Introduction
Proteinuria is one of the most important risk factors for chronic kidney disease (CKD) progression. Approximately 5% to 10% of pediatric nephrotic syndrome is resistant to steroids and other immunosuppressants, and up to one-third of them are caused by monogenic disorders. Blockers of the renin-angiotensin-aldosterone system are considered as the basic treatment for proteinuric CKD. However, these agents often cannot achieve sufficient proteinuria reduction. Recently, sodium-glucose cotransporter 2 inhibitors (SGLT2i) have demonstrated benefits in reducing proteinuria and improving kidney outcomes in patients with proteinuric CKD with and without type 2 diabetes in adults.1 Several mechanisms have been proposed to underlie the renal benefits of SGLT2i, and indirect hemodynamic effect is thought to play a major role in the renoprotective effects of SGLT2i: inhibition of proximal sodium reabsorption leads to increased delivery of sodium to the macula densa, which stimulates tubuloglomerular feedback and afferent arterial vasoconstriction and reduces glomerular hyperfiltration.2,3
In view of the renoprotective effect of SGLT2 inhibitors in adult CKD cohort without diabetes, we hypothesize that they have similar proteinuria-lowering responses and renoprotective effects in children. The purpose of this study was to investigate the antiproteinuric effect and safety of dapagliflozin in children with proteinuric CKD.
Results
Study Population
Between September 1, 2020, and March 1, 2021, we screened 15 patients, and 9 of them were enrolled. One patient was lost to follow-up during the first 4 weeks (Supplementary Figure S1). The full inclusion and exclusion criteria are described in Supplementary Methods and Supplementary Table S1. After enrolment, patients were prescribed dapagliflozin 5 mg per day (body weight ≤30 kg) or 10 mg per day (body weight >30 kg) for 12 weeks. Their clinical characteristics are listed in Table 1. Among them, the mean age was 10.4 years, the mean weight was 34.9 kg, the mean body mass index (BMI) was 17.8 kg/m2, and the estimated glomerular filtration rate (eGFR) was 104.9 ml/min per 1.73 m2. The primary kidney disease diagnoses were Alport syndrome (n = 5), Dent disease (n = 1), and others (n = 3). Among them, 6 patients (66.6%) had a previous kidney biopsy, 9 (100%) experienced gene test, and 8 (88.9%) confirmed their primary disease. All of the participants received a stable dose of fosinopril. The mean fasting plasma glucose was 5.2 mmol/ at enrolment.
Table 1.
Characteristics of the participants at baseline and follow-up
| No. | Sex | Clinical characteristics |
At baseline |
12 wk after treatment |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (yr) | BW (kg) | Diagnosis | Genetic diagnosis | 24-h proteinuria (g/m2) | Plasma albumin (g/l) | eGFR (ml/minper 1.73 m2) | BMI (kg/m2) | 24-h proteinuria (g/m2) | Plasma albumin (g/l) | eGFR (ml/minper 1.73 m2) | BMI (kg/m2) | ||
| 1 | F | 11.2 | 47.5 | Proteinuria | PAX2 | 2.35 | 38.7 | 72.8 | 25.1 | 1.45 | 43.0 | 73.2 | 24.8 |
| 2 | F | 13.8 | 43.7 | Proteinuria | NUP160 | 2.63 | 33.4 | 126.2 | 16.6 | 2.29 | 34.8 | 110.9 | 16.4 |
| 3 | M | 9.8 | 33.6 | Dent disease | CLCN5 | 1.64 | 47.5 | 102.7 | 18.4 | 1.26 | 51.6 | 97.6 | 18.1 |
| 4a | M | 11.9 | 42.2 | Alport syndrome | COL4A5 | 6.21 | 24.9 | 60.8 | 17.2 | - | - | - | - |
| 5 | M | 8.1 | 24 | Alport syndrome | COL4A5 | 2.42 | 34.2 | 113.1 | 15.6 | 1.75 | 35.3 | 112.5 | 15.3 |
| 6 | F | 14.2 | 43.4 | Alport syndrome | COL4A3 | 1.84 | 31.5 | 128.9 | 18.8 | 1.56 | 31.7 | 123.9 | 20.3 |
| 7 | M | 6.4 | 24.2 | Alport syndrome | COL4A5 | 1.23 | 38.0 | 103.1 | 15.7 | 1.22 | 37.0 | 100.7 | 15.5 |
| 8 | M | 8.4 | 28.2 | Alport syndrome | COL4A5 | 6.18 | 24.1 | 163.6 | 19.3 | 4.28 | 25.3 | 150.6 | 18.9 |
| 9b | F | 9.4 | 27 | FSGS | Negative | 1.28 | 34.9 | 63.3 | 13.4 | 0.55 | 41.1 | 60.8 | 14.2 |
BMI, body mass index; BW, body weight; eGFR, estimated glomerular filtration rate; F, female; FSGS, focal segmental glomerular sclerosis; M, male.
Patient 4 was loss to follow-up at the first 4 wk.
