Abstract
Background:
Abnormalities in cardiac energy metabolism occur in heart failure (HF) and contribute to contractile dysfunction, but their role, if any, in HF-related pathologic remodeling is much less established. Creatine kinase (CK), the primary muscle energy reserve reaction which rapidly provides ATP at the myofibrils and regenerates mitochondrial ADP, is down-regulated in experimental and human HF. To test the hypotheses that pathologic remodeling in human HF is related to impaired cardiac CK energy metabolism and that rescuing CK attenuates maladaptive hypertrophy in experimental HF.
Methods:
First, in twenty-seven HF patients and fourteen healthy subjects, we measured cardiac energetics and left ventricular (LV) remodeling using noninvasive magnetic resonance 31P spectroscopy and MRI, respectively. Second, we tested the impact of metabolic rescue with cardiac-specific overexpression of either myofibrillar CK (CKmyofib) or mitochondrial CK (CKmito) on HF-related maladaptive hypertrophy in mice.
Results:
In people, pathologic LV hypertrophy and dilatation correlate closely with reduced myocardial ATP levels and rates of ATP synthesis through CK. In mice, transverse aortic constriction (TAC)-induced LV hypertrophy and dilatation are attenuated by overexpression of CKmito, but not by overexpression of CKmyofib. CKmito overexpression also attenuates hypertrophy after chronic isoproterenol stimulation. CKmito lowers mitochondrial ROS, tissue ROS levels, and upregulates antioxidants and their promoters. When the CK capacity of CKmito-overexpressing mice is limited by creatine substrate depletion, the protection against pathologic remodeling is lost, suggesting the ADP regenerating capacity of the CKmito reaction rather than CK protein per se is critical in limiting adverse HF remodeling.
Conclusions:
In the failing human heart, pathologic hypertrophy and adverse remodeling are closely related to deficits in ATP levels and in the CK energy reserve reaction. CKmito, sitting at the intersection of cardiac energetics and redox balance, plays a crucial role in attenuating pathologic remodeling in HF.
Clinical Trial Registration:
Keywords: Metabolism, Clinical Studies, Animal Models of Human Disease, Basic Science Research
INTRODUCTION:
Heart failure (HF) remains a significant, growing worldwide public health problem. More Medicare dollars are spent on the care of HF than on any other diagnosis.1 Adverse outcomes in HF are associated with two related but separate clinical features: contractile dysfunction and adverse remodeling, the latter characterized by the pathologic ventricular hypertrophy and dilatation. Contractile dysfunction in experimental and human HF has been extensively studied and is related, at least in part, to inadequate ATP energy metabolism.2-4 However, the role of energetic abnormalities, if any, in contributing to adverse remodeling is far less understood. On the one hand, reductions in energy-related metabolites in the failing heart may be a consequence of adverse remodeling since ventricular dilatation increases wall tension, a determinant of energetic demand. On the other hand, energetic abnormalities precede adverse remodeling in experimental HF, suggesting but not proving, that impaired energetics may cause adverse remodeling.5,6
The energy liberated during ATP hydrolysis fuels nearly all myocellular functions. Most myocardial ATP is generated primarily via mitochondrial oxidative phosphorylation.6 Yet the creatine kinase (CK) reaction is the primary short-term high-energy phosphate energy supply2,7 in that it rapidly and reversibly generates ATP by phosphoryl transfer from creatine phosphate (PCr) to adenosine diphosphate (ADP) in the cytosol and the reverse reaction in the mitochondria.7,8 Abnormalities in CK activity and metabolites occur in experimental and human HF9-13, and reductions in cardiac function and work are closely related to impaired CK ATP transfer in human HF.14 Surprisingly, little is known about the role that impaired CK energy metabolism plays in adverse cardiac remodeling. Transgenic over-expression of the cytosolic, myofibrillar CK (CKmyofib), rescues depressed cardiac CK energy metabolism, improves contractile function, and prolongs survival in several murine models of HF.15,16 Yet, CKmyofib overexpression does not attenuate the development of left ventricular hypertrophy (LVH) or pathologic dilatation.15 The mitochondrial isoform of CK (CKmito) is less abundant and has been far less investigated in HF, despite its strategic location in the mitochondrial intermembrane space where it plays a critical role in energy transfer and stimulating oxidative phosphorylation by regenerating mitochondrial ADP.17,18 In theory, CKmito could enhance ATP production and limit the formation of reactive oxygen species (ROS), which can contribute to pathologic hypertrophy and remodeling.19
We set out to determine whether abnormalities in cardiac energetics and specifically in mitochondrial CK, contribute to adverse HF remodeling. We find that both LVH and dilatation in human HF are closely associated with reduced in vivo myocardial ATP concentrations, [ATP], and the rate of ATP synthesis through CK. In a mouse model of HF induced by transverse aortic constriction (TAC), cardiac-specific overexpression of CKmito prevents TAC-induced decline in in vivo cardiac energetics, modestly enhances contractile function, and significantly limits the development of LVH and ventricular dilatation. In contrast, overexpression of the cytosolic, myofibrillar CK isoform did not limit LVH or ventricular dilatation. Mitochondrial CK overexpression reduces mitochondrial ROS, attenuates the increase in left ventricular (LV) ROS following TAC, upregulates several antioxidant enzymes in failing murine hearts, and increases myocyte tolerance to exogenous oxidative stress. The expression of several pro-hypertrophic signaling molecules is attenuated with CKmito overexpression while that of the regulator, protooncogene, cMyc, is suppressed by CKmito but not by CKmyofib overexpression. When ATP flux through the CK reaction is reduced by depleting creatine, a CK substrate, CKmito overexpressing mice are no longer protected from pathologic hypertrophy and adverse remodeling following TAC. The results demonstrate that the mitochondrial ADP regenerating role of CKmito and not the protein, per se, is vital for attenuating pathologic cardiac remodeling. Taken together, the data indicate that CKmito resides at the interface of subcellular energetics, redox balance, and adverse remodeling in the failing heart. Thus, strategies that maintain mitochondrial CK activity may offer a metabolic approach for reducing pathologic HF remodeling, its consequences, and attendant morbidity and mortality.
METHODS
Data availability:
The data that support the findings of this study are available from the corresponding author upon reasonable request. The authors have full access to all the data in the study and take responsibility for its integrity and the data analysis.
Study subjects:
The study was performed at the Johns Hopkins Hospital, approved by its Institutional Review Board, and all participants provided written consent. Twenty-seven patients (age = 45±14 yrs, mean±SD) with a history of HF (New York Heart Association, NYHA, class I-III) and reduced LV EF (≤45%) were enrolled, as previously described.14 Fourteen age-matched healthy subjects (age = 42±18 yrs) with no heart disease history, diabetes, or hypertension were enrolled as controls. The initial report of these subjects focused on cardiac work and contractile abnormalities and contained additional demographic information.14 After publication, the data were subsequently analyzed to test for a relationship between altered energy metabolism and adverse remodeling. That previously unpublished analysis appears here. Further details appear in the Supplement.
Transgenic mouse lines:
Two types of mice were generated: first, those with a transgene for the regulatory protein tTA (tetracycline-controlled transactivator) under the control of the α-MHC promoter; and second, mice expressing the CKmito transgene under the control of tetracycline-responsive element (TRE) following microinjection of the CKmito construct into fertilized mouse embryos (C57BL/6 strain). After crossing of TRE-CKmito mice with α-MHCtTA mice, double transgenic mice (CKmito-tTA) were confirmed by genotyping, and CKmito transgene induction was achieved by a diet free of doxycycline (designated CKmito overexpressors). Control mice (hereafter called “WT”) were littermates containing either the tTA or CKmito transgene alone. Guanidinoaceate N-methyltransferase knock-out (GAMT−/−) mice were created as previously described and provided as a gift.20 Animals were assigned an alphanumeric code so that investigators were blinded to the genetic background and group assignment of each mouse. Additional details appear in the Supplement.
Interventions to induce in vivo hypertrophy/heart failure:
Eight to 16-week-old male mice underwent sham or TAC surgery as previously described.5,15 Chronic isoproterenol administration was accomplished by subcutaneous implantation of Alzet osmotic mini-pumps containing either 0.0125mg/kg of isoproterenol or saline. Details appear in the Supplement.
Animal experiments:
The methods for all experiments are described in detail in the Supplement. A statistical power analysis identified the target number of animals to demonstrate significance at an expected difference of roughly 25% and a 15% variation (based in part on CK flux and LV mass differences between groups).
Statistical Analysis:
All statistical analyses are summarized in the Online Supplement and specific tests and results show in Supplement Table SI.
RESULTS
Left ventricular hypertrophy and adverse remodeling in human heart failure are associated with reduced myocardial ATP levels and rates of ATP synthesis through cardiac CK
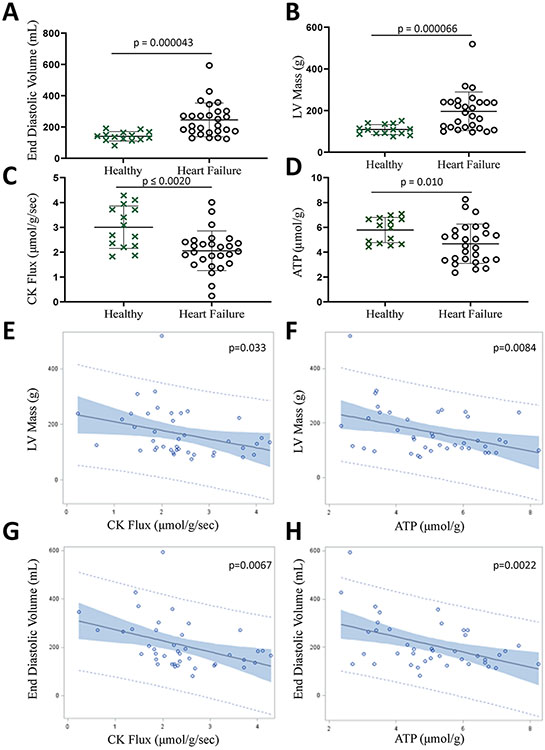
Contractile dysfunction in human HF is associated with impaired myocardial energy metabolism14. However, a link between adverse remodeling, another well-recognized consequence of myocardial injury, and impaired myocardial energy metabolism has not been studied in the failing human heart. First, we measured cardiac energetics and LV remodeling in twenty-seven patients with HF using noninvasive phosphorus 31P MRS and 1H cine MRI, respectively, and compared the results with those of fourteen healthy subjects. HF patients had a clinical history of HF, contractile dysfunction with LV ejection fraction (EF)≤45%, and no history or findings of significant ischemic heart disease or infarction, factors known to disrupt mitochondrial function and potentially exaggerate any perceived role of impaired energy metabolism. As previously reported in these subjects,14 myocardial mass and end-diastolic volumes (EDV) were increased, and [ATP] and the rate of ATP synthesis through CK were reduced in HF patients as compared to healthy subjects (Fig 1A-D). The novel and previously unpublished observations here are that critical indices of pathologic hypertrophy (measured by LV mass) and of ventricular dilatation (measured by EDV) correlated significantly with lower in vivo myocardial [ATP] and rates of ATP synthesis through myocardial CK (Fig 1E-H). This new evidence that established indices of adverse remodeling in human HF are closely correlated with reductions in simultaneous in vivo measures of ATP and myocardial CK energetics suggests a role for reduced CK energy metabolism in adverse remodeling in human HF.
