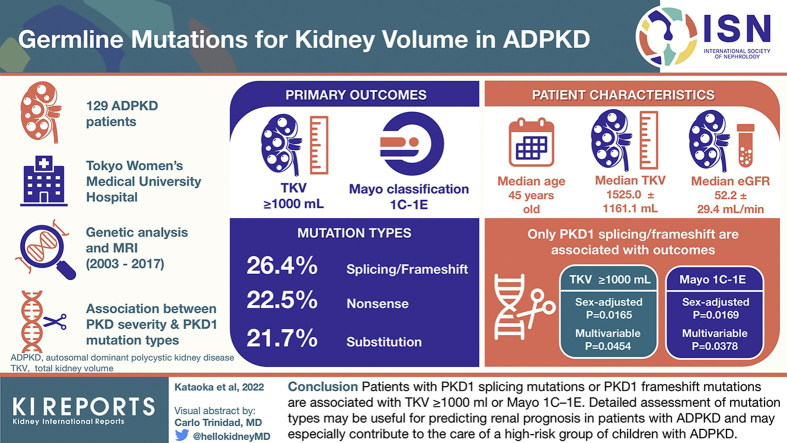
Abstract
Introduction
Valid prediction models or predictors of disease progression in children and young patients with autosomal dominant polycystic kidney disease (ADPKD) are lacking. Although total kidney volume (TKV) and Mayo imaging classification are generally used to predict disease progression in patients with ADPKD, it remains unclear whether germline mutation types are associated with these factors. We therefore investigated the association between mutation type and TKV and Mayo imaging classification among patients with ADPKD.
Methods
A total of 129 patients with ADPKD who underwent genetic analyses were enrolled in the study. The associations between the severity of PKD (TKV ≥ 1000 ml and Mayo classes 1C–1E) and the PKD1 mutation types (nonsense mutation, frameshift or splicing mutation, and substitution) were evaluated.
Results
Among the mutation types, only PKD1 splicing/frameshift mutation had significant associations with TKV ≥ 1000 ml in sex-adjusted and multivariable logistic analyses. Similarly, only the PKD1 splicing/frameshift mutation was significantly associated with Mayo 1C–1E in sex-adjusted and multivariable logistic analyses. PKD1 nonsense mutation, PKD1 substitution, or PKD1 mutation position had no significant association with TKV ≥ 1000 ml or Mayo 1C–1E.
Conclusion
Kidney cyst severity differs according to the mutation types in PKD1. Patients with PKD1 splicing mutations or PKD1 frameshift mutations are associated with TKV ≥ 1000 ml or Mayo 1C–1E. Detailed assessment of mutation types may be useful for predicting renal prognosis in patients with ADPKD and may especially contribute to the care of a high-risk group of children with ADPKD.
Keywords: autosomal dominant polycystic kidney disease, frameshift mutation, germline mutation, kidney volume, Mayo imaging classification, splicing mutation
Graphical abstract
ADPKD is the most common progressive hereditary kidney disease.1 At present, kidney disease progression in patients with ADPKD is generally predicted using estimated glomerular filtration rate (eGFR),2,3 TKV,4, 5, 6 and the Mayo imaging classification.7, 8, 9 eGFR, as a representative predictor of chronic kidney disease, is strong but less sensitive in the early stages of ADPKD because the eGFR sometimes declines in a nonlinear pattern10 and generally remains in the normal range (eGFR ≥ 90 ml/min per 1.73 m2) before the age of 30 years, despite the progressive formation of cysts.4 Therefore, in early stage disease, kidney volume has been used as a predictor5,7,11,12 and has already been used as the end point in clinical trials.13 Perrone et al.5 reported that the risk of progression to a 30% decline in eGFR or end-stage renal disease in patients with a larger TKV of ≥1000 ml was significantly greater than that in patients with a smaller TKV (<1000 ml), regardless of kidney function. The Mayo imaging classification divides typical ADPKD into 5 groups (Mayo image classes 1A–1E) according to age- and height-adjusted TKV to predict renal outcome.7 Patients with Mayo image classes 1C–1E (Mayo 1C–1E) had a faster decline in renal function compared with those with classes 1A–1B7; Mayo image classes 1C–1E are defined as “rapidly progressing disease,” and for which, tolvaptan treatments are recommended.8,9
Although TKV and the Mayo imaging classification are clinically important, valid prediction models to identify children with ADPKD who therefore likely to suffer kidney failure are still lacking, as the radiological features in children are different from those in adult patients.14 As TKV changes with aging, the Mayo imaging classification is only applicable from 16 years of age.7 This situation is unfavorable because 20% of children with ADPKD have hypertension,15 and the pediatric stages of ADPKD have been recognized as important stages for disease understanding and treatment.14 Considering that beneficial effects of early treatment for slowing the increase in TKV have been reported in children with ADPKD16 and that valid prediction models to identify children with ADPKD likely to suffer kidney failure are lacking,14 it is important to identify a high-risk group among patients with ADPKD, who are candidates for early intervention. The lack of early prognostic markers for kidney prognosis is still a concern for both physicians and patients17; additional indicators other than eGFR, TKV, and Mayo 1C–1E are clinically desired in children with ADPKD.
Mutations in PKD1 and PKD2 are responsible for ADPKD.18,19 We believe that detailed information on germline mutations could be helpful in predicting the severity of ADPKD. Indeed, many reports have indicated that patients with a PKD1 mutation, especially truncating mutations, have a faster decline in kidney function than patients with a PKD2 mutation.20, 21, 22, 23, 24, 25 Similarly, patients with PKD1 mutations, especially truncating mutations, have significantly larger kidneys26, 27, 28 and more cysts26 than those with PKD2 mutations. As a result, genotypic factors such as truncating PKD1 mutations, nontruncating PKD1 mutations, and PKD2 mutations have been adopted in scoring systems (PROPKD Score) to predict kidney failure.29 Although the PROPKD Score contributes to the clinical setting, it has limited value in patients who are <35 years old and who do not have complications.30 In addition, the genetic variables used in the PROPKD Score are limited to only 3 mutation types (truncating PKD1, nontruncating PKD1, and PKD2). Therefore, useful genetic information for determining the prognosis of a patient is yet to be determined. In ADPKD, 4 mutation types (splicing mutation, frameshift mutation, nonsense mutation, and substitution) are reported to account for >90% of patients.30,31 Of these gene mutations, 3 (splicing mutations, frameshift mutations, and nonsense mutations) are classified as truncating mutations, but they have recently been reported to have different effects on disease severity in patients with ADPKD.32,33 In particular, eGFR decline is reported to be associated with PKD1 splicing mutations and PKD1 frameshift mutations.33 At present, the relationship between TKV ≥ 1000 ml, Mayo imaging classification of 1C–1E, and detailed gene mutation types in PKD has not been reported. In this study, we hypothesized that PKD1 splicing and frameshift mutations could be predictors for a TKV ≥ 1000 ml and Mayo imaging class of 1C–1E; in addition, we investigated the relationship between these 2 predictors and the detailed gene mutation types.
