is characterized by intermittent fever, massive hepatosplenomegaly, anemia, thrombocytopenia, and polyclonal B-cell activation with hypergammaglobulinemia. Visceral disease is often fatal. A notable feature of clinical visceral leishmaniasis is the apparent cellular anergy to parasite antigens (119). This anergy may result from inappropriate antigen presentation and communication between the antigen-presenting cells and T cells and from the induction of cytokines with macrophage-inactivating properties (39).
Nonhealing and systemic leishmaniasis.
As mentioned above, some Leishmania species which usually cause self-healing infections can also cause visceralizing infection. In addition to these, there are three species noted for causing nonhealing cutaneous disease: L. tropica, L. aethiopica, and L. mexicana amazonensis. In leishmaniasis recidiva or lupoid leishmaniasis caused by L. tropica, parasites are scarce in the lesions but persist in the presence of strong cell-mediated immunity. The cause of the extensive dermal pathological response is thought to be the inappropriate host responses to the parasite. Diffuse cutaneous leishmaniasis lesions which do not heal have been described in Ethiopia and South America and have been attributed to L. aethiopica and L. mexicana amazonenesis, respectively. Most of these patients fail to display Leishmania-specific cell-mediated immunity. Histologically, the lesions contain numerous macrophages laden with parasites; they also have few lymphocytes and plasma cells.
An additional form of nonhealing leishmaniasis is espundia or mucocutaneous leishmaniasis, where the initial skin lesion may cure but metastatic lesions develop in the mucosa of the nasopharynx. Both cell-mediated immune responses and antibody responses are higher in these individuals than in patients with simple cutaneous leishmaniasis, suggesting that the host responses contribute greatly to the observed tissue damage. The granuloma in these cases is a mixture of lymphocytes and macrophages carrying few parasites (139). Tapia et al. have proposed that a defect in accessory signals and the secretion of cytokines in the skin lead to an impaired immune response to the parasite and also to tissue damage (139).
Relationships between pathogenesis and genetics of the host and parasite.
The clinical manifestations of leishmaniasis depend on the interaction between the genetics of the parasite and the genetics of the host. In human infections, the host population is heterogeneous and the parasites are not clonal, and this makes it difficult to dissect out the relative contributions of the parasite and the host.
Much of our knowledge of the contribution of host genetics to the pathogenesis of leishmaniasis comes from mouse models using cloned parasite lines and inbred mice, but these systems have obvious limitations and serve only as guides for exploration in humans. The elucidation of the complete sequence of the human genome should facilitate the mapping of human genes controlling susceptibility to leishmaniasis. On the other hand, the elucidation of the complete sequence of the Leishmania genome, which is well under way, should facilitate the discovery of genes which determine parasite virulence (18, 58).
Several host genes have been identified using genetic approaches in both mice and humans. The early discovery that susceptibility to L. donovani, Salmonella enterica serovar Typhimurium, and Mycobacterium bovis was partly controlled by a single gene on mouse chromosome 1 led to the isolation from humans and mice of the gene encoding natural resistance-associated macrophage protein 1 (NRAMP1) (15–17, 44). Disappointingly, recent data tend to indicate that this gene may not play a role in human leishmaniasis, in contrast to the wealth of data obtained in mice. The precise biological function of NRAMP1 is not yet known, but a closely related protein, NRAMP2, is a transporter of iron from endosomes to the cytoplasm (41).
The pathogenesis of mucocutaneous leishmaniasis remains a puzzle. Only a small percentage of infected individuals develop this grossly disfiguring complication. A study in a Venezuelan population demonstrated that particular alleles encoding the cytokines tumor necrosis factor alpha (TNF-α) and TNF-β were associated with significantly increased relative risks of mucocutaneous disease. TNF-α has also been implicated in susceptibility of mice. Mice lacking the TNF-α receptor could not heal cutaneous ulcers despite being able to control parasite replication (101). Obviously, molecules other than the TNF receptors must be involved in susceptibility to disease.
For a disease in which healing depends on the induction of T-cell immunity, it is not surprising that the major histocompatibility complex (MHC) has been implicated in susceptibility. It was shown that different MHC haplotypes in mice were associated with different degrees of susceptibility to visceral leishmaniasis (17). A role for the MHC in human cutaneous leishmaniasis has also been described in (71) and is supported by a genetic linkage study in mice (73, 148). These data add to the body of evidence supporting a role for the MHC in resistance to a variety of infectious diseases including leprosy, schistosomiasis, malaria, hepatitis B infection, and the progression of HIV infection to AIDS (76).
In summary, the current data indicate that susceptibility to leishmaniasis is controlled by many genes, including TNF, the MHC, NRAMP1, and others of unknown function. Susceptibility, resistance, and disease patterns probably depend on complex interactions between these genes (115).
Experimental Leishmaniasis
Many experimental models of leishmaniasis have been developed. These models have the major attraction of allowing control over the genetics of both the parasite and the host, but none entirely reproduces the disease in humans. One of the factors contributing to differences between humans and animal models is the size and nature of the parasite inoculum. In natural infections, the sandfly introduces into the skin a very small number (possibly as few as 100 to 1,000) metacyclic promastigotes together with strongly bioactive saliva, whereas in laboratory infections thousands to millions of culture-derived promastigotes or tissue-derived amastigotes are injected. The sandfly is a blood pool feeder, using its mandibles to cut a wound in the skin and sucking up the blood that accumulates. It is in this superficial pool that the infective parasite inoculum is deposited, most probably in a very small volume. In contrast, the laboratory infection is commonly done in relatively large volumes of 50 μl or more. In addition, in the laboratory the syringe-delivered parasites are deposited mostly subcutaneously or, in visceral leishmaniasis models, intravenously.
A better understanding of the molecular mechanisms involved in parasite maturation in the sandfly and the ability to mimic some of these in the laboratory are leading to much improved protocols for infection (36). Investigators are now using small numbers of in vitro-derived metacyclic promastigotes and intradermal rather than subcutaneous infection into the ears of mice (36).
Experimental models of cutaneous leishmaniasis.
L. enriettii infection of guinea pigs was the first model to be well characterized. It established the requirement for cell-mediated immunity for recovery from cutaneous disease. Guinea pigs develop T-cell responses to parasite antigens within 2 weeks of infection, and the lesions heal within about 10 weeks (79). A major attraction of this animal model is the fact that the host-parasite combination is a natural one and that the disease pattern is similar to that observed in human cutaneous leishmaniasis caused by L. major.
The L. enriettii guinea pig model has now been superseded by infection of inbred mice with Leishmania species pathogenic for humans. Although not perfect, the spectrum of disease manifestations observed in human leishmaniasis can be mimicked in the laboratory by infection of different inbred strains of mice with L. major. The mouse model reproduces many aspects of the human disease, including a range of susceptibility states depending on the strain of mouse used.
In this animal model, the use of a clonal parasite population eliminates the contribution of the genetic diversity of the parasites and allows analysis of the host factors which determine disease manifestations. BALB/c mice are highly susceptible; upon infection they develop large skin ulcers, which expand and metastasize, leading to death. On the other hand, C57BL/6 and CBA/N mice are resistant, develop small lesions which cure in 10 to 12 weeks, and are resistant to reinfection. Most other strains of mice are intermediate in susceptibility (112).
In mice, the outcome of infection depends on the polarized activation of one of two subsets of CD4+ T cells, Th1 or Th2 (Fig. 3). The subdivision into Th1 and Th2 cells is based on the pattern of cytokines that they produce (Fig. 3). Th1 cells produce gamma interferon (IFN-γ) and interleukin-2 (IL-2), whereas Th2 cells produce IL-4, IL-5, and IL-10. Protective immunity depends on the induction of T cells producing Th1 cytokines which activate macrophages to kill the intracellular organisms primarily through a nitric oxide-mediated mechanism (74). BALB/c mice produce mainly Th2 cytokines, in particular IL-4, and this pattern is established within hours of infection (19, 129). However, during the period of active lesion development, both susceptible and resistant mice produce a wave of Th2 cytokines (97, 98). An important difference between susceptible and resistant mice is that the resistant mice are able to switch to a Th1 profile and control the disease (51, 129). An important factor in the “decision” to form a Th1 or Th2 phenotype is the early cytokine environment, and IL-12 is one of the cytokines that contributes significantly to the establishment of the Th1 phenotype (129).
FIG. 3.
Schematic representation of the immune regulation by Th cell types, the cytokines they produce, and their effects on immune responses. These cytokines are classified as type 1, type 2, or shared. Type 1 and type 2 cytokines activate different types of effector cells and lead to different responses. Abbreviations: LT-α, lymphotoxin alpha; GM-CSF, granulocyte-macrophage colony-stimulating factor; IgG and IgE, immunoglobulins G and E. Adapted from reference 68 with permission from the publisher.
While it is useful in many ways, one must remember that the mouse model for leishmaniasis is just a model and that the mechanisms of pathogenesis and immunity may be a little different in humans. Extrapolation from mouse to human requires much care (67, 68).
Experimental models of systemic leishmaniasis.
As mentioned above, visceral leishmaniasis in humans has generally been ascribed to members of the L. donovani species, L. donovani, L. infantum, and L. chagasi, although some dermatotropic strains such as L. tropica and L. aethiopica strains may also visceralize, presumably depending on host factors.
The golden hamster was used in one of the early animal models for the study of visceral leishmaniasis. Infection with L. donovani leads to visceral disease and death. Anemia, hyperglobulinemia, and cachexia are aspects of the human disease mimicked in the hamster, making it a useful tool for the characterization of molecules and mechanisms involved in pathogenesis (53). However, in recent years, interest in it has waned and the hamster is now used primarily as a source of L. donovani amastigotes, which seem to be the required life cycle stage for infection of mice, the currently preferred model animal for visceral leishmaniasis.
Outbred mice are generally resistant to infection with L. donovani, but inbred strains display marked differences in susceptibility, which led, in the early 1970s, to the isolation of the Lsh susceptibility gene (subsequently designated NRAMP1) (20). NRAMP1 determines the degree of early expansion of the parasites in the liver and spleen (17, 21). Studies with the mouse model also led to the characterization of the immune mechanisms important for the development of organ-specific immune responses which cause the clearance of the parasites from the liver but not the spleen (39, 63). Another important contribution of the mouse model has been the discovery that chemotherapy is ineffective in the absence of intact T-cell-mediated responses (63). These experimental studies pointed to the need to activate the immune system for successful chemotherapy and led to the successful trial in India which combined IFN-γ and antimony treatment (137, 138).