Figure 1. Pathologic hypertrophy and adverse remodeling are associated with decreased cardiac energy metabolism in HF patients.
Cardiac end-diastolic volume (A) and left ventricular (LV) mass (B) measured by in vivo MRI were higher, and cardiac CK flux (C) and ATP levels (D) measured by 31P MRS were lower in HF patients as compared to healthy subjects. Pathologic remodeling was associated with energy deficits as noted by correlations between LV mass and CK flux (E, p=0.033); LV mass and ATP levels (F, p=0.0084); end-diastolic volume and CK flux (G, p=0.0067); and end-diastolic volume and ATP levels (H, p=0.0022). Graphs show data points for individual participants. Data were tested for normality using the Kolmogorov-Smirnov test for normality and analyzed by two-tailed Student’s t-test (A-D) or a linear regression model (E-H) (n = 14 (healthy controls), 27 (heart failure patients). The error bars represent ±SEM.
Mitochondrial CK counters maladaptive cardiac hypertrophy
These human data do not distinguish the contributions of different CK isoforms to in vivo CK activity (as is the case for all prior in vivo 31P MRS studies) and they do not demonstrate whether impaired cardiac energetics (lower ATP and/or CK flux) contribute to pathologic hypertrophy and adverse remodeling or is simply one of many consequences. To probe this relationship, we studied a murine model of HF induced by TAC that results in progressive LVH, contractile dysfunction, and eventual dilatation, as well as energetic and CK abnormalities similar to those observed in human HF.12,13 The goal was to determine whether attenuating the decline of mitochondrial CK in the failing heart by transgenic CKmito overexpression improves energetics and limits adverse remodeling.
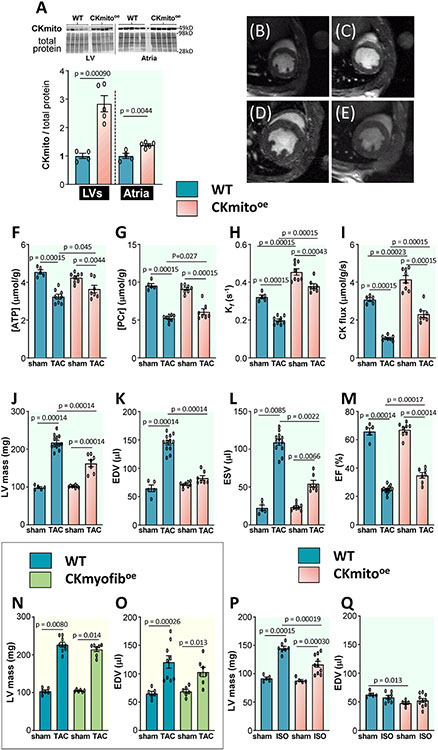
Mice conditionally overexpressing cardiac-specific mitochondrial CK were created and exhibited CKmito protein levels nearly three-fold in wild-type (WT) mice (Fig 2A). The additional CKmito was confirmed to be co-localized to the mitochondria, and the levels of CKmyofib and CKB isoenzymes were unchanged (Supplement S1A-C). CKmito hearts at baseline exhibited no changes in myocardial mass, dimensions, or energetics, apart from increased in vivo rates of ATP synthesis through CK (Fig 2). However, 9-10 weeks following TAC surgery, CKmito hearts exhibited less energetic decline in vivo and higher ATP synthesis rates through CK than did WT TAC hearts (Fig 2F-I). This improved energetic status in CKmito TAC hearts occurred with modestly better in vivo contractile function and significantly less LVH and dilatation than in WT TAC (Fig 2J-M). Specifically, mean LV mass and EDV following TAC were about 30% and 50% lower, respectively, in CKmito than in WT hearts (Fig 2JK). In contrast, the overexpression of the myofibrillar CK isoform did not attenuate the increase in LV mass or adverse remodeling (EDV) after TAC as previously reported, but the data are shown here for the first time (Fig 2NO).15
Figure 2. Cardiac-specific overexpression of mitochondrial creatine kinase (CKmito) improves in vivo cardiac energetics and attenuates pathologic remodeling in failing murine hearts.
Tissues were isolated from WT and cardiac-specific CKmito overexpressing (CKmitooe) mice. (A) Expression levels of CKmito were normalized to total protein and presented as relative to the amount of CKmito detected in WT hearts (experimental replicates: n=4 (WT LV or atria), n=5 (CKmito LV or atria)). Representative transverse in vivo 1H MRI of mice at the mid-left ventricle showing (B) WT sham, (C) CKmitooe sham, (D) WT TAC, and (E) CKmitooe TAC. In vivo cardiac 31P MRS and MRI measures of (F) ATP and (G) phosphocreatine (PCr) levels; (H) CK forward pseudo-first-order rate constant (Kf); (I) CK flux; (J) left ventricular (LV) mass; (K) end-diastolic volume (EDV); (L) end-systolic volume (ESV); and (M) ejection fraction (EF) for WT or CKmitooe mice with or without TAC (experimental replicates: n=5 (WT sham), n=8 (WT TAC or CKmitooe sham), and n=7 (CKmitooe TAC) for F-I and n=5 (WT sham), n=12 (WT TAC), n=8 (CKmitooe sham), and n=7 (CKmitooe TAC) for J-M), (N) LV mass and (O) EDV for WT or cardiac-specific myofibrillar CK overexpressing (CKmyofiboe) mice with or without TAC (experimental replicates: n=6 (WT sham or CKmyofiboe sham), n=9 (WT TAC), n=7 (CKmyofiboe TAC)); and (P) LV mass, and (Q) EDV for WT or CKmitooe mice following chronic isoproterenol (ISO) or saline infusion (experimental replicates: n=5 (WT saline or CKmitooe saline), n=7 (WT ISO), and n=10 (CKmitooe ISO)). Graphs show data points for individual mice. Data were tested for normality using the Kolmogorov-Smirnov test for normality and analyzed by two-tailed Student’s t-test (A), two-way ANOVA followed by Tukey’s post-hoc multiple comparison tests (F-K, M, O-Q), or Wilcoxon signed rank test followed by pair-wise, two-sided multiple comparison analysis (Dwass, Steel, Crichlow-Fligner Method) (L, N). The error bars represent ±SEM.
At 9-10 weeks post-TAC (Fig 2), WT TAC mice exhibit cardiac dysfunction (EF), LV remodeling, and reductions in cardiac CK flux similar to that present in ambulatory patients with NYHA Class II-III heart failure (Fig 1). To gain insight into the role of CKmito overexpression at an earlier stage of pathologic remodeling, we also studied mice just 2-3 weeks following TAC. In these studies, we observed less hypertrophy (LV mass/body weight) and remodeling (LVID/body weight) and a trend for higher EF (p=0.080) in CKmito TAC versus control TAC (Supplement S1D-H). Thus, there is a signal for CKmito overexpression protection even at the earliest stages of pathologic remodeling in 2-3 week TAC hearts (Supplement S1D-H) and significant attenuation of pathological remodeling and improved function at 9-10 weeks post-TAC (Fig 2) when control animals exhibit remodeling, function, and metabolism similar to the findings of patients with heart failure.
To determine whether CKmito overexpression also attenuates hypertrophy in other pathologic settings, we observed that the increase in LV mass resulting from the chronic infusion of the β1/β2 agonist, isoproterenol (ISO), was also significantly attenuated by CKmito overexpression (Fig 2P). Because ISO did not cause LV dilatation in WT over the time interval studied (Fig 2Q), the remainder of the experiments focused on the 9-10 week TAC model that manifests both pathologic hypertrophy and ventricular dilatation (remodeling) akin to that observed in human HF. Nevertheless, the ISO experiments are important because ISO is an established model of LVH and the findings demonstrate that attenuation of LVH by CKmito preservation is not limited to a single model. Thus, attenuating the energetic decline in failing hearts by overexpressing CKmito improves in vivo ATP synthesis through cardiac CK, high-energy phosphate levels, and LV function and significantly mitigates LV hypertrophy development in two models and limits pathologic dilatation after TAC. Attenuation of hypertrophy and dilatation after TAC was not observed with myofibrillar CK overexpression. Taken together, we interpret these findings to indicate a specific role for CKmito in limiting pathologic hypertrophy and adverse remodeling.
Mitochondrial CK limits myocardial redox imbalance in HF
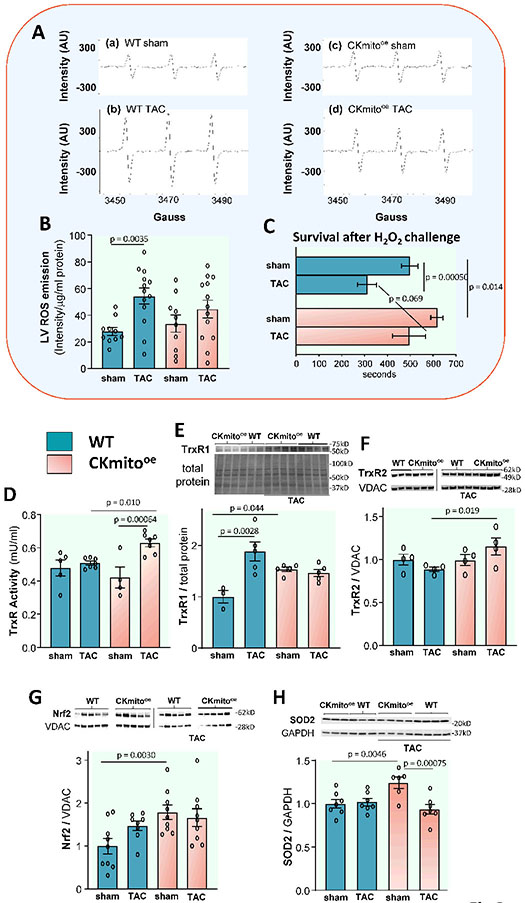
Oxidative stress can be a significant determinant of pathologic remodeling, and the mitochondria are a substantial source of endogenous ROS. To determine whether TAC exacerbates ROS production in the heart, and if so, whether CKmito overexpression counters this effect, we assessed myocardial tissue oxygen-derived free radicals using electron paramagnetic resonance (EPR) (Fig 3A). We observed a significant increase in ROS following TAC in WT LV but not in mice overexpressing CKmito (Fig 3AB). To determine whether overexpressing CKmito renders the cardiac myocyte more resistant to ROS, we exposed myocytes isolated from WT and CKmito mice to exogenous H2O2. We found that survival after H2O2 exposure was shortened by TAC in WT myocytes. However, survival after H2O2 in TAC CKmito myocytes was not shortened and was not significantly different than that of sham myocytes (Fig 3C). Next, we measured major cellular antioxidants, starting from the thioredoxin (Trx) system, since thioredoxin 1 is also a well-known endogenous negative regulator of cardiac hypertrophy.21 Because the efficiency of Trx in quenching H2O2 via peroxiredoxins is determined by the activity of thioredoxin reductase (TrxR),22 we measured the levels of TrxR protein abundance and total TrxR activity. We found both TrxR2 expression and total TrxR activity significantly enhanced after TAC in the CKmito hearts (Fig 3D-F). To better understand why TrxR protein and activity are increased in CKmito hearts, we assessed nuclear factor erythroid-2 related factor 2 (Nrf2), a master regulator of the endogenous antioxidant defense system that transcriptionally regulates the cellular Trx system (i.e. Trx and TrxR, in particular TrxR1).23 We observe that Nrf2 is significantly upregulated even at baseline in CKmito hearts (Fig 3G). Superoxide dismutase (SOD) content was higher in CKmito hearts before TAC (Fig 3H). This set of data shows that resistance to ROS is lower in TAC-failing myocytes but maintained in those from CKmito overexpressing hearts and that the latter occurs with, and likely results from, an increase in antioxidant proteins. In concert, there is a significant increase in tissue ROS levels with TAC in WT failing hearts but not in CKmito overexpressors.
