Abstract
Schistosomiasis japonica is a serious communicable disease and a major disease risk for more than 30 million people living in the tropical and subtropical zones of China. Infection remains a major public health concern despite 45 years of intensive control efforts. It is estimated that 865,000 people and 100,250 bovines are today infected in the provinces where the disease is endemic, and its transmission continues. Unlike the other schistosome species known to infect humans, the oriental schistosome, Schistosoma japonicum, is a true zoonotic organism, with a range of mammalian reservoirs, making control efforts extremely difficult. Clinical features of schistosomiasis range from fever, headache, and lethargy to severe fibro-obstructive pathology leading to portal hypertension, ascites, and hepatosplenomegaly, which can cause premature death. Infected children are stunted and have cognitive defects impairing memory and learning ability. Current control programs are heavily based on community chemotherapy with a single dose of the drug praziquantel, but vaccines (for use in bovines and humans) in combination with other control strategies are needed to make elimination of the disease possible. In this article, we provide an overview of the biology, epidemiology, clinical features, and prospects for control of oriental schistosomiasis in the People's Republic of China.
Schistosomiasis (also termed bilharzia) is caused by adult blood flukes (trematode worms) depositing eggs in blood vessels surrounding the bladder or gut of the infected host. Three major schistosome species are known to infect humans. Urinary schistosomiasis, in which the bladder is affected, is caused by Schistosoma haematobium and occurs in Africa and the eastern Mediterranean. Intestinal schistosomiasis results from infection with Schistosoma mansoni (endemic to Africa, the eastern Mediterranean, the Caribbean, and South America) or Schistosoma japonicum, the Asian or oriental schistosome (endemic mainly in China and to a lesser extent the Philippines).
Here we examine the ecology and epidemiology of schistosomiasis japonica. We emphasize the importance of the snail host, the zoonotic component of transmission, and the occupational water contact that exposes residents of areas where the disease is endemic to repeated and unavoidable infection. We note the advances made over the last half century due to the extraordinary historic emphasis on controlling this disease in the People's Republic of China (PRC). The clinical features of the acute and chronic diseases caused by the parasite together with their diagnosis and treatment will also be reviewed, as will the rapidly expanding knowledge concerning the complex immunoepidemiology of infection and disease. The results of control technology advances, including the success of the recent World Bank inputs, are brought together to mathematically model prospects for the future. These data show that a new integrated approach to control is needed that could lead to eradication of infection in areas where it is endemic. Finally, the threat posed by the giant Three Gorges Dam is considered because it will change the Yangtze basin ecology and associated schistosomiasis transmission risks over the next 10 years.
Schistosomiasis in China
Archeological studies have revealed that schistosomiasis japonica has a very long history in China. S. japonicum eggs were identified in a female corpse dating back to the Western Han dynasty some 2,100 years ago (111, 146) that was exhumed in 1971 in Hunan province. Schistosome eggs were also found in the liver of another corpse buried 100 years earlier in Jianglin Hsien, Hubei province (227). In old volumes of traditional Chinese medicine (111), a description of clinical symptoms resembling Katayama fever (acute schistosomiasis) can be traced back to 400 B.C. The first reported clinical diagnosis in modern China was made by an American physician in 1905 in Hunan province (105).
After the founding of the People's Republic of China in 1949, large-scale epidemiological surveys were carried out by Chinese scientists to determine the incidence, prevalence, and intensity of S. japonicum infections. The results revealed that schistosomiasis was endemic in 380 counties comprising 12 provinces south of the Yangtze River. Approximately 12 million people were infected, with an additional 100 million people at serious risk. A total of 14,000 square kilometers of infected Oncomelania flood plains were identified as potential transmission zones despite remarkable successes in schistosomiasis control achieved over the previous four decades (31, 34, 110).
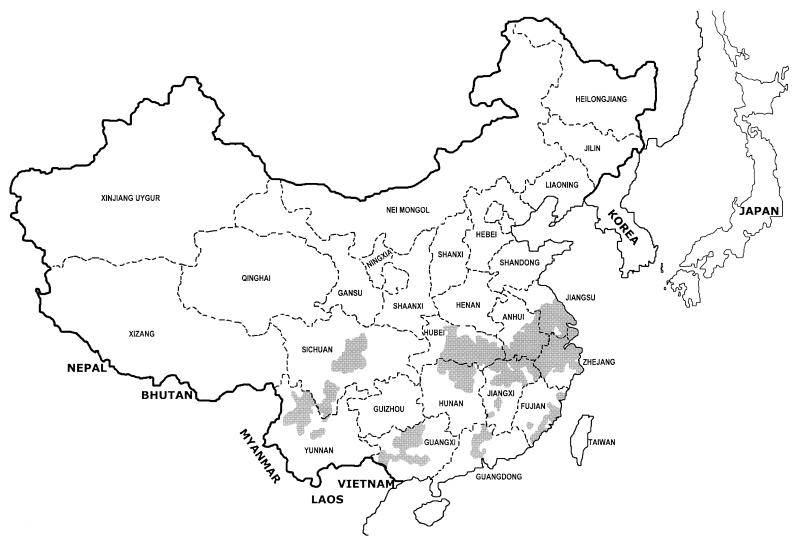
Schistosomiasis japonica remains a major public health problem in China today. In a nationwide sample survey conducted in 1989, the number of people infected was estimated to be 1.52 million (223). The major endemic foci are the marsh and lake regions of southern China, which cover a vast area of five provinces (Jiangsu, Anhui, Hubei, Jiangxi, and Hunan); cases within the area account for 86% of the total number of people infected in the whole of China (Fig. 1). Since 1985, the rural Chinese economy has been boosted (resulting in an increased standard of living), but the prevalence of S. japonicum and its associated morbidity have also risen slightly in focus areas (217).
FIG. 1.
Map illustrating the current and former areas of endemic schistosomiasis (shaded) in China. The disease has now been eradicated from three important zones: the whole Pearl River system (Guangxi and Guangdong), the isolated coastal focus (Fujian), and the Yangtze delta (Shanghai, Zhejang, and Jiangsu). Schistosomiasis distribution has been substantially reduced in the other provinces but remains a major problem in the marshland and lake areas of Hubei, Hunan, Anhui, and Jiangxi and in some mountainous areas of Sichuan and Yunnan.
Results from the 1995 nationwide sampling survey indicate that prevalence among both human and bovine populations has decreased further since the late 1980s. It is estimated that 865,000 humans and 100,250 bovines are presently infected (31). However, construction of the giant Three Gorges Dam (due to be completed by 2009) across the Yangtze River could substantially alter transmission of schistosomiasis both above and below the dam. The Ministry of Health is currently investigating the risk associated with the dam, and no schistosomiasis data exist to indicate the probable outcomes.
LIFE CYCLE
Schistosomes are digenetic trematodes that are transmitted through fresh water containing free-swimming larval forms of the parasite called cercariae. The cercariae utilize a proteolytic enzyme (elastase), produced in specialized glands in the head region, to penetrate the skin of humans or, in the case of S. japonicum, other mammals (buffaloes, pigs, dogs, etc.), which act as reservoirs for human transmission. The cercariae shed their bifurcated tails and transform their trilaminate tegument into the heptalaminate form adapted to a mammalian environment. Now known as schistosomula, they leave the skin via the blood vessels and draining lymphatics and reach the lungs. After several days, the male and female worms exit the lungs and arrive in the hepatic portal system, where they mature, pair up, and migrate downstream.
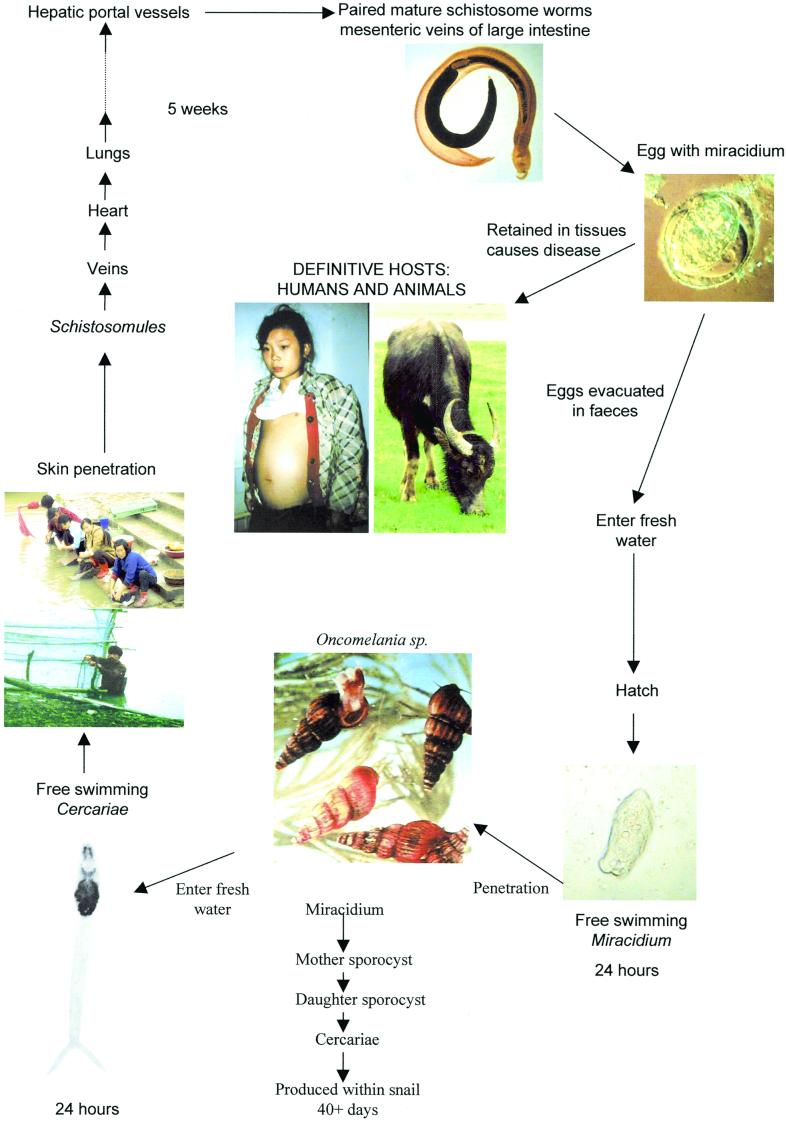
The worm pairs reach mucosal branches of the inferior mesenteric and superior hemorrhoidal veins (S. japonicum) or the superior mesenteric veins (S. mansoni) or pass through portosystemic anastamoses to reach veins surrounding the bladder and ureters (S. haematobium). The females then begin egg production. The process of migration and maturation may take 4 to 6 weeks, depending on the host and the parasite species involved. Many eggs pass through the intestinal or bladder wall and are discharged in the feces (S. mansoni and S. japonicum) or urine (S. haematobium). The schistosome lifecycle is completed when the eggs hatch and release free-swimming miracidia, which in turn reinfect receptive freshwater snails (in the case of S. japonicum, snails of the genus Oncomelania) (Fig. 2.). The miracidium forms a sporocyst at the site of penetration, and this produces daughter sporocysts that migrate to the snail hepatopancreas and asexually produce larval cercariae for daily release into the surrounding water. Snails may remain infected for several months. However, some eggs lodge in the tissues of the mammalian host instead of being excreted. It is the presence of these retained eggs and, in particular, the host inflammatory responses to the eggs lodged in the liver, bladder, or ureters rather than the worms themselves that cause the principal pathology associated with all forms of schistosomiasis. The shape and size of the eggs of the three major schistosome species are useful diagnostic features. The eggs of S. japonicum are round with a reduced lateral spine and are smaller in size (60 by 100 μm) than those of S. mansoni (61 by 140 μm; prominent lateral spine) and S. haematobium (62 by 150 μm; prominent terminal spine), which are ovoid.
FIG. 2.
Life cycle of S. japonicum.
INTERMEDIATE HOST AND TRANSMISSION
This section updates the classification of snails transmitting S. japonicum, summarizes recent findings about the ecology of transmission, and discusses aspects of the population genetics of Oncomelania relative to disease transmission. Throughout, it is important to understand that there are patterns and processes of coevolution between the snails and parasites (39, 40).
Molecular data (mitochondrial cytochrome oxidase I [COI] sequences and allozymes) (41, 43, 44, 192) and detailed anatomical data are congruent; the classification (Table 1) of relevant snails transmitting schistosomes in Asia is thus supported. It is noteworthy that no members of the family Hydrobiidae, sometimes misused in China and elsewhere to classify Oncomelania and related taxa, exist in China. The Hydrobiidae are highly divergent genetically from the Pomatiopsidae (42).
TABLE 1.
Classification of snails transmitting schistosomiasis in Asia
| Family | Species | Schistosoma species transmitted or geographic origin |
|---|---|---|
| Superfamily Rissooidea | ||
| Family Pomatiopsidae | China | |
| Subfamily Pomatiopsinae | ||
| O. hupensis hupensis | S. japonicum | |
| O. hupensis robertsoni | S. japonicum | |
| P. hupensis tangi | S. japonicum | |
| Subfamily Triculinae | ||
| Tribe Triculini | ||
| Tricula | S. sinensium complex | |
| Delavaya | S. sinensium complex | |
| Tribe Pachydrobiini | ||
| Neotricula | S. mekongi, Laos | |
| Robertsiella | S. malayensis, Malaysia | |
| Jinhongia | S. sinensium complex |
Historical data strongly support coevolution between S. japonicum and Oncomelania throughout Asia operating at the local and even population level (39, 40). The direction and timing of this coevolution have been reviewed (44). In mainland China there are three subspecies of Oncomelania hupensis, widely separated biogeographically. O. hupensis subsp. robertsoni is found in Sichuan and Yunnan provinces at high elevations (>500 m, most >1,000 m above sea level) above the Three Gorges of the Yangtze River. It has the ancestral (pleisiomorphic) small, smooth shell without varix (thickening of the outer lip of the shell). O. hupensis subsp. tangi is found in Fujian Province along the southern coast of China (opposite Taiwan), separated from the Yangtze River by high mountain ranges. The shells are smooth but have a doubly thick varix, and their allometry differs from that of the other two subspecies because their shells are proportionally much wider for their length. Extensive control measures have all but brought this subspecies to extinction, with only one marginal coastal population allowed to survive to provide living specimens of this genetic type. O. hupensis subsp. hupensis thrives throughout the Yangtze River drainage below the Three Gorges to Shanghai, Jiangsu, and Zhejiang provinces. It is also found distributed to Guangxi Province, which is connected to Hunan (and hence to the Yangtze River) by rivers and a major canal.
O. hupensis subsp. hupensis lives primarily at low altitude, from sea level to 200 m. A few populations live in mountain valleys such as are found in Zhejiang and Anhui provinces at an altitude of about 1,000 m. Other populations in Guangxi Province live along the Yu Jiang and Hongshui rivers at altitudes of 200 to 400 m, a few at 1,000 m (104). It inhabits horizontal or nearly horizontal habitats, along irrigation ditches or natural streams where the soil pH is close to neutral or alkaline and the humidity is high due to proximity to water and dense shade. Adults are amphibious, living at the edge of water. The young live in water or float upside down on the surface of the water. The three most critical factors affecting Oncomelania are water, dense vegetation providing shade and humidity, and temperature. If the environment is too wet, O. hupensis will not live there. Flooding is used as a control measure in some areas of China, as flooding drowns adult snails (43, 44).
In China, O. hupensis occurs in six ecological zones: high elevation (>500 m); the flood plains and islands of the Yangtze River; Dongting and Poyang lakes; the vast canal system in Hubei Province; the lowland network of canals found in Jiangsu Province and to a lesser extent in other provinces; and elevation less than 500 m but sufficient to be above the effects of the annual floods of the Yangtze River. Where Oncomelania is affected by flooding, the shell is ribbed; at elevations above the effects of flooding, the shell is smooth. The ribbed and smooth states are controlled by a single gene with multiple alleles; ribbing is dominant (38). Davis et al. (44) argue that ribbing enables greater survival during the annual floods. All non-Chinese Oncomelania have smooth shells. In the same river system, where downstream populations are affected by flooding and upstream populations are not, the ribbed-shelled and smooth-shelled snails are not significantly different genetically (43). Unlike O. hupensis subsp. robertsoni, O. hupensis subsp. hupensis has a varix whether the shell is smooth or ribbed. Varix formation is presumably controlled by a second gene. O. hupensis subsp. hupensis has the same shell growth allometry as O. hupensis subsp. robertsoni but, on average, has a longer shell. Oncomelania reached the extreme eastern edge of China by dispersal down the Yangtze River (the genus is found as a fossil in Burma and Yunnan, China). In the water networks in Jiangsu Province, close to the Yellow Sea, O. hupensis subsp. hupensis (smooth shelled) lives on what was once a flat sea bed, only recently emerged. The agricultural land on this exposed shelf is still slightly salty. This is an amazing bit of adaptation for Oncomelania, but then, one of Oncomelania's sister genera, Cecina, has gone back to a marine environment in Japan and the northwest United States. Oncomelania reached Zhejiang province by dispersal from the Yangtze through Lake Tai Hu, which is connected to the Yangtze by water networks.
Two of the largest lakes in China are Poyang Lake (the largest) and Dongting Lake. Both are major areas of endemicity for S. japonicum but differ significantly with regard to schistosome transmission. The great annual floods of the Yangtze affect these lakes in different ways. Dongting Lake is part of a vast marsh-lake-lowland system connected to the Yangtze. It is virtually impossible to control snails in this system. Unlike Hubei Province, across the Yangtze to the north of Hunan Province, Hunan has no continuous system of dikes that contain water from moving inland. Accordingly, the area covered by floods is immense. Together, these two provinces are punchbowl-like in topography, with a lowland area that was once a vast lake. Hubei Province is protected by immense dikes that hold back the Yangtze, thus controlling flooding of the vast plains.
Poyang Lake in Jiangxi Province is unique. It is connected to the Yangtze River by a narrow passage. People live behind dikes, whether on high islands in the lake basin or outside the basin. The lake is completely surrounded by dikes, so that with the annual floods (beginning in late May or June and ending in October or November), the lake fills up like a bathtub. At high flood season, the lake is an inland sea available only to fishermen. When the flood subsides, the lake loses as much as 75% of its water, exposing vast flat marshlands. These marshlands constitute a major area of endemicity for schistosomiasis. The marshlands are used for grazing cattle. The epidemiology of schistosomiasis in the Poyang Lake has four unique features. First, cattle are considered to be responsible for more than 85% of the transmission from snails to humans in the lake basin. This hypothesis is currently being tested by the Tropical Medical Research Center, Institute of Parasitic Diseases, Shanghai, China. Second, there are no snails living outside the lake basin behind the dikes. Third, all transmission occurs in the lake basin. Finally, the annual floods drown snails, and presumably the life cycle of the snails is reduced to 1 year or less (220). Most reproduction must then occur in the spring, when the soil temperature rises above 10°C (March or April, depending on the year). The very young snails can withstand the flood and live as aquatic snails.
Elsewhere in China, the life expectancy of Oncomelania exceeds 2 years. Oncomelania can live over 5 years, and a female, once fertilized, can lay eggs throughout her lifetime (43, 44). Finally, Oncomelania can bury deep into the soil and estivate when the temperature becomes too cold and when the environment experiences a drought.
The annual floods move snails around to a considerable degree. Islands and flood plains are swept, and snails drown, climb trees to above the flood line and estivate, or float down rivers. With the subsidence of flooding, snails are deposited in aggregates that are not natural populations. We have called such genetically unstable aggregates unstable populations (43, 44). Researchers in Hubei have used nets to assess the influx of snails into the canal systems when the flood gates are opened to let Yangtze River water flow in for agricultural use (210, 211, 213). Schistosomiasis control experts in Nanjing, Jiangsu Province, have noted the yearly import of infected snails to the flood plains along Nanjing. Areas in the suburbs of Shanghai, once cleared of snails, become reinvaded.
A sign of the genetic stability of a population is that it is in Hardy-Weinberg equilibrium. Polymorphoic loci of O. hupensis are not, for the most part, in such equilibrium. Instead, they often show heterozygote deficiencies. Two causes for this genetic instability are most likely. Routine molluscuciding is used to control snails and reduce the abundance of schistosome-infected snails. Only a few surviving females can revive a population, and the resulting genetic instability would be due to extensive inbreeding. As O. hupensis is under continual assault in efforts to control schistosomiasis, habitats for these snails are ever changing due to land use changes in China. These changes disrupt population, and thus it may be difficult to locate genetically stable populations using allozymes as markers. Also, the annual floods move snails around, as discussed above, creating aggregates of snails (43, 44).
