Abstract
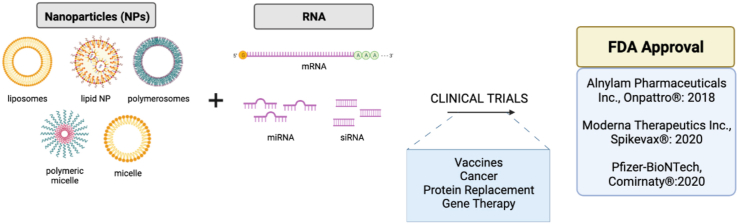
The clinical application of nanoparticles (NPs) to deliver RNA for therapy has progressed rapidly since the FDA approval of Onpattro® in 2018 for the treatment of polyneuropathy associated with hereditary transthyretin amyloidosis. The emergency use authorization or approval and widespread global use of two mRNA-NP based vaccines developed by Moderna Therapeutics Inc. and Pfizer-BioNTech in 2021 has highlighted the translatability of NP technology for RNA delivery. Furthermore, in clinical trials, a wide variety of NP formulations have been found to extend the half-life of RNA molecules such as microRNA, small interfering RNA, and messenger RNA, with limited safety issues. In this review, we discuss the NP formulations that are already used in the clinic to deliver therapeutic RNA and highlight examples of RNA-NPs which are currently under evaluation for human use. We also detail NP formulations that failed to progress through clinical trials, in hopes of guiding future successful translation of nanomedicine-based RNA therapeutics into the clinic.
Keywords: Nanoparticles, RNA therapy, Drug delivery, Nanomedicine, Clinical trials, COVID-19
Graphical abstract
Highlights
-
•
Discuss the RNA-Nanoparticle (RNA-NP) formulations available in clinic.
-
•
Detail RNA-NPs currently under assessment in clinic for human use.
-
•
Describe the RNA-NPs that failed to progress through clinical trials.
1. Introduction
The extensive characterization of ribonucleic acid (RNA) since the initial discovery of nucleic acids in 1868 has demonstrated its essential role in both health and disease [1]. Following the identification of messenger RNA (mRNA), microRNA (miRNA), and small interfering RNA (siRNA), these functionally and structurally diverse molecules have been explored as preventative vaccines, drug treatments, and immunomodulatory agents to treat a wide variety of diseases, including cancer, atherosclerosis, and Alzheimer's disease, via induction or silencing of protein production (Fig. 1) [2,3].
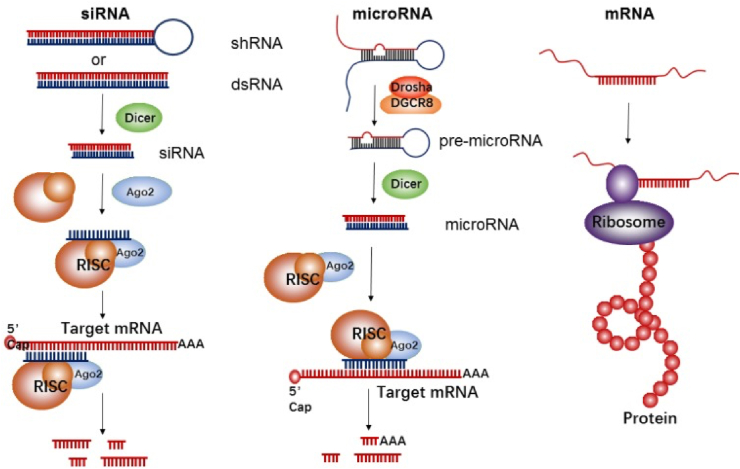
Fig. 1.
Mechanism of action of short interfering RNA (siRNA), microRNA (miRNA), and messenger RNA (mRNA), reprinted from Lin et al. [3] under the Creative Commons Attribution 4.0 International License (CC-BY license). siRNA: The precursor of siRNA, short hairpin RNA (shRNA), or double-stranded RNA (dsRNA), is recognized by Dicer and then incorporated into the RNA-induced silencing complex (RISC). The siRNA-RISC complex then binds to one target mRNA, inducing cleavage by the argonuate-2 endonuclease (Ago2) and reducing target protein expression. microRNA: The enzymes Drosha and DGCR8 convert primary miRNA (pri-miRNA) into precursor miRNA (pre-miRNA), measuring ∼70 nucleotides. Cleavage by Dicer then creates mature miRNA which is incorporated into RISC and reduces protein expression via mRNA cleavage in the 3′-untranslated regions (UTR). Unlike siRNA, miRNA can bind multiple mRNA targets. mRNA: The UTR at the 5′ and 3′ ends of mRNA are recognized by the ribosome, which facilitates protein translation.
However, although the use of RNA for therapy has multiple advantages, including ease of production and dose dependent, transient mechanism of action in vivo, clear disadvantages have limited their clinical success. These disadvantages include the short half-life of RNA molecules caused by reticuloendothelial system (RES) clearance and nuclease degradation [4,5]. Therapeutic efficacy is also limited by poor RNA delivery into the cellular cytoplasm, where RNA acts, which has been associated with RNA's negative charge and size. For example, the small size of miRNA and siRNA promotes increased renal clearance, while the potentially large size of mRNA limits cellular uptake [6,7]. Cellular binding and endocytosis of RNA is also inhibited by the stiff structure of RNA molecules, especially siRNAs, a feature that has been shown to limit receptor-ligand interactions on the cell surface and impede the ability of the cell membrane to engulf RNA [8,9]. Additionally, following cell membrane internalization, RNA is trapped in endosomes, with only ∼1–2% of siRNAs that are taken up by cells escaping the endosome [1]. Even when these hurdles are overcome, RNA administration can induce toxicity at high doses, particularly with double stranded structures such as siRNA, which can trigger an anti-viral innate immune response [8,10]. Furthermore, the lack of specific RNA targeting to the disease site results in off-target accumulation in healthy cells, thereby decreasing the therapeutic efficacy of RNA and necessitating the administration of high doses for therapy [9]. Additionally, this increased dosage increases the risk of developing off-target side effects [11].
In order to overcome the inherent limitations of using RNA molecules as therapeutics, nanoparticles (NPs) have been successfully used in the clinic and in clinical trials to facilitate RNA delivery for therapy and disease prevention [7,12]. Depending on their size, NPs can avoid macrophage clearance and extend RNA half-life, thereby increasing in vivo circulation time and cellular uptake [13,14]. Additionally, targeted NPs can reduce RNA toxicity at off-target sites, and multiple RNA types can be incorporated in one NP, allowing multi-target therapy for complex pathologies [15].
Many types of NPs have been applied for RNA delivery, although lipid- and polymer-based formulations remain the most widely used [16]. Non-toxic and biodegradable components can be used to create biocompatible NPs for clinical use with low host immunogenicity [17,18]. Cationic and ionizable lipids are often employed to produce solid lipid NPs, bi-layered liposomes, or micelles for RNA delivery in order to optimize complexation with negatively charged RNA and condense large mRNA molecules that would otherwise be unable to enter the cell. Ionizable lipids, e.g. 1,2-dioleoyl-3-dimethylammonium propane (DODAP) and 1,2-dioleyloxy-N,N-dimethyl-3-aminopropane (DODMA), are particularly useful as they have a neutral or low positive charge at physiological pH (e.g., in the extracellular space) and high positive charge at low pH (e.g., in endosomes), preventing non-specific interactions and reducing the risk of toxicity [7,19]. “Stealth” molecules, including polyethylene glycol (PEG), are also commonly incorporated in NPs to prolong circulation time by reducing serum protein attachment and subsequent aggregation, and thereby avoiding clearance by the mononuclear phagocyte system [20]. Considering the wide variety of materials available, each with numerous potential modifications, NP formulations can easily be optimized for the specific RNA type to be delivered, as detailed previously [[21], [22], [23], [24], [25]].
Here, we discuss the RNA-NP therapies that are approved by the United States (US) Food and Drug Administration (FDA) for clinical use, along with those that are currently in clinical trials. Finally, we examine some common reasons for the therapeutic failure of RNA-NPs and how these can be combatted to optimize the translational potential of emerging RNA-NP based therapies.
2. FDA approved RNA-NP therapy
FDA approval is required for the marketing and sale of drugs in the US [26]. In order to receive approval by the FDA, there are multiple factors that are considered during regulatory review. Outside of the physiochemical properties of the RNA-NP— such as charge, size, and reaction of the NP to environmental factors (e.g. pH, salt concentration, and temperature)—, manufacturing processes and controls (e.g. stability, sensitivity, and purity/quality of the RNA-NPs during production and storage) must be evaluated [27]. For clinical evaluation, the RNA-NP pharmacokinetics/pharmacodynamics, dosage and dose frequency, administration route, immunogenicity, bioavailability, biodistribution, biodegradation, and elimination pathway must be monitored in clinical trials and provided to the FDA for review [28]. Safety and efficacy of the drug varies depending on the disease which the RNA-NP is targeting. However, the treatment should be able to induce a significant therapeutic effect with minimal adverse side effects. The environmental impact of the RNA-NP, particularly in terms of its manufacturing process, must also be submitted to the FDA [28]. The low number of FDA approved RNA-NPs can be partially explained by the numerous determinants that must be assessed preclinically and clinically before approval for public use, along with the variability in acceptable efficacy or safety parameters. For example, lower drug efficacy is normally considered acceptable for FDA approval in the case of rare diseases where no alternative therapy is available [29,30].
