Abstract
Introduction
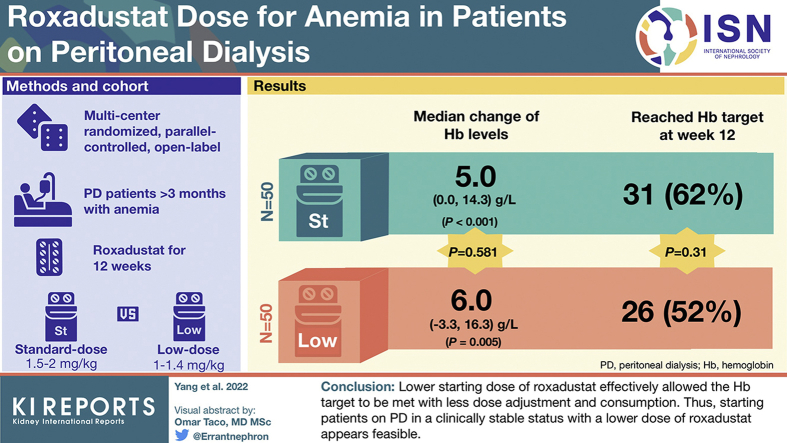
We aimed to investigate whether a lower starting dose of roxadustat (∼1–1.4 mg/kg) converted from erythropoiesis-stimulating agent (ESA) could achieve a comparable hemoglobin (Hb) target (≥100 and ≤120 g/l) compared with the standard weight-based dose (∼1.5–2 mg/kg) at week 12 through a peritoneal dialysis (PD) cohort.
Methods
A 12-week multicenter randomized, parallel-controlled, open-label, pilot clinical trial enrolled adult patients who had undergone PD treatment for >3 months with renal anemia. Participants were randomized in blocks of 4 in a 1:1 ratio to either the standard-dose group (n = 50) or the low-dose group (n = 50). The primary end point was the proportion of patients achieving the Hb target at week 12.
Results
Baseline demographic and clinical characteristics of the 2 groups were comparable. There was no difference in the proportion of patients who met the Hb target at week 12, that is, 26 patients (52%) versus 31 patients (62%) in the low-dose group and standard-dose group, respectively (P = 0.31). The Hb levels significantly increased in both groups from baseline to week 12; the median change of Hb levels was 5.0 (0.0–14.3) g/l (P < 0.001) for the standard-dose group and 6.0 (−3.3 to 16.3) g/l for the low-dose group (P = 0.005) (P = 0.581 for between groups).
Conclusion
This study suggests that a lower starting dose of roxadustat effectively achieves the Hb target as standard-dose does among patients on PD. (ClinicalTrials.gov number, NCT04454879).
Keywords: hypoxia-inducible factor prolyl hydroxylase inhibitor, peritoneal dialysis, renal anemia, roxadustat
Graphical abstract
Renal anemia is a common complication in patients with chronic kidney disease (CKD)1 and is associated with increased risks of mortality and comorbidity.2 Recent studies have reported on the management of renal anemia using a hypoxia-inducible factor prolyl hydroxylase inhibitor, such as roxadustat.3, 4, 5, 6, 7 Several phase Ⅲ clinical trials have verified the efficacy and safety of roxadustat as a therapy for anemia in non–dialysis-dependent and dialysis-dependent patients.5,8, 9, 10, 11 Of note, previous phase Ⅱ and Ⅲ trials indicated the dose-effect relationship of roxadustat and correction of anemia,6,12 where Hb levels rapidly increased by approximately 10 g/l within the first 4 to 5 weeks with a weight-based initial dose, that is, 1.5 to 2 mg/kg 3 times per week.6,7,13 This phenomenon in turn explained why the mean weekly dose of roxadustat was reduced after 4 weeks of treatment in some dialysis-dependent recipients with 100 to 110 g/l of Hb at baseline, indicating dose reduction due to Hb overshooting.5
In the PD patients, it was noticed that ESA requirement to obtain the same Hb level was much less as compared with their hemodialysis (HD) counterparts.14, 15, 16 Explanations include better ESA responsiveness and slower declining rate of residual kidney function in PD patients, and that HD patients experience more chronic blood loss because of red blood cell destruction or catheter blood coagulation. The recent worldwide data showed that the proportions of PD patients achieving Hb between 100 g/l and 130 g/l were 46% to 62%, and even 4% to 12% of patients had an Hb level ≥130 g/l.17 It is hypothesized that roxadustat requirement might be less in the relatively good condition of anemia management in PD patients with ESA maintenance therapy.
We aimed to investigate whether a lower weight-based dose (∼1–1.4 mg/kg) of roxadustat could achieve a comparable Hb target compared with the standard weight-based dose (∼1.5–2 mg/kg) as per the recommendations. Therefore, we undertook a 12-week multicenter randomized control pilot study involving patients on PD to investigate this issue.
Methods
Study Design and Setting
This multicenter, parallel-controlled, open-label randomized clinical trial (RCT) exclusively involving patients on PD was conducted at Peking University First Hospital, Beijing Haidian Hospital, and Beijing Hospital of Traditional Chinese Medicine. The study protocol adhered to the “Standard Protocol Items: Recommendations for Interventional Trials” statement.18
Ethical Approval
Our study was approved by the Ethics Committees of Peking University First Hospital and accepted by the other 2 hospitals and registered on ClinicalTrials.gov on July 2, 2020, and updated on April 3, 2021 (NCT04454879).
Study Population
We screened all adult patients who had undergone PD treatment >3 months owing to end stage kidney disease between July 6, 2020, and December 28, 2020. Patients with an Hb level ≥90 g/l and <120 g/l were enrolled. Patients using ESAs of 3000 U and 10,000 U single strength treatment were required to discontinue ESAs for at least 3 days and 7 days, respectively, and were then converted to roxadustat. Patients not using ESAs were enrolled directly. We excluded patients with a body weight (BW) >110 kg or <45 kg and patients with a diagnosis of active hepatitis or hepatic failure, uncontrolled hypertension, a history of tumor and radiotherapy or chemotherapy in the past 6 months, a life expectancy of <6 months, anemia not related to CKD, or a recognized allergy to roxadustat. Pregnant or breastfeeding women were also excluded. All patients provided written informed consent.