COI gene sequences have proved ideal for evaluating the degree of genetic instability (44). For example, in collections of snail aggregates around Dongting Lake impacted by annual floods, as many as 10 different haplotypes in a sample of 10 snails have been found, at a single locality. The array of different haplotypes can be used to assess the probable origin and direction of dispersion to any one aggregate. At elevations above the impact of flooding, one finds genetically stable natural populations in which there are only one or two haplotypes per 10 individuals (0 to 0.5% nucleotide differences, or 0 to 3 nucleotide differences). Genetic instability is rampant throughout the Yangtze River drainage. As S. japonicum must track genetically receptive snails, the mass swirling and mixing of snails means that schistosomes from diverse areas are likewise being intermixed. The churning and intermixing of snails throughout the Yangtze River basin with ensuing genetic instability is of more than merely academic interest. The coevolutionary linkage between O. hupensis and S. japonicum is a tight genetic linkage. As snails are intermixed, so also are the schistosomes that depend on these snails for survival. This constant intermixing and dispersal of genotypes has considerable implications for the spread and evolution of schistosomes and schistosomiasis. Considering the effects of the Three Gorges Dam across the Yangtze River, Davis et al. (44) have hypothesized that passive transport of snails will occur both up into the reservoir behind the dam and, for the first time, from upstream down below the dam.
Considering all of the above factors, there are four discrete modes of schistosome transmission (Table 2). These modes are important to consider when designing control programs for schistosomiasis in China.
TABLE 2.
Four modes of schistosomiasis transmission in Chinaa
| Mode | Snail species | COI gene divergence | Present above Three Gorges | Environment | Annual floods | Genetically stable population structure | Life span (yr) | Bovines responsible for maintaining 80% of schistosome transmission |
|---|---|---|---|---|---|---|---|---|
| I | O. hupensis subsp. robertsoni | Yes | Yes | High elevation | No | Yes | >2 | No |
| II | O. hupensis subsp. hupensis | Yes | No | Poyang Lake | Yes | No | 1 | Yes |
| III | O. hupensis subsp. hupensis | Yes | No | Yangtze flood plains, Dongting Lake | Yes | No | >2 | No |
| IV | O. hupensis subsp. hupensis | Yes | No | Canals, water networks, low hills | No | Yes | >2 | No |
Reproduced from reference 44 with permission from the publisher.
CLINICAL FEATURES OF SCHISTOSOMIASIS
Historical Importance of the Disease
Schistosomiasis disabled and killed millions of Chinese peasants before the new government of the People's Republic of China began systematic control programs in the 1950s (31). Some village areas of hyperendemicity were depopulated, and in others only a few widows survived. In severely affected areas, a notable proportion of the surviving population had dwarfism (up to 5%) and a smaller proportion would be expected to have epilepsy—both consequences of S. japonicum infection (33, 127). Wasting, weakness, and ascites were much more frequent manifestations and incapacitated a large proportion of the rural population before they succumbed to premature deaths in their third or fourth decades. Soldiers were often infected during the wars before liberation in 1949, and snail fever (acute infection) affected many persons involved in flood relief in the early 1950s. Political leaders, especially Chairman Mao Zedong, were aware of the wide distribution of the disease and its importance in populous rural areas drained by the Pearl and Yangtze rivers. It was also known that infection in endemic areas decreased rural production, compounding the extreme poverty that confronted postrevolutionary China. There were adverse social effects as well: women would not move to areas of endemicity to marry, and infected men could not join the People's Liberation Army and obtain skills that would enable them to escape the drudgery and poverty of rural life (172).
Given these observations, it is not surprising that the new Chinese government made schistosomiasis control its top priority for the public health aspects of reconstruction. This led to substantial research on the disease, its treatment, and its control. The numerous published Chinese reports on morbidity became accessible when Chen and Mott (33) published a critical review of that literature in English in 1989. We were thus able to incorporate much of the valuable Chinese experience in this review.
Role of Schistosome Eggs in Development of Disease
The worms themselves are thought not to be responsible for disease. However, host responses to various secreted worm products are incompletely understood and could account for some pathological effects. Most of the disease manifestations arise from host responses to the larval miracidia contained within schistosome eggs. Each female worm of S. japonicum produces up to 3,500 eggs per day, and a large proportion are trapped within intestinal and hepatic tissues. Retained eggs induce a cell-mediated granulomatous reaction that accumulates to produce the pathology of chronic disease. The colon, especially the rectosigmoid area, and the left lobe of the liver are usually the most affected, and the number of eggs accumulating can be huge. In one Chinese autopsy report, nearly 1 billion eggs were recovered from liver tissue of a schistosomiasis victim—an impressive number, given that most eggs are reabsorbed and most people die after many years of infection (33). Among 15 autopsy cases for which eggs were counted, the next most egg-dense site was the rectum, and the ratio of eggs in the liver to that in the rectum was 20:1.
Trapped eggs contain the miracidium larva, which matures over 5 days and remains alive for up to 20 days, secreting enzymes and other toxic products that elicit intense inflammatory responses. A characteristic perioval granuloma forms, with a necrotic center containing the egg or egg cluster surrounded by epithelioid cells, giant cells, and lymphocytes and an outer layer of plasma cells, eosinophils, and fibroblasts. Single eggs are usually reabsorbed, but the tissue damage leads to fibrosis. Large egg clusters tend to calcify. Perioval granulomas have been found in many tissues, including skin, lung, brain, and muscle. They have also been noted in the adrenal glands and the urogenital system of both sexes. Most granulomas develop at the sites of maximum egg accumulation, the intestine and liver.
Numerous reports (27, 28, 52, 85, 204) from animal experiments show that host responses to schistosome eggs are modified over time and vary according to host and schistosome species, tissue, and infection history. When the volume of granulomas decreases around recently laid eggs containing viable mature miracidia, the phenomenon is referred to as modulation. Little is known of modulation in humans, but animal models reveal a complex interplay of regulatory cytokines, tumor necrosis factor, various immune effector cells, and fibroblasts, all subject to genetic influence. The balance of cellular and humoral influences on modulation may vary with the chronicity of the infection, with humoral activity dominating in the later stages of disease.
Two intriguing additional hypotheses explaining the decrease in the size of perioval granulomas are provided by the work of Mitchell et al. (120, 121). They noted that female S. japonicum miracidia induce a greater immunological response from mammalian hosts and that male eggs (and male worms) predominate in chronic infections. If female eggs survive less in chronic infections, the granulomatous response, now directed mostly at relatively immunodominant male eggs, would modulate. Their other hypothesis derives from antibody-mediated antiembryonation immunity detected in murine schistosomiasis japonica. This phenomenon could also produce modulation, because antibody-damaged eggs do not mature and are less potent as immunopathological agents. An understanding of the actual determinants of modulation in human schistosomiasis is an important focus of research because it could lead to drugs or vaccines for disease control or to spin-off benefits for other granulomatous diseases.
Cercarial Dermatitis
A maculopapular eruption may arise at the site of cercarial penetration of the skin. This condition is infrequent among those living in areas of endemicity, but migrants or visitors who are infected may develop it within a few hours of exposure. The resulting dermatitis is similar to swimmer's itch, noted in persons sensitized and reexposed to avian or other nonhuman schistosomes found in freshwater bodies all over the world (184). It is not known if reexposure is a feature of cercarial dermatitis for S. japonicum.
Acute Schistosomiasis
Acute schistosomiasis, first described in Japan as Katayama fever (184), is common throughout areas of high transmission risk in China. The disease appears an average of 41.5 days after individuals are exposed to a first infection or a large reinfection or superinfection (33, 89). The timing of disease onset appears to relate to initiation of egg laying by young female worms. Individuals present with nocturnal fever peaks, coughing, generalized muscle pain, headache, and a tender enlarged liver. Schistosome egg excretion is often detected, and splenomegaly occurs in a third of cases. Diffuse pulmonary infiltrates are found clinically and radiologically, and a few cases have signs of meningoencephalitis. All cases have eosinophilia and a history of water contact 14 to 84 days before and respond well to a 6-day course of 20-mg/kg doses of praziquantel (169).
Acute cases often occur as epidemics during the rainy season, sometimes in cities, and are especially common at flood times, when many relief workers are affected. The frequency and distribution of acute cases in China can be used to monitor progress in schistosomiasis control. For example, a resurgence of schistosomiasis transmission in China was noted throughout the 1980s, probably reflecting increased rural activity (irrigation, agriculture, animal husbandry, fishing, and water contact) due to economic reforms introducing incentives for household production (169). The number of reported acute schistosomiasis cases more than doubled, and in 1989 acute cases recorded in the five worst provinces totaled 13,000, with 38% occurring in children less than 16 years old. As a result, the Chinese government teamed up with the World Bank to boost schistosomiasis control in the 1990s. The number of acute cases fell quickly, providing evidence of success.
Intestinal Disease
The worms migrate frequently within the mesenteric veins but are thought to favor certain locations to lay eggs, usually in clusters. Egg clusters aggregate and induce mucosal inflammation, hyperplasia, ulceration, microabscess formation, blood loss, and pseudopolyposis (26, 30, 35, 60). Lower abdominal pain is frequent, often colicky, and usually referred to the left lower quadrant. Diarrhea is common, usually with occult blood. Sometimes blood is visible in the stools, and diarrhea may alternate with constipation. Diarrhea is particularly notable in children, and despite the well-known link with other infections (viral and bacterial), its presence remains a powerful predictor of chronic schistosome infection (228). In severe cases of chronic disease, bowel fibrosis and stenosis result, most often in the lower colon and rectum, and the mesentery may thicken to form an abdominal mass. There is evidence to suggest that the most serious consequence of intestinal schistosomiasis is colorectal cancer (see section below on cancer).
Hepatosplenic Disease
Schistosome eggs that do not pass through the mucosa to reach the intestinal lumen are trapped in situ or swept up in the portal blood flow. Eggs reaching the liver are too large to reach the sinusoidal plexus and accumulate in presinusoidal venules within the portal triads, especially in the left lobe. There they induce granulomatous inflammation, fibrosis, venous obstruction, portal hypertension, and splenomegaly. Liver enlargement initially tends to correspond in size to the intensity of concurrent or recent infections. Thus, hepatomegaly reflects granulomatous perioval inflammation rather than consequent fibrosis and occurs early in the evolution of chronic disease (137). Modulation of the inflammatory response has been observed in murine models and is expected to occur in humans. If so, it may partly account for the decrease in liver size that is often observed after the initial enlargement.
Splenic enlargement may also appear early in the course of chronic infection but is usually minor and reflects cellular hyperplasia without granulomatous inflammation. If portal hypertension ensues, passive congestion enlarges the spleen to Hackett grade II or III, and it is easily palpated clinically. Enlarged spleens rarely contain schistosome eggs. In some cases, severe hypersplenism is noted and is the usual indication for splenectomy, a procedure that has been used frequently in China for treatment of chronic schistosomiasis.
Perioval granulomas in the liver lead to fibrosis (Fig. 3). The links between granulomatous inflammation, subsequent fibrosis and its persistence are complex (28). Collagen deposition, cross-linking, contraction, and reabsorption are in dynamic balance and each component is subject to immunoregulation. Most of the information available for S. japonicum comes from murine models and indicates that the determinants of granuloma size may frequently dissociate from those of hepatic fibrosis. Some animal models show well how chemotherapy and parasitological cure can lead to reversal of gross periportal fibrosis (6). Longitudinal studies of endemic schistosomiasis mansoni in Brazil indicate that reversal of human liver pathology does occur and relates to falling worm burdens, whether they fall spontaneously (166) or as result of repeated chemotherapy (168). Such reversal also occurs for human schistosomiasis japonica but is slow and may be aborted by reinfection (137).
FIG. 3.
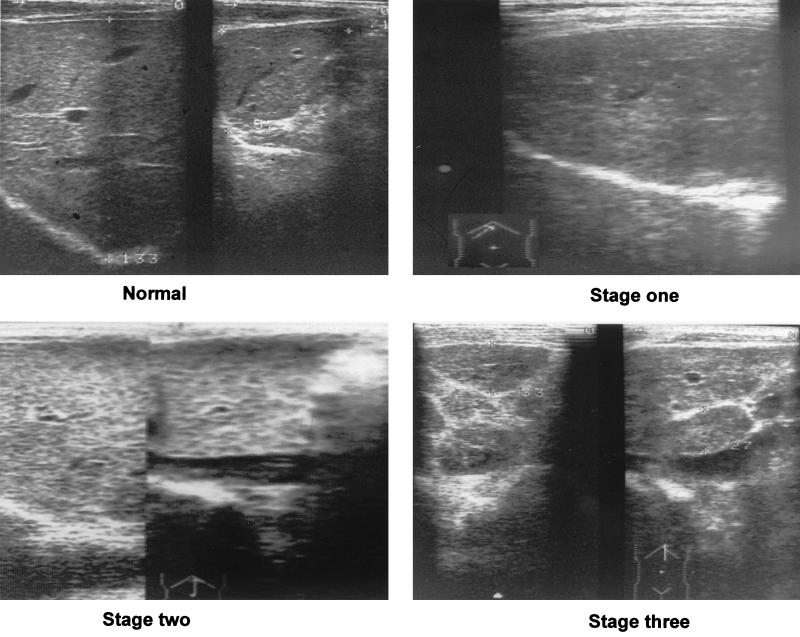
Ultrasound B photographs depicting normal and schistosomiasis-diseased liver parenchyma. Normal image (top left) of the left and right lobe. Stage one fibrosis (top right; right lobe) with focal echodense areas scattered within the parenchyma and no definite borders. Stage two fibrosis (lower left; both lobes) with typical fish-scale pattern and a few echodense areas <20 mm in diameter. Stage three fibrosis (lower right; left lobe) with echodense bands forming contiguous network, multiple focal echodense areas >20 mm in diameter, and masses of central fibrosis.
The hepatosplenic end product of chronic schistosomal infection is the final result of the immunoregulated host response to a sustained intravascular egg assault on the liver. Eventually, after years or decades, gross periportal fibrosis appears along with presinusoidal portal hypertension and secondary gastroesophageal varices. On section, the liver surface resembles clay pipestems due to the wide bands of fibrous tissue extending along the portal tracts. This appearance is only found for hepatic schistosomiasis and was first described in Egypt (174) for what we now know as schistosomiasis mansoni. The severely affected liver is macroscopically similar for schistosomiasis japonica. However, a given worm burden of S. japonicum induces more severe hepatic disease, probably because the daily egg production of the female worms is much higher for this species and the eggs tend to occur in clusters. S. japonicum also has another hepatic effect not seen with Manson's schistosomiasis, widespread septal fibrosis and calcification, that produces parenchymal abnormalities detectable on ultrasound examination (175), magnetic resonance imaging (126), and computerized tomography (36).
Symmer's liver fibrosis is now known as fibro-obstructive hepatic schistosomiasis. The former use of the term schistosomal cirrhosis was inappropriate because essential features of cirrhosis are lacking: lobular architecture is not disrupted, and nodular hepatocellular hyperplasia does not occur. Alcoholic and/or postviral cirrhosis may coincide with schistosomiasis, and pathologic interactions have been investigated with no definite conclusions (33). The parenchymal form of hepatic fibrosis is the most prevalent in schistosomiasis japonica and may persist as a common subclinical finding in populations treated so frequently that overt liver disease has resolved (153).
Li et al. (102) selected a cohort of 193 such individuals and showed that persisting parenchymal fibrosis responds to parasitological cure: the fibrosis substantially reversed over the next 2 years, but alcohol intake or schistosome reinfection impeded this effect. Periportal fibrosis and enlarged portal vein diameter also improved, but less so, and without interaction with alcohol intake. There was no interaction of schistosomal fibrosis and hepatitis B virus infection, although the latter was also common in this community. Serum levels of fibrosis markers provided biochemical support for the ultrasound results; laminin and collagen IV were significantly higher in reinfected persons, and both markers, together with hyaluronic acid, correlated with the ultrasound staging of end-point parenchymal fibrosis. Periportal fibrosis also correlated with fibrosis markers, but patterns were less clear-cut.
In the past, as many as 20 to 30% of the population in affected communities had detectable hepatosplenic schistosomiasis. Half were symptomatic, with fatigue, weakness, abdominal pain, and diarrhea the most frequent complaints. Hepatocellular function was usually well maintained, but those with portal hypertension often developed ascites and were prone to gastroesophageal hemorrhage, the common cause of death. Hepatic coma was a frequent and usually fatal consequence of upper intestinal bleeding, and it also occurred in some postsurgical patients (33). These forms of severe disease are now uncommon. However, weakness, diarrhea, growth retardation, hepatomegaly, and liver fibrosis are still common in areas of endemicity, especially in the lake and marshland zones (103, 137, 153). All improve with chemotherapy but recur with reinfection.
Cerebral Schistosomiasis
Cerebral disease is caused by the host reaction to schistosome eggs. Some persons with eggs in the central nervous system develop no symptoms. The mechanism of egg deposition is unknown, but their presence suggests that eggs may cross the blood-brain barrier or that some worm pairs may reach the venous side of the cerebral circulation (184). There is no evidence to support either theory.
Symptoms of cerebral schistosomiasis were found in American soldiers infected in the Philippines during World War II (89). Among this group, epilepsy usually appeared within the first year. The common forms were Jacksonian convulsions and grand mal seizures. Among groups of adult Chinese hospital patients with schistosomiasis, up to 4.3% had cerebral schistosomiasis. The prevalence of epilepsy in infected communities has been estimated at 1 to 4%, against a baseline rate of 0.3 to 0.5% (33, 89).
Praziquantel (see above) is better than older drugs for treating cerebral schistosomiasis and has been found to be safe and effective, leading to resolution of symptoms for more than 75% of cases (33, 185). In a few patients, cerebral schistosomiasis and cysticercosis may occur together. Fortunately, praziquantel therapy also cures seizures caused by neurocysticercosis (178). Several other reports, such as that of Bang et al. (7), noting infarction after treatment, and Garg (64), reporting other transient adverse effects, including seizures, raise concern about the safety of praziquantel for treatment of cerebral cysticercosis. Concomitant administration of corticosteroids has been advocated, but convincing evidence of benefit is not available. Therefore, if cerebral schistosomiasis and cerebral cysticercosis coexist, especially if cysticerci are present in vital neural structures such as the medulla or retina, the risks of treatment must be weighed against the risks of symptomatic management without attempting parasitological cure.
S. japonicum and Cancer
The evidence connecting schistosomiasis japonica to esophagogastric cancer or carcinoma of the liver is not convincing (33). These diseases occur together but are probably unrelated. The same authors also reviewed the extensive Chinese literature relating colorectal cancer and schistosomiasis. On aggregated data, the incidence of this cancer correlates with prevalence and intensity of schistosome infection. Affected individuals usually have a long history of inflammatory large bowel symptoms and schistosome infection. The cancers correspond in anatomic location to the large bowel area most affected by schistosomiasis, the age of onset is lower in areas of schistosome endemicity, and the length of survival is shorter. Correlation of schistosomiasis and colorectal cancer has also been reported from Japan (114) but was not found in one Philippine study (1). In patients with colonic schistosomiasis, epithelial dysplasia is common and may be severe, but the mechanism relating that or schistosome infection to colorectal cancer remains unknown.
Possible Associations with Viral Hepatitis, Alcoholic Cirrhosis, and Typhoid
Viral hepatitis B has been reported to be more frequent among those infected with S. mansoni. Cooccurrence of these diseases among the rural poor is expected and has been noted in China. The evidence of increased cosusceptibility or synergism is not strong, and statistical associations may be an example of ecological confounding. The same holds for putative associations between alcoholic cirrhosis and hepatic fibrosis due to schistosomiasis. However, as noted above, there is evidence that alcohol intake can interact with septal fibrosis and impede its reversal after treatment (103), but no mechanism for this effect is known. Prolonged or recurrent septicemic typhoid is well known for African and South American schistosomiasis, and although described for S. japonicum (184), it appears to be rare. The typhoid fever recurs until the schistosomacidal therapy removes the parasite. It is thought that typhoid organisms are sequestered inside the schistosomes and thus protected from antibacterial drugs.
Growth Retardation and Cognitive Defects
Fifty years ago, dwarfism affected up to 5% of the population in areas of hyperendemicity. It has now virtually disappeared. The pathogenesis is unknown, but the clinical presentation indicated pituitary growth failure. Schistosomiasis dwarfs had normal mental abilities but remained sexually immature, with atrophy of the pituitary and gonads. This dwarfism is thought to have been caused by heavy infection in early childhood. Cure of infection and early hormonal treatment improved the condition substantially.
Schistosome infection in childhood also causes substantial wasting and growth retardation without pituitary dwarfism (115, 137). Chemotherapeutic cure resulted in catch-up growth, but this did not equal the original deficit. Thus, schistosome-related childhood malnutrition is probably a factor limiting human potential in areas of endemic infection, and persistent schistosomiasis transmission may contribute to stunting among adult rural Chinese populations. As well, recent research in Sichuan, China, indicates that infected children suffer cognitive impairment, with significant adverse effects on memory (131).