One of the difficulties with the mouse as a model for human disease is the need to inject amastigotes intravenously in order to induce a reproducible pattern of colonization of the liver and spleen. This route of administration does not mimic the natural infection by the sandfly. In addition, there is no evidence of wasting, as in the human disease, and the infection is chronic but not fatal.
As mentioned above, the outcome (cutaneous or visceral) may depend on interactions between the genotype of the parasite and that of the host rather than exclusively on the parasite.
Because of the limitations of the mouse experimental system using human visceralizing strains such as L. donovani and L. infantum, it has been proposed that visceral leishmaniasis induced in BALB/c mice by the otherwise “dermatotropic” L. major may be a better model of human visceral leishmaniasis.
The dog is the major reservoir of L. infantum in the Middle East and the Mediterranean region and L. donovani chagasi in South America. The disease pattern in dogs and humans is similar, with a long period of asymptomatic infection followed by wasting, anemia, enlarged lymph nodes, and fever. As in humans, the infection remains asymptomatic in some dogs (111). One of the few differences is the presence of skin lesions in the dogs, rarely detected in humans (53). The dog may be the best animal model for visceral leishmaniasis in which relevant immunological studies and vaccine development could be performed (90, 96). With the recent cloning of several dog genes encoding cytokines and immunologically important cell markers, as well as the development of monoclonal antibodies to these molecules, there is hope for a more sustained exploitation of this excellent animal model.
HISTORY OF LEISHMANIA VACCINES
Historically, cutaneous leishmaniasis has been the focus of vaccination attempts, probably because it has been known since antiquity that individuals who had healed their skin lesions were protected from further infections. Bedouin or some Kurdistani tribal societies traditionally expose their babies' bottoms to sandfly bites in order to protect them from facial lesions. Another ancient technique practised in the Middle East has been the use of a thorn to transfer infectious material from lesions to uninfected individuals.
With the establishment by Nicolle and Manceau in 1908 (102) of culture conditions able to support the growth of promastigotes, live organisms started to be used for vaccination (or, to be precise, for controlled infections). Large-scale vaccination trials (controlled infection) using live promastigotes were carried out in the Soviet Union and Israel (43, 66) with a high percentage of successful lesion development. The success of this strategy depended critically on the viability and infectivity of the injected organisms. Organisms which had lost virulence were shown to induce delayed-type hypersensitivity but did not protect from subsequent natural infection (65).
The use of live vaccines has had many problems, including the development of large uncontrolled skin lesions, exacerbation of psoriasis and other skin diseases, and even immunosuppression as determined by low responses to the diphtheria, pertussis, and tetanus triple vaccine (94, 123). Consequently, the use of live virulent organisms for vaccination was discontinued, and in the 1990s the focus shifted to killed organisms.
The concept of a Leishmania killed vaccine was neglected for many years, possibly because of conflicting results obtained in the 1940s. Vaccination with killed organisms failed to protect persons in the Middle East (14), whereas a Brazilian trial showed excellent protection (108, 109). The tide turned when studies performed in the 1980s showed that injection of irradiated parasites induced excellent protection in mice provided that they were injected intravenously or intraperitoneally but not subcutaneously. These experiments paved the way for a reassessment of the use of killed vaccines and led to the successful development and field trials of several formulations of killed vaccines (7, 55, 56, 80, 81).
A summary of vaccination studies in humans and experimental models is given in Table 2.
TABLE 2.
Summary of vaccination studies in humans and experimental models
| Antigen | Mode of immunization (country) | Protection | Host | Reference(s) |
|---|---|---|---|---|
| Live promastigotes | Prophylactic (Russia, Israel) | Dependent on virulence | Humans | 43, 65, 66 |
| Killed promastigotes | Prophylactic (Middle East, Brazil) | Variable | Humans | 7, 11, 14, 37, 55, 56, 77, 80, 81, 90, 94, 108, 109 |
| Killed promastigotes with BCG | Therapeutic (Brazil) | High cure rate | Humans | 23, 25 |
| Killed promastigotes with BCG | Prophylactic (Iran) | No protection, transient stimulation | Humans | 95, 124 |
| Killed promastigotes with IL-12 | Prophylactic | Good | Primates, mice, dogs | 2, 69, 72, 114 |
| Irradiated promastigotes | Prophylactic | Good | Mice | 113 |
| Live attenuated promastigotes | Prophylactic | Good | Mice | 8, 116, 141, 144 |
| Recombinant or native gp63 and synthetic peptides | Prophylactic | Good | Mice, primates | 61, 103, 114, 116, 131 |
| Recombinant or native gp46/M2/PSA-2 | Prophylactic | Excellent but dependent on conformation and adjuvant | Mice | 27, 49, 87 |
| Recombinant LACK | Prophylactic | Good, enhanced by IL-12 | Mice | 45, 100 |
| A2, P4, and P8 | Prophylactic | Good | Mice | 130 |
| Flagellar antigen LCR1 | Prophylactic | Good | Mice | 134 |
| Naked DNA gp63, PSA-2, and LACK | Prophylactic or therapeutic | Good | Mice | 45, 127, 147 |
Current Vaccines
To date, there is no vaccine against Leishmania in routine use anywhere in the world. Several vaccine preparations are in more or less advanced stages of testing.
Killed Vaccines
Extensive vaccination trials in Brazil and Ecuador have demonstrated that a cocktail of five killed Leishmania stocks or a single strain of L. amazonensis induces significant protection from natural infection (11, 37, 77, 94). These studies also indicated that delayed-type hypersensitivity skin test conversion can be used as a surrogate marker for protective immunity. Moreover, the immunized individuals developed long-lasting specific T-cell responses of the Th1 type, which may indicate a potential to protect from infection (26, 90).
Convit and colleagues, some of the early pioneers of killed vaccines, used a combination of killed L. mexicana or L. braziliensis promastigotes and M. bovis BCG both prophylactically and therapeutically against South American leishmaniasis (25). When used in the therapeutic mode, vaccination appeared to induce a high cure rate even in patients with severe cases. Cure was accompanied by the development of Th1-type immune responses in the recipients, with the production of IFN-γ and the absence of IL-4 (23, 25).
In Iran, a mixed BCG-L. major killed vaccine has also undergone clinical trials for safety and efficacy. In one study there was little difference in disease incidence between the group vaccinated with BCG alone and the group given BCG and vaccine. A second study showed that in the longer term, the vaccine combination provided better efficacy than BCG alone, suggesting that BCG may have had only a transient immunostimulatory effect (95, 124). Vaccination with a single dose of 1 mg of L. major protein and BCG is the simplest vaccine tested so far. Although it proved safe, only about 35% of vaccinated individuals became skin test positive. If skin test conversion is a surrogate marker for protection, the efficacy of this vaccine is not remarkable. It may require multiple doses to increase immunogenicity.
In a monkey model of cutaneous leishmaniasis, protective immunity was achieved using killed L. amazonensis coadministered with recombinant IL-12 as adjuvant (69). This study extends the data obtained with the mouse and dog models (2, 72, 82, 83, 114) and provides a basis for further human trials. However, because delayed-type hypersensitivity was not predictive of protection in this monkey model, the value of delayed-type hypersensitivity as a surrogate marker of protection in humans needs to be reassessed.
Live-Attenuated Vaccines
The relative merits of live-attenuated vaccines versus killed vaccines has been a constant subject of debate in relation to many antimicrobial and viral vaccines. As discussed above for Leishmania, the most notable arguments have been those concerned with immunogenicity, efficacy, safety, ease of production and distribution, and stability.
Early data from our laboratory indicated (surprisingly) that most parasites cloned directly from a skin lesion in mice were avirulent (47). This suggested that the parasite population present in the lesion may be heterogeneous and that the avirulent organisms (which are rapidly killed by the host and provide antigens), rather than the virulent organisms, contribute most to the immune response observed in the infected mice. More recent data indicate that, indeed, L. mexicana antigens can be presented to T cells by macrophages harboring dead organisms but not by cells harboring live parasites (104). In line with this argument, mice injected with cloned avirulent lines were protected from challenge infection with a virulent clone derived from the same lesion (47). However, in the absence of a clear genetic profile of any avirulent cloned organisms available at the time, their use for human vaccination would have been unacceptable because of the risk of reversion to a virulent phenotype. Other data showed that mice injected with irradiated parasites were also protected from infection (113). Taken together, these data strongly supported prophylactic vaccination with attenuated organisms as a useful approach to human vaccine development.
Recent advances in the ability to manipulate the Leishmania genome by introducing or eliminating genes has the potential to make live-attenuated vaccines much more feasible. It is now possible to generate parasites lacking genes essential for long-term survival in the mammalian host, such as the gene encoding the enzyme dihydrofolate reductase-thymidylate synthetase (DHFR-TS) (141). These organisms can invade and undergo a limited number of replications in macrophages without producing disease. In a mouse model, L. major parasites lacking DHFR-TS induced protection against infection with either L. major or L. amazonensis (141, 144). An attenuated line of L. mexicana was also used successfully to protect against homologous infection. This mutant lacked two genes encoding the cysteine proteases cpa and cpb (8, 116).
In summary, the use of attenuated organisms is very attractive because they are the closest mimic to the natural course of infection and may therefore lead to similar immune responses. Moreover, because of the small load of antigen delivered by the transient infection, the immune responses may be skewed even more toward a Th1 protective response than in natural infection (32, 92). Such immunization will also deliver many more parasite antigens than the limited number possible with subunit or recombinant antigens. Summarizing a large amount of experimental evidence, Rivier et al. (114) concluded that injection of attenuated organisms achieved better protection than any method involving recombinant gp63 as test antigen delivered with a variety of adjuvants and delivery systems. If this conclusion is shown to be generally applicable to other vaccine candidates, the prospect of using attenuated Leishmania vaccines in preference to subunit or recombinant approaches will gain favor. The disadvantages of such vaccines are the logistics of their large-scale production and distribution in the field.
Recombinant and Synthetic Vaccines
The newer vaccines under consideration comprise recombinant DNA-derived antigens and peptides. Some of the target antigens are species and life cycle stage specific, while others are shared by promastigotes and amastigotes. Some are conserved among Leishmania species, while others are not. Since T cells recognize peptides derived from cytosolic proteins bound in the MHC class I groove or peptides derived from the lysosomal compartment bound in the MHC class II groove on the antigen-presenting cell surface, it would appear that virtually any parasite protein might function as an antigen, regardless of its location in the parasite. At the effector stage, in the lesion, it may not be important if the antigens are presented on the surface of infected or bystander antigen-presenting cells. As long as the appropriate proinflammatory Th1 cytokines are generated in the lesion, macrophage activation and parasite killing should occur.
