Abstract
Objective:
The last decade has seen improved outcomes for children requiring Extracorporeal Life Support (ECLS) as well as for children undergoing Hematopoietic Cell Transplantation (HCT). Thus, given the historically poor survival of HCT patients using ECLS, the Pediatric Acute Lung Injury and Sepsis Investigators’ (PALISI) HCT and Cancer Immunotherapy (CI) subgroup aimed to characterize the utility of ECLS in facilitating recovery from critical cardiorespiratory illnesses in pediatric HCT patients.
Data Sources:
All available published data were identified using a set of PubMed search terms for pediatric ECLS and HCT.
Study Selection:
All articles that provided original reports of pediatric HCT patients who underwent ECLS support were included. Sixty-four manuscripts met search criteria. Twenty-four were included as primary reports of pediatric HCT patients who underwent ECLS (11 were single case reports, 4 single institution case series, 2 multi-institution case series, and 7 registry reports from ELSO, PHIS and VPS).
Data Extraction:
All 24 articles were reviewed by 1st and last authors and a spread sheet was constructed including sample size, potential biases and conclusions.
Data Synthesis:
Discussions regarding incorporation of available evidence into our clinical practice were held at biannual meetings, as well as through email and virtual meetings. An expert consensus was determined through these discussions and confirmed through a modified Delphi process.
Conclusion:
ECLS in HCT patients is being used with increasing frequency and potentially improving survival. The PALISI HCT-CI subgroup has developed a framework to guide physicians in decision making surrounding ECLS candidacy in pediatric HCT patients. In addition to standard ECLS considerations, candidacy in the HCT population should consider the following 6 factors in order of cencensus agreement: 1) patient comorbidities; 2) underlying disease necessitating HCT; 3) HCT toxicities, 4) family and patient desires for goals of care; 5) HCT preparatory regimen; and 6) graft characteristics. Although risk assessment may be individualized, data are currently insufficient to clearly delineate ideal candidacy. Therefore, we urge the onco-critical care community to collaborate and capture data to provide better evidence to guide physicians’ decision making in the future.
Keywords: Extracorporeal life support, Hematopoietic stem cell transplant, Pediatric, Intensive Care
INTRODUCTION
HCT offers the potential for curative therapy for children with a variety of diseases and survival for those requiring intensive care during their course is improving over time (1–4). Although malignancy is the most common indication for HCT, currently over one third of pediatric allogeneic transplants are performed for non-malignant disorders (5). Results for these patients with non-malignant conditions vary, but over 80% of them will become long-term HCT survivors and the majority will enjoy a very good quality of life. (6)
Patients undergoing allogeneic or autologous transplantation are at risk for a number of complications that result in cardiac or respiratory failure (7) (Figure 1). Once deadly complications now have the potential for cure with agents such as defibrotide for veno-occlusive disease (VOD) (8), eculizumab for transplant associated-thrombotic microangiopathy (TA-TMA) (9, 10), new generation immunomodulators to control graft versus host disease (GVHD) and idiopathic pneumonia syndrome (IPS) (11), and topical agents such as inhaled tranexamic acid (TXA) and recombinant Factor VIIa to control pulmonary hemorrhage (12).
Figure 1: Critical Complications after Pediatric HCT.
