Abstract
Objective:
Obsessive-compulsive disorder (OCD) is known to be substantially heritable; however, the contribution of common genetic variation across the allele frequency spectrum to this heritability remains uncertain. We use two new, homogenous cohorts to estimate heritability of OCD from common genetic variation and contrast results with prior studies.
Methods:
The sample consisted of 2090 Swedish-born individuals diagnosed with OCD and 4567 controls, all genotyped for common genetic variants, specifically >400,000 single nucleotide polymorphisms (SNPs) with minor allele frequency (MAF) ≥ 0.01. Using genotypes of these SNPs to estimate distant familial relationships among individuals, we estimated heritability of OCD, both overall and partitioned according to MAF bins.
Results:
We estimated narrow-sense heritability of 29% (SE=4%). The estimate was robust, varying only modestly under different models. Contrary to an earlier study, however, SNPs with MAF between 0.01 and 0.05 accounted for 10% of heritability and estimated heritability per bin roughly follows expectations based on a simple model for SNP-based heritability.
Conclusions:
These results indicate that common inherited risk variation (MAF ≥ 0.01) accounts for most of the heritable variation in OCD. SNPs with low MAF contribute meaningfully to the heritability of OCD and the results are consistent with expectation under the “infinitesimal model,” where risk is influenced by a large number of loci across the genome and across MAF bins.
1. Introduction
Obsessive-compulsive disorder (OCD) is a serious and often long-lasting psychiatric disorder characterized by intrusive and unwanted thoughts, images, or urges (obsessions) that are typically linked to ritualized behaviors (compulsions) (1–4). OCD affects 1–3% of the population and multiple studies provide reliable evidence for a significant genetic contribution to risk (1, 3–6), as well as a role for environmental factors impacting risk (7, 8). The heritability of OCD, historically estimated by analysis of twin and family studies and within the context of the ACE model (additive genetic, also known as narrow-sense heritability, A; shared environment, C; and nonshared environment, E), is reported to be 35–50% (1, 4, 8–14).
As an alternative to the analysis of recurrence risk for OCD within pedigrees, heritability can also be estimated from individuals drawn from a population who have no obvious familial relationships, as long as they have been characterized for genetic variation across their genomes. Usually, this genetic characterization employs genotypes of single nucleotide polymorphisms (SNPs) for which alleles are common in the population. In this approach, which we will call SNP-based, the central idea is that the multiplicity of SNP genotypes allows estimation of familial relationships, albeit distant, among subjects as well as the covariance of their phenotypes, and these are the key elements for estimating heritability. When the heritability of OCD is computed in this manner, estimates range from 25–43% (5, 14–16).
It is useful to compare the heritability results from family-based and SNP-based approaches. Family-based studies, being more direct, typically yield estimates of heritability with lower standard errors, whereas the inaccuracy of estimating distant relationships from genetic data tends to produce fuzzier estimates. Family-based estimates also tend to yield higher estimates of heritability because the familial covariance traces to both rare and common genetic variation, whereas SNP-based estimates mostly arise from covariance due to common genetic variants. Looking at the results summarized above, one might conclude that this is also operating for OCD, i.e., that family-based studies are producing higher heritability estimates than SNP-based studies.
However, in an influential paper by Davis and colleagues (number of cases: 1,061; number of controls: 4,236; number of SNPs: 373,846) (5), there was no evidence for heritability from SNPs with minor allele frequency (MAF) < 0.05 and over 60% of total heritability mapped to the most common variants (MAF > 0.3). In addition, in a meta-analysis of data from OCD Collaborative Genetics Association Study (OCGAS) and Davis et al., ~60% of heritability was accounted for by SNPs with MAF > 0.4 in both the OCGAS sample alone and in the combined sample (16). If this observation were true, it could have profound implications for which evolutionary forces shaped this unusual mapping of risk alleles to their population frequency distribution. For example, balancing selection, where multiple alleles are maintained in the gene pool of a population at frequencies larger than expected from genetic drift alone may play a role in OCD.
At the same time, other studies have implicated rare variants in risk for OCD (17–20). Thus, the contribution of inherited genetic variation across the allelic frequency spectrum to the risk of OCD remains uncertain and worthy of further study, as it impacts both our understanding of processes underlying OCD risk architecture and rational study design. Here, using a substantially larger sample compared to previous studies and new genetic data from the Swedish population, we estimate SNP-based heritability for OCD.
2. Methods
2.1. Study population
Ethical approvals were obtained from the Institutional Review Board (IRB) at the Icahn School of Medicine at Mount Sinai, New York, NY, and the Regional Ethical Review Board in Stockholm. We used Swedish OCD cases collected through two studies: the EGOS cohort (Epidemiology and Genetics of Obsessive-compulsive disorder and chronic tic disorders in Sweden) (21) and the NORDiC cohort (Nordic OCD and Related Disorders Consortium) (22).
In the EGOS cohort, individuals born between 1954 and 1998, with at least two clinical diagnoses of OCD in the Swedish National Patient Register (NPR), were eligible for inclusion (21). In the Swedish site of the NORDiC cohort, individuals with OCD were recruited from specialty OCD and related disorder clinics across Sweden (22). Genotype data on the global screen array (GSA) were collected for 1108 individuals from the EGOS cohort and 1107 individuals from the NORDiC cohort.
A sample of 4738 controls from the LifeGene cohort was available for this study. LifeGene is a prospective population-based cohort of around 50,000 individuals in Sweden (23). The samples were available in four batches: LifeGene-EGOS (n=1444), LifeGene-NORDiC (n=500), LifeGene-ANGI-Wave-1 (n=1500), and LifeGene-ANGI-Wave-2 (n=1500). LifeGene-ANGI controls were previously used in a study of anorexia nervosa (AN) (24); they were mostly females (2935 females and 65 males), and all individuals with a diagnosis of AN were previously removed from this batch. All controls were genotyped using GSA.