Randomization and Blinding
Patients from 3 hospitals were assigned in blocks of 4 in a 1:1 ratio to receive either a standard initial dose (standard-dose group) or a lower initial dose (low-dose group) in a consecutive BW range. An external statistical consultant undertook randomization via SAS 9.4 software package (SAS 9.4 Institute Inc., Cary, NC) and put the grouping information in the sealed envelopes. When the patient was enrolled, sealed envelopes would be opened by someone who was blinded to this assignment. As a pilot study, each group enrolled 50 patients. All laboratory parameters and evaluations in the case report form for each patient were measured and recorded by medical workers who were blinded to treatment assignment. Blinding of the treatment assignment to investigators and patients was not possible because of the dose adjustment of roxadustat.
Intervention
Both groups of patients received roxadustat for 12 weeks. In the standard-dose group, patients with a BW 45 kg to 60 kg and ≥60 kg received an initial dose of roxadustat 100 mg and 120 mg 3 times per week, respectively. Accordingly, patients with a BW between 60 kg and 80 kg (25th–75th percentile) actually received an initial dose of ∼1.5 to 2 mg/kg.
In the low-dose group, patients with a BW 45 kg to 50 kg, 50 kg to 70 kg, 70 kg to 90 kg, and 90 kg to 110 kg received an initial roxadustat dose of 50 mg, 70 mg, 90 mg, and 110 mg 3 times per week, respectively, at ∼1 to 1.4 mg/kg. All patients were required to follow the dose adjustment rule according to the Hb response outlined in the study by Chen et al.5 (Supplementary Table S1).
For both groups, dialysis prescription and treatment for hypertension, diabetes mellitus, and mineral bone disease were adjusted according to current guidelines and recommendations. Oral or i.v. iron supplementation was permitted during the study period, and total iron element intake from iron supplementation was calculated for each patient.
Baseline Evaluation and Follow-Up
Data collection comprised 2 phases: a baseline evaluation and a follow-up. All data were recorded by a responsible physician using a uniform case report form. An overview of the data collection schedule during baseline evaluation and follow-up was shown in Supplementary Table S2.
Baseline Evaluation
Demographics were recorded, including age, sex, duration of PD, height, BW, body mass index, primary disease, and the Charlson comorbidities index.19 Medication regimens concerning the management of anemia, hypertension, diabetes and dyslipidemia, and mineral bone disease were recorded. Pre-enrollment ESA dosages were expressed as U/kg/d. Laboratory variables included complete blood counts, reticulocyte counts, and biochemical indexes such as liver enzymes, serum albumin, lipids spectrum, uric acid, urea, creatinine, serum iron, ferritin, total iron binding capacity, transferrin saturation, high-sensitive C-reactive protein, and intact parathyroid hormone. Biochemical profiles were investigated using an automatic Hitachi chemistry analyzer. High-sensitive C-reactive protein was measured using immune rate nephelometric analysis. Small solute clearance and residual kidney function were measured through collecting 24-hour urine and dialysate. Small solute clearance was defined as total urea clearance (total Kt/V) and total creatinine clearance. Residual kidney function was estimated using the average kidney clearance of urea and creatinine.
Follow-Up
Patients were followed up for 12 weeks, with in-person visits at weeks 2, 4, 8, and 12. According to the Hb level at each time point, all patients were required to adjust the roxadustat dose according to the Hb response outlined in the study by Chen et al.5 (Supplementary Table S1). Serial complete blood and reticulocyte counts were tested at each time point. Biochemical indexes were collected at baseline and at week 12. Adherence to roxadustat was investigated through a check of dispensed and remaining tablets. Details concerning other clinical information and medications were collected. All outcomes and adverse events were recorded.
End Points
The primary end point was the proportion of patients achieving the Hb target (i.e., ≥100 g/l and ≤120 g/l) at week 12. Secondary end points were the change of average Hb level of weeks 12 from baseline, the proportion of patients achieving Hb >120 g/l and <100 g/l, variations in Hb levels during follow-up (SD divided by the mean Hb level), and the ratio of Hb overshooting (Hb >130g/l during the follow-up). Comparisons between groups and within each group concerning changing trends in Hb levels, roxadustat dose, iron parameters, and lipid spectrum over the 12-week period were investigated.
Adverse Events
We obtained information regarding adverse events at each visit including but not limited to symptoms, clinically significant laboratory results, and physical examination findings. Potential causes of each adverse event in relation to roxadustat were assessed and categorized into 6 grades (unrelated, unlikely-related, possibly-related, most-likely-related, definitely-related, and unable-to-assess). Patients in undetermined categories were discussed, and their status was confirmed by principal investigators and pharmacists from the study hospitals.
Statistical Analysis
Parametric data are presented as mean ± SD. Nonparametric data are presented as median values with an interquartile range. Categorical variables are expressed as percentages or ratios. Missing data were otherwise imputed using last-observation-carried-forward method. Student t, Mann–Whitney U, and the χ2 tests were used to compare differences in baseline characteristics between the groups as appropriate. Missing data due to the drop out patients were imputed using last-observation-carried-forward imputation. A χ2 test was used to compare between the groups in terms of the proportions of patients achieving the Hb target (target Hb level, ≥100 g/l and ≤120 g/l) and those with Hb >120 g/l or <100 g/l and was performed on an intention-to-treat analysis, which comprised patients who completed randomization. A Student t test was utilized to compare the rate of Hb variation over the 12-week period between groups. A paired t test was used to compare the iron parameters and lipid spectrums between baseline and week 12 in each group. Changing trends in Hb levels, roxadustat dose, iron parameters, and lipid spectrums between baseline and week 12 were compared between groups and within each group using a mixed-model analysis of variance, adjusting for baseline values of the outcome variables and additionally, total elemental iron or defined daily dose of β-Hydroxy β-methylglutaryl-coenzyme A reductase inhibitors,20 as appropriate. The primary and second analysis were also performed on per-protocol population as sensitivity analysis. The per-protocol population comprised patients who completed randomization and had baseline and each time point Hb values assessed.
