Abstract
Polycystic ovary syndrome (PCOS) is characterized by reproductive and metabolic dysfunction, and elevated blood pressure (BP). The cardiometabolic consequences of maternal hyperandrogenemia on offspring, either as adults or with aging, have not been well studied. We previously found that male offspring of hyperandrogenemic female (HAF) rats, a model of PCOS, are normotensive but have an exaggerated pressor response to angiotensin (Ang) II. In this study, the hypothesis was tested that adult and aging female offspring of HAF rats develop a metabolic and hypertensive phenotype. Control and HAF rats were implanted prepubertally with placebo or dihydrotestosterone pellets, that continued throughout pregnancy and lactation. Female offspring of HAF dams had lower birth weight than female control offspring. While female HAF offspring (aged 16–24 wks) had no differences in intrarenal Ang II, plasma lipids or proteinuria, they did have lower intrarenal Ang(1–7) and lower nitrate/nitrite excretion than controls. Adult HAF offspring had similar baseline BP as controls, but had an attenuated pressor response to Ang II. With aging (16–20 months), female HAF offspring remained normotensive with an attenuated pressor response to Ang II and high salt diet but more proteinuria and higher intrarenal Ang (1–7) than controls. Taken together, these data suggest that female HAF offspring are protected from developing hypertension, but may be at risk for renal injury with aging. Future studies are necessary to determine whether adult and postmenopausal offspring of PCOS women are at increased risk for cardiovascular dysfunction.
Keywords: Hyperandrogenemic female, pregnancy, blood pressure, female offspring, aging, angiotensin
Graphical Abstract

Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine and reproductive disorder in women, affecting approximately 10% of women worldwide. PCOS is characterized by hyperandrogenemia, obesity (80% in the US1), insulin resistance, dyslipidemia, endothelial dysfunction, and elevated blood pressure (BP)2, 3. Despite these risk factors, it is still not clear whether PCOS women are at increased risk of cardiovascular disease (CVD) later in life4–7.
One of the complications of PCOS is difficulty becoming pregnant8, 9. Children of PCOS women are often born either large or small for gestational age10–12, both conditions that have been shown to be associated with increased risk for CVD later in life in other groups13–16. However, the consequences of PCOS pregnancy on the health of adult or aging offspring have not been studied. This is partly because the accepted Rotterdam Criteria for diagnosis of PCOS was only put in place in the early 2000s17. Thus, children of PCOS women under this diagnosis paradigm would only now be in their late teens and early 20s. In addition, most studies in female PCOS offspring have focused on their reproductive health rather than CV health, since the incidence of PCOS in daughters of PCOS women is not clear, with some studies showing PCOS daughters have symptoms of PCOS themselves18–22, and other studies not supporting this contention23–25.
To our knowledge, the only CV study in female offspring (aged 20 years) of PCOS women, who were not diagnosed according to the Rotterdam criteria, showed they had an increase in ambulatory BP and a decrease in plasma nitrate/nitrite, an index of nitric oxide. They were also insulin-resistant and glucose-intolerant26, which, taken together, may indicate an increased risk of CVD, especially with aging. However, these PCOS daughters did not have PCOS themselves since they had normal testosterone levels, body weight and menstrual cycles. In contrast, Legro et al. reported that postpubertal PCOS daughters with hirsutism, suggesting they may have PCOS themselves, had normal BP as measured by the cuff method24.
There are CV and metabolic data reported for young children of PCOS women diagnosed with the Rotterdam criteria, however. For example, de Wilde and colleagues reported that children of PCOS women, aged 2.5–8 years, had significantly lower diastolic BP, but higher pulse pressure, compared to controls27. They also had increased left ventricular internal diameter and increased carotid intima-media thickness, compared to control offspring27. This study did not separate the findings by sex, however, nor was BP measured by 24-hour ambulatory monitoring27. Gunning, et al., performed meta-analyses on data from 298 PCOS offspring, aged 2.5 to 17 years, from the Netherlands, Chile, and the United States28. The female PCOS offspring had higher fasting insulin, higher HDL-cholesterol, and lower LDL-cholesterol than male PCOS offspring. However, BP was not included in these analyses28.
Over the last several years, our laboratory has extensively characterized a model of PCOS, the hyperandrogenemic female (HAF) rat, produced by implantation of dihydrotestosterone (DHT) pellets, starting at 4 weeks of age (prepubertal) and continuously replacing the pellet every 85 days throughout their lives29–33. As adults, HAF rats exhibit many of the characteristics of PCOS women, such as increased body weight, insulin resistance, dyslipidemia, and elevated BP29. BP increases even further with aging in HAF rats33. Just as in PCOS women10, HAF rats have difficulty becoming pregnant and pregnancy rates in HAF rats are approximately 60%, compared to 99% in controls33. HAF rats give birth to similar numbers of pups per litter compared to control dams, but the male and female pups from HAF dams are born with intrauterine growth restriction (IUGR)33, 34. We previously reported that male offspring of the HAF rat model are normotensive as adults, but have increased proteinuria, hypercholesterolemia, and an exaggerated pressor response to angiotensin (Ang) II infusion34, suggesting they may develop CVD and metabolic disease as they age.
The present studies were done to evaluate the CV and metabolic effects of hyperandrogenemic pregnancy on female offspring, using our well-characterized and clinically-relevant model of PCOS, the HAF rat. The hypothesis tested was that female offspring of HAF dams will develop CVD as adults that will be exacerbated with aging.
Methods
HAF Rat Model and Offspring Generation:
Female Sprague Dawley (SD) rats, obtained at 3 wks of age (Envigo, Indianapolis, IN) were randomly assigned to either control or HAF groups. Rats were implanted with 5α-DHT pellets (7.5 mg/90day, s.c.; Innovative Research of America, FL) (HAF model) or placebo pellets (controls), beginning at 4 wks of age, that replaced every 85 days throughout pregnancy and lactation, as previously described29, 32, 33. All rats were maintained on standard chow (Teklad #8640) and tap water in a controlled environment (22°C, 50% humidity) with 12 hr:12 hr light (0600–1800 hr):dark (1800–0600 hr) cycle. All protocols followed the ARRIVE Guidelines and were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center and complied with the Guidelines for the Care and Use of Laboratory Animals by the National Institutes of Health.
