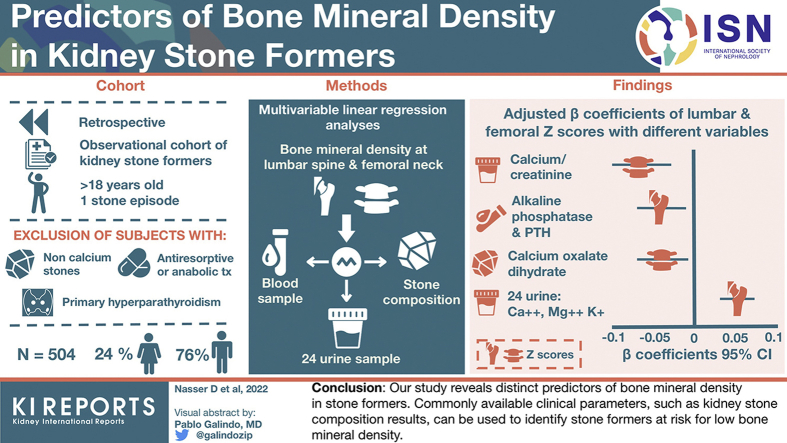
Abstract
Introduction
Nephrolithiasis is associated with an increased fracture risk, but predictors of bone mineral density (BMD) in stone formers (SFs) remain poorly defined.
Methods
We conducted a retrospective analysis in the Bern Kidney Stone Registry (BKSR), an observational cohort of kidney SFs. Inclusion criteria were age ≥18 years and ≥1 past stone episode. Participants with non–calcium (Ca)-containing kidney stones, a history of primary hyperparathyroidism or antiresorptive or anabolic bone treatment were excluded. Multivariable linear regression analyses were used to assess the association of blood and 24-hours urine parameters and stone composition with BMD at the lumbar spine and femoral neck.
Results
In the analysis, 504 participants were included, mean age was 46 years, and 76% were male. In multivariable analyses, fasting (β: −0.031; P = 0.042), postload (β: −0.059; P = 0.0028) and Δ postload − fasting (β: −0.053; P = 0.0029) urine Ca-to-creatinine ratios after 1 week of a sodium- and Ca- restricted diet and Ca oxalate dihydrate stone content (β: −0.042; P = 0.011) were negatively associated with z scores at the lumbar spine. At the femoral neck, alkaline phosphatase (β: −0.035; P = 0.0034) and parathyroid hormone (PTH) (β: −0.035; P = 0.0026) were negatively associated with z scores, whereas 24-hours urine Ca (β: 0.033; P = 0.0085), magnesium (β: 0.043; P = 3.5 × 10−4), and potassium (β: 0.032; P = 0.012) correlated positively with z scores at the femoral neck.
Conclusion
Our study reveals distinct predictors of BMD in SFs. Commonly available clinical parameters, such as kidney stone composition results, can be used to identify SFs at risk for low BMD.
Keywords: bone mineral density, calcium oxalate, femoral neck, kidney stones, lumbar spine, urine calcium
Graphical abstract
Nephrolithiasis is a global health care problem, and both incidence and prevalence are on the rise.1 Previous cross-sectional studies demonstrated a high prevalence of low BMD in kidney SFs as a manifestation of this systemic disease.2, 3, 4 In support of these studies, a population-based retrospective cohort study with a mean and maximum follow-up of 19 and 30 years, respectively, revealed a nearly 4-fold increased risk of vertebral fractures in SFs.5 Fractures affected both sexes and were more likely to affect cancellous than cortical bone. The incidence of fractures increased with time, and cumulative incidence of fractures was 45% in women and 30% in men at 30 years of follow-up. An increased fracture risk associated with a history of kidney stones was also observed in other population-based studies.6, 7, 8 In one of these studies, median time from urolithiasis diagnosis to fracture was 10 years.6 Risk to develop low BMD in SFs seems to depend on the activity of the underlying kidney stone disease, being higher in recurrent SFs compared with individuals with a history of a single stone event.9 Hypercalciuria has long been considered a metabolic trait associated with BMD in SFs, but previous studies yielded conflicting results, and reduced BMD was also reported in SFs without hypercalciuria.2,10, 11, 12, 13, 14, 15, 16
Bone mineral loss in SFs affects all skeletal sites. A comprehensive analysis of previously published BMD studies in SFs showed that 40% of patients exhibit reduced BMD at the vertebral spine, 31% at the femoral neck, and 65% at the radius.2 Bone histomorphometry studies suggested reduced bone formation as the primary defect, but the underlying mechanisms and the connection of bone defects to kidney stones remain incompletely understood.17, 18, 19, 20, 21, 22, 23 On the basis of these findings and the fact that bone fractures are a cause of significant morbidity and mortality in affected patients, expert panels strongly recommend BMD measurements as surrogates for fracture risk in SFs.4 However, BMD measurements are not routinely performed during metabolic work-up of SF, likely because of the high prevalence of nephrolithiasis, the lack of well-defined screening algorithms, and the cost associated with BMD measurements.4 Hence, a better understanding of nephrolithiasis-related risk factors associated with low BMD in SFs is clearly needed. Knowledge of these factors would allow a personalized diagnostic work-up and facilitate treatment decisions in SFs.
To address this issue, we conducted a retrospective analysis in the BKSR, a large Swiss cohort of SF with detailed phenotype, including a low Ca and sodium dietary intervention and bone density measurement by dual-energy X-ray absorptiometry (DEXA) during work-up. On the basis of current pathophysiological concepts of bone homeostasis and kidney stone formation, we hypothesized that parameters of mineral metabolism and stone composition would be associated with BMD in SF.
Methods
Study Population
The BKSR is a single-center, observational cohort of SF at the Division of Nephrology and Hypertension, Bern University Hospital, Bern, Switzerland. The BKSR adheres to the Declaration of Helsinki and was approved by the ethical committee of the Kanton of Bern (approval #BE 95/06). Inclusion criteria for the BKSR are (i) written informed consent, (ii) age ≥18 years, and (iii) at least 1 past stone episode.
For this study, 607 of 941 BKSR participants who had a bone density measurement by DEXA available were selected. All 941 BKSR participants who underwent metabolic work-up for kidney stone disease were scheduled for a bone density measurement by DEXA. The 334 participants without bone density measurement available are BKSR participants who either actively declined a DEXA measurements or missed the scheduled DEXA appointment during work-up. From this selection of 607 BKSR participants with available DEXA, we excluded participants on the basis of the following criteria: history of kidney stones containing uric acid (n = 37), cystine (n = 10), or struvite (n = 12); primary hyperparathyroidism or history of parathyroidectomy (n = 15); total plasma Ca >2.5 mmol/l with a PTH >65 pg/ml (n = 23); history of anorexia nervosa or bulimia (n = 4); active malignant diseases (n = 12); history of solid organ transplantation (n = 2); current or past administration of antiresorptive or anabolic bone treatment: bisphosphonates (n = 9), denosumab (n = 2), teriparatide (n = 1). We included 504 BKSR participants in the final analysis.