Recombinant antigens can be delivered as purified proteins, as the naked DNA encoding them, or as bacteria manufacturing the proteins in situ. Manipulations now allow targeting of the antigen to specific locations or to particular antigen-presenting cells, such as dendritic cells or Langerhans cells, which are considered essential for the initiation of primary T-cell responses. Injection of bacteria or naked DNA may have the added advantage of providing an adjuvant effect, which may “activate” or “licence” these antigen-presenting cells (78).
Expression of Immunogens in Bacteria and Viruses
The first recombinant antigen used to vaccinate against leishmaniasis was leishmaniolysin or gp63. This is an Mr 65,000 membrane protease present in promastigotes of all species. gp63 is one of the parasite receptors for host macrophages, and parasite mutants lacking the protein are avirulent (29). gp63 belongs to a multigene family, with different members being expressed in promastigotes and amastigotes. Interestingly, both the recombinant and native proteins seem to protect better against infection with L. amazonensis than against infection with L. major, suggesting species-specific epitopes, at least in animal models (116, 103). It is unfortunate that in humans and animal models the T-cell responses to gp63 have been variable. However, when detected, they appeared to be of the Th1 type (59, 89, 117). Overall, gp63 is still considered a promising vaccine candidate. The gene has been engineered in a number of delivery systems (BCG, vaccinia virus, and S. enterica serovar Typhimurium) in the hope of inducing the appropriate Th1 immune response (see below).
A second vaccine candidate tested in animal models is a membrane antigen of unknown function, gp46/M2 or parasite surface antigen 2 (PSA-2) (27, 49, 75, 87, 127). As with gp63, PSA-2 belongs to a multigene family expressed in all Leishmania species except L. braziliensis. Similar but distinct gene products are found in amastigotes and promastigotes of L. major and L. donovani, but in L. mexicana expression seems to be restricted to promastigotes (86).
Its presence in most species makes PSA-2 an attractive candidate for a pan-Leishmania vaccine. PSA-2 protects against L. major (49) as well as L. mexicana when administered as purified protein or expressed in vaccinia virus (27, 87). Data from our laboratory showed that immunization with the L. donovani PSA-2 protects mice against infection with L. major and that, conversely, immunization with the L. major proteins afforded partial protection against infection with L. donovani (E. Handman, C. L. Jaffe, C. R. Engwerda, and P. M. Kaye, unpublished data). Recombinant DNA-derived PSA-2 protein was variable in its ability to confer protection, while the protein derived from the yeast Pichia pastoris provided good protection. These data suggested that the native conformation of the protein may be important for processing and presentation by antigen-presenting cells. These difficulties may be overcome by the development of a DNA-based vaccine (see below).
The leishmanial eukaryotic ribosomal protein (LeIF), a homologue of the ribosomal protein cIF4A, is being considered as a vaccine candidate based on its ability to induce Th1-type cytokines in humans (128). This protein is highly conserved in evolution, but assuming that specific parasite epitopes will be used for vaccination such that autoimmune responses will be avoided, it may be useful as a component in a pan-Leishmania vaccine.
A similarly conserved antigen, the Leishmania homologue of the receptor for activated C kinase (LACK), which is expressed by both promastigotes and amastigotes has been shown to protect mice from infection, in particular when administered with IL-12 as an adjuvant (45, 100). Interestingly, LACK is also the major target for Th2 responses in susceptible BALB/c mice, and BALB/c mice made tolerant to LACK are resistant to infection (62). The significance of this finding for the use of LACK as a vaccine in humans remains to be elucidated.
Several other vaccine candidates identified in the last few years are in the process of being characterized. Some are amastigote specific, such as A2, P4, and P8 of L. mexicana pifanoi (130). Another vaccine candidate is a flagellar antigen, lcr1, from L. donovani chagasi (134). In view of the fact that the target of host protection is the amastigote, which has only a rudimentary flagellum, the mechanism by which host protection is achieved with this antigen is not obvious.
A most interesting approach to the identification of potential vaccine candidates has been the elution of antigenic peptides from antigen-presenting cells (24). Several peptides were identified, and the sequences were used to clone the cognate genes. One of these genes encodes a membrane polypeptide expressed in promastigotes and amastigotes. This polypeptide induced Th1-type responses in immunized mice (24). Surprisingly, in view of its potential, there have been few new data published since its discovery.
Synthetic Peptides
The 1980s were marked by a wave of enthusiasm concerning the use of peptide vaccines, in particular those considered to be T-cell epitopes (60, 70, 118, 131). This enthusiasm seems to have waned in recent times, and the focus appears to have moved to the use of recombinant DNA-produced polypeptides and to naked DNA. Several considerations make the peptide antigens less attractive: the magnitude of the T-cell memory induced, the inability of all individuals in the population to respond to the peptide, and the economics of production. Since the antigenic peptide is processed and presented to T cells in the context of MHC class I or class II and since not all peptides associate with all MHC types, some peptides will not be recognized by all individuals in the population. There are additional “holes” in the ability to respond to individual peptides due to failure of processing, cleavage, transport or due to deletion of certain T-cell specificities due to self-tolerance (57). Despite these caveats, several Leishmania gp63 peptides have been tested successfully in animal models (61, 131). Importantly, host protection was long-lasting, indicating the induction of long-term T-cell memory (131).
In general, the success of subunit vaccines based on recombinant proteins or peptides has been variable to poor. Several factors may account for this. Some polypeptides, such as PSA-2, need to be in their native conformation for antigen processing, and Escherichia coli-derived recombinant proteins may not fulfil this requirement (126). This problem may be overcome by exploitation of the parasites themselves by overexpression of parasite antigens in transfected nonpathogenic Leishmania strains or the related trypanosomatid Crithidia (64). Presumably, polypeptides expressed in these systems will be abundant, correctly folded, and glycosylated (31, 96, 110).
Another reason for the low success rate of subunit vaccines is that some polypeptides may be minor immunogens and so even though they may be excellent in a cocktail vaccine, individually they may provide only partial protection. The immune responses in leishmaniasis can range from protective to positively harmful, as described above. These differences in the quality of the response are at least partly due to predominance of Th1 or Th2 cytokines and may be greatly influenced by antigen dose (22). Accordingly, the amount of antigen and possibly the route of its administration may be important issues.
Another thorny issue concerns adjuvants. The delivery system may be critical in biasing the type of T-cell responses induced, and this can determine whether protection is achieved or, indeed, whether immunization makes the disease worse (52, 54). Delivery of PSA-2 packaged in immunostimulating complexes induced a strong but mixed Th1/Th2 response and no protection, whereas its delivery as a DNA vaccine induced a low but exclusive Th1 response and protection (126, 127). In most experimental systems, adjuvants are essential to provoke protective immunity. However, the most effective adjuvants generally cause strong inflammation, which may be essential for adjuvanticity but may preclude their use in humans because of unacceptable side effects.
Nonprotein Antigens
Early studies on vaccine development indicated that glycolipids such as the Leishmania lipophosphoglycan (LPG) provided excellent protection (48, 84, 116). Protection depended on the use of adjuvants such as liposomes or Corynebacterium parvum and on the integrity of the molecule. Not only was the water-soluble form of LPG lacking the glycosylphosphatidylinositol anchor not protective, but it exacerbated disease (93). At the time when that work was published, the immune mechanism leading to host protection by such a nonprotein molecule was totally mysterious. Immunity was known to be T-cell mediated, but T cells were not thought to recognize or present nonprotein antigens. Today, it is accepted that many novel and interesting microbial antigens including mycobacterial glycolipids can be recognized by T cells and that these antigens are presented to T cells by a special subset of MHC class I proteins known as CD1 (96, 125, 135). In this context, it may be rewarding to reevaluate the potential of LPG as a vaccine candidate.
Naked DNA Vaccines
Immunization with naked DNA is a new approach, which promises to revolutionize the prevention and treatment of infectious diseases (6, 46, 122, 145). The gene encoding the vaccine candidate is cloned in a mammalian expression vector, and the DNA is injected directly into muscle or skin (38, 143, 145, 146). Surprisingly, the plasmid DNA is taken up by cells and translocated to the nucleus, where it is transcribed into RNA and then translated in the cytoplasm. The efficiency of uptake and the expression of plasmid DNA must be extremely low, but there is abundant evidence that it is sufficient to provoke immune responses in both T and B cells (46, 50).
How is the antigen encoded by the injected plasmid presented to the immune system? There are convincing data that antigen presentation is not done directly via skin or muscle cells but, rather, is done via either class I or class II MHC molecules on professional antigen-presenting cells (38, 50). A large body of literature indicates that both CD4+- and CD8+-mediated responses are induced, making a DNA vaccine attractive for a Leishmania vaccine (107). In addition to being able to induce the appropriate immune responses, DNA vaccines are attractive because they ensure appropriate folding of the polypeptide, produce the antigen over long periods, and do not require adjuvants. Another advantage is that the technology for production is very simple. DNA is stable, has a long shelf life, and does not require a strict cold chain for distribution. Concerns raised in relation to safety, such as integration of the DNA into the mammalian genome and induction of autoimmune disease or cancer, have not been substantiated to date. Several DNA vaccines are in advanced clinical trials; these include a malaria vaccine, a mycobacterial vaccine, and an HIV vaccine (reviewed in references 6 and 50).
Vaccinations with DNA encoding gp63, LACK, and PSA-2 all protected both genetically resistant and susceptible mice from infection with L. major (45, 127, 146). Protection was accompanied by Th1 immune responses. Unexpectedly, protection induced by LACK depended on CD8+ T cells, and depletion of this population abrogated protection (45).
PROBLEMS TO BE SOLVED BEFORE A LEISHMANIA VACCINE BECOMES A REALITY
Protective Antigens
When complex organisms such as protozoa interact with the immune system, most of their components are immunogenic to the host, as evidenced by the induction of antibodies or cell-mediated immune responses. However, responses to some antigens have no value in terms of host protection. Some may even contribute to pathological responses, for example by cross-reactivity with host molecules.
In the early days of vaccine development, when live or killed whole parasites were considered, identification of the antigens which induce the appropriate immune responses leading to host protection was only a dream. The advent of gene cloning and monoclonal antibodies opened up the possibility of identifying and characterizing relevant protein antigens and, most importantly, producing them in unlimited amounts. It is now clear that many antigens may combine to elicit protective immune responses, and it is likely that many more will be discovered when the sequencing of the Leishmania genome is completed. It is somewhat ironic that the same gene-cloning technology which allowed the isolation of genes encoding vaccine candidates can now be used to engineer the ablation of essential genes in Leishmania, thus making the use of live-attenuated vaccines a much more attractive proposition than ever before. The wheel seems to have turned full circle; we may not need to know which are the most highly protective antigens.