With regard to sample size calculation, we did not find any data on the efficacy of low-dose Roxadustat in PD patients in previous literatures, which preclude us from calculating the size for the present trial on comparison of two-dosing strategies.
All probabilities were 2-tailed, and the level of significance was set at 0.05. Statistical analysis was performed using SPSS software package version 20.0 (SPSS, Chicago, IL).
Results
Baseline Characteristics and Follow-Up
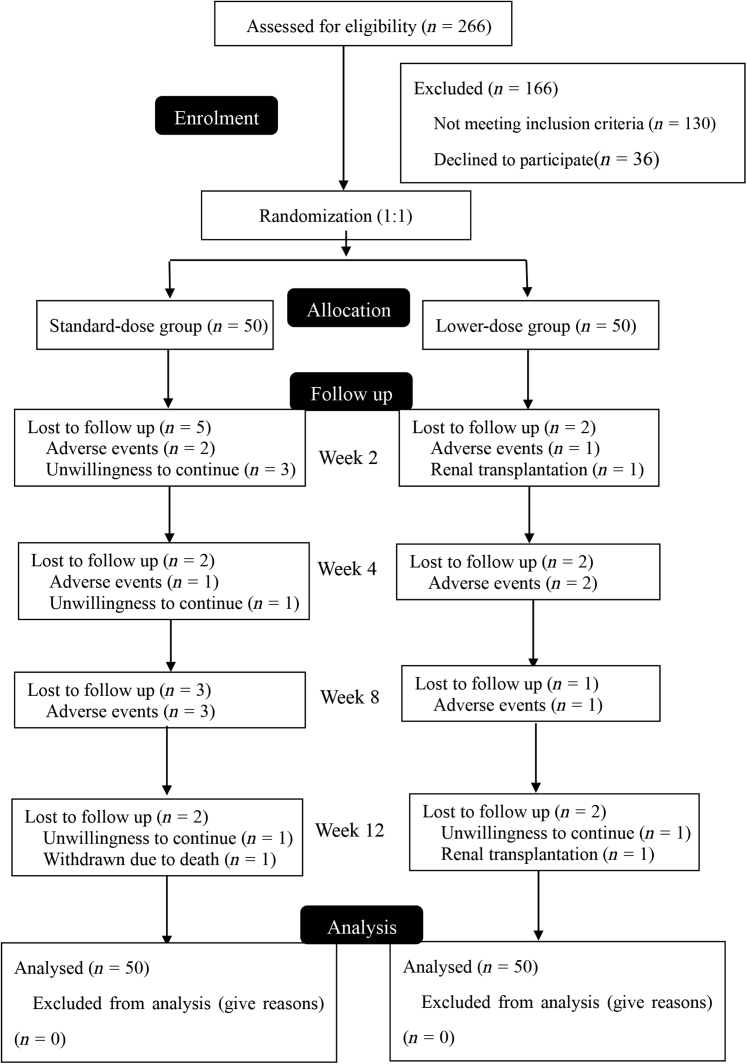
We screened 266 patients between July 2020 and December 2020 and recruited 100 patients in the study (Figure 1). Demographic data and baseline characteristics were compared between the standard-dose and low-dose groups (Table 1 and Supplementary Table S3). Ninety-six patients used ESAs before administrating roxadustat, with a median dosage of 15.6 U/kg/d. There were 2 patients temporarily not using ESAs in each group. Baseline Hb levels, iron status, total iron element supplementation, and epoetin dosages were comparable between the groups (P > 0.05). No significant differences were found between the groups in other variables including sex, age, weight, primary kidney disease, Charlson index, PD duration, serum albumin, lipids spectrums, residual kidney function, and high-sensitive C-reactive protein (P > 0.05).
Figure 1.
Flowchart of the study. Standard-dose, 100 mg and 120 mg thrice-weekly respectively for patients <60 kg and ≥60 kg; low-dose, initial dose was 50 mg, 70 mg, 90 mg, and 110 mg thrice-weekly for patients weighing ≥45 kg and ≤50 kg, >50 kg, and ≤70 kg, >70 kg and ≤90 kg, and >90 kg and ≤110 kg, respectively. ∗(Reasons for patients) not meeting inclusion criteria (n = 130): PD duration <3 months (n = 20); Hb >130 g/l or <90 g/l (n = 59); weight <45 kg (n = 4); with history of malignancy (n = 1); anemia not related to CKD (n = 1); uncontrolled hypertension (n = 2); taking roxadustat or allergy to roxadustat (n = 43).
Table 1.