Methods
Study Design
A total of 129 patients with ADPKD who presented at the Kidney Center at the Tokyo Women’s Medical University Hospital (Tokyo, Japan) and underwent genetic analysis34 between 2003 and 2017, including magnetic resonance imaging or computed tomography to evaluate TKV and Mayo imaging classification, were included in the study (Supplementary Figure S1). All procedures were approved by the research ethics committee of Tokyo Women’s Medical University (number 196 B) in accordance with the 1964 Declaration of Helsinki and its later amendments or with comparable ethical standards. Written informed consent was obtained from all the participants. A detailed description of the methods can be found in the Supplementary Material (Supplementary Methods: mutation analysis, measurement of kidney volume and kidney cyst, definition of comorbidities). The participants were assessed up to October 31, 2020.
Outcome Evaluation
The primary outcomes were TKV ≥ 1000 ml and Mayo imaging classification 1C–1E.
Statistical Analyses
Continuous variables are reported as mean ± SD or as median (minimum, maximum). Categorical variables are reported as percentages, unless otherwise stated. Group differences were evaluated using unpaired t tests, Mann-Whitney U tests, χ2 tests, or Fisher exact tests, as appropriate. Logistic regression analyses were performed to determine the factors associated with outcomes.35,36 Variables of interest, including general risk factors for outcomes based on existing knowledge, were included in the multivariable model. Standard methods were applied to estimate sample size for multivariable logistic regression, with at least 5 outcomes needed for each independent variable.36 Discriminatory ability was measured using the area under the receiver operating characteristic curve. The goodness-of-fit was evaluated using McFadden’s pseudo-R-squared (pseudo-R2).37 All statistical tests were 2-tailed, and statistical significance was set at P < 0.05. All statistical analyses were performed using JMP Pro version 15.0.0 software program (SAS Institute, Cary, NC).
Results
Patient Characteristics
The characteristics of the entire patient group are found in Table 1 and Supplementary Table S1. Regarding mutation type, 34 patients harbored PKD1 splicing mutations or frameshift mutations owing to the insertion or deletion of nucleotides (26.4%), 29 patients harbored PKD1 nonsense mutations (22.5%), and 28 patients harbored PKD1 substitutions (21.7%). At the time of evaluating TKV ≥ 1000 ml/Mayo imaging classification, the median age was 45 years (minimum–maximum, 15–77 years), eGFR was 52.2 ± 29.4 ml/min per 1.73 m2, TKV was 1525.0 ± 1161.1 ml, and maximum liver cyst diameter was 3.95 ± 3.55 cm. Hypertension affected 81 patients (62.8%).
Table 1.
Patient characteristics according to TKV and Mayo classification (entire cohort, N = 129)
| Variables | Total, N = 129 | Patients with TKV <1000 ml, n = 55 | Patients with TKV ≥1000 ml, n = 74 | P value | Total, N = 121 | Mayo imaging classification 1A–1B, n = 48 | Mayo imaging classification 1C–1E, n = 73 | P value |
|---|---|---|---|---|---|---|---|---|
| Clinical findings | ||||||||
| Age (yr) | 45 (15–77) [129] | 43 (15–74) | 47 (22–77) | 0.0709 | 45 (15–77) [121] | 50.5 (21–77) | 44 (15–75) | 0.0019a |
| Sex (men), n (%) | 55 (42.6) [129] | 14 (25.5) | 41 (55.4) | 0.0007a | 52 (43.0) [121] | 15 (31.3) | 37 (50.7) | 0.0346a |
| Smoking, current or former, n (%) | 32 (24.8) [129] | 9 (16.4) | 23 (31.1) | 0.0556 | 31 (25.6) [121] | 8 (16.7) | 23 (31.5) | 0.0673 |
| PKD1/PKD2/unknown, n (%) | 99 (76.7)/21 (16.3)/9 (7.0) [129] | 42 (76.4)/8 (14.6)/5 (9.1) | 57 (77.0)/13 (17.6)/4 (5.4) | 0.6726 | 93 (76.9)/21 (17.4)/7 (5.8) [121] | 34 (70.8)/10 (20.8)/4 (8.3) | 59 (80.8)/11 (15.1)/3 (4.1) | 0.4018 |
| PKD1 truncating mutation, n (%) | 68 (52.7) [129] | 25 (45.5) | 43 (58.1) | 0.1546 | 63 (52.1) [121] | 21 (43.8) | 42 (57.5) | 0.1376 |
| PKD1 splicing mutation or frameshift mutation, n (%) | 34 (26.4) [129] | 8 (14.6) | 26 (35.1) | 0.0087a | 33 (27.3) [121] | 7 (14.6) | 26 (35.6) | 0.0110a |
| PKD1 nonsense mutation, n (%) | 29 (22.5) [129] | 13 (23.6) | 16 (21.6) | 0.7863 | 27 (22.3) [121] | 11 (22.9) | 16 (21.9) | 0.8973 |
| PKD1 substitution, n (%) | 28 (21.7) [129] | 14 (25.5) | 14 (18.9) | 0.3732 | 27 (22.3) [121] | 11 (22.9) | 16 (21.9) | 0.8973 |
| PKD1 mutation position (cDNA) | 7816 (1–12,721) [99] | 7546 (1–12,577) | 8309 (529–12,721) | 0.0834 | 8068 (1–12,721) [93] | 7546 (1–12,145) | 8515 (529–12,721) | 0.0665 |
| PKD2 mutation position (cDNA) | 1249 (1–2614) [19] | 1249 (181–2614) | 1249 (1–2507) | 0.5497 | 1249 (1–2614) [19] | 1249 (181–2614) | 1249 (1–2507) | 0.5589 |