Other Clinical Manifestations
The African schistosomes sometimes cause pulmonary hypertension, cor pulmonale, glomerulonephritis, or transverse myelitis, but none of these are noteworthy features of schistosomiasis japonica. It has been speculated that the relative lack of pulmonary disease reflects a reduced inflammatory response for S. japonicum granulomas in the lungs (33). It is not known why glomerulonephritis has no apparent association with schistosomiasis in China. It is also not known why Chinese schistosomes associate with ectopic cerebral lesions, while African schistosomes tend to produce ectopic spinal lesions.
Typical Laboratory Findings
In acute cases, eosinophilia and leukocytosis are always found. In hepatosplenic patients, anemia is common, usually normochromic and often macrocytic. In chronic cases, pancytopenia may reflect hypersplenism associated with splenomegaly. Hepatocellular liver function is usually relatively normal, but mild elevations of serum levels of liver enzymes can occur. In chronic cases, albumin production is low and polyclonal elevation of gamma globulin is found, with an inverted albumin-to-globulin ratio. Renal abnormalities result from severe fibroobstructive liver disease and include proteinuria and impaired electrolyte excretion and creatinine clearance.
Common radiological features include a large liver and spleen and, in acute cases, diffuse or patchy bilateral midzone infiltrates in the lungs. Ultrasound is very useful to diagnose and stage liver fibrosis (parenchymal and periportal) and measure portal vein diameters (175). For experienced workers, schistosomiasis japonica is easily distinguished from other forms of liver disease. Chinese ultrasonographers have used portable machines as diagnostic aids for many years.
DIAGNOSING SCHISTOSOMIASIS
Diagnosis is central to treating schistosomiasis. Case finding and community treatment, assessment of morbidity, and evaluations of control strategies all build on the results from diagnostic tests. In general, there are three different approaches to the diagnosis of schistosomiasis: to detect schistosome eggs in stool samples by direct parasitological methods and disclose eggs in tissue biopsies by histological methods; to measure pathological morbidity associated with schistosome infection by clinical, subclinical, and biochemical markers; and to test immunological responses to certain schistosome antigens and the levels of parasite-derived antigens in blood and urine. Traditionally, the diagnosis of schistosomiasis has been made by direct parasitological techniques, which are now well standardized and useful in areas with moderate to high intensity of infection.
Parasitological Examination
The miracidium hatching test is a traditional approach to assessing S. japonicum infection and has been used widely in China for more than four decades (32). This test is initiated by concentration of ova through a nylon tissue bag and suspension in distilled water. Three consecutive hatching tests are generally accepted by Chinese public health workers to rule out infection. This method is simple, and its potential for high sensitivity has been recognized, as this approach can process and examine 50 g of feces per sample. However, this method has not been standardized for quantitative measurement; more importantly, even under optimal conditions, only 50 to 70% of eggs will hatch, with light infections being missed.
The Kato-Katz thick smear stool examination, based on the examination of a calibrated amount of feces, is the most widely applied method to determine fecal egg counts in field surveys (91, 199, 224). Several studies have demonstrated that egg load is correlated with the severity of disease (90, 98), at least at the community level. However, egg excretion in the feces may show strong day-to-day fluctuations (intraindividual variation), especially with low intensities of infection (49, 50, 75). Epidemiological studies also show considerable interindividual variation in egg counts. Several authors have reported that, because of this variation, a large fraction of infected individuals will remain undetected if only one examination by the conventional diagnosis method is performed (68, 224). Therefore, the relative insensitivity of stool examination makes it less reliable for well-controlled areas where both the prevalence and the intensity of infection are generally low. Although multiple stool examinations should ideally be performed in order to reduce the number of false-negative results, repeated stool collection and examination require a lot of time and manpower (153). Thus, it is impractical to initiate such a strategy at the national level for routine schistosomiasis control programs. Repeated fecal smear examinations, usually from a single stool specimen, are useful for boosting the accuracy of diagnosis for field research (11, 153).
Microscopic examination of rectal biopsy specimens is a sensitive clinical diagnostic technique and was used in China during the 1970s, but this invasive procedure is neither simple nor convenient for population-based surveys. Furthermore, it is not possible to differentiate recently dead ova from those that have been dead for a long time, and this may lead to unnecessary repeated treatment.
As an ideal field method for the detection of schistosome ova in stool is still not available, there has been continuing interest in developing serological methods for the diagnosis of light infections.
Serological Detection
Immunological diagnosis of schistosomiasis has been available for the better part of this century and was first applied to S. japonicum infection in 1910 by Yoshimoto (14, 215). However, it was not until the advent of modern biotechnology that these assays were improved sufficiently to offer an alternative to parasitological diagnosis. Immunological diagnosis is divided into specific antibody and circulating antigen measurements. Various antibody detection methods have been implemented as adjuncts to fecal examinations, especially in China. Since antibody titers diminish in the absence of reinfection and eventually disappear or reach very low levels, this approach could be a useful diagnostic tool in the maintenance phase of control programs. Although antibody detection is not suitable for the assessment of active infections, this approach can yield sufficiently accurate results in controlled areas where the prevalence is less than 3% according to parasitological examination. In some studies which used crude S. japonicum soluble egg antigen, the data have shown a high sensitivity and an adequate specificity compared to standard parasitological techniques (100, 215). In addition, the quantitative seroreactivity of the characterized S. japonicum egg antigen correlated directly with the intensity of infection in all age groups. Antibody responses to proteinases (Sj32) and cysteine proteinases extracted from schistosomes have revealed that a strong antibody response is consistent with the early reactivity of S. japonicum-infected mice, and these proteinases may prove useful for the detection of schistosome infections in humans (165).
Keyhole limpet hemocyanin (KLH), synthesized by the marine mollusk Megathura crenulata, has a protective carbohydrate epitope similar to that found in schistosomes. Several studies have shown that KLH can be used to differentiate acute from chronic infections of both S. japonicum and S. mansoni (99, 100, 147). However, the slow reduction in specific antibody levels after treatment diminishes the value of such detection in areas of endemicity. The differentiation between past and current infection will continue to pose a problem until improved immunological or new biochemical tests are widely available. Cross-reactions with other infectious agents constitute another obstacle yet to be overcome. However, Wei et al. (189) reported that specific antiworm immunoglobulin G4 (IgG4) disappeared quickly after treatment; 98% of subjects become negative in 12 months after treatment. Therefore, antibody detection of short-term duration may still play an important role in assessing successful chemotherapy.
During the past decade, there has been much interest in understanding the molecular biology and immunology of the Asian schistosome (17, 58, 95, 116, 117). Furthermore, a number of recombinant antigens have been purified using modern molecular techniques during this period. These include 26-, 28-, and 31-kDa proteins of S. japonicum (Sj26, Sj28, and Sj31, respectively), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), triose-phosphate isomerase, paramyosin, fatty acid binding proteins, aspartic proteinases, and others (116, 180). Some of these antigens (e.g., Sj31, Sj32, and proteinases) have proved to be immunogenic and have been used as potential serological diagnostic markers in schistosomiasis (97, 165, 189).
It is generally accepted that antigen detection is a useful diagnostic approach for assessing the incidence of new infection in areas where schistosomiasis is currently under control. This approach could potentially be important in evaluating vaccine efficacy following human trials. For chronically infected persons with comparatively low infection intensities, parasitological documentation augmented by immunodiagnosis in the detection of circulating antigens did show a number of advantages over traditional antibody detection. Therefore, detection of circulating antigens in schistosomiasis is increasingly used as a diagnostic tool because the level of antigen can be correlated with worm burden and schistosome antigens are expected to disappear relatively rapidly from the circulation after successful chemotherapy. Since 1993, three national collaborative studies on immunodiagnosis, especially the detection of circulating antigens of S. japonicum, have been organized by an advisory committee for schistosomiasis control in China. Several diagnostic kits for measuring circulating antigens, developed by various institutes from the national to the provincial level, have been blindly assessed (71, 214). The sensitivity of most kits is usually higher than that of stool examination, but the specificity is lower. Recently, a rapid one-step enzyme immunoassay to detect circulating schistosome antigen with 95% sensitivity and 100% specificity was reported (183). Although this method is of high sensitivity and specificity, most institutes at the provincial level still prefer to sell their own diagnostic kits and use them in their province. It seems appropriate that a national organization should be established in order to reduce kit production costs and promote their wide distribution.
Taking into account the many advantages that antigen detection assays have over antibody detection assays, it seems paradoxical that these methods are still implemented in only a few research laboratories in China. There are major obstacles that limit the field application of diagnostic procedures based on detection of circulating antigens. Some of these are the high cost associated with detection for each case; complicated protocols for circulating antigen assays; expensive biochemical reagents with a short half-life, as well as the lack of trained and experienced technicians; small amounts of detectable circulating schistosome antigens in the serum and urine of infected patients; and the kinetics of circulating schistosome antigens and factors poorly understood associated with the immune response. These are limitations that are not readily overcome by laboratories in developing countries such as China. It seems likely that the wide application of antigen detection in community diagnosis will require a very simple dipstick-like test, standardized and manufactured by a commercial or public health agency at the national level.
Measurement of Pathological Lesions
The morbidity associated with schistosomiasis japonica has a broad spectrum of presentation. Hepatosplenic involvement with symptomatic portal hypertension is very often seen in schistosomiasis japonica (16, 33, 136, 173). Ultrasonography, in addition to clinical examination, is used to detect and quantify hepatosplenic schistosomal disease based on World Health Organization (WHO) protocols (21, 87, 103). Decreases in the thickness of the portal vein wall and in echogenic bands have been detected after praziquantel treatment (22, 191). Recent studies in the Dongting Lake region, Hunan Province, showed that changes in the liver parenchyma are characteristic of S. japonicum infection, and there was a significant improvement in ultrasound parenchyma and periportal images after a 2-year treatment cycle (102) (see above). However, the grading standard of ultrasound measurement needs to be improved to make grading easier in practice. In China, assessment of submorbidity related to S. japonicum infection by ultrasound has been limited to hospital and medical research surveys. Ultrasound measurement has not been used for evaluation of the success of control programs due to the high examination costs. There is no report of the use of computerized tomography to diagnose schistosomiasis at the community level in China. However, Cheung et al. (36) studied a group of patients with schistosomiasis in a hospital setting using computerized tomography and reported images thought to result from progressive septal fibrosis and related clusters of calcified eggs.
The most direct way to diagnose and quantify fibrosis in schistosomiasis is by histological examination of the liver. However, a liver biopsy specimen is difficult to obtain and subject to sampling error and reflects a static picture of hepatic fibrosis. As a result, noninvasive biochemical markers for assessing liver fibrosis are being actively sought to help to evaluate histological damage and monitor the progression of fibrosis. Several studies have reported that serum levels of procollagen peptide (types III and IV), the P1 fragment of laminin, hyaluronic acid, and fibrosin are elevated in severe hepatosplenic cases and decrease shortly after praziquantel treatment (90, 161, 201). Shahin et al. (161) reported that a serum elevation of procollagen type III N-propeptide may indicate early fibrogenic activity in patients with schistosome infection, and the combined measurement of procollagen type III N-propeptide with procollagen type IV C-propeptide and collagen VI seems to provide additional information to predict progressive hepatic fibrosis. Cai et al. (22) reported that parameters of hepatic fibrosis detected either by ultrasonography or by procollagen III and hyaluronic acid levels showed a significant improvement in a group of Chinese subjects 1 year after treatment. A recent study from the Dongting Lake region of China showed that hyaluronic acid levels correlate with the ultrasound findings, while the presence of laminin and collagen IV correlates with reinfection (103). A more sensitive marker for reflecting hepatic fibrosis is required, as the detection of biochemical markers in patients with schistosomiasis is of importance when monitoring liver fibrosis and morbidity in patients with more advanced schistosomiasis.
DRUG TREATMENT
Trivalent antimonial drugs were developed in 1918 and became the first of a long list of somewhat toxic drugs used to treat schistosomiasis in China (171). In the 1970s, the Bayer AG and E Merck drug companies developed a new antischistosomal pyrazino-isoquinolin drug known later as praziquantel. The drug was not related to previous antischistosomals and proved to be safe and well absorbed orally and to have a plasma half-life of 1 to 1.5 h. Absorbed praziquantel is subjected to a pronounced first-pass biotransformation, and 90% of the ingested drug is converted by the liver to inactive metabolites that are excreted by the kidneys. The drug diffuses into breast milk. The mechanism of action of praziquantel is unknown, although the active form causes schistosomes to develop tetanic contractions and tegumental vacuoles; affected worms lose their hold on the vein wall, are washed upstream to the liver, and die.
A series of standardized double-blind trials involved a historic collaboration between the industry (Bayer), WHO (A. Davis), and national health agencies in Brazil, Kenya, South America, Japan, and the Philippines (93). Trials also proceeded in China (212). The birth of praziquantel was smooth and a model for other drugs that are likely to benefit the poor. Rapid worldwide use of the drug followed once it was shown to have a high efficacy against all forms of schistosomiasis and to be suitable for single-dose oral therapy. Agreements were forged enabling subsidized praziquantel supply to Third World governments and profit taking through sales in rich countries. Later, China, Korea, and Thailand manufactured the drug, and the prices in developing countries fell below $1 per dose.
Since 1984, millions of people have been treated with praziquantel. No deaths have been reported, and schistosomes have not yet been proven unequivocally to develop drug resistance. For schistosomiasis japonica, cure rates of 70 to 90% have been recorded. Those not cured also benefit because their egg counts fall to one fifth or less of pretreatment levels. The single dose for community-based treatment in China has been standardized at 40 mg/kg. For optimal treatment of chronic cases in hospital, two 30-mg/kg doses are given 4 h apart. Side effects are thought to relate to worm death and include transient nausea, dizziness, rash, and pruritus. They are reported more frequently among patients with heavy infection and when the drug is taken on an empty stomach. Some heavily infected patients develop transient bloody diarrhea, but this resolves quickly without sequelae (144, 186).
Chemoprophylaxis
Praziquantel is not useful as a preventive drug because its actions only last a few hours. It is effective against schistosomula for the first 2 days of their life but is unable to kill them as they mature from days 3 to 21. A recently developed antimalarial drug, artemether, does kill schistosomula over the first 21 days after their entry into the body. Thus, it would be expected to kill all immature schistosome infections if given every 2 weeks, and evidence is emerging that this is indeed the case. The drug was synthesized as beta-methyl ether artemisinin by the Shanghai Institute of Materia Medica of the Chinese Academy of Sciences (205). Although artemether first attracted attention for its excellent antimalarial properties, it is now known to succeed as a chemoprophylactic in the field. In a trial with residents of an area of endemicity, the drug was administered every 15 days throughout the transmission season at a dose of 6 mg/kg (206). Acute cases were prevented, and incident infections were less than half as frequent as in the control group and were of lower intensity. Artemether is also active against other schistosome species (176, 207), and its overall effect is enhanced when it is combined with praziquantel (208). The prospects seem good for this drug to be used as a chemoprophylactic among high-risk groups in areas of endemicity, such as flood relief workers, tourists known to have been exposed recently, and fishermen (209). It will probably not be deployed in areas with malaria because of fears that the low doses required for schistosomiasis prevention could enable coincident malaria organisms to develop resistance.
EPIDEMIOLOGY AND CONTROL
Human Exposure
Human water contact studies to date have estimated exposure at the population level with either direct observational studies or interviews. When transmission areas are focal, observational studies are an ideal way to measure an individual's degree of water contact. However, in the lake and marshland regions of China, where 85% of the human cases reside, the transmission zones are vast and thus observational studies are of limited value. As a result a new method has been developed to quantify individual exposure in square meter-minutes, based on daily activity diaries (154). This method allowed examination of the pathways to exposure and infection among fishing communities in the Dongting Lake region (156). Moreover, this knowledge assisted in epidemiologically categorizing patients as either putatively susceptible or insusceptible (resistant) to disease, which has important implications for vaccine development (101, 157).
Human water contact studies are limited for S. japonicum, but recent work in the Dongting Lake region (154) has shown that most contact occurs in males aged 18 to 49 years, in the early afternoon (coinciding with peak cercarial shedding) (109, 129), while fishing. Thus, most human exposure to schistosomiasis in a typical Chinese lake region is occupationally driven. Females, to a far lesser extent, are exposed in the morning while bathing or washing clothes. Male-dominated exposure has been reported elsewhere in China (200). In Jiangxi Province, China, most of the reported contact for males occurred while washing or fishing (200). In contrast to these observations, other studies from Africa (59, 61, 83) have reported higher degrees of water contact in young (10 to 20 years) females. Most of this domestic and recreational activity occurred in the afternoon, as was observed for the Chinese subjects.
Overall, there is a general trend for adolescents (<21 years) to have greater exposure levels, but in China, this appears not to be the case, as most of the human water contact occurs in adults (>21 years) (154, 156, 200). The contrasting exposure patterns are ultimately a reflection of social, economic, and ecological variation from one area to another (81).
Human Prevalence and Intensity of Infection
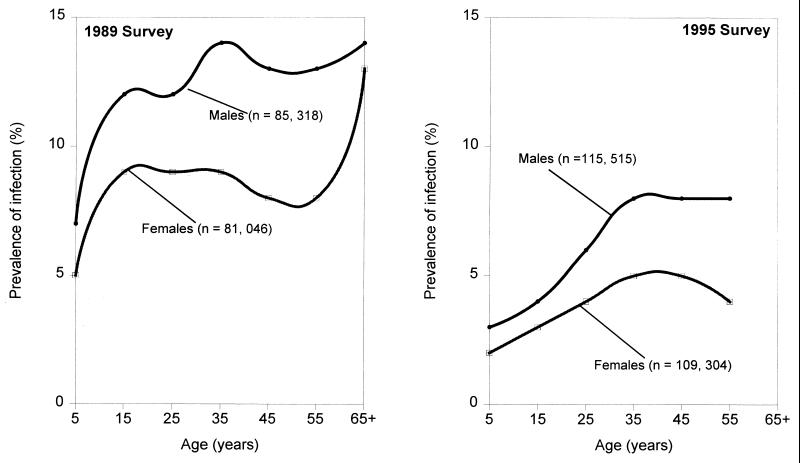
Age-prevalence (Fig. 4) and age-intensity data (134, 135) profiles for S. japonicum have revealed that males are twice as likely to become infected as females and that the praziquantel-based World Bank-Chinese government control activities from 1992 to 1995 halved the infection rates, as noted in the report of the second survey undertaken in 1995 (135). Rates for adult males and females in two national surveys (1989 and 1995) tended to exceed those found for children and adolescents, but one cannot attribute this infection pattern to underlying biological mechanisms without additional information. However, drug therapy directed especially at school-age children has been a feature of Chinese control programs for several decades, and praziquantel was introduced widely in 1986, 3 years before the first national survey. Moreover, the one-child policy for limiting the population in China dates from the early 1980s, and this could have increased the precautions exercised in areas of endemicity to limit exposure of children, especially given the extensive community education about schistosomiasis conducted since the 1950s. It is also known that occupational exposure is unavoidable for a large proportion of the adult population, and as mentioned earlier, human transmission of S. japonicum has a zoonotic component that is very difficult to control. Separating these influences on age trends for infection from influences of biological protection is not possible despite the size and accuracy of the national surveys.
FIG. 4.
Age-sex distribution of S. japonicum in China based on the 1989 and 1995 national parasitic surveys which comprised eight provinces. The data are from stratified random cluster samples and are derived from the same study populations using the same stool and serological methods. Results for each survey were pooled for all the populations included. Source: Office of Endemic Disease Control, China (1989 and 1997). n, number of subjects involved in the surveys.
The population-based data from discrete Chinese and Filipino communities (82, 138, 139, 152, 193) are potentially more informative. In three of the four discrete community studies, the adult prevalence remained high relative to corresponding estimates for children and adolescents. This was also noted for intensity in two communities and could reflect the influence of child-centered past control activities, which had occurred for many years before in Hunan (China) and Leyte (the Philippines). The Chinese community on Jishan Island had been treated only once with praziquantel, 3 years before the survey, and this could mean that these data reflect a less perturbed parasite distribution in an area of very high transmission. The age patterns for Jishan Island showed a sharp fall from adolescent peaks, a pattern considered typical for S. haematobium and partly attributed for the latter to age-dependent immunity (167). If the above data are taken together, it can be said that extensive examination of age patterns for prevalence and intensity of S. japonicum infection has not yielded curves typical of those for the other human schistosomes (158).
