Abstract
Introduction:
Pernicious anemia (PA) is a risk factor for gastric cancer. Other autoimmune conditions may also contribute.
Methods:
In a case-control study, we evaluated 47 autoimmune conditions among 39,125 gastric cancers and 200,000 cancer-free controls.
Results:
Six conditions were associated with increased gastric cancer risk (range of adjusted odds ratios [ORs]: 1.28-1.93, p<0.05): PA, membranous nephropathy, primary biliary cirrhosis, pure red cell aplasia, primary sclerosing cholangitis, and Graves’ disease. PA was associated with 8 other autoimmune conditions (adjusted ORs: 1.57-4.54, p<0.05).
Conclusions:
Autoimmune conditions associated with gastric cancer or PA may reflect effects of autoimmune gastritis or other carcinogenic pathways.
Keywords: Autoimmunity, Gastric Cancer, Epidemiology, Elderly
INTRODUCTION
Pernicious anemia (PA) has been associated with gastric cancer (1, 2). With waning prevalence of Helicobacter pylori infection, the relative significance of this alternative etiologic pathway involving autoimmunity may grow (3). Notably, recent increases in gastric cancer among certain groups implicate autoimmunity as a possible cause (4). Several autoimmune diseases besides PA have been associated with gastric cancer risk (5).
The global prevalence of autoimmune conditions is rising (6). Therefore, it is important to assess their impact on cancer incidence. We comprehensively evaluated associations between autoimmune conditions and gastric cancer among elderly adults in the United States. We further explored associations of autoimmune conditions with PA, which we hypothesize may mediate associations between some conditions and gastric cancer.
MATERIALS AND METHODS
In a population-based case-control study using the Surveillance Epidemiology and End Results (SEER)-Medicare linked database (7), we identified gastric cancer cases with a first cancer diagnosis during 1992-2015. A total of 200,000 cancer-free controls were selected from the 5% random sample of Medicare beneficiaries included in SEER-Medicare, frequency-matched to cases by calendar year of selection, age category (66-69, 70-74, 75-79, 80-84, 85-99 years), sex and race (non-Hispanic White, non-Hispanic Black, other/unknown). We identified autoimmune conditions using Medicare claims, requiring documentation of autoimmune conditions in one hospital claim or two provider or outpatient claims at least 30 days apart. We evaluated 47 different autoimmune conditions but the results for 11 conditions with ≤10 affected cases are not presented (Supplementary Table 1).
Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using unconditional logistic regression (7), adjusting for matching variables, average number of physician visits per year, zip code-based median income, smoking, and alcohol abuse (Supplementary Table 2). We used a Bonferroni correction to determine statistical significance (p-value cutoff 0.05/47=0.001). Significant associations were assessed for heterogeneity by sex. We conducted exploratory analyses for anatomical subsites, for which p<0.05 was considered significant (nominal significance). Furthermore, we assessed the association of conditions with PA among controls. See the Supplementary Methods for additional details.
RESULTS
The study included 39,125 gastric cancer cases and 200,000 cancer-free controls (Supplementary Table 3). Having any autoimmune conditions was positively associated with gastric cancer (OR 1.16, 95%CI 1.13-1.20). Six autoimmune conditions were nominally associated with increased risk of gastric cancer: PA, membranous nephropathy, primary biliary cirrhosis, pure red cell aplasia (PRCA), primary sclerosing cholangitis, and Graves’ disease (OR range 1.28-1.93, Figure 1). Two were associated with reduced gastric cancer risk: Takayasu arteritis and celiac disease (ORs 0.58-0.73). After Bonferroni correction, only PA and PRCA remained significant. The association between PRCA and gastric cancer was similar after additional adjustments for PA (Supplementary Table 4).
Figure 1. Association of autoimmune conditions with gastric cancer.
