Summary
Objective.
To support patient centered care and the collaboration of patients and clinicians, we developed and pilot tested a conversation aid for patients with thyroid nodules.
Methods.
We developed a web-based Thyroid NOdule Conversation aid (TNOC) following a human-centered design. A proof of concept observational pre–post study was conducted [TNOC vs. usual care (UC)] to assess the impact of TNOC on the quality of conversations. Data sources included recordings of clinical visits, post encounter surveys, and review of electronic health records. Summary statistics and group comparisons are reported.
Results.
Sixty five patients were analyzed (32 in the UC and 33 in the TNOC cohort). Most patients were women (89%) with a median age of 57 years and were incidentally found to have a thyroid nodule (62%). Most thyroid nodules were at low risk for thyroid cancer (71%) and the median size was 1.4 cm. At baseline, the groups were similar except for higher numeracy in the TNOC cohort. The use of TNOC was associated with increased involvement of patients in the decision making process, clinician satisfaction, and discussion of relevant topics for decision making. In addition, decreased decisional conflict and fewer thyroid biopsies as next management step was noted in the TNOC cohort. No differences in terms of knowledge transfer, length of consultation, thyroid cancer risk perception or concern for thyroid cancer diagnosis were found.
Conclusion.
In this pilot observational study, using TNOC in clinical practice was feasible and seemed to help the collaboration of patients and clinicians.
Keywords: thyroid nodules, thyroid cancer, shared decision making, conversation aids
Introduction
Caring for patients with thyroid nodules often includes deciding when to proceed with thyroid biopsy or monitoring with thyroid ultrasound (US). This decision is based on thyroid cancer risk, presence of symptoms associated with nodular enlargement, or hyperthyroidism secondary to toxic thyroid nodules.1,2 Additionally, the decision of how to proceed depends on the patient’s preferences, values, and context.1,2 In fact, the American Thyroid Association (ATA) suggests consideration of patient factors that can affect the expected risks and benefits of a thyroid biopsy, such as high surgical risk or limited life expectancy.2 Ideally, the end result of this process is a decision that makes sense to patients intellectually (i.e., I understand what I am doing), practically (i.e., I can do it), and emotionally (i.e., it feels right).3
Yet, in practice, these conversations are challenging. Clinicians often have difficulty communicating thyroid cancer risk, the risks/benefits of biopsy, and eliciting patients’ preferences. As evidence of the complexity of these conversations, a survey of 196 patients that had just undergone a thyroid biopsy showed that patients had a limited understanding about the thyroid biopsy outcomes.4 In fact, one third were not aware that their biopsy could be reported as non-diagnostic or indeterminate, and half did not know their risk of thyroid cancer. These results suggest a gap in the quality of decision making and the need to support conversations in clinical practice related to thyroid nodule diagnosis.4
Shared decision making (SDM) is a care approach that supports conversations between patients and clinicians about treatment or diagnostic decisions.3 Tools that support SDM have been found to increase knowledge, accurate risk perceptions, satisfaction with the decision, and the number of patients achieving decisions that were informed and consistent with their values.5 To support SDM between patients with thyroid nodules considering thyroid biopsy and their clinicians, we developed a Thyroid NOdule Conversation Aid (TNOC). Here, we report the development of TNOC and the results of the initial (pilot) evaluation of its impact on the quality of conversation and diagnostic decisions.
Material and Methods
Development and field testing of TNOC
We established a multidisciplinary team consisting of a senior design researcher, SDM experts, and clinicians with expertise in the care of patients with thyroid nodules to design a conversation aid prototype. The team followed a human-centered design approach that involved: 1) review of the clinical evidence, 2) observation and analysis of usual practice, 3) development of an initial prototype, and 4) field testing in real encounters between patients and their clinicians with successive iteration of the prototype. The input from patients and clinicians was incorporated in this process. (Figure1) The development process continued until there was observable evidence that the conversation aid prototype supported the creation of a conversation consistently between patients and clinicians in which patients verbalized “trying on” the different options and testing the hypotheses that the option considered would be the best fit for them.6–8 The evidence review focused on the evaluation of clinical practice guidelines recommendations, and clinical studies that guide the care patients with thyroid nodules receive.2,9–11
Figure 1.
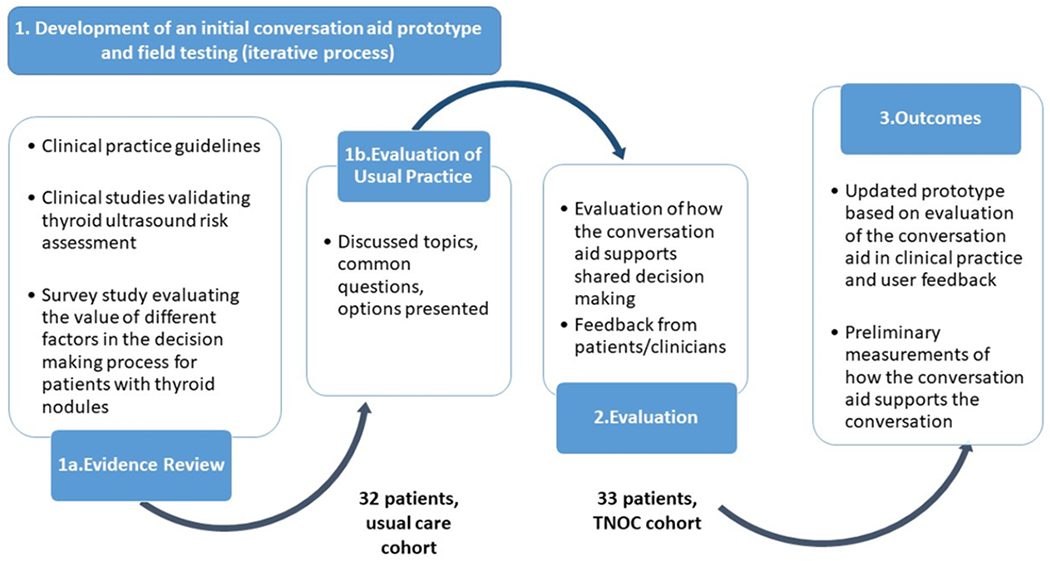
TNOC development process and study design.