Data Collection and Measurements
All patients underwent a 3-visit mineral metabolism work-up, including two 24-hours urines on random outpatient diet and one 24-hours urine after 1 week of instructed low Ca and sodium diet according to a protocol first established by Pak et al.24, 25, 26 In addition, in the morning-after collection of the 24-hours urine on a diet restricted in Ca and sodium, a 2 hours fasting urine collection and a 4 hours urine collection after an oral load of 1 g Ca carbonate were conducted to measure fasting, postload, and ΔCa: postload − fasting urine Ca-to-creatinine ratios.
A blood draw was performed during the day after the second 24-hours urine collection. All blood parameters were determined from the same blood draw. All other urine and blood analyses were performed at the Central Laboratory of the Bern University Hospital as single measurements immediately after sampling. Assay characteristics for the measurements of PTH, 25(OH) Vitamin D3, and 1,25(OH)2 Vitamin D3 were described previously.26, 27, 28 The glomerular filtration rate was estimated by the creatinine-based Chronic Kidney Disease-Epidemiology Collaboration 2009 equation.29 Urine creatinine excretion was used as the criterion for completeness of 24-hours urine collections.30,31 Percentiles 2.5 and 97.5 of the 24-hours creatinine excretion were calculated for each 24-hours urine collection using a linear regression model recently published.32 Completeness of 24-hours urine collections was assumed for each subject if the total 24-hours creatinine excretion was within percentiles 2.5 and 97.5. The mean value of both 24-hours urine collections on random outpatient diet was used for the calculation of 24-hours urinary excretions.
Corrected plasma Ca was calculated by the following formula: Cacorr (mmol/l) = plasma Ca measured (mmol/l) + 0.025 × (40 – plasma albumin (g/l) in case of a plasma albumin ≤40 g/l. Diabetes was defined as reported, treated, fasting glycemia ≥7 mmol/l (≥126.13 mg/dl), or random glycemia ≥11.1 mmol/l (≥200 mg/dl). Hypertension was defined as reported, treated, a mean systolic blood pressure ≥140 mmHg, or a mean diastolic blood pressure ≥90 mmHg. Osteodensitometry was performed at the Department of Osteoporosis at the Bern University Hospital of Bern at the time of the metabolic work-up (maximally 3 months apart from the 24-h urine collections) by DEXA (Hologic QDR 4500A, Hologic, Bedford, MA) at the lumbar spine and the femoral neck, as described.33,34 Kidney stone analysis was performed at the Central Laboratory of the Bern University Hospital by Fourier transform infrared spectroscopy.35
Statistical Analysis
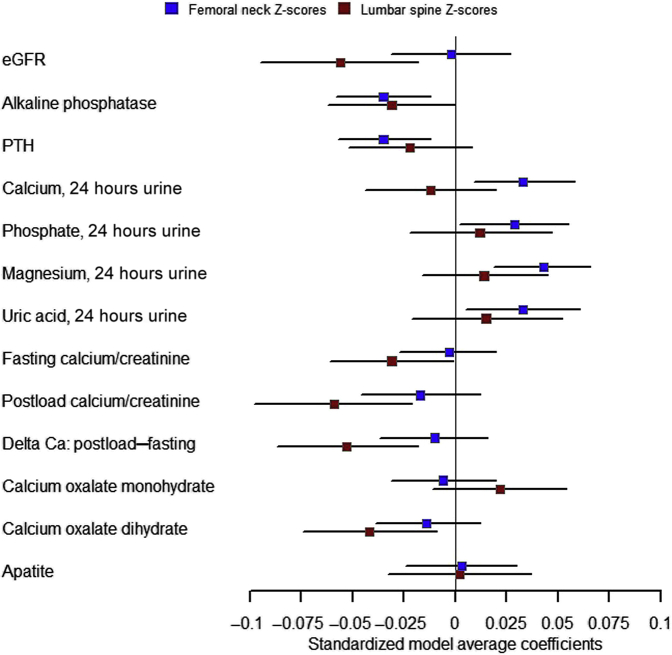
Statistical analyses were conducted using the R software, version 4.0.2.36 All statistical tests were 2-sided, and a P < 0.05 was considered statistically significant. Unadjusted and adjusted linear regression analyses of clinical characteristics, kidney stone history, kidney stone composition and laboratory parameters as predictor variables with appropriately transformed z scores at the lumbar spine and at the femoral neck as outcome variables were conducted. Adjusted analyses considered sex, age, body mass index (BMI), estimated glomerular filtration rate (eGFR), and tobacco consumption in pack years as co-variables and potential confounders. All continuous predictor variables were scaled by being converted to z scores. Models were validated for homogeneity of variance by plotting residuals versus fitted values, for normality of residuals by histograms of the residuals and by quantile-quantile plots, for homoscedasticity by plotting residuals against each explanatory variable used in the final models, and for highly influential observations by plotting the Cook’s distance for each data point. Visualization of key findings of the association analysis in Figure 1 was done using the R software package forestplot (Max Gordon and Thomas Lumley, 2021. forestplot: Advanced Forest Plot Using “grid” Graphics. R package version 2.0.1. https://CRAN.R-project.org/package=forestplot).
Figure 1.
Visualization of key findings from the association analysis. Explanatory variables are indicated on the left side. Boxes indicate adjusted β-coefficients; horizontal lines indicate the corresponding 95% CIs. For abbreviations and units, please refer to Tables 3 and 4. Ca, calcium; eGFR, estimated glomerular filtration rate; PTH, parathyroid hormone.
In a sensitivity analysis, unadjusted and adjusted models were further assessed after exclusion of further potential confounders: intake of Ca and/or vitamin D supplements (n = 26), thiazide (n = 39) or loop diuretics (n = 5), alkali supplements (n = 10).
Results
Characteristics of Study Population
A total of 504 BKSR participants met the predefined eligibility criteria and were included in this study (for details see Materials and Methods section). Clinical characteristics of study participants, including kidney stone history, kidney stone composition and BMD measurements are shown in Table 1. Age of study participants ranged from 18 to 81 years, mean age ± SD was 46.2 ± 13.5 years. The majority of participants were male (76.4%, n = 385); mean BMI was 26.5 ± 4.8 kg/m2. Of the 23.6% (n = 119) female participants, 42.9% (n = 51) were self-reported postmenopausal. Most participants were recurrent SF (84.2%, n = 422), that is, had >1 past stone episode. A kidney stone analysis was available in 79.2% (n = 397) of participants: 95.7% of stones (n = 380) contained Ca oxalate; 32.5% (n = 129) of stones contained Ca phosphate in the form of apatite (31.2%, n = 124) or brushite (2.5%, n = 10). Mean lumbar spine z score of the cohort was −0.19 ± 1.36, and mean femoral neck z score was 0.11 ± 0.95. Blood and urine parameters of participants are shown in Table 2.
Table 1.