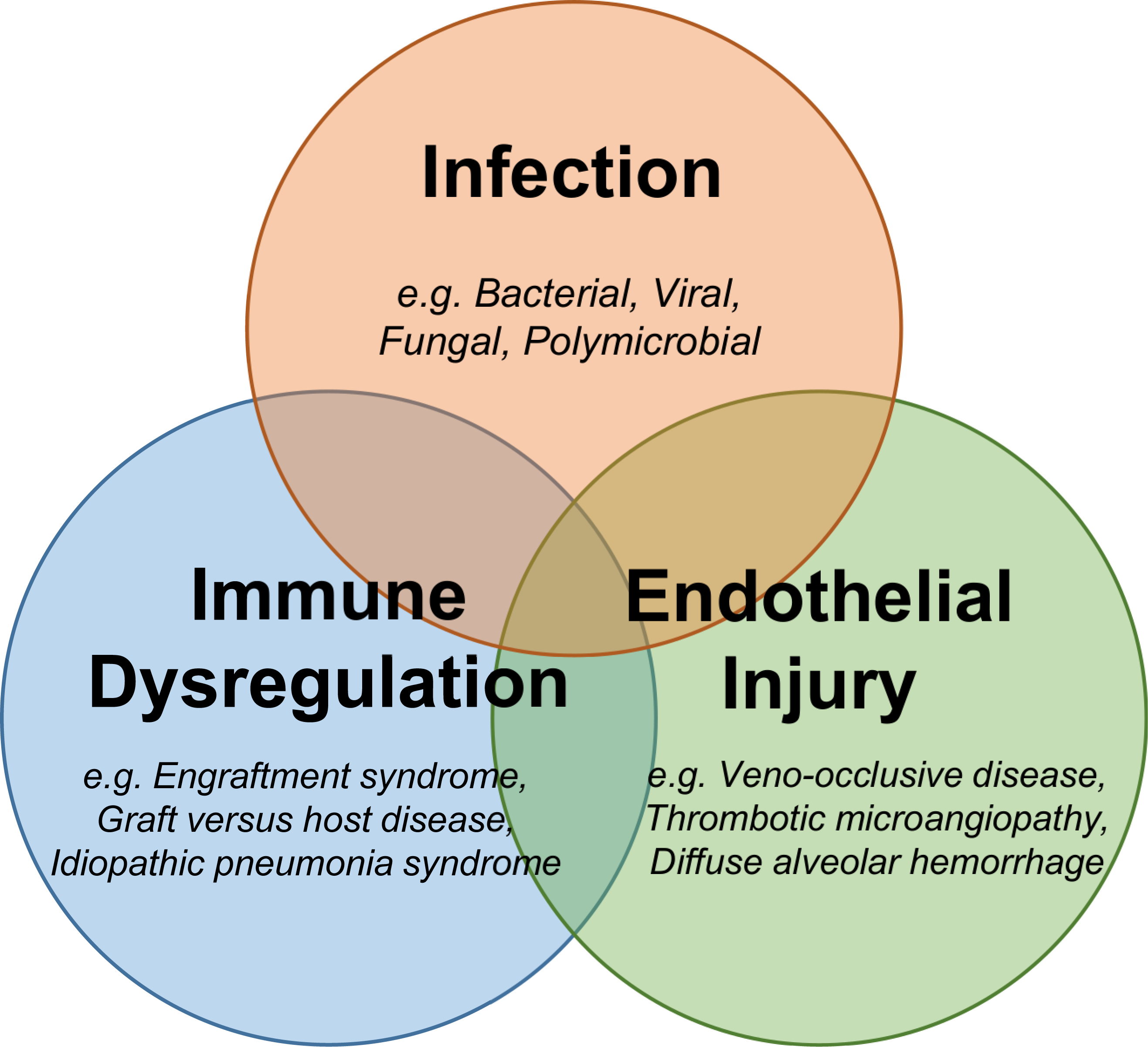
Pediatric HCT patients can experience cardiopulmonary and multiorgan failure from infection, immune dysregulation, endothelial injury, and other systemic insults. Many syndromes overlap to precipitate multiorgan failure.
ECLS rescue has been used in a limited number of HCT patients. With historically grim survival rates, ECLS rescue for HCT patients with cardiorespiratory failure was previously viewed as futile by many intensivists (13). However, more recent reports have published increasing numbers of survivors with ECLS rescue after HCT (14,15).
The HCT and Cancer Immunotherapy (CI) subgroup of the Pediatric Acute Lung Injury and Sepsis Investigators’ (PALISI) Network was established in 2005. We are a clinical research network composed primarily of pediatric critical care and pediatric hematopoietic cell transplant physicians with notable involvement from other fields such as pediatric hematology, nephrology, infectious disease, cardiology, pulmonology and transfusion medicine. The group has collaborated on over 20 publications focused on pediatric onco-critical care. The paucity of existing data thus encouraged the development of guidance to aid the physician at the bedside.
METHODS
Literature Search:
We searched PubMed for the terms (“extracorporeal membrane oxygenation” OR “extra-corporeal membrane oxygenation” OR “extracorporeal life support” OR “extra-corporeal life support”) AND (“pediatric” OR “paediatric” OR “child” or “infant” OR “neonate” OR “neonatal”) AND (“bone marrow transplant” OR “bone marrow transplants” OR “bone marrow transplantation” OR “stem cell transplant” OR “stem cell transplants” OR “stem cell transplantation” OR “hematopoietic cell transplant” OR “hematopoietic cell transplants” OR “hematopoietic cell transplantation” OR “cord blood transplant” OR “cord blood transplants” OR “cord blood transplantation” OR “BMT” or “HCT” or “HSCT” or “PBSCT” or “UCB”). All articles were reviewed and articles were included if they contained original reports of pediatric HCT patients who underwent ECLS. Reports of adult HCT patients undergoing ECLS were excluded due to significant differences in reason for HCT and outcomes between pediatric and adult HCT patients.