At 10–12 wks of age, HAF and control rats were paired with male SD rats to induce pregnancy. Pregnancy occurred in 60% of HAF rats compared to 99% in control females33. Offspring were counted and weighed within 12 hours of birth, and culled at 48 h after birth to 8 pups/litter (4 males and 4 females), in order to ensure equal nutrition for all pups. HAF and control dams were allowed to lactate, and pups were weaned at postnatal day 21. Only female offspring (from 28 HAF dams and 27 control dams) were used for the present studies. All studies were done with one rat/per litter/group. Different litters of rats were used for adult and aging studies.
Female HAF and control offspring were weighed daily for 1 wk and then weekly until 2 months of age. HAF and control offspring were left untreated until they were studied at either 16–24 wks (adults) or 16–20 months (aging). Adult HAF rats had a 4-day estrous cycle, just as controls. However, by 16–18 mos of age, all rats had ceased cycling.
Adult rats:
A more detailed Methods section is available in the online-only supplement. At 16–24 wks of age, female HAF and control offspring (n=4–14/group) were weighed and body composition was measured by EchoMRI33. Lipid profile, including total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were measured in plasma after 5 hours of fasting (0800 – 1300 hr), as we previously described33. Serum estradiol and testosterone were measured by ELISA from morning blood samples (collected between 0800 – 1000 hr-- start of the light cycle was 0600 hr), by the Ligand Assay and Analysis Core, University of Virginia. Renal cortical Ang II and Ang (1–7) peptides were measured by the Hypertension Assay Core, Wake Forest University, as described35. Urinary protein and nitrate/nitrite excretion were measured in 24 hr urine collections, as we previously described29, 30, 32, 36.
Pressor response to Ang II:
In a separate set of female HAF and control offspring (n = 3–4/group), radiotelemetry transmitters (HD-S10, Data Sciences International) were implanted29–31, 33, 37, and rats were allowed to recover for 2 wks. Baseline mean arterial pressure (MAP), systolic BP (SBP), diastolic BP (DBP), pulse pressure, heart rate (HR), and hourly MAP (to identify circadian rhythm) were measured. Rats were given enalapril, an Ang I converting enzyme (ACE) inhibitor (25 mg/kg/day)38 in their drinking water throughout the study, with water intake measured daily to ensure consistent dosing. After 10 days on enalapril, rats were implanted with osmotic minipumps to deliver Ang II (50 ng/kg/min)39, and MAP was recorded for 18 days. Osmotic minipumps were then replaced to provide Ang II (200 ng/kg/min)40 for 7 days. Proteinuria (24 hours collection) was measured at the conclusion of the study.
Aging rats
As described above, at 16–20 months of age, female HAF and control offspring (n=4–9/group) were weighed, and body composition was measured by EchoMRI, along with lipid profile, urinary protein and nitrate/ nitrite excretion, as described above.
Response to chronic Ang II in the presence of high salt diet:
Radiotelemetry transmitters were implanted in female HAF and control offspring (n=4–5 rats/group), as described above. Following recovery, baseline MAP, SBP, DBP, pulse pressure, HR, and circadian rhythm of MAP were measured. Rats were then given enalapril for 7 days. On day 15, rats were implanted with osmotic minipumps to deliver Ang II (50 ng/kg/min)28 or saline (“No Ang II”). MAP was recorded for 7 days. On day 24, rats were given a 4% NaCl diet for an additional 6 days. On day 30, rats were placed in metabolism cages for 24 hrs for urine collection for protein and nitrate/nitrite excretion. Osmotic minipumps were then replaced to deliver Ang II (200 ng/kg/min)40 or saline, and MAP was measured for an additional 5 days. Urine collection (24 hrs) was repeated for proteinuria, nitrate/nitrite, and urinary creatinine41. Rats were euthanized, blood samples were taken for plasma creatinine41, and kidney, heart and left ventricular weights were measured. Renal cortical levels of Ang II and Ang (1–7) were measured, as described above.
Statistical Analyses
All data are expressed as means ± S.E.M. Two-way ANOVA was used to determine differences among groups in most studies. MAP was compared using repeated measures 2-way ANOVA. In some studies, unpaired Student’s t test was used to determine differences in two groups. Post hoc tests were used as specified in figure legends and tables. Values of p ≤ 0.05 were considered significant. Statistical analyses were performed using GraphPad Prism software (GraphPad Software Inc., V8.4.3, San Diego, CA).
Results
Characteristics of female HAF and control neonates:
As shown in Figure 1, litter sizes were similar for HAF and control dams, as we previously showed33. As we also previously described, birth weights were significantly lower in female HAF offspring compared to control offspring (Figure 1B). As shown in Supplemental Table 1, body lengths, abdominal and head circumferences were similar between female HAF and control offspring at 12 and 48 hr postnatal. Body weights remained lower in HAF female offspring, up to approximately 42 days of age (Figures 1B and 1C).
Fig. 1:

Number of pups per litter and body weights (BWs) of female HAF and control offspring. [1A]. Total number of pups per pregnancy. [1B]. Average BWs of female offspring daily until weaning. [1C]. Average BWs of female offspring weekly until 2 mos of age. Values represent mean ± S.E.M (n = 16–25, litters/group). Statistical analyses were performed by unpaired student t test (A) or repeated measures 2-way ANOVA with Bonferroni multiple comparisons post hoc test (B and C); significance was defined as P ≤ 0.05. a, P ≤ 0.05, compared with Control off. Key: HAF: hyperandrogenemic female; off.: offspring
Characteristics of female adult HAF offspring :
As shown in Figure 2, there were no differences in body weight, lean mass, fat mass, or lean mass or fat mass factored for body weight between adult female HAF or control offspring. As shown in Table 1 and Figure 3, respectively, there were also no differences in lipid profiles and proteinuria between adult groups, but nitrate/nitrite excretion was significantly lower in HAF offspring than controls (Figure 3B).