Despite these limitations, there are currently three RNA-NPs that have fulfilled these requirements and have been approved by the FDA for public use in the US. In 2018, both the FDA and the European Medicines Agency (EMA) approved an RNA-NP for the first time [31]. Onpattro® (or patisiran) was developed by Alnylam Pharmaceuticals Inc. to treat polyneuropathy associated with autosomal dominant hereditary transthyretin amyloidosis (hATTR). This type of amyloidosis is characterized by abnormal deposition of bone marrow-derived amyloid protein in the liver, thus inhibiting proper organ function. Onpattro® consists of liposome-encapsulated siRNA that silences expression of transthyretin (TTR), a protein that is released by the liver to transport thyroid hormone, and can undergo mutations causing hATTR [31]. siRNA is a double stranded, 20–25 nucleotide molecule that binds to and inhibits a specific mRNA [5,32]. This specificity offers therapeutic benefits by preventing off-target binding and associated negative side effects, although it also limits its versatility [20]. The Onpattro® liposomes contain DLin-MC3-DMA (MC3), cholesterol, 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), and PEG, and have a diameter range of 60–100 nm [33]. Following intravenous (IV) administration, Onpattro® NPs exploit the RES to localize in the liver, where they release siRNA in hepatocytes to inhibit TTR production and secretion. One of the major reasons for Onpattro's® therapeutic success is its incorporation of the pH sensitive ionizable lipid MC3 (Fig. 2). As the pKa of MC3 is ∼6.5, the same pH as the late endosome/lysosome [34], the liposome can fuse to the endosomal membrane following uptake and release therapeutic siRNA into the cytoplasm [35]. However, although Onpattro® can suppress TTR expression by >80% [36], the requirement for IV infusion every three weeks limits the ease and widespread use of this RNA-NP therapy.
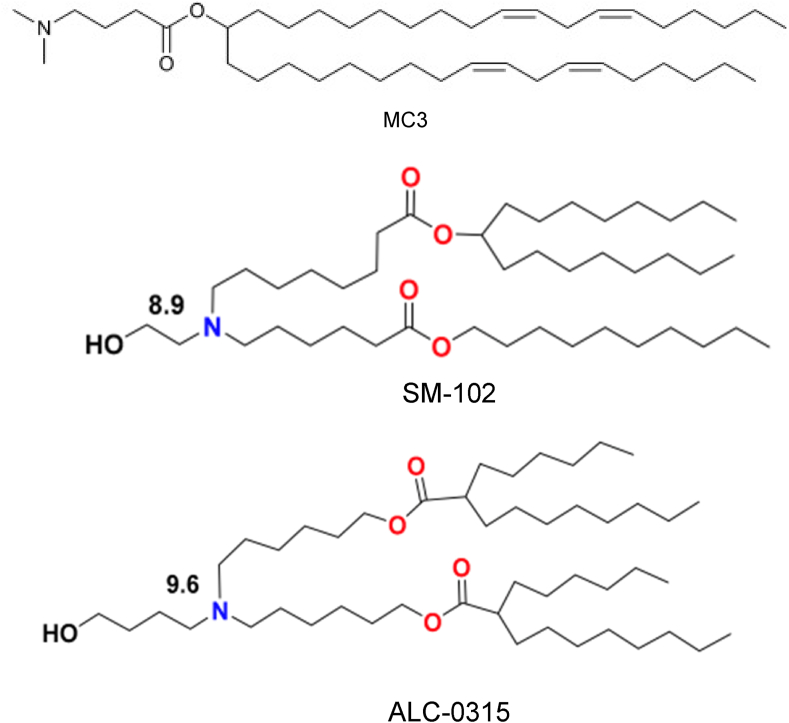
Fig. 2.
Chemical compositions of the ionizable lipids (top) DLin-MC3-DMA (MC3) used in Onpattro® liposomes to deliver siRNA targeting transthyretin for the treatment of polyneuropathy associated with hereditary transthyretin amyloidosis (hATTR), republished with permission from Jayaraman et al. [43], (middle) sphingomyelin-102 (SM-102) used in liposomes to deliver mRNA for vaccination against COVID-19 developed by Moderna Therapeutics Inc., and (bottom) ALC-0315 used in liposomes to deliver mRNA for vaccination against COVID-19 developed by Pfizer-BioNTech, both reprinted from Buschmann et al. [36] under the Creative Common CC BY License.
The most recently approved/authorized and widely publicized RNA-NP therapies are the two RNA-NP vaccines produced by Pfizer-BioNTech and Moderna Therapeutics Inc. (Moderna) in 2020 to prevent severe coronavirus disease 2019 (COVID-19) infection and hospitalization caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [7]. These vaccines use mRNA, a single-stranded molecule that is complementary to a single DNA strand. mRNA is translated to a protein in the cytoplasm in a rapid, transient, and dose dependent manner, and is subsequently degraded, often in mere minutes, therefore limiting long-term protein expression and potential associated adverse effects [9,[37], [38], [39]]. mRNA vaccines induce cells to transiently express a specific antigen in order to elicit a strong immune response that provides long-term protection against the target virus [17]. While Moderna has previously developed mRNA vaccines for cytomegalovirus (CMV), Pfizer's COVID-19 vaccine is the company's first mRNA-based vaccine. Both COVID-19 vaccines progressed from phase I to phase III clinical trials in <7 months and in 2020, both received emergency use authorization (EUA) from the FDA [40,41]. The Pfizer-BioNTech vaccine received full FDA approval in 2021. Furthermore, the EMA approved the Pfizer-BioNTech and Moderna vaccines in 2020 and 2021, respectively. PEG and cholesterol are incorporated in both COVID-19 vaccine liposome formulations, with Moderna using the ionizable lipid sphingomyelin-102 (SM-102) and Pfizer-BioNTech including the ionizable lipid ALC-0315 from Acuitas [36,42] (Fig. 1).
The Pfizer-BioNTech vaccine uses mRNA BNT162b2, which is 4.3 kb long and encodes for the prefusion full-length SARS-CoV-2 membrane-bound spike protein. The protein is stabilized by two prolines (to prevent viral fusion) in the C-terminal furin (an enzyme responsible for proteolytic cleavage during viral protein assembly) cleavage fragment, which encodes for the viral fusion machinery (Fig. 3) [36,44]. This antigen can induce antibody production against SARS-CoV-2 along with a strong Th-1 T-cell response, showing 94% efficacy in preventing infection in phase III clinical trials [36,40]. The Moderna vaccine incorporates a similar mRNA sequence, mRNA-1273, which encodes for the transmembrane-anchored prefusion spike protein with a native furin cleavage site, similarly stabilized with two prolines [36]. The Moderna vaccine also showed a strong Th-1 T-cell response and demonstrated 94.5% efficacy in interim phase III trials. However, Moderna's vaccine induced a higher incidence of side effects than the Pfizer-BioNTech vaccine, including fatigue (9.7% vs. 3.8%) and headaches (4.5% vs. 2%) [36]. While both COVID-19 vaccines show high efficacy, one of their major limitations is temperature instability, with both vaccines requiring storage at −20 °C (Moderna) or −80 °C (Pfizer-BioNTech), a factor that is particularly detrimental when distributing vaccines to under-resourced communities [45]. A summary of these three FDA approved/authorized RNA-NPs is given in Table 1.
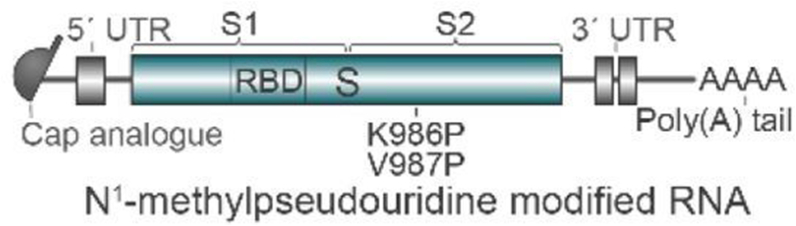
Fig. 3.
The structure of BNT162b2 mRNA used in the COVID-19 vaccine developed by Pfizer-BioNTech, reprinted from Vogel et al. [44]. UTR-untranslated region.
Table 1.
FDA approved RNA-NP therapies.
| Company, drug name | Disease target | Mechanism of action | NP carrier components |
RNA | Method, frequency of administration | Year approved by the FDA |
|---|---|---|---|---|---|---|
| Alnylam Pharmaceuticals Inc., Onpattro® | Polyneuropathy associated with hereditary transthyretin-mediated amyloidosis | Gene silencing of transthyretin | Lipid NP (DLin-MC3-DMA, DSPC, cholesterol, PEG2000-C-DMG) | siRNA targeting transthyretin | IV infusion, every three weeks | 2018 |
| Moderna Therapeutics Inc., Spikevax® | COVID-19 | Induces antibody production against SARS-CoV-2 | Lipid NP (cholesterol, SM-102, PEG2000-DMG, DSPC) | Synthetic mRNA-1273, encoding stabilized prefusion SARS-CoV-2 spike protein | IM (deltoid), two doses | 2020 Emergency use authorization |
| Pfizer-BioNTech, Comirnaty® | COVID-19 | Induces antibody production against SARS-CoV-2 | Lipid NP (cholesterol, PEG2000, ALC-0315, DSPC) | Nucleoside modified mRNA BNT162b2, encoding SARS-CoV-2 spike protein | IM (deltoid), two doses | 2021 |
Legend: NP- nanoparticle, DSPC- 1,2-distearoyl-sn-glycero-3-phosphocholine, PEG-polyethylene glycol, IV-intravenous, COVID-19- coronavirus disease 2019, SARS-CoV-2- severe acute respiratory syndrome coronavirus 2, SM-102- sphingomyelin 102, IM- intramuscular.