TNOC, thyroid nodule conversation aid
Preliminary (pilot) evaluation of TNOC’s impact on quality of conversation and diagnostic decision
Study design, setting, and participants
We conducted a proof of concept pre-post observational study (non randomized) at the Endocrinology and Ear, Nose and Throat (ENT) outpatient clinics at the University of Florida between 2018–2019. Four endocrinologists and two ENT clinicians with expertise in the management of patients with thyroid nodules participated in the study. Eligible patients were ≥18 years and presented for the evaluation of a thyroid nodule. Pregnant patients, those with evidence of hyperthyroidism, or with available records of a previous biopsy of the nodule of interest were excluded. The study was approved by the University of Florida Institutional Review Board and informed consent was obtained at the time of enrollment.
We grouped participants into two temporal sequential cohorts, a methodology previously used by our group for the development and pilot testing of a conversation aid for patients with Graves’ Disease.12 The first cohort included patients presenting for the evaluation of a thyroid nodule and receiving counseling following usual care (UC). In other words, patients were evaluated and counseled as routinely done by the clinical team. This cohort allowed us to evaluate the usual practice for patients with thyroid nodules (information needed to develop TNOC) and was used as a comparison group. The second cohort included patients presenting for the evaluation of a thyroid nodule and receiving counseling that was supplemented by the use of TNOC. In other words, clinicians used TNOC to support their routine clinical discussion. TNOC was displayed and used during the clinical visit, as deemed fit by clinicians in their interaction with their patients. Patients who participated in the UC cohort were ineligible to participate in the TNOC cohort. (Figure 1)
Outcomes and data sources
Data sources for this study included clinical visit recordings, post visit surveys by patients, and baseline and post visit surveys by clinicians. See (Appendix 1) for a detailed description of measurements used.
Recordings of clinical visits:
Video or audio recordings were assessed independently by two authors (NSO, DB) and disagreements were resolved by consensus to assess for: visit duration (minutes), fidelity of TNOC use (by scoring the presence of 10 elements included in the conversation aid and calculating a score of 1–100, with a higher number indicating higher fidelity of use), frequency of topics discussed related to the diagnosis of thyroid nodules, proportion of encounters in which patients expressed a management preference, or either the patient or the clinician delegated the decision to the other party.
Furthermore, we evaluated the extent to which clinicians engaged patients in decision making using the OPTION score. This score, includes 12 items scored 0–4 and transformed into a 1–100 scale, with higher levels indicating higher patient involvement.13,14
In our practice, patients might first undergo an initial evaluation by a trainee focused on data gathering, followed by ultrasound assessment and counseling performed by an attending. Due to this dynamic process, it was feasible to only include the counseling section for some patients.
Patients post visit survey:
Patients completed a post visit survey. This included demographic information, health status, literacy and numeracy (8 questions) 15–18, an assessment of knowledge related to thyroid nodules (12 statements to be evaluated as True/False/I don’t know) and participant thyroid cancer risk perception (number from 0% to 100% or I don’t know). We evaluated the percentage of patients that provided a risk estimate and whether this risk was correct. We followed the American College of Radiology Thyroid Imaging Reporting and Data System (ACR-TIRADS) to estimate this risk, as the initial prototype allowed clinicians to enter their estimated thyroid cancer risk and/or follow the ATA framework.1,9 Furthermore, patients rated their concern for a thyroid cancer diagnosis on a scale of 1–5 (not concerned to very concerned) and the degree of uncertainty about a particular course of action using the decisional conflict scale. This scale includes 16 statements, evaluated from 0–4, with higher numbers associated with higher degree of decisional conflict (reported as 0 −100 scale).19 Finally, we evaluated quality of communication and satisfaction with the encounter using Likert score scales. 20
Clinicians baseline and post visit survey:
Clinicians completed a baseline survey that included questions related to their clinical experience and demographics and a post visit survey after each clinical visit including questions related to their satisfaction (Likert score, with higher numbers indicating increased satisfaction) and decision making during the encounter.
Electronic health record:
We used health records to obtain clinical characteristics related to the thyroid nodule, final management choice, and patients’ demographics.
Statistical analysis and data management
Clinical, survey, and video-analysis data were uploaded into piloted electronic forms in RedCap.21 Continuous variables were summarized as median and interquartile range (IQR). Categorical variables were summarized as frequencies and proportions. For demographic and baseline clinical characteristics, Wilcoxon rank sum test was used to compare continuous variables, and Chi-squared test or Fisher exact test to compare categorical variables.
As multiple participants may share one clinician, we calculated intraclass correlation (ICC) for each outcome. Wilcoxon rank sum test was used to compare continuous outcomes if its ICC was 0; univariable linear mixed model with random intercept was used if ICC was non-zero. Chi-squared test or Fisher exact test was used to compare categorical outcomes if its ICC is 0; univariable generalized linear mixed model with random intercept was used if ICC was non-zero.
A multivariable generalized linear mixed model was fitted for outcome biopsy (received biopsy vs. not received biopsy) to evaluate the effect of group (TNOC vs UC), adjusting for nodule size and TIRADS (TR4/5 vs TR1–3). All statistical analyses were conducted using R 3.6.3.