Adjudication:
After summarizing all available data, consensus statements were generated through group discussion and voted on by all active members of the PALISI HCT-CI subgroup. Specifically, patient characteristics important for consideration in decisions surrounding ECLS eligibility in HCT patients were voted upon through modified Delphi methodology. Votes were returned from 24 of 29 active members.
RESULTS
64 manuscripts met search criteria and 24 were included as primary reports of pediatric HCT patients who underwent ECLS. These 24 reports included 11 single case reports (16–26), 4 single institution case series (27–30), 2 multi-institution case series (31,32) and 7 registry reports from ELSO, PHIS, and VPS (13, 14, 33–37). As patients included in these case reports, series and registries are variably de-identified, we cannot exclude the possibility of patient overlap in these multiple manuscript formats.
Registry Reports:
In 2014, Di Nardo et al (35) reported n=29 pediatric HCT patients treated with ECLS between 1991–2012 within the Extracorporeal Life Support Organization (ELSO) registry. Approximately 80% had primary respiratory failure but 20% required ECLS for cardiac indications. Most patients had microbiologic evidence of infection prior to the initiation of ECLS. Six patients survived to decannulation (20%) and three survived to hospital discharge. The authors commented that compared with survivors, non-survivors began ECLS later in their course of illness with worse metrics of illness severity at initiation of ECLS. Oxygenation Index (OI) below 38 was most strongly associated with survival. Most recently, Olsen et al updated the ELSO registry with a report of n=90 patients treated from 1990–2019 (15). While overall survival to hospital discharge was achieved in n=17 patients (19%), it was notable that survival rates were 1/28 (4%) in the 1991–2009 epoch vs 16/62 (26%) in the 2010–2019 epoch. Other registry reports include a report of n=9 allogeneic pediatric HCT patients treated with ECLS between 2009–2014 in the Virtual Pediatric Systems (VPS) registry; survival to PICU discharge was achieved in only 2 of the 9 (21%) (37). Also, in 2020, Coleman et al reported n=31 pediatric HCT patients who received ECLS between 2004–2013 in the Pediatric Heath Information System (PHIS) registry (36). They found that HCT patients had a median ECMO duration of 13 days (IQR 1–31) and 6 of 31 patients survived to hospital discharge (19%). In 2020, Steppan et al reported a cohort of n=8 pediatric HCT patients treated with ECLS between 2011–2018 in the PedECOR registry (14). Using propensity score matching, the authors found that oncology and HCT ECMO patients had greater platelet transfusion requirement but similar overall bleeding complications relative to their matched non-oncology/HCT counterparts. Survival to hospital discharge was achieved in n=4 of the 8 patients. Taken together, these registry reports suggest an approximately 75% mortality rate for pediatric HCT patients requiring ECLS, although patients included in these reports were treated between 5–30 years ago. None of the registry reports provide sufficient granularity regarding transplant characteristics to aid physicians at the bedside in making ECLS candidacy decisions for a specific HCT patient with their own unique characteristics. In particular, use of VA vs. VV ECLS strategy varied widely according to center, patient type, ECLS indication, and comorbidities and thus recommendations about cannulation strategy cannot be made based on these data alone.
Case Series:
In an effort to obtain more detailed patient information, 6 single- and multi-institutional case series have been reviewed. Jensen et al reported a cohort of n=4 pediatric HCT patients treated with ECLS between 2000–2017 with 3 of the 4 surviving to hospital discharge (29). In 2021, Bridges et al and Potratz et al each reported an additional n=9 children, of whom n=3 and n=2 survived, respectively (32, 30). Di Nardo reviewed a single center experience with 10 children, 2 surviving to hospital discharge (27). Friedman (31), Maue (28), and Bridges (32) all combined oncology and HCT patients thus precluding HCT-specific conclusions. Overall these case series suggest that there may be more contemporaneous cohorts of pediatric HCT patients with improving ECLS survival, although we cannot exclude a selection and reporting bias. While these case series provided slightly more granularity regarding HCT characteristics, the numbers of patients reported were very small making generalizability difficult.