| CKD1–2/CKD3/CKD4–5, n (%) | 50 (39.4)/45 (35.4)/32 (25.2) [127] | 33 (60.0)/16 (29.1)/6 (10.9) | 17 (23.6)/29 (40.3)/26 (36.1) | <0.0001a | 48 (39.7)/41 (33.9)/32 (26.5) [121] | 21 (43.8)/18 (37.5)/9 (18.8) | 27 (37.0)/23 (31.5)/23 (31.5) | 0.2978 |
| Mayo imaging classification class 1A–1B/ class 1C–1E, n (%) | 48 (39.7)/73 (60.3) [121] | 38 (76.0)/12 (24.0) | 10 (14.1)/61 (85.9) | <0.0001a | NA | NA | NA | NA |
| eGFR (ml/min per 1.73m2) | 52.2 ± 29.4 [127] | 66.9 ± 26.4 | 41.0 ± 26.7 | <0.0001a | 52.0 ± 29.7 [121] | 56.9 ± 27.7 | 48.7 ± 30.7 | 0.1384 |
| U-Prot (g/g・Cre) | 0.00 (0.00–7.14) [104] | 0.00 (0.00–0.59) | 0.08 (0.00–7.14) | 0.0059a | 0.00 (0.00–7.14) [99] | 0.00 (0.00–7.14) | 0.00 (0.00–1.76) | 0.2151 |
| TKV (ml) | 1525.0 ± 1161.1 [129] | 665.1 ± 195.1 | 2164.1 ± 1168.1 | <0.0001a | 1532.7 ± 1154.6 [121] | 765.6 ± 369.5 | 2037.0 ± 1217.6 | <0.0001a |
| TKV ≥1000 ml, n (%) | 74 (57.4) [129] | NA | NA | NA | 71 (58.7) [121] | 10 (20.8) | 61 (83.6) | <0.0001a |
| htTKV (ml/m) | 923.2 ± 677.3 [121] | 410.9 ± 122.1 | 1283.9 ± 675.6 | <0.0001a | 923.2 ± 677.3 [121] | 472.5 ± 223.6 | 1219.5 ± 712.3 | <0.0001a |
| Maximum kidney cyst diameter (cm) | 6.54 ± 2.09 [129] | 5.54 ± 2.03 | 7.28 ± 1.82 | <0.0001a | 6.56 ± 2.04 [121] | 5.66 ± 1.94 | 7.15 ± 1.89 | <0.0001a |
| Maximum liver cyst diameter (cm) | 3.95 ± 3.55 [129] | 3.63 ± 3.21 | 4.18 ± 4.79 | 0.3905 | 3.80 ± 3.49 [121] | 3.87 ± 3.30 | 3.76 ± 3.64 | 0.8627 |
| Intracranial aneurysm, n (%) | 19 (14.7) [129] | 2 (3.6) | 17 (23.0) | 0.0021a | 19 (15.7) [121] | 3 (6.3) | 16 (21.9) | 0.0224a |
| Comorbidities | ||||||||
| Hypertension, n (%) | 81 (62.8) [129] | 23 (41.8) | 58 (78.4) | <0.0001a | 79 (65.3) [121] | 25 (52.1) | 54 (74.0) | 0.0133a |
| Hyperuricemia, n (%) | 44 (34.1) [129] | 9 (16.4) | 35 (47.3) | 0.0002a | 43 (35.5) [121] | 10 (20.8) | 33 (45.2) | 0.0061a |
| Low HDL cholesterol, n (%) | 19 (14.7) [129] | 4 (7.3) | 15 (20.3) | 0.0463a | 18 (14.9) [121] | 2 (4.2) | 16 (21.9) | 0.0081a |
CKD, chronic kidney disease; Cre, creatinine; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; htTKV, height-adjusted total kidney volume; mutation position (cDNA), the location number of PKD1 or PKD2 mutation position in the nucleotide sequence of cDNA; NA, not applicable; PKD, polycystic kidney disease; TKV, total kidney volume; U-Prot, urinary protein excretion.
Continuous values are expressed as the mean ± SD or median (minimum–maximum). Count data are expressed as n (%). Values for number of subjects are in brackets.
P < 0.05.
Comparative analysis of the patients within the group revealed that 85.9% of the patients with TKV ≥1000 ml had a higher Mayo image classification (Mayo1C–1E) (P < 0.0001), compared with those with TKV < 1000 ml (24.0%). Furthermore, we determined the following characteristics: male sex (55.4% in patients with TKV ≥ 1000 ml vs. 25.5% in patients with TKV < 1000 ml, P = 0.0007), PKD1 splicing mutation or frameshift mutation (35.1% in patients with TKV ≥1000 ml vs. 14.6% in patients with TKV <1000 ml, P = 0.0087), intracranial aneurysm (23.0% in patients with TKV ≥1000 ml vs. 3.6% in patients with TKV <1000 ml, P = 0.0021), hypertension (78.4% in patients with TKV ≥1000 ml vs. 41.8% in patients with TKV < 1000 ml, P < 0.0001), hyperuricemia (47.3% in patients with TKV ≥1000 ml vs. 16.4% in patients with TKV <1000 ml, P = 0.0002), and low HDL cholesterol (20.3% in patients with TKV ≥1000 ml vs. 7.3% in patients with TKV <1000 ml, P = 0.0463).
Drawing a comparative analysis between the patients with and without a Mayo classification of 1C–1E revealed that 83.6% of patients with Mayo classes 1C–1E compared with 20.8% of those with Mayo classes 1A–1B had higher rates of TKV ≥ 1000 ml (P < 0.0001). We also determined the following characteristics: male sex (50.7% in patients with Mayo 1C–1E vs. 31.3% in patients with Mayo 1A–1B, P = 0.0346), PKD1 splicing mutations or frameshift mutations (35.6% in patients with Mayo 1C–1E vs. 14.6% in patients with Mayo 1A–1B, P = 0.0110), intracranial aneurysm (21.9% in patients with Mayo 1C–1E vs. 6.3% in patients with Mayo 1A–1B, P = 0.0224), hypertension (74.0% in patients with Mayo 1C–1E vs. 52.1% in patients with Mayo 1A–1B, P = 0.0133), hyperuricemia (45.2% in patients with Mayo 1C–1E vs. 20.8% in patients with Mayo 1A–1B, P = 0.0061), and low HDL cholesterol (21.9% in patients with Mayo 1C–1E vs. 4.2% in patients with Mayo 1A–1B, P = 0.0081).
PKD1 Splicing/Frameshift Mutation as a Predictive Indicator of Both TKV ≥ 1000 ml and Mayo 1C–1E
Univariable and multivariable logistic regression analyses were performed for TKV ≥ 1000 ml and Mayo imaging classification 1C–1E (univariable analyses, Supplementary Tables S2 and S3; multivariable analyses, Tables 2 and 3). PKD1 or PKD2 mutation positions were not associated with TKV ≥ 1000 ml/Mayo 1C–1E (Supplementary Tables S2 and S3).
Table 2.
