Abstract
Nitric oxide (NO), a free radical molecule synthesized by nitric oxide synthases (NOS), regulates multiple cellular functions in a variety of cell types. These NOS, including endothelial NOS (eNOS), inducible NOS (iNOS) and neural NOS (nNOS), are expressed in keratinocytes. Expression levels of both iNOS and nNOS decrease with aging, and insufficient NO has been linked to the development of a number of disorders such as diabetes and hypertension, and to the severity of atherosclerosis. Conversely, excessive NO levels can induce cellular oxidative stress, but physiological levels of NO are required to maintain the normal functioning of cells, including keratinocytes. NO also regulates cutaneous functions, including epidermal permeability barrier homeostasis and wound healing, through its stimulation of keratinocyte proliferation, differentiation and lipid metabolism. Topical applications of a diverse group of agents which generate nitric oxide (called NO donors) such as S-nitroso-N-acetyl-D,L-penicillamine (SNAP) can delay permeability barrier recovery in barrier-disrupted skin, but iNOS is still required for epidermal permeability barrier homeostasis. This review summarizes the regulatory role that NO plays in epidermal permeability barrier functions and the underlying mechanisms involved.
Keywords: Nitric oxide, differentiation, keratinocyte, permeability barrier
Introduction
Nitric oxide (NO), a free radical molecule, is synthesized in almost all tissues, including the skin. While excessive NO can provoke oxidative stress, leading to the development of a variety of systemic disorders, physiological levels of NO are required to maintain normal cellular function. Insufficiency of NO is linked to the development of a number of disorders, including hyperlipidemia, diabetes, hypertension, and to the severity of atherosclerosis.1 For example, deficiency in endothelial nitric oxide synthase (eNOS) results in increased blood pressure in mice.2,3 Accordingly, knockout of eNOS alone lowers survival rates by ≈50%, while knockout of all three NOS isoforms, inducible NOS (iNOS), neuro NOS (nNOS) and eNOS, reduces 10-month survival rate of mice by 80%.4 In premature lambs, inhalation of low doses of NO decreases neutrophil infiltration and myeloperoxidase activity, while increasing pulmonary blood flow.5 NO also exerts antimicrobial properties.6,7 Topical applications of a NO donor increase erythropoietin production in the kidney,8 and inhalation of NO improves pulmonary hypertension in premature neonates.9 In contrast, blockade of NO synthesis decreases T regulatory cells, worsens renal damage, and increases blood pressure in rats.10–12 Studies have also demonstrated an important regulatory role for NO in cutaneous functions. Previous studies showed that NO stimulates keratinocyte migration in vitro,13 while deficiency in iNOS delays cutaneous wound healing.14 Conversely, either topical applications or peritoneal injections of a NO donor accelerate cutaneous wound healing.15–18 This evidence demonstrates a regulatory role of NO in the biological functioning of multiple systems/organs.
Epidermal permeability barrier, residing in the stratum corneum, protects excessive water and water-soluble substances in and out of the body. Formation of epidermal permeability barrier is largely regulated by epidermal lipid production and expression of keratinocyte differentiation-marker related proteins.19 Any factors that regulate keratinocyte differentiation and lipid production can affect epidermal permeability barrier. Previous studies showed that NO regulates keratinocyte proliferation and differentiation as well as epidermal permeability barrier homeostasis.20–22
1. Production and Regulation of Nitric Oxide
NO synthases (NOS) (EC 1.14.13.39) convert arginine to citrulline, generating NO (Figure 1). Flavin mononucleotide, flavin adenine dinucleotide, nicotinamide adenine-dinucleotide phosphate, and (6R-)5,6,7,8-tetrahydro-L-biopterin are cofactors (which help catalyst activity) of NOS. The three major isoforms of NOS; i.e., nNOS, iNOS, and eNOS, are preferentially expressed in different tissues. nNOS, also termed NOS1, is mainly expressed in neurons and the brain, while eNOS, also referred to as NOS3, is primarily expressed in endothelial cells. iNOS (NOS2) is normally expressed at low levels in almost all tissues. Both eNOS and nNOS are constitutively expressed in all tissues, while keratinocytes notably express all three NOS isoforms.23,24 In general, the physiological functions of nNOS-generated NO include synaptic plasticity in the central nervous system, central regulation of blood pressure, smooth muscle relaxation, and vasodilatation, while eNOS-generated NO positively regulates vasodilation and angiogenesis, and negatively regulates platelet aggregation and leucocyte adhesion.25 Essential role of NO generated by iNOS is non-specific defense against microorganisms. The exact functions of these NOSs in the epidermis have not been well defined yet.
Figure 1. Schematic Diagram of Nitric Oxide Synthesis.
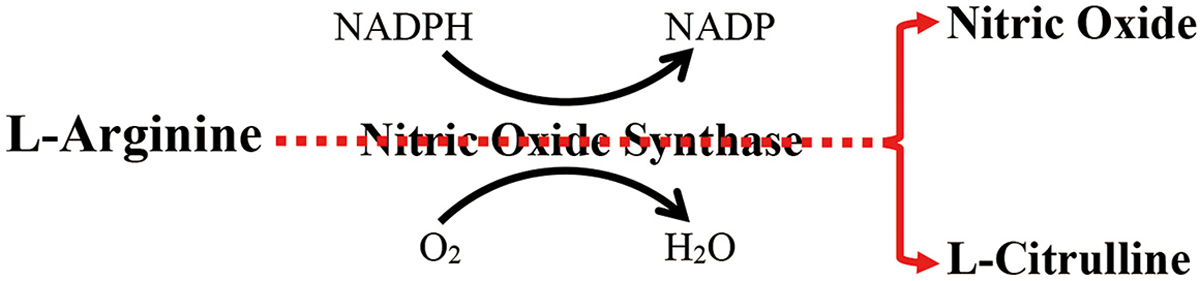
A number of factors regulate NOS expression and activity. While physiologic levels of intracellular calcium regulate activity of eNOS and nNOS via calmodulin-calcium interaction,26 high calcium levels increase the binding of calmodulin to NOS, leading to an increase in NO synthesis. But in certain instances, eNOS can synthesize NO independently of calcium in response to certain stimuli.20 Moreover, expression levels of eNOS and nNOS are also regulated by physical stimuli (such as heat and light exposure), irritant and allergic agents, sex hormones, cytokines, growth factors, and bacterial lipopolysaccharides.26 While iNOS activity is regulated by calcium-dependent and -independent signaling pathways in some cell types,27 bacterial infection can increase both iNOS expression and NO production.28 Regulation of NOS expression by certain stimuli is organ specific. For instance, bone fractures upregulate expression levels of mRNA for all three NOS;29 gamma irradiation increases iNOS expression in the ileum, but not in the colon;30 and either intravenous or intraperitoneal injection of lipopolysaccharide markedly increases iNOS expression in the ileum, but not in the duodenum.31, 32
In the epidermis, cutaneous wounding and certain growth factors can increase nNOS expression in keratinocytes.33 Mechanical stimulation of the skin increases NO production by both nNOS and eNOS in the epidermis,34 and both UVB and UVA irradiation increase iNOS expression in keratinocytes.35,36 The potency of interferon gamma-induced iNOS expression in keratinocytes is dependent on tissue origins. Thus, a more dramatic increase in iNOS expression was observed in epidermal keratinocytes than in either HaCat or CaSki cells.37 A recent study demonstrated an elevation in iNOS mRNA expression following acute disruption of epidermal permeability barrier.22 In addition to enzymatic production of NO, non-enzymatic decomposition of photo-reactive nitrogen oxides can produce NO in the skin. For example, UVA irradiation (40 J/cm2) of either human skin or skin homogenates can increase NO content by ≈90%.38 Because inhibition of NOS does not attenuate UVA-induced increase in NO production in skin homogenates, UVA-irradiation induced production of NO is independent of NOS in skin homogenates. Thus, UVA-induced NO production can also be independent of NOS in the skin. Collectively, many endogenous and exogenous factors can regulate NO production in keratinocytes.
