Abstract
The postrhinal cortex (POR) serves as a key input area to the hippocampal system. It receives highly processed information from the ventral visual stream and other limbic areas including the retrosplenial cortex, parahippocampal areas, and portions of the limbic thalamus. The POR was studied early on by David Bucci and colleagues who first postulated that the POR plays a major role in contextual learning. Here we review a number of approaches and experimental studies that have explored POR’s role in contextual processing. We discuss POR lesion studies that monitored deficits in fear conditioning tasks and the effects that these lesions had on processing visual landmark information. We then review the types of spatial correlates encoded by POR cells. A large number of head direction (HD) cells are present, although recent findings suggest that they are more accurately characterized as landmark modulated-HD cells as opposed to classic HD cells. A significant number of POR cells are also tuned to egocentric properties of the environment, such as the spatial relationship of the animal to the center of its environment, or the distance between the animal and either the environment’s center or its boundaries. We suggest potential frameworks through which these functional cell types might support contextual processing. We then discuss deficits seen in humans who have damage to the homologous parahippocampal cortex, and we finish by reviewing functional imaging studies that found activation of this area while human subjects performed various tasks. A preponderance of evidence suggests that the POR, along with its interactions with retrosplenial cortex, plays a key role in contextual information processing.
Introduction
This article is a tribute to David Bucci and his passion for understanding the functional roles that various brain areas play in animal behavior, particularly as they relate to attention and mechanisms of learning and memory. A major focus of Dave’s scientific career during his post-doc years was understanding the functional role of the postrhinal cortex (POR) in behavior. Here we review Dave’s initial work on the POR and continue by describing our recent efforts toward understanding its functional role in spatial and contextual processing. We also review the human neuropsychological and functional imaging literature on the functional role of the POR and the homologous parahippocampal cortex (PHC) in humans. Our take-home message is that Dave and colleagues initially suggested that the POR serves to support contextual learning – the encoding of environmental features that accompany the occurrence of external events – and this view continues to be supported by experiments conducted across species and experimental paradigms. Moreover, our recent experiments have uncovered some of the functional properties of POR neurons that may support the neural manifestation of this process.
POR and the Learning of Environmental Context
Dave’s two main studies in the early 2000s explored the effects of contextual fear conditioning in rats with POR lesions. In his first study (Bucci et al., 2000), Dave showed that lesions of the POR or perirhinal cortex, either before or after acquisition, impaired performance in a contextual fear conditioning task. In a follow-up study (Bucci et al., 2002), he trained rats in different contexts that depended on discriminating between different configurations of cues. He reported that animals with POR lesions had difficulty distinguishing the different configural contexts and concluded that the POR plays a role in learning about the configural and contextual elements of the environment. Consistent with this view, Norman & Eacott (2005) tested rats in a spontaneous object recognition paradigm. Rats were exposed to familiar objects in either the same place or in a different location within the same environmental context. The authors reported that control rats, but not rats with POR lesions, explored the familiar object more when it was placed in a novel location than when the object was placed in the same location, indicating that the POR lesioned rats could not distinguish the two conditions and were therefore impaired at discriminating different contexts. Together, these studies were the first to demonstrate the important role the postrhinal cortex plays in processing information about the contextual elements of the environment. More recent work using immediate early gene expression (ARC) have found results that are consistent with this view. Sethumadhavan et al. (2020) reported ARC expression in the POR following an item place learning task that involved processing large, easily visible, global items in the environment (macro-scale), such as surrounding landmarks, but not when processing small, nearby items that were partially hidden from view (micro-scale). Working in his own lab, Dave’s later studies have also shown how the retrosplenial cortex, which has strong connectivity with the POR, functionally interacts with the POR during contextual fear conditioning (Robinson et al., 2012).
While many labs have pointed to POR’s role in contributing to environmental context, other labs have noted POR’s role in processing spatial information. Some of the performance deficits described above following POR lesions, which were attributed to deficits in understanding the environmental context, occurred in tasks considered spatial by nature. For example, the object recognition task conducted by Norman & Eacott (2005) can be considered a spatial task because the rat has to learn and keep track of where particular objects are in the environment. Unfortunately, many of the studies that have examined the effects of POR lesions on spatial tasks used lesions that combined both the POR and the perirhinal cortex (e.g., rodents: Bussey et al., 1999, 2000; Winters et al., 2004; monkeys: Malkova & Mishkin, 2003), thus making it difficult to determine whether the effects reported were due to POR or perirhinal cortex lesions. Two studies that confined the lesions to the POR both reported performance deficits on the reference memory version of the radial arm maze task (Liu & Bilkey, 2002; Ramos, 2013), and one of the studies also found deficits on the water maze task (Liu & Bilkey, 2002; but see Burwell et al., 2004). Despite these reports on spatial tasks, most researchers currently consider POR’s functional role to be more closely associated with aspects of environmental context than processing spatial information per se. Nonetheless, many POR neurons contain strong spatial correlates to both egocentric and allocentric reference frames (LaChance et al., 2019, 2022; Gofman et al., 2019), which we will return to below. However, when considering the functional role of these POR cells, we believe they may be encoding the spatial aspects of the subject’s environmental context from both an egocentric and allocentric point of view.
POR Anatomy
In rodents, the postrhinal cortex is situated on the lateral surface of the posterior cortex and wraps around its caudal pole (Burwell, 2001; Sugar et al., 2011). In a sagittal view, the POR is bounded dorsally by the temporal association area (TEA) and ventrally by the lateral entorhinal area at is anterior extent and the medial entorhinal area at its posterior extent. Anterior to the POR lies the perirhinal cortex and medially lies the subiculum. In coronal sections, it appears adjacent to the medial and lateral entorhinal areas ventrally and is bounded dorsally by TEA. Anatomically, the rodent POR is positioned upstream in the flow of information from parahippocampal areas to the hippocampus. Thus, it receives projections from polymodal association areas including the retrosplenial, posterior parietal, and visual association cortex and sends efferents most prominently to the perirhinal cortex (area 36), and visual and visuospatial regions; it also sends projections to a lesser extent to widespread cortical areas (Agster & Burwell, 2009; Burwell & Amaral, 1998; Furtak et al., 2007). Consistent with its association to processing visual information, there is also a dense projection from the lateral posterior thalamic nucleus to the POR (Furtak et al., 2007). Other major thalamic inputs include the anterior thalamus and the nucleus reuniens (Pereira et al., 2016).
The POR is usually considered to be the homolog of the primate PHC (Burwell et al., 1995). One functionally defined brain region that shows overlap with the PHC in humans is the parahippocampal place area (PPA), which is activated in imaging studies when subjects view images of scenes that contain visual landmarks (Epstein & Kanwisher, 1998). A similar area has also been identified in macaques (Kornblith et al. 2013). It is important to consider, however, that the same homology that the POR shares with the PHC cannot be fully extended to the PPA, which is more defined functionally than anatomically. The PPA appears to include the most caudal portion of the PHC, but extends even more caudally beyond the caudal PHC border (Weiner et al., 2018). It is tempting to relate the location of the PPA to the anatomical organization of the POR; for example, POR afferents show rostrocaudal topography such that inputs from canonically spatial areas (e.g., dorsal presubiculum and CA1; Agster and Burwell, 2013) and visual areas (e.g., lateral posterior thalamic nucleus; Pereira et al., 2016) preferentially target caudal instead of rostral POR. Outputs from POR to the medial entorhinal cortex (MEC) are also topographically organized (Koganezawa et al., 2015) such that caudal regions of POR project ventrally to caudal regions of MEC (where spatially modulated/grid cells are most abundant; Hafting et al., 2005). In addition, recordings done in our lab, which reveal spatial firing correlates among POR neurons (LaChance et al., 2019; LaChance et al., 2022), have been restricted to the most caudal region of POR. However, even if a rostrocaudal gradient of spatial coding exists in POR (which has yet to be demonstrated), the question of whether rodents possess a functionally defined homolog of the PPA, unrestricted by the anatomical borders of the POR, remains unanswered. Thus, while we discuss findings related to the PPA later in this review, these findings should not be related directly to the POR, but rather indirectly through its homology with the PHC.