Acquired Immunity in Humans
With this in mind, more useful data bearing on age-acquired resistance to infection were obtained by conducting reinfection studies in a cohort of at-risk residents in the Dongting Lake region who were cured of infection and then monitored from 1996 to 1998. Great care was exercised to obtain accurate measurements of reinfection and water exposure (101, 154, 156, 157). The central finding was that reinfection intensity was highest among adolescents but that exposure was highest among adults (158). This feature was quite pronounced and substantially exceeded differences that could be accounted for solely by chance. The conclusion from the epidemiological evidence is that Chinese adults appear to become less infected than they should given their exposure and that such insusceptibility (resistance) could reflect age-dependent acquired immunity. Additional explanations include postpubertal hormonal influences (adrenarche) or increased mechanical barriers to cercarial penetration, but there is, as yet, no biological evidence for either effect among humans (62).
Animal Reservoirs
Domestic cattle and buffalo are the most important reservoir hosts for human schistosomiasis in China. Wild rats and rabbits can carry the infection but may be relatively unimportant to the human cycle because of their small size and feral range. Although goats and sheep are very susceptible, they are usually few in number and cannot contribute much to overall transmission (171). Horses, donkeys, and mules are also susceptible but are usually confined to the mountainous zones. Until recently, dogs were uncommon in rural China and were not important hosts. Pigs are potentially important hosts, but they are relatively short-lived and are often restricted to pens to fatten faster and provide feces for biogas production.
Cattle and buffaloes are used to plough and carry loads and are allowed to forage freely in many areas of endemicity, including mountain canyons, marshlands, and islands in the lake districts (103, 226). The buffalo herds ranging across marshland areas can be very large (several hundred animals), and they are brought back to shelter in the villages overnight. In many areas the bovines and humans converge at water bodies near the village, making cross-transmission inevitable. The most populous zones are the marshlands and lake districts of the middle reaches of the Yangzte, and there the overall ratio of people to bovines and of buffaloes to cattle is approximately 10:1.
Bovines are large animals (500 kg), produce 100 times more feces than humans (25 kg/day versus 250 g/day), deposit their excreta near or in surface water, and live 10 to 12 years. They can carry large numbers of schistosomes and develop hepatointestinal disease. In marshland areas where S. japonicum is endemic, 5 to 40% of bovines are infected. Praziquantel is curative, but the animals become reinfected. In most areas where schistosomiasis is endemic, a large proportion (>70%) of the environmental contamination may be traced back to bovine defecation (162, 225, 226). For these reasons, periodic bovine treatment with praziquantel (25 mg/kg) is included in control programs and managed by veterinarians employed in the provincial Agriculture Bureau. The dose is lower than that used for humans because the collateral effects may be fatal, thought to result from portal occlusion by dead worms in heavily infected animals, as noted for other forms of cattle schistosomiasis (45).
Chinese veterinarians have noticed that older buffaloes tend to excrete fewer viable eggs than those less than 18 months old. This could reflect self-cure, decreased egg viability, or decreased egg production by female worms, and all have been described for other forms of bovine schistosomiasis (45). Several experiments with S. japonicum infection have reported these phenomena in studies involving a total of 39 water buffalo. There was considerable variation in the timing and method of establishing infections and outcomes. However, worm burdens, worm size, and worm fecundity all appeared to decrease 1 to 1.5 years after infection (106, 107).
Current Control Strategies and Their Problems
Today, there are approximately 17,000 (1992 census) individuals working nationally at various levels to control schistosomiasis within China (63). Appropriate and stable management from the central to township levels has been established, with leading groups for schistosomiasis control at each administrative level. Each group consists of responsible officers from the departments of public health, water resources, agriculture, planning, and finance who jointly set up a schistosomiasis control office to draft laws and regulations, plan and carry out programs, and monitor progress (227). Technical aspects are taken care of at national and provincial levels by Institutes of Parasitic Diseases, while antischistosomiasis stations at prefecture and county levels are responsible for carrying out particular activities of the control program (141).
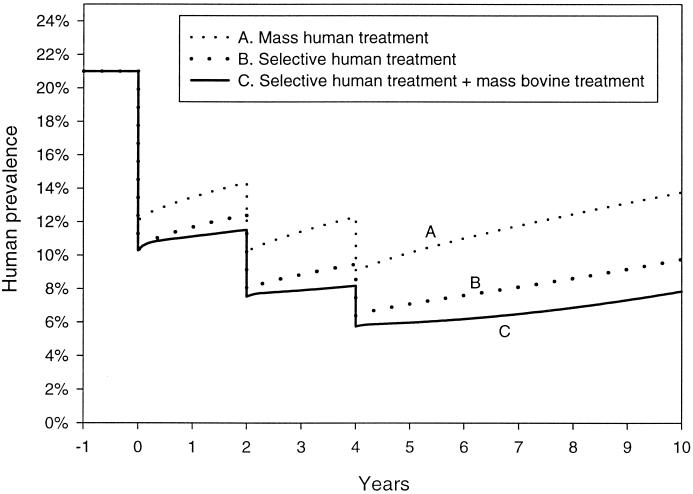
The World Bank committed a $71 million loan (with a complementary $82 million from the PRC government) to China for schistosomiasis control. The main goal of the World Bank loan was to reduce the prevalence of schistosomiasis in both humans and bovines (cattle and water buffaloes) by approximately 40%. This ambitious task relied primarily on two approaches. The first involves large-scale chemotherapy for humans and bovines with praziquantel, which has been produced in China since 1978, and the other involves selective treatment of humans (praziquantel) and snail control with molluscicidal programs and/or environmental modification (63). Mass chemotherapy was used when significant flooding occurred, as in Hunan Province in July 1996, or when the prevalence in a community was higher than 15%. Selective treatment was employed when the prevalence ranged between 3 and 15% or when seropositive individuals aged 7 to 14 years were identified when the prevalence was less than 3%.
The World Bank was also involved in a number of research-related activities throughout China, including impact and cost analysis of praziquantel, economic assessment of different control strategies, development of new procedures in the diagnosis of schistosomiasis (antigen or antibody detection), cercaria detection, liver examination with the aid of ultrasound, and health education. Each term, children were instructed for 3 to 4 hours on the life cycle of schistosomiasis and what precautions should be taken. Videos, textbooks, and posters were used as teaching aids to ensure that the message was clearly understood. Thousands of children have received invaluable information, which improves compliance with programs that aim to lower the prevalence of schistosomiasis in transmission areas (37).
South Korea, with financial backing from the World Bank, is the major supplier of praziquantel to China. However, two factories in China (in Shanghai and Nanjing) are able to produce this product should a crisis arise. In the Dongting Lake region alone, approximately 150,000 infected people receive praziquantel treatment annually (182). Despite the fact that praziquantel can reduce the incidence of severe forms of the disease and reverse much of the existing liver pathology (103), it does not prevent reinfection and has little effect on advanced hepatosplenic effects (98). It should be reemphasized that, to date, there are no reports of praziquantel resistance in schistosomiasis japonica.
There is a further and major complication of control of S. japonicum, which (as mentioned earlier), unlike the other human schistosomes, causes a true zoonosis and occurs as a natural parasite of a large number of mammalian species which act as reservoirs for the infection, rendering all chemotherapy-based control inadequate. Domestic animals, especially bovines and pigs, play a very important role in the transmission and epidemiology of this diseases in China (29, 218). The prevalence of schistosomiasis in many local Chinese communities is still high as a consequence, especially in the Dongting and Poyang lake regions.
Other strategies are required to complement existing control measures, and the development and effective application of an anti-S. japonicum vaccine, applicable in the first instance in bovines, especially water buffaloes, are warranted. The aim of such a vaccine would be to eliminate or markedly reduce disease transmission to humans, achievable by a decrease in intensity of infection and/or a reduction in worm fecundity and egg viability in bovines. Vaccine development is discussed further below.
Modeling Schistosomiasis Japonica
The statistical modeling of schistosomiasis has important implications when considering options for control. Models can predict the spread of disease, the utility of various strategies for treatment coverage, and the impact of vaccines. The future eradication of schistosomiasis in China will no doubt rely on a combination of various control options, such as drug treatment regimens, snail killing, vaccination, environmental modification, and sanitation. Mathematical models will assist in estimating the effects of these combined strategies and the costs of control.
According to Barbour (9), the first attempt to model the occurrence of schistosomiasis in humans was made by Hairston (74) in 1965, and the first dynamic model was made by MacDonald in 1965 (108). Barbour (10) concluded that MacDonald's model did not provide a good description of observed data and thus failed to explain the stability of the transmission cycle. The modeling of S. japonicum infection in China is especially complex because of the role that other mammalian hosts, specifically bovines, play in transmission. As a result, Barbour (10) has proposed a three-host general model based on a simple Ross model and suggests its appropriateness for modeling the transmission of S. japonicum. The key assumptions of the Ross model are that an infected definitive host has an infectivity that is not influenced by the number of times it is subsequently infected or by its parasite burden and a fixed recovery rate whatever its infection history; a recovered definitive host has the same susceptibility to infection as a definitive host not previously infected; and host lifetimes are negatively and exponentially distributed, and births of susceptible hosts compensate for host mortality.
With these assumptions, the prevalence of disease in each host is modeled as a function of time. Barbour extended the single-definitive-host model by allowing for two such hosts. He used this to determine bounds for the net reproductive parameter, which is the expected number of new parasites arising from a single parasite in its lifetime and entering the definitive host population. He compared qualitatively the likely success of various control measures in eradicating schistosomiasis when the population is only partially covered by a control strategy. Furthermore, he suggested that this model can be extended to more than two definitive hosts.
This model may be further extended to allow heterogeneity in definitive hosts. If it is parametrized according to known features of schistosomiasis epidemiology in the lake and marshland areas of China, it is possible to simulate the impact of competing control strategies. In particular, the model may be used to compare targeted chemotherapy (with high coverage) and mass chemotherapy (with poorer coverage). To achieve this, each of the two mammalian hosts may be subdivided into groups that are characterized by baseline prevalence of infection in humans (40, 30, 20, 10, and 5%) and bovines (25, 20, 15, 10, and 5%) or degree of compliance with the control strategy (50 and 90%). The prevalence of infection for snails is set at 1%. These prevalences are based on reports from lake and marshland communities in the 1990s (135). The duration of infection is set at 1.4 months for snails, 1.5 years for bovines, and 4 years for humans. The model assumes that snail, bovine, and human populations remain in a steady state, with replacements numerically equal to those leaving or dying.
Under the assumption that a system is in equilibrium, the transmission parameters of Barbour's model are estimated from endemic disease prevalences, together with an additional assumption about relative stool contamination for humans and bovines in snail breeding areas. Data from the Poyang Lake region of China (216, 219) indicate that in this population, taking into account the numbers of humans and bovines and their stool mass and infection rates, overall egg outputs of bovines and humans were approximately equal. Once model parameters are estimated, the impact of chemotherapy at one point in time is determined by incorporating an immediate reduction in prevalence in those treated by an amount determined by the efficacy of treatment (10). Over a subsequent period of time, the impact of periodic human and/or bovine treatment may also be simulated. Ten-year model simulations with 5 years of control (Fig. 5) compare results for biennial mass human treatment only (85% efficacy and 50% coverage), selected human treatment (targeting 40% of the population with the highest prevalence [35% compared to a prevalence of 10% in the remaining 60%]), and selected human and mass bovine treatment. Under these assumptions, the net reproduction rate for all three scenarios is 1.45, indicating that eradication cannot occur and that, once treatment is discontinued, prevalence will rise to former endemic disease levels. It is evident from Fig. 5 that selective human treatment together with mass treatment of bovines is the best treatment-based control option.
FIG. 5.
Effect of schistosomiasis on human prevalence in China based on three treatment scenarios. Key assumptions are (A) mass human treatment with 50% coverage; (B) selected or targeted human treatment with 90% coverage; and (C) selected or targeted human treatment with 90% coverage plus mass bovine treatment with 50% coverage.
IMMUNOLOGICAL CONSIDERATIONS
Immunology of Schistosomiasis
It would be impossible to report on progress towards development of antischistosome vaccines without a description of some basic details of the immunology of schistosomiasis. A number of valuable reports cover this topic in animal models and in humans (20, 48, 142, 143). An important point to make is that schistosomes are nonreplicating organisms in their mammalian hosts. As a result, a partial, nonsterilizing, naturally acquired or vaccine-induced immunity could potentially decrease human pathology and transmission in areas where schistosomiasis is endemic. It has been estimated that a reduction in worm intensity of 50% or more would significantly reduce pathology and degree of morbidity and decrease transmission rates (116). A putative human vaccine would be given to infants or small children in order to prevent severe infection in later years of high risk. Booster vaccine doses may be unnecessary, as subsequent trickle infections with cercariae could provide continuous restimulation of immunity. Successful attenuated cercarial vaccines have been tested in rodents and, with S. japonicum, larger animals, including buffaloes under field conditions and experimentally in pigs (116). Clearly, however, the antigen(s) involved in protection needs to be defined and produced by synthetic or recombinant means for use as a practical vaccine, aims central to the current research effort.
Important information facilitating the development of practical vaccines includes whether humans develop resistance against schistosomes and, if so, whether effective protective immunity can be induced with defined molecules in rodents, in natural hosts such as buffaloes in the case of S. japonicum, and in humans. These questions are considered below.
Immunology of Schistosomiasis in Rodent Models
The most recent studies of the immunological basis of high-level resistance to infection in rodent models generally involve immunization with UV or γ-irradiated cercariae or schistosomula, which fail to mature into adult worms and do not lay eggs, so that egg-induced liver pathology can play no part (37). It appears that lung stage schistosomula are the target of this protective immunity in mice, but whether parasite killing in humans follows the same course remains to be established. Nevertheless, it seems likely that antigens present at this developmental stage can be a potential source of novel vaccine candidates (76). It is important also to emphasize that a key obstacle to vaccine development in schistosomiasis is a lack of understanding of what type of an immune response should be invoked. As a consequence, a number of groups have used gene-disrupted mice to study immune responses in general (16, 198) and to dissect the mechanisms involved in immune killing of schistosomes following immunization by radiation-attenuated cercariae (79, 197).
The immune response is regulated by a network of cross-regulatory cytokines released by T helper (Th) cells. Extensive early studies indicated the preeminence of T-cell-mediated immunity in the acquisition of resistance by mice. Evidence that activated macrophages play a role as effector cells in protection against murine schistosomiasis, together with studies of the role of cytokines, suggested that a vaccine which preferentially induces macrophage-activating Th1 cytokines (e.g., gamma interferon [IFN-γ] and interleukin-2 [IL-2]) might be beneficial in controlling schistosomiasis (140, 202). More recent studies have demonstrated that IL-12, which promotes Th1 responses and suppresses Th2 responses, enhances attenuated cercaria-induced immunity in terms of reduced worm burden (202). Furthermore, this cytokine dramatically reduces tissue fibrosis induced by natural infection (203), and IFN-γ, IL-12, and tumor necrosis factor alpha are required to maintain the reduced liver pathology in mice vaccinated with this IL-12-based vaccine.
While activated macrophages associated with Th1 responses appear to be the major effector in mice protectively vaccinated once with attenuated cercariae, other experiments have demonstrated that repeated vaccination, while still protective, is associated with Th2 cell responses (25), suggesting that both Th1 and Th2 responses may contribute to protection. However, IgE does not appear to play an essential role in the murine response to schistosome infection (56), as appears to be the case in the rat model and probably in humans (see below). Other work has demonstrated that protection in mice multiply vaccinated with attenuated cercariae can be mediated by humoral factors transferable in serum to naive recipients (125) and that an increased level of protection is transferable by increasing the number of vaccinations (54). Studies using B-cell-deficient and IFN-γ knockout mice (5, 86) or double (IL-10 and IL-4 deficient or IL-10 and IL-12 deficient) knockout mice (79) have strongly reinforced the belief that effective vaccination against schistosomes depends on the simultaneous induction of both type 1- and type 2-associated immune responses. This conclusion may explain the limited success of most subunit vaccine protocols designed to preferentially induce either B-cell- or IFN-γ-dependent protective mechanisms. These new and important findings will clearly impact on future antischistosome vaccine development.
Although the concept has been questioned (196), antibody-dependent cell-mediated cytotoxicity (ADCC) mechanisms have been shown to efficiently kill newly transformed schistosomula in vitro (24), with IgE and subclasses of IgG or IgA acting in conjunction with effector cells such as eosinophils [with selectin and Lewis(x) antigen acting as coreceptors with human eosinophils (132)], macrophages, and platelets. This occurs in primate and human schistosomiasis as well as in the rat model. Rats are semipermissive hosts which eliminate the primary schistosome burden about 4 weeks postinfection and subsequently develop protective immunity to reinfection. Recent findings have shown that S. mansoni infection can drive Th2 responses in rats in the absence of egg production, which is required to induce a Th2 response in mice, and are in favor of the role of Th2-type cytokines (e.g., IL-4, IL-5, and IL-10) in protective immunity against reinfection (116). Adoptive transfer of eosinophils or platelets bearing cytophilic IgE was able to induce resistance to infection in rats (23). In addition, monoclonal antischistosome antibodies of the rat IgE and IgG2a isotypes could passively transfer protection (69, 179), while immunity was diminished following anti-μ and anti-IgE antibody treatment of neonatal rats or when IgE-depleted immune rat serum was passively transferred (12, 92). Potentially, antischistosome vaccines capable of replicating ADCC mechanisms could be very potent, but it is fair to say that evidence for ADCC efficacy in vivo is somewhat limited.
Immunity in Humans
Longitudinal treatment and reinfection studies of human schistosomiasis mansoni (19, 88) and schistosomiasis haematobia (194) suggest that acquired immunity develops gradually with age, a phenomenon that is particularly evident at or following puberty. Associations suggesting acquisition of anti-S. japonicum infection immunity have also been noted in preliminary studies in China and the Philippines (158). Knowledge of such processes could prove invaluable in identifying and targeting those immune responses required for the development of effective antischistosome vaccines. The exact role played by cytokines in regulating susceptibility or resistance to schistosome infection in humans remains unclear. Taking the available data as a whole suggests, however, that both Th1-type and Th2-type cytokines are involved in the human immune response to schistosomes and that they can be downregulated by active infection (112, 116).
Allergic-type immune responses, particularly IgE, correlate with the development of human resistance to reinfection for both S. haematobium (73) and S. mansoni (46, 53, 148, 149). In baboons also, it has been shown (130) that adult worm-specific IgE is associated with acquired immunity to S. mansoni infection. In contrast, IgG4 (53, 149) and IgG2 (149) were found to correlate with susceptibility, and it has been suggested that a specific function of IgG4 in serum might be to control antigen recognition by IgE and consequently to regulate anaphylactic reactions and IgE-mediated immunity (133).
Antibodies recognizing specific parasite antigens have also been associated with resistance to reinfection in humans. These include IgG antibodies against a 37-kDa molecule, GAPDH (47, 67), and IgA antibodies against a schistosome-derived 28-kDa glutathione S-transferase (GST) (66). Furthermore, there has been much recent interest in defining the molecular targets of IgE antibodies from resistant subjects, as these may provide important candidates for a vaccine. Accordingly, IgE recognition of an S. mansoni 22.6-kDa tegumental antigen was shown to correlate with low levels of reinfection after drug treatment, suggesting that IgE responses to this antigen may be protective (70). It has been proposed that IgE antibodies may also play a significant role in immunity to human schistosomiasis japonica and that an antigen(s) in the 22-kDa range also appears to be the target of this response, at least in studies carried out in the Philippines (72, 159, 187). There is evidence as well that IgE directed towards glycolipids could play an important role in resistance to schistosome reinfection (188).
A recent study based in Brazil reports that the IgE and IgG4 responses to defined S. mansoni antigens aggregates within families (177). This reinforces other kinship and family studies of genetic susceptibility, which have indicated the presence of a codominant major gene (SM1) controlling the intensity of S. mansoni infection (2). SM1 has been localized (2, 3, 113, 128) to a region of chromosome 5 (5q31-q33) close to the colony-stimulating factor-1 receptor gene and other genes encoding cytokines or cytokine receptors implicated in regulating the immune response against schistosomiasis and other pathogens. There is evidence (150) that the SM1 locus controls the differentiation of Th2 lymphocytes and schistosome infection. No studies have yet been undertaken on S. japonicum, and this is clearly an area for future research.