2. Regulation of Epidermal Permeability Barrier by NO
The role of NO in regulating epidermal permeability barrier homeostasis was first shown by the acceleration of epidermal permeability barrier recovery following topical application of either a specific nNOS inhibitor (Nω-propyl-l-arginine), or a broad NOS inhibitor (l-NG-nitro-L-arginine methyl ester) immediately after barrier disruption with tape-stripping, while in contrast, topical application of a NO donor to barrier-disrupted skin delayed epidermal permeability barrier recovery.39 This negative impact of NO on epidermal permeability barrier recovery was further demonstrated by the acceleration of permeability barrier recovery in both nNOS and eNOS knockout mice, but not in iNOS knockout mice.39,40 However, recent studies by Dang et al. demonstrated a need for iNOS in epidermal permeability barrier homeostasis.22 First, disruption of the epidermal permeability barrier increased expression levels of iNOS mRNA by over 1-fold in mouse epidermis, consistent with previous findings that barrier disruption increases NO release in the epidermis.39 While iNOS deficiency delays permeability barrier recovery, topical applications of NO donors largely corrected the permeability barrier abnormality in iNOS knockout mice.22 The discrepant results between various studies in iNOS knockout mice likely can be attributed to the differences in methodology. In one study which showed no alterations in barrier recovery in iNOS knockout mice,40 ears were used to assess barrier recovery. Because inflammatory reactions to the same stimuli are more severe on the ears than on the flanks of normal mice,41 tape-stripping-induced inflammation and NO release could be less in the ear of iNOS deficient mice than in that of normal mice. Reduced inflammation and NO production could benefit epidermal function in the skin of iNOS knockout mice, because excessive inflammation and/or excessive NO levels can compromise epidermal function and induce more inflammation.42,43. Thus, barrier recovery can be normal or accelerated on the ears of NOS-deficient mice. In the other study, barrier recovery was assessed on the mouse flanks,22 where inflammation and NO are less prominent than on the ears. Although too much NO can be harmful, lower levels of NO benefit lipid processing and keratinocyte differentiation,20,44–46 which are both required for epidermal permeability barrier function. But, because of lower-than-normal NO levels, delayed barrier recovery can be observed on the flanks of iNOS knockout mice. However, a side-by-side comparison of barrier recovery between the ears and the flanks of iNOS knockout mice would be needed to validate the above speculations. Moreover, the topical NO donor-induced delay in barrier recovery in barrier-disrupted mouse skin could also be ascribed to excessive NO, leading to impaired epidermal function. Because barrier disruption alone already increases NOS mRNA expression and NO release,22,39 topical application of NO to barrier-disrupted skin will further elevate NO levels, likely resulting in a deterioration of epidermal function. Yet, another study revealed that addition of the NO donor, 3-ethyl-3-(ethylaminoethyl)-1-hydroxy-2-oxo-1-triazene (10μM), to cultured keratinocytes decreased transepithelial electrical resistance (TEER) by ≈60%, indicating transepithelial permeability barrier dysfunction, while increasing lucifer yellow paracellular flux by 75% to 100%.47,48
In support of negative impact of NO on the epidermal permeability barrier in barrier disrupted skin (a condition already with excessive NO level in the skin), Ormerod et al. showed that a topical NOS inhibitor (NG-nitro-L-arginine methyl ester) lowered transepidermal water loss rates in human skin irritated with sodium lauryl sulfate.49 UV-B is another inducer of NO production, and it compromises the transepithelial barrier.50,51 Inhibition of NOS by NG-nitro-L-arginine methyl ester also improved the tight junction barrier in UVB-irradiated keratinocytes.47 Irradiation of keratinocytes with UVB (5mJ/cm2) reduced claudin 1 expression by ≈50% while inducing an increase in expression levels of eNOS protein by ≈75%, accompanied by significant increases in lucifer yellow paracellular flux and reduction in TEER, indicative of a defective transepithelial permeability barrier. In addition, inhibition of eNOS attenuated UVB-irradiation-induced changes in both lucifer yellow paracellular flux and TEER.47 Together, these data suggest that NO is required for normal permeability barrier function, while excessive NO can compromise epidermal permeability barrier. Although certain inflammatory dermatoses such as eczematous dermatitis and psoriasis exhibit infiltrates of neutrophils, macrophages and T cells, which all can produce NO, in the epidermis.52–55 However, whether excessive production of NO by inflammatory cells contributes to dysfunction in epidermal permeability barrier in these inflammatory skin disorders remains unknown.
3. Mechanisms by Which NO Regulates Epidermal Permeability Barrier
3.1. Normal Skin
Because keratinocytes account for 95% of all cells in the epidermis,56 epidermal functions are largely dictated by keratinocyte functions. NO is vital as a signaling molecule regulating multiple epidermal functions, including keratinocyte proliferation and differentiation, apoptosis, migration, and oxidative stress, as well as cytokine production.13,20,45,57,58
3.1.1. Keratinocyte Proliferation and Differentiation
Keratinocyte proliferation is required for the formation of the epidermal permeability barrier. Accordingly, a number of studies have demonstrated the importance of NO in regulating keratinocyte proliferation: i) treatment of primary keratinocytes with a NO donor,1-Hydroxy-2-oxo-3,3-bis (3-aminoethyl)-1-triazene (DETA/NO), at concentrations of 0.01 to 0.25 mM, for 48 hours induced a dose-dependent increase in Ki67 positive cells;20 ii) incubation of keratinocytes with either S-nitrosoglutathione (GSNO) or DETA/NO for 24 hours increased proliferation rates by ≈40%;59 and iii) conversely, inhibition of either iNOS or eNOS decreased Ki67 positive cells and proliferating cell nuclear antigen expression in cutaneous wound healing model of both mice and rats.60,61 Likewise, topical applications of an nNOS inhibitor also prevented epidermal hyperproliferation induced by repeated disruption of the epidermal permeability barrier.39 Following cutaneous wounding, reductions in Ki67 positive cells in eNOS knockout mice further support a requirement for NO in regulating keratinocyte proliferation.62 Yet, the impact of NO on keratinocyte proliferation depends on the concentration of NO donors. For example, S-nitroso-N-acetylpenicillamine (SNAP) at concentrations of 0.001 to 0.5mM dose-dependently increased the number of Ki67 positive cells, while the concentrations of SNAP>0.5 mM decreased the number of Ki67 positive cells.20 Likewise, GSNO at a concentration of 500 μM inhibited keratinocyte proliferation.59 Evidence also suggests that the epidermal hyperproliferation in psoriasis could be linked to insufficient NO levels.63
In addition to proliferation, NO also regulates keratinocyte terminal differentiation, a crucial event to generate structural proteins that contribute to the epidermal permeability barrier. Incubation of keratinocytes with NO donors (either DETA/NO or SNAP) for 48 hours induced a dose-dependent increase in cytokeratin 6-positive cells.20 Similarly, sodium nitroprusside (SNP) at concentrations of 0.05 to 1mM dose-dependently upregulated expression levels of involucrin and K16 in keratinocyte cultures.33 Expression levels of keratin 14 mRNA also increased by over 1-fold in the presence of SNAP following 48-hour incubation under high calcium condition.45 Conversely, iNOS-deficient mice display significantly lower expression levels of mRNA for epidermal differentiation marker-related proteins both under basal conditions and 2 hours after barrier disruption.22. But topical applications of a NO donor to iNOS knockout mice significantly upregulated expression levels of mRNA for filaggrin, loricrin and involucrin, indicating that NO is required for keratinocyte differentiation. Stimulation of keratinocyte differentiation is mediated by reactive nitrogen species (peroxynitrite), rather than NO.64 However, Rossi, et al. have demonstrated that incubation of keratinocytes with 1 mM SNAP for 1 week induced 3-fold reductions in the formation of cornified envelopes, along with decreased activities of transglutaminase 1 and 3, possibly due to long-term incubation with a high concentration of this NO donor, again because the extent of reductions in cornified envelope formation and transglutaminase activities induced by NO were dose- and time-dependent.65 Other studies showed that either SNAP (0.2 mM) or L-NAME (10 mM) can lower expression levels of keratin 10 and profilaggrin in keratinocytes cultured under the high calcium conditions (1.1 mM).45 Although the underlying mechanisms contributing to these contradictory results remain unclear, these results nevertheless indicate that NO donors regulate keratinocyte proliferation and differentiation.