Case Reports:
Detailed reports of at least 11 pediatric HCT patients using ECLS are publicly available (16–26). ECLS was initiated in a variety of HCT patients including those with primary immunodeficiencies, malignancies, and hemoglobinopathies. The indication for ECLS also varied but reports document inciting conditions of bacterial sepsis, disseminated viremia, idiopathic pneumonia syndrome, diffuse alveolar hemorrhage, myocarditis, and others. Duration between HCT and ECLS cannulation range from days to months, highlighting the uniqueness of each case. Each report shares a common theme of early deployment of ECLS in candidates with potentially reversible etiologies, ideally allowing specific delineation of cardiac/hemodynamic and respiratory pathologies. Of the 11 unique patient reports, 10/11 survived to decannulation with 9/11 surviving to hospital discharge. As always, these case reports may reflect a selection bias towards more successful ECLS courses. Unfortunately, the existing data do not allow reliable determination of other factors predictive of ECLS outcome due to small numbers, a heterogeneous population, and the inability to easily link data between multiple specialized databases.
Criteria for Discussion:
After review of available publications and discussion amongst the group, 6 criteria important in the decision making process for ECLS candidacy were determined by the group and then voted upon using a modified Delphi process (38) (see Table 1). Twenty four of 29 members of the group in regular attendance of the subgroup meetings voted. These 6 criteria included 1) patient comorbidities; 2) underlying disease necessitating HCT; 3) HCT preparatory regimen; 4) HCT toxicities; 5) allograft characteristics; and 6) family and patient desires for goals of care with input from other care teams. Four of the 6 reached 100% consensus agreement. Considerations regarding allograft characteristics and conditioning regimen did not reach consensus. However, a majority of votes were in agreement with the statement with the remainder of the members voting neutral (see below).
Table 1.
Considerations when Discussing ECLS Candidacy in Pediatric HCT Patients
| Considerations when Discussing ECLS Candidacy in Pediatric HCT Patients |
|---|
|
Factor 1) Current Comorbidities • What current infections are present or suspected and how have they responded to therapy? What is their prognosis in light of forecasted immune reconstitution? • What end-organ toxicities exist (including significant neurological injury)? Can these be quantified/staged? What available therapies exist to address these toxicities and what is the ultimate prognosis? Is the cardiac and/or respiratory failure which requires ECLS potentially reversible? • What bleeding & hemostatic complications are present? Can these be corrected with transfusional/other support? Are there deep venous thromboses complicating cannulation? |
|
Factor 2) Underlying Disease and Prognosis • If the underlying disease is malignant, what is the chance of post-HCT relapse? (This is affected by disease status, remission, and other characteristics at the time of HCT) • If the underlying disease is non-malignant, what is the chance of cure due to HCT? • What disease specific toxicities were present prior to HCT? (i.e.: chronic transfusion related iron overload, chemotherapy-related toxicities, chronic infections, chronic cardiac dysfunction, etc) |
|
Factor 3) HCT-Specific Comorbidities • What is the current graft function? Has the patient achieved neutrophil/platelet engraftment? Have T-cells reconstituted? What is the donor chimerism? (These can inform the prognosis for immune reconstitution) • Does the patient have acute or chronic GVHD? What organs are involved and of what severity? Has the patient been responsive to therapy, and if not, what additional therapies are available? • Has the patient developed an endothelial injury syndrome such as VOD/SOS, TMA, or IPS? How has this responded to therapy? • Has the patient developed other HCT-specific comorbidities? |
|
Factor 4) Family and patient desires and expectations • What are the expectations regarding ongoing maximally intensive care in the setting of severe critical illness? • What are the cares desired vs. declined? • Has an advance directive been discussed with the patient? • Are those who provided longitudinal care and the psychosocial and palliative care teams involved? |
|
Factor 5) HCT Conditioning Regimen • What conditioning regimen was used and what toxicities were caused by it? (This is particularly relevant for TBI and myeloablative conditioning regimens) • How will the conditioning regimen affect the rate of immune reconstitution (Use of serotherapy such as ATG, alemtuzumab) |
|
Factor 6) HCT Graft Characteristics • Was the graft from the patient (autologous) or a donor (allogeneic)? • Was the graft HLA-matched? • Was the graft modified, depleted, or enriched in certain cell types that might affect the patient’s immune reconstitution? (The restoration of the patient’s immune system is key for short-term survival) |
DISCUSSION
In the absence of sufficient data to develop true guidelines, the HCT-CI subgroup of PALISI has developed a position statement regarding the determination of ECLS candidacy in HCT patients with cardiac and/or respiratory failure. The overarching theme of the statement is that patients should be considered in the context of their unique characteristics and not exclusively as “a HCT patient”, incorporating input from the multi-disciplinary spectrum of the care team.