3. RNA-NPs in clinical trials
While there are only a few RNA-NPs that are authorized for public use by the FDA and EMA as of 2021, there are many that are being investigated in ongoing clinical trials. Considering the rapid development and approval of the two RNA-NP vaccines against COVID-19, there is a reasonable expectation that more successful RNA-NP based treatments will attain FDA and EMA approval in the coming years. Here, we describe NP formulations that are currently in clinical trials as potential vaccines, cancer therapies, protein replacement therapies, and gene editing therapies.
3.1. Vaccines for infectious diseases
Similar to the FDA approved COVID-19 vaccines described in section 2, many RNA-NP vaccines are under investigation for disease prevention in humans. Most RNA-NP vaccines in clinical trials incorporate ionizable lipids, such as MC3, to facilitate cellular uptake and/or endosomal escape, along with mRNAs that encode for virus-specific structural proteins that can stimulate antibody production (e.g., NCT04232280 targeting CMV, NCT03713086 for rabies, NCT04144348 for human metapneumovirus (hMPV) and human parainfluenza virus type 3 (PIV3) infection) or directly encode for disease-targeting antibodies (e.g., NCT03829384 targeting Chikungunya virus, a trial which concluded in July 2021). These ionizable lipid-based NPs often exploit charge-mediated targeting, with cationic lipids preferentially accumulating in the lungs [36]. For example, a phase I clinical trial (NCT04844268) by Senai Cimatec is currently investigating an RNA-NP to be used as a COVID-19 vaccine. This formulation contains the cationic lipid 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), which enables surface adsorption of an mRNA encoding the spike protein of SARS-CoV-2. The final mRNA-NP product is produced by mixing the mRNA and NPs at a 1:1 M ratio. The liposomes are stabilized by encapsulating superparamagnetic iron oxide NPs [46]. Pre-clinical trials showed this RNA-NP formulation elicited an effective T-cell response against SARS-CoV-2 in mice and macaques [46]. This formulation has the advantage of large scalability, and is stable for 3 months at both 4 °C and 25 °C, conferring an advantage over the currently approved COVID-19 vaccines [46]. Similarly, other companies have produced vaccines utilizing a lipid-based NP with RNA, incorporating mRNA encoding for SARS-CoV-2 antigens such as the viral spike protein (e.g., NCT04847102, NCT04785144, NCT04566276).
Outside of COVID-19 prevention, RNA-NP vaccines have also been developed to target other pathogenic viruses such as the zika virus and rabies lyssavirus. Moderna used its proprietary ionizable lipid MC3 along with DSPC, cholesterol, and PEG in a 50:38:5:1.5 ratio to form lipid NPs which deliver modified mRNA-1893 encoding for the both the envelope and pre-membrane protein of the zika virus [47]. Interim results from the completed phase I trial (NCT04064905) showed no serious side effects related to the vaccine. Furthermore, a majority of the patients who had previously tested seropositive (either by having been previously vaccinated or infected) for the zika virus produced a four-fold increase in the number of disease neutralizing antibodies present following vaccination [48]. Like Moderna's zika virus vaccine NP formulation, CureVac AG's rabies vaccine (NCT03713086) also uses a proprietary ionizable lipid from Acuitas along with cholesterol, PEG, and DSPC to deliver mRNA encoding a rabies virus glycoprotein which can induce an antibody response [49,50]. Phase I results from this clinical trial have shown no vaccine-related adverse effects at low doses (1 or 2 μg), and induced functional neutralizing antibody production against the rabies virus glycoprotein that were comparable to licensed rabies vaccines [49]. A list of ongoing clinical trials involving RNA-NP based vaccines is presented in Table 2.
Table 2.
| Company/Investigator | Disease Target | RNA | NP carrier | Method of administration | Phase | NCT number |
|---|---|---|---|---|---|---|
| CureVac AG | Rabies | mRNA CV7202 encoding the rabies virus glycoprotein | Liposomes (cholesterol, PEG2000, Acuitas proprietary ionizable lipid, DSPC) | IM, 1–2 doses administered at days 1, 29 in the deltoid | I | NCT03713086 |
| Moderna Therapeutics Inc. | Cytomegalovirus (CMV) infection | mRNA-1647 encoding the pentamer complex and the full-length membrane-bound glycoprotein B and mRNA-1443 encoding the pp65 T cell antigen of CMV | Liposomes (DLin-MC3-DMA, DSPC, cholesterol, PEG2000-C-DMG) | IM, on days 1, 56, 168 | II | NCT04232280 |
| Moderna Therapeutics Inc. | Human metapneumovirus (hMPV) and human parainfluenza virus type 3 (PIV3) infection | mRNA-1653 encoding the fusion proteins of hMPV and PIV3 | Liposomes (DLin-MC3-DMA, DSPC, cholesterol, PEG2000-C-DMG) | IM, on day 1 and 57 | I | NCT04144348 |
| Senai Cimatec | COVID-19 | mRNA encoding for the full-length spike protein of the SARS-CoV-2 virus (HD-301) | Lipid-inorganic NP (LION™) | IM, on day 1 and 29 or day 1 and 57 | I | NCT04844268 |
| Walvax Biotechnology Co., Ltd. | mRNA encoding for the receptor binding domain of the spike glycoprotein of the SARS-CoV-2 virus | Liposomes (Lipid 9001, cholesterol, DSPC, DMG-PEG2000) | IM, on day 1 and day 29 | III | NCT04847102 | |
| National Institute of Allergy and Infectious Diseases (NIAID) | mRNA-1273.351 encoding for full-length spike protein of SARS-CoV-2 B.1.351 variant | Proprietary lipid NP | IM, single dose | I | NCT04785144 | |
| Chulalongkorn University | mRNA encoding SARS-Cov2 wild-type spike protein mRNA | Proprietary Lipid NP | IM, escalating dose regimen 21 days apart | I | NCT04566276 |
Legend: NP- nanoparticle, NCT- national clinical trials, PEG- polyethylene glycol, DSPC- 1,2-distearoyl-sn-glycero-3-phosphocholine, IM- intramuscular, ID- intradermal, IV- intravenous, COVID-19- coronavirus disease 2019, SARS-CoV-2- severe acute respiratory syndrome coronavirus 2.
3.2. Cancer
Outside of vaccines, cancer therapies using RNA-NPs have shown great potential. This is likely influenced by the success and FDA approval of NP-based cancer therapies such as Doxil® (liposomal doxorubicin to treat Kaposi's sarcoma, breast cancer, ovarian cancer, and other solid tumors) and Onivyde® (liposomal irinotecan to treat metastatic pancreatic cancer). Although these two therapies do not use RNA, they do demonstrate the feasibility of NP use to target and treat cancer.
Many of the mRNA-NPs currently under clinical investigation for cancer treatment have vaccine-like properties, as they increase the production of tumor-specific and tumor-associated antigens on cancer cell surfaces, thus allowing for increased recognition by the host immune system (e.g., NCT02410733 targeting melanoma-associated antigens). A major advantage of mRNA cancer vaccines is that they have the potential to be personalized to a patient by generating mRNA that corresponds to specific tumor antigens that are over-expressed in individual patients, thus increasing the vaccines efficacy (e.g., NCT03897881, NCT03313778, NCT02316457, NCT03289962). These personalized cancer treatments are generated by isolating patient cancer cells through a biopsy, and sequencing their DNA or RNA, most often using next generation sequencing (NGS) [53]. NGS is used to identify the tumor-related genes, proteins, and mutations that could be used as possible therapeutic targets. For example, Moderna's ongoing phase II trial NCT03897881 is investigating IV infusion of lipid NPs that deliver mRNA-4157 encoding for 20 neoantigen epitopes identified in individual patients with high-risk melanoma to generate a strong immune response following tumor resection. Phase I studies using mRNA-4157 in combination with pembrolizumab (a humanized immunotherapy used to treat melanoma) showed an adequate safety profile and a high T-cell response specific to the encoded neoantigens [54]. Although these initial clinical results are promising, development of this personalized therapy can take 3–4 months from the moment a patient sample is obtained to sequencing and mRNA production, therefore delaying initiation of therapy. However, the production timeline is expected to be shortened to as little as four weeks in the near future as NGS technology and analysis techniques continue to advance [55].
In addition to mRNA-based RNA-NP therapeutics, siRNA and miRNA-based RNA-NPs are being used in clinical trials in order to knockdown tumorigenic genes and inhibit cancer progression (e.g., NCT02716012, NCT04675996, NCT01591356). miRNAs are small (∼22 nucleotides), single stranded, endogenous, noncoding RNAs that regulate post transcriptional gene expression by binding to the 3′-untranslated regions (UTR) of multiple target mRNAs and repressing protein production by mRNA destabilization or cleavage and translational silencing [[56], [57], [58]]. Interestingly, miRNAs show evolutionarily conservation across species, prompting extensive mammalian research that has implicated miRNAs in a wide variety of human pathologies including kidney disease, cancer, Parkinson's disease, and Alzheimer's disease [56,59,60]. They are now commonly listed as disease markers, with up- or down-regulation of specific miRNAs being associated with various pathologies. This association has enabled their use as therapeutic targets via multiple strategies including replacement with synthetic or biomimetic miRNAs, or targeting with synthetic anti-miRNAs, which bind to and inhibit miRNAs by steric hindrance, thereby upregulating gene expression and subsequently altering protein translation [[61], [62], [63]]. Pre-clinical data using miRNA-based therapy is promising, and their smaller molecular weight in comparison to mRNA lends themselves to easier and more efficient delivery in vivo via improved cellular uptake [9].
