Abstract
The origins of the chiroptical activities of inorganic nanostructures have perplexed scientists, and deracemization of high-nuclearity metal nanoclusters (NCs) remains challenging. Here, we report a single-crystal structure of Rac-Ag70 that contains enantiomeric pairs of 70-nuclearity silver clusters with 20 free valence electrons (Ag70), and each of these clusters is a doubly truncated tetrahedron with pseudo-T symmetry. A deracemization method using a chiral metal precursor not only stabilizes Ag70 in solution but also enables monitoring of the gradual enlargement of the electronic circular dichroism (CD) responses and anisotropy factor gabs. The chiral crystals of R/S-Ag70 in space group P21 containing a pseudo-T-symmetric enantiomeric NC show significant kernel-based and shell-based CD responses. The small symmetry breaking of Td symmetry arising from local distortion of Ag−S motifs and rotation of the apical Ag3 trigons results in large chiroptical responses. This work opens an avenue to construct chiral medium/large-sized NCs and nanoparticles, which are promising for asymmetric catalysis, nonlinear optics, chiral sensing, and biomedicine.
Subject terms: Coordination chemistry, Inorganic chemistry
Having control over the chirality of metal nanoclusters is challenging. Here, the authors report the deracemization of silver nanoclusters and monitor the chiroptical responses.
Introduction
Chirality, an essential attribute in nature, is especially fascinating at nanosize level1, including metal nanoclusters2–16, quantum dots17, one-dimensional spiral18,19, and two-dimensional surface20. Chiral metal nanoclusters with atomically precise structures are the ideal models for understanding the origin of chirality at nanoscale3. Thus far, ultrasmall homochiral metal nanoclusters containing ca. less than 50 metal atoms have been more easily prepared and separated due to their rigid metal skeletons2,5–16. Well-defined optically pure high-nuclearity metal clusters remain scarce because of the difficulty in separating and crystallizing enantiomers. Although a few chiral medium-sized nanoclusters have been structurally resolved by single-crystal X-ray diffraction analysis3,21–23, they crystallized in racemates, which have pairs of enantiomers in a unit cell of the crystal. Only one case of enantiomerically pure single crystals of a medium-sized (>50 metal atoms) silver nanocluster, Ag78, protected by a chiral diphosphine ligand, has been characterized16, yet its deracemization process is unclear, and the CD origins are not well assigned.
Herein, we report the preparation and structural characterization, including X-ray crystallography, of {NH2(CH3)2}2{Ag70S4(SiPr)24(CF3COO)20(DMF)3}·4DMF (Rac-Ag70). It crystallizes in the Pc1 space group (No. 165) and contains enantiomeric pairs of 70-nuclearity silver clusters, each of which is a pseudo-T-symmetric doubly truncated tetrahedron (Fig. 1), lacking a C3 axis (and thus strictly speaking should be called C1 symmetry). By using a small Ag(I) cluster as a stabilizer, we obtain regularly Td-symmetric Ag70 (Fig. 1a) in the structure of achiral cocrystal Ag70·Ag12. By controlling the chiral metal precursor, we monitor a gradual increase in CD responses and gabs in solution, demonstrating that the chiral carboxylate enters the coordination layer step-by-step and causes a progressive deracemization of the Ag70 racemates. Interestingly, the process can be reversed. Moreover, the chiral crystals of R/S-Ag70 in space group P21 only contain pseudo-T-symmetric enantiomeric nanoclusters similar to but with more deviation from those in Rac-Ag70 (Fig. 1b). The CD responses of crystalline R/S-Ag70 are consistent with those in solution, which originate from kernel-based and shell-based electronic transitions, as evidenced by theoretical calculations. Structural analysis reveals that the small symmetry breaking of Td symmetry arises from local distortion of Ag–S motifs and rotation of the apical Ag3 trigons, resulting in large chiroptical responses. This work opens an avenue to construct chiral medium-sized or large-sized nanoclusters and nanoparticles.
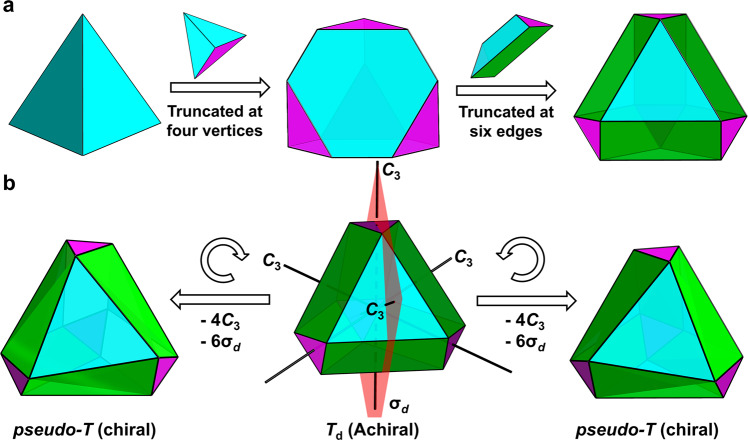
Fig. 1. Schematic evolution of the Td-symmetric doubly truncated tetrahedron and its symmetry breaking.
a Tetrahedron (one of the Platonic solids, left); truncated tetrahedron (one of the Archimedean solids, middle); doubly truncated tetrahedron (right). b Symmetry breaking of the Td-symmetric doubly truncated tetrahedron by rotation and twisting of the apical trigons, leading to pseudo-T symmetry lacking mirror planes (σd) and rotation axes (C3).
Results
Synthesis and characterization
The synthesis of Rac-Ag70 was achieved by an acid reduction method. {Ag(SiPr)}n and CF3COOAg together with CF3COOH, an indispensable acid, reacted in a mixed solvent containing N,N′-dimethylformamide (DMF) and isopropanol (iPrOH) under solvothermal conditions at 80 °C for 24 h. The reaction solution changed from colourless to black-red, indicating that slow reduction (AgI to Ag0) had occurred during the solvothermal process. Subsequently, black crystals of Rac-Ag70 were deposited after the filtrate evaporated in the dark for 1 week (Supplementary Fig. 1).
According to previous reports, Ag+ ions can be reduced to Ag0 atoms by heating DMF solution in a neutral or alkaline environment24–29. However, the silver clusters prepared under these conditions bear a maximum of two free electrons24–27. Here, we changed the environment from basic to acidic (CF3COOH) for the preparation of Rac-Ag70 nanoclusters (NCs) under solvothermal conditions. At high temperature, the addition of more CF3COOH may slow down the DMF reduction and the nucleation of Ag nanoparticles, and the S2− anions slowly generated in situ simultaneously capture an appropriate aggregation state, resulting in a high-nuclearity structure with a distinct Ag core. Thus, a reduction method was established for the synthesis of Ag NCs possessing multiple free electrons.