Fig. 2:
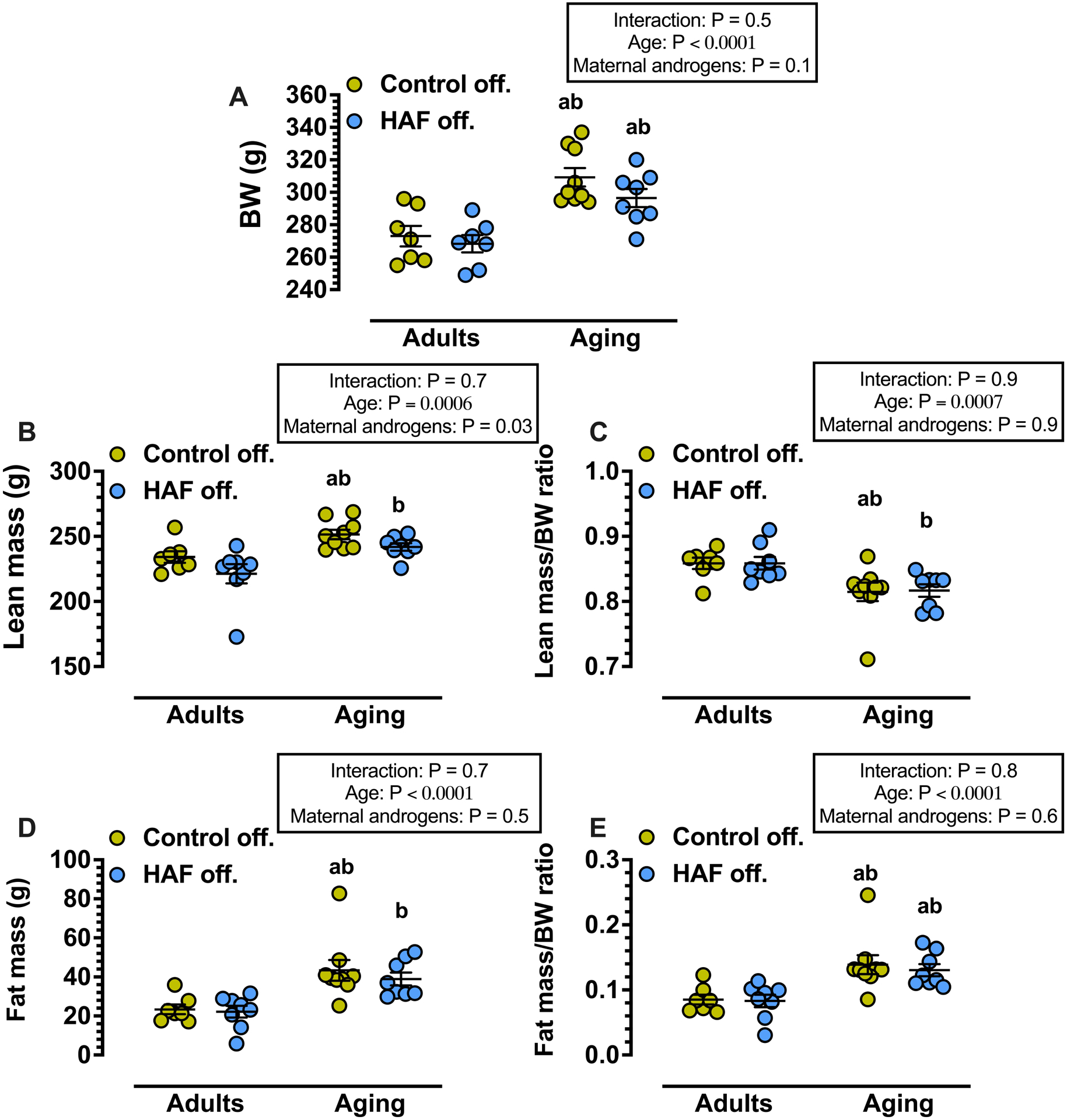
Body weights (BWs) and Body Composition of adult (16–24 wks) and aging (16–18 mos) female HAF and control offspring. Values represent mean ± S.E.M (n = 7–9, 1 rat/litter/group). Fat and lean masses were determined by Echo-magnetic resonance imaging, as described in Methods. Statistical analyses were performed by 2-way ANOVA with Bonferroni multiple comparisons post hoc test; significance was defined as p ≤ 0.05. a, P ≤ 0.05, compared with adult Control off.; b, p ≤ 0.05, compared with adult HAF off. Key: HAF: hyperandrogenemic female; off.: offspring.
Table 1:
Lipid profile in adult (16–24 wks) and aging (16–18 mos) female HAF and control offspring
| Adult | Aging | P value (age) | P value (maternal androgens) | Interaction | |||
|---|---|---|---|---|---|---|---|
| Control off. (n=4) | HAF off. (n=8) | Control off. (n=4) | HAF off. (n=4) | ||||
| TC (mg/dl) | 91.5 ± 9.2 | 97.1 ± 3.3 | 104.5 ± 7.8 | 129.5 ± 5.1abc | 0.001 | 0.02 | 0.1 |
| TG (mg/dl) | 69 ± 11 | 71.3 ± 7.9 | 105.5 ± 17.5 | 118.8 ± 8.9b | 0.002 | 0.5 | 0.6 |
| HDL-C (mg/dl) | 32.8 ± 2.8 | 33.3 ± 1.4 | 44.8 ± 4.2ab | 44.8 ± 2.9b | 0.0005 | 0.9 | 0.9 |
| LDL-C (mg/dl) | 8.3 ± 1.3 | 9.9 ± 0.5 | 10.4 ± 1.3 | 10.2 ± 0.8 | 0.2 | 0.5 | 0.3 |
Key: HAF: hyperandrogenemic female; off.: offspring; TC: total cholesterol; TG: triglycerides; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol. Values represent mean ± S.E.M (n = 4–8, 1 rat/litter). Statistical analyses were performed by 2-way ANOVA with Bonferroni multiple comparison`s post hoc test; significance was defined as P ≤ 0.05.
P ≤ 0.05, compared with adult control off.;
P ≤ 0.05, compared with adult HAF off.;
P ≤ 0.05, compared with aging control off.
Fig. 3:

Urinary protein and nitrate/nitrite excretion (UNOx) in adult (16–24 wks) and aging (16–18 mos) female HAF and control offspring. Values represent mean ± S.E.M (n = 4–14, 1 rat/litter/group). Statistical analyses were performed by 2-way ANOVA with Bonferroni multiple comparisons post hoc test; significance was defined as p ≤ 0.05. a, P ≤ 0.05, compared with adult Control off.; b, p ≤ 0.05, compared with adult HAF off.; c, p ≤ 0.05, compared with aging Control off. Key: HAF: hyperandrogenemic female; off.: offspring.
At baseline, there were no differences between adult HAF and control offspring in systolic or diastolic BP, or pulse pressure (Supplemental Figure 1), nor were their differences in circadian rhythm of BP (Supplemental Figure 2). As shown in Figure 4A, heart rate at this age was also similar between the groups. Serum estradiol (4.9 ± 0.7 vs 4.6 ± 0.5 pg/ml, n = 4–5/group) and testosterone (48.1 ± 3.9 vs 41.5 ± 3.6 ng/dl, n = 4–5/group) in female HAF offspring and controls, respectively, were also similar. Finally, at baseline, kidney cortical Ang II levels were similar between adult female HAF and control offspring (Figure 5A), but Ang (1–7) was significantly lower in HAF offspring than controls (Figure 5B).