Characteristics of study population
| Characteristics | N | % or mean ± SD |
|---|---|---|
| Age, yr | 504 | 46.2 ± 13.5 |
| Male, % | 385 | 76.4 |
| Postmenopausal status, % | 51 | 42.9 |
| BMI, kg/m2 | 504 | 26.5 ± 4.8 |
| Diabetes, % | 39 | 7.7 |
| Hypertension, % | 252 | 50.0 |
| Current or former smoker | 248 | 53.9 |
| Kidney stone history | ||
| Age at first stone event, yr | 476 | 35.3 ± 12.8 |
| Recurrent stone former, % | 422 | 84.2 |
| Kidney stone analysis available | 397 | 79.2 |
| Kidney stone composition (containing) | ||
| Calcium, % | 397 | 100 |
| Calcium oxalate, % | 380 | 95.7 |
| Calcium oxalate monohydrate, % | 314 | 82.8 |
| Calcium oxalate dihydrate, % | 289 | 76.3 |
| Apatite, % | 124 | 31.2 |
| Brushite, % | 10 | 2.5 |
| Bone mineral density | ||
| Lumbar spine, z score | 498 | −0.19 ± 1.36 |
| Femoral neck, z score | 500 | 0.11 ± 0.95 |
BMI, body mass index.
Categorical variables are reported as number (N) and percentage (%) of the 504 study participants. For continuous variables, the available number (N) is indicated and they are described as mean ± SD.
Table 2.
Blood and urine parameters of study population
| Parameters | N | % or mean ± SD |
|---|---|---|
| Blood | ||
| eGFR, ml/min per 1.73 m2 BSA | 494 | 95.4 ± 19.1 |
| Ca total, mmol/l | 494 | 2.3 ± 0.10 |
| Phosphate, mmol/l | 492 | 1.0 ± 0.17 |
| Magnesium, mmol/l | 489 | 0.83 ± 0.064 |
| Uric acid, μmol/l | 491 | 329 ± 200 |
| Bicarbonate, mmol/l | 481 | 26 ± 2.1 |
| Glucose, mmol/l | 482 | 5.2 ± 1.1 |
| Alkaline phosphatase, IU/ml | 492 | 67 ± 18 |
| PTH, pg/ml | 491 | 42 ± 18 |
| 25(OH) Vitamin D3, ng/ml | 319 | 44 ± 27 |
| 1,25(OH)2 Vitamin D3, pg/ml | 487 | 102 ± 42 |
| Urine | ||
| Sodium, mmol/24 h | 475 | 192 ± 75 |
| Sodium, mmol/24 h – restricted diet | 415 | 94 ± 58 |
| Potassium, mmol/24 h | 474 | 65 ± 23 |
| Ca, mmol/24 h | 473 | 6.4 ± 3.1 |
| Ca, mmol/24 h – restricted diet | 418 | 3.6 ± 2.2 |
| Phosphate, mmol/24 h | 473 | 30 ± 10 |
| Magnesium, mmol/24 h | 473 | 4.3 ± 1.6 |
| Uric acid, μmol/24 h | 473 | 3392 ± 1080 |
| Urea, mmol/24 h | 473 | 409 ± 129 |
| Citrate, mmol/24 h | 471 | 2.8 ± 1.6 |
| Oxalate, mmol/24 h | 471 | 0.51 ± 0.65 |
| Sulfate, mmol/24 h | 463 | 24 ± 22 |
| NGIA, mmol/24 h | 434 | 33 ± 30 |
| pH, 24 h | 463 | 6.0 ± 0.72 |
| Fasting Ca/creatinine, mmol/mmol | 455 | 0.26 ± 0.3 |
| Postload Ca/creatinine, mmol/mmol | 458 | 0.80 ± 0.58 |
| ΔCa: postload − fasting, mmol/mmol | 397 | 0.21 ± 0.17 |
BSA, body surface area; Ca, calcium; eGFR, estimated glomerular filtration rate; NGIA, net gastrointestinal absorption; PTH, parathyroid hormone.
The number (N) of available participants and mean ± SD are indicated for each parameter. Unless indicated, 24-h urine parameters were measured in the 24-h urine collections under random outpatient diet. Restricted diet corresponds to 24-h urine collection performed after 1 wk of instructed low calcium and sodium diet.
Univariable and Adjusted Association Analyses With z Score at the Lumbar Spine as Outcome Variable
In a first step, we performed univariable linear regression analyses with z score at the lumbar spine as outcome variable and clinical characteristics, kidney stone history, kidney stone analysis, and laboratory parameters as predictor variables (Table 3). Established factors such as age (β: 0.096; P = 9.5 × 10−11), BMI (β: 0.090; P = 1.7 × 10−9), eGFR (β: −0.095; P = 2.4 × 10−10), sex (women vs. men, β: 0.207; P = 3.2 × 10−9), and tobacco consumption (β: −0.035; P = 0.034) were significantly associated with z score at the lumbar spine in unadjusted analyses. Unadjusted, 24-hours urine Ca excretion under both random (β: −0.054; P = 5.4 × 10−4) and restricted diet (β: −0.059; P = 5.2 × 10−4) was negatively associated with z scores at the lumbar spine. In addition, fasting (β: −0.055; P = 3.9 × 10−4), postload (β: −0.082; P = 5.6 × 10−8), and ΔCa: postload − fasting (β: −0.074; P = 3.6 × 10−6) urine Ca-to-creatinine ratios were all negatively associated with lumbar spine z scores. There was a negative association of 24-hours urine uric acid (β: −0.030; P = 0.030) and 24-hours urine pH (β: −0.042; P = 0.0069) with lumbar spine z scores. Other parameters of acid-base homeostasis, including plasma bicarbonate, 24-hours urine citrate, and sulfate excretion were not significantly associated with z scores at the lumbar spine. 1,25(OH)2 Vitamin D3 (β: −0.037; P = 0.015) was negatively associated with z scores at the lumbar spine, but we observed no significant association of 25(OH) Vitamin D3 or PTH with z scores at the lumbar spine. Furthermore, the analysis revealed a strong negative association of Ca oxalate dihydrate stone content (β: −0.057; P = 6.3 × 10−4) with z scores at the lumbar spine. In contrast, Ca oxalate monohydrate, apatite, or brushite content in the stone analysis were not significantly associated with z scores at the lumbar spine.
Table 3.
