Abstract
Introduction:
The parasympathetically derived marker of heart rate variability, root mean square of successive R-R differences (RMSSD), and the daily fluctuations as measured by the coefficient of variation (RMSSDCV) may be useful for tracking training adaptations in athletic populations. These vagally derived markers of heart rate variability may be especially pertinent when simultaneously considering a female athlete’s menstrual cycle.
Purpose:
The purpose of this study was to observe the perturbations in RMSSDCV, while considering RMSSD, across a season in the presence and absence of menses with training load in female collegiate rowers.
Methods:
Thirty-six (20 [1] y, 25.6 [3.4] kg·m−2) National Collegiate Athletic Association Division I female rowers were monitored for 18 consecutive weeks across a full season. Seated, ultrashortened RMSSD measurements were obtained by the rowers on at least 3 mornings per week using a smartphone photoplethysmography device. Following the RMSSD measurement, athletes indicated the presence or absence of menstruation within the application. Individual meters rowed that week and sessions rate of perceived exertion were obtained to quantify training load.
Results:
Longitudinal mixed-effects modeling demonstrated a significant effect of menses and time, while also considering RMSSD, such that those who were on their period had a significantly greater RMSSDCV than those who were not (11.2% vs 7.5%, respectively; P < .001). These changes were independent of meters rowed, sessions rate of perceived exertion, body mass index, birth-control use, and years of rowing experience, which were all nonsignificant predictors of RMSSDCgV (P > .05).
Conclusion:
The presence of menses appears to significantly impact RMSSDCV when also considering RMSSD, which may allow coaches to consider individualized training plans accordingly.
Keywords: female athletes, RMSSD, athlete monitoring, longitudinal data analysis, sport performance
Heart rate variability (HRV) has evolved as a noninvasive physiological marker which sport scientists and coaches can utilize to assess and optimize training adaptations and performance in their athletes.1–6 HRV reflects global cardiac-autonomic modulation at the individual level, and proper balance of vagal (ie, parasympathetic) modulation is paramount for peak performance with chronic exercise.1,7 Analysis of root mean square of successive R-R differences (RMSSD) reflects vagal activity independent of breathing rate, particularly in response to ambulatory conditions such as dynamic exercise.1,6 For meaningful interpretation of adaptation to training using RMSSD data, it is important to consider the normal, day-to-day perturbations in RMSSD (assessed by the coefficient of variation, CV; %) from a variety of potential stimuli.3,8,9 As such, significant daily fluctuations of vagal activity (CV of RMSSD) have been associated with greater fatigue during training sessions.9 Thus, RMSSD and CV of RMSSD (RMSSDCV) are the preferred metric for daily measurement by athletes in the field to indicate specific perturbations to training stimuli.1,6 Although previous research has established a connection between HRV and fatigue accumulated from training, a majority of these studies did not include female athletes, nor investigate potential female-specific RMSSD responses.
Female reproductive hormones, such as estrogen and progesterone, are introduced in a cyclic physiological cascade in premenopausal women. The normal rhythm of the menstrual cycle (MC) “begins” at the onset of menses during the early-follicular phase (days 1–6) when the hormones estrogen and progesterone are at the lowest concentration levels.10 Increases in estrogen are evident in the middle of the MC during ovulation (~ day 14) and remain above follicular baseline levels during the subsequent luteal phase, coinciding with peak progesterone levels on days 19 to 25.10 Previous research in female rowers has demonstrated no effect of MC phase on endurance performance.11,12 While most studies13 report no changes in various determinants of performance across the MC, some have shown higher exercising heart rate during prolonged endurance activity performed in the luteal phase.14,15 Phase-related differences in core body temperature are a potential mechanism underlying the higher exercising heart rate, and thereby cardiovascular strain in the luteal versus follicular phase.16 Increased sinoatrial node firing from increased sympathetic activity and elevated intrinsic heart rate are direct effects of elevated body temperature, and all of these elevate heart rate.17 Furthermore, elevated progesterone increases resting core temperature ~0.5°C versus the follicular phase,16 and this temperature difference between phases persists throughout prolonged exercise.18
Several studies10–12,19 have reported decreased vagally derived HRV at rest in eumenorrheic women during the luteal phase, which could be attributed to estrogenic effects on increased blood volume coupled with increased vasoconstriction.16,17,19 However, much of this work indicating parasympathetic predominance during the follicular phase of the MC is based on HRV trends observed from a snapshot of only one or two cycles of menses.11–13,19 It reasonable to then suggest that combined accumulation of prolonged athletic activities during a high-hormone state may result in greater strain and thereby greater perturbations to RMSSDCV. Furthermore, it could be that there are other external factors contributing to cardiac-autonomic perturbations across the MC, such as pain, emotional distress, or chronic training load.14,15 These equivocal findings indicate the lack of a thorough understanding of both the direct and indirect influences of such pulsatile reproductive hormones on physical functioning of premenopausal female athletes. Isolating individual responses to cyclic cardiac-autonomic modulation across multiple MCs that inevitably occur in an athletic season is particularly important for elite female athletes who rely on individualized training plans to perform at optimal levels on competition days.
The RMSSD has capabilities to reflect global parasympathetic modulation in athletic populations, but there is currently no available literature regarding MC effects spanning an athletic season. We conducted this investigation because using vagally derived HRV indices to understand the individual fluctuations indicating a favorable training response could prove to be a useful tool in premenopausal athletes, operating independently of MC status.11 Therefore, the purpose of this study was: (1) to observe the fluctuations in RMSSDCV across a full sports season in the presence and absence of menses in female collegiate rowers, while considering RMSSD; (2) to determine if the presence of menses, when considering RMSSD, modified the relationship between RMSSDCV and training load as measured by meters rowed that week and session ratings of perceived exertion (sRPE). Considering the recent evidence which indicates a nonlinear decrease of RMSSD measures in female athletes across the MC, we hypothesized that RMSSDCV, when considering RMSSD, would demonstrate significant perturbations in the presence of menses throughout the entire collegiate rowing season.19 Furthermore, daily perturbations to cardiac-autonomic modulation, as expressed by RMSSDCV, would be subsequentially modified by the consideration of training load.