The most crucial factor determining ECLS success for any patient is the potential reversibility of the underlying process(es) necessitating ECLS support. With advances in novel therapies, the list of HCT complications that can be considered absolutely irreversible is rapidly decreasing. We identified several specific issues in addition to therapy-related toxicities that are important to consider prior to ECLS after HCT. Descriptions of these considerations were distributed as a survey to the subgroup members and consensus responses were tabulated (Agree 9,8,7; Neutral 6,5,4; Disagree 3,2,1). Major criteria to be considered in evaluating disease reversibility are included in Figure 2. First, as with all pediatric ECLS candidates, current comorbidities are important to consider. Current infections, poor graft function and multi-organ dysfunction must be thoroughly investigated and staged. Special emphasis on the reversibility of infections in the HCT recipient is warranted and discussion with numerous specialists regarding the utility of novel antimicrobial and immunomodulatory therapies is mandatory. The severity of renal, pulmonary, hepatic, and cardiac injury must be considered with special focus on bleeding and hemostasis (consensus agreement 100%, median 9, IQR 9–9, n=24). Second, the patient’s underlying disease must be considered, as HCT for malignant and non-malignant disorders can present different challenges. For example, patients with inherited non-malignant disorders may come to HCT with significant comorbidities. However, if HCT is successfully navigated, these patients are treated without the concern of disease relapse omnipresent in the malignant setting (consensus agreement 100%, median 9, IQR 8.5–9, n=24). Third, the presence of any HCT-specific toxicities or complications needs to be evaluated. As discussed above, these may include GVHD, immune reconstitution syndromes, endothelial injury syndromes, non-infectious pneumonia syndromes, and other HCT-specific complications (consensus agreement 100%, median 9, IQR 8.5–9, n=24). No specific toxicities are uniformly absolute contraindications for ECLS candidacy, but rather the potential reversibility of each complication as best predicted by a multidisciplinary team remains the key variable. Fourth, family and patient (if applicable) desires and expectations regarding providing ongoing maximally intensive care in the setting of severe critical illness must be assessed. Input from those who have provided longitudinal care for the patient and the psychosocial and palliative care teams is essential to ensure families are appropriately informed and included in the decision making process (consensus agreement 100%, median 9, IQR 9–9, n=24). The remaining considerations were less widely endorsed but felt by the majority to still be important to the decision to offer ECLS. Fifth, for allogeneic HCT recipients, allograft characteristics should be considered, as they strongly impact the rate of immune reconstitution as well as the risk for GVHD or malignancy relapse. These characteristics include donor source, cell dose, and HLA match (consensus agreement 75%, median 7.5, IQR 6.5–7.5, n=24). Sixth, the patient’s preparative conditioning regimen should be considered, particularly in the acute post-HCT period. Myriad conditioning regimens are used and individualized to provide the optimal degree of myeloablation while minimizing organ toxicity and supporting graft reconstitution. Some regimens are associated with acute risks of cardiopulmonary toxicity, particularly fully ablative regimens incorporating total body irradiation (TBI). Others involving depletion of patient/donor lymphocytes (with anti-thymocyte globulin for example) can cause delayed immune reconstitution (consensus agreement 63%, median 7.5, IQR 5–7.5, n=24).
Figure 2: Dynamic Considerations of Pediatric HCT Patients for Trial of ECLS.

A proposed framework for considering HCT patients with cardiopulmonary failure for a trial of ECLS.
We emphasize the need for serial evaluation by a multidisciplinary team of intensivists, transplant physicians, surgeons, perfusionists, other consultants and the bedside care team as the cornerstone of a systematic approach to patient selection and timing of ECLS. This multi-disciplinary approach is also important to ensure prospective discussions of limitations of support occur if no improvement is noted after a defined period of time.
These recommendations are based on review of the pediatric literature and may not be congruent with recommendations and evidence put forth in adult HCT recipients. For example, Wolfharth et al noted better ECLS outcome among adult patients further from HCT (40). Data to support this observation in pediatrics do not currently exist, and thus were not included in the statements above. In addition, we note that transplant-related mortality in children is significantly lower than for adults and so the generalizability of adult HCT ECLS experience to children remains unclear.