Results
TNOC prototype development
The research team evaluated the clinical practice guidelines and associated clinical evidence guiding the care of patients with thyroid nodules, topics considered important by patients with thyroid nodules during the decision making process, and the clinical visit recordings of patients in the UC cohort, in an iterative process and with input from patients and clinicians to develop a conversation aid prototype. (Figure 1) This prototype included: 1) a description of the thyroid cancer risk displayed as a graph that allowed the clinician to enter the expected risk or follow the ATA US risk classification, 2) a discussion of the management options (e.g., US surveillance, thyroid biopsy, surgery), 3) a description of what each option means for the patient and potential risks for each option, and 4) an estimation of the cost related to each management option. In addition, clinicians were provided with a template to document the conversation in the electronic medical record. (Figure 2 and Appendix 2)
Figure 2, TNOC components.
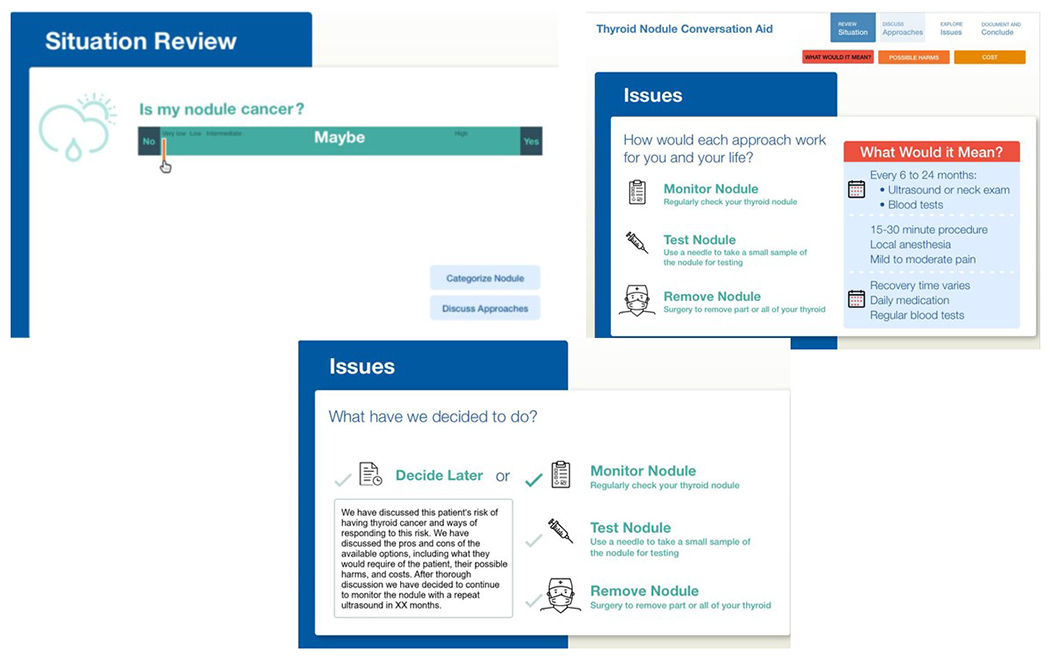
TNOC, thyroid nodule conversation aid
TNOC pilot testing
Participants demographics and clinical characteristics
Seventy-nine patients were enrolled in the study and 14 patients were excluded (e.g., technical issues related to recordings, previous biopsy, no identification of thyroid nodule on repeat US, no discussion about management options). The UC cohort included 32 patients and the TNOC cohort included 33 patients (Figure 1).
Most patients, were women (89.2%), middle aged (57 years), had completed graduate education (61.8%), and a minority had difficulty reading medical forms (10.9%) or inadequate health literacy (1.7%). There were no differences between groups, except for higher numeracy in the TNOC cohort (5.2 vs 4.6, p=0.027). There was a non statistically significant higher proportion of low risk nodules in TNOC (76% vs 66%, p= 0.532). Similarly, nodules in the TNOC cohort were smaller, although the difference was not statistically significant (1.2 cm vs 1.5 cm, p=0.279). Table 1 and Table 2 summarize other demographic and clinical characteristics between counseling groups.
Table1.