VACCINE DEVELOPMENT
Rationale
Evidence supporting the feasibility of a vaccine for schistosomiasis has been provided by successful vaccination-challenge experiments using attenuated cercariae in animal models of infection. Work on mice has demonstrated that it is possible to evoke protective immunity against a challenge infection with schistosomes by immunization and subsequent exposure of the mice to cercariae previously attenuated using either UV or γ-irradiation (123, 150). In the case of S. japonicum, however, it has been shown that certain mouse strains may not be suitable hosts for studies involving γ-irradiated vaccines (124, 140). Furthermore, protection against schistosome parasites appears to be species specific (124, 150) and, in the case of the Chinese and Philippine strains of S. japonicum, even strain specific (15, 124). UV-attenuated S. japonicum cercarial vaccines have been tested successfully in buffaloes (222) and in pigs (163) under field conditions in China, but due to the difficulty and impracticality of producing quality-controlled, reproducible batches of these vaccines on a large scale and the associated safety considerations, they are clearly not suitable for use in humans or for wide veterinary application. As a result, considerable attention has focused on investigating the protective mechanisms of the anti-schistosome immune response and identifying relevant parasite antigens that may be involved in inducing protective immune responses, with a view to developing a recombinant synthetic-peptide-based (164) or DNA (4) vaccine. The following section provides a summary of the most encouraging vaccine antigens that have been shown to confer protection in animals against schistosomiasis japonica.
Vaccine Candidates
Recent encouraging studies testing S. japonicum vaccine antigens have provided cause for optimism for the development of a vaccine against schistosomiasis japonica to be applied both in the veterinary context (to impact on human transmission) and clinically (to prevent or reduce disease). The research has involved studies on the cellular proliferation (51) and cytokine production (181) by human peripheral blood mononuclear cells stimulated by recombinant S. japonicum antigens in subjects residing in the schistosomiasis-endemic Dongting Lake region of China, together with the testing of native and recombinant antigens in vaccination-challenge trials in animals. A number of molecules are currently of interest and include 26-kDa and 28-kDa GSTs, paramyosin, triose-phosphate isomerase, a 23-kDa integral membrane protein, GAPDH, calpain, calreticulin, and tegumental antigens in the 22.6-kDa range (116, 117, 118). A series of vaccination studies with a recombinant form of the homologue of the S. mansoni myosin fragment LrV-5 have also been carried out, but the results proved to be generally disappointing (180). A summary of the protective efficacy of the leading S. japonicum vaccine candidates is presented in Table 3. As with S. mansoni GST (and S. haematobium GST, which has recently undergone a phase I clinical trial), the logical way forward is to produce GMP formulations of suitable S. japonicum vaccine candidates and their efficacy in extensive field trials on domestic livestock, particularly bovines, probably in China, and later in well-controlled phase I/II human clinical trials.
TABLE 3.
Protective efficacy of leading S. japonicum vaccine candidatesa
| Antigen | Host | Vaccine protocolb | Efficacy |
|---|---|---|---|
| Unfractionated GST, 26/28 kDa (native) | Mouse, BALB/c strain | FCA/FIC, s.c. | 26–30% worm reduction |
| Sheep | FCA/FIC plus KLH, i.m. | 30% worm reduction, no liver egg reduction, 52% fecal egg reduction | |
| GST, 26 kDa (native) | Rat | Unknown | Up to 53% worm reduction |
| GST, 26 kDa (recombinant) | Mouse, BALB/c strain | FCA/FIC, s.c. | 26–30% worm reduction |
| Mouse, NIH strain | FCA/FIC, s.c. | 24–26% worm reduction | |
| Pig | Alum, i.m. | 25–29% worm reduction, 47–54% liver egg reduction | |
| Buffalo | FCA/FIC, s.c. | 22% worm reduction, 48% liver egg reduction | |
| Sheep | FCA/FIC, i.m. | 30–62% worm reduction, 19–55% liver egg reduction, 11–37% fecal egg reduction | |
| GST, 28 kDa (native) | Rat | Unknown | Up to 43% worm reduction |
| GST, 28 kDa (recombinant) | Mouse, BALB/c strainc | FCA/FIC, s.c. | No worm or egg reduction |
| Mouse, CBA strainc | FCA/FIC, s.c. | No worm or egg reduction | |
| Mouse, C57BL/6 strainc | FCA/FIC, s.c. | 0–35% worm reduction,d no egg reduction | |
| Sheep | FCA/FIC, s.c. or i.m. | 34–69% worm reduction, 56–69% liver egg reduction, 43–60% fecal egg reduction | |
| Buffaloe | FCA/FIC, route unknown | 33% worm reduction, 36% fecal egg reduction | |
| Cattlee | FCA/FIC, route unknown | No worm reduction | |
| Paramyosin (native) | Mouse, NIH strain | FCA/FIC, s.c. | 27–28% worm reduction |
| Mouse, NIH strain | No adjuvant, i.p. | No worm reduction | |
| Mouse, ICR strainc | No adjuvant, i.p. | 62–86% worm reduction | |
| Sheep | BCG, i.d. | 44–48% worm reduction, 53–56% liver egg reduction, 63% fecal egg reduction | |
| Sheep | No adjuvant, i.d. | 43% worm reduction, 43% liver egg reduction, 24% fecal egg reduction | |
| Buffaloe | BCG, route unknown | No worm reduction | |
| Cattlee | BCG, route unknown | 31% worm reduction, 36% fecal egg reduction | |
| Paramyosin (recombinant) | Mouse, CBA strain | Quil adjuvant, s.c. | 32% worm reduction, 35% liver egg reduction |
| Mouse, Kunming strain | Quil adjuvant, s.c. | 32% worm reduction, 66% liver egg reduction | |
| Mouse, strain unknown | Unknown | 20–60% worm reduction | |
| Pig | Alum, i.d. | 25–33% worm reduction, some fecal egg reduction | |
| Pig | Titermax, i.d. | 35% worm reduction | |
| Paramyosin fragment (recombinant) | Sheep | BCG, i.d. | 17% worm reduction, no liver egg reduction, 16% fecal egg reduction |
| Buffaloe | BCG, route unknown | 35% worm reduction, 16% fecal egg reduction | |
| Cattlee | BCG, route unknown | 19% worm reduction | |
| TPI (native) | Mouse, NIH strain | FCA/FIC, s.c. | 21–24% worm reduction, 57–60% liver egg reduction |
| Integral membrane protein fragment (Sj23) (recombinant) | Sheep | FCA/FIC, i.m. | 59% worm reduction, 55% liver egg reduction, 35% fecal egg reduction |
| Buffaloe | FCA/FIC, route unknown | 33% worm reduction, 51% fecal egg reduction | |
| Cattlee | FCA/FIC, route unknown | 32% worm reduction, 71% fecal egg reduction |
Reprinted from reference 117 with permission from the publisher.
FCA, Freund's complete adjuvant; FIC, Freund's incomplete adjuvant; s.c., subcutaneous injection; i.m., intramuscular injection; i.d., intradermal injection; i.p., intraperitoneal injection; BCG, bacillus Calmette Guérin; TPI, triose-phosphate isomerase.
Challenged with Philippine strain S. japonicum.
Inconsistent efficacy results, as the 35% worm reduction could not be repeated in a follow-up experiment.
Natural field challenge.
New Approaches
A series of novel techniques have been applied by a number of groups in attempts to identify new schistosome vaccine candidates and to enhance the immunogenicity and effectiveness of available schistosome vaccines. These approaches include the use of Bordetella pertussis filamentous hemagglutinin in conjunction with S. mansoni 28-kDa GST (145, 221), recombinant Mycobacterium bovis bacillus Calmette-Guérin expressing S. haemotobium 28-kDa GST (119), S. mansoni 28-kDa GST entrapped by biodegradable microparticles (94), recombinant vaccinia viruses and gene gun vectors expressing S. mansoni calpain (8), the coupling of a major B-cell epitope of GAPDH to ovalbumin coadsorbed with granulocyte-macrophage colony-stimulating factor or IL-12 on alum (57), putative identification of novel vaccine candidates by isolation of T-cell antigens from a recombinant protein library (80), the expression of an epitope of the protective S. mansoni surface antigen 9B in the flagellin of Salmonella serovars (55), and the combination of praziquantel treatment and S. mansoni 28-kDa GST DNA immunization (13). Results with these strategies have been generally encouraging, and they can provide the basis for more effective vaccines in the future. Indeed, several of the approaches have yielded outcomes that provide cautious optimism for the development of a successful oral vaccine against schistosomiasis, which, if successful, would be a major advance.
Furthermore, the Schistosoma genome has become the subject of a major characterization initiative, through support from the WHO Tropical Diseases Research Program and other agencies. This has resulted in rapid expansion of knowledge of the schistosome genome, including the development of cDNA libraries from different developmental life cycle stages and the generation of approximately 10,000 expressed sequence tags from S. mansoni and S. japonicum (58, 195). As this database increases, it is likely that a number of novel vaccine candidates will be identified for future study. In addition, the recent construction and characterization of an S. mansoni bacterial artificial chromosome library (96) provide a new resource for the physical mapping and sequencing of the schistosome genome.
HUMAN GENETICS AND SUSCEPTIBILITY
The host genome has a profound influence on susceptibility or resistance to disease induced by infectious agents (193). Previous genetic studies of humans living in regions where schistosomiasis is endemic have identified at least two genomic regions which are associated with infection intensity or clinical manifestation. As was discussed above, segregation and linkage analysis of Brazilian pedigrees resulted in the detection (2) of a codominant major gene (referred to as SM1) controlling the intensity of infection by S. mansoni and located near a region containing the colony-stimulating factor-1 receptor marker gene on human chromosome 5q31-q33 (113). This result was confirmed in a Senegalese population (128).
Hepatosplenic disease (advanced schistosomiasis) is characterized by periportal liver fibrosis, spleen enlargement and congestion, portal hypertension, and other serious sequelae. These are the end result of CD4+ T-cell-dependent granuloma formation, caused by live parasite eggs lodged in hepatic portal venules. CD4+ T-cell responses are dependent on antigen presentation in the context of class II major histocompatibility complex (MHC) antigens, and therefore it is likely that host MHC class II phenotypes may influence susceptibility and resistance to hepatosplenic disease. Indeed, previous work in an area where S. mansoni is endemic in northeastern Brazil indicated a significant (P < 0.005) association between the presence of the HLA class II allele DQB1*0201 and hepatosplenic disease (160). In direct contrast, a study by Mohamed-Ali et al. (122) indicated that severe fibrosis in human schistosomiasis mansoni is under the control of a genetic locus that is not linked to the HLA locus.
Work by Waine et al. (181) in Jiangsu Province, China, has shown that two alleles, HLA-DRB1∗1202 and HLA-DQA1∗0601, are statistically associated with failure to develop advanced schistosomiasis. In contrast, HLA-DQB1∗05031 was associated with susceptibilty to advanced disease. It was interesting that allele DRB1∗1202 occurred with allele DQA1∗0601 and thus their independent protective effects could not be ascertained. In contrast, alleles DQA1∗0601 and DQB1∗05031 never occurred together and had opposite and significant effects on the occurrence of disease. In another study undertaken in Jiangxi Province, it was demonstrated that patients with HLA-DRB1 alleles DRB1∗1202, DRB1∗1404, and DRB1∗1405, associated with increased fibrosis (susceptible), all encoded a valine at amino acid residue 86, whereas alleles associated with resistance, DRB1∗1101, DRB1∗0409, and DRB1∗0701, all encoded glycine at position 86. The presence of the valine-86 allele was significantly increased in patients with fibrosis (77). A more extensive follow-up HLA-class I/II allele typing study on an expanded number of individuals from the same village indicated that the HLA-DRB1∗1101-DQA1∗0501-DQB1∗0301 and HLA-DRB1∗1501-DRB5∗0101 haplotypes are associated with protection from and susceptibility to grade 1 fibrosis, respectively, and that the HLA-DPA1∗0103-DPB1∗0201 haplotype is associated with protection against more severe disease (78).
The reasons for the differences in HLA class II associations observed for the Chinese populations studied are noteworthy and probably reflect many influences, acting independently or synergistically, on the genetic analyses. Genetic differences inherent between the S. japonicum populations and/or the human populations surveyed may have contributed to the different susceptibility-protection HLA II associations obtained. As discussed by Hirayama et al. (77), the presence or absence of hepatitis B in these communities and the influence of smoking and alcohol intake, which are known to influence the progression of liver fibrosis, may partly explain the disparity. Furthermore, variation in the intensity of S. japonicum infection in individuals from the different areas, differing exposure patterns to infection in the three communities, different praziquantel treatment doses, coverage, and frequency, and the immune status of the subjects investigated may also have affected the analysis.
NEW THREAT TO CONTROL
The ecology of central China will soon change substantially in ways that could affect the distribution and transmission of schistosomiasis (170). The Chinese government has begun work on a giant impoundment across the Yangtze River, and by 2003 this Three Gorges Dam will be closed to a height of 135 m. Six years later it will reach its full height of 185 m and begin to generate 18,600 MW of power for the whole of China (84). It will also help to control the lower Yangtze floods, which cause periodic disasters and are much feared. But it will create a lake behind the dam that will stretch 600 km upriver, with shorelines suitable in many areas for schistosomiasis. The lake will be located between the two key transmission zones of S. japonicum, Sichuan and Hubei-Hunan provinces. The downstream schistosomiasis-free buffer is only 40 km, and the upstream buffer is 500 km. Dislocations of over 1 million people, immigration into areas settled by 19 million people along the lake, and increased river traffic could introduce both parasite and intermediate host into the lake. The lake will provide an opportunity for hybridization by two subspecies of the intermediate host, O. hupensis subsp. robertsoni in Sichuan and O. hupensis subsp. hupensis in Hubei, with unpredictable consequences (see above). Once schistosomes are established in the lake, they would be difficult to eradicate.
In addition to the problems posed by the new lake, the downstream area will undergo massive change in ways that could also affect schistosomiasis. Downstream flows will be higher in winter and lower in summer. The marshlands will expand. Silt deposition will change; some areas will become more suitable for intermediate-host snails, and other areas will become less suitable. This is certain to alter the distribution and endemicity of schistosome infection in unpredictable ways.
CONCLUSIONS
The collective effort to understand and control schistosomiasis japonica in China over the last 50 years has been enormous. In areas where it was possible, the transmission environment was modified to make it resistant to the snail hosts. Toxic drugs were used to cure infection, and those infected were monitored for years until cure was certain. Great progress was made, and the awful toll taken on affected human populations has now been reduced greatly. Dwarfism, impoverishing incapacity to work in rural areas, and widespread premature death are now gone. We hope they will not return, and we salute the tenacity and dedication of the thousands of schistosomiasis control workers, farmers, government cadres, and others who made this possible.
In some areas, eradication was not successful. However, advances in chemotherapy with praziquantel and (probably) artemether now make it possible to cure and prevent both parasite infection and associated fibro-obstructive disease. But in areas of continued transmission, it is not possible to remove the parasite from the environment. Thus, chemotherapy needs to be given repeatedly to protect communities, a process that must eventually lead to community fatigue and possibly to drug resistance (65).
The inability to eradicate the parasite from large areas of the lake and marshland zones of the middle Yangtze and from the upper reaches of that river, together with ecological changes soon to arise throughout the Yangzte basin due to construction of the Three Gorges Dam, make it imperative to develop new strategies for control (155). Foremost among these is the development of human and animal vaccines.
Considering the breadth of consolidated, international efforts to generate schistosomiasis vaccines, we can be hopeful that these endeavors will one day have a successful outcome. The path to eventual success is, nevertheless, likely to be demanding, and we may have to wait some time before the ultimate final product, possibly comprising a combination of two or more antigens, is available. To date, however, there has been limited interest in the use of a multivalent antigen or multiepitope approaches. It is worth emphasizing that even when it becomes available, a schistosome vaccine in China will probably be used most effectively as one component of an integrated program of schistosomiasis control that would include effective and well-tested approaches currently in use in China.
ACKNOWLEDGMENTS
Our studies of the development of an antischistosomiasis vaccine have received financial support from various sources but particularly the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases, the National Health and Medical Research Council of Australia, and the National Institute of Allergy and Infectious Diseases (Tropical Medicine Research Center grant) (NIH 1 P50 AI39461).
We thank all our colleagues in Australia and our collaborators in China, the Philippines, and elsewhere who have provided us with much invaluable support during the course of our research. We much appreciate the excellent typing and formatting skills of Tracey Checkley.