2.2. Quality control
All OCD cases, LifeGene-EGOS controls, and LifeGene-NORDiC controls were genotyped in the same laboratory but in different batches. GenomeStudio’s genotyping module was used to re-call genotypes on the joint data.
Quality control (QC) was first carried out on three batches of samples that may differ in key variables: 1) all cases, LifeGene-EGOS controls, and LifeGene-NORDiC, 2) LifeGene-ANGI-Wave-1 controls, and 3) LifeGene-ANGI-Wave-2 controls. We employed the following QC steps using PLINK 2.0 (Supplementary Materials Tables S1–S3): individuals were removed who had a genotype non-call rate > 0.05, were discrepant for nominal versus genetically-determined sex, or had low heterozygosity (< −3SD or > +3SD from the mean); a SNP was removed if its non-call rate for genotypes was > 0.05, its MAF < 0.01, or it had Hardy–Weinberg equilibrium (HW) p-value < 0.00125. Gemtools was used to choose individuals with European ancestry where indicated (Supplementary Materials Figure S1).
We next used the McCarthy tool to match the SNPs to 1000 Genomes, and Genotype Harmonizer software (automatic strand alignment software) to align the different cohorts (25). After QC, we merged the cohorts based on the set of all intersecting SNPs and performed additional QC as noted in Supplementary Materials. The final data set included 2090 cases and 4567 controls, with 412,813 SNPs (Table 2).
Table 2.
Summary of data before and after quality control.
| Study | Before QC | After QC | |||
|---|---|---|---|---|---|
|
|
|
||||
| #Individuals | #SNP | #Individuals | #Females (%) | ||
| EGOS, cases | 1108 | 759993 | 1066 | 667 | (63%) |
| NORDiC, cases | 1107 | 759993 | 1024 | 596 | (58%) |
| LifeGene-EGOS, controls | 1444 | 759993 | 1238 | 452 | (36%) |
| LifeGene-ANGI-Wave-1, controls | 1500 | 688032 | 1432 | 1378 | (96%) |
| LifeGene-ANGI-Wave-2, controls | 1500 | 688032 | 1442 | 1432 | (99%) |
| LifeGene-NORDiC, controls | 500 | 759993 | 455 | 228 | (50%) |
|
| |||||
| Total (merged) 1 | 7059 | 6657 | 4753 | (71%) | |
| Cases | 2115 | 2090 | 1263 | (60%) | |
| Controls | 4944 | 4567 | 3490 | (76%) | |
After QC, the final data had 412813 SNP, 406120 on autosomes
2.3. Statistical analysis
We used the Genome-wide Complex Trait Analysis (GCTA) program version 1.26.0 to estimate the genetic relationship matrix (GRM) between all pairs of individuals from SNPs (26). Then, we used PLINK 2.0 to extract the top principal components (PCAs) from the variance-standardized relationship matrix (for more details, see Supplementary Materials). We performed restricted maximum likelihood (REML) analysis, implemented in GCTA, to estimate heritability of OCD attributable to SNP genotypes. Because the OCD diagnosis is dichotomous, we scaled the phenotypic variance to an underlying liability scale using the population prevalence of 1%, similar to our most recent estimate of population prevalence in Sweden using data from the Swedish national registers (1) (for more details, see Supplementary Materials, where we also provide results for 2% prevalence).
To evaluate the sensitivity of estimates of SNP-based heritability to modeling approaches, we assessed the data in multiple ways. The first assessment of data included all affected and unaffected individuals born in Sweden, of whom most, but not all, were of Swedish/European genetic ancestry; use all 405,105 high quality, genotyped SNPs for analysis. The sampling in this first assessment of data is consistent with our previously-published, family-based analyses and will be our primary analytical approach. The second assessment of data pruned SNPs according to linkage disequilibrium (LD) to obtain a smaller set of 184,296 largely independent SNPs. The third assessment of data limited the sample to individuals of European genetic ancestry. The fourth assessment of data removed all individuals for whom there is also a fifth degree or greater relative in the sample. The fifth assessment of data analyzed only pairs of affected and unaffected individuals, matched on two dimensions of genetic ancestry using the function pairmatch in the package optmatch in R (1-to-1 fullmatch) (Supplementary Materials). The sixth assessment of data was conducted as was done for the fifth, using only individuals of European ancestry. Pair matching, as done in the fifth and sixth assessments, is a common epidemiological approach for controlling confounding (here, differences in ancestry in cases versus controls) and has been shown to be useful for genetic studies (27–29). Note assessments 3–6 use all high-quality SNPs.
We also estimated heritability partitioned by chromosomes and MAF bins and compared the results with those from Davis et al. (5). Following Davis, we created six MAF bins: 0.01–0.05, 0.05–0.1, 0.1–0.2, 0.2–0.3, 0.3–0.4, and 0.4–0.5. For each bin, we computed a GRM, and then additive genetic variance attributed to each subset was jointly estimated with multiple GRM (using --mgrm in GCTA). This allows for the effects of LD to be partitioned by the REML.
3. Results
Our study population included 2090 cases and 4567 controls after quality control was completed. Among our cases, 60% were female, while 76% of controls were female. Based on Principal Component Analysis (PCA; Figure S2), we used the first six PCAs as covariates to adjust for variation in ancestry in all heritability analyses. As a check for compatibility of cohorts, we first estimated heritability by treating EGOS and NORDiC controls as cases and LifeGene-ANGI controls as controls. Heritability was estimated at 0.0001% (SE = 5%). These results show that the control cohorts were homogeneous. Next, we estimated heritability of OCD for the full sample, contrasting OCD cases to controls and yielding an estimate of 29% (SE=4%) for a population prevalence of 1%.