Demographics according to counseling group
| UC cohort | TNOC cohort | All | p-value | |
|---|---|---|---|---|
| Sex (women), n (%) | 29 (90.6%) | 29 (87.9%) | 58 (89.2%) | 1.000 |
| Age (years), n, median (IQR) | 3250 (39.2 – 68.2) | 3359 (38 - 70) | 6557 (38 - 69) | 0.276 |
| Marital status, n, (%) | 0.855 | |||
| Married | 11 (44.0%) | 16 (50.0%) | 27 (47.4%) | |
| Hispanic/Latino/Spanish origin, n, (%) | 6 (24.0%) | 5 (15.6%) | 11 (19.3%) | 0.508 |
| Race, n, (%) | 1.000 | |||
| White | 21 (84.0%) | 27 (84.4%) | 48 (84.2%) | |
| Education, n, (%) | 0.955 | |||
| Graduate or professional degree | 15 (65.2%) | 19 (59.4%) | 34 (61.9%) | |
| Some college, no degree | 5 (21.7%) | 9 (28.1%) | 14 (25.5%) | |
| High school graduate | 2 (8.7%) | 3 (9.4%) | 5 (9.1%) | |
| Less than high school | 1 (4.3%) | 1 (3.1%) | 2 (3.6%) | |
| Household income, n, (%) | 0.148 | |||
| > $100,000 | 3 (13.0%) | 11 (34.4%) | 14 (25.5%) | |
| $40,000 -$100,000 | 10 (43.5%) | 8 (25.0%) | 18 (32.7%) | |
| Less than $40,000 | 10 (43.5%) | 13 (40.6%) | 23 (41.8%) | |
| Difficulty reading forms, n, (%) | 0.686 | |||
| Always, often, or sometimes | 3 (13.0%) | 3 (9.4%) | 6 (10.9%) | |
| Rarely or never | 20 (87.0%) | 29 (90.6%) | 49 (89.1%) | |
| Overall health, n, (%) | 0.778 | |||
| Excellent or very good | 12 (44.4%) | 16 (51.6%) | 28 (48.3%) | |
| Good, fair, or poor | 15 (55.6%) | 15 (48.4%) | 30 (51.7%) | |
| Daily medicines n, median, (IQR) | 274 (1 - 7) | 315 (0 - 7) | 584.5 (0.2 - 7) | 0.608 |
| Confidence filling medical forms (health literacy) , n, (%) | 1.000 | |||
| Extremely, quite a bit, somewhat confident | 26 (100%) | 31 (96.9%) | 57 (98.3%) | |
| A little bit confident | 0 | 1 (3.1%) | 1 (1.7%) | |
| Numeracy n, median, (IQR) | 264.6 (3.8 - 5.5) | 325.2 (4.7 - 6.0) | 584.8 (4.2 - 5.8) | 0.027 |
Abbreviations: UC, usual care; TNOC, thyroid nodule conversation aid; IQR, interquartile range
Number of missingness: Marital status, n=8; Hispanic/Latino/Spanish origin, n=8; Race, n=8; Education, n=10; Household income, n=10; Difficulty reading forms, n=10; Overall health, n=7; Daily medicines, n=7; Confidence filling medical forms (health literacy), n=7; Numeracy, n=7.
Table 2.
Clinical and recording characteristics according to counseling group
| Clinical Features | ||||
|---|---|---|---|---|
| UC cohort | TNOC cohort | All | p-value | |
| Nodule discovery, n, (%) | 0.04 | |||
| Diagnostic work up | 8 (25.0%) | 1 (3.0%) | 9 (13.8%) | |
| Incidental (exam) | 5 (15.6%) | 12 (36.4%) | 17 (26.2%) | |
| Incidental (imaging) | 13 (40.6%) | 10 (30.3%) | 23 (35.4%) | |
| Other | 3 (9.4%) | 6 (18.2%) | 9 (13.8%) | |
| Patient’s complaint | 3 (9.4%) | 3 (9.1%) | 6 (9.2%) | |
| Screening | 0 | 1 (3.0%) | 1 (1.5%) | |
| History of radiation therapy to the neck, n, (%) | 0 | 2 (6.1%) | 2 (3.1%) | 0.492 |
| Family history of thyroid cancer, n, (%) | 2 (6.2%) | 4 (12.1%) | 6 (9.2%) | 0.672 |
| History of Hypothyroidism, n, (%) | 6 (18.8%) | 4 (12.1%) | 10 (15.4%) | 0.511 |
| MNG, n, (%) | 26 (81.2%) | 21 (63.6%) | 47 (72.3%) | 0.190 |
| ATA US risk classification, n, (%) | 0.391 | |||
| High suspicion | 2 (6.2%) | 2 (6.1%) | 4 (6.2%) | |
| Intermediate suspicion | 7 (21.9%) | 2 (6.1%) | 9 (13.8%) | |
| Low suspicion | 13 (40.6%) | 12 (36.4%) | 25 (38.5%) | |
| Very low suspicion | 7 (21.9%) | 12 (36.4%) | 19 (29.2%) | |
| Benign | 0 | 1 (3.0%) | 1 (1.5%) | |
| No class | 3 (9.4%) | 4 (12.1%) | 7 (10.8%) | |
| ACR-TIRADS, n, (%) | 0.559 | |||
| TR1 | 0 | 1 (3.0%) | 1 (1.5%) | |
| TR2 | 7 (21.9%) | 12 (36.4%) | 19 (29.2%) | |
| TR3 | 14 (43.8%) | 12 (36.4%) | 26 (40.0%) | |
| TR4 | 9 (28.1%) | 6 (18.2%) | 15 (23.1%) | |
| TR5 | 2 (6.2%) | 2 (6.1%) | 4 (6.2%) | |
| Nodule Size (cm) median (IQR) | 1.5 (1.3 – 1.8) | 1.2 (1.0 - 2.0) | 1.4 (1.1-1.9) | 0.279 |
| Clinical Recording Features | ||||
| Type of clinical visit recording (video or audio), n, (%) | ||||
| Video | 3 (9.4%) | 14 (42.4%) | 17 (26.2%) | 0.006 |
| Number of clinicians that evaluated the patient (senior clinician only or trainee and clinician), n, (%) | ||||
| Trainee and clinician | 8 (25.0%) | 9 (27.3%) | 17 (26.2%) | 1.000 |
| Type of clinical visit recording (complete visit history and counseling, or counseling only), n, (%) | ||||
| Complete History and counseling | 28(87.5%) | 22 (66.7%) | 50 (76.9%) | 0.089 |
Abbreviations: UC, usual care; TNOC, thyroid nodule conversation aid; MNG, multinodular goiter; ATA US, American Thyroid Association Ultrasound Risk classification; ACR TIRADS, American College of Radiology Thyroid Imaging Reporting and Data System; IQR, interquartile range. Number of missingness: Nodule Size, n=1.
Six clinicians participated in the study; most were women (83%), 67% were endocrinologists, and 33% were ENT. The median time in clinical practice after completing medical training was 3 years (IQR 1–10) with a median of 2 new patients with thyroid nodules evaluated weekly (IQR 2- 3).