A series of characterizations (detailed discussions in the Methods section and Supplementary Figs. 2–6 in Supplementary Information), including single-crystal X-ray diffraction (SCXRD), elemental analyses, powder X-ray diffraction (PXRD), thermogravimetric (TG) analyses, X-ray photoelectron spectroscopy (XPS), energy dispersive spectroscopy (EDS), elemental mapping, UV-Vis-NIR spectroscopy, and electrospray ionization mass spectrometry (ESI-MS), were applied to elucidate the structures, compositions, phase purities, and electronic states.
Single-crystal structure of Rac-Ag70
Based on the SCXRD structural analysis (detailed below), 70-nuclearity Ag NCs of Rac-Ag70 were determined to be −2 valence-state anionic clusters with 20 free valence electrons [20 = 70(Ag+) − 4 × 2(S2−) − 24(SiPr−) − 20(CF3COO−) + 2], which can be described using the jellium model (1S21P61D102S2)30–32. The valence number was further corroborated by ESI-MS experiments and the well-consistent electronic transition spectra between the experimental and theoretically calculated results (vide infra).
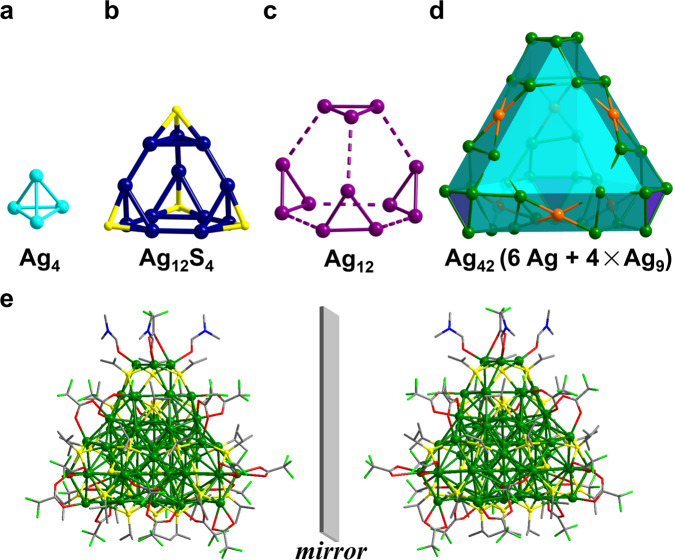
SCXRD analysis reveals that Rac-Ag70 possesses a fascinating core-shell skeleton with a Ag28S4 inner kernel (Ag4@Ag12S4@Ag12, Fig. 2a–c) enveloped by a larger doubly truncated tetrahedral Ag42 shell (Fig. 2d) that is covered and stabilized by SiPr− and CF3COO− ligands together with DMF solvent molecules. The 70-Ag atoms can be divided into concentric tetrahedral multishells (Fig. 2a–d): Ag4 (core)@Ag12S4 (1st shell)@Ag12 (2nd shell)@Ag42 (3rd shell). The Ag···Ag distances in Rac-Ag70 range from 2.770(2) to 3.385(2) Å, indicating the intermetallic interactions (Supplementary Figs. 7–8 and Supplementary Table 1). The detailed silver coordination modes and local structural features are discussed in the Supplementary Information (Supplementary Figs. 7–19).
Fig. 2. Single-crystal X-ray structure of Rac-Ag70.
a–d Innermost Ag4 tetrahedron; first-shell Ag12S4 tetrahedron; second-shell truncated Ag12 tetrahedron; outermost-shell doubly truncated Ag42 tetrahedron. e Enantiomers in a unit cell of Rac-Ag70. Atom colour codes: turquoise/dark blue/violet/green/orange, Ag; yellow, S; red, O; bright green, F; blue, N; grey, C. All hydrogen atoms are omitted for clarity.
From the innermost core outward (Supplementary Fig. 13), the 70-Ag atom NC, denoted Ag4@Ag12@Ag12@Ag6@Ag24@Ag12, features a tetrahedron of four Ag atoms (an idealized Platonic solid), a truncated tetrahedron of twelve Ag atoms (an idealized Archimedean solid), a truncated tetrahedron of 12 Ag atoms (an Archimedean solid), an octahedron of six Ag atoms (an idealized Platonic solid), a truncated octahedron of 24 Ag atoms (an Archimedean solid), and a doubly truncated tetrahedron of twelve Ag atoms, with each of the vertices occupied by a Ag atom. This 70-Ag atom aggregate possesses tetrahedral topology12,21,22,33–35, which incorporates S2−-passivated FCC-based Ag16 inner core (Ag−μ3-S bond lengths: 2.469(5)−2.489(6) Å; Supplementary Fig. 8). This kernel may provide a valuable clue for a deep insight into the nucleation and evolution of FCC-based Ag NCs and silver bulk materials. From single ion to three-layer FCC close-packing (Ag+→Ag6→Ag16, Supplementary Fig. 14), the regular aggregation states of small Ag species captured by four S2− anion-templates may represent the early-growth stage of Ag bulk or Ag nanoparticles with FCC close-packing. Based on the ideal tetrahedron growth pattern, the next larger member of this family should have a Ag31S4 core with FCC four-layer close-packing (Supplementary Fig. 14).
The 24 μ4-SiPr− ligands are evenly distributed and anchored on the surface of the cluster with Ag−S bond lengths in the range of 2.432(8)−2.616(5) Å. These organic components can be divided into two groups according to tetrahedral arrangement characteristics (Supplementary Fig. 15). The 20 CF3COO− ligands, which are located on the rectangular or triangular faces of the double truncated tetrahedron protect the Ag70 in μ2-η1,η1 or μ1−η1,η1 coordination mode (Ag−O bond lengths: 2.29(2)−2.44(4) Å, Fig. 2e and Supplementary Fig. 13a). Three DMF molecules are located at a Ag3 vertex of polyhedra (Ag−O bond lengths: 2.35(2) Å). In addition, it was found that there are multiple C − H···F hydrogen bonds between CF3COO− and –CH3 groups of SiPr− and DMF ligands (Supplementary Fig. 16), suggesting the strong interactions between the clusters.