Early POR Recording Studies
The first recording studies by Burwell and Hafeman (2003) showed some interesting findings compared to place cell recordings in the hippocampus. While location-specific firing was identified in the majority of POR neurons in a four-arm radial maze task, the vast majority of cells contained split or multiple subfields. Further, when the prominent local and distal cues were rotated in opposite directions, most neurons developed new place fields that did not correspond to either of the rotated cues, which suggests that the cells remapped their environment. The authors suggested that the POR “participates in visuospatial functions by monitoring changes in environmental stimuli rather than encoding stable spatial cues.” A second study that recorded POR neurons in rats performing a two-choice visual discrimination task found that many cells appeared to link specific objects with particular places while other cells discharged in relation to the rat’s egocentric motor responses when making a behavioral choice (i.e., turn left or right) (Furtak et al., 2012). Taken together with the behavioral lesions studies described above, the POR appears to play a strong role in representing the context of the surrounding environment. We will return to this issue below when we describe the responses from other types of spatial cells in the POR.
POR is not involved in landmark control over canonical HD cells
One connection of the POR is with the postsubiculum (PoS; dorsal presubiculum). This connection is reciprocal, although the efferent projection from POR to the postsubiculum is weaker than the afferent projection from the postsubiculum (Furtak et al., 2007). Yoder et al. (2011) suggested that the postsubiculum plays a key role in the direct transfer of visual landmark information to spatial signals within the limbic system. Previous studies have shown that lesions of the postsubiculum lead to a deficit in landmark control in both place cells and head direction cells. Thus, when a salient visual landmark cue is rotated, neither place fields of place cells or the preferred firing directions of HD cells rotate an equivalent amount as the cue in PoS lesioned animals (Goodridge & Taube, 1997; Calton et al., 2003; Yoder et al., 2015), as they do in control animals (Muller & Kubie, 1987; Taube et al., 1990b). This landmark control of cell firing is thought to be mediated in the rodent by direct projections from visual areas 17 and/or 18 to the PoS (Vogt & Miller, 1983). Alternatively, because of the connectivity between PoS and POR, and POR’s involvement in the ventral visual stream pathway to the hippocampus and its probable role in processing environmental context, it is possible that landmark cue control could be mediated by the connections with POR rather than areas 17/18. To address this possibility, we lesioned the POR and recorded HD cells in the anterodorsal thalamus (ADN) (Peck & Taube, 2017). We found that POR lesions neither disrupted HD cell firing in ADN, nor did they disrupt cue control following rotation of the salient visual landmark. Further, despite the intact landmark control displayed in these lesioned animals, these same animals displayed the usual deficits associated with POR lesions in a fear conditioning task (similar to the ones described above by Bucci et al.). Thus, HD cells from POR lesioned animals, with demonstrated impairments in contextual fear conditioning, were able to use a visual landmark to control their preferred directions. Thus, despite its location at the tail end of the ventral visual processing stream and its importance in processing visual landmark information in primates, the POR, at least in rats, does not appear to play a pivotal role in controlling visual landmark information in the canonical HD system.
Recent Recordings from POR Neurons
Our recent work recording from POR neurons reveals that there are a variety of different spatial correlates, both egocentric and allocentric, that respond to landmark cues in a very different way than has been shown previously for hippocampal place cells and for head direction cells throughout the limbic system. In particular, in addition to identifying HD cells in POR, our 2019 study (LaChance et al., 2019) revealed that approximately 39% of POR neurons showed tuning to the egocentric bearing of the geometric centroid or boundaries of the animal’s local environment (a square enclosure), with egocentric bearing defined as the angle between the animal’s head direction and an external reference point (in this case, the environment centroid; Fig. 1A, B). We called these cells ‘center-bearing’ cells, although neurons with similar tuning properties in POR have been referred to as ‘bearingboundary’ or ‘egocentric boundary cells’ (Gofman et al., 2019). Center-bearing cells have also been identified in several brain regions connected with the POR, including the lateral entorhinal cortex (Wang et al., 2018), dorsal striatum (Hinman et al., 2019), retrosplenial cortex (Alexander et al., 2020), and parietal cortex (Wilber et al., 2014). In addition, we found that approximately 17% of POR cells encoded the egocentric distance of the centroid (i.e., the distance between the rat and the environment’s center, Fig. 1C), revealing an egocentric vector representation of local space. These ‘center-distance’ cells exhibited an approximately linear change in firing rate with distance from the environment centroid, with both positive (cells that increased their firing with increasing distance from the centroid) and negative (cells that decreased their firing with increasing distance from the centroid) tuning slopes present in similar proportions. Based on testing these cells in different sized environments, center-distance cells did not appear to scale their tuning slopes to fit enclosures with different sizes, suggesting that their firing reflects absolute instead of relative distances.
Figure 1 –
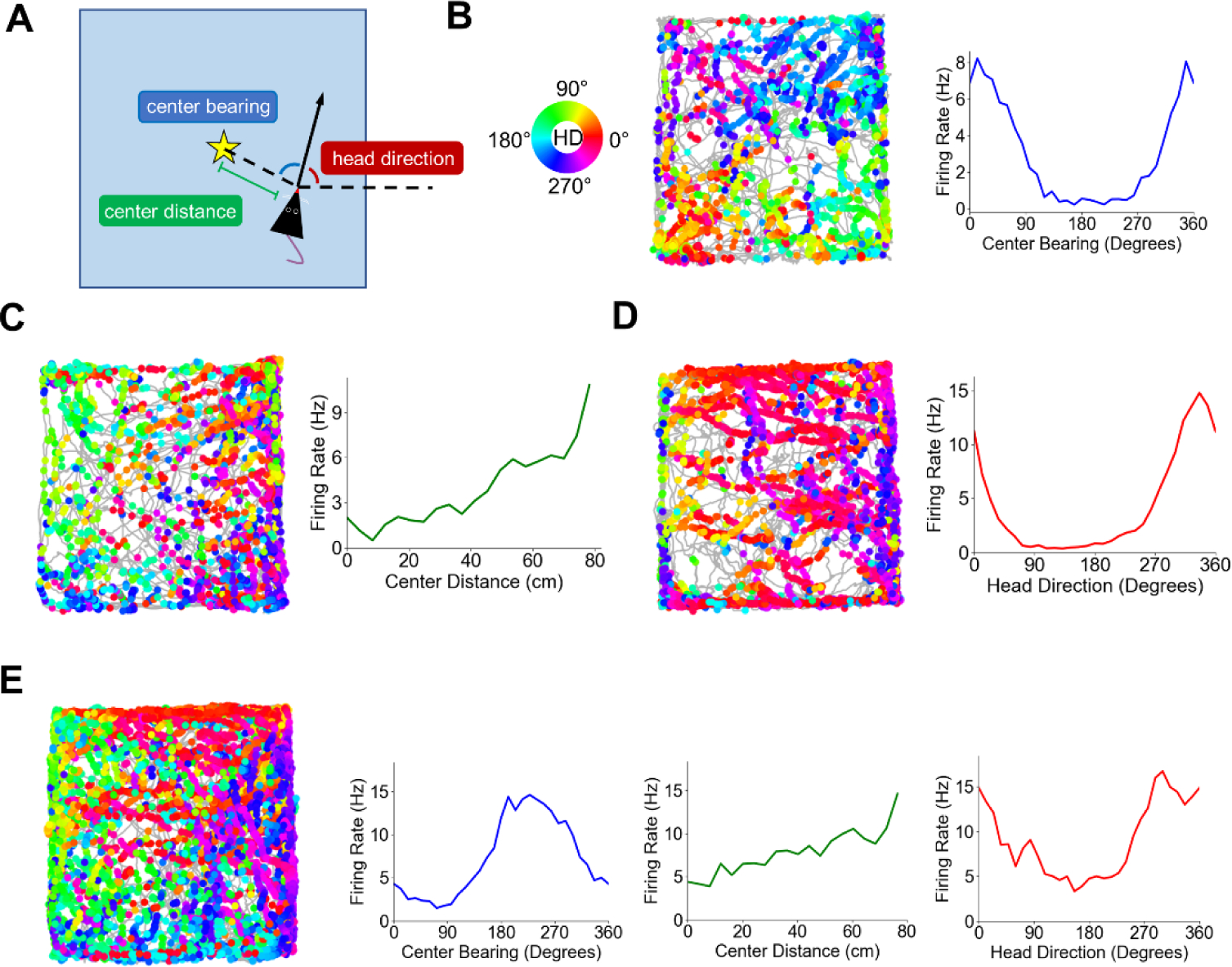
Egocentric and allocentric spatial correlates in POR. A) Schematic top-down view of the recording arena defining egocentric and allocentric spatial variables encoded by POR cells. Star represents the geometric centroid of the environment. B) Directional spike plot and center-bearing tuning curve for an example POR center-bearing cell. The gray trace represents the path of the animal during the recording session, while the dots represent locations where the cell fired a spike. Dots are colored according to the animal’s allocentric head direction when the spike was fired (color wheel on left). C) Directional spike plot and center-distance tuning curve for an example POR center-distance cell. Note the approximately linear increase in firing rate with distance from the centroid. D) Directional spike plot and head direction tuning curve for an example POR HD cell. E) Directional spike plot and tuning curves for a POR cell that showed conjunctive tuning to multiple variables. Figure modified from LaChance et al., 2019.