Sex-adjusted and multivariable logistic regression for correlations between the TKV ≥1000 ml and risk factors (entire cohort, N = 129)
| Variables A. Sex-adjusted logistic regression analyses |
Model for PKD1 truncating mutation (R2 = 0.08, AUC = 0.68) |
Model for PKD1 splicing/frameshift mutation (R2 = 0.10, AUC = 0.70) |
Model for PKD1 nonsense mutation (R2 = 0.07, AUC = 0.66) |
Model for PKD1 substitution (R2 = 0.07, AUC = 0.66) |
||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Men (vs. women) | 3.59 (1.67–7.71) | 0.0001a | 3.56 (1.64–7.76) | 0.0014a | 3.65 (1.71–7.83) | 0.0008a | 3.59 (1.67–7.69) | 0.0010a |
| PKD1 truncating mutation (vs. no) | 1.61 (0.77–3.36) | 0.2057 | — | — | — | — | — | — |
| PKD1 splicing mutation or frameshift mutation (vs. no) | — | — | 3.09 (1.23–7.76) | 0.0165a | — | — | — | — |
| PKD1 nonsense mutation (vs. no) | — | — | — | — | 0.85 (0.35–2.03) | 0.7115 | — | — |
| PKD1 substitution (vs. no) | — | — | — | — | — | — | 0.74 (0.31–1.79) | 0.5048 |
| B. Multivariable logistic regression analyses | Model for PKD1 truncating mutation (R2 = 0.15, AUC = 0.75) |
Model for PKD1 splicing/frameshift mutation (R2 = 0.17, AUC = 0.77) |
Model for PKD1 nonsense mutation (R2 = 0.15, AUC = 0.76) |
Model for PKD1 substitution (R2 = 0.15, AUC = 0.75) |
||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Men (vs. women) | 1.84 (0.76–4.45) | 0.1771 | 1.88 (0.77–4.61) | 0.1685 | 1.82 (0.75–4.40) | 0.1826 | 1.82 (0.75–4.39) | 0.1843 |
| Hypertension (vs. no) | 3.01 (1.29–7.00) | 0.0107a | 3.00 (1.27–7.09) | 0.0122a | 3.21 (1.37–7.53) | 0.0074a | 3.09 (1.33–7.18) | 0.0087a |
| Hyperuricemia (vs. no) | 1.99 (0.73–5.41) | 0.1777 | 1.91 (0.70–5.26) | 0.2081 | 2.14 (0.79–5.77) | 0.1346 | 2.01 (0.74–5.45) | 0.1713 |
| Low high-density lipoprotein cholesterol (vs. no) | 1.82 (0.49–6.71) | 0.3675 | 1.69 (0.45–6.32) | 0.4386 | 1.68 (0.46–6.15) | 0.4338 | 1.83 (0.50–6.75) | 0.3634 |
| PKD1 truncating mutation (vs. no) | 1.36 (0.62–2.99) | 0.4377 | — | — | — | — | — | — |
| PKD1 splicing mutation or frameshift mutation (vs. no) | — | — | 2.69 (1.02–7.10) | 0.0454a | — | — | — | — |
| PKD1 nonsense mutation (vs. no) | — | — | — | — | 0.69 (0.27–1.76) | 0.4308 | — | — |
| PKD1 substitution (vs. no) | — | — | — | — | — | — | 0.79 (0.31–2.03) | 0.6262 |
AUC, area under the receiver operating characteristic curve; PKD, polycystic kidney disease; R2, McFadden’s pseudo-R-squared; TKV, total kidney volume.
Each mutation type, hypertension, hyperuricemia, and low high-density lipoprotein cholesterol were included in the multivariable model.
P < 0.05.
Table 3.
Sex-adjusted and multivariable logistic regression analyses for correlations between the Mayo imaging classification 1C–1E and mutation types (entire cohort, N = 121)
| Variables A. Sex-adjusted logistic regression analyses |
Model for PKD1 truncating mutation (R2 = 0.04, AUC = 0.63) |
Model for PKD1 splicing/frameshift mutation (R2 = 0.07, AUC = 0.67) |
Model for PKD1 nonsense mutation (R2 = 0.03, AUC = 0.60) |
Model for PKD1 substitution (R2 = 0.03, AUC = 0.60) |
||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Men (vs. women) | 2.21 (1.03–4.78) | 0.0430a | 2.21 (1.01–4.83) | 0.0474a | 2.27 (1.06–4.89) | 0.0353a | 2.26 (1.05–4.86) | 0.0366a |
| PKD1 truncating mutation (vs. no) | 1.69 (0.80–3.57) | 0.1700 | — | — | — | — | — | — |
| PKD1 splicing mutation or frameshift mutation (vs. no) | — | — | 3.17 (1.23–8.17) | 0.0169a | — | — | — | — |
| PKD1 nonsense mutation (vs. no) | — | — | — | — | 0.89 (0.37–2.17) | 0.6912 | — | — |
| PKD1 substitution (vs. no) | — | — | — | — | — | — | 1.00 (0.41–2.44) | 0.9945 |
| B. Multivariable logistic regression analyses | Model for PKD1 truncating mutation (R2 = 0.10, AUC = 0.70) |
Model for PKD1 splicing/frameshift mutation (R2 = 0.12, AUC = 0.74) |
Model for PKD1 nonsense mutation (R2 = 0.10, AUC = 0.69) |
Model for PKD1 substitution (R2 = 0.10, AUC = 0.68) |
||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Men (vs. women) | 1.31 (0.53–3.23) | 0.5625 | 1.35 (0.54–3.39) | 0.5165 | 1.27 (0.52–3.12) | 0.5980 | 1.27 (0.52–3.11) | 0.6039 |
| Hypertension (vs. no) | 1.67 (0.71–3.97) | 0.2423 | 1.72 (0.72–4.13) | 0.2230 | 1.80 (0.76–4.27) | 0.1847 | 1.76 (0.75–4.15) | 0.1967 |
| Hyperuricemia (vs. no) | 1.75 (0.65–4.71) | 0.2669 | 1.66 (0.61–4.50) | 0.3219 | 1.89 (0.71–5.02) | 0.2013 | 1.88 (0.70–5.03) | 0.2081 |
| Low high-density lipoprotein cholesterol (vs. no) | 4.85 (1.01–23.35) | 0.0487a | 4.56 (0.94–22.23) | 0.0602 | 4.58 (0.96–21.91) | 0.0570 | 4.66 (0.97–22.36) | 0.0543 |
| PKD1 truncating mutation (vs. no) | 1.49 (0.68–3.29) | 0.3230 | — | — | — | — | — | — |
| PKD1 splicing mutation or frameshift mutation (vs. no) | — | — | 2.84 (1.06–7.59) | 0.0378a | — | — | — | — |
| PKD1 nonsense mutation (vs. no) | — | — | — | — | 0.83 (0.32–2.12) | 0.6912 | — | — |
| PKD1 substitution (vs. no) | — | — | — | — | — | — | 1.04 (0.40–2.65) | 0.9425 |
AUC, area under the receiver operating characteristic curve; PKD, polycystic kidney disease; R2, McFadden’s pseudo-R-squared.