Fidelity and decision making process
Most patients were evaluated only by an attending (senior clinician) (74%) and the complete clinical visit recording was available (77%).The duration of clinical visits was similar between groups (18 minutes TNOC cohort vs 15 minutes UC cohort, ICC= 0.16, p= 0.240) (Table3).
Table3.
Outcomes according to counseling group (patient and clinician post visit survey)
| UC cohort | TNOC Cohort | ICC | p-value | |
|---|---|---|---|---|
| Clinical Visit Recordings | ||||
| Fidelity of use (conversation aid), median (IQR) | 90 (80 - 90) | |||
| Patient expressed a preference for a management option, n, (%) | 2 (6.2%) | 15, (45.5%) | 0.03 | 0.002 |
| Patient delegated decision to the clinician, n, (%) | 0 | 4 (12.1%) | 0 | 0.114 |
| Clinician delegated the decision to the patient | 1 (3.1%) | 0 | 0 | 0.492 |
| OPTION Score, median (IQR) | 20.8 (16.7 – 25.5) | 33.3 (31.2 – 37.5) | 0.01 | <0.001 |
| Duration clinical visit (minutes), median (IQR) | 15 (11.5 - 21.2) | 18 (11 - 25) | 0.16 | 0.240 |
| Patient Survey | ||||
| Knowledge all questions, median (IQR) | 66.7 (52.1 – 81.2) | 66.7 (50 – 77.1) | 0 | 0.969 |
| Knowledge 7 questions included in TNOC, median (IQR) | 71.4 (57.1 – 82.1) | 71.4 (57.1 – 85.7) | 0 | 0.687 |
| Knowledge 5 questions not in TNOC, median (IQR) | 60 (60 - 80) | 60 (60 - 80) | 0 | 0.580 |
| Provided a risk estimate for thyroid cancer, n, (%) | 14 (51.9%) | 21 (65.6%) | 0 | 0.420 |
| Correct risk estimate for thyroid cancer*, n, (%) | 12 (85.7%) | 16 (76.2%) | 0 | 0.676 |
| Very Concerned for a diagnosis of Thyroid Cancer diagnosis, n, (%) | 17 (65.4%) | 21 (67.7%) | 0 | 1.000 |
| Decisional conflict scale, median (IQR) | 17.8 (2.0 – 37.1) | 2.3 (0 – 20.3) | 0.01 | 0.007 |
| Would you recommend the way you and your clinician shared information about your thyroid nodule to other patients?, median (IQR) | 1 (1 - 1.5) | 1 (1 - 1) | 0.79 | 0.104 |
| Clinicians Survey | ||||
| Satisfaction with the discussion you had with your patient?, median (IQR) | 5 (5 - 6) | 6 (5 - 6) | 0.36 | 0.003 |
| Likelihood of recommending the way you and your patient worked together to make a decision?, median (IQR) | 7(6 – 7.2) | 8(7 - 8) | 0.09 | <0.001 |
Abbreviations: UC, usual care; TNOC, thyroid nodule conversation aid; ICC, intraclass correlation coefficient; IQR, interquartile range.
This is calculated among those 35 participants who provided risk estimate for thyroid cancer, therefore, the proportion denominator is 35 (14 for UC group and 21for TNOC group).
Number of missingness: Knowledge all questions, n=7; Knowledge 7 questions included in the CA, n=7; Knowledge 5 questions not included in the CA, n=7; Provided a risk estimate for thyroid cancer, n=6; Very Concerned for a diagnosis of Thyroid Cancer diagnosis, n=8; Decisional conflict scale, n=7; Would you recommend the way you and your clinician shared information about your thyroid nodule to other patients, n=7; Satisfaction with the discussion you had with your patient, n=4; Likelihood of recommending the way you and your patient worked together to make a decision, n=4.
The fidelity of use of TNOC was high (90%, IQR 80%- 90%). The use of TNOC led to increased discussion of topics related to diagnostic management options and thyroid cancer risk. (Figure 3). The OPTION score was higher in the TNOC cohort compared to the UC cohort (33.3 vs 20.8, p<0.001) and more patients in the TNOC expressed a preference for a management option (45.5% vs 6.2%, p=0.002). Table 3 summarizes outcomes according to study group.
Figure 3.