Technically, heritability is first estimated on the observed scale, namely dichotomous OCD diagnosis; however, heritability on the continuous liability scale is more interpretable and so is usually reported. Heritability can be transformed from the observed to the continuous liability scales because they are functions of prevalence (30). To determine how sensitive our heritability estimate was to prevalence, we varied it between 0.5%−3% and found heritability to vary between 25%−38% (Supplementary Materials Table S4).
We next performed a set of sensitivity analyses by different treatments of the data, as described in Methods, and found the estimates to be quite robust (Table 3). Notably, although analyses suggested that EGOS and NORDiC cases had slightly different ancestry distributions, the results in Table 3 show that our adjustments for ancestry were sufficient to compensate for these differences (Figures S4–S9, Table S5). In addition, we did not observe a significant difference in heritability between the EGOS and NORDiC cases (Table S5).
Table 3.
Estimates of heritability of OCD under various treatments of the data.
| Data | #Cases | #Controls | Heritability (SE) | |
|---|---|---|---|---|
| All cases and controls | 2090 | 1263 | 29% | (4%) |
| All cases and controls (based on 184296 SNPs after LD pruning) | 2090 | 1263 | 28% | (4%) |
| Individuals with European ancestry | 1831 | 4065 | 28% | (5%) |
| All third cousins or closer relatives removed | 1822 | 3954 | 30% | (5%) |
| Ancestry-matched (1-to-1 fullmatch) | 2090 | 2090 | 26% | (6%) |
| Ancestry and sex matched (1-to-1 fullmatch) | 2090 | 2090 | 29% | (6%) |
| Ancestry-matched (1-to-1 fullmatch), European ancestry | 1831 | 1831 | 23% | (7%) |
3.1. Heritability analysis partitioned by MAF bins
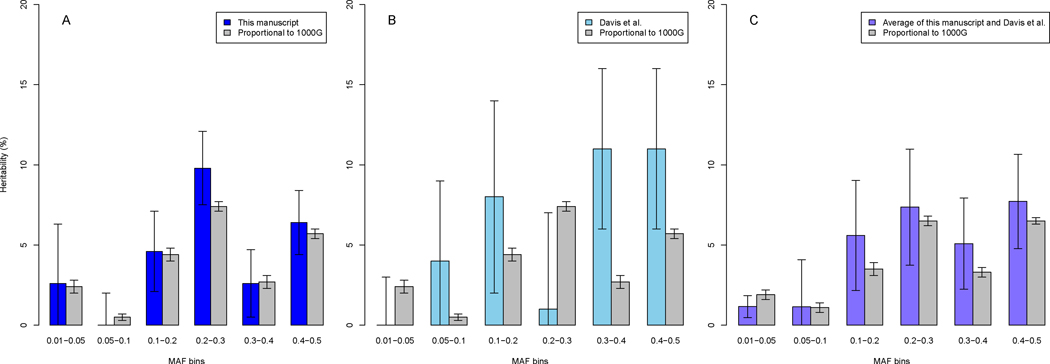
Having established that a substantial portion of OCD traces to common variation, we next addressed an important issue about its nature. Specifically, in an earlier study, Davis et al. (5) found that alleles with MAF < 0.05 did not contribute meaningfully to the heritability of OCD (0.0001% of total heritability). To compare our results to those in Davis et al. (5), we estimated the portion of total heritability for groups of autosomal SNPs with distinct allele frequencies, grouping the SNPs into six bins based on their MAF (Figure 1; Table S9): 0.01–0.05, 0.05–0.1, 0.1–0.2, 0.2–0.3, 0.3–0.4, and 0.4–0.5. For all the bins, we included the first six PCAs as covariates and set population prevalence to 0.01. Estimates of the portion of total heritability for the bins were distributed differently between these two studies (Figure 1; Table S9). Curiously, although the total heritability of the first two bins (MAF < 0.1) was similar across studies, 2.6% for our study versus 4% for Davis, estimates for specific bins were not similar; in the Davis et al. study, the MAF bin from 0.01–0.05 accounted for essentially no heritability (0.0001%) whereas our estimate was much larger (2.6%) (Figure 1; Table S9). A portion of the difference could be due to the number and nature of the SNPs falling in this bin: there were approximately ten times more genotyped SNPs falling into this bin in the current study compared with Davis et al. (Table S9); however, Davis also imputed genotypes for over 2 million SNPs for this bin and those genotypes did not alter their heritability estimate in that bin (see their Table 2).
Figure 1.
Estimates of heritability partitioned by MAF bins from the results in A) this study, B) Davis et al. (5) and, C) weighted averages (weights proportional to the inverse of variance) of this study and Davis et al. In each panel, we also show the estimate of heritability for each bin from 1000G data, presented as the mean of heritability for that bin for ten samples of size 180K SNP, where sampling from each bin was proportional to the percentage of SNPs in that bin from 1000G data. Note that the SE for this latter analysis is the standard error of the sample mean for the ten samples and is not directly comparable to the SNP-based SE. Correlations with 1000G data were 0.99, p-value<0.001, for panel A; 0.04, p-value=0.94, for panel B; and 0.94, p-value=0.005, for panel C.
To investigate these differences, we estimated what the expected portion of total heritability in these bins should be. First, we observed that the percentages of the total SNPs in each bin were distributed differently in comparison to 1000 Genomes data (for SNPs with MAF > 0.01) (Tables S6 and S7), which we would expect is more representative of variation in the general population. For example, 45.2% of the SNPs in our study had MAF between 0.01 and 0.05, while 29.5% of SNPs in 1000 Genomes data had MAF between 0.01 and 0.05. Under the standard quantitative genetic “infinitesimal model” (also referred to as the “polygenic model”) (31), it is reasonable to assume the effect of all risk SNPs is equal. With this assumption, we then explored various models to predict the expected heritability in each MAF bin (Figure 1; Tables S6–S8; Figures S10 and S11).
