Abstract
BACKGROUND:
There is growing interest in using glycated albumin for the diagnosis of diabetes, especially when standard tests (glucose and hemoglobin A1c [Hb A1c]) are unavailable. However, it is unknown how well glycated albumin identifies diabetes in the general population.
METHODS:
We measured glycated albumin in stored serum samples from the 1999–2004 National Health and Nutrition Examination Survey. We evaluated the ability of glycated albumin to identify undiagnosed diabetes in US adults aged ≥20 (n = 4785), overall and at thresholds corresponding to clinical cut points for Hb A1c and fasting plasma glucose (FPG). We assessed 4 reference definitions for undiagnosed diabetes: increased FPG (≥126 mg/dL) [≥6.99 mmol/L), increased Hb A1c (≥6.5%), either FPG or Hb A1c increased, or both FPG and Hb A1c increased.
RESULTS:
Among US adults, glycated albumin had excellent diagnostic accuracy across all 4 definitions of undiagnosed diabetes, with the area under the receiver operating characteristic curve (AUC) ranging from 0.824 to 0.951. Performance was generally consistent across patient demographic and clinical characteristics. Glycated albumin cut points of 16.5% and 17.8% were equivalent to an FPG of 126 mg/dL (6.99 mmol/L; 97th percentile) and Hb A1c of 6.5% (98th percentile) and had low to moderate sensitivity (0.273 to 0.707) but high specificity (0.980 to 0.992) for detecting undiagnosed diabetes.
CONCLUSION:
The excellent diagnostic performance of glycated albumin to identify diabetes defined by either FPG or Hb A1c suggests that glycated albumin may be useful for identifying adults with undiagnosed diabetes when standard tests are unavailable.
Introduction
Fasting plasma glucose (FPG) and glycated hemoglobin (Hb A1c) are standard measures used to screen and diagnose diabetes (1), but both have limitations. FPG requires an overnight fast and has high within-person variability, while Hb A1c is unreliable in certain patients, including those with altered red blood cell turnover, anemia, some hemoglobinopathies, or chronic kidney disease (2).
Glycated albumin is formed when glucose binds to albumin and reflects intermediate (2 to 3 week) hyperglycemia. Because glycated albumin is unaffected by erythrocyte lifespan or alterations in hemoglobin concentration, there is growing interest using it as an alternative or complementary test for diagnosis of diabetes (1, 3). Glycated albumin is used in clinical practice in some parts of Asia and recently received clearance from the Food and Drug Administration for clinical use in the United States. However, the clinical utility of glycated albumin in the general population remains unclear and diagnostic cut points for glycated albumin have not been established (3–5).
We conducted the first study of the diagnostic performance of glycated albumin in a nationally representative sample of US adults. Our primary objective was to assess the performance of glycated albumin for the identification of diabetes, overall and in populations where standard markers may be less reliable. Our secondary objective was to evaluate potential diagnostic cut points for glycated albumin.
Materials and Methods
STUDY POPULATION
The National Health and Nutrition Examination Survey (NHANES) is a nationally representative, cross-sectional study designed to monitor the health of the US population. Participants are selected from the US noninstitutionalized, civilian population using a complex, stratified, multistage probability cluster sampling design for in-home interviews and visits to a mobile examination center for laboratory testing (6). A randomly selected subsample of participants fasted overnight prior to their examination.
We included all nonpregnant adults aged ≥ 20-years with no history of diagnosed diabetes from the 1999–2004 NHANES who completed the medical examination, attended the fasting morning examination, and had valid data for all 3 measures of glycemia (glycated albumin, Hb A1c, and FPG) available (see online Supplemental Fig. S1, n = 4785).
MEASURES OF GLYCEMIA
Whole blood specimens were collected and immediately stored at 4 to 8 °C. Samples were shipped at the same temperature to the University of Missouri for processing. Hb A1c was measured using HPLC methods (7) in whole blood samples and calibrated to account for changes in laboratory methods over time (8).
A 3 to 5 mL sample of whole blood was collected from fasting participants and immediately centrifuged at 1500g for 10 min. Plasma was transferred into vials then frozen at −70 °C and shipped in dry ice to the University of Missouri for processing. Upon arrival, specimens were placed in −70 °C freezers until analyzed. Glucose was measured in plasma specimens using the hexokinase method.
We measured glycated albumin in surplus serum samples using a method developed by Asahi Kasei Pharma that was adapted to the Siemens Dimension Vista 1500. Measurements were conducted between 2018 and 2020 at the University of Maryland (Baltimore, MD, USA) (9). Glycated albumin was expressed as a percentage of total albumin using the following formula: [(glycated albumin concentration in g/dL/serum albumin concentration in g/dL) × 100/1.14] + 2.9 (10). We restricted our sample to measurement of glycated albumin in samples that had not previously undergone 2 or more freeze-thaw cycles. We also excluded one participant with an implausible glycated albumin value (>100%). Reagents for the glycated albumin assay were donated by Asahi Kasei.
DEFINITION OF UNDIAGNOSED DIABETES
We evaluated the ability of glycated albumin to identify 4 different definitions of undiagnosed diabetes: FPG ≥ 126 mg/dL (≥6.99 mmol/L); Hb A1c ≥6.5%; FPG ≥ 126 mg/dL (≥6.99 mmol/L) or Hb A1c ≥6.5%; FPG ≥ 126 mg/dL (≥6.99 mmol/L) and Hb A1c ≥6.5% (1).