REFERENCES
- 1.Abanilla L M. A report on the association of colonic cancer with Schistosoma japonicum infection. J Philipp Med Assoc. 1986;62:30–32. [Google Scholar]
- 2.Abel L, Demenasi F, Prata A, Souza A E, Dessein A J. Evidence for the segregation of a major gene in human susceptibility/resistance to infection by Schistosoma mansoni. Am J Hum Genet. 1991;48:959–970. [PMC free article] [PubMed] [Google Scholar]
- 3.Abel L, Dessein A J. Genetic epidemiology of infectious diseases in humans: design of population-based studies. Emerg Infect Dis. 1998;4:1–13. doi: 10.3201/eid0404.980409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alarcon J B, Waine G W, McManus D P. DNA vaccines: technology and application as anti-parasite and antimicrobial agents. Adv Parasitol. 1999;42:343–410. doi: 10.1016/s0065-308x(08)60152-9. [DOI] [PubMed] [Google Scholar]
- 5.Anderson S, Coulson P S, Ljubojevic S, Mountford A P, Wilson R A. The radiation-attenuated schistosome vaccine induces high levels of protective immunity in the absence of B cells. Immunology. 1999;96:22–28. doi: 10.1046/j.1365-2567.1999.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrade Z. An experimental approach to the pathogenesis of pipestem fibrosis (Symmers' fibrosis of the liver) Mem Inst Oswaldo Cruz. 1997;92:699–706. doi: 10.1590/s0074-02761997000500026. [DOI] [PubMed] [Google Scholar]
- 7.Bang O Y, Heo J H, Choi S A, Kim D I. Large cerebral infarction during praziquantel therapy in neurocysticercosis. Stroke. 1997;28:211–213. doi: 10.1161/01.str.28.1.211. [DOI] [PubMed] [Google Scholar]
- 8.Baras B, Benoit M A, Dupre L, Poulain-Godefroy O, Schacht A M, Capron A, Gillard J, Riveau G. Single-dose mucosal immunization with biodegradable microparticles containing a Schistosoma mansoni antigen. Infect Immun. 1999;67:2643–2648. doi: 10.1128/iai.67.5.2643-2648.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbour A D. Schistosomiasis. In: Anderson R M, editor. Population dynamics of infectious diseases: theory and applications. London, United Kingdom: Chapman and Hall; 1982. pp. 180–208. [Google Scholar]
- 10.Barbour A D. Modeling the transmission of schistosomiasis: an introductory view. Am J Trop Med Hyg. 1996;55:S135–S143. doi: 10.4269/ajtmh.1996.55.135. [DOI] [PubMed] [Google Scholar]
- 11.Barreto M L, Smith D, Sleigh A C. Implications of faecal egg count variation when using the Kato Katz method to assess S. mansoni infections. Trans R Soc Trop Med Hyg. 1990;84:554–555. doi: 10.1016/0035-9203(90)90037-f. [DOI] [PubMed] [Google Scholar]
- 12.Bazin H, Capron A, Capron M, Joseph M, Dessaint J P, Pauwels R. Effect of neonatal injection of anti-antibodies on immunity to schistosomes (Schistosoma mansoni) in the rat. J Immunol. 1980;124:2373–2377. [PubMed] [Google Scholar]
- 13.Ben-Yedidia T, Tarrab-Hazdai R, Schechtman D, Arnon R. Intranasal administration of synthetic recombinant peptide-based vaccine protects mice from infection by Schistosoma mansoni. Infect Immun. 1999;67:4360–4366. doi: 10.1128/iai.67.9.4360-4366.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergquist N R. Immunodiagnosis approaches in schistosomiasis. Chichester, England: John Wiley & Sons Ltd; 1992. [Google Scholar]
- 15.Bickle Q D, Andrews B J, Doenhoff M J, Ford M J, Taylor M G. Resistance against Schistosoma mansoni induced by highly irradiated infections: studies on species specificity of immunization and attempts to transfer resistance. Parasitology. 1985;90:301–312. doi: 10.1017/s0031182000051003. [DOI] [PubMed] [Google Scholar]
- 16.Booth M, Guyatt H L, Li Y S, Tanner M. The morbidity attributable to S. japonicum infection in 3 villages in the Dongting lake region, Hunan province, China. Trop Med Int Health. 1996;1:646–654. doi: 10.1111/j.1365-3156.1996.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 17.Brindley P J, Ramirez B, Tiu W, Wu G L, Wu H W. Networking on Schistosomiasis japonica. Parasitol Today. 1995;11:1–5. [Google Scholar]
- 18.Brunet L R, Kopf M A, Pearce E J. Schistosoma mansoni: IL-4 is necessary for concomitant immunity in mice. J Parasitol. 1999;85:734–736. [PubMed] [Google Scholar]
- 19.Butterworth A E. Immunity in human schistosomiasis. Acta Trop Suppl. 1987;12:31–40. [PubMed] [Google Scholar]
- 20.Butterworth A E. Immunological aspects of human schistosomiasis. Br Med Bull. 1998;54:357–368. doi: 10.1093/oxfordjournals.bmb.a011693. [DOI] [PubMed] [Google Scholar]
- 21.Cai W M, Qiu D C, Hatz C. Studies on ultrasonographic diagnosis of schistosomiasis japonica in China—a review of selected Chinese studies. Acta Trop. 1992;51:37–43. doi: 10.1016/0001-706x(92)90019-t. [DOI] [PubMed] [Google Scholar]
- 22.Cai W M, Chen Z, Chen F, Zhou C, Liu R, Wang J X. Changes of ultrasonography and two serum biochemical indices for hepatic fibrosis in schistosomiasis japonica patients one year after praziquantel treatment. Chin Med J (Peking) 1997;110:797–800. [PubMed] [Google Scholar]
- 23.Capron A, Dessaint J P. Effector and regulatory mechanisms in immunity to schistosomes: a heuristic view. Annu Rev Immunol. 1985;3:455–476. doi: 10.1146/annurev.iy.03.040185.002323. [DOI] [PubMed] [Google Scholar]
- 24.Capron M, Capron A. Immunoglobulin E and effector cells in schistosomiasis. Science. 1994;264:1876–1877. doi: 10.1126/science.8009216. [DOI] [PubMed] [Google Scholar]
- 25.Caulada-Benedetti Z, Al-Zamel F, Sher A, James S. Comparison of Th1 and Th2-associated immune reactivities stimulated by single versus multiple vaccination of mice with irradiated Schistosoma mansoni cercariae. J Immunol. 1991;146:1655–1660. [PubMed] [Google Scholar]
- 26.Cheever A W, Duvall R H. Schistosoma japonicum: migration of adult worm pairs in mesenteric veins of mice. Trans R Soc Trop Med Hyg. 1982;76:641–645. doi: 10.1016/0035-9203(82)90231-0. [DOI] [PubMed] [Google Scholar]
- 27.Cheever A W, Williams M E, Wynn T A, Finkelman F D, Seder R A, Cox T M, Hieny S, Caspar P, Sher A. Anti-IL-4 treatment of Schistosoma mansoni-infected mice inhibits development of T cells and non-B, non-T cells expressing Th2 cytokines while decreasing egg-induced hepatic fibrosis. J Immunol. 1994;153:753–759. [PubMed] [Google Scholar]
- 28.Cheever A W. Differential regulation of granuloma size and hepatic fibrosis in schistosome infections. Mem Inst Oswaldo Cruz. 1997;92:689–692. doi: 10.1590/s0074-02761997000500024. [DOI] [PubMed] [Google Scholar]
- 29.Chen M G. Progress and problems in schistosomiasis control in China. Trop Med Parasitol. 1989;40:174–176. [PubMed] [Google Scholar]
- 30.Chen M G. Relative distribution of Schistosoma japonicum eggs in the intestine of man: a subject of inconsistencies. Acta Trop. 1991;48:163–171. doi: 10.1016/0001-706x(91)90044-k. [DOI] [PubMed] [Google Scholar]
- 31.Chen M G. Progress in schistosomiasis control in China. Chin Med J (Peking) 1999;112:930–933. [PubMed] [Google Scholar]
- 32.Chen M G. Schistosomiasis control program in the People's Republic of China: a review. Southeast Asian J Trop Med Public Health. 1989;20:511–517. [PubMed] [Google Scholar]
- 33.Chen M G, Mott K E. Progress in the assessment of morbidity due to Schistosoma japonicum infection: a review of recent literature. Trop Dis Bull. 1989;85:R1–R56. [Google Scholar]
- 34.Chen M G, Feng Z. Schistosomiasis control in China. Parasitol Int. 1999;48:11–19. doi: 10.1016/s1383-5769(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 35.Chen M G, Wang S C, Chang P Y, Chuang C Y, Chen Y J, Tang Y C, Chou S C. Granulomatous disease of large intestine secondary to schistosome infection: a study of 229 cases. Chin Med J (Peking) 1978;91:371–378. [PubMed] [Google Scholar]
- 36.Cheung H, Lai Y M, Loke L L, Lee K C, Ho W C, Choi C H, Metreweli C. The imaging diagnosis of hepatic schistosomiasis japonica sequelae. Clin Radiol. 1996;51:51–55. doi: 10.1016/s0009-9260(96)80220-0. [DOI] [PubMed] [Google Scholar]
- 37.Dai C Q, Wei W Y. Mid-term assessment of health education on schistosomiasis control in Hunan province. Chin J Schistosomiasis Control. 1998;10:124–125. [Google Scholar]
- 38.Davis G M, Ruff M D. Oncomelania hupensis (Gastropoda: Hydrobiidae): hybridization, genetics, and transmission of Schistosoma japonicum. Malacol Rev. 1973;6:181–197. [Google Scholar]
- 39.Davis G M. Snail hosts of Asian Schistosoma infecting man: origin and coevolution. Malacol Rev Suppl. 1980;2:195–238. [Google Scholar]
- 40.Davis G M. Evolution of prosobranch snails transmitting Asian Schistosoma; coevolution with Schistosoma: a review. Prog Clin Parasitol. 1992;3:145–204. doi: 10.1007/978-1-4612-2732-8_6. [DOI] [PubMed] [Google Scholar]
- 41.Davis G M, Zhang Y, Guo Y H, Spolsky C M. Population genetics and systematic status of Oncomelania hupensis (Gastropoda: Pomatiopsidae) throughout China. Malacologia. 1995;37:133–156. [Google Scholar]
- 42.Davis G M, Wilke T, Spolsky C M, Ch-Ping Q, Qui D, Xia M Y, Zhang Y, Rosenberg G. Cytochrome oxidase I-based phylogenetic relationships among the Pomatiopsidae, Hydrobiidae, Rissoidae and Truncatellidae (Gastropoda: Caenogastropoda: Rissoacea) Malacologia. 1998;40:251–266. [Google Scholar]
- 43.Davis G M, Zhang Y, Xu X J, Yang X X. Allozyme analyses test the taxonomic relevance of ribbing in Chinese Oncomelania (Gastropoda: Rissoacea: Pomatiopsidae) Malacologia. 1999;41:297–317. [Google Scholar]
- 44.Davis G M, Wilke T, Zhang Y, Xu X, Chi-Ping Qiu, Spolsky C M, Qiu D, Li Y S, Xia M Y, Zheng F. Snail-Schistosoma, Paragonimus interactions in China: population ecology, genetic diversity, coevolution and emerging diseases. Malacologia. 1999;41:355–377. [Google Scholar]
- 45.De Bont J, Vercruysse J. Epidemiology and control of cattle schistosomiasis. Parasitol Today. 1997;13:255–262. doi: 10.1016/s0169-4758(97)01057-0. [DOI] [PubMed] [Google Scholar]
- 46.Demeure C E, Rihet P, Abel L, Ouattara M, Bourgois A, Dessein A J. Resistance to Schistosoma mansoni in humans: influence of the IgE/IgG4 balance and IgG2 in immunity to reinfection after chemotherapy. J Infect Dis. 1993;168:1000–1008. doi: 10.1093/infdis/168.4.1000. [DOI] [PubMed] [Google Scholar]
- 47.Dessein A J, Begley M, Demeure C, Caillol D, Fueri J, dos Reis M G, Andrade, Prata A, Bina J C. Human resistance to schistosomiasis is associated with IgG reactivity to a 37-kDa larval surface antigen. J Immunol. 1988;140:2727–2736. [PubMed] [Google Scholar]
- 48.Dessein A J, Coussinier P, Demeure C, Rihet P, Kohlstaedt S, Carneiro-Carvalho D, Ouattara M, Goudot-Crozel V, Dessein H, Bourgois A. Environmental, genetic and immunological factors in human resistance to Schistosoma mansoni. Immunol Investig. 1992;21:423–453. doi: 10.3109/08820139209069383. [DOI] [PubMed] [Google Scholar]
- 49.De Vlas S J, Gryseels B, Van Oortmarssen G J, Polderman A M, Habbema J D F. A model for variations in single and repeated egg count in Schistosoma mansoni infections. Parasitology. 1992;104:451–460. doi: 10.1017/s003118200006371x. [DOI] [PubMed] [Google Scholar]
- 50.Doehring E, Feldmeier H, Daffala A A. Day-to-day variation and circadian rhythm of egg excretion in urinary schistosomiasis in the Sudan. Ann Trop Med Parasitol. 1983;77:587–594. doi: 10.1080/00034983.1983.11811757. [DOI] [PubMed] [Google Scholar]
- 51.Doenhoff M J. A vaccine for schistosomiasis: alternative approaches. Parasitol Today. 1998;14:105–107. doi: 10.1016/s0169-4758(97)01204-0. [DOI] [PubMed] [Google Scholar]
- 52.Dunn M A, Cheever A W, Paglia L M, Kelly E P, Duvall R H, Andrade Z A, Goldner F H. Reversal of advanced liver fibrosis in rabbits with schistosomiasis japonica. Am J Trop Med Hyg. 1994;50:499–505. doi: 10.4269/ajtmh.1994.50.499. [DOI] [PubMed] [Google Scholar]
- 53.Dunne D W, Butterworth A E, Fulford A J, Kariuki H C, Langley J G, Ouma J H, Capron A, Pierce R J, Sturrock R F. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worms and resistance to reinfection. Eur J Immunol. 1992;22:1483–1494. doi: 10.1002/eji.1830220622. [DOI] [PubMed] [Google Scholar]
- 54.Dunne D W, Jones F M, Cook L, Moloney N A. Passively transferable protection against Schistosoma japonicum induced in the mouse by multiple vaccination with attenuated larvae: the development of immunity, antibody isotype responses and antigen recognition. Parasite Immunol. 1994;16:655–688. doi: 10.1111/j.1365-3024.1994.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 55.Eberi M, Mountford A P, Jankovic D, Beck E. Isolation of T-cell antigens by using a recombinant protein library and its application to the identification of novel vaccine candidates against schistosomiasis. Infect Immun. 1999;67:3383–3389. doi: 10.1128/iai.67.7.3383-3389.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El Ridi E, Ozaki T, Kamiya H. Schistosoma mansoni infection in IgE-producing and IgE-deficient mice. J Parasitol. 1998;84:171–174. [PubMed] [Google Scholar]
- 57.El Ridi R, Farouk F, Sherif M, Al-Sherbiny M, Osman A, El Gengehi N, Shoemaker C B. T and B cell reactivity to a 42-kDa protein is associated with human resistance to both schistosomiasis mansoni and haematobia. J Infect Dis. 1998;177:1364–1372. doi: 10.1086/515274. [DOI] [PubMed] [Google Scholar]
- 58.Fan J, Minchella D J, Day S R, McManus D P, Tiu W U, Brindley P J. Generation, identification, and evaluation of expressed sequence tags from different developmental stages of the Asian bloodfluke Schistosoma japonicum. Biochem Biophys Res Commun. 1998;252:348–356. doi: 10.1006/bbrc.1998.9491. [DOI] [PubMed] [Google Scholar]
- 59.Farooq M, Mallah M B. The behavioural pattern of social and religious water-contact activities in the Egypt-49 bilharziasis project area. Bull WHO. 1966;35:377–387. [PMC free article] [PubMed] [Google Scholar]
- 60.Faust E C, Meleney H E. Studies on schistosomiasis japonica. Am J Hyg Monogr Ser. 1924;3:1–339. [Google Scholar]
- 61.Fulford A J C, Ouma J H, Kariuki H C, Thiongo F W, Klumpp R, Kloos H, Sturrock R F, Butterworth A E. Water-contact observations in Kenya communities endemic for schistosomiasis: methodology and patterns of behaviour. Parasitology. 1996;113:223–241. doi: 10.1017/s0031182000082007. [DOI] [PubMed] [Google Scholar]
- 62.Fulford A J C, Ouma J H, Kariuki H C, Thiongo F W, Klumpp R, Kloos H, Sturrock R F, Butterworth A E. Puberty and age-related changes in susceptibility to schistosome infection. Parasitol Today. 1998;14:23–26. doi: 10.1016/s0169-4758(97)01168-x. [DOI] [PubMed] [Google Scholar]
- 63.Gao S F. World Bank Loan guidelines for Chinese schistosomiasis control programmes. Shanghai People's Republic of China: Shanghai Publishing House of Science and Technology; 1994. pp. 15–84. . (In Chinese). [Google Scholar]
- 64.Garg R K. Drug treatment of neurocysticercosis. Nat Med J India. 1997;10:173–177. [PubMed] [Google Scholar]
- 65.Geerts S, Gryseels B. Drug resistance in human helminths: current situation and lessons from livestock. Clin Microbiol Rev. 2000;13:207–222. doi: 10.1128/cmr.13.2.207-222.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goudot-Crozel V, Caillol D, Djabali M, Dessein A J. The major parasite surface antigen associated with human resistance to schistosomiasis is a 37-kD glyceraldehyde-3P-dehydrogenase. J Exp Med. 1989;170:2065–2080. doi: 10.1084/jem.170.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gounni A S, Lamkhioued B, Ochiaia K, Tanaka Y, Delaporte E, Capron A, Kinet J P, Capron M. High-affinity IgE receptor on eosinophils is involved in defence against parasites. Nature. 1994;367:183–186. doi: 10.1038/367183a0. [DOI] [PubMed] [Google Scholar]
- 68.Gryseels B, Nkulikyinka L, Engels D. Repeated community-based chemotherapy for control of Schistosoma mansoni: effect of screening and selective treatment on prevalence and intensities of infection. Am J Trop Med Hyg. 1991;45:509–517. doi: 10.4269/ajtmh.1991.45.509. [DOI] [PubMed] [Google Scholar]
- 69.Grzych J M, Capron M, Brazin H, Capron A. In vitro and in vivo effector function of rat IgG2a monoclonal anti-S. mansoni antibodies. J Immunol. 1982;129:2739–2743. [PubMed] [Google Scholar]
- 70.Grzych J M, Grezel D, Xu C B, Neyrinck J L, Capron M, Ouma J H, Butterworth A E, Capron A. IgA antibodies to a protective antigen in human schistosomiasis mansoni. J Immunol. 1993;150:527–535. [PubMed] [Google Scholar]
- 71.Guang X H, Shi Y E. Collaborative study on evaluation of immunodiagnostic assays in schistosomiasis japonica by treatment efficacy assessment. Chin Med J (Peking) 1997;109:659–664. [PubMed] [Google Scholar]
- 72.Hafalla J C R, Alamares II J G, Acosta L P, Dunne D W, Ramirez B L, Santiago M L. Molecular identification of a 21.7 kDa Schistosoma japonicum antigen as a target of human IgE response. Mol Biochem Parasitol. 1999;98:157–161. doi: 10.1016/s0166-6851(98)00160-1. [DOI] [PubMed] [Google Scholar]
- 73.Hagan P, Blumenthal U J, Dunne D W, Simpson A J G, Wilkins H A. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991;349:243–245. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- 74.Hairston N G. On the mathematical analysis of schistosome populations. Bull WHO. 1965;33:45–62. [PMC free article] [PubMed] [Google Scholar]
- 75.Hall A. Intestinal helminths of man: the interpretation of egg counts. Parasitology. 1982;85:605–613. doi: 10.1017/s0031182000056389. [DOI] [PubMed] [Google Scholar]
- 76.Harrop R, Coulson P S, Wilson R A. Characterization, cloning and immunogenicity of antigens released by lung-stage larvae of Schistosoma mansoni. Parasitology. 1999;118:583–594. doi: 10.1017/s003118209900431x. [DOI] [PubMed] [Google Scholar]
- 77.Hirayama K, Chen H, Kikuchi M, Yin T, Itoh M, Gu X, Zhang S, Yuan H. Glycine-valine dimorphism at the 86th amino acid of HLA-DRB1 influenced the prognosis of postschistosomal hepatic fibrosis. J Infect Dis. 1998;177:1682–1686. doi: 10.1086/515299. [DOI] [PubMed] [Google Scholar]
- 78.Hirayama K, Chen H, Kikuchi M, Yin T, Gu X, Zhang S, Yuan H. HLA-DR-DQ alleles and HLA-DP alleles are independently associated with susceptibility to different stages of post-schistosomal hepatic fibrosis in the Chinese population. Tissue Antigens. 1999;53:269–274. doi: 10.1034/j.1399-0039.1999.530307.x. [DOI] [PubMed] [Google Scholar]
- 79.Hoffmann K F, James S L, Cheever A W, Wynn T A. Studies with double cytokine-deficient mice reveal that highly polarized Th1- and Th2-type cytokine and antibody responses contribute equally to vaccine-induced immunity to Schistosoma mansoni. J Immunol. 1999;163:927–938. [PubMed] [Google Scholar]
- 80.Hota-Mitchell S, Clarke M W, Podesta R B, Dekaban G A. Recombinant vaccinia viruses and gene gun vectors expressing the large subunit of Schistosoma mansoni calpain used in a murine immunization-challenge model. Vaccine. 1999;17:1338–1354. doi: 10.1016/s0264-410x(98)00391-0. [DOI] [PubMed] [Google Scholar]
- 81.Huang Y, Manderson L. Schistosomiasis and the social patterning of infection. Acta Trop. 1992;51:75–194. doi: 10.1016/0001-706x(92)90037-x. [DOI] [PubMed] [Google Scholar]