Methods
Participants
Thirty-nine (n = 39) female, National Collegiate Athletic Association Division I Collegiate Rowers from the University of Alabama (Tuscaloosa, AL) Women’s Rowing Team volunteered to participate in this study. Each of the athletes passed a medical examination from university physicians prior to participation in rowing-related activities, and pertinent health histories (eg, height, weight, contractive method, medication use) were collected from the team’s medical staff. Specific exclusion criteria included: (1) any diagnosed disease, syndrome, or disability that could influence response to training, heart rate, and/or MC status; (2) women with irregular MC or amenorrhea; (3) medications that alter heart rate or metabolic responses; (4) asthma or respiratory/breathing disorder; (5) postmenopausal women; and (6) women who have been or become pregnant. Participants refrained from consumption of alcohol (24 h) and caffeine (4 h) prior to practice.
Informed consent was obtained from each participant following approval from the institutional review board at the University of Alabama. The study was performed in accordance with the Declaration of Helsinki, as well as National Collegiate Athletic Association Compliance personnel.
Study Design
Data collection for this observational study took place across 18 weeks, which included the preparatory phase of training preceding the spring racing season for collegiate women’s rowing (January to May 2018; Table 1). This study was a secondary analysis of a larger study investigating time course of HRV monitoring within female athletes; more detailed methods are described elsewhere.20
Table 1.
Average Training Load per Week for the 36 Rowers
| Total weekly training minutes |
||||||||
|---|---|---|---|---|---|---|---|---|
| Week | Description of training | % compliance | Water | Rowing ergometer | Land | Resistance training | sRPE | Meters rowed |
| Preparatory phase | ||||||||
| 1 | Winter break training camp | 100.0 | 1110 | 280 | 172 | 0 | 395 (156) | 56,015 (12,672) |
| 2 | Winter training period | 100.0 | 292 | 208 | 48 | 120e | 492 (160) | 79,433 (10,657) |
| 3 | 100.0 | 381 | 81 | 82 | 120e | 604 (163) | 91,752 (12,424) | |
| 4 | 97.2 | 261 | 83 | 72 | 120e | 668 (185) | 67,327 (9202) | |
| 5 | 97.2 | 371 | 119 | 60 | 120e | 625 (175) | 89,901 (11,604) | |
| 6 | 97.2 | 356 | 34 | 72 | 120e | 527 (193) | 77,223 (14,376) | |
| 7 | Regatta; 4000a | 88.9 | 477 | 55 | 60 | 120e | 626 (170) | 73,895 (12,116) |
| 8 | Regatta; 4000b | 86.1 | 587 | 32 | 30 | 120e | 525 (155) | 116,012 (25,758) |
| 9 | 80.6 | 400 | 10 | 0 | 120e | 546 (177) | 116,200 (27,305) | |
| Spring racing season | ||||||||
| 10 | 63.9 | 290 | 212 | 0 | 0 | 585 (175) | 83,432 (23,785) | |
| 11 | Regatta; 4000c | 83.3 | 433 | 0 | 0 | 80f | 552 (207) | 114,543 (40,302) |
| 12 | 63.9 | 548 | 15 | 0 | 120e | 742 (293) | 40,659 (6047) | |
| 13 | Regatta; 4000c,* | 66.7 | 436 | 15 | 12 | 80f | 551 (180) | 33,993 (6776) |
| 14 | Regatta; 6000d,* | 55.6 | 485 | 0 | 12 | 80f | 546 (270) | 69,724 (22,940) |
| 15 | 52.8 | 639 | 0 | 90 | 80f | 960 (199) | 22,391 (6795) | |
| 16 | Regatta; 4000c,* | 38.9 | 561 | 0 | 12 | 80f | 985 (207) | 173,732 (3865) |
| 17 | 19.4§ | 460 | 0 | 12 | 80f | 900 (380) | 83,867 (4563) | |
| 18 | Championship Regatta; 2000* | 8.3§ | 378 | 0 | 0 | 0 | 626 (315) | 23,768 (1256) |
Abbreviations: RPE, rating of perceived exertion; sRPE, session RPE (duration of exercise × average RPE per individual) as a marker of training load. Note: Data are presented as mean (SD).
Four 1000-m races were conducted, with rest in between pieces.
One 2000-m race, followed by two 1000-m races with rest in between pieces.
Two 2000-m races were conducted, with rest in between pieces.
Three 2000-m races were conducted, 2 on Saturday and 1 on Sunday, with rest in between pieces.
Sixty-minute resistance training sessions were conducted twice per week (Tuesday and Thursday).
Reduced load, 40-minute resistance training sessions were conducted twice per week (Tuesday and Thursday).
Travel to out-of-town regatta during this week.
Only top 18 rowers continue to practice/compete.
Collegiate women’s rowing programs focus on the shift from long and continuous rowing sessions on the water and the rowing ergometer during the early training period of the spring season (January to March), before competing in spring races which consist primarily of 2000-m sprints.21 This training stimulus must significantly load the cardiopulmonary system in order for the athletes to be successful in a 2000-m race, which requires rowers to achieve an aerobic steady state that exceeds that of their anaerobic threshold as early as possible.22 Thus, the first 9-week period generally consists of longer endurance training both on and off the water, combined with anaerobic threshold training sessions to build a solid foundation for the second 9-week spring racing program. These specifics are detailed further in Table 1.21
HRV in the Time Domain
All HRV variables were collected using the HRV4Training application (https://www.hrv4training.com/) upon arrival at the team’s boathouse for regularly scheduled morning practice, as described elsewhere.20 Briefly, athletes were instructed to limit bodily movement and practice spontaneous breathing while they sat in a backed chair with their arms and legs uncrossed.6 Then they opened the HRV4Training application on their own personal mobile device, removed any phone covering, and placed their left index finger directly on the posterior camera sensor. The rowers then initiated a 1-minute stabilization period, followed by a 1-minute data acquisition period using the rear camera sensor as a photoplethysmography device.6 Ultrashortened (ie, 2-min) measures of RMSSD are an accurate and preferred surrogate to the 10-minute criterion measure, which is impractical in such athletic environments.4,7 In order to be considered ecologically valid, each athlete must have taken at least 3 ultrashortened measurements at any point throughout the training week upon arrival at the team’s boathouse for morning practice (Monday to Saturday).4,5 This rolling weekly average method has been shown to accurately indicate cardiac-autonomic adaptations in response to a training stimulus.4,5
Covariate Measures
Daily meters rowed and time spent in each training session (Monday to Saturday) were compiled for each rower and summed across each week in an Excel spreadsheet. This data was collected from ergometer workouts (Model D; Concept2® Rowing Ergometers, Morrisville, VT), boat meters rowed (SpeedCoach® GPS Stoke Coaches; Nielsen-Kellerman, Boothwyn, PA), and biweekly resistance training sessions (Table 1). All of these data were captured by the coaches and team leaders before being transferred to the research team at the end of each training day (Monday to Saturday) for the 18 weeks using the same encrypted USB flash drive mentioned above.