Association of laboratory and kidney stone history parameters as main explanatory variables with z scores at the lumbar spine as outcome variable
| Explanatory variables | Unadjusted model |
Multivariable model |
||||
|---|---|---|---|---|---|---|
| N | β;95% CI | P-value | N | β;95% CI | P-value | |
| Blood | ||||||
| eGFR, ml/min per 1.73 m2 BSA | 489 | −0.095;−0.123 to −0.066 | 2.4E-10 | 387 | −0.056;−0.095 to −0.018 | 0.0041 |
| Calcium total, mmol/l | 489 | −0.032;−0.062 to −0.003 | 0.033 | 387 | −0.025;−0.056 to 0.005 | 0.11 |
| Phosphate, mmol/l | 487 | 0.005;−0.025 to 0.035 | 0.74 | 387 | −0.009;−0.039 to 0.02 | 0.54 |
| Magnesium, mmol/l | 484 | −0.03;−0.059 to 0 | 0.052 | 384 | −0.027;−0.056 to 0.001 | 0.060 |
| Uric acid, μmol/l | 486 | 0.019;−0.011 to 0.048 | 0.22 | 384 | 0.015;−0.012 to 0.043 | 0.27 |
| Bicarbonate, mmol/l | 477 | −0.027;−0.057 to 0.002 | 0.072 | 378 | 0.004;−0.026 to 0.035 | 0.78 |
| Glucose, mmol/l | 477 | 0.023;−0.008 to 0.053 | 0.14 | 379 | 0.001;−0.028 to 0.03 | 0.94 |
| Alkaline phosphatase, IU/ml | 487 | −0.023;−0.053 to 0.007 | 0.13 | 385 | −0.031;−0.062 to 0 | 0.053 |
| PTH, pg/ml | 486 | 0.002;−0.027 to 0.032 | 0.88 | 383 | −0.022;−0.052 to 0.008 | 0.15 |
| 25-OH-Vitamin D3, ng/ml | 314 | 0.021;−0.018 to 0.059 | 0.29 | 235 | 0.034;−0.005 to 0.072 | 0.088 |
| 1,25-OH-Vitamin D3, pg/ml | 482 | −0.037;−0.067 to −0.007 | 0.015 | 380 | 0.001;−0.028 to 0.031 | 0.93 |
| Urine | ||||||
| Sodium, mmol/24 h | 471 | −0.008;−0.039 to 0.022 | 0.60 | 371 | 0.017;−0.017 to 0.051 | 0.33 |
| Sodium, mmol/24 h – restricted diet | 411 | −0.016;−0.05 to 0.017 | 0.34 | 326 | 0.011;−0.022 to 0.044 | 0.52 |
| Potassium, mmol/24 h | 470 | 0.013;−0.017 to 0.043 | 0.40 | 370 | 0.009;−0.023 to 0.042 | 0.58 |
| Ca, mmol/24 h | 469 | −0.054;−0.084 to −0.023 | 5.4E-04 | 371 | −0.012;−0.044 to 0.02 | 0.46 |
| Ca, mmol/24 h – restricted diet | 414 | −0.059;−0.092 to −0.026 | 5.2E-04 | 328 | −0.02;−0.054 to 0.015 | 0.26 |
| Phosphate, mmol/24 h | 469 | −0.028;−0.058 to 0.003 | 0.075 | 371 | 0.012;−0.022 to 0.047 | 0.49 |
| Magnesium, mmol/24 h | 469 | −0.015;−0.045 to 0.016 | 0.34 | 371 | 0.014;−0.016 to 0.045 | 0.36 |
| Uric acid, μmol/24 h | 469 | −0.034;−0.064 to −0.003 | 0.030 | 371 | 0.015;−0.021 to 0.052 | 0.41 |
| Urea, mmol/24 h | 469 | −0.007;−0.037 to 0.023 | 0.65 | 370 | 0.023;−0.012 to 0.058 | 0.19 |
| Citrate, mmol/24 h | 467 | 0.011;−0.019 to 0.042 | 0.46 | 369 | 0.005;−0.025 to 0.034 | 0.76 |
| Oxalate, mmol/24 h | 467 | −0.019;−0.05 to 0.011 | 0.21 | 369 | −0.015;−0.042 to 0.012 | 0.29 |
| Sulfate, mmol/24 h | 459 | −0.014;−0.045 to 0.017 | 0.37 | 363 | 0.04;−0.044 to 0.124 | 0.35 |
| NGIA, mmol/24 h | 430 | 0.024;−0.008 to 0.056 | 0.14 | 342 | 0.008;−0.028 to 0.044 | 0.65 |
| pH, 24 h | 457 | −0.042;−0.073 to −0.012 | 0.0069 | 358 | −0.017;−0.048 to 0.014 | 0.28 |
| Fasting calcium/creatinine, mmol/mmol | 452 | −0.055;−0.085 to −0.025 | 3.9E-04 | 356 | −0.031;−0.061 to −0.001 | 0.042 |
| Postload calcium/creatinine, mmol/mmol | 454 | −0.082;−0.112 to −0.053 | 5.6E-08 | 357 | −0.059;−0.098 to -0.021 | 0.0028 |
| ΔCa: postload − fasting, mmol/mmol | 394 | −0.074;−0.106 to −0.043 | 3.6E-06 | 313 | −0.053;−0.087 to -0.018 | 0.0029 |
| Kidney stone history and composition | ||||||
| Age at first stone event, yr | 471 | 0.063;0.033 to 0.093 | 3.9E-05 | 369 | −0.006;−0.046 to 0.034 | 0.77 |
| Number of stone events | 495 | 0.021;−0.008 to 0.051 | 0.15 | 387 | 0.01;−0.017 to 0.037 | 0.46 |
| Recurrent stone former (yes) | 495 | 0.009;−0.021 to 0.038 | 0.58 | 387 | 0.025;−0.006 to 0.056 | 0.11 |
| Ca oxalate monohydrate content, % | 392 | 0.031;−0.002 to 0.063 | 0.066 | 310 | 0.022;−0.011 to 0.054 | 0.19 |
| Ca oxalate dihydrate content, % | 392 | −0.057;−0.089 to −0.024 | 6.3E-04 | 310 | −0.042;−0.074 to −0.009 | 0.011 |
| Apatite, % | 394 | 0.011;−0.022 to 0.044 | 0.52 | 310 | 0.002;−0.033 to 0.037 | 0.92 |
BMI, body mass index; BSA, body surface area; Ca, calcium; eGFR, estimated glomerular filtration rate; NGIA, net gastrointestinal absorption; PTH, parathyroid hormone.
Multivariable models are adjusted for the covariables sex (women versus men), age, BMI, eGFR, and tobacco consumption. The number (N) of participants, unadjusted and adjusted beta coefficients (β), their 95% CI, and the corresponding P values are indicated for each model. All continuous explanatory variables in the models were scaled to SD, and every 1 SD increase in the continuous explanatory variable results in an average increase of the β value in z scores at the lumbar spine as outcome variable.
In a next step, we performed multivariable linear regression analyses, adjusting for known factors associated with BMD, including sex, age, BMI, eGFR, and tobacco consumption (Table 3 and Fig. 1). Established factors such as BMI (β: 0.082; P = 7.7 × 10−7), eGFR (β: −0.056; P = 0.0041), age (β: 0.042; P = 0.048) or sex (women vs. men, β: 0.227; P = 3.5 × 10−10) remained significantly associated with z score at the lumbar spine in these analyses, whereas no significant association was found for tobacco consumption. Fasting (β: −0.031; P = 0.042), postload (β: −0.059; P = 0.0028) and Δ postload − fasting (β: −0.053; P = 0.0029) urine Ca-to-creatinine ratios, but not absolute 24 hours Ca excretion under random or restricted diet, remained negatively associated with lumbar spine z scores in adjusted analyses. Kidney stone Ca oxalate dihydrate content (β: −0.042; P = 0.011) also remained negatively associated with z scores at the lumbar spine. The associations of 1,25(OH)2 Vitamin D3 and urine pH with z scores at the lumbar spine did not remain robust in the adjusted analysis.