Fig. 4:

Heart rate (HR) at baseline in adult (16–24 wks) and aging (17–20 mos) female HAF and control offspring. [4A]. HR in adult HAF and control offspring. [4B]. HR in aging HAF and control offspring. [4C]. HR in adult and aging control offspring. [4D]. HR in adult and aging HAF offspring. Values represent mean ± S.E.M (n = 4–8, 1 rat/litter/group). Statistical analyses were performed by repeated measures 2-way ANOVA with Bonferroni multiple comparisons post hoc test; significance was defined as P ≤ 0.05. b, P ≤ 0.05, compared with adult HAF offspring. Key: HAF: hyperandrogenemic female; off.: offspring.
Fig. 5:

Angiotensin (Ang) II and Ang (1–7) in kidney cortexes from adult (16–24 wks) female HAF and control offspring. Values represent mean ± S.E.M (n = 4–8, 1 rat/litter/group). Statistical analyses were performed by unpaired student t test; significance was defined as P ≤ 0.05. a, P ≤ 0.05, compared with adult control offspring. Key: HAF: hyperandrogenemic female; off.: offspring.
Response to Ang II infusion in adult female offspring:
As shown in Figure 6, baseline MAP was similar between HAF and control offspring, and enalapril reduced MAP to similar levels in both. Low dose Ang II (50 ng/kg/min) increased MAP in control offspring to baseline levels, but not in HAF offspring. Ang II (200 ng/kg/min) increased MAP in both groups, but the response was attenuated in HAF offspring compared to controls. Urinary protein excretion on high dose Ang II was similar between control and HAF offspring.
Fig. 6:

Mean arterial pressure (MAP) in response to Angiotensin (Ang) II in adult (16–24 wks) female HAF and control offspring. [6A]. MAP measured at baseline, after treatment with enalapril (E; 25 mg/kg/d), after starting Ang II (50 ng/kg/min), and after increasing Ang II (to 200 ng/kg/min). [6B]. Tabulated values of MAP on the last day of each treatment period and proteinuria at the end of experiment. Values represent mean ± S.E.M (n = 3–4, 1 rat/litter/group). Statistical analyses were performed by repeated measures 2-way ANOVA with Bonferroni multiple comparisons post hoc test for comparisons between groups and with Dunnett`s multiple comparisons post hoc test for comparisons between different treatment periods within the same group; significance was defined as P ≤ 0.05. a, P ≤ 0.05, compared with Control offspring.; #, P ≤ 0.05, compared with its baseline; *, P ≤ 0.05, compared with its Enalapril; $, P ≤ 0.05, compared with its E + Ang (50). Key: HAF: hyperandrogenemic female; off.: offspring.
Characteristics of aging female HAF offspring :
As shown in Figure 2, body weights were higher in aging offspring groups than adult groups, but were similar between aging control and HAF offspring. Aging also affected body composition with both control and HAF offspring having higher lean and fat masses, higher fat mass/body weight ratio, but lower lean mass/body weight, compared to adult groups. As shown in Table 1, HDL-Cholesterol was increased in aging offspring compared with adult groups, and aging HAF offspring had higher total cholesterol than the other groups; triglycerides were increased in aging HAF offspring compared to adult HAF offspring.
As shown in Figure 3A, proteinuria increased significantly with aging only in HAF offspring. Urinary nitrate/nitrite excretion was not affected by aging in HAF offspring, but was lower in aging control offspring than adult control offspring.
As shown in Supplemental Figure 1, there were no differences in systolic or diastolic BP, between aging control or HAF offspring. However, pulse pressure was different between the two groups. As shown in Supplemental Figure 2, there were no differences in circadian rhythm between aging HAF and control offspring. Heart rates were also similar between aging HAF and control offspring (Figure 4B). However, when compared with adult offspring, heart rate decreased significantly in aging HAF offspring (Figure 4D), whereas heart rate was not different between adult and aging control offspring (Figure 4C).
Response to Ang II and high (4%) salt diet in aging rats:
As shown in Figure 7A–C, baseline MAP was similar between aging control and HAF offspring. Enalapril decreased MAP to similar levels in all groups. Low dose Ang II increased MAP back to baseline levels in aging control offspring, but not in HAF offspring. Addition of 4% salt diet to enalapril alone in “No Ang II” groups, increased MAP to baseline levels in both HAF and control offspring. High salt diet further increased MAP above baseline in Ang II-treated control offspring and increased MAP to baseline levels in HAF offspring. Increasing Ang II to 200 ng/kg/min, in the presence of high salt diet, caused further increases in MAP in both offspring groups, but MAP remained significantly lower in the HAF offspring compared to controls.
Fig. 7:

Mean arterial pressure (MAP) in aging (17–20 mos) female HAF and control offspring in response to Ang II and high salt diet. [7A]. MAP at baseline and after treatment with enalapril (E; 25 mg/kg/d); [7B]. MAP with Ang II (50 ng/kg/min), addition of 4% salt diet, and increasing Ang II (to 200 ng/kg/min); [7C]. Tabulated values of MAP on the last day of each treatment period. Values represent mean ± S.E.M (n = 3–5, 1 rat/litter/group). Statistical analyses were performed by 2-way ANOVA with Bonferroni multiple comparisons post hoc test for comparisons between groups and with Dunnett`s multiple comparisons post hoc test for comparisons between different treatment periods within the same group (C); significance was defined as P ≤ 0.05. a, P ≤ 0.05, compared with Control off. - No Ang II; b, P ≤ 0.05, compared with HAF off. - No Ang II; c, P ≤ 0.05, compared with Control off. - Ang II; #, P ≤ 0.05, compared with its baseline; *, P ≤ 0.05, compared with its Enalapril; $, P ≤ 0.05, compared with its E + Ang (50); &, P ≤ 0.05, compared with its E + Ang (50) + salt D. Key: HAF: hyperandrogenemic female; off.: offspring.