An egocentric vector-based map of local space may be useful for guiding spatial behavior – particularly for elements within the subject’s ‘personal’ space that are readily available to interact with, but it is inherently different from the allocentric spatial maps exemplified by downstream place and grid cells in the hippocampus (O’Keefe and Nadel, 1978) and medial entorhinal cortex (MEC; Hafting et al., 2005), respectively. How might egocentric firing correlates in POR interact with downstream allocentric representations? O’Keefe’s centroid hypothesis (1991) proposed that an egocentric vector representation of the centroid of local cues could be combined with an allocentric HD signal to anchor it to an allocentric reference frame and create an allocentric map of space. Indeed, allocentric HD cells have been found in POR (Kornienko et al., 2018; LaChance et al., 2019; Gofman et al., 2020; Fig. 1D), with our study finding that ~38% of POR cells exhibited HD tuning. Much like center-bearing and center-distance cells, the firing of POR HD cells was tied to the layout of local cues, such that a 45° rotation of the local environment (a square enclosure with an orienting visual cue along one wall) resulted in a 45° rotation of each HD cell’s preferred firing direction (PFD). In addition, over half of the POR HD cells were conjunctively tuned to center-bearing, and 20% were conjunctively tuned to center-distance, suggesting that integration of these egocentric and allocentric variables is present at the single-cell level (Fig. 1E). We also demonstrated through pseudo-population decoding that firing properties of center-bearing, center-distance, and HD cells in POR could be used to accurately determine an animal’s position (location and orientation) within the local environment (LaChance et al., 2019), which is consistent with the centroid hypothesis (O’Keefe, 1991). Thus, it appears that the egocentric and allocentric firing properties of POR cells may constitute a template for mapping local allocentric space that could provide a foundation for higher-level spatial maps in the downstream hippocampus and MEC.
More recently, we have focused on the influence of local visual landmarks on the firing of POR HD cells (LaChance et al., 2022), compelled by the discovery of HD cells in POR (Kornienko et al., 2018; LaChance et al., 2019; Gofman et al., 2019) and Dave Bucci’s finding that POR lesions impaired attentional orienting toward a visual cue (Bucci & Burwell, 2004). After animals were habituated to a gray square enclosure with a large white cue card (cue A) placed along one wall, we found that the population of POR HD cells showed a bias in the distribution of their PFDs, with an increase in the number of PFDs where the animal’s head pointed approximately toward or away from the visual cue. Analyzing the widths of individual tuning curves suggested that, while cells with PFDs oriented toward the visual cue showed a sharp peak in firing at that HD (‘peak-locked cells’), cells with PFDs oriented away from the visual cue actually tended to show a sharp firing trough when the animal faced toward the visual cue (‘trough-locked’ or ‘anti-HD’ cells). Critically, when a second identical cue card (cue B) was placed along the wall 180° opposite the first cue (AB condition), the HD cells tended to become bidirectionally tuned, with peak-locked cells adding a second tuning peak 180° opposite the first peak, and trough-locked cells adding a second trough 180° opposite the first trough (Fig. 2A, B). The new peak or trough was generally smaller in amplitude than the first, suggesting that, despite their identical appearances, POR cells weighted the visual cues according to their stability or familiarity. In contrast, ADN HD cells failed to show bidirectional tuning under these conditions, highlighting that POR HD cells are more visually modulated and their firing is less constrained to representing a single HD than the canonical ADN HD signal, which is thought to be governed by strict attractor dynamics (Skaggs et al., 1995; Redish et al., 1996). Because of the clear differences between the landmark-modulated POR HD cells and more classic HD cells early in the HD signal generation circuit, we refer to these POR cells as landmark modulated-head direction (LM-HD) cells.
Figure 2 –
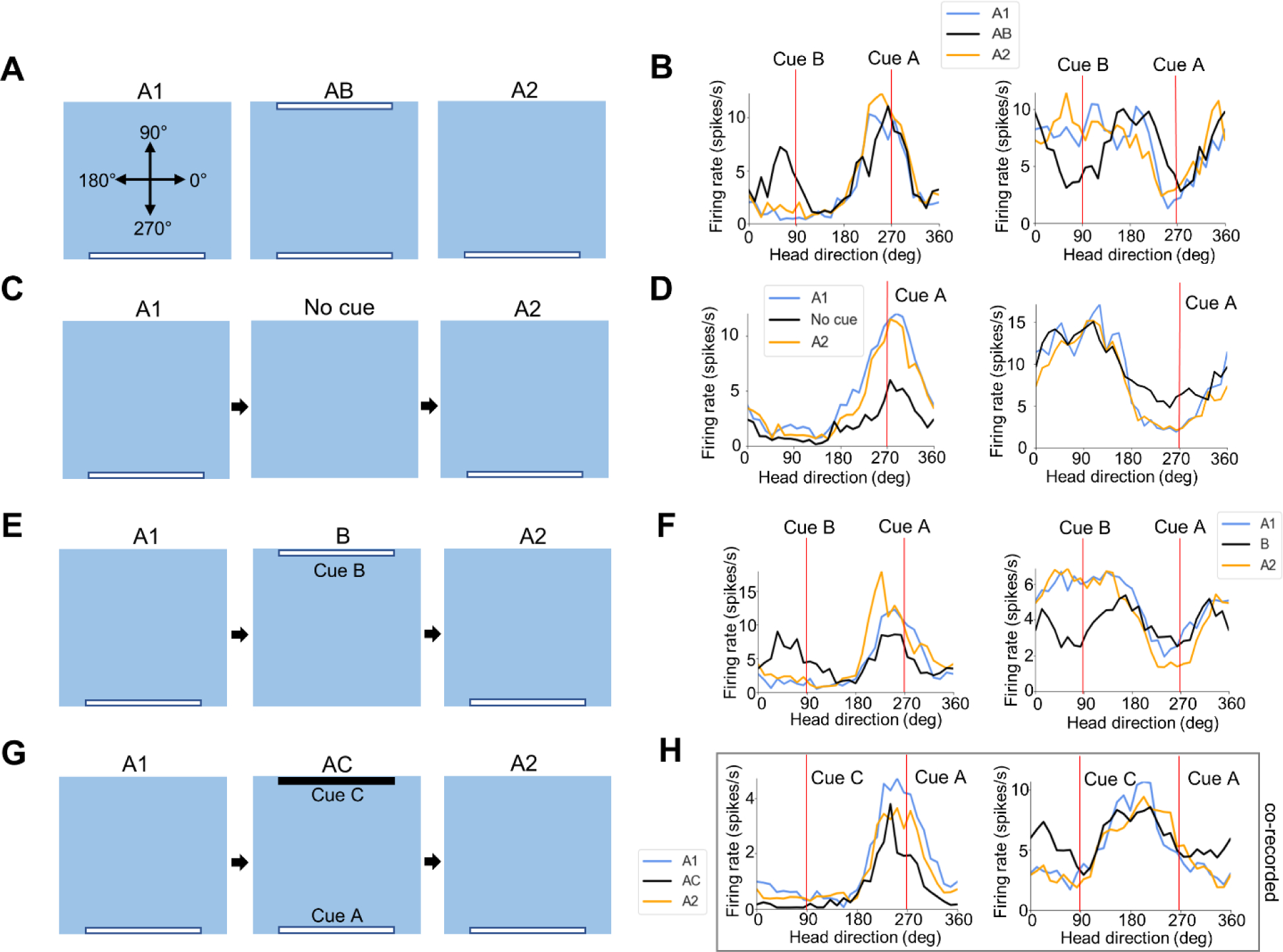
Modulation of POR LM-HD cell firing by visual landmarks. A) Schematic top-down view of the recording arena across the three sessions of the AB experiment, including the reference frame for measuring HD. B) Tuning curves for two example POR LM-HD cells (left peak-locked, right trough-locked) across the three sessions of the AB experiment. Cue positions in an allocentric reference frame are indicated by red lines. Note that both cells became bidirectionally tuned during the AB session. C) Same as (A) but for the no cue experiment. D) Tuning curves for two example POR LM-HD cells (left peak-locked, right trough-locked) across the three sessions of the no cue experiment. Note that both cells became less strongly tuned when cue A was removed. E) Same as (A) but for the B experiment. F) Tuning curves for two example POR LM-HD cells (left peak-locked, right trough-locked) across the three sessions of the B experiment. Note that both cells became bidirectionally tuned when cue B was introduced and cue A was removed. G) Same as (A) but for the AC experiment. H) Tuning curves for two example co-recorded peak-locked POR LM-HD cells across the three sessions of the AC experiment. Note that, while the cell on the left did not become bidirectional in response to the addition of cue C (the most typical response), the cell on the right did, suggesting that both types responses are simultaneously possible among POR cells. Figure modified from LaChance et al., 2022.