A quick survey, built into the application, followed the ultrashortened measurement, which allowed the athletes to note the presence of menstruation in a simple “yes” or “no” fashion. The postmeasurement survey also included questions regarding the athlete’s ratings of perceived exertion (RPE) by means of a 10-point Borg scale from the whole prior day’s training.23 Training load was calculated using sRPE, which considers RPE multiplied by the total training time of the prior day’s session, as this has been shown to be suitable for training quantification in team-sports athletes.24 The weekly averaged sRPE values were used for the final analysis. The use and method of contraception, along with pertinent descriptive variables, were collected from the health histories and confirmed by the athletes. For the purposes of this study, the presence of menses, or menstrual bleeding, was indicative of a low hormone state, regardless of exogenous or endogenous hormonal fluctuations. Thus, endogenous estrogen levels during menses (ie, withdrawal phase for women on oral contraceptives [OC]) were assumedly similar to the levels of women with ovulatory cycles during menses (ie, follicular phase). This is based on recent reports that endogenous estrogen levels are similar between women taking an OC and those with ovulatory cycles.18 Due to the observational nature of this study intended to reflect real-world fluctuations in female athletes’ vagal modulations, we included and covaried for these athletes who were taking OCs if they had regular menstrual bleeding at least once per month, which was verbally confirmed, as well as indicated within the application.
Data and Statistical Analyses
All data collected from the HRV4Training application were downloaded using the coach’ s platform on Sunday morning for each week, combined with the performance data, anonymized, and stored on an encrypted USB flash drive. The RMSSD mean (RMSSDM) and RMSSDCV were calculated (CV = [SD/mean] × 100; in percentage) for each individual across each of the 18 weeks using Microsoft® Excel 365 software (Microsoft® Corporation, Redmond, WA). RMSSDCV values represent the SE of the estimate (absolute reliability) within interday RMSSDM assessments.3,8,9 By observing RMSSDCV from at least 3 morning HRV recordings, users can assess cardiac-autonomic modulations that inevitably occur throughout a week of training instead of a single, isolated value compressed over the full week.2,8,9
Shapiro–Wilk test was performed to identify nonnormal data. Due to the skewed nature of vagally derived indices of HRV, all daily RMSSD values were log-transformed (LnRMSSDM and LnRMSSDCV), and these values were used for subsequent analyses.1,2,6 We used longitudinal mixed-effects models to examine the effects of menses on LnRMSSDCV, according to the proposed theories. The presence of menses (MC status) was dummy coded and entered into each of the models. Model 0 contained no fixed effects and was termed the null model. The first model (model 1) investigated LnRMSSDCV across time in the presence of menstruation, while also considering LnRMSSDM. Model 2 investigated the same relationship as model 1, with MC status and LnRMSSDM as predictors of LnRMSSDCV across the 18 weeks, with the addition of training load (measured as individual meters rowed in the water and/or on the erg and sRPE) in order to fulfill the second purpose, which investigates how quantitative training load would influence the observed fluctuations in LnRMSSDCV when considering LnRMSSDM and menses. A third model was calculated to investigate the relationships of the first and second purpose, while controlling for potential confounding factors such as OC use, body mass index (fixed variable), and years of rowing experience (fixed variable) by each athlete.
As seen in Figure 1, we included random intercepts for each individual rower and the random slopes of time (linear effects) effects relative to their MC status to control for individual variation and report a more generalizable effect that is less dependent on these specific precipitants.25 Since slopes are estimated per rower, athletes were included for analysis so long as they had 3 weeks of measurements, with at least one menses recorded. P values were generated using Satterthwaite approximations. Analyses were performed using R (version 3.6.3; R Core Team; Vienna, Austria) and RStudio (version 1.2.5033; RStudio, Inc; Boston, MA) using the packages lmer,26 ggplot2,27 effects,28 sjPlot,29 lmerTest,30 and afex31 libraries. A priori α was set to P < .05, and all data are presented as mean SD unless otherwise stated.
Figure 1 —
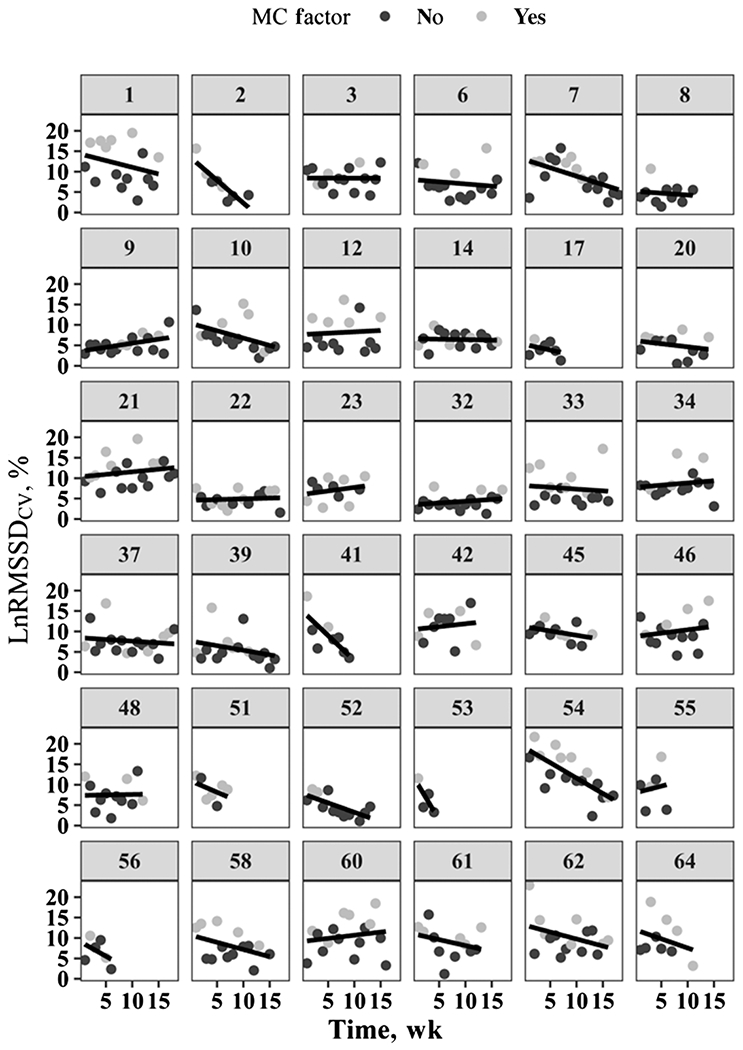
Individual mean LnRMSSDCV values per week across entire spring season for 36 rowing athletes. CV indicates coefficient of variation (measures the daily perturbations); LnRMSSD, natural logarithm of the root mean square of successive R-R differences (in milliseconds); MC, menstrual cycle.