The intrarenal Ang II and Ang (1–7), plasma aldosterone and salt diet intake were similar between HAF and control offspring in the high salt “No Ang II” groups, and between HAF and control offspring in the high salt/high Ang II groups. Intrarenal Ang II and plasma aldosterone were higher and salt diet intake was lower in both HAF and control offspring in high salt/high Ang II groups compared to the high salt “No Ang II” groups (Table 2 and Supplemental Figure 3). However, intrarenal Ang (1–7) in control offspring with high salt/ high Ang II was similar to rats in high salt, “No Ang II” groups, but was increased in HAF offspring with high salt/ high Ang II (Table 2).
Table 2:
Intrarenal angiotensin (Ang) II and Ang (1–7) in aging (17–20 mos) female HAF and control offspring, in response to 4 % salt diet with or without high dose Ang II.
| Control off. - No Ang II (n=5) | HAF off. - No Ang II (n=5) | Control off. - Ang II (n=4) | HAF off. - Ang II (n= 3) | P value (Ang II) | P value (maternal androgens) | P Interaction | |
|---|---|---|---|---|---|---|---|
| Cortical Ang II (pg/mg protein) | 27.8 ± 7.9 | 18.1 ± 4.3 | 129.4 ± 14.9ab | 105.0 ± 33.8ab | <0.0001 | 0.3 | 0.6 |
| Cortical Ang (1–7) (pg/mg protein) | 19.4 ± 2.9 | 23.0 ± 3.1 | 29.8 ± 6.1 | 45.3 ± 5.3ab | 0.002 | 0.04 | 0.2 |
Key: HAF: hyperandrogenemic female; off.: offspring. Values represent mean ± S.E.M (n = 3–5, 1 rat/litter). Statistical analyses were performed by 2-way ANOVA with Bonferroni multiple comparison`s post hoc test; significance was defined as P ≤ 0.05.
P ≤ 0.05 compared with Control off. - No Ang II;
P ≤ 0.05 compared with HAF off. - No Ang II.
With high dose Ang II and high salt diet, proteinuria increased in both control and HAF offspring, but to higher levels in HAF offspring than controls (Table 3). There were no differences in plasma or urinary creatinine among the groups (Supplemental Table 2). Nitrate/nitrate excretion was also similar among groups (Supplemental Table 3), and there were also no differences among groups in heart, left ventricle or kidney weights factored for body weights (Supplemental Table 2).
Table 3:
Proteinuria in aging (17–20 mos) female HAF and control offspring, in response to 4 % salt diet without (Day 31) or with (Day 37) angiotensin (Ang) II.
| Day of assay | Control off. - No Ang II (n=4) | HAF off. - No Ang II (n=5) | Control off. - Ang II (n=5) | HAF off. - Ang II (n= 3–4) | |
|---|---|---|---|---|---|
| Proteinuria (mg/24 h) | 31 | 7.7 ± 1.7 | 12.9 ± 4.2 | 9.3 ± 2.6 | 11.7 ± 4.3 |
| 37 | 7.7 ± 1.3 | 13.1 ± 4.9 | 30.8 ± 7.1 | 69.4 ± 27.1abcd |
Key: HAF: hyperandrogenemic female; off.: offspring. Values represent mean ± S.E.M (n = 4–5, 1 rat/litter). 24-h urine samples were collected on day 31 (before starting Ang (200 ng/kg/min) in Ang-treated groups) and on day 37 (the end of experiment). Statistical analyses were performed by repeated measures 2-way ANOVA with Bonferroni multiple comparison`s post hoc test; significance was defined as P ≤ 0.05.
P ≤ 0.05 compared with Control off. - No Ang II;
P ≤ 0.05 compared with HAF off. - No Ang II;
P ≤ 0.05 compared with Control off. - Ang II;
P ≤ 0.05 compared with same group on day 31.
Discussion
In this study the major findings in female HAF offspring are: 1) as adults and with aging, female HAF offspring have similar baseline BPs as female control offspring, despite having higher proteinuria with aging. 2) Female HAF offspring have an attenuated pressor response to Ang II alone (adults) or when given with high salt diet (aging). 3) The attenuated pressor responses to Ang II in HAF offspring were independent of body weights, body composition, sex steroids, intrarenal Ang II or nitrate/nitrite excretion. 4) Intrarenal Ang (1–7) was increased with high salt/Ang II in aging HAF offspring. 5) Aging HAF offspring developed significantly higher proteinuria with high dose Ang II/high salt compared to controls, despite their lower MAP. 6) Although heart rates were similar between adult and aging HAF and control offspring, with aging heart rates decreased in HAF offspring, but not control offspring. Taken together, these data show that while baseline BP was similar between female control and HAF offspring, HAF offspring were protected from Ang II hypertension, but they may be at increased risk of renal injury, especially with aging.
PCOS offspring are frequently born with IUGR12, 42. IUGR in humans is associated with increased risk of CVD in adulthood and with aging14, 43. We showed previously that both male and female HAF offspring are born with IUGR33. IUGR in other animal models of developmental programming is also associated with increased CVD and hypertension although there are sex differences. For example, using a rat model of preeclampsia, the reduced uterine perfusion pressure (RUPP) model, in which the offspring are born with IUGR44, Alexander and colleagues reported that the male offspring of RUPP dams, but not the female offspring, are hypertensive as adults45, 46. The female offspring only develop hypertension after reproductive senescence (approximately 12 months of age)47. These data are consistent with other models of developmental programming in which female offspring born with IUGR or low birth weight are protected against hypertension and even the pressor response to Ang II in young adulthood46, 48–50, but develop hypertension with aging47.
While the HAF offspring are born with IUGR, we showed previously that adult male HAF offspring are normotensive34, just as we found in adult female HAF offspring in the present study. Despite no hypertension, adult male HAF offspring have elevated levels of proteinuria, compared to controls, and they exhibit an exaggerated pressor response to Ang II (at both 50 and 200 ng/kg/min), compared to controls34. In contrast to males, in the present study we found that female HAF offspring did not have increased proteinuria, and they had an attenuated pressor response to Ang II, even at doses up to 200 ng/kg/min. For this reason, we decided to determine whether the pressor response to Ang II would change with aging in female HAF offspring, studies we did not perform in male HAF offspring. However, in aging female HAF offspring, low dose Ang II alone also failed to increase MAP, compared to controls, and while addition of high salt diet increased MAP back to baseline levels, it did so independent of Ang II. It was only when the dose of Ang II was increased to 200 ng/kg/min with high salt diet, that MAP increased in female HAF offspring, but the pressor response was still attenuated compared to control offspring.