OTHER VARIABLES OF INTEREST
Participants self-reported age, sex (male/female), race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American, other). Body mass index (BMI) was defined as weight in kilograms divided by height in meters squared. Participants were classified into one of three weight status groups (normal, BMI <25 kg/m2; overweight, BMI 25 to 29.9 kg/m2; obese, BMI ≥30 kg/m2). Anemia was defined as hemoglobin <13.5 g/dL for men and <12 g/dL for women (11). Iron deficiency was defined as abnormal values for at least 2 of the 3 measures: free erythrocyte protoporphyrin (>70 μg/dL), transferrin saturation (<16%), or serum ferritin (≤15 μg/l) (11). Hypertension was defined as mean blood pressure ≥140/90 mm Hg or current use of blood pressure-lowering medication (12). High cholesterol was defined as total cholestrol ≥240 mg/dL (6.21 mmol/L) or use of cholesterol-lowering medication. Glomerular filtration rate was estimated (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration formula (13), and chronic kidney disease was defined as eGFR <60 ml/min/1.73 m2 (14). Urine albumin and creatinine were measured in a random urine sample. Albuminuria was defined as an albumin-to-creatinine ratio ≥30 mg/g (14).
STATISTICAL ANALYSIS
We assessed the distribution of glycated albumin, Hb A1c, and FPG. To derive potential diabetes cut points, we determined the percentile of glycated albumin corresponding to an Hb A1c of 6.5% or an FPG of 126 mg/dL (6.99 mmol/L). This approach is consistent with prior work and identifies glycated albumin values that approximate cut points currently used in clinical practice (15). We examined the associations between glycated albumin, Hb A1c, and FPG using weighted Pearson correlations and scatterplots with lowess-smoothed curves. We evaluated the performance of glycated albumin to identify different definitions of diabetes by computing the area under the receiver operating curve (AUC), overall and across subgroups. We calculated the specificity, sensitivity, positive predictive value, and negative predictive value of glycated albumin at values that corresponded to Hb A1c and FPG cut points. We compared the performance of glycated albumin to Hb A1c and FPG and examined the factors associated with increases in glycated albumin, Hb A1c, and FPG with logistic regression models. We explored the association between body mass index and all 3 glycemic markers using weighted Pearson correlations and scatterplots with lowess-smoothed curves.
All estimates were generated using Stata 16.0 (StataCorp) and recommended sample weights, making our results representative of the noninstitutionalized, civilian US adult population. A two-sided P value less than 0.05 was considered statistically significant.
The National Center for Health Statistics (NCHS) ethics review board approved study protocols and approved the measurement of glycated albumin in stored serum samples. All participants provided written informed consent.
Results
During the period 1999 to 2004, the mean age of US adults without a history of diabetes was 45.5 years, 51.3% were women, and 73.6% were non-Hispanic Whites (see online Supplemental Table S1). Glycated albumin was modestly correlated with total albumin (see online Supplemental Fig. S2, r=0.33). Levels of glycated albumin ranged from 10.5% to 17.8% (2nd percentile to 98th percentile) in US adults without a history of diabetes (Table 1). The glycated albumin value corresponding to the equivalent percentile as Hb A1c 6.5% (98th percentile) was 17.8%. The glycated albumin value corresponding to fasting glucose of 126 mg/dL (6.99 mmol/L; 97th percentile) was 16.5%.
Table 1.
Equipercentile values of Hb A1c, fasting plasma glucose, and glycine albumin in US adults without diagnosed diabetes, NHANES 1999–2004 (n = 4785).
| Hb A1c, % | FPG, mg/dLb | Glycated albumin, % | Percentile |
|---|---|---|---|
| 4.7 | 79 | 10.5 | 2nd |
| 5.2 | 89 | 12.2 | 25th |
| 5.4 | 95 | 13.0 | 50th |
| 5.5 | 100 | 13.8 | 69th |
| 5.6 | 102 | 14.0 | 75th |
| 5.7 | 105 | 14.4 | 82nd |
| 6.2 | 126a | 16.5 | 97th |
| 6.5a | 138 | 17.8 | 98th |
Hb A1c of 6.5% and FPG of 126 mg/dL (6.99 mmol/L) are the clinical cut points for diabetes diagnosis.
To convert glucose concentrations from mg/dL to mmol/L, multiply by 0.05551.
There was a moderate correlation between glycated albumin and Hb A1c (Fig. 1, A, r=0.52), though the association was stronger at higher values. For instance, at glycated albumin ≥16.5%, the correlation between HbA1c and glycated albumin was 0.78, compared to 0.13 for values <16.5%. There was a similar nonlinear association between glycated albumin and FPG (Fig. 1, B). While the overall correlation between FPG and glycated albumin was 0.51, the correlation was 0.78 at glycated albumin values ≥16.5% and 0.09 at values <16.5%. In contrast, there was a strong linear association between Hb A1c and FPG (Fig. 1, C, r=0.78).