An example of miRNA delivery via NPs is in the ongoing phase I clinical trial NCT04675996 which employs a modified miR-193a-3p mimic. This miRNA inhibits expression of the cyclin D1 protein, which is involved in tumor progression. This inhibition can induce tumor cell apoptosis and inhibit cell migration and proliferation in advanced solid tumors found in the colon, liver, skin, pancreatic, and breast cancer [64]. The addition of 2′-O-methyl nucleotide on the passenger strand of this miRNA mimic enabled it to impede inflammatory pathway activation and reduce adverse side effects, as well as increase binding efficacy of the miRNA strand to cyclin D1 mRNA in vivo [64].
As mentioned with the clinical trial NCT03897881 above, many ongoing clinical trials assess dual cancer therapy by applying RNA-NPs in combination with traditional cancer therapies, such as chemotherapy or immunotherapy. Examples of these trials include NCT03289962 (mRNA-NPs used with atezolizumab, an anti-PD-L1 antibody), NCT03739931 (mRNA-NPs used to stimulate T-cell response with durvalumab, an anti-PD-L1 antibody) and NCT03323398 (liposomal mRNA-2416 used with durvalumab to treat multiple advanced malignancies). Synergistic treatments combining these RNA-NPs and a chemo- or immune-therapeutic agent can reduce tumor recurrence, sensitize cancer cells, and reduce therapeutic resistance. A summary of ongoing clinical trials using RNA-NP therapy for cancer is given in Table 3.
Table 3.
| Company/Investigator | Cancer target | RNA | NP carrier | Method of administration | Phase | NCT number |
|---|---|---|---|---|---|---|
| Moderna Therapeutics Inc. | Melanoma | mRNA-4157 modified to encode for patient specific tumor-associated antigens or neoantigens | Proprietary lipid NP formulation | IV infusion | II | NCT03897881 |
| BioNTech RNA Pharmaceuticals GmbH | RNAs (RBL001.1, RBL002.2, RBL003.1, RBL004.1) that induce a CD8+ and CD4+ response against melanoma-associated antigens | Liposomes (cholesterol, DOPE, DOTMA) | ID, 7 dose escalation cohorts | II | NCT02410733 | |
| M.D. Anderson Cancer Center | Solid tumors | siRNA against Ephrin type-A receptor 2 (EphA2) | Liposomes (DOPC) |
IV infusion over 120 min on days 1 and 4, repeated every 21 days. | I | NCT01591356 |
| Moderna Therapeutics Inc. | mRNA-4157 modified to encode for patient specific tumor-associated antigens or neoantigens | Proprietary liposome formulation | IM, 9 doses administered every 21 days | I | NCT03313778 | |
| InteRNA Technologies B.V. | miRNA-193a-3p mimic | Proprietary lipid NP formulation | IV infusion for 60 min twice per week | I | NCT04675996 | |
| Genentech Inc. | mRNA RO7198457 encoding patient specific tumor-associated antigens | Proprietary lipoplex formulation | IV infusion in 21-day cycles | I | NCT03289962 | |
| Mina Alpha Limited | Advanced liver cancer | Double stranded RNA to activate CEBPA gene | Amphoteric liposomes (SMARTICLES®) | IV, 1–3 times weekly for 21 days | I | NCT02716012 |
| University Medical Center Groningen | Ovarian cancer | mRNAs encoding for three ovarian cancer tumor associated antigens | Liposomes | IV | I | NCT04163094 |
| BioNTech SE | Triple negative breast cancer | IVAC_WAREHOUSE_bre1_uID and IVAC® MUTANOME _uID, which produce RNAs de novo targeting patient specific tumor-associated antigens/neoantigens | Liposomes | IV | I | NCT02316457 |
| Moderna Therapeutics Inc. | Solid tumors and lymphoma | mRNA-2752 encoding for the OX40 ligand T-cell costimulator, IL-23, and IL-36γ | Proprietary lipid NP | Intratumoral, escalating dose every 2 weeks | I | NCT03739931 |
| Moderna Therapeutics Inc. | Melanoma, colon cancer, gastrointestinal cancer, genitourinary cancer, hepatocellular cancer, relapsed/refractory solid tumor malignancies, lymphoma, ovarian cancer |
mRNA NCI-4650 encoding patient specific tumor-associated antigens mRNA-2416 encoding for a OX40 ligand |
Liposomes (SM-102, DSPC, PEG2000-DMG, cholesterol) |
Intratumoral injection on days 1 and 15 for six 28-day cycles |
I/II | NCT03323398 |
Legend: IM- intramuscular, ID- intradermal, IV- intravenous, DOPE- 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, DOTMA- 1,2-di-O-octadecenyl-3-trimethylammonium propane, DOPC- 1,2-dioleoyl-sn-glycero-3-phosphocholine, SM-102- sphingomyelin 102, DSPE- 1,2-Distearoyl-sn-glycero-3-phosphorylethanolamine, PEG- polyethylene glycol.
3.3. Protein replacement and gene editing therapies
Outside of vaccine development and cancer therapy, NPs that deliver RNA molecules for protein replacement therapy and gene editing have also progressed to clinical trials (Table 4). mRNA has been extensively researched for therapeutic protein replacement, whereby a specific protein that is absent or mutated in a diseased state can be produced in vivo [65]. Clinical trials addressing protein replacement in patients have mainly incorporated non-replicating mRNA in order to overcome protein deficiencies (e.g., NCT04442347 to treat ornithine transcarbamylase (OTC) deficiency by producing OTC enzyme) or to produce healthy functional versions of a specific physiological protein (e.g. NCT03375047 to treat cystic fibrosis by producing functional human cystic fibrosis transmembrane regulator protein). For example, Moderna's phase I/II clinical trial NCT04159103 explores the use of mRNA-NPs to treat propionic acidemia. In this rare metabolic disorder, the breakdown of proteins and lipids is inhibited due to propionyl-CoA carboxylase (PCC) mitochondrial enzyme deficiency, thus leading to a build-up of toxins such as urine organic acids [66]. The trial uses lipid NPs to deliver mRNA-3297, which translates PCC. This mRNA-NP formulation, which includes the proprietary MC3-based ionizable lipid “Moderna lipid 5”, received fast track designation by the FDA in 2019, allowing for more rapid review and development of the therapy [67,68].
Table 4.
Examples of ongoing clinical trials for mRNA-based protein replacement therapy and RNA-NP based gene editing applications [19,51,72,73].
| Company/Investigator | Disease target | Type of therapy | RNA | NP carrier | Method of administration | Phase | NCT Number |
|---|---|---|---|---|---|---|---|
| Moderna Therapeutics Inc. | Propionic acidemia | Protein replacement | mRNA 3927 encoding for and subunits of mitochondrial propionyl-CoA carboxylase protein | Lipid NP (Moderna lipid 5, cholesterol, PEG2000, DSPC) | IV infusion, up to 10 doses over 30 weeks | I/II | NCT04159103 |
| Translate Bio Inc. | Cystic fibrosis (CF) | Protein replacement | mRNA MRT 5005 for human CF transmembrane regulator protein | Lipid NP (Poly(β-amino esters, DOPE, PEG2000, cholesterol) | Nebulization | I/II | NCT03375047 |
| Nitto Denko Corporation | Hepatic and pulmonary fibrosis | Gene editing | siRNA for heat shock protein (HSP) 47 | Lipid NP (diretinamide-PEG-diretinamide conjugated to Vitamin A) | IV, every 2 weeks | II | NCT03538301 |
| Arcturus Therapeutics, Inc. | Ornithine Transcarb-amylase (OTC) deficiency | Protein replacement | ARCT-810 mRNA encoding for OTC enzyme | Proprietary lipid NP (LUNAR®) | IV | I | NCT04442347 |
Legend: NP- nanoparticle, PEG-polyethylene glycol, DSPC- 1,2-distearoyl-sn-glycero-3-phosphocholine, IM-intramuscular, ID- intradermal, IV- intravenous, SQ- subcutaneous, DOPE- 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine.
Another application of RNA-NPs is gene editing therapy, which utilizes siRNA, miRNA, and/or anti-miRNA to suppress or promote protein production. For example, the ongoing phase II clinical trial NCT03538301 employs an siRNA-NP (ND-L02-s0201) to target hepatic stellate cells or lung myofibroblasts for treatment of liver or lung fibrosis respectively [69,70]. Following chronic liver or lung damage, excessive activation of hepatic stellate cells or lung myofibroblasts respectively causes fibrosis and excessive collagen accumulation, thereby diminishing organ function [69]. ND-L02-s0201 incorporates six lipids along with the retinoid-conjugating molecule di-retinamide-PEG-di-retinamide for targeting of hepatic stellate cells or lung myofibroblasts. These lipid NPs deliver siRNA that reversibly inhibits production of the heat shock protein 47 (HSP47), which facilitates the correct folding of procollagen, thereby reducing collagen deposition [69,71]. In preclinical trials, ND-L02-s0201 was shown to reduce expression of HSP47 and inhibit fibrosis progression in silica-induced lung fibrosis rat models [69].