Comparison of baseline characteristics between the 2 roxadustat initial-dose groups
| Variables | Total (N = 100) | Standard-dose groupa (n = 50) | Low-dose groupb (n = 50) | P |
|---|---|---|---|---|
| Male, n (%) | 61 (61.0) | 27 (54.0) | 34(68.0) | 0.15 |
| Age, years | 51.4 ± 13.0 | 52.0 ± 12.9 | 50.7 ± 13.2 | 0.62 |
| Weight, kg | 69.0 ± 13.6 | 66.8 ± 14.1 | 71.3 ± 12.8 | 0.10 |
| Height, cm | 166.9 ± 8.6 | 166.2 ± 7.9 | 167.5 ± 9.3 | 0.45 |
| Body mass index, kg/m2 | 24.7 ± 4.7 | 24.0 ± 4.0 | 25.5 ± 5.2 | 0.11 |
| Primary kidney disease, n (%) | ||||
| Glomerulonephritis | 44 (44.0) | 21 (42.0) | 23 (46.0) | 0.82 |
| Diabetic nephropathy | 28 (28.0) | 14(28.0) | 14(28.0) | |
| Hypertension | 14 (14.0) | 9(18.0) | 5(10.0) | |
| Miscellaneous | 5 (5.0) | 2 (4.0) | 3 (6.0) | |
| Unknown | 9 (9.0) | 4 (8.0) | 5 (10.0) | |
| Diabetes mellitus, n (%) | 33(33.0) | 16(32.0) | 17(34.0) | 0.83 |
| Charlson index | 3.5 (2.0–6.0) | 4.0 (2.0–6.0) | 3.0 (2.0–5.3) | 0.93 |
| PD duration, mo | 26.0 (14.5–59.3) | 30.5 (18.8–64.3) | 23.0 (11.0–51.0) | 0.17 |
| SBP, mmHg | 137.3 ± 16.0 | 136.2 ± 16.8 | 138.3 ± 15.3 | 0.52 |
| DBP, mmHg | 81.7 ± 11.3 | 80.8 ± 12.0 | 82.6 ± 10.6 | 0.41 |
| Hemoglobin, g/l | 108.0 ± 6.6 | 107.8 ± 6.8 | 108.1± 6.5 | 0.85 |
| Reticulocyte, 109/l | 65.6 ± 32.1 | 62.2 ± 30.1 | 68.8 ± 33.9 | 0.31 |
| Albumin, g/l | 37.5 ± 3.4 | 37.8 ± 3.6 | 37.2 ± 3.3 | 0.39 |
| Hs-CRP, mg/l | 1.4 (0.5–5.0) | 1.3 (0.4–5.1) | 1.7 (0.7–5.1) | 0.42 |
| Urea, mmol/l | 21.5 ± 5.8 | 22.2 ± 5.4 | 20.7 ± 6.1 | 0.21 |
| Serum creatinine, μmol/l | 991.0 ± 252.3 | 948.0 ± 227.0 | 1033.9 ± 270.9 | 0.09 |
| RKF, ml/min | 0.4 (0–1.0) | 0.2 (0–0.9) | 0.4 (0–1.1) | 0.38 |
| Triglyceride, mmol/l | 1.7 (1.2–2.3) | 1.6 (1.3–2.1) | 1.7 (1.2–2.8) | 0.51 |
| Total cholesterol, mmol/l | 4.2 ± 1.0 | 4.1 ± 0.9 | 4.3 ± 1.0 | 0.30 |
| HDL, mmol/l | 0.9 ± 0.3 | 1.0 ± 0.3 | 0.9 ± 0.3 | 0.22 |
| LDL, mmol/l | 2.2 ± 0.7 | 2.2 ± 0.6 | 2.2 ± 0.7 | 0.98 |
| Ferritin, ng/ml | 279.1 (147.0–396.4) | 291.0 (151.7–386.9) | 273.7 (129.8–405.2) | 0.57 |
| Serum iron, μmol/l | 15.2 ± 6.5 | 14.5 ± 7.1 | 15.8 ± 5.9 | 0.33 |
| TIBC, μmol/l | 45.7 ± 7.1 | 45.7 ± 7.5 | 45.7 ± 6.7 | 0.95 |
| TSAT, % | 34.0 ± 15.1 | 33.0 ± 17.1 | 35.0 ± 13.0 | 0.52 |
| Iron supplementation, n (%) | 65 (65.0) | 31 (62.0) | 34 (68.0) | 0.53 |
| Total iron element, mg/wk | 420.0 (0–840.0) | 420.0 (0–840.0) | 630.0 (0–840.0) | 0.71 |
| ESA administration, n (%) | 96 (96.0) | 48 (96.0) | 48 (96.0) | 1.00 |
| Daily epoetin dosage, U/kg/d | 15.6 ± 10.1 | 16.0 ± 10.2 | 15.2 ± 10.2 | 0.71 |
DBP, diastolic blood pressure; ESA, erythropoiesis-stimulating agent; HDL, high density lipoprotein; Hs-CRP, high-sensitive C-reactive protein; LDL, low density lipoprotein; PD, peritoneal dialysis; RKF, residual kidney function; SBP, systolic blood pressure; TIBC, Total iron binding capacity; TSAT, Transferring saturation.
Standard-dose, 100 mg and 120 mg thrice-weekly, respectively, for patients <60 kg and ≥60 kg.
Low-dose, initial dose was 50 mg, 70 mg, 90 mg, and 110 mg thrice-weekly for patients weighing ≥45 kg and ≤50 kg, >50 kg and ≤70 kg, >70 kg, and ≤90 kg, >90 kg and ≤110 kg, respectively.
During the 12-week study period (Figure 1), 10 patients discontinued roxadustat owing to adverse events, 6 patients withdrew their consent, 2 patients had kidney transplantation, and 1 patient died of cardiovascular disease. Totally, 24 patients (24%) reported adverse events (Supplementary Table S4), including 14 patients (28%) in the standard-dose group and 10 patients (20%) in the low-dose group. Adverse events were considered to be related to the drug in 17 patients, among which gastrointestinal symptoms (n = 10) and fatigue (n = 3) were reported most often, and 7 patients discontinued roxadustat.
Hb Response to Roxadustat
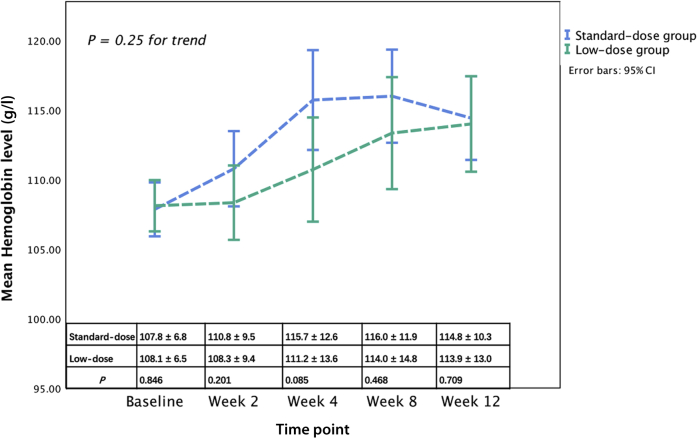
At baseline, no difference was found in the Hb level between the standard-dose group and low-dose group (107.8 ± 6.8 g/l vs. 108.1 ± 6.5 g/l, P = 0.85) (Figure 2). Compared with baseline, Hb levels significantly increased in both groups at week 12. The median change of Hb levels of weeks 12 from baseline was 5.0 (0.0–14.3) g/l in the standard-dose group (P < 0.001) and 6.0 (−3.3 to 16.3) g/l in the low-dose group (P = 0.005). The mean change of Hb at week 12 from baseline was not significantly different between the 2 groups (P = 0.581). Overall, there was no difference in the changing trend of Hb after adjusting for baseline Hb levels between the groups (P = 0.25) or when comparing on Hb levels at each visit (P > 0.05) in the intention-to-treat population (Figure 2). And in the per-protocol population, main results were consistent with the results of intention-to-treat analysis, except that the Hb level was significantly higher in the standard-dose group than low-dose group at week 4 (117.3 ± 12.8 g/l vs. 111.2 ± 13.3 g/l, P = 0.029) (Supplementary Figure S1).