The model that best fit the data was one in which risk alleles were sampled proportional to their occurrence in 1000 Genomes data, with a goodness-of-fit adjusted R2 = 0.49. Notably, the largest proportion of expected heritability was not explained by SNPs in the higher frequency allele bins (0.3–0.4 and 0.4–0.5), contrary to what was observed in Davis et al. (5). In addition, we observed that SNPs with low MAF (0.01–0.05) are expected to account for 10.4% of the heritability under this model, similar to the 10% that we observed and in contrast to Davis where low MAF SNPs accounted for almost no heritability. These discrepancies and the smaller ones observed in our study track with sample size. For example, the sample size for Davis et al. (1061 cases and 4236 controls) was smaller than our current study and variance of estimates are a direct function of sample size. Combining results from both studies demonstrated strong concordance with expectation (Figure 1C). In addition, prior studies (5,16) are likely more ancestrally heterogeneous than our present Swedish sample which can lead to increased variance.
3.2. Heritability analysis partitioned by chromosomes
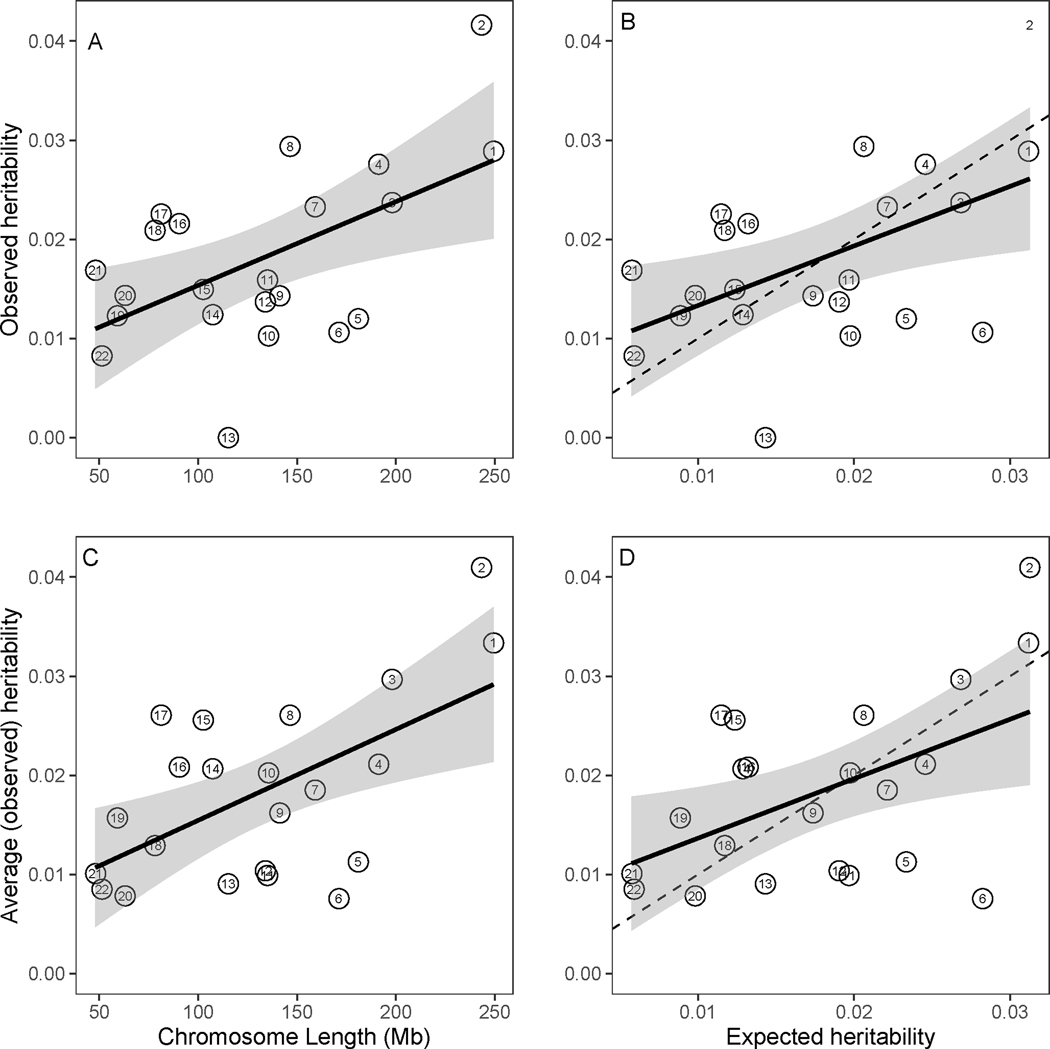
Under the infinitesimal model, SNPs affecting heritability of OCD (or any trait) should be scattered randomly across chromosomes, so that heritability per chromosome should track with chromosome length. This is observed in our study (Figure 2) and there is a significant correlation between heritability per chromosome and length (r = 0.55, p-value = 0.008). Chromosome 13 had the lowest heritability, significantly lower than what would be expected under the uniform distribution model.
Figure 2.
Estimates of heritability partitioned by chromosome. A) The observed heritability by chromosome length and the 95% confidence interval (CI) for the regressed line (adjusted R2=0.27, p-value=0.008); B) The observed heritability by expected heritability and the 95% CI for the regressed line (adjusted R2=0.23, p-value=0.014); C) The weighted average observed heritability by chromosome length and the 95% CI for the regressed line (average over this manuscript and Davis et al. study) (adjusted R2=0.31, p-value=0.004), the results for chromosome 21 and 22 are overlapping; and D) The weighted average heritability by expected heritability and the 95% CI for the regressed line (adjusted R2=0.22, p-value=0.0161). The dashed lines have slope one and intercept zero (observed=expected).