Careful analysis of the geometric structure of an individual cluster in Rac-Ag70 indicated that it lacks a mirror plane (σ) and inversion (i). Meanwhile, breakage of Td symmetry occurred due to distortion of the surface Ag−S motifs3, although the rotation angle along the pseudo-C3 axes is slight (Supplementary Fig. 17). Notably, the inner kernel (Ag4 (core) and Ag12S4 (1st shell), Supplementary Fig. 18) presents achiral structural characteristics due to the existence of the three mirror planes. This slight change based on surface motifs could be an important cause of large chiroptical responses of inorganic nanoparticles, which will be further verified by homochiral single crystals. As a result, left- and right-handed enantiomers coexist in the unit cell of Rac-Ag70 (Fig. 2e and Supplementary Fig. 17).
Optical properties and stability of Rac-Ag70 in solution
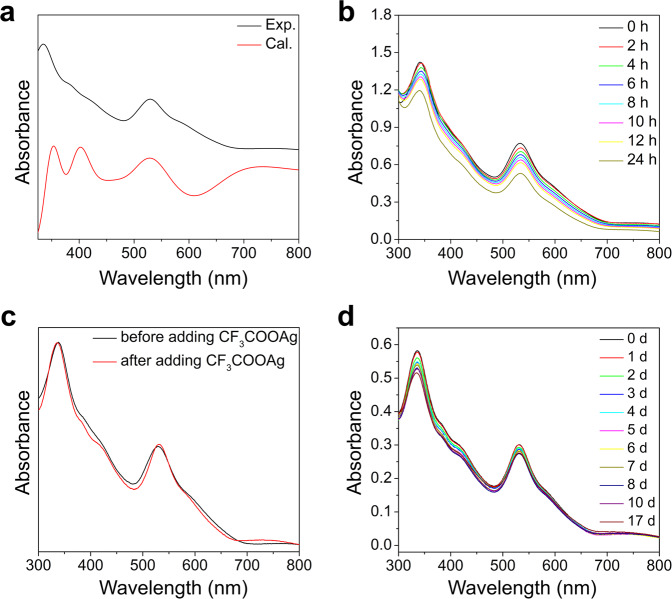
The UV-Vis-NIR absorption spectrum of the Rac-Ag70 solution exhibits multiple molecular-like peaks: two sharp peaks (335 nm, 530 nm), one broad peak (ca. 767 nm), some shoulder peaks (ca. 386 nm, 426 nm, and 595 nm) and the lowest absorption peak at ∼1450 nm (0.855 eV) (Fig. 3a and Supplementary Figs. 20–21). The main peaks that appear in the UV-Vis absorption spectrum of Rac-Ag70 (Fig. 3a) are well reproduced in the theoretically simulated spectrum (the calculations are described in the Methods section and Supplementary Information), further confirming the structure and the number of valence electrons of this NC7,13. The efficient transitions of the low-energy absorption peaks mainly involve metal-based transitions (Supplementary Figs. 22–26), and partial ligands (CF3COO−) contribute to the high-energy peaks at 353 nm (Supplementary Fig. 25). The solution of Rac-Ag70 shows broad emission peaks centred at 1300 nm in the NIR-II region (Supplementary Fig. 27), which may arise from core-based transitions with low energy14,36.
Fig. 3. UV-Vis absorption spectra of Rac-Ag70.
a Experimental (black) and calculated (red) absorption spectra of Rac-Ag70 in EtOH. b Time-dependent UV-Vis absorption spectra of Rac-Ag70 in EtOH (ca. 5 × 10−6 M) under ambient conditions. c UV-Vis absorption spectra of the EtOH solution of Rac-Ag70 before and after adding silver trifluoroacetate (250 eq.). d Time-dependent UV-Vis absorption spectra of Rac-Ag70 in EtOH together with silver trifluoroacetate (250 eq.).
Furthermore, ESI-MS was used to investigate the composition, charge state, and solution behaviour of Rac-Ag70 in detail (Supplementary Figs. 28–29). Crystals of Rac-Ag70 were dissolved in EtOH and measured in negative-ion mode with varied declustering potential and collision energy, showing three main grouped peaks in the mass-to-charge ratio (m/z) ranges of 3500–4000 (−3 charge state), 4200–4600 (−2 charge state), and 5500–6100 (−2 charge state). As shown in Supplementary Fig. 29, the peaks (Rac-Ag70: 2 h − 2 l) corresponding to the complete cluster skeleton (Ag70S4) are easy to find, confirming that the entire cluster molecule can stably exist in solution. The correlations between the species mainly involve the same Ag70S4 skeleton and the exchange of molecules outside the shell (SiPr− and CF3COO−), in which the weakly bound CF3COO− molecules are easily removed from the surface of the cluster under the prevailing ionization conditions. When a collision energy is applied to the system (from 0 to −15 V), fragment peaks 1a − 1j appear, and their intensity gradually increases; these peaks may be ascribed to some large fragments (Ag(48−51)S4, Supplementary Fig. 29b and Supplementary Table 2), suggesting that Rac-Ag70 is unstable in solution, consistent with the results of time-dependent UV-Vis (Fig. 3b). Meanwhile, the presence of peaks 2a − 2c (Ag68S4), and 2d − 2 g (Ag69S4) indicates that the dissociation of Rac-Ag70 starts with the loss of CF3COOAg small molecules step-by-step. The appearance of the peak corresponding to the Ag12 cluster ([Ag12(SiPr)6(CF3COO)7]−, Supplementary Fig. 29d) indicates that this stable small cluster formed immediately after Rac-Ag70 decomposition.
Inspired by the relatively strong interactions between the anionic cluster and the small molecules (CF3COOAg), as revealed by ESI − MS (2 m − 2t, Supplementary Fig. 29c and Supplementary Table 2), we proposed that CF3COOAg could stabilize the Rac-Ag70 cluster and that dissociation-coordination equilibrium could occur between the anionic NC and CF3COOAg. This speculation was confirmed by time-dependent UV-Vis absorption spectra and ESI-MS experiments. When 250 equivalents of CF3COOAg were added to the solution of Rac-Ag70, Ag70 remained stable for dozens of days (Fig. 3c–d). As depicted in Supplementary Fig. 29h–i, supramolecular assembly was carried out in a solution of Rac-Ag70 after the introduction of 250 eq. of CF3COOAg to form a more stable hybrid product, Ag70·(1–4)CF3COOAg (Supplementary Table 3), which completely inhibited the removal of CF3COO− and the dissociation of clusters. When C2F5COOAg was used, we obtained the same stability trend of Rac-Ag70 in solution (Supplementary Fig. 30 and Supplementary Tables 4–5). This direct and sufficient evidence suggested that stable and rapid dissociation-coordination equilibrium occurred between the anionic NC and the small molecules (RCOOAg) in the solution. Based on the above experimental data, a reasonable stability mechanism in solution is proposed. For the solution of Rac-Ag70, NCs decompose and form small molecules (such as CF3COOAg) and fragments (such as Ag12 clusters) until equilibrium is reached. Note that when the concentration of Rac-Ag70 is too low, the NCs will be completely destroyed. If there is a large number of small molecules in the Rac-Ag70 solution, then the decomposition of the NCs will be inhibited, and some stable products (Ag70·(1–4)CF3COOAg) will be formed. Note that excessive RCOOAg (more than 500 eq.) is detrimental to these NCs.