Figure 2.
Comparison of hemoglobin responses between the standard-dose group and the low-dose group.
In terms of the proportion of patients who met the Hb target (≥100 g/l and ≤120 g/l), there was no difference in the proportion of patients who met the Hb target at baseline and week 2, 4, and 12 (P range = 0.31–0.84; Table 2). At week 12, a total of 26 patients (52%) in the low-dose group versus 31 patients (62%) in the standard-dose group achieved the Hb target (P = 0.31). The ratio of Hb overshooting was similar between these 2 groups at week 2, 4, 8, and 12 (P > 0.05). In addition, the proportions of patients with Hb >120 g/l at each time point (P range = 0.190–0.668) were comparable between the groups, although the proportions of patients with Hb <100 g/l were higher in low-dose group at week 8 and 12 (P range = 0.014–0.037) in the intention-to-treat population. No significant difference was found between the variation in Hb levels in the standard- and the low-dose groups (6.3% and 6.7%, respectively; P = 0.57). There was no significant correlation between baseline ESA dose and roxadustat dose at week 12 (r = 0.245, P = 0.05). Proportion of patients with Hb at different levels in the two roxadustat dose groups, either conversion from ESA treatment (Supplementary Table S5) or the per-protocol analysis had similar results (Supplementary Table S6).
Table 2.
Number and proportion of patients with Hb at different levels in the 2 roxadustat dose groups
| Time point | Hb (g/dl) | Standard-dose group n (%) | Low-dose group n (%) | Pa |
|---|---|---|---|---|
| Baseline | ||||
| <100 | 7 (14.0) | 7 (14.0) | 1.00 | |
| ≥100 and ≤120 | 43 (86) | 43 (86) | 1.00 | |
| >120 | 0 (0) | 0 (0) | — | |
| Week 2 | ||||
| <100 | 4 (8) | 10 (20) | 0.08 | |
| ≥100 and ≤120 | 38 (76) | 36 (72) | 0.65 | |
| >120 | 8 (16) | 4 (8) | 0.22 | |
| >130 | 2(4) | 1(2) | 0.56 | |
| Week 4 | ||||
| <100 | 4 (8) | 9 (18) | 0.14 | |
| ≥100 and ≤120 | 28 (56) | 29 (58) | 0.84 | |
| >120 | 18 (36) | 12 (24) | 0.19 | |
| >130 | 5(10) | 3(6) | 0.46 | |
| Week 8 | ||||
| <100 | 2 (4) | 10 (20) | 0.01 | |
| ≥100&≤120 | 33 (66) | 23 (46) | 0.04 | |
| >120 | 15 (30) | 17 (34) | 0.67 | |
| >130 | 5(10) | 6(12) | 0.75 | |
| Week 12 | ||||
| <100 | 3 (6) | 10 (20) | 0.04 | |
| ≥100 and ≤120 | 31 (62) | 26 (52) | 0.31 | |
| >120 | 16 (32) | 14 (28) | 0.66 | |
| >130 | 2(4) | 5(10) | 0.24 |
Hb, hemoglobin.
At each time point, comparisons between groups were performed at each category of Hb level range by 2 × 2 crosstabs.
Changing Trends of Actual Roxadustat Dose
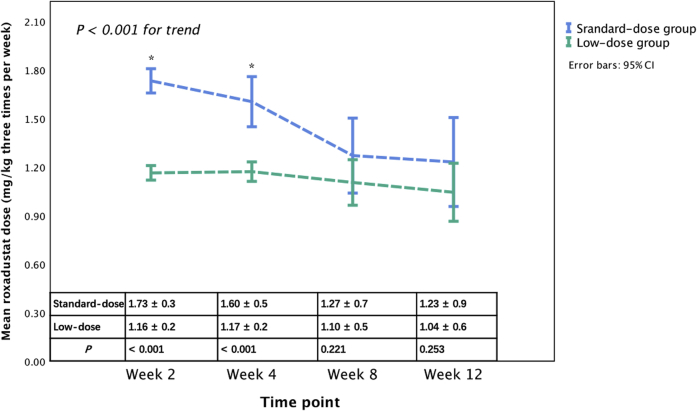
Changing trends in the actual roxadustat dose in both groups are shown in Figure 3. A significantly different trend was observed between the groups after adjusting for the baseline roxadustat dose (P < 0.001). There was a significant roxadustat dose decrease in the standard-dose group (P < 0.001), whereas no significant change in dose in the low-dose group was observed (P = 0.32). Compared with the low-dose group, roxadustat doses at baseline and at week 4 and average roxadustat dose during the study in the standard-dose group were significantly higher (P < 0.001), while roxadustat doses were comparable at week 8 and 12 (P > 0.05).
Figure 3.
Comparison of actual roxadustat dose between the standard-dose group and the low-dose group. ∗P < 0.001 between the standard-dose group and the low-dose group.