Fig. 1. Weighted scatterplots (with lowess curves) of Hb A1c, FPG, and glycated albumin in US adults without diagnosed diabetes, NHANES 1999–2004.
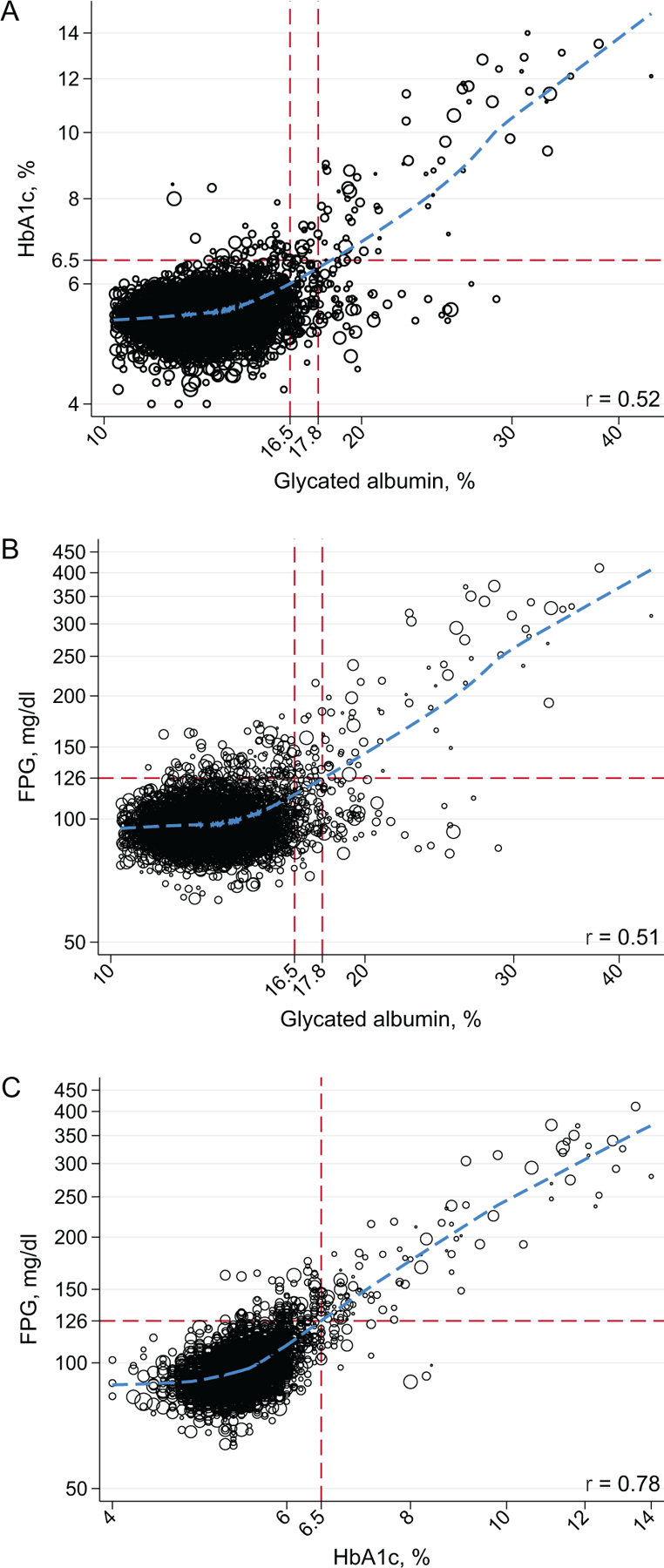
Glycated albumin values of 16.5% and 17.8% are “equivalent” (i.e., the same percentile) as a FPG of 126 mg/dL (6.99 mmol/L) and Hb A1c of 6.5% in US adult without diagnosed diabetes. Glycated albumin values were truncated at the 1st percentile in (A) and (B) for clarity. All figures are presented on the log scale.
Glycated albumin had excellent diagnostic accuracy across all 4 definitions of undiagnosed diabetes, with AUCs ranging from 0.824 to 0.951 (Table 2, Fig. 2). However, glycated albumin performed better when more specific definitions of diabetes were used. For instance, the AUC was 0.951 (95% CI, 0.910–0.974) when defined as having an increased Hb A1c and FPG compared to 0.824 (95% CI, 0.780–0.861) when diabetes was defined having an increased Hb A1c or FPG. In head-to-head comparisons, glycated albumin did not perform as well as Hb A1c in identifying individuals with single increased FPG (AUC of 0.824 vs 0.940, P<0.001). Glycated albumin also did not perform as well as FPG in identifying individuals with single increased Hb A1c (AUC of 0.921 vs 0.956, P=0.01).
Table 2.