Future Directions
As occurred in the earliest days of ECLS use in the sickest infants (41), in-depth and longitudinal multidisciplinary input and robust data collection will optimize decision making for individual pediatric HCT patients and ultimately allow for better prognostication in the future.
Establishing criteria for ECLS in pediatric HCT patients is particularly challenging given the heterogeneity of the group with respect to pre-HCT disease, accumulated toxicity during HCT, and potential for ongoing HCT-related complications. While no robust predictors of outcomes for this population exist, two crucial considerations include identifying a potentially reversible process and initiating ECLS before irreversible organ damage occurs. Current data are too limited to determine if ECLS use in the early post-HCT period is more or less morbid than use of ECLS rescue for later onset HCT complications. Because these patients are rare and the ability to link current registries internationally is limited, additional detailed data collection is necessary to improve our knowledge of factors associated with ECLS survival in pediatric HCT patients. Centers providing ECLS should consider reporting patient data to a database that collects HCT-specific information such as the Pediatric ECMO outcomes Registry (PEDECOR). Transplant specific factors such as donor source, degree of HLA match, conditioning regimen and transplant related complications such as TA-TMA, VOD and GVHD are potentially relevant factors that should be collected in future studies. Transplant, patient, and ECLS specific factors as well as short and long term outcomes should be collected internationally in order to guide patient care, resource utilization and future research initiatives.
Acknowledgments
Copyright Form Disclosure: Drs. Zinter and Rowan received support for article research from the National Institutes of Health. Dr. Zinter received support for article resaerch from the National Heart, Lung, and Blood Institute (NHLBI) (K23HL146936). Dr. Dalton received funding from Innovative ECMO Concepts and Entegrion; she disclosed the off-label product use of ECMO equiptment. Dr. Rowan’s institution received funding from the NHLBI (K23HL150244. Dr. Steiner received funding from DSMB for the PumpKIN trial. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Brissot E, Rialland F, Cahu X, et al. : Improvement of overall survival after allogeneic hematopoietic stem cell transplantation for children and adolescents: a three-decade experience of a single institution. Bone Marrow Transplant 2015; 51:267–272 [DOI] [PubMed] [Google Scholar]
- 2.Chima RS, Daniels RC, Kim MO, et al. : Improved outcomes for stem cell transplant recipients requiring pediatric intensive care. Pediatr Crit Care Med 2012;13:336–42 [DOI] [PubMed] [Google Scholar]
- 3.Duncan C, Lehmann L, Cheifetz I, et al. : Clinical outcomes of children receiving intensive cardiopulmonary support during hematopoietic stem cell transplant. Pediatr Crit Care Med 2013;14:261–267 [DOI] [PubMed] [Google Scholar]
- 4.Elbahlawan L, Srinivasan A, Morrison RR. A critical care and transplantation-based approach to acute respiratory failure after hematopoietic stem cell transplantation in children. Biol Blood Marrow Transplant 2016; 22:617–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Souza A, Lee S, Zhu X, et al. : Current use and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant 2017; 23(9):1417–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrera M, Atenafu E. Cognitive Educational, psychosocial adjustment and quality of life of children who survive hematopoietic SCT and their siblings. Bone Marrow Transplant 2008; 42(1):15–21 [DOI] [PubMed] [Google Scholar]
- 7.Holmquist A, Chen Y, Wu J, et al. : Late mortality after autologous blood or marrow transplantation in childhood: a blood or marrow transplant survivor study-2 report. Blood Journal 2018; 131(24):2720–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahadeo K, Bajwa R, Abdel-Azim H, et al. : Diagnosis, grading, and treatment recommendations for children, adolescents, and young adults with sinusoidal obstructive syndrome: an international expert position statement. Lancet Haematology 2020; 7(1):61–72 [DOI] [PubMed] [Google Scholar]