Univariable and Adjusted Association Analyses With z Score at the Femoral Neck as Outcome Variable
We then performed univariable linear regression analyses with z score at the femoral neck as outcome variable and clinical characteristics, kidney stone history, kidney stone analysis, and laboratory parameters as predictor variables (Table 4). Similar to the lumbar spine, age (β: 0.033; P = 0.0014), BMI (β: 0.069; P = 1.1×10−11), and eGFR (β: −0.026; P = 0.013) but not sex (β: 0.022; P = 0.37) were significantly associated with z scores at the femoral neck. In the unadjusted analysis, ΔCa: postload − fasting urine Ca-to-creatinine ratios (β: −0.031; P = 0.0066) but not fasting or postload urine Ca-to-creatinine ratio or absolute 24-hours urine Ca excretion under both random and restricted diet significantly associated with z scores at the femoral neck. No significant association was observed with stone composition. Alkaline phosphatase was negatively associated with z scores at the femoral neck (β: −0.023; P = 0.029). The only mineral metabolism hormone associated with femoral neck z scores in the unadjusted analysis was 1,25(OH)2 Vitamin D3 (β: −0.025; P = 0.021). Furthermore, 24-hours urine potassium (β: 0.040; P = 2.1 × 10−4), phosphate (β: 0.027; P = 0.012), magnesium (β: 0.029; P = 0.0065), urea (β: 0.038; P = 3.3E-04), and citrate (β: 0.030; P = 0.0053) displayed all a significant positive association with z scores at the femoral neck in unadjusted analyses.
Table 4.
Association of laboratory and kidney stone history parameters as main explanatory variables with z scores at the femoral neck as outcome variable
| Explanatory variables | Unadjusted Model |
Multivariable Model |
||||
|---|---|---|---|---|---|---|
| N | β; 95% CI | P-value | N | β; 95% CI | P-value | |
| Blood | ||||||
| eGFR, ml/min per 1.73 m2 BSA | 490 | −0.026;−0.047 to -0.005 | 0.013 | 387 | −0.002;−0.031 to 0.027 | 0.90 |
| Ca total, mmol/l | 490 | −0.008;−0.029 to 0.013 | 0.45 | 387 | −0.008;−0.031 to 0.016 | 0.51 |
| Phosphate, mmol/l | 488 | 0.006;−0.015 to 0.027 | 0.56 | 387 | 0.006;−0.017 to 0.028 | 0.63 |
| Magnesium, mmol/l | 485 | −0.004;−0.024 to 0.017 | 0.74 | 384 | −0.005;−0.027 to 0.017 | 0.64 |
| Uric acid, μmol/l | 488 | 0.004;−0.017 to 0.025 | 0.72 | 385 | 0;−0.021 to 0.021 | 1.00 |
| Bicarbonate, mmol/l | 477 | −0.007;−0.027 to 0.014 | 0.54 | 377 | 0.015;−0.008 to 0.038 | 0.21 |
| Glucose, mmol/l | 478 | 0.022;0.001 to 0.043 | 0.036 | 379 | 0.009;−0.013 to 0.031 | 0.44 |
| Alkaline phosphatase, IU/ml | 489 | −0.023;−0.044 to −0.002 | 0.029 | 386 | −0.035;−0.058 to −0.012 | 0.0034 |
| PTH, pg/ml | 487 | −0.019;−0.04 to 0.001 | 0.066 | 383 | −0.035;−0.057 to −0.012 | 0.0026 |
| 25-OH-Vitamin D3, ng/ml | 316 | 0.016;−0.011 to 0.043 | 0.26 | 236 | 0.018;−0.014 to 0.049 | 0.28 |
| 1,25-OH-Vitamin D3, pg/ml | 483 | −0.025;−0.045 to −0.004 | 0.021 | 380 | −0.009;−0.032 to 0.014 | 0.44 |
| Urine | ||||||
| Sodium, mmol/24 h | 472 | 0.02;−0.001 to 0.041 | 0.061 | 371 | 0.019;−0.007 to 0.045 | 0.14 |
| Sodium, mmol/24 h – restricted diet | 412 | 0.018;−0.004 to 0.041 | 0.104 | 326 | 0.014;−0.011 to 0.038 | 0.28 |
| Potassium, mmol/24 h | 471 | 0.04;0.019 to 0.061 | 2.1E-04 | 370 | 0.032;0.007 to 0.057 | 0.012 |
| Ca, mmol/24 h | 470 | 0.02;−0.001 to 0.041 | 0.068 | 371 | 0.033;0.009 to 0.058 | 0.0085 |
| Ca, mmol/24 h – restricted diet | 415 | 0.016;−0.007 to 0.038 | 0.17 | 328 | 0.019;−0.006 to 0.045 | 0.14 |
| Phosphate, mmol/24 h | 470 | 0.027;0.006–0.048 | 0.012 | 371 | 0.029;0.002–0.055 | 0.035 |
| Magnesium, mmol/24 h | 470 | 0.029;0.008–0.05 | 0.0065 | 371 | 0.043;0.019–0.066 | 3.5E-04 |
| Uric acid, μmol/24 h | 470 | 0.021;0–0.042 | 0.054 | 371 | 0.033;0.005–0.061 | 0.020 |
| Urea, mmol/24 h | 470 | 0.038;0.018–0.059 | 3.3E-04 | 370 | 0.037;0.011–0.063 | 0.0061 |
| Citrate, mmol/24 h | 468 | 0.03;0.009–0.052 | 0.0053 | 369 | 0.022;−0.001 to 0.046 | 0.057 |
| Oxalate, mmol/24 h | 468 | −0.003;−0.024 to 0.018 | 0.79 | 369 | −0.004;−0.025 to 0.017 | 0.73 |
| Sulfate, mmol/24 h | 460 | 0.011;−0.01 to 0.033 | 0.30 | 363 | 0.059;−0.005 to 0.124 | 0.070 |
| NGIA, mmol/24 h | 432 | 0.02;−0.003 to 0.042 | 0.088 | 343 | 0.016;−0.012 to 0.044 | 0.26 |
| pH, 24 h | 460 | −0.014;−0.036 to 0.007 | 0.19 | 359 | 0.004;−0.02 to 0.027 | 0.77 |
| Fasting calcium/creatinine, mmol/mmol | 451 | −0.004;−0.026 to 0.017 | 0.68 | 354 | −0.003;−0.027 to 0.02 | 0.77 |
| Postload calcium/creatinine, mmol/mmol | 454 | −0.019;−0.039 to 0.002 | 0.080 | 356 | −0.017;−0.046 to 0.012 | 0.26 |
| ΔCa: postload − fasting, mmol/mmol | 395 | −0.031;−0.054 to −0.009 | 0.0066 | 313 | −0.01;−0.037 to 0.016 | 0.45 |
| Kidney stone history and composition | ||||||
| Age at first stone event, yr | 473 | 0.014;−0.007 to 0.035 | 0.21 | 369 | −0.014;−0.044 to 0.016 | 0.37 |
| Number of stone events | 497 | 0.014;−0.006 to 0.035 | 0.18 | 387 | 0.009;−0.011 to 0.03 | 0.36 |
| Recurrent stone former (yes) | 497 | 0.001;−0.02 to 0.021 | 0.94 | 387 | 0.006;−0.017 to 0.029 | 0.61 |
| Ca oxalate monohydrate content, % | 393 | 0;−0.024 to 0.023 | 0.98 | 309 | −0.006;−0.031 to 0.02 | 0.67 |
| Ca oxalate dihydrate content, % | 393 | −0.014;−0.037 to 0.009 | 0.25 | 309 | −0.014;−0.039 to 0.012 | 0.29 |
| Apatite content, % | 395 | 0.006;−0.018 to 0.029 | 0.63 | 309 | 0.003;−0.024 to 0.03 | 0.85 |
BMI, body mass index; BSA, body surface area; Ca, calcium; eGFR, estimated glomerular filtration rate; NGIA, net gastrointestinal absorption; PTH, parathyroid hormone.