Results
Across the 18 weeks of data collection, 467 observations were recorded (156 of which occurred during menses) from 36 rowing athletes. Their descriptive characteristics are included in Table 2, Table 3, and Figure 1. There were 181 observations (ie, LnRMSSDCV & MC status) not collected as the individuals did not complete enough HRV recordings throughout that respective week to be considered ecologically valid indications of weekly rolling HRV.4,5 Two individuals did not meet the minimum requirements to be included in the longitudinal models (ie, record the minimum HRV measurements for at least 3 weeks throughout the season) and/or did not record their MC status on any of the weeks within the HRV4Training application. One individual was removed from analyses as her method of contraception did not allow her to have a physical period, and without the use of more invasive measures, her MC status could not be confirmed. Fifteen women were taking triphasic OCs, wherein 3 different doses of exogenous estrogen and progesterone fluctuated approximately every 7 days with a withdrawal bleed evident during a placebo phase once per cycle, which was recorded as menses for this study.
Table 2.
Descriptive Characteristics of the 36 Female Rowers Who Completed the Study
| Variable | Mean (SD) |
|---|---|
| Age, y | 20 (1) |
| Height, in | 67.7 (2.0) |
| Weight, kg | 76.2 (10.8) |
| Body mass index, kg·m−2 | 25.6 (3.4) |
| Rowing experience, y | 4 (3) |
| 2000-m performance, s | 454 (15) |
Table 3.
HRV Data for the 36 Female Rowers
| Week | % of women on menses | LnRMSSDM, ms | LnRMSSDCV, % |
|---|---|---|---|
| 1 | 42 | 4.56 (0.6) | 9.09 (5.0) |
| 2 | 43 | 4.47 (0.5) | 9.05 (4.0) |
| 3 | 42 | 4.53 (0.5) | 8.51 (4.1) |
| 4 | 26 | 4.55 (0.5) | 7.68 (3.5) |
| 5 | 34 | 4.54 (0.5) | 8.95 (4.0) |
| 6 | 29 | 4.53 (0.5) | 7.84 (4.5) |
| 7 | 22 | 4.49 (0.5) | 6.99 (3.7) |
| 8 | 35 | 4.52 (0.5) | 7.48 (3.9) |
| 9 | 52 | 4.54 (0.6) | 8.25 (4.2) |
| 10 | 30 | 4.50 (0.6) | 8.47 (4.7) |
| 11 | 23 | 4.49 (0.6) | 7.73 (4.5) |
| 12 | 26 | 4.33 (0.5) | 7.21 (3.5) |
| 13 | 29 | 4.44 (0.7) | 7.28 (3.6) |
| 14 | 30 | 4.42 (0.6) | 8.03 (4.8) |
| 15 | 37 | 4.53 (0.6) | 7.87 (4.3) |
| 16 | 36 | 4.62 (0.7) | 6.16 (3.2) |
| 17 | 29 | 4.33 (0.7) | 7.36 (3.3) |
| 18 | 0 | 4.07 (0.2) | 8.66 (3.8) |
Abbreviations: CV, coefficient of variation (measures the daily perturbations); HRV, heart rate variability; LnRMSSD, Natural logarithm of the root mean square of successive R-R differences. Note: Data are presented as mean (SD).
As seen in Table 4, model 1 showed significant effects of time (Figure 2) and the presence of menses (Figure 3), while also considering LnRMSSDM. This suggests that rowers were adapting well to the training plan but showed increased LnRMSSDCV during menses. The covariates of meters rowed per day, sRPE, triphasic OC use, BMI, and years of rowing experience did not significantly influence LnRMSSDCV, as displayed in models 2 and 3. Simply put, the aforementioned covariates, including triphasic OC use, did not affect the magnitude of the slopes of time or menses and the overall model fits remain roughly equivalent. Interactions were tested for the 2 significant effects of time and menses but were not significant and are not reported in Table 4. Model 1 was found to be the best fit and was used for the final analyses.
Table 4.