Figure 3. Cardiac-specific overexpression of mitochondrial creatine kinase (CKmito) attenuates reactive oxygen species (ROS) due to TAC heart failure.
(A) Representative electron paramagnetic resonance (EPR) spectra showing oxygen-derived free radicals detected in left ventricles (LV) from WT (a) sham or (b) TAC hearts, and LVs from cardiac-specific CKmito overexpressing (CKmitooe) (c) sham or (d) TAC hearts. (B) ROS production measured by EPR in LVs of WT or CKmitooe sham or TAC hearts (experimental replicates: n=10 (WT sham or CKmitooe sham) and n=13 (WT TAC or CKmitooe TAC)). (C) Cardiomyocytes isolated from WT‡ or CKmitooe sham or TAC hearts were exposed to H2O2 (50 μM) for 700 s, and the time to irreversible arrhythmia/cell death was monitored (experimental replicates: n=20 cells isolated from 6 mice (WT sham), n=6 cells isolated from 2 mice (WT TAC), n=20 cells isolated from 5 mice (CKmitooe sham), and n=10 cells isolated from 4 mice (CKmitooe TAC)). Note that the same WT cardiomyocytes were employed for Fig 3C and Supplement Figure S4E since the experiments were conducted on the same day and under the same experimental conditions. (D) Thioredoxin reductase (TrxR) activities determined in WT or CKmitooe sham or TAC heart LV homogenates in the presence of excess NADPH (experimental replicates: n=5 (WT sham), n=7 (WT TAC or CKmito TAC), and n=4 (CKmito sham)). Representative immunoblots and summary of data showing LV expression levels of: (E) TrxR1; (F) TrxR2; (G) Nrf2 (nuclear factor erythroid 2-related factor 2); and (H) SOD2 (superoxide dismutase 2) normalized to total protein, GAPDH (glyceraldehyde 3-phosphate dehydrogenase), or VDAC (voltage-dependent anion channel) and presented as relative to the amount of protein detected in sham WT hearts (experimental replicates: n=3 (WT sham) and n=5 (WT TAC, CKmito sham, or CKmito TAC) (E); n=4 per group (F); n=9 (WT sham, CKmito sham, or CKmito TAC) and n=8 (WT TAC) (G); n=7 (WT sham, WT TAC, or CKmito TAC) and n=6 (CKmito sham) (H). Graphs show data points for individual mice (B, D-H). Data were tested for normality using the Kolmogorov-Smirnov test and analyzed by two-way ANOVA followed by Tukey’s post-hoc multiple comparison test (B, D-H) while a generalized estimating equation model was used to take into account the correlation of within-subject data (C). The error bars represent ±SEM.
CKmito overexpression improves mitochondrial function and reduces proton leak
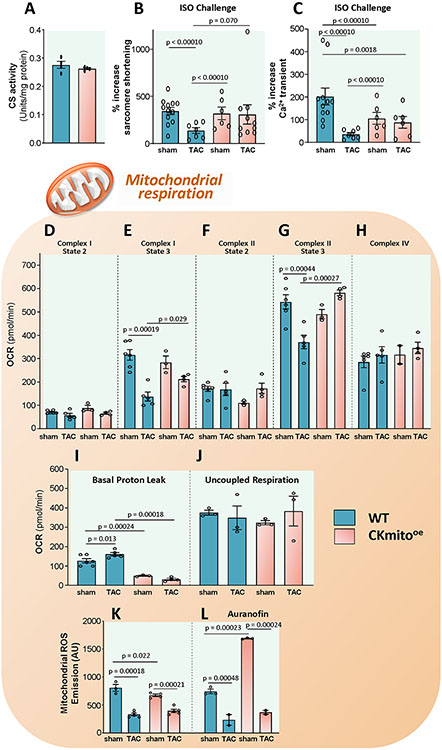
Mitochondria are a primary source of intracellular ROS and host the mitochondrial CK isoform. The in vivo data above demonstrate improved energy balance in CKmito overexpressing failing hearts (Fig.2), suggesting a central role for mitochondria. Therefore, we investigated isolated mitochondrial function, also to rule out possible confounding extra-mitochondrial factors, such as energy demand differences under hemodynamic load. We observed that total mitochondrial mass is likely unchanged in CKmito overexpressing mice, as evidenced by unchanged citrate synthase activity (Fig 4A). We assessed mitochondrial respiration supported by substrates of Complex I (Glutamate/Malate), Complex II (Succinate/Rotenone) and Complex IV (TMPD). TAC reduced ADP-stimulated (State 3) respiration in WT mitochondria for both Complexes I and II. However, there was no statistically significant decline in Complex I or Complex II after TAC in CKmito overexpressing mitochondria. There was no statistically significant difference in Complex IV activity among the groups, ruling out any artifacts related to aberrant loading or mitochondrial damage during isolation. In addition, mitochondrial proton leak (in the presence of Oligomycin to block the ATP Synthase) was significantly lower, at baseline and with TAC, in mitochondria from CKmito overexpressors (Fig 4I) while maximal uncoupled respiration also did not differ statistically across groups (Fig 4J). Thus, CKmito overexpression prevents TAC-induced mitochondrial function decline and improves coupled respiration.
Figure 4. Cardiac-specific overexpression of mitochondrial creatine kinase (CKmito) improves isolated mitochondrial function and reduces proton leak.
(A) Citrate synthase (CS) activities, an index of total mitochondrial mass, analyzed in WT, and cardiac-specific CKmito overexpressing (CKmitooe) heart lysates (n=4 per group). Cardiomyocytes isolated from WT or CKmitooe sham or TAC hearts were stimulated with isoproterenol (ISO, 2.5 nM, 10 min) and percent increase in: (B) sarcomere shortening and (C) Ca2+ transients were monitored (experimental replicates: n=12 cells isolated from 3 mice (WT sham), n=7 cells isolated from 2 mice (WT TAC), n=6 cells isolated from 2 mice (CKmitooe sham), and n=10 cells isolated from 6 mice (CKmitooe TAC) cells (B) and n=11 cells isolated from 3 mice (WT sham), n=7 cells isolated from 2 mice (WT TAC), n=6 cells isolated from 2 mice (CKmitooe sham), and n=6 cells isolated from 2 mice (CKmitooe TAC) (C)). Mitochondria were isolated from WT and CKmitooe sham or TAC hearts. Summary of data showing oxygen consumption rate (OCR) for: (D) complex I state 2; (E) complex I state 3; (F) complex II state 2; (G) complex II state 3; and (H) complex IV; (I) basal proton leak; and (J) uncoupled respiration (experimental replicates: n=6 (WT sham), n=5 (WT TAC), n=3 (CKmito sham), and n=4 (CKmito TAC) (D-G, I); n=6 (WT sham), n=5 (WT TAC), n=2 (CKmito sham), and n=4 (CKmito TAC) (H); n=3 per group (J)). Mitochondrial reactive oxygen species (ROS) emission in the (K) absence or (L) presence of auranofin (20nM) (experimental replicates: n=3 (WT sham), n=5 (WT TAC or CKmito TAC), and n=4 (CKmito sham) (K); n=3 (WT sham or CKmito sham) and n=2 (WT TAC or CKmito TAC) (L)). Graphs show data points for individual mice (A, D-L) or individual cells (B, C). Data were tested for normality using the Kolmogorov-Smirnov test for normality and analyzed using a two-tailed Student’s t-test (A), a generalized estimating equation model was used to take into account the correlation of within-subject data (B, C), or two-way ANOVA followed by Tukey’s post-hoc multiple comparison test (D-L). The error bars represent ±SEM.
Next, we directly measured ROS in isolated mitochondria. Mitochondrial ROS emission was significantly lower at baseline in CKmito overexpressors than in WT mitochondria and declined with TAC in both CKmito and WT (Fig 4K). Of note, when the TrxR inhibitor, auranofin, was administered, mitochondrial ROS emission increased dramatically in CKmito OE but not WT mitochondria, indicating a critical role for augmented TrxR in CKmito overexpressing mitochondria in buffering mitochondrial ROS (Fig 4L).
To assess the functional implications of these biochemical findings, we challenged myocytes obtained from WT and CKmito mice, at baseline or after TAC, with isoproterenol (ISO). We found that ISO-induced contractile response was severely blunted in isolated WT TAC myocytes but not in the CKmito overexpressing TAC cells (Fig 4B). Since ROS can oxidatively modify proteins implicated in myocyte Ca2+ handling and thereby reduce Ca2+ cycling, we also measured myocyte whole-cell Ca2+ transients after ISO stimulation. The Ca2+ transient response to ISO declined in WT TAC myocytes (Fig 4C) but was preserved in CKmito TAC myocytes, compared to their respective non-failing controls. In summary, the overexpression of mitochondrial CK does not result in a statistically significant change in mitochondrial mass but preserves Complex I and II-supported oxidative phosphorylation after TAC and reduces proton leak, offsetting the energetic deficits observed in HF.24,25 These favorable events likely account for the preserved Ca2+-cycling and contractile reserve seen in TAC CKmito myocytes upon ß-adrenergic challenge. In addition, CKmito overexpression significantly reduces mitochondrial ROS at baseline, through enhanced TrxR mechanisms.