As noted above, the noisy nature of these results can likely be attributed to relatively small sample size for this type of analysis. We conjectured that if this were the case, and assuming both study samples were homogeneous, combining the Davis et al. heritability estimates and our heritability estimates, per chromosome, would produce a somewhat better fit between heritability per chromosomes and length. This result is confirmed in Figure 2C–D; the fit of the regression for this weighted average heritability (weights proportional to the inverse of variance), adjusted R2 = 0.31, is better than the fit for our sample alone, adjusted R2=0.27. Furthermore, note that chromosome 6, which had very low heritability in Davis et al., shows reasonable heritability in both our analyses and in the combined data, again suggesting small sample sizes are driving some of the results.
4. Discussion
Common genetic variation – variants shared among many individuals in a population and most frequently SNPs – has been found to play a role in liability for most psychiatric disorders, including OCD. Open questions remain about the impact on risk due to common variation, including how much of the heritability of OCD it accounts for and how it is partitioned across the frequency spectrum of alleles. These are important questions for a variety of reasons. For example, both schizophrenia and autism spectrum disorder demonstrate high heritability (32, 33) and much of it traces to common genetic variation. Yet rare variation with a damaging impact on gene function, especially de novo variation, plays a larger role in overall autism spectrum disorder risk than in overall schizophrenia risk (32, 34, 35); e.g., in Singh et al. (35), de novo protein truncating variants were found to be fourfold more common in individuals with autism than schizophrenia when they evaluated evolutionarily-constrained genes. This difference is critical for clinical genetics, genetic counseling, and possibly treatment. It also could be relevant for disentangling evolutionary processes underlying different psychiatric disorders, consistent with stronger natural selection on autism than schizophrenia. Finally, it would impact study design (if, for example, rare variants contribute little to OCD heritability).
Here we evaluate whether a substantial portion of the heritability of OCD traces to common variation, as it does for autism and schizophrenia, and characterize its frequency spectrum, which is directly relevant to evolutionary processes. For example, in an early study estimating heritability of OCD from common variation, results in Davis et al. (5) suggested that alleles with the highest frequencies, i.e., those with MAF > 0.3, account for the bulk of SNP-based heritability of OCD. Similar findings were reported using meta-analysis of data from OCGAS and Davis et al. (16). Such a strong pattern would suggest that OCD was under strong balancing selection.
By sampling individuals with OCD from the Swedish population, as well as a larger sample of unaffected (control) individuals, we were able to address these questions. Our analyses of over 2000 individuals diagnosed with OCD and twofold more unaffected individuals, each genotyped across their genome via > 400,000 SNPs, yielded an OCD heritability estimate of 29% (SE=4%), a robust estimate (Table 3).
Moreover, when we assumed SNPs contributed equally to risk for OCD, regardless of MAF, we obtained good fit between estimated OCD heritabilities from MAF bins of our sample and what was expected based on the distribution of MAF in 1000 Genomes data (Figure 1). SNPs affecting risk appear to be distributed at random over chromosomes because size was a good predictor of a chromosome’s contribution to total heritability (Figure 2). Chromosome 13 showed the poorest fit to this model, which may be partially explained by it having one of the lowest gene densities (6.5 genes per Mb) among human chromosomes. All of these results fit expectations of the infinitesimal quantitative genetics model.
In terms of estimated heritability from common variation, our results compare favorably with previous studies of OCD. Published estimates of SNP-based heritability, based on different samples from different populations, range from 25–43% (5, 15, 16). Thus, all studies have converged on a substantial contribution of common variation to the heritability of OCD, showing notable consistency. There are some differences, however. Notably, the recent study by Davis and colleagues suggest that only SNPs with substantial frequency in their population sample (MAF > 0.05) contribute to this heritability and the contribution to heritability tends to increase with increasing MAF.
In light of our findings, we found their results intriguing: an increasing heritability associated with MAF is appealing because the contribution to heritability of any SNP of frequency p is 2p(1-p)a2, where the SNP’s effect a can be assumed to be roughly equal over all SNPs under the infinitesimal model; on the other hand, it seems unlikely that low MAF SNPs have no contribution to heritability because there are so many of them in the human genome (Table S9). Our results from Sweden argue that these low MAF SNPs do contribute to OCD heritability, their contribution is roughly in proportion to the frequency spectrum of alleles, and can be assumed to be of similar effect (i.e., a) across the frequency spectrum. Thus, our results show that future studies of less common and even rare alleles are also informative for OCD etiology, with the caveat that effects of risk alleles of very low frequency can be difficult to detect by case-control methods.
Another interesting contrast is the evidence for heritability across chromosomes. Davis et al. observed essentially no heritability for OCD on chromosome 6, which encodes both the HLA and histone gene clusters, and extremely high heritability on chromosome 15. In discussing these results, the authors suggest that chromosome 15 has an outsized contribution to OCD risk and that the HLA locus is effectively excluded from OCD risk. Given the contrasting results in our study, and in our analyses combining results from both studies, we again conclude that the data are consistent with the infinitesimal model and that smaller sample sizes might account for results that diverge from expectation.
The previous work by Davis and colleagues involved about 50% fewer OCD cases and the variance in any estimate is a direct function of sample size. It is also possible that the Davis study had a different distribution of distantly related individuals than our relatively homogeneous sample from Sweden. Accuracy of SNP-based heritability diminishes as the fraction of very distantly related pairs, relative to all relative pairs, increases. Consistent with estimates from both studies being noisy, when we combined the Davis et al. results to obtain new estimates of average heritability per allele bin and heritability per chromosome, the average fit expectation was better than in either study alone.
Prior to the advent of dense genotyping, the heritability of a trait was typically estimated from its distribution within pedigrees. These kinds of studies continue to this day, in large part because they capture heritability due to both common and rare inherited genetic variation. It is thus interesting to compare our SNP-based heritability estimate from common variation, 29%, to that from Swedish families, 35–50% (1, 4). This comparison suggests that while the majority of inherited liability for OCD in Sweden traces to common genetic variation, rare variation contributes to OCD liability as well, but to a lesser degree, consistent with the findings to date regarding rare variation and risk for OCD (17–20).