When POR LM-HD cells were recorded with the original visual cue only and then again with the cue removed (‘no cue’ condition), they generally maintained their PFDs but showed an overall reduction in tuning strength (Fig. 2C, D). Their retained tuning preferences suggest that they could use memory or remaining sensory properties of the environment to maintain their firing properties, though the absence of direct perception of the cue disrupted the strength of the signal. However, on an individual cell level, POR LM-HD cells fell on a spectrum between completely retaining and completely losing their tuning properties, suggesting a heterogeneity of inputs to each cell. When the original cue was removed from the environment and the second cue alone introduced (B condition), POR LM-HD cells overall showed bidirectional tuning, firing both to the previous location of the original cue and the actual location of the new cue (Fig. 2E, F). Note that although this manipulation is similar to a cue rotation session, it differs from it in that the animals were not disoriented prior to placement in the enclosure of the B session, and ADN HD cells did not rotate their PFDs in this condition. Again, however, individual cell responses were heterogeneous; while some cells showed similar degrees of firing rate modulation relative to each cue, others appeared to ‘choose’ one cue over the other. Importantly, we simultaneously recorded cells that exhibited different response types, indicating that a single property could not be driving the entire network of cells. Finally, when a novel black cue card (cue C) was introduced opposite the original white cue card (AC condition), POR LM-HD cells generally did not become bidirectionally tuned, suggesting that they distinguished between the visually disparate cues (Fig. 2G, H left). A very small proportion of cells did become bidirectional, however, and could be co-recorded with cells that did not become bidirectional (Fig. 2H), suggesting that some POR LM-HD cells have the capacity to generalize across visually disparate cues.
Importantly, further experiments showed that the landmark-tuned responses of POR LM-HD cells were found to be tied only to landmarks that had been previously established as a stable part of a given environment (i.e., associated with a specific environmental context). For example, when rats were trained with a single black cue card instead of a white cue card, HD-responsive cells became bidirectional when a second black cue card was added to the environment, suggesting that the familiarity of the landmark was responsible for causing bidirectional tuning, not the color or luminance of the card (see Fig. 6A-E in LaChance et al., 2022). When animals were habituated to an environment with no salient landmark cues, HD-responsive cells were still found in POR, but these failed to become bidirectionally tuned when two identical cue cards were introduced to the environment at the same time (see Fig. 6K-O in LaChance et al., 2022), presumably because they had not been established as stable elements of the environment. Perhaps most importantly, when two identical white cue cards were present from the beginning of training, HD-responsive cells in POR were largely unidirectional (see Fig. 6F-J in LaChance et al., 2022). Thus, the POR LM-HD representation does not appear to communicate a separate directional signal relative to each cue, but rather an estimate of the animal’s HD based on the constellation of available cues. When the two cues are present from the beginning, the LM-HD signal references both to form a single stable representation. However, when only one cue is present from the beginning, the signal becomes locked to the position of that one cue, such that duplicating that cue along the opposite wall causes the cells to fire in both directions. Thus, directional firing relative to visual landmarks in POR appears to be contextually modulated, such that the number and stability of landmarks associated with given environmental context dictates how the cells will respond to landmark manipulations.
Recent POR Recordings: Implications for Environmental Context Encoding
In addition to providing a template for mapping allocentric space and determining orientation relative to visual landmarks, egocentric and allocentric spatial correlates among POR neurons may also be useful for mapping the spatial layout of the local environment to help identify the current environmental context (Julian et al., 2018). For example, the center-bearing, center-distance, and HD signals may be combined with an explicit boundary representation (such as border cells in downstream MEC; Solstad et al., 2008) in order to establish an efficient representation of environmental geometry. A potential coding scheme for this representation comes from the field of visual pattern recognition, and defines a “shape” (e.g., the geometry of an enclosure) based on the distance of points along the shape’s boundary from its geometric centroid (Davies, 1997), which we will refer to as the ‘centroid-boundary distance’ (CBD). Distances of points along the boundary are indexed according to their allocentric bearing relative to the centroid. During navigation, the allocentric bearing of the centroid relative to the animal can be computed by summing the animal’s egocentric center-bearing and allocentric HD (indicated by the firing of center-bearing and HD cells; Fig. 3A), and 180° can be subtracted from this value to obtain the allocentric bearing of the animal with respect to the centroid. Note that, at any one location in the environment, the animal can face any direction and its egocentric center-bearing and HD will always sum to the same allocentric center-bearing. When the animal is adjacent to a boundary (possibly indicated by the firing of a border cell), the portion of the boundary at that particular location can be indexed according to its allocentric bearing with respect to the centroid, with its distance from the centroid indicated by the firing rate of a center-distance cell that increases its firing rate linearly with distance from the centroid. Cataloging the centroid-boundary distance at each index along the length of the extended boundary would create a unique geometric signature for each uniquely shaped environment as shown in Figure 3. In addition, if center-distance cells truly indicate absolute distances from the centroid, then environments with the same geometry but different sizes would be represented uniquely as well (Fig. 3B, C). Whether such a scheme is actually used by the brain remains unknown, but it provides a potential unifying account for how these disparate neural signals may work in concert to produce an efficient geometric representation of the local environment. In agreement with this idea, a recent theoretical modeling study has suggested a similar geometric encoding framework, in which different boundary-related cell types (including center-bearing/distance cells) may combine their signals to form an efficient representation of local geometry measured with respect to the environment center. The authors of this study predict the existence of a new cell type called the ‘geometry cell,’ which may reside in POR and would encode the distance of environmental boundaries from the centroid at different allocentric bearings relative to the centroid (Zeng et al., 2022). It will be important for future recording studies to investigate the existence and location of this hypothesized cell type.