Results From the 3 Longitudinal Mixed-Effects Models for the 36 Female Rowing Athletes Who Completed the Study
| Model 1 LnRMSSDCV |
Model 2 LnRMSSDCV |
Model 3 LnRMSSDCV |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor | Estimate | SE | P | Estimate | SE | P | Estimate | SE | P |
| (Intercept) | 7.50 | 0.41 | <.001 | 7.53 | 0.46 | <.001 | 7.65 | 0.51 | <.001 |
| Time | −0.11 | 0.04 | .004 | −0.08 | 0.04 | .066 | −0.12 | 0.03 | .001 |
| Presence of menses | 3.68 | 0.41 | <.001 | 3.41 | 0.53 | <.001 | 3.66 | 0.41 | <.001 |
| LnRMSSDM | −0.56 | 0.23 | .016 | −0.72 | 0.26 | .006 | −0.60 | 0.23 | .011 |
| Meters rowed per week | −0.04 | 0.18 | .805 | ||||||
| sRPE | −0.15 | 0.20 | .456 | ||||||
| Athlete taking birth control | −0.26 | 0.68 | .703 | ||||||
| BMI | −0.19 | 0.30 | .545 | ||||||
| Years of rowing experience | −0.36 | 0.34 | .294 | ||||||
| Random effects | |||||||||
| Residual | 8.45 | 8.08 | 8.55 | ||||||
| Intercept | subject | 2.77 | 2.65 | 2.75 | ||||||
| Time | subject | 0.01 | 0.01 | 0.00 | ||||||
| MC | subject | 2.65 | 4.54 | 2.72 | ||||||
| ICC | 0.25 | 0.25 | 0.24 | ||||||
| n | 36 | 31 | 36 | ||||||
| Observations | 467 | 344 | 451 | ||||||
| Marginal R2/conditional R2 | .255/.439 | .245/.432 | .259/.439 | ||||||
| AIC | 2433.403 | 1795.324 | 2351.092 | ||||||
| Log likelihood | −1208.701 | −887.662 | −1164.546 | ||||||
Abbreviations: AIC, Akaike information criterion; BMI, body mass index; CV, coefficient of variation (measures the daily perturbations); ICC, intraclass correlation coefficient; LnRMSSD, natural logarithm of the root mean square of successive R-R differences; MC, menstrual cycle (menses); sRPE, session rating of perceived exertion. α = .05.
Figure 2 —
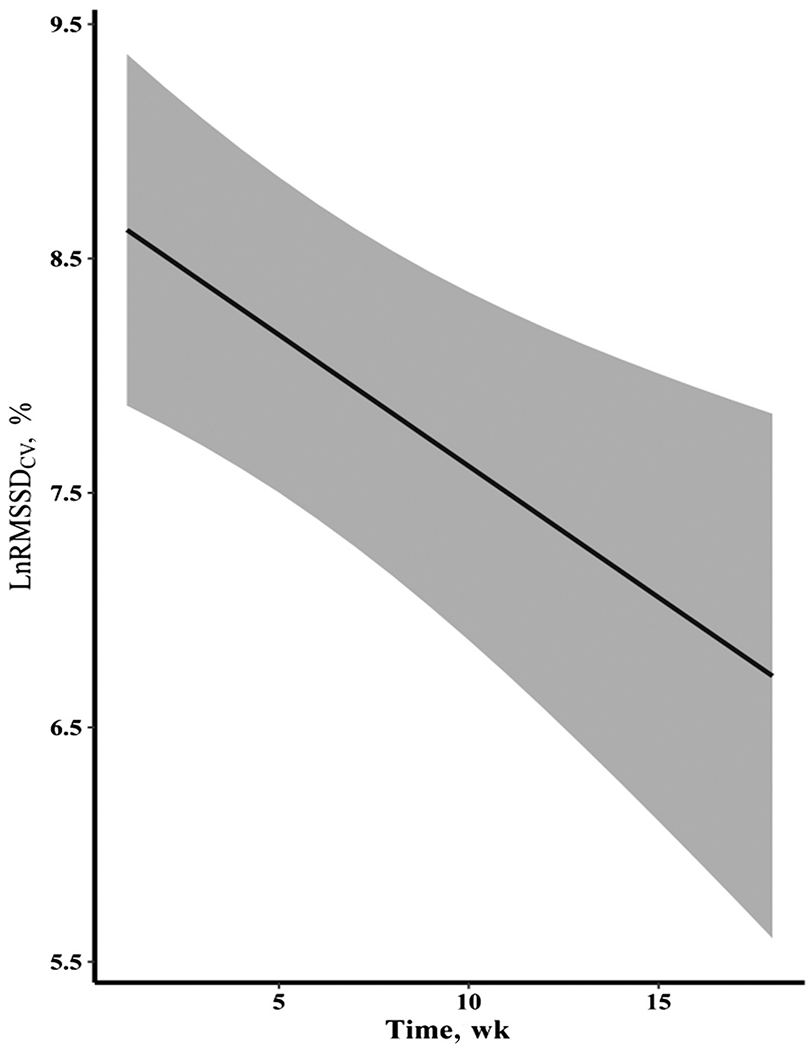
LnRMSSDCV across the entire spring rowing season, according to model 2. Data are presented as mean ± 95% confidence interval. CV indicates coefficient of variation (measures the daily perturbations); LnRMSSD, natural logarithm of the root mean square of successive R-R differences (in milliseconds).