Deracemization of Rac-Ag70 solution
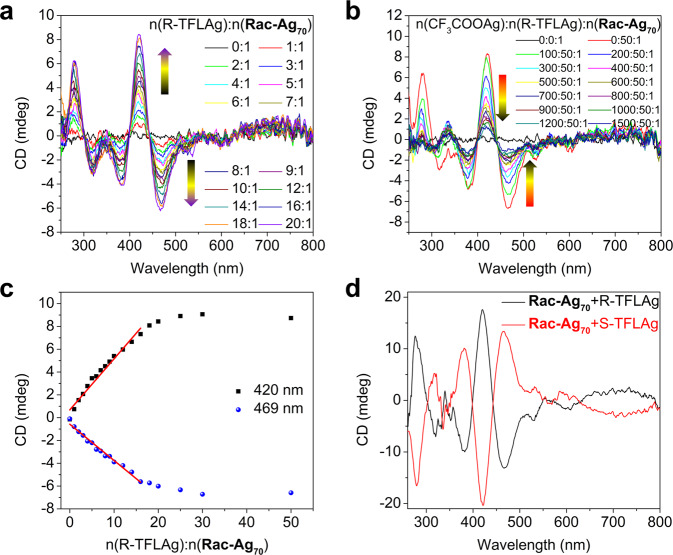
Considering that each cluster in Rac-Ag70 is slightly distorted and thereafter chiral and that RCOOAg not only stabilized the NC in solution but also replaced the auxiliary CF3COO− ligands on the constant metal framework of Ag70, chiral trifluorolactic acid (HTFL) was used to deracemize Rac-Ag70. The as-prepared solution of Rac-Ag70 was treated with different amounts of silver R-/S-trifluorolactate (denoted R-/S-TFLAg) in EtOH. The resulting mixture was monitored by UV-Vis, CD, ESI − MS and 19F-NMR spectra (Fig. 4 and Supplementary Figs. 31–40). As shown in Fig. 4a and Supplementary Figs. 31–32, the as-prepared solution of Rac-Ag70 is CD silent. With the continuous introduction of R-/S-TFLAg, Cotton effects of the solution appear (with weak peaks at 320, 530, 600, and 700 nm (broad) and strong peaks at 280, 383, 420, and 469 nm) (Fig. 4a, d). The Cotton effects and dissymmetry factor gabs (Supplementary Fig. 33) gradually increase with an increasing ratio of n(R-/S-TFLAg):n(Rac-Ag70), while the UV-Vis absorption behaviour is basically unchanged (Supplementary Figs. 31–32). Note that R-/S-TFLAg in EtOH exhibits only CD optical activity below 250 nm (Supplementary Fig. 34).
Fig. 4. Deracemization and Cotton effect amplification of Rac-Ag70 in solution.
a CD titration of Rac-Ag70 using a chiral metal precursor (R-TFLAg) in EtOH. b Back CD titration of deracemization solution using an achiral metal precursor (CF3COOAg) in EtOH. c Cotton effects at 420 nm and 469 nm plotted vs. the concentration ratio of R-TFLAg and Rac-Ag70. d CD spectra of deracemization of Rac-Ag70 solution.
The corresponding ESI-MS spectrum (Supplementary Fig. 35 and Supplementary Tables 6–7) shows the grouped peaks assigned to {Ag70S4(SiPr)24(TFL/CF3COO)21}3−·(0–3)TFLAg and {Ag70S4(SiPr)24(TFL/CF3COO)20}2−·(0–4)TFLAg, suggesting that the increase of Cotton effects is positively associated with gradual ligand exchange (CF3COO−→TFL−). In addition, the corresponding UV-Vis absorption does not change within a certain period of time (Supplementary Fig. 31d), indicating that the main metal skeleton of the cluster remains unchanged, and dynamic equilibrium and long-term stability of the system are achieved. Interestingly, in the range of n(R-/S-TFLAg):n(Rac-Ag70) = 0–15, the Cotton effects are almost linearly enhanced, while beyond the ratio of 15, these effects are basically unchanged (Fig. 4c and Supplementary Fig. 32c). The amplification of chiral signals may stem from the fact that the achiral ligands (CF3COO−) are continuously replaced by chiral ligands (TFL−) on the cluster surface, and the symmetry breaking of the whole structure is gradually amplified. A reversible decrease in Cotton effects is recovered by using CF3COOAg titration (Fig. 4b). In addition, the reversible CD titration does not change the nature of the cluster (Supplementary Figs. 31–32, Supplementary Figs. 36–37 and Supplementary Tables 8–9), which could find applications in chiral sensing fields.
Cocrystals of Ag70·Ag12 evidencing the proposed stability mechanism
Aiming to further verify the above-proposed stability mechanism in solution, we attempted to prepare single crystals that contained an anionic Ag70 cluster and a small Ag(I)12 cluster ({Ag12(SiPr)6(CF3COO)6}) as a stabilizer, which was named Ag70·Ag12 (Supplementary Figs. 41–57)37–43. The appearance of Ag(I)12 cluster could be attributed to the remainder of the unreduced silver(I) fragments in the EtOH-DMF system. The smaller Ag(I)12 cluster possesses a slightly distorted cuboctahedral metal framework (Ag···Ag distances: 3.06(1)–3.09(2) Å, Supplementary Figs. 47–49). The six μ4-SiPr− ligands are anchored on the quadrilateral surface of Ag(I)12 with Ag−S bond lengths in the range of 2.44(4)–2.50(4) Å, and the CF3COO− ligands on the edges in μ2−η1, η1 coordination mode (Ag−O bond lengths: 2.29(2)–2.44(4) Å).