The present study had strengths and limitations. We used OCD cases from the EGOS and NORDiC cohorts, the two largest OCD studies in Sweden to examine the role of genetic and environmental factors. The EGOS cohort utilized the NPR for its sampling frame, thus it is an epidemiological cohort minimizing selection biases, while the NORDiC recruited through specialty OCD clinics across Sweden, a sampling frame more typical of case-control studies. This difference in sampling frames could introduce heterogeneity into our study. Nonetheless, when we evaluated this possibility by estimating the heritability induced by contrasting OCD cases from EGOS to OCD cases from NORDiC, and doing the same for controls, both estimated heritabilities were not significantly different from zero. Hence, while there could be subtle heterogeneity between the cohorts, it must be small. Furthermore, for both cohorts, reliance on inclusion as a result of individuals seeking care at mental health hospitals/clinics can inadvertently exclude those with milder forms of the disorder who may seek treatment from primary care providers and/or those who do not present to clinical services at all. If such individuals were included and if their genetic architecture were different from our current OCD case sample, it would impact the estimated heritability. By restricting cases to individuals in Sweden, we had a genetically homogeneous sample, which minimized the risk of confounding due to population stratification and facilitated the combining of the cohorts. Nonetheless, it does limit the generalizability of our results. However, after combining our results with those of Davis et al., we observe results that fit expectation, thus suggesting that the results are likely to relevant for most populations.
Our results provide new insights into the genomics architecture of OCD, impacting research design for genomic discovery and the ultimate clinical impact of such studies. While there is no doubt that rare and common genetic variation contributes to risk for OCD, the balance of their contributions has remained uncertain. Results from earlier studies (5, 16) implied an unexpectedly large role for very common variation in OCD risk and no evidence for heritability related to rarer variation (MAF < .05). This would be quite distinct from what is known about other psychiatric disorders, and consistent with some form of evolutionary selection, such as balancing selection. Our results differ substantially with those of the earlier studies, specifically we observe that the contribution to risk from common SNP variation follows expectations. Hence our results do not support a role for unusual evolutionary forces playing a role in OCD risk and do support a role for rare variation in risk.
Assessing the contribution of rare variants in OCD has the additional benefit of uncovering variation of major effect, which can lead to direct insight into OCD biology and potentially pave the way for family counseling. In addition, these high-effect genes represent tools to create animal models of OCD to study pathobiology and also may represent targets for developing novel therapeutics.
As datasets get larger, risk prediction will improve as will our ability to characterize the balance and effects of common and rare risk variation. We conjecture that the liability arising from common and rare risk variation likely combines additively to determine risk for individuals diagnosed with OCD, similar to the risk patterns for ASD (36, 37). This knowledge can be translated into a deeper etiological understanding of OCD subtypes and their treatment and, in the future, at better predictors of OCD risk. OCD is a clinically and etiologically heterogeneous condition (38) with a complex symptom structure (39). Studies suggest that the burden of common risk alleles of OCD may differ based on OCD symptom type. For example, although not yet replicated, in one study compulsive symptoms rather than obsessive symptoms showed higher SNP heritability and genetic correlations with OCD (40). However, it is still unclear to what extent rare genetic variation, and the joint effect with common variation, differs between the subtypes of OCD, and how this balance may depend on age of onset and sex. Such findings could encourage a reconsideration of key clinical features of OCD as a means of defining subtypes. Defining clinical subtypes that differ in rare and common variation could accelerate research into biomarkers and novel treatments, eventually helping clinicians offer patients optimal prognosis and treatment.
Pharmacogenetic studies of OCD have focused on the role of common genetic variants in treatment response (41). However, to date, no replicated significant GWAS variant has been reported for OCD - likely due to the small sample sizes - and therefore it has been challenging to contextualize the results of pharmacogenetic studies. Future studies examining predictors of treatment response will shift the focus from select common genetic variants to genome-wide studies that also estimate how rare and common risk variants jointly affect liability and optimal interventions (42).
The heterogeneity of OCD should always be considered in the light of psychiatric comorbidity, an approach that is facilitated in samples such as EGOS and NORDiC that are linked to national health registries. For example, in EGOS, using an epidemiological frame, approximately 40% of individuals with OCD have more than one psychiatric comorbidity, with anxiety disorders and major depressive disorder being most common (43). In addition, the severity of OCD was significantly higher in individuals with at least one additional psychiatric comorbidity compared to individuals with no psychiatric comorbidity: higher symptoms of obsessing and ordering, measured using the OCI-R, were observed in individuals with OCD and at least one additional psychiatric comorbidity (43). In future studies, it will be important to investigate how the combination of rare and common genetic variants differ in their relationship with the comorbid conditions.
In summary, our results demonstrate that the majority of inherited liability for OCD in Sweden traces to common genetic variation. Moreover, our results show that the distribution of risk as a function of allele frequency is consistent with expectations, indicating that balancing selection, or other more complex evolutionary forces, are not strongly at play in OCD. Furthermore, our results indicate that risk for OCD is distributed across the genome as expected and that results presented here and in prior studies are consistent with the infinitesimal model for OCD. Finally, our results support the continued study of rare variation, both inherited and de novo, in OCD risk.
Supplementary Material
Table 1.