The mechanisms responsible for the attenuated Ang II response in the female HAF offspring are not entirely clear from our studies. Intrarenal Ang II levels prior to Ang II infusion were similar between adult control and HAF offspring. In addition, there were no differences in body weights, body composition, or plasma lipids. Furthermore, while Ang II levels were similar between the two adult offspring groups, Ang (1–7) and nitrate/nitrite excretion, an index of total body NO, were lower in adult female HAF offspring than control offspring, which should have caused an increase in their BP. Ang (1–7) has been linked to NO formation in the kidneys51. In contrast, with aging, Ang(1–7) was significantly higher in HAF offspring than control offspring receiving Ang II and high salt diet, suggesting that this may be one mechanism by which the pressor response in aging female HAF offspring was attenuated. The increased Ang (1–7) that occurred in aging HAF offspring with high dose Ang II/salt diet was independent of an increase in nitrate/nitrite excretion, thus suggesting that that Ang(1–7) did not work through NO to attenuate the pressor response to high dose Ang II/high salt. Elevated ACE2, the enzyme responsible for conversion of Ang II to Ang (1–7), may be a “compensatory response” to the hypertension, resulting in increased expression of anti-hypertensive components of renin-angiotensin system (RAS) (Ang (1–7), Mas R and AT2R), thus attenuating the elevated BP52–54. It is also possible that high dose Ang II/high salt increased oxidative stress and thus attenuated NO production in the HAF offspring, but not enough to increase BP to the same level as in control offspring. Thus future studies are necessary to determine the role of the vasodilatory RAS, NO and, perhaps oxidative stress, in mediating the attenuated pressor response to Ang II in aging HAF offspring.
Female HAF offspring develop proteinuria with aging that is exacerbated with Ang II/high salt. Whether this is indicative of developing renal disease with aging is not clear since neither plasma or urinary creatinine were increased with high dose Ang II/salt. This may suggest that with Ang II and high salt, glomerular capillary pressure may increase, thus causing increased proteinuria. In future studies glomerular filtration rate and even glomerular capillary pressure will need to be measured to determine the consequences of aging on female HAF offspring.
It is also possible that female HAF offspring may exhibit some measure of heart failure or left ventricular dysfunction55, that precludes their ability to increase BP in response to typical stimuli. In support of this contention, while there were no differences in systolic or diastolic BP between adult and aging HAF offspring, compared to controls, heart rate significantly decreased with aging in female HAF offspring, but did not change in control offspring. Future studies will be necessary to determine the contribution of cardiac function or perhaps baroreflex sensitivity, to BP control in aging female HAF offspring.
Perspectives:
The data in the present study do not lend themselves to specific predictions as to the potential CVD risk in female offspring of PCOS women, either as adults or with aging. On the one hand, the female HAF offspring are not hypertensive and are resistant to Ang II hypertension, and with aging, this may be due to increased Ang (1–7) and activation of the vasodilator arm of the RAS which should be protective against CVD risk. However, aging HAF offspring have increased proteinuria, also suggesting evolving renal injury and the potential for future increases in BP. Most importantly, future studies will need to be done in adult and postmenopausal women whose mothers had PCOS to determine their susceptibility to CVD.
Supplementary Material
Novelty and Significance.
What is New?
This is the first study to evaluate the baseline BP and response to Ang II in adult and aging female offspring of dams exposed to androgens prior to and throughout pregnancy and lactation as seen in PCOS women.
What is Relevant?
PCOS is a syndrome that affects ~ 10% of reproductive age women, who become pregnant and their female offspring may be at increased risk of high BP or CVD as well.
Summary
Female HAF offspring have similar baseline BP as age-matched control offspring, but have attenuated pressor responses to Ang II (as adults) and Ang II/salt (with aging), suggesting potential protection from Ang II hypertension. On the other hand, female HAF offspring also have enhanced renal injury with aging. Future studies are clearly warranted to expand these studies to explore additional BP control mechanisms.
Acknowledgements
We acknowledge the excellent technical support of Ruth M. Vinson and Kacey Davenport for these studies. The graphical abstract was partly generated by BioRender.com. We would like to thank Dr. Seth Lirette for his assistance with statistical analyses.
Funding Sources
This work was supported by the National Institutes of Health (NIH) grants: R01HL66072 (JFR), R01HL135089 (JFR, NS), P01HL051971 (JFR, BTA), P20GM121334 (JFR, BTA), P20GM104357 (JFR, BTA), R01HL143459 (BTA), and American Heart Association 20POST35150001 (NMS).
References
- [1].Sam S: Obesity and Polycystic Ovary Syndrome. Obes Manag 2007, 3:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS: Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr Rev 2015, 36:487–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Holte J, Gennarelli G, Berne C, Bergh T, Lithell H: Elevated ambulatory day-time blood pressure in women with polycystic ovary syndrome: a sign of a pre-hypertensive state? Human reproduction (Oxford, England) 1996, 11:23–8. [DOI] [PubMed] [Google Scholar]
- [4].Gunning MN, Fauser B: Are women with polycystic ovary syndrome at increased cardiovascular disease risk later in life? Climacteric 2017, 20:222–7. [DOI] [PubMed] [Google Scholar]
- [5].Lambrinoudaki I: Cardiovascular risk in postmenopausal women with the polycystic ovary syndrome. Maturitas 2011, 68:13–6. [DOI] [PubMed] [Google Scholar]
- [6].Schmidt J, Landin-Wilhelmsen K, Brannstrom M, Dahlgren E: Cardiovascular disease and risk factors in PCOS women of postmenopausal age: a 21-year controlled follow-up study. J Clin Endocrinol Metab 2011, 96:3794–803. [DOI] [PubMed] [Google Scholar]