Performance of glycated albumin to detect undiagnosed diabetes in US adults, overall and according to subgroups, NHANES 1999–2004.
| FPG≥126 mg/dL (≥6.99 mmol/L) (reference) |
Hb A1c≥6.5% (reference) |
FPG≥126 mg/dL (≥6.99 mmol/L) and Hb A1c≥6.5% (reference) Glycated albumin, AUC (95% CI) | FPG≥126 mg/dL (≥6.99 mmol/L) or Hb A1c≥6.5% (reference) Glycated albumin, AUC (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Glycated albumin, AUC (95% CI) | HbA1c, AUC (95% CI) | P value | Glycated albumin, AUC (95% CI) | FPG, AUC (95% CI) | P value | |||
| Overall | 0.824 (0.779–0.861) | 0.940 (0.908–0.961) | <0.001 | 0.921 (0.862–0.956) | 0.956 (0.896–0.982) | 0.01 | 0.951 (0.910–0.974) | 0.824 (0.780–0.861) |
| Age categories | ||||||||
| 20 to 44 | 0.815 (0.724–0.881) | 0.966 (0.903–0.989) | <0.001 | 0.854 (0.653–0.948) | 0.896 (0.685–0.972) | 0.03 | 0.960 (0.882–0.987) | 0.788 (0.677–0.868) |
| 45 to 64 | 0.835 (0.759–0.891) | 0.924 (0.850–0.963) | 0.009 | 0.951 (0.879–0.981) | 0.979 (0.934–0.993) | 0.30 | 0.948 (0.869–0.980) | 0.843 (0.769–0.896) |
| 65+ | 0.746 (0.659–0.817) | 0.888 (0.831–0.928) | <0.001 | 0.899 (0.822–0.945) | 0.961 (0.932–0.977) | 0.04 | 0.923 (0.823–0.969) | 0.759 (0.681–0.823) |
| Sex | ||||||||
| Male | 0.846 (0.792–0.888) | 0.935 (0.891–0.962) | <0.001 | 0.951 (0.911–0.974) | 0.972 (0.931–0.989) | 0.21 | 0.961 (0.912–0.983) | 0.851 (0.800–0.890) |
| Female | 0.803 (0.723–0.864) | 0.946 (0.890–0.974) | <0.001 | 0.883 (0.750–0.950) | 0.941 (0.814–0.983) | 0.02 | 0.939 (0.850–0.977) | 0.796 (0.713–0.859) |
| Race/ethnicity | ||||||||
| Non-Hispanic White | 0.803 (0.746–0.849) | 0.932 (0.890–0.958) | <0.001 | 0.896 (0.798–0.950) | 0.953 (0.830–0.988) | 0.006 | 0.931 (0.864–0.966) | 0.800 (0.743–0.847) |
| Non-Hispanic Black | 0.852 (0.742–0.920) | 0.952 (0.889–0.980) | 0.03 | 0.938 (0.852–0.975) | 0.971 (0.941–0.986) | 0.21 | 0.983 (0.967–0.991) | 0.850 (0.757–0.911) |
| Mexican-American | 0.905 (0.795–0.959) | 0.983 (0.959–0.993) | 0.04 | 0.935 (0.856–0.972) | 0.925 (0.681–0.986) | 0.80 | 0.977 (0.933–0.992) | 0.884 (0.791–0.939) |
| Chronic kidney disease (eGFRa <60) | ||||||||
| No | 0.825 (0.776–0.865) | 0.943 (0.908–0.965) | <0.001 | 0.922 (0.857–0.959) | 0.954 (0.886–0.983) | 0.03 | 0.958 (0.916–0.979) | 0.820 (0.772–0.861) |
| Yes | 0.760 (0.630–0.855) | 0.872 (0.775–0.930) | 0.02 | 0.898 (0.677–0.973) | 0.960 (0.927–0.978) | 0.37 | 0.855 (0.550–0.966) | 0.801 (0.682–0.884) |
| Albuminuria (ACRb≥30) | ||||||||
| No | 0.801 (0.745–0.847) | 0.945 (0.913–0.966) | <0.001 | 0.897 (0.811–0.946) | 0.938 (0.848–0.976) | 0.04 | 0.941 (0.879–0.972) | 0.799 (0.743–0.845) |
| Yes | 0.870 (0.782–0.926) | 0.924 (0.818–0.970) | 0.05 | 0.962 (0.900–0.986) | 0.985 (0.967–0.993) | 0.23 | 0.962 (0.885–0.988) | 0.881 (0.799–0.933) |
| Obesity (BMI≥30) | ||||||||
| No | 0.776 (0.699–0.838) | 0.938 (0.892–0.965) | <0.001 | 0.902 (0.733–0.969) | 0.926 (0.776–0.978) | 0.25 | 0.971 (0.896–0.992) | 0.767 (0.686–0.832) |
| Yes | 0.896 (0.851–0.929) | 0.943 (0.898–0.969) | 0.01 | 0.952 (0.918–0.973) | 0.969 (0.946–0.982) | 0.31 | 0.956 (0.911–0.979) | 0.904 (0.864–0.933) |
| Anemia | ||||||||
| No | 0.828 (0.783–0.865) | 0.940 (0.908–0.961) | <0.001 | 0.925 (0.863–0.960) | 0.958 (0.892–0.984) | 0.02 | 0.953 (0.911–0.976) | 0.827 (0.782–0.865) |
| Yes | 0.819 (0.608–0.930) | 0.954 (0.858–0.986) | 0.08 | 0.833 (0.600–0.944) | 0.919 (0.807–0.969) | 0.30 | 0.957 (0.918–0.978) | 0.786 (0.612–0.896) |
| Iron deficiency | ||||||||
| No | 0.827 (0.769–0.872) | 0.933 (0.890–0.960) | <0.001 | 0.920 (0.825–0.965) | 0.962 (0.848–0.991) | 0.003 | 0.965 (0.913–0.986) | 0.819 (0.760–0.866) |
| Yes | 0.884 (0.663–0.967) | 0.987 (0.940–0.997) | 0.14 | 0.969 (0.912–0.989) | 0.955 (0.817–0.990) | 0.71 | 0.981 (0.957–0.992) | 0.896 (0.711–0.968) |
eGFR, estimated glomerular filtration rate.