If one were to consider a subunit vaccine, how many antigens would be necessary or sufficient? The contribution of each antigen may be only partial, as expected from interactions between two complex genomes, those of the host and the parasite. Data obtained using different strains of inbred mice indicate that genetic variation in the host has a major influence in determining the outcome of infection. Part of this variation probably reflects differences in the ability of the host to respond to individual antigens. A high responder to one antigen may be a low responder to another antigen. Accordingly, a vaccine to be used in an outbred population, such as humans, will probably require several different antigens to guarantee a satisfactory overall response by most if not all of the population. One could envisage the design of polyvalent vaccines incorporating as many polypeptide and glycolipid antigens as possible. While large-scale production of polypeptides may not be a problem, large-scale production of complex glycolipid antigens may be difficult. Production of strings of antigenic protein epitopes (“polytopes”) might be considered, as has been done for Ebna virus (40, 99, 156). Such combinations may be necessary to ensure that all individuals in the population, including low responders to some antigens, will get the benefit of the vaccine.
Safety
One of the nightmares in vaccine development is the possibility that a particular vaccine may exacerbate the disease associated with infection (54) or cause pathological reactions due to cross-reactivity between host and parasite antigens. Another nightmare is the possibility of unleashing the disease due to incomplete attenuation or ineffective killing of the organisms. There is also the possibility that attenuated organisms may cause disease in immunocompromised individuals. In the current worldwide AIDS pandemic, such individuals are no longer rare (151). People in tropical countries rarely suffer from a single disease, and many have lower immunocompetence, as already demonstrated by low responses to various other vaccines. Many are malnourished or starving, a significant risk factor for visceral leishmaniasis.
Delivery and Adjuvants
Adjuvants, from the Latin word meaning “to help,” have been used to improve vaccine efficacy from the early 1920s (33, 34, 120). While the number and type of adjuvants have expanded, their mechanism of action has remained largely mysterious and empirical. Can we use new knowledge about the cells and molecules involved in the initiation and potentiation of immune responses to make better vaccines?
Recent work from many laboratories has changed our thinking about the cells and molecules which initiate immune responses. The current concept is that primary T-cell responses must be initiated by specialized “professional” antigen-presenting cells. These cells are the dendritic cells or the Langerhans cells in the skin (13, 35). Antigen is taken up by dendritic cells via receptor-mediated endocytosis or fluid-phase pinocytosis. The interaction with antigen drives dendritic cells to mature and migrate out of the tissue into the draining lymph nodes, where the antigen is processed into peptides and where the peptides meet the MHC class II molecules as they are assembled and transported through the endoplasmic reticulum and the Golgi apparatus. The complex of peptide with MHC class II is transported to the cell surface and displayed for recognition by T cells. The antigen-presenting cells also secrete cytokines which attract CD4+ T cells to the area, such that T cells with cognate receptors for the peptide MHC class II complex undergo clonal expansion. It is likely that the Th1/Th2 switch will occur at this point, thus determining the course and outcome of the immune response. It is at this level that adjuvants are believed to contribute to the amplitude of the immune response and to its quality. This scenario underlines the importance of a judicious choice of adjuvant for vaccines that require Th1 responses for protection.
The ability of bacteria and other particles to be taken up by dendritic cells, coupled with the ability to express foreign genes in bacteria, has made them attractive delivery vehicles for vaccines. Such a vaccine could exploit attenuated bacteria such as Salmonella or BCG, which are already in use as vaccines with demonstrated safety and immunogenicity in their own right. BCG has been used successfully for anti-Leishmania immunotherapy in South American patients without side effects. BCG vectors carrying gp63 have also been used successfully to induce protection in the L. major system (1, 2). S. enterica serovar Typhimurium expressing gp63 has also been used in the mouse model. Unfortunately, the vaccine induced variable protection, ranging from minimal to significant, despite the induction of apparently appropriate T-cell responses (88, 152, 155). It is possible that the use of “adjuvanted” bacteria carrying the genes for TNF-α and IFN-γ, which appear to potentiate the immune response (see below), may improve the performance of a Salmonella-delivered vaccine (153). An added advantage of a Salmonella-delivered vaccine is its oral administration. Problems with the Salmonella vectors include instability of the plasmids carrying the genes of interest and the need for multiple administrations.
Several other types of particulate adjuvants or delivery systems have been tested for use with leishmania vaccines; they include liposomes, microparticles, immunostimulating complexes, and micelles formed by intrinsically adjuvanted lipopeptides. The outcomes have been variable (3, 42, 84, 106, 126). Most of this work currently involves animal models, and the use of these systems in humans is still somewhat distant.
Use of Cytokines as Adjuvant
One of the most promising adjuvants involves the use of some of “nature's adjuvants” (Fig. 3), i.e., soluble cytokines which are known to promote Th1 immune responses. Among these, IL-12 is essential for the induction and maintenance of Th1 immune responses by Leishmania vaccines, including DNA vaccines (121). It appears that the persistence of IL-12 delivered as DNA may be an important contributor to the long-term memory induced by the vaccinating DNA-encoded LACK antigen.
There is a possibility that the timing of administration of the cytokine in relation to the vaccine has a major effect on the quality and maintenance of the response. In experiments testing the ability of IL-12 to amplify the vaccinating effect of PSA-2 DNA, we observed a paradoxical effect of IL-12. While mice injected with DNA encoding IL-12 alone showed protection from infection, protection was abrogated in mice injected with a combination of IL-12 and PSA-2-encoding DNA (A. H. Noormohamaddi, H. Hochrein, J. M. Curtis, T. M. Baldwin, and E. Handman, submitted for publication).
An interesting approach to the targeting of the immune response has been the coupling of the potent antigen-presenting ability of dendritic cells to the delivery of proinflammatory cytokines to the local site of response. For this purpose, adoptive transfer of dendritic cells engineered by retroviral infection to secrete IL-12 has been used to augment the effect of vaccination with dendritic cells pulsed with L. donovani antigen (4, 5). This regimen led to an increase in parasite-specific IFN-γ production and 1 to 3-log-unit lower parasite burdens in mice with murine visceral leishmaniasis.
Immunostimulatory Effects of Bacterial DNA
Certain sequences of bacterial DNA seem to have immunostimulatory effects (91, 132, 154). Specific DNA sequences containing unmethylated dinucleotides (CpG motifs) activate B cells and dendritic cells and induce cytokine production by macrophages. The ability of CpG motifs to induce the production of IL-12 and TNF-α and to lead to a polarized Th1 response makes them particularly attractive as adjuvants for Leishmania vaccines. CpG-containing DNA has immunostimulatory effects in vaccination against L. major (133, 147, 157). If these promising results can be confirmed and extended, immunostimulatory DNA may become an important and relatively nontoxic adjuvant for Leishmania vaccines.
Drugs or Vaccines for Disease Control?
Significant advances have been made in the development of anti-Leishmania drugs. In addition to the traditional pentavalent antimonials, new drugs such as aminosidine and liposome-delivered amphotericin B have been introduced (28). Other drugs such as allopurinol and ketoconazole, targeting parasite-specific biosynthetic pathways, are in advanced stages of production and large-scale use. There are advantages to the use of chemotherapy for control of leishmaniasis. Drugs are not affected by parasite heterogeneity, they can be administered orally, and, most importantly, once they are developed, better formulations are relatively easy to produce. Unfortunately, drugs are much less effective in immunocompromised individuals, and drug resistance, although not yet a major problem, is looming on the horizon (30, 142).
Unlike chemotherapy, vaccination is usually a “one-shot” affair. This makes it cheaper, and the easier logistics of administration lead to much better compliance. Vaccines have the advantage that they can be administered both in the prophylactic and therapeutic modes. As described above, immunotherapy has proved successful in both human and experimental leishmaniasis. An additional advantage of vaccination is its long-lasting effect and the fact that it avoids problems with drug resistance. A combination of chemotherapy, immunotherapy, and immunoprophylaxis may be the ideal to strive for in the battle against leishmaniasis.
CONCLUSIONS
The main scientific issues in the design of a Leishmania vaccine are no different from those for any other vaccine. They include specificity, the class or type of response induced, and the induction of long-term immunological memory. As is the case for many other vaccines, the “rules of the game” for achieving these goals are still far from clear. On a more positive note, there is currently rapid progress in our understanding of the molecular nature of potential vaccine candidates and of the mechanisms that determine disease-preventing immune responses. However, our ability to control these responses in a reliable way is still at an early stage. Notwithstanding these caveats, there is a feeling of renewed optimism in the scientific community that a Leishmania vaccine is achievable.
ACKNOWLEDGMENTS
I was supported by the Australian National Health and Medical Research Council and the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases.
I am grateful to J. W. Goding and S. Henri for insightful and critical advice.