Each mutation type, hypertension, hyperuricemia, and low high-density lipoprotein cholesterol were included in the multivariable model.
P < 0.05.
Among the mutation types, only the PKD1 splicing/frameshift mutation had significant associations with TKV ≥ 1000 ml in sex-adjusted (P = 0.0165) and multivariable (P = 0.0454) logistic analyses (Figure 1a and Table 2). Similarly, only the PKD1 splicing/frameshift mutation was significantly associated with Mayo 1C–1E in sex-adjusted (P = 0.0169) and multivariable (P = 0.0378) logistic analyses (Figure 1b and Table 3). In contrary, PKD1 truncating mutation, PKD1 nonsense mutation, and PKD1 substitution had no significant associations with TKV ≥ 1000 ml/Mayo 1C–1E in sex-adjusted and multivariable logistic analyses (Figure 1 and Table 2, Table 3).
Figure 1.
Odds ratios for TKV ≥ 1000 ml and the Mayo imaging classification 1C–1E in the entire cohort. (a) Mutation type for TKV ≥ 1000 ml. (b) Mutation type for the Mayo imaging classification 1C–1E. The circles represent odds ratios, and the bars represent 95% CI for the association of mutation types with TKV ≥ 1000 ml (derived from A in Table 2) and Mayo imaging classification 1C–1E (derived from A in Table 3). PKD, polycystic kidney disease; PKD1 nonsense, PKD1 nonsense mutation; PKD1 splicing/frameshift, PKD1 splicing mutation or PKD1 frameshift mutation owing to the insertion or deletion of nucleotides; TKV, total kidney volume.
Discussion
Chronic kidney disease, especially hereditary kidney disease, results in a lifelong fight against illness. Therefore, we believe that providing useful predictive information to patients fighting this illness is important. Recently, the significance of a detailed mutation type for patients with ADPKD regarding cerebral aneurysm, severity of polycystic liver disease,32 and renal prognosis33 has been reported. Nevertheless, the association between TKV/Mayo classification and germline mutation types has not been clearly elucidated. To the best of our knowledge, the present study is the first of its kind to perform a detailed analysis of patients with ADPKD, whereby the association between TKV ≥ 1000 ml/Mayo 1C–1E and genetic factors, including genotype, mutation type, and mutation position, was investigated. The results have revealed that the detailed mutation type of PKD1 splicing/frameshift had a significant association with TKV ≥ 1000 ml and the Mayo 1C–1E classification.
As intrafamilial phenotypic variability exists among patients with the same mutation, somatic inactivation of the remaining wild-type PKD1 or PKD2 allele is thought to be required to initiate ADPKD and to play a key role in patients with ADPKD (the 2-hit model of ADPKD).38, 39, 40 As a result, most previous studies on ADPKD have focused on the second hit mechanism and have made remarkable progress in the genome studies of ADPKD; however, research on germline mutations or genetic background has not progressed extensively. Although the 2-hit model is an important mechanism of ADPKD, recent evidence has suggested that PKD progression or severity is influenced by the level of functional polycystins (haploinsufficiency/loss of function model).41,42
In human genetic diseases, haploinsufficiency or loss of function is caused by the nonsense-mediated decay (NMD) process.43, 44, 45 The degradation of transcripts containing premature termination codons through NMD46, 47, 48 prevents the synthesis of aberrantly truncated proteins with potentially harmful dominant-negative effects.49, 50, 51 Nevertheless, various additional determinants of NMD have been recently proposed52,53; NMD efficacy and escape from NMD have been attracting research attention.52,54 It is possible that premature termination codon-containing mRNAs escaping NMD produce aberrant transcripts/truncated proteins with dominant-negative effects/gain of function that in turn contribute to phenotypic variation.47,55,56
In this study, TKV ≥ 1000 ml/Mayo 1C–1E had associations with PKD1 splicing/frameshift mutations, which was not observed in patients with PKD1 nonsense mutations (Figure 1). Transcripts with germline frameshift mutations and splicing mutations that escape NMD are reported in various genetic diseases56, 57, 58, 59 and experimental researches.60, 61, 62 These transcripts can substantially change the amino acid sequences of the encoded proteins, exert a more dramatic effect on the protein 3-dimensional structure than a single amino acid change,63,64 and form aberrant transcripts of the mutated genes.56,57,65 In contrast, nonsense mutations that generate in-frame premature termination codons generally do not produce transcripts with extra aberrant amino acids and tend to cause haploinsufficiency/loss of function.56,66 Indeed, Malan et al.56 elucidated the phenotypic difference between Marshall-Smith Syndrome and Sotos-like overgrowth syndrome based on the difference between nonsense mutations/large deletions and frameshift/splice-site mutations. Patients with Marshall-Smith Syndrome had expression of both the normal and mutant alleles, indicating transcripts with frameshift and splice-site mutations that escape the NMD yield mutant proteins that exert a dominant-negative effect and cause a more severe phenotype of Marshall-Smith Syndrome. In contrast, patients with Sotos-like overgrowth syndrome had expression of only a single wild-type allele. This indicated that transcripts with large deletions and nonsense mutations undergoing NMD lead to haploinsufficiency in patients with Sotos-like overgrowth syndrome with mild intellectual deficits.65 We consider that a similar underlying mechanism affected the patients with ADPKD in this study, resulting in no association between nonsense mutations and TKV ≥ 1000 ml/Mayo 1C–1E. The phenotypic difference according to mutation type in patients with ADPKD (illustrated in in Table 4) might be affected by haploinsufficiency/loss of function model or dominant-negative effects/gain of function.
Table 4.
Relationship between mutation types and phenotypes in kidney cysts, liver cysts, and intracranial aneurysms
| Mutation type | Kidney dysfunction33 | Kidney cysts (the present study) | Liver cysts32 | Intracranial aneurysms (submitted) |
|---|---|---|---|---|
| Splicing mutation | ● | ● | ● (younger age) | |
| Frameshift mutation | ● | ● | ▲ (younger age) | |
| Nonsense mutation | ● | |||
| Substitution | ▲ (older age, low GFR) |
GFR, glomerular filtration rate; ●, high risk; ▲, moderate risk.