Cocrystallization with Ag(I)12 leads to a more regular stacking of Ag70 (Supplementary Figs. 50–51); thus, Ag70·Ag12 crystallizes in a higher-symmetry space group (Fdm, number 227) compared to Rac-Ag70 (Pc1, number 165). More interestingly, the 70-nuclearity Ag NC in Ag70·Ag12 exhibits nearly perfect Td symmetry (Supplementary Fig. 47b), and its polyhedral skeleton is similar to that in Rac-Ag70, except that the slight symmetry breaking leads to different Ag···Ag distances (Supplementary Figs. 48–49 and Supplementary Table 1). Therefore, the small Ag(I)12 cluster not only stabilizes Ag70 but also changes the crystal packing, which probably weakens the intercluster strain in Rac-Ag70 and results in Td-symmetric Ag70 in Ag70·Ag12.
Ag70·Ag12 exhibits UV-Vis-NIR absorption (Supplementary Figs. 52–53), NIR-II photoluminescence emission (Supplementary Fig. 27) and ESI-MS results (Supplementary Figs. 54–55 and Supplementary Table 10) similar to those of Rac-Ag70. The Ag(I)12 cluster truly stabilizes Ag70 in solution: Ag70·Ag12 remains stable in EtOH for more than 7 days, as revealed by time-dependent UV-Vis absorption spectra (Supplementary Fig. 56); Ag70·Ag12 is also more thermally stable than Rac-Ag70 in solution (Supplementary Fig. 57).
Single crystal of chiral Ag70 verifying the proposed deracemization mechanism
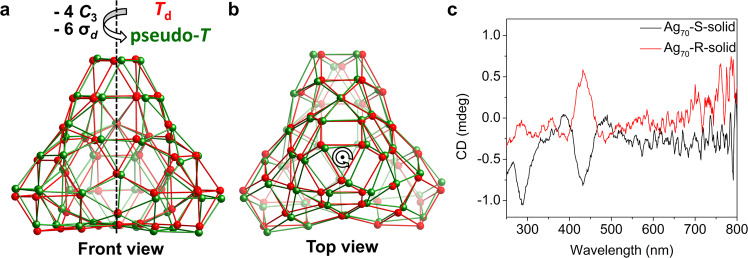
We subsequently attempted to prepare single crystals of homochiral Ag70. This is considerably difficult because homochiral high-nuclearity NCs remain an open challenge9,13,16. After continuous attempts, we obtained enantiomeric R/S-Ag70 crystals (Supplementary Fig. 58) in chiral space group P21 in the presence of R/S-TFLAg. The main composition of R/S-Ag70 was determined as {Ag70S4(SiPr)24(CF3COO)12(R/S-TFL)8}2− based on SCXRD, ESI-MS (Supplementary Figs. 35–37) and 19F-NMR (Supplementary Fig. 59). Although the peripheral ligands of R-Ag70 could not be positioned, its Ag−S skeleton was well resolved (see the Methods for the refinement of the structure of R-Ag70). Structural analysis indicates that the pseudo-T-symmetric metal skeleton of R-Ag70 (Fig. 5a–b) is more severely distorted than that of Rac-Ag70 (Supplementary Figs. 60–63 and Supplementary Table 1). Therefore, the absence of mirror planes in the Ag70 cluster molecule leads to the obvious shell-based and metal kernel-based Cotton effects of R-Ag70 and S-Ag70 crystals (Fig. 5c and Supplementary Fig. 64). The CD spectra of R-Ag70 and S-Ag70 are consistent with those of the above deracemized Rac-Ag70 solution with R/S-TFLAg, verifying the small symmetry breaking related to the considerable Cotton effects. Combining the single-crystal structure analysis, and the nearly identical CD signals of solution and solid state, we propose that the incoming TFL− (chiral organic ligand) induces the structural deformation, which plays a critical role in chiroptical response and the deracemization process. In addition, TFLAg (chiral complex), which was used to trigger the reaction in our experiment, is found to be the concomitants of the silver cluster in ESI-MS (Supplementary Figs. 35–37 and Supplementary Tables 6–9), and also plays a role in stabilizing the clusters (Supplementary Fig. 31d and Supplementary Fig. 40).
Fig. 5. Symmetry breaking of the Td-symmetric shell of Ag70 and solid-state CD spectra.
a, b Compared with the Td M54 doubly truncated tetrahedron (red), an illustration of the symmetry breaking in the Ag54 shell (green) of R-Ag70. c CD spectra of R/S-Ag70 crystallites.
Discussion
In summary, we prepared a medium-sized silver NC racemate (Rac-Ag70) through acid reduction synthesis, in which each NC featured the largest doubly truncated tetrahedron with pseudo-T symmetry. The dissociation-coordination mechanism between ligands and the silver framework enables stabilization of Ag70 by cocrystallization with Ag(I)12 clusters, deracemization of Rac-Ag70 in solution, and achievement of enantiomeric crystals of R/S-Ag70 through chiral metal precursors. SCXRD analysis revealed that small symmetry breaking from Td symmetry is responsible for the large chiroptical response of chiral clusters. This work provides not only an insight into the stabilization mechanism of high-nuclearity metal clusters and symmetry breaking related to the chiroptical response but also a significant case for the exploration of growth of truncated tetrahedron-shaped noble-metal NCs.
Methods
Synthesis of Rac-Ag70
{Ag(SiPr)}n (0.05 mmol, 9 mg) and CF3COOAg (0.10 mmol, 22.1 mg) were dissolved together in a mixed solvent of iPrOH and DMF (5.0 mL, v:v = 7:3), and then, 50 μL of CF3COOH was added to the above solution. The mixture was sealed in a 15 mL Teflon-lined reaction vessel and kept at 80 °C for 24 h. After cooling to room temperature, the black-red solution was filtered and evaporated in the dark for 1 week. Black needle crystals of Rac-Ag70 were isolated and washed with dichloride/n-hexane at a yield of 35% (based on {Ag(SiPr)}n). Elemental analysis (found (calcd), %; based on C137H233O47Ag70N9S28F60): C, 13.67 (13.33); H, 1.94 (1.90); N, 0.85 (1.02).