REFERENCES
- 1.Abdelhak S, Louzir H, Timm J, Blel L, Benlasfar Z, Lagranderie M, Gheorghiu M, Dellagi K, Gicquel B. Recombinant BCG expressing the leishmania surface antigen Gp63 induces protective immuity against Leishmania major infection in BALB/c mice. Microbiology. 1995;141:1585–1592. doi: 10.1099/13500872-141-7-1585. [DOI] [PubMed] [Google Scholar]
- 2.Aebischer T, Wolfram M, Patzer S I, Ilg T, Wiese M, Overath P. Subunit vaccination of mice against new world cutaneous leishmaniasis: comparison of three proteins expressed in amastigotes and six adjuvants. Infect Immun. 2000;68:1328–1336. doi: 10.1128/iai.68.3.1328-1336.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afrin F, Ali N. Adjuvanticity and protective immunity elicited by Leishmania donovani antigens encapsulated in positively charged liposomes. Infect Immun. 1997;65:2371–2377. doi: 10.1128/iai.65.6.2371-2377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahuja S S, Mummidi S, Malech H L, Ahuja S K. Human dendritic cell (DC)-based anti-infective therapy: engineering DCs to secrete functional IFN-gamma and IL-12. J Immunol. 1998;161:868–876. [PubMed] [Google Scholar]
- 5.Ahuja S S, Reddick R L, Sato N, Montalbo E, Kostecki V, Zhao W, Dolan M J, Melby P C, Ahuja S K. Dendritic cell (DC)-based anti-infective strategies: DCs engineered to secrete IL-12 are a potent vaccine in a murine model of an intracellular infection. J Immunol. 1999;163:3890–3897. [PubMed] [Google Scholar]
- 6.Alarcon J B, Waine G W, McManus D P. DNA vaccines: technology and application as anti-parasite and anti-microbial agents. Adv Parasitol. 1999;42:344–410. doi: 10.1016/s0065-308x(08)60152-9. [DOI] [PubMed] [Google Scholar]
- 7.Alexander J. A radioattenuated Leishmania major vaccine markedly increases the resistance to CBA mice to subsequent infection with Leishmania mexicana mexicana. Trans R Soc Trop Med Hyg. 1982;76:646–649. doi: 10.1016/0035-9203(82)90232-2. [DOI] [PubMed] [Google Scholar]
- 8.Alexander J, Coombs G H, Mottram J C. Leishmania mexicana cysteine proteinase-deficient mutants have attenuated virulence for mice and potentiate a Th1 response. J Immunol. 1998;161:6794–6801. [PubMed] [Google Scholar]
- 9.Altges J, Salas A, Riera M, Udina M, Galmges A, Balanzat J, Ballesteros A, Buades J, Salvga F, Villalonga C. Visceral leishmaniasis: another HIV-associated opportunistic infection? Report of eight new cases and review of the literature. AIDS. 1991;5:201–207. [PubMed] [Google Scholar]
- 10.Alvar J. Leishmaniasis and AIDS co-infection: the Spanish example. Parasitol Today. 1994;10:160–163. doi: 10.1016/0169-4758(94)90270-4. [DOI] [PubMed] [Google Scholar]
- 11.Armijos R X, Weigel M M, Aviles H, Maldonado R, Racines J. Field trial of a vaccine against New World cutaneous leishmaniasis in an at-risk child population: safety, immunogenicity, and efficacy during the first 12 months of follow-up. J Infect Dis. 1998;177:1352–1357. doi: 10.1086/515265. [DOI] [PubMed] [Google Scholar]
- 12.Badaro R, Carvalho E M, Rocha H, Queiroz A C, Jones T C. Leishmania donovani: an opportunistic microbe associated with progressive disease in three immunocompromised patients. Lancet. 1986;1:647–649. doi: 10.1016/s0140-6736(86)91725-3. [DOI] [PubMed] [Google Scholar]
- 13.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 14.Berberian D A. Cutaneous leishmaniasis (oriental sore). IV. Vaccination against oriental sore with suspensions of killed Leishmania tropica. Arch Dermatol Syphilol. 1944;50:234. [Google Scholar]
- 15.Blackwell J M, Barton C H, White J K, Roach T I A, Shaw M-A, Whitehead S H, Mock B A, Searle S, Williams H, Baker A-M. Genetic regulation of leishmanial and mycobacterial infections: the Lsh/Ity/Bcg gene story continues. Immunol Lett. 1994;43:99–107. doi: 10.1016/0165-2478(94)00161-8. [DOI] [PubMed] [Google Scholar]
- 16.Blackwell J M. Structure and function of the natural-resistance-associated macrophage protein (Nramp1), a candidate protein for infectious and autoimmune disease susceptibility. Mol Med Today. 1996;2:205–211. doi: 10.1016/1357-4310(96)88773-9. [DOI] [PubMed] [Google Scholar]
- 17.Blackwell J M. Genetic susceptibility to leishmanial infections: studies in mice and man. Parasitology. 1996;112:S67–S74. [PubMed] [Google Scholar]
- 18.Blackwell J M. Parasite genome analysis. Progress in the Leishmania genome project. Trans R Soc Trop Med Hyg. 1997;91:107–110. doi: 10.1016/s0035-9203(97)90187-5. [DOI] [PubMed] [Google Scholar]
- 19.Bogdan C. The multiplex function of nitric oxide in (auto)immunity. J Exp Med. 1998;187:1361–1365. doi: 10.1084/jem.187.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley D J. Genetic control of natural resistance to Leishmania donovani. Nature. 1974;250:353. doi: 10.1038/250353a0. [DOI] [PubMed] [Google Scholar]
- 21.Bradley D J. Lsh: origins and growth of a cottage industry. Res Immunol. 1989;140:827–828. doi: 10.1016/0923-2494(89)90041-2. [DOI] [PubMed] [Google Scholar]
- 22.Bretscher, P. A., O. Ogunremi, and J. N. Menon. 1997. Distinct immunological states in murine cutaneous leishmaniasis by immunising with different amounts of antigen: the generation of beneficial, potentially harmful, harmful and potentially extremely harmful states. Behring Inst. Mitt. 153–159. [PubMed]
- 23.Cabrera M, Blackwell J M, Castes M, Trujillo D, Convit J, Shaw M A. Immunotherapy with live BCG plus heat killed Leishmania induces a T helper 1-like response in American cutaneous leishmaniasis patients. Parasite Immunol. 2000;22:73–79. doi: 10.1046/j.1365-3024.2000.00278.x. [DOI] [PubMed] [Google Scholar]
- 24.Campos-Neto A, Soong L, Cordova J L, Sant'Angelo D, Skeiky Y A W, Ruddle N H, Reed S G, Janeway C, Jr, McMahon-Pratt D. Cloning and expression of a Leishmania donovani gene industructed by a peptide isolated from major histocompatibility complex class II molecules of infected macrophages. J Exp Med. 1995;182:1423–1433. doi: 10.1084/jem.182.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castes M, Moros Z, Martinez A, Trujillo D, Castellanos P L, Rondon A J, Convit J. Cell-mediated immunity in localized cutaneous leishmaniasis patients before and after treatment with immunotherapy or chemotherapy. Parasite Immunol. 1989;11:211–222. doi: 10.1111/j.1365-3024.1989.tb00660.x. [DOI] [PubMed] [Google Scholar]
- 26.Castes M, Blackwell J, Trujillo D, Formica S, Cabrera M, Zorrilla G, Rodas A, Castellanos P L, Convit J. Immune response in healthy volunteers vaccinated with killed leishmanial promastigotes plus BCG. I. Skin-test reactivity, T-cell proliferation and interferon-gamma production. Vaccine. 1994;12:1041–1051. doi: 10.1016/0264-410x(94)90342-5. [DOI] [PubMed] [Google Scholar]
- 27.Champsi J, McMahon-Pratt D. Membrane glycoprotein M-2 protects against Leishmania amazonensis infection. Infect Immun. 1988;56:3272–3279. doi: 10.1128/iai.56.12.3272-3279.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chance M L. New developments in the chemotherapy of leishmaniasis. Ann Trop Med Parasitol. 1995;89(Suppl.):37–43. doi: 10.1080/00034983.1995.11813013. [DOI] [PubMed] [Google Scholar]
- 29.Chang K-P, Chaudhuri G, Fong D. Molecular determinants of Leishmania virulence. Ann Rev Microbiol. 1990;44:499–529. doi: 10.1146/annurev.mi.44.100190.002435. [DOI] [PubMed] [Google Scholar]
- 30.Chow L M, Volkman S K. Plasmodium and Leishmania: the role of mdr genes in mediating drug resistance. Exp Parasitol. 1998;90:135–141. doi: 10.1006/expr.1998.4311. [DOI] [PubMed] [Google Scholar]
- 31.Constant P, Davodeau F, Peyrat M-A, Poquet Y, Puzo G, Bonneville M, Fournie J-J. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 32.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med. 1995;182:1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox J C, Culter A R. Advances in adjuvant technology and application. In: Yong W K, editor. Animal parasite control utilizing biotechnology. Boca Raton, Fla: CRC Press, Inc; 1992. pp. 49–112. [Google Scholar]
- 34.Cox J C, Coulter A R. Adjuvants—a classification and review of their modes of action. Vaccine. 1997;15:248–256. doi: 10.1016/s0264-410x(96)00183-1. [DOI] [PubMed] [Google Scholar]
- 35.Davoust J, Banchereau J. Naked antigen-presenting molecules on dendritic cells. Nat Cell Biol. 2000;2:E46–E48. doi: 10.1038/35004075. [DOI] [PubMed] [Google Scholar]
- 36.DeKrey G K, Titus R G. A method for the isolation and analysis of leucocytic cells from leishmanial ear lesions in mice. J Immunol Methods. 1999;228:1–11. doi: 10.1016/s0022-1759(99)00085-x. [DOI] [PubMed] [Google Scholar]
- 37.De Luca P M, Mayrink W, Alves C R, Coutinho S G, Oliveira M P, Bertho A L, Toledo V P, Costa C A, Genaro O, Mendonca S C. Evaluation of the stability and immunogenicity of autoclaved and nonautoclaved preparations of a vaccine against American tegumentary leishmaniasis. Vaccine. 1999;17:1179–1185. doi: 10.1016/s0264-410x(98)00338-7. [DOI] [PubMed] [Google Scholar]
- 38.Donnelly J J, Ulmer J B, Shiver J W, Liu M A. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 39.Engwerda C R, Kaye P M. Organ-specific immune responses associated with infectious disease. Immunol Today. 2000;21:73–78. doi: 10.1016/s0167-5699(99)01549-2. [DOI] [PubMed] [Google Scholar]
- 40.Fitzmaurice C J, Brown L E, Kronin V, Jackson D C. The geometry of synthetic peptide-based immunogens affects the efficiency of T cell stimulation by professional antigen-presenting cells. Int Immunol. 2000;12:527–535. doi: 10.1093/intimm/12.4.527. [DOI] [PubMed] [Google Scholar]