3.1.2. Lipid Production and Post-Secretory Processing
Formation of a competent epidermal permeability barrier requires epidermal lipid synthesis and post-secretory lipolytic processing to generate ceramides and free fatty acids, crucial steps in forming intercellular membrane bilayers in the stratum corneum.66–69 Our previous studies showed that inhibition of either phospholipase A2 or β-glucocerebrosidase activities delayed epidermal permeability barrier recovery.66–69 Although incubation of keratinocytes with SNAP induced a transit reduction in ceramide synthesis at early time points (24 and 48 hours), the rates of ceramide synthesis were comparable between SNAP- and vehicle-treated keratinocytes at 96-hour incubation.45 However, 96-hour incubation of keratinocytes with SNAP induced significant increases in β-glucocerebrosidase activity and its mRNA expression levels, with a further increase following 144- and 192-hour incubation (≈2-fold increase), suggesting that NO can stimulate lipid processing, potentially explaining the observation that NO accelerates permeability barrier recovery. Our recent studies demonstrated significant reductions in expression levels of mRNA for lipid synthetic enzymes, including 3-hydroxy-3-methyl-glutaryl-CoA reductase, serine palmitoyltransferase 1 and fatty acid synthase in iNOS deficient mice,22 each of which is required for epidermal permeability barrier homeostasis. The possible role of NO in regulating lipid production is demonstrated by the significant upregulation of mRNA expression levels for epidermal lipid synthetic enzymes (3-hydroxy-3-methyl-glutaryl-CoA reductase, serine palmitoyltransferase 1 and fatty acid synthase) after topical applications of a NO donor in iNOS knockout mice.22 Yet, other studies showed that NO negatively regulated lipid production in other tissues.70–72 Thus, further studies are needed to further illuminate the role of NO in epidermal lipid synthesis.
3.1.3. Keratinocyte Apoptosis
As mentioned earlier, keratinocyte differentiation is required for the formation of the epidermal permeability barrier. Previous studies from our group and others have demonstrated the necessity of caspases 3 and 14 for keratinocyte differentiation and apoptosis,73–77 and pertinently, NO increases caspases 3 and 14 activities and their expression levels. For example, treatment of cardiomyocytes with 500 μM 3-morpholinosydnonimine hydrochloride, a NO donor, increased expression levels of caspase 14 by over 2-fold.78 Similarly, treatment of keratinocytes with 3 mM SNP, another NO donor, for 48 hours, induced condensed and fragmented nuclei, indicators of apoptosis. In parallel, pro-caspase 3 activity also was upregulated by incubation of keratinocytes with SNP.44 Conversely, treatment of keratinocytes with a NOS inhibitor, NG-methyl-L-arginine, decreased apoptotic cells, while inhibiting the cleavage of poly (ADP-ribose) polymerase 1,57 a process that is required for keratinocyte differentiation.79 Thus, NO-induced apoptosis could benefit the formation of epidermal permeability barrier.
In UVB-irradiated keratinocytes, the results of NO in regulating apoptosis were inconclusive. UVB irradiation of keratinocytes can induce NO release, resulting in keratinocyte apoptosis.58,80 Likewise, addition of NOC18 (1 mM) (a diazeniumdiolate slow-releasing NO donor) to culture medium immediately after UVB irradiation enhanced UVB-induced apoptosis.81 In contrast, other studies have demonstrated that inhibition of nNOS with L-NAME (10 mM) increased caspase 3 activity, while a NO donor (0.2 mM SNAP) inhibited caspase 3 activity.45 Likewise, low concentrations of a NO donor (250 μM or 500 μM NOC18) inhibited apoptosis, caspase 3 activity, and expression levels of p53, while upregulating Bcl-2 expression in UVB-irradiated murine keratinocytes.81 SNAP also prevented the UVB-irradiation-induced increase in apoptosis and caspase activity.82 Conversely, addition of a NOS inhibitor (NG-nitro-L-arginine methyl ester) prior to UVB irradiation increased apoptosis in comparison to UVB irradiation alone.83 Deficiency in either iNOS or eNOS enhanced apoptosis following irradiation with UVB. Interestingly, eNOS deficient mice are more sensitive than iNOS deficient mice to UVB-induced apoptosis.82 Hence, the impact of NO on apoptosis in UVB-irradiated keratinocytes is possibly attributable to variations in experimental conditions. Nonetheless, this line of evidence indicates that the influence of NO on keratinocyte apoptosis depends on the NO concentration and the condition of keratinocytes, and that NO-induced stimulation of keratinocyte apoptosis can benefit epidermal permeability barrier function at least in normal skin.
3.1.4. Other NO-Induced Mechanisms
Additional mechanisms could also contribute to the improved epidermal permeability barrier induced by NO. Frank, et al. showed that addition of 500 μM s-nitrosoglutathione, a NO donor, to keratinocyte cultures rapidly increased expression levels of the vascular endothelial growth factor (VEGF) mRNA by 6-fold, in addition to augmenting keratinocyte growth factor- and pro-inflammatory cytokine-induced elevations in VEGF expression.84 The positive effect of NO on VEGF expression was also evidenced by intraperitoneal injection of 2.5 mg L-N6-(1-iminoethyl)lysine (L-NIL), a selective inhibitor of iNOS, twice-daily for only one day markedly decreased expression levels of cutaneous VEGF mRNA, and a more profound reduction was observed on day 7 (≈3-fold reduction vs. vehicle).84 Because VEGF is required for epidermal permeability homeostasis,85 NO-induced upregulation of VEGF expression could be another one of its operating mechanisms that promotes epidermal permeability barrier homeostasis.