- [7].Doroszewska K, Milewicz T, Mrozińska S, Janeczko J, Rokicki R, Janeczko M, Warzecha D, Marianowski P: Blood pressure in postmenopausal women with a history of polycystic ovary syndrome. Prz Menopauzalny 2019, 18:94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Persson S, Elenis E, Turkmen S, Kramer MS, Yong EL, Sundstrom-Poromaa I: Fecundity among women with polycystic ovary syndrome (PCOS)-a population-based study. Human reproduction (Oxford, England) 2019, 34:2052–60. [DOI] [PubMed] [Google Scholar]
- [9].He Y, Lu Y, Zhu Q, Wang Y, Lindheim SR, Qi J, Li X, Ding Y, Shi Y, Wei D, Chen ZJ, Sun Y: Influence of metabolic syndrome on female fertility and in vitro fertilization outcomes in PCOS women. Am J Obstet Gynecol 2019, 221:138.e1–.e12. [DOI] [PubMed] [Google Scholar]
- [10].Roos N, Kieler H, Sahlin L, Ekman-Ordeberg G, Falconer H, Stephansson O: Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: population based cohort study. Bmj 2011, 343:d6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kjerulff LE, Sanchez-Ramos L, Duffy D: Pregnancy outcomes in women with polycystic ovary syndrome: a metaanalysis. American Journal of Obstetrics and Gynecology 2011, 204:558.e1–.e6. [DOI] [PubMed] [Google Scholar]
- [12].Sir-Petermann T, Hitchsfeld C, Maliqueo M, Codner E, Echiburú Br, Gazitúa R, Recabarren S, Cassorla F: Birth weight in offspring of mothers with polycystic ovarian syndrome. Human Reproduction 2005, 20:2122–6. [DOI] [PubMed] [Google Scholar]
- [13].Barker DJ: The developmental origins of adult disease. J Am Coll Nutr 2004, 23:588s–95s. [DOI] [PubMed] [Google Scholar]
- [14].Calkins K, Devaskar SU: Fetal origins of adult disease. Curr Probl Pediatr Adolesc Health Care 2011, 41:158–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Boney CM, Verma A, Tucker R, Vohr BR: Metabolic Syndrome in Childhood: Association With Birth Weight, Maternal Obesity, and Gestational Diabetes Mellitus. Pediatrics 2005, 115:e290–e6. [DOI] [PubMed] [Google Scholar]
- [16].Evagelidou EN, Giapros VI, Challa AS, Cholevas VK, Vartholomatos GA, Siomou EC, Kolaitis NI, Bairaktari ET, Andronikou SK: Prothrombotic State, Cardiovascular, and Metabolic Syndrome Risk Factors in Prepubertal Children Born Large for Gestational Age. Diabetes Care 2010, 33:2468–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group: Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004, 19:41–7. [DOI] [PubMed] [Google Scholar]
- [18].Kent SC, Gnatuk CL, Kunselman AR, Demers LM, Lee PA, Legro RS: Hyperandrogenism and hyperinsulinism in children of women with polycystic ovary syndrome: a controlled study. The Journal of clinical endocrinology and metabolism 2008, 93:1662–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sir-Petermann T, Maliqueo M, Codner E, Echiburu B, Crisosto N, Perez V, Perez-Bravo F, Cassorla F: Early metabolic derangements in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 2007, 92:4637–42. [DOI] [PubMed] [Google Scholar]
- [20].Sir-Petermann T, Codner E, Pérez V, Echiburú Br, Maliqueo M, Ladrón de Guevara A, Preisler J, Crisosto Ns, Sánchez F, Cassorla F, Bhasin S: Metabolic and Reproductive Features before and during Puberty in Daughters of Women with Polycystic Ovary Syndrome. The Journal of Clinical Endocrinology & Metabolism 2009, 94:1923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Torchen LC, Legro RS, Dunaif A: Distinctive Reproductive Phenotypes in Peripubertal Girls at Risk for Polycystic Ovary Syndrome. J Clin Endocrinol Metab 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Olszanecka-Glinianowicz M, Zachurzok A, Drosdzol-Cop A, Bozetowicz-Wikarek M, Owczarek A, Gawlik A, Chudek J, Skrzypulec-Plinta V, Malecka-Tendera E: Circulating Anti-Mullerian Hormone Levels in Daughters of Women with and without Polycystic Ovary Syndrome. Hormone research in paediatrics 2016, 85:372–8. [DOI] [PubMed] [Google Scholar]
- [23].Sir-Petermann T, Codner E, Maliqueo M, Echiburú Br, Hitschfeld C, Crisosto Ns, Pérez-Bravo F, Recabarren SE, Cassorla F: Increased Anti-Müllerian Hormone Serum Concentrations in Prepubertal Daughters of Women with Polycystic Ovary Syndrome. The Journal of Clinical Endocrinology & Metabolism 2006, 91:3105–9. [DOI] [PubMed] [Google Scholar]
- [24].Legro RS, Kunselman AR, Stetter CM, Gnatuk CL, Estes SJ, Brindle E, Vesper HW, Botelho JC, Lee PA, Dodson WC: Normal Pubertal Development in Daughters of Women With PCOS: A Controlled Study. J Clin Endocrinol Metab 2017, 102:122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Harnois-Leblanc S, Trottier A, Leblanc S, Battista MC, Geller DH, Baillargeon JP: Evolution of metabolic alterations 5 Years after early puberty in a cohort of girls predisposed to polycystic ovary syndrome. Reproductive biology and endocrinology : RB&E 2017, 15:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Battaglia C, Mancini F, Cianciosi A, Busacchi P, Persico N, Paradisi R, Facchinetti F, de Aloysio D: Cardiovascular risk in normal weight, eumenorrheic, nonhirsute daughters of patients with polycystic ovary syndrome: a pilot study. Fertility and sterility 2009, 92:240–9. [DOI] [PubMed] [Google Scholar]
- [27].de Wilde MA, Eising JB, Gunning MN, Koster MPH, Evelein AMV, Dalmeijer GW, Uiterwaal CSPM, Eijkemans MJC, van der Ent CK, Meijboom FJ, Fauser BCJM: Cardiovascular and Metabolic Health of 74 Children From Women Previously Diagnosed With Polycystic Ovary Syndrome in Comparison With a Population-Based Reference Cohort. Reprod Sci 2018, 25:1492–500. [DOI] [PubMed] [Google Scholar]