- 41.Fleming M D, Trenor III C C, Su M A, Foernzler D, Beier D R, Dietrich W F, Andrews N C. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16:383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- 42.Frankenburg S, Axelrod O, Kutner S, Greenblatt C L, Klaus S N, Pirak E A, McMaster R, Lowell G H. Effective immunization of mice against cutaneous leishmaniasis using an intrinsically adjuvanted synthetic lipopeptide vaccine. Vaccine. 1996;14:923–929. doi: 10.1016/0264-410x(95)00245-v. [DOI] [PubMed] [Google Scholar]
- 43.Greenblatt C L. The present and future of vaccination for cutaneous leishmaniasis. Prog Clin Biol Res. 1980;47:259–285. [PubMed] [Google Scholar]
- 44.Gros P, Malo D. A reverse genetics approach to Bcg/Ity/Lsh gene cloning. Res Immunol. 1989;140:774–777. doi: 10.1016/0923-2494(89)90031-x. [DOI] [PubMed] [Google Scholar]
- 45.Gurunathan S, Sacks D L, Brown D R, Reiner S L, Charest H, Glaichenhaus N, Seder R A. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J Exp Med. 1997;186:1137–1147. doi: 10.1084/jem.186.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gurunathan S, Wu C, Freidag B L, Seder R A. DNA vaccines: a key for inducing long-term cellular immunity. Curr Opin Immunol. 2000;12:442–447. doi: 10.1016/s0952-7915(00)00118-7. [DOI] [PubMed] [Google Scholar]
- 47.Handman E, Hocking R E, Mitchell G F, Spithill T W. Isolation and characterization of infective and non-infective clones of Leishmania tropica. Mol Biochem Parasitol. 1983;7:111–126. doi: 10.1016/0166-6851(83)90039-7. [DOI] [PubMed] [Google Scholar]
- 48.Handman E, Mitchell G F. Immunization with Leishmania receptor for macrophages protects mice against cutaneous leishmaniasis. Proc Natl Acad Sci USA. 1985;82:5910–5914. doi: 10.1073/pnas.82.17.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Handman E, Symons F M, Baldwin T M, Curtis J M, Scheerlinck J-P Y. Protective vaccination with promastigote surface antigen 2 from Leishmania major is mediated by a TH1 type of immune response. Infect Immun. 1995;63:4261–4267. doi: 10.1128/iai.63.11.4261-4267.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hasan U A, Abai A M, Harper D R, Wren B W, Morrow W J. Nucleic acid immunization: concepts and techniques associated with third generation vaccines. J Immunol Methods. 1999;229:1–22. doi: 10.1016/s0022-1759(99)00104-0. [DOI] [PubMed] [Google Scholar]
- 51.Heinzel F P, Sadick M D, Mutha S S, Locksley R M. Production of interferon γ, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc Natl Acad Sci USA. 1991;88:7011–7015. doi: 10.1073/pnas.88.16.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hewlett E L, Cherry J D. New and improved vaccines against pertussis. In: Woodrow C C, Levine M M, editors. New generation vaccines. New York, N.Y: Marcel Dekker, Inc; 1990. pp. 231–250. [Google Scholar]
- 53.Hommel M, Jaffe C L, Travi B, Milon G. Experimental models for leishmaniasis and for testing anti-leishmanial vaccines. Ann Trop Med Parasitol. 1995;89(Suppl. 1):55–73. doi: 10.1080/00034983.1995.11813015. [DOI] [PubMed] [Google Scholar]
- 54.Hoskins T W, Davies J R, Smith A J, Miller C L, Allchin A. Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at Christ's Hospital. Lancet. 1979;i:33–35. doi: 10.1016/s0140-6736(79)90468-9. [DOI] [PubMed] [Google Scholar]
- 55.Howard J G, Nicklin S, Hale C, Liew F Y. Prophylactic immunization against experimental leishmaniasis. I. Protection induced in mice genetically vulnerable to fatal Leishmania tropica infection. J Immunol. 1982;129:2206–2212. [PubMed] [Google Scholar]
- 56.Howard J G, Liew F Y, Hale C, Nicklin S. Prophylactic immunization against experimental leishmaniasis. III. Further characterization of the protective immunity against fatal Leishmania tropica infection induced by irradiated promastigotes. J Immunol. 1984;132:450–455. [PubMed] [Google Scholar]
- 57.Howard J C. Restrictions on the use of antigenic peptides by the immune system. Proc Natl Acad Sci USA. 1993;90:3777–3779. doi: 10.1073/pnas.90.9.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ivens A C, Smith D F. Parasite genome analysis. A global map of the Leishmania major genome: prelude to genomic sequencing. Trans R Soc Trop Med Hyg. 1997;91:111–115. doi: 10.1016/s0035-9203(97)90188-7. [DOI] [PubMed] [Google Scholar]
- 59.Jaffe C L, Perez L, Schnur L F. Lipophosphoglycan and secreted acid phosphatase of Leishmania tropica share species-specific epitopes. Mol Biochem Parasitol. 1990;41:233–240. doi: 10.1016/0166-6851(90)90186-p. [DOI] [PubMed] [Google Scholar]
- 60.Jardim A, Alexander J, Teh H S, Ou D W, Olafson R W. Immunoprotective Leishmania major synthetic T cell epitopes. J Exp Med. 1990;172:645–648. doi: 10.1084/jem.172.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jardim A, Alexander J, Teh H S, Ou D, Olafson R W. Immunoprotective Leishmania major synthetic T cell epitopes. J Exp Med. 1991;172:645–648. doi: 10.1084/jem.172.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Julia V, Rassoulzadegan M, Glaichenhaus N. Resistance to Leishmania major induced by tolerance to a single antigen. Science. 1996;274:421–423. doi: 10.1126/science.274.5286.421. [DOI] [PubMed] [Google Scholar]
- 63.Kaye P M, Gorak P, Murphy M, Ross S. Strategies for immune intervention in visceral leishmaniasis. Ann Trop Med Parasitol. 1995;89(Suppl. 1):75–81. doi: 10.1080/00034983.1995.11813016. [DOI] [PubMed] [Google Scholar]
- 64.Kelley J M. Genetic transformation of parasitic protozoa. Adv Parasitol. 1997;39:227–270. doi: 10.1016/s0065-308x(08)60047-0. [DOI] [PubMed] [Google Scholar]
- 65.Kellina O I. Changes in virulence of strains of Leishmania tropica major. Med Parasitol. 1965;6:701–708. [PubMed] [Google Scholar]
- 66.Kellina O I. Problems and current lines in investigations on the epidemiology of leishmaniasis and its control in the USSR. Bull Soc Pathol Exot. 1981;74:306–318. [PubMed] [Google Scholar]
- 67.Kelso A. Th1 and Th2 subsets: paradigms lost? Immunol Today. 1995;16:374–379. doi: 10.1016/0167-5699(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 68.Kelso A. Cytokines: principles and prospects. Immunol Cell Biol. 1998;76:300–317. doi: 10.1046/j.1440-1711.1998.00757.x. [DOI] [PubMed] [Google Scholar]
- 69.Kenney R T, Sacks D L, Sypek J P, Vilela L, Gam A A, Evans-Davis K. Protective immunity using recombinant human IL-12 and alum as adjuvants in a primate model of cutaneous leishmaniasis. J Immunol. 1999;163:4481–4488. [PubMed] [Google Scholar]
- 70.Lamb J R, Zanders E D, Lake P, Webster R G, Eckels D D, Woody J N, Green N, Lerner R A, Feldmann M. Inhibition of T cell proliferation by antibodies to synthetic peptides. Eur J Immunol. 1984;14:153–157. doi: 10.1002/eji.1830140209. [DOI] [PubMed] [Google Scholar]
- 71.Lara M L, Layrisse Z, Scorza J V, Garcia E, Stoikow Z, Granados J, Bias W. Immunogenetics of human American cutaneous leishmaniasis. Study of HLA haplotypes in 24 families from Venezuela. Hum Immunol. 1991;30:129–135. doi: 10.1016/0198-8859(91)90081-j. [DOI] [PubMed] [Google Scholar]
- 72.Lasri S, Sahibi H, Sadak A, Jaffe C L, Rhalem A. Immune responses in vaccinated dogs with autoclaved Leishmania major promastigotes. Vet Res. 1999;30:441–449. [PubMed] [Google Scholar]
- 73.Leclercq V, Lebastard M, Belkaid Y, Louis J, Milon G. The outcome of the parasitic process initiated by Leishmania infantum in laboratory mice: a tissue-dependent pattern controlled by the Lsh and MHC loci. J Immunol. 1996;157:4537–4545. [PubMed] [Google Scholar]
- 74.Liew F Y, Li Y, Millott S. Tumor necrosis factor-α synergizes with IFN-γ mediating killing of Leishmania major through the induction of nitric oxide. J Immunol. 1990;145:4306–4310. [PubMed] [Google Scholar]
- 75.Lohman K L, Langer P J, McMahon-Pratt D. Molecular cloning and characterization of the immunologically protective surface glycoprotein GP46/M-2 of Leishmania amazonensis. Proc Natl Acad Sci USA. 1990;87:8393–8397. doi: 10.1073/pnas.87.21.8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mann D L, Garner R P, Dayhoff D E, Cao K, Fernandez-Vina M A, Davis C, Aronson N, Ruiz N, Birx D L, Michael N L. Major histocompatibility complex genotype is associated with disease progression and virus load levels in a cohort of human immunodeficiency virus type 1-infected Caucasians and African Americans. J Infect Dis. 1998;178:1799–1802. doi: 10.1086/314519. [DOI] [PubMed] [Google Scholar]
- 77.Marzochi K B, Marzochi M A, Silva A F, Grativol N, Duarte R, Confort E M, Modabber F. Phase 1 study of an inactivated vaccine against American tegumentary leishmaniasis in normal volunteers in Brazil. Mem Inst Oswaldo Cruz. 1998;93:205–212. doi: 10.1590/s0074-02761998000200014. [DOI] [PubMed] [Google Scholar]
- 78.Matzinger P. An innate sense of danger. Semin Immunol. 1998;10:399–415. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- 79.Mauel J, Behin R, Louis M. Leishmania enriettii immune induction of macrophage activation in an experimental model of immunoprophylaxis in the mouse. Exp Parasitol. 1981;148:393–407. doi: 10.1016/0014-4894(81)90091-6. [DOI] [PubMed] [Google Scholar]
- 80.Mayrink W, da Costa C A, Magalhaes P A, Melo M N, Dias M, Lima A O, Michalick M S, Williams P. A field trial of a vaccine against American dermal leishmaniasis. Trans R Soc Trop Med Hyg. 1979;73:385–387. doi: 10.1016/0035-9203(79)90159-7. [DOI] [PubMed] [Google Scholar]