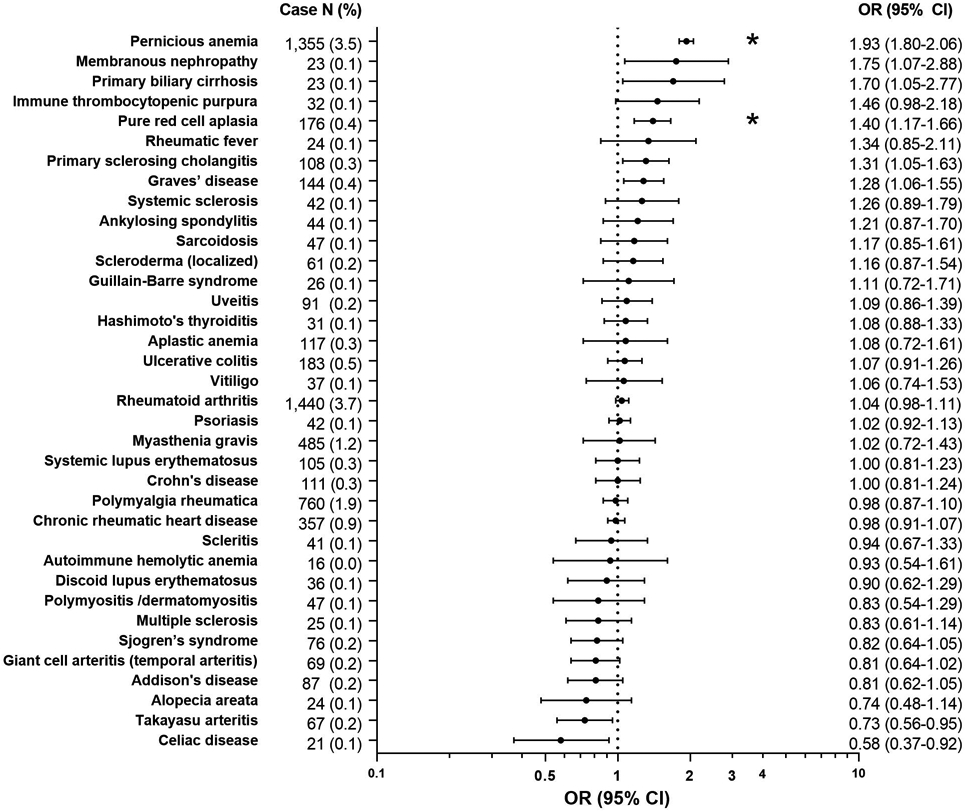
Odds ratios are adjusted for age, sex, race/ethnicity, calendar year of cancer diagnosis/control selection, average number of physician visits per year, zip code median income, smoking, and alcohol abuse. Asterisk (*) indicates association is significant at the Bonferroni p-value threshold.
Abbreviations: CI, confidence interval; OR, odds ratio
A sex-stratified analysis revealed stronger PA and gastric cancer association in women (OR 2.17, 95%CI 1.99-2.37) than men (1.67, 1.51-1.85, Pheterogeneity<0.0001), but not for PRCA (Pheterogeneity=0.47). As shown in Table 1, the magnitude of association was stronger for noncardia than cardia gastric cancers with any autoimmune condition (OR 1.16 vs 1.08, respectively; Pheterogeneity=0.0004) and PA (2.03 vs 1.27; Pheterogeneity < 0.0001) but not PRCA (1.50 vs 1.00; Pheterogeneity=0.07).
Table 1.
Associations between autoimmune conditions and gastric cancer risk, according to cancer subsite
| Conditions | Control | Cardia (n=10,169) | Noncardia (n=19,453) | Others/Unspecified (n=9,503) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | OR (95%CI) | N | % | OR (95%CI) | N | % | OR (95%CI) | |
| Addison's disease | 406 | 0.2 | 14 | 0.1 | 0.62 (0.36-1.06) | 34 | 0.2 | 0.80 (0.56-1.15) | 21 | 0.2 | 1.06 (0.68-1.64) |
| Alopecia areata | 165 | 0.1 | 17 | 0.1 | 0.94 (0.57-1.56) | ||||||
| Ankylosing spondylitis | 175 | 0.1 | 11 | 0.1 | 0.96 (0.52-1.78) | 20 | 0.1 | 1.18 (0.74-1.88) | 13 | 0.1 | 1.61 (0.91-2.86) |
| Aplastic anemia | 138 | 0.1 | 17 | 0.1 | 1.09 (0.65-1.84) | ||||||
| Celiac disease | 179 | 0.1 | 11 | 0.1 | 0.67 (0.36-1.23) | ||||||
| Chronic rheumatic heart disease | 3,580 | 1.8 | 229 | 2.3 | 1.11 (0.96-1.27) | 341 | 1.8 | 0.89 (0.79-1.00) | 190 | 2.0 | 1.06 (0.91-1.23) |
| Crohn's disease | 507 | 0.3 | 25 | 0.2 | 0.78 (0.52-1.18) | 57 | 0.3 | 1.16 (0.88-1.53) | 23 | 0.2 | 0.95 (0.62-1.45) |
| Discoid lupus erythematosus | 197 | 0.1 | 15 | 0.1 | 0.71 (0.42-1.21) | 12 | 0.1 | 1.21 (0.67-2.19) | |||
| Giant cell arteritis (temporal arteritis) | 522 | 0.3 | 25 | 0.2 | 1.00 (0.66-1.51) | 46 | 0.2 | 0.84 (0.61-1.14) | 16 | 0.2 | 0.60 (0.36-0.99) |
| Graves’ disease | 553 | 0.3 | 26 | 0.3 | 0.98 (0.66-1.46) | 86 | 0.4 | 1.48 (1.17-1.86) | 32 | 0.3 | 1.17 (0.82-1.68) |
| Guillain-Barré syndrome | 112 | 0.1 | 13 | 0.1 | 1.12 (0.63-1.99) | ||||||
| Hashimoto's thyroiditis | 538 | 0.3 | 30 | 0.3 | 1.07 (0.73-1.55) | 61 | 0.3 | 1.15 (0.88-1.51) | 26 | 0.3 | 1.00 (0.67-1.50) |
| Immune thrombocytopenic purpura | 107 | 0.1 | 16 | 0.1 | 1.60 (0.94-2.73) | ||||||
| Membranous nephropathy | 65 | 0.0 | 11 | 0.1 | 1.67 (0.86-3.23) | ||||||