Local energy recycling mechanisms are required to explain CKmito anti-hypertrophic effects: evidence from creatine deficient mice
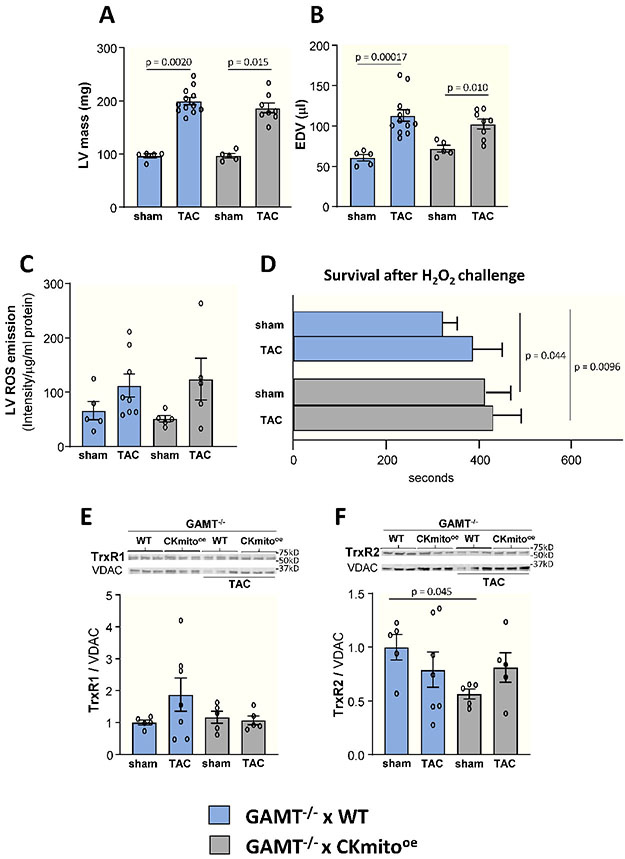
First, to test the hypothesis that limiting the ADP regenerating capacity of CKmito worsens pathologic remodeling, we used mice deficient in creatine, a substrate for the CK reaction, and compared creatine-deficient guanidinoacetate N-methyltransferase knock-out (GAMT−/−) mice with control mice following TAC. Creatine-deficient GAMT−/− mice were smaller than WT mice and exhibited worse LV function following TAC (EF, p=0.060) and more pathological remodeling with higher LV mass (p=0.0028) and EDV (p=0.072) than WT TAC mice when corrected for body weight (Supplement S2D-E). LV ROS levels were also higher in creatine-deficient GAMT−/− mice than in WT both at baseline and following TAC (Supplement S2G).
Second, to distinguish whether the impact of CKmito overexpression to limit pathological LV remodeling is tied to its well-known energetic effects of regenerating ADP for mitochondrial ATP synthesis or due to other “non-metabolic” stabilizing effects of the CKmito protein per se, we pursued orthogonal experiments. We speculated that, given its compartmentalization, CKmito might have protein membrane-stabilizing and other effects that contribute to the attenuation of pathologic hypertrophy and adverse remodeling, independent of its ability to regenerate ADP.26 To answer this question, we crossed CKmito-overexpressing mice with creatine-deficient guanidinoacetate N-methyltransferase knock-out (GAMT−/−) mice. The resultant double transgenic mice (GAMT−/−xCKmito) exhibited increased CKmito protein content but lower ATP flux through the CK reaction due to CK substrate depletion. ATP synthesis through cardiac CK in double transgenics was not detectable by in vivo 31P MRS studies, as anticipated. Unlike the observations in CKmito-overexpressing mice (Fig 2JK), CKmito overexpression in GMAT−/− mice did not attenuate the development of pathologic hypertrophy or adverse remodeling when the CK reaction was inhibited by creatine substrate depletion (Fig 5AB) in that in vivo LV mass and EDV did not differ between GAMT−/−xWT vs. GAMT−/−xCKmito hearts following TAC (Fig 5BC). Likewise, the TAC-induced increase in tissue ROS was no longer attenuated, and intrinsic antioxidants were no longer augmented when the CK energy reaction was substrate-limited in CKmito-overexpressors (GAMT−/−xCKmito vs. GAMT−/−xWT hearts, Fig 5). Thus, overexpressing the CKmito protein was ineffective in countering ROS bursts and preventing hypertrophy and adverse remodeling when ADP regeneration and ATP recycling through mitochondrial CK were attenuated by creatine substrate deprivation. Local energy production and recycling by CKmito in the failing heart, and not just the presence of the protein, is required for attenuating pathologic hypertrophy and adverse remodeling.
Figure 5. Cardiac-specific overexpression of mitochondrial creatine kinase (CKmito) does not attenuate pathologic remodeling and ROS burden in creatine-deficient mice.
Creatine-deficient guanidinoacetate N-methyltransferase (GAMT) knock-out mice were crossed with cardiac-specific CKmito overexpressing (GAMT−/− x CKmitooe) or WT (GAMT−/− x WT) mice. Cardiac (A) left ventricular (LV) mass and (B) end-diastolic volume (EDV) for GAMT−/− x CKmitooe and GAMT−/− x WT mice with or without TAC were determined by in vivo MRI (experimental replicates: n=5 (GAMT−/− x WT sham or GAMT−/− x CKmitooe sham), n=12 (GAMT−/− x WT TAC), and n=8 (GAMT−/− x CKmitooe TAC) (A-B). (C) Reactive oxygen species (ROS) production measured by EPR spectroscopy in LVs of GAMT−/− x CKmitooe and GAMT−/− x WT sham or TAC hearts (experimental replicates: n=5 (GAMT−/− x WT sham, GAMT−/− x CKmitooe sham, GAMT−/− x CKmitooe TAC) and n=8 (GAMT−/− x WT TAC). (D) Cardiomyocytes were isolated from GAMT−/− x CKmitooe, and GAMT−/− x WT sham or TAC hearts were exposed to H2O2 (50 μM) for 700 s and time to irreversible arrhythmia/cell death was monitored (experimental replicates: n=19 cells isolated from 3 mice (GAMT−/− x WT sham), n=12 cells isolated from 2 mice (GAMT−/− x WT TAC or GAMT−/− x CKmitooe TAC), and n=18 cells isolated from 3 mice (GAMT−/− x CKmitooe sham)). Representative immunoblots and summary of data showing LV expression levels of (E) TrxR1 (thioredoxin reductase 1) and (F) TrxR2 (thioredoxin reductase 2) normalized to VDAC (voltage-dependent anion channel) and presented as relative to the amount of protein detected in sham GAMT−/− x WT hearts (experimental replicates: n=5 (GAMT−/− x WT sham, GAMT−/− x CKmitooe sham, GAMT−/− x CKmitooe TAC) and n=7 (GAMT−/− x WT TAC) (E-F)). Graphs show data points for individual mice (A-C, E-F). Data were tested for normality using the Kolmogorov-Smirnov test for normality and analyzed by Wilcoxon signed rank test followed by pair-wise, two-sided multiple comparison analysis (Dwass, Steel, Crichlow-Fligner Method) (A), two-way ANOVA followed by Tukey’s post-hoc multiple comparison test (B-C,E-F), or a generalized estimating equation model was used to take into account the correlation of within-subject data. (D). The error bars represent ±SEM.
CKmito enhances the balance of pro-/anti-hypertrophic transcription factor in pressure overloaded hearts
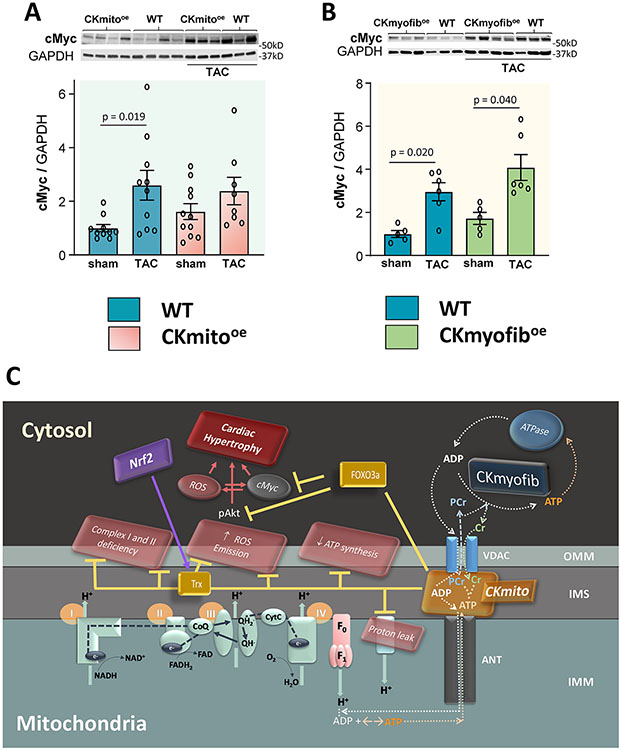
The links between cardiac energetics, redox balance and transcription factors previously reported to regulate myocardial hypertrophy are likely complex and incompletely understood. Thus, as both a hypothesis-generating endeavor and to place in context how the new CKmito energetic observations above relate to the considerable prior literature on molecular signaling in the development of hypertrophy, we evaluated changes in several hypertrophic signaling candidates. Besides the Trx system reported above, at least two other systems are known to sit at the intersection between hypertrophy and cardiac redox conditions. These are forkhead box O3 (FOXO3) and the protooncogene, cMyc. We observed that several hypertrophic transcription factors tracked in similar directions in both CKmito and CKmyofib overexpressing hearts despite the differing impact of CKmito and CKmyofib on pathologic hypertrophy, including protective FOXO3a and Mad1 which were both higher in CKmito and CKmyofib overexpressors than in WT following TAC (Supplement S3). Likewise, we examined the status of another ROS-sensitive transcription factor, GATA-4, and found that, in agreement with previous reports,27,28 GATA-4 expression is markedly upregulated in WT TAC hearts but not in CKmito or CKmyofib mice (Supplement S3). In contrast, we observed that the expression of cMyc is increased in WT hearts after TAC, consistent with previous findings,29-34 but that this up-regulation is attenuated in the presence of CKmito overexpression (Fig 6A) and not attenuated by CKmyofib overexpression (Fig 6B).
Figure 6. Overexpression of mitochondrial creatine kinase (CKmito) maintains pro-/anti-hypertrophic transcription factor balance in TAC failing hearts.