Multivariable models are adjusted for the covariables sex (women versus men), age, BMI, eGFR, and tobacco consumption. The number (N) of participants, unadjusted and adjusted beta coefficients (β), their 95% CI, and the corresponding P-values are indicated for each model. All continuous explanatory variables in the models were scaled to SD, and every 1 SD increase in the continuous explanatory variable results in an average increase of the β value in z scores at the femoral neck as outcome variable.
In multivariable linear regression analyses, adjusted for sex, age, BMI, eGFR, and tobacco consumption, only BMI (β: 0.066; P = 1.3 × 10−7) was significantly associated with z scores at the femoral neck. Plasma alkaline phosphatase (β: −0.035; P = 0.0034) and PTH (β: −0.035; P = 0.0026) were negatively associated with z scores at the femoral neck (Table 4 and Fig. 1). The association of 1,25(OH)2 Vitamin D3 and ΔCa: postload − fasting Ca-to-creatinine ratios with z scores at the femoral neck did not remain robust after multivariable adjustment. However, absolute 24-hours random urine excretion of Ca (β: 0.033; P = 0.0085), magnesium (β: 0.043; P = 3.5 × 10−4), phosphate (β: 0.029; P = 0.035), potassium (β: 0.032; P = 0.012), urea (β: 0.037; P = 0.0061), and uric acid (β: 0.033; P = 0.020) excretion remained positively associated with z scores at the femoral neck.
In a sensitivity analysis, unadjusted and adjusted models were further assessed after exclusion of 72 participants with potentially confounding medication intake (Ca and/or vitamin D supplements, thiazide or loop diuretics, and alkali supplements). Adjusted results obtained from this analysis (Supplementary Tables S1 and S2) with the remaining 432 participants were very similar to those from the primary analysis except for the following associations: a new negative association was found between PTH (β: −0.031; P = 0.048) and z score at the lumbar spine, and a new positive association was found between net gastrointestinal absorption (β: 0.034; P = 0.039) and z score at the femoral neck. In contrast, the association between z score at the lumbar spine with fasting Ca-to-creatinine ratio and the association between z score at the femoral neck and 24 hours urine phosphate excretion were no longer significant.
Discussion
Our results surprisingly reveal distinct predictors of BMD at the lumbar spine and the femoral neck in SF. To the best of our knowledge, this is also the first study that identified kidney stone constituents as predictors of BMD. We find that Ca oxalate dihydrate stone content negatively associates with BMD at the lumbar spine. Ca oxalate dihydrate stones are typically found in patients with hypercalciuria or high urine Ca-to-oxalate ratios.37 In support of these findings, fasting, postload, and Δ postload − fasting urine Ca-to-creatinine ratios after 1 week of restricted diet in Ca and sodium were negatively associated with BMD at the lumbar spine. In contrast, absolute 24-hours urine Ca excretion rates under random or restricted diet were not associated with BMD at the lumbar spine. The reason for this is not entirely clear but likely at least partially explained by the large interindividual variations in diet.
Interestingly, however, 24-hours urine excretion rates of Ca as well as magnesium, phosphate, potassium, urea, and uric acid were positively associated with BMD at the femoral neck. The association with urinary magnesium was very robust and, together with the positive association observed with urinary potassium, suggests that magnesium- and potassium-rich foods, such as vegetables and fruits, are beneficial for BMD at the femoral neck in SFs. However, at the same time, markers of protein intake such as urea and uric acid also correlated positively with BMD at the femoral neck. Together, these results may be interpreted that a diet rich in protein, balanced with a high intake of alkali-rich foods optimally sustains bone health at the femoral neck. Clearly, prospective studies are needed to further explore the nature of these associations. Despite the lack of a clear explanation for the associations observed, both 24-hours urinary potassium and magnesium excretion, which displayed strong positive associations with BMD at the femoral neck in both unadjusted and multivariable analyses, maybe useful clinical markers to identify patients at high risk of low BMD at the femoral neck.
The importance of urinary Ca excretion as a predictor of BMD in SFs is unclear at the moment.2,10, 11, 12, 13, 14, 15, 16 We find that both random and restricted-diet urinary Ca excretion were not associated with BMD at the lumbar spine. This finding is in agreement with other cross-sectional studies in patients with Ca nephrolithiasis15 or idiopathic hypercalciuria16 but in apparent contradiction to a prospective study in SF with idiopathic hypercalciuria.10 In this study, the association with a change in BMD and urinary Ca excretion only marginally correlated with the change of BMD at the lumbar spine. Because of the cross-sectional design, we may have missed this weak association. However, our analysis revealed a negative association of fasting, postload and Δ postload − fasting urinary Ca excretion after a 1 week of a diet restricted with BMD at the lumbar spine. These findings align with the results of a previous study.16 Taken together, our data demonstrate that high 24-hours urinary Ca excretion is not a predictor of low BMD in SFs. Furthermore, our study reveals that urinary Ca differentially associates with BMD at the lumbar spine versus femoral neck. Whereas 24-hours urine Ca excretion is not associated with BMD at the lumbar spine, we find that 24-hours urinary Ca excretion is positively associated with BMD at the femoral neck.
PTH was negatively associated with BMD at the femoral neck and in sensitivity analyses after exclusion of potentially confounding medications also negatively associated with BMD at the lumbar spine. PTH excess causes loss of BMD and is an established risk factor for vertebral and nonvertebral fractures.38 The stronger association at the femoral neck compared with the lumbar spine may be explained by the predominance of cortical bone in the former and trabecular bone in the latter.39 In addition to PTH, alkaline phosphatase correlated negatively with BMD at the femoral neck. In the bone, alkaline phosphatase is synthesized by osteoblasts, involved in the calcification of bone matrix, and circulating alkaline phosphatase levels correlate with bone turnover. Increased levels have been associated with rapid bone loss, and antiresorptive treatments in postmenopausal women decrease circulating alkaline phosphatase levels.40, 41, 42 In our study, we measured total plasma alkaline phosphatase, a mixture of mainly liver and bone isoenzymes.43,44 There is a strong positive correlation between total and bone-specific plasma alkaline phosphatase,44 and in most clinical situations, total plasma alkaline phosphatase appears to provide equivalent information compared with bone-specific alkaline phosphatase.43 It thus seems likely that the negative association of total plasma alkaline phosphatase with BMD observed in our study is driven by bone-specific plasma alkaline phosphatase levels. However, we cannot rule out at the moment the possibility that other alkaline phosphatase isoforms are involved, and this will require further study.