4. Reasons for therapeutic failure of RNA-NPs
Despite the promising clinical research occurring in the field of RNA-NP therapeutics recently, there have been many setbacks in RNA-NP translation. Most commonly, NPs and/or their RNA load are incapable of escaping the endosome, or they induce toxic immune responses and/or off-target side effects, leading to failure of clinical trials. Safety and efficacy remain vital in the clinical success of RNA-NPs and there are multiple methods for evaluation of these factors. Confirmation of in vitro safety should be evaluated using relevant cell types, including diseased cells, and should aim to use complex 3D models to better reflect the in vivo disease [74,75]. Similarly, appropriate animal models that present similar biological signs and symptoms to those displayed in humans, and appropriately age matched, should be utilized in preclinical studies [74]. In terms of clinical research, the FDA requires information on both short- and long-term side effects of RNA-NPs when reviewing them for general use. Therefore, recording minor adverse reactions, such as pain or inflammation at injection sites or mild/transient nausea following oral administration, or major side effects that dramatically affect the health or quality of life of the patient, is in an integral part of clinical trials. Major adverse reactions can lead to the rejection of RNA-NP use by the FDA. Likewise, a lack of efficacy is a major reason for failure to receive FDA approval. The definition of treatment efficacy varies based on disease, but broadly refers to the induction of factors that ameliorate or prevent a disease, e.g., the induction of antibody production by administration of the COVID vaccines that prevents development of severe disease and reduces hospitalization rates [17]. Here, we discuss some examples of clinical trials that were withdrawn and broadly categorized as failures, either due to issues with NP formulation or due to the structure of the RNA construct incorporated in NPs for therapy.
4.1. Limitations due to NP formulation
The use of NP formulations that have not been optimized either for RNA delivery or for use in humans is one of the reasons for failure or withdrawal of RNA-NPs from clinical trials. Most commonly, the use of generic NP formulations that are incapable of escaping the endosome following cellular uptake prevents the release of RNA cargo, leading to limited therapeutic response. Currently, many NPs utilize ionizable lipids to induce endosomal escape, as the positive charges on their headgroups can destabilize the endosomal membrane. A study in 2006 highlights the importance of endosomal escape in the clinical success of RNA-NPs. A lipid that lacks a cationic headgroup, N,N-dimethyl-2,3-bis[(9Z,12Z)-octadeca-9,12-dienoxy]propan-1-amine (DLinDMA), was used to create NPs for siRNA delivery. When administered to non-human primates with hypercholesterolemia, this formulation reduced expression of apolipoprotein B (ApoB) protein [76,77]. However, the primates exhibited low tolerance of the treatment and ApoB protein expression was not sufficiently reduced to induce a therapeutic response, factors which prevented advancement to clinical trials [76]. To address this failure, Alnylam Pharmaceuticals reformulated the siRNA-NP to include a potent cationic headgroup, (6Z,9Z,28Z,31Z)-Heptatriaconta-6,9,28,31-tetraen-19-yl 4-(dimethylamino)butanoate (DLin-MC3-DMA) [34]. Addition of MC3 as part of DLin-MC3-DMA increased the therapeutic and protein knockdown efficacy of the NP compared to DLinDMA NPs by two orders of magnitude, demonstrating the importance of enhancing RNA-NP endosomal escape [76]. This formulation was later used in the FDA-approved Onpattro®, as described in section 2.
Additionally, insufficient localization of the RNA-NP in the target cells can limit therapeutic effects, and associated accumulation in off-target cells can generate toxicity. For example, in September 2019, the clinical trial NCT03767270 run by Translate Bio was withdrawn as preclinical results showed poor pharmacokinetics and concerning safety profiles of their RNA-NP formulation. The trial used IV-administered NPs loaded with mRNA encoding OTC enzyme. These RNA-NPs were delivered to the liver to treat OTC deficiency, although a detailed mechanism of targeting has not been published [78].
Similarly, Calando Pharmaceutical's phase Ia/Ib clinical trial (NCT00689065) targeting multiple types of solid tumor cancer cells (including melanoma, prostate cancer, breast cancer, colorectal cancer, and cervical cancer), was terminated in 2013 due to dose-limiting toxic effects, likely due to the NP carrier [79]. In this trial, siRNA was delivered to inhibit expression of ribonucleotide reductase subunit M2, which plays a role in tumor progression [79]. The NP was comprised of adamantane PEG (AD-PEG), along with a human transferrin protein which acted as a targeting ligand to transferrin receptors present on the surface of cancer cells. Unfortunately, the transferrin-targeting ligand may have caused adverse effects due to misfolding and aggregation that took place during storage before administration [79]. Five of the 24 enrolled patients discontinued treatment due to adverse side effects, including bradycardia, hematuria, and pericardial effusion [79]. This study emphasizes the need for detailed RNA-NP stability studies, including monitoring aggregation behavior over time under various storage conditions (e.g., temperature), before progression to clinical studies.
Low targeting efficacy may also be improved through alteration of the RNA-NP delivery route rather than modification of the NP formulation. Although many RNA-NPs are administered IV to avoid bioavailability limitations associated with other administration routes, IV injections can lead to adverse effects due to accumulation in off-target tissues, commonly the heart and lungs where NP accumulation can cause clot formation [79,80]. As an alternative, many researchers explore localized delivery of RNA-NPs to the tissue or organ of interest, including injection directly into the heart [81,82], eyes [83,84], lungs [85], or tumors [86,87], in an attempt to minimize systemic side effects and to improve therapeutic efficacy. AstraZeneca's phase II clinical trial NCT03370887, for example, is looking into the safety and tolerability of their RNA-NPs (AZD8601) after being administered through an epicardial injection in patients that undergo coronary artery bypass grafting surgery. AZD8601 contains an mRNA encoding for VEGF-A, which can enhance blood vessel and myocardial growth at the wound site [88]. Injection of this RNA-NP could improve recovery after bypass surgery and enhance cardiac function. Although the results of this clinical trial have not yet been released, pre-clinical in vivo results of epicardial injection of AZD8601 in pigs have shown augmented cardiac activity after myocardial infarction, with greater vessel density and decreased myocardial fibrosis [89].
The failures highlighted in this section emphasize the need to optimize NP formulations in vitro and in vivo, including the choice of appropriate administration route, before use in clinical trials. This must include analysis and optimization of (1) NP stability under different storage conditions (e.g., temperature) and at different pH levels, including those present in the endosome, (2) quantification of targeting ability in order to determine if incorporation of specific targeting moieties is necessary, and (3) an efficient endosomal escape mechanism. The addition of stability enhancers, e.g. trehalose [90], targeting ligands, e.g. peptides, sugars, or antibodies [[91], [92], [93]], and/or endosomal escape moieties, e.g. trileucine [94], can potentially overcome these common issues.
4.2. Withdrawal of RNA-NPs from clinical trials due to issues with the RNA complex
The choice of therapeutic RNA molecule to be incorporated in the RNA-NP may be another reason for a lack of clinical success. Before loading into the NP, many RNA molecules are chemically modified. For example, 5′ capping can prevent innate immune sensing, addition of a poly(A) tail can prevent degradation by exonucleases, and incorporation of beta-globin 5′- or 3′-UTRs can enhance translation efficiency. Furthermore, the addition of 2′-O-methyl and 2′-fluoro to RNA can increase its stability, reduce immunogenicity, improve efficacy, and reduce off-target side effects [95]. 2′-O-methyl modifications have been shown to change the conformation of RNA to protect it from nucleases [96]. Similarly, 2′-fluoro modifications have allowed siRNA to evade detection by RNases, thus protecting siRNA from degradation [97]. However, RNA modifications have sometimes been shown to lessen their therapeutic effect inside the target cell. For example, including the 2′-fluoro modification on both the sense and anti-sense strands of siRNA can decrease its binding efficacy and thereby inhibits its ability to silence its target gene [95].
Along with RNA modifications, new technologies have been employed to engineer alternative RNA types to the traditionally used mRNA, siRNA, and miRNA molecules in order to improve the efficiency and efficacy of RNA therapeutics. For example, self-amplifying mRNA (saRNA) has been extensively investigated in vivo as a therapeutic [24,98]. Unlike non-replicating mRNA, which consists of mature mRNA with one open reading frame (ORF) that produces a target protein/antigen, saRNA comprises alphavirus replicons with two ORFs. saRNAs allow for production of the target antigen and amplification of the mRNA itself and have been shown to produce a larger number of antigens than non-replicating mRNA. However, the generally large size of replicons (∼9500 nucleotides) limits their potential application as a therapeutic [24]. An alternative promising technology is circular RNA (circRNAs) [99]. circRNAs are long (ranging in size from 100 nucleotides to over 4 kb) closed non-coding RNAs developed by covalently linking the 5′ and 3’ ends of pre-mRNA via back-splicing of exons. These molecules are expressed in numerous mammalian cell types and can regulate cellular processes by binding to and inhibiting miRNAs, thereby controlling gene expression and subsequent protein translation. Though this regulatory capacity indicates that circRNAs are a promising therapeutic technology, the full details of their physiological mechanisms and interactions require further elucidation before their clinical application [100].