Darkly pigmented skin displays a superior epidermal permeability barrier in comparison to lightly pigmented skin.86–88 Previous studies demonstrated that NO increased melanogenesis in melanocyte cultures, while inhibition of NO production decreased melanogenesis in mice.89–91 Stimulation of melanogenesis may well represent yet another mechanism by which NO benefits the epidermal permeability barrier.
3.2. Barrier Disrupted Skin.
While NO is a signaling molecule that regulates cellular functions in various cell types, excessive levels of NO can cause oxidative stress, which can negatively impact cellular functions.92,93 Reactive oxygen species increase the expression levels of both eNOS and iNOS,94 leading to an increase in NO levels. Both UVB-irradiation and cutaneous barrier disruption increase NO release,39,47, 95 which can activate soluble guanylyl cyclase.39,96 The latter can further increase NO production,97 leading to amplification of NO levels, consequently resulting in increased oxidative stress. Conversely, inhibition of guanylyl cyclase accelerates permeability barrier recovery, while activation of guanylyl cyclase delays barrier recovery in tape-stripped skin.39 Moreover, NO-induced activation of guanylyl cyclase increases intracellular calcium, which can inhibit lamellar body secretion, which is critically required for the repair of the epidermal permeability barrier.39,98–100 Furthermore, NO can react with superoxide to form peroxynitrite, resulting in an increase in endocytosis of claudin 1.47 Because inhibition of endocytosis overcame UVB-irradiation-induced alterations in TEER, claudin 1 expression and paracellular influx,47,48 NO-induced endocytosis of claudin 1 is another mechanism attributable to NO’s negative influence on epidermal permeability barrier function. Thus, NO-induced dysfunction in epidermal permeability barrier homeostasis can be largely attributed to: i) oxidative stress, ii) inhibition of lamellar body secretion, and iii) reduction in claudin 1 expression in barrier-disrupted and UVB-irradiated skin (Figure 2).
Figure 2. Putative Mechanisms by Which Nitric Oxide Regulates the Epidermal Permeability Barrier.
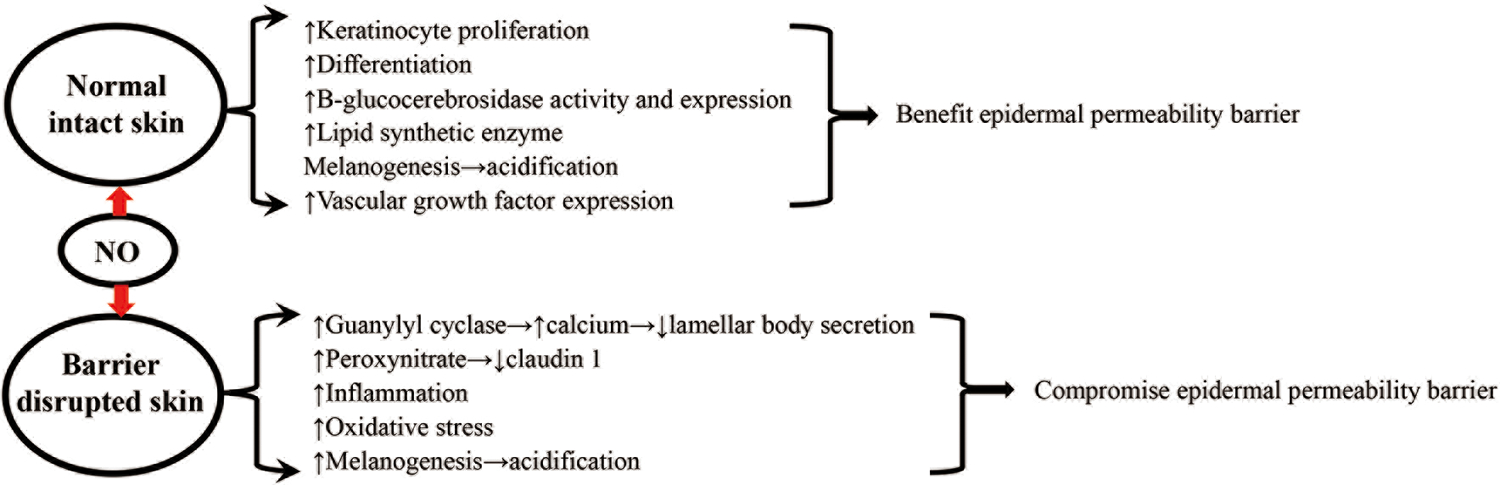
Finally, while NO exhibits anti-inflammatory properties under normal physiological conditions,43 evidence nonetheless indicates a pathogenic role of NO in inflammatory skin disorders,101,102 suggesting that NO could compromise epidermal permeability barrier in inflamed skin. Studies have shown that NO-releasing glucocorticoids enhance anti-inflammatory efficacy in comparison to glucocorticoids alone.103–105 However, whether topical applications of NO can improve epidermal permeability barrier in eczematous dermatitis in clinical settings remains to be determined, although the extent of the abnormality in the epidermal permeability barrier correlates directly with the severity of dermatitis.106–108 The putative mechanisms whereby NO regulates epidermal permeability barrier are illustrated in Figure 2.
Conclusions
Keratinocytes express nitric oxide synthases (i.e., nNOS, iNOS and eNOS). Upon stimulation by pro-inflammatory cytokines, infection, injury or infection, these NOS synthesize and release nitric oxide (NO). NO can influence epidermal permeability barrier via divergent mechanisms, including regulation of keratinocyte proliferation and differentiation, apoptosis, lipid processing and melanogenesis. Although evidence indicates a definite requirement for NO for the maintenance of epidermal permeability barrier, whether NO positively or negatively regulates the epidermal permeability barrier largely depends on the cutaneous conditions involved and NO content levels. Generally, low NO levels are required to maintain a normal epidermal permeability barrier, while excessive NO compromises this barrier. The impact of NO on the epidermal permeability barrier in clinical settings still needs to be evaluated.
Funding Sources:
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Disease of the National Institutes of Health under award number R01 AR061106, administered by the Northern California Institute for Research and Education, with resources from the Research and Development Service, Department of Veterans Affairs Medical Center, San Francisco. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: All authors declare no conflicts of interest.