- 9.Jodele S, Dandoy CE, Lane A et al. : Complement blockade for TA-TMA: lessons learned from a large pediatric cohort treated with eculizumab. Blood. 2020; 135(13): 1049–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dandoy CE, Rotz S, Alonso PB, et al. : A pragmatic multi-institutional approach to understanding transplant-associated thrombotic microangiopathy after stem cell transplant. Blood Advances 2021; 5(1): 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamburro RF, Cooke KR, Davies SM, et al. : Pulmonary complications of pediatric hematopoietic cell transplantation: A National Institutes of Health Workshop Summary. Ann American Thorac Society 2021; 18: 381–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan K, McArthur J, Morrison RR, Ghafoor S. Diffuse alveolar hemorrhage after pediatric hematopoietic stem cell transplantation. Frontiers in Oncology 2020; 10:1757–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta M, Shanley T, Moler F. Extracorporeal life support for severe respiratory failure in children with immunecompromised conditions. Pediatric Crit Care Med 2008; 9(4):380–385 [DOI] [PubMed] [Google Scholar]
- 14.Steppan DA, Coleman RD, Viamonte HK, et al. : Outcomes of pediatric patients with oncologic disease or following hematopoietic stem cell transplant supported on extracorporeal membrane oxygenation: The PEDECOR experience. Pediatric Blood and Cancer 2020; 67:e28403. [DOI] [PubMed] [Google Scholar]
- 15.Olson TL, O’Neil ER, Kurtz KJ, MacLaren G, Anders MM. Improving outcomes for children requiring extracorporeal membrane oxygenation therapy following hematopoietic stem cell transplantation. Crit Care Med, 2021; 1:49(4):e381–e393 [DOI] [PubMed] [Google Scholar]
- 16.Leahey AM, Bunin NJ, Schears GJ, et al. : Successful use of extracorporeal membrane oxygenation (ECMO) during BMT for SCID. Bone Marrow Transplant 1998; 21(8):839–840 [DOI] [PubMed] [Google Scholar]
- 17.Wolfson RK, Kahana MD, Nachman JB, et al. : Extracorporeal membrane oxygenation after stem cell transplant: clinical decision-making in the absence of evidence. Pediatr Crit Care Med 2005; 6(2):200–203 [DOI] [PubMed] [Google Scholar]
- 18.Morris S, Haight A, Kamat P, et al. : Successful use of extracorporeal life support in a hematopoietic stem cell transplant patient with diffuse alveolar hemorrhage. Pediatr Crit Care Med 2010; 11(1):e4–e7 [DOI] [PubMed] [Google Scholar]
- 19.DiNardo M, LiPira G, Amodeo A, et al. : Adoptive immunotherapy with antigen-specific T-cells during extracorporeal membrane oxygenation (ECMO) for adenovirus-related respiratory failure in a child given haploidentical stem cell transplantation. Pediatr Blood Cancer 2014; 61:376–379 [DOI] [PubMed] [Google Scholar]
- 20.Meserve EE, Lehmann LE, Perez-Atayde AR, Labelle JL. Cyclophosphamide-associated cardiotoxicity in a child after stem cell transplantation for B-thalassemia major: case report and review of the literature. Pediatr Dev Pathol 2014;17(1):50–4 [DOI] [PubMed] [Google Scholar]
- 21.Di Nardo M, Locatelli F, Di Florio F, Cecchetti C, Amodeo A, Rutella S, Bertaina A. Extracorporeal membrane oxygenation as a bridge to allogeneic T-cell depleted hematopoietic stem cell transplant in infants with severe combined immunodeficiency: is it feasible? Intensive Care Medicine 2014; 40(10):1600–1 [DOI] [PubMed] [Google Scholar]
- 22.Anton-Martin P, Darnell-Bowens C, Aquino VM, Jones T. Raman L. Successful engraftment after hematopoietic stem cell transplant with infusion of donor stem cells through the extracorporeal membrane oxygenation circuit. Indian J Crit Care Med 2016; 20(10):617–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zinter MS, Barrows BD, Ursell PC, et al. : Extracorporeal life support survival in a pediatric hematopoietic cellular transplant recipient with presumed GvHD-related fulminant myocarditis. Bone Marrow Transplant 2017; 52:1330–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams FZ, Vats A, Cash T, Fortenberry JD. Successful use of extracorporeal life support in a hematopoietic cell transplant patient with neuroblastoma. J Extra Corp Technol 2018;50:60–61 [PMC free article] [PubMed] [Google Scholar]
- 25.Potratz J, Ahlmann M, Rossig C, et al. : Successful extracorporeal life support in a pediatric hematopoietic stem cell transplant recipient with peri engraftment respiratory failure. J Pediatr Hematol Oncol 2018; 40(4):e256–e259 [DOI] [PubMed] [Google Scholar]