The present study has certain limitations. First, as an observational study, the causal relationships associated with our observations were not proven. Second, the sample size was relatively small; hence, further studies are required to confirm our findings in a larger patient cluster. Third, our results do not necessarily exclude a second-hit theory by somatic mutations. Nevertheless, the results of the present study suggest that the pathology of ADPKD can also develop when germline mutations are present. Genetic diagnosis can improve the clinical management of patients and has the potential to benefit patients with ADPKD (especially for a high-risk group of children, such as those with young-onset hypertension) by providing novel therapeutic options.67,68
In conclusion, this study revealed that patients with ADKPD exhibited an association between PKD1 splicing mutations or PKD1 frameshift mutations and TKV ≥ 1000 ml and Mayo classification of 1C–1E. The novel finding that the differences in these germline mutations affect the severity of kidney cysts may provide prognostic benefits for patients with ADPKD.
Disclosure
TM and KT report receiving travel fees and honoraria for lectures from Otsuka Pharmaceutical Co. TM and HK belong to an endowed department sponsored by Otsuka Pharmaceutical Co., Chugai Pharmaceutical Co., Kyowa Hakko Kirin Co., and JMS Co. All the other authors declared no competing interests.
Acknowledgments
This study was supported in part by JSPS KAKENHI grant number JP 15K09279 and by a Grant-in-Aid for Intractable Renal Diseases Research, Research on rare and intractable diseases, Health and Labour Sciences Research Grants from the Ministry of Health, Labour and Welfare of Japan.
Footnotes
Supplementary Methods. Mutation analysis, classification of mutation types, and classification of mutation positions; measurement of maximum liver cyst diameter, total kidney volume, maximum kidney cyst, and intracranial aneurysms; definitions of comorbidities.
Figure S1. Patient selection flowchart.
Table S1. Patient characteristics according to TKV and Mayo classification (entire cohort, n = 129).
Table S2. Univariable logistic regression analyses for correlations between the TKV ≥ 1000 ml and risk factors (entire cohort and subcohorts).
Table S3. Univariable logistic regression analyses for correlations between the Mayo imaging classification 1C–1E and risk factors (entire cohort and subcohorts).
Supplementary Material
Supplementary Methods. Mutation analysis, classification of mutation types, and classification of mutation positions; measurement of maximum liver cyst diameter, total kidney volume, maximum kidney cyst, and intracranial aneurysms; definitions of comorbidities.
Figure S1. Patient selection flowchart.
Table S1. Patient characteristics according to TKV and Mayo classification (entire cohort, n = 129).
Table S2. Univariable logistic regression analyses for correlations between the TKV ≥ 1000 ml and risk factors (entire cohort and subcohorts).
Table S3. Univariable logistic regression analyses for correlations between the Mayo imaging classification 1C–1E and risk factors (entire cohort and subcohorts).
STROBE Statement (PDF)
References
- 1.Mochizuki T., Tsuchiya K., Nitta K. Autosomal dominant polycystic kidney disease: recent advances in pathogenesis and potential therapies. Clin Exp Nephrol. 2013;17:317–326. doi: 10.1007/s10157-012-0741-0. [DOI] [PubMed] [Google Scholar]
- 2.Ushio Y., Kataoka H., Sato M., et al. Association between anemia and renal prognosis in autosomal dominant polycystic kidney disease: a retrospective study. Clin Exp Nephrol. 2020;24:500–508. doi: 10.1007/s10157-020-01856-1. [DOI] [PubMed] [Google Scholar]
- 3.Uchiyama K., Mochizuki T., Shimada Y., et al. Factors predicting decline in renal function and kidney volume growth in autosomal dominant polycystic kidney disease: a prospective cohort study (Japanese Polycystic Kidney Disease registry: J-PKD) Clin Exp Nephrol. 2021;25:970–980. doi: 10.1007/s10157-021-02068-x. [DOI] [PubMed] [Google Scholar]
- 4.Grantham J.J., Torres V.E. The importance of total kidney volume in evaluating progression of polycystic kidney disease. Nat Rev Nephrol. 2016;12:667–677. doi: 10.1038/nrneph.2016.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perrone R.D., Mouksassi M.S., Romero K., et al. Total kidney volume is a prognostic biomarker of renal function decline and progression to end-stage renal disease in patients with autosomal dominant polycystic kidney disease [published correction appears in Kidney Int Rep. 2018;3:1015] Kidney Int Rep. 2017;2:442–450. doi: 10.1016/j.ekir.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tangri N., Hougen I., Alam A., Perrone R., McFarlane P., Pei Y. Total kidney volume as a biomarker of disease progression in autosomal dominant polycystic kidney disease. Can J Kidney Health Dis. 2017;4 doi: 10.1177/2054358117693355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irazabal M.V., Rangel L.J., Bergstralh E.J., et al. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol. 2015;26:160–172. doi: 10.1681/ASN.2013101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chebib F.T., Perrone R.D., Chapman A.B., et al. A practical guide for treatment of rapidly progressive ADPKD with tolvaptan. J Am Soc Nephrol. 2018;29:2458–2470. doi: 10.1681/ASN.2018060590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gansevoort R.T., Arici M., Benzing T., et al. Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice. Nephrol Dial Transplant. 2016;31:337–348. doi: 10.1093/ndt/gfv456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brosnahan G.M., Abebe K.Z., Moore C.G., et al. Patterns of kidney function decline in autosomal dominant polycystic kidney disease: a post hoc analysis from the HALT-PKD trials. Am J Kidney Dis. 2018;71:666–676. doi: 10.1053/j.ajkd.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grantham J.J., Torres V.E., Chapman A.B., et al. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354:2122–2130. doi: 10.1056/NEJMoa054341. [DOI] [PubMed] [Google Scholar]
- 12.Bhutani H., Smith V., Rahbari-Oskoui F., et al. A comparison of ultrasound and magnetic resonance imaging shows that kidney length predicts chronic kidney disease in autosomal dominant polycystic kidney disease. Kidney Int. 2015;88:146–151. doi: 10.1038/ki.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres V.E., Chapman A.B., Devuyst O., et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gimpel C, Bergmann C, Mekahli D. The wind of change in the management of autosomal dominant polycystic kidney disease in childhood. Pediatr Nephrol. Published online March 7, 2021. https://doi.org/10.1007/s00467-021-04974-4 [DOI] [PMC free article] [PubMed]