One clinical trial that failed due to problems with the incorporated RNA complex is NCT01829971, which was terminated in 2016 [80]. This trial used a mimic miR-34a (MRX34) encapsulated in liposomes named SMARTICLES® [80,101]. miR-34a has been shown to downregulate the expression of multiple oncogenes and inhibit tumor growth, progression, and metastasis [79]. At least one dose of the RNA-NP formulation was administered to 85 patients presenting with an assortment of cancers such as melanoma, small cell lung cancer, hepatocellular carcinoma, sarcoma, and ovarian cancer. Approximately 50% of these patients discontinued the study due to persisting cancer progression and five patients withdrew due to serious side effects, including sepsis, hypoxia, systemic inflammatory response syndrome, cytokine release syndrome, and liver failure along with enterocolitis and pneumonitis. Furthermore, there were four treatment-related patient deaths [80]. Due to a lack of serious side effects in other clinical trials utilizing SMARTICLES®, the authors concluded that adverse effects in the patients were not due to the NP carrier, but due to MRX34 [80,101]. Chemical modification of the miRNA construct, such as 2′-O-methylation (which can suppress immune activation against the miRNA), will likely be necessary in order to minimize immunogenicity if MRX34-based treatment is to be explored in the future [102].
5. Conclusion
RNA-NP therapy has proven to be safe and effective for both disease treatment and prevention in the clinic, as demonstrated with Onpattro® and the two COVID-19 vaccines developed by Pfizer-BioNTech and Moderna. These RNA-NP formulations can be produced rapidly and on a large scale, facilitating the incorporation of a variety of RNA molecules, including mRNA, miRNA and/or siRNA, for treatment and prevention of many diseases, ranging from multiple cancer types and viral diseases to pathologies characterized by functional protein deficiencies such as hATTR.
RNA-NPs are versatile due to the wide array of materials that can be used for NP synthesis, from biological lipids to biodegradable polymers. However, it should be noted that the three RNA-NPs authorized by the FDA for use in humans consist of cholesterol-PEG based lipid NPs, with almost identical structures being broadly applied in clinical trials currently. The success of lipid NPs thus far over other formulations may guide future research and could indicate that biologically derived and biocompatible lipids, such as cholesterol, may be the safest and most efficient way to deliver RNA in humans. The common incorporation of PEG in the FDA approved RNA-NPs also underlines the importance of using materials that prolong the in vivo half-life of drugs, in this case incorporating PEG to limit serum protein adsorption to NPs.
Despite some RNA-NP success in the clinic, most RNA-NPs do not enter or complete clinical trials, indicating safety and toxicity issues in non-human primates and humans. In order to overcome these issues and improve effective translation to clinical use, RNA-NPs for translational purposes require effective and stable RNA incorporation methods, a low surface charge following RNA incorporation, high stability, a diameter measuring <100 nm to maximize cellular uptake, and a well-established, large scale, sterile manufacturing process [103].
With continued research into the links between RNA molecules and disease, RNA may offer more hope for therapy for rare or previously untreatable diseases. The rapid increase in emergent knowledge related to NP processing in vivo will facilitate more advanced modifications to effectively deliver RNA. By following the examples of clinically successful NPs, the field of RNA therapy can expand dramatically for years to come.
Declaration of competing interest
There are no conflicts of interest.
Acknowledgements
This work was supported by the University of Southern California, New Innovator Award (NIH, DP2-DK121328), NSF EAGER from DMR BMAT 2132744 and WISE Major Support Award granted to E.J.C, and by the PKD Foundation postdoctoral fellowship 839636 to A.C.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Siyoung A. Lim, Email: limsa@usc.edu.
Alysia Cox, Email: alysiaco@usc.edu.
Madelynn Tung, Email: madelynt@usc.edu.
Eun Ji Chung, Email: eunchung@usc.edu.
References
- 1.Kim Y.-K. RNA therapy: current status and future potential. Chonnam Medical Journal. 2020;56(2):87. doi: 10.4068/cmj.2020.56.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cullis P.R., Hope M.J. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 2017;25(7):1467–1475. doi: 10.1016/j.ymthe.2017.03.013. (in eng) 07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin Y.X., et al. RNA nanotechnology-mediated cancer immunotherapy. Theranostics. 2020;10(1):281–299. doi: 10.7150/thno.35568. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orlandini von Niessen A.G., et al. Improving mRNA-based therapeutic gene delivery by expression-augmenting 3' UTRs identified by cellular library screening. Mol. Ther. 2019;27(4):824–836. doi: 10.1016/j.ymthe.2018.12.011. (in eng) 04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valle A., Gualberto A., Pittella F. Short interfering RNA (siRNA) based Medicines and the future of RNAi therapy: a mini review. Current Trends in Biomedical Engineering & Biosciences. 2020;19 [Google Scholar]
- 6.Tatiparti K., Sau S., Kashaw S.K., Iyer A.K. siRNA delivery strategies: a comprehensive review of recent developments. Nanomaterials. Apr 2017;7(4) doi: 10.3390/nano7040077. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park K.S., Sun X., Aikins M.E., Moon J.J. Non-viral COVID-19 vaccine delivery systems. Adv. Drug Deliv. Rev. 2021;169:137–151. doi: 10.1016/j.addr.2020.12.008. (in eng) 02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong C.A., Nam Y.S. Functional nanostructures for effective delivery of small interfering RNA therapeutics. Theranostics. 2014;4(12):1211–1232. doi: 10.7150/thno.8491. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wadhwa A., Aljabbari A., Lokras A., Foged C., Thakur A. Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics. Jan 2020;12(2) doi: 10.3390/pharmaceutics12020102. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freund I., Eigenbrod T., Helm M., Dalpke A.H. RNA modifications modulate activation of innate toll-like receptors. Genes. 2019;10:2. doi: 10.3390/genes10020092. (in eng) 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim T., et al. Dual-targeting RNA nanoparticles for efficient delivery of polymeric siRNA to cancer cells. Chem Commun (Camb) Jun 2020;56(49):6624–6627. doi: 10.1039/d0cc01848a. (in eng) [DOI] [PubMed] [Google Scholar]
- 12.Chung Y.H., Beiss V., Fiering S.N., Steinmetz N.F. COVID-19 vaccine frontrunners and their nanotechnology design. ACS Nano. 2020;14(10):12522–12537. doi: 10.1021/acsnano.0c07197. (in eng) [DOI] [PubMed] [Google Scholar]
- 13.Yoshinaga N., et al. Bundling mRNA strands to prepare nano-assemblies with enhanced stability towards RNase for InVivoDelivery. Angew Chem. Int. Ed. Engl. 2019;58(33):11360–11363. doi: 10.1002/anie.201905203. ( ineng ) 08. [DOI] [PubMed] [Google Scholar]
- 14.Xu P.Y., Kankala R.K., Pan Y.J., Yuan H., Wang S.B., Chen A.Z. Overcoming multidrug resistance through inhalable siRNA nanoparticles-decorated porous microparticles based on supercritical fluid technology. Int. J. Nanomed. 2018;13:4685–4698. doi: 10.2147/IJN.S169399. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halbur C., Choudhury N., Chen M., Kim J.H., Chung E.J. siRNA-conjugated nanoparticles to treat ovarian cancer. SLAS Technol. 2019;24(2):137–150. doi: 10.1177/2472630318816668. (in eng) 04. [DOI] [PubMed] [Google Scholar]
- 16.Uchida S., Perche F., Pichon C., Cabral H. Nanomedicine-based approaches for mRNA delivery. Mol. Pharm. 2020;17(10):3654–3684. doi: 10.1021/acs.molpharmaceut.0c00618. (in eng) 10. [DOI] [PubMed] [Google Scholar]
- 17.Ho W., Gao M., Li F., Li Z., Zhang X.Q., Xu X. Next-generation vaccines: nanoparticle-mediated DNA and mRNA delivery. Adv Healthc Mater. Jan 2021:e2001812. doi: 10.1002/adhm.202001812. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato Y., Nakamura T., Yamada Y., Harashima H. The nanomedicine rush: new strategies for unmet medical needs based on innovative nano DDS. J. Contr. Release. Dec 2020;330:305–316. doi: 10.1016/j.jconrel.2020.12.032. (in eng) [DOI] [PubMed] [Google Scholar]
- 19.Gómez-Aguado I., Rodríguez-Castejón J., Vicente-Pascual M., Rodríguez-Gascón A., Solinís M., Del Pozo-Rodríguez A. Nanomedicines to deliver mRNA: state of the art and future perspectives. Nanomaterials. Feb 2020;10:2. doi: 10.3390/nano10020364. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nosova A.S., et al. Diversity of PEGylation methods of liposomes and their influence on RNA delivery. Medchemcomm. Mar 2019;10(3):369–377. doi: 10.1039/c8md00515j. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajj K.A., Whitehead K.A. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nature Reviews Materials. 2017;2(10):1–17. [Google Scholar]