- 26.Fan K, Hurley C, McNeil MJ, Agulnik A, Qudeimet A, Saini A, McArthur J, Morrison RR, Sandhu H, Shah S, Ghafoor S. Case report: management approach and use of extracorporeal membrane oxygenation for diffuse alveolar hemorrhage after pediatric hematopoietic cell transplantation. Front Pediatr 2021; Jan 13;8:587601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiNardo M, Merli P, Ceccheet C, Pasotti E, Bertaina A Locatelli F. Progressive increase in D-dimer levels during extracorporeal membrane oxygenation can predict membrane oxygenator failure in children given hematopoietic stem cell transplantation. J Crit Care 2016; 31(1): 262–263 [DOI] [PubMed] [Google Scholar]
- 28.Maue DK, Hobson MJ, Friedman ML, et al. : Outcomes of pediatric oncology and hematopoietic cell transplant patients receiving extracorporeal membrane oxygenation. Perfusion 2019; 34(7):598–604 [DOI] [PubMed] [Google Scholar]
- 29.Jensen ML, Nielson JS, Nielson J et al. : Declining mortality rates in children admitted to ICU following HCT. Pediatric Transplantation 2020; Dec 12; e13946. [DOI] [PubMed] [Google Scholar]
- 30.Potratz JC, Guddorf S, Ahlmann M, et al. : Extracorporeal membrane oxygenation in children with cancer of hematopoietic cell transplantation: Single-center experience in 20 consecutive patients. Frontiers in Pediatrics. 2021; 11:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedman ML, Barbaro RP, Bembea MM, et al. : Mechanical ventilation in children on venovenous ECMO. Respiratory Care. 2020; 65(3): 271–280 [DOI] [PubMed] [Google Scholar]
- 32.Bridges BC, Kilbaugh TJ, Barbaro RP, et al. : Veno-venous extracorporeal membrane oxygenation for children with cancer or hematopoietic cell transplant. ASAIO Journal 2021; ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gow KW, Wulkan ML, Heiss KF, Haight AE, Heard ML, Rycus P, Fortenberry JD. Extracorporeal membrane oxygenation for support of children after hematopoietic stem cell transplantation: the Extracorporeal Life Support Organization experience. Journal of Ped Surgery. 2006; 41:662–667 [DOI] [PubMed] [Google Scholar]
- 34.Zabrocki L, Brogan T, Statler K, et al. : Extracorporeal membrane oxygenation for pediatric respiratory failure: survival and predictors of mortality. Crit Care Med 2011; 39(2):364–370 [DOI] [PubMed] [Google Scholar]
- 35.DiNardo M, Locatelli F, Palmer K, et al. : Extracorporeal membrane oxygenation in pediatric recipients of hematopoietic stem cell transplantation: an updated analysis of the Extracorporeal Life Support Organization experience. Intensive Care Med 2014; 40(5):754–6 [DOI] [PubMed] [Google Scholar]
- 36.Coleman RD, Goldman J, Moffett B, Guffey D, Loftis L, Thomas J, Shekerdemian LS. Extracorporeal membrane oxygenation mortality in high-risk populations: An analysis of the Pediatric Health Information System database. ASAIO Journal 2020; 66(3):327–331 [DOI] [PubMed] [Google Scholar]
- 37.Zinter MS, Logan BR, Fretham C, et al. : Comprehensive prognostication in critically ill hematopoietic cell transplant patients: results from merging the Center for International Blood and Marrow Transplant Research (CIBMTR) and Virtual Pediatric Systems (VPS) registries. Biology of Blood and Marrow Transplantation 2020; 26:333–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khodyakov D, Grant S, Denger B, Kinnett K, Martin A, Peay H, Coulter I. Practical considerations in using online modified-Delphi approaches to engage patients and other stakeholders in clinical practice guideline development. The Patient-Patient-Centered Outcomes research. 2020; 13:11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panoskaltsis-Mortari A, Griese M, Madtes D, et al. : An official American Thoracic Society Research Statement: non-infectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Resp Critical Care Medicine 2011; 183(9):1262–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolfharth P, Beutel G, Lebiedz P, et al. Characteristics and Outcome of Patients After Allogeneic Hematopoietic Stem Cell Transplantation Treated With Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome. Crit Care Med 2017; 45(5):e500–507. [DOI] [PubMed] [Google Scholar]
- 41.Lantos J, Frader J. Extracorporeal membrane oxygenation and the ethics of clinical research in pediatrics. N Engl J Med 1990; 323(6):409–413 [DOI] [PubMed] [Google Scholar]