- 15.Marlais M., Cuthell O., Langan D., Dudley J., Sinha M.D., Winyard P.J. Hypertension in autosomal dominant polycystic kidney disease: a meta-analysis. Arch Dis Child. 2016;101:1142–1147. doi: 10.1136/archdischild-2015-310221. [DOI] [PubMed] [Google Scholar]
- 16.Cadnapaphornchai M.A., George D.M., McFann K., et al. Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2014;9:889–896. doi: 10.2215/CJN.08350813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho Y., Tong A., Craig J.C., et al. Establishing a core outcome set for autosomal dominant polycystic kidney disease: report of the standardized outcomes in nephrology-polycystic kidney disease (SONG-PKD) consensus workshop. Am J Kidney Dis. 2021;77:255–263. doi: 10.1053/j.ajkd.2020.05.024. [DOI] [PubMed] [Google Scholar]
- 18.The polycystic kidney disease 1 gene encodes a 14-kb transcript and lies within a duplicated region on chromosome 16. The European Polycystic Kidney Disease Consortium [published correction appears in Cell. 1994;78:725] Cell. 1994;77:881–894. doi: 10.1016/0092-8674(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 19.Mochizuki T., Wu G., Hayashi T., et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 20.Hateboer N., v Dijk M.A., Bogdanova N., et al. Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet. 1999;353:103–107. doi: 10.1016/s0140-6736(98)03495-3. [DOI] [PubMed] [Google Scholar]
- 21.Cornec-Le Gall E., Audrézet M.P., Chen J.M., et al. Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol. 2013;24:1006–1013. doi: 10.1681/ASN.2012070650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higashihara E., Horie S., Kinoshita M., et al. A potentially crucial role of the PKD1 C-terminal tail in renal prognosis. Clin Exp Nephrol. 2018;22:395–404. doi: 10.1007/s10157-017-1477-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barua M., Cil O., Paterson A.D., et al. Family history of renal disease severity predicts the mutated gene in ADPKD. J Am Soc Nephrol. 2009;20:1833–1838. doi: 10.1681/ASN.2009020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornec-Le Gall E., Audrézet M.P., Renaudineau E., et al. PKD2-related autosomal dominant polycystic kidney disease: prevalence, clinical presentation, mutation spectrum, and prognosis. Am J Kidney Dis. 2017;70:476–485. doi: 10.1053/j.ajkd.2017.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossetti S., Burton S., Strmecki L., et al. The position of the polycystic kidney disease 1 (PKD1) gene mutation correlates with the severity of renal disease. J Am Soc Nephrol. 2002;13:1230–1237. doi: 10.1097/01.asn.0000013300.11876.37. [DOI] [PubMed] [Google Scholar]
- 26.Harris P.C., Bae K.T., Rossetti S., et al. Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2006;17:3013–3019. doi: 10.1681/ASN.2006080835. [DOI] [PubMed] [Google Scholar]
- 27.Hwang Y.H., Conklin J., Chan W., et al. Refining genotype-phenotype correlation in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2016;27:1861–1868. doi: 10.1681/ASN.2015060648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heyer C.M., Sundsbak J.L., Abebe K.Z., et al. Predicted mutation strength of nontruncating PKD1 mutations aids genotype-phenotype correlations in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2016;27:2872–2884. doi: 10.1681/ASN.2015050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cornec-Le Gall E., Audrézet M.P., Rousseau A., et al. The PROPKD score: A new algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2016;27:942–951. doi: 10.1681/ASN.2015010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nobakht N., Hanna R.M., Al-Baghdadi M., et al. Advances in autosomal dominant polycystic kidney disease: a clinical review. Kidney Med. 2020;2:196–208. doi: 10.1016/j.xkme.2019.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossetti S., Consugar M.B., Chapman A.B., et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2007;18:2143–2160. doi: 10.1681/ASN.2006121387. [DOI] [PubMed] [Google Scholar]
- 32.Kataoka H., Watanabe S., Sato M., et al. Predicting liver cyst severity by mutations in patients with autosomal-dominant polycystic kidney disease. Hepatol Int. 2021;15:791–803. doi: 10.1007/s12072-021-10176-9. [DOI] [PubMed] [Google Scholar]
- 33.Kataoka H., Fukuoka H., Makabe S., et al. Prediction of renal prognosis in patients with autosomal dominant polycystic kidney disease using PKD1/PKD2 mutations. J Clin Med. 2020;9:146. doi: 10.3390/jcm9010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mochizuki T., Teraoka A., Akagawa H., et al. Mutation analyses by next-generation sequencing and multiplex ligation-dependent probe amplification in Japanese autosomal dominant polycystic kidney disease patients. Clin Exp Nephrol. 2019;23:1022–1030. doi: 10.1007/s10157-019-01736-3. [DOI] [PubMed] [Google Scholar]
- 35.Curtis M.J., Bond R.A., Spina D., et al. Experimental design and analysis and their reporting: new guidance for publication in BJP [published correction appears in Br J Pharmacol. 2015;172:4600] Br J Pharmacol. 2015;172:3461–3471. doi: 10.1111/bph.12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vittinghoff E., McCulloch C.E. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165:710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 37.Hauber A.B., González J.M., Groothuis-Oudshoorn C.G., et al. Statistical methods for the analysis of discrete choice experiments: A report of the ISPOR conjoint analysis good research practices task force. Value Health. 2016;19:300–315. doi: 10.1016/j.jval.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Qian F., Watnick T.J., Onuchic L.F., Germino G.G. The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell. 1996;87:979–987. doi: 10.1016/s0092-8674(00)81793-6. [DOI] [PubMed] [Google Scholar]
- 39.Pei Y., Watnick T., He N., et al. Somatic PKD2 mutations in individual kidney and liver cysts support a “two-hit” model of cystogenesis in type 2 autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1999;10:1524–1529. doi: 10.1681/ASN.V1071524. [DOI] [PubMed] [Google Scholar]
- 40.Wu G., D’Agati V., Cai Y., et al. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell. 1998;93:177–188. doi: 10.1016/s0092-8674(00)81570-6. [DOI] [PubMed] [Google Scholar]
- 41.Lantinga-van Leeuwen I.S., Leonhard W.N., van der Wal A., Breuning M.H., de Heer E., Peters D.J. Kidney-specific inactivation of the Pkd1 gene induces rapid cyst formation in developing kidneys and a slow onset of disease in adult mice. Hum Mol Genet. 2007;16:3188–3196. doi: 10.1093/hmg/ddm299. [DOI] [PubMed] [Google Scholar]