- 82.Hunan Institute of Parasitic Diseases. Results of sampling surveys from four villages in West Dongting Lake, Hunan Province, 1991–1995. Yueyang, People's Republic of China: Hunan Institute of Parasitic Diseases; 1995. . (In Chinese). [Google Scholar]
- 83.Husting E I. Human water contact activities related to the transmission of bilharziasis (schistosomiasis) J Trop Med Hyg. 1983;86:23–35. [PubMed] [Google Scholar]
- 84.Jackson S, Sleigh A C. Resettlement for China's Three Gorges Dam: socio-economic impact and institutional tensions. Communist Post-Communist Studies. 2000;33:223–241. [Google Scholar]
- 85.Jankovic D, Cheever A W, Kullberg M C, Wynn T A, Yap T A, Caspar P, Lewis F A, Clynes R, Ravetch J V, Sher A. CD4+ T cell-mediated granulomatous pathology in schistosomiasis is downregulated by a B cell-dependent mechanism requiring Fc receptor signaling. J Exp Med. 1998;187:619–629. doi: 10.1084/jem.187.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jankovic D, Wynn T A, Kullberger M C, Hieny S, Caspar P, James S, Cheever A W, Sher A. Optimal vaccination against Schistosoma mansoni requires the induction of both B cell and IFN-y-dependent effector mechanisms. J Immunol. 1999;162:345–351. [PubMed] [Google Scholar]
- 87.Jenkins J M, Hatz C The Cairo Working Group. The use of diagnosis ultrasound in schistosomiasis—attempts at standardization of methodology. Acta Trop. 1992;51:45–63. doi: 10.1016/0001-706x(92)90020-x. [DOI] [PubMed] [Google Scholar]
- 88.Kabatereine N B, Vennervald B J, Ouma J H, Kemijumbi J, Butterworth A E, Dunne D W, Fulford A J. Adult resistance to schistosomiasis mansoni: age-dependence of reinfection remains constant in communities with diverse exposure patterns. Parasitology. 1999;118:101–105. doi: 10.1017/s0031182098003576. [DOI] [PubMed] [Google Scholar]
- 89.Kane C A, Most H. Schistosomiasis of the central nervous system: experiences in World War II and review of literature. Arch Neurol Psychiatr. 1948;59:141–183. doi: 10.1001/archneurpsyc.1948.02300370003001. [DOI] [PubMed] [Google Scholar]
- 90.Kardorff R, Gabone R M, Mugashe C, Obiga D, Ramarokoto C E, Mahlert C, Spannbrucker N, Lang A, Gunzler V, Gryseels B, Ehrich J H, Doehring E. Schistosoma mansoni-related morbidity on Ukerewe Island, Tanzania: clinical, ultrasonographical and biochemical parameters. Trop Med Int Health. 1997;3:230–239. doi: 10.1046/j.1365-3156.1997.d01-269.x. [DOI] [PubMed] [Google Scholar]
- 91.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 92.Kigoni E P, Elsas P, Lenzi H L, Dessein A J. IgE antibody and resistance to infection. II. Effect of IgE suppression on the early and late skin reaction and resistance of rats to Schistosoma mansoni infection. Eur J Immunol. 1986;16:589–595. doi: 10.1002/eji.1830160602. [DOI] [PubMed] [Google Scholar]
- 93.King C H, Mahmoud A A F. Drugs five years later: praziquantel. Ann Intern Med. 1989;110:290–296. doi: 10.7326/0003-4819-110-4-290. [DOI] [PubMed] [Google Scholar]
- 94.Kremer L, Dupre L, Riveau G, Capron A, Locht C. Systemic and mucosal immune responses after intranasal administration of recombinant Mycobacterium bovis bacillus Calmette-Guérin expressing glutathione S-transferase from Schistosoma haematobium. Infect Immun. 1998;66:5669–5676. doi: 10.1128/iai.66.12.5669-5676.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Le T H, Blair D, McManus D P. Mitochondrial DNA sequences of human schistosomes: the current status. Int J Parasitol. 2000;30:283–290. doi: 10.1016/s0020-7519(99)00204-0. [DOI] [PubMed] [Google Scholar]
- 96.Le Paslier M-C, Pierce R J, Merlin F, Hirai H, Wu W, Williams D L, Johnston D, LoVerde P T, Le Paslier D. Construction and characterization of a Schistosoma mansoni bacterial artificial chromosome library. Genomics. 2000;65:87–94. doi: 10.1006/geno.2000.6147. [DOI] [PubMed] [Google Scholar]
- 97.Li Y L, Lio S Y, Han J J, Ning C X, Ruppel A. Evaluation of a monoclonal antibody-based sandwich ELISA for detection of circulating S. japonicum antigen (Sj31/32) in an endemic area of China. Trop Med Int Health. 1996;1:847–850. doi: 10.1111/j.1365-3156.1996.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 98.Li Y S, Li Y, Yu D B, Hu S Q, Xiang Y, Zhong Z N. A multivariate analysis of the relationship between the workability and S. japonicum infection in China. Rev Inst Med Trop Sao Paulo. 1993;35:347–353. [PubMed] [Google Scholar]
- 99.Li Y S, Rabello A L T, Simpson A J G, Katz N. The serological differentiation of acute and chronic S. japonicum infection by ELISA using keyhole limpet haemocyanin as antigen. Trans R Soc Trop Med Hyg. 1994;88:249–251. doi: 10.1016/0035-9203(94)90319-0. [DOI] [PubMed] [Google Scholar]
- 100.Li Y S, Ross A G P, Li Y, He Y, Luo X, McManus D P. The serological diagnosis of Schistosoma japonicum infection in China: the dot-ELISA technique employing keyhole limpet haemocyanin as the specific antigen. Trans R Soc Trop Med Hyg. 1997;91:19–21. doi: 10.1016/s0035-9203(97)90378-3. [DOI] [PubMed] [Google Scholar]
- 101.Li Y S, Sleigh A C, Ross A G, Li Y, Williams G M, Forsyth S J, Tanner M, McManus D P. A 2-year prospective study in China provides epidemiological evidence for resistance in humans to re-infection with Schistosoma japonicum. Ann Trop Med Parasitol. 1999;93:629–642. doi: 10.1080/00034989958131. [DOI] [PubMed] [Google Scholar]
- 102.Li Y S, Sleigh A C, Ross A G P, Li Y, Williams G M, Forsyth S J, Tanner M, McManus D P. Two year impact of praziquantel for Schistosoma japonicum infection in China: re-infection, sub-clinical disease and fibrosis marker measurements. Trans R Soc Trop Med Hyg. 2000;94:191–197. doi: 10.1016/s0035-9203(00)90274-8. [DOI] [PubMed] [Google Scholar]
- 103.Li Y S, Sleigh A C, Ross A G P, Li Y, Williams G M, Tanner M, McManus D P. Epidemiology of Schistosoma japonicum in China: morbidity and strategies for control in the Dongting Lake region. Int J Parasitol. 2000;30:273–281. doi: 10.1016/s0020-7519(99)00201-5. [DOI] [PubMed] [Google Scholar]
- 104.Liu Y Y, Lou T K, Wang Y X, Zhang W Z. Subspecific differentiation of oncomelanid snails. Acta Hydrobiol Sin. 1981;6:253–266. [Google Scholar]
- 105.Logan O T. A case of dysentery in Hunan province caused by the trematode Schistosoma japonicum. Chin Med J (Peking) 1905;19:243–245. [Google Scholar]
- 106.Lu Y L, Pan S D. Experimental study on self-cure phenomenon in water buffaloes infected with Schistosoma japonicum. In Selection on schistosomiasis research (1961–1979) Beijing, People's Republic of China: Research Committee of Schistosomiasis, Ministry of Public Health; 1980. p. 453. [Google Scholar]
- 107.Luo X F, Zhang C, Wu W M. Observation of the self-cure phenomenon in water buffaloes infected with Schistosoma japonicum. Chin Vet Sci. 1988;8:42. . (In Chinese.) [Google Scholar]
- 108.MacDonald G. The dynamics of helminth infections with special reference to schistosomes. Trans R Soc Trop Med Hyg. 1965;59:489–506. doi: 10.1016/0035-9203(65)90152-5. [DOI] [PubMed] [Google Scholar]
- 109.Mao S P, Li L, Wu C C. Studies on the emergence of cercariae of Schistosoma japonicum from their Chinese snail host, Oncomelania hupensis. Am J Trop Med. 1949;29:937–944. doi: 10.4269/ajtmh.1949.s1-29.937. [DOI] [PubMed] [Google Scholar]
- 110.Mao S P. Recent progress in the control of schistosomiasis in China. Chin Med J (Peking) 1986;99:439–443. [PubMed] [Google Scholar]
- 111.Mao S P, Shao B R. Schistosomiasis control in the People's Republic of China. Am J Trop Med Hyg. 1982;31:92–99. [PubMed] [Google Scholar]
- 112.Marguerite M, Gallissot M C, Diagne M, Moreau C, Diakkhate M M, Roberts M, Remoue F, Thiam A, Decam C, Rogerie F, Cottrez F, Neyrinck J L, Butterworth A E, Sturrock R F, Piau J P, Daff B, Niang M, Wolowczuk I, Riveau G, Auriault C, Capron A. Cellular immune responses of a Senegalese community recently exposed to Schistosoma mansoni: correlations of infection level with age and inflammatory cytokine production by soluble egg antigen-specific T cells. Trop Med Int Health. 1999;4:530–543. doi: 10.1046/j.1365-3156.1999.00443.x. [DOI] [PubMed] [Google Scholar]
- 113.Marquet S, Abel L, Hillair D, Dessein H, Kalil J, Feingold J, Weissenbach J, Dessein A J. Genetic localization of a locus controlling the intensity of infection by Schistosoma mansoni on chromosome 5q31–q33. Nat Genet. 1996;14:181–184. doi: 10.1038/ng1096-181. [DOI] [PubMed] [Google Scholar]
- 114.Matsuda K, Masaki T, Ishii S, Yamashita H, Watanabe T, Nagawa H, Muto T, Hirata Y, Kimura K, Kojima S. Possible associations of rectal carcinoma with Schistosoma japonicum infection and membranous nephropathy: a case report with a review. Jpn J Clin Oncol. 1999;29:576–581. doi: 10.1093/jjco/29.11.576. [DOI] [PubMed] [Google Scholar]
- 115.McGarvey S T, Wu G L, Zhang S, Wang Y, Peters P, Olds G R, Wiest P M. Child growth, nutritional status and schistosomiasis japonica in Jiangxi, People's Republic of China. Am J Trop Med Hyg. 1993;48:547–553. doi: 10.4269/ajtmh.1993.48.547. [DOI] [PubMed] [Google Scholar]
- 116.McManus D P. The search for a vaccine against schistosomiasis—a difficult path but an achievable goal. Immunol Rev. 1999;171:149–161. doi: 10.1111/j.1600-065x.1999.tb01346.x. [DOI] [PubMed] [Google Scholar]
- 117.McManus D P. A vaccine against Asian schistosomiasis: the story unfolds. Int J Parasitol. 2000;30:265–271. doi: 10.1016/s0020-7519(99)00200-3. [DOI] [PubMed] [Google Scholar]
- 118.McManus D P, Ross A G P, Sleigh A C, Williams G M, Yang W, Li Y S, Li Y, Acosta L, Waine G J. Production of interleukin-10 by peripheral blood mononuclear cells from residents of a marshland area in China endemic for Schistosoma japonicum. Parasitol Int. 1999;48:169–177. doi: 10.1016/s1383-5769(99)00015-x. [DOI] [PubMed] [Google Scholar]
- 119.Mielcarek N, Riveau G, Remoue F, Antoine R, Capron A, Locht C. Homologous and heterologous protection after single intranasal administration of live attenuated recombinant Bordetella pertussis. Nat Biotechnol. 1998;16:454–457. doi: 10.1038/nbt0598-454. [DOI] [PubMed] [Google Scholar]
- 120.Mitchell G F, Garcia E G, Wood S M, Diasanta R, Almonte R, Calica E, Davern K M, Tiu W U. Studies on the sex ratio of worms in schistosome infections. Parasitology. 1990;101:27–34. doi: 10.1017/s0031182000079713. [DOI] [PubMed] [Google Scholar]
- 121.Mitchell G F, Garcia E G, Davern K M, Tiu W U. Anti-embryonation immunity in murine schistosomiasis japonica (Philippines) Mem Inst Oswaldo Cruz. 1995;90:293–295. doi: 10.1590/s0074-02761995000200030. [DOI] [PubMed] [Google Scholar]
- 122.Mohamed-Ali Q, Elwali N E, Abdelhameed A A, Mergani A, Rahoud S, Elagib K E, Saeed O K, Abel L, Magzoub M M, Dessein A J. Susceptibility to periportal (Symmers) fibrosis in human Schistosoma mansoni infections: evidence that intensity and duration of infection, gender, and inherited factors are critical in disease progression. J Infect Dis. 1999;180:1298–1306. doi: 10.1086/314999. [DOI] [PubMed] [Google Scholar]
- 123.Moloney N A, Bickle Q D, Webbe G. The induction of specific immunity against Schistosoma japonicum by exposure of mice to ultraviolet attenuated cercariae. Parasitology. 1985;90:313–323. doi: 10.1017/s0031182000051015. [DOI] [PubMed] [Google Scholar]
- 124.Moloney N A, Garcia E G, Webbe G. The strain specificity of vaccination with ultraviolet attenuated cercariae of the Chinese strain of Schistosoma japonicum. Trans R Soc Trop Med Hyg. 1985;79:245–247. doi: 10.1016/0035-9203(85)90347-5. [DOI] [PubMed] [Google Scholar]
- 125.Moloney N A, Webbe G. Antibody is responsible for the passive transfer of immunity to mice from rabbits, rats or mice vaccinated with attenuated S. japonicum cercariae. Parasitology. 1990;100:235–239. doi: 10.1017/s0031182000061230. [DOI] [PubMed] [Google Scholar]
- 126.Monzawa S, Ohtomo K, Oba H, Nogata Y, Kachi K, Uchiyama G. Septa in the liver of patients with chronic hepatic schistosomiasis japonica: MR appearance. Am J Roentgenol. 1994;162:1347–1351. doi: 10.2214/ajr.162.6.8191996. [DOI] [PubMed] [Google Scholar]
- 127.Most H, Kane C A, Lavietes P H, Schroeder E F, Behm A, Blum L, Katzin B, Hayman J M. Schistosomiasis japonica in American military personnel: clinical studies of 600 cases during the first year after infection. Am J Trop Med Hyg. 1950;5:831–840. doi: 10.4269/ajtmh.1950.s1-30.239. [DOI] [PubMed] [Google Scholar]
- 128.Müller-Myhsok B, Stelma F F, Guisse-Sow F, Muntau B, Thye T, Burchard G D, Gryseels B, Horstman R D. Further evidence suggesting the presence of a locus on human chromosome 5q31–q33 influencing the intensity of infection with Schistosoma mansoni. Am J Hum Genet. 1997;61:452–454. doi: 10.1016/S0002-9297(07)64073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.National Schistosomiasis Research Committee. Studies on Schistosomiasis japonica in New China. Chin Med J (Peking) 1959;78:368–379. [PubMed] [Google Scholar]
- 130.Naus C W, Kimani G, Ouma J H, Fulford A J, Webster M, van Dam G J, Deelder A M, Butterworth A E, Dunne D W. Development of antibody isotype responses to Schistosoma mansoni in an immunologically naive immigrant population: influence of infection duration, infection intensity, and host age. Infect Immun. 1999;67:3444–3451. doi: 10.1128/iai.67.7.3444-3451.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nokes C, McGarvey S T, Shiue L, Wu G, Wu H, Bundy D A, Olds G R. Evidence for an improvement in cognitive function following treatment of Schistosoma japonicum infection in Chinese primary schoolchildren. Am J Trop Med Hyg. 1999;60:556–565. doi: 10.4269/ajtmh.1999.60.556. [DOI] [PubMed] [Google Scholar]
- 132.Nutten S, Papin J P, Woerly G, Dunne D W, MacGregor J, Trottein F, Capron M. Selectin and Lewis(x) are required as co-receptors in antibody-dependent cell-mediated cytotoxicity of human eosinophils to Schistosoma mansoni schistosomula. Eur J Immunol. 1999;29:799–808. doi: 10.1002/(SICI)1521-4141(199903)29:03<799::AID-IMMU799>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 133.Nyindo M, Kariuki T M, Mola P W, Farah I O, Elson L, Blanton R E, King C L. Role of adult worm antigen-specific immunoglobulin E in acquired immunity to Schistosoma mansoni infection in baboons. Infect Immun. 1999;67:636–642. doi: 10.1128/iai.67.2.636-642.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Office of Endemic Diseases Control. Epidemiological situation of schistosomiasis in China—results of a nation-wide sampling survey in 1989. Beijing, People's Republic of China: Ministry of Public Health; 1989. [Google Scholar]
- 135.Office of Endemic Diseases Control. Epidemiological situation of schistosomiasis in China—results of a nation-wide sampling survey in 1995. Beijing, People's Republic of China: Ministry of Public Health; 1997. [Google Scholar]
- 136.Ohmae H, Tanaka M, Hayashi M. Ultrasonographic and serologic abnormalities in Schistosoma japonicum infection in Leyte, the Philippines. Am J Trop Med Hyg. 1992;46:89–98. doi: 10.4269/ajtmh.1992.46.89. [DOI] [PubMed] [Google Scholar]
- 137.Olds G R, Olveda R, Wu G, Wiest P, McGarvey S, Aligui G, Zhang S, Ramirez B, Daniel B, Peters P, Romulo R, Fevidal P, Tiu W, Yuan J, Domingo E, Blas B. Immunity and morbidity in schistosomiasis japonica infection. Am J Trop Med Hyg. 1996;55:121–126. doi: 10.4269/ajtmh.1996.55.121. [DOI] [PubMed] [Google Scholar]
- 138.Olveda R M, Tiu E, Fevidal P, Jr, de Veyra F, Jr, Icatio F C, Jr, Domingo E O. Relationship of prevalence and intensity of infection to morbidity in schistosomiasis japonica: a study of three communities in Leyte, Philippines. Am J Trop Med Hyg. 1983;32:1312–1321. doi: 10.4269/ajtmh.1983.32.1312. [DOI] [PubMed] [Google Scholar]
- 139.Olveda R M, Daniel B L, Ramirez B D, Aligui G D, Acosta L P, Fevidal P, Tiu E, de Veyra F, Peters P A, Romulo R, Domingo E, Wiest P M, Olds G R. Schistosomiasis japonica in the Philippines: the long-term impact of population-based chemotherapy on infection, transmission, and morbidity. J Infect Dis. 1996;174:163–172. doi: 10.1093/infdis/174.1.163. [DOI] [PubMed] [Google Scholar]
- 140.Osada Y, Chen X, Mohamed R T, Shin E-H, Horie T, Kojima S. The involvement of Th1/2 cytokines in protective immunity against Schistosoma japonicum infection. In: Tada I, Kojima S, Tsuji M, editors. Parasitology into the 21st century. Proceedings of the Ninth International Congress of Parasitology. Monduzzi Editore, Bologna, Italy. 1998. pp. 495–502. [Google Scholar]
- 141.Pan H. Review of eradicated schistosomiasis in Shanghai. People's Republic of China: Shanghai Publishing House of Science and Technology, Shanghai; 1988. pp. 1–23. . (In Chinese.) [Google Scholar]
- 142.Pearce E. The immunology of schistosomiasis. In: Boothroyd J C, Komuniecki R, editors. Molecular approaches to parasitology. New York, N.Y: Wiley-Liss, Inc.; 1995. pp. 497–510. [Google Scholar]
- 143.Pearce E, Sher A. Functional dichotomy in the CD4+ T cell response to Schistosoma mansoni. Exp Parasitol. 1991;73:110–116. doi: 10.1016/0014-4894(91)90014-n. [DOI] [PubMed] [Google Scholar]
- 144.Polderman A M, Gryseels B, Gerold J L, Mpamila K, Manshande J P. Side effects of praziquantel in the treatment of Schistosoma mansoni in Maniema, Zaire. Trans R Soc Trop Med Hyg. 1984;78:752–754. doi: 10.1016/0035-9203(84)90007-5. [DOI] [PubMed] [Google Scholar]
- 145.Poulain-Godefroy O, Mielcarek N, Ivanoff N, Remoue F, Schacht A M, Phillips N, Locht C, Capron A, Riveau G. Bordetella pertussis filamentous hemagglutinin enhances the immunogenicity of liposome-delivered antigen administered intranasally. Infect Immun. 1998;66:1764–1767. doi: 10.1128/iai.66.4.1764-1767.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Qian X Z. Office of Control of the Central Communist Party Committee, Beijing. Shanghai, People's Republic of China: Shanghai Publishing House of Science & Technology; 1986. Review of controlling schistosomiasis in the past 30 years; pp. 15–16. . (In Chinese.) [Google Scholar]
- 147.Rabello A L T, Garcia M M A, Dias Neto E, Rocha R S, Katz N. Dot-dye-immunoassay and dot-ELISA for the serological differentiation of acute and chronic schistosomiasis mansoni using keyhole limpet haemocyanin as antigen. Trans R Soc Trop Med Hyg. 1993;87:279–281. doi: 10.1016/0035-9203(93)90127-c. [DOI] [PubMed] [Google Scholar]
- 148.Rihet P, Demeure C E, Bourgois A, Prata A, Dessein A J. Evidence for an association between human resistance to Schistosoma mansoni and high anti-larval IgE levels. Eur J Immunol. 1991;21:2679–2686. doi: 10.1002/eji.1830211106. [DOI] [PubMed] [Google Scholar]
- 149.Rihet P, Demeure C E, Dessein A J, Bourgois A. Strong serum inhibition of specific IgE correlated to competing IgG4, revealed by a new methodology in subjects from a S. mansoni endemic area. Eur J Immunol. 1992;22:2063–2070. doi: 10.1002/eji.1830220816. [DOI] [PubMed] [Google Scholar]