| Multiple sclerosis | 282 | 0.1 | 16 | 0.2 | 0.97 (0.58-1.62) | 19 | 0.1 | 0.72 (0.45-1.16) | 12 | 0.1 | 0.91 (0.51-1.63) |
| Myasthenia gravis | 203 | 0.1 | 13 | 0.1 | 1.00 (0.56-1.77) | 18 | 0.1 | 0.94 (0.57-1.53) | 11 | 0.1 | 1.19 (0.64-2.19) |
| Pernicious anemia | 3,532 | 1.8 | 225 | 2.2 | 1.27 (1.10-1.46) | 717 | 3.7 | 2.03 (1.86-2.21) | 413 | 4.3 | 2.46 (2.21-2.74) |
| Polymyalgia rheumatica | 1,802 | 0.9 | 91 | 0.9 | 0.98 (0.79-1.22) | 176 | 0.9 | 0.97 (0.83-1.14) | 90 | 0.9 | 1.00 (0.81-1.25) |
| Primary sclerosing cholangitis | 399 | 0.2 | 17 | 0.2 | 0.85 (0.52-1.39) | 71 | 0.4 | 1.64 (1.26-2.13) | 20 | 0.2 | 1.03 (0.66-1.63) |
| Psoriasis | 2,352 | 1.2 | 164 | 1.6 | 1.15 (0.98-1.36) | 211 | 1.1 | 0.93 (0.81-1.08) | 110 | 1.2 | 1.01 (0.83-1.23) |
| Pure red cell aplasia | 597 | 0.3 | 30 | 0.3 | 1.00 (0.69-1.46) | 99 | 0.5 | 1.50 (1.20-1.87) | 47 | 0.5 | 1.56 (1.15-2.11) |
| Rheumatic fever | 87 | 0.0 | 12 | 0.1 | 1.32 (0.72-2.43) | ||||||
| Rheumatoid arthritis | 6,731 | 3.4 | 293 | 2.9 | 0.93 (0.82-1.05) | 774 | 4.0 | 1.06 (0.98-1.14) | 373 | 3.9 | 1.12 (1.01-1.25) |
| Sarcoidosis | 198 | 0.1 | 29 | 0.1 | 1.38 (0.93-2.05) | 15 | 0.2 | 1.56 (0.92-2.67) | |||
| Scleritis | 209 | 0.1 | 11 | 0.1 | 1.13 (0.60-2.12) | 18 | 0.1 | 0.76 (0.47-1.25) | 12 | 0.1 | 1.19 (0.66-2.15) |
| Scleroderma (localized) | 260 | 0.1 | 18 | 0.2 | 1.54 (0.95-2.48) | 30 | 0.2 | 1.13 (0.77-1.66) | 13 | 0.1 | 0.98 (0.56-1.72) |
| Sjögren’s syndrome | 456 | 0.2 | 19 | 0.2 | 0.92 (0.58-1.46) | 39 | 0.2 | 0.80 (0.58-1.12) | 18 | 0.2 | 0.79 (0.49-1.27) |
| Systemic lupus erythematosus | 542 | 0.3 | 21 | 0.2 | 0.82 (0.53-1.28) | 55 | 0.3 | 0.95 (0.71-1.26) | 35 | 0.4 | 1.29 (0.91-1.83) |
| Systemic sclerosis | 159 | 0.1 | 12 | 0.1 | 1.71 (0.94-3.10) | 18 | 0.1 | 1.03 (0.62-1.69) | 12 | 0.1 | 1.46 (0.80-2.65) |
| Takayasu arteritis | 438 | 0.2 | 16 | 0.2 | 0.66 (0.40-1.10) | 38 | 0.2 | 0.83 (0.59-1.16) | 13 | 0.1 | 0.60 (0.34-1.04) |
| Ulcerative colitis | 841 | 0.4 | 60 | 0.6 | 1.19 (0.91-1.56) | 83 | 0.4 | 1.02 (0.81-1.29) | 40 | 0.4 | 1.02 (0.74-1.40) |
| Uveitis | 411 | 0.2 | 14 | 0.1 | 0.82 (0.48-1.40) | 50 | 0.3 | 1.10 (0.81-1.49) | 27 | 0.3 | 1.32 (0.88-1.96) |
| Vitiligo | 174 | 0.1 | 21 | 0.1 | 1.13 (0.71-1.80) | ||||||
| Any autoimmune condition | 24,459 | 12.2 | 1,346 | 13.2 | 1.08 (1.02-1.15) | 2,862 | 14.7 | 1.16 (1.11-1.21) | 1,455 | 15.3 | 1.28 (1.20-1.36) |
Entries for the number of affected cases are not presented when the number is ≤10, in accordance with the SEER-Medicare data use agreement. Odds ratios are adjusted for age, sex, race/ethnicity, calendar year of cancer diagnosis/control selection, average number of physician visits per year, zip code median income, smoking, and alcohol abuse.