Patient 9 was with familial FSGS.
Clinical End Points
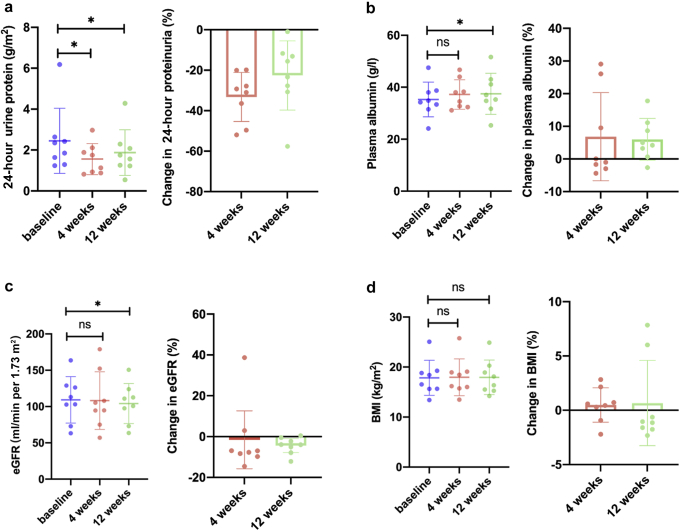
At the end of 12 weeks after treatment, 8 patients showed a reduction in 24-hour proteinuria, ranging from 0.02 to 1.90 g/m2. Dapagliflozin treatment led to significant reductions in 24-hour proteinuria levels both at 4 weeks and 12 weeks versus baseline (1.4 [0.9–2.1] vs. 2.1 [1.4–2.6] g/m2; 1.5 [1.2–2.2] vs. 2.1 [1.4–2.6] g/m2; P < 0.05, respectively). The percentage change of 24-hour proteinuria from baseline was decreased by 33.3% (95% CI 23.1–43.4) at 4 weeks of treatment and 22.6% (95% CI 8.3–36.9) at 12 weeks (Figure 1a). Changes in the plasma albumin, eGFR, and BMI, compared with baseline at week 4 and 12 are shown in Figure 1b to d. Although there was no significant change in plasma albumin levels at 4 weeks (37.2 ± 5.7 vs. 35.3 ± 6.7 g/l, P > 0.05), the plasma albumin level was increased significantly at 12 weeks compared with baseline (37.5 ± 7.9 vs. 35.3 ± 6.7 g/l, P < 0.05) (Figure 1b). No significant change of eGFR was observed at 4 weeks (108.2 ± 39.7 vs. 109.2 ± 32.0 ml/min per 1.73 m2, P > 0.05), whereas it decreased slightly at 12 weeks compared with baseline (103.8 ± 28.2 vs. 109.2 ± 32.0 ml/min per 1.73 m2, P = 0.048) (Figure 1c). The BMI remain stable during the 12 weeks treatment period (Figure 1d).
Figure 1.
Changes in (a) 24-hour urine protein, (b) plasma albumin, (c) eGFR, and (d) BMI during dapagliflozin treatment. Absolute values were shown as mean, error bars were shown as SD. Data were from 8 patient who completed the treatment period. The baseline and posttreatment parameters were compared using paired t test; ∗P < 0.05. BMI, body mass index; eGFR, estimated glomerular filtration rate; ns, no significant difference.
No patient discontinued dapagliflozin owing to an adverse event. One patient experienced an asymptomatic bacteriuria during dapagliflozin treatment. The exploratory biochemical parameters were at normal range during the treatment (Supplementary Table S2).
Discussion
Our results showed that dapagliflozin resulted in a mean reduction in baseline proteinuria by 33.3% at 4 weeks and 22.6% at 12 weeks in children with proteinuric CKD. Similarly, a recent case series also showed the effect of SGLT2i in reducing proteinuria in adults with hereditary podocytopathies.4 The decrease in proteinuria in children with proteinuric CKD after dapagliflozin treatment is consistent with previous reports in adult cohort of CKD with type 2 diabetes, which showed SGLT-2i resulting in a 30% to 50% reduction in proteinuria after treatment.5 However, the recent DIAMOND trial6 failed to detect a significant decrease in proteinuria in the overall study population of CKD without diabetes, whereas the subgroup analysis demonstrated that proteinuria decreased significantly among patients with a measured GFR >60 ml/min per 1.73 m2. In our study, we enrolled patients with an eGFR >60 ml/min per 1.73 m2, and the result showed an efficient antiproteinuric effect of dapagliflozin. This might be due to a sufficient delivery of sodium to the macula densa that stimulated tubulo-glomerular feedback and reduced glomerular hyperfiltration in patients with a relative higher eGFR.3
Similar to the previous studies,1,6 our data also showed a slight fall in eGFR during the dapagliflozin treatment. Previous meta-analyses have demonstrated that SGLT2i do not cause acute kidney injury, and in fact may even reduce the likelihood of it occurring.7 As to this, reviews have demonstrated that SGLT2i preserve kidney function regardless of the initial dip in eGFR,7,8 and the reduction in eGFR reflects a decrease in hyperfiltration.6 That is, the limited fall in eGFR in the short term cannot be taken to definitely exclude the possibility of specific renoprotective properties of dapagliflozin in children with proteinuric CKD.