Left ventricular (LV) tissue from WT, cardiac-specific CKmito overexpressing (CKmitooe), or cardiac-specific myofibrillar CK overexpressing (CKmyofiboe) sham or TAC hearts was isolated and analyzed. Representative immunoblots and summary of data showing expression levels of cMyc (Myc protooncogene protein) in: WT, CKmitooe (A); or CKmyofiboe (B) sham or TAC hearts normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and presented as relative to the amount of protein detected in sham WT hearts (experimental replicates: n=10 (WT sham or WT TAC), n=11 (CKmitooe sham), and n=8 (CKmitooe TAC) (A); n=5 (WT sham or CKmyofiboe sham) and n=6 (WT TAC or CKmyofiboe TAC) (B). Data were tested for normality using the Kolmogorov-Smirnov test for normality and analyzed by non-parametric Kruskal-Wallis test, followed by pair-wise, two-sided multiple comparison analysis (Dunn method with Benjamini-Hochberg adjustment). The error bars represent ±SEM. (C) Scheme summarizing the attenuation of cardiac hypertrophy and dysfunction during pressure overload in CKmitooe hearts. Abbreviations: OMM (outer mitochondrial membrane), IMM (inner mitochondrial membrane), Trx (thioredoxin), ROS (reactive oxygen species), Nrf2 (nuclear factor erythroid 2-related factor 2), CoQ (coenzyme Q), QH• (semiquinone), QH2• (ubiquinol), CytC (cytochrome C), F1F0 (F1F0-ATP synthase), ANT (adenine nucleotide translocator), VDAC (voltage-dependent anion channel), CKmyofib (myofibrillar creatine kinase), CKmito (mitochondrial creatine kinase)
Finally, we tested whether, in addition to the direct modulation of pro- and anti-hypertrophic redox-sensitive genes, CKmito could also interfere with post-translational modifications of kinases involved in the regulation of myocardial growth in response to stress. Phosphorylated and total mammalian target of rapamycin (mTOR) and mitogen-activated protein kinase (ERK), did not differ between WT and CKmito hearts (Supplement S4). Thus, CKmito overexpression attenuates the TAC-induced increase in redox-dependent activation of pro-hypertrophic signaling (c-Myc and GATA-4), boosts key antioxidant defenses with anti-hypertrophic properties (Trx), and attenuates the decline in protective FOXO3 levels, factors at the crossroads of oxidant stress and nutrient signaling.
DISCUSSION
This work demonstrates for the first time to our knowledge that pathologic hypertrophy and adverse remodeling in human HF directly correlate with decreased in vivo myocardial ATP levels and reduced rates of ATP synthesis through CK, the primary muscle energy reserve reaction. While such energetic changes could, in theory, be a consequence of hypertrophy and adverse remodeling, more basic murine studies here demonstrate that reductions in the mitochondrial isoform, but not the myofibrillar isoform, of CK, contribute to pathologic hypertrophy and remodeling. The mitochondrial CK isoform was previously reported to be reduced in experimental HF across species and in human HF.8,35,36 We now show that augmenting mitochondrial CK limits murine pathologic hypertrophy resulting from either chronic adrenergic stimulation or that due to TAC. The murine TAC HF model manifests similar contractile and energetic characteristics to those of human HF,5,12,15 and transgenic overexpression of cardiac mitochondrial CK limits the declines in cardiac ATP and the rate of ATP synthesis through CK. Importantly, it mitigates the development of pathologic hypertrophy and adverse ventricular dilatation. This is in contrast to attenuating the loss of the cytosolic myofibrillar form of CK (CKmyofib) which, in a prior report, did not alter pathologic remodeling.15 These new data also signal that CKmito overexpression attenuates earlier stages of pathologic hypertrophy, such as those observed after only 2-3 weeks after TAC (Supplement S1D-H).
To better understand the mechanism by which the mitochondrial CK isoform limits pathologic hypertrophy and remodeling, we first measured tissue ROS, a potent stimulus of pathologic hypertrophy often produced in mitochondria and increased by a dysfunctional mitochondrial mismatch of oxidative metabolism and ATP synthesis. We observed that CKmito overexpression attenuates the increase in left ventricular tissue ROS during HF and affords isolated myocytes increased resistance to exogenous ROS-induced cell death. Our data also show that CKmito augments antioxidant defenses, as demonstrated by augmented TrxR protein and activity and increased SOD, but also lowers mitochondrial ROS emission and improves in vivo and in vitro mitochondrial function in the failing heart. The importance of metabolic function is demonstrated by the observation that limiting CKmito ADP regeneration by depleting creatine, worsens pathologic remodeling (Supplement S2) and negates the ability of CKmito overexpression per se to attenuate pathologic hypertrophy in the failing heart (Fig 5A-B). The intersection of cardiac CK and several established pro-hypertrophic signaling pathways were studied. Although several transcription factors were unchanged by CKmito overexpression, the increase in pro-hypertrophic and ROS sensitive c-Myc was attenuated by CKmito overexpression, but not by CKmyofib overexpression in failing hearts. We interpret all of these data taken together to indicate that pathologic hypertrophy and adverse remodeling, established predictors of adverse clinical HF outcomes and mortality, are closely associated with reduced ATP levels and synthesis through CK in the failing human heart. Moreover, CKmito, which sits at the intersection of cardiac mitochondrial ADP regeneration and pathologic ROS generation, plays a critical role in maintaining energy metabolism, limiting ROS, and mitigating pathologic hypertrophy and adverse remodeling in the failing heart.
Multiple myocardial energetic metabolic pathways are altered in failing hearts, and several offer potential metabolic treatment targets.4,37 The hydrolysis of ATP is the biochemical energy utilized to sustain normal myocardial contractile and relaxation function and to fuel most myocellular processes. Cardiac ATP synthesis rates are the highest per gram of any organ in the body2, but because cardiac ATP stores are small relative to the demand, increases in energy demand due to augmented myocardial work or hypertrophy must be tightly matched by increased ATP synthesis.38 Most ATP is generated by phosphorylation of ADP, primarily via mitochondrial oxidative phosphorylation.39 The CK reaction is a more rapid source of ATP than that provided by oxidative phosphorylation. It is often considered the primary myocardial high-energy phosphate reserve reaction called upon during exercise and other forms of stress.2 CK rapidly and reversibly generates ATP at the myofibrils by phosphoryl transfer from creatine phosphate (PCr) to ADP and the reverse reaction generates ADP in the mitochondria with as much as 90% of ATP moving through CK.
Prior lines of experimental and human evidence link reduced ATP generation by the CK reaction to systolic and diastolic dysfunction, but the role of CK, if any, in adverse remodeling in HF was far less established. The former include observations that inhibiting or reducing CK in healthy hearts adversely limits contractile reserve,40,41 that reductions in CK metabolites and/or ATP synthesis through CK (hereafter termed “CK flux”) impair diastolic function in healthy, non-failing hearts,42,43 and that reduced CK metabolism (i.e., decreased CK flux, [ATP], [PCr], [Cr] and total CK activity) is observed in nearly all experimental HF models and in human HF.44-46 Most of our understanding of human myocardial high energy phosphate metabolism comes from studies using 31P MRS, the only noninvasive means to quantify high energy phosphates in the beating heart. In vivo 31P MRS saturation transfer studies identified more severe decreases in CK flux than in PCr/ATP in HF patients.11-13,47 However, those prior reports did not study remodeling12 or studied it in a different patient population (patients with hypertrophic cardiomyopathy of which only five had reduced ejection fraction) and did not find a significant relationship of CK flux with remodeling.13 Reductions in cardiac CK flux in the failing human heart are now recognized as independent predictors of adverse clinical HF outcomes48 and were more recently shown to have a close association with reduced contractile work and performance.14 In terms of the potential link between reduced CK energetics and adverse remodeling in HF, adverse remodeling following coronary ligation in pigs in the absence of HF was associated with reduced PCr/ATP whereas pigs with both adverse remodeling and HF had the lowest cardiac PCr/ATP.45 Moreover, in a porcine model of pressure-overload, the degree of hypertrophy was inversely related to cardiac PCr/ATP and the amount of both the myofibrillar and mitochondrial isoforms of CK, while the mitochondrial isoform content correlated directly with the rate of ATP synthesis through CK.49 Finally, reductions in cardiac PCr/ATP in the murine TAC model predict subsequent pathologic remodeling as evidenced by serial changes in EDV.5 In addition, it should be noted that myocardial PCr and ATP concentrations were directly measured with 31P MRS in both the human and murine studies in the current manuscript. Contemporary human 31P MRS studies that assume ATP concentrations or rely solely on PCr/ATP could underestimate the extent of metabolic abnormalities and potentially miss ATP depletion, here closely linked to pathologic remodeling.
Reductions in each CK isozyme with HF may have different consequences, although they catalyze the same reaction and have extensive sequence homology. The predominant CK isozymes in the healthy adult heart, CKmyofib and CKmito, are expressed in a tissue-specific fashion, have distinct intracellular locations, and differ in their activity.50,51 CKmyofib is a dimer that occurs in the cytosol, binds tightly to the M-band, and as such, is thought to represent a functional micro-compartment that facilitates myofibrillar ATP transfer and under physiologic conditions produces ATP.51 CKmito occurs predominantly as an octamer found in the mitochondrial intermembrane space in close proximity with the adenine nucleotide translocator and the mitochondrial periphery with the mitochondrial permeability transition pore complex (Fig 6C).50 CKmito under physiologic conditions results in ADP regeneration and is particularly sensitive to inactivation by oxidative injury50,52-54, which is associated with apoptosis.50 In HF models and human HF, the amount of CKmyofib and CKmito both decrease from normal, non-failing levels8,55. As noted above, the reduction in CKmito is associated with reductions in CK flux in experimental HF.49 Overexpression of cytosolic CKmyofib in the same TAC HF model used in the present studies improved in vivo cardiac energetics, LV systolic function, and survival15 but did not attenuate adverse remodeling (Fig 2NO). Conversely, overexpression of CKmito, shown here for the first time, attenuated adverse remodeling by limiting the increase in LV mass (Fig 2J) and ultimately attenuating an increase in EDV (Fig 2K) in hearts 9-10 week post TAC. CKmito overexpression also limited early pathologic remodeling after only 2-3 weeks following TAC (Supplement S1D-IH) and significantly attenuated the increase in LV mass following chronic adrenergic stimulation as well (Fig 2P). The ability of CKmito overexpression to differentially attenuate pathologic hypertrophy and adverse remodeling, as compared to CKmyofib overexpressors, is not due to an enhanced ability to increase in vivo CK flux to higher levels at baseline (4.49±1.20 vs 4.15±0.57 umol/g/sec, CKmyofib vs CKmito respectively, p=.48) or following TAC (2.70±0.86 vs 2.31±0.46, umol/g/sec, p=.32, CKmyofib vs CKmito respectively, Fig 2 and ref. 15).
The mechanism by which CKmito attenuates pathologic hypertrophy and adverse remodeling is related, at least in part, to its ability to regenerate ADP in the mitochondria. Depletion of the CK substrate, creatine, worsened pathologic remodeling in GAMT−/− TAC vs WT TAC mice (Supplement Figure S2A-E). Further, inhibition of the ADP regenerating capacity of the CK system by creatine substrate depletion completely abrogated the ability of CKmito overexpression to limit hypertrophy and ventricular dilatation in vivo (Fig 5AB, GAMT−/−). Thus, there was no evidence that “non-metabolic” or mitochondrial stabilizing effects of CKmito protein, per se, contributed to the attenuation of pathologic hypertrophy and adverse remodeling, despite its location spanning the inner and outer mitochondrial membranes. There is prior evidence that at least in the brain, CKmito plays a vital role as an antioxidant to reduce oxidative stress, and this ability is related to its capacity for ADP regeneration.26 Moreover, ROS and oxidative stress are well-established contributors to pathologic cardiac hypertrophy.56 We report here for the first time that CKmito overexpression attenuates the increase in tissue ROS that occurs in WT hearts following TAC and, importantly, improves myocyte survival following exogenous ROS exposure (Fig 3). We also observe that CKmito impacts myocardial redox balance in at least three ways. First, boosting CKmito helps maintain better mitochondrial coupling and less proton leak in the failing heart, as evidenced by the preserved Complex I/enhanced Complex II activities and reduced proton leak (Figure 4). The additional CKmito enhances ADP availability for ATP synthesis and along with improved Complex I/II and reduced proton leak improves coupling efficiency between respiratory oxygen to ADP phosphorylation and ATP generation, while reducing ROS generation (Fig 6C, scheme). Second, we show that CKmito overexpression reduces mitochondrial ROS (Fig 4K). Third, several major antioxidant pathways are increased with CKmito overexpression at baseline or in response to TAC, including Trx and SOD. In addition, several ROS-related pro-hypertrophic and anti-hypertrophic signaling molecules are down- or upregulated in CKmito hearts, respectively, as discussed below.