Strengths of our study include the large sample size allowing adjustment for confounders, a diverse patient collective, a detailed phenotype including stone analysis in most patients, and a 1-week dietary intervention. Our study has also several limitations, including the observational and monocentric design, an almost exclusive Caucasian study population, and lack of detailed dietary data. Despite these limitations, our analyses conducted in a large and deeply phenotyped cohort of SFs significantly extend our knowledge on factors associated with BMD in SFs. Our results indicate that parameters commonly available in SFs such as kidney stone analysis, plasma alkaline phosphatase, PTH, and 24-hours urinary potassium and magnesium excretion can be used to personalize diagnostic work-up for low BMD in SF.
Disclosure
DGF has served as a consultant for Otsuka Pharmaceuticals and Alnylam Pharmaceuticals and has received unrestricted research funding from Novartis, AbbVie, Otsuka Pharmaceuticals, and Boehringer Ingelheim. All the other authors declared no competing interests.
Acknowledgments
DGF was supported by the Swiss National Centre of Competence in Research NCCR TransCure, the Swiss National Centre of Competence in Research NCCR Kidney.CH, and the Swiss National Science Foundation (grants #31003A_135503, 31003A_152829, and 33IC30_166785/1).
Footnotes
Table S1. Association of laboratory and kidney stone history parameters as main explanatory variables with z scores at the lumbar spine as outcome variable after exclusion of potentially confounding medication intake.
Table S2. Association of laboratory and kidney stone history parameters as main explanatory variables with z scores at the femoral neck as outcome variable after exclusion of potentially confounding medication intake.
STROBE Statement.
Supplementary Material
Table S1. Association of laboratory and kidney stone history parameters as main explanatory variables with z scores at the lumbar spine as outcome variable after exclusion of potentially confounding medication intake.
Table S2. Association of laboratory and kidney stone history parameters as main explanatory variables with z scores at the femoral neck as outcome variable after exclusion of potentially confounding medication intake.
STROBE Statement (PDF)
References
- 1.Scales C.D., Jr., Smith A.C., Hanley J.M., Saigal C.S. Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160–165. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakhaee K., Maalouf N.M., Kumar R., Pasch A., Moe O.W. Nephrolithiasis-associated bone disease: pathogenesis and treatment options. Kidney Int. 2011;79:393–403. doi: 10.1038/ki.2010.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucato P., Trevisan C., Stubbs B., et al. Nephrolithiasis, bone mineral density, osteoporosis, and fractures: a systematic review and comparative meta-analysis. Osteoporos Int. 2016;27:3155–3164. doi: 10.1007/s00198-016-3658-8. [DOI] [PubMed] [Google Scholar]
- 4.Gambaro G., Croppi E., Coe F., et al. Metabolic diagnosis and medical prevention of calcium nephrolithiasis and its systemic manifestations: a consensus statement. J Nephrol. 2016;29:715–734. doi: 10.1007/s40620-016-0329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melton L.J., 3rd, Crowson C.S., Khosla S., Wilson D.M., O’Fallon W.M. Fracture risk among patients with urolithiasis: a population-based cohort study. Kidney Int. 1998;53:459–464. doi: 10.1046/j.1523-1755.1998.00779.x. [DOI] [PubMed] [Google Scholar]
- 6.Denburg M.R., Leonard M.B., Haynes K., et al. Risk of fracture in urolithiasis: a population-based cohort study using the health improvement network. Clin J Am Soc Nephrol. 2014;9:2133–2140. doi: 10.2215/CJN.04340514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauderdale D.S., Thisted R.A., Wen M., Favus M.J. Bone mineral density and fracture among prevalent kidney stone cases in the Third National Health and Nutrition Examination Survey. J Bone Miner Res. 2001;16:1893–1898. doi: 10.1359/jbmr.2001.16.10.1893. [DOI] [PubMed] [Google Scholar]
- 8.Taylor E.N., Feskanich D., Paik J.M., Curhan G.C. Nephrolithiasis and risk of incident bone fracture. J Urol. 2016;195:1482–1486. doi: 10.1016/j.juro.2015.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arrabal-Polo M.A., Arrabal-Martin M., de Haro-Munoz T., et al. Mineral density and bone remodelling markers in patients with calcium lithiasis. BJU Int. 2011;108:1903–1908. doi: 10.1111/j.1464-410X.2011.10167.x. [DOI] [PubMed] [Google Scholar]
- 10.Asplin J.R., Donahue S., Kinder J., Coe F.L. Urine calcium excretion predicts bone loss in idiopathic hypercalciuria. Kidney Int. 2006;70:1463–1467. doi: 10.1038/sj.ki.5001778. [DOI] [PubMed] [Google Scholar]
- 11.Pietschmann F., Breslau N.A., Pak C.Y. Reduced vertebral bone density in hypercalciuric nephrolithiasis. J Bone Miner Res. 1992;7:1383–1388. doi: 10.1002/jbmr.5650071205. [DOI] [PubMed] [Google Scholar]
- 12.Fuss M., Gillet C., Simon J., Vandewalle J.C., Schoutens A., Bergmann P. Bone mineral content in idiopathic renal stone disease and in primary hyperparathyroidism. Eur Urol. 1983;9:32–34. doi: 10.1159/000474039. [DOI] [PubMed] [Google Scholar]
- 13.Pacifici R., Rothstein M., Rifas L., et al. Increased monocyte interleukin-1 activity and decreased vertebral bone density in patients with fasting idiopathic hypercalciuria. J Clin Endocrinol Metab. 1990;71:138–145. doi: 10.1210/jcem-71-1-138. [DOI] [PubMed] [Google Scholar]
- 14.Jaeger P., Lippuner K., Casez J.P., Hess B., Ackermann D., Hug C. Low bone mass in idiopathic renal stone formers: magnitude and significance. J Bone Miner Res. 1994;9:1525–1532. doi: 10.1002/jbmr.5650091004. [DOI] [PubMed] [Google Scholar]
- 15.Sakhaee K., Maalouf N.M., Poindexter J., Adams-Huet B., Moe O.W. Relationship between urinary calcium and bone mineral density in patients with calcium nephrolithiasis. J Urol. 2017;197:1472–1477. doi: 10.1016/j.juro.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Letavernier E., Traxer O., Daudon M., et al. Determinants of osteopenia in male renal-stone-disease patients with idiopathic hypercalciuria. Clin J Am Soc Nephrol. 2011;6:1149–1154. doi: 10.2215/CJN.10191110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bordier P., Ryckewart A., Gueris J., Rasmussen H. On the pathogenesis of so-called idiopathic hypercalciuria. Am J Med. 1977;63:398–409. doi: 10.1016/0002-9343(77)90278-9. [DOI] [PubMed] [Google Scholar]
- 18.Malluche H.H., Tschoepe W., Ritz E., Meyer-Sabellek W., Massry S.G. Abnormal bone histology in idiopathic hypercalciuria. J Clin Endocrinol Metab. 1980;50:654–658. doi: 10.1210/jcem-50-4-654. [DOI] [PubMed] [Google Scholar]