Synthesis of Ag70·Ag12
{Ag(SiPr)}n (0.05 mmol, 9 mg) and CF3COOAg (0.10 mmol, 22.1 mg) were dissolved together in a mixed solvent of EtOH and DMF (5.0 mL, v:v = 7:3), and then, 50 μL of CF3COOH was added to the above solution. The mixture was sealed in a 15 mL Teflon-lined reaction vessel and kept at 80 °C for 24 h. After cooling to room temperature, the black-red solution was filtered and evaporated in the dark for 1 week. Black octahedral block crystals of Ag70·Ag12 ({NH2(CH3)2}2{Ag70S4(SiPr)24(CF3COO)20(DMF)x}·{Ag12(SiPr)6 (CF3COO)6}·(DMF)12−x) were isolated and washed with dichloride/n-hexane at a yield of 20% (based on {Ag(SiPr)}n). Elemental analysis (found (calcd), %; based on C188H324O66Ag82N16S34F78): C, 14.49 (14.44); H, 1.99 (2.06); N, 0.98 (1.29).
Synthesis of R/S-Ag70
Chiral R/S-Ag70 was synthesized by a ligand-exchange method from Ag70·Ag12 NCs. Herein, the synthetic method for {Ag70S4(SiPr)24(CF3COO)20−n(R-TFL)n}2− (R-Ag70, n = 8 based on 19F-NMR (Supplementary Fig. 59)) is used as an example. Three milligrams of Ag70·Ag12 were dissolved in 2 mL DMF. One milligram of R-TFLAg was added to the above solution under stirring and stirred for 2 min. The black-red solution was filtered and evaporated in the dark for 1 week. The black block crystals of R-Ag70 were isolated and washed with dichloride/n-hexane (yield: 70 %).
Crystallographic data collection and refinement of the structure
SCXRD measurements of Rac-Ag70, Ag70·Ag12 and R-Ag70 were performed at 200 K on a Rigaku XtaLAB Pro diffractometer with Cu-Kα radiation (λ = 1.54184 Å). Data collection and reduction were performed using the program CrysAlisPro44,45. All structures were solved with direct methods (SHELXS)46 and refined by full-matrix least squares on F2 using OLEX247, which utilizes the SHELXL-2018/3 module48. All non-hydrogen atoms were refined anisotropically, and hydrogen atoms were placed in calculated positions with idealized geometries and assigned fixed isotropic displacement parameters. Appropriate restraints and/or constraints were applied to the geometry, and the atomic displacement parameters of the atoms in the clusters were determined. The absence of {NH2(CH3)2}+, CF3COO−, and DMF molecules in the SCXRD data of Ag70·Ag12 could be caused by weak diffraction, high symmetry, and a highly disordered state in the lattice.
Due to the extremely large unit cell and high disorder on the ligands, it is not very easy to solve the structure of the whole cluster for R-Ag70, particularly for the organic ligands. Even, to be honest the space group cannot be confidently confirmed from the X-ray diffraction data at 100 K. According to the results from XPREP, other than the suggested “A” lattice by default, primitive lattice should be the more suitable one since there are in total 15139 exceptions of I > 3σ and the mean intensity is 10.2 with the man I/σ of 1.9. The exceptions for other lattices show similar total reflection number, and only slightly high mean intensity (21.4–27.7) and mean I/σ (2.4–2.6). The high statistic mean |E*E−1| value (1.019 in this case) normally points to the centrosymmetry. However, a large number of reflection exceptions tend to suggest the absence of any glide planes (a, n, c) in the structure. In detail, comparing with that only 30 weak reflections (Imean = 1.1, I/σ = 0.5) violate the 21 axis, 1835 reflections where 86 have intensity stronger than 3σ don’t support the existence of c glide. The exceptions for a and n symmetries show similar total number as that for c (1835 for a and 1843 for n) (Supplementary Fig. 65). Therefore, the possible space group might be P21. The confusing part is that much more strong (I/3σ) reflections violate the a and c plane, which suggests the P21/c as an alternative. Considering the ligand that we used to synthesize the cluster is chiral with analytical pure (97%) and the relatively high yield (70%) of the single-crystal product, the correct space group of R-Ag70 should be P21. Finally, we solved the structure in P21 space group. Due to the bad disorder on the organic ligands, only the Ag70S28 core can be assigned and freely refined with anisotropic displacement parameters. With this incomplete model, a Flack parameter of 0.42(6) was obtained, indicating the twinning. However, the apparently high Flack parameter is also possibly caused by the lack of the organic part of the structure, particularly the chiral ligand.
Similar cell parameters (a = 37.478(3) Å, b = 33.6617(11) Å, c = 23.8119(11) Å, α = 90°, β = 90.938(6)°, γ = 90°) were also collected for S-Ag70 through the SCXRD. Unfortunately, we didn’t obtain the single crystals of S-Ag70 with enough quality.
Detailed information about the X-ray crystal data, intensity collection procedure, and refinement results for Rac-Ag70, Ag70·Ag12 and R-Ag70 are summarized in Supplementary Table 11.
Quantum chemical calculations
The ground state of the metal cluster for Ag70 was optimized by the semiempirical tight binding method (GFN-xTB) with the GBSA model for methanol49, and all excitations up to 6 eV were calculated with the simplified Tamm-Dancoff approach (sTDA)50–53. The molecular orbitals were extracted from Molden format by Multiwfn54 and then rendered and virtualized by the VMD program55.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 92061201, 21825106, U21A20277, and 21975065) and Zhengzhou University. We thank Prof. Stefan Grimme and Dr. Marc de Wergifosse for their helpful comments on the part of computational methods, and thank for the support of parallel high performance computing of National Supercomputing Center in Zhengzhou.
Author contributions
S.Q.Z. conceived and designed the experiments. X.M.L., C.H.G., and J.W.Y. conducted the synthesis and characterization. X.M.L. drew pictures in the manuscript. S.Q.Z., X.Y.D., and X.M.L. analyzed the experimental results. X.M.L. and F.P. completed crystallographic data collection and refinement of the structure. Y.S. performed the calculations. M.A. helped to revise the writings. X.M.L., X.Y.D., S.Q.Z., and T.C.W.M. co-wrote the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
Data supporting the findings of this manuscript are available from the corresponding authors upon request. The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC: 2072308 (Rac-Ag70), 2072309 (Ag70·Ag12), and 2104598 (R-Ag70).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xi-Ming Luo, Chun-Hua Gong.
Contributor Information
Xi-Yan Dong, Email: dongxiyan0720@hpu.edu.cn.
Shuang-Quan Zang, Email: zangsqzg@zzu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-28893-6.