- 22.Eygeris Y., Patel S., Jozic A., Sahay G. Deconvoluting lipid nanoparticle structure for messenger RNA delivery. Nano Lett. 2020;20(6):4543–4549. doi: 10.1021/acs.nanolett.0c01386. (in eng) 06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato Y., et al. Hydrophobic scaffolds of pH-sensitive cationic lipids contribute to miscibility with phospholipids and improve the efficiency of delivering short interfering RNA by small-sized lipid nanoparticles. Acta Biomater. 2020;102 doi: 10.1016/j.actbio.2019.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Blakney A.K., McKay P.F., Yus B.I., Aldon Y., Shattock R.J. Inside out: optimization of lipid nanoparticle formulations for exterior complexation and in vivo delivery of saRNA. Gene Ther. 2019;26(9):363–372. doi: 10.1038/s41434-019-0095-2. (in eng) 09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox A., Lim S.A., Chung E.J. Strategies to deliver RNA by nanoparticles for therapeutic potential. Mol. Aspect. Med. 2021:100991. doi: 10.1016/j.mam.2021.100991. 2021/08/05/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciociola A.A., et al. How drugs are developed and approved by the FDA: current process and future directions. Official journal of the American College of Gastroenterology | ACG. 2014;109(5) doi: 10.1038/ajg.2013.407. [DOI] [PubMed] [Google Scholar]
- 27.U. S. D. o. H. a. H. Services, F. a. D. Administration, and C.f.D. E. a. R. (CDER) 2018. Liposome Drug Products: Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation. [Google Scholar]
- 28.U. S. D. o. H. a. H. Services, F. a. D. Administration, C. f. D. E. a. R. (CDER), and C. f. B. E. a. R. (CBER) 2018. Drug Products, Including Biological Products, that Contain Nanomaterials: Guidance for Industry. [Google Scholar]
- 29.Mahase E. FDA allows drugs without proven clinical benefit to languish for years on accelerated pathway. BMJ. 2021;374:n1898. doi: 10.1136/bmj.n1898. [DOI] [PubMed] [Google Scholar]
- 30.Brown D.G., Wobst H.J. A decade of FDA-approved drugs (2010–2019): trends and future directions. J. Med. Chem. 2021;64(5):2312–2338. doi: 10.1021/acs.jmedchem.0c01516. 2021/03/11. [DOI] [PubMed] [Google Scholar]
- 31.Urits I., et al. A review of patisiran (ONPATTRO®) for the treatment of polyneuropathy in people with hereditary transthyretin amyloidosis. Neurol Ther. Dec 2020;9(2):301–315. doi: 10.1007/s40120-020-00208-1. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dammes N., Peer D. Paving the road for RNA therapeutics. Trends Pharmacol. Sci. 2021;41 doi: 10.1016/j.tips.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agency E.M. Assessment report for Onpattro. 2018. https://www.ema.europa.eu/en/documents/assessment-report/onpattro-epar-public-assessment-report_.pdf [Online]. Available:
- 34.Kulkarni J.A., Cullis P.R., van der Meel R. Lipid nanoparticles enabling gene therapies: from concepts to clinical utility. Nucleic Acid Therapeut. 2018;28(3):146–157. doi: 10.1089/nat.2018.0721. 2018/06/01. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X., Goel V., Attarwala H., Sweetser M.T., Clausen V.A., Robbie G.J. Patisiran pharmacokinetics, pharmacodynamics, and exposure-response analyses in the phase 3 APOLLO trial in patients with hereditary transthyretin-mediated (hATTR) amyloidosis. J. Clin. Pharmacol. 2020;60(1):37–49. doi: 10.1002/jcph.1480. 2020/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buschmann M.D., Carrasco M.J., Alishetty S., Paige M., Alameh M.G., Weissman D. Nanomaterial delivery systems for mRNA vaccines. Vaccines. 2021;9(no. 1) doi: 10.3390/vaccines9010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Islam M.A., et al. Restoration of tumour-growth suppression in vivo via systemic nanoparticle-mediated delivery of PTEN mRNA. Nat Biomed Eng. 2018;2(11):850–864. doi: 10.1038/s41551-018-0284-0. (in eng) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golan-Lavi R., et al. Coordinated pulses of mRNA and of protein translation or degradation produce EGF-induced protein bursts. Cell Rep. Mar 28 2017;18(13):3129–3142. doi: 10.1016/j.celrep.2017.03.014. (in eng) [DOI] [PubMed] [Google Scholar]
- 39.Kawaguchi D., et al. Phosphorothioate modification of mRNA accelerates the rate of translation initiation to provide more efficient protein synthesis. Angew. Chem. 2020;132(40):17556–17560. doi: 10.1002/anie.202007111. [DOI] [PubMed] [Google Scholar]
- 40.Polack F.P., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. 2020/12/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baden L.R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2020;384(5):403–416. doi: 10.1056/NEJMoa2035389. 2021/02/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khurana A., et al. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today. 2021:101142. doi: 10.1016/j.nantod.2021.101142. 2021/03/26/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jayaraman M., et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chem. 2012;51(34):8529–8533. doi: 10.1002/anie.201203263. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vogel A.B., et al. A prefusion SARS-CoV-2 spike RNA vaccine is highly immunogenic and prevents lung infection in non-human primates. bioRxiv. 2020:2020. doi: 10.1101/2020.09.08.280818. 09.08.280818. [DOI] [Google Scholar]
- 45.Meo S.A., Bukhari I.A., Akram J., Meo A.S., Klonoff D.C. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021;25(3):1663–1669. doi: 10.26355/eurrev_202102_24877. (in eng) 02. [DOI] [PubMed] [Google Scholar]
- 46.Erasmus J.H., et al. An <em>Alphavirus</em>-derived replicon RNA vaccine induces SARS-CoV-2 neutralizing antibody and T cell responses in mice and nonhuman primates. Sci. Transl. Med. 2020;12(555):eabc9396. doi: 10.1126/scitranslmed.abc9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richner J.M., et al. Modified mRNA vaccines protect against zika virus infection. Cell. 2017;168(6):1114–1125. doi: 10.1016/j.cell.2017.02.017. 2020. e10, 2017/03/09/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castanha P.M.S., Marques E.T.A. A glimmer of hope: recent updates and future challenges in zika vaccine development. Viruses. 2020;12(12):1371. doi: 10.3390/v12121371. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aldrich C., et al. Proof-of-concept of a low-dose unmodified mRNA-based rabies vaccine formulated with lipid nanoparticles in human volunteers: a phase 1 trial. Vaccine. 2021;39(8):1310–1318. doi: 10.1016/j.vaccine.2020.12.070. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lutz J., et al. Unmodified mRNA in LNPs constitutes a competitive technology for prophylactic vaccines. npj Vaccines. 2017;2(1):29. doi: 10.1038/s41541-017-0032-6. 2017/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anselmo A.C., Mitragotri S. Nanoparticles in the clinic: an update. Bioengineering & translational medicine. 2019;4(3) doi: 10.1002/btm2.10143. (in eng) e10143-e10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Didierlaurent A.M., Laupèze B., Di Pasquale A., Hergli N., Collignon C., Garçon N. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expet Rev. Vaccine. 2017;16(1):55–63. doi: 10.1080/14760584.2016.1213632. 2017/01/02. [DOI] [PubMed] [Google Scholar]
- 53.Shemesh C.S., et al. Personalized cancer vaccines: clinical landscape, challenges, and opportunities. Mol. Ther. 2021;29(2):555–570. doi: 10.1016/j.ymthe.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miao L., Zhang Y., Huang L. mRNA vaccine for cancer immunotherapy. Mol. Cancer. 2021;20(1):41. doi: 10.1186/s12943-021-01335-5. 2021/02/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sahin U., Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;359(6382):1355. doi: 10.1126/science.aar7112. [DOI] [PubMed] [Google Scholar]
- 56.Titze-de-Almeida S.S., Soto-Sánchez C., Fernandez E., Koprich J.B., Brotchie J.M., Titze-de-Almeida R. The promise and challenges of developing miRNA-based therapeutics for Parkinson's disease. Cells. 2020;9(4) doi: 10.3390/cells9040841. (in eng) 03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Z., Qin Y.W., Brewer G., Jing Q. MicroRNA degradation and turnover: regulating the regulators. Wiley Interdiscip Rev RNA. 2012;3(4):593–600. doi: 10.1002/wrna.1114. (in eng) 2012 Jul-Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zlotorynski E. Insights into the kinetics of microRNA biogenesis and turnover. Nat. Rev. Mol. Cell Biol. 2019;20(9):511. doi: 10.1038/s41580-019-0164-9. (in eng) 09. [DOI] [PubMed] [Google Scholar]
- 59.Yheskel M., Patel V. Therapeutic microRNAs in polycystic kidney disease. Curr. Opin. Nephrol. Hypertens. 2017;26(4):282–289. doi: 10.1097/MNH.0000000000000333. (in eng) 07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo H.Y., et al. Enzymatically synthesized poly(amino-co-ester) polyplexes for systemic delivery of pcDNA-miRNA-214 to suppress colorectal cancer liver metastasis. J. Mater. Chem. B. Oct 2018;6(40):6365–6376. doi: 10.1039/c8tb01932k. (in eng) [DOI] [PubMed] [Google Scholar]