Figure 3 –
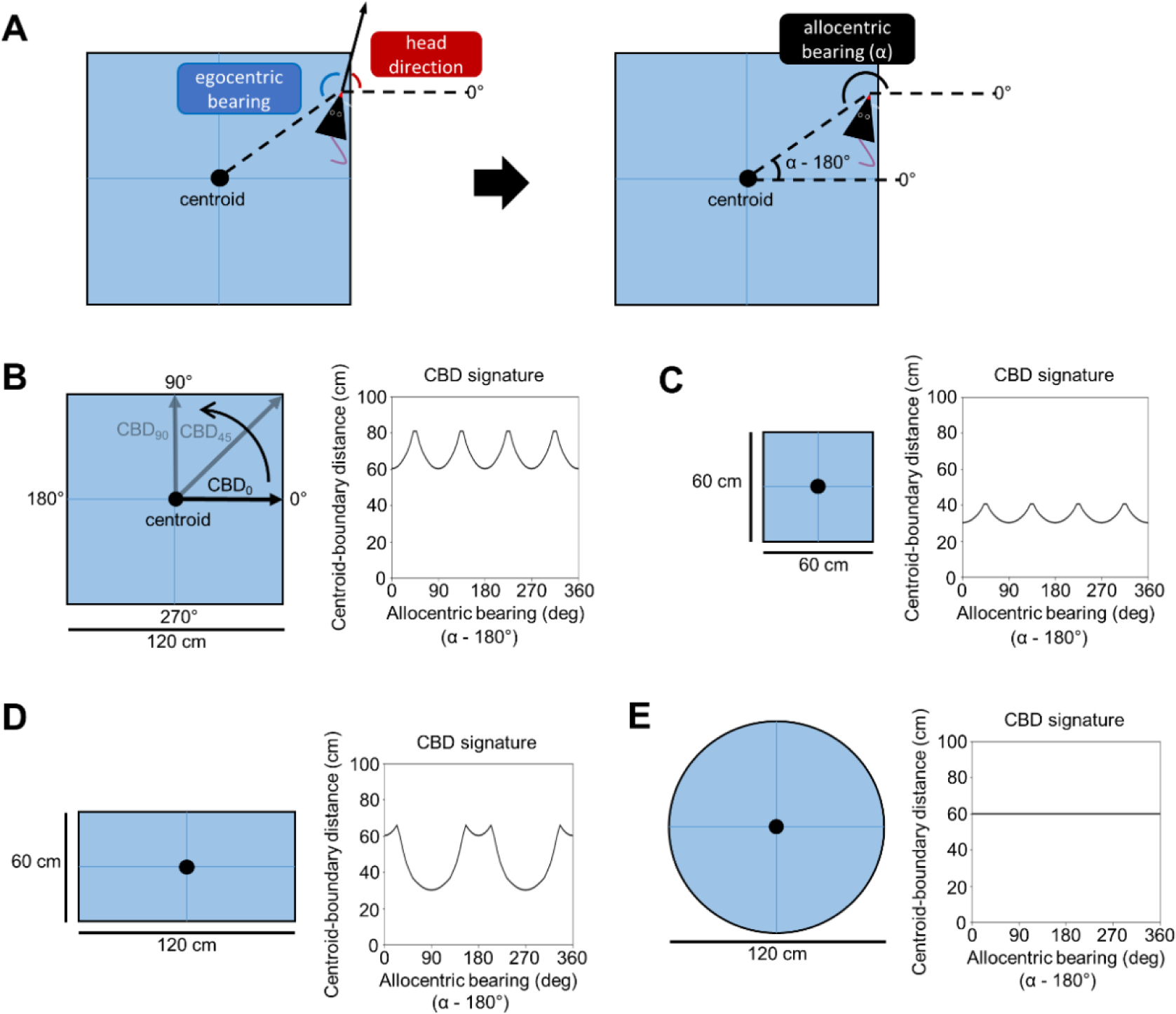
Centroid-boundary distance encoding of environmental geometry. A) left, top-down schematic view of the recording arena showing measurement of egocentric center-bearing and HD. right, egocentric center-bearing and HD are summed to compute allocentric center-bearing (α). This calculation can be reversed to compute the allocentric bearing of the animal’s location with respect to the centroid (1- α). B) left, top-down schematic view of a 120 × 120 cm square recording arena. Arrows pointing from the arena centroid to the boundary at specific allocentric bearings indicate measurement of centroid-boundary distance (CBD) along that angle. right, CBDs plotted as a function of allocentric bearing relative to the centroid. The result is a CBD signature that is unique to the shape and size of the arena. C-E) Same as B but for a 60 × 60 cm square, 120 × 60 cm rectangle, and 120 cm diameter circle, respectively. Note that while the arenas in B and C are both squares, they have unique CBD signatures because of their different sizes.
Our more recent findings regarding the modulation of POR LM-HD cell firing by the spatial distribution and visual properties of landmarks (LaChance et al., 2022) indicate another potential method for determining the current environmental context. Specifically, POR LM-HD cells appear to define their HD signal based on the positions, visual properties, and relative familiarity or stability of surrounding visual landmarks. Duchon (1996), building on O’Keefe’s centroid hypothesis (1991), proposed a spatial processing framework that combined two separate but interacting representations of space; one that involved computing one’s position relative to the overall centroid of all cues in the environment (c), and a second that involved computing the centroid of all cues that were first weighted by their salience (s). This second centroid was referred to as the ‘salience centroid’. By computing the distance and direction from the unweighted centroid to the salience centroid, one would establish a vector (the ‘salience vector’;) that was unique to environments with different cue layouts, even if their geometries were identical (Fig. 4A). POR LM-HD cells could contribute to the computation of such a salience centroid by weighting their response to each cue by multiple factors including visual appearance, familiarity, and stability. The resulting signal would provide an estimate of the animal’s orientation relative to the salience vector. This signal is similar to what O’Keefe called the ‘slope’ of the environment. Because the salience vector would differ between environments with different established landmark configurations, the POR HD signal might be expected to ‘remap’ between different environmental contexts depending on the location of the salience centroid, although further recording studies with multiple separate environmental contexts (i.e., completely different enclosures instead of different cue configurations in the same enclosure) will be necessary to test this possibility. This is just one potential framework by which egocentric and allocentric signals in POR could contribute to the encoding of environmental context by indicating visual cue layout of different environments.
Figure 4 –
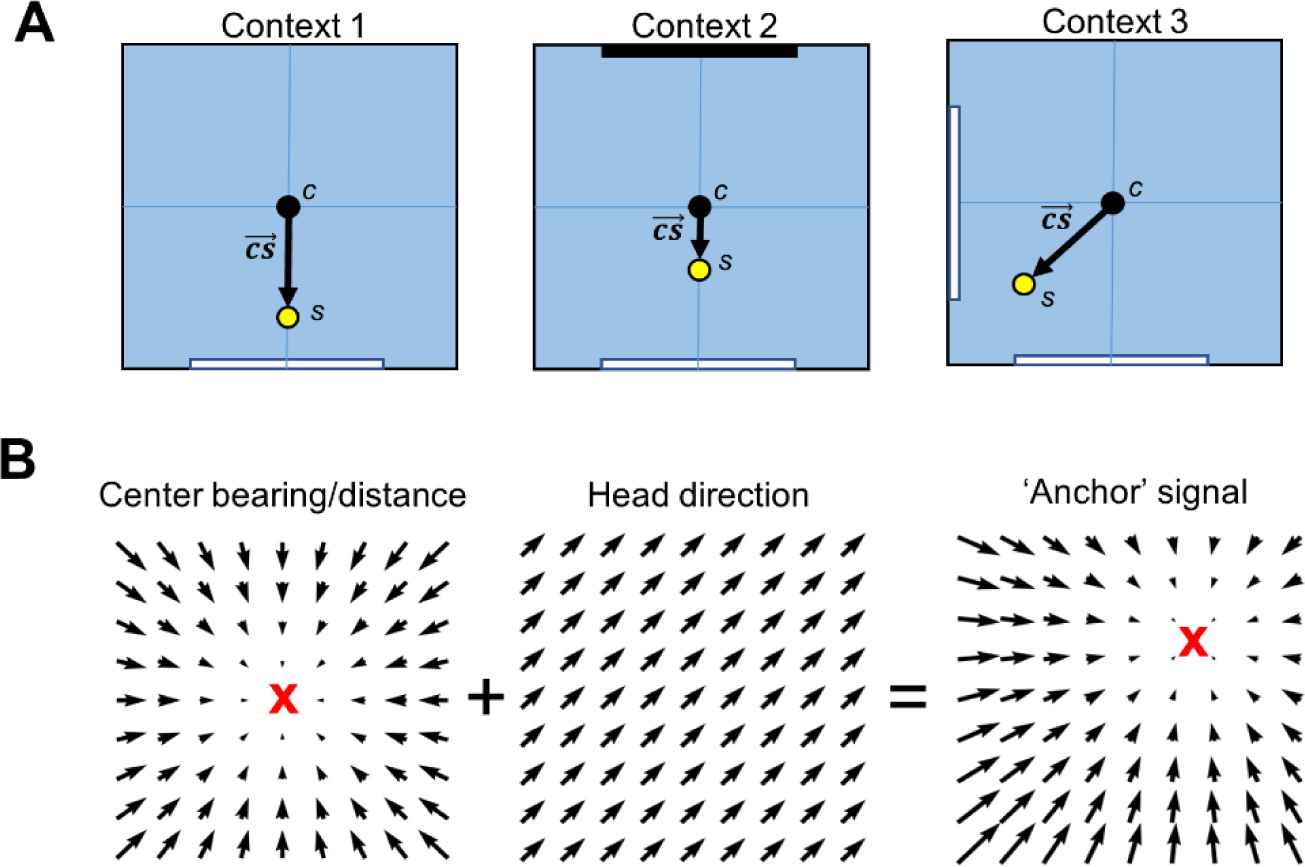
Potential interactions between egocentric and HD signals in representing spatial context. A) Top-down schematic views of a recording arena with identical geometries but different visual landmarks, along with the locations of the unweighted centroid c based on all physical cues of the environment and the salience centroid s which is weighted by the salience of each physical cue. Note that s is displaced preferentially toward the location of a white cue card in each context, and to a lesser extent toward the black cue card in context 2. The salience vector connects the unweighted centroid to the salience centroid and is unique for each context. B) Left, vector field showing the firing preferences of a center-bearing by center-distance cell; middle, vector field showing the firing preferences of an HD cell with PFD pointing northeast; right, resultant vector field after summing the center-bearing/distance and HD fields. Note that the focal point of the center-bearing/distance field (anchor point; indicated by a red X) has shifted toward the northeast after summing with an HD signal.