ACR, albumin-to-creatinine ratio.
Fig. 2. Overall performance of glycated albumin to detect undiagnosed diabetes in US adults, NHANES 1999–2004.
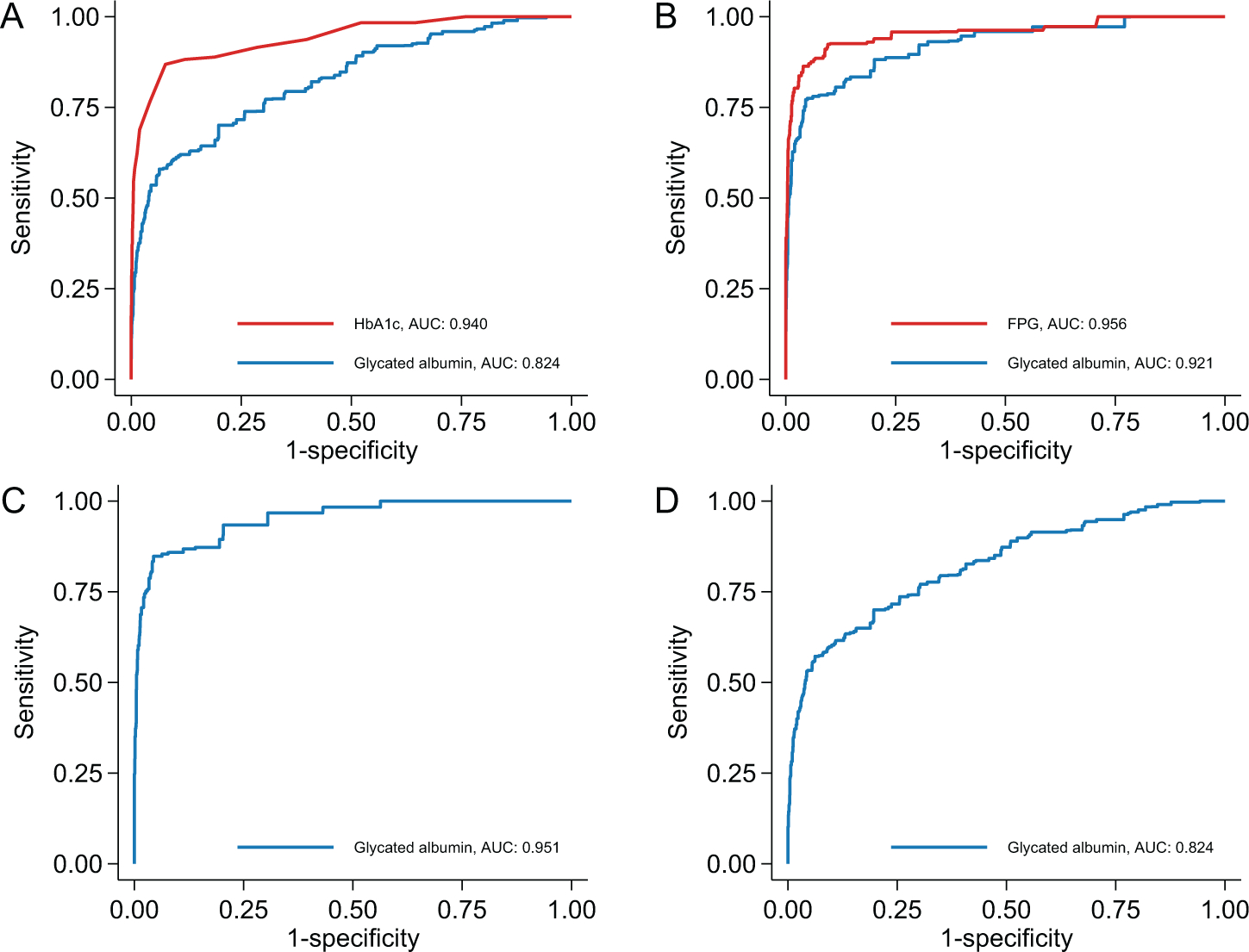
Reference definition of diabetes was: (A), FPG ≥126 mg/dL; (B), Hb A1c ≥6.5%; (C), FPG ≥126 mg/dL and Hb A1c ≥6.5%; and (D), FPG ≥126 mg/dL or Hb A1c ≥6.5%.
The performance of glycated albumin was generally excellent and consistent across subgroups, regardless of the definition of diabetes. Glycated albumin did not outperform Hb A1c or fasting glucose in any subgroup, including those with anemia, iron deficiency, or chronic kidney disease (Table 2).