- 42.Hopp K., Ward C.J., Hommerding C.J., et al. Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J Clin Invest. 2012;122:4257–4273. doi: 10.1172/JCI64313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rio Frio T., Wade N.M., Ransijn A., Berson E.L., Beckmann J.S., Rivolta C. Premature termination codons in PRPF31 cause retinitis pigmentosa via haploinsufficiency due to nonsense-mediated mRNA decay. J Clin Invest. 2008;118:1519–1531. doi: 10.1172/JCI34211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bateman J.F., Freddi S., Nattrass G., Savarirayan R. Tissue-specific RNA surveillance? Nonsense-mediated mRNA decay causes collagen X haploinsufficiency in Schmid metaphyseal chondrodysplasia cartilage. Hum Mol Genet. 2003;12:217–225. doi: 10.1093/hmg/ddg054. [DOI] [PubMed] [Google Scholar]
- 45.Nogueira G., Fernandes R., Garcia-Moreno J.F., Romão L. Nonsense-mediated RNA decay and its bipolar function in cancer. Mol Cancer. 2021;20:72. doi: 10.1186/s12943-021-01364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurosaki T., Maquat L.E. Nonsense-mediated mRNA decay in humans at a glance. J Cell Sci. 2016;129:461–467. doi: 10.1242/jcs.181008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen L.S., Wilkinson M.F., Gecz J. Nonsense-mediated mRNA decay: inter-individual variability and human disease. Neurosci Biobehav Rev. 2014;46:175–186. doi: 10.1016/j.neubiorev.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller J.N., Pearce D.A. Nonsense-mediated decay in genetic disease: friend or foe? Mutat Res Rev Mutat Res. 2014;762:52–64. doi: 10.1016/j.mrrev.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anna A., Monika G. Splicing mutations in human genetic disorders: examples, detection, and confirmation [published correction appears in J Appl Genet. 2019;60:231] J Appl Genet. 2018;59:253–268. doi: 10.1007/s13353-018-0444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicholson P., Yepiskoposyan H., Metze S., Zamudio Orozco R., Kleinschmidt N., Mühlemann O. Nonsense-mediated mRNA decay in human cells: mechanistic insights, functions beyond quality control and the double-life of NMD factors. Cell Mol Life Sci. 2010;67:677–700. doi: 10.1007/s00018-009-0177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khajavi M., Inoue K., Lupski J.R. Nonsense-mediated mRNA decay modulates clinical outcome of genetic disease. Eur J Hum Genet. 2006;14:1074–1081. doi: 10.1038/sj.ejhg.5201649. [DOI] [PubMed] [Google Scholar]
- 52.Litchfield K., Reading J.L., Lim E.L., et al. Escape from nonsense-mediated decay associates with anti-tumor immunogenicity. Nat Commun. 2020;11:3800. doi: 10.1038/s41467-020-17526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindeboom R.G., Supek F., Lehner B. The rules and impact of nonsense-mediated mRNA decay in human cancers. Nat Genet. 2016;48:1112–1118. doi: 10.1038/ng.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dyle M.C., Kolakada D., Cortazar M.A., Jagannathan S. How to get away with nonsense: mechanisms and consequences of escape from nonsense-mediated RNA decay. Wiley Interdiscip Rev RNA. 2020;11:e1560. doi: 10.1002/wrna.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coban-Akdemir Z., White J.J., Song X., et al. Identifying genes whose mutant transcripts cause dominant disease traits by potential gain-of-function alleles. Am J Hum Genet. 2018;103:171–187. doi: 10.1016/j.ajhg.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malan V., Rajan D., Thomas S., et al. Distinct effects of allelic NFIX mutations on nonsense-mediated mRNA decay engender either a Sotos-like or a Marshall–Smith syndrome. Am J Hum Genet. 2010;87:189–198. doi: 10.1016/j.ajhg.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujiwara T., Takeda N., Hara H., et al. Distinct variants affecting differential splicing of TGFBR1 exon 5 cause either Loeys–Dietz syndrome or multiple self-healing squamous epithelioma. Eur J Hum Genet. 2018;26:1151–1158. doi: 10.1038/s41431-018-0127-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khajavi M., Inoue K., Wiszniewski W., Ohyama T., Snipes G.J., Lupski J.R. Curcumin treatment abrogates endoplasmic reticulum retention and aggregation-induced apoptosis associated with neuropathy-causing myelin protein zero-truncating mutants. Am J Hum Genet. 2005;77:841–850. doi: 10.1086/497541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Inoue K., Khajavi M., Ohyama T., et al. Molecular mechanism for distinct neurological phenotypes conveyed by allelic truncating mutations. Nat Genet. 2004;36:361–369. doi: 10.1038/ng1322. [DOI] [PubMed] [Google Scholar]
- 60.Ajiboye A.S., Esopi D., Yegnasubramanian S., Denmeade S.R. Androgen receptor splice variants are not substrates of nonsense-mediated decay. Prostate. 2017;77:829–837. doi: 10.1002/pros.23323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pastor F., Kolonias D., Giangrande P.H., Gilboa E. Induction of tumour immunity by targeted inhibition of nonsense-mediated mRNA decay. Nature. 2010;465:227–230. doi: 10.1038/nature08999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lindeboom R.G.H., Vermeulen M., Lehner B., Supek F. The impact of nonsense-mediated mRNA decay on genetic disease, gene editing and cancer immunotherapy. Nat Genet. 2019;51:1645–1651. doi: 10.1038/s41588-019-0517-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.López-Bigas N., Audit B., Ouzounis C., Parra G., Guigó R. Are splicing mutations the most frequent cause of hereditary disease? FEBS Lett. 2005;579:1900–1903. doi: 10.1016/j.febslet.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 64.Walsh S., Gösswein S.S., Rump A., et al. Novel dominant-negative NR2F1 frameshift mutation and a phenotypic expansion of the Bosch-Boonstra-Schaaf optic atrophy syndrome. Eur J Med Genet. 2020;63:104019. doi: 10.1016/j.ejmg.2020.104019. [DOI] [PubMed] [Google Scholar]
- 65.Schanze D., Neubauer D., Cormier-Daire V., et al. Deletions in the 3' part of the NFIX gene including a recurrent Alu-mediated deletion of exon 6 and 7 account for previously unexplained cases of Marshall–Smith syndrome. Hum Mutat. 2014;35:1092–1100. doi: 10.1002/humu.22603. [DOI] [PubMed] [Google Scholar]
- 66.Lek M., Karczewski K.J., Minikel E.V., et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bergmann C., Guay-Woodford L.M., Harris P.C., Horie S., Peters D.J.M., Torres V.E. Polycystic kidney disease. Nat Rev Dis Primers. 2018;4:50. doi: 10.1038/s41572-018-0047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Makabe S., Manabe S., Kataoka H., et al. Urinary aquaporin 2 as a potential indicator predicting tolvaptan response in patients with ADPKD. Kidney Int Rep. 2021;6:2436–2444. doi: 10.1016/j.ekir.2021.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.