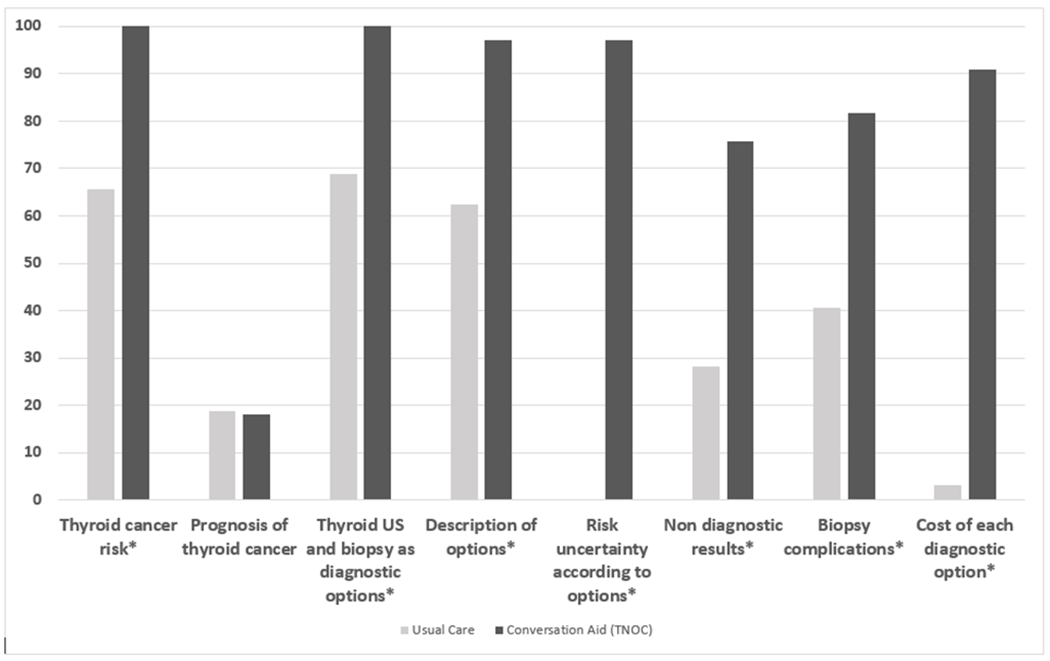
Topics discussed according to counseling group. X axis, topics (%); Y axis, proportion of encounters. * Statistically significant difference
Knowledge, thyroid cancer risk estimate, level of concern, and conflict
There was no difference in knowledge transfer based on the percentage of correct answers between the two groups. More participants in the TNOC cohort compared to the UC cohort provided an estimate risk for thyroid cancer (65.6% vs 51.9%, p=0.42).
Furthermore, the TNOC cohort had a lower decisional conflict scale score (lower numbers indicate less conflict) compared to the UC cohort (2.3 vs 17.8, p=0.007). Similarly, patients in both groups felt their clinicians explained things in a way that was easy to understand (96.9% TNOC vs 85.2% UC, p=0.169) and felt their clinician showed respect (100% TNOC vs 92.6% UC, p=0.205) and listened carefully to them (100% TNOC vs 92.6% UC, p=0.205). Finally, clinicians in the TNOC cohort were more satisfied with their discussion and likely to recommend the way they worked together to others, when compared to those in the UC cohort. (Table 3)
Diagnostic pathway choice
Reviewed medical records showed more patients deciding for biopsy as the next step (15/32, 46.9%) in the UC cohort vs. (5/33, 15.2%) in TNOC cohort, p=0.021, ICC 0.44. In the UC cohort, out of the 15 patients that underwent thyroid biopsy 2 (13.3%) were in the ATA high risk category and 8 (53.3%) in the ACR-TIRADS 4–5 category. In the TNOC cohort, out of the 5 patients that underwent thyroid biopsy 1 (20%) was in the ATA high risk category and 4 (80%) in ACR-TIRADS 4–5 category. (Supplemental Table 1) Other management options included follow up US at different time intervals and deferring the decision (e.g., until review of previous medical records, discussion with other family members).
Multivariable model for thyroid biopsy
The odds of moving forward with thyroid biopsy as the next management step among patients in the UC group was 46.1 times higher of those in the TNOC group (OR 95% CI: 4.3–2057.9). Similarly, on average, for one cm increase of nodule size, there was 21.3 times higher odds of moving forward with a thyroid biopsy (OR 95% CI: 4.5 – 293.9). Finally, on average, the odds of receiving thyroid biopsy among patients with nodules TIRADS 4–5 was 18.4 times higher of those with nodules considered TIRADS 1–3 (OR 95% 3.0–269.8).
Discussion
We developed and field tested a conversation aid for patients with thyroid nodules (TNOC) following a human centered approach. In a pilot study evaluating a population consistent mostly of women presenting for the evaluation of incidentally discovered thyroid nodules (most low risk for thyroid cancer), the use TNOC during clinical practice was feasible and seemed to support the conversation of diagnostic strategies for patients with thyroid nodules. We found positive effects on the decisional conflict scale and OPTION score and higher levels of clinician satisfaction in the TNOC cohort when compared to the UC cohort. Moreover, the proportion of encounters in which important decision making topics were discussed was higher in the TNOC cohort. The proportion of patients who underwent biopsy as the next management step, was lower in the TNOC group. We found no statistical differences in terms of knowledge or accurate thyroid cancer risk assessment.