In this study population, glycated albumin values of 16.5% and 17.8% corresponded to clinical cut points for FPG (126 mg/dL [6.99 mmol/L]) and Hb A1c (6.5%). Both glycated albumin thresholds had nearly perfect specificity and negative predictive value across all 4 definitions of diabetes (Table 3). However, the sensitivity and positive predictive value of glycated albumin at these cut points varied widely, with better performance observed for more specific definitions of diabetes. In head-to-head comparisons, Hb A1c ≥6.5% and FPG ≥126 mg/dL (≥6.99 mmol/L) had similar specificity but higher sensitivity than glycated albumin ≥16.5% and ≥17.8% for identifying adults with single increased values of FPG and Hb A1c, respectively.
Table 3.
Performance of glycated albumin to identify undiagnosed diabetes at selected thresholds in US adults, NHANES 1999–2004.a
| Threshold | Sensitivity (95% CI) | Specificity (95% CI) | Positive predictive value (95% CI) | Negative predictive value (95% CI) |
|---|---|---|---|---|
| FPG ≥126 mg/dL (≥6.99 mmol/L) (reference) | ||||
| Glycated albumin ≥16.5% | 0.376 (0.282–0.480) | 0.981 (0.974–0.986) | 0.381 (0.269–0.507) | 0.980 (0.975–0.985) |
| Glycated albumin ≥17.8% | 0.284 (0.198–0.389) | 0.992 (0.985–0.995) | 0.514 (0.338–0.685) | 0.978 (0.972–0.982) |
| Hb A1c ≥6.5% | 0.463 (0.368–0.561) | 0.996 (0.994–0.997) | 0.782 (0.675–0.861) | 0.983 (0.979–0.987) |
| HbA 1c ≥6.5% (reference) | ||||
| Glycated albumin ≥16.5% | 0.628 (0.485–0.751) | 0.981 (0.975–0.986) | 0.377 (0.278–0.488) | 0.993 (0.989–0.996) |
| Glycated albumin ≥17.8% | 0.502 (0.375–0.629) | 0.992 (0.986–0.996) | 0.538 (0.371–0.697) | 0.991 (0.987–0.994) |
| FPG ≥126 mg/dL | 0.782 (0.675–0.861) | 0.983 (0.979–0.987) | 0.463 (0.368–0.561) | 0.996 (0.994–0.997) |
| Hb A1c ≥6.5% and FPG ≥126 mg/dL (≥6.99 mmol/L) (reference) | ||||
| Glycated albumin ≥16.5% | 0.707 (0.568–0.815) | 0.980 (0.973–0.985) | 0.332 (0.236–0.445) | 0.996 (0.993–0.997) |
| Glycated albumin ≥17.8% | 0.591 (0.450–0.719) | 0.991 (0.985–0.995) | 0.495 (0.332–0.659) | 0.994 (0.991–0.996) |
| Hb A1c ≥6.5% or FPG ≥126 mg/dL (≥6.99 mmol/L) (reference) | ||||
| Glycated albumin ≥16.5% | 0.372 (0.280–0.475) | 0.982 (0.975–0.987) | 0.426 (0.311–0.551) | 0.978 (0.972–0.982) |
| Glycated albumin ≥17.8% | 0.273 (0.195–0.367) | 0.992 (0.986–0.996) | 0.556 (0.379–0.721) | 0.975 (0.968–0.980) |
Glycated albumin values of 16.5% and 17.8% are “equivalent” (i.e., the same percentile) as a FPG of 126 mg/dL (≥6.99 mmol/L) and Hb A1c of 6.5% in US adult without di- agnosed diabetes.
Older adults and non-Hispanic Blacks were more likely than young adults and non-Hispanic Whites to have increased glycated albumin, whether defined as ≥16.5% or ≥17.8% (see online Supplemental Table S2). Overweight and obesity were significantly associated with glycated albumin ≥17.8%, but not ≥16.5%. Age, race/ethnicity, and weight status were also risk factors for increased FPG and increased Hb A1c. However, body mass index was strongly nonlinearly related to glycated albumin, but linearly associated with Hb A1c and FPG (see online Supplemental Fig. S3).
Discussion
Among US adults, glycated albumin performed well to identify individuals with undiagnosed diabetes in the population. The diagnostic accuracy of glycated albumin was high for all reference definitions of diabetes, overall and across different population subgroups. Glycated albumin performed best when more specific criteria (e.g., both Hb A1c and FPG increased) were used to define diabetes. This confirmatory definition is consistent with clinical guidelines (1) and strongly associated with clinical outcomes (16). Our results suggest that glycated albumin may be a suitable alternative or complementary test for diabetes diagnosis in the general population.
Our findings are consistent with smaller studies and those in more select populations. In a previous analysis of the community-based Atherosclerosis Risk in Communities (ARIC) Study (mean age 70), we showed glycated albumin performed well for detection of undiagnosed diabetes (defined as increased FPG or Hb A1c), with AUCs ranging from 0.71 to 0.80 (4). A community study of Japanese adults (mean age 50) found glycated albumin had excellent ability to identify diabetes defined by FPG or Hb A1c (AUC, 0.91) (5). Smaller studies of Asian (17, 18) and African (19) populations reported that glycated albumin had very good diagnostic accuracy to detect diabetes based on 2-hr glucose criteria, with AUCs ranging from 0.83 to 0.87. As the first nationally representative US study of glycated albumin, our study extends the existing research by demonstrating the clinical utility of glycated albumin in the general adult population.