Dapagliflozin has been reported an acceptable safety profile in the adult cohort with CKD,1 and the safety data of dapagliflozin in the CKD children is limited. A previous study of dapagliflozin in pediatric patients with type 2 diabetes mellitus was generally well tolerated and was not associated with any unexpected or clinically significant safety findings.9 In our study, the only reported adverse event was asymptomatic bacteriuria and no patient discontinued dapagliflozin owing to an adverse event. No clinically meaningful changes in laboratory data were noted. Given the small number of participants in our study, safety data should be validated in a larger population.
To best of our knowledge, this is the first report on the use of dapagliflozin in children with inherited proteinuric CKD. The results provide a new option for the proteinuric CKD in children, especially for the monogenetic kidney disease. In our limited experience, dapagliflozin is effective for use in children with inherited proteinuric CKD with an eGFR >60 ml/min per 1.73 m2. Further research involving more patients and a longer duration of treatment are necessary to confirm the efficacy of dapagliflozin in mitigating proteinuria and in slowing CKD progression in children.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This study was supported by the National Science and Technology Major Project of China (grant number: 2020ZX09201-011). The authors thank all the patients and their family for participating in the study. The authors are grateful to Yin Wang for his contribution in statistical analysis.
Author Contributions
JL and JCu contributed to the data collection and data analysis and drafted the manuscript. XF and JCh contributed to the patient follow-up. WY contributed to the data analysis. HX and QS contributed to the study design and critically revised the manuscript for important intellectual content. HX obtained the funding. All authors have given their approval for the final version of the manuscript to be published. Each author participated sufficiently in the work to be responsible for the content.
Availability of Data and Materials
Data and materials were included in the manuscript and Supplementary Material.
Footnotes
Supplementary Methods.
Supplementary References.
Figure S1. Study flowchart.
Table S1. The full inclusion and exclusion criteria.
Table S2. Changes in exploratory biochemical parameters during dapagliflozin treatment
Contributor Information
Qian Shen, Email: shenqian@shmu.edu.cn.
Hong Xu, Email: hxu@shmu.edu.cn.
Supplementary Material
Supplementary Methods.
Supplementary References.
Figure S1. Study flowchart.
Table S1. The full inclusion and exclusion criteria.
Table S2. Changes in exploratory biochemical parameters during dapagliflozin treatment.
References
- 1.Heerspink H.J.L., Stefansson B.V., Correa-Rotter R., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 2.Ghezzi C., Loo D.D.F., Wright E.M. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia. 2018;61:2087–2097. doi: 10.1007/s00125-018-4656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fioretto P., Zambon A., Rossato M., Busetto L., Vettor R. SGLT2 inhibitors and the diabetic kidney. Diabetes Care. 2016;39(suppl 2):S165–S171. doi: 10.2337/dcS15-3006. [DOI] [PubMed] [Google Scholar]
- 4.Boeckhaus J., Gross O. Sodium-glucose Cotransporter-2 inhibitors in patients with hereditary podocytopathies, Alport syndrome, and FSGS: a case series to better plan a large-scale study. Cells. 2021;10:1815. doi: 10.3390/cells10071815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piperidou A., Sarafidis P., Boutou A., et al. The effect of SGLT-2 inhibitors on albuminuria and proteinuria in diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. J Hypertens. 2019;37:1334–1343. doi: 10.1097/HJH.0000000000002050. [DOI] [PubMed] [Google Scholar]
- 6.Cherney D.Z.I., Dekkers C.C.J., Barbour S.J., et al. Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): a randomised, double-blind, crossover trial [published correction appears in Lancet Diabetes Endocrinol. 2020;8:582-593] Lancet Diabetes Endocrinol. 2020;8:582–593. doi: 10.1016/S2213-8587(20)30162-5. [DOI] [PubMed] [Google Scholar]
- 7.Zhao M., Sun S., Huang Z., Wang T., Tang H. Network meta-analysis of novel glucose-lowering drugs on risk of acute kidney injury. Clin J Am Soc Nephrol. 2020;16:70–78. doi: 10.2215/CJN.11220720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heerspink H.J.L., Cherney D.Z.I. Clinical implications of an acute dip in eGFR after SGLT2 inhibitor initiation. Clin J Am Soc Nephrol. 2021;16:1278–1280. doi: 10.2215/CJN.02480221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tirucherai G.S., LaCreta F., Ismat F.A., Tang W., Boulton D.W. Pharmacokinetics and pharmacodynamics of dapagliflozin in children and adolescents with type 2 diabetes mellitus. Diabetes Obes Metab. 2016;18:678–684. doi: 10.1111/dom.12638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and materials were included in the manuscript and Supplementary Material.