We are not aware of other conversation aids designed to support SDM in the initial evaluation of patients with thyroid nodules. In fact, although a large group of SDM tools have been developed to assist patients and clinicians in treatment decisions, fewer are available to help patients facing diagnostic decisions (outside of screening).5,22–24 This is to some extent paradoxical, as conversations about diagnostic options are sometimes more challenging than treatment decisions, given diagnostic decisions usually involve a greater level of uncertainty and a larger decision tree (multiple hypothetical treatment options and prognosis based on different test results).22 For patients with thyroid nodules, these challenges are evident given the need to consider variable risks for thyroid cancer, associated uncertainty, and the possibility of subsequent treatment decisions depending on the results of a biopsy or clinical changes during thyroid US surveillance. Moreover, “the stakes” associated with these clinical decisions are significant given that an incorrect/delayed diagnosis might lead to increased morbidity/unnecessary worry, but at the same time, excessive diagnostic interventions can have harmful consequences from overdiagnosis.22,25,26
We observed a low proportion of conversations including discussions about cost in the UC cohort, which was increased by the inclusion of cost as a variable for consideration in the TNOC prototype. Our previous survey found that 13% of participants considered cost as a very important variable when deciding between medical options. We included this variable in this initial TNOC prototype, given literature supporting cost as a variable of interest in medical decision making.4,27,28 Field testing suggested limited value of cost when deciding between diagnostic options, as it rarely led to any significant conversation. The value of cost conversations emerges as another possible distinction between treatment and diagnostic decisions. However, is possible that contextual factors related healthcare coverage and access can affect the value of including cost in diagnostic conversations.27
The explicit discussion of management alternatives and clarifying the need to make a choice are important components of SDM.29,30 Our results suggest that the use of TNOC increased the likelihood that management alternatives were explicitly discussed, in this case, thyroid biopsy and surveillance with US. We also found a lower proportion of participants in the TNOC cohort undergoing thyroid biopsy as the next step in management. Diagnostic cascades of incidental findings have been associated with harm.25,31 For many asymptomatic patients incidentally diagnosed with a thyroid nodule, the decision to perform a thyroid biopsy versus surveillance with US might represent an adequate reflection point to avoid further downstream consequences of this seemingly automatic diagnostic cascade.1,32,33
Similarly, the use of TNOC increased the proportion of encounters in which thyroid cancer risk was discussed, although we did not find a statistically significant effect on thyroid cancer risk perception. The TNOC prototype pilot tested in this study, allowed the clinician to directly entered an estimated thyroid cancer risk or to choose the risk according to the ATA classification. In addition, we used the ACR-TIRADS to determine if the thyroid cancer risk recalled by the patient was accurate or not (given it allows for classification of all nodules). The impact on accurate thyroid cancer risk recall by patients, might be affected by the risk stratification system used during the visit or as the gold standard and will require further evaluation. We have previously showed that most patients that had just undergone a thyroid biopsy were aware that evaluation for thyroid cancer was the main reason for thyroid biopsy. However, 56% were unaware of their risk for thyroid cancer.4 Clinically, objective stratification of thyroid nodules according to their risk of thyroid cancer is extremely important. However, only a few studies have evaluated how patients undergoing evaluation for thyroid cancer use the risk for thyroid cancer when making management decisions. 2,9
We found a non statistically significant difference in encounter duration, with longer duration of the visits where TNOC was used. Nonetheless, the overall visit time is likely within the allowed time for clinical consultations for patients with new thyroid nodules.34,35
The current study is limited by a single center observational design. In addition, it included a small number of clinicians, mostly endocrinologists and evaluated patients following a before and after design. Therefore, our evaluation of clinical outcomes can be affected by imbalances in clinical features and other unknown confounders and provides only preliminary information of the proximal effects of using TNOC when discussing diagnostic management options for patients with thyroid nodules. Similarly, TNOC was developed and pilot tested in an English speaking population with reasonable education and numeracy level. Further studies will need to evaluate the feasibility and acceptability of using TNOC to support the care of patients with thyroid nodules in other clinical settings, where linguistic and cultural adaptations might be required.36 However, field testing of TNOC allowed for further refinement of the tool that now includes: 1) a pictogram in addition to a risk bar when displaying the risk for thyroid cancer, 2) the option for clinicians of using ACR-TIRADS for calculating a thyroid cancer risk, 3) simplified/staged decision tree, by including surgery as a secondary management option, 4) a printable summary for patients, and 5) increased attention to the prognosis/treatment of thyroid cancer. Additional field testing and evaluation on a pilot randomized clinical trial (NCT04472026; NCT04463719), will help further tailor TNOC to the needs of its users and assess the clinical impact, within the safeguards against bias of a randomized design.
Conclusion
Using TNOC during clinical visits was feasible and seemed to help patients with thyroid nodules and their clinicians collaborate when deciding the next diagnostic step and can serve as a reflection point for many patients that have incidentally entered the diagnostic cascade of thyroid nodules. The results of this feasibility and proof of concept study, supports the need for larger and randomized studies to increase our understanding of the effects of using TNOC in conversations with patients with thyroid nodules on the quality of diagnostic conversations and other proximal and distal patient important outcomes.
Supplementary Material
Appendix 1.Measurements
Appendix 2. Thyroid Nodule Conversation Aid
Supplemental Table 1. Distribution of patients who underwent thyroid biopsy according to thyroid cancer ultrasound risk category and counseling group
Funding:
This work was supported by the Gatorade Trust through funds distributed by the University of Florida, Department of Medicine and the Clinical and Translational Science Institute (CTSI) grant support (NIH National Center for Advancing Translational Sciences (NCATS) grant UL1 TR000064).