Finally, POR cells with conjunctive egocentric and allocentric spatial correlates may contribute to contextual processing by behaving similarly to ‘anchor’ cells identified in the rodent hippocampus (Shahi et al., 2018; Jercog et al., 2019) and egocentric bearing cells in the human PHC (Kunz et al., 2021). Anchor or egocentric bearing cells are tuned to the egocentric bearing of a specific location in the environment, called the anchor point. POR center-bearing cells may be considered a special case of anchor cell whose anchor point lies at the center of the environment (Kunz et al., 2021). Importantly, a center-bearing signal can be transformed into an anchor signal by adding an allocentric HD signal. Imagine the firing preferences of a center-bearing by center-distance cell as a vector field distributed across the environment, whose vector directions point toward the environment center and whose lengths change gradually with distance from the center (Fig. 4B). If the response to distance is linear and the response to bearing is sinusoidal (evident in POR; LaChance et al., 2019), then adding a sinusoidal HD signal creates a new gradient field that points to a different location (Fig. 4B). This new location, the anchor point, depends on the preferred direction of the HD signal (controls the direction of the shift) and the amplitude of the HD signal (controls the distance of the shift). If POR HD cells change their firing properties based on the spatial distribution and salience of landmark cues in each unique environment, then conjunctive center-bearing by HD cells would be expected to represent unique anchor points in each environment, much like how hippocampal place cells adopt unique place field locations in each unique environment. Especially considering that POR LM-HD cells base their representations on the visual landmarks associated with a specific environmental context, and the fact that over half of POR LM-HD cells are also tuned to center-bearing which may result in tuning to specific anchor locations (Fig. 4B), it seems likely that POR already contains a context-dependent anchor code.
Relationship to Patients with Damage to the PHC and Related Areas
Collectively, how do these findings relate to the characteristics seen in humans who have damage to the PHC and related areas? Epstein and colleagues described impairments from two patients that had vascular incidents in the PPA (Epstein et al., 2001). Both patients were unable to navigate unfamiliar environments and were impaired at generating accurate maps of new places. They were, however, able to recognize famous landmarks as well as analyze the spatial geometry within a visual scene. On an N-back memory task, they were unimpaired when the stimuli involved novel objects, but had difficulty when the stimuli were novel scenes. Taken together with the early imaging studies showing activation of the PPA when the subject viewed scenes containing objects that could be used as landmarks, studies with humans suggest the PPA is important for processing spatial information concerning landmarks, particularly when the information is novel. It is possible that the PPA is important for integrating new landmarks into the framework of an existing cognitive map.
This view is consistent with older descriptions in the literature concerning patients who had damage to the PHC. Such patients were typically described as having topographical amnesia – a deficit where there is a loss in the familiarity of landmarks (Aguirre et al., 1996). These patients usually report they are unfamiliar with a particular place because they have never seen it or been there before. The memory loss appears confined to the realm of landmarks and patients usually are not impaired in their familiarity for other categories of information. An inability to recognize landmarks for what they represent would prevent the utilization of them as spatial reference points. In turn, this deficit would result in patients’ poor sense of spatial self-cognizance. They would have difficulty in perceiving their location and directional heading and this impairment would lead to a loss in navigational skills. Even if subjects are capable of processing the spatial relationships amongst items for the purpose of navigation, if they cannot recognize which objects to use as spatial reference points, they will be unable to navigate successfully. A major factor in the process of landmark recognition is understanding the environmental context that the subjects find themselves in, and this process is where the POR may play a critical role.
The older studies concerning cases with topographic disorientation emphasize the inability of the subjects to integrate knowledge about novel spatial relationships into an existing cognitive map. For example, Whiteley & Warrington (1978) reported patients who had difficulty in recognizing and recalling landmarks, but had no difficulty in drawing or describing a particular route to a destination. However, when they were confronted with the actual route, they were unable to perform it, presumably because they could not recognize the landmarks. Similarly, Habib & Sirigu (1987) reported four cases where all the patients had difficulty navigating unfamiliar environments, and in each case there was damage to the right parahippocampal area (also see Barrash et al., 2000).
Functional Imaging Studies and the PHC
More recent functional imaging studies have postulated different roles for the PHC and related areas in navigation; these roles fall into three broad categories: 1) representing spatial relationships amongst items; 2) scene categorization where objects of landmark significance are identified and distinguished from non-landmark objects; and 3) forming contextual associations across individual objects, although not necessarily across items belonging to scenes and landmark categorization. Each hypothesis is championed by different research groups, each of whom provide evidence promoting their theories.
In one study supporting the spatial relationships view, Kravitz et al. (2011) had subjects view images that varied across a number of dimensions including distance from the observer, whether the viewed items were natural or fabricated, or whether the scene was closed or expansive. The authors reported that activation of brain areas believed integral to the scene/landmark processing network (e.g., PPA, early visual cortex [EVC], occipital place area), did not differ based on the amount of landmark information contained within the image. Further, they were able to decode individual scenes in both EVC and PPA, although the PPA was activated in particular by expanse, while EVC activation was related more to relative distance. Based on these findings the authors concluded that the PPA reflected spatial information rather than categorical information.
In contrast, other imaging studies have argued for just the opposite – that the PPA was important for identifying objects as belonging to a membership group of significant items or places. These items and places contained high informational value for acting as landmarks, but the PPA did not necessarily encode the spatial relationships about the items. One of the first functional imaging studies to suggest this view was conducted by Janzen and van Turennout (2004). They had subjects explore a virtual museum and found that neural activity in the PPA reflected the navigational relevance of the object’s location in the museum. This increased activity occurred at decision points and was not dependent on attentional demands. Another study showed that the PPA can differentiate natural scenes into categories that either include or exclude information relevant for processing landmarks (Walther et al., 2009). Similarly, Persichetti and Dilks (2019) argue that the PPA does not encode specific spatial information about landmarks, but rather is involved more in the categorization process because they found that the pattern of activation in PPA “represents two buildings from the same category, but in different locations, as more similar than two buildings from different categories, but in the same location.” In sum, these studies support the view that the role of the PPA is to identify items that would be useful as landmarks.
The last view of the PHC’s functional role is that it is important for monitoring environmental context. Using fMRI, Bar et al. (2008) showed that the PHC was more activated when scenes contained rich contextual information compared to scenes that contained equal amounts of visual information, but were devoid of information for placing the scene in a particular time and space. This view appears to meld both the spatial and categorization views into a single comprehensive one, where aspects of episodic memory, like time and space, come together to form a framework for linking events that occur at a particular location as denoted by landmarks - in other words, environmental context (also see Gronau et al., 2008). Consistent with this view are two recent studies. First, Park and Park (2017) used multi-voxel pattern analysis (MVPA) and repetition suppression analyses to show that each method activated a particular type of information pattern depending on whether the neurons activated were contained within a single voxel (repetition suppression) or across voxels (MVPA). Using different visual images that contained either solely textural features within a scene (e.g., a sandy dune vs. a forested hillside vs. a rocky mountain) or both textural features and an associated spatial location, the authors showed that the textural features were represented at multiple levels within the PPA. Based on MVPA, different patterns of activity could be distinguished depending on the textural features in the scene. Images that shared the same texture, regardless of where the texture occurred in the scene, led to a similar level of activation for a given voxel. Thus, unique sets of voxels encoded different types of textures. In contrast, based on repetition suppression analyses, conjoint information about both texture and its location within the scene was represented at the within voxel level. Taken together, the authors suggested that information beyond just the spatial aspects of a scene, such as the surface features in the scene, was encoded in the PPA. Second, Sun et al., (2021) recently monitored activity in subjects who viewed different objects in either the same or different rooms and found that the PPA distinguished objects that were in different rooms more than objects that were in the same room. From these results they concluded that the PPA encoded the spatial significance of landmark objects. Recordings of single neurons from the occipitotemporal sulcus of macaques, an analogous area to the PPA in monkeys, showed preferential activation to images of scenes (Kornblith et al., 2013). Collectively, activation of PPA neurons appears to be particularly sensitive to features concerning the suitability of objects as landmarks and aligns with the view that the PPA is involved with processing environmental context (also see Troiani et al., 2014).