The link between transcription factors regulating myocardial hypertrophy, cardiac energetics and redox (i.e., ROS/antioxidant balance) conditions is complex and has yet to be fully elucidated. Besides the Trx system, at least two other systems are at the intersection between hypertrophy and cardiac redox conditions, and both of these involve cardiac metabolism as well: forkhead box O3 (FOXO3) and the protooncogene, cMyc. FOXO3a limits myocardial hypertrophy57, whereas cMyc promotes it.31,32 Of note, an inter-relationship exists between cMyc and ROS/tissue redox conditions, while FOXO3a is a well-known suppressor of myocardial hypertrophy58 and cMyc activity.59 FOXO3a directly antagonizes cMyc at promoters of nuclear-encoded mitochondrial genes,59 and via the suppression of cMyc-dependent genes, such as Mxi1 which belongs to the Mad/Mxd family.60 Moreover, FOXO3a sits at the intersection of oxidant stress61 and insulin/growth, and enhances antioxidant signaling.61 Here, we find that CKmito overexpression limits the TAC-induced rise in GATA-4 and cMyc. At the same time, CKmito nearly abrogates TAC-induced decline in FOXO3a. Taken together, the present data indicate that preserving CKmito in a heart prone to failure blunts ROS levels, increases tolerance to exogenous ROS, and likely does so by ameliorating mitochondrial dysfunction at different sites, enhancing mitochondrial ADP regeneration and reducing mitochondrial ROS. It should be emphasized that CKmito and CKmyofib overexpressing hearts exhibit no significant differences in ROS attenuation (Fig 3B and Supplement S5) and increased ROS tolerance (Fig 3C and Supplement S5) as well as FOXO3 and GATA4 changes (Supplement S3). Still, only CKmito attenuates increases in cMyc following TAC (Fig 6). This suggests that cMyc, the protooncogene sitting at the intersection of metabolism, redox, and hypertrophy, may contribute to mitochondrial CK attenuation of pathologic hypertrophy and remodeling. This latter evidence calls for more in-depth studies to define how CKmito differentially regulates various transcription factors.
CKmito overexpression in murine TAC hearts was recently reported to improve in vivo cardiac energetics (PCr/ATP) and show a trend of increased survival (p=0.08) but did not attenuate hypertrophy as measured by two-dimensional echocardiography six-weeks after TAC.62 There are three main differences between that study and the present work that may explain why we identified attenuated adverse remodeling with CKmito overexpression. First, the degree of hypertrophy and extent of adverse remodeling were higher in the current study, which may have afforded a higher likelihood of detecting differences. The echocardiography measures in the prior investigation were obtained only six weeks after TAC when the LVH had increased by ~60% and the ventricular dilatation by only ~25% in WT animals. However, in the current study, these were several-fold higher at 9-10 weeks post-TAC in WT animals, with an increase in LV mass by ~120% and ventricular dilatation by ~100% (Fig 2). Second, anatomic LV measures by MRI may be more sensitive and reproducible in HF than those by echocardiography since the former are volumetric and don’t rely on geometric assumptions. Thus the combination of more significant hypertrophy and remodeling measured with a volumetric imaging modality in the present study may have improved the detection of attenuated pathologic hypertrophy and remodeling in CKmito overexpressing hearts. Even so, we were able to detect a signal for attenuation of hypertrophy with CKmito overexpression at only 2-3 weeks after TAC by echocardiography (Supplement S1HI). Third, the amount of CKmito protein overexpression was higher in the current study (~3x WT) compared to the prior report (~1.5x WT).62,63 Thus robust, not marginal, CKmito-expression (~3x WT), as shown here, attenuates pathologic hypertrophy and adverse remodeling in the failing heart.
The present findings are novel, but there are limitations to acknowledge. The human studies were performed in a relatively small sample size (41 subjects). However, the cohort was still sufficient to detect significant reductions in cardiac [ATP] and CK flux between HF and healthy subjects and to identify their relationship with increased LV mass and dilatation. Because there are no demonstrated metabolic interventions which specifically and safely manipulate only a single CK isoform in the human heart, we performed studies in mice where specific CK isoform overexpression can be accomplished. Even though overexpression of CKmito improved cardiac energetics in TAC hearts, it did not fully restore ATP levels or rates of flux through CK to those of non-failing hearts. Thus the full impact of CKmito on pathologic hypertrophy and adverse remodeling may be underestimated by these studies. Alternative strategies to better limit the decline and oxidative damage to CKmito should be investigated. Although we characterized the ADP regenerating and ATP exchange properties of CKmito in protecting against adverse remodeling in HF, more basic work is needed to identify which of the signaling cascades or others are critical for the protective effect. Although one could question whether creatine deficiency per se induces an insult, prior studies reported unchanged mitochondrial organization, compartmentation of high-energy phosphates and more than 500 proteins, including those of the respiratory complexes in creatine-deficient GAMT−/− as compared to wildtype mouse hearts.64,65 Thus the loss of protection from adverse remodeling in CKmito hearts crossed with GAMT−/− creatine-deficient mice is best attributed to reduced ADP regeneration capacity rather than a potential insult not identified in several prior studies. Similar studies of loss of protection from adverse remodeling were not performed in CKmyofib hearts crossed with GAMT−/− mice since CKmyofib overexpression does not protect from pathologic remodeling (Figure 2N,O) like CKmito (Figure 2J,K). CKmito increases certain basal antioxidant activities and survival in response to H2O2 exposure and this may indicate that CKmito overexpression induces a stress response that may also contribute to benefit. We note that normality testing may not have been sufficiently powered for the smaller data sets and thus we provide both parametric and nonparametric testing in the data supplement for those data sets.
In summary, pathologic hypertrophy and adverse remodeling are well-established, clinically important predictors of adverse outcomes in patients with HF. Abnormalities in energy metabolism occur in the failing heart that were previously associated with contractile abnormalities, but the role, if any, of altered energy metabolism in human HF remodeling was less well characterized. The present observations are the first to identify a close relationship between pathologic hypertrophy and adverse remodeling with reduced cardiac ATP levels and synthesis through CK in the failing human heart. Moreover, it is not the cytosolic myofibrillar CK isoform, but the mitochondrial CK isoform, sitting at the intersection of cardiac mitochondrial ADP regeneration-ATP transfer and pathologic ROS generation, that plays a critical role in mitigating pathologic hypertrophy and adverse remodeling in the failing heart. Thus, mitochondrial CK offers a potential new target for improving depressed metabolism, reducing ROS and their consequences, and limiting adverse remodeling in heart failure.
Supplementary Material
Novelty and Significance:
What is known?
Heart failure is characterized by abnormalities in contraction/relaxation and pathologic ventricular remodeling, whereby the heart wall hypertrophies (thickens) and eventually dilates. The latter has been associated with adverse heart failure outcomes and increased mortality in heart failure patients.
Impaired cardiac energy metabolism occurs in heart failure and is closely related to contractile abnormalities. However, the role of impaired energy metabolism in heart failure-associated pathologic remodeling is not known.
What new information does this article contribute?
In patients with heart failure, pathologic ventricular hypertrophy and dilatation are closely associated with low myocardial ATP levels and reduced rates of ATP synthesis through creatine kinase, the primary energy reserve reaction.
In mouse models of hypertrophy and heart failure, rescuing the mitochondrial isoform of creatine kinase, rather than the cytosolic myofibrillar isoform, significantly limits the development of pathologic hypertrophy and adverse remodeling.
The ability of mitochondrial creatine kinase, sitting at the crossroads of myocardial energy production and redox balance, to regenerate ADP for mitochondrial ATP synthesis is critical for this protection from pathologic remodeling.
The findings demonstrate that metabolic strategies and interventions to improve mitochondrial health and mitochondrial creatine kinase activity are critical players in reducing pathologic remodeling in heart failure and the closely linked consequences of adverse clinical outcomes and increased mortality.
Cardiac mitochondrial energy metabolism is impaired in heart failure (HF), but its role in HF-related pathologic remodeling is poorly understood. Creatine kinase (CK), the primary muscle energy reserve reaction which rapidly provides ATP at the myofibrils (via a myofibrillar isoform, CKmyofib) and regenerates mitochondrial ADP (via a mitochondrial isoform, CKmito), is down-regulated in HF. We measured in vivo cardiac energetics and remodeling in people and observed that pathologic LV hypertrophy and dilatation correlate closely with reduced ATP levels and rates of ATP synthesis through CK. Metabolic rescue with cardiac-specific overexpression of either CKmyofib or CKmito was studied in mice for its impact on maladaptive hypertrophy. TAC-induced LV hypertrophy and dilatation were attenuated by overexpression of CKmito, but not by CKmyofib. CKmito overexpression also attenuated isoproterenol-induced hypertrophy. When the CK reaction was limited by creatine substrate depletion, protection was lost in CKmito mice, indicating ADP regeneration and not the protein per se is critical in limiting adverse HF remodeling. In human HF, pathologic hypertrophy and adverse remodeling are closely related to ATP levels and CK energy reaction deficits. Sitting at the intersection of cardiac energetics and redox balance, CK mito plays a crucial role in attenuating pathologic remodeling in HF.
Sources of Funding:
This work was supported the National Institutes of Health (HL63030, HL61912, HL136918, HL134821 and T32AG058527), and by funds from the Johns Hopkins University Older Americans Independence Center of the National Institute on Aging (NIA) (under award number P30AG021334), the Magic that Matters Fund, the Russell Morgan Professorship in Radiology, and the Clarence Doodeman Endowment in Cardiology at Johns Hopkins University School of Medicine.