- [28].Gunning MN, Sir Petermann T, Crisosto N, van Rijn BB, de Wilde MA, Christ JP, Uiterwaal C, de Jager W, Eijkemans MJC, Kunselman AR, Legro RS, Fauser B: Cardiometabolic health in offspring of women with PCOS compared to healthy controls: a systematic review and individual participant data meta-analysis. Hum Reprod Update 2020, 26:103–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yanes LL, Romero DG, Moulana M, Lima R, Davis DD, Zhang H, Lockhart R, Racusen LC, Reckelhoff JF: Cardiovascular-renal and metabolic characterization of a rat model of polycystic ovary syndrome. Gend Med 2011, 8:103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dalmasso C, Maranon R, Patil C, Bui E, Moulana M, Zhang H, Smith A, Yanes Cardozo LL, Reckelhoff JF: Cardiometabolic Effects of Chronic Hyperandrogenemia in a New Model of Postmenopausal Polycystic Ovary Syndrome. Endocrinology 2016, 157:2920–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Maranon R, Lima R, Spradley FT, do Carmo JM, Zhang H, Smith AD, Bui E, Thomas RL, Moulana M, Hall JE, Granger JP, Reckelhoff JF: Roles for the sympathetic nervous system, renal nerves, and CNS melanocortin-4 receptor in the elevated blood pressure in hyperandrogenemic female rats. American journal of physiology Regulatory, integrative and comparative physiology 2015, 308:R708–R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Patil CN, Racusen LC, Reckelhoff JF: Consequences of advanced aging on renal function in chronic hyperandrogenemic female rat model: implications for aging women with polycystic ovary syndrome. Physiol Rep 2017, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shawky NM, Patil CN, Dalmasso C, Maranon RO, Romero DG, Drummond H, Reckelhoff JF: Pregnancy Protects Hyperandrogenemic Female Rats From Postmenopausal Hypertension. Hypertension (Dallas, Tex : 1979) 2020, 76:943–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zuchowski Y, Dalmasso C, Shawky NM, Reckelhoff JF: Cardiometabolic consequences of maternal hyperandrogenemia in male offspring. Physiological Reports 2021, 9:e14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Allred AJ, Chappell MC, Ferrario CM, Diz DI: Differential actions of renal ischemic injury on the intrarenal angiotensin system. Am J Physiol Renal Physiol 2000, 279:F636–45. [DOI] [PubMed] [Google Scholar]
- [36].Reckelhoff JF, Kellum JA, Blanchard EJ, Bacon EE, Wesley AJ, Kruckeberg WC: Changes in nitric oxide precursor, L-arginine, and metabolites, nitrate and nitrite, with aging. Life sciences 1994, 55:1895–902. [DOI] [PubMed] [Google Scholar]
- [37].Dalmasso C, Maranon R, Patil C, Moulana M, Romero DG, Reckelhoff JF: 20-HETE and CYP4A2 omega-hydroxylase contribute to the elevated blood pressure in hyperandrogenemic female rats. Am J Physiol Renal Physiol 2016, 311:F71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hirsch AT, Talsness CE, Smith AD, Schunkert H, Ingelfinger JR, Dzau VJ: Differential effects of captopril and enalapril on tissue renin-angiotensin systems in experimental heart failure. Circulation 1992, 86:1566–74. [DOI] [PubMed] [Google Scholar]
- [39].Hirawa N, Uehara Y, Kawabata Y, Numabe A, Ohshima N, Ono H, Gomi T, Ikeda T, Yagi S, Toyo-oka T, et al. : Subpressor dose of angiotensin II increases susceptibility to the haemodynamic injury of blood pressure in Dahl salt-sensitive rats. Journal of hypertension 1995, 13:81–90. [PubMed] [Google Scholar]
- [40].Simon G, Cserep G, Limas C: Development of structural vascular changes with subpressor angiotensin II administration in rats. Am J Hypertens 1995, 8:67–73. [DOI] [PubMed] [Google Scholar]
- [41].Soljancic A, Ruiz AL, Chandrashekar K, Maranon R, Liu R, Reckelhoff JF, Juncos LA: Protective role of testosterone in ischemia-reperfusion-induced acute kidney injury. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 2013, 304:R951–R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS: A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update 2006, 12:673–83. [DOI] [PubMed] [Google Scholar]
- [43].Barker DJ: Fetal origins of cardiovascular disease. Annals of medicine 1999, 31 Suppl 1:3–6. [PubMed] [Google Scholar]
- [44].Alexander BT: Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension (Dallas, Tex : 1979) 2003, 41:457–62. [DOI] [PubMed] [Google Scholar]
- [45].Ojeda NB, Grigore D, Yanes LL, Iliescu R, Robertson EB, Zhang H, Alexander BT: Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. American journal of physiology Regulatory, integrative and comparative physiology 2007, 292:R758–63. [DOI] [PubMed] [Google Scholar]
- [46].Ojeda NB, Grigore D, Robertson EB, Alexander BT: Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension (Dallas, Tex : 1979) 2007, 50:679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Intapad S, Tull FL, Brown AD, Dasinger JH, Ojeda NB, Fahling JM, Alexander BT: Renal denervation abolishes the age-dependent increase in blood pressure in female intrauterine growth-restricted rats at 12 months of age. Hypertension (Dallas, Tex : 1979) 2013, 61:828–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R: Maternal Protein Restriction Suppresses the Newborn Renin-Angiotensin System and Programs Adult Hypertension in Rats. Pediatric research 2001, 49:460–7. [DOI] [PubMed] [Google Scholar]
- [49].Woods LL, Ingelfinger JR, Rasch R: Modest maternal protein restriction fails to program adult hypertension in female rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 2005, 289:R1131–R6. [DOI] [PubMed] [Google Scholar]
- [50].Moritz KM, Mazzuca MQ, Siebel AL, Mibus A, Arena D, Tare M, Owens JA, Wlodek ME: Uteroplacental insufficiency causes a nephron deficit, modest renal insufficiency but no hypertension with ageing in female rats. J Physiol 2009, 587:2635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gwathmey TM, Westwood BM, Pirro NT, Tang L, Rose JC, Diz DI, Chappell MC: Nuclear angiotensin-(1–7) receptor is functionally coupled to the formation of nitric oxide. Am J Physiol Renal Physiol 2010, 299:F983–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lo J, Patel VB, Wang Z, Levasseur J, Kaufman S, Penninger JM, Oudit GY: Angiotensin-converting enzyme 2 antagonizes angiotensin II-induced pressor response and NADPH oxidase activation in Wistar-Kyoto rats and spontaneously hypertensive rats. Exp Physiol 2013, 98:109–22. [DOI] [PubMed] [Google Scholar]
- [53].Díez-Freire C, Vázquez J, Correa de Adjounian MF, Ferrari MF, Yuan L, Silver X, Torres R, Raizada MK: ACE2 gene transfer attenuates hypertension-linked pathophysiological changes in the SHR. Physiol Genomics 2006, 27:12–9. [DOI] [PubMed] [Google Scholar]
- [54].Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK: Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension (Dallas, Tex : 1979) 2007, 49:926–31. [DOI] [PubMed] [Google Scholar]
- [55].Manti M, Fornes R, Pironti G, McCann Haworth S, Zhengbing Z, Benrick A, Carlström M, Andersson D, Stener-Victorin E: Maternal androgen excess induces cardiac hypertrophy and left ventricular dysfunction in female mice offspring. Cardiovascular research 2019, 116:619–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