References
- 1.Torregrossa AC, Aranke M, Bryan NS. Nitric oxide and geriatrics: Implications in diagnostics and treatment of the elderly. J Geriatr Cardiol. 2011;8:230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1996;93:13176–13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyamoto Y, Saito Y, Kajiyama N, Yoshimura M, Shimasaki Y, Nakayama M, Kamitani S, Harada M, Ishikawa M, Kuwahara K, Ogawa E, Hamanaka I, Takahashi N, Kaneshige T, Teraoka H, Akamizu T, Azuma N, Yoshimasa Y, Yoshimasa T, Itoh H, Masuda I, Yasue H, Nakao K. Endothelial nitric oxide synthase gene is positively associated with essential hypertension. Hypertension. 1998;32:3–8. [DOI] [PubMed] [Google Scholar]
- 4.Cau SB, Carneiro FS, Tostes RC. Differential modulation of nitric oxide synthases in aging: therapeutic opportunities. Front Physiol. 2012;3:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinsella JP, Parker TA, Galan H, Sheridan BC, Halbower AC, Abman SH. Effects of inhaled nitric oxide on pulmonary edema and lung neutrophil accumulation in severe experimental hyaline membrane disease. Pediatr Res. 1997;41:457–463. [DOI] [PubMed] [Google Scholar]
- 6.Vazquez-Torres A, Stevanin T, Jones-Carson J, Castor M, Read RC, Fang FC. Analysis of nitric oxide-dependent antimicrobial actions in macrophages and mice. Methods Enzymol. 2008;437:521–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Svensson L, Poljakovic M, Demirel I, Sahlberg C, Persson K. Host-Derived Nitric Oxide and Its Antibacterial Effects in the Urinary Tract. Adv Microb Physiol. 2018;73:1–62. [DOI] [PubMed] [Google Scholar]
- 8.Boutin AT, Weidemann A, Fu Z, Mesropian L, Gradin K, Jamora C, Wiesener M, Eckardt KU, Koch CJ, Ellies LG, Haddad G, Haase VH, Simon MC, Poellinger L, Powell FL, Johnson RS. Epidermal sensing of oxygen is essential for systemic hypoxic response. Cell. 2008;133:223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozłowska Z, Owsiańska Z, Wroblewska JP, et al. Genotype-phenotype correlation in two Polish neonates with alveolar capillary dysplasia. BMC Pediatr. 2020;20:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramirez LA, Gillis EE, Musall JB, Mohamed R, Snyder E, El-Marakby A, Sullivan JC. Hypertensive female Sprague-Dawley rats require an intact nitric oxide synthase system for compensatory increases in renal regulatory T cells. Am J Physiol Renal Physiol. 2020;319:F192–F201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brinson KN, Elmarakby AA, Tipton AJ, Crislip GR, Yamamoto T, Baban B, Sullivan JC. Female SHR have greater blood pressure sensitivity and renal T cell infiltration following chronic NOS inhibition than males. Am J Physiol Regul Integr Comp Physiol. 2013;305:R701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanecková I, Kramer HJ, Novotná J, Kazdová L, Opocenský M, Bader M, Ganten D, Cervenka L. Roles of nitric oxide and oxidative stress in the regulation of blood pressure and renal function in prehypertensive Ren-2 transgenic rats. Kidney Blood Press Res. 2005;28:117–126. [DOI] [PubMed] [Google Scholar]
- 13.Zhan R, Yang S, He W, Wang F, Tan J, Zhou J, Yang S, Yao Z, Wu J, Luo G. Nitric oxide enhances keratinocyte cell migration by regulating Rho GTPase via cGMP-PKG signalling. PLoS One. 2015;10:e0121551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitano T, Yamada H, Kida M, Okada Y, Saika S, Yoshida M. Impaired Healing of a Cutaneous Wound in an Inducible Nitric Oxide Synthase-Knockout Mouse. Dermatol Res Pract. 2017;2017:2184040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malone-Povolny MJ, Maloney SE, Schoenfisch MH. Nitric Oxide Therapy for Diabetic Wound Healing. Adv Healthc Mater. 2019;8:e1801210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben-Yehuda Greenwald M, Tacconi C, Jukic M, Joshi N, Hiebert P, Brinckmann J, Tenor H, Naef R, Werner S. A Dual-Acting Nitric Oxide Donor and Phosphodiesterase 5 Inhibitor Promotes Wound Healing in Normal Mice and Mice with Diabetes. J Invest Dermatol. 2021;141:415–426. [DOI] [PubMed] [Google Scholar]
- 17.Choi M, Hasan N, Cao J, Lee J, Hlaing SP, Yoo JW. Chitosan-based nitric oxide-releasing dressing for anti-biofilm and in vivo healing activities in MRSA biofilm-infected wounds. Int J Biol Macromol. 2020;142:680–692. [DOI] [PubMed] [Google Scholar]
- 18.Shi HP, Wang SM, Zhang GX, Zhang YJ, Barbul A. Supplemental L-arginine enhances wound healing following trauma/hemorrhagic shock. Wound Repair Regen. 2007;15:66–70. [DOI] [PubMed] [Google Scholar]
- 19.Feingold KR, Elias PM. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim Biophys Acta. 2014;1841:280–94. [DOI] [PubMed] [Google Scholar]
- 20.Krischel V, Bruch-Gerharz D, Suschek C, Kröncke KD, Ruzicka T, Kolb-Bachofen V. Biphasic effect of exogenous nitric oxide on proliferation and differentiation in skin derived keratinocytes but not fibroblasts. J Invest Dermatol. 1998;111:286–91. [DOI] [PubMed] [Google Scholar]
- 21.Feingold KR, Denda M. Regulation of permeability barrier homeostasis. Clin Dermatol. 2012;30:263–8. [DOI] [PubMed] [Google Scholar]
- 22.Dang E, Man G, Zhang J, Lee D, Mauro TM, Elias PM, Man MQ. Inducible Nitric Oxide Synthase Is Required for Epidermal Permeability Barrier Homeostasis in Mice. Exp Dermatol. Exp Dermatol. 2020;29:1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimizu Y, Sakai M, Umemura Y, Ueda H. Immunohistochemical localization of nitric oxide synthase in normal human skin: expression of endothelial-type and inducible-type nitric oxide synthase in keratinocytes. J Dermatol. 1997;24:80–7. [DOI] [PubMed] [Google Scholar]
- 24.Vaccaro M, Irrera N, Cutroneo G, Rizzo G, Vaccaro F, Anastasi GP, Borgia F, Cannavò SP, Altavilla D, Squadrito F. Differential Expression of Nitric Oxide Synthase Isoforms nNOS and iNOS in Patients with Non-Segmental Generalized Vitiligo. Int J Mol Sci. 2017;18:2533. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–37, 837a–837d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Förstermann U, Boissel JP, Kleinert H. Expressional control of the ‘constitutive’ isoforms of nitric oxide synthase (NOS I and NOS III). FASEB J. 1998;12:773–90. [PubMed] [Google Scholar]
- 27.Zhang B, Crankshaw W, Nesemeier R, Patel J, Nweze I, Lakshmanan J, Harbrecht BG. Calcium-mediated signaling and calmodulin-dependent kinase regulate hepatocyte-inducible nitric oxide synthase expression. J Surg Res. 2015;193:795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wienerroither S, Rauch I, Rosebrock F, Jamieson AM, Bradner J, Muhar M, Zuber J, Müller M, Decker T. Regulation of NO synthesis, local inflammation, and innate immunity to pathogens by BET family proteins. Mol Cell Biol. 2014;34:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu W, Diwan AD, Lin JH, Murrell GA. Nitric oxide synthase isoforms during fracture healing. J Bone Miner Res. 2001;16:535–40. [DOI] [PubMed] [Google Scholar]