- 150.Rodrigues V, Jr, Piper K, Couissinier-Paris P, Bacelar O, Dessein H, Dessein A J. Genetic control of schistosome infections by the SM1 locus of the 5q31–q33 region is linked to differentiation of type 2 helper T lymphocytes. Infect Immun. 1999;67:4689–4692. doi: 10.1128/iai.67.9.4689-4692.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ross A G P, Li Y S, Sleigh A C, McManus D P. Schistosomiasis control in the People's Republic of China. Parasitol Today. 1997;13:152–155. doi: 10.1016/s0169-4758(97)01026-0. [DOI] [PubMed] [Google Scholar]
- 152.Ross A G P, Li Y S, Sleigh A C, Li Y, Williams G M, Wu W Z, Lou X, He Y, McManus D P. Epidemiologic features of Schistosoma japonicum among fishermen and other occupational groups in the Dongting Lake region (Hunan Province) of China. Am J Trop Med Hyg. 1997;57:302–308. doi: 10.4269/ajtmh.1997.57.302. [DOI] [PubMed] [Google Scholar]
- 153.Ross A G P, Li Y S, Sleigh A C, Williams G M, McManus D P. Faecal egg aggregation in humans infected with Schistosoma japonicum in China. Acta Trop. 1998;70:205–210. doi: 10.1016/s0001-706x(98)00022-9. [DOI] [PubMed] [Google Scholar]
- 154.Ross A G P, Li Y S, Sleigh A C, Williams G M, Hartel G F, Forsyth S J, Li Y, McManus D P. Activity diaries to assess human water contact patterns for schistosomiasis japonica in the Dongting Lake region of China. I. A comparison with other exposure instruments. Acta Trop. 1998;71:213–228. doi: 10.1016/s0001-706x(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 155.Ross, A. G. P., Y. S. Li, G. M. Williams, J. Zheng, and D. P. McManus. Dam worms. Biologist, in press. [PubMed]
- 156.Ross A G P, Sleigh A C, Li Y S, Williams G M, Waine G, Forsyth S J, Li Y, Hartel G F, McManus D P. Activity diaries to assess human water contact patterns for schistosomiasis japonica in the Dongting Lake region of China. II. Activity diaries and path analysis to predict infection. Acta Trop. 1998;71:229–236. [Google Scholar]
- 157.Ross A G P, Sleigh A C, Li Y S, Williams G, Li Y, Waine G J, Tang G T, Forsyth S J, McManus D P. Epidemiologic identification of Chinese individuals putatively susceptible or insusceptible to Schistosoma japonicum: a prelude to immunogenetic study of human resistance to Asian schistosomiasis. Ann Trop Med Parasitol. 1998;92:765–774. doi: 10.1080/00034989859005. [DOI] [PubMed] [Google Scholar]
- 158.Ross A G P, Sleigh A C, Li Y S, Williams G M, Aligui G D L, McManus D P. Is there immunity to Schistosoma japonicum? Parasitol Today. 2000;16:159–164. doi: 10.1016/s0169-4758(99)01621-x. [DOI] [PubMed] [Google Scholar]
- 159.Santiago M L, Hafalla J C R, Kurtls J D, Aligui G L, Wiest P M, Olveda R M, Olds G R, Dunne D W, Ramirez B L. Identification of the Schistosoma japonicum 22.6-kDa antigen as a major target of the human IgE response: similarity of IgE-binding epitopes to allergen peptides. Allergy Immunol. 1998;117:94–104. doi: 10.1159/000023995. [DOI] [PubMed] [Google Scholar]
- 160.Secor W E, del Corral H, dos Reis M G, Ramos E A, Zimon A E, Matos E P, Reis E A, do Carmo T M, Hirayama K, David R A, David J R, Harn D A., Jr Association of hepatosplenic schistosomiasis with HLA-DQB1∗0201. J Infect Dis. 1996;174:1131–1135. doi: 10.1093/infdis/174.5.1131. [DOI] [PubMed] [Google Scholar]
- 161.Shahin M, Schuppan D, Waldherr R, Ristell J, Risteli L, Savolainen E R, Oesterling C, Rahman H M A, Sahly A M, Razek S M A, Ruby O L, Koch A, Seitz H K. Serum procollagen peptides and collagen VI for the assessment of activity and degrees of hepatic fibrosis in schistosomiasis and alcoholic liver disease. Hepatology. 1992;15:637–644. doi: 10.1002/hep.1840150414. [DOI] [PubMed] [Google Scholar]
- 162.Shen W. Review and suggestions of control of animal schistosomiasis in China. Chin J Schistosomiasis Control. 1992;4:82–84. . (English abstract.) [Google Scholar]
- 163.Shi Y E, Jiang C, Han J, Li, Ruppel A. Schistosoma japonicum: an ultraviolet-attenuated cercarial vaccine applicable in the field for water buffaloes. Exp Parasitol. 1990;71:100–106. doi: 10.1016/0014-4894(90)90012-2. [DOI] [PubMed] [Google Scholar]
- 164.Shi Y E, Jiang C F, Han J J, Li Y Y, Ruppel A. Immunization of pigs against infection with Schistosoma japonicum using ultraviolet-attenuated cercariae. Parasitology. 1993;106:459–462. doi: 10.1017/s0031182000076745. [DOI] [PubMed] [Google Scholar]
- 165.Shu X H, Zhou J C, Cai C, Zeng X F, Zeng Q R, Yi X Y. Schistosoma japonicum: purification and diagnostic application of recombinant 32kD antigen. Bull Hum Med Univ. 1998;23:331–334. [PubMed] [Google Scholar]
- 166.Sleigh A C, Mott K E, Hoff R, Barreto M L, Mota E A, Maguire J H, Sherlock I, Weller T H. Three-year prospective study of the evolution of Manson's schistosomiasis in North-East Brazil. Lancet. 1985;ii:63–66. doi: 10.1016/s0140-6736(85)90177-1. [DOI] [PubMed] [Google Scholar]
- 167.Sleigh A C, Mott K E. Schistosomiasis. In: Gilles H M, editor. Epidemiology and control of tropical diseases (Clinics in Tropical Medicine and Communicable Diseases, Vol. 1). London, U.K: W. B. Saunders Co.; 1986. pp. 643–670. [Google Scholar]
- 168.Sleigh A C, Hoff R, Mott K E, Maguire J H, Franca Silva J T. Manson's schistosomiasis in Brazil: 11-year evaluation of successful disease-control with oxamniquine. Lancet. 1986;i:635–637. doi: 10.1016/s0140-6736(86)91721-6. [DOI] [PubMed] [Google Scholar]
- 169.Sleigh A C. Mission Report to WHO/WPRO National Workshop on Schistosomiasis in Lake Regions, Nanchang, China. WHO, Manila, The Philippines. 1990. Schistosomiasis research and control; p. 15. [Google Scholar]
- 170.Sleigh A C, Jackson S. Public health and public choice: dammed off at China's Three Gorges. Lancet. 1998;351:1449–1450. doi: 10.1016/S0140-6736(05)78866-8. [DOI] [PubMed] [Google Scholar]
- 171.Sleigh A C, Li X, Jackson S, Huang K. Eradication of schistosomiasis in Guangxi, China. 1. Setting, strategies, operations and outcomes, 1953–1992. Bull WHO. 1998;76:359–370. [PMC free article] [PubMed] [Google Scholar]
- 172.Sleigh A C, Li X, Jackson S, Huang K. Eradication of schistosomiasis in Guangxi, China. 2. Political economy, management strategy and costs, 1953–92. Bull WHO. 1998;76:497–508. [PMC free article] [PubMed] [Google Scholar]
- 173.Sturrock R F. The parasites and their life cycles. In: Jordan P, Webber G, Sturrock R F, editors. Human schistosomiasis. Cambridge, U.K: CAB International; 1993. pp. 1–4. [Google Scholar]
- 174.Symmers W. Note on a new form of liver cirrhosis due to the presence of ova of Bilharzia haematobia. J Pathol Bacteriol. 1903;9:237–239. [Google Scholar]
- 175.The Cairo Working Group. The use of diagnostic ultrasound in schistosomiasis—attempts at standardization of methodology. Acta Trop. 1992;51:45–63. doi: 10.1016/0001-706x(92)90020-x. [DOI] [PubMed] [Google Scholar]
- 176.Utzinger J, N'Goran E, N'Dri A, Lengeler C, Xiao S, Tanner M. Randomized controlled trial of oral artemether for the prevention of Schistosoma mansoni infection. Lancet. 2000;355:1320–1325. doi: 10.1016/s0140-6736(00)02114-0. [DOI] [PubMed] [Google Scholar]
- 177.van Der Kleij D, Tielens A G, Yazdanbakhsh M. Recognition of schistosome glycolipids by immunoglobulin E: possible role in immunity. Infect Immun. 1999;67:5946–5950. doi: 10.1128/iai.67.11.5946-5950.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Vazquez V, Sotelo J. The course of seizures after treatment for cerebral cysticercosis. N Engl J Med. 1992;327:696–701. doi: 10.1056/NEJM199209033271005. [DOI] [PubMed] [Google Scholar]
- 179.Verwaerde C, Joseph M, Capron M, Pierce R J, Dammonville M, Velge F, Auriault C, Capron A. Functional properties of a rat monoclonal IgE antibody specific for Schistosoma mansoni. J Immunol. 1987;138:4441–4446. [PubMed] [Google Scholar]
- 180.Waine G J, McManus D P. Schistosomiasis vaccine development—the current picture. BioEssays. 1997;19:435–443. doi: 10.1002/bies.950190511. [DOI] [PubMed] [Google Scholar]
- 181.Waine G J, Wen Y, Ross A G P, Li Y S, Sleigh A C, Kalinna B, Scott J C, Mazzer D, Li Y, McManus D P. Differential antigen-stimulated proliferation of human mononuclear cells by recombinant Schistosoma japonicum antigens in a Chinese population. Clin Exp Immunol. 1998;112:69–73. doi: 10.1046/j.1365-2249.1998.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang H Z. A national epidemiological survey on Schistosomiasis japonica in the People's Republic of China. Shanghai, People's Republic of China: Shanghai Institute of Parasitic Diseases; 1995. pp. 15–84. . (In Chinese.) [Google Scholar]
- 183.Wang X Z, Li S T, Zhou Z X. A rapid one-step method of EIA for detection of circulating antigen of Schistosoma japonicum. Chin Med J (Peking) 1999;112:124–128. [PubMed] [Google Scholar]
- 184.Warren K S. The pathology of schistosome infections. Helminthol Abstr Ser A. 1973;42:590–633. [Google Scholar]
- 185.Watt G, Baldovino P C, Castro J T, Fernando M T, Ranoa C P, Cross J H. Praziquantel in treatment of cerebral schistosomiasis. Lancet. 1986;ii:529–532. doi: 10.1016/s0140-6736(86)90110-8. [DOI] [PubMed] [Google Scholar]
- 186.Watt G, Baldovino P C, Castro J T, Fernando M T, Ranoa C P. Bloody diarrhoea after praziquantel therapy. Trans R Soc Trop Med Hyg. 1986;80:345. doi: 10.1016/0035-9203(86)90053-2. [DOI] [PubMed] [Google Scholar]
- 187.Webster M, Fulford A J C, Braun G. Human immunoglobulin E responses to a recombinant 22.6-kilodalton antigen from Schistosoma mansoni adult worms are associated with low intensities of reinfection after treatment. Infect Immun. 1996;64:4042–4046. doi: 10.1128/iai.64.10.4042-4046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Webster M, Ramirez B L, Aligui G D, Olveda R M, Ouma J H, Kariuki H C, Kimani G, Olds G R, Fulford A J C, Butterworth A E, Dunne D W. The influence of sex and age on antibody isotype responses to Schistosoma mansoni and Schistosoma japonicum in human populations in Kenya and the Philippines. Parasitology. 1997;114:383–393. doi: 10.1017/s003118209600858x. [DOI] [PubMed] [Google Scholar]
- 189.Wei C D, Shi Y E. Studies on the detection of specific IgG subclasses and its evaluation of therapeutic efficacy in Schistosoma japonicum. Chin J Zoonosis. 1998;14:30–32. . (In Chinese.) [Google Scholar]
- 190.Wiest P M, Wu G, Zhang S, Yuan J, Peters P A S, McGarvey S T, Tso M, Olveda R, Olds G R. Morbidity due to schistosomiasis japonica in the People's Republic of China. Trans R Soc Trop Med Hyg. 1992;86:47–50. doi: 10.1016/0035-9203(92)90437-h. [DOI] [PubMed] [Google Scholar]
- 191.Wiest P M, Wu G, Zhong S, McGarvey S T, Yuan J, Olveda R M, Peters P A, Olds G R. Impact of annual screening and chemotherapy with praziquantel on schistosomiasis japonica on Jishan Island, People's Republic of China. Am J Trop Med Hyg. 1994;51:162–169. doi: 10.4269/ajtmh.1994.51.162. [DOI] [PubMed] [Google Scholar]
- 192.Wilke, T., G. M. Davis, G. Xin, and H. X. Liu.Erhaia (Gastropoda:Rissooidea): phylogenetic relationships and the question of Paragonimus coevolution in Asia. Am. J. Trop. Med. Hyg., in press. [DOI] [PubMed]
- 193.Wilkins A S. Genetics of the susceptibility to infectious diseases (First Louis Pasteur Conference on Infectious Disease) BioEssays. 1996;19:85–86. doi: 10.1002/bies.950190113. [DOI] [PubMed] [Google Scholar]
- 194.Wilkins H A, Blumenthal U J, Hagan P, Hayes R J, Tulloch S. Resistance to reinfection after treatment of urinary schistosomiasis. Trans R Soc Trop Med Hyg. 1987;81:29–35. doi: 10.1016/0035-9203(87)90273-2. [DOI] [PubMed] [Google Scholar]
- 195.Williams S A, Johnston D A. Helminth genome analysis: the current status of the filarial and schistosome genome projects. Parasitology. 1999;118:S19–S38. doi: 10.1017/s0031182099004473. [DOI] [PubMed] [Google Scholar]
- 196.Wilson R A, Coulson P S. Why don't we have a schistosomiasis vaccine? Parasitol Today. 1998;14:97–99. doi: 10.1016/s0169-4758(97)01198-8. [DOI] [PubMed] [Google Scholar]
- 197.Wilson R A, Coulson P S, Mountford A P. Immune responses to the radiation-attenuated schistosome vaccine: what can we learn from knock-out mice? Immunol Lett. 1999;65:117–123. doi: 10.1016/s0165-2478(98)00134-5. [DOI] [PubMed] [Google Scholar]
- 198.Wolowczuk I, Roye O, Nutten S, Delacre M, Trottein F, Auriault C. Role of interleukin-7 in the relation between Schistosoma mansoni and its definitive vertebrate host. Microbes Infect. 1999;1:545–551. doi: 10.1016/s1286-4579(99)80094-x. [DOI] [PubMed] [Google Scholar]
- 199.World Health Organization. Cellophane faecal thick examination technique (Kato) for diagnosis of intestinal schistosomiasis and gastro intestinal helminth infections. Document PDP/83.3. Geneva, Switzerland: World Health Organization; 1983. [Google Scholar]
- 200.Wu Z D, Zhang S J, Gao Z, Xie Z W, Hu L S, Zhao G M, Jiang Q W, Uwe B. Reinfection with Schistosoma japonicum after treatment with praziquantel in the Poyang Lake region, China. SE Asian J Trop Med Public Health. 1994;25:163–169. [PubMed] [Google Scholar]
- 201.Wyler D J, Tabebian P. A quantitative assay to detect circulating fibrosin and its application in experimental schistosomiasis. Am J Trop Med Hyg. 1997;56:66–70. doi: 10.4269/ajtmh.1997.56.66. [DOI] [PubMed] [Google Scholar]
- 202.Wynn T A, Jankovic D, Hieny S, Cheever A, Sher A. IL-12 enhances vaccine-induced immunity to Schistosoma mansoni in mice and decreases T helper 2 cytokine expression, IgE production, and tissue eosinophilia. J Immunol. 1995;154:4701–4709. [PubMed] [Google Scholar]
- 203.Wynn T A, Cheever A W, Jankovic D, Poindexter R W, Caspar P, Lewis F A, Sher A. An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature. 1995;376:594–596. doi: 10.1038/376594a0. [DOI] [PubMed] [Google Scholar]
- 204.Wynn T A, Cheever A W, Williams M E, Hieny S, Caspar P, Kuhn R, Muller W, Sher A. IL-10 regulates liver pathology in acute murine schistosomiasis mansoni but is not required for immune down-modulation of chronic diseases. J Immunol. 1998;160:4473–4480. [PubMed] [Google Scholar]
- 205.Xiao S H, You J Q, Mei J Y, Jiao P Y. Early treatment of schistosomal infection with artemether and praziquantel in rabbits. Acta Pharmacol Sin. 1994;15:447–452. [PubMed] [Google Scholar]
- 206.Xiao S H, Shi Z G, Zhuo S J, Wang C Z, Zhang Z G, Chu B, Zheng J, Chen M G. Field studies on the preventive effect of oral artemether against schistosomal infection. Chin Med J (Peking) 1996;109:272–275. [PubMed] [Google Scholar]
- 207.Xiao S H, Chollet J, Weiss N A, Bergquist R N, Tanner M. Preventive effect of artemether in experimental animals infected with Schistosoma mansoni. Int J Parasitol. 2000;49:19–24. doi: 10.1016/s1383-5769(00)00028-3. [DOI] [PubMed] [Google Scholar]
- 208.Xiao S H, You J Q, Mei J Y, Guo H F, Jiao P Y, Tanner M. Effect of praziquantel together with artemether on Schistosoma japonicum parasites of different ages in rabbits. Int J Parasitol. 2000;49:25–30. doi: 10.1016/s1383-5769(00)00029-5. [DOI] [PubMed] [Google Scholar]
- 209.Xiao S H, Booth M, Tanner M. The prophylactic effects of artemether against Schistosoma japonicum infections. Parasitol Today. 2000;16:122–126. doi: 10.1016/s0169-4758(99)01601-4. [DOI] [PubMed] [Google Scholar]
- 210.Xu X, Fang T. Observation on collecting Oncomelania hupensis when opening the sluices during flood season in Yangtze River. Kasetsart J (Nat Sci) 1988;22:251–260. [Google Scholar]
- 211.Xu X, Fang T, Yu C, Jiang Y, Cao J. Relationship between sluicing for irrigation and spreading of Oncomelania hupensis in Hubei Province. Kasetsart J (Nat Sci) 1989;23:281–286. [Google Scholar]
- 212.Xuexhang Z. Clinical evaluation of praziquantel treatment of Schistosomiasis japonica. Chin Med J (Peking) 1980;93:375–384. [Google Scholar]
- 213.Yang X, Xu X, Fang T, Yu C. Experimental observation on the prevention of snail spreading by reverse siphoning. Chin J Zool. 1992;27:12–14. [Google Scholar]
- 214.Yi X Y, He Y K, Li Y S. A collaborative study on the detection of circulating antigens in Schistosomiasis japonica. Chin J Parasitol Parasitic Dis. 1995;13:277–283. [PubMed] [Google Scholar]
- 215.Yu S H, Qiu L S, Yang S J, Jiang G D, Miao D Q, Hong J W, Hu Y D, Xu Z Q, Tsang V I W, Dixon H, Bergquist N. Collaborative study on antigens and methods for immunodiagnosis of schistosomiasis in China. In: Bergquist N, editor. Immunodiagnosis approaches in schistosomiasis. Chichester, England: John Wiley & Sons Ltd.; 1992. pp. 29–38. [Google Scholar]
- 216.Yuan H C. Epidemic situation and strategies for schistosomiasis control in marshland and lake regions. Chin J Schistosomiasis Control. 1989;1:2–6. [Google Scholar]
- 217.Yuan H C. Epidemiological features and control of schistosomiasis japonica in China. Mem Inst Oswaldo Cruz. 1992;87:241. [PubMed] [Google Scholar]
- 218.Yuan H C. Epidemiological features and control strategies of schistosomiasis japonica in China. Chin Med J (Peking) 1993;106:563–568. [PubMed] [Google Scholar]
- 219.Zhang S, Liu Z, Li G, Zhong J, Cheng Y. Snail distribution and susceptible zones of schistosomiasis in endemic areas around Poyang Lake. Chin J Parasitol Parasitic Dis. 1990;8:8–12. [PubMed] [Google Scholar]
- 220.Zhang S J, Liu Z D, Hu L S. Studies on snail ecology in the marshlands of Poyang Lake region. J Nanchang Univ (Nat Sci) 1996;20(Suppl. Dec):25–34. [Google Scholar]
- 221.Zhang Y, Taylor M G, Bickle Q D. Schistosoma japonicum myosin: cloning, expression and vaccination studies with the homologue of the S. mansoni myosin fragment IrV-5. Parasite Immunol. 1998;20:583–594. doi: 10.1046/j.1365-3024.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- 222.Zhang Y, Taylor M G, Bickle Q D, Wang H, Ge J. Vaccination of mice with gamma-irradiated Schistosoma japonicum cercariae. Parasite Immunol. 1999;21:111–117. doi: 10.1046/j.1365-3024.1999.00208.x. [DOI] [PubMed] [Google Scholar]
- 223.Zhen J. A brief introduction to nation-wide sampling survey on schistosomiasis. Chin Med J (Peking) 1993;106:569–575. [PubMed] [Google Scholar]
- 224.Zheng J, Wu X H, Li Y S. Kato-Katz stool examinations in schistosomiasis control of China. Chin J Schistosomiasis Control. 1995;7:382–384. . (In Chinese.) [Google Scholar]
- 225.Zheng J, Gu X G, Qiu Z L, Hua Z H. Transmission factors of schistosomiasis japonica in the mountainous regions with type of plateau canyon and plateau basin. Chin Med J (Peking) 1997;110:86–89. [PubMed] [Google Scholar]
- 226.Zheng J, Zheng Q S, Wang X F, Hua Z H. Influence of livestock husbandry on schistosomiasis transmission in mountainous regions of Yunnan province. SE Asian J Trop Med Public Health. 1997;28:291–295. [PubMed] [Google Scholar]
- 227.Zhou D, Li Y S, Yang X. Schistosomiasis control in China. World Health Forum. 1994;15:387–389. [PubMed] [Google Scholar]
- 228.Zhou H, Ross A G P, Hartel G F, Sleigh A C, Williams G F, McManus D P, Luo X S, He Y, Li Y S. Diagnosis of schistosomiasis japonica in Chinese schoolchildren by administration of a questionnaire. Trans R Soc Trop Med Hyg. 1998;92:245–250. doi: 10.1016/s0035-9203(98)90997-x. [DOI] [PubMed] [Google Scholar]