The single-unit responses we observed in POR cells correspond most closely to the last hypothesis above – specifically that the PHC is responsible for forming contextual associations and identifying items that can be classified and used as landmarks. With this view in mind, we have now come full circle with the ideas first suggested by David Bucci and his colleagues in the early 2000s – namely that the functional role of the PHC is concerned with establishing the environmental context. Navigation is a complex activity that involves many sub-processes. The ability to select individual items or aspects from a scene that are to be used as spatial reference points is fundamental for successful navigation. The process of utilizing landmarks for navigation requires a high level of cognitive capacity involving multiple processes, including recognition of visual stimuli, understanding the meaning of the item in terms of its size, stability (does it move around), and function. Performing these processes likely requires multiple brain areas, and indeed Steel et al. (2021) recently showed how a distributed network lying anterior to the scene perception region in the posterior cortex, including the PPA, performs these functions when subjects recall familiar places. This network interacts closely with associated mnemonic areas in the hippocampal system and culminates in the situational awareness of the current context.
Conclusions
Overall, the POR contains a rich representation of surrounding space in both allocentric and egocentric reference frames, potentially contributing to the higher-order allocentric maps of space found downstream in the hippocampal system. Instead of explicitly indicating an animal’s position or orientation relative to local landmarks, however, spatial correlates in the POR may instead provide a means for processing the contextual aspects of a scene. This may include representing the geometry of the local environment as well as the spatial distribution, visual properties, and salience of local landmarks. Thus, even for the PPA, which was originally thought of as processing information about the landmarks in a scene, processing may be more devoted to the contextual aspects of scenes and not spatial information per se. This view can also account for the topographical disorientation deficits seen with damage to the PPA and the PHC in general. If subjects cannot comprehend the context they are in, it will be difficult for them to fully understand their spatial orientation in that environment. This view would also account for why POR lesions do not affect cue control in ADN HD cells because that type of spatial processing occurs more in the direct pathway from visual cortex to postsubiculum. We end by noting that David Bucci’s work was seminal in identifying the POR as an integral brain area involved in processing contextual information. His subsequent studies demonstrated that the POR is both anatomically and functionally interconnected with the retrosplenial cortex, and together they form a circuit within the limbic system that is critically important for learning about the environmental context of an event (Keene & Bucci, 2008; Robinson et al., 2012; also see review by Smith et al., 2021). Overall, David Bucci’s findings have provided valuable insights into the neural mechanisms underlying contextual information processing and the role of the POR and related brain areas in supporting animal behavior.
Acknowledgements:
The authors were supported through grants from the National Institute of Health RO1-NS053907, UF1-NS111695.
Footnotes
Credit Author Statement
Patrick A. LaChance: Conceptualization, Investigation, Writing.
Jeffrey S. Taube: Conceptualization, Funding acquisition, Supervision, Writing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agster KL, & Burwell RD (2009). Cortical efferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Hippocampus 19, 1159–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre GK, Ketre JA, Alsop DC, & D’Esposito M (1996). The parahippocampus subserves topographical learning in man. Cerebral Cortex 6, 823–829. [DOI] [PubMed] [Google Scholar]
- Alexander AS, Carstensen LC, Hinman JR, Raudies F, Chapman GW, & Hasselmo ME (2020). Egocentric boundary vector tuning of the retrosplenial cortex. Science Advances 6, eaaz2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Aminoff E, & Schacter DL (2008). Scenes unseen: the parahippocampal cortex intrinsically subserves contextual associations, not scenes or places per se. Journal of Neuroscience 28, 8539–8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrash J, Damasio H, Adolphs R, & Tranel D (2000). The neuroanatomical correlates of route learning impairment. Neuropsychologia 38, 820–836. [DOI] [PubMed] [Google Scholar]
- Bucci DB, Phillips RG, & Burwell RD (2000). Contributions of postrhinal and perirhinal cortex to contextual information processing. Behavioral Neuroscience 114, 882–894. [DOI] [PubMed] [Google Scholar]
- Bucci DB, Saddoris MP, & Burwell RD (2002). Contextual fear discrimination is impaired by damage to the postrhinal or perirhinal cortex. Behavioral Neuroscience 116, 479–488. [PubMed] [Google Scholar]
- Bucci DB, & Burwell RD (2004). Deficits in attentional orienting following damage to the perirhinal or postrhinal cortices. Behavioral Neuroscience 118, 1117–1122. [DOI] [PubMed] [Google Scholar]
- Burwell RD (2001). Borders and cytoarchitecture of the perirhinal and postrhinal cortices in the rat. Journal of Comparative Neurology 437, 17–41. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Saddoris MP, Bucci DB, & Wiig KA (2004). Corticohippocampal contributions to spatial and contextual learning. Journal of Neuroscience 24, 3826–3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, & Amaral DG (1998). Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. Journal of Comparative Neurology 391, 293–321. [DOI] [PubMed] [Google Scholar]
- Burwell RD, & Hafeman DM (2003). Positional firing properties of postrhinal cortex neurons. Neuroscience 119, 577–588. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Duck J, Muir JL, & John P Aggleton JP (2000). Distinct patterns of behavioural impairments resulting from fornix transection or neurotoxic lesions of the perirhinal and postrhinal cortices in the rat. Behavioural Brain Research 111, 187–202. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, & John P Aggleton JP (1999). Functionally dissociating aspects of event memory: the effects of combined perirhinal and postrhinal cortex lesions on object and place memory in the rat. Journal of Neuroscience 19, 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calton JL, Stackman RW, Goodridge JP, Archey WB, Dudchenko PA, & Taube JS (2003). Hippocampal place cell instability following lesions of the head direction cell network. Journal of Neuroscience 23, 9719–9731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies ER (1997). Machine Vision: Theory, Algorithms, Practicalities. Academic Press: New York. [Google Scholar]
- Duchon AP Maze navigation using optical flow (1996). In From Animals to Animats 4: Proceedings of the Fourth International Conference on Adaptive Behavior (eds. Maes P, Mataric MJ, Meyer J-A, Pollack J, & Wilson SW; MIT Press/Bradford Books; ), pp. 224–232. [Google Scholar]
- Epstein R, Deyoe EA, Press DZ, Rosen AC, & Kanwisher N (2001). Neuropsychological evidence for a topographical learning mechanism in parahippocampal cortex. Cognitive Neuropsychology 18, 481–508. [DOI] [PubMed] [Google Scholar]
- Epstein R, & Kanwisher N (1998). A cortical representation of the local visual environment. Nature 392, 598–601. [DOI] [PubMed] [Google Scholar]
- Furtak SC, Ahmed OJ, & Burwell RD (2012). Single neuron activity and theta modulation in postrhinal cortex during visual object discrimination. Neuron 76, 976–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtak SC, Wei S-M, Agster KL, & Burwell RD (2007). Functional neuroanatomy of the parahippocampal region in the rat: the perirhinal and postrhinal cortices. Hippocampus 17, 709–722. [DOI] [PubMed] [Google Scholar]
- Gofman X, Tocker G, Weiss S, Boccara CN, Lu L, Moser M-B, Moser EI, Morris G, & Derdikman D (2019). Dissociation between postrhinal cortex and downstream parahippocampal regions in the representation of egocentric boundaries. Current Biology 29, 2751–2757.e4. [DOI] [PubMed] [Google Scholar]
- Goodridge JP, & Taube JS (1997). Interaction between the postsubiculum and anterior thalamus in the generation of head direction cell activity. Journal of Neuroscience 17, 9315–9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronau N, Neta M, & Bar M (2008). Integrated contextual representation for objects’ identities and their locations. Journal of Cognitive Neuroscience 20, 371–388. [DOI] [PubMed] [Google Scholar]
- Habib M, & Sirigu A (1987). Pure topographical disorientation: a definition and anatomical basis. Cortex 23, 73–85. [DOI] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser M-B, & Moser EI (2005). Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806. [DOI] [PubMed] [Google Scholar]
- Hinman JR, Chapman GW, & Hasselmo ME (2019). Neuronal representation of environmental boundaries in egocentric coordinates. Nature Communications 10, 2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen G, & van Turennout M (2004). Selective neural representation of objects relevant for navigation. Nature Neuroscience 7, 673–677. [DOI] [PubMed] [Google Scholar]