- 30.MacNaughton WK, Aurora AR, Bhamra J, Sharkey KA, Miller MJ. Expression, activity and cellular localization of inducible nitric oxide synthase in rat ileum and colon post-irradiation. Int J Radiat Biol. 1998;74:255–64. [DOI] [PubMed] [Google Scholar]
- 31.Morin MJ, Unno N, Hodin RA, Fink MP. Differential expression of inducible nitric oxide synthase messenger RNA along the longitudinal and crypt-villus axes of the intestine in endotoxemic rats. Crit Care Med. 1998;26:1258–64. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi T, Fujii H, Shimizu H, Omori E, Uehara K, Takeuchi M, Matsumi M, Yokoyama M, Akagi R, Morita K. Site- and cell-type- specific induction of intestinal inducible nitric oxide synthase in a rat model of endotoxemia. Med Chem. 2005;1:643–7. [DOI] [PubMed] [Google Scholar]
- 33.Boissel JP, Ohly D, Bros M, Gödtel-Armbrust U, Förstermann U, Frank S. The neuronal nitric oxide synthase is upregulated in mouse skin repair and in response to epidermal growth factor in human HaCaT keratinocytes. J Invest Dermatol. 2004;123:132–139. [DOI] [PubMed] [Google Scholar]
- 34.Ikeyama K, Denda S, Tsutsumi M, Denda M. Neuronal nitric oxide synthase in epidermis is involved in cutaneous circulatory response to mechanical stimulation. J Invest Dermatol. 2010;130:1158–1166. [DOI] [PubMed] [Google Scholar]
- 35.Kuhn A, Fehsel K, Lehmann P, Krutmann J, Ruzicka T, Kolb-Bachofen V. Aberrant timing in epidermal expression of inducible nitric oxide synthase after UV irradiation in cutaneous lupus erythematosus. J Invest Dermatol. 1998;111:149–153. [DOI] [PubMed] [Google Scholar]
- 36.Roméro-Graillet C, Aberdam E, Clément M, Ortonne JP, Ballotti R. Nitric oxide produced by ultraviolet-irradiated keratinocytes stimulates melanogenesis. J Clin Invest. 1997;99:635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arany I, Brysk MM, Brysk H, Tyring SK. Induction of iNOS mRNA by interferon-gamma in epithelial cells is associated with growth arrest and differentiation. Cancer Lett. 1996;110:93–6. [DOI] [PubMed] [Google Scholar]
- 38.Paunel AN, Dejam A, Thelen S, Kirsch M, Horstjann M, Gharini P, Mürtz M, Kelm M, de Groot H, Kolb-Bachofen V, Suschek CV. Enzyme-independent nitric oxide formation during UVA challenge of human skin: characterization, molecular sources, and mechanisms. Free Radic Biol Med. 2005;38:606–15. [DOI] [PubMed] [Google Scholar]
- 39.Ikeyama K, Fuziwara S, Denda M. Topical application of neuronal nitric oxide synthase inhibitor accelerates cutaneous barrier recovery and prevents epidermal hyperplasia induced by barrier disruption. J Invest Dermatol. 2007;127:1713–9. [DOI] [PubMed] [Google Scholar]
- 40.Ikeyama K, Denda M. Effect of endothelial nitric oxide synthase on epidermal permeability barrier recovery after disruption. Br J Dermatol. 2010;163:915–9. [DOI] [PubMed] [Google Scholar]
- 41.Kostiala AA. Delayed-type hypersensitivity measured as an increase of ear thickness in guinea pigs. Int Arch Allergy Appl Immunol. 1979;60:207–215. [DOI] [PubMed] [Google Scholar]
- 42.Terra VA, Souza-Neto FP, Pereira RC, et al. Nitric oxide is responsible for oxidative skin injury and modulation of cell proliferation after 24 hours of UVB exposures. Free Radic Res. 2012;46:872–882. [DOI] [PubMed] [Google Scholar]
- 43.Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007;15:252–259. [DOI] [PubMed] [Google Scholar]
- 44.Lee SK, Kim HS, Lee HJ, Lee J, Jeon BH, Jun CD, Lee SK, Kim EC. Dual effect of nitric oxide in immortalized and malignant human oral keratinocytes: induction of apoptosis and differentiation. J Oral Pathol Med. 2006;35:352–60. [DOI] [PubMed] [Google Scholar]
- 45.Gallala H, Macheleidt O, Doering T, Schreiner V, Sandhoff K. Nitric oxide regulates synthesis of gene products involved in keratinocyte differentiation and ceramide metabolism. Eur J Cell Biol. 2004;83:667–79. [DOI] [PubMed] [Google Scholar]
- 46.Goren I, Linke A, Müller E, Pfeilschifter J, Frank S. The suppressor of cytokine signaling-3 is upregulated in impaired skin repair: implications for keratinocyte proliferation. J Invest Dermatol. 2006;126:477–85. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi M, Shu S, Marunaka K, Matsunaga T, Ikari A. Weak Ultraviolet B Enhances the Mislocalization of Claudin-1 Mediated by Nitric Oxide and Peroxynitrite Production in Human Keratinocyte-Derived HaCaT Cells. Int J Mol Sci. 2020;21:7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marunaka K, Kobayashi M, Shu S, Matsunaga T, Ikari A. Brazilian Green Propolis Rescues Oxidative Stress-Induced Mislocalization of Claudin-1 in Human Keratinocyte-Derived HaCaT Cells. Int J Mol Sci. 2019;20:3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ormerod AD, Woo PN, Islam J, Moran J, Cals-Grierson MM. An Investigation into the Effect of the Nitric Oxide Synthase Antagonist L-NAME and Plant Extracts on the Irritability and Barrier Function of the Skin. Exog Dermatol 2003;2:295–300. [Google Scholar]
- 50.Holliman G, Lowe D, Cohen H, Felton S, Raj K. Ultraviolet Radiation-Induced Production of Nitric Oxide:A multi-cell and multi-donor analysis. Sci Rep. 2017;7:11105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tu Y, Wu W, Guo Y, Lu F, Xu D, Li X, Zhao Y, He L. Upregulation of hsa-miR-31–3p induced by ultraviolet affects keratinocytes permeability barrier by targeting CLDN1. Biochem Biophys Res Commun. 2020;532:626–632. [DOI] [PubMed] [Google Scholar]
- 52.Chiang CC, Cheng WJ, Korinek M, Lin CY, Hwang TL. Neutrophils in Psoriasis. Front Immunol. 2019;10:2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsepkolenko A, Tsepkolenko V, Dash S, Mishra A, Bader A, Melerzanov A, Giri S. The regenerative potential of skin and the immune system. Clin Cosmet Investig Dermatol. 2019;12:519–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dubey M, Nagarkoti S, Awasthi D, Singh AK, Chandra T, Kumaravelu J, Barthwal MK, Dikshit M. Nitric oxide-mediated apoptosis of neutrophils through caspase-8 and caspase-3-dependent mechanism. Cell Death Dis. 2016;7:e2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chi DS, Qui M, Krishnaswamy G, Li C, Stone W. Regulation of nitric oxide production from macrophages by lipopolysaccharide and catecholamines. Nitric Oxide. 2003;8:127–32. [DOI] [PubMed] [Google Scholar]