- 81.Mayrink, W., C. M. Antunes, C. A. Da Costa, M. N. Melo, M. Dias, M. S. Michalick, P. A. Magalhaes, A. De Oliveira Lima, and P. Williams. 1986. Further trials of a vaccine against American cutaneous leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. p80. [DOI] [PubMed]
- 82.Mayrink W, Genaro O, Dias M, da Costa C A, Michalick M S, Melo M N, Williams P, da Costa R T, Nascimento E, Oliveira L A. Vaccination of dogs against Leishmania (Viannia) braziliensis. Rev Inst Med Trop Sao Paulo. 1990;32:67–69. doi: 10.1590/s0036-46651990000100012. [DOI] [PubMed] [Google Scholar]
- 83.Mayrink W, Genaro O, Silva J C, da Costa R T, Tafuri W L, Toledo V P, da Silva A R, Reis A B, Williams P, da Costa P W. Phase I and II open clinical trials of a vaccine against Leishmania chagasi infections in dogs. Mem Inst Oswaldo Cruz. 1996;91:695–697. doi: 10.1590/s0074-02761996000600006. [DOI] [PubMed] [Google Scholar]
- 84.McConville M J, Bacic A, Mitchell G F, Handman E. Lipophosphoglycan of Leishmania major that vaccinates against cutaneous leishmaniasis contains an alkylglycerophosphoinositol lipid anchor. Proc Natl Acad Sci USA. 1987;84:8941–8945. doi: 10.1073/pnas.84.24.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McGregor A. WHO warns of epidemic leishmania. Lancet. 1998;351:575. doi: 10.1016/S0140-6736(05)78567-6. [DOI] [PubMed] [Google Scholar]
- 86.McMahon-Pratt D, Traub-Cseko Y, Lohman K L, Rogers D D, Beverley S M. Loss of the GP46/M2 surface membrane glycoprotein gene family in the Leishmania braziliensis complex. Mol Biochem Parasitol. 1992;50:151–160. doi: 10.1016/0166-6851(92)90252-f. [DOI] [PubMed] [Google Scholar]
- 87.McMahon-Pratt D, Rodriguez D, Rodriguez J-R, Zhang Y, Manson K, Bergman C, Rivas L, Rodriguez J F, Lohman K L, Ruddle N H, Esteban M. Recombinant vaccinia viruses expressing GP46/M-2 protect against Leishmania infection. Infect Immun. 1993;61:3351–3359. doi: 10.1128/iai.61.8.3351-3359.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McSorley S J, Xu D, Liew F Y. Vaccine efficacy of Salmonella strains expressing glycoprotein 63 with different promoters. Infect Immun. 1997;65:171–178. doi: 10.1128/iai.65.1.171-178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mendonca S C F, Russell D G, Coutinho S G. Analysis of the human T cell responsiveness to purified antigens of Leishmania: lipophosphoglycan (LPG) and glycoprotein 63 (GP63) Clin Exp Immunol. 1991;83:472–478. doi: 10.1111/j.1365-2249.1991.tb05663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mendonca S C F, de Luca P M, Mayrink W, Restom T G, Conceicao-Silva F, Da-Cruz A M, Bertho A L, Da Costa C A, Genaro O, Toledo V P C P, Coutinho S G. Characterization of human T lymphocyte-mediated immune responses induced by a vaccine against American tegumentary leishmaniasis. Am J Trop Med Hyg. 1995;53:195–201. doi: 10.4269/ajtmh.1995.53.195. [DOI] [PubMed] [Google Scholar]
- 91.Messina J P, Gilkeson G S, Pisetsky D S. Stimulation of in vitro murine lymphocyte proliferation by bacterial DNA. J Immunol. 1991;147:1759–1764. [PubMed] [Google Scholar]
- 92.Metz D P, Bottomly K. Function and regulation of memory CD4 T cells. Immunol Res. 1999;19:127–141. doi: 10.1007/BF02786482. [DOI] [PubMed] [Google Scholar]
- 93.Mitchell G F, Handman E. The glycoconjugate derived from a Leishmania major receptor for macrophages is a suppressogenic, disease-promoting antigen in murine cutaneous leishmaniasis. Parasite Immunol. 1986;8:255–263. doi: 10.1111/j.1365-3024.1986.tb01037.x. [DOI] [PubMed] [Google Scholar]
- 94.Modabber F. Vaccines against leishmaniasis. Ann Trop Med Parasitol. 1995;89:83–88. doi: 10.1080/00034983.1995.11813017. [DOI] [PubMed] [Google Scholar]
- 95.Momeni A Z, Jalayer T, Emamjomeh M, Khamesipour A, Zicker F, Ghassemi R L, Dowlati Y, Sharifi I, Aminjavaheri M, Shafiei A, Alimohammadian M H, Hashemi-Fesharki R, Nasseri K, Godal T, Smith P G, Modabber F. A randomised, double-blind, controlled trial of a killed L. major vaccine plus BCG against zoonotic cutaneous leishmaniasis in Iran. Vaccine. 1999;17:466–472. doi: 10.1016/s0264-410x(98)00220-5. [DOI] [PubMed] [Google Scholar]
- 96.Moody D B, Ulrichs T, Muhlecker W, Young D C, Gurcha S S, Grant E, Rosat J P, Brenner M B, Costello C E, Besra G S, Porcelli S A. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature. 2000;404:884–888. doi: 10.1038/35009119. [DOI] [PubMed] [Google Scholar]
- 97.Morris L, Troutt A B, Handman E, Kelso A. Changes in the precursor frequencies of IL-4 and IFN-γ secreting CD4+ cells correlate with resolution of lesions in murine cutaneous leishmaniasis. J Immunol. 1992;149:2715–2721. [PubMed] [Google Scholar]
- 98.Morris L, Troutt A B, McLeod K S, Kelso A, Handman E, Aebischer T. Interleukin-4 but not interferon-γ production correlates with the severity of disease in murine cutaneous leishmaniasis. Infect Immun. 1993;61:3459–3465. doi: 10.1128/iai.61.8.3459-3465.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moss D J, Schmidt C, Elliott S, Suhrbier A, Burrows S, Khanna R. Strategies involved in developing an effective vaccine for EBV-associated diseases. Adv Cancer Res. 1996;69:213–245. doi: 10.1016/s0065-230x(08)60864-7. [DOI] [PubMed] [Google Scholar]
- 100.Mougneau E, Altare F, Wakil A E, Zheng S, Coppola T, Wang Z-E, Waldmann R, Locksley R M, Glaichenhaus N. Expression cloning of a protective Leishmania antigen. Science. 1995;268:563–566. doi: 10.1126/science.7725103. [DOI] [PubMed] [Google Scholar]
- 101.Nashleanas M, Kanaly S, Scott P. Control of Leishmania major infection in mice lacking TNF receptors. J Immunol. 1998;160:5506–5513. [PubMed] [Google Scholar]
- 102.Nicolle C H. Cultures des corps de Leishman isoles de la rate dans trois cas d'anemic sptenique infantile. Bull Soc Pathol Exot. 1908;1:121. [Google Scholar]
- 103.Olobo J O, Anjili C O, Gicheru M M, Mbati P A, Kariuki T M, Githure J I, Koech D K, McMaster W R. Vaccination of vervet monkeys against cutaneous leishmaniosis using recombinant Leishmania ‘major surface glycoprotein’ (gp63) Vet Parasitol. 1995;60:199–212. doi: 10.1016/0304-4017(95)00788-6. [DOI] [PubMed] [Google Scholar]
- 104.Overath P, Aebischer T. Antigen presentation by macrophages harboring intravesicular pathogens. Parasitol Today. 1999;15:325–332. doi: 10.1016/s0169-4758(99)01473-8. [DOI] [PubMed] [Google Scholar]
- 105.Ozbel Y, Turgay N, Ozensoy S, Ozbilgin A, Alkan M Z, Ozcel M A, Jaffe C L, Schnur L, Oskam L, Abranches P. Epidemiology, diagnosis and control of leishmaniasis in the Mediterranean region. Ann Trop Med Parasitol. 1995;89(Suppl. 1):89–93. doi: 10.1080/00034983.1995.11813018. [DOI] [PubMed] [Google Scholar]
- 106.Papadopoulou G, Karagouni E, Dotsika E. ISCOMs vaccine against experimental leishmaniasis. Vaccine. 1998;16:885–892. doi: 10.1016/s0264-410x(97)00308-3. [DOI] [PubMed] [Google Scholar]
- 107.Pardoll D M, Beckerleg A M. Exposing the immunology of naked DNA vaccines. Immunity. 1995;3:165–169. doi: 10.1016/1074-7613(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 108.Pessoa S B, Pestana B R. Enseaio sobre vacinacao preventiva na leishmaniose tegumentar americana com germenes mortos. Arq Hig Saude Publ. 1941;6:141–147. [Google Scholar]
- 109.Pessoa S B. Segunda nota sobre a vacinacao preventiva na leishmaniose tegumentar americana com leptomones mortas. Rev Paul Med. 1941;19:106. [Google Scholar]
- 110.Pfeffer K, Schoel B, Gulle H, Kaufmann S H E, Wagner H. Primary responses of human T cells to mycobacteria: a frequent set of γδ T cells are stimulated by protease-resistant ligands. Eur J Immunol. 1990;20:1175–1179. doi: 10.1002/eji.1830200534. [DOI] [PubMed] [Google Scholar]
- 111.Pinelli E, Gonzalo R M, Boog C J P, Rutten V P M G, Gebhard D, de Real G, Ruitenberg E J. Leishmania infantum-specific T cell lines derived from asymptomatic dogs that lyse infected macrophages in a major histocopatibility complex-restricted manner. Eur J Immunol. 1995;25:1594–1600. doi: 10.1002/eji.1830250619. [DOI] [PubMed] [Google Scholar]
- 112.Preston P M, Dumonde D C. Experimental cutaneous leishmaniasis. V. Protective immunity in subclinical and selfhealing infection in the mouse. Clin Exp Immunol. 1976;23:126–138. [PMC free article] [PubMed] [Google Scholar]
- 113.Rivier D, Shah R, Bovay P, Mauel J. Vaccine development against cutaneous leishmaniasis. Subcutaneous administration of radioattenuated parasites protects CBA mice against virulent Leishmania major challenge. Parasite Immunol. 1993;15:75–84. doi: 10.1111/j.1365-3024.1993.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 114.Rivier D, Bovay P, Shah R, Didisheim S, Mauel J. Vaccination against Leishmania major in a CBA mouse model of infection: role of adjuvants and mechanism of protection. Parasite Immunol. 1999;21:461–473. doi: 10.1046/j.1365-3024.1999.00244.x. [DOI] [PubMed] [Google Scholar]
- 115.Roberts L J, Handman E, Foote S J. Leishmaniasis in the new genetic era. Br Med J. 2000;321:801–804. doi: 10.1136/bmj.321.7264.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Russell D G, Alexander J. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J Immunol. 1988;140:1274–1279. [PubMed] [Google Scholar]
- 117.Russo D M, Burns J M J, Carvalho E M, Armitage R J, Grabstein K H, Button L L, McMaster W R, Reed S G. Human T cell responses to gp63, a surface antigen of Leishmania. J Immunol. 1991;147:3575–3580. [PubMed] [Google Scholar]