There is growing evidence that glycated albumin is a useful secondary test of glycemia. However, it is important to note that we found that standard tests, Hb A1c in particular, had better diagnostic performance than glycated albumin overall. These results are consistent with our prior work, which found that Hb A1c was more predictive of incident diabetes than glycated albumin in middle-aged US adults (20). Our findings suggest that glucose and Hb A1c should remain the preferred tests for diabetes diagnosis, and that glycated albumin can serve as an adjunct test when standard tests are unreliable.
Glycated albumin is believed to be a more accurate diagnostic test for patients with health conditions that may cause Hb A1c to be unreliable (3). However, we did not find that glycated albumin outperformed Hb A1c significantly in identifying undiagnosed diabetes in adults with anemia, iron deficiency, or chronic kidney disease. Differences in disease severity may explain why our results differ from existing research. For example, prior studies comparing glycated albumin with Hb A1c in the setting of kidney disease have focused largely on dialysis patients (21). In contrast, kidney disease in NHANES participants was mostly early stage. Nonetheless, the performance of glycated albumin was excellent across all subgroups, highlighting its potential utility as an adjunct test across a broad range of patients.
We found that glycated albumin levels of 16.5% and 17.8% were “equivalent” to clinical cut points for FPG and Hb A1c and thus may be useful for identifying individuals with diabetes. Both glycated albumin thresholds had nearly perfect specificity, but low to moderate sensitivity for detecting diabetes. Increased glycated albumin has been shown to predict the onset of microvascular disease, suggesting that these thresholds identify individuals with the highest risk for complications (20). Nonetheless, some caution is warranted, as the lower sensitivity of these glycated albumin thresholds suggests that patients with milder hyperglycemia may be missed. Our glycated albumin cut points differ from prior studies, which have identified “optimal” thresholds by computing the Youden index (5, 18, 19). However, our goal was not to simultaneously maximize specificity and sensitivity; we chose 16.5% and 17.8% to put glycated albumin on roughly equal footing with current diagnostic biomarkers.
Traditional diabetes risk factors such as older age were risk factors for increased glycated albumin, Hb A1c, and FPG. However, overweight and obesity were inconsistently associated with increased glycated albumin, but robustly and positively associated with increased Hb A1c and FPG. This nonlinear association of body mass index with glycated albumin is consistent with prior studies (22–24). The reasons for the more complicated association of body mass index with glycated albumin are unknown but may be partly due to inflammation, which is higher in individuals with obesity, and increased albumin turnover (24). The association of adiposity with glycated albumin may make glycated albumin a less reliable measure of glycemia at lower levels. Consistent with this hypothesis, we found that glycated albumin was weakly correlated with Hb A1c and FPG at normal levels but strongly correlated at high (diabetic) levels (25). Additional work is needed to understand how this differential association with body mass index may affect the clinical performance of glycated albumin.
There were important limitations to our study. First, oral glucose tolerance tests were not administered in the NHANES 1999–2004. We relied on FPG and Hb A1c to assess the diagnostic performance of glycated albumin. Second, glycated albumin was measured in blood samples that were stored for over a decade. While glycated albumin is known to be stable in long-term stored samples (26), the correlation between glycated albumin and Hb A1c or FPG may have been stronger if glycated albumin was measured in fresh or short-term stored samples. Third, because the NHANES is a cross-sectional study, we were not able to evaluate the ability of glycated albumin to predict risk of future diabetes or its complications.
Our study had notable strengths. We are the first to assess the performance of glycated albumin in a nationally representative sample of adults. Our large sample size allowed us to conduct subgroup analyses with a high level of precision. Key measures, including glycated albumin, Hb A1c and FPG, were systemically measured by trained personnel using rigorous and standardized methods.
Conclusion
In conclusion, glycated albumin performed well as a diagnostic test for diabetes in the general US adult population and in important subgroups. However, glucose and Hb A1c demonstrated better diagnostic performance than glycated albumin. Our results suggest that in settings where glucose and Hb A1c are unavailable, glycated albumin may be a useful alternative. Glycated albumin values of 17% to 18% are roughly equivalent to established cut points for standard tests and may be useful thresholds for identifying adults with diabetes.
Supplementary Material
Research Funding:
This work was funded by a grant from the Foundation for the National Institutes of Health Biomarkers Consortium to the Johns Hopkins Bloomberg School of Public Health (PI: E. Selvin). The Foundation for the National Institutes of Health received support for this project from Abbott Laboratories, AstraZeneca, Johnson & Johnson, the National Dairy Council, Ortho Clinical Diagnostics, Roche Diagnostics, and Siemens Healthcare Diagnostics. E. Selvin was also supported by NIH/NHLBI grant K24 HL152440. M. Fang was supported by NIH/NHLBI grant T32 HL007024. Reagents for the glycated albumin assay were donated by Asahi Kasei.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of manuscript.
Nonstandard Abbreviations:
- Hb A1c
hemoglobin A1c
- FPG
fasting plasma glucose
- AUC
area under the receiver operating curve
- NHANES
National Health and Nutrition Examination Survey
- BMI
body mass index
Footnotes
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: R.H. Christenson, The Journal of Applied Laboratory Medicine, AACC.