- 61.Kim J., Yoon H., Chung D.E., Brown J.L., Belmonte K.C. miR-186 is decreased in aged brain and suppresses BACE1 expression. J. Neurochem. May 2016;137(3):436–445. doi: 10.1111/jnc.13507. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tong F., Ying Y., Pan H., Zhao W., Li H., Zhan X. MicroRNA-466 (miR-466) functions as a tumor suppressor and prognostic factor in colorectal cancer (CRC) Bosn. J. Basic Med. Sci. Aug 2018;18(3):252–259. doi: 10.17305/bjbms.2018.2376. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yheskel M., Lakhia R., Cobo-Stark P., Flaten A., Patel V. Anti-microRNA screen uncovers miR-17 family within miR-17∼92 cluster as the primary driver of kidney cyst growth. Sci. Rep. 2019;9(1):1920. doi: 10.1038/s41598-019-38566-y. (in eng) 02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Telford B.J., et al. Multi-modal effects of 1B3, a novel synthetic miR-193a-3p mimic, support strong potential for therapeutic intervention in oncology. Oncotarget. 2021;12(No 5) doi: 10.18632/oncotarget.27894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Magadum A., Kaur K., Zangi L. mRNA-based protein replacement therapy for the heart. Mol. Ther. 2019;27(4):785–793. doi: 10.1016/j.ymthe.2018.11.018. (in eng) 04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shchelochkov O.A., Carrillo N., Venditti C. University of Washington; 2016. Propionic Acidemia.https://www.ncbi.nlm.nih.gov/books/NBK92946/ [Online]. Available: [Google Scholar]
- 67.An D., et al. Systemic messenger RNA therapy as a treatment for methylmalonic acidemia. Cell Rep. 2017;21(12):3548–3558. doi: 10.1016/j.celrep.2017.11.081. 2017/12/19/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang L., et al. Dual mRNA therapy restores metabolic function in long-term studies in mice with propionic acidemia. Nat. Commun. 2020;11(1):5339. doi: 10.1038/s41467-020-19156-3. 2020/10/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y., et al. Anti-HSP47 siRNA lipid nanoparticle ND-L02-s0201 reverses interstitial pulmonary fibrosis in preclinical rat models. ERJ Open Research. 2021:733–2020. doi: 10.1183/23120541.00733-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yin C., Evason K.J., Asahina K., Stainier D.Y.R. Hepatic stellate cells in liver development, regeneration, and cancer. J. Clin. Invest. 2013;123(5):1902–1910. doi: 10.1172/JCI66369. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anselmo A.C., Mitragotri S. Nanoparticles in the clinic. Bioengineering & translational medicine. 2016;1(1):10–29. doi: 10.1002/btm2.10003. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang X., et al. Lipid nanoparticle–mediated delivery of anti-miR-17 family oligonucleotide suppresses hepatocellular carcinoma growth. Mol. Cancer Therapeut. 2017;16(5):905. doi: 10.1158/1535-7163.MCT-16-0613. [DOI] [PubMed] [Google Scholar]
- 73.Chakraborty C., Sharma A.R., Sharma G., Lee S.-S. Therapeutic advances of miRNAs: a preclinical and clinical update. J. Adv. Res. 2021;28:127–138. doi: 10.1016/j.jare.2020.08.012. 2021/02/01/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Magro R.D., Cox A., Zambelli V., Mancini S., Masserini M., Re F. The ability of liposomes, tailored for blood-brain barrier targeting, to reach the brain is dramatically affected by the disease state. Nanomedicine. 2018;13(6):585–594. doi: 10.2217/nnm-2017-0317. (in eng) 03. [DOI] [PubMed] [Google Scholar]
- 75.Takai K., Le A., Weaver V.M., Werb Z. Targeting the cancer-associated fibroblasts as a treatment in triple-negative breast cancer. Oncotarget. Dec 2016;7(50):82889–82901. doi: 10.18632/oncotarget.12658. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schlich M., et al. Cytosolic delivery of nucleic acids: the case of ionizable lipid nanoparticles. Bioengineering & Translational Medicine. 2021;6(2):e10213. doi: 10.1002/btm2.10213. 2021/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zimmermann T.S., et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441(7089):111–114. doi: 10.1038/nature04688. 2006/05/01. [DOI] [PubMed] [Google Scholar]
- 78.Translate Bio, Inc. Translate Bio Pipeline Program Update. 2019. Translate Bio. [Google Scholar]
- 79.Zuckerman J.E., et al. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc. Natl. Acad. Sci. U.S.A. 2014;111(31):11449–11454. doi: 10.1073/pnas.1411393111. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hong D.S., et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer. 2020;122(11):1630–1637. doi: 10.1038/s41416-020-0802-1. 2020/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Turnbull I.C., et al. Myocardial delivery of lipidoid nanoparticle carrying modRNA induces rapid and transient expression. Mol. Ther. : the journal of the American Society of Gene Therapy. 2016;24(1):66–75. doi: 10.1038/mt.2015.193. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zangi L., et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol. 2013;31(10):898–907. doi: 10.1038/nbt.2682. 2013/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Devoldere J., et al. Non-viral delivery of chemically modified mRNA to the retina: subretinal versus intravitreal administration. J. Contr. Release. 2019;307:315–330. doi: 10.1016/j.jconrel.2019.06.042. 2019/08/10/ [DOI] [PubMed] [Google Scholar]
- 84.Patel S., Ryals R.C., Weller K.K., Pennesi M.E., Sahay G. Lipid nanoparticles for delivery of messenger RNA to the back of the eye. J. Contr. Release. 2019;303:91–100. doi: 10.1016/j.jconrel.2019.04.015. (in eng) 06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang H., Leal J., Soto M.R., Smyth H.D.C., Ghosh D. Aerosolizable lipid nanoparticles for pulmonary delivery of mRNA through design of experiments. Pharmaceutics. 2020;12(11) doi: 10.3390/pharmaceutics12111042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hewitt Susannah L., et al. Durable anticancer immunity from intratumoral administration of IL-23, IL-36γ, and OX40L mRNAs. Sci. Transl. Med. 2019;11(477):eaat9143. doi: 10.1126/scitranslmed.aat9143. 2019/01/30. [DOI] [PubMed] [Google Scholar]
- 87.Li Y., et al. Multifunctional oncolytic nanoparticles deliver self-replicating IL-12 RNA to eliminate established tumors and prime systemic immunity. Nat. Can. 2020;1(9):882–893. doi: 10.1038/s43018-020-0095-6. 2020/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anttila V., et al. Synthetic mRNA encoding VEGF-A in patients undergoing coronary artery bypass grafting: design of a phase 2a clinical trial. Mol. Ther. 2020;18:464–472. doi: 10.1016/j.omtm.2020.05.030. (in eng) Methods & clinical development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carlsson L., et al. Biocompatible, purified VEGF-A mRNA improves cardiac function after intracardiac injection 1 Week post-myocardial infarction in swine. Mol. Ther. 2018;9:330–346. doi: 10.1016/j.omtm.2018.04.003. (in eng) Methods & clinical development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao P., et al. Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioact Mater. Jun 2020;5(2):358–363. doi: 10.1016/j.bioactmat.2020.03.001. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiong S., et al. Gold nanoparticle-based nanoprobes with enhanced tumor targeting and photothermal/photodynamic response for therapy of osteosarcoma. Nanotechnology. 2021;32 doi: 10.1088/1361-6528/abd816. [DOI] [PubMed] [Google Scholar]
- 92.Pei M., Xu R., Zhang C., Wang X., Li C., Hu Y. Mannose-functionalized antigen nanoparticles for targeted dendritic cells, accelerated endosomal escape and enhanced MHC-I antigen presentation. Colloids Surf. B Biointerfaces. 2021;197:111378. doi: 10.1016/j.colsurfb.2020.111378. 2021/01/01/ [DOI] [PubMed] [Google Scholar]
- 93.Corti R., et al. The clustering of mApoE anti-amyloidogenic peptide on nanoparticle surface does not alter its performance in controlling beta-amyloid aggregation. Int. J. Mol. Sci. Feb 5 2020;21(3) doi: 10.3390/ijms21031066. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ullah I., et al. Trileucine residues in a ligand-CPP-based siRNA delivery platform improve endosomal escape of siRNA. J. Drug Target. 2017;25(4):320–329. doi: 10.1080/1061186X.2016.1258566. [DOI] [PubMed] [Google Scholar]
- 95.Kwok A., Raulf N., Habib N. Developing small activating RNA as a therapeutic: current challenges and promises. Ther. Deliv. 2019;10(3):151–164. doi: 10.4155/tde-2018-0061. 2019/03/01. [DOI] [PubMed] [Google Scholar]
- 96.Kraynack B.A., Baker B.F. Small interfering RNAs containing full 2'-O-methylribonucleotide-modified sense strands display Argonaute2/eIF2C2-dependent activity. RNA (New York, N.Y.) 2006;12(1):163–176. doi: 10.1261/rna.2150806. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chiu Y.-L., Rana T.M. siRNA function in RNAi: a chemical modification analysis. RNA (New York, N.Y.) 2003;9(9):1034–1048. doi: 10.1261/rna.5103703. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anderluzzi G., et al. Investigating the impact of delivery system design on the efficacy of self-amplifying RNA vaccines. Vaccines. 2020;8 doi: 10.3390/vaccines8020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu C.-Y., Kuo H.-C. The emerging roles and functions of circular RNAs and their generation. J. Biomed. Sci. 2019;26(1):29. doi: 10.1186/s12929-019-0523-z. 2019/04/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu M. Circular RNA: functions, applications and prospects. ExRNA. 2020;2(1) doi: 10.1186/s41544-019-0046-5. 1, 2020/03/02. [DOI] [Google Scholar]
- 101.Tolcher A.W., et al. A phase 1 study of the BCL2-targeted deoxyribonucleic acid inhibitor (DNAi) PNT2258 in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2014;73(2):363–371. doi: 10.1007/s00280-013-2361-0. 2014/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang X., Cozen A.E., Liu Y., Chen Q., Lowe T.M. Small RNA modifications: integral to function and disease. Trends Mol. Med. 2016;22(12):1025–1034. doi: 10.1016/j.molmed.2016.10.009. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Akinc A., et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019;14(12):1084–1087. doi: 10.1038/s41565-019-0591-y. 2019/12/01. [DOI] [PubMed] [Google Scholar]