- 19.de Vernejoul M.C., Kuntz D., Miravet L., Goutallier D., Ryckewaert A. Bone histomorphometric reproducibility in normal patients. Calcif Tissue Int. 1981;33:369–374. doi: 10.1007/BF02409458. [DOI] [PubMed] [Google Scholar]
- 20.Steiniche T., Mosekilde L., Christensen M.S., Melsen F. A histomorphometric determination of iliac bone remodeling in patients with recurrent renal stone formation and idiopathic hypercalciuria. APMIS. 1989;97:309–316. doi: 10.1111/j.1699-0463.1989.tb00793.x. [DOI] [PubMed] [Google Scholar]
- 21.Heilberg I.P., Martini L.A., Szejnfeld V.L., et al. Bone disease in calcium stone forming patients. Clin Nephrol. 1994;42:175–182. [PubMed] [Google Scholar]
- 22.Heller H.J., Zerwekh J.E., Gottschalk F.A., Pak C.Y. Reduced bone formation and relatively increased bone resorption in absorptive hypercalciuria. Kidney Int. 2007;71:808–815. doi: 10.1038/sj.ki.5002181. [DOI] [PubMed] [Google Scholar]
- 23.Misael da Silva A.M., dos Reis L.M., Pereira R.C., et al. Bone involvement in idiopathic hypercalciuria. Clin Nephrol. 2002;57:183–191. doi: 10.5414/cnp57183. [DOI] [PubMed] [Google Scholar]
- 24.Pak C.Y., Kaplan R., Bone H., Townsend J., Waters O. A simple test for the diagnosis of absorptive, resorptive and renal hypercalciurias. N Engl J Med. 1975;292:497–500. doi: 10.1056/NEJM197503062921002. [DOI] [PubMed] [Google Scholar]
- 25.Pak C.Y., Britton F., Peterson R., et al. Ambulatory evaluation of nephrolithiasis. Classification, clinical presentation and diagnostic criteria. Am J Med. 1980;69:19–30. doi: 10.1016/0002-9343(80)90495-7. [DOI] [PubMed] [Google Scholar]
- 26.Dhayat N.A., Schaller A., Albano G., et al. The vacuolar H+-ATPase B1 subunit polymorphism p.E161K Associates with impaired urinary acidification in recurrent stone formers. J Am Soc Nephrol. 2016;27:1544–1554. doi: 10.1681/ASN.2015040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhayat N.A., Ackermann D., Pruijm M., et al. Fibroblast growth factor 23 and markers of mineral metabolism in individuals with preserved renal function. Kidney Int. 2016;90:648–657. doi: 10.1016/j.kint.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 28.Dhayat N.A., Lüthi D., Schneider L., Mattmann C., Vogt B., Fuster D.G. Distinct phenotype of kidney stone formers with renal phosphate leak. Nephrol Dial Transplant. 2019;34:129–137. doi: 10.1093/ndt/gfy170. [DOI] [PubMed] [Google Scholar]
- 29.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med. 2011;155:408] Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bingham S.A., Cummings J.H. The use of creatinine output as a check on the completeness of 24-hour urine collections. Hum Nutr Clin Nutr. 1985;39:343–353. [PubMed] [Google Scholar]
- 31.Côté A.M., Firoz T., Mattman A., Lam E.M., von Dadelszen P., Magee L.A. The 24-hour urine collection: gold standard or historical practice? Am J Obstet Gynecol. 2008;199:625.e1–625.e6256. doi: 10.1016/j.ajog.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Forni Ogna V., Ogna A., Vuistiner P., et al. New anthropometry-based age- and sex-specific reference values for urinary 24-hour creatinine excretion based on the adult Swiss population. BMC Med. 2015;13:40. doi: 10.1186/s12916-015-0275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casez J.P., Troendle A., Lippuner K., Jaeger P. Bone mineral density at distal tibia using dual-energy X-ray absorptiometry in normal women and in patients with vertebral osteoporosis or primary hyperparathyroidism. J Bone Miner Res. 1994;9:1851–1857. doi: 10.1002/jbmr.5650091203. [DOI] [PubMed] [Google Scholar]
- 34.Popp A.W., Senn C., Franta O., Krieg M.A., Perrelet R., Lippuner K. Tibial or hip BMD predict clinical fracture risk equally well: results from a prospective study in 700 elderly Swiss women. Osteoporos Int. 2009;20:1393–1399. doi: 10.1007/s00198-008-0808-7. [DOI] [PubMed] [Google Scholar]
- 35.Cloutier J., Villa L., Traxer O., Daudon M. Kidney stone analysis: “Give me your stone, I will tell you who you are!”. World J Urol. 2015;33:157–169. doi: 10.1007/s00345-014-1444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kimura S., Zhang G.X., Nishiyama A., et al. Mitochondria-derived reactive oxygen species and vascular MAP kinases: comparison of angiotensin II and diazoxide. Hypertension. 2005;45:438–444. doi: 10.1161/01.HYP.0000157169.27818.ae. [DOI] [PubMed] [Google Scholar]
- 37.Daudon M., Letavernier E., Frochot V., et al. Respective influence of calcium and oxalate urine concentration on the formation of calcium oxalate monohydrate or dihydrate crystals. C R Chimie. 2016;19:1504–1513. doi: 10.1016/j.crci.2016.08.009. [DOI] [Google Scholar]
- 38.Khosla S., Melton L.J., 3rd, Wermers R.A., Crowson C.S., O’Fallon Wm, Riggs Bl. Primary hyperparathyroidism and the risk of fracture: a population-based study. J Bone Miner Res. 1999;14:1700–1707. doi: 10.1359/jbmr.1999.14.10.1700. [DOI] [PubMed] [Google Scholar]
- 39.Duan Y., De Luca V., Seeman E. Parathyroid hormone deficiency and excess: similar effects on trabecular bone but differing effects on cortical bone. J Clin Endocrinol Metab. 1999;84:718–722. doi: 10.1210/jcem.84.2.5498. [DOI] [PubMed] [Google Scholar]
- 40.Ross P.D., Knowlton W. Rapid bone loss is associated with increased levels of biochemical markers. J Bone Miner Res. 1998;13:297–302. doi: 10.1359/jbmr.1998.13.2.297. [DOI] [PubMed] [Google Scholar]
- 41.Delmas P.D., Eastell R., Garnero P., Seibel M.J., Stepan J. Committee of Scientific Advisors of the International Osteoporosis Foundation. The use of biochemical markers of bone turnover in osteoporosis. Committee of Scientific Advisors of the International Osteoporosis Foundation. Osteoporos Int. 2000;11(suppl 6):S2–S17. doi: 10.1007/s001980070002. [DOI] [PubMed] [Google Scholar]
- 42.Glover S.J., Gall M., Schoenborn-Kellenberger O., et al. Establishing a reference interval for bone turnover markers in 637 healthy, young, premenopausal women from the United Kingdom, France, Belgium, and the United States. J Bone Miner Res. 2009;24:389–397. doi: 10.1359/jbmr.080703. [DOI] [PubMed] [Google Scholar]
- 43.Woitge H.W., Seibel M.J., Ziegler R. Comparison of total and bone-specific alkaline phosphatase in patients with nonskeletal disorder or metabolic bone diseases. Clin Chem. 1996;42:1796–1804. [PubMed] [Google Scholar]
- 44.Gomez B., Jr., Ardakani S., Ju J., et al. Monoclonal antibody assay for measuring bone-specific alkaline phosphatase activity in serum. Clin Chem. 1995;41:1560–1566. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.