Bolded results indicate nominal significance (p<0.05).
Abbreviations: CI, confidence interval; OR, odds ratio
Among controls, PA was most strongly associated with aplastic anemia, followed by Crohn’s disease, PRCA, polymyositis/dermatomyositis, celiac disease, Addison’s disease, rheumatoid arthritis, and ulcerative colitis (OR range 1.75-4.54, Figure 2).
Figure 2. Associations of autoimmune conditions with pernicious anemia among control individuals.
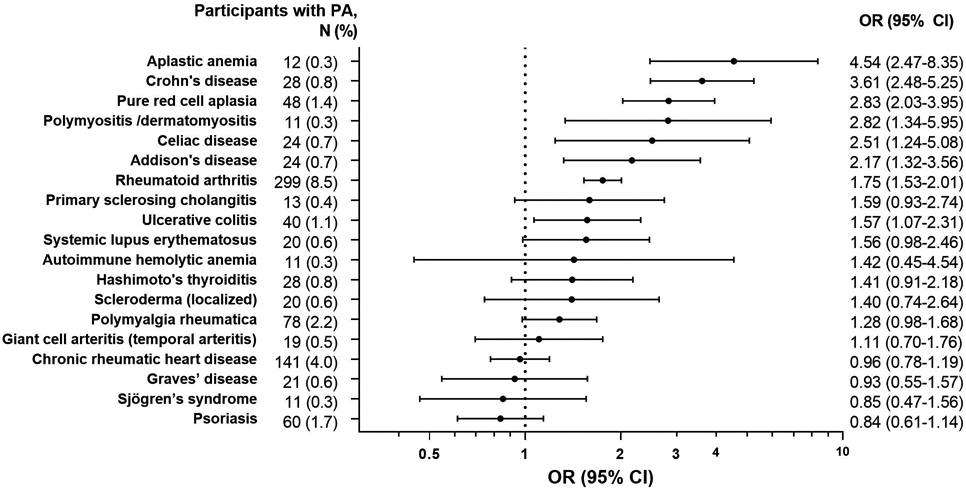
Odds ratios are adjusted for age, sex, race/ethnicity, calendar year of cancer diagnosis/control selection, average number of physician visits per year, zip code median income, smoking, and alcohol abuse.
Abbreviations: CI, confidence interval; OR, odds ratio; PA, pernicious anemia
DISCUSSION
To our knowledge, this is the largest, most comprehensive study evaluating associations between autoimmune conditions and gastric cancer. Having any autoimmune condition was associated with a small increased risk of gastric cancer. We confirmed the known strong association with PA and found a novel independent association with PRCA.
PA is caused by autoimmune gastritis, which may increase gastric cancer risk through chronic increase in gastric pH and gastrin secretion (8). The association with PA was strongest for noncardia gastric cancer, which matches the anatomy of autoimmune gastritis (9). We are the first to report a difference in the association between PA and gastric cancer by sex. This stronger association among women may reflect a higher prevalence of other cofactors in men, such as cigarette smoking or H. pylori infection.
Notably, we found PRCA to be associated with both gastric cancer and PA. There are limited case studies of co-occurrence of PRCA and PA (10), and PRCA with gastric cancer (11). The restricted association of PRCA with noncardia gastric cancer supports that autoimmune gastritis may mediate this association.