Figure 3 —
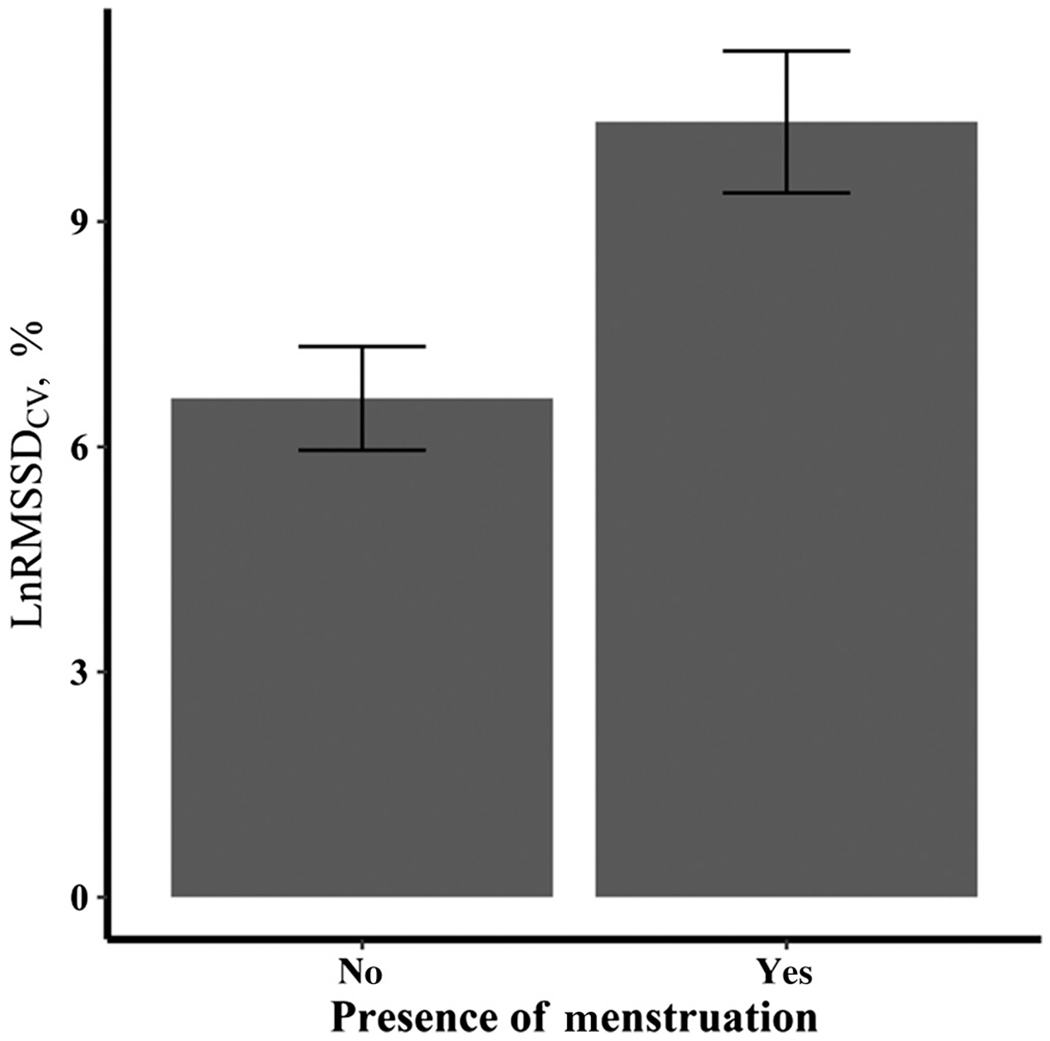
LnRMSSDCV in the presence and absence of menstruation, according to model 2. Data are presented as mean ± 95% confidence interval. CV indicates coefficient of variation (measures the daily perturbations); LnRMSSD, natural logarithm of the root mean square of successive R-R differences (in milliseconds).
Discussion
Our initial hypothesis that LnRMSSDCV values would be unaffected by the presence of menses, but would instead be attributable to training load, was not supported by the data in the present study. We found that LnRMSSDCV significantly decreased across the season, but the presence of menstruation seemed to significantly increase LnRMSSDCV values. Individuals with higher LnRMSSDCV values had significantly lower LnRMSSDM values, indicating a decrease in parasympathetic modulation during the week for which menses was recorded. These findings occurred even when controlling for training load as measured by meters rowed and sRPE. Other covariates such as BMI, triphasic OC use, and years of rowing experience did not significantly influence this relationship between LnRMSSDCV when considering LnRMSSDM.
Menses, or menstrual bleeding, occurs about 450 times throughout the female’s lifetime, and is a critical component of female physiology.10 This is the first study to longitudinally address the presence of MC on RMSSD across an entire sports season in this underrepresented population of female athletes. Studies utilizing a few data points or on a daily basis across only one full MC have demonstrated that women have the most stable vagal modulation during menses, but this was not the case in the current study.11,12,19 The discrepancies among the studies are not entirely clear but highlight the need to longitudinally investigate the cyclic hormonal changes, which influence adaptation to training in female athletes. Weekly averages of parasympathetically derived HRV indices have provided better methodological validity for detecting training adaptations versus single-day values.6 It could be that isolating the presence of menses into a week, versus identifying individual days for which the athletes recorded the presence of menses, overshadowed the true trends in parasympathetic modulation as noted by LnRMSSDCV. Furthermore, sympathetic activity is highest the week before menses, which could have been combined and minimized in the weeks where menses was one day, but by design was designated as menses.32 Nonetheless, the strong within-week effect that the presence of menses seems to have on LnRMSSDCV should not be overlooked.
A decrease in parasympathetic modulation throughout a MC, beginning with menses, was recently noted by Kokts-Porietis et al19 using ultra-short-term resting measures taken on the same HRV4Training application. Though Kokts-Porietis et al19 did not specifically investigate LnRMSSDCV, rather raw change values, they found that the daily fluctuations of HRV occurred in a nonlinear fashion. Flatt and Esco3 suggest that ultrashortened LnRMSSDCV values may be a more suitable marker than LnRMSSDM for reflecting acute adjustment of weekly training load in female team-sport athletes. These findings stress the importance of investigating day-to-day fluctuations in cardiac-autonomic activity (ie, LnRMSSDCV) as opposed to singular daily values.9,19
Distraction, training monotony, and strain are often cited as associated symptoms of menses in elite female athletes, especially before competitions.14 Regardless, relatively low absenteeism due to menses may be explained by internal and external pressures to perform, particularly in scholarship athletes.14 It is not entirely clear, then, why sRPE was not associated with LnRMSSDCV in the current study. This contradicts previous findings showing that sRPE is a sensitive indicator of changes in external training load, particularly when overreaching was the training goal.33 To note, the original research that provided data for the present study was observational and did not implement a training intervention. The significant HRV findings during menses despite the lack of sRPE significance could indicate that the heightened arousal and/or greater attentional focus during practice and competition may override the distraction from the symptoms, which may occur during menses in this population.14 As such, some literature has noted a reduced awareness of MC on daily life in female athletes as compared to either practice or competition.14 Another study found that the weeks with higher perceived training load resulted in lower LnRMSSDM and higher LnRMSSDCV, respectively, when compared with those with lower perceived training loads in a group of female soccer players.34 It could be that the use of sRPE or meters rowed per day was not the most appropriate method of measuring training load in this unique population. However, previous studies employing more traditional measures of performance (eg, VO2max) are not the ideal corollary to the present study because HRV was not evaluated.35,36
Limitations and Future Research
It is currently unclear if endogenous and exogenous hormones have the same mechanism of action, and thus, may have different physiological effects within the same physiological system. Two studies have noted no significant differences in HRV variables between women taking OCs and healthy controls, but these results are difficult to interpret given the overall paucity of this research.37,38 Vaiksaar et al36 found no effect of OC use on endurance performance in eumenorrheic rowers, supporting our supposition that OCs did not confound the present results. The inclusion of women taking OCs in the current study provides more data in women to fill this gap in exercise literature and a potential basis for examining OC effects on HRV through future empirical studies.