- 56.Lai-Cheong JE, McGrath JA. Structure and function of skin, hair and nails[J]. Medicine, 2013, 41: 317–320. [Google Scholar]
- 57.Wu S, Wang L, Jacoby AM, Jasinski K, Kubant R, Malinski T. Ultraviolet B light-induced nitric oxide/peroxynitrite imbalance in keratinocytes--implications for apoptosis and necrosis. Photochem Photobiol. 2010;86:389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bécherel PA, Mossalayi MD, Ouaaz F, Le Goff L, Dugas B, Paul-Eugène N, Frances C, Chosidow O, Kilchherr E, Guillosson JJ, et al. Involvement of cyclic AMP and nitric oxide in immunoglobulin E-dependent activation of Fc epsilon RII/CD23+ normal human keratinocytes. J Clin Invest. 1994;93:2275–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frank S, Kämpfer H, Podda M, Kaufmann R, Pfeilschifter J. Identification of copper/zinc superoxide dismutase as a nitric oxide-regulated gene in human (HaCaT) keratinocytes: implications for keratinocyte proliferation. Biochem J. 2000;346 Pt 3:719–28. [PMC free article] [PubMed] [Google Scholar]
- 60.Stallmeyer B, Kämpfer H, Kolb N, Pfeilschifter J, Frank S. The function of nitric oxide in wound repair: inhibition of inducible nitric oxide-synthase severely impairs wound reepithelialization. J Invest Dermatol. 1999;113:1090–8. [DOI] [PubMed] [Google Scholar]
- 61.Benrath J, Zimmermann M, Gillardon F. Substance P and nitric oxide mediate would healing of ultraviolet photodamaged rat skin: evidence for an effect of nitric oxide on keratinocyte proliferation. Neurosci Lett. 1995;200:17–20. [DOI] [PubMed] [Google Scholar]
- 62.Stallmeyer B, Anhold M, Wetzler C, Kahlina K, Pfeilschifter J, Frank S. Regulation of eNOS in normal and diabetes-impaired skin repair: implications for tissue regeneration. Nitric Oxide. 2002;6:168–77. [DOI] [PubMed] [Google Scholar]
- 63.Bruch-Gerharz D, Schnorr O, Suschek C, Beck KF, Pfeilschifter J, Ruzicka T, Kolb-Bachofen V. Arginase 1 overexpression in psoriasis: limitation of inducible nitric oxide synthase activity as a molecular mechanism for keratinocyte hyperproliferation. Am J Pathol. 2003;162:203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vallette G, Tenaud I, Branka JE, Jarry A, Sainte-Marie I, Dreno B, Laboisse CL. Control of growth and differentiation of normal human epithelial cells through the manipulation of reactive nitrogen species. Biochem J. 1998;331:713–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rossi A, Catani MV, Candi E, Bernassola F, Puddu P, Melino G. Nitric oxide inhibits cornified envelope formation in human keratinocytes by inactivating transglutaminases and activating protein 1. J Invest Dermatol. 2000;115:731–9. [DOI] [PubMed] [Google Scholar]
- 66.Mao-Qiang M, Feingold KR, Jain M, Elias PM. Extracellular processing of phospholipids is required for permeability barrier homeostasis. J Lipid Res. 1995;36:1925–35. [PubMed] [Google Scholar]
- 67.Mao-Qiang M, Jain M, Feingold KR, Elias PM. Secretory phospholipase A2 activity is required for permeability barrier homeostasis. J Invest Dermatol. 1996;106:57–63. [DOI] [PubMed] [Google Scholar]
- 68.Holleran WM, Takagi Y, Menon GK, Legler G, Feingold KR, Elias PM. Processing of epidermal glucosylceramides is required for optimal mammalian cutaneous permeability barrier function. J Clin Invest. 1993;91:1656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uchida Y, Hara M, Nishio H, Sidransky E, Inoue S, Otsuka F, Suzuki A, Elias PM, Holleran WM, Hamanaka S. Epidermal sphingomyelins are precursors for selected stratum corneum ceramides. J Lipid Res. 2000;41:2071–82. [PubMed] [Google Scholar]
- 70.Kanuri BN, Kanshana JS, Rebello SC, Pathak P, Gupta AP, Gayen JR, Jagavelu K, Dikshit M. Altered glucose and lipid homeostasis in liver and adipose tissue pre-dispose inducible NOS knockout mice to insulin resistance. Sci Rep. 2017;7:41009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roediger WE, Hems R, Wiggins D, Gibbons GF. Inhibition of hepatocyte lipogenesis by nitric oxide donor: could nitric oxide regulate lipid synthesis? IUBMB Life. 2004;56:35–40. [DOI] [PubMed] [Google Scholar]
- 72.Schild L, Dombrowski F, Lendeckel U, Schulz C, Gardemann A, Keilhoff G. Impairment of endothelial nitric oxide synthase causes abnormal fat and glycogen deposition in liver. Biochim Biophys Acta. 2008;1782:180–7. [DOI] [PubMed] [Google Scholar]
- 73.Wu NL, Lee TA, Tsai TL, Lin WW. TRAIL-induced keratinocyte differentiation requires caspase activation and p63 expression. J Invest Dermatol. 2011;131:874–83. [DOI] [PubMed] [Google Scholar]
- 74.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. [DOI] [PubMed] [Google Scholar]
- 75.Weil M, Raff MC, Braga VM. Caspase activation in the terminal differentiation of human epidermal keratinocytes. Curr Biol. 1999;9:361–4. [DOI] [PubMed] [Google Scholar]
- 76.Demerjian M, Hachem JP, Tschachler E, Denecker G, Declercq W, Vandenabeele P, Mauro T, Hupe M, Crumrine D, Roelandt T, Houben E, Elias PM, Feingold KR. Acute modulations in permeability barrier function regulate epidermal cornification: role of caspase-14 and the protease-activated receptor type 2. Am J Pathol. 2008;172:86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Denecker G, Ovaere P, Vandenabeele P, Declercq W. Caspase-14 reveals its secrets. J Cell Biol. 2008;180:451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rabkin SW, Klassen SS. Nitric oxide differentially regulates the gene expression of caspase genes but not some autophagic genes. Nitric Oxide. 2007;16:339–47. [DOI] [PubMed] [Google Scholar]
- 79.Kiss B, Szántó M, Hegedűs C, Antal D, Szödényi A, Márton J, Méhes G, Virág L, Szegedi A, Bai P. Poly(ADP-ribose) polymerase-1 depletion enhances the severity of inflammation in an imiquimod-induced model of psoriasis. Exp Dermatol. 2020;29:79–85. [DOI] [PubMed] [Google Scholar]
- 80.Deliconstantinos G, Villiotou V, Stravrides JC. Release by ultraviolet B (u.v.B) radiation of nitric oxide (NO) from human keratinocytes: a potential role for nitric oxide in erythema production. Br J Pharmacol. 1995;114:1257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamaoka J, Kawana S, Miyachi Y. Nitric oxide inhibits ultraviolet B-induced murine keratinocyte apoptosis by regulating apoptotic signaling cascades. Free Radic Res. 2004;38:943–50. [DOI] [PubMed] [Google Scholar]
- 82.Weller R, Schwentker A, Billiar TR, Vodovotz Y. Autologous nitric oxide protects mouse and human keratinocytes from ultraviolet B radiation-induced apoptosis. Am J Physiol Cell Physiol. 2003;284:C1140–8. [DOI] [PubMed] [Google Scholar]