Changing Trends in Iron Parameters and in the Serum Lipid Spectrums
There were no significant differences in terms of changing trends in iron parameters including serum iron, ferritin, total iron binding capacity, and transferrin saturation between the groups after adjusting for baseline corresponding iron parameters and total iron element consumption (P > 0.05) (Table 3). Compared with the baseline, the total iron binding capacity value markedly increased in both groups (P < 0.001), but transferrin saturation value significantly decreased only in the low-dose group (P = 0.02). No significant differences in serum iron and ferritin levels between baseline and week 12 were found in either group (P > 0.05).
Table 3.
Changes in the iron parameters between baseline and week 12 in the 2 roxadustat initial-dose groups
| Variable | Standard-dose group |
Low-dose group |
P for trend | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 12 | P | Baseline | Week 12 | P | ||
| Serum iron | 14.5 ± 7.1 | 15.2 ± 6.0 | 0.54 | 15.8 ± 5.9 | 15.2 ± 5.0 | 0.56 | 0.42 |
| Ferritin | 313.3 ± 240.1 | 286.6 ± 234.8 | 0.11 | 288.1 ± 200.5 | 267.6 ± 194.8 | 0.16 | 0.61 |
| TIBC | 45.7 ± 7.5 | 50.2 ± 8.2 | <0.001 | 45.7 ± 6.7 | 53.3 ± 9.9 | <0.001 | 0.26 |
| TSAT | 32.9 ± 17.1 | 31.0 ± 12.9 | 0.46 | 35.0 ± 13.0 | 29.8 ± 12.2 | 0.02 | 0.82 |
TIBC, total iron binding capacity; TSAT, transferring saturation.
P for trend compared the change trend between roxadustat initial-dose groups; P, compared between baseline and week 12.
There was significantly different changing trend in triglyceride level between groups after adjustment for baseline value and the defined daily dose of β-Hydroxy β-methylglutaryl-coenzyme A reductase inhibitors (P = 0.04) (Table 4). A mildly significant decrease in triglyceride level was only observed in the standard-dose group (P = 0.03). Total cholesterol and high density lipoprotein levels at week 12 significantly decreased from baseline in both groups (P < 0.05). No differences were found in the changing trends in total cholesterol and high density lipoprotein levels between the groups after adjustment (P > 0.05).
Table 4.
Changes in the lipid levels tween baseline and week 12 in the 2 roxadustat initial-dose groups
| Variable | Standard-dose group |
Low-dose group |
P for trend | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 12 | P | Baseline | Week 12 | P | ||
| Triglyceride | 1.6 (1.3–2.1) | 1.3 (1.1–2.2) | 0.03 | 1.7 (1.2–2.8) | 1.7 (1.2–3.4) | 0.95 | 0.04 |
| Total cholesterol | 4.1 ± 0.8 | 3.6 ± 0.9 | 0.01 | 4.3 ± 1.0 | 4.0 ± 1.1 | 0.03 | 0.29 |
| HDL | 1.0 ± 0.3 | 0.9 ± 0.2 | 0.01 | 0.9 ± 0.3 | 0.8 ± 0.3 | 0.002 | 0.33 |
| LDL | 2.0 ± 0.5 | 2.0 ± 0.6 | 0.37 | 2.2 ± 0.7 | 2.0 ± 0.7 | 0.051 | 0.31 |
HDL, high density lipoprotein; LDL, low density lipoprotein.
P for trend compared the change trend between roxadustat initial-dose groups; p, compared between baseline and week 12.
Discussion
This open-label 12-week pilot RCT was the first comparative study on different roxadustat regimens exclusively involving patients on PD. Among our subjects converted from ESA treatment, standard-dose and low-dose regimens could achieve comparable proportions of Hb-target met. The mean change of Hb at week 12 from baseline was not significantly different between the 2 groups.
Till now, phase Ⅱ and Ⅲ studies of roxadustat have mainly focused on patients with non–dialysis-dependent CKD or HD, and only limited data in patients undergoing PD have been reported.21, 22, 23 These studies verified that roxadustat was well tolerated and effective in maintaining target Hb levels for those who were previously treated or not treated with ESA but did not provide the evidence on the comparisons of different dose regimens of roxadustat. Our study thus provided the first-hand evidence on the effectiveness and feasibility of low-dose regimen in the PD population.
The dose-effect relationship between roxadustat and Hb response has been repeatedly reported.5,7,13 A phase Ⅱ clinical trial involving nondialyzing patients with CKD showed that Hb response rates (>100 g /l) within 6 weeks were 58%, 40%, 91%, and 100% with 0.7 mg/kg, 1 mg/kg, 1.5 mg/kg, and 2 mg/kg of roxadustat, respectively.12 At a higher dose of roxadustat (1.5 mg/kg–2.5 mg/kg), the Hb level could increase by 10 to 20 g/l during 4 weeks in patients with CKD and HD.6 In our study, PD patients had a good condition of anemia management with relatively lower dose of ESA at baseline than their HD counterparts.14,24 This is why patients in the low-dose group could achieve as high a proportion of Hb-target met and Hb response rate (if defined by Hb >100 g/l as previous studies) as that of patients in the standard-dose group. Of note, their Hb level (108 g/l) and mean ESA dose (15.6 U/kg/d) were also comparable to their PD peers as the Peritoneal Dialysis Telemedicine-assisted Platform cohort including 27 centers’ data in PR China reported (15.4 U/kg/d).17 This clue indicates that low-dose regimen verified through our preliminary study is possibly applicable to more PD patients.
Of note, despite a comparable proportion of Hb-target met, patients in the low-dose group were more likely to have Hb <100 g/l at weeks 8 and 12. This finding should not be considered as a dissenting opinion of low-dose regimen owing to several reasons. First, on the basis of a preset dose adjustment rule according to Hb response, we found a significant decrease in the roxadustat dose in the standard-dose group. In a phase Ⅲ clinical trial involving patients on dialysis, a group that was administrated the protocol same to the standard-dose group in our study also reported a 0.5 mg/kg of reduction in roxadustat dose at the fifth week.5 We considered that the low-dose group underwent less dose adjustment and consumption may lead to a smoother and cost-efficient strategy. Second, we cannot preclude the inappropriateness of current dosing-adjustment strategy. At 120 to 130 of Hb levels, it is recommended to decrease the drug dose no matter the change of Hb was >10 g/l or −10∼10 g/l. This regimen led to a quick reduction in Hb levels from the peak in some individuals, even in the low-dose group. Third, recent meta-analysis indicated that standard-dose roxadustat therapy could increase serious incidences of treatment-emergent adverse events compared with ESA in non–dialysis-dependent CKD population.25,26 Taken together, more heterogeneous and long-term interventional studies should be performed to observe the effectiveness and safety of different initial-dose regimens and dosing-adjustment strategies for anemia correction.