Non-Standard Abbreviations:
- ADP
adenosine diphosphate
- Akt
protein kinase B
- ATP
adenosine triphosphate
- CK
creatine kinase
- CKmyofib
myofibrillar isoform of creatine kinase
- CKmito
mitochondrial isoform of creatine kinase
- c-Myc
protooncogene c-Myc
- EDV
end-diastolic volume
- EF
ejection fraction
- EPR
electron paramagnetic resonance
- FOXO3
forkhead box O3
- GAMT−/− mice
Guanidinoaceate N-metthyltransferase knock-out mice
- GATA-4
transcription factor GATA-4
- HF
heart failure
- ISO
isoproterenol
- LV
left ventricle
- LVH
left ventricular hypertrophy
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- PCr
creatine phosphate
- ROS
reactive oxygen species
- TAC
transverse aortic constriction
- TrxR
thioredoxin reductase
Footnotes
Disclosures:
Under a licensing agreement with NanoCor Therapeutics, Inc., Dr. Weiss is entitled to a share of royalty on sales of technology related to creatine kinase gene therapy. The terms of this arrangement, classified as a “modest” conflict, are being managed by the Johns Hopkins University in accordance with its conflict of interest policies. The other authors declare no financial interests.
Bibliography:
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, et al. Heart disease and stroke statistics—2010 update Circulation. 2010;121:e46–e215. [DOI] [PubMed] [Google Scholar]
- 2.Ingwall JS and Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res. 2004;95:135–145. [DOI] [PubMed] [Google Scholar]
- 3.Stanley WC, Recchia FA and Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. [DOI] [PubMed] [Google Scholar]
- 4.Tian R, Colucci WS, Arany Z, Bachschmid MM, Ballinger SW, Boudina S, Bruce JE, Busija DW, Dikalov S, Dorn GW II, et al. Unlocking the secrets of mitochondria in the cardiovascular system: Path to a cure in heart failure—A report from the 2018 National Heart, Lung, and Blood Institute Workshop. Circulation. 2019;140:1205–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maslov MY, Chacko VP, Stuber M, Moens AL, Kass DA, Champion HC and Weiss RG. Altered high-energy phosphate metabolism predicts contractile dysfunction and subsequent ventricular remodeling in pressure-overload hypertrophy mice. Am J Physiol Heart Circ Physiol. 2007;292:H387–H391. [DOI] [PubMed] [Google Scholar]
- 6.Maslov MY, Chacko VP, Hirsch GA, Akki A, Leppo MK, Steenbergen C and Weiss RG. Reduced in vivo high-energy phosphates precede adriamycin-induced cardiac dysfunction. Am J Physiol Heart Circ Physiol. 2010;299:H332–H337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlattner U, Tokarska-Schlattner M and Wallimann T. Mitochondrial creatine kinase in human health and disease. Biochim Biophys Acta. 2006;1762:164–180. [DOI] [PubMed] [Google Scholar]
- 8.Nascimben L, Ingwall JS, Pauletto P, Friedrich J, Gwathmey JK, Saks V, Pessina AC and Allen PD. Creatine kinase system in failing and nonfailing human myocardium. Circulation. 1996;94:1894–1901. [DOI] [PubMed] [Google Scholar]
- 9.Murakami Y, Zhang J, Eijgelshoven MH, Chen W, Carlyle WC, Zhang Y, Gong G and Bache RJ. Myocardial creatine kinase kinetics in hearts with postinfarction left ventricular remodeling. Am J Physiol. 1999;276:H892–H900. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Chacko VP and Weiss RG. Abnormal energetics and ATP depletion in pressure-overload mouse hearts: in vivo high-energy phosphate concentration measures by noninvasive magnetic resonance. Am J Physiol Heart Circ Physiol. 2009;297:H59–H64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardy CJ, Weiss RG, Bottomley PA and Gerstenblith G. Altered myocardial high-energy phosphate metabolites in patients with dilated cardiomyopathy. Am Heart J. 1991;122:795–801. [DOI] [PubMed] [Google Scholar]
- 12.Weiss RG, Gerstenblith G and Bottomley PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci U S A. 2005;102:808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith CS, Bottomley PA, Schulman SP, Gerstenblith G and Weiss RG. Altered creatine kinase adenosine triphosphate kinetics in failing hypertrophied human myocardium. Circulation. 2006;114:1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabr RE, El-Sharkawy AM, Schar M, Panjrath GS, Gerstenblith G, Weiss RG and Bottomley PA. Cardiac work is related to creatine kinase energy supply in human heart failure: a cardiovascular magnetic resonance spectroscopy study. J Cardiovasc Magn Reson. 2018;20:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta A, Akki A, Wang Y, Leppo MK, Chacko VP, Foster DB, Caceres V, Shi S, Kirk JA, Su J, et al. Creatine kinase-mediated improvement of function in failing mouse hearts provides causal evidence the failing heart is energy starved. J Clin Invest. 2012;122:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta A, Rohlfsen C, Leppo MK, Chacko VP, Wang Y, Steenbergen C and Weiss RG. Creatine kinase-overexpression improves myocardial energetics, contractile dysfunction and survival in murine doxorubicin cardiotoxicity. PLoS ONE. 2013;8:e74675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fritz-Wolf K, Schnyder T, Wallimann T and Kabsch W. Structure of mitochondrial creatine kinase. Nature. 1996;381:341–345. [DOI] [PubMed] [Google Scholar]
- 18.Speer O, Back N, Buerklen T, Brdiczka D, Koretsky A, Wallimann T and Eriksson O. Octameric mitochondrial creatine kinase induces and stabilizes contact sites between the inner and outer membrane. Biochem J. 2005;385:445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlattner U, Kay L and Tokarska-Schlattner M. Mitochondrial proteolipid complexes of creatine kinase. In: Harris JR and Boekema E, eds. Membrane Protein Complexes: Structure and Function. 1 ed. Singapore: Springer; 2018: 365–408. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt A, Marescau B, Boehm EA, Renema WKJ, Peco R, Das A, Steinfeld R, Chan S, Wallis J, Davidoff M, et al. Severely altered guanidino compound levels, disturbed body weight homeostasis and impaired fertility in a mouse model of guanidinoacetate N-methyltransferase (GAMT) deficiency Hum Mol Genet. 2004;13:905–921. [DOI] [PubMed] [Google Scholar]
- 21.Hardt SE and Sadoshima J. Negative regulators of cardiac hypertrophy. Cardiovasc Res. 2004;63:500–509. [DOI] [PubMed] [Google Scholar]
- 22.Stanley BA, Sivakumaran V, Shi S, McDonald I, Lloyd D, Watson WH, Aon MA and Paolocci N. Thioredoxin reductase-2 is essential for keeping low levels of H2O2 emission from isolated heart mitochondria. J Biol Chem. 2011;286:33669–33677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakurai A, Nishimoto M, Himeno S, Imura N, Tsujimoto M, Kunimoto M and Hara S. Transcriptional regulation of thioredoxin reductase 1 expression by cadmium in vascular endothelial cells: role of NF-E2-related factor-2. J Cell Physiol. 2005;203:529–537. [DOI] [PubMed] [Google Scholar]
- 24.Park S-Y, Trinity JD, Gifford JR, Diakos NA, McCreath L, Drakos S and Richardson RS. Mitochondrial function in heart failure: The impact of ischemic and non-ischemic etiology. Int J Cardiol. 2016;220:711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akhmedov AT, Rybin V and Marin-Garcia J. Mitochondrial oxidative metabolism and uncoupling proteins in the failing heart. Heart Fail Rev. 2015;20:227–249. [DOI] [PubMed] [Google Scholar]
- 26.Meyer LE, Machado LB, Santiago AP, S. A, da-Silva WS, De Felice FG, Holub O, Oliveira MF and Galina A. Mitochondrial creatine kinase activity prevents reactive oxygen species generation. J Biol Chem. 2006;281:37361–37371. [DOI] [PubMed] [Google Scholar]
- 27.Oka T, Maillet M, Watt AJ, Schwartz RJ, Aronow BJ, Duncan SA and Molkentin JD. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ Res. 2006;98:837–845. [DOI] [PubMed] [Google Scholar]
- 28.van Berlo JH, Aronow BJ and Molkentin JD. Parsing the roles of the transcription factors GATA-4 and GATA-6 in the adult cardiac hypertrophic response. PLoS ONE. 2013;8:e84591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong W, Mao S, Tobis S, Angelis E, Jordan MC, Roos KP, Fishbein MC, de Alboran IM and MacLellan WR. Hypertrophic growth in cardiac myocytes is mediated by Myc through a Cyclin D2-dependent pathway. EMBO J. 2006;25:3869–3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izumo S, Nadal-Ginard B and Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988;85:339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Starksen NF, Simpson PC, Bishopric N, Coughlin SR, Lee WM, Escobedo JA and Williams LT. Cardiac myocyte hypertrophy is associated with c-myc protooncogene expression. Proc Natl Acad Sci U S A. 1986;83:8348–8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao G, Mao S, Baumgarten G, Serrano J, Jordan MC, Roos KP, Fishbein MC and MacLellan WR. Inducible activation of c-Myc in adult myocardium In vivo provokes cardiac myocyte hypertrophy and reactivation of DNA synthesis. Circ Res. 2001;89:1122–1129. [DOI] [PubMed] [Google Scholar]
- 33.Taketani S, Sawa Y, Ichikawa H, Ohtake S, Nishimura M, Kawaguchi N and Matsuda H. Change of c-Myc expression and cardiac hypertrophy in patients with aortic valve replacement. Ann Thorac Surg. 2001;71:1154–1159. [DOI] [PubMed] [Google Scholar]
- 34.Kolbeck-Ruhmkorff C, Horban A and Zimmer H-G. Effect of pressure and volume overload on proto-oncogene expression in the isolated working rat heart Cardiovasc Res. 1993;27:1998–2004. [DOI] [PubMed] [Google Scholar]
- 35.Park SJ, Zhang J, Ye Y, Ormaza S, Liang P, Bank AJ, Miller LW and Bache RJ. Myocardial creatine kinase expression after left ventricular assist device support. J Am Coll Cardiol. 2002;39:1773–1779. [DOI] [PubMed] [Google Scholar]
- 36.Spindler M, Engelhardt S, Niebler R, Wagner H, Hein L, Lohse MJ and Neubauer S. Alterations in the myocardial creatine kinase system precede the development of contractile dysfunction in β1-adrenergic receptor transgenic mice. J Mol Cell Cardiol. 2003;35:389–397. [DOI] [PubMed] [Google Scholar]
- 37.De Jong KA and Lopaschuk GD. Complex energy metabolic changes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. Can J Cardiol. 2017;33:860–871. [DOI] [PubMed] [Google Scholar]
- 38.Ingwall JS. Is cardiac failure a consequence of decreased energy reserve? Circulation. 1993;87:VII-58–VII-62. [Google Scholar]
- 39.Kobayashi K and Neely JR. Control of maximum rates of glycolysis in rat cardiac muscle. Circ Res. 1979;44:166–175. [DOI] [PubMed] [Google Scholar]
- 40.Tian R and Ingwall JS. Energetic basis for reduced contractile reserve in isolated rat hearts. Am J Physiol. 1996;270:H1207–H1216. [DOI] [PubMed] [Google Scholar]