Menstrual cycle status was self-reported in this study to avoid interrupting the athletes’ daily regimen, but additional information regarding their normal MC was not collected. Physiological markers of menses (eg, core temperature, blood hormone levels) are the most reliable indicators; however, invasive measures are often limited by time, resources, and cost. Reporting menses as “yes” or “no” using a smartphone application is a feasible alternative for coaches to gain insight that can be used to adjust training plans. Future research should assess the agreement between physiological markers and self-reported measures of menses in conjunction with daily HRV monitoring. Furthermore, researchers did not implicitly induce hemostatic perturbations to elicit a particular response. Thus, it is possible that other measures of performance could have been more appropriate than rank or meters rowed as implicated in this study. Given that both sRPE and meters rowed were nonsignificant predictors of LnRMSSDCV, the potential impact of other performance measures is unclear.
Practical Applications
These data could impart essential knowledge of how an inevitable physiological cycle will influence training adaptations and/or performance of many female athletes throughout an entire season. By tracking patterns of vagal modulation combined with the presence of an apparent and noninvasive sign of menstruation such as menses, a female athlete can be empowered with the awareness of her specific adaptations and how this might affect her performance and/or training throughout the season.
Conclusion
Large perturbations of LnRMSSDCV occurred during the weeks which these female athletes indicated the presence of menses, despite a decrease in weekly rolling LnRMSSDM. These results were observed independent of BC use, sRPE, meters rowed per day, BMI, and years of rowing experience, and could potentially suggest a maladaptation to the training stimulus that is specific to the presence of menses observed in eumenorrheic female athletes.
Acknowledgments
We would like to thank the University of Alabama National Collegiate Athletic Association Division I Women’s Rowing Team athletes, coaches, and training staff for allowing the success of this longitudinal study. Your time and dedication to this science and sport, which is the first of any female collegiate rowing team of your caliber, will undoubtedly help to inspire other young scientists to narrow the gap in female athletics and research. Thank you to Ward Dobbs (University of Alabama), Zack Cicone (University of Alabama), and Andrea Frankenstein (University of Illinois Chicago) for your unparalleled guidance and mentorship throughout this study and beyond. Thank you to Dr Marco Altini of HRV4Training for walking us through your smartphone application and providing almost instant insight at any hour of the morning.
Contributor Information
Sara R. Sherman, Dept of Kinesiology, University of Alabama, Tuscaloosa, AL, USA; Integrative Physiology Laboratory, College of Applied Health Sciences, University of Illinois at Chicago, Chicago, IL, USA
Clifton J. Holmes, Dept of Kinesiology, University of Alabama, Tuscaloosa, AL, USA; Dept of Physical Therapy, Washington University School of Medicine, St Louis, MO, USA
Alexander P. Demos, Dept of Psychology, University of Illinois at Chicago, Chicago, IL, USA
Tori Stone, Dept of Kinesiology, University of Alabama, Tuscaloosa, AL, USA; Dept of Obstetrics, Gynecology and Reproductive Sciences, Yale School of Medicine, New Haven, CT, USA.
Bjoern Hornikel, Dept of Kinesiology, University of Alabama, Tuscaloosa, AL, USA.
Hayley V. MacDonald, Dept of Kinesiology, University of Alabama, Tuscaloosa, AL, USA
Michael V. Fedewa, Dept of Kinesiology, University of Alabama, Tuscaloosa, AL, USA
Michael R. Esco, Dept of Kinesiology, University of Alabama, Tuscaloosa, AL, USA
References
- 1.Buchheit M Monitoring training status with HR measures: do all roads lead to Rome? Front Physiol. 2014;5:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esco MR, Flatt AA. Ultra-short-term heart rate variability indexes at rest and post-exercise in athletes: evaluating the agreement with accepted recommendations. J Sport Sci Med. 2014;13(3):535–541. [PMC free article] [PubMed] [Google Scholar]
- 3.Flatt A, Esco M. Smartphone-derived heart-rate variability and training load in a women’s soccer team. Int J Sports Physiol Perform. 2015;10(8):994–1000. [DOI] [PubMed] [Google Scholar]
- 4.Pereira L, Flatt A, Ramirez-Campillo R, Loturco I, Nakamura F. Assessing shortened field-based heart rate variability data acquisition in team-sport athletes. Int J Sports Physiol Perform. 2016;11(2):154–158. doi: 10.1123/ijspp.2015-0038 [DOI] [PubMed] [Google Scholar]
- 5.Plews D, Laursen P, Kilding A, Buchheit M. Evaluating training adaptation with heart-rate measures: a methodological comparison. Int J Sports Physiol Perform. 2013;8(6):688–691. doi: 10.1123/ijspp.8.6.688 [DOI] [PubMed] [Google Scholar]
- 6.Plews D, Laursen P, Stanley J, Kilding A, Buchheit M. Training adaptation and heart rate variability in elite endurance athletes: opening the door to effective monitoring. Sports Med. 2013;43(9): 773–781. doi: 10.1007/s40279-013-0071-8 [DOI] [PubMed] [Google Scholar]
- 7.Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Task force of the European society of cardiology and the North American society of pacing and electrophysiology. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 8.Flatt AA, Esco MR, Allen JR, et al. Heart rate variability and training load among national collegiate athletic association division 1 college football players throughout spring camp. J Strength Cond Res. 2018; 32(11):3127–3134. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura FY, Pereira LA, Rabelo FN, et al. Monitoring weekly heart rate variability in futsal players during the preseason: the importance of maintaining high vagal activity. J Sports Sci. 2016; 34(24):2262–2268. doi: 10.1080/02640414.2016.1186282 [DOI] [PubMed] [Google Scholar]