From baseline to week 12, we found an increasing trend in the total iron binding capacity levels in both groups and a comparable changing trend in all iron parameters after adjusting for total iron element supplementation between groups, suggesting mildly improvement in iron metabolism in both groups. These findings supported previous studies proving that hypoxia-inducible factor prolyl hydroxylase inhibitor stimulates endogenous erythropoietin production while simultaneously coordinating iron bioavailability.5,6,8,12,13,23,27 Without measurement of hepcidin, iron metabolism associated with hepcidin was not provided.
Moreover, significant decreasing trends in total cholesterol and high density lipoprotein in both groups and a decreasing trend in triglyceride levels in the standard-dose group were found over the 12-week period. These findings again supported that roxadustat has some metabolic benefits.5,6 One previous study even showed a decreasing trend in these 4 components of the lipid panel over a 27-week follow-up,5 suggesting a possibly prolonged effect on lipid of roxadustat.
Our study is the first postmarketing RCT on dose-regimen exploration of roxadustat in PD population, adding to the body of knowledge concerning initiating roxadustat administration in the setting of ESA conversion. A tight study design with a closely monitored follow-up period undertaken in a multicenter setting is likely to enhance reliability and completeness of data. The findings indicated a necessity for designing larger and more dedicated RCTs involving different dosing strategies for individuals with varied characteristics, such as different Hb levels, iron status, inflammation status, and EPO responsiveness at baseline.
This study had some limitations. First, this small-size and short-term interventional study had weak power to identify the performance of different dose regimes. Second, despite multicenter RCT design and comparable baseline characteristics, the study enrolled relatively stable, young, and nonobese patients on PD. We should improve the generalizability through further interventional trials. Third, nearly all patients used ESAs before the study and converted to roxadustat with different initial-dose regimens. Although the participants were required to have stopped ESA administration for 3 to 7 days, effect of ESA was likely to lag behind. However, baseline characteristics including ESA administration were even between groups on the basis of the RCT design. Third, it is worth noting that about 30% patients had Hb >120 g/l or 130 g/l during the research. We were not sure whether there would be some long-term effects of ESA. Last, while our findings suggested that drug costs on roxadustat might be reduced in the low-dose group, more pharmaco-economic indicators such as whole medical expenditure and healthcare resource utilization should be considered in future studies.
In terms of practical clinical interest, our findings suggest that a lower starting dose of roxadustat effectively allowed the Hb target to be met with less dose adjustment and consumption. This finding provided a possibility of low-dose regimen except for the current weight-based protocol. Starting patients on PD in a clinically stable status with a lower dose of roxadustat appears feasible.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors extend our gratitude to all patients and staff of the peritoneal dialysis center of Peking University First Hospital, Beijing Haidian Hospital, and Beijing Hospital of Traditional Chinese Medicine, for their continuing contribution to this study. Scientific Research Project of Capital Health Development (2020-2-4079); CAMS Innovation Fund for Medical Sciences (2019-I2M-5-046); Youth Clinical Research Project Of Peking University First Hospital (2017CR03, 2019CR25)
Availability of Data and Material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
Research idea and study design: ZY, TTM, JCL, JD; data acquisition: XX, YX, BY, DS, TTM, GF, JZ; statistical analysis: ZY, XX, TTM, SNZ, JD; manuscript drafting or revision: ZY, XX, TTM, JD; supervision or mentorship: JD. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Footnotes
Table S1. Roxadustat Dose Adjustment rule.
Table S2. Data collection schedule during baseline evaluation and follow-up in the study.
Table S3. Comparison of Baseline characteristics between the 2 roxadustat initial-dose groups (appendix).
Table S4. Adverse events during the study period.
Table S5. Number and proportion of patients with Hb at different levels in the 2 roxadustat dose groups (only patients conversion from ESA treatment).
Table S6. Number and proportion of patients with Hb at different levels in the 2 roxadustat dose groups (per-protocol population).
Figure S1. Comparison of hemoglobin responses between the standard-dose group and the low-dose group (per-protocol population).
CONSORT checklist.
Supplementary Material
Table S1. Roxadustat Dose Adjustment rule.
Table S2. Data collection schedule during baseline evaluation and follow-up in the study.
Table S3. Comparison of Baseline characteristics between the two roxadustat initial-dose groups (appendix).
Table S4. Adverse events during the study period.
Table S5. Number and proportion of patients with Hb at different levels in the two roxadustat dose groups (only patients conversion from ESA treatment).
Table S6. Number and proportion of patients with Hb at different levels in the two roxadustat dose groups (per-protocol population).
Figure S1. Comparison of hemoglobin responses between the standard-dose group and the low-dose group (per-protocol population).
CONSORT checklist.