Consultant or Advisory Role: J. Coresh, Scientific Advisory Board for Healthy.io; R.H. Christenson, Scientific Advisory Board for Healthy.io.
Stock Ownership: None declared.
Honoraria: E. Selvin receives payments from Wolters Kluwer for chapters and laboratory monographs in UpToDate on measurements of glycemic control and screening tests for type 2 diabetes.
Expert Testimony: None declared.
Patents: None declared.
Supplemental Material
Supplemental material is available at Clinical Chemistry online.
References
- 1.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care 2021;44(Suppl 1):S15–S33. [DOI] [PubMed] [Google Scholar]
- 2.Sacks DB. A1C versus glucose testing: a comparison. Diabetes Care 2011;34:518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parrinello CM, Selvin E. Beyond HbA1c and glucose: the role of nontraditional glycemic markers in diabetes diagnosis, prognosis, and management. Curr Diab Rep 2014;14:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juraschek SP, Steffes MW, Selvin E. Associations of alternative markers of glycemia with hemoglobin A1c and fasting glucose. Clin Chem 2012;58:1648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furusyo N, Koga T, Ai M, Otokozawa S, Kohzuma T, Ikezaki H, et al. Utility of glycated albumin for the diagnosis of diabetes mellitus in a Japanese population study: results from the Kyushu and Okinawa Population Study (KOPS). Diabetologia 2011;54:3028–36. [DOI] [PubMed] [Google Scholar]
- 6.Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszan-Moran D, Dohrmann SM, Curtin LR. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat 2 2013;161:1–24. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. National health and nutrition examination laboratory protocol 2015–2016. https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/GHB_I_MET.pdf (Accessed June 2021).
- 8.Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in US adults, 1999–2018. N Engl J Med 2021;384:2219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Center for Health Statistics. National Health and Nutrition Examination Survey Data Documentation - Glycated albumin, Beta-2 Microglobulin, Cystatin C. https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/SSCARD_A.htm (Accessed June 2021).
- 10.Kohzuma T, Yamamoto T, Uematsu Y, Shihabi ZK, Freedman BI. Basic performance of an enzymatic method for glycated albumin and reference range determination. J Diabetes Sci Technol 2011;5:1455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA 1997;277:973–6. [DOI] [PubMed] [Google Scholar]
- 12.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. ; for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Eckardt K-U, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005;67:2089–100. [DOI] [PubMed] [Google Scholar]
- 15.Selvin E, Warren B, He X, Sacks DB, Saenger AK. Establishment of community-based reference intervals for fructosamine, glycated albumin, and 1,5-anhydroglucitol. Clin Chem 2018;64:843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selvin E, Wang D, Matsushita K, Grams ME, Coresh J. Prognostic implications of single-sample confirmatory testing for undiagnosed diabetes: a prospective cohort study. Ann Intern Med 2018;169: 156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang Y-C, Jung CH, Ahn H-Y, Jeon WS, Jin S-M, Woo J-T, et al. Optimal glycated albumin cutoff value to diagnose diabetes in Korean adults: a retrospective study based on the oral glucose tolerance test. Clin Chim Acta 2014;437:1–5. [DOI] [PubMed] [Google Scholar]
- 18.Wu W-C, Ma W-Y, Wei J-N, Yu T-Y, Lin M-S, Shih S-R, et al. Serum glycated albumin to guide the diagnosis of diabetes mellitus. PLoS One 2016;11:e0146780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zemlin AE, Barkhuizen M, Kengne AP, Erasmus RT, Matsha TE. Performance of glycated albumin for type 2 diabetes and prediabetes diagnosis in a South African population. Clin Chim Acta 2019;488:122–8. [DOI] [PubMed] [Google Scholar]
- 20.Selvin E, Rawlings AM, Grams M, Klein R, Sharrett AR, Steffes M, Coresh J. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol 2014;2:279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gan T, Liu X, Xu G. Glycated albumin versus HbA1c in the evaluation of glycemic control in patients with diabetes and CKD. Kidney Int Rep 2018;3:542–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poon AK, Juraschek SP, Ballantyne CM, Steffes MW, Selvin E. Comparative associations of diabetes risk factors with five measures of hyperglycemia. BMJ Open Diabetes Res Care 2014;2:e000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyashita Y, Nishimura R, Morimoto A, Matsudaira T, Sano H, Tajima N. Glycated albumin is low in obese, type 2 diabetic patients. Diabetes Res Clin Pract 2007;78:51–5. [DOI] [PubMed] [Google Scholar]
- 24.Koga M, Otsuki M, Matsumoto S, Saito H, Mukai M, Kasayama S. Negative association of obesity and its related chronic inflammation with serum glycated albumin but not glycated hemoglobin levels. Clin Chim Acta 2007;378:48–52. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro RT, Macedo MP, Raposo JF. HbA1c, fructosamine, and glycated albumin in the detection of dysglycaemic conditions. Curr Diabetes Rev 2016;12:14–9. [DOI] [PubMed] [Google Scholar]
- 26.Nathan DM, Steffes MW, Sun W, Rynders GP, Lachin JM. Determining stability of stored samples retrospectively: the validation of glycated albumin. Clin Chem 2011;57:286–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