- 118.Russo D M, Jardim A, Carvalho E M, Sleath P R, Armitage R J, Olafson R W, Reed S G. Mapping human T cell epitopes in Leishmania gp63. J Immunol. 1993;150:932–939. [PubMed] [Google Scholar]
- 119.Sacks D L, Lal S L, Shrivastava S N, Blackwell J, Neva F A. An analysis of T cell responsiveness in Indian kala-azar. J Immunol. 1987;138:908–913. [PubMed] [Google Scholar]
- 120.Schijns V E J C. Immunological concepts of vaccine adjuvant activity. Curr Opin Immunol. 2000;12:456–463. doi: 10.1016/s0952-7915(00)00120-5. [DOI] [PubMed] [Google Scholar]
- 121.Scott P, Trinchieri G. IL-12 as an adjuvant for cell-mediated immunity. Semin Immunol. 1997;9:285–291. doi: 10.1006/smim.1997.0084. [DOI] [PubMed] [Google Scholar]
- 122.Seder R A, Gurunathan S. DNA vaccines—designer vaccines for the 21st century. N Engl J Med. 1999;341:277–278. doi: 10.1056/NEJM199907223410410. [DOI] [PubMed] [Google Scholar]
- 123.Serebryakov V A, Karakhodznaeva S K H, Dzhumaev M D. Effect of leishmanial vaccinations on the dynamics of immunity to diphteria in conditions of secondary revaccination with adsorbed pertussis-diphteria-tetanus vaccine. Med Parasit Mosk. 1972;41:303–309. [PubMed] [Google Scholar]
- 124.Sharifi I, FeKri A R, Aflatonian M R, Khamesipour A, Nadim A, Mousavi M R, Momeni A Z, Dowlati Y, Godal T, Zicker F, Smith P G, Modabber F. Randomised vaccine trial of single dose of killed Leishmania major plus BCG against anthroponotic cutaneous leishmaniasis in Bam, Iran. Lancet. 1998;351:1540–1543. doi: 10.1016/S0140-6736(98)09552-X. [DOI] [PubMed] [Google Scholar]
- 125.Sieling P A, Ochoa M T, Jullien D, Leslie D S, Sabet S, Rosat J P, Burdick A E, Rea T H, Brenner M B, Porcelli S A, Modlin R L. Evidence for human CD4+ T cells in the CD1-restricted repertoire: derivation of mycobacteria-reactive T cells from leprosy lesions. J Immunol. 2000;164:4790–4796. doi: 10.4049/jimmunol.164.9.4790. [DOI] [PubMed] [Google Scholar]
- 126.Sjölander A, Baldwin T M, Curtis J M, Lövgren Bengtsson K, Handman E. Vaccination with recombinant parasite surface antigen 2 from Leishmania major induces a Th1 type of immune response but does not protect against infection. Vaccine. 1998;16:2077–2084. doi: 10.1016/s0264-410x(98)00075-9. [DOI] [PubMed] [Google Scholar]
- 127.Sjölander A, Baldwin T M, Curtis J M, Handman E. Induction of a Th1 immune response and simultaneous lack of activation of a Th2 response is required for generation of immunity to leishmaniasis. J Immunol. 1998;160:3949–3957. [PubMed] [Google Scholar]
- 128.Skeiky Y A, Kennedy M, Kaufman D, Borges M M, Guderian J A, Scholler J K, Ovendale P J, Picha K S, Morrissey P J, Grabstein K H, Campos-Neto A, Reed S G. LeIF: a recombinant Leishmania protein that induces an IL-12-mediated Th1 cytokine profile. J Immunol. 1998;161:6171–6179. [PubMed] [Google Scholar]
- 129.Solbach W, Laskay T. The host response to Leishmania infection. Adv Immunol. 2000;74:275–317. doi: 10.1016/s0065-2776(08)60912-8. [DOI] [PubMed] [Google Scholar]
- 130.Soong L, Duboise S M, Kima P, McMahon-Pratt D. Leishmania pifanoi amastigote antigens protect mice against cutaneous leishmaniasis. Infect Immun. 1995;63:3559–3566. doi: 10.1128/iai.63.9.3559-3566.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Spitzer N, Jardim A, Lippert D, Olafson R W. Long-term protection of mice against Leishmania major with a synthetic peptide vaccine. Vaccine. 1999;17:1298–1300. doi: 10.1016/s0264-410x(98)00363-6. [DOI] [PubMed] [Google Scholar]
- 132.Stacey K J, Sweet M J, Hume D A. Macrophages ingest and are activated by bacterial DNA. J Immunol. 1996;157:2116–2122. [PubMed] [Google Scholar]
- 133.Stacey K J, Blackwell J M. Immunostimulatory DNA as an adjuvant in vaccination against Leishmania major. Infect Immun. 1999;67:3719–3726. doi: 10.1128/iai.67.8.3719-3726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Streit J A, Recker T J, Donelson J E, Wilson M E. BCG expressing LCR1 of Leishmania chagasi induces protective immunity in susceptible mice. Exp Parasitol. 2000;94:33–41. doi: 10.1006/expr.1999.4459. [DOI] [PubMed] [Google Scholar]
- 135.Sugita M, Moody D B, Jackman R M, Grant E P, Rosat J P, Behar S M, Peters P J, Porcelli S A, Brenner M B. CD1—a new paradigm for antigen presentation and T cell activation. Clin Immunol Immunopathol. 1998;87:8–14. doi: 10.1006/clin.1997.4500. [DOI] [PubMed] [Google Scholar]
- 136.Sukkar F. Leishmaniasis in the Middle East. In: Chang K-P, Bray R S, editors. Leishmaniasis. Amsterdam, The Netherlands: Elsevier; 1985. pp. 394–411. [Google Scholar]
- 137.Sundar S, Murray H W. Effect of treatment with interferon-gamma alone in visceral leishmaniasis. J Infect Dis. 1995;172:1627–1629. doi: 10.1093/infdis/172.6.1627. [DOI] [PubMed] [Google Scholar]
- 138.Sundar S, Rosenkaimer F, Lesser M L, Murray H W. Immunochemotherapy for a systemic intracellular infection: accelerated response using interferon-gamma in visceral leishmaniasis. J Infect Dis. 1995;171:992–996. doi: 10.1093/infdis/171.4.992. [DOI] [PubMed] [Google Scholar]
- 139.Tapia F J, Caceres-Dittmar G, Sanchez M A. Inadequate epidermal homing leads to tissue damage in human cutaneous leishmaniasis. Immunol Today. 1994;15:160–165. doi: 10.1016/0167-5699(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 140.Reference deleted.
- 141.Titus R G, Gueiros-Filho F J, de Freitas L A, Beverley S M. Development of a safe live Leishmania vaccine line by gene replacement. Proc Natl Acad Sci USA. 1995;92:10267–10271. doi: 10.1073/pnas.92.22.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ullman B. Multidrug resistance and P-glycoproteins in parasitic protozoa. J Bioenerg Biomembr. 1995;27:77–84. doi: 10.1007/BF02110334. [DOI] [PubMed] [Google Scholar]
- 143.Ulmer J B, Sadoff J C, Liu M A. DNA vaccines. Curr Opin Immunol. 1996;8:531–536. doi: 10.1016/s0952-7915(96)80042-2. [DOI] [PubMed] [Google Scholar]
- 144.Veras P, Brodskyn C, Balestieri F, Freitas L, Ramos A, Queiroz A, Barral A, Beverley S, Barral-Netto M. A dhfr−ts− Leishmania major knockout mutant cross-protects against Leishmania amazonensis. Mem Inst Oswaldo Cruz. 1999;94:491–496. doi: 10.1590/s0074-02761999000400011. [DOI] [PubMed] [Google Scholar]
- 145.Wahren B. Gene vaccines. Immunotechnology. 1996;2:77–83. doi: 10.1016/1380-2933(96)00043-7. [DOI] [PubMed] [Google Scholar]
- 146.Walker P S, Scharton-Kersten T, Rowton E D, Hengge U, Bouloc A, Udey M C, Vogel J C. Genetic immunization with glycoprotein 63 cDNA results in a helper T cell type 1 immune response and protection in a murine model of leishmaniasis. Hum Gene Ther. 1998;9:1899–1907. doi: 10.1089/hum.1998.9.13-1899. [DOI] [PubMed] [Google Scholar]
- 147.Walker P S, Scharton-Kersten T, Krieg A M, Love-Homan L, Rowton E D, Udey M C, Vogel J C. Immunostimulatory oligodeoxynucleotides promote protective immunity and provide systemic therapy for leishmaniasis via IL-12- and IFN-gamma-dependent mechanisms. Proc Natl Acad Sci USA. 1999;96:6970–6975. doi: 10.1073/pnas.96.12.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wassom D L, Kelly E A. The role of the major histocompatibility complex in resistance to parasite infections. Crit Rev Immunol. 1990;10:31–52. [PubMed] [Google Scholar]
- 149.World Health Organization. AIDS, leishmaniasis dangers of clash highlighted. TDR News. 1991;36:1. , 11. [Google Scholar]
- 150.World Health Organization. Advances in the battle against leishmaniasis. TDR News. 1998;57:2. [PubMed] [Google Scholar]
- 151.Wright F J. Exacerbation of dimorphous leprosy apparently due to BCG vaccination. Lepr Rev. 1971;42:219–221. doi: 10.5935/0305-7518.19710026. [DOI] [PubMed] [Google Scholar]
- 152.Xu D, McSorley S J, Chatfield S N, Dougan G, Liew F Y. Protection against Leishmania major infection in genetically susceptible BALB/c mice by GP63 delivered orally in attenuated Salmonella typhimurium (AroA−AroD−) Immunology. 1995;85:1–7. [PMC free article] [PubMed] [Google Scholar]
- 153.Xu D, McSorley S J, Tetley L, Chatfield S, Dougan G, Chan W L, Satoskar A, David J R, Liew F Y. Protective effect on Leishmania major infection of migration inhibitory factor, TNF-alpha, and IFN-gamma administered orally via attenuated Salmonella typhimurium. J Immunol. 1998;160:1285–1289. [PubMed] [Google Scholar]
- 154.Yamamoto S, Kuramoto E, Shimada S, Tokunaga T. In vitro augmentation of natural killer cell activity and production of interferon-alpha/beta and -gamma with deoxyribonucleic acid fraction from Mycobacterium bovis BCG. Jpn J Cancer Res. 1988;79:866–873. doi: 10.1111/j.1349-7006.1988.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang D M, Fairweather N, Button L L, McMaster W R, Kahl L P, Liew F Y. Oral Salmonella typhimurium (AroA−) vaccine expressing a major leishmanial surface protein (gp63) preferentially induces T helper 1 cells and protective immunity against leishmaniasis. J Immunol. 1990;145:2281–2285. [PubMed] [Google Scholar]
- 156.Zeng W, Jackson D C, Murray J, Rose K, Brown L E. Totally synthetic lipid-containing polyoxime peptide constructs are potent immunogens. Vaccine. 2000;18:1031–1039. doi: 10.1016/s0264-410x(99)00346-1. [DOI] [PubMed] [Google Scholar]
- 157.Zimmermann S, Egeter O, Hausmann S, Lipford G B, Rocken M, Wagner H, Heeg K. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J Immunol. 1998;160:3627–3630. [PubMed] [Google Scholar]