Characteristics of the cohorts.
| Characteristics | Category | EGOS | NORDiC | ||
|---|---|---|---|---|---|
| Number of OCD cases | 1108 | 1107 | |||
|
| |||||
| Sex, count (%) | Females | 692 | (63%) | 651 | (59%) |
|
| |||||
| Comorbidities, count (%) | CTD (ICD-10: F95) | 100 | (9%) | 43 | (5%)1 |
| ADHD (ICD-10: F90) | 40 | (4%) | 83 | (10%)1 | |
| Bipolar Disorder (ICD-10: F31) | 33 | (3%) | 93 | (11%)1 | |
| Phobic anxiety disorders (ICD-10: F40) | 19 | (2%) | 98 | (12%)1 | |
| Other anxiety disorders (ICD-10: F41) | 112 | (10%) | 106 | (13%)1 | |
| Autistic disorder (ICD-10: F84.0) | 3 | (0.3%) | 8 | (1%)1 | |
| Asperger’s syndrome (ICD-10: F84.5) | 40 | (4%) | 51 | (6%)1 | |
| Intellectual disability (ICD-10: F71–73) | 7 | (0.6%) | 2 | (0.2%)1 | |
| At least one psychiatric comorbidity | 417 | (37%) | 424 | (53%)1 | |
|
| |||||
| Diagnosis age/Age at first symptom (p5, Median, p95)2 | (12,21,34) | (5,12,30)3 | |||
283 individuals have missing values (n=804).
p5 and p95 are the 5th and 95th percentiles.
437 missing values (n=670).
OCD: obsessive-compulsive disorder, CTD: chronic tic disorders, ADHD: attention-deficit/hyperactivity disorder.
Acknowledgments:
This study was supported by a grant from the Friedman Brain Institute (DEG), and the Beatrice and Samuel A. Seaver Foundation (DEG, SS, JDB, BM); the Mindworks Charitable Lead Trust (DEG); the Stanley Center for Psychiatric Research (DEG and JDB); and NIMH grant R01MH124679 (DEG), R37MH057881 (BD/KR) and R01MH110427 (JC); the Swedish Research Council (grant numbers 2015-02271, 2018-02487) (DM-C and CR), CIMED and Region Stockholm (CR).
Funding for some of the control samples was from the Anorexia Nervosa Genetics Initiative (ANGI), an initiative of the Klarman Family Foundation (PI: Bulik). Dr. Bulik is also supported by (R01MH120170, R01MH119084 PI: Bulik; U01 MH109528 PI: Sullivan); the Swedish Research Council (Vetenskapsrådet, award: 538-2013-8864); Brain and Behavior Research Foundation Distinguished Investigator Grant; and Lundbeck Foundation (Grant no. R276-2018-4581).
The computation was performed on resources provided by SNIC through Uppsala Multidisciplinary Center for Advanced Computational Science (UPPMAX) under Project sens2018605.
LifeGene was supported by the Torsten and Ragnar Söderbergs Foundation, Karolinska Institutet, Stockholm County Council, and AFA Insurance. LifeGene is a core facility at Karolinska Institutet.
Footnotes
Disclosures: Authors report no financial relationships with commercial interests.
References
- 1.Mahjani B, Klei L, Hultman CM, et al. : Maternal Effects as Causes of Risk for Obsessive-Compulsive Disorder, in Biological Psychiatry. 2020, pp 1045–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruscio AM, Stein DJ, Chiu WT, et al. : The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry 2010; 15:53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicolini H, Arnold P, Nestadt G, et al. : Overview of genetics and obsessive-compulsive disorder. Psychiatry Res 2009; 170:7–14 [DOI] [PubMed] [Google Scholar]
- 4.Mataix-Cols D, Boman M, Monzani B, et al. : Population-based, multigenerational family clustering study of obsessive-compulsive disorder. JAMA Psychiatry 2013; 70:709–717 [DOI] [PubMed] [Google Scholar]
- 5.Davis LK, Yu D, Keenan CL, et al. : Partitioning the Heritability of Tourette Syndrome and Obsessive Compulsive Disorder Reveals Differences in Genetic Architecture. PLoS Genet 2013; 9:e1003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brander G, Rydell M, Kuja-Halkola R, et al. : Association of Perinatal Risk Factors With Obsessive-Compulsive Disorder: A Population-Based Birth Cohort, Sibling Control Study. JAMA psychiatry 2016; 73:1135–1144 [DOI] [PubMed] [Google Scholar]
- 7.Brander G, Pérez-Vigil A, Larsson H, et al. : Systematic review of environmental risk factors for Obsessive-Compulsive Disorder: A proposed roadmap from association to causation. Neurosci Biobehav Rev 2016; 65:36–62 [DOI] [PubMed] [Google Scholar]
- 8.Clifford CA, Murray RM, Fulker DW: Genetic and environmental influences on obsessional traits and symptoms. Psychol Med 1984; 14:791–800 [DOI] [PubMed] [Google Scholar]
- 9.Jonnal AH, Gardner CO, Prescott CA, et al. : Obsessive and compulsive symptoms in a general population sample of female twins. Am J Med Genet - Neuropsychiatr Genet 2000; 96:791–796 [DOI] [PubMed] [Google Scholar]
- 10.Eley TC, Bolton D, O’Connor TG, et al. : A twin study of anxiety-related behaviours in pre-school children. J Child Psychol Psychiatry Allied Discip 2003; 44:945–960 [DOI] [PubMed] [Google Scholar]
- 11.Hudziak JJ, Van Beijsterveldt CEM, Althoff RR, et al. : Genetic and environmental contributions to the child behavior checklist obsessive-compulsive scale: A cross-cultural twin study. Arch Gen Psychiatry 2004; 61:608–616 [DOI] [PubMed] [Google Scholar]
- 12.Taylor S: Etiology of obsessions and compulsions: A meta-analysis and narrative review of twin studies. Clin Psychol Rev 2011; 31:1361–1372 [DOI] [PubMed] [Google Scholar]
- 13.Monzani B, Rijsdijk F, Harris J, et al. : The structure of genetic and environmental risk factors for dimensional representations of DSM-5 obsessive-compulsive spectrum disorders. JAMA Psychiatry 2014; 71:182–189 [DOI] [PubMed] [Google Scholar]
- 14.Mahjani B, Bey K, Boberg J, et al. : Genetics of obsessive-compulsive disorder. Psychol Med 2021; 1–13 Available from: 10.1017/S0033291721001744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattheisen M, Samuels JF, Wang Y, et al. : Genome-wide association study in obsessive-compulsive disorder: Results from the OCGAS. Mol Psychiatry 2015; 20:337–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnold PD, Askland KD, Barlassina C, et al. : Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol Psychiatry 2018; 23:1181–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halvorsen M, Samuels J, Wang Y, et al. : RARE AND DE NOVO VARIANTS IN OBSESSIVE COMPULSIVE DISORDER. Eur Neuropsychopharmacol 2019; 29:S860–S861 [Google Scholar]