STROBE Statement (PDF)
References
- 1.McClellan W., Aronoff S.L., Bolton W.K., et al. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin. 2004;20:1501–1510. doi: 10.1185/030079904X2763. [DOI] [PubMed] [Google Scholar]
- 2.Hörl W.H. Anaemia management and mortality risk in chronic kidney disease. Nat Rev Nephrol. 2013;9:291–301. doi: 10.1038/nrneph.2013.21. [DOI] [PubMed] [Google Scholar]
- 3.Provenzano R., Shutov E., Eremeeva L., et al. Roxadustat for anemia in patients with end-stage renal disease incident to dialysis. Nephrol Dial Transplant. 2021;36:1717–1730. doi: 10.1093/ndt/gfab051. [DOI] [PubMed] [Google Scholar]
- 4.Shutov E., Sułowicz W., Esposito C., et al. Roxadustat for the treatment of anemia in chronic kidney disease patients not on dialysis: a phase 3, randomized, double-blind, placebo-controlled study (ALPS) Nephrol Dial Transplant. 2021;36:1629–1639. doi: 10.1093/ndt/gfab057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N., Hao C., Liu B.C., et al. Roxadustat treatment for anemia in patients undergoing long-term dialysis. N Engl J Med. 2019;381:1011–1022. doi: 10.1056/NEJMoa1901713. [DOI] [PubMed] [Google Scholar]
- 6.Chen N., Qian J., Chen J., et al. Phase 2 studies of oral hypoxia-inducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China. Nephrol Dial Transplant. 2017;32:1373–1386. doi: 10.1093/ndt/gfx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Provenzano R., Besarab A., Wright S., et al. Roxadustat (FG-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: a phase 2, randomized, 6- to 19-week, open-label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am J Kidney Dis. 2016;67:912–924. doi: 10.1053/j.ajkd.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Chen N., Hao C., Peng X., et al. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N Engl J Med. 2019;381:1001–1010. doi: 10.1056/NEJMoa1813599. [DOI] [PubMed] [Google Scholar]
- 9.Akizawa T., Iwasaki M., Otsuka T., Yamaguchi Y., Reusch M. Phase 3 study of roxadustat to treat anemia in non-dialysis-dependent CKD. Kidney Int Rep. 2021;6:1810–1828. doi: 10.1016/j.ekir.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fishbane S., El-Shahawy M.A., Pecoits-Filho R., et al. Roxadustat for treating anemia in patients with CKD not on dialysis: results from a randomized phase 3 study. J Am Soc Nephrol. 2021;32:737–755. doi: 10.1681/ASN.2020081150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akizawa T., Iwasaki M., Yamaguchi Y., Majikawa Y., Reusch M. Phase 3, randomized, double-blind, active-comparator (darbepoetin alfa) study of oral roxadustat in CKD patients with anemia on hemodialysis in Japan. J Am Soc Nephrol. 2020;31:1628–1639. doi: 10.1681/ASN.2019060623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Besarab A., Provenzano R., Hertel J., et al. Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol Dial Transplant. 2015;30:1665–1673. doi: 10.1093/ndt/gfv302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Besarab A., Chernyavskaya E., Motylev I., et al. Roxadustat (FG-4592): correction of anemia in incident dialysis patients. J Am Soc Nephrol. 2016;27:1225–1233. doi: 10.1681/ASN.2015030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bae M.N., Kim S.H., Kim Y.O., et al. Association of erythropoietin-stimulating agent responsiveness with mortality in hemodialysis and peritoneal dialysis patients. PLoS One. 2015;10 doi: 10.1371/journal.pone.0143348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duong U., Kalantar-Zadeh K., Molnar M.Z., et al. Mortality associated with dose response of erythropoiesis-stimulating agents in hemodialysis versus peritoneal dialysis patients. Am J Nephrol. 2012;35:198–208. doi: 10.1159/000335685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frei U., Kwan J.T., Spinowitz B.S. Epoetin Delta 3002 Study Group. Anaemia management with subcutaneous epoetin delta in patients with chronic kidney disease (predialysis, haemodialysis, peritoneal dialysis): results of an open-label, 1-year study. BMC Nephrol. 2009;10:5. doi: 10.1186/1471-2369-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perlman R.L., Zhao J., Fuller D.S., et al. International anemia prevalence and management in peritoneal dialysis patients. Perit Dial Int. 2019;39:539–546. doi: 10.3747/pdi.2018.00249. [DOI] [PubMed] [Google Scholar]
- 18.Chan A.W., Tetzlaff J.M., Altman D.G., et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.WHO Collaborating Centre for Drug Statistics Methodology ATC/DDD Index 2022. WHO Collaborating Centre for Drug Statistics Methodology. https://www.whocc.no/atc_ddd_index/ Accessed March 3, 2021.
- 21.Hirai K., Nonaka H., Ueda M., et al. Effects of roxadustat on the anemia and iron metabolism of patients undergoing peritoneal dialysis. Front Med (Lausanne) 2021;8:667117. doi: 10.3389/fmed.2021.667117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou Y.P., Mao X.Y., Wang C., et al. Roxadustat treatment for anemia in peritoneal dialysis patients: a randomized controlled trial. J Formos Med Assoc. https://doi.org/10.1016/j.jfma.2021.06.004 Published online June 21, 2021. [DOI] [PubMed]
- 23.Akizawa T., Otsuka T., Reusch M., Ueno M. Intermittent oral dosing of roxadustat in peritoneal dialysis chronic kidney disease patients with anemia: a randomized, phase 3, multicenter, open-label study. Ther Apher Dial. 2020;24:115–125. doi: 10.1111/1744-9987.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wetmore J.B., Peng Y., Monda K.L., et al. Trends in anemia management practices in patients receiving hemodialysis and peritoneal dialysis: a retrospective cohort analysis. Am J Nephrol. 2015;41:354–361. doi: 10.1159/000431335. [DOI] [PubMed] [Google Scholar]
- 25.Tang M., Zhu C., Yan T., Zhou Y., Lv Q., Chuan J. Safe and effective treatment for anemic patients with chronic kidney disease: an updated systematic review and meta-analysis on roxadustat. Front Pharmacol. 2021;12:658079. doi: 10.3389/fphar.2021.658079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng L., Tian J., Liu D., et al. Efficacy and safety of roxadustat for anaemia in dialysis-dependent and non-dialysis-dependent chronic kidney disease patients: a systematic review and meta-analysis. Br J Clin Pharmacol. https://doi.org/10.1111/bcp.15055 Published online August 24, 2021. [DOI] [PubMed]
- 27.Shah Y.M., Matsubara T., Ito S., Yim S.H., Gonzalez F.J. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009;9:152–164. doi: 10.1016/j.cmet.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.