- 10.Mihm M, Gangooly S, Muttukrishna S. The normal menstrual cycle in women. Anim Reprod Sci. 2011;124(3):229–236. doi: 10.1016/j.anireprosci.2010.08.030 [DOI] [PubMed] [Google Scholar]
- 11.McKinley PS, King AR, Shapiro PA, et al. The impact of menstrual cycle phase on cardiac autonomic regulation. Psychophysiology. 2009;46(4):904–911. doi: 10.1111/j.1469-8986.2009.00811.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tenan MS, Brothers RM, Tweedell AJ, Hackney AC, Griffin L. Changes in resting heart rate variability across the menstrual cycle. Psychophysiology. 2014;51(10):996–1004. doi: 10.1111/psyp.12250 [DOI] [PubMed] [Google Scholar]
- 13.Forsyth JJ, Reilly T. The effect of menstrual cycle on 2000-m rowing ergometry performance. Eur J Sport Sci. 2008;8(6):351–357. doi: 10.1080/17461390802308644 [DOI] [Google Scholar]
- 14.Findlay RJ, Macrae EHR, Whyte IY, Easton C, Forrest Nee Whyte LJ. How the menstrual cycle and menstruation affect sporting performance: experiences and perceptions of elite female rugby players. Br J Sports Med. 2020;54(18):1108–1113. [DOI] [PubMed] [Google Scholar]
- 15.Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13(12):859–866. doi: 10.1038/nrn3360 [DOI] [PubMed] [Google Scholar]
- 16.Charkoudian N, Stachenfeld NS. Reproductive hormone influences on thermoregulation in women. Compr Physiol. 2014;4(2):793–804. [DOI] [PubMed] [Google Scholar]
- 17.Gorman AJ, Proppe DW. Mechanisms producing tachycardia in conscious baboons during environmental heat stress. J Appl Physiol. 1984; 56(2):441–446. doi: 10.1152/jappl.1984.56.2.441 [DOI] [PubMed] [Google Scholar]
- 18.Stone T, Earley RL, Burnash SG, Wingo JE. Menstrual cycle effects on cardiovascular drift and maximal oxygen uptake during exercise heat stress. Eur J Appl Physiol. 2021;121(2):561–572. doi: 10.1007/s00421-020-04542-y [DOI] [PubMed] [Google Scholar]
- 19.Kokts-Porietis RL, Minichiello NR, Doyle-Baker PK. The effect of the menstrual cycle on daily measures of heart rate variability in athletic women. J Psychophysiol. 2020;34(1):60–68. doi: 10.1027/0269-8803/a000237 [DOI] [Google Scholar]
- 20.Sherman SR, Holmes CJ, Hornikel B, MacDonald HV, Fedewa MV, Esco MR. Heart-rate variability recording time and performance in collegiate female rowers. Int J Sports Physiol Perform. 2021;16(4): 550–556. doi: 10.1123/ijspp.2019-0587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mäestu J, Jürimäe J, Jürimäe T. Monitoring of performance and training in rowing. Sports Med. 2005;35(7):597–617. [DOI] [PubMed] [Google Scholar]
- 22.Hagerman FC. Applied physiology of rowing. Sports Med. 1984; 1(4):303–326. doi: 10.2165/00007256-198401040-00005 [DOI] [PubMed] [Google Scholar]
- 23.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. doi: 10.1249/00005768-198205000-00012 [DOI] [PubMed] [Google Scholar]
- 24.Foster C, Florhaug JA, Franklin J, et al. A new approach to monitoring exercise training. J Strength Cond Res. 2001;15(1):109–115. [PubMed] [Google Scholar]
- 25.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 26.Bates D, Maechler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48. [Google Scholar]
- 27.Wickham H ggplot2: Elegant Graphics for Data Analysis [computer program]. New York, NY: Springer-Verlag; 2016. [Google Scholar]
- 28.Fox J, Weisberg S. An R Companion to Applied Regression [computer program]. Thousand Oaks, CA: Sage; 2019. [Google Scholar]
- 29.Lüdecke D sjPlot: Data Visualization for Statistics in Social Science [computer program]. R-package version 2.8.9; 2020. [Google Scholar]
- 30.Kuznetsova A, Brockhoff PB, Christensen RH. lmerTest package: tests in linear mixed effects models. J Stat Softw. 2017;82(13):1–26. [Google Scholar]
- 31.Singmann H, Bolker B, Westfall J, Aust F, Ben-Shachar M. afex: Analysis of Factorial Experiments [computer program]. R-package version 0.27-2; 2020. [Google Scholar]
- 32.Brar TK, Singh KD, Kumar A. Effect of different phases of menstrual cycle on heart rate variability (HRV). J Clin Diagn Res. 2015;9(10): CC01–CC04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freitas VH, Nakamura FY, Miloski B, Samulski D, Bara-Filho MG. Sensitivity of physiological and psychological markers to training load intensification in volleyball players. J Sport Sci Med. 2014; 13(3):571–579. [PMC free article] [PubMed] [Google Scholar]
- 34.Flatt AA, Esco MR, Nakamura FY, Plews DJ. Interpreting daily heart rate variability changes in collegiate female soccer players. J Sports Med Phys Fitness. 2017;57(6):907–915. doi: 10.23736/S0022-4707.16.06322-2 [DOI] [PubMed] [Google Scholar]
- 35.Lebrun CM, McKenzie DC, Prior JC, Taunton JE. Effects of menstrual cycle phase on athletic performance. Med Sci Sports Exerc. 1995;27(3):437–444. doi: 10.1249/00005768-199503000-00022 [DOI] [PubMed] [Google Scholar]
- 36.Vaiksaar S, Jürimäe J, Mäestu J, et al. No effect of menstrual cycle phase and oral contraceptive use on endurance performance in rowers. J Strength Cond Res. 2011;25(6):1571–1578. doi: 10.1519/JSC.0b013e3181df7fd2 [DOI] [PubMed] [Google Scholar]
- 37.Wilczak A, Marciniak K, Kłapciński M, Rydlewska A, Danel D, Jankowska EA. Relations between combined oral contraceptive therapy and indices of autonomic balance (baroreflex sensitivity and heart rate variability) in young healthy women. Ginekol Pol. 2013;84(11):915–921. doi: 10.17772/gp/1660 [DOI] [PubMed] [Google Scholar]
- 38.Nisenbaum MG, de Melo NR, Giribela CR, et al. Effects of a contraceptive containing drospirenone and ethinyl estradiol on blood pressure and autonomic tone: a prospective controlled clinical trial. Eur J Obstet Gynecol Reprod Biol. 2014;175:62–66. doi: 10.1016/j.ejogrb.2014.01.006 [DOI] [PubMed] [Google Scholar]