- 18.Purty A, Nestadt G, Samuels J, et al. : Genetics of obsessive-compulsive disorder. Indian J Psychiatry 2019; 61:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGrath LM, Yu D, Marshall C, et al. : Copy number variation in obsessive-compulsive disorder and tourette syndrome: A cross-disorder study. J Am Acad Child Adolesc Psychiatry 2014; 53:910–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cappi C, Brentani H, Lima L, et al. : Whole-exome sequencing in obsessive-compulsive disorder identifies rare mutations in immunological and neurodevelopmental pathways. Transl Psychiatry 2016; 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahjani B, Dellenvall K, Grahnat ACS, et al. : Cohort profile: Epidemiology and Genetics of Obsessive–compulsive disorder and chronic tic disorders in Sweden (EGOS). Soc Psychiatry Psychiatr Epidemiol 2020; 55:1383–1393 [DOI] [PubMed] [Google Scholar]
- 22.Mataix-Cols D, Hansen B, Mattheisen M, et al. : Nordic OCD & Related Disorders Consortium: Rationale, design, and methods. Am J Med Genet Part B Neuropsychiatr Genet 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almqvist C, Adami HO, Franks PW, et al. : LifeGene - A large prospective population-based study of global relevance. Eur J Epidemiol 2011; 26:67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thornton LM, Munn-Chernoff MA, Baker JH, et al. : The Anorexia Nervosa Genetics Initiative (ANGI): Overview and methods. Contemp Clin Trials 2018; 74:61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deelen P, Bonder MJ, Van Der Velde KJ, et al. : Genotype harmonizer: Automatic strand alignment and format conversion for genotype data integration. BMC Res Notes 2014; 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Lee SH, Goddard ME, et al. : GCTA: A tool for genome-wide complex trait analysis. Am J Hum Genet 2011; 88:76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bodea CA, Neale BM, Ripke S, et al. : A Method to Exploit the Structure of Genetic Ancestry Space to Enhance Case-Control Studies. Am J Hum Genet 2016; 98:857–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crossett A, Kent BP, Klei L, et al. : Using ancestry matching to combine family-based and unrelated samples for genome-wide association studies. Stat Med 2010; 29:2932–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luca D, Ringquist S, Klei L, et al. : On the Use of General Control Samples for Genome-wide Association Studies: Genetic Matching Highlights Causal Variants. Am J Hum Genet 2008; 82:453–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Lee SH, Goddard ME, et al. : Genome-Wide Complex Trait Analysis (GCTA): Methods, Data Analyses, and Interpretations 2013; 215–236 [DOI] [PubMed] [Google Scholar]
- 31.Barton NH, Etheridge AM, Véber A: The infinitesimal model: Definition, derivation, and implications. Theor Popul Biol 2017; 118:50–73 [DOI] [PubMed] [Google Scholar]
- 32.Gaugler T, Klei L, Sanders SJ, et al. : Most genetic risk for autism resides with common variation. Nat Genet 2014; 46:881–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SH, Decandia TR, Ripke S, et al. : Estimating the proportion of variation in susceptibility to schizophrenia captured by common SNPs. Nat Genet 2012; 44:247–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satterstrom FK, Kosmicki JA, Wang J, et al. : Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 2020; 180:568–584.e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh T, Poterba T, Curtis D, et al. : Exome sequencing identifies rare coding variants in 10 genes which confer substantial risk for schizophrenia [Internet]. medRxiv 2020; 2020.09.18.20192815; Available from: 10.1101/2020.09.18.20192815 [DOI] [Google Scholar]
- 36.Klei L, Mcclain LL, Mahjani B, et al. : How rare and common risk variation jointly affect liability for autism spectrum disorder [Internet]. medRxiv 2020; 2020.10.27.20220095 Available from: 10.1101/2020.10.27.20220095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiner D, Wigdor E, Ripke S, et al. : Polygenic transmission disequilibrium confirms that common and rare variation act additively to create risk for autism spectrum disorders. Nat Genet. 2017. Jul;49(7):978–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cervin M, Miguel EC, Güler AS, et al. : Towards a definitive symptom structure of obsessive-compulsive disorder: A factor and network analysis of 87 distinct symptoms in 1366 individuals. Psychol Med 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bloch MH, Landeros-Weisenberger A, Rosario MC, et al. : Meta-analysis of the symptom structure of obsessive-compulsive disorder. Am J Psychiatry 2008; 165:1532–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smit DJA, Cath D, Zilhão NR, et al. : Genetic meta-analysis of obsessive–compulsive disorder and self-report compulsive symptoms. Am J Med Genet Part B Neuropsychiatr Genet 2020; 183:208–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zai G, Brandl EJ, Müller DJ, et al. : Pharmacogenetics of antidepressant treatment in obsessive-compulsive disorder: An update and implications for clinicians. Pharmacogenomics 2014; 15:1147–1157 [DOI] [PubMed] [Google Scholar]
- 42.Murray GK, Lin T, Austin J, et al. : Could Polygenic Risk Scores Be Useful in Psychiatry?: A Review. JAMA Psychiatry 2021; 78:210–219 [DOI] [PubMed] [Google Scholar]
- 43.Mahjani B, Gustavsson Mahjani C, Reichenberg A, et al. : OCD symptom severity and comorbid psychiatric diagnoses in a Swedish genetic epidemiological obsessive-compulsive disorder cohort. [Internet]. medRxiv 2020; 2021.06.28.21259652; Available from: 10.1101/2021.06.28.21259652 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.