- 41.Hamman BL, Bittl JA, Jacobus WE, Allen PD, Spencer RS, Tian R and Ingwall JS. Inhibition of the creatine kinase reaction decreases the contractile reserve of isolated rat hearts. Am J Physiol. 1995;269:H1030–H1036. [DOI] [PubMed] [Google Scholar]
- 42.Tian R, Christe ME, Spindler M, Hopkins JC, Halow JM, Camacho SA and Ingwall JS. Role of MgADP in the development of diastolic dysfunction in the intact beating rat heart. J Clin Invest. 1997;99:745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian R, Nascimben L, Ingwall JS and Lorell BH. Failure to maintain a low ADP concentration impairs diastolic function in hypertrophied rat hearts. Circulation. 1997;96:1313–1319. [DOI] [PubMed] [Google Scholar]
- 44.Liao R, Nascimben L, Friedrich J, Gwathmey JK and Ingwall JS. Decreased energy reserve in an animal model of dilated cardiomyopathy. Relationship to contractile performance. Circ Res. 1996;78:893–902. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J, Wilke N, Wang Y, Zhang Y, Wang C, Eijgelshoven MH, Cho YK, Murakami Y, Ugurbil K, Bache RJ, et al. Functional and bioenergetic consequences of postinfarction left ventricular remodeling in a new porcine model. MRI and 31P-MRS study. Circulation. 1996;94. [DOI] [PubMed] [Google Scholar]
- 46.Shen W, Asai K, Uechi M, Mathier MA, Shannon RP, Vatner SF and Ingwall JS. Progressive loss of myocardial ATP due to a loss of total purines during the development of heart failure in dogs: a compensatory role for the parallel loss of creatine. Circulation. 1999;100:2113–2118. [DOI] [PubMed] [Google Scholar]
- 47.Hirsch GA, Bottomley PA, Gerstenblith G and Weiss RG. Allopurinol acutely increases adenosine triphospate energy delivery in failing human hearts. J Am Coll Cardiol. 2012;59:802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bottomley PA, Panjrath GS, Lai S, Hirsch GA, Wu K, Najjar SS, Steinberg A, Gerstenblith G and Weiss RG. Metabolic rates of ATP transfer through creatine kinase (CK Flux) predict clinical heart failure events and death. Sci Transl Med. 2013;5:215re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye Y, Gong G, Ochiai K, Liu J and Zhang J. High-energy phosphate metabolism and creatine kinase in failing hearts: a new porcine model. Circulation. 2001;103:1570–1576. [DOI] [PubMed] [Google Scholar]
- 50.Wallimann T, Tokarska-Schlattner M and Schlattner U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids. 2011;40:1271–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stolz M and Wallimann T. Myofibrillar interaction of cytosolic creatine kinase (CK) isoenzymes: allocation of N-terminal binding epitope in MM-CK and BB-CK. J Cell Sci. 1998;111:1207–1216. [DOI] [PubMed] [Google Scholar]
- 52.Wendt S, Schlattner U and Wallimann T. Differential effects of peroxynitrite on human mitochondrial creatine kinase isoenzymes. Inactivation, octamer destabilization, and identification of involved residues. J Biol Chem. 2003;278:1125–1130. [DOI] [PubMed] [Google Scholar]
- 53.Konorev EA, Hogg N and Kalyanaraman B. Rapid and irreversible inhibition of creatine kinase by peroxynitrite. FEBS Lett. 1998;427:171–174. [DOI] [PubMed] [Google Scholar]
- 54.Yuan G, Kaneko M, Masuda H, Hon RB, Kobayashi A and Yamazaki N. Decrease in heart mitochondrial creatine kinase activity due to oxygen free radicals. Biochim Biophys Acta. 1992;1140:78–84. [DOI] [PubMed] [Google Scholar]
- 55.Ingwall JS, Kramer MF, Fifer MA, Lorell BH, Shemin R, Grossman W and Allen PD. The creatine kinase system in normal and diseased human myocardium. N Engl J Med. 1985;313:1050–1054. [DOI] [PubMed] [Google Scholar]
- 56.Seddon M, Looi YH and Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93:903–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, Cheng J, Lu G, Morris DJ, Castrillon DH, et al. Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signalin. Circulation. 2006;114:1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skurk C, Izumiya Y, Maatz H, Razeghi P, Shiojima I, Sandri M, Sato K, Zeng L, Schiekofer S, Pimentel D, et al. The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling. J Biol Chem. 2005;280:20814–20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jensen KS, Binderup T, Jensen KT, Therkelsen I, Borup R, Nilsson E, Multhaupt H, Bouchard C, Quistorff B, Kjaer A, et al. FoxO3A promotes metabolic adaptation to hypoxia by antagonizing Myc function. EMBO J. 2011;30:4554–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Delpuech O, Griffiths B, East P, Essafi A, Lam EW, Burgering B, Downward J and Schulze A. Induction of Mxi1-SR alpha by FOXO3a contributes to repression of Myc-dependent gene expression. Mol Cell Biol. 2007;27:4917–4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klotz LO, Sanchez-Ramos C, Prieto-Arroyo I, Urbanek P, Steinbrenner H and Monsalve M. Redox regulation of FoxO transcription factors. Redox Biology. 2015;6:51–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cao F, Maguire ML, McAndrew DJ, Lake HA, Neubauer S, Zervou S, Schneider JE and Lygate CA. Overexpression of mitochondrial creatine kinase preserves cardiac energetics without ameliorating murine chronic heart failure. Basic Res Cardiol. 2020;115:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whittington HJ, Ostrowski PJ, McAndrew DJ, Cao F, Shaw A, Eykyn TR, Lake HA, Tyler J, Schneider JE, Neubauer S, et al. Over-expression of mitochondrial creatine kinase in the murine heart improves functional recovery and protects against injury following ischaemia-reperfusion. Cardiovasc Res. 2018;114:858–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Branovets J, Sepp M, Kotlyarova S, Jepihhina N, Sokolova N, Aksentijevic D, Lygate CA, Neubauer S, Vendelin M and Birkedal R. Unchanged mitochondrial organization and compartmentation of high-energy phosphates in creatine-deficient GAMT−/− mouse hearts Am J Physiol Heart Circ Physiol. 2013;305:H506–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lygate CA, Aksentijevic D, Dawson D, ten Hove M, Phillips D, de Bono JP, Medway DJ, Sebag-Montefiore L, Hunyor I, Channon KM, et al. Living without creatine: unchanged exercise capacity and response to chronic myocardial infarction in creatine-deficient mice Circ Res. 2013;112:945–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.El-Sharkawy A-MM, Gabr RE, Schar M, Weiss RG and Bottomley PA. Quantification of human high-energy phosphate metabolite concentrations at 3 t with partial volume and sensitivity corrections NMR Biomed. 2013;26:1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schar M, El-Sharkawy A-MM, Weiss RG and Bottomley PA. Triple repetition time saturation transfer (TRiST) 31P spectroscopy for measuring human creatine kinase reaction kinetics. Magn Reson Med. 2010;63:1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gabr RE, Weiss RG and Bottomley PA. Correcting reaction rates measured by saturation-transfer magnetic resonance spectroscopy. J Magn Reson. 2008;191:248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schar M, Gabr RE, El-Sharkawy A-MM, Steinberg A, Bottomley PA and Weiss RG. Two repetition time saturation transfer (TwiST) with spill-over correction to measure creatine kinase reaction rates in human hearts J Cardiovasc Magn Reson. 2015;17:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chacko VP, Aresta F, Chacko SM and Weiss RG. MRI/ MRS assessment of in vivo murine cardiac metabolism, morphology, and function at physiological heart rates. Am J Physiol Heart Circ Physiol. 2000;279:H2218–H2224. [DOI] [PubMed] [Google Scholar]
- 71.Weiss RG, Chatham JC, Georgakopolous D, Charron MJ, Wallimann T, Kay L, Walzel B, Wang Y, Kass DA, Gerstenblith G, et al. An increase in the myocardial PCr/ATP ratio in GLUT4 null mice FASEB J. 2002;16:613–615. [DOI] [PubMed] [Google Scholar]
- 72.Ryding AD, Sharp MG and Mullins JJ. Conditional transgenic technologies J Endocrinol. 2001;171:1–14. [DOI] [PubMed] [Google Scholar]
- 73.Glasenapp A, Derlin K, Gutberlet M, Hess A, Ross TL, Wester HJ, Bengel FM and Thackeray JT. Molecular imaging of inflammation and fibrosis in pressure overload heart failure. . Circ Res. 2021;129:369–382. [DOI] [PubMed] [Google Scholar]
- 74.Lygate CA, Fischer A, Sebag-Montefiore L, Wallis J, ten Hove M and Neubauer S. The creatine kinase energy transport system in the failing mouse heart. J Mol Cell Cardiol. 2007;42:1129–1136. [DOI] [PubMed] [Google Scholar]
- 75.Lygate CA, Schneider JE, Hulbert K, ten Hove M, Sebag-Montefiore LM, Cassidy PJ, Clarke K and Neubauer S. Serial high resolution 3D-MRI after aortic banding in mice: band internalization is a source of variability in the hypertrophic response. Basic Res Cardiol. 2006;101:8–16. [DOI] [PubMed] [Google Scholar]
- 76.Feng N, Huke S, Zhu G, Tocchetti CG, Shi S, Aiba T, Kaludercic N, Hoover DB, Beck SE, Mankowski JL, et al. Constitutive BDNF/TrkB signaling is required for normal cardiac contraction and relaxation. Proc Natl Acad Sci U S A. 2015;112:1880–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keceli G, Majumdar A, Thorpe CN, Jun S, Tocchetti CG, Lee DI, Mahaney JE, Paolocci N and Toscano JP. Nitroxyl (HNO) targets phospholamban cysteines 41 and 46 to enhance cardiac function. J Gen Physiol. 2019;151:758–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chelko SP, Keceli G, Carpi A, Doti N, Agrimi J, Asimaki A, Beti CB, Miyamoto M, Amat-Codina N, Bedja D, et al. Exercise triggers CAPN1-mediated AIF truncation inducing myocyte necroptosis in arrhythmogenic cardiomyopathy. Sci Transl Med. 2021;13:eabf0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aon MA, Cortassa S, Wei A-C, Grunnet M and O'Rourke B. Energetic performance is improved by specific activation of K+ fluxes through KCa channels in heart mitochondria. Biochim Biophys Acta. 2010;1797:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tocchetti CG, Caceres V, Stanley BA, Xie C, Shi S, Watson WH, O'Rourke B, Spadari-Bratfisch RC, Cortassa S, Akar FG, et al. GSH or palmitate preserves mitochondrial energetic/redox balance, preventing mechanical dysfunction in metabolically challenged myocytes/hearts from type 2 diabetic mice. Diabetes. 2012;61:3094–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Srere PA. Citrate synthase: [EC 4.1.3.7. Citrate oxaloacetate-lyase (CoA-acetylating)]. Methods Enzymol. 1969;13:3–11. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The authors have full access to all the data in the study and take responsibility for its integrity and the data analysis.