References
- 1.Ma W, et al. Chiral inorganic nanostructures. Chem. Rev. 2017;117:8041–8093. doi: 10.1021/acs.chemrev.6b00755. [DOI] [PubMed] [Google Scholar]
- 2.Huang J-H, Wang Z-Y, Zang S-Q, Mak TCW. Spontaneous resolution of chiral multi-thiolate-protected Ag30 nanoclusters. ACS Cent. Sci. 2020;6:1971–1976. doi: 10.1021/acscentsci.0c01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Higaki T, Du X, Jin R. Chirality and surface bonding correlation in atomically precise metal nanoclusters. Adv. Mater. 2020;32:1905488. doi: 10.1002/adma.201905488. [DOI] [PubMed] [Google Scholar]
- 4.Zhang C, Li S, Dong X-Y, Zang S-Q. Circularly polarized luminescence of agglomerate emitters. Aggregate. 2021;2:e48. [Google Scholar]
- 5.Deng G, et al. From symmetry breaking to unraveling the origin of the chirality of ligated Au13Cu2 nanoclusters. Angew. Chem. Int. Ed. 2018;57:3421–3425. doi: 10.1002/anie.201800327. [DOI] [PubMed] [Google Scholar]
- 6.Deng G, et al. Enhanced surface ligands reactivity of metal clusters by bulky ligands for controlling optical and chiral properties. Angew. Chem. Int. Ed. 2021;60:12897–12903. doi: 10.1002/anie.202101141. [DOI] [PubMed] [Google Scholar]
- 7.Liu W-D, Wang J-Q, Yuan S-F, Chen X, Wang Q-M. Chiral superatomic nanoclusters Ag47 induced by the ligation of amino acids. Angew. Chem. Int. Ed. 2021;60:11430–11435. doi: 10.1002/anie.202100972. [DOI] [PubMed] [Google Scholar]
- 8.Shen H, et al. Tertiary chiral nanostructures from C−H⋅⋅⋅F directed assembly of chiroptical superatoms. Angew. Chem. Int. Ed. 2021;60:22411–22416. doi: 10.1002/anie.202108141. [DOI] [PubMed] [Google Scholar]
- 9.Chai J, et al. Chiral inversion and conservation of clusters: a case study of racemic Ag32Cu12 nanocluster. Inorg. Chem. 2021;60:9050–9056. doi: 10.1021/acs.inorgchem.1c01049. [DOI] [PubMed] [Google Scholar]
- 10.Deng G, Teo BK, Zheng N. Assembly of chiral cluster-based metal–organic frameworks and the chirality memory effect during their disassembly. J. Am. Chem. Soc. 2021;143:10214–10220. doi: 10.1021/jacs.1c03251. [DOI] [PubMed] [Google Scholar]
- 11.Huang J-H, et al. Symmetry breaking of atomically precise fullerene-like metal nanoclusters. J. Am. Chem. Soc. 2021;143:12439–12444. doi: 10.1021/jacs.1c05568. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, et al. Chalcogens-induced Ag6Z4@Ag36 (Z = S or Se) core-shell nanoclusters: enlarged tetrahedral core and homochiral crystallization. J. Am. Chem. Soc. 2019;141:17884–17890. doi: 10.1021/jacs.9b09460. [DOI] [PubMed] [Google Scholar]
- 13.Yan J, et al. Asymmetric synthesis of chiral bimetallic [Ag28Cu12(SR)24]4- nanoclusters via ion pairing. J. Am. Chem. Soc. 2016;138:12751–12754. doi: 10.1021/jacs.6b08100. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M-M, et al. Alkynyl-stabilized superatomic silver clusters showing circularly polarized luminescence. J. Am. Chem. Soc. 2021;143:6048–6053. doi: 10.1021/jacs.1c02098. [DOI] [PubMed] [Google Scholar]
- 15.Liang X-Q, et al. Revealing the chirality origin and homochirality crystallization of Ag14 nanocluster at the molecular level. Nat. Commun. 2021;12:4966. doi: 10.1038/s41467-021-25275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang H, et al. From racemic metal nanoparticles to optically pure enantiomers in one pot. J. Am. Chem. Soc. 2017;139:16113–16116. doi: 10.1021/jacs.7b10448. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Moshe A, Maoz BM, Govorov AO, Markovich G. Chirality and chiroptical effects in inorganic nanocrystal systems with plasmon and exciton resonances. Chem. Soc. Rev. 2013;42:7028–7041. doi: 10.1039/c3cs60139k. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu T, Ding W, Kameta N. Soft-matter nanotubes: a platform for diverse functions and applications. Chem. Rev. 2020;120:2347–2407. doi: 10.1021/acs.chemrev.9b00509. [DOI] [PubMed] [Google Scholar]
- 19.Yashima E, et al. Supramolecular helical systems: helical assemblies of small molecules, foldamers, and polymers with chiral amplification and their functions. Chem. Rev. 2016;116:13752–13990. doi: 10.1021/acs.chemrev.6b00354. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Hoffman JM, Kanatzidis MG. The 2D halide perovskite rulebook: how the spacer influences everything from the structure to optoelectronic device efficiency. Chem. Rev. 2021;121:2230–2291. doi: 10.1021/acs.chemrev.0c01006. [DOI] [PubMed] [Google Scholar]
- 21.Gao M-Y, et al. Tetrahedral geometry induction of stable Ag–Ti nanoclusters by flexible trifurcate TiL3 metalloligand. J. Am. Chem. Soc. 2020;142:12784–12790. doi: 10.1021/jacs.0c05199. [DOI] [PubMed] [Google Scholar]
- 22.Li XY, et al. A platonic solid templating Archimedean solid: an unprecedented nanometre-sized Ag37 cluster. Nanoscale. 2015;7:8284–8288. doi: 10.1039/c5nr01222h. [DOI] [PubMed] [Google Scholar]
- 23.Zeng C, et al. Gold tetrahedra coil up: Kekulé-like and double helical superstructures. Sci. Adv. 2015;1:e1500425. doi: 10.1126/sciadv.1500425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo X-M, Gong C-H, Dong X-Y, Zhang L, Zang S-Q. Evolution of all-carboxylate-protected superatomic Ag clusters confined in Ti-organic cages. Nano Res. 2021;14:2309–2313. [Google Scholar]
- 25.Liu KG, Gao XM, Liu T, Hu ML, Jiang DE. All-carboxylate-protected superatomic silver nanocluster with an unprecedented rhombohedral Ag8 core. J. Am. Chem. Soc. 2020;142:16905–16909. doi: 10.1021/jacs.0c06682. [DOI] [PubMed] [Google Scholar]