- 83.Weller R, Billiar T, Vodovotz Y. Pro- and anti-apoptotic effects of nitric oxide in irradiated keratinocytes: the role of superoxide. Skin Pharmacol Appl Skin Physiol. 2002;15:348–52. [DOI] [PubMed] [Google Scholar]
- 84.Frank S, Stallmeyer B, Kämpfer H, Kolb N, Pfeilschifter J. Nitric oxide triggers enhanced induction of vascular endothelial growth factor expression in cultured keratinocytes (HaCaT) and during cutaneous wound repair. FASEB J. 1999;13:2002–14. [PubMed] [Google Scholar]
- 85.Elias PM, Arbiser J, Brown BE, Rossiter H, Man MQ, Cerimele F, Crumrine D, Gunathilake R, Choi EH, Uchida Y, Tschachler E, Feingold KR. Epidermal vascular endothelial growth factor production is required for permeability barrier homeostasis, dermal angiogenesis, and the development of epidermal hyperplasia: implications for the pathogenesis of psoriasis. Am J Pathol. 2008;173:689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin TK, Man MQ, Abuabara K, Wakefield JS, Sheu HM, Tsai JC, Lee CH, Elias PM. By protecting against cutaneous inflammation, epidermal pigmentation provided an additional advantage for ancestral humans. Evol Appl. 2019;12:1960–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Man MQ, Lin TK, Santiago JL, Celli A, Zhong L, Huang ZM, Roelandt T, Hupe M, Sundberg JP, Silva KA, Crumrine D, Martin-Ezquerra G, Trullas C, Sun R, Wakefield JS, Wei ML, Feingold KR, Mauro TM, Elias PM. Basis for enhanced barrier function of pigmented skin. J Invest Dermatol. 2014;134:2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gunathilake R, Schurer NY, Shoo BA, Celli A, Hachem JP, Crumrine D, Sirimanna G, Feingold KR, Mauro TM, Elias PM. pH-regulated mechanisms account for pigment-type differences in epidermal barrier function. J Invest Dermatol. 2009;129:1719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lassalle MW, Igarashi S, Sasaki M, Wakamatsu K, Ito S, Horikoshi T. Effects of melanogenesis-inducing nitric oxide and histamine on the production of eumelanin and pheomelanin in cultured human melanocytes. Pigment Cell Res. 2003;16:81–4. [DOI] [PubMed] [Google Scholar]
- 90.Dong Y, Wang H, Cao J, Ren J, Fan R, He X, Smith GW, Dong C. Nitric oxide enhances melanogenesis of alpaca skin melanocytes in vitro by activating the MITF phosphorylation. Mol Cell Biochem. 2011;352:255–60. [DOI] [PubMed] [Google Scholar]
- 91.Choi YJ, Uehara Y, Park JY, Chung KW, Ha YM, Kim JM, Song YM, Chun P, Park JW, Moon HR, Chung HY. Suppression of melanogenesis by a newly synthesized compound, MHY966 via the nitric oxide/protein kinase G signaling pathway in murine skin. J Dermatol Sci. 2012;68:164–71. [DOI] [PubMed] [Google Scholar]
- 92.Förstermann U Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. 2010;459:923–39. [DOI] [PubMed] [Google Scholar]
- 93.Wink DA, Kasprzak KS, Maragos CM, Elespuru RK, Misra M, Dunams TM, Cebula TA, Koch WH, Andrews AW, Allen JS, et al. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science. 1991;254:1001–3. [DOI] [PubMed] [Google Scholar]
- 94.Zhen J, Lu H, Wang XQ, Vaziri ND, Zhou XJ. Upregulation of endothelial and inducible nitric oxide synthase expression by reactive oxygen species. Am J Hypertens. 2008;21:28–34. [DOI] [PubMed] [Google Scholar]
- 95.Elias PM, Wood LC, Feingold KR. Epidermal pathogenesis of inflammatory dermatoses. Am J Contact Dermat. 1999;10:119–26. [PubMed] [Google Scholar]
- 96.Bellamy TC, Wood J, Garthwaite J. On the activation of soluble guanylyl cyclase by nitric oxide. Proc Natl Acad Sci U S A. 2002;99:507–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Martinelli AM, Rodrigues CNDS, Moraes TF, Rodrigues GJ. In Endothelial Cells, the Activation or Stimulation of Soluble Guanylyl Cyclase Induces the Nitric Oxide Production by a Mechanism Dependent of Nitric Oxide Synthase Activation. J Pharm Pharm Sci. 2018;21:38–45. [DOI] [PubMed] [Google Scholar]
- 98.Mao-Qiang M, Mauro T, Bench G, Warren R, Elias PM, Feingold KR. Calcium and potassium inhibit barrier recovery after disruption, independent of the type of insult in hairless mice. Exp Dermatol. 1997;6:36–40. [DOI] [PubMed] [Google Scholar]
- 99.Lee SH, Elias PM, Proksch E, Menon GK, Mao-Quiang M, Feingold KR. Calcium and potassium are important regulators of barrier homeostasis in murine epidermis. J Clin Invest. 1992;89:530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mao-Qiang M, Brown BE, Wu-Pong S, Feingold KR, Elias PM. Exogenous nonphysiologic vs physiologic lipids. Divergent mechanisms for correction of permeability barrier dysfunction. Arch Dermatol. 1995;131:809–16. [DOI] [PubMed] [Google Scholar]
- 101.Bruch-Gerharz D, Ruzicka T, Kolb-Bachofen V. Nitric oxide in human skin: current status and future prospects. J Invest Dermatol. 1998;110:1–7. [DOI] [PubMed] [Google Scholar]
- 102.Bécherel PA, Chosidow O, Le Goff L, Francès C, Debré P, Mossalayi MD, Arock M. Inducible nitric oxide synthase and proinflammatory cytokine expression by human keratinocytes during acute urticaria. Mol Med. 1997;3:686–94. [PMC free article] [PubMed] [Google Scholar]
- 103.Hyun E, Bolla M, Steinhoff M, Wallace JL, Soldato PD, Vergnolle N. Anti-inflammatory effects of nitric oxide-releasing hydrocortisone NCX 1022, in a murine model of contact dermatitis. Br J Pharmacol. 2004;143:618–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Turesin F, del Soldato P, Wallace JL. Enhanced anti-inflammatory potency of a nitric oxide-releasing prednisolone derivative in the rat. Br J Pharmacol. 2003;139:966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wallace JL, Rizzo G, Cirino G, Del Soldato P, Fiorucci S. Enhanced anti-inflammatory potency of a nitric oxide-releasing derivative of flunisolide: role of nuclear factor-kappaB. J Pharmacol Exp Ther. 2004;310:1096–102. [DOI] [PubMed] [Google Scholar]
- 106.Gupta J, Grube E, Ericksen MB, Stevenson MD, Lucky AW, Sheth AP, Assa’ad AH, Khurana Hershey GK. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. 2008;121:725–730.e2. [DOI] [PubMed] [Google Scholar]
- 107.Kim DW, Park JY, Na GY, Lee SJ, Lee WJ. Correlation of clinical features and skin barrier function in adolescent and adult patients with atopic dermatitis. Int J Dermatol. 2006;45:698–701. [DOI] [PubMed] [Google Scholar]
- 108.Sugarman JL, Fluhr JW, Fowler AJ, Bruckner T, Diepgen TL, Williams ML. The objective severity assessment of atopic dermatitis score: an objective measure using permeability barrier function and stratum corneum hydration with computer-assisted estimates for extent of disease. Arch Dermatol. 2003;139:1417–22. [DOI] [PubMed] [Google Scholar]