- Jercog PE, Ahmadian Y, Woodruff C, Deb-Sen R, Abbott LF, & Kandel ER (2019). Heading direction with respect to a reference point modulates place-cell activity. Nature Communications 10, 2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian JB, Keinath AT, Marchette SA, & Epstein RA (2018). The neurocognitive basis of spatial reorientation. Current Biology 28, R1059–R1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene CS, & Bucci DJ (2008). Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behavioral Neuroscience 122, 89–97. [DOI] [PubMed] [Google Scholar]
- Koganezawa N, Gisetstad R, Husby E, Doan TP, & Witter MP (2015). Excitatory postrhinal projections to principal cells in the medial entorhinal cortex. Journal of Neuroscience 35, 15860–15874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblith DJ, Cheng X, Ohayon S, & Tsao DY (2013). A network for scene processing in the macaque temporal lobe. Neuron 79, 766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornienko O, Latuske P, Bassler B, Kohler L, & Allen K (2018). Non-rhythmic head-direction cells in the parahippocampal region are not constrained by attractor network dynamics. eLife 7, e35949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz DJ, Peng CS & Baker CJ (2011). Real-world scene representations in high-level visual cortex: It’s the spaces more than the places. Journal of Neuroscience 31, 7322–7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz L, Brandt A, Reinacher PC, Staresina BP, Reifenstein ET, Weidemann CT, Herweg NA, Patel A, Tsitsiklis M, Kempter R, Kahana MJ, Schulze-Bonhage A, & Jacobs J (2021). A neural code for egocentric spatial maps in the human medial temporal lobe. Neuron 109: 2781–2796.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaChance PA, Graham J, Shapiro BL,. Morris AJ, & Taube JS (2022). Landmark-modulated directional coding in postrhinal cortex. Science Advances 8, eabg8404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaChance PA, Todd TP, & Taube JS (2019). A sense of space in postrhinal cortex. Science 365: eaax4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova L, & Mishkin M (2003). One-trial memory for object-place associations after separate lesions of hippocampus and posterior parahippocampal region in the monkey. Journal of Neuroscience 23, 1956–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, & Bilkey DK (2002). The effects of NMDA lesions centered on the postrhinal cortex on spatial memory tasks in the rat. Behavioral Neuroscience 116, 860–873. [DOI] [PubMed] [Google Scholar]
- Muller RU, & Kubie JL (1987). The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. Journal of Neuroscience 7, 1951–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman G, & Eacott MJ (2005). Dissociable effects of lesions to the perirhinal cortex and the postrhinal cortex on memory for context and objects in rats. Behavioral Neuroscience 119, 557–566. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, & Nadel L (1978). The Hippocampus as a Cognitive Map. Oxford University Press. [Google Scholar]
- O’Keefe J (1991). An allocentric spatial model for the hippocampal cognitive map. Hippocampus 1, 230–235. [DOI] [PubMed] [Google Scholar]
- Park J, & Park S (2017). Conjoint representation of texture ensemble and location in the parahippocampal place area. Journal of Neurophysiology 117, 1595–1607, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck JR, & Taube JS (2017). The postrhinal cortex is not involved in landmark control in rat head direction cells. Hippocampus 27: 156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persichetti AS, & Dilks DD (2019). Distinct representations of spatial and categorical relationships across human scene-selective cortex. Proceedings of the National Academy of Sciences (USA) 116, 21312–21317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira IT, Agster KL, & Burwell RD (2016) Subcortical connections of the perirhinal, postrhinal, and entorhinal cortices of the rat. I. afferents. Hippocampus 26, 1189–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos JMJ (2013). Differential contribution of hippocampus, perirhinal cortex and postrhinal cortex to allocentric spatial memory in the radial maze. Behavioural Brain Research 247, 59–64. [DOI] [PubMed] [Google Scholar]
- Redish AD, Elga AN, & Touretzky DS (1996). A coupled attractor model of the rodent head direction system. Network: Computation in Neural Systems 4, 671–685. [Google Scholar]
- Robinson S, Poorman CE, Marder TJ, & Bucci DJ (2012). Identification of functional circuitry between retrosplenial and postrhinal cortices during fear conditioning. Journal of Neuroscience 32, 12076–12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethumadhavan N, Hoang T-H, Strauch C, & Manahan-Vaughan D (2020). Involvement of the postrhinal and perirhinal cortices in microscale and macroscale visuospatial information encoding. Frontiers in Behavioural Neuroscience 14, 556645. doi: 10.3389/fnbeh.2020.556645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahi M, Dhingra S, Sandler R, Rios R, Vuong C, Acharya L, & Mehta MR (2018). A statistical model based approach to decipher the mechanisms governing spatial and directional tuning of hippocampal neurons. Program No. 508.15.2018 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience. [Google Scholar]
- Skaggs WE, Knierim JJ, Kudrimoti HS, & McNaughton BL (1995). A model of the neural basis of the rat’s sense of direction. In Advances in Neural Information Processing Systems, Vol. 7 (eds. Tesauro G, Touretzky DS, & Leen TK; MA: MIT Press; ), pp. 173–178. [PubMed] [Google Scholar]
- Smith DM, Yang YY, Subramanian DL, Miller AMP, Bulkin DA, & Law LM (2021). The limbic memory circuit and the neural basis of contextual memory. Neurobiology of Learning & Memory, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solstad T, Boccara CN, Kropff E, Moser M-B, & Moser EI (2008). Representation of geometric borders in the entorhinal cortex. Science 322, 1865–1868. [DOI] [PubMed] [Google Scholar]
- Steel A, Billings MM, Silson EH, & Robertson CE (2021). A network linking scene perception and spatial memory systems in posterior cerebral cortex. Nature Communications 12: 2632. 10.1038/s41467-021-22848-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugar J, Witter MP, van Strien NM, & Cappaert NLM (2011). The retrosplenial cortex intrinsic connectivity and connections with the (para).hippocampal region in the rat. An interactive connectome. Frontiers Neuroinformatics, 10.3389/fninf.2011.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Frank SM, Epstein RA, & Tse PU (2021). The parahippocampal place area and hippocampus encode the spatial significance of landmark objects. NeuroImage 236, 118081. [DOI] [PubMed] [Google Scholar]
- Taube JS (1995). Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. Journal of Neuroscience 15, 70–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS (2007). The head direction signal: Origins and sensory–motor integration. Annual Review of Neuroscience 30, 181–207. [DOI] [PubMed] [Google Scholar]
- Taube JS, Muller RU, & Ranck JB Jr. (1990a). Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. Journal of Neuroscience 10, 420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS, Muller RU, & Ranck JB Jr. (1990b). Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. Journal of Neuroscience 10, 436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiani V, Stigliani A, Smith ME, & Epstein RA (2014). Multiple object properties drive scene-selective regions. Cerebral Cortex 24, 883–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, & Miller MW (1983). Cortical connections between rat cingulate cortex and visual, motor and postsubicular cortices. Journal of Comparative Neurology 216, 192–210. [DOI] [PubMed] [Google Scholar]
- Walther DB, Caddigan E, Fei-Fei L, & Beck DM (2009). Natural scene categories revealed in distributed patterns of activity in the human brain. Journal of Neuroscience 29, 10573–10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Chen X, Lee H, Deshmukh SS, Yoganarasimha D, Savelli F, & Knierim JJ (2018). Egocentric coding of external items in the lateral entorhinal cortex. Science 362, 945–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner KS, Barnett M, Witthoft N, Golarai G, Stigliani A, Kay KN, Gomez J, Natu VS, Amunts K, Zilles K, & Grill-Spector K (2018). Defining the most probable location of the parahippocampal place area using cortex-based alignment and cross-validation. Neuroimage 170, 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley AM, & Warrington EK (1978). Selective impairment of topographical memory: a single case study. Journal of Neurology, Neurosurgery, Psychiatry 41, 575–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber AA, Clark BJ, Forster TC, Tatsuno M, McNaughton BL (2014). Interaction of egocentric and world-centered reference frames in the rat posterior parietal cortex. Journal of Neuroscience 34, 5431–5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, & Bussey TJ (2004). Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. Journal of Neuroscience 24, 5901–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, Clark BJ, & Taube JS (2011). Origins of landmark encoding in the brain. Trends in Neuroscience 34, 567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, Peck JR, & Taube JS (2015). Visual landmark information gains control of the head direction signal at the lateral mammillary nuclei. Journal of Neuroscience 35, 1354–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng T, Si B, & Feng J (2022). A theory of geometry representations for spatial navigation. Progress in Neurobiology, 102228. [DOI] [PubMed] [Google Scholar]