- 26.Liu JW, et al. Core modulation of 70-nuclei core-shell silver nanoclusters. Angew. Chem. Int. Ed. 2019;58:6276–6279. doi: 10.1002/anie.201900568. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, et al. Trapping an octahedral Ag6 kernel in a seven-fold symmetric Ag56 nanowheel. Nat. Commun. 2018;9:2094. doi: 10.1038/s41467-018-04499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pastoriza-Santos I, Liz-Marzán LM. Formation and stabilization of silver nanoparticles through reduction by N,N-dimethylformamide. Langmuir. 1999;15:948–951. [Google Scholar]
- 29.Pastoriza-Santos I, Liz-Marzán LM. Synthesis of silver nanoprisms in DMF. Nano Lett. 2002;2:903–905. [Google Scholar]
- 30.Knight WD, et al. Electronic shell structure and abundances of sodium clusters. Phys. Rev. Lett. 1984;52:2141–2143. [Google Scholar]
- 31.Reveles JU, Khanna SN, Roach PJ, Castleman AW. Multiple valence superatoms. Proc. Natl Acad. Sci. USA. 2006;103:18405. doi: 10.1073/pnas.0608781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walter M, et al. A unified view of ligand-protected gold clusters as superatom complexes. Proc. Natl Acad. Sci. USA. 2008;105:9157. doi: 10.1073/pnas.0801001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teo BK, Sloane NJA. Magic numbers in polygonal and polyhedral clusters. Inorg. Chem. 1985;24:4545–4558. [Google Scholar]
- 34.Kang X, et al. Rational construction of a library of M29 nanoclusters from monometallic to tetrametallic. Proc. Natl Acad. Sci. USA. 2019;116:18834–18840. doi: 10.1073/pnas.1912719116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang X, et al. The tetrahedral structure and luminescence properties of bi-metallic Pt1Ag28(SR)18(PPh3)4 nanocluster. Chem. Sci. 2017;8:2581–2587. doi: 10.1039/c6sc05104a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weerawardene KLDM, Aikens CM. Theoretical insights into the origin of photoluminescence of Au25(SR)18– nanoparticles. J. Am. Chem. Soc. 2016;138:11202–11210. doi: 10.1021/jacs.6b05293. [DOI] [PubMed] [Google Scholar]
- 37.Yan J, et al. Co-crystallization of atomically precise metal nanoparticles driven by magic atomic and electronic shells. Nat. Commun. 2018;9:3357. doi: 10.1038/s41467-018-05584-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bodiuzzaman M, et al. Camouflaging structural diversity: co-crystallization of two different nanoparticles having different cores but the same shell. Angew. Chem. Int. Ed. 2019;58:189–194. doi: 10.1002/anie.201809469. [DOI] [PubMed] [Google Scholar]
- 39.Dar WA, et al. Interparticle reactions between silver nanoclusters leading to product cocrystals by selective cocrystallization. ACS Nano. 2019;13:13365–13373. doi: 10.1021/acsnano.9b06740. [DOI] [PubMed] [Google Scholar]
- 40.He L, Gan Z, Xia N, Liao L, Wu Z. Alternating array stacking of Ag26Au and Ag24Au nanoclusters. Angew. Chem. Int. Ed. 2019;58:9897–9901. doi: 10.1002/anie.201900831. [DOI] [PubMed] [Google Scholar]
- 41.Kang X, Xu F, Wei X, Wang S, Zhu M. Valence self-regulation of sulfur in nanoclusters. Sci. Adv. 2019;5:eaax7863. doi: 10.1126/sciadv.aax7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu JY, et al. Different silver nanoparticles in one crystal: Ag210(iPrPhS)71(Ph3P)5Cl and Ag211(iPrPhS)71(Ph3P)6Cl. Angew. Chem. Int. Ed. 2019;58:195–199. doi: 10.1002/anie.201810772. [DOI] [PubMed] [Google Scholar]
- 43.Kang X, Zhu M. Cocrystallization of atomically precise nanoclusters. ACS Mater. Lett. 2020;2:1303–1314. [Google Scholar]
- 44.Rigaku, O. D. CrysAlisPro Software System, Version 1.171.38.41k. https://www.rigaku.com (Rigaku Coorporation, Oxford, 2015).
- 45.Agilent Technologies Inc. CrysAlisPro, Version 1.171.36.31. https://www.rigaku.com (Agilent Technologies Inc., Santa Clara, 2012).
- 46.Sheldrick G. A short history of SHELX. Acta Cryst. A. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 47.Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009;42:339–341. [Google Scholar]
- 48.Sheldrick G. Crystal structure refinement with SHELXL. Acta Cryst. C. 2015;71:3–8. doi: 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grimme S, Bannwarth C, Shushkov P. A robust and accurate tight-binding quantum chemical method for structures, vibrational frequencies, and noncovalent interactions of large molecular systems parametrized for all spd-block elements (Z = 1–86) J. Chem. Theory Comput. 2017;13:1989–2009. doi: 10.1021/acs.jctc.7b00118. [DOI] [PubMed] [Google Scholar]
- 50.de Wergifosse M, Seibert J, Grimme S. Simplified time-dependent density functional theory (sTD-DFT) for molecular optical rotation. J. Chem. Phys. 2020;153:084116. doi: 10.1063/5.0020543. [DOI] [PubMed] [Google Scholar]
- 51.Grimme S, Bannwarth C. Ultra-fast computation of electronic spectra for large systems by tight-binding based simplified Tamm-Dancoff approximation (sTDA-xTB) J. Chem. Phys. 2016;145:054103. doi: 10.1063/1.4959605. [DOI] [PubMed] [Google Scholar]
- 52.Bannwarth C, Grimme S. A simplified time-dependent density functional theory approach for electronic ultraviolet and circular dichroism spectra of very large molecules. Comput. Theor. Chem. 2014;1040−1041:45–53. [Google Scholar]
- 53.Grimme S. A simplified Tamm-Dancoff density functional approach for the electronic excitation spectra of very large molecules. J. Chem. Phys. 2013;138:244104. doi: 10.1063/1.4811331. [DOI] [PubMed] [Google Scholar]
- 54.Lu T, Chen F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012;33:580–592. doi: 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- 55.Humphrey W, Dalke A, Schulten K. VMD-Visual Molecular Dynamics. J. Molec. Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this manuscript are available from the corresponding authors upon request. The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC: 2072308 (Rac-Ag70), 2072309 (Ag70·Ag12), and 2104598 (R-Ag70).