NSO was supported by the National Cancer Institute of the National Institutes of Health under Award Number K08CA248972. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: the authors have no conflict of interest
Availability of data and material:
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
References
- 1.Singh Ospina N, Iniguez-Ariza NM, Castro MR. Thyroid nodules: diagnostic evaluation based on thyroid cancer risk assessment. BMJ 2020;368:l6670. [DOI] [PubMed] [Google Scholar]
- 2.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hargraves I, LeBlanc A, Shah ND, Montori VM. Shared Decision Making: The Need For Patient-Clinician Conversation, Not Just Information. Health Aff (Millwood) 2016;35:627–9. [DOI] [PubMed] [Google Scholar]
- 4.Singh Ospina N, Castaneda-Guarderas A, Ward R, et al. Patients’ knowledge about the outcomes of thyroid biopsy: a patient survey. Endocrine 2018;61:482–8. [DOI] [PubMed] [Google Scholar]
- 5.Stacey D, Legare F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2017;4:CD001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brito JP, Moon JH, Zeuren R, et al. Thyroid Cancer Treatment Choice: A Pilot Study of a Tool to Facilitate Conversations with Patients with Papillary Microcarcinomas Considering Treatment Options. Thyroid 2018;28:1325–31. [DOI] [PubMed] [Google Scholar]
- 7.Hargraves IG, Montori VM, Brito JP, et al. Purposeful SDM: A problem-based approach to caring for patients with shared decision making. Patient Educ Couns 2019;102:1786–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeballos-Palacios CL, Hargraves IG, Noseworthy PA, et al. Developing a Conversation Aid to Support Shared Decision Making: Reflections on Designing Anticoagulation Choice. Mayo Clin Proc 2019;94:686–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tessler FN, Middleton WD, Grant EG, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J Am Coll Radiol 2017;14:587–95. [DOI] [PubMed] [Google Scholar]
- 10.Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur Thyroid J 2017;6:225–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castellana M, Castellana C, Treglia G, et al. Performance of Five Ultrasound Risk Stratification Systems in Selecting Thyroid Nodules for FNA. J Clin Endocrinol Metab 2020;105. [DOI] [PubMed] [Google Scholar]
- 12.Brito JP, Castaneda-Guarderas A, Gionfriddo MR, et al. Development and Pilot Testing of an Encounter Tool for Shared Decision Making About the Treatment of Graves’ Disease. Thyroid 2015;25:1191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elwyn G, Edwards A, Wensing M, Hood K, Atwell C, Grol R. Shared decision making: developing the OPTION scale for measuring patient involvement. Qual Saf Health Care 2003;12:93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elwyn G, Hutchings H, Edwards A, et al. The OPTION scale: measuring the extent that clinicians involve patients in decision-making tasks. Health Expect 2005;8:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med 2004;36:588–94. [PubMed] [Google Scholar]
- 16.Chew LD, Griffin JM, Partin MR, et al. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med 2008;23:561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunny KA, Perri M 3rd. Single-item vs multiple-item measures of health-related quality of life. Psychol Rep 1991;69:127–30. [DOI] [PubMed] [Google Scholar]
- 18.Fagerlin A, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Derry HA, Smith DM. Measuring numeracy without a math test: development of the Subjective Numeracy Scale. Med Decis Making 2007;27:672–80. [DOI] [PubMed] [Google Scholar]
- 19.O’Connor AM. Validation of a decisional conflict scale. Med Decis Making 1995;15:25–30. [DOI] [PubMed] [Google Scholar]
- 20.CAHPS for Merit-Based Incentive Payment System (MIPS) Survey. last reviewed February 2019. at https://www.ahrq.gov/cahps/surveys-guidance/cg/cahps-mips.html.)
- 21.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger ZD, Brito JP, Ospina NS, et al. Patient centred diagnosis: sharing diagnostic decisions with patients in clinical practice. BMJ 2017;359:j4218. [DOI] [PubMed] [Google Scholar]
- 23.Hess EP, Homme JL, Kharbanda AB, et al. Effect of the Head Computed Tomography Choice Decision Aid in Parents of Children With Minor Head Trauma: A Cluster Randomized Trial. JAMA Netw Open 2018;1:e182430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hess EP, Knoedler MA, Shah ND, et al. The chest pain choice decision aid: a randomized trial. Circ Cardiovasc Qual Outcomes 2012;5:251–9. [DOI] [PubMed] [Google Scholar]
- 25.Hoang JK, Nguyen XV. Understanding the Risks and Harms of Management of Incidental Thyroid Nodules: A Review. JAMA Otolaryngol Head Neck Surg 2017;143:718–24. [DOI] [PubMed] [Google Scholar]
- 26.Hoang JK, Nguyen XV, Davies L. Overdiagnosis of thyroid cancer: answers to five key questions. Acad Radiol 2015;22:1024–9. [DOI] [PubMed] [Google Scholar]
- 27.Brick DJ, Scherr KA, Ubel PA. The Impact of Cost Conversations on the Patient-Physician Relationship. Health Commun 2019;34:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez SL, Weissman A, Read S, et al. U.S. Internists’ Perspectives on Discussing Cost of Care With Patients: Structured Interviews and a Survey. Ann Intern Med 2019;170:S39–S45. [DOI] [PubMed] [Google Scholar]
- 29.Kunneman M, Branda ME, Noseworthy PA, et al. Shared decision making for stroke prevention in atrial fibrillation: study protocol for a randomized controlled trial. Trials 2017;18:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunneman M, Engelhardt EG, Ten Hove FL, et al. Deciding about (neo-)adjuvant rectal and breast cancer treatment: Missed opportunities for shared decision making. Acta Oncol 2016;55:134–9. [DOI] [PubMed] [Google Scholar]
- 31.Ganguli I, Simpkin AL, Lupo C, et al. Cascades of Care After Incidental Findings in a US National Survey of Physicians. JAMA Netw Open 2019;2:e1913325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganguli I, Simpkin AL, Colla CH, et al. Why Do Physicians Pursue Cascades of Care After Incidental Findings? A National Survey. J Gen Intern Med 2020;35:1352–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh Ospina N, Maraka S, Espinosa De Ycaza AE, et al. Physical exam in asymptomatic people drivers the detection of thyroid nodules undergoing ultrasound guided fine needle aspiration biopsy. Endocrine 2016;54:433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaw MK, Davis SA, Fleischer AB, Feldman SR. The duration of office visits in the United States, 1993 to 2010. Am J Manag Care 2014;20:820–6. [PubMed] [Google Scholar]
- 35.Dobler CC, Sanchez M, Gionfriddo MR, et al. Impact of decision aids used during clinical encounters on clinician outcomes and consultation length: a systematic review. BMJ Qual Saf 2019;28:499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chenel V, Mortenson WB, Guay M, Jutai JW, Auger C. Cultural adaptation and validation of patient decision aids: a scoping review. Patient Prefer Adherence 2018;12:321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1.Measurements
Appendix 2. Thyroid Nodule Conversation Aid
Supplemental Table 1. Distribution of patients who underwent thyroid biopsy according to thyroid cancer ultrasound risk category and counseling group
Data Availability Statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.