Conditions that showed associations with gastric cancer but not with PA may act through pathways that do not involve autoimmune gastritis. These autoimmune conditions may exacerbate gastritis caused by H. pylori, or H. pylori may cause these autoimmune conditions (12). Autoimmune conditions associated only with PA probably do not have direct mechanistic links to gastric carcinogenesis, and positive results in published literature may reflect their associations with PA (5).
The strengths of our study include its population-based design and large sample size. Our study is larger than an earlier SEER-Medicare study (2), and we included 7-10 times more gastric cancers with autoimmune diseases than studies in Sweden (13) and among US veterans (14). The large size enabled us to explore associations between rare autoimmune diseases and gastric cancer, and with its anatomical subsites.
However, we could not assess some factors related to gastric cancer risks, such as H. pylori infection, family history of gastric cancer, salty food consumption, and autoimmune disease treatments. The results may not be generalizable to younger people or non-White individuals (who were under-represented). Medical claims data are inherently limited in diagnostic accuracy. Clinical conditions could not be ascertained before age 65, so some autoimmune conditions may have been underdiagnosed. Finally, the rarity of autoimmune conditions and gastric cancer limited our ability to detect associations with small effect sizes.
Rising trends in autoimmunity may account for the increasing incidence of gastric cancer among certain populations. Additional research may provide evidence for developing strategies in assessing gastric cancer risk, which may facilitate early detection and improve cancer outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
This study used the linked SEER-Medicare database. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors would also like to acknowledge Winnie Ricker at IMS for providing analytical support. This study was supported by the Intramural Research Program of the National Cancer Institute.
ABBREVIATIONS
- CI
confidence interval
- OR
odds ratios
- PA
pernicious anemia
- PRCA
pure red cell aplasia
- SEER
Surveillance Epidemiology and End Results
Footnotes
Potential competing interests: None.
Writing Assistance: None.
REFERENCES
- 1.Vannella L, Lahner E, Osborn J, et al. Systematic review: gastric cancer incidence in pernicious anaemia. Aliment Pharmacol Ther 2013;37:375–82. [DOI] [PubMed] [Google Scholar]
- 2.Murphy G, Dawsey SM, Engels EA, et al. Cancer Risk After Pernicious Anemia in the US Elderly Population. Clin Gastroenterol Hepatol 2015;13:2282–9 e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song M, Rabkin CS, Camargo MC. Gastric Cancer: an Evolving Disease. Curr Treat Options Gastroenterol 2018;16:561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson WF, Rabkin CS, Turner N, et al. The Changing Face of Noncardia Gastric Cancer Incidence Among US Non-Hispanic Whites. J Natl Cancer Inst 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song M, Latorre G, Ivanovic-Zuvic D, et al. Autoimmune Diseases and Gastric Cancer Risk: A Systematic Review and Meta-Analysis. Cancer Res Treat 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lerner A, Jeremias P, Matthias T. The World Incidence and Prevalence of Autoimmune Diseases is Increasing. International Journal of Celiac Disease 2015;3:151–155. [Google Scholar]
- 7.Engels EA, Pfeiffer RM, Ricker W, et al. Use of surveillance, epidemiology, and end results-medicare data to conduct case-control studies of cancer among the US elderly. Am J Epidemiol 2011;174:860–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hakanson R, Sundler F. Trophic effects of gastrin. Scand J Gastroenterol Suppl 1991;180:130–6. [DOI] [PubMed] [Google Scholar]
- 9.Neumann WL, Coss E, Rugge M, et al. Autoimmune atrophic gastritis--pathogenesis, pathology and management. Nat Rev Gastroenterol Hepatol 2013;10:529–41. [DOI] [PubMed] [Google Scholar]
- 10.Dan K, Ito T, Nomura T. Pure red cell aplasia following pernicious anemia. Am J Hematol 1990;33:148–50. [DOI] [PubMed] [Google Scholar]
- 11.Gajwani B, Zinner EN. Pure red-cell aplasia. Associated with adenocarcinoma of stomach. N Y State J Med 1976;76:2177–9. [PubMed] [Google Scholar]
- 12.Hasni S, Ippolito A, Illei GG. Helicobacter pylori and autoimmune diseases. Oral Dis 2011;17:621–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemminki K, Liu X, Ji J, et al. Autoimmune disease and subsequent digestive tract cancer by histology. Ann Oncol 2012;23:927–33. [DOI] [PubMed] [Google Scholar]
- 14.Landgren AM, Landgren O, Gridley G, et al. Autoimmune disease and subsequent risk of developing alimentary tract cancers among 4.5 million US male veterans. Cancer